Abstract
Background
Dementia is a syndrome of acquired cognitive impairment which is severe enough to interfere with independent living. Over the course of the illness, people with dementia also experience changes in emotions, behaviour and social relationships. According to Alzheimer's Disease International, dementia affects approximately 55 million people worldwide. The latest NICE guideline for dementia highlights the value of diverse treatment options for the different stages and symptoms of dementia, including non‐pharmacological treatments. Relevant literature also argues for the value of interventions that acknowledge the complexity of the condition and address the person as a whole, including their physical, emotional, social and cognitive processes. A growing literature highlights the capacity of the arts and has embodied practices to address this complexity. Dance movement therapy (DMT) is an embodied psychological intervention that can address complexity and thus may be useful for people with dementia, but its effectiveness remains unclear.
Objectives
To assess the effects of dance movement therapy on behavioural, social, cognitive and emotional symptoms of people with dementia in comparison to no treatment, standard care or any other treatment. Also, to compare different forms of dance movement therapy (e.g. Laban‐based dance movement therapy, Chacian dance movement therapy or Authentic Movement)
Search methods
We searched the Cochrane Dementia and Cognitive Improvement Group's register, MEDLINE (Ovid SP), Embase (Ovid SP), PsycINFO (Ovid SP), CINAHL (EBSCOhost), Web of Science Core Collection (Clarivate), LILACS (BIREME), ClinicalTrials.gov and the World Health Organization's meta‐register of the International Clinical Trials Registry Portal until 8 December 2022.
Selection criteria
We included randomised controlled trials (RCTs) that included people with dementia, of any age and in any setting. The DMT intervention had to be delivered by a dance movement therapy practitioner who (i) had received formal training (ii) was a dance movement therapist in training or (iii) was otherwise recognised as a dance movement therapist in the country in which the study was conducted.
Data collection and analysis
Two review authors independently assessed studies for inclusion, extracted data and evaluated methodological quality. We expressed effect estimates using the mean difference (MD) between intervention groups and presented associated confidence intervals (CIs). We used GRADE methods to rate our certainty in the results.
Main results
We found only one study eligible for inclusion in this review. This was a 3‐arm parallel‐group RCT conducted in Hong Kong involving 204 adults with mild neurocognitive disorder or dementia. The study examined the effects of short‐term (12 weeks) group DMT in comparison with exercise and a waiting‐list control group immediately post‐intervention and three and nine months later.
We found that, at the end of the intervention, DMT may result in little to no difference in neuropsychiatric symptoms assessed with the 12‐item Neuropsychiatric Inventory when compared with waiting list (MD 0.3, 95% CI ‐0.96 to 1.56; low‐certainty evidence) or exercise (MD ‐0.30, 95% CI ‐1.83 to 1.23; low‐certainty evidence). Nor was there any evidence of effects at later time points.
Cognitive functioning was assessed with a variety of instruments and there were no statistically significant between‐group differences (low‐certainty evidence). When compared to exercise or waiting list, DMT may result in little to no difference in cognitive function immediately after the intervention or at follow‐up.
In comparison to waiting list, DMT may result in a slight reduction in depression assessed with the 4‐item Geriatric Depression Scale at the end of therapy (MD ‐0.60, 95% CI ‐0.96 to ‐0.24; low‐certainty evidence). This slight positive effect of DMT on depression scores was sustained at three and nine months after the completion of the intervention. DMT may also reduce depression slightly in comparison with exercise at the end of therapy (MD ‐0.40, 95% CI ‐0.76 to ‐0.04, low‐certainty evidence), an effect also sustained at three and nine months.
Our fourth primary outcome, quality of life, was not assessed in the included study.
There were data for two of our secondary outcomes, social and occupational functioning and dropouts (which we used as a proxy for acceptability), but in both cases the evidence was of very low certainty and hence our confidence in the results was very low.
For all outcomes, we considered the certainty of the evidence in relation to our review objectives to be low or very low in GRADE terms due to indirectness (because not all participants in the included study had a diagnosis of dementia) and imprecision.
