Abstract
Bulbar dysfunction is common in Parkinson’s disease (PD) with more than 80% of affected individuals developing dysphagia during the course of the disease. Symptoms can begin in the preclinical stage and individuals may remain clinically asymptomatic for years into the disease course. Furthermore, patients are not always aware of swallowing changes, which may contribute to the difference between the prevalence of self-reported dysphagia and the findings during instrumental evaluations. Dysphagia is underrecognized and contributes to the development of aspiration pneumonia which is the leading cause of death in PD. Dysphagia in PD is complex and not completely understood as both dopaminergic and non-dopaminergic pathways seem to underpin this symptom.
Areas covered:
This comprehensive review will cover the epidemiology, pathophysiology, clinical evaluation, and the expert management of dysphagia and aspiration in patients with PD.
Expert Opinion:
A multidisciplinary team approach is important to properly identify and to manage PD dysphagia. Regular screenings using swallowing-specific questionnaires and a clinical bedside swallow evaluation (CSE) along with objective instrumental assessments using videofluoroscopic swallow study (VFSS) or fiberoptic endoscopic evaluation (FEES) are necessary for the detection of dysphagia. Future studies are needed to better understand the mechanism(s) involved in PD dysphagia, establish markers for early detection and progression, and develop evidence-based treatment options.
Keywords: aspiration, dysphagia, evaluation, management, Parkinson’s disease, pathophysiology, swallowing, treatment
1. Introduction
Parkinson’s disease (PD) is one of the most common neurological disorders and is characterized by motor symptoms including tremor, rigidity, bradykinesia, and postural instability. Dysphagia is a significant problem in PD and swallowing has been reported to affect morbidity and mortality (1). Current reports estimate that 30% to greater than 80% of patients with PD will develop dysphagia during the course of their disease (2,3). The development of dysphagia contributes to the risk of aspiration pneumonia, which is the leading cause of death in this population (4,5). If an aspiration event occurs, the risk of developing aspiration pneumonia depends on the type and volume of material aspirated, bacterial load of aspirated material, and the individual’s response to aspiration such as cough, mucociliary action of the lungs, and immune response (6). In addition to swallowing dysfunction, factors that increase the risk for aspiration pneumonia include a compromised immune system, respiratory dysfunction, poor oral hygiene, poor functional status, polypharmacy, and enteral feeding (7–10). Furthermore, dysphagia can have a significant impact on PD related quality of life specifically social interactions, fatigue, and the ability to maintain a reasonable body weight (11–15).
Often swallowing dysfunction goes unrecognized in early PD. Dysphagia can occur during any stage of the disease including preclinical or prodromal stages (16,17). Clinical predictors of PD dysphagia include Hoehn and Yahr greater than 3 (advanced PD), dementia, anterior spillage of food and/or liquid from oral cavity (i.e. drooling), weight loss, or a Body Mass Index of <20 kg/m2(18–21). Patients with PD can have impaired swallowing safety and efficiency across the 3 stages of swallowing: oral, pharyngeal, and esophageal; however the pathologic mechanisms remain somewhat unclear (22). Early detection, assessment, and management of dysphagia in patients with PD by a multidisciplinary team can improve swallowing safety and efficiency, quality of life, nutrition, and hydration (23–29).
1.1. Definition
The stages of swallowing include oral, pharyngeal, and esophageal. Oral phase is defined by mastication and tongue movements that propel the bolus to the pharynx. The pharyngeal phase consists of moving the bolus through the pharynx, which includes closure of the laryngeal vestibule and glottis, pharyngeal peristalsis, and relaxation of the upper esophageal sphincter. The esophageal phase includes contraction of the upper esophageal sphincter, esophageal peristalsis, and relaxation of the lower esophageal sphincter resulting in the transport of the bolus into the stomach (30).
Swallow safety describes how well an individual is able to protect their airway during the act of swallowing. Impaired swallow safety includes penetration and aspiration. Penetration is defined by material entering the airway above or to the level of the vocal folds. Aspiration occurs when material enters the airway below the level of the true vocal folds. Swallow efficiency describes the individual’s ability to move the material (i.e. bolus) from the oral cavity through the pharynx and esophagus. Impaired swallow efficiency includes residue within the oral cavity, pharynx, or esophagus.
1.2. Epidemiology
One meta-analysis reported a worldwide prevalence of PD that was increasing with age from 41 per 100,000 in subjects 40–49 years old to approximately 1900 per 100,000 in subjects over 80 years of age (31). Estimates have revealed that by the year 2030, approximately 9 million people globally will be diagnosed with PD (11). This has been referred to as a PD pandemic (32).
The reported prevalence of dysphagia in patients with PD varies widely. This is likely due to the mismatch between patient reported swallowing difficulties and findings during instrumental evaluations. Another meta-analysis revealed the prevalence of dysphagia based on patient reports was 35%, whereas in objective clinician rated measurements it increased to 82% (2). Pflug et al. (2018) found more than 50% of patients who did not report swallowing dysfunction were confirmed to have dysphagia using flexible endoscopic evaluation of swallowing (FEES) (33).
For most patients with PD, dysphagia is present during advanced stages, on average 10–15 years after symptom onset (21,34). The rate of clinical dysphagia increases after 15 years from the onset of PD symptoms (11). Aspiration pneumonia is the most common cause for hospitalization in patients with PD with a disease duration of more than 5 years (35). Additionally, the majority of these patients had abnormal videofluoroscopic swallow study/modified barium swallow study (VFSS/MBSS) findings, cognitive impairment, and a history of psychiatric symptoms (36). Predictors of patients with PD developing aspiration pneumonia include impaired mastication, poor lingual motility prior to transfer, aspiration, and increased total swallow time (37).
1.3. Neural control of swallowing
All phases of swallowing have distinct neural control mechanisms that include sensory and motor integration by cortical and subcortical structures (24). Swallowing is a complex behavior that involves a highly coordinated sensorimotor sequence consisting of the activation and suppression of more than 30 muscles bilaterally (38).
The oral phase of the swallow is considered predominately volitional, while pharyngeal and esophageal phases are involuntary. The cranial nerves involved include the trigeminal (V), facial (VII), glossopharyngeal (IX), vagus (X), accessory (XI), and hypoglossal (XII). Information is relayed to the brainstem, with afferent pathways to the bilateral prefrontal, frontal, and parietal cortices (39). The efferent pathways from the cortex to the brainstem motor nuclei are modulated by the cerebellum. The upper pharynx and the esophagus are controlled by the vagus nerve (40).
The central pattern generator for swallowing (CPGs) produces the coordinated sequential motor events required (41,42). CPGs areas exist bilaterally in the dorsomedial and ventrolateral medulla oblongata (43,44). In the dorsomedial medulla, the generator neurons involved in the triggering, shaping, and timing of the sequential or rhythmic swallow pattern reside within the nucleus tractus solitarii. In the ventrolateral medulla, the switching neurons are involved in distributing incoming sensory information regarding bolus size, taste, location, and viscosity to the appropriate motor nuclei, including the trigeminal nucleus, facial nucleus, nucleus ambiguous, and hypoglossal nucleus (45,46). There is also supramedullary modulation of the CPGs from cortical and subcortical areas (42,43).
2. Pathophysiology
The cardinal symptoms of PD include tremor, rigidity, bradykinesia, and postural instability which are associated with a gradual loss of dopaminergic neurons in the substantia nigra, and deposition of alpha-synuclein across many motor and non-motor circuitries (47). The pathophysiology of dysphagia in PD is not fully understood, however the disturbance of dopaminergic and non-dopaminergic mechanisms, brainstem dysfunction, and muscle atrophy are likely contributing factors (48). The specific circuitry within the basal ganglia systems has been elusive, but there is agreement that it must include more than the typical dopaminergic loss. Studies have shown basal ganglia dysfunction caused by degeneration of dopaminergic neurons in the substantia nigra likely plays an important role (11,49,50). Lewy bodies (alpha-synuclein aggregates) are deposited in various non-dopaminergic cortical areas and in the brainstem, including the CPGs in the medulla, which can impair the sequential swallowing pattern (21).
Chronic denervation and reinnervation secondary to neurodegeneration associated with PD can lead to atrophy of the muscles which are utilized during the pharyngeal phase of swallowing (51,52). Research has identified possible associated changes in the morphology of pharyngeal muscle fibers and changes in enzymatic activity (28,39). Alpha-synuclein in peripheral nerves innervating the pharyngeal muscles has been found in patients with PD exhibiting dysphagia (53). Bradykinesia and rigidity can contribute to delayed onset of the oropharyngeal phase of swallowing and impaired cricopharyngeal opening (54–56). There is increasing evidence that alpha-synuclein accumulation occurs outside of the central nervous system, such as the enteric nervous system, likely contributing to the observed esophageal impairments (57,58). It is unclear which changes are biological and which may account for swallowing dysfunction (59).
Patients with PD can experience difficulty during any or all stages of swallowing (Table 1). It is important to note that normal variation in swallowing and age-related swallowing changes (presbyphagia) exist and should not be labeled as dysphagia (60–64).
Table 1.
| Phase of swallowing | Observed impairments |
|---|---|
| Oral preparatory/transit | Impaired mandible movement |
| Jaw rigidity | |
| Xerostomia | |
| Sialorrhea | |
| Impaired lingual movement/pressure generation | |
| Tongue pumping/rocking | |
| Premature spillage/reduced posterior bolus containment | |
| Reduced spontaneous swallowing | |
| Piecemealed deglutition | |
| Oral residue | |
| Pharyngeal | Delayed swallow reflex |
| Decreased hyolaryngeal elevation | |
| Impaired laryngeal/pharyngeal movement due to anterocollis | |
| Impaired pharyngeal contraction | |
| Aspiration | |
| Diminished/absent cough response to aspiration | |
| Decreased cricopharyngeal opening | |
| Residue in the valleculae and pyriform sinuses | |
| Esophageal | Dysfunction of the upper esophageal sphincter |
| Impaired esophageal peristalsis/non-propulsive contractions | |
| Esophageal spasms Repetitive contractions |
3. Swallowing impairments in PD
3.1. Oral phase
Oral phase deficits include tongue pumping/rocking (a repetitive, rocking, non-propulsive movement of the tongue), impaired bolus formation resulting in the presence of oral residue, piecemeal deglutition, and/or anterior spillage due to reduced lip closure (65–67). Reduced tongue strength and pressure generation between the tongue and the palate have been found in subjects with PD compared to healthy controls (68). Subjects with PD who exhibit dysphagia on VFSS (aspiration and/or moderate amounts of residue in the oral cavity) were found to have reduced maximum tongue pressure, particularly at the anterior part of the tongue, compared to non-dysphagic PD subjects (69). However, Fukuoka et al. (2018) found no significant difference in maximum tongue pressures between PD subjects with and without dysphagia. Their study revealed PD subjects with dysphagia exhibited prolonged duration of tongue pressure, increased time to peak pressure, and reduced pressure gradient compared to the PD subjects without dysphagia (70).
3.2. Pharyngeal phase
Pharyngeal phase deficits include delayed initiation of the pharyngeal phase of swallowing, reduced hyolaryngeal excursion, reduced base of tongue retraction, reduced pharyngeal constriction (creating variable swallowing pressures), delayed airway closure, reduced duration of airway closure, and decreased pharyngeal/laryngeal sensitivity (16,71,72). Overall, these deficits can result in aspiration or pharyngeal residue. A recent retrospective analysis of patients with PD during VFSS revealed predictors of penetration and aspiration included reduced hyolaryngeal excursion, delayed initiation of the pharyngeal swallow, and increased volume of liquid bolus (73). Studies have reported diminished or absent cough behaviors in patients with PD, particularly in those with dysphagia, which poses a risk for uncompensated aspiration (i.e. material entering the airway with an inappropriate response to expel the material) (12,74,75). Discoordination of breathing and swallowing has also been found in patients with PD. Inhalation versus typical exhalation after swallowing may reduce swallow safety (76). Additionally, a recent study of 20 patients with PD revealed lower saliva Substance P concentrations in individuals with mild pharyngeal dysphagia compared to individuals without dysphagia (77). Substance P is a neuropeptide involved in the regulation of the response by the pharyngeal mucosa to stimuli (77–80).
