Fungi are microeukaryotes, estimated to encompass between 2.2 to 3.9 million species that inhabit various anatomic sites within the human body with relatively stable colonization – collectively forming the mycobiome1, 2. Despite their low abundance in the human microbiome1, 2, emerging evidence highlights the significant influence of mycobiome on the host’s health, including nutrition, metabolism, immunity, and the physiological functions of various body organs3, 4. More specifically, the associations between mycobiome and human cancer have garnered increasing attention in recent years due to the significant enrichment of specific fungi in patients with malignant tumors. However, the biological underpinnings of human mycobiome in cancer pathogenesis and disease progression continue to evolve and remain unclear.
A recent pre-clinical and clinical study revealed that pancreatic ductal adenocarcinomas (PDACs) harbor significant enrichment of one such specific fungi in mice models and human specimens relative to normal pancreatic tissues5. Furthermore, this study revealed that the commensal gut fungi – Malassezia globosa (M. globosa) – migrates from the gut to the pancreas and promotes pancreatic carcinogenesis via the activation of the complement cascade5, 6. More interestingly, this study elegantly demonstrated that antifungal drugs inhibited the oncogenic progression of PDAC, and the combination of an antifungal drug and chemotherapy exhibited a synergistic anti-cancer effect against PDAC in animal models5. Likewise, another commensal fungus, Candida tropicalis, was implicated in the pathogenesis of colorectal cancer (CRC) through the accumulation of myeloid-derived suppressor cells7. In light of such groundbreaking studies, alterations in the mycobiome have gained increasing attention as functional determinants of disease and potential diagnostic and prognostic biomarkers in various cancers. For instance, as fecal biomarkers, a higher abundance of multiple fungi, including M. globosa, has been shown to distinguish CRC patients from healthy individuals accurately8. Furthermore, more recent analytical studies demonstrated that tumor-specific mycobiome could predict prognosis in multiple gastrointestinal malignancies9. Given this evidence, we hypothesized that high M. globosa levels in tissue specimens from patients with PDAC might serve as critical prognostic biomarkers.
Accordingly, this study explored the feasibility of tissue-based fungal biomarkers for predicting prognosis in patients with PDAC. Using quantitative nested polymerase chain reaction (PCR) assays in PDAC patient cohorts, we first determined the frequency of M. globosa-positivity in PDACs, followed by the quantitative associations between the levels of this fungus and its potential in predicting cancer-related death and tumor recurrence in patients with this malignancy.
To examine the M. globosa levels, surgically resected tissue specimens from a cohort of 180 PDAC patients with stages I-III disease were analyzed using quantitative nested PCR assays. Following extraction of DNA, the specific target amplification for the internal transcribed spacer (ITS) regions of M. globosa ribosomal DNA was confirmed using the BigDye Terminator Cycle Sequencing (Fig. 1A). Subsequently, in the quantitative nested PCR assays normalized by the HBB gene expression, 78 of 180 (43.3%) cases exhibited a Ct value of less than 33, indicating high levels of this fungus, and were defined as M. globosa-positive. In contrast, no signal was observed for the remaining 102 cases, even after 50 PCR cycles (Fig. 1B), and they were defined as M. globosa-negative. Interestingly, this frequency of M. globosa-positivity in PDAC tissues was similar to that in stool samples of healthy individuals (36–53%), which were reported in a previous sequencing-based human gut mycobiome project10.
Figure 1.