Authors' conclusions
This review included one RCT with a low risk of bias. Due to the low certainty of the evidence, the true effects of DMT as an intervention for dementia may be substantially different from those found. More RCTs are needed to determine with any confidence whether DMT has beneficial effects on dementia.
Keywords: Adult, Aged, Humans, Alzheimer Disease, Alzheimer Disease/therapy, Cognitive Dysfunction, Cognitive Dysfunction/therapy, Dance Therapy, Dancing, Depression, Depression/therapy, Quality of Life
Plain language summary
Is dance movement therapy an effective intervention for dementia? A review of the evidence
Plain language summary title
Are there any benefits of dance movement therapy for people with dementia?
Key messages
We do not know if dance movement therapy is an effective intervention for dementia. More research is needed in this field especially regarding the impact of dance movement therapy on depression.
What is dementia?
Dementia affects thinking and memory and how people are able to manage daily tasks. People with dementia may also struggle to follow conversations, be confused and change moods at different times. These symptoms can affect communication, and lead to loneliness, consequently causing depression and increased stress levels.
How is dementia treated?
Dementia may be treated through drugs to reduce symptoms. However, there are also complex interventions that are starting to emerge that address the person as a whole. There is also a growing interest in the use of dance and other forms of the arts for people with dementia.
What did we want to find out?
We wanted to assess the impact of dance movement therapy on different aspects of a person's life in comparison to no treatment, standard care or any other treatment. The main outcomes we were interested in were overall problems with behaviour and mental well‐being, cognition (thinking and remembering), depression and quality of life. We also wanted to compare different forms of dance movement therapy.
What did we do?
We searched the literature carefully for studies which compared a group of people with dementia who had dance movement therapy with another group of people with dementia (the control group). For the comparison to be fair, the assignment of a person to a particular group had to be decided randomly. We found only one study to include in our review. The study took place in Hong Kong and involved 204 people. Some of them had mild dementia and some had even milder problems with thinking and memory. In this study, the researchers compared dance movement therapy with exercise and with a waiting list. They compared the groups at the end of the therapy and then again three and nine months later.
What did we find?
We did not find any difference between dance movement therapy and either exercise or waiting list for overall behaviour and mental well‐being or for cognition. For depression, we found that there may be a small beneficial effect of dance movement therapy compared with exercise or waiting list, and this effect was still present three and nine months after the end of the therapy. However, we are not sure whether or not the effect was large enough to be really noticeable to the people with dementia. The study did not measure the participants' quality of life.
What are the limitations of the evidence?
There was only one study, so the amount of evidence was small. The study was well‐conducted, but not all the participants had dementia (some had milder problems) and we do not know how well the results apply only to people with dementia. For these reasons, we are not certain if dance movement therapy is effective in supporting people with mild dementia and we cannot say anything about its effects in moderate or severe dementia. More studies are needed to be able to say for certain if dance movement therapy is beneficial for people with dementia of any severity.
How up‐to‐date is this evidence?
The last search was on 8 December 2022.
Summary of findings
Summary of findings 1. Summary of findings table ‐ DMT compared to waiting list for dementia (end of intervention).