3.3. Esophageal phase
Esophageal phase deficits include impaired peristalsis, incomplete bolus transit, repetitive contractions, and inconsistent findings of reduced opening duration and/or extent of the upper esophageal sphincter (UES) (25,28,81,82). Sung et al. (2010) completed pharyngoesophageal manometry on subjects with early-stage PD and found reduced UES resting pressure compared to age-matched healthy controls (16). Abnormal esophageal manometry results were found in 40.7% of subjects for liquids and 57.4% of subjects for viscous material (16). Suttrup et al. (2016) analyzed oropharyngeal dysphagia with FEES and esophageal swallowing using high-resolution manometry (HRM) in 65 subjects with PD across disease severity. The study revealed major esophageal disorders in nearly 1/3 of subjects and minor impairments in 95% of subjects. Impairments observed were esophageal dysmotility, and increased intrabolus pressure with advanced disease. No association between FEES and esophageal HRM were found (28).
4. Evaluation
During the initial stages of PD, dysphagia is frequently unrecognized. Use of subjective in addition to objective measures are required to accurately detect the presence of dysphagia. Screening for dysphagia may include swallowing-specific questionnaires, CSE, and/or water tests. A baseline instrumental evaluation is still recommended to assess oropharyngeal swallowing safety and efficiency, and to allow for a comparison of oropharyngeal swallow function as the disease progresses. Instrumental evaluations such as VFSS/MBSS and FEES are considered to be the gold standard for the evaluation of oropharyngeal dysphagia (11,33,83). Moreover, these studies provide vital information for the management of dysphagia by analyzing the effects of bolus volume, bolus consistency (liquids, pudding/puree, solids, and pills), swallow timing, response (i.e. cough, throat clearing), effectiveness of the response to penetration/aspiration, material and/or residue clearance, and impact of compensatory strategies.
4.1. Swallowing-specific questionnaires
Swallowing-specific questionnaires such as the Swallowing Quality of Life questionnaire (SWAL-QOL) or Eating Assessment Tool (EAT-10) can help identify patients at risk for swallowing dysfunction (84–87). The PILL-5 is a self-reported validated questionnaire for pill dysphagia (88). There are PD-specific swallowing questionnaires that are validated including the Swallowing Disturbance Questionnaire (SDQ) and Munich Dysphagia test- Parkinson’s disease (MDT-PD) (19,89). Buhmann et al. (2019) found the MDT-PD questionnaire did not reliably detect aspiration in patients with PD and concluded that the questionnaire should not be solely used as a screening tool for dysphagia (90).
4.2. Clinical bedside swallow evaluation (CSE)
A clinical bedside swallow evaluation (CSE) performed by a speech-language pathologist or logopedic can help identify patients who need additional testing. The CSE includes: review of past medical history, examination of cranial nerves and cognition, and signs of dysphagia during oral trials (including labial spillage, oral residue/pocketing, mastication efficiency, vocal quality, presence of cough, or throat clearing) (91–93). Additionally a modified water test can be performed to determine maximum swallow volume or maximum swallowing speed to identify individuals with PD dysphagia (11,34,94–98). Of note, the water test is not a validated tool, and may underestimate the severity of dysphagia.
4.3. Video fluoroscopic swallow study (VFSS)/modified barium swallow study (MBSS)
A VFSS/MBSS is completed by a speech-language pathologist and a physician (most commonly a radiologist) to assess the oral and pharyngeal phases of the swallow. It consists of a radiographic video taken place in the lateral view, at a minimum of 30 frames per second (99,100). The patient is seated in a chair and swallows varying consistencies of barium. In the lateral view, the following can be assessed: mastication, bolus containment, bolus formation, bolus transport, tongue pumping, oral residue, soft palate elevation/retraction, base of tongue retraction, hyolaryngeal excursion, epiglottic inversion, laryngeal vestibule closure, quantification of penetration or aspiration (timing, depth, estimated volume, patient response), pharyngeal contraction (also known as pharyngeal stripping wave), pharyngeal residue, and upper esophageal opening extent and duration (101). It also provides a view of the proximal esophagus to assess esophageal clearance (101). An anterior-posterior (A-P) view can be obtained to assess pharyngeal constriction symmetry, bolus flow, and scan for esophageal clearance in an upright position (101). Images captured from the A-P angle can help determine if additional evaluations by gastroenterology are necessary to assess the esophageal stage of the swallow.
4.4. Fiberoptic endoscopic evaluation of swallowing (FEES)
FEES is completed by trained physicians (ENTs, phoneticians, or neurologists) or speech-language pathologists to assess the pharyngeal phase of the swallow while the patient consumes varying consistencies of liquids and/or solids as well as pills (83). This evaluation is more invasive than VFSS and involves passing an endoscope through the nose into the oropharynx to visualize the base of the tongue, pharynx, larynx, and part of the trachea. It is taken from a superior (rostral to caudal) viewpoint. FEES can be completed at the bedside and can assess secretion management along with structural changes, which are not possible with VFSS. Additional advantages include the absence of radiation exposure, ease of frequent follow-up examination (if needed), flexibility with patient positioning, sensory testing (FEESST), visualization of vocal cord mobility, and biofeedback (102). During the FEES, a “white out” period due to the reflection of light from the endoscope against structures prohibits the visualization of anatomy and bolus flow during the height of the swallow. This could translate into missing penetration or aspiration.
Interpretation of the instrumental evaluations can be variable amongst professionals. The Penetration-Aspiration Scale (PAS) is an 8-point scale commonly used to quantify the severity of airway invasion during swallowing (103). Scales to quantify residue using VFSS include the Normalized Residue Ratio Scale (104), the Vallecular Residue Ratio Scale (105), and the Bolus Residue Scale (106). When evaluating with FEES, the Yale Pharyngeal Residue Severity Rating Scale can be utilized (107). Additionally the MBS Impairment tool (MBSImP™) developed by Martin-Harris and colleagues (108) is a standardized approach to evaluate VFSS by a trained speech-language pathologist who follows a protocol to rate seventeen components of swallowing physiology and bolus flow measures.
4.5. High Resolution Manometry (HRM)
HRM can be used to assess pharyngeal or esophageal contraction in patients with PD (28,52,82). During this procedure a small catheter is passed through the nose into the pharynx and esophagus in order to measure pressures generated along the pathway.
4.6. Other swallowing evaluation modalities
Other modalities have been utilized in research studies to assess dysphagia including surface EMG or ultrasonography (US) (109–112) as well as previously mentioned tongue strength assessments (68,70).
5. Management of dysphagia in individuals with PD
A multidisciplinary team approach for the evaluation and management of dysphagia to prevent significant consequences of dysphagia such as malnutrition, dehydration, aspiration pneumonia, or choking, which could result in death, is critical for optimal care in PD (113). Weight monitoring and nutritional evaluations should be implemented early to reduce the likelihood of malnutrition and dehydration. Also, given the occurrence of subclinical dysphagia and silent aspiration, it is important to counsel patients on signs and symptoms of swallowing difficulties. Dysphagia management varies based on the underlying deficits noted during the instrumental evaluation. Treatment approaches to improve swallow safety and efficiency can be divided into compensatory behavioral strategies, dietary modifications, or rehabilitative exercises.
5.1. Compensatory behavioral strategies
Compensatory strategies to improve swallow safety and efficiency are prescribed by a speech-language pathologist or logopedic. Examples include: smaller bites/sips, larger sips, alternating bites and sips, pacing, varying head positions, swallowing twice per bite/sip, or pills taken with pureed consistency (ie: apple sauce or pudding) (48,114–116). For example, smaller sips may be used to reduce aspiration while larger sips can increase sensory input to improve oral and pharyngeal swallow initiation, increase pharyngeal pressure generation, and/or provide weight to assist with passive inversion of the epiglottis to help with laryngeal vestibule closure (73,117–120). Additionally, impulsive feeding exhibited by some individuals with PD can result in food collection in the oral cavity, increasing the risk of aspiration and choking (114). Reducing distractions and dual-tasking for patients with cognitive and attention deficits during meals can increase swallow safety (121). A speech-language pathologist may suggest pacing strategies or adaptive equipment to limit the size of each bite or sip. Timing meals, in the context of achieving the maximal benefit of levodopa therapy, could facilitate improved upper extremity control for feeding during mealtime (114,122).
5.2. Dietary modifications
Results from instrumental evaluations are used to determine the least restrictive, but safest diet for patients with PD. Dietary modifications may include changing the viscosity of liquids by using an artificial thickener, chopped, or pureed food (73,120,123). Foods of high viscosity may be difficult for patients with reduced tongue base retraction and pharyngeal constriction to efficiently swallow (114,119). There is also evidence that increasing sensory input with sour or cold foods could help trigger the oral and pharyngeal swallow (119,124–127). Patients given a modified diet should receive skilled speech-language therapy in an attempt to rehabilitate their swallow function and upgrade their diet. Diet modifications can have a significant impact on a patient’s quality of life, thus repeated instrumental evaluations are encouraged to evaluate for an improvement in swallowing that safely allows for a diet upgrade.
The Frazier Free Water Protocol may be appropriate for patients on modified diets identified to be at risk for aspirating thin liquids (128,129). This protocol allows patients to consume water in a structured manner, and is associated with improvements in fluid intake, oral health, quality of life, compliance with dietary restrictions for dysphagia management and xerostomia.
In advanced PD, a feeding tube may be considered for short or long-term use if a patient demonstrates an unsafe swallow, inability to consume pills or maintain adequate hydration or nutrition despite using available techniques. As part of providing patient-centered care, patients and their families should be educated regarding the risk of unsafe swallowing and nutrition/hydration, along with the risks/benefits of a feeding tube. A feeding tube may reduce, but does not eliminate, the risk of aspiration or aspiration pneumonia, as aspiration can still occur due to reflux or saliva (130–134). At times, patients with severe dysphagia choose to consume liquids or solids by mouth for pleasure. These patients can receive their vital nutrition and hydration by an enteral tube. Ultimately, the decision made by the patient or their healthcare proxy should be documented and their wishes should be followed as they have the right to refuse life-sustaining treatment (135). In this case, treatment will include decreasing aspiration risk as much as possible, patient comfort, and quality of life.
5.3. Rehabilitative strategies
Many patients with PD dysphagia will benefit from rehabilitative techniques including exercises prescribed to strengthen the expiratory muscles (136–138). Troche and colleagues (2010) have shown Level 1 evidence supporting the use of expiratory muscle strength training (EMST) to improve airway protection in PD (139). The application of EMST consists of a forceful exhale into a pressure threshold device which opens a valve depending on the pressure generated (140). The device maintains a constant pressure load against exhalation, and this improves respiratory muscle strength (141). The training protocol involves completing 5 sets of 5 successful repetitions, 5 days/week for at least 4 weeks (139). The device is periodically reset as the patient gets stronger. EMST at 75% of a participant’s average maximum expiratory pressure was found to improve hyolaryngeal function, PAS during VFSS, and voluntary cough effectiveness (139,142). Wheeler et al. (2008) found EMST may strengthen the suprahyoid muscles using surface EMG. These muscles aid in hyolaryngeal elevation and laryngeal vestibule closure for improved airway protection (143).
The Lee Silverman Voice Treatment (LSVT®) is an exercise based behavioral treatment program for speech symptoms of PD (e.g. hypokinetic dysarthria) (144–146). While LSVT® is mainly used to target speech, research has shown positive benefits on swallowing for a subset of patients (147,148). Its effects on swallowing include improvement in tongue base function and bolus control during the oropharyngeal swallow, along with reduced oral transit time and oral residue, and increase upper esophageal sphincter opening extent and duration (147,148).
Additional exercises may include effortful swallow, Mendelsohn maneuver, or falsetto exercises. For an effortful swallow, patients are encouraged to squeeze hard with their muscles when swallowing, promoting posterior tongue movement for bolus clearance. This exercise can be combined with surface EMG to provide biofeedback (149,150). Adequate effortful swallow exercises have demonstrated increased duration of hyolaryngeal excursion, laryngeal vestibule closure, and upper esophageal sphincter opening (151,152). The Mendelsohn maneuver can also improve laryngeal excursion (152–155). This maneuver requires the patient to hold their larynx in an elevated position (at the highest point) for 2–3 seconds at the height of the swallow to facilitate opening of the esophagus (154). During falsetto exercises, an individual is asked to slide their voice up to a high pitch and sustain it for several seconds to improve hyolaryngeal elevation (156,157).
Another tool available to speech-language pathologists is neuromuscular electrical stimulation (NMES or e-stim) which uses surface electrodes to deliver an electrical current to peripheral nerves to cause muscle contraction. It is typically used in addition to voluntary exercise, however there is insufficient evidence to show its impact on dysphagia (158–160). The majority of studies on NMES and dysphagia have been completed in the stroke population. Park et al. (2018) evaluated the use of NMES (placed in the infrahyoid region) vs sham stimulation combined with an effortful swallow in 18 patients with PD. Participants underwent five, 30-minute sessions per week for 4 weeks (161). They found a difference in the PAS scores and increased hyoid movement in the experimental group compared to the placebo group, however there was no significant change in the oral or pharyngeal phase on the Videofluoroscopic Dysphagia Scale (161).
