Letter to the Editor
Here, we report the first documented case of long-term remission in a patient with refractory AML after graft failure of allogeneic cord blood stem cells, and subsequent remission of his AML, along with an expansion of autologous, CD8+ cyctotoxic T cells.
This 67-year-old Caucasian male developed progressive pancytopenias and was referred to our institution in August 2007. The patient’s bone marrow aspirate revealed 43% blasts with the morphology and an immunophenotype consistent with acute myeloid leukemia (AML), M4 according to the French-American-British (FAB) classification (see Fig. 1 A, B). Cytogenetic and molecular tests showed a normal diploid karyotype, and the FLT3 mutation status was negative. His other significant past medical history included seronegative rheumatoid arthritis, and a history of prostate cancer, treated with radical prostatectomy. The patient’s AML initially was treated with 2 courses of clofarabine between August and October of 2007. However, he was found to be primary refractory to this treatment with persistent bone marrow blasts, accounting for 14% of the marrow cellularity in November 2007. Therefore, the patient then received salvage treatment with 3 courses of idarubicin, cytarabine, and XIAP antisense (AEG-53156) between November 2007 and February 2008, and again he was found to have persistent AML cells, accounting for about 7% of the marrow cells in bone marrow specimens from March and May of 2008. The patient therefore proceeded to a double cord blood stem cell transplantation (CB-SCT) in June 2008 after nonmyeloablative conditioning with fludarabine (40 mg/m2/day x 4) plus cyclophosphamide (50 mg/kg x 1), antithymocyte globulin (ATG), and total body irradiation (TBI) (2 Gy). He received supportive red blood cell and platelet transfusions in the peri-transplantation period as well as filgrastim. The patient did not suffer any significant infectious complications after the transplantation. One month after the transplantation a bone marrow biopsy with PCR-based microsatellite polymorphism analysis showed 100% host chimerism, indicating graft failure. However, despite the failure to engraft, the patient achieved a complete remission with clearance of marrow blasts by morphology and flow cytometry, and subsequent normalization of his peripheral counts. Fig. 1 C and D show bone marrow aspirate smears after CB-SCT that are consistent with a complete remission and depict scattered large granular lymphocytes.
Figure 1:
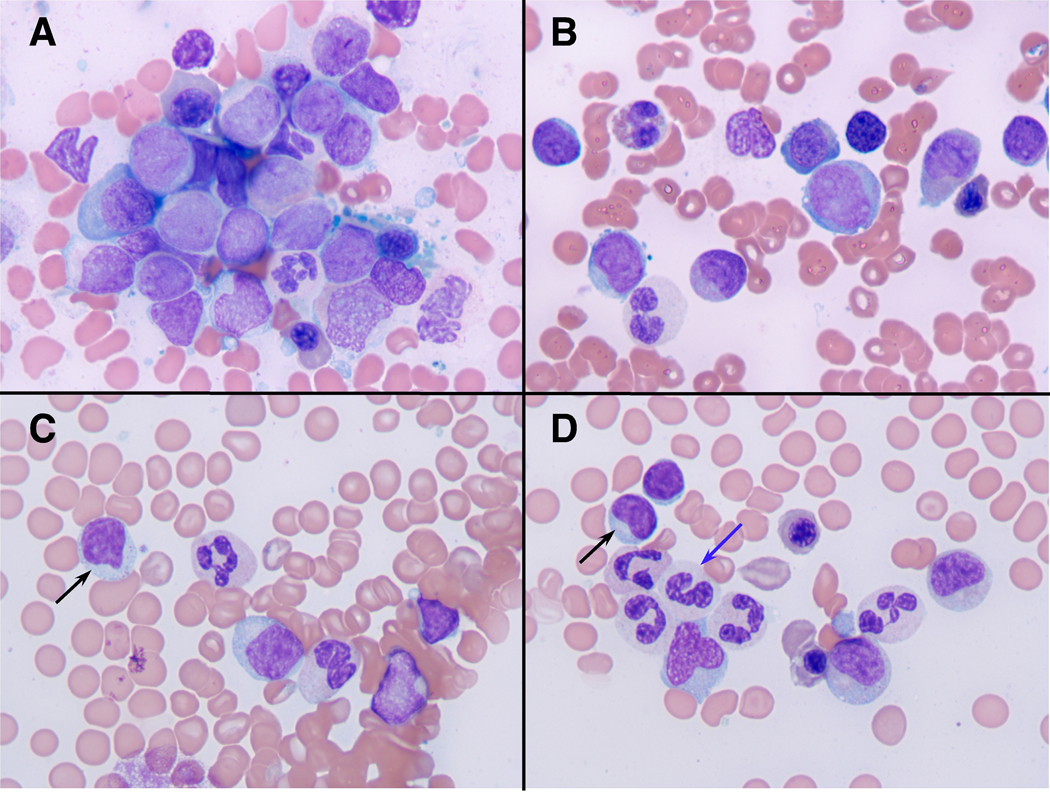
Bone marrow morphology before (A, B) and after cord blood stem cell transplantation (C, D). Fig. 1 A and B depict bone marrow aspirate smears at the initial presentation with myeloblasts in a background of dysplastic erythroid and myeloid elements (Wright-Giemsa stains, photographed at 1000x). Fig. 1 C and D display photomicrographs of bone marrow aspirate smears after CB-SCT that are consistent with a complete remission and that depict scattered large granular lymphocytes (black arrows) in a background of mildly dysplastic myeloid cells, some with hypogranular cytoplasm (blue arrow).
