ABSTRACT
This study systematically reviewed the literature reporting the changes in rats’ core body temperature (TCORE) induced by either incremental- or constant-speed running to fatigue or exhaustion. In addition, multiple linear regression analyses were used to determine the factors contributing to the TCORE values attained when exercise was interrupted. Four databases (EMBASE, PubMed, SPORTDiscus, and Web of Science) were searched in October 2021, and this search was updated in August 2022. Seventy-two studies (n = 1,538 rats) were included in the systematic review. These studies described heterogeneous experimental conditions; for example, the ambient temperature ranged from 5 to 40°C. The rats quit exercising with TCORE values varying more than 8°C among studies, with the lowest and highest values corresponding to 34.9°C and 43.4°C, respectively. Multiple linear regression analyses indicated that the ambient temperature (p < 0.001), initial TCORE (p < 0.001), distance traveled (p < 0.001; only incremental exercises), and running speed and duration (p < 0.001; only constant exercises) contributed significantly to explaining the variance in the TCORE at the end of the exercise. In conclusion, rats subjected to treadmill running exhibit heterogeneous TCORE when fatigued or exhausted. Moreover, it is not possible to determine a narrow range of TCORE associated with exercise cessation in hyperthermic rats. Ambient temperature, initial TCORE, and physical performance-related variables are the best predictors of TCORE at fatigue or exhaustion. From a broader perspective, this systematic review provides relevant information for selecting appropriate methods in future studies designed to investigate exercise thermoregulation in rats.
KEYWORDS: Body mass, environment, heat, hyperthermia, performance, physical exercise, regression analysis, thermoregulation
Introduction
Rats are widely used to study the physiological mechanisms underlying the regulation of physical performance, including the thermoregulatory mechanisms [1]. In particular, they represent an experimental model for investigating exercise performance and physiology when the use of human subjects is neither feasible nor desirable [2]. For example, rats are used in studies involving invasive procedures that cannot be carried out in humans for ethical reasons [2]. These invasive procedures include brain cannulation [3,4], identification of exercise-induced activation of hypothalamic areas [5,6], and surgery for denervating peripheral receptors [7,8]. Utilizing rats is also an alternative when studies involve addressing physiological parameters in human subjects throughout their lifetimes, which is often impractical [2]. From the thermal biology perspective, studies of running rats can, with certain limitations (e.g. differences in evaporative heat loss), help understand some features of exercise thermoregulation in humans [1].
Physical exercise increases whole-body metabolic rate due to augmented chemical-to-mechanical energy transformation in contracting skeletal muscles [9]. In rats, the running-induced increase in metabolic rate can attain levels 2 to 4 times greater than baseline values [10–13], leading to higher heat production. Thus, augmented heat loss to the environment is necessary to counterbalance enhanced heat production and avoid exaggerated core body hyperthermia [14,15]. Interestingly, the increase in heat production precedes the increase in cutaneous heat loss, favoring body heat accumulation and the consequent core body temperature (TCORE) rise at the beginning of the exercise [1]. After that, the TCORE level attained by the rats will significantly depend on the ambient temperature (TAMB) and exercise intensity, duration, and protocol [1,16–18].
Augmented body temperature – either TCORE or muscular temperature – is associated with physiological benefits during exercise, including higher enzymatic activity [19], reduced saturation of hemoglobin and myoglobin with O2 molecules [20,21], augmented local blood flow [22], and attenuated increase in blood viscosity [23]. Therefore, some level of hyperthermia is required to promote adequate metabolic responses to physical exertion (e.g. adequate O2 supply to contracting muscles). However, severe hyperthermia influences the functioning of many physiological systems (e.g. cardiovascular and gastrointestinal systems) [24,25] and is associated with the occurrence of heat-related disorders, including heatstroke [26,27].
Alongside undesirable effects on health, severe hyperthermia might also favor the occurrence of fatigue or exhaustion during prolonged exercises, thus impairing endurance [28–30]. The increase in TCORE, particularly brain temperature, changes the electroencephalographic activity, increases perceived exertion, and ultimately impairs the ability of the central nervous system to stimulate the contraction of skeletal muscles [31,32]. Moreover, other indirect effects of augmented TCORE may influence endurance, such as reduced cerebral blood flow [33], enhanced cardiovascular strain [34,35], altered thermal perception [36], and muscle/systemic inflammation [37].
Considering the impacts of severe hyperthermia on an animal’s physical performance and health, we considered it essential to carefully assess the extensive literature reporting the changes in TCORE in rats exercised to fatigue or exhaustion. By systematically reviewing this literature, we expect to provide relevant insight into the factors (e.g. TAMB, body mass, and exercise intensity and duration) that may affect the TCORE value when rats quit exercising on a treadmill. Moreover, identifying what factors affect the increase in TCORE during exercise will allow the development of more effective strategies to mitigate the hyperthermia-induced degradation of endurance performance and minimize the incidence of severe heat illnesses.
Therefore, we aimed to systematically review the studies that recorded TCORE at fatigue or exhaustion in rats subjected to incremental- or constant-speed treadmill running. In addition, we analyzed the data extracted from these studies using multiple linear regression analysis to understand what factors affect the TCORE attained by the rats when they quit exercising.
Methods
Search strategy
The present systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [38]. The searched databases were: PubMed (U.S. National Institutes of Health), Web of Science (Clarivate), EMBASE (Elsevier), and SPORTDiscus (EBSCO). The following search terms were combined using Boolean operators: (exercise OR running) AND (thermoregulation OR hyperthermia) AND (fatigue OR exhaustion) AND rat.
The search was conducted in October 2021, with no date restrictions, and all included studies were manuscripts written in English that contained original data. An update search was conducted in August 2022. Books and book chapters, theses, dissertations, review articles, points of view, essays, editorials, and scientific meeting proceedings were not included, though their bibliographic reference lists were consulted during the screening process. We also checked the reference list of all selected manuscripts to ensure that our search strategy has not missed relevant studies.
The following inclusion criteria were set: a) the study sample should consist of rats; b) the intervention should be a running exercise on a treadmill; c) the exercise should have been performed until the rats were fatigued or exhausted; d) the study should have measured any TCORE index (e.g. abdominal, colonic, or brain temperature), at least when rats were fatigued or exhausted.
Methodological considerations
This systematic review focused on studies involving rats subjected to physical exercise. Although rats and humans have developed distinct strategies to address environmental challenges (e.g. rats have limited evaporative heat loss compared to humans), the exercise-induced adjustments in heat production and dissipation occur in the same direction in both species. Heat production sharply increases with exercise initiation, whereas the convective cutaneous heat loss decreases until the attainment of a threshold TCORE that triggers skin vasodilation. Thus, the increase in heat production is always faster than the increase in heat loss, raising TCORE at the beginning of the exercise in both species [1,39].
Treadmill running was chosen because this exercise modality allows for the continuous measurement of thermoregulatory parameters, such as the TCORE and skin temperature (TSKIN) [1], and the precise quantification of external work performed by rats [12,40]. Moreover, we focused on the TCORE at fatigue and exhaustion because it usually corresponds to the highest value attained during exercise and because this was the most reproducible thermoregulatory parameter when rats were subjected to repeated incremental running [41].
The terms “fatigue” and “exhaustion” are often used interchangeably in the literature. However, many authors differentiate them by stating that exhaustion is synonymous with fatigue but more intense [42]; i.e. exhaustion is considered extreme fatigue [1]. In addition, from a medical perspective, fatigue indicates a declining ability to respond to stressors, while exhaustion indicates an almost complete inability to respond to stressors [43].
In experiments with exercising rats, fatigue is usually determined as the moment when the animals cannot keep pace with the treadmill [44,45] and/or expose themselves to electrical stimulation during a predetermined time, which corresponds to 10 s in our experiments [3,46,47]. Using this criterion, we observed that fatigued rats could right themselves when placed on their backs. In contrast, exhaustion is usually confirmed by observing that exhausted rats lose their righting reflex [48–50]. Nevertheless, different criteria exist for defining exhaustion; for instance, in the study performed by Hasegawa et al. [51], “exhaustion was considered to have occurred when the rat was unable to keep pace with the treadmill and lay flat on, and stayed on the grid positioned at the back of the treadmill for a period of 30 s despite gently being pushed with sticks or breathed on.”
The TCORE (or deep body temperature) would ideally represent the mean temperature of the thermal core [52]. In practice, TCORE is represented by a specified temperature, such as the abdominal, arterial blood, brain, colonic, or rectal temperature in rats. Of note, the depth at which thermocouples are inserted past the anal sphincter determines whether the rectal or colonic temperature is measured. The thermal core corresponds to those inner tissues of the body whose temperatures are not altered in their relationship to each other by circulatory adjustments and changes in heat dissipation to the environment that affect the thermal shell of the body [52]. Brain temperatures have been considered a TCORE index [52], although evidence showing the independence of brain temperatures from other indices of TCORE is available [53]. Rats’ TCORE can be measured through different methods, including thermocouples, thermistors [54], and telemetry sensors [55].
The thermoregulation of small animal species, including rats, depends largely on environmental conditions [56], and, therefore, these conditions are another methodological aspect deserving attention. In this sense, it is crucial to introduce the thermoneutral zone (TNZ) concept. There are many definitions of the TNZ (for a detailed review, see ref [57]). According to the third Glossary of Terms for Thermal Physiology, the TNZ is defined as “the range of ambient temperature at which temperature regulation is achieved only by control of sensible heat loss, i.e. without regulatory changes in metabolic heat production or evaporative heat loss” [52], and this definition is currently prevalent [57]. Using this definition, Romanovsky et al. [58] developed a practical way to determine the TNZ of rats by measuring the TSKIN of their principal “heat-loss organ,” the tail, and then calculating the heat loss index (HLI). Briefly, the HLI is a ratio of two temperature gradients (TSKIN – TAMB and TCORE – TAMB) [59] and varies from 0 (maximal vasoconstriction) to 1 (maximal vasodilation). Within the TNZ, tail-skin vasoconstriction constantly changes for tail-skin vasodilation, and, consequently, high magnitude changes in tail TSKIN and HLI are observed [58].
When Wistar rats are resting in the treadmill setup at TAMBs below 24°C, their tails are vasoconstricted [46,60], and the HLI stabilizes at levels below < 0.1 (sub-neutral conditions). On the other hand, we observed HLI values ranging from 0.2 to 0.3 in resting rats maintained inside the chamber that contained the treadmill belt at a local temperature of 24–26°C [61–63], thus suggesting this TAMB range corresponds to the lower end of the TNZ of rats inside the treadmill setup. Moreover, at 30°C, the HLI varies largely between 0.2 to 0.4, indicating thermoneutral conditions [60]. Finally, at 35°C, rats’ tails are fully vasodilated [61,64], with HLI values consistently above 0.5 (supra-neutral conditions). Therefore, the TNZ of Wistar rats resting inside the treadmill setup is located between 23°C and 35°C, most likely close to 30°C.
It is noteworthy that exercise-induced TCORE increase is exaggerated at 31–32°C, as compared to 23–24°C [65–67], and no steady-state TCORE is attained while running under these warm environmental conditions (i.e. 31–32°C). Indeed, no previous study tried to determine a range of TAMB at which TCORE regulation in an exercising rat is achieved only by control of sensible heat loss. Even when TCORE remained unaltered throughout a running session in the cold, rats’ tails were clearly vasoconstricted [46]. Moreover, environmental factors other than TAMB may influence the TNZ inside the treadmill chamber, such as the wind speed (e.g. whether the equipment has an electrical fan in front of the treadmill belt) or relative humidity (e.g. rats increase evaporative heat loss from the respiratory tract in proportion to exercise intensity [17]). However, because wind speed and relative humidity were not reported in most experiments we reviewed, the TAMB was the only environmental factor included as an independent variable in the regression analyses.
Study selection
Studies were searched and inserted into the Rayyan web application (https://rayyan.qcri.org). The selection was carried out in two stages that involved reading the titles/abstracts and full texts. Next, studies were screened for inclusion by three researchers (MA, NB, and SW), with all cases of disagreement discussed until consensus was reached. Finally, inclusion and exclusion decisions were duly labeled in the Rayyan web application [68].
The search process retrieved a total of 329 articles. One hundred and eighty-four of these articles were excluded for being duplicates. Then, the title and abstract of the 145 remaining articles were read, and 53 were excluded at this stage. Next, 92 articles were selected for full reading, with 20 of these articles being excluded at this final stage; the reasons for exclusions are presented in Figure 1. In the end, 72 studies published between 1968 and 2022 were included in the systematic review (Figure 1).
Figure 1.
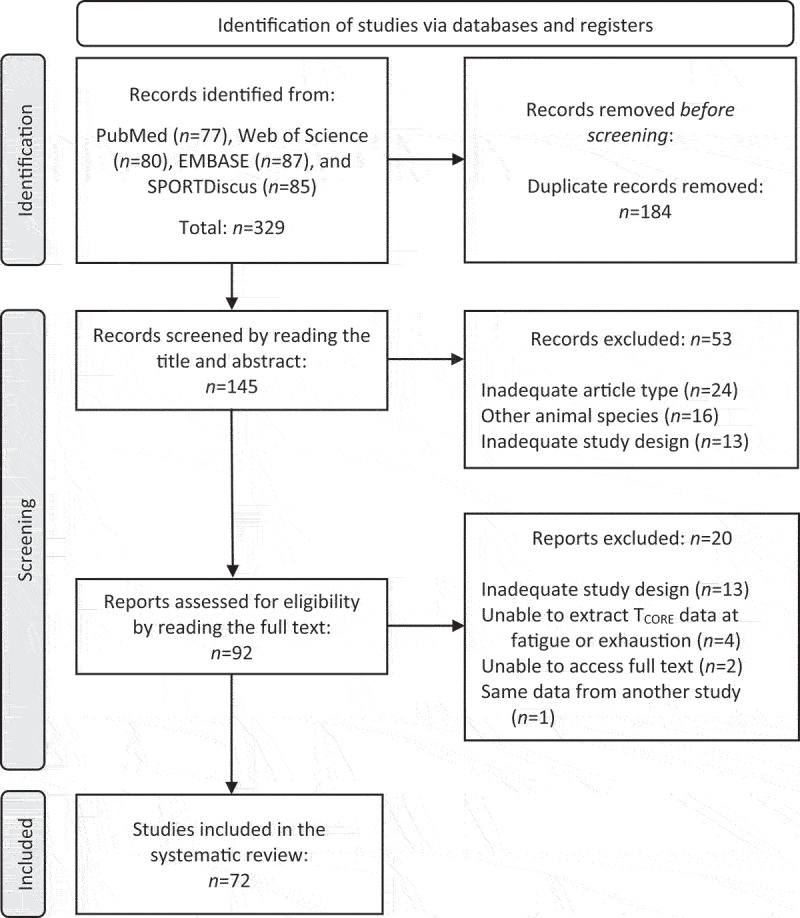
Flowchart of the process of identification, selection, eligibility, and inclusion of studies investigating the core body temperature in rats subjected to treadmill running until they were fatigued or exhausted. The screening process is based on the PRISMA flow diagram. Briefly, inadequate publication type included review articles and proceedings abstracts, whereas inadequate study design included other exercise types (i.e. swimming and running wheel), exercise with predetermined duration (not to fatigue or exhaustion), experiments with isolated tissues, and using heat-acclimated rats.