5.4. Additional considerations
Xerostomia (subjective dry mouth or hyposalivation) can also be a symptom of PD due to autonomic dysfunction or a medication side-effect, and can have a profound impact on swallowing (162–164). Strategies to improve this symptom can include throat lozenges, synthetic saliva, or frequent sips of water.
Another non-motor symptom commonly encountered is sialorrhea (drooling or excessive salivation). Sialorrhea appears to be related to reduced frequency of spontaneously swallowing saliva rather than an overproduction of saliva (165). It can result in the inability to maintain saliva in the oral cavity. Non-pharmacologic management strategies include wiping with a cloth, encouraging an upright posture, and frequent reminders to swallow. Off-label use of anticholinergics to reduce salivary secretions may be considered, however there is insufficient data regarding its safety (166). Commonly used anticholinergics include glycopyrrolate and sublingual atropine drops. The most effective intervention for drooling is local injections with botulinum toxin serotypes A or B to the parotid and/or submandibular glands (167–171). Currently incobotulinumtoxinA (Xeomin®) and rimabotulinumtoxinB (Myobloc®) are the only approved botulinum toxins for sialorrhea (172,173). A marked reduction in oral secretions can lead to dental caries, thus these strategies should be implemented with caution.
Patients with PD are at an increased risk for reduced oral health due to difficulty executing routine oral care secondary to motor symptoms, xerostomia, dysphagia, and/or drooling (174–178). Poor oral health can increase the risk of dental caries, periodontal disease, and tooth loss (179). It also strongly correlated with an increased risk of developing aspiration pneumonia (180). Interestingly, one study found that only 38% of known aspirators (with varying medical conditions) developed pneumonia stating “…dysphagia and aspiration are necessary, but not sufficient conditions for development of pneumonia. Other risk factors must be present as well” (7). Research has shown a reduction of pneumonia in nursing home residents receiving oral care (8,181–183). In fact, Bassim et al. (2008) found nursing home residents who did not receive oral care were three times more likely to die from pneumonia than those who received oral care after adjusting for other risk factors causing pneumonia (183). Therefore, routine oral care is critical in optimal management of dysphagia and the prevention of aspiration pneumonia. Oral care should be completed regardless of dental status (natural teeth, dentures, or edentulous) or feeding status (oral or enteral). It is recommended oral care be completed at a minimum of 2x/day with regular dental appointments at least every 6 months.
At this time, there is inconclusive evidence that swallowing improves with levodopa therapy (184–186). Studies completed have included a small sample size, patients in “On”, “Off”, or incomplete On/Off states, have been open-label studies, or used varying methods of dysphagia diagnosis for patient enrollment and outcome measurements of dysphagia. Rigidity and bradykinesia may affect the oral phase of swallowing, thus it could be impacted by dopaminergic medications (187,188). Occasionally some individuals will report an improvement after medication adjustment (14). Additionally, no significant differences in swallowing dysfunction have been observed in patients with dyskinesia compared to those without (189).
Deep brain stimulation (DBS) therapy of the subthalamic nucleus (STN) or the globus pallidus internus (GPi) is another option for treating motor symptoms in PD, but it has not resulted in clinically significant improvement in swallowing when comparing “On” and “Off” stimulation states (48,190). Troche et al. (2014) indicated that STN DBS may be associated with increased swallowing dysfunction compared to GPi DBS, at least with unilateral lead placement, however a head to head randomized comparison has not been performed (191). Low frequency stimulation (60Hz) compared to high frequency (130Hz) STN-DBS revealed a decrease in aspiration frequency by 57% and reduced swallowing difficulty by 80% (192). In patients who can tolerate low frequency DBS, this may be an approach favorable to swallowing, although it does not affect tremor and other motor symptoms as effectively as high frequency stimulation. At this time, the evidence regarding DBS and swallowing outcomes remains inconclusive.
6. Conclusion
The majority of patients with PD will experience dysphagia during the course of their disease, however clinical diagnosis remains a challenge especially in detecting subclinical dysphagia. Decreased patient awareness of bulbar dysfunction can result in delayed evaluation and management, as well as serious health concerns associated with aspiration pneumonia. At this time, the pathophysiology of dysphagia in PD is not fully understood, however disturbance of dopaminergic and non-dopaminergic mechanisms, brainstem dysfunction, and muscle atrophy are likely contributing factors (193). PD dysphagia does not respond to conventional treatments used for motor symptoms such as dopaminergic medications or DBS. Early and ongoing assessments by a multidisciplinary team using swallowing specific questionnaires, CSE, and instrumental evaluations are critical for identification of PD dysphagia. Management of PD dysphagia includes compensatory strategies, diet modifications, and/or rehabilitative exercises to improve quality of life and reduce the risk of morbidity and mortality. Additional research is needed to better understand the pathologic mechanisms underlying PD dysphagia and to optimize treatment outcomes.
7. Expert Opinion
This article presents the current knowledge, diagnostic evaluation, and management options for PD dysphagia and aspiration. As the number of individuals with PD rise, PD dysphagia will continue to be a symptom in need of early identification and novel treatment options. A multidisciplinary team approach including a speech-language pathologist, physicians (ENT, phonetician, movement disorder trained neurologist), nutritionist, and occupational therapist is important to properly identify and manage PD dysphagia. Regular screenings using subjective swallowing specific questionnaires and a clinical bedside swallow evaluation, in addition to objective instrumental evaluations (videofluoroscopic swallow study or fiberoptic endoscopic evaluation of swallowing) are necessary for detection of dysphagia. Dysphagia in PD impacts quality of life, social participation, fatigue, nutrition, hydration, morbidity, and mortality, and is likely a contributing factor in the development of aspiration pneumonia, which is the leading cause of death in this patient population.
A comprehensive understanding of why aspiration pneumonia occurs in a disproportionately high rate in PD is needed. Ongoing investigations to better understand the mechanism(s) of PD dysphagia and the impact of alpha-synuclein in both the central and peripheral nervous systems is warranted. Limited information is known about the neural networks involved in swallowing including the inhibitory and excitatory pathways (42). Additionally, supramedullary influences on swallowing need additional investigation. These findings could potentially elucidate the underlying pathologic mechanism of various causes of dysphagia. A recent pilot study revealed reduced saliva concentrations of substance P, a neuropeptide that is involved in the swallowing reflex, in PD patients with early pharyngeal dysphagia (77). Although this observation needs deeper investigation, agents increasing substance P may be targets for future studies.
The development of a marker for the early detection and progression of dysphagia will allow for earlier implementation of rehabilitative strategies aimed to maintain safe swallowing function, nutrition, and hydration. The current gold standard for identification of dysphagia is VFSS or FEES. Current management of dysphagia varies based on the underlying deficits noted during the instrumental evaluation.
Treatment approaches can be divided into compensatory behavioral strategies, dietary modifications, or rehabilitative exercises, which are prescribed by a speech-language pathologist or logopedic. Many of the current management methods for dysphagia are effective but may be cost prohibitive for some patients as it requires frequent therapy sessions with repeated instrumental evaluations. Frequent visits also pose an additional challenge given the mobility issues patients with PD often face. Current research is developing portable monitoring devices to collect biofeedback data in real-time during swallowing and swallowing exercises. Previous portable devices have been limited by ergonomics when placed on the skin in the submental area, which effects data quality and patient comfort. Using recent advances in electronics, researchers have developed flexible wearable technology to mitigate these issues. A flexible sensor sticker is placed on the patient’s submental area and is connected to a small wireless transmitter worn on the patient’s shirt. Muscle activity during swallowing is recorded and wirelessly transmitted to software, which is then analysed by a speech-language pathologist or physician. These devices are reported to be more comfortable, cost-effective, and can be used about 10 times before disposing (194). Furthermore, the increasing use of telemedicine and the ease of data transmission to a secure server can allow their speech-language pathologist to remotely monitor their therapy progress.
Lastly, it is important to emphasize that the presence of aspiration alone does not cause aspiration pneumonia, and typically additional factors such as a compromised immune system, respiratory dysfunction, poor oral hygiene or functional status, polypharmacy, or enteral nutrition are associated with developing aspiration pneumonia (7,8,195,196). A comprehensive evaluation of not only swallowing function, but also cough, oral hygiene, and general activity level is critical to managing dysphagia in patients with PD.
Figure 1.
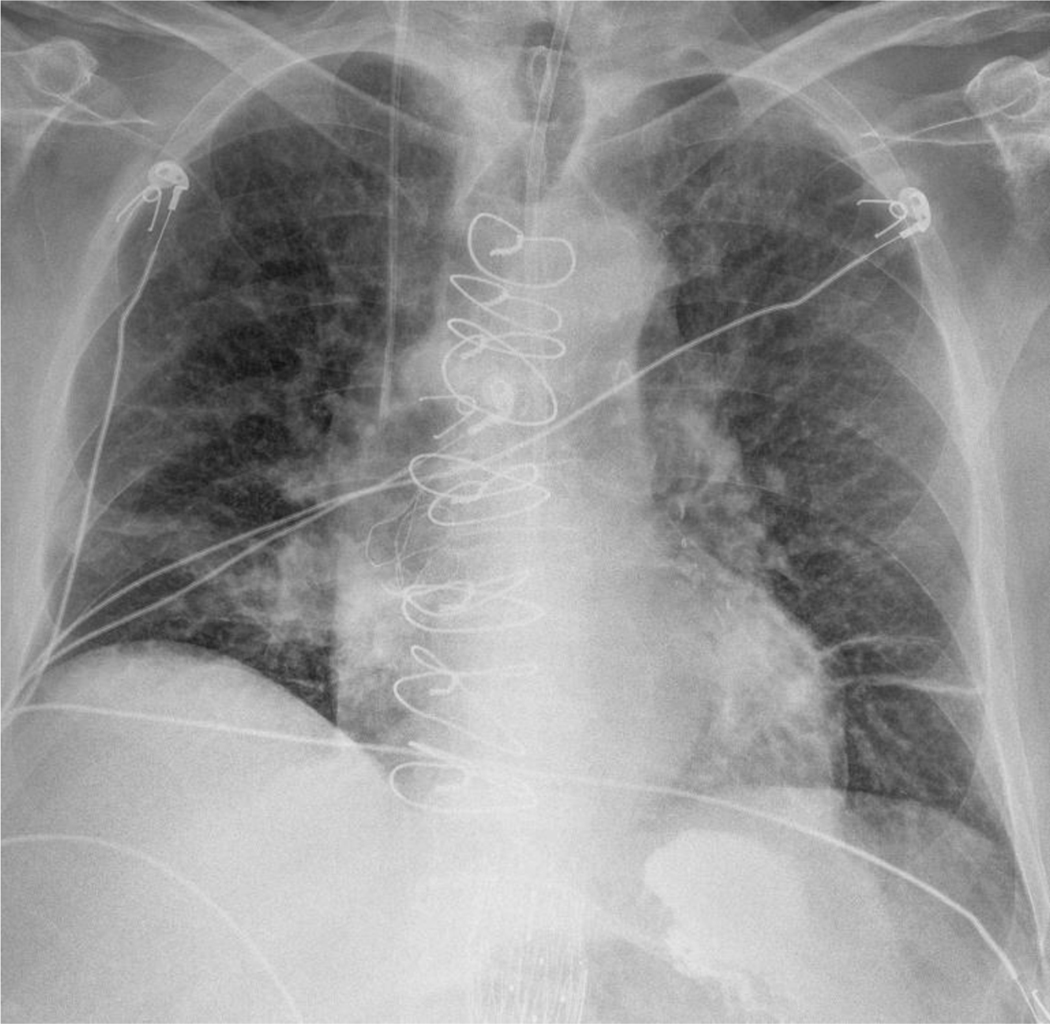
Chest radiograph with features of aspiration pneumonia. This image shows classic right lobar consolidation typically seen in aspiration. Electrocardiogram leads and suture wires from a previous sternotomy are also seen.
Figure 2.