| DMT compared to waiting list for dementia (end of intervention) | ||||||
| Patient or population: dementia or mild neurocognitive disorder Setting: Outpatient department and community centres Intervention: DMT Comparison: waiting list | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with waiting list | Risk with DMT | |||||
| Neuropsychiatric symptoms assessed with: 12‐Item Neuropsychiatric Inventory Scale from: 0 to 36 follow‐up: mean 3 months | The mean neuropsychiatric symptoms was 2.2 | MD 0.3 higher (0.96 lower to 1.56 higher) | ‐ | 137 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | There may be little or no effect of DMT on neuropsychiatric symptoms. Higher scores indicate greater behavioural challenges. |
| Cognitive outcome assessed with: the Fuld object memory evaluation (total retrieval and delayed recall); semantic retrieval (verbal fluency); Digit Span test (forward and backward digit span); Trail‐Making test follow‐up: mean 3 months | The seven outcome variables on cognitive functioning did not show any statistically significant effects immediately after the DMT intervention in comparison with the waiting‐list control group. | 137 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | There may be little or no effect of DMT on cognitive functioning. | ||
| Depression assessed with: Brief Geriatric Depression Scale Scale from: 0 to 4 follow‐up: mean 3 months | The mean depression was 1.2 | MD 0.6 lower (0.96 lower to 0.24 lower) | ‐ | 137 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | There may be little or no effect of DMT on depression. Higher scores indicate increased severity of depression. |
| Social and occupational functioning assessed with: Instrumental Activities of Daily Living (IADL) Scale Scale from: 0 to 18 follow‐up: mean 3 months | The mean social and occupational functioning was 12.6 | MD 0.6 higher (1.22 lower to 2.42 higher) | ‐ | 137 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | The evidence is very uncertain about the effect of DMT on social and occupational functioning. Higher scores suggest better functioning. |
| Dropout follow‐up: mean 3 months | 88 per 1000 | 14 per 1000 (2 to 112) | OR 0.15 (0.02 to 1.30) | 137 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c,d | The evidence is very uncertain on whether dropouts were due to the type of intervention participants attended. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_434553657932634084. | ||||||
a Indirectness: downgraded by one level because the reviewed study included an unknown number of participants with mild neurocognitive disorder b Imprecision: downgraded by one level because the evidence comes from a study with not enough participants to meet the optimal information size c Imprecision: downgraded by one level due to wide confidence intervals d Indirectness: downgraded by one level because dropout during intervention was used as a proxy outcome for acceptability
Summary of findings 2. Summary of findings table ‐ DMT vs. exercise for dementia or mild neurocognitive disorder.
| Patient or population: dementia or mild neurocognitive disorder Setting: Outpatient department or community centres Intervention: DMT Comparison: exercise | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with exercise | Risk with DMT | |||||
| Neuropsychiatric symptoms assessed with: 12‐item Neuropsychiatric Inventory Scale from: 0 to 36 follow‐up: mean 3 months | The mean neuropsychiatric symptoms was 2.8 | MD 0.3 lower (1.83 lower to 1.23 higher) | ‐ | 136 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | There may be little or no effect of DMT on neuropsychiatric symptoms. Higher scores indicate greater behavioural challenges. |
| Cognitive functioning assessed with: Fuld Object Memory Evaluation (total retrieval and delayed recall); semantic retrieval (verbal fluency); Digit Span test (forward and backward digit span); Trail‐Making test follow‐up: mean 3 months | The seven outcome variables on cognitive functioning did not show any statistically significant effect immediately after the DMT intervention in comparison with the exercise group. | 136 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | There may be little or no effect of DMT on cognitive functioning. | ||
| Depression assessed with: Brief Geriatric Depression Scale Scale from: 0 to 4 follow‐up: mean 3 months | The mean depression was 1 | MD 0.4 lower (0.76 lower to 0.04 lower) | ‐ | 136 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | There may be little or no effect of DMT on depression. Higher scores indicate increased severity of depression. |
| Social and occupational functioning assessed with: Instrumental Activities of Daily Living (IADL) Scale from: 0 to 18 follow‐up: mean 3 months | The mean social and occupational functioning was 11.8 | MD 1.4 higher (0.33 lower to 3.13 higher) | ‐ | 136 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | The evidence is very uncertain about the effect of DMT on social and occupational functioning. Higher scores indicate better functioning. |
| Dropouts | 45 per 1000 | 14 per 1000 (1 to 127) | OR 0.31 (0.03 to 3.09) | 136 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c,d | The evidence is very uncertain on whether dropouts were due to the type of intervention participants attended. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_434557322563418890. | ||||||
a Indirectness: downgraded by one level because study also included an unknown number of participants with mild neurocognitive disorder b Imprecision: downgraded by one level because the evidence comes from a study with not enough participants to meet the optimal information size c Imprecision: downgraded by one level because of wide confidence interval d Indirectness: downgraded by one level because dropout during the intervention was used as a proxy outcome for acceptability
Background
Description of the condition
Dementia is a collective name for a number of different progressive degenerative brain syndromes. According to the International Statistical Classification of Diseases and Related Health problems (WHO 2019), Alzheimer's disease remains the most common of these syndromes, followed by vascular dementia, Lewy body dementia and frontotemporal dementia. Alzheimer's Disease International (Gauthier 2021) indicates that over 55 million people are affected by dementia worldwide which could be seen to triple by 2050 due to an increase in the aged population.