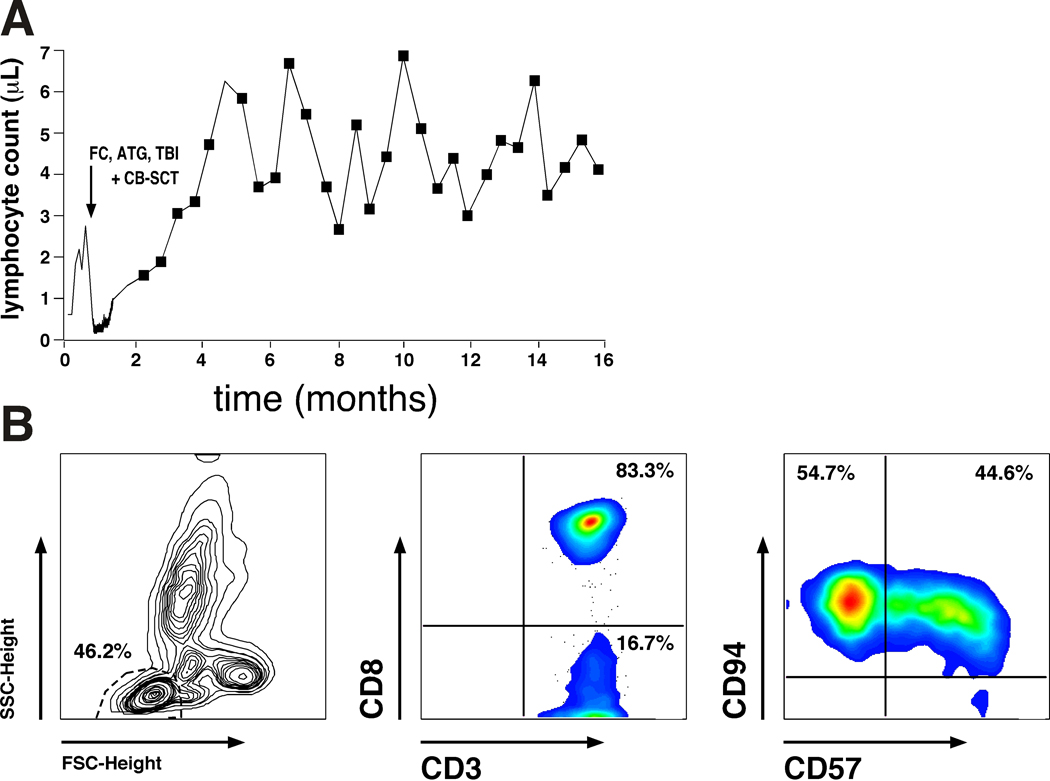
About 6–7 weeks after the CB-SCT, the patient gradually developed a lymphocytosis that is illustrated in Fig. 2A. The lymphocytes were 100% of host origin by microsatellite polymorphism analysis, and they were oligoclonal CD8+ T-cells, as demonstrated by the oligoclonal pattern of T-cell receptor gamma chain gene rearrangements by PCR analysis, and flow cytometry, respectively. Fig. 2B illustrates the immunophenotype of the expanded T cells. Now sixteen months after his cord blood transplant, the patient has been without any evidence of leukemia both on peripheral blood and bone marrow tests, and has been managed only with observation. Clinically, the patients has become fully asymptomatic and doing very well over the course of the last year, and his arthritis, which was a (possibly paraneoplastic) major clinical issue at diagnosis also is in remission.
Figure 2:
Expansion of autologous, CD8+ T cells after CB-SCT. Fig. 2A depicts the kinetics of the lymphocytosis after non-myeloablative conditioning with FC, ATG and TBI, and subsequent CB-SCT (indicated by the arrow). Fig. 2B illustrates the immunophenotype of the expanded T cells. Displayed are the forward (FSC) and sideward scatter (SSC) characteristics with gating on the lymphocyte population (left hand box), and staining with anti-CD3 and anti-CD8 mAbs, revealing that 83.3% of the lymphocytes were CD8 positive (center box). Staining with anti-CD57 and anti-CD94 mAbs (right hand box) also revealed that these CD8+ T cells displayed a phenotype consistent with large granular lymphocytes (LGL), which is in keeping with the morphology of these activated lymphocytes (Fig. 1C, D).
The clinical course and the laboratory studies in this case suggest that the allogeneic cord blood cells triggered the oligoclonal expansion of autologous cytotoxic T cells that cross-react with the patient’s AML clone. To our knowledge, this is the first case of a remission after an unsuccessful engraftment of CB-SCs, and therefore we will discuss these findings in the context of the graft-versus-leukemia (GvL) effect and spontaneous remission (SR) in AML.