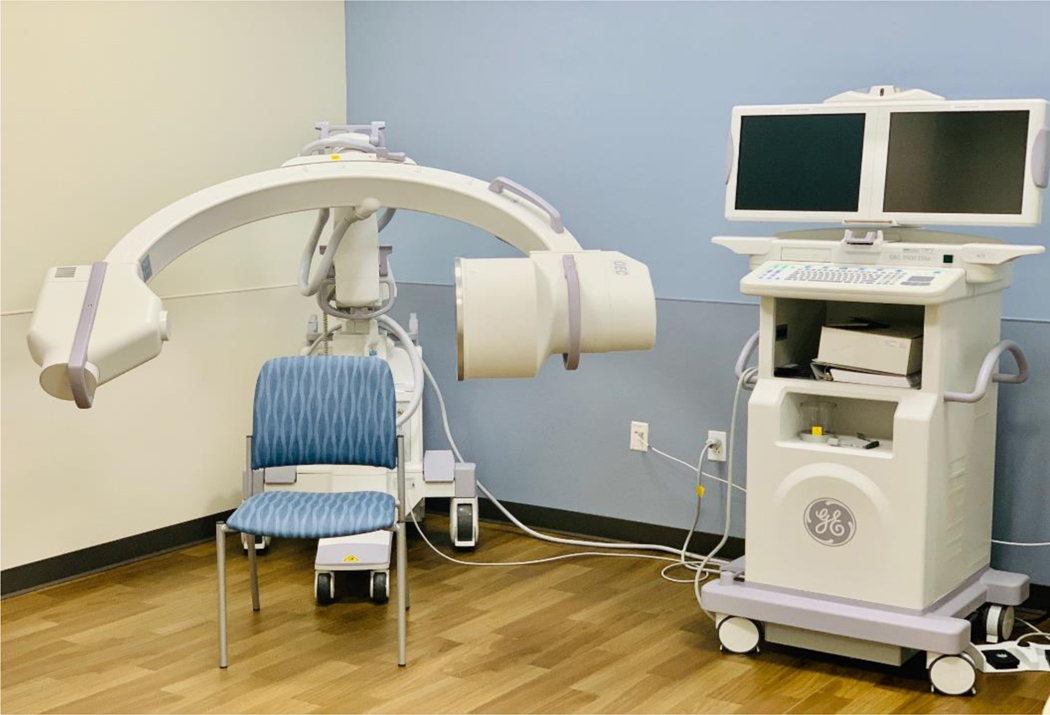
Mobile C-Arm Fluoroscopy machine. The patient sits in a chair while a VFSS is conducted to evaluate oropharyngeal dysphagia.
Figure 3.
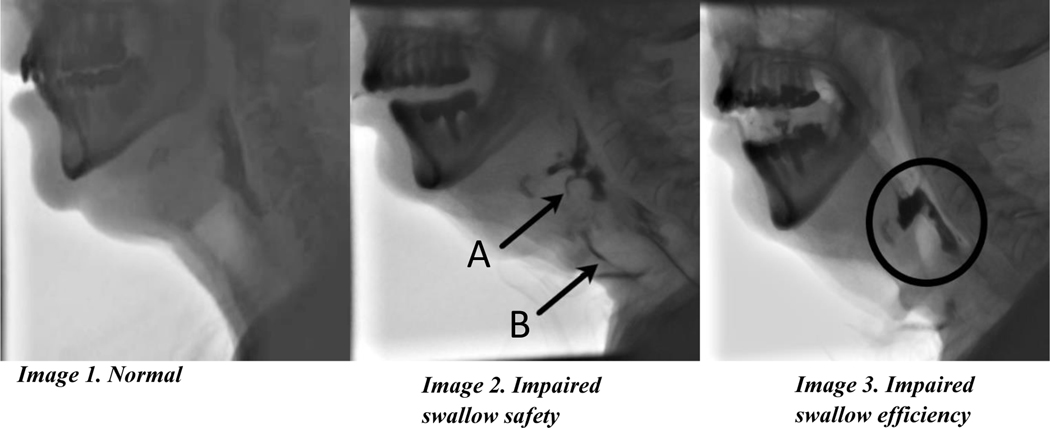
Videofluoroscopic swallow study (VFSS). Image 1: Normal swallow with thin liquid with complete laryngeal vestibule closure. Image 2: Reduced swallow safety with thin liquid indicated by aspiration along the anterior tracheal wall (A) and penetration into the laryngeal vestibule above the level of the vocal folds (B). Image 3: Reduced swallow efficiency with solid consistency with marked residue seen in the valleculae, and aryepiglottic folds into the pyriform sinuses.
Figure 4.
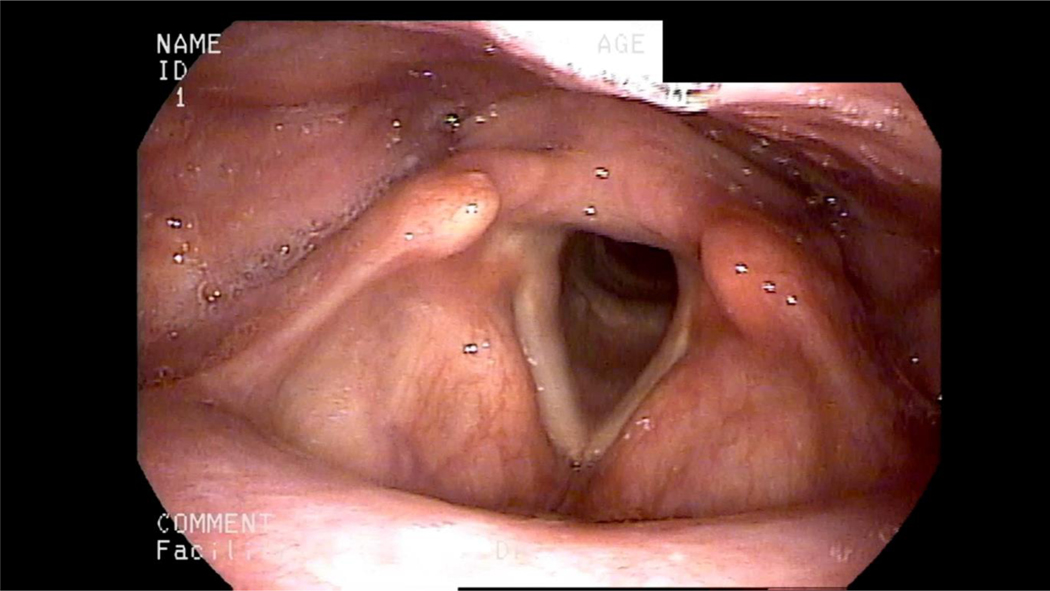
Endoscopic view of oropharynx, pharynx, and larynx. A laryngoscope is passed transnasally to directly visualize the oropharynx, pharynx, and larynx during swallowing.
Figure 5.
Example of an expiratory muscle strength training device. Image of the EMST 150® pressure-threshold device; (Aspire, LLC; Atlanta, GA) used as a rehabilitative strategy to strengthen expiratory muscles. The device consists of a calibrated, one-way, spring-loaded valve and pressure resistance can be set from 30–150 cmH2O.
Table 2.
Penetration-aspiration scale (PAS): An 8-point ordinal scale used to indicate the depth to which material enters the airway and whether penetrated or aspirated material is ejected from the airway. (Reprinted with permission from from SpringerNature: SpringerNature, Dysphagia, A penetration-aspiration scale, Rosenbek et al. [1996]). [103]
| Score | Airway Safety | Description |
|---|---|---|
| 1 | No penetration | Contrast material does not enter airway |
| 2 | Penetration | Contrast material enters airway, remains above vocal folds, ejected from airway |
| 3 | Penetration | Contrast material enters airway, remains above vocal folds, not ejected from airway |
| 4 | Penetration | Contrast material contacts vocal folds, ejected from airway, no visible residue |
| 5 | Penetration | Contrast material contacts vocal folds, not ejected from airway, visible residue |
| 6 | Aspiration | Contrast material passes below vocal folds, ejected from airway or into larynx |
| 7 | Aspiration | Contrast material passes below vocal folds, not ejected from airway despite patient effort |
| 8 | Aspiration | Contrast material passes below vocal folds, absence of patient effort to eject |
Article Highlights:
More than 80% of patients with PD develop dysphagia during the course of their disease, and it may occur even in pre-symptomatic stages.
PD dysphagia is associated with aspiration pneumonia, which is the most frequent cause of PD related death.
Routine screening for dysphagia with swallowing-specific questionnaires and/or clinical bedside swallow evaluation, as well as formal instrumental evaluations utilizing either videofluoroscopic swallow study (also known as modified barium swallow study) or fiberoptic endoscopic evaluation of swallowing are necessary for detection of dysphagia.
Management of dysphagia includes compensatory strategies (i.e. postural changes, pacing of bites and sips, bolus volume, etc.), diet modifications, and exercises aimed to strengthen muscles, improve airway safety, and swallow efficiency.
Clinical predictors of dysphagia in patients with PD include Hoehn and Yahr greater than 3 (advanced PD), dementia, anterior spillage of food and/or liquid from oral cavity (i.e. drooling), weight loss, and a Body Mass Index of <20 kg/m2.
There is one randomized clinical study revealing that expiratory muscle strength training (EMST) may be helpful in prevention of aspiration in PD.
Early detection and management of dysphagia in patients with PD through the use of a multidisciplinary team can improve quality of life, swallowing safety and efficiency, nutrition, and hydration.
Funding
This paper was supported by a NIH grant on cough with overlap on aspiration, R01 HD091658 (Hegland).
The Fixel Institute is supported by a Center of Excellence grant from the Parkinson’s Foundation.
M S Okun serves as a consultant for the Parkinson’s Foundation, and has received research grants from NIH, Parkinson’s Foundation, the Michael J. Fox Foundation, the Parkinson Alliance, Smallwood Foundation, the Bachmann-Strauss Foundation, the Tourette Syndrome Association, and the UF Foundation. M S Okun’s DBS research is supported by: NIH R01 NR014852 and R01NS096008. M Okun is PI of the NIH R25NS108939 Training Grant. M S Okun has received royalties for publications with Demos, Manson, Amazon, Smashwords, Books4Patients, Perseus, Robert Rose, Oxford and Cambridge (movement disorders books). M S Okun is an associate editor for New England Journal of Medicine Journal Watch Neurology. M S Okun has participated in CME and educational activities on movement disorders sponsored by the Academy for Healthcare Learning, PeerView, Prime, QuantiaMD, WebMD/Medscape, Medicus, MedNet, Einstein, MedNet, Henry Stewart, American Academy of Neurology, Movement Disorders Society and by Vanderbilt University. The institution and not M S Okun receives grants from Medtronic, Abbvie, Boston Scientific, Abbott and Allergan and the PI has no financial interest in these grants. M S Okun has participated as a site PI and/or co-I for several NIH, foundation, and industry sponsored trials over the years but has not received honoraria. Research projects at the University of Florida receive device and drug donations.