Data extraction
After selection, the following information was extracted from each article included in the systematic review: author, year of publication, rat strain, sex, body mass, age, exercise protocol, TAMB, daytime/period when experiments were carried out, TCORE index measured, time to fatigue/exhaustion, distance traveled, initial TCORE, and TCORE at fatigue or exhaustion. The treadmill speed was also extracted from the manuscripts involving constant-speed exercises. We always extracted the information as provided by the authors, even though literature provides different definitions for fatigue and exhaustion and for the site of TCORE measurement (e.g. rectal vs. colonic).
The data were mainly extracted from the control trials. We did not use data from rats subjected to pharmacological and behavioral interventions (e.g. manipulation of brain neurotransmission and sleep deprivation, respectively) or from trained or heat-acclimated rats because these conditions affect exercise thermoregulation. For example, aerobic training improves thermoregulatory efficiency during treadmill running, as trained rats run farther distances to attain a similar TCORE at fatigue compared to untrained rats [67,69]. Experiments with fasted rats were included in the systematic review if they were not fasted for more than 24 h. As reported earlier, fasting was used to standardize the metabolic and hormonal parameters before exercise and minimize their influence on performance [70]. Moreover, 24-h fasting did not influence endurance in low-intensity exercise under environmental heat stress [71].
When not available in the text or tables, the temperature and performance data were extracted from figures using the WebPlotDigitizer (version 4.5, https://automeris.io/WebPlotDigitizer; Pacifica, CA, USA) or were obtained through contact with the correspondent authors via electronic message.
The extracted data were divided into two tables: data from constant-speed (Table 1) and incremental-speed (Table 2) exercises, both to fatigue/exhaustion. The exercises with at least three different speed stages were considered incremental exercises. For example, Lubbe et al. [72] subjected the rats to an exercise starting at a speed of 10 m/min for 5 min, after which the speed was suddenly increased to 30 m/min; therefore, we considered the initial 5 min as a warm-up period and running at 30 m/min as a constant-speed exercise to exhaustion. We separated the data from these two protocols due to the marked differences in the evolution of running speed, which impacts the evolution of exercise intensity and, ultimately, the mechanisms underpinning fatigue/exhaustion. In this sense, metabolic factors are likely more critical for regulating fatigue than thermoregulatory factors during incremental exercises [18].
Table 1.
Studies that investigated the core body temperature at fatigue/exhaustion in rats subjected to incremental-speed exercises on a treadmill.
| Authors(year) | Number, sex, and strain | Body mass(g) | Time to fatigue (min) | Distance traveled (m) | Ambient temperature (°C) | Time of the day(h) | TCORE measured | TCORE at exercise initiation (°C) | TCORE at fatigue(°C) |
|---|---|---|---|---|---|---|---|---|---|
| Gollnick & Ianuzzo (1968) | 5 maleSprague-Dawley | 332–343 | – | – | 24 ± 1 | 13 to 16 | Colonic | 39.4 ± 0.2 | 41.8 ± 0.3 |
| Fruth & Gisolfi (1983) | 20 male Sprague-Dawley (survivors) | 250–400 | 68.0 ± 5.3 | 1521 | 27.1 (from 23 to 35) | NR | Colonic | 37.8 | 41.4 ± 0.1 |
| 14 male Sprague-Dawley(fatalities) | 250–400 | 79.9 ± 2.8 | 1842 | 28.2 (from 23 to 35) | NR | Colonic | 37.9 | 42.4 ± 0.2 | |
| Ardévol et al. (1999) | 6 female lean Zucker | 175–220 | 9.1 ± 0.4 | 255 | 27.8 | NR | Aortic | 36.7 ± 0.1 | 37.4 ± 0.1 |
| Balthazar et al. (2009) | 6 male Wistar | 250–300 | 17 ± 3 | 210 | 22 ± 1 | 13 to 17 | Abdominal | 37.7 ± 0.2 | 39.1 |
| Balthazar et al. (2010) | 6 male Wistar | 250–300 | 22.0 ± 3.1 | 290 | 22 ± 1 | 13 to 17 | Abdominal | 37.6 ± 0.4 | 38.5 |
| Fonseca et al. (2014) | 8 male Wistar | 250–365 | 42.9 ± 1.5 | 868 | 12 | 13 to 18 | Abdominal | 37.4 ± 0.1 | 37.3 ± 0.2 |
| 8 male Wistar | 250–365 | 33.2 ± 3.6 | 591 | 25 | 13 to 18 | Abdominal | 37.3 ± 0.1 | 38.6 ± 0.2 | |
| Kunstetter et al. (2014) | 7 male Wistar | 250–350 | 59.5 ± 4.4 | 1156 | 25.2 ± 0.2 | 10 to 16 | Brain (cortical) | 37.2 ± 0.1 | 39.3 ± 0.3 |
| Machado et al. (2015) | 11 male Wistar | 297 ± 5 | 52.1 ± 2.5 | 948 | 23 | 6 | Abdominal | 37.0 ± 0.1 | 40.0 ± 0.1 |
| 11 male Wistar | 297 ± 5 | 47.0 ± 2.0 | 815 | 23 | 20 | Abdominal | 38.4 ± 0.1 | 40.3 ± 0.2 | |
| Drummond et al. (2016) | 8 male Wistar | 383 ± 11 | 41.0 ± 3.4 | 670 | 25 | 7 to 13 | Abdominal/Brain (cortical) | 36.8 ± 0.1 /36.9 ± 0.1 | 39.4 ± 0.1 /38.6 ± 0.2 |
| 8 male Wistar | 383 ± 11 | 28.5 ± 1.6 | 407 | 32 | 7 to 13 | Abdominal/Brain (cortical) | 37.0 ± 0.1 /36.9 ± 0.1 | 40.0 ± 0.1 /39.5 ± 0.2 | |
| Machado et al. (2016) | 11 male Wistar | 342 ± 11 | 46.5 ± 2.3 | 803 | 24 ± 1 | 6 | Abdominal | 37.1 ± 0.2 | 39.8 ± 0.3 |
| 11 male Wistar | 340 ± 10 | 40.5 ± 2.3 | 659 | 24 ± 1 | 20 | Abdominal | 38.2 ± 0.2 | 40.1 ± 0.2 | |
| Morozova et al. (2016) | 6 maleSprague-Dawley | 300 ± 20 | 11.9 | 163 | 24 | 10 to 16 | Abdominal | 38.3 | 39.4 |
| 6 maleSprague-Dawley | 300 ± 20 | 14.8 ± 0.8 | 235 | 24 | 10 to 16 | Abdominal | 38.3 | 39.9 ± 0.2 | |
| Santiago et al. (2016) |
8 male Wistar |
350–370 |
38.6 ± 1.9 |
615 |
23 ± 1 |
NR |
Abdominal |
37.6 ± 0.1 |
38.7 ± 0.1 |
| Authors(year) |
Number, sex, and strain |
Body mass(g) |
Time to fatigue (min) |
Distance traveled (m) |
Ambient temperature (°C) |
Time of the day(h) |
TCORE measured |
TCORE at exercise initiation (°C) |
TCORE at fatigue(°C) |
| Kunstetter et al. (2018) | 6 male Wistar | 344 ± 18 | 43.8 ± 2.4 | 736 | 23 to 24 | NR | Abdominal | 37.0 ± 0.2 | 39.7 ± 0.2 |
| 6 male Wistar | 340 ± 16 | 45.2 ± 2.7 | 770 | 23 to 24 | NR | Abdominal | 38.0 ± 0.3 | 40.1 ± 0.2 | |
| Bittencourt et al. (2020) | 8 male Wistar | 287 ± 7 | 45.9 ± 2.9 | 788 | 32.1 ± 0.0 | NR | Colonic | 37.3 ± 0.1 | 42.1 ± 0.2 |
| 8 male Wistar | 287 ± 7 | 47.3 ± 2.4 | 823 | 32.1 ± 0.0 | NR | Colonic | 37.3 ± 0.1 | 41.9 ± 0.3 | |
| 8 male Wistar | 287 ± 7 | 49.5 ± 4.3 | 879 | 32.1 ± 0.1 | NR | Colonic | 37.0 ± 0.1 | 41.6 ± 0.3 | |
| Nunan et al. (2021) | 12 male Wistar | 270–300 g | 37.9 ± 1.9 | 600 | 25 | Light phase | Abdominal | 37.0 ± 0.1 | 39.4 ± 0.2 |
| Shang et al. (2021) | 9 male Wistar | 250–350 | 41.6 ± 2.6 | 889 | 24 | 19 to 21 | Colonic | 37.9 ± 0.1 | 40.3 ± 0.3 |
| 9 male Wistar | 250–350 | 43.6 ± 2.5 | 951 | 24 | 19 to 21 | Colonic | 38.0 ± 0.1 | 40.4 ± 0.2 | |
| 9 male Wistar | 250–350 | 32.7 ± 2.3 | 636 | 31 | 19 to 21 | Colonic | 38.1 ± 0.2 | 41.7 ± 0.3 | |
| 9 male Wistar | 250–350 | 39.1 ± 2.4 | 814 | 24 | 19 to 21 | Colonic | 38.1 ± 0.2 | 40.4 ± 0.1 | |
| 9 male Wistar | 250–350 | 31.8 ± 1.8 | 612 | 31 | 19 to 21 | Colonic | 38.3 ± 0.1 | 41.8 ± 0.2 | |
| Teixeira-Coelho et al. (2021) | 8 male Wistar | 220–250 g | 39.3 ± 2.8 | 630 | 23 | NR | Abdominal | 37.1 ± 0.1 | 39.5 ± 0.2 |
| 8 male Wistar | 220–250 g | 33.6 ± 2.5 | 508 | 32 | NR | Abdominal | 37.1 ± 0.1 | 40.4 ± 0.3 | |
| Andrade et al. (2022) | 20 male Wistar | 266.1 ± 19.8 | 55.1 ± 8.3 | 1041 | 24 | 12:30 to 17:30 | Abdominal | 36.7 ± 0.4 | 39.6 ± 0.8 |
| 20 male Wistar | 272.0 ± 18.8 | 57.1 ± 8.6 | 1099 | 24 | 12:30 to 17:30 | Abdominal | 36.9 ± 0.5 | 39.9 ± 0.8 | |
| 8 male Wistar | 339.4 ± 30.0 | 57.4 ± 5.5 | 1099 | 24 | 12:30 to 17:30 | Abdominal | 37.0 ± 0.3 | 40.1 ± 1.0 | |
| 8 male Wistar | 340.9 ± 29.0 | 47.6 ± 7.1 | 838 | 31 | 12:30 to 17:30 | Abdominal | 36.9 ± 0.3 | 40.9 ± 0.7 |
Data are expressed as means ± standard deviations or standard errors. Legend: NR = not reported. The data in italics were kindly provided by the corresponding/first authors, correspond to estimated values calculated from data included in the manuscripts, or were extracted from figures using the WebPlotDigitizer software.
Table 2.
Studies that investigated the core body temperature at fatigue/exhaustion in rats subjected to constant-speed exercises on a treadmill.