People living with dementia may have symptoms such as:
memory loss
difficulty concentrating
finding it hard to carry out familiar daily tasks, such as getting confused over the correct change when shopping
struggling to follow a conversation or find the right word
being confused about time and place
mood changes (Gauthier 2021)
Communication under these circumstances can be very challenging and, as communication deteriorates, it can lead to unpleasant feelings and stress for the person with dementia (Banovic 2018). A further decline in cognitive function in dementia patients may increase mental and behavioural problems such as delusions, aggression, agitation, and violence which can affect their ability to remain autonomous resulting in a need for care delivery (Terada 2019).
Earlier intervention and care of risk factors such as lifestyle changes can reduce these numbers, However, increased age remains a risk factor for the development of dementia (Livingston 2020).
Increasingly within dementia literature, a focus on the value of maintaining a dementia patients’ autonomy and quality of life is frequently mentioned and enabling strategies are being highlighted (Yates 2019).
Similarly, Alzheimers Society 2022 recognises that there are ‘stages’ of dementia that depend on the progression of the disease, with each stage requiring different care needs and treatment strategies. To promote cognition and independence, Sikkes 2020 recommends management strategies including non‐pharmacological interventions amongst others. Furthermore, there is a growing literature that argues for the value of the arts and has embodied practices for this population; it is thought that these practices may be capable of bypassing impairments, connecting with people at a pre‐cognitive level and slowing down the progressive nature of the disease (Karkou 2017). Dance movement therapy (DMT) is one of these interventions that has been considered a useful intervention for people with dementia.
Description of the intervention
DMT is a form of psychotherapy, one of the arts therapies, and a treatment option for people with dementia as reported by practitioners in the field (Hill 2017; Hayes 2011).
DMT is also known as dance therapy, movement therapy, dance movement psychotherapy, movement psychotherapy, dance/movement therapy or dance‐movement therapy. As a profession, in the UK, it is regulated by the Association for Dance Movement Psychotherapy UK (ADMP UK 2021) and more recently also from the UK Council for Psychotherapy (UKCP 2021). In Europe, the European Association for Dance Movement Therapy (EADMT 2021) represents national professional Dance Movement Therapy associations, supporting professional development while, in the USA, dance/movement therapists receive national certification from the Dance/Movement Therapy Certification Board, an independent affiliate of the American Dance Therapy Association (ADTA 2020). In the UK, the Association for Dance Movement Psychotherapy UK defines the discipline as follows:
"Dance Movement Psychotherapy (DMP) is a relational process in which client(s) and therapist use body movement and dance as an instrument of communication during the therapy process. DMP is an empathic creative process practised as individual and group therapy in clinical, community, and educational settings, as well as in private practice." (ADMP UK 2021).
DMT is regarded as a useful and appropriate intervention for people with a range of conditions, diagnoses and presenting problems, and especially those for whom words can be difficult, those with a cognitive impairment, or who just find it difficult to express and explore their emotions through words (ADMP UK 2021).
This type of therapy can, depending on the group of people who are accessing it, take place in a number of different settings including health services, schools, social services, voluntary organisations, prisons and care homes. Sessions can last from 30 to 90 minutes and often take place weekly at an agreed place and time. Interventions may last from a few weeks to several months, depending on client needs, and can be delivered as one‐to‐one, pair‐ or group‐therapy.
The practice of DMT in the 21st century stems in part from pioneering work that took place in the USA during the middle and latter part of the last century. However, pioneers in other countries also developed approaches that initially were independent of American influences (Meekums 2008; Payne 1992).
Karkou 2006 identifies three main models in the field.