Footnotes
Declaration of Interests
All authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References:
- 1.Mehanna R, Jankovic J. Respiratory problems in neurologic movement disorders. Parkinsonism and Related Disorders. 2010. [DOI] [PubMed] [Google Scholar]
- 2.Kalf JG, de Swart BJM, Bloem BR, Munneke M. Prevalence of oropharyngeal dysphagia in Parkinson’s disease: A meta-analysis. Vol. 18, Parkinsonism and Related Disorders. 2012. p. 311–5. [DOI] [PubMed] [Google Scholar]
- 3.Ding X, Gao J, Xie C, Xiong B, Wu S, Cen Z, et al. Prevalence and clinical correlation of dysphagia in Parkinson disease: A study on Chinese patients. Eur J Clin Nutr. 2018; [DOI] [PubMed] [Google Scholar]
- 4.Fall PA, Saleh A, Fredrickson M, Olsson JE, Granérus AK. Survival time, mortality, and cause of death in elderly patients with Parkinson’s disease: A 9-year follow-up. Mov Disord. 2003. Nov;18(11):1312–6. [DOI] [PubMed] [Google Scholar]
- 5.Mogensen PH, Jakobsen J. Causes of death in a community-based study of Parkinson’s disease. Acta Neurol Scand. 2001; [DOI] [PubMed] [Google Scholar]
- 6.Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001; [DOI] [PubMed] [Google Scholar]
- 7.Langmore SE, Terpenning MS, Schork A, Chen Y, Murray JT, Lopatin D, et al. Predictors of aspiration pneumonia: How important is dysphagia? Dysphagia. 1998;13(2):69–81. [DOI] [PubMed] [Google Scholar]
- 8.Yoneyama T, Yoshida M, Ohrui T, Mukaiyama H, Okamoto H, Hoshiba K, et al. Oral care reduces pneumonia in older patients in nursing homes. J Am Geriatr Soc. 2002;50(3):430–3. [DOI] [PubMed] [Google Scholar]
- 9.Ortega O, Parra C, Zarcero S, Nart J, Sakwinska O, Clavé P. Oral health in older patients with oropharyngeal dysphagia. Age Ageing [Internet]. 2014. Jan [cited 2020 Feb 12];43(1):132–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24190874 [DOI] [PubMed] [Google Scholar]
- 10.Manabe T, Teramoto S, Tamiya N, Okochi J, Hizawa N. Risk factors for aspiration pneumonia in older adults. PLoS One [Internet]. 2015. Oct 7 [cited 2020 Feb 12];10(10):e0140060. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26444916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suttrup I, Warnecke T. Dysphagia in Parkinson’s Disease. Vol. 31, Dysphagia. 2016. p. 24–32. [DOI] [PubMed] [Google Scholar]
- 12.Troche MS, Schumann B, Brandimore AE, Okun MS, Hegland KW. Reflex Cough and Disease Duration as Predictors of Swallowing Dysfunction in Parkinson’s Disease. Dysphagia. 2016. Dec 1;31(6):757–64. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez HH, Lapane KL. Predictors of mortality among nursing home residents with a diagnosis of Parkinson’s disease. Med Sci Monit. 2002;8(4). [PubMed] [Google Scholar]
- 14.Melo A, Monteiro L. Swallowing improvement after levodopa treatment in idiopathic Parkinson’s disease: Lack of evidence. Vol. 19, Parkinsonism and Related Disorders. 2013. p. 279–81. [DOI] [PubMed] [Google Scholar]
- 15.Plowman-Prine EK, Sapienza CM, Okun MS, Pollock SL, Jacobson C, Wu SS, et al. The relationship between quality of life and swallowing in Parkinson’s disease. Mov Disord. 2009. Jul 15;24(9):1352–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sung HY, Kim JS, Lee KS, Kim YI, Song IU, Chung SW, et al. The prevalence and patterns of pharyngoesophageal dysmotility in patients with early stage Parkinson’s disease. Mov Disord [Internet]. 2010. Oct 30 [cited 2020 Feb 13];25(14):2361–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20669313 [DOI] [PubMed] [Google Scholar]
- 17.Durcan R, Wiblin L, Lawson RA, Khoo TK, Yarnall AJ, Duncan GW, et al. Prevalence and duration of non-motor symptoms in prodromal Parkinson’s disease. Eur J Neurol [Internet]. 2019. Jul 1 [cited 2020 Feb 13];26(7):979–85. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30706593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cereda E, Cilia R, Klersy C, Canesi M, Zecchinelli AL, Mariani CB, et al. Swallowing disturbances in Parkinson’s disease: A multivariate analysis of contributing factors. Park Relat Disord. 2014. Dec 1;20(12):1382–7. [DOI] [PubMed] [Google Scholar]
- 19.Simons JA, Fietzek UM, Waldmann A, Warnecke T, Schuster T, Ceballos-Baumann AO. Development and validation of a new screening questionnaire for dysphagia in early stages of Parkinson’s disease. Park Relat Disord. 2014;20(9):992–8. [DOI] [PubMed] [Google Scholar]
- 20.Lam K, Lam FKY, Kwok KL, Yiu KC, Kan EYL, Woo J, et al. Simple clinical tests may predict severe oropharyngeal dysphagia in Parkinson’s disease. Mov Disord. 2007. Apr 15;22(5):640–4. [DOI] [PubMed] [Google Scholar]
- 21.Umemoto G, Furuya H. Management of Dysphagia in Patients with Parkinson’s Disease and Related Disorders. Intern Med [Internet]. 2019. [cited 2019 Dec 7]; Available from: https://www.jstage.jst.go.jp/article/internalmedicine/advpub/0/advpub_2373-18/_article [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curtis JA, Molfenter S, Troche MS. Predictors of Residue and Airway Invasion in Parkinson’s Disease. Dysphagia [Internet]. 2019; Available from: 10.1007/s00455-019-10014-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barbagelata E, Nicolini A, Tognetti P. Swallowing dysfunctions in Parkinson’s disease patients: A novel challenge for the internist. Ital J Med. 2019;13(2):91–4. [Google Scholar]
- 24.Simons JA. Swallowing Dysfunctions in Parkinson’s Disease. In: International Review of Neurobiology [Internet]. Academic Press; 2017. [cited 2019 Dec 6]. p. 1207–38. Available from: https://www.sciencedirect.com/science/article/pii/S0074774217300831?via%3Dihub [DOI] [PubMed] [Google Scholar]
- 25.Kwon M, Lee J-H. Oro-Pharyngeal Dysphagia in Parkinson’s Disease and Related Movement Disorders [Internet]. 2019. [cited 2019 Dec 8]. Available from: 10.14802/jmd.19048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finger ME, Madden LL, Haq IU, McLouth CJ, Siddiqui MS. Analysis of the prevalence and onset of dysphonia and dysphagia symptoms in movement disorders at an academic medical center. J Clin Neurosci. 2019. Jun 1;64:111–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fabbri M, Coelho M, Abreu D, Guedes LC, Rosa MM, Godinho C, et al. Dysphagia predicts poor outcome in late-stage Parkinson’s disease. Parkinsonism Relat Disord [Internet]. 2019. Jul 1 [cited 2019 Dec 7];64:73–81. Available from: https://www.sciencedirect.com/science/article/pii/S1353802019300896 [DOI] [PubMed] [Google Scholar]
- 28.Suttrup I, Suttrup J, Suntrup-Krueger S, Siemer ML, Bauer J, Hamacher C, et al. Esophageal dysfunction in different stages of Parkinson’s disease. Neurogastroenterol Motil. 2017. Jan 1;29(1). [DOI] [PubMed] [Google Scholar]
- 29.Plowman-Prine EK, Sapienza CM, Okun MS, Pollock SL, Jacobson C, Wu SS, et al. The Relationship Between Quality of Life and Swallowing in Parkinson’s Disease NIH Public Access Author Manuscript. Mov Disord. 2009;24(9):1352–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lang IM. Brain stem control of the phases of swallowing. Vol. 24, Dysphagia. Springer; 2009. p. 333–48. [DOI] [PubMed] [Google Scholar]
- 31.Pringsheim T, Jette N, Frolkis A, Steeves TDL. The prevalence of Parkinson’s disease: A systematic review and meta-analysis [Internet]. Vol. 29, Movement Disorders. 2014. [cited 2020 Jan 23]. p. 1583–90. Available from: http://doi.wiley.com/10.1002/mds.25945 [DOI] [PubMed] [Google Scholar]
- 32.Dorsey ER, Sherer T, Okun MS, Bloemd BR. The emerging evidence of the Parkinson pandemic. Vol. 8, Journal of Parkinson’s Disease. IOS Press; 2018. p. S3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pflug C, Bihler M, Emich K, Niessen A, Nienstedt JC, Flügel T, et al. Critical Dysphagia is Common in Parkinson Disease and Occurs Even in Early Stages: A Prospective Cohort Study. Dysphagia [Internet]. 2018. Feb 1 [cited 2019 Dec 8];33(1):41–50. Available from: 10.1007/s00455-017-9831-1 [DOI] [PubMed] [Google Scholar]
- 34.Miller N, Allcock L, Hildreth AJ, Jones D, Noble E, Burn DJ. Swallowing problems in Parkinson disease: Frequency and clinical correlates. J Neurol Neurosurg Psychiatry. 2009;80(9):1047–9. [DOI] [PubMed] [Google Scholar]
- 35.Fujioka S, Fukae J, Ogura H, Mishima T, Yanamoto S, Higuchi MA, et al. Hospital-based study on emergency admission of patients with Parkinson’s disease. eNeurologicalSci. 2016. Sep 1;4:19–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujioka S, Fukae J, Ogura H, Mishima T, Yanamoto S, Higuchi MA, et al. Hospital-based study on emergency admission of patients with Parkinson’s disease. eNeurologicalSci. 2016. Sep;4:19–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomita S, Oeda T, Umemura A, Kohsaka M, Park K, Yamamoto K, et al. Video-fluoroscopic swallowing study scale for predicting aspiration pneumonia in Parkinson’s disease. PLoS One. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones B. Normal and abnormal swallowing : imaging in diagnosis and therapy. Springer; 2003. 287 p. [Google Scholar]
- 39.Mu L, Sobotka S, Chen J, Su H, Sanders I, Nyirenda T, et al. Parkinson disease affects peripheral sensory nerves in the pharynx. J Neuropathol Exp Neurol. 2013. Jul;72(7):614–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Costa MMB. Neural control of swallowing. Vol. 55, Arquivos de Gastroenterologia. IBEPEGE - Inst. Bras. Estudos Pesquisas Gastroent.; 2018. p. 61–75. [DOI] [PubMed] [Google Scholar]
- 41.Doty R. Neural organization of deglutition. American Physiological Society; 1968. p. 1861–902. [Google Scholar]
- 42.Jean A. Brain Stem Control of Swallowing: Neuronal Network and Cellular Mechanisms [Internet]. 2001. [cited 2020 Feb 7]. Available from: http://physrev.physiology.org [DOI] [PubMed] [Google Scholar]
- 43.Jean A. Brainstem Control of Swallowing: Localization and Organization of the Central Pattern Generator for Swallowing. In: Neurophysiology of the Jaws and Teeth. 1990. [Google Scholar]
- 44.Jean A, Kessler J-P TF. Nucleus tractus solitarii and deglutition: monoamines, excitatory amino acids and cellular properties. In: Barraco IRA, editor. Nucleus of the Solitary Tract [Internet]. 1st ed. Boca Raton: CRC Press; 1994. p. 361–75. Available from: https://www.taylorfrancis.com/books/9781000005950 [Google Scholar]
- 45.Jean A. Brain Stem Control of Swallowing: Neuronal Network and Cellular Mechanisms. 2001. [DOI] [PubMed] [Google Scholar]
- 46.Jean A, Dallaporta M. Electrophysiologic characterization of the swallowing pattern generator in the brainstem. GI Motil online. 2006; [Google Scholar]
- 47.Jankovic J. Parkinson’s disease: Clinical features and diagnosis. Journal of Neurology, Neurosurgery and Psychiatry. 2008. [DOI] [PubMed] [Google Scholar]
- 48.van Hooren MRA, Baijens LWJ, Voskuilen S, Oosterloo M, Kremer B. Treatment effects for dysphagia in Parkinson’s disease: A systematic review. Vol. 20, Parkinsonism and Related Disorders. Elsevier Ltd; 2014. p. 800–7. [DOI] [PubMed] [Google Scholar]
- 49.Brabenec L, Mekyska J, Galaz Z, Rektorova I. Speech disorders in Parkinson’s disease: early diagnostics and effects of medication and brain stimulation. J Neural Transm. 2017. Mar;124(3):303–34. [DOI] [PubMed] [Google Scholar]
- 50.Chaudhuri KR, Healy DG, Schapira AHV. Non-motor symptoms of Parkinson’s disease: Diagnosis and management. Vol. 5, Lancet Neurology. 2006. p. 235–45. [DOI] [PubMed] [Google Scholar]