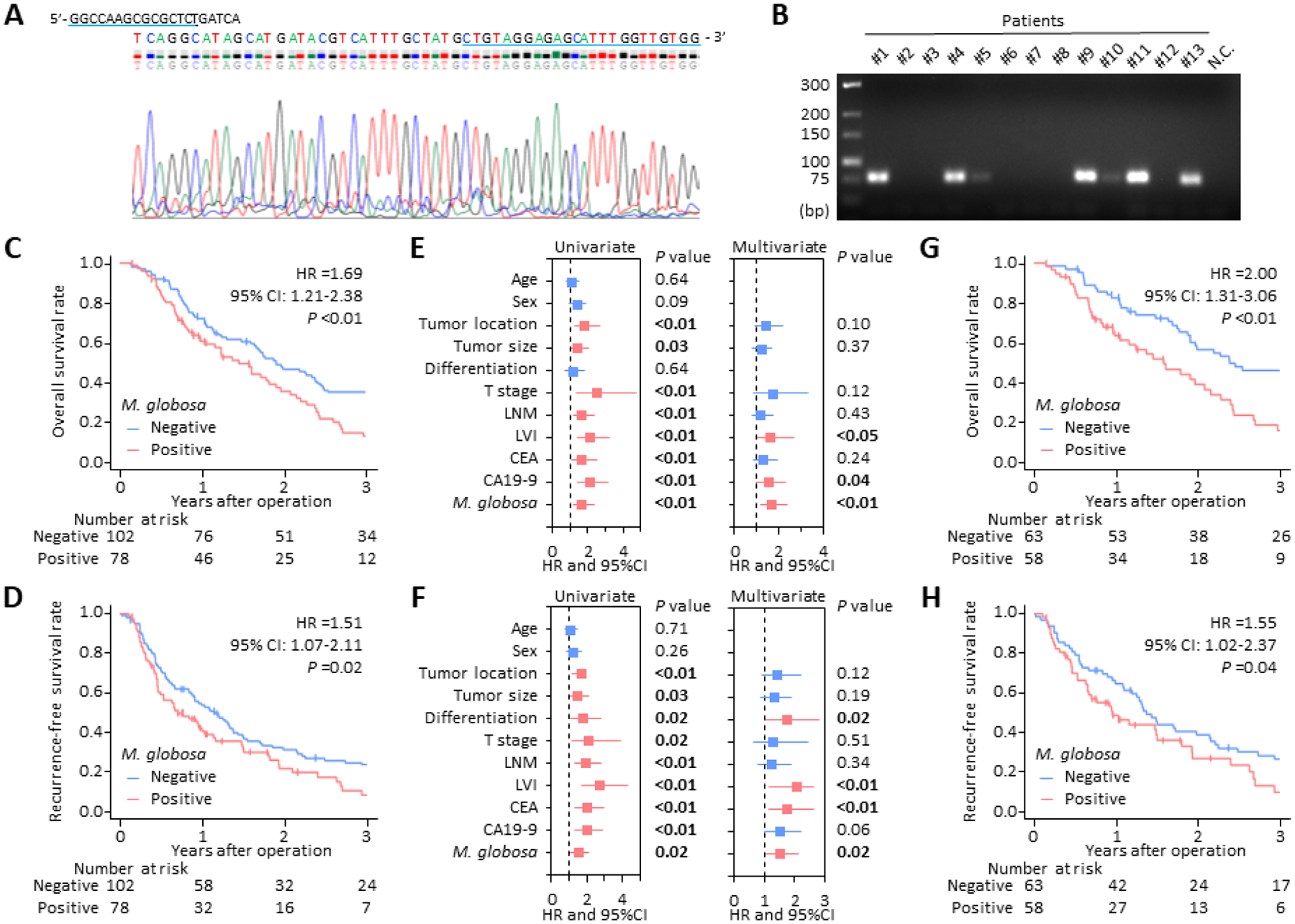
Intratumoral Malassezia globosa levels and prognostic significance in PDAC. (A) Representative sequences of PCR amplified products in nested PCR assays for detecting M. globosa in PDAC specimens. Forward and reverse primer location is indicated by a blue underline. (B) Representative agarose gel electrophoresis (4% agarose) of PCR amplified products in nested PCR assays. Lane 1 is a low-range DNA ladder, lanes 2–14 are PCR amplified products from PDAC tissue specimens, and lane 15 is negative control. (C, D) Kaplan-Meier curves depicting the overall survival (C) and recurrence-free survival (D) for patients with M. globosa negative (n =102) and positive (n =78) tumors. (E, F) Forest plots with HR for each key clinicopathological factor and M. globosa level in univariate and multivariate Cox regression analysis for overall survival (E) and recurrence-free survival (F) in the clinical cohort (n =180) (G, H) Kaplan-Meier curves depicting the overall survival (G) and recurrence-free survival (H) for PDAC patients who were treated with adjuvant chemotherapy (n = 121). PDAC, pancreatic ductal adenocarcinoma; PCR, polymerase chain reaction; M. globosa, Malassezia globosa; N.C., negative control; HR, hazard ratio; CI, confidence interval; LNM, lymph node metastases; LVI, lymphovascular invasion; CEA, carcinoembryonic antigen; CA19–9, carbohydrate antigen 19–9.
First, the correlation between M. globosa levels and clinicopathological factors was assessed to determine their prognostic potential. In these analyses, M. globosa levels did not significantly correlate with other key clinicopathological factors, including patient demographics, tumor characteristics, and serum tumor markers (Supplementary Table. S1). However, male patients tended to have a higher burden of M. globosa-positive cases vs. female patients (P =0.09). This higher abundance of Malassezia species in male patients was also found in previous studies that quantified Malassezia colonization of the skin11, 12. It was unclear whether this higher abundance of M. globosa in male patients may affect differential gender burden and prognostic outcomes in PDAC. Therefore, further studies are warranted on this topic, including investigating larger global populations to confirm the findings further.