Approaches that rely primarily upon dance/movement engagement and aim to explore specific movement themes and qualities, with or without active movement by the therapist. Examples of these are often, though not exclusively, informed by Rudolf Laban, an early proponent of therapeutic applications of dance movement (Laban 1975).
Approaches that prioritise the non‐verbal interaction between client(s) and therapist. A good example of this is the interactive model developed by the American DMT pioneer Marian Chace, as described by Chaiklin 1986, in which mirroring of movement is a key technique, that is, movement qualities used by the client are reflected back by the therapist and at times other group members.
Approaches that concentrate on movement improvisation are associated with a focus on internal experience, in the presence of the therapist. An example of this type of approach is Authentic Movement, a practice developed by the American choreographer Mary Whitehouse (Whitehouse 1979). In this form of DMT, the therapist stays still and observes, using the self as an empathic witness, usually without music to influence the movement.
Meekums 2008 suggests that the feature that marks out contemporary DMT practice is the emphasis on it as a form of psychotherapy. For many therapists, theories relating to the psychodynamic school of thought are used to guide practice (Karkou 2006). For example, Authentic Movement is associated with Jungian psychology (Whitehouse 1979). For others, humanistic approaches are more relevant (Karkou 2006); the interactive model of Marian Chace is an example of an approach that is often closely connected with humanistic thinking. For others, developmental, behavioural or eclectic and integrative models are valued (Karkou 2006). For example, in her work with mothers and young children Meekums used a behavioural approach combined with attachment theory (Meekums 1991). More recently, Meekums suggested an integrative framework based on the symbolic power of the 'movement metaphor’, which transcends such theoretical divisions (Meekums 2002).
In all cases, the therapist is concerned with developing an embodied therapeutic relationship. Within this relationship, the therapist's body may be seen as holding projections from individual clients, or from the group as a whole. These projections may be worked through verbally, or non‐verbally, or both; more or less verbal reflection may take place, depending on the level of cognitive functioning of the participants.
In dementia care, the systematic review by Lyons 2018 suggests that an overall person‐centred framework with some elements of psychodynamic thinking are particularly relevant to working with people with dementia, with Marian Chase's approach frequently reported in the reviewed papers.
How the intervention might work
DMT may have positive effects for people with dementias that include delaying cognitive deterioration, improving mood, and increasing social interaction for a number of reasons such as:
the use of movement as exercise and as dance;
the use of music;
the therapeutic relationship; and
DMT‐specific features.
The use of movement
Since DMT uses the body and encourages movement amongst participants, it is expected that there will be some physiological changes associated with exercise. These are well documented in the generic literature on the physiology of exercise, and some studies include positive effects on cognitive function (Groot 2016). However, the more recent Cochrane Review on exercise programmes for people with dementia reports that there is no evidence of the value of exercise on cognition, depression or neuropsychiatric symptoms (Forbes 2015).
The dance literature related to this review reveals that some dance forms, such as tango, may have the effect of over‐riding problems in the brain associated with balance and gait in Parkinson's Disease (Hackney 2007; Hackney 2010). Tango has many of the elements also found in a DMT session, including: " . . . frequent movement initiation and cessation, a range of speeds, rhythmic variation. . . " (Hackney 2010; p. 682). There is also growing research evidence to suggest that those individuals who dance regularly can improve their cognitive functioning and memory (Bek 2020), counteract cognitive decline (Chen 2020), having a positive impact on neuroplasticity, depression and quality of life (Wu 2021).
It is also possible that the use of dance within the intervention can connect with older people’s experiences of social dancing when younger and the pleasures that this brought, while encouraging people to engage in rhythmic movement and body action, both concepts and associated practices that are extensively used within the discipline on dance movement therapy (Chaiklin 1986).
The use of music
Although not essential, it is common for dance movement therapists to use music when working with people with dementias. Music of all styles and types can be used, often with particular attention to the corresponding rhythm. Studies included in the Cochrane Review by Van der Steen 2018 indicate that music‐based therapeutic interventions (sometimes combined with movement activities) may reduce depression and anxiety, improve well‐being and quality of life as well as generic behavioural problems. This review did not find any impact of music on cognitive outcomes, agitation or aggression. However, similarly to other systematic reviews that have evaluated emerging treatment options, the authors of the review concluded that further research is needed to establish the impact of music on dementia.