- 51.Mu L, Sobotka S, Chen J, Su H, Sanders I, Adler CH, et al. Altered pharyngeal muscles in parkinson disease. J Neuropathol Exp Neurol [Internet]. 2012. Jun [cited 2020 Feb 19];71(6):520–30. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22588389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones CA, Hoffman MR, Lin L, Abdelhalim S, Jiang JJ, McCulloch TM. Identification of swallowing disorders in early and mid-stage Parkinson’s disease using pattern recognition of pharyngeal high-resolution manometry data. Neurogastroenterol Motil. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mu L, Sobotka S, Chen J, Su H, Sanders I, Adler CH, et al. Alpha-synuclein pathology and axonal degeneration of the peripheral motor nerves innervating pharyngeal muscles in parkinson disease. J Neuropathol Exp Neurol. 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hunker CJ, Abbs JH, Barlow SM. The relationship between parkinsonian rigidity and hypokinesia in the orofacial system: A quantitative analysis. Neurology. 1982; [DOI] [PubMed] [Google Scholar]
- 55.Baradaran N, Tan SN, Liu A, Ashoori A, Palmer SJ, Wang ZJ, et al. Parkinson’s disease rigidity: Relation to brain connectivity and motor performance. Front Neurol. 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Higo R, Tayama N, Watanabe T, Niimi S. Abnormal elevation of resting pressure at the upper esophageal sphincter of Parkinson’s disease patients. Eur Arch Oto-Rhino-Laryngology. 2001; [DOI] [PubMed] [Google Scholar]
- 57.Visanji NP, Marras C, Hazrati LN, Liu LWC, Lang AE. Alimentary, my dear Watson? The challenges of enteric α-synuclein as a Parkinson’s disease biomarker [Internet]. Vol. 29, Movement Disorders. John Wiley and Sons Inc; 2014. [cited 2020 Feb 19]. p. 444–50. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24375496 [DOI] [PubMed] [Google Scholar]
- 58.Fasano A, Visanji NP, Liu LWC, Lang AE, Pfeiffer RF. Gastrointestinal dysfunction in Parkinson’s disease. Vol. 14, The Lancet Neurology. Lancet Publishing Group; 2015. p. 625–39. [DOI] [PubMed] [Google Scholar]
- 59.Umay E, Ozturk E, Gurcay E, Delibas O, Celikel F. Swallowing in Parkinson’s disease: How is it affected? Clin Neurol Neurosurg [Internet]. 2019. Feb 1 [cited 2019 Dec 7];177:37–41. Available from: https://www.sciencedirect.com/science/article/pii/S0303846718304785 [DOI] [PubMed] [Google Scholar]
- 60.Barrera MA, O’Connor Wells B. Presbyphagia Versus Dysphagia: Normal Versus Abnormal Swallowing Symptoms in Older Adults with Parkinson Disease and Multiple Sclerosis. Top Geriatr Rehabil. 2019; [Google Scholar]
- 61.Muhle P, Wirth R, Glahn J, Dziewas R. Age-related changes in swallowing: Physiology and pathophysiology. Nervenarzt. 2015; [DOI] [PubMed] [Google Scholar]
- 62.Daggett A, Logemann J, Rademaker A, Pauloski B. Laryngeal penetration during deglutition in normal subjects of various ages. Dysphagia. 2006; [DOI] [PubMed] [Google Scholar]
- 63.Robbins J, Bridges AD, Taylor A. Oral, pharyngeal and esophageal motor function in aging. GI Motil online. 2006; [Google Scholar]
- 64.Malandraki GA, Robbins JA. Effects of aging on the oral phase of deglutition. In: Principles of Deglutition: A Multidisciplinary Text for Swallowing and its Disorders. 2013. [Google Scholar]
- 65.Umemoto G, Tsuboi Y, Kitashima A, Furuya H, Kikuta T. Impaired food transportation in parkinson’s disease related to lingual bradykinesia. Dysphagia [Internet]. 2011. Sep [cited 2020 Feb 13];26(3):250–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20803220 [DOI] [PubMed] [Google Scholar]
- 66.Wakasugi Y, Yamamoto T, Oda C, Murata M, Tohara H, Minakuchi S. Effect of an impaired oral stage on swallowing in patients with Parkinson’s disease. J Oral Rehabil. 2017. Oct 1;44(10):756–62. [DOI] [PubMed] [Google Scholar]
- 67.Ali GN, Wallace KL, Schwartz R, DeCarle DJ, Zagami AS, Cook IJ. Mechanisms of oral-pharyngeal dysphagia in patients with Parkinson’s disease. Gastroenterology. 1996;110(2):383–92. [DOI] [PubMed] [Google Scholar]
- 68.Pitts LL, Morales S, Stierwalt JAG. Lingual pressure as a clinical indicator of swallowing function in Parkinson’s disease. J Speech, Lang Hear Res. 2018; [DOI] [PubMed] [Google Scholar]
- 69.Minagi Y, Ono T, Hori K, Fujiwara S, Tokuda Y, Murakami K, et al. Relationships between dysphagia and tongue pressure during swallowing in Parkinson’s disease patients. J Oral Rehabil [Internet]. 2018. Jun 1 [cited 2020 Apr 9];45(6):459–66. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29575051 [DOI] [PubMed] [Google Scholar]
- 70.Fukuoka T, Ono T, Hori K, Wada Y, Uchiyama Y, Kasama S, et al. Tongue Pressure Measurement and Videofluoroscopic Study of Swallowing in Patients with Parkinson’s Disease. Dysphagia [Internet]. 2019. Feb 15 [cited 2020 Apr 9];34(1):80–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29948261 [DOI] [PubMed] [Google Scholar]
- 71.Curtis JA, Molfenter S, Troche MS. Predictors of Residue and Airway Invasion in Parkinson’s Disease. Dysphagia [Internet]. 2019. [cited 2019 Dec 7];1:3. Available from: 10.1007/s00455-019-10014-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Curtis JA, Molfenter SM, Troche MS. Pharyngeal Area Changes in Parkinson’s Disease and Its Effect on Swallowing Safety, Efficiency, and Kinematics. Dysphagia. 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gaeckle M, Domahs F, Kartmann A, Tomandl B, Frank U. Predictors of Penetration-Aspiration in Parkinson’s Disease Patients With Dysphagia: A Retrospective Analysis. Ann Otol Rhinol Laryngol. 2019; [DOI] [PubMed] [Google Scholar]
- 74.Hegland KW, Okun MS, Troche MS. Sequential voluntary cough and aspiration or aspiration risk in Parkinson’s disease. Lung. 2014;192(4):601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Silverman EP, Carnaby G, Singletary F, Hoffman-Ruddy B, Yeager J, Sapienza C. Measurement of voluntary cough production and airway protection in parkinson disease. Arch Phys Med Rehabil. 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gross RD, Atwood CW, Ross SB, Eichhorn KA, Olszewski JW, Doyle PJ. The coordination of breathing and swallowing in Parkinson’s disease. Dysphagia [Internet]. 2008. Jun [cited 2020 Feb 19];23(2):136–45. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18027027 [DOI] [PubMed] [Google Scholar]
- 77.Schröder JB, Marian T, Claus I, Muhle P, Pawlowski M, Wiendl H, et al. Substance P Saliva Reduction Predicts Pharyngeal Dysphagia in Parkinson’s Disease. Front Neurol [Internet]. 2019. Apr 16 [cited 2020 Jan 25];10. Available from: https://www.frontiersin.org/article/10.3389/fneur.2019.00386/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Renner B, Ahne G, Grosan E, Kettenmann B, Kobal G, Shephard A. Tonic stimulation of the pharyngeal mucosa causes pain and a reversible increase of inflammatory mediators. Inflamm Res. 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Imoto Y, KojiM AA, Osawa Y, Sunaga H, Fujieda S. Cough reflex induced by capsaicin inhalation in patients with dysphagia. Acta Otolaryngol. 2010; [DOI] [PubMed] [Google Scholar]
- 80.Niel JP. [Role of substance P in the nervous system control of digestive motility]. Arch Int Physiol Biochim Biophys. 1991; [DOI] [PubMed] [Google Scholar]
- 81.Kooi AHJ, Boo JPL, Ng SYE, Acharyya S, Goh KH, Tay KY, et al. The Modified Barium Swallow Impairment Profile as a Predictor of Clinical Outcomes of Admission for Pneumonia or Choking in Dysphagic Patients with Parkinson’s Disease. Dysphagia. 2019. Dec 1;34(6):896–903. [DOI] [PubMed] [Google Scholar]
- 82.Bassotti G, Germani U, Pagliaricci S, Plesa A, Giulietti O, Mannarino E, et al. Esophageal manometric abnormalities in Parkinson’s disease. Dysphagia. 1998;13(1):28–31. [DOI] [PubMed] [Google Scholar]
- 83.Langmore SE, Kenneth SMA, Olsen N. Fiberoptic endoscopic examination of swallowing safety: A new procedure. Dysphagia. 1988. Dec;2(4):216–9. [DOI] [PubMed] [Google Scholar]
- 84.McHorney CA, Bricker DE, Kramer AE, Rosenbek JC, Robbins JA, Chignell KA, et al. The SWAL-QOL outcomes tool for oropharyngeal dysphagia in adults: I. Conceptual foundation and item development. Dysphagia. 2000;15(3):115–21. [DOI] [PubMed] [Google Scholar]
- 85.McHorney CA, Earl Bricker D, Robbins J, Kramer AE, Rosenbek JC, Chignell KA. The SWAL-QOL outcomes tool for oropharyngeal dysphagia in adults: II. Item reduction and preliminary scaling. Dysphagia. 2000;15(3):122–33. [DOI] [PubMed] [Google Scholar]
- 86.McHorney CA, Robbins JA, Lomax K, Rosenbek JC, Chignell K, Kramer AE, et al. The SWAL-QOL and SWAL-CARE outcomes tool for oropharyngeal dysphagia in adults: III. Documentation of reliability and validity. Dysphagia. 2002. Mar;17(2):97–114. [DOI] [PubMed] [Google Scholar]
- 87.Belafsky PC, Mouadeb DA, Rees CJ, Pryor JC, Postma GN, Allen J, et al. Validity and reliability of the eating assessment tool (EAT-10). Ann Otol Rhinol Laryngol. 2008;117(12):919–24. [DOI] [PubMed] [Google Scholar]
- 88.Nativ-Zeltzer N, Bayoumi A, Mandin VP, Kaufman M, Seeni I, Kuhn MA, et al. Validation of the PILL-5: A 5-Item Patient Reported Outcome Measure for Pill Dysphagia. Front Surg. 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Manor Y, Giladi N, Cohen A, Fliss DM, Cohen JT. Validation of a swallowing disturbance questionnaire for detecting dysphagia in patients with Parkinson’s disease. Mov Disord. 2007. Oct 15;22(13):1917–21. [DOI] [PubMed] [Google Scholar]
- 90.Buhmann C, Bihler M, Emich K, Hidding U, Pötter-Nerger M, Gerloff C, et al. Pill swallowing in Parkinson’s disease: A prospective study based on flexible endoscopic evaluation of swallowing. Park Relat Disord. 2019. May 1;62:51–6. [DOI] [PubMed] [Google Scholar]
- 91.Ott DJ, Hodge RG, Pikna LA, Chen MYM, Gelfand DW. Modified barium swallowclinical and radiographic correlation and relation to feeding recommendations. Dysphagia. 1996; [DOI] [PubMed] [Google Scholar]
- 92.Langmore SE, Logemann JA. After the Clinical Bedside Swallowing Examination. Am J Speech-Language Pathol. 1991; [Google Scholar]
- 93.McCullough GH, Martino R. Clinical Evaluation of Patients with Dysphagia: Importance of History Taking and Physical Exam. In: Manual of Diagnostic and Therapeutic Techniques for Disorders of Deglutition. 2013. [Google Scholar]
- 94.Kalf H, De Swart B, Bonnier-Baars M, Kanters J, Hofman M, Kocken J, et al. Guidelines for Speech-Language Therapy in Parkinson’s Disease [Internet]. 2008. [cited 2020 Jan 25]. Available from: www.ParkinsonNet.nl [Google Scholar]
- 95.Ertekin C, Aydogdu I, Yüceyar N, Tarlaci S, Kiylioglu N, Pehlivan M, et al. Electrodiagnostic methods for neurogenic dysphagia. Electroencephalogr Clin Neurophysiol - Electromyogr Mot Control. 1998; [DOI] [PubMed] [Google Scholar]
- 96.Potulska A, Friedman A, Królicki L, Spychala A. Swallowing disorders in Parkinson’s disease. Park Relat Disord. 2003; [DOI] [PubMed] [Google Scholar]
- 97.Nathadwarawala KM, McGroary A, Wiles CM. Swallowing in neurological outpatients: Use of a timed test. Dysphagia. 1994; [DOI] [PubMed] [Google Scholar]
- 98.Brodsky MB, Suiter DM, González-Fernández M, Michtalik HJ, Frymark TB, Venediktov R, et al. Screening Accuracy for Aspiration Using Bedside Water Swallow Tests: A Systematic Review and Meta-Analysis. Chest. 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mulheren RW, Azola A, González-Fernández M. Do Ratings of Swallowing Function Differ by Videofluoroscopic Rate? An Exploratory Analysis in Patients After Acute Stroke. Arch Phys Med Rehabil [Internet]. 2019. Jun 1 [cited 2020 Feb 7];100(6):1085–90. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30452891 [DOI] [PubMed] [Google Scholar]