Next, to determine whether the intratumoral M. globosa levels might serve as predictors of prognosis in patients with PDAC, Kaplan-Meier analyses were performed for overall survival (OS) and recurrence-free survival (RFS). Interestingly, M. globosa-positive PDACs exhibited a significantly poor OS (hazard ratio [HR]: 1.69; 95% confidence interval [CI]: 1.21–2.38; log-rank P <0.01; Fig. 1C), as well as markedly poor RFS (HR: 1.51; 95% CI: 1.07–2.11; P =0.02; Fig. 1D). Of note, multivariate Cox regression analyses revealed that the M. globosa levels were significant and independent predictors for both OS (HR: 1.66; 95% CI: 1.17–2.35; P <0.01; Fig. 1E) and RFS (HR: 1.51; 95% CI: 1.07–2.13; P =0.02; Fig. 1F), highlighting that intratumoral M. globosa levels have the potential to serve as prognostic biomarkers in patients with PDAC.
Finally, we examined the impact of intratumoral M. globosa levels on treatment response in PDAC patients treated with adjuvant chemotherapy following curative resection of their tumors, using Kaplan-Meier analyses. In line with our findings for all cases, M. globosa-positive PDACs exhibited a significantly poor OS (HR: 2.00; 95% CI: 1.31–3.06; P <0.01; Fig. 1G) and RFS (HR: 1.55; 95% CI: 1.02–2.37; P =0.04; Fig. 1H) in the subset of patients treated with adjuvant chemotherapy. These results illustrate that patients with M. globosa-positive PDAC might have greater resistance to adjuvant chemotherapy, rendering an overall poor prognosis in this malignancy.
In summary, we, for the first time, demonstrate that intratumoral M. globosa levels are associated with cancer-related survival and tumor recurrence in patients with PDAC. In addition, our study provides novel evidence that M. globosa levels are a significant predictor of therapeutic response to adjuvant chemotherapy in PDAC patients. This study highlights that M. globosa levels are a significant predictor of prognosis in PDAC patients and might be a potential target of antifungal intervention in patients with PDAC.
Supplementary Material
Acknowledgments:
The PDAC Biomarker Working Group: Naoki Takahashi (Department of Gastroenterology, Saitama Cancer Center, Saitama, Japan), Yasuhide Yamada (Comprehensive Cancer Center, National Center for Global Health and Medicine, Tokyo, Japan), Mitsuro Kanda (Department of Gastroenterological Surgery, Nagoya University Graduate School of Medicine, Nagoya, Japan), Yasuhiro Kodera (Department of Gastroenterological Surgery, Nagoya University Graduate School of Medicine, Nagoya, Japan), Hideo Baba (Department of Gastroenterological Surgery, Graduate School of Medical Sciences, Kumamoto University, Kumamoto, Japan).
Funding:
This work was supported by the CA072851, CA184792, CA187956, CA202797, CA227602, and CA214254 grants from the National Cancer Institute and National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors disclosed no potential conflicts of interest.
Data availability:
Data is contained within the article.
REFERENCES
- 1.Cui L, Morris A, Ghedin E. The human mycobiome in health and disease. Genome Med 2013;5:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huffnagle GB, Noverr MC. The emerging world of the fungal microbiome. Trends Microbiol 2013;21:334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu X, Xia Y, He F, et al. Intestinal mycobiota in health and diseases: from a disrupted equilibrium to clinical opportunities. Microbiome 2021;9:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kong HH, Segre JA. Cultivating fungal research. Science 2020;368:365–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aykut B, Pushalkar S, Chen R, et al. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature 2019;574:264–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pushalkar S, Hundeyin M, Daley D, et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov 2018;8:403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang T, Fan C, Yao A, et al. The Adaptor Protein CARD9 Protects against Colon Cancer by Restricting Mycobiota-Mediated Expansion of Myeloid-Derived Suppressor Cells. Immunity 2018;49:504–514.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coker OO, Nakatsu G, Dai RZ, et al. Enteric fungal microbiota dysbiosis and ecological alterations in colorectal cancer. Gut 2019;68:654–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Narunsky-Haziza L, Sepich-Poore GD, Livyatan I, et al. Pan-cancer analyses reveal cancer-type-specific fungal ecologies and bacteriome interactions. Cell 2022;185:3789–3806.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nash AK, Auchtung TA, Wong MC, et al. The gut mycobiome of the Human Microbiome Project healthy cohort. Microbiome 2017;5:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugita T, Suzuki M, Goto S, et al. Quantitative analysis of the cutaneous Malassezia microbiota in 770 healthy Japanese by age and gender using a real-time PCR assay. Med Mycol. 2010;48:229–233. [DOI] [PubMed] [Google Scholar]
- 12.Hobi S, Cafarchia C, Romano V, et al. Malassezia: Zoonotic Implications, Parallels and Differences in Colonization and Disease in Humans and Animals. J Fungi (Basel). 2022;8:708. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is contained within the article.