| Authors(year) | Number, sex, and strain | Body mass(g) | Time to fatigue (min) | Distance traveled (m) | Ambient temperature (°C) | Time of the day(h) | TCORE measured | TCORE at exercise initiation (°C) | TCORE at fatigue(°C) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Hubbard et al. (1976) | 30 male Sprague-Dawley | 484 ± 15 | 100 ± 30 | 1,100 | 5 | NR | Rectal | 38.4 ± 0.6 | 39.7 ± 0.7 | |
| 22 male Sprague-Dawley | 488 ± 17 | 57 ± 23 | 627 | 20, 23 or 26 | NR | Rectal | 38.1 ± 0.7 | 41.5 ± 0.2 | ||
| 41 male Sprague-Dawley | 486 ± 15 | 55 ± 22 | 605 | 20, 23 or 26 | NR | Rectal | 38.4 ± 0.6 | 41.9 ± 0.4 | ||
| Hubbard et al. (1977) | 21 male Sprague-Dawley | 485–545 | 57 ± 30 | 627 | 5, 20, 23 or 26 | NR | Rectal | NR | 41.1 ± 0.4 | |
| 16 male Sprague-Dawley | 485–545 | 58 ± 19 | 638 | 5, 20, 23 or 26 | NR | Rectal | NR | 41.5 ± 0.3 | ||
| 19 male Sprague-Dawley | 485–545 | 50 ± 16 | 550 | 5, 20, 23 or 26 | NR | Rectal | NR | 41.8 ± 0.3 | ||
| 22 male Sprague-Dawley | 485–545 | 50 ± 12 | 550 | 5, 20, 23 or 26 | NR | Rectal | NR | 42.0 ± 0.4 | ||
| 40 male Sprague-Dawley | 485–545 | 65 ± 23 | 715 | 5, 20, 23 or 26 | NR | Rectal | NR | 42.3 ± 0.5 | ||
| Francesconi & Mager (1979a) | 6 male Sprague-Dawley | 280–350 | 25.8 ± 1.4 | 236 | 35 ± 1 | NR | Rectal | 38.2 | 42.9 ± 0.1 | |
| 6 male Sprague-Dawley | 280–350 | 25.1 ± 1.1 | 229 | 35 ± 1 | NR | Rectal | 37.4 | 42.7 ± 0.1 | ||
| Francesconi & Mager (1979b) | 27 male Sprague-Dawley | 300–400 | NR | NR | 34–35 | NR | Rectal | NR | 42.7 ± 0.1 | |
| Hubbard et al. (1979) | 13 male Sprague-Dawley | 485 ± 12 | 145.3 | 1,598 | 5 | 8 | Rectal | NR | 38.2 ± 1.1 | |
| 57 male Sprague-Dawley | 485 ± 12 | 68.1 | 749 | 20, 26 or 30 | 8 | Rectal | NR | 41.5 ± 1.0 | ||
| Francesconi & Mager (1980) | 13 male Sprague-Dawley | 280–340 | 32.9 ± 1.7 | 301 | 35 | NR | Rectal | 37.0 | 42.6 ± 0.1 | |
| Francesconi & Mager (1981a) | 13 male Sprague-Dawley | 250–350 | 32.9 ± 1.7 | 301 | 35 ± 0.5 | NR | Rectal | 37.6 | 42.6 ± 0.1 | |
| Francesconi & Mager (1981b) | 16 male Sprague-Dawley | 250–350 | 30.5 ± 2.3 | 279 | 35 ± 0.5 | NR | Rectal | 38.4 | 42.9 ± 0.3 | |
| Francesconi & Mager (1981c) | 12 male Sprague-Dawley | 275–350 | 32.3 | 245 | 35 ± 0.5 | NR | Rectal | 37.4 | 42.41 | |
| Hubbard et al. (1981) |
12 male Sprague-Dawley |
250–300 |
113.3 |
1,246 |
15 |
NR |
Rectal |
37.9 ± 0.7 |
39.9 ± 0.5 |
|
| Authors(year) |
Number, sex, and strain |
Body mass(g) |
Time to fatigue (min) |
Distance traveled (m) |
Ambient temperature (°C) |
Time of the day(h) |
TCORE measured |
TCORE at exercise initiation (°C) |
TCORE at fatigue(°C) |
|
| Hubbard et al. (1981) | 12 male Sprague-Dawley | 250–300 | 117.7 | 1,295 | 20 | NR | Rectal | 37.9 ± 0.7 | 41.1 ± 0.5 | |
| Francesconi et al. (1983a) | 12 male Sprague-Dawley | 310–325 | 37.0 | 338 | 35 | NR | Rectal | NR | 43.0 | |
| Francesconi et al. (1983b) | 22 males | 300–325 | 34.7 ± 1.4 | 317 | 35 ± 0.5 | NR | Rectal | 38.7 | 43.4 | |
| Francesconi & Hubbard (1985) | 16 male Sprague-Dawley | 322.7 ± 4.5 | 31.6 | 289 | 35.5 | NR | Rectal | 38.3 | 43.0 | |
| 16 male Sprague-Dawley | 297.3 ± 3.7 | 35.1 | 321 | 35.5 | NR | Rectal | 38.5 | 42.1 | ||
| Durkot et al. (1986) | 16 male Sprague-Dawley | 237 ± 3.0 | 221 ± 26 | 2,431 | 26 | 6 to 12 | Rectal | 37.5 | 41.7 ± 0.2 | |
| 16 male Sprague-Dawley | 256 ± 3.0 | 108 ± 10 | 1,188 | 26 | 6 to 12 | Rectal | 38.0 | 42.3 ± 0.1 | ||
| 16 male Sprague-Dawley | 503 ± 3.0 | 67 ± 7 | 737 | 26 | 6 to 12 | Rectal | 38.2 | 42.0 ± 0.1 | ||
| 16 male Sprague-Dawley | 504 ± 3.0 | 54 ± 4 | 594 | 26 | 6 to 12 | Rectal | 38.6 | 41.9 ± 0.1 | ||
| Francesconi & Hubbard (1986) | 16 male Sprague-Dawley | 320 ± 4.7 | 31.9 ± 1.1 | 292 | 35.5 ± 0.5 | NR | Rectal | 38.4 ± 0.1 | 43.0 | |
| Matthew et al. (1987) | 10 male Sprague-Dawley | 510–530 | 53 | 583 | 26 | NR | Rectal | 38.6 ± 0.2 | 41.6 ± 0.2 | |
| Matthew et al. (1990) | 8 male Sprague-Dawley | 510–530 | 62 ± 8 | 682 | 26 | NR | NR | 38.8 ± 0.2 | 42.1 | |
| Caputa & Kamari (1991) | 12 male/female Wistar | NR | 62.1 ± 5.4 | 1,552 | 18 → 5 | NR | Rectal | 37.2 | 37.7 | |
| 12 male/female Wistar | NR | 62.1 ± 5.4 | 1,552 | 18 → 5 | NR | Brain | 37.5 | 37.8 | ||
| 12 male/female Wistar | NR | 16.2 ± 1.2 | 405 | 45 → 27 | NR | Rectal | 40.4 | 41.6 | ||
| 12 male/female Wistar | NR | 16.2 ± 1.2 | 405 | 45 → 27 | NR | Brain | 40.3 | 41.4 | ||
| Durkot et al. (1992) | 12 male Sprague-Dawley | 362 ± 7 | 70.3 ± 3.3 | 773 | 30 | NR | Rectal | 37.2 | 42.1 | |
| Matthew et al. (1992) | 12 male Sprague-Dawley | 510–530 | 67 ± 6 | 737 | 26 | NR | Rectal | 38.8 ± 0.1 | 41.8 ± 0.2 | |
| Matthew et al. (1993) |
12 male Sprague-Dawley |
510–530 |
80.1 |
881 |
10 |
NR |
Rectal |
38.8 ± 0.1 |
39.4 ± 0.3 |
|
| Authors(year) |
Number, sex, and strain |
Body mass(g) |
Time to fatigue (min) |
Distance traveled (m) |
Ambient temperature (°C) |
Time of the day(h) |
TCORE measured |
TCORE at exercise initiation (°C) |
TCORE at fatigue(°C) |
|
| Matthew et al. (1993) | 12 male Sprague-Dawley | 510–530 | 90.4 | 994 | 15 | NR | Rectal | 39.0 ± 0.1 | 40.5 ± 0.3 | |
| 12 male Sprague-Dawley | 510–530 | 67.6 | 744 | 26 | NR | Rectal | 38.8 ± 0.1 | 41.8 ± 0.2 | ||
| 12 male Sprague-Dawley | 510–530 | 30.0 | 330 | 30 | NR | Rectal | 39.0 ± 0.1 | 42.8 ± 0.1 | ||
| Durkot et al. (1995) | 20 male Sprague-Dawley | 300–400 | 101.3 ± 5.0 | 1,114 | 26 | NR | Rectal | 38.5 ± 0.1 | 41.3 ± 0.2 | |
| Fuller et al. (1998) | 14 male Sprague-Dawley | 350–450 | 29.4 ± 5.9 | 441 | 33 | 15.5 | Abdominal | 37.7 ± 0.6 | 39.9 ± 0.3 | |
| 14 male Sprague-Dawley | 350–450 | 29.4 ± 5.9 | 441 | 33 | 15.5 | Brain | 37.8 ± 0.6 | 40.2 ± 0.4 | ||
| 14 male Sprague-Dawley | 350–450 | 22.1 ± 3.7 | 332 | 38 | 15.5 | Abdominal | 37.8 ± 0.5 | 39.9 ± 0.3 | ||
| 14 male Sprague-Dawley | 350–450 | 22.1 ± 3.7 | 332 | 38 | 15.5 | Brain | 37.9 ± 0.6 | 40.2 ± 0.4 | ||
| 14 male Sprague-Dawley | 350–450 | 14.3 ± 2.9 | 215 | 38 | 15.5 | Abdominal | 38.2 ± 0.2 | 39.8 ± 0.3 | ||
| 14 male Sprague-Dawley | 350–450 | 14.3 ± 2.9 | 215 | 38 | 15.5 | Brain | 38.3 ± 0.2 | 40.1 ± 0.4 | ||
| Moran et al. (1999) | 8 male Zabar | 300–350 | 14 ± 1 | 350 | 40 | NR | Colonic | NR | 40.9 ± 0.2 | |
| Durkot et al. (2000) | 20 male Sprague-Dawley | 350 | 41 ± 4 | 451 | 30 | NR | Rectal | 38.1 ± 0.1 | 41.3 ± 0.2 | |
| Walters et al. (2000) | 11 male Sprague-Dawley | 380–390 | 38.1 ± 5.5 | 686 | 35 ± 1 | 12 to 13 | Rectal | 39.5 ± 0.1 | 42.4 ± 0.1 | |
| 11 male Sprague-Dawley | 380–390 | 38.1 ± 5.5 | 686 | 35 ± 1 | 12 to 13 | Brain | 39.4 ± 0.1 | 42.2 ± 0.2 | ||
| 11 male Sprague-Dawley | 380–390 | 35.8 ± 3.9 | 644 | 35 ± 1 | 12 to 13 | Rectal | 39.0 ± 0.2 | 42.5 ± 0.1 | ||
| 11 male Sprague-Dawley | 380–390 | 35.8 ± 3.9 | 644 | 35 ± 1 | 12 to 13 | Brain | 39.0 ± 0.2 | 42.2 ± 0.2 | ||
| Rodrigues et al. (2003) | 6 male Wistar | 260–360 | 57.7 ± 8.6 | 1,212 | 22 | NR | Abdominal | 37.2 ± 0.2 | 39.3 ± 0.3 | |
| 6 male Wistar | 260–360 | 41.6 ± 8.1 | 874 | 28 | NR | Abdominal | 37.6 ± 0.2 | 39.9 ± 0.3 | ||
| 6 male Wistar | 260–360 | 28.3 ± 5.4 | 594 | 35 | NR | Abdominal | 37.4 ± 0.1 | 41.3 ± 0.2 | ||
| 6 male Wistar | 260–360 | 38.3 ± 5.8 | 919 | 22 | NR | Abdominal | 37.2 ± 0.1 | 39.0 ± 0.2 | ||
| |
6 male Wistar |
260–360 |
25.8 ± 3.2 |
619 |
28 |
NR |
Abdominal |
37.7 ± 0.2 |
40.2 ± 0.2 |
|
| Authors(year) |
Number, sex, and strain |
Body mass(g) |
Time to fatigue (min) |
Distance traveled (m) |
Ambient temperature (°C) |
Time of the day(h) |
TCORE measured |
TCORE at exercise initiation (°C) |
TCORE at fatigue(°C) |
|
| Rodrigues et al. (2003) | 6 male Wistar | 260–360 | 17.2 ± 2.2 | 413 | 35 | NR | Abdominal | 37.3 ± 0.2 | 40.5 ± 0.1 | |
| Rodrigues et al. (2004) | 8 male Wistar | 250–300 | 52.5 ± 1.8 | 1,050 | 23 ± 2 | 10 to 14 | Colonic | 37.3 ± 0.1 | 39.1 ± 0.1 | |
| Soares et al. (2004) | 6 male Wistar | 270 ± 20 | 40 ± 3 | 720 | 23 ± 2 | 10 to 14 | Abdominal | 37.5 ± 0.2 | 38.0 ± 0.3 | |
| Lacerda et al. (2005) | 6 male Wistar | 250–340 | 92.7 | 1,667 | 21 ± 2 | 13 to 17 | Colonic | 37.3 | 39.2 ± 0.1 | |
| Leite et al. (2006) | 15 male Wistar | 240–330 | 52.5 ± 6.7 | 945 | 22 ± 2 | 10 to 14 | Abdominal | 37.6 | 38.7 ± 0.1 | |
| Pires et al. (2007) | 8 male Wistar | 250–300 | 27.8 ± 5.4 | 667 | 26 ± 1 | 9 to 16 | Abdominal | 37.5 ± 0.2 | 38.5 ± 0.4 | |
| Wanner et al. (2007) | 8 male Wistar | 250 − 300 | 33.5 ± 3.4 | 804 | 23 ± 1 | NR | Abdominal | 36.8 ± 0.4 | 38.0 | |
| 4 male Wistar | 250 − 300 | 23.7 ± 2.5 | 569 | 23 ± 1 | NR | Abdominal | 36.8 ± 0.2 | 37.8 | ||
| Hasegawa et al. (2008) | 8 male Wistar | 300–350 | 143.6 ± 21 | 3,734 | 18 | NR | Abdominal | 37.8 ± 0.8 | 39.6 ± 0.6 | |
| 8 male Wistar | 300–350 | 143.6 ± 21 | 3,734 | 18 | NR | Brain | 37.9 | 39.1 ± 0.8 | ||
| 8 male Wistar | 300–350 | 65.8 ± 13 | 1,711 | 30 | NR | Abdominal | 37.6 ± 0.8 | 41.0 ± 0.7 | ||
| 8 male Wistar | 300–350 | 65.8 ± 13 | 1,711 | 30 | NR | Brain | 37.8 | 40.5 ± 0.7 | ||
| Rodrigues et al. (2008) | 7 male Wistar | 300 ± 20 | 53.2 ± 2.5 | 1,064 | 23 ± 2 | 10 to 14 | Colonic | 37.3 ± 0.1 | 38.9 ± 0.1 | |
| Leite et al. (2010) | 6 male Wistar | 290 ± 10 | 70.7 ± 6.6 | 1,273 | 22 ± 2 | 10 to 14 | Abdominal | 37.3 | 38.7 ± 0.1 | |
| Pires et al. (2010) | 23 male Wistar | 250–350 | 51 ± 4 | 918 | 26 | 9 to 16 | Abdominal | 38.0 ± 0.1 | 38.8 ± 0.1 | |
| 5 male Wistar | 250–350 | 45.8 | 824 | 26 | 9 to 16 | Abdominal | 38.0 | 38.8 | ||
| Guimarães et al. (2013) | 9 male Wistar | 250–350 | 74.7 ± 12.8 | 1,494 | 8 | NR | Abdominal | 37.2 ± 0.4 | 36.5 ± 0.4 | |
| 9 male Wistar | 250–350 | 63.9 ± 7.1 | 1,278 | 12 | NR | Abdominal | 37.2 ± 0.3 | 37.1 ± 0.4 | ||
| 9 male Wistar | 250–350 | 61.3 ± 6.8 | 1,226 | 15 | NR | Abdominal | 37.0 ± 0.3 | 37.6 ± 0.4 | ||
| 7 male Wistar | 250–350 | 53.7 ± 6.7 | 1,074 | 5 | NR | Abdominal | 36.0 ± 0.3 | 34.9 | ||
| 7 male Wistar | 250–350 | 49.3 ± 3.7 | 986 | 12 | NR | Abdominal | 37.1 ± 0.3 | 37.3 ± 0.3 | ||
| 6 male Wistar | 250–350 | 50.7 ± 4.6 | 1,014 | 15 | NR | Abdominal | 36.8 ± 0.2 | 37.4 | ||
| Pires et al. (2013) | 6 male Wistar | 280–350 | 66 ± 11 | 1,188 | 25 | 8 to 16 | Abdominal | 37.5 | 38.5 | |
| 6 male Wistar | 280–350 | 23 ± 2.0 | 414 | 35 | 8 to 16 | Abdominal | 37.7 | 40.9 ± 0.1 | ||
| Campos et al. (2014) | 8 male Wistar | 350–450 | 47.0 ± 7.1 | 630 | 25 | 7 to 12 | Abdominal | 36.9 | 38.9 | |
| |
8 male Wistar |
350–450 |
15.8 ± 1.0 |
211 |
32 |
7 to 12 |
Abdominal |
37.1 |
39.1 |
|
| Authors(year) |
Number, sex, and strain |
Body mass(g) |
Time to fatigue (min) |
Distance traveled (m) |
Ambient temperature (°C) |
Time of the day(h) |
TCORE measured |
TCORE at exercise initiation (°C) |
TCORE at fatigue(°C) |
|
| Cordeiro et al. (2014) | 6 male Wistar | 250–300 | 70.7 ± 20.8 | 1,273 | 23 | NR | Abdominal | 37.5 ± 0.1 | 38.5 ± 0.1 | |
| Fonseca et al. (2014) | 6 male Wistar | 250–365 | 67.1 ± 13.7 | 1,342 | 12 | 13 to 18 | Abdominal | 37.3 ± 0.1 | 37.7 ± 0.2 | |
| 6 male Wistar | 250–365 | 67.1 ± 13.7 | 1,342 | 12 | 13 to 18 | Brain | 37.4 ± 0.3 | 37.7 ± 0.2 | ||
| 7 male Wistar | 250–365 | 75.8 ± 13.2 | 1,516 | 12 | 13 to 18 | Abdominal | 37.2 | 37.4 | ||
| 7 male Wistar | 250–365 | 75.8 ± 13.2 | 1,516 | 12 | 13 to 18 | Brain | 37.4 | 37.5 | ||
| 7 male Wistar | 250–365 | 31.5 ± 5.6 | 630 | 25 | 13 to 18 | Abdominal | 37.4 ± 0.1 | 38.8 ± 0.2 | ||