- 100.Bonilha HS, Blair J, Carnes B, Huda W, Humphries K, McGrattan K, et al. Preliminary investigation of the effect of pulse rate on judgments of swallowing impairment and treatment recommendations. Dysphagia [Internet]. 2013. Dec [cited 2020 Feb 7];28(4):528–38. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23559454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Martin-Harris B, Jones B. The Videofluorographic Swallowing Study. Vol. 19, Physical Medicine and Rehabilitation Clinics of North America. 2008. p. 769–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Langmore SE. History of Fiberoptic Endoscopic Evaluation of Swallowing for Evaluation and Management of Pharyngeal Dysphagia: Changes over the Years. Vol. 32, Dysphagia. Springer; New York LLC; 2017. p. 27–38. [DOI] [PubMed] [Google Scholar]
- 103.Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11(2):93–8. [DOI] [PubMed] [Google Scholar]
- 104.Pearson WG, Molfenter SM, Smith ZM, Steele CM. Image-based measurement of post-swallow residue: The normalized residue ratio scale. Dysphagia. 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dyer JC, Leslie P, Drinnan MJ. Objective computer-based assessment of valleculae residue - Is it useful? Dysphagia. 2008; [DOI] [PubMed] [Google Scholar]
- 106.Rommel N, Borgers C, Van Beckevoort D, Goeleven A, Dejaeger E, Omari TI. Bolus Residue Scale: An Easy-to-Use and Reliable Videofluoroscopic Analysis Tool to Score Bolus Residue in Patients with Dysphagia. Int J Otolaryngol. 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Neubauer PD, Rademaker AW, Leder SB. The Yale Pharyngeal Residue Severity Rating Scale: An Anatomically Defined and Image-Based Tool. Dysphagia. 2015; [DOI] [PubMed] [Google Scholar]
- 108.Martin-Harris B, Brodsky MB, Michel Y, Castell DO, Schleicher M, Sandidge J, et al. MBS measurement tool for swallow impairment-MBSimp: Establishing a standard. Dysphagia. 2008; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ertekin C. Electrophysiological Evaluation of Oropharyngeal Dysphagia in Parkinson’s Disease. J Mov Disord. 2014. Oct 30;7(2):31–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Monteiro L, Souza-Machado A, Pinho P, Sampaio M, Nóbrega AC, Melo A. Swallowing impairment and pulmonary dysfunction in Parkinson’s disease: The silent threats. J Neurol Sci. 2014. Apr 15;339(1–2):149–52. [DOI] [PubMed] [Google Scholar]
- 111.Oh DH, Park JS, Kim WJ. Effect of neuromuscular electrical stimulation on lip strength and closure function in patients with dysphagia after stroke. J Phys Ther Sci. 2017;29(11):1974–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hsiao M-Y, Karunia Wahyuni L, Wang T-G. Ultrasonography in Assessing Oropharyngeal Dysphagia. 2013. [cited 2020 Jan 27]; Available from: 10.1016/j.jmu.2013.10.008 [DOI] [Google Scholar]
- 113.Fabbri M, Coelho M, Abreu D, Guedes LC, Rosa MM, Godinho C, et al. Dysphagia predicts poor outcome in late-stage Parkinson’s disease. Park Relat Disord. 2019. Jul 1;64:73–81. [DOI] [PubMed] [Google Scholar]
- 114.Tjaden K. Speech and swallowing in Parkinson’s disease. Vol. 24, Topics in Geriatric Rehabilitation. 2008. p. 115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ra JY, Hyun JK, Ko KR, Lee SJ. Chin tuck for prevention of aspiration: Effectiveness and appropriate posture. Dysphagia. 2014. Sep 28;29(5):603–9. [DOI] [PubMed] [Google Scholar]
- 116.Rosenbek J, Jones H. Dysphagia in movement disorders [Internet]. 2008. [cited 2020 Jan 24]. Available from: https://books.google.com/books?hl=en&lr=&id=HVo0BwAAQBAJ&oi=fnd&pg=PR5&ots=Q7IMEl7aZZ&sig=ZP3-zQWrKWX_-VUR-_-JGCrbd68 [Google Scholar]
- 117.Ryu JS, Park D, Oh Y, Lee ST, Kang JY. The effects of bolus volume and texture on pharyngeal pressure events using high-resolution manometry and its comparison with videofluoroscopic swallowing study. J Neurogastroenterol Motil. 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Park JW, Sim GJ, Yang DC, Lee KH, Chang JH, Nam KY, et al. Increased bolus volume effect on delayed pharyngeal swallowing response in post-stroke oropharyngeal dysphagia: A pilot study. Ann Rehabil Med. 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bisch EM, Logemann JA, Rademaker AW, Kahrilas PJ, Lazarus CL. Pharyngeal effects of bolus volume, viscosity, and temperature in patients with dysphagia resulting from neurologic impairment and in normal subjects. J Speech Hear Res. 1994;37(5):1041–9. [DOI] [PubMed] [Google Scholar]
- 120.Butler SG, Stuart A, Markley L, Feng X, Kritchevsky SB. Aspiration as a Function of Age, Sex, Liquid Type, Bolus Volume, and Bolus Delivery Across the Healthy Adult Life Span. Ann Otol Rhinol Laryngol. 2018; [DOI] [PubMed] [Google Scholar]
- 121.Troche MS, Okun MS, Rosenbek JC, Altmann LJ, Sapienza CM. Attentional resource allocation and swallowing safety in Parkinson’s disease: A dual task study. Park Relat Disord. 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Logemann JA. The evaluation and treatment of swallowing disorders. Vol. 6, Current Opinion in Otolaryngology and Head and Neck Surgery. Lippincott Williams and Wilkins; 1998. p. 395–400. [Google Scholar]
- 123.Clavé P, De Kraa M, Arreola V, Girvent M, Farré R, Palomera E, et al. The effect of bolus viscosity on swallowing function in neurogenic dysphagia. Aliment Pharmacol Ther. 2006; [DOI] [PubMed] [Google Scholar]
- 124.Cola PC, Gatto AR, Da Silva RG, Spadotto AA, Schelp AO, Henry MACDA. The influence of sour taste and cold temperature in pharyngeal transit duration in patients with stroke. Arq Gastroenterol. 2010; [DOI] [PubMed] [Google Scholar]
- 125.Gatto AR, Cola PC, Da Silva RG, Spadotto AA, Ribeiro PW, Schelp AO, et al. Sour taste and cold temperature in the oral phase of swallowing in patients after stroke. CODAS. 2013; [DOI] [PubMed] [Google Scholar]
- 126.Logemann JA, Pauloski BR, Colangelo L, Lazarus C, Fujiu M, Kahrilas PJ. Effects of a sour bolus on oropharyngeal swallowing measures in patients with neurogenic dysphagia. J Speech Hear Res. 1995; [DOI] [PubMed] [Google Scholar]
- 127.Logemann JA, Gensler G, Robbins JA, Lindblad AS, Brandt D, Hind JA, et al. A randomized study of three interventions for aspiration of thin liquids in patients with dementia or Parkinson’s disease. J Speech, Lang Hear Res. 2008; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gillman A, Winkler R, Taylor NF. Implementing the Free Water Protocol does not Result in Aspiration Pneumonia in Carefully Selected Patients with Dysphagia: A Systematic Review. Dysphagia. 2017. Jun 1;32(3):345–61. [DOI] [PubMed] [Google Scholar]
- 129.Panther K. The Frazier Free Water Protocol. Perspect Swallowing Swallowing Disord. 2005. Mar 1;14(1):4. [Google Scholar]
- 130.Roche V. Percutaneous endoscopic gastrostomy. Clinical care of PEG tubes in older adults. Geriatrics [Internet]. 2003. Nov [cited 2020 Jan 28];58(11):22–6, 28–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14628393 [PubMed] [Google Scholar]
- 131.Loeb M, McGeer A, McArthur M, Walter S, Simor AE. Risk factors for pneumonia and other lower respiratory tract infections in elderly residents of long-term care facilities. Arch Intern Med. 1999. Sep 27;159(17):2058–64. [DOI] [PubMed] [Google Scholar]
- 132.Opilla M. Aspiration risk and enteral feeding: A clinical approach. Pract Gastroenterol. 2003. Apr 1;27:89–96. [Google Scholar]
- 133.Marik PE, Kaplan D. Aspiration pneumonia and dysphagia in the elderly. Vol. 124, Chest. American College of Chest Physicians; 2003. p. 328–36. [DOI] [PubMed] [Google Scholar]
- 134.Ciocon JO, Silverstone FA, Graver LM, Foley CJ. Tube Feedings in Elderly Patients: Indications, Benefits, and Complications. Arch Intern Med. 1988;148(2):429–33. [PubMed] [Google Scholar]
- 135.Koch KA. Patient Self-Determination Act. J Fla Med Assoc [Internet]. 1992. Apr [cited 2020 Jan 27];79(4):240–3. Available from: http://www.ncbi.nlm.nih.gov/pubmed/1588296 [PubMed] [Google Scholar]
- 136.Pitts T, Bolser D, Rosenbek J, Troche M, Okun MS, Sapienza C. Impact of expiratory muscle strength training on voluntary cough and swallow function in Parkinson disease. Chest. 2009. May 1;135(5):1301–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sapienza C, Wheeler K. Respiratory Muscle Strength Training: Functional Outcomes versus Plasticity. Semin Speech Lang [Internet]. 2006. Nov [cited 2020 Jan 27];27(4):236–44. Available from: http://www.thieme-connect.de/DOI/DOI?10.1055/s-2006-955114 [DOI] [PubMed] [Google Scholar]
- 138.Saleem AF, Sapienza CM, Okun MS. Respiratory muscle strength training: Treatment and response duration in a patient with early idiopathic Parkinson’s disease. NeuroRehabilitation. 2005;20(4):323–33. [PubMed] [Google Scholar]
- 139.Troche MS, Okun MS, Rosenbek JC, Musson N, Fernandez HH, Rodriguez R, et al. Aspiration and swallowing in Parkinson disease and rehabilitation with EMST: A randomized trial. Neurology. 2010. Nov 23;75(21):1912–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hegland KW, Davenport PW, Brandimore AE, Singletary FF, Troche MS. Rehabilitation of Swallowing and Cough Functions Following Stroke: An Expiratory Muscle Strength Training Trial. Arch Phys Med Rehabil. 2016;97(8):1345–51. [DOI] [PubMed] [Google Scholar]
- 141.Miles A, Jardine M, Johnston F, de Lisle M, Friary P, Allen J. Effect of Lee Silverman Voice Treatment (LSVT LOUD®) on swallowing and cough in Parkinson’s disease: A pilot study. J Neurol Sci. 2017. Dec 15;383:180–7. [DOI] [PubMed] [Google Scholar]
- 142.Pitts T, Bolser D, Rosenbek J, Troche M, Okun MS, Sapienza C. Impact of expiratory muscle strength training on voluntary cough and swallow function in Parkinson disease. Chest. 2009. May;135(5):1301–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wheeler-Hegland KM, Rosenbek JC, Sapienza CM. Submental sEMG and hyoid movement during mendelsohn maneuver, effortful swallow, and expiratory muscle strength training. J Speech, Lang Hear Res. 2008. Oct 1;51(5):1072–87. [DOI] [PubMed] [Google Scholar]
- 144.Ramig LO, Fox C, Sapir S. Speech treatment for Parkinson’s disease. Vol. 8, Expert Review of Neurotherapeutics. 2008. p. 297–309. [DOI] [PubMed] [Google Scholar]
- 145.Ramig LO, Sapir S, Countryman S, Pawlas AA, O’Brien C, Hoehn M, et al. Intensive voice treatment (LSVT®) for patients with Parkinson’s disease: A 2 year follow up. J Neurol Neurosurg Psychiatry. 2001. Oct;71(4):493–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sapir S, Spielman JL, Ramig LO, Story BH, Fox C. Effects of intensive voice treatment (the Lee Silverman Voice Treatment [LSVT]) on vowel articulation in dysarthric individuals with idiopathic Parkinson disease: Acoustic and perceptual findings. J Speech, Lang Hear Res. 2007. Aug 1;50(4):899–912. [DOI] [PubMed] [Google Scholar]
- 147.El Sharkawi A, Ramig L, Logemann JA, Pauloski BR, Rademaker AW, Smith CH, et al. Swallowing and voice effects of Lee Silverman Voice Treatment (LSVT®): A pilot study. J Neurol Neurosurg Psychiatry. 2002;72(1):31–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Miles A, Jardine M, Johnston F, de Lisle M, Friary P, Allen J. Effect of Lee Silverman Voice Treatment (LSVT LOUD®) on swallowing and cough in Parkinson’s disease: A pilot study. J Neurol Sci. 2017. Dec;383:180–7. [DOI] [PubMed] [Google Scholar]