The therapeutic relationship
As a form of psychotherapy, the therapeutic relationship can be seen as a key agent for change (Macaskie 2012; Norcross 2011) that needs to be considered within case formulations (Vilkin 2022). For example, in verbal psychotherapy, and with regard to intersubjective therapeutic relationships in particular, Macaskie 2012 concludes that the relationship is an embodied one, drawing on implicit relational knowing, implicit body memory and embodied participatory sense‐making. Within DMT, embodied relational knowing, body memory and embodied sense‐making are highly developed. Furthermore, empathy, a core component of the therapeutic relationship, is extensively utilised in DMT in the form of a sophisticated understanding and use of kinaesthetic empathy (Meekums 2012). The development of a therapeutic relationship is a key aspect of the work, and its embodied/relational nature in this context is of particular importance when cognition, communication, self‐confidence, self‐identity, self‐worth, orientation in space and time and more, are all under particular threat for the person with dementia. Also, it is accurate to say that, ‘maintaining relationships’ are at the heart of best practice in dementia care. The development of a therapeutic relationship is also one of the main differences between DMT and dance practice.
DMT‐specific features
While DMT participants are encouraged to engage in movement initiation and cessation, rhythmic variation and a range of speeds, they are encouraged to engage in movement that is primarily creative and takes place within an embodied therapeutic relationship, rather than learning steps to music. Additional benefits, therefore, might reasonably be expected beyond those associated with exercise or dance classes. As a complex and holistic intervention, it has been argued that DMT can stimulate different domains including physical, emotional, and cognitive functioning and, as such, it is a useful, economical and impactful approach to dementia care (Goldstein‐Levitas 2016).
Karkou 2006 argues that therapeutic change may be related to some of the unique features of DMT specifically: embodiment, creativity and improvisation, movement‐based imagination, the use of symbolic movement, and the use of movement as a metaphor. The embodied nature of DMT makes it potentially relevant to those clients for whom body image or body memory may be a particular issue requiring exploration and working through, for example, for people who are overweight and have emotional eating patterns (Vaverniece 2012). In the case of dementia, engaging body memories may assist the goals of reminiscence therapy (Woods 2018). Metaphors inherent in symbolic movement offer a way to understand communication (Meekums 2002), and may be important for communication by people with dementia (Young 2011). Creativity and improvised movement work are also seen as enabling participants to develop new ways of being in the world in themselves and in interactions with others (Karkou 2006); this could potentially lead to a better emotional and social life. The value of activating imagination through movement was extensively discussed by Dosamanstes‐Alperson as early as 1981 (Dosamantes‐Alperson 1981), who offers specific guidance on how this can be facilitated and encouraged in a body‐based manner. Furthermore, Meekums 2002 and Karkou 2006 argue that symbols and metaphors can increase emotional distance from distressing memories and feelings, while they can allow for safe explorations that may change cognitions and feelings.
One of the central aspects of DMT for which there is an evidence base relates to the use of non‐verbal communication, and kinaesthetic empathy in particular (Berrol 2006; Brooks 1989; Meekums 2012), which is an important aspect of the therapeutic relationship discussed above. 'Movement mirroring', derived from the Chace approach to DMT mentioned earlier, is a technique extensively used by dance movement therapists as a way of engaging patients and achieving non‐verbal empathy. Within DMT literature (Berrol 2006; Meekums 2012), empathic engagement through mirroring is often seen as linked with the activation of mirror neurons in the brain (Gazzola 2006; Rizzolatti 1996), which fire as a kind of body memory when an individual observes another person engaged in either purposeful movement or emotional expression that is within the repertoire of the observer. However, the significance of mirror neurons within therapeutic kinaesthetic empathy is unclear; emotional engagement through a deliberate attempt to imagine oneself into another's experience appears to be an important additional requirement, used by dance movement therapists alongside their embodied engagement (Meekums 2012). Moreover, mirror neuron activity is associated with watching movements; different processes may be involved in actively mirroring movements and so, while some aspects of kinaesthetic empathy may involve mirror neuron activity, the processes involved in experiencing and conveying empathy are likely to be different.