| 7 male Wistar | 250–365 | 31.5 ± 5.6 | 630 | 25 | 13 to 18 | Brain | 37.7 ± 0.1 | 39.2 ± 0.1 | ||
| 7 male Wistar | 250–365 | 38.4 ± 6.1 | 768 | 25 | 13 to 18 | Abdominal | 37.5 | 39.0 | ||
| 7 male Wistar | 250–365 | 38.4 ± 6.1 | 768 | 25 | 13 to 18 | Brain | 37.7 | 39.2 | ||
| Kunstetter et al. (2014) | 9 male Wistar | 250–350 | 207 ± 15 | 3,726 | 25.2 ± 0.2 | 10 to 16 | Brain | 37.2 ± 0.1 | 40.5 ± 0.3 | |
| 9 male Wistar | 250–350 | 149 ± 14 | 3,129 | 25.2 ± 0.2 | 10 to 16 | Brain | 37.3 ± 0.1 | 40.3 ± 0.3 | ||
| 9 male Wistar | 250–350 | 62 ± 11 | 1,488 | 25.2 ± 0.2 | 10 to 16 | Brain | 37.2 ± 0.1 | 40.1 ± 0.2 | ||
| Lima et al. (2014) | 10 male Wistar | 240–330 | 61.2 ± 8.4 | 1,102 | 23 ± 1 | 7 to 12 | Abdominal | 37.4 ± 0.1 | 39.5 ± 0.2 | |
| Zheng et al. (2014) | 10 male Wistar | 300–350 | 104.4 ± 30.9 | 1,879 | 23 | NR | Abdominal | 37.6 ± 0.2 | 39.8 ± 0.6 | |
| Damasceno et al. (2015) | 6 male Wistar | 250–350 | 113.3 ± 25.0 | 2,379 | 24 ± 1 | 7 to 11 | Abdominal | 36.9 | 39.5 | |
| 6 male Wistar | 250–350 | 113.3 ± 25.0 | 2,379 | 24 ± 1 | 7 to 11 | Brain | 37.6 | 39.6 | ||
| Wanner et al. (2015) | 14 male Wistar | 250–280 | 27.2 ± 1.8 | 490 | 24.1 ± 0.4 | NR | Abdominal | 37.9 ± 0.1 | 38.9 ± 0.1 | |
| Drummond et al. (2016) | 8 male Wistar | 383 ± 11 | 177.5 ± 8.6 | 2,485 | 25 | 7 to 13 | Abdominal | 36.8 | 38.9 ± 0.2 | |
| 8 male Wistar | 383 ± 11 | 177.5 ± 8.6 | 2,485 | 25 | 7 to 13 | Brain | 36.8 | 38.4 ± 0.2 | ||
| 8 male Wistar | 383 ± 11 | 78.8 ± 11.8 | 1,103 | 32 | 7 to 13 | Abdominal | 36.9 | 40.7 | ||
| 8 male Wistar | 383 ± 11 | 78.8 ± 11.8 | 1,103 | 32 | 7 to 13 | Brain | 36.9 | 40.2 | ||
| Zheng et al. (2016) | 10 male Wistar | 300–350 | 91 ± 14.1 | 1,638 | 23 | NR | Abdominal | 37.8 | 39.7 ± 0.5 | |
| Malheiros-Lima et al. (2018) | 14 male Wistar | 307 | 39 ± 3 | 702 | 25 | NR | Abdominal | 37.1 | 38.7 ± 0.2 | |
| Zaretsky et al. (2018) | 7 male Sprague-Dawley | 300 ± 20 | 15.2 ± 1.8 | 274 | 32 ± 1 | 10 to 16 | Abdominal | 37.6 ± 0.1 | 40.0 ± 0.4 | |
| Gomes et al. (2019) | 8 male Wistar | 390–463 | 21.9 ± 1.91 | 350 | 25 | NR | Abdominal | 36.4 | 39.8 | |
| Lima et al. (2019) | 7 male Wistar | 250–350 | 287.0 ± 14.7 | 4,908 | 23 ± 1 | 8 to 12 | Abdominal | 37.0 ± 0.5 | 38.6 ± 0.3 | |
| Rabelo et al. (2019) | 7 male Wistar | 240–280 | 49.0 ± 8.4 | 490 | 24 ± 1 | 8 to 12 | Abdominal | 36.2 ± 0.1 | 38.1 ± 0.3 | |
| 6 male Wistar | 240–280 | 109.4 ± 8.3 | 1,422 | 24 ± 1 | 8 to 12 | Abdominal | 36.3 ± 0.1 | 38.1 ± 0.5 | ||
| 7 male Wistar | 240–280 | 224.6 ± 26.7 | 4,267 | 24 ± 1 | 8 to 12 | Abdominal | 36.6 ± 0.4 | 39.1 ± 0.2 | ||
| Wang et al. (2019) | 8 male Wistar | 270 ± 30 | 68.9 ± 10.0 | 1,446 | 38 to 40 | NR | Rectal | 37.1 | 40.8 ± 0.4 | |
| Rodovalho et al. (2020) | 7 male Wistar | 280–330 | 37.9 ± 10.0 | 682 | NR | NR | Abdominal | 37.9 | 38.9 | |
| Lubbe et al. (2021) | 12 male Sprague-Dawley | 150 ± 10 | 5.59 | 168 | NR | NR | Rectal | 33.5 ± 0.5 | 35.4 ± 1.0 | |
| 12 female Sprague-Dawley | 150 ± 10 | 8.83 | 265 | NR | NR | Rectal | 34.8 ± 1.0 | 37.1 ± 0.8 | ||
Data are expressed as means ± standard deviations or standard errors. Legend: NR = not reported. The data in italics were kindly provided by the corresponding/first authors, correspond to estimated values calculated from data included in the manuscripts, or were extracted from figures using the WebPlotDigitizer software.
Analysis of the studies’ quality
The quality of the articles included in the systematic review was determined using a qualitative assessment consisting of 13 questions, with answer categories of “no” and “yes,” scoring 0 and 1, respectively. Some questions also had an intermediate answer category (i.e. “partial”), corresponding to a score of 0.5. All questions and the assessment criteria are described in detail in Supplementary file 1. This quality assessment was elaborated by the first (MA) and the last (SW) authors and then improved by the other collaborators; it is worth noting that some of the current study’s authors have extensive experience studying exercise thermoregulation and performance in rats. Pearson’s correlation coefficient was used to assess the strength of the association between the years of publication and the studies’ quality.
Multiple linear regression analysis
This analysis was performed to understand the contribution of different parameters on the TCORE attained by rats at fatigue or exhaustion during treadmill running. Variables related to the regression model (adjusted R2 and standard error of the estimate), the regression coefficients, and the beta weights are reported in the Results section. It is noteworthy that the regression coefficients cannot be used to establish the relative importance of specific variables within a regression equation because they are based on different units of measurement [73]. Therefore, the standardized regression coefficients (i.e. converted to z-scores), also called beta weights, were presented to provide the reader with a complete and practical interpretation of the observed relationships [73]. The analyses were performed using the IBM SPSS Statistics software (version 19.0, International Business Machines Corporation, Armonk – NY, USA). The significance level was set at p < 0.05.
The following parameters were evaluated when analyzing the incremental exercises: body mass, TAMB, distance traveled, and initial TCORE. When the manuscript reported a range for rats’ body mass, the highest value was used in the regression analysis because most studies exercised the rats at the end of the experimental design. The model did not include daytime and time to fatigue because these parameters directly affect the initial TCORE and distance traveled, respectively. In addition, strain, sex, exercise protocol, and TCORE index were not included because these are categorical parameters. We performed an additional analysis that considered only the most common conditions observed in the systematic review to ensure that some categorical parameters (e.g. rat strain and TCORE index) have not influenced our results. Therefore, the additional analysis included male Wistar rats with their abdominal temperature measured while being subjected to an incremental exercise with an initial speed of 10 m/min and increases of 1 m/min every 3 min.
When analyzing the constant-speed exercises, we included treadmill speed and exercise duration instead of distance traveled in the multiple linear regression analysis. This is because faster treadmill speeds are commonly associated with shorter durations and lower distances traveled during constant exercises, whereas faster speeds are usually a prerequisite to traveling farther distances during incremental exercises. In addition, although the rats ran at 18 m/min in 16 studies (please see the Results section), the experimental conditions were highly variable between these investigations (e.g. rat strain, treadmill incline, and measured TCORE). This heterogeneity precluded us from performing an additional analysis with sufficient trials and considering only the most common conditions in the studies involving constant-speed exercises.
Results
Incremental-speed exercise
Eighteen studies, including 35 experimental trials, investigated the TCORE of rats subjected to incremental exercises until they were fatigued/exhausted (Table 1). Among them, seven studies provided data from one experimental trial [18,69,74–78], eight studies from two trials [47,67,79–84], and one study from three [85], four [41] or five trials [66].
Data from 324 rats were included in this analysis, with an average of 9 ± 4 (mean ± SD) rats per experimental trial. The animals subjected to exercise were Wistar male rats in most investigations, except for three studies that used Sprague-Dawley male rats [74,79,83] and one study that used lean Zucker female rats [75]. Of note, the latter study [75] was the only one investigating the thermoregulatory responses of female rats subjected to an incremental exercise to fatigue/exhaustion. The average body mass corresponded to 328 ± 45 g, but it is not always clear whether these values were recorded on the day rats exercised (Table 3).
Table 3.
Data extracted from studies assessing the core body temperatures of rats subjected to an exercise session to fatigue or exhaustion.
| Incremental exercises |
Constant exercises |
|||
|---|---|---|---|---|
| Parameter | Mean ± SD | Min. – Max. | Mean ± SD | Min. – Max. |
| Body mass (g) | 328 ± 45 | 220–400 | 375 ± 83 | 150–545 |
| Time to fatigue (min) | 40.8 ± 14.9 | 9.1–79.9 | 63.7 ± 47.8 | 5.6–287.0 |
| Running speed (m/min) | NA | NA | 17 ± 5 | 9–30 |
| Distance traveled (m) | 749 ± 344 | 163–1,842 | 1,037 ± 869 | 168–4,908 |
| TAMB (°C) | 25.7 ± 4.2 | 12.0–32.1 | 25.4 ± 8.2 | 5.0–40.0 |
| Initial TCORE (°C) | 37.5 ± 0.6 | 36.7–39.4 | 37.6 ± 0.9 | 33.5–40.4 |
| TCORE at fatigue/exh. (°C) | 40.0 ± 1.2 | 37.3–42.4 | 40.0 ± 1.8 | 34.9–43.4 |
Legend: exh. = exhaustion; Max. = maximum value; Min. = minimum value; NA = not applicable; SD = standard deviation; TAMB = ambient temperature; TCORE = core temperature. n = 35 and 101 trials for the incremental and constant exercises, respectively.
Concerning the exercise protocol, 12 studies reported incremental treadmill running with an initial speed of 10 m/min and 1 m/min increments every 3 min (Supplementary Table 1). However, Fonseca et al. [47] and Shang et al. [66] used slightly different protocols. In the first manuscript, the initial speed and the increments were the same; however, the speed was increased every 2 min [47]. In the second manuscript, the rats were subjected to a treadmill running with an initial speed of 13 m/min and 1.3 m/min increments every 3 min [66]. Finally, Gollnick & Ianuzzo [74] did not precisely describe the time to exhaustion and the duration of the running stages, which corresponded in their experiments to the time elapsed until the first and second plateau of TCORE. Therefore, this study was not included in the multiple linear regression analysis.
The rats ran on average for 40.8 ± 14.9 min and traveled a distance of 749 ± 344 m. Fruth & Gisolfi [79] and Ardévol et al. [75] reported the longest and shortest exercise duration (79.9 and 9.1 min), respectively. The farthest distance traveled corresponded to 1,842 m and was observed by Fruth & Gisolfi [79], whereas rats covered the shortest distance (i.e. 163 m) in the study by Morozova et al. [83]. The dry TAMB ranged from 12°C [47] to 32°C [67,85], with the average temperature corresponding to 25.7 ± 4.2°C.
The abdominal temperature was the TCORE index in most studies included in this systematic review. In contrast, Gollnick & Ianuzzo [74], Fruth & Gisolfi [79], Bittencourt et al. [85], and Shang et al. [66] measured the colonic temperature, whereas Ardévol et al. [75] and Kunstetter et al. [18] measured, respectively, the aortic and brain cortex temperatures. Interestingly, Drummond et al. [82] simultaneously measured the abdominal and brain cortex temperatures. The initial temperature (i.e. at the beginning of the exercise) ranged from 36.7°C [41] to 39.4°C [74], with average values of 37.5 ± 0.6°C. Finally, the TCORE at fatigue or exhaustion corresponded to 40.0 ± 1.2°C, being 37.3°C [47] and 42.4°C [79], the lowest and highest temperature values recorded (Figure 2a).
Figure 2.