- 149.Crary MA, Carnaby GD, Groher ME, Helseth E. Functional benefits of dysphagia therapy using adjunctive sEMG biofeedback. Dysphagia. 2004; [DOI] [PubMed] [Google Scholar]
- 150.Park JS, Hwang NK, Kim HH, Lee G, Jung YJ. Effect of neuromuscular electrical stimulation combined with effortful swallowing using electromyographic biofeedback on oropharyngeal swallowing function in stroke patients with dysphagia: A pilot study. Medicine (Baltimore). 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hind JA, Nicosia MA, Roecker EB, Carnes ML, Robbins JA. Comparison of effortful and noneffortful swallows in healthy middle-aged and older adults. Arch Phys Med Rehabil. 2001;82(12):1661–5. [DOI] [PubMed] [Google Scholar]
- 152.Wheeler-Hegland KM, Rosenbek JC, Sapienza CM. Submental sEMG and hyoid movement during mendelsohn maneuver, effortful swallow, and expiratory muscle strength training. J Speech, Lang Hear Res. 2008. Oct;51(5):1072–87. [DOI] [PubMed] [Google Scholar]
- 153.Logemann JA. Evaluation and treatment of swallowing disorders. PRO-ED; 1998. 406 p. [Google Scholar]
- 154.McCullough GH, Kim Y. Effects of the mendelsohn maneuver on extent of hyoid movement and UES opening post-stroke. Dysphagia. 2013. Dec;28(4):511–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.McCullough GH, Kamarunas E, Mann GC, Schmidley JW, Robbins JAA, Crary MA. Effects of Mendelsohn maneuver on measures of swallowing duration post stroke. Top Stroke Rehabil. 2012. Jan 1;19(3):234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Miloro KV, Pearson WG, Langmore SE. Effortful pitch glide: A potential new exercise evaluated by dynamic MRI. J Speech, Lang Hear Res. 2014;57(4):1243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Logemann JA, Pauloski BR, Rademaker AW, Colangelo LA. Speech and Swallowing Rehabilitation for Head and Neck Cancer Patients. Oncology. 1997;11(5):651–9. [PubMed] [Google Scholar]
- 158.Baijens LWJ, Speyer R, Passos VL, Pilz W, Roodenburg N, Clavé P. The effect of surface electrical stimulation on swallowing in dysphagic parkinson patients. Dysphagia. 2012; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chen YW, Chang KH, Chen HC, Liang WM, Wang YH, Lin YN. The effects of surface neuromuscular electrical stimulation on post-stroke dysphagia: A systemic review and meta-analysis. Clinical Rehabilitation. 2016. [DOI] [PubMed] [Google Scholar]
- 160.Sun Y, Chen X, Qiao J, Song G, Xu Y, Zhang Y, Xu D, Gao W, Li Y XC. Effects of transcutaneous neuromuscular electrical stimulation on swallowing disorders: a systematic review and meta-analysis. Am J Phys Med Rehabil. 2020;Publish Ah. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Park JS, Oh DH, Hwang NK, Lee JH. Effects of neuromuscular electrical stimulation in patients with Parkinson’s disease and dysphagia: A randomized, single-blind, placebo-controlled trial. NeuroRehabilitation. 2018; [DOI] [PubMed] [Google Scholar]
- 162.Kieser J, Jones G, Borlase G, MacFadyen E. Dental treatment of patients with neurodegenerative disease. Vol. 95, The New Zealand dental journal. 1999. p. 130–4. [PubMed] [Google Scholar]
- 163.Cersósimo MG, Perandones C, Micheli FE, Raina GB, Beron AM, Nasswetter G, et al. Alpha-synuclein immunoreactivity in minor salivary gland biopsies of Parkinson’s disease patients. Mov Disord [Internet]. 2011. Jan [cited 2020 Jan 27];26(1):188–90. Available from: http://doi.wiley.com/10.1002/mds.23344 [DOI] [PubMed] [Google Scholar]
- 164.Barbe AG, Bock N, Derman SHM, Felsch M, Timmermann L, Noack MJ. Self-assessment of oral health, dental health care and oral health-related quality of life among Parkinson’s disease patients. Gerodontology [Internet]. 2017. Mar [cited 2020 Jan 27];34(1):135–43. Available from: http://doi.wiley.com/10.1111/ger.12237 [DOI] [PubMed] [Google Scholar]
- 165.Proulx M, de Courval FP, Wiseman MA, Panisset M. Salivary production in Parkinson’s disease. Mov Disord. 2005. Feb;20(2):204–7. [DOI] [PubMed] [Google Scholar]
- 166.Srivanitchapoom P, Pandey S, Hallett M. Drooling in Parkinson’s disease: A review. Parkinsonism and Related Disorders. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dogu O, Apaydin D, Sevim S, Talas DU, Aral M. Ultrasound-guided versus “blind” intraparotid injections of botulinum toxin-A for the treatment of sialorrhoea in patients with Parkinson’s disease. Clin Neurol Neurosurg. 2004. Mar 1;106(2):93–6. [DOI] [PubMed] [Google Scholar]
- 168.Lagalla G, Millevolte M, Capecci M, Provinciali L, Ceravolo MG. Botulinum toxin type A for drooling in Parkinson’s disease: A double-blind, randomized, placebo-controlled study. Mov Disord [Internet]. 2006. May 1 [cited 2020 Feb 13];21(5):704–7. Available from: http://doi.wiley.com/10.1002/mds.20793 [DOI] [PubMed] [Google Scholar]
- 169.Seppi K, Weintraub D, Coelho M, Perez-Lloret S, Fox SH, Katzenschlager R, et al. The Movement Disorder Society Evidence-Based Medicine Review Update: Treatments for the non-motor symptoms of Parkinson’s disease. Mov Disord [Internet]. 2011. Oct 1 [cited 2020 Feb 13];26(S3):S42–80. Available from: http://doi.wiley.com/10.1002/mds.23884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Srivanitchapoom P, Pandey S, Hallett M. Drooling in Parkinson’s disease: A review. Vol. 20, Parkinsonism and Related Disorders. Elsevier Ltd; 2014. p. 1109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jost WH, Friedman A, Michel O, Oehlwein C, Slawek J, Bogucki A, et al. SIAXI: Placebo-controlled, randomized, double-blind study of incobotulinumtoxinA for sialorrhea. Neurology. 2019. Apr 23;92(17):E1982–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Accessdata.fda.gov [Internet]. Maryland: FDA; 2000. [updated 2019 Aug; cited 2020 April 1]. Available from https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125360s073lbl.pdf.. [Google Scholar]
- 173.Accessdata.fda.gov [Internet]. Maryland: FDA; 2010. [updated 2019 July; cited 2020 April 1]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125360s073lbl.pdf.173. [Google Scholar]
- 174.Jolly DE, Paulson RB, Paulson GW, Pike JA. Parkinson’s disease: a review and recommendations for dental management. Spec Care Dent [Internet]. 1989. May [cited 2020 Jan 27];9(3):74–8. Available from: http://doi.wiley.com/10.1111/j.1754-4505.1989.tb01032.x [DOI] [PubMed] [Google Scholar]
- 175.Johnston BT, Li Q, Castell JA, Castell DO. Swallowing and esophageal function in Parkinson’s disease. Am J Gastroenterol [Internet]. 1995. Oct [cited 2020 Jan 27];90(10):1741–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7572887 [PubMed] [Google Scholar]
- 176.Einarsdóttir ER, Gunnsteinsdóttir H, Hallsdóttir MH, Sveinsson S, Jónsdóttir SR, Olafsson VG, et al. Dental health of patients with Parkinson’s disease in Iceland. Spec Care Dent [Internet]. 2009. May [cited 2020 Jan 27];29(3):123–7. Available from: http://doi.wiley.com/10.1111/j.1754-4505.2009.00075.x [DOI] [PubMed] [Google Scholar]
- 177.Fukayo S, Nonaka K, Shimizu T, Yano E. Oral health of patients with Parkinson’s disease: Factors related to their better dental status. Tohoku J Exp Med. 2003. Nov;201(3):171–9. [DOI] [PubMed] [Google Scholar]
- 178.Friedlander AH, Mahler M, Norman KM, Ettinger RL. Parkinson disease: Parkinson disease systemic and orofacial manifestations, medical and dental management. J Am Dent Assoc. 2009;140(6):658–69. [DOI] [PubMed] [Google Scholar]
- 179.Baijens LWJ, Speyer R. Effects of therapy for dysphagia in parkinson’s disease: Systematic review. Vol. 24, Dysphagia. 2009. p. 91–102. [DOI] [PubMed] [Google Scholar]
- 180.Tada A, Miura H. Prevention of aspiration pneumonia (AP) with oral care. Arch Gerontol Geriatr. 2012; [DOI] [PubMed] [Google Scholar]
- 181.Yoneyama T, Yoshida M, Matsui T, Sasaki H. Oral care and pneumonia [8]. Lancet. 1999. [DOI] [PubMed] [Google Scholar]
- 182.Adachi M, Ishihara K, Abe S, Okuda K. Professional oral health care by dental hygienists reduced respiratory infections in elderly persons requiring nursing care. International journal of dental hygiene. 2007. [DOI] [PubMed] [Google Scholar]
- 183.Bassim CW, Gibson G, Ward T, Paphides BM, DeNucci DJ. Modification of the risk of mortality from pneumonia with oral hygiene care. J Am Geriatr Soc. 2008; [DOI] [PubMed] [Google Scholar]
- 184.Sutton JP. Dysphagia in Parkinson’s disease is responsive to levodopa. Vol. 19, Parkinsonism and Related Disorders. 2013. p. 282–4. [DOI] [PubMed] [Google Scholar]
- 185.Lim A, Leow LP, Huckabee ML, Frampton C, Anderson T. A pilot study of respiration and swallowing integration in Parkinson’s disease: “On” and “Off” levodopa. Dysphagia. 2008. Mar;23(1):76–81. [DOI] [PubMed] [Google Scholar]
- 186.Warnecke T, Suttrup I, Schröder JB, Osada N, Oelenberg S, Hamacher C, et al. Levodopa responsiveness of dysphagia in advanced Parkinson’s disease and reliability testing of the FEES-Levodopa-test. Park Relat Disord. 2016. Jul 1;28:100–6. [DOI] [PubMed] [Google Scholar]
- 187.Johnston BT, Li Q, Castell JA, Castell DO. Swallowing and esophageal function in Parkinson’s disease. Am J Gastroenterol. 1995. Oct;90(10):1741–6. [PubMed] [Google Scholar]
- 188.Tison F, Wiart L, Guatterie M, Fouillet N, Lozano V, Henry P, et al. Effects of central dopaminergic stimulation by apomorphine on swallowing disorders in Parkinson’s disease. Mov Disord. 1996;11(6):729–32. [DOI] [PubMed] [Google Scholar]
- 189.Monte FS, da Silva FP, Braga-Neto P, Nobre e Souza MÂ, Sales de Bruin VM. Swallowing abnormalities and dyskinesia in Parkinson’s disease. Mov Disord. 2005. Apr;20(4):457–62. [DOI] [PubMed] [Google Scholar]
- 190.Troche MS, Brandimore AE, Foote KD, Okun MS. Swallowing and deep brain stimulation in Parkinson’s disease: A systematic review. Vol. 19, Parkinsonism and Related Disorders. 2013. p. 783–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Troche MS, Brandimore AE, Foote KD, Morishita T, Chen D, Hegland KW, et al. Swallowing outcomes following unilateral STN vs. GPi surgery: A retrospective analysis. Dysphagia. 2014;29(4):425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Xie T, Vigil J, MacCracken E, Gasparaitis A, Young J, Kang W, et al. Low-frequency stimulation of STN-DBS reduces aspiration and freezing of gait in patients with PD. Neurology. 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Costa MMB. Neural control of swallowing. Vol. 55, Arquivos de Gastroenterologia. IBEPEGE - Inst. Bras. Estudos Pesquisas Gastroent.; 2018. p. 61–75. [DOI] [PubMed] [Google Scholar]
- 194.Kim MK, Kantarcigil C, Kim B, Baruah RK, Maity S, Park Y, et al. Flexible submental sensor patch with remote monitoring controls for management of oropharyngeal swallowing disorders. Sci Adv. 2019. Dec 13;5(12):eaay3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ortega O, Parra C, Zarcero S, Nart J, Sakwinska O, Clavé P. Oral health in older patients with oropharyngeal dysphagia. Age Ageing. 2014. Jan;43(1):132–7. [DOI] [PubMed] [Google Scholar]
- 196.Manabe T, Teramoto S, Tamiya N, Okochi J, Hizawa N. Risk factors for aspiration pneumonia in older adults. PLoS One. 2015. Oct;10(10):e0140060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Argolo N, Sampaio M, Pinho P, Melo A, Nóbrega AC. Swallowing disorders in Parkinson’s disease: impact of lingual pumping. Int J Lang Commun Disord [Internet]. 2015. Sep [cited 2020 Jan 23];50(5):659–64. Available from: http://doi.wiley.com/10.1111/1460-6984.12158 [DOI] [PubMed] [Google Scholar]