SR is an infrequent but recurrent and often well-documented phenomenon in patients with renal cell carcinoma or malignant melanoma, but a rarity in cancers of the hematopoietic system. The published cases of spontaneous remission in patients with acute myeloid leukemia (AML) indicate that this is an exceedingly rare and generally temporary event, with a mean remission duration of approximately 6–7 months (1, 2). The mechanism responsible for inducing these remissions is largely unknown, although the reported cases suggest that there may be a correlation with infections or blood transfusions (3). It has been speculated that these events trigger immune responses responsible for SR. Natural killer cells and cytotoxic T cells transfused with blood products, or cytokines such as tumor necrosis factor (TNF) and interleukin-2 (IL-2), released during infections have been proposed to participate in the development of SR. Previous reports of successful re-induction of a second SR by BCG vaccination support the hypothesis that SR in AML is a T-cell mediated process (4, 5). In hematopoietic stem cell transplantation (SCT), the importance of T cell-mediated immune responses for induction of remissions via the “graft-versus-leukemia” (GVL) effect is well established (6). Furthermore, it is increasingly recognized that the induction of an effective allo-reactive T cell responses against leukemia stem and progenitor cells is essential for complete eradication of AML after SCT. Several studies have demonstrated a correlation between the presence of T cells recognizing leukemia CD34+ progenitor cells and the occurrence of complete remissions in vivo (7). Furthermore, distinct minor histocompatibility antigens (MiHA) expressed on AML progenitor cells, that are recognized by CD8+ T cells, are emerging, and have been proposed as target antigens for immunotherapy (8).
In summary, we report the fascinating case of a primary refractory AML patient that achieved a complete remission after an allogeneic SCT with graft failure, lasting now for over 16 months. This remission coincided with the oligoclonal expansion of autologous, CD8+ T cells, suggesting that the CB-SCT triggered the induction and expansion of autologous cytotoxic T cells that cross-react with the AML clone. This unique case illustrates that failure of a therapeutic intervention (here: the cord blood graft loss) can sometimes trigger events that benefit the patient, and also encourages our hopes that our immune system, if properly primed and targeted, has the capacity to overcome relapsed/refractory AML, clinically one of the most dismal settings. In Winston Churchill’s words: “Success is not final, failure is not fatal: it is the courage to continue that counts”.
Footnotes
Disclaimer: The authors state that they have no conflict of interest related to this article
References
- 1.Daccache A, Kizhakekuttu T, Siebert J, Veeder M. Hematologic and cytogenetic spontaneous remission in acute monocytic leukemia (FAB M5b) with trisomy 8. J Clin Oncol 2007. Jan 20; 25(3): 344–346. [DOI] [PubMed] [Google Scholar]
- 2.Delmer A, Heron E, Marie JP, Zittoun R. Spontaneous remission in acute myeloid leukaemia. Br J Haematol 1994. Aug; 87(4): 880–882. [DOI] [PubMed] [Google Scholar]
- 3.Mitterbauer M, Fritzer-Szekeres M, Mitterbauer G, Simonitsch I, Knobl P, Rintelen C, et al. Spontaneous remission of acute myeloid leukemia after infection and blood transfusion associated with hypergammaglobulinaemia. Ann Hematol 1996. Oct; 73(4): 189–193. [DOI] [PubMed] [Google Scholar]
- 4.Narayanan MN, Lewis MJ. Spontaneous complete remission of acute myeloid leukaemia with interstitial deletion of chromosome 5. Clinical and laboratory haematology 1991; 13(4): 391–395. [DOI] [PubMed] [Google Scholar]
- 5.Muller CI, Trepel M, Kunzmann R, Lais A, Engelhardt R, Lubbert M. Hematologic and molecular spontaneous remission following sepsis in acute monoblastic leukemia with translocation (9;11): a case report and review of the literature. European journal of haematology 2004. Jul; 73(1): 62–66. [DOI] [PubMed] [Google Scholar]
- 6.Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood 2008. Dec 1; 112(12): 4371–4383. [DOI] [PubMed] [Google Scholar]
- 7.Kloosterboer FM, van Luxemburg-Heijs SA, van Soest RA, van Egmond HM, Barbui AM, Strijbosch MP, et al. Minor histocompatibility antigen-specific T cells with multiple distinct specificities can be isolated by direct cloning of IFNgamma-secreting T cells from patients with relapsed leukemia responding to donor lymphocyte infusion. Leukemia 2005. Jan; 19(1): 83–90. [DOI] [PubMed] [Google Scholar]
- 8.Norde WJ, Overes IM, Maas F, Fredrix H, Vos JC, Kester MG, et al. Myeloid leukemic progenitor cells can be specifically targeted by minor histocompatibility antigen LRH-1-reactive cytotoxic T cells. Blood 2009. Mar 5;113(10): 2312–23 [DOI] [PubMed] [Google Scholar]