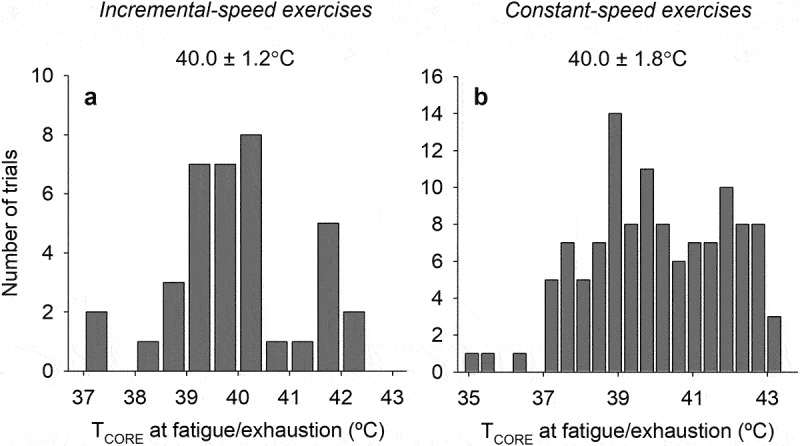
Histograms showing the distribution of the core body temperature (TCORE) attained at fatigue or exhaustion in rats subjected to incremental-speed (A) or constant-speed (B) exercises. The numeric values on the top indicate the mean ± SD for TCORE at fatigue/exhaustion in each exercise condition.
We performed a multiple regression analysis to understand the determinants of TCORE attained by rats at fatigue and exhaustion during incremental exercises (n = 34 trials). Three of the four parameters included in the analysis contributed significantly to explaining the variance in the TCORE at fatigue/exhaustion: distance traveled (t = 6.863; p < 0.001), TAMB (t = 9.401; p < 0.001), and initial temperature (t = 6.192; p < 0.001); the contribution of the body mass (t = −1.790; p = 0.083) was close to reaching statistical significance. This analysis led to the following regression equation, with an adjusted R2 = 0.813 and standard error of the estimate = 0.521:
TCORE at fatigue/exhaustion = −4.631 – (0.004 × Body mass) + (0.002 × Distance traveled) + (0.198 × TAMB) + (1.051 × Initial TCORE)
Standardized beta coefficients corresponded to −0.144, 0.540, 0.693, and 0.469 for the body mass, distance traveled, TAMB, and initial TCORE, respectively (Figure 3a). These data indicate that TAMB and body mass were, respectively, the variables with the best and worst predictive values used in the model. Moreover, these beta values mean that TCORE at fatigue/exhaustion increases by 0.693 (in standard deviations) when TAMB increases by one standard deviation – assuming other variables in the model are held constant. Finally, the negative values for the t-value and standardized beta coefficient regarding the body mass indicate an inverse association between this parameter and TCORE.
Figure 3.
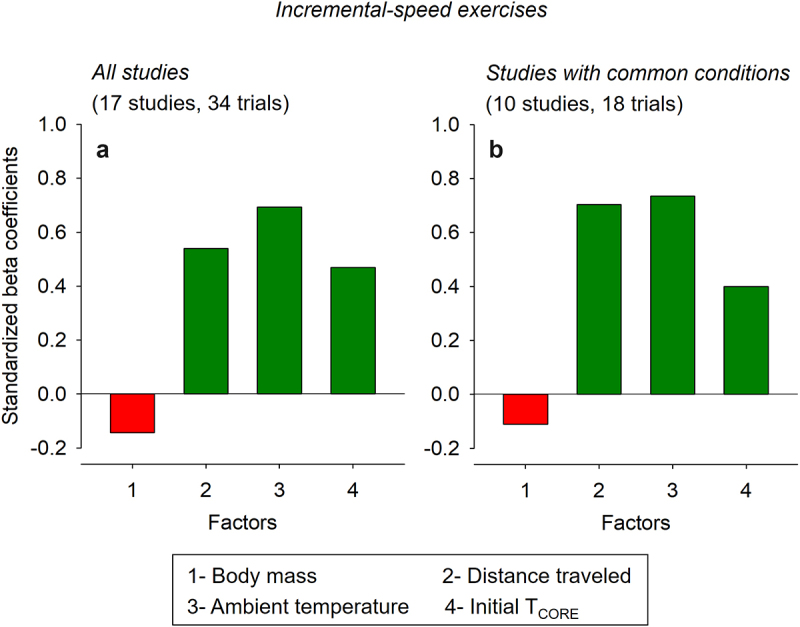
Standardized beta coefficients for the different factors included in the multiple linear regression analysis regarding the core body temperature (TCORE) of rats subjected to incremental-speed exercises to fatigue or exhaustion. Panel A shows the results from all studies included in the analysis. In contrast, panel B shows the results from studies with common conditions: the measurement of abdominal temperature in male Wistar rats subjected to an incremental exercise to fatigue, with an initial speed of 10 m/min and increases of 1 m/min every 3 min. The bars in red mean negative coefficients (i.e. inverse effects), while the bars in dark green mean positive coefficients (i.e. direct effects). In addition, the variables with greater coefficients are the most important for predicting TCORE at fatigue or exhaustion.
An additional analysis, considering only the 18 trials using the most common conditions observed in the systematic review, was conducted to ensure that excluding strain, exercise protocol, and TCORE index from the original regression analysis has not influenced our results. These most common conditions included measuring abdominal temperature in male Wistar rats subjected to an incremental exercise to fatigue (not exhaustion), with an initial speed of 10 m/min and increases of 1 m/min every 3 min. In this case, the same three parameters contributed significantly to explaining the variance in the TCORE at fatigue – distance traveled (t = 5.143; p < 0.001), TAMB (t = 5.762; p < 0.001), and initial TCORE (t = 3.292; p = 0.006) – whereas the body mass did not (t = −0.859; p = 0.406). This analysis led to the following regression equation, with an adjusted R2 = 0.727 and standard error of the estimate = 0.308:
TCORE at fatigue = 14.064 – (0.002 × Body mass) + (0.002 × Distance traveled) + (0.147 × TAMB) + (0.572 × Initial TCORE)
Standardized beta coefficients corresponded to −0.111, 0.709, 0.808, and 0.484 for the body mass, distance traveled, TAMB, and initial TCORE, respectively (Figure 3b). Again, these data indicate that TAMB and body mass were, respectively, the variables with the best and worst predictive values used in the model.
Constant-speed exercise
Fifty-seven studies, including 101 experimental trials, investigated the TCORE of rats subjected to constant exercise to fatigue or exhaustion (Table 2). Among them, 34 studies provided data from one experimental trial [4,6,44,45,62,63,86–113], 13 studies from two trials [3,8,51,64,70–72,82,114–118], four studies from three trials [13,18,48,119], three studies from four trials [47,120,121], one study from five trials [122], and two studies from six trials [46,123].
Data from 1,214 rats were included in this analysis, with an average of 12 ± 8 (mean ± SD) rats per experimental trial. In most investigations, the animals subjected to exercise were Wistar and Sprague-Dawley rats, except Moran et al. [99], which used Zabar rats, and Francesconi et al. [92], which did not report the strain. Only two studies investigated the thermoregulatory responses of female rats subjected to constant exercise [72,116]. The average body mass corresponded to 375 ± 83 g (Table 3).
Concerning the exercise protocol, 16 of the 57 studies reported constant treadmill running at 18 m/min (Supplementary Table 2). This was the most common protocol found in the literature, possibly because 18 m/min corresponds to approximately 65–80% of the maximum aerobic speed attained by untrained rats [41,124] and thus allows elevated metabolic heat production for long periods. The fastest running speed corresponded to 30 m/min [72], whereas the slowest speed was 9.14 m/min, used in a series of studies by Hubbard’s group in the 1970s and 1980s [48,70,115,122].
The rats ran on average for 63.7 ± 47.8 min and traveled a distance of 1,037 ± 869 m. Lima et al. [6] and Lubbe et al. [72] reported the longest and shortest exercise duration (287.0 and 5.6 min), respectively. Therefore, the farthest distance traveled corresponded to 4,908 m and was observed by Lima et al. [6], whereas rats covered the shortest distance (i.e. 168 m) in the study by Lubbe et al. [72]. The dry TAMB ranged from 5°C [46] to 40°C [99], with the average temperature corresponding to 25.4 ± 8.2°C. Of note, Caputa & Kamari [116] initiated an experimental trial at a TAMB of 45°C but gradually decreased it to 27°C; this strategy aimed to maintain hypothalamic temperature around 41°C while rats were running.
In most studies included in this systematic review, the rectal and abdominal temperature were the TCORE index measured. Seven studies simultaneously recorded the rectal/abdominal and brain temperatures in the same rats [47,51,82,109,116,117,119]. The initial pre-exercise temperature ranged from 33.5°C [72] to 40.4°C [116], with average values of 37.6 ± 0.9°C. Finally, the TCORE at fatigue or exhaustion corresponded to 40.0 ± 1.8°C, being 34.9°C [46] and 43.4°C [92], the lowest and highest temperature values recorded (Figure 2b).
Multiple linear regression analysis was done to understand the determinants of TCORE values when rats quit running during constant exercises. Four of the five parameters included in the analysis contributed significantly to explaining the variance in the TCORE at fatigue/exhaustion: running speed (t = −5.610; p < 0.001), time to fatigue or exhaustion (t = 2.728; p = 0.008), TAMB (t = 10.022; p < 0.001), and initial TCORE (t = 7.756; p < 0.001). In contrast, the body mass (t = −0.714; p = 0.477) did not contribute significantly to explaining the variance of TCORE. This analysis led to the following regression equation, with an adjusted R2 = 0.786 and standard error of the estimate = 0.808:
TCORE at fatigue/exhaustion = −2.627 – (0.001 × Body mass) – (0.105 × Running speed) + (0.005 × Time to fatigue) + (0.120 × TAMB) + (1.097 × Initial TCORE)
Standardized beta coefficients corresponded to −0.040, −0.294, 0.139, 0.535, and 0.443 for the body mass, running speed, time to fatigue/exhaustion, TAMB, and initial TCORE, respectively (Figure 4). Again, TAMB and body mass were the variables with the best and worst predictive values used in the model. Finally, the negative values for the t-value and standardized beta coefficient regarding the body mass and running speed indicate the existence of inverse associations between these parameters and TCORE.
Figure 4.
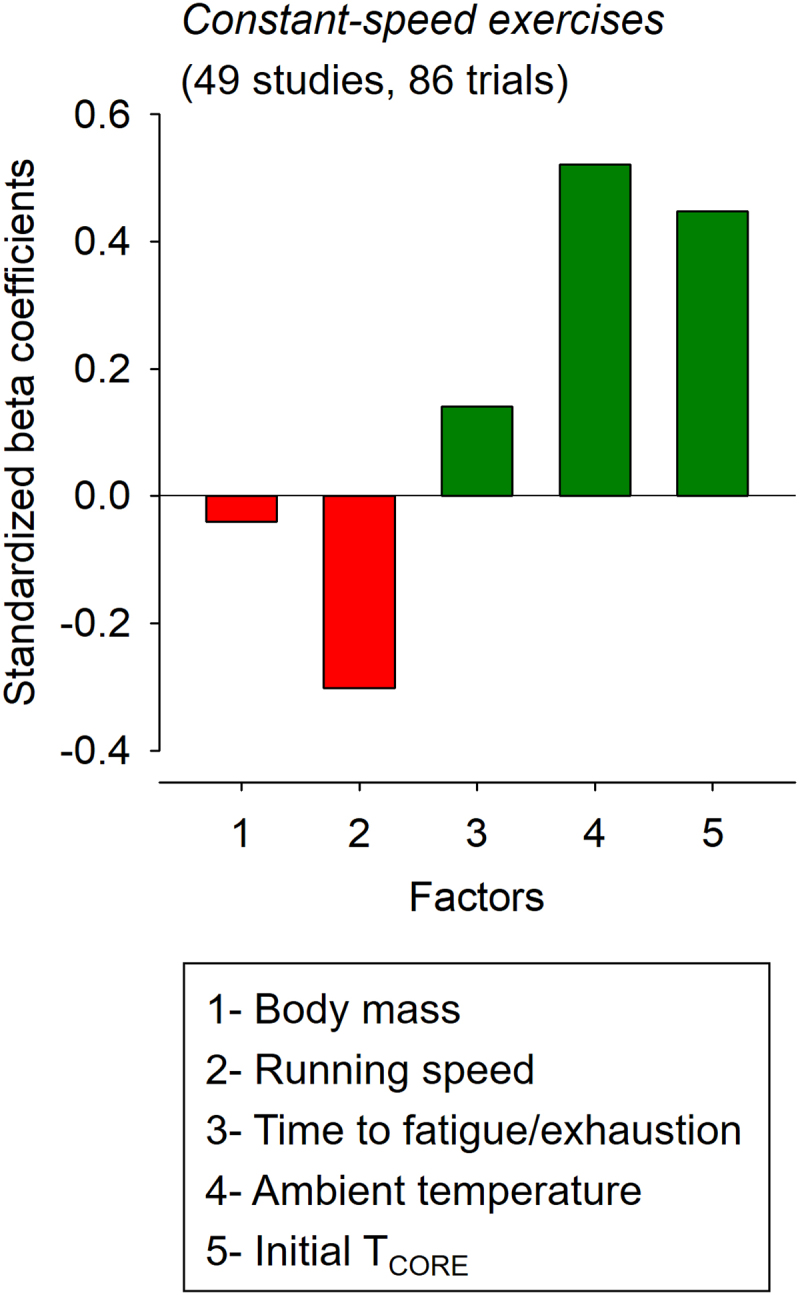
Standardized beta coefficients for the different factors included in the multiple linear regression analysis regarding the core body temperature (TCORE) of rats subjected to constant-speed exercises to fatigue or exhaustion. The bars in red mean negative coefficients (i.e. inverse effects), while the bars in dark green mean positive coefficients (i.e. direct effects). In addition, the variables with greater coefficients are the most important for predicting TCORE at fatigue or exhaustion.
Quality assessment
The quality assessment of the studies in which rats were subjected to incremental and constant-speed exercises is presented in Table 4.
Table 4.
Quality assessment results of the studies in which the rats were subjected to incremental- or constant-speed exercises to fatigue or exhaustion.
| Item | Incremental | Constant |
|---|---|---|
| 1- – Was the purpose of the study clearly stated? | 1.0 ± 0.0 | 1.0 ± 0.2 |
| 2- Was the information on the rats’ sex/strain and origin provided? | 0.7 ± 0.2 | 0.7 ± 0.3 |
| 3- Was the information on the age and body mass of the rats provided? | 0.9 ± 0.2 | 0.9 ± 0.2 |
| 4- Were the rats familiarized with running on a treadmill prior to the experiments? | 0.9 ± 0.3 | 0.6 ± 0.5 |
| 5- Was the method used to measure core body temperature described in detail? | 1.0 ± 0.0 | 0.9 ± 0.2 |
| 6- Was the exercise protocol described in detail? | 0.9 ± 0.2 | 0.7 ± 0.3 |
| 7- Was the core body temperature continuously measured during exercise? | 1.0 ± 0.1 | 0.9 ± 0.2 |
| 8- Were the criteria used to determine fatigue/exhaustion described in detail? | 0.8 ± 0.4 | 0.6 ± 0.4 |
| 9- Were the environmental conditions described in detail? | 0.5 ± 0.1 | 0.7 ± 0.3 |
| 10- Was the daytime/period when experiments were carried out described? | 0.5 ± 0.5 | 0.3 ± 0.4 |
| 11- Were the exercise-induced changes in core temperature analyzed with appropriate statistical tests? | 0.7 ± 0.3 | 0.6 ± 0.2 |
| 12- Was a limitation paragraph included in the study? | 0.2 ± 0.4 | 0.4 ± 0.5 |
| 13- Were the conclusions appropriate considering the objectives and methods of the study? | 1.0 ± 0.0 | 0.9 ± 0.3 |
| TOTAL | 10.1 ± 1.1 | 9.3 ± 1.5 |
Concerning the studies with incremental exercises, the average score was 10.1 ± 1.1 (mean ± SD) in 13.0 maximum possible points. All manuscripts attained the maximum score in the questions related to the objective, method for measuring TCORE, and conclusions. In contrast, the lowest scores were related to the lack of a limitations paragraph and inadequate information on the environmental conditions and daytime when experiments were conducted (more details in Supplementary Table 3). Interestingly, the publication date was positively associated with the quality score given to a manuscript (R2 = 0.300, p = 0.019; Pearson’s coefficient), thus indicating that the more recent studies were evaluated better than older ones.
With respect to the studies with constant-speed exercises, their average score was 9.3 ± 1.5 points (mean ± SD). The highest scores were obtained in the questions regarding the study objectives, information on age and body mass, method for measuring TCORE, continuous measurement of TCORE during the exercise (i.e. more than two time points), and conclusions. In contrast, the lowest scores were related to the lack of a limitations paragraph and insufficient information on daytime when experiments were conducted (more details in Supplementary Table 4). Again, the publication date was positively associated with the quality score given to a manuscript (R2 = 0.426, p < 0.001), thus indicating that the more recent manuscripts received better scores than older ones.
Discussion
The present systematic review provides extensive information regarding the TCORE attained by rats when exercising to fatigue or exhaustion and relevant information on the factors modulating the running-induced TCORE increase. The studies mainly investigated exercise thermoregulation in male Wistar or Sprague-Dawley rats and described heterogeneous experimental conditions; for example, TAMB ranged from 5 to 40°C, whereas the initial (pre-exercise) TCORE ranged from 33.5 to 40.4°C. Quite fascinating, TCORE values reported at exercise cessation varied approximately 8°C among the studies reviewed. The present systematic review also revealed that higher values of TAMB, initial TCORE, and physical performance-related variables (e.g. exercise duration for constant running or distance traveled for incremental running) are associated with higher TCORE at fatigue or exhaustion.
The lowest TCORE value (i.e. 34.9°C) at fatigue/exhaustion was observed in rats running in a cold environment [46]. Although this value is similar to hypothermic states induced experimentally by causing severe aseptic inflammation [125,126], Guimarães et al. [46] did not report fatalities following treadmill running, suggesting that hypothermia was reversed after the exercise. Interestingly, rats seem to have greater endurance under cold than temperate conditions [47,127]. On the other hand, the highest TCORE values were generally associated with fatalities. For example, almost every sedentary (i.e. non-trained) rat attaining more than 43.0°C did not survive [79]. Under these conditions, animal death results from heatstroke, a life-treating condition associated with uncontrolled systemic inflammatory response syndrome and disseminated intravascular coagulation, which combine to induce multi-organ system dysfunction and failure [27,128].
TAMB was the primary parameter determining the TCORE attained at fatigue or exhaustion during incremental and constant exercises, with augmented environmental heat stress inducing more significant hyperthermia. This finding confirms previous observations, including experiments when different TAMBs were used to induce different levels of hyperthermia [46,47,65]. Indeed, the apparent dependence of exercise hyperthermia on TAMB reinforces the notion that the regulation of TCORE in small mammals (e.g. rats and mice), which have a higher body mass-to-body surface ratio than bigger mammals, is susceptible to slight variations in TAMB [56,125,129,130]. Therefore, high TAMB limits the ability of rats to dissipate heat through dry pathways (e.g. convection and radiation), thus favoring marked increases in TCORE. Interestingly, while running in the cold, the facilitated heat exchange with the environment due to the high body mass-to-body surface ratio is not compensated by increased metabolism, and, therefore, rats become hypothermic [46].
The initial TCORE also determined its level when exercise was interrupted; the rats starting the treadmill running with higher TCORE were the ones that became fatigued or exhausted with more severe hyperthermia. Indirectly, this observation confirms the experimental evidence showing a circadian influence [80,81] or the influence of prior exposure to stress [84] or hot environments [119] on thermoregulation during exercise. The prominent role played by the initial TCORE also highlights the importance of developing rigorous designs that control the daytime at which experiments are conducted and ensure that rats are tested under stress-free conditions.
The distance traveled also determined TCORE at fatigue or exhaustion; therefore, the rats that ran farther attained higher temperatures during incremental exercise. In the case of this protocol, the distance traveled is a performance index that provides information on exercise duration but primarily on intensity. As speed gradually increases, the rats will cover longer distances within an exact time interval. Interestingly, the prominent role played by exercise intensity in increasing TCORE corroborates with data obtained from athletes. For example, the women cyclists winning gold medals in a team time trial (i.e. the ones with the highest power output) under environmental heat stress were among the athletes with higher hyperthermia levels (up to 41.5°C) during this competition [131]. Taken together, our current data in rats and data obtained from athletes suggest that the intensity of physical exertion determines the hyperthermia level attained during incremental exercise in rats or self-paced intermittent exercise in humans.
During constant-speed exercises, the TCORE at exercise interruption was positively associated with running duration while negatively associated with treadmill speed. Although a faster treadmill speed produces more intense increases in metabolic rate, tolerance to exercise under these conditions is very restricted. For example, male rats could run for less than 6 min at 30 m/min; this speed is greater than the maximum aerobic speed attained by untrained rats reported in some studies included herein [82,111,118]. On the other hand, several studies that subjected rats to hyperthermic exhaustion consisted of slow treadmill speeds (i.e. 9.14 and 11 m/min) during prolonged periods under environmental heat stress [48,114,120].
Finally, the body mass was the factor analyzed that less predicted the TCORE at fatigue or exhaustion, regardless of the exercise protocol. Moreover, the associations between the body mass and TCORE level were consistently negative, with lighter rats more susceptive to heating up. In this sense, lighter animals will heat up faster than heavier animals, provided the same amount of heat is stored in the core body region, mainly if heat originates from external sources. In contrast, it is expected that a lower body mass would exert a protective thermoregulatory role, as previously shown in humans. For instance, treadmill walking at a fixed external workload elicits a lower rate of metabolic heat production in lighter than heavier individuals [132]. Despite the divergent effects observed in rat and human experiments, body mass is a concern for interpreting data in weight-bearing exercises. Therefore, caution should be exercised in interpreting thermoregulatory data in rat experiments when body mass is different between experimental groups, such as the lower body mass in spontaneously hypertensive rats than in normotensive Wistar rats [82] and the reduction in body mass caused by aerobic training in rats subjected to a high-fat diet [133]. Lastly, whether the body mass indeed contributes inversely to determining the rats’ TCORE at fatigue or exhaustion is an issue to be investigated carefully in future studies.
This systematic review included 72 articles published between 1968 and 2022. Among these studies, only three subjected female rats to treadmill running [72,75,116]. The lack of data on female rats reproduces the underrepresentation of women in research dealing with exercise thermoregulation. While Hutchins et al. [134] reported that women accounted for 30% of the human subjects in the exercise thermoregulation research published in 2019, we observed that females represented only 2% of the rats investigated since 1968. The underrepresentation of women/females is undesirable because it significantly limits the generalization ability of the findings obtained only in men/males. This issue must be resolved soon, considering the increased number of women involved in physically demanding work activities [135] and their growing participation in elite sports [136,137].
The manuscripts included in the present review had their quality assessed as recommended by the PRISMA guidelines [38]. Although the average score was higher than 9 in 13 maximum possible points for the studies involving incremental- or constant-speed exercises, some relevant points should be highlighted. First, the authors of the current review elaborated themselves the checklist for quality assessment; therefore, some criteria seen as necessary by other research groups may have been left aside from the assessment. Second, positive and significant correlations were observed between the publication date and quality score, indicating that the more recent manuscripts received better scores than older ones. This observation is expected because the requirements for scientific publishing have become more stringent over time. Indeed, the recent manuscripts have method sections, particularly the statistical analyses, which are much more extensive and detailed than the older ones.
Despite the criticisms presented earlier, the quality assessment provided some crucial information for enhancing the quality of future experiments on the topic, including the necessity of more comprehensive control of methodological issues, such as the daytime when experiments are performed and the environmental factors other than TAMB. The current findings also encourage inserting the studies’ limitations at the end of the discussion section. Furthermore, it is worth noting that while the more recent studies received better scores in most criteria (e.g. description/use of familiarization sessions with treadmill running), this statement is not valid for all criteria. For example, the description of environmental conditions followed the opposite way, suggesting that the authors are not controlling these factors (e.g. relative humidity and artificial airflow inside the treadmill) as effectively as in the past. Indeed, the airflow generated by electric fans may facilitate convective cutaneous heat loss [2], and rats can evaporate water from their respiratory tract while running [17].
The present systematic review is not free of limitations. First, the data used in the multiple linear regression analysis consisted of average data extracted from the manuscripts included in the review. This is not an ideal procedure because average TCORE may be associated with different levels of heterogeneity or result from different sample sizes in the studies we reviewed. Ideally, data from individual rats should be used to run the regression analysis; however, these data are not available because several manuscripts were published more than 30 years ago. Second, data related to cutaneous heat loss and metabolic heat production were not extracted from the manuscripts included in the review. Because the changes in TCORE result from imbalances between the rates of heat production and dissipation [1,39], these aspects should be considered in future investigations.
Third, body mass data were reported as a range in several studies, and we did not have access to the body mass measured on the day of the treadmill exercise. In this case, we considered the greatest mass, which may have added imprecision to our analysis. Fourth, we included data from different TCORE indices, namely the abdominal, brain cortex, and colonic temperatures. The temperatures measured in specific body compartments are not homogeneous and do not respond in a similar way (particularly regarding their time course) to several arousing stimuli [138] and physical exercise [139]. Nonetheless, a sub-analysis using only the abdominal temperature measured during incremental exercises reproduced most findings of the analysis using the three TCORE indices, thus suggesting that the site of TCORE measurement was possibly not a confounding factor in the outcomes of the multiple linear regression analyses. Despite all these limitations, essential patterns have emerged from regression analyses, consistent with some existing ideas in the literature, as discussed earlier.
In conclusion, when fatigued or exhausted, rats subjected to treadmill running exhibit heterogeneous TCORE values. Moreover, it is not possible to determine a single TCORE or a narrow range of TCORE associated with exercise cessation in hyperthermic rats. More importantly, the present systematic review helps understand the parameters that determine the TCORE values attained at fatigue/exhaustion in two physical exercise protocols, with a particular reference to TAMB, initial TCORE, and physical performance-related parameters (i.e. distance traveled in the incremental exercises and duration in the constant exercises). In contrast, among the factors analyzed, the body mass was the one that least predicted the level of TCORE attained in both exercise protocols. Finally, from a broader perspective, this systematic review provides relevant information for selecting appropriate methods in future studies designed to investigate exercise thermoregulation in rats.
Supplementary Material
Funding Statement
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. NHSB was the recipient of post-graduate fellowship from CAPES, whereas KNOG received a post-doctoral fellowship from CAPES/Print. SPW receives a fellowship from the Conselho Nacional de Desenvolvimento Científico e Tecnologico (CNPq) for being a productive researcher (grant number 315199/2021–0). DAPG is currently funded by the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG, grant number APQ-01268-21). DDS, DAPG, TTM, and SPW are researchers of the MEDIANTAR group, which is supported by CNPq/MCTIC/CAPES/FNDCT/PROANTAR (grant number 442645/2018-0).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/23328940.2022.2115274
References
- [1].Wanner SP, Prímola-Gomes TN, Pires W, et al. Thermoregulatory responses in exercising rats: methodological aspects and relevance to human physiology. Temperature. 2015b;2(4):457–475. doi: 10.1080/23328940.2015.1119615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kregel KC, Allen DL, Booth FW, et al. Resource book for the design of animal exercise protocols. 1st ed. Bethesda (MA): American Physiological Society; 2006. [Google Scholar]
- [3].Wanner SP, Guimarães JB, Rodrigues LOC, et al. Muscarinic cholinoceptors in the ventromedial hypothalamic nucleus facilitate tail heat loss during physical exercise. Brain Res Bull. 2007;73(1–3):28–33. [DOI] [PubMed] [Google Scholar]
- [4].Zaretsky DV, Kline H, Zaretskaia MV, et al. Disinhibiting neurons in the dorsomedial hypothalamus delays the onset of exertional fatigue and exhaustion in rats exercising in a warm environment. Brain Res. 2018a;1689:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Soya H, Mukai A, Deocaris CC, et al. Threshold-like pattern of neuronal activation in the hypothalamus during treadmill running: establishment of a minimum running stress (MRS) rat model. Neurosci Res. 2007;58(4):341–348. [DOI] [PubMed] [Google Scholar]
- [6].Lima PMA, Campos HO, Fóscolo DRC, et al. The time-course of thermoregulatory responses during treadmill running is associated with running duration-dependent hypothalamic neuronal activation in rats. Brain Struct Funct. 2019;224(8):2775–2786. [DOI] [PubMed] [Google Scholar]
- [7].Ceroni A, Chaar LJ, Bombein RL, et al. Chronic absence of baroreceptor inputs prevents training-induced cardiovascular adjustments in normotensive and spontaneously hypertensive rats. Exp Physiol. 2009;94(6):630–640. [DOI] [PubMed] [Google Scholar]
- [8].Pires W, Wanner SP, Lima MRM, et al. Sinoaortic denervation prevents enhanced heat loss induced by central cholinergic stimulation during physical exercise. Brain Res. 2010;1366:120–128. [DOI] [PubMed] [Google Scholar]
- [9].González‐Alonso J, Quistorff B, Krustrup P, et al. Heat production in human skeletal muscle at the onset of intense dynamic exercise. J Physiol. 2000;524(2):603–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lacerda ACR, Rodrigues-Machado Mda G, Mendes PL, et al. Paraquat (PQ)-induced pulmonary fibrosis increases exercise metabolic cost, reducing aerobic performance in rats. J Toxicol Sci. 2009;34(6):671–679. [DOI] [PubMed] [Google Scholar]
- [11].Wanner SP, Guimarães JB, Pires W, et al. Muscarinic receptors within the ventromedial hypothalamic nuclei modulate metabolic rate during physical exercise. Neurosci Lett. 2011;488(2):210–214. [DOI] [PubMed] [Google Scholar]
- [12].Teixeira-Coelho F, Fonseca CG, Barbosa NHS, et al. Effects of manipulating the duration and intensity of aerobic training sessions on the physical performance of rats. PLoS One. 2017;12(8):e0183763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rabelo PCR, Cordeiro LMS, Aquino NSS, et al. Rats with higher intrinsic exercise capacities exhibit greater preoptic dopamine levels and greater mechanical and thermoregulatory efficiencies while running. J Appl Physiol. 2019;126(2):393–402. [DOI] [PubMed] [Google Scholar]
- [14].Gleeson M. Temperature regulation during exercise. Int J Sports Med. 1998;19(Suppl S 2):S96–S99. [DOI] [PubMed] [Google Scholar]
- [15].Sawka MN, Leon LR, Montain SJ, et al. Integrated physiological mechanisms of exercise performance, adaptation, and maladaptation to heat stress. Compr Physiol. 2011;1(4):1883–1928. [DOI] [PubMed] [Google Scholar]
- [16].Sonne B, Galbo H. Simultaneous determinations of metabolic and hormonal responses, heart rate, temperature and oxygen uptake in running rats. Acta Physiol Scand. 1980;109(2):201–209. [DOI] [PubMed] [Google Scholar]
- [17].Tanaka H, Yanase M, Nakayama T. Body temperature regulation in rats during exercise of various intensities at different ambient temperatures. Jpn J Physiol. 1988;38(2):167–177. [DOI] [PubMed] [Google Scholar]
- [18].Kunstetter AC, Wanner SP, Madeira LG, et al. Association between the increase in brain temperature and physical performance at different exercise intensities and protocols in a temperate environment. Braz J Med Biol Res. 2014;47(8):679–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Koga S, Shiojiri T, Kondo N, et al. Effect of increased muscle temperature on oxygen uptake kinetics during exercise. J Appl Physiol. 1997;83(4):1333–1338. [DOI] [PubMed] [Google Scholar]
- [20].Barcroft J, King WOR. The effect of temperature on the dissociation curve of blood. J Physiol. 1909;39(5):374–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Theorell H. The effect of temperature on myoglobin. Biochem Z. 1934;73:268. [Google Scholar]
- [22].Barcroft H, Edholm OG. The effect of temperature on blood flow and deep temperature in the human forearm. J Physiol. 1943;102(1):5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Buono MJ, Cabrales P. Hyperthermia during exercise - a double-edged sword. Temperature. 2016;3(4):512–513. doi: 10.1080/23328940.2016.1194954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Crandall CG, González-Alonso J. Cardiovascular function in the heat-stressed human. Acta Physiol. 2010;199(4):407–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pires W, Veneroso CE, Wanner SP, et al. Association between exercise-induced hyperthermia and intestinal permeability: a systematic review. Sports Med. 2017;47(7):1389–1403. [DOI] [PubMed] [Google Scholar]
- [26].Armstrong LE, Casa DJ, Millard-Stafford M, et al. American college of sports medicine position stand. Exertional heat illness during training and competition. Med Sci Sports Exerc. 2007;39(3):556–572. [DOI] [PubMed] [Google Scholar]
- [27].Bouchama A, Abuyassin B, Lehe C, et al. Classic and exertional heatstroke. Nat Rev Dis Primers. 2022;8(1):8. [DOI] [PubMed] [Google Scholar]
- [28].Cheung SS, Sleivert GG. Multiple triggers for hyperthermic fatigue and exhaustion. Exerc Sport Sci Rev. 2004;32(3):100–106. [DOI] [PubMed] [Google Scholar]
- [29].Racinais S, Cocking S, Périard JD. Sports and environmental temperature: from warming-up to heating-up. Temperature. 2017;4(3):227–257. doi: 10.1080/23328940.2017.1356427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].van Delden M, Bongers CCWG, Broekens D, et al. Thermoregulatory burden of elite sailing athletes during exercise in the heat: a pilot study. Temperature. 2019;6(1):66–76. doi: 10.1080/23328940.2018.1540964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Nybo L, Nielsen B. Hyperthermia and central fatigue during prolonged exercise in humans. J Appl Physiol. 2001a;91(3):1055–1060. [DOI] [PubMed] [Google Scholar]
- [32].Nybo L, Nielsen B. Perceived exertion is associated with an altered brain activity during exercise with progressive hyperthermia. J Appl Physiol. 2001b;91(5):2017–2023. [DOI] [PubMed] [Google Scholar]
- [33].Nybo L, Møller K, Volianitis S, et al. Effects of hyperthermia on cerebral blood flow and metabolism during prolonged exercise in humans. J Appl Physiol. 2002;93(1):58–64. [DOI] [PubMed] [Google Scholar]
- [34].González-Alonso J, Crandall CG, Johnson JM. The cardiovascular challenge of exercising in the heat. J Physiol. 2008;586(1):45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sawka MN, Cheuvront SN, Kenefick RW. High skin temperature and hypohydration impair aerobic performance. Exp Physiol. 2012;97(3):327–332. [DOI] [PubMed] [Google Scholar]
- [36].Flouris AD, Schlader ZJ. Human behavioral thermoregulation during exercise in the heat. Scand J Med Sci Sports. 2015;25(Suppl. 1):52–64. [DOI] [PubMed] [Google Scholar]
- [37].Vargas N, Marino F. Heat stress, gastrointestinal permeability and interleukin-6 signaling - Implications for exercise performance and fatigue. Temperature. 2016;3(2):240–251. doi: 10.1080/23328940.2016.1179380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLOS Med. 2021;18(3):e1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Webb P. The physiology of heat regulation. Am J Physiol. 1995;268(4 Pt 2):R838–R850. [DOI] [PubMed] [Google Scholar]
- [40].Brooks GA, Donovan CM, White TP. Estimation of anaerobic energy production and efficiency in rats during exercise. J Appl Physiol Respir Environ Exerc Physiol. 1984;56(2):520–525. [DOI] [PubMed] [Google Scholar]
- [41].Andrade PVR, Damasceno WC, Hudson ASR, et al. Reliability of physical performance and thermoregulatory parameters in rats subjected to an incremental treadmill running. J Therm Biol. 2022;108:103270. [DOI] [PubMed] [Google Scholar]
- [42].Zaretsky DV, Kline H, Zaretskaia MV, et al. Automatic analysis of treadmill running to estimate times to fatigue and exhaustion in rodents. PeerJ. 2018b;6:e5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Olson K, Turner AR, Courneya KS, et al. Possible links between behavioral and physiological indices of tiredness, fatigue, and exhaustion in advanced cancer. Support Care Cancer. 2008;16(3):241–249. [DOI] [PubMed] [Google Scholar]
- [44].Soares DD, Lima NR, Coimbra CC, et al. Intracerebroventricular tryptophan increases heating and heat storage rate in exercising rats. Pharmacol Biochem Behav. 2004;78(2):255–261. [DOI] [PubMed] [Google Scholar]
- [45].Leite LH, Lacerda AC, Marubayashi U, et al. Central angiotensin AT1-receptor blockade affects thermoregulation and running performance in rats. Am J Physiol. 2006;291(3):R603–R607. [DOI] [PubMed] [Google Scholar]
- [46].Guimarães JB, Wanner SP, Machado SC, et al. Fatigue is mediated by cholinoceptors within the ventromedial hypothalamus independent of changes in core temperature. Scand J Med Sci Sports. 2013;23(1):46–56. [DOI] [PubMed] [Google Scholar]
- [47].Fonseca CG, Pires W, Lima MR, et al. Hypothalamic temperature of rats subjected to treadmill running in a cold environment. PLoS One. 2014;9(11):e111501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hubbard RW, Matthew WT, Linduska JD, et al. The laboratory rat as a model for hyperthermic syndromes in humans. Am J Physiol. 1976;231(4):1119–1123. [DOI] [PubMed] [Google Scholar]
- [49].Terblanche SE, Gohil K, Packer L, et al. The effects of endurance training and exhaustive exercise on mitochondrial enzymes in tissues of the rat (Rattus norvegicus). Comp Biochem Physiol Part A Mol Integr Physiol. 2001;128(4):889–896. [DOI] [PubMed] [Google Scholar]
- [50].Dousset E, Marqueste T, Decherchi P, et al. Effects of neonatal capsaicin deafferentation on neuromuscular adjustments, performance, and afferent activities from adult tibialis anterior muscle during exercise. J Neurosci Res. 2004;76(5):734–741. [DOI] [PubMed] [Google Scholar]
- [51].Hasegawa H, Piacentini MF, Sarre S, et al. Influence of brain catecholamines on the development of fatigue in exercising rats in the heat. J Physiol. 2008;586:141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].IUPS Thermal Commission, Blatteis C, Boulant J, Cabanac M, et al. Glossary of terms for thermal physiology: 3rd edition. Jpn J Physiol. 2001;51(2):245–280. [Google Scholar]
- [53].Kiyatkin EA. Brain temperature and its role in physiology and pathophysiology: lessons from 20 years of thermorecording. Temperature. 2019;6(4):271–333. doi: 10.1080/23328940.2019.1691896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Dyer I, Srinivasa L. Measuring temperature. Anaesth Intensive Care Med. 2009;10(5):256–260. [Google Scholar]
- [55].Yang Y, Gordon CJ. Ambient temperature limits and stability of temperature regulation in telemetered male and female rats. J Therm Biol. 1996;21(5–6):353–363. [Google Scholar]
- [56].Gordon CJ. 1993. Temperature regulation in laboratory rodents. 1st ed. Cambridge (UK): Cambridge University Press; DOI: 10.1017/CBO9780511565595 [DOI] [Google Scholar]
- [57].Romanovsky AA. The thermoregulation system and how it works. Handb Clin Neurol. 2018;156:3–43. [DOI] [PubMed] [Google Scholar]
- [58].Romanovsky AA, Ivanov AI, Shimansky YP. Selected contribution: ambient temperature for experiments in rats: a new method for determining the zone of thermal neutrality. J Appl Physiol. 2002;92(6):2667–2679. [DOI] [PubMed] [Google Scholar]
- [59].Székely M. Skin temperature-skin blood flow: assessment of thermoregulatory changes (Abstract). Acta Physiol Hung. 1986;68:284. [Google Scholar]
- [60].Moraes MM. Post-exercise hypotension at 23°C and 30°C: cardiovascular and thermoregulatory responses in Wistar rats and spontaneously hypertensive rats (SHR). Doctoral thesis in Sports Sciences. Universidade Federal de Minas Gerais, 2016. [Google Scholar]
- [61].Lima MR, Pires W, Fonseca IA, et al. Chronic sympathectomy of the caudal artery delays cutaneous heat loss during passive heating. Neurosci Lett. 2013;537:11–16. [DOI] [PubMed] [Google Scholar]
- [62].Wanner SP, Leite LHR, Guimarães JB, et al. Increased brain L-arginine availability facilitates cutaneous heat loss induced by running exercise. Clin Exp Pharmacol Physiol. 2015a;42(6):609–616. [DOI] [PubMed] [Google Scholar]
- [63].Malheiros-Lima MR, Pires W, Fonseca IAT, et al. Physical exercise-induced cardiovascular and thermoregulatory adjustments are impaired in rats subjected to cutaneous artery denervation. Front Physiol. 2018;9:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Pires W, Wanner SP, Lima MR, et al. Physical exercise performance in temperate and warm environments is decreased by an impaired arterial baroreflex. PLoS One. 2013;8(8):e72005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Hudson ASR, Soares ADN, Horta NAC, et al. The magnitude of physical exercise-induced hyperthermia is associated with changes in the intestinal permeability and expression of tight junction genes in rats. J Therm Biol. 2020;91:102610. [DOI] [PubMed] [Google Scholar]
- [66].Shang FLT, Wanner SP, Damasceno WC, et al. Independent effects of rapid eye movement sleep deprivation and exposure to environmental heat stress on aerobic performance and thermoregulatory responses in exercising rats. Temperature. 2021;8(2):188–201. doi: 10.1080/23328940.2020.1829939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Teixeira-Coelho F, Fonseca CG, Vaz FF, et al. Physical exercise-induced thermoregulatory responses in trained rats: effects of manipulating the duration and intensity of aerobic training sessions. J Therm Biol. 2021;97:102878. [DOI] [PubMed] [Google Scholar]
- [68].Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):e210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Santiago HP, Leite LH, Lima PM, et al. The improvement of exercise performance by physical training is related to increased hypothalamic neuronal activation. Clin Exp Pharmacol Physiol. 2016;43(1):116–124. [DOI] [PubMed] [Google Scholar]
- [70].Hubbard RW, Criss RE, Elliott LP, et al. Diagnostic significance of selected serum enzymes in a rat heatstroke model. J Appl Physiol Respir Environ Exerc Physiol. 1979;46(2):334–339. [DOI] [PubMed] [Google Scholar]
- [71].Francesconi RP, Hubbard RW. Food deprivation and exercise in the heat: thermoregulatory and metabolic effects. Aviat Space Environ Med. 1985;56(8):771–776. [PubMed] [Google Scholar]
- [72].Lubbe C, Harvey BH, Viljoen FP, et al. Forced running-induced rhabdomyolysis in the Sprague-Dawley rat: towards a rodent model of capture myopathy. Vet Res Commun. 2021;45(4):459–465. [DOI] [PubMed] [Google Scholar]
- [73].Portney LG, Watkins MP. Foundations of clinical research: applications to practice. 3rd ed. Philadelphia PA: FA Davis Company; 2015. [Google Scholar]
- [74].Gollnick PD, Ianuzzo CD. Colonic temperature response of rats during exercise. J Appl Physiol. 1968;24(6):747–750. [DOI] [PubMed] [Google Scholar]
- [75].Ardévol A, Adán C, Remesar X, et al. Hind leg heat balance in obese Zucker rats during exercise. Pflugers Arch. 1998;435(4):454–464. [DOI] [PubMed] [Google Scholar]
- [76].Balthazar CH, Leite LH, Rodrigues AG, et al. Performance-enhancing and thermoregulatory effects of intracerebroventricular dopamine in running rats. Pharmacol Biochem Behav. 2009;93(4):465–469. [DOI] [PubMed] [Google Scholar]
- [77].Balthazar CH, Leite LH, Ribeiro RM, et al. Effects of blockade of central dopamine D1 and D2 receptors on thermoregulation, metabolic rate and running performance. Pharmacol Rep. 2010;62(1):54–61. [DOI] [PubMed] [Google Scholar]
- [78].Nunan BLCZ, Drummond LR, Rodrigues QT, et al. Inhibition of nNOS in the paraventricular nucleus of hypothalamus decreases exercise-induced hyperthermia. Brain Res Bull. 2021;177:64–72. [DOI] [PubMed] [Google Scholar]
- [79].Fruth JM, Gisolfi CV. Work-heat tolerance in endurance-trained rats. J Appl Physiol Respir Environ Exerc Physiol. 1983;54(1):249–253. [DOI] [PubMed] [Google Scholar]
- [80].Machado FS, Rodovalho GV, Coimbra CC. The time of day differently influences fatigue and locomotor activity: is body temperature a key factor?. Physiol Behav. 2015;140:8–14. [DOI] [PubMed] [Google Scholar]
- [81].Machado FS, Fóscolo DR, Poletini MO, et al. Influence of time-of-day on maximal exercise capacity is related to daily thermal balance but not to induced neuronal activity in rats. Front Physiol. 2016;7:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Drummond LR, Kunstetter AC, Vaz FF, et al. Brain temperature in spontaneously hypertensive rats during physical exercise in temperate and warm environments. PLoS One. 2016;11(5):e0155919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Morozova E, Yoo Y, Behrouzvaziri A, et al. Amphetamine enhances endurance by increasing heat dissipation. Physiol Rep. 2016;4(17):e12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Kunstetter AC, Barbosa NHS, Moraes MM, et al. Pre-exercise exposure to the treadmill setup changes the cardiovascular and thermoregulatory responses induced by subsequent treadmill running in rats. Temperature. 2018;5(2):109–122. doi: 10.1080/23328940.2017.1388343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Bittencourt MA, Wanner SP, Kunstetter AC, et al. Comparative effects of two heat acclimation protocols consisting of high-intensity interval training in the heat on aerobic performance and thermoregulatory responses in exercising rats. PLoS One. 2020;15(2):e0229335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Francesconi R, Mager M. Heat- and exercise-induced hyperthermia: effects on high-energy phosphates. Aviat Space Environ Med. 1979b;50(8):799–802. [PubMed] [Google Scholar]
- [87].Francesconi RP, Mager M. Hypothermia induced by 5-thio-D-glucose: effects on treadmill performance in the heat. Aviat Space Environ Med. 1980;51(8):754–758. [PubMed] [Google Scholar]
- [88].Francesconi R, Mager M. Alcohol consumption in rats: effects on work capacity in the heat. J Appl Physiol Respir Environ Exerc Physiol. 1981a;50(5):1006–1010. [DOI] [PubMed] [Google Scholar]
- [89].Francesconi R, Mager M. Chronic chlorpromazine administration in rats: effects on ability to work in the heat. J Appl Physiol Respir Environ Exerc Physiol. 1981b;50(3):509–512. [DOI] [PubMed] [Google Scholar]
- [90].Francesconi R, Mager M. Prostaglandin E1 hyperthermia: effects on ability to work in the heat. J Appl Physiol Respir Environ Exerc Physiol. 1981c;51(1):62–67. [DOI] [PubMed] [Google Scholar]
- [91].Francesconi R, Hubbard R, Mager M. Malathion administration: effects on physiological and physical performance in the heat. Pharmacol Biochem Behav. 1983a;19(6):1031–1035. [DOI] [PubMed] [Google Scholar]
- [92].Francesconi R, Hubbard R, Mager M. Effects of pyridostigmine on ability of rats to work in the heat. J Appl Physiol Respir Environ Exerc Physiol. 1983b;56(4):891–895. [DOI] [PubMed] [Google Scholar]
- [93].Francesconi RP, Hubbard RW. Dietary manipulation and exercise in the heat: thermoregulatory and metabolic effects in rats. Aviat Space Environ Med. 1986;57(1):31–35. [PubMed] [Google Scholar]
- [94].Matthew CB, Hubbard RW, Francesconi RP, et al. Carbamate-induced performance and thermoregulatory decrements restored with diazepam and atropine. Aviat Space Environ Med. 1987;58(12):1183–1187. [PubMed] [Google Scholar]
- [95].Matthew CB, Francesconi RP, Bowers WD, et al. Chronic vs acute carbamate administration in exercising rats. Life Sci. 1990;47(4):335–343. [DOI] [PubMed] [Google Scholar]
- [96].Durkot MJ, Francesconi R, Hubbard R. The relationship of plasma catecholamines to peripheral blood flow and thermoregulation during exercise in the heat. J Therm Biol. 1992;17(3):155–159. [Google Scholar]
- [97].Matthew CB, Francesconi RP, Hubbard RW. Physostigmine: dose-response effects on endurance and thermoregulation during exercise. Life Sci. 1992;50(n.1):39–44. [DOI] [PubMed] [Google Scholar]
- [98].Durkot MJ, de Garavilla L, Caretti D, et al. The effects of dichloroacetate on lactate accumulation and endurance in an exercising rat model. Int J Sports Med. 1995;16(3):167–171. [DOI] [PubMed] [Google Scholar]
- [99].Moran D, Epstein Y, Wiener M, et al. Dantrolene and recovery from heat stroke. Aviat Space Environ Med. 1999;70(10):987–989. [PubMed] [Google Scholar]
- [100].Durkot MJ, de Garavilla L. Exercise in the heat: effects of an adenosine antagonist. Int J Sports Med. 2000;21(4):270–274. [DOI] [PubMed] [Google Scholar]
- [101].Rodrigues AG, Lima NRV, Coimbra CC, et al. Intracerebroventricular physostigmine facilitates heat loss mechanisms in running rats. J Appl Physiol. 2004;97(1):333–338. [DOI] [PubMed] [Google Scholar]
- [102].Lacerda ACR, Marubayashi U, Coimbra CC. Nitric oxide pathway is an important modulator of heat loss in rats during exercise. Brain Res Bull. 2015;67(1–2):110–116. [DOI] [PubMed] [Google Scholar]
- [103].Pires W, Wanner SP, La Guardia RB, et al. Intracerebroventricular physostigmine enhances blood pressure and heat loss in running rats. J Physiol Pharmacol. 2007;58(1):3–17. [PubMed] [Google Scholar]
- [104].Rodrigues AG, Lima NRV, Coimbra CC, et al. Evidence that exercise-induced heat storage is dependent on adrenomedullary secretion. Physiol Behav. 2008;94(3):463–467. [DOI] [PubMed] [Google Scholar]
- [105].Leite LHR, Rodrigues AG, Soares DD, et al. Central fatigue induced by losartan involves brain serotonin and dopamine content. Med Sci Sports Exerc. 2010;42(8):1469–1476. [DOI] [PubMed] [Google Scholar]
- [106].Cordeiro LMS, Guimarães JB, Wanner SP, et al. Inhibition of tryptophan hydroxylase abolishes fatigue induced by central tryptophan in exercising rats. Scand J Med Sci Sports. 2014;24(1):80–88. [DOI] [PubMed] [Google Scholar]
- [107].Lima PMA, Santiago HP, Szawka RE, et al. Central blockade of nitric oxide transmission impairs exercise-induced neuronal activation in the PVN and reduces physical performance. Brain Res Bull. 2014;108:80–87. [DOI] [PubMed] [Google Scholar]
- [108].Zheng X, Takatsu S, Wang H, et al. Acute intraperitoneal injection of caffeine improves endurance exercise performance in association with increasing brain dopamine release during exercise. Pharmacol Biochem Behav. 2014;122:136–143. [DOI] [PubMed] [Google Scholar]
- [109].Damasceno WC, Pires W, Lima MRM, et al. The dynamics of physical exercise-induced increases in thalamic and abdominal temperatures are modified by central cholinergic stimulation. Neurosci Lett. 2015;590:193–198. [DOI] [PubMed] [Google Scholar]
- [110].Zheng X, Hasegawa H. Administration of caffeine inhibited adenosine receptor agonist-induced decreases in motor performance, thermoregulation, and brain neurotransmitter release in exercising. Pharmacol Biochem Behav. 2016;140:82–89. [DOI] [PubMed] [Google Scholar]
- [111].Gomes LHLS, Drummond LR, Campos HO, et al. Thermoregulation in hypertensive rats during exercise: effects of physical training. Arq Bras Cardiol. 2019;112(n.5):534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Wang D, Ripley-Gonzalez JW, Hu Y. Aerobic Physical training protects the rat brain against exercise-heat related oxidative damage through the increased expression of HSP70. Neurophysiology. 2019;51:66–71. [Google Scholar]
- [113].Rodovalho GV, Drummond LR, Coimbra CC. Involvement of brainstem noradrenergic system in cutaneous heat loss during exercise. Brain Res Bull. 2020;164:372–379. [DOI] [PubMed] [Google Scholar]
- [114].Francesconi R, Mager M. Hypothermia induced by chlorpromazine or L-tryptophan: effects on treadmill performance in the heat. J Appl Physiol Respir Environ Exerc Physiol. 1979a;47(4):813–817. [DOI] [PubMed] [Google Scholar]
- [115].Hubbard RW, Mager M, Bowers WD, et al. Effect of low-potassium diet on rat exercise hyperthermia and heatstroke mortality. J Appl Physiol Respir Environ Exerc Physiol. 1981;51(1):8–13. [DOI] [PubMed] [Google Scholar]
- [116].Caputa M, Kamari A. Exercise performance of normothermic and hyperthermic rats: effect of warm rearing. J Therm Biol. 1991;16(6):363–366. [Google Scholar]
- [117].Walters TJ, Ryan KL, Tate LM, et al. Exercise in the heat is limited by a critical internal temperature. J Appl Physiol. 2000;89(2):799–806. [DOI] [PubMed] [Google Scholar]
- [118].Campos HO, Leite LHR, Drummond LR, et al. Temperature control of hypertensive rats during moderate exercise in warm environment. J Sports Sci Med. 2014;13(3):695–701. [PMC free article] [PubMed] [Google Scholar]
- [119].Fuller A, Carter RN, Mitchell D. Brain and abdominal temperatures at fatigue in rats exercising in the heat. J Appl Physiol. 1998;84(3):877–883. [DOI] [PubMed] [Google Scholar]
- [120].Durkot MJ, Francesconi RP, Hubbard RW. Effect of age, weight, and metabolic rate on endurance, hyperthermia, and heatstroke mortality in a small animal model. Aviat Space Environ Med. 1986;57(10 Pt 1):974–979. [PubMed] [Google Scholar]
- [121].Matthew CB. Ambient temperature effects on thermoregulation and endurance in anticholinesterase-treated rats. Life Sci. 1993;52(16):1343–1349. [DOI] [PubMed] [Google Scholar]
- [122].Hubbard RW, Bowers WD, Matthew WT, et al. Rat model of acute heatstroke mortality. J Appl Physiol Respir Environ Exerc Physiol. 1977;42(6):809–816. [DOI] [PubMed] [Google Scholar]
- [123].Rodrigues LO, Oliveira A, Lima NR, et al. Heat storage rate and acute fatigue in rats. Braz J Med Biol Res. 2003;36(1):131–135. [DOI] [PubMed] [Google Scholar]
- [124].Rabelo PC, Almeida TF, Guimarães JB, et al. Intrinsic exercise capacity is related to differential monoaminergic activity in the rat forebrain. Brain Res Bull. 2015;112:7–13. [DOI] [PubMed] [Google Scholar]
- [125].Wanner SP, Yoshida K, Kulchitsky VA, et al. Lipopolysaccharide-induced neuronal activation in the paraventricular and dorsomedial hypothalamus depends on ambient temperature. PLoS One. 2013;8(9):e75733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Wanner SP, Almeida MC, Shimansky YP, et al. Cold-induced thermogenesis and inflammation-associated cold-seeking behavior are represented by different dorsomedial hypothalamic sites: a three-dimensional functional topography study in conscious rats. J Neurosci. 2017;37(29):6956–6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Molkov YI, Zaretsky DV. Why is it easier to run in the cold? Temperature. 2016;3(4):509–511. doi: 10.1080/23328940.2016.1201182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Leon LR, Helwig BG. Heat stroke: role of the systemic inflammatory response. J Appl Physiol. 2010;109(6):1980–1988. [DOI] [PubMed] [Google Scholar]
- [129].Wanner SP, Costa KA, Soares AD, et al. Physical exercise-induced changes in the core body temperature of mice depend more on ambient temperature than on exercise protocol or intensity. Int J Biometeorol. 2014;58(6):1077–1085. [DOI] [PubMed] [Google Scholar]
- [130].Fischer AW, Cannon B, Nedergaard J. Optimal housing temperatures for mice to mimic the thermal environment of humans: an experimental study. Mol Metab. 2018;7:161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Racinais S, Moussay S, Nichols D, et al. Core temperature up to 41.5°C during the UCI road cycling world championships in the heat. Br J Sports Med. 2018;53(7):426–429. [DOI] [PubMed] [Google Scholar]
- [132].Havenith G. Human surface to mass ratio and body core temperature in exercise heat stress—a concept revisited. J Therm Biol. 2001;26(4–5):387–393. [Google Scholar]
- [133].Melo BP, Zacarias AC, Oliveira JCC, et al. Combination of aerobic training and cocoa flavanols as effective therapies to reduce metabolic and inflammatory disruptions in insulin-resistant rats: the exercise, cocoa, and diabetes study. Int J Sport Nutr Exerc Metab. 2022;32(2):89–101. [DOI] [PubMed] [Google Scholar]
- [134].Hutchins KP, Borg DN, Bon AJE, et al. Female (Under) Representation in exercise thermoregulation research. Sports Med Open. 2021;7(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Jahnke SA, Poston WS, Haddock CK, et al. The health of women in the US fire service. BMC Womens Health. 2012;12:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Pfister G. Women in sport – gender relations and future perspectives. Sport Soc. 2010;13(2):234–248. [Google Scholar]
- [137].Fink JS. Female athletes, women’s sport, and the sport media commercial complex: have we really “come a long way, baby”?. Sport Manage Rev. 2015;18(3):331–342. [Google Scholar]
- [138].Kiyatkin EA. Brain temperature fluctuations during physiological and pathological conditions. Eur J Appl Physiol. 2007;101(1):3–17. [DOI] [PubMed] [Google Scholar]
- [139].Kunstetter AC, Damasceno WC, Fonseca CG, et al. Physical exercise-induced changes in brain temperature. In: Watson RR (eds.) Physical activity and the aging brain: effects of exercise on neurological function. 1st ed. Amsterdam (Netherlands):Elsevier.2017;29–38. doi: 10.1016/B978-0-12-805094-1.00004-6. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