Finally, in the review of the literature by Lyons 2018 on DMT in dementia care, some important therapeutic components that were valued in the reviewed studies were opportunities for improvisation and spontaneity of expression, the use of touch, the use of rhythm and symbolism.
Further investigation is needed to establish the role of both specific and nonspecific factors in therapeutic change associated with DMT for people with dementia.
Why it is important to do this review
DMT is widely practised around the world in both statutory and non‐statutory sectors with a range of client populations indicating large effects on outcomes relating to depression (Karkou 2019) as well as anxiety, quality of life, and interpersonal and cognitive skills (Koch 2019), embodied cognition and introception (Millman 2021). The current review will also add to other completed Cochrane Reviews of studies in DMT that have reviewed research evidence concerning the effects of DMT on depression (Karkou 2022), schizophrenia (Ren 2013) [https://Ren 2013] and cancer care (Bradt 2015).
Furthermore, there is a growing need to offer appropriate services to people with dementias who are faced with multiple issues beyond traditional pharmacological treatment. Given that medication focuses primarily on reducing cognitive deterioration, and that there is a growing number of people with dementias, the search for effective interventions that aim to address the person as a whole is particularly timely. Dance movement therapists do insist that they treat the person as a whole, tapping into cognitive areas of functioning but also addressing emotional, social, spiritual and physical aspects (Lyons 2018). The current review, therefore, will add to existing literature of non‐pharmacological treatment options for dementia, the closest Cochrane review to this review being on exercise (Forbes 2015), music‐based interventions (Van der Steen 2018) and art therapy (Deshmukh 2018).
Objectives
To assess the effects of dance movement therapy on behavioural, social, cognitive and emotional symptoms of people with dementia in comparison to no treatment, standard care or any other treatment. Also, to compare different forms of dance movement therapy (e.g. Laban‐based dance movement therapy, Chacian dance movement therapy or Authentic Movement).
Methods
Criteria for considering studies for this review
Types of studies
We included published or unpublished randomised controlled trials (RCTs) in any language. Studies with cross‐over designs and cluster‐RCTs were eligible.
Types of participants
The review aimed to include studies of people who were formally diagnosed as having any type of dementia of any severity, according to the International Statistical Classification of Diseases and Related Health Problems (ICD‐11, WHO 2019), Diagnostic and Statistical Manual of Mental Disorders (DSM‐5, American Psychiatric Association 2022) or other comparable diagnostic criteria. Women and men in all age groups and in all settings were eligible for inclusion in the review.
Types of interventions
Experimental interventions
We defined eligible dance therapy interventions as interventions delivered by DMT practitioners who: (i) had received formal training; (ii) were a dance movement therapist in training or; (iii) were otherwise recognised as dance movement therapists in the country in which the study was conducted.
DMT could be delivered to groups, individuals or families/couples. There were no restrictions on the number or duration of sessions.
Sessions could include active involvement in dance/movement in the presence of a dance movement therapist, or dance/movement interaction with a dance movement therapist with or without other group members, or both. Dance/movement could be improvisatory or structured. Sessions would have clear therapeutic intent and a clear description of the intervention would be reported or available on request. All approaches to DMT were considered.
We considered the presence or absence of verbal interaction and reflection as a factor to be examined in subgroup analysis. In all cases, however, creative movement work needed to be the main means of working through cognitive, behavioural, emotional or social issues faced by the participants.
Comparators
No treatment or standard care;
Other psychological therapies: for example, psychodynamic psychotherapy, humanistic, cognitive behavioural or integrative therapies;
Pharmacological interventions: medications such as cholinesterase inhibitors (CgEIs) or memantine;
Other interventions: for example, exercise, dance (not DMT) or music;
Different types of DMT, as defined above.
Types of outcome measures
Primary outcomes
The primary outcomes were changes in behaviours often seen as challenging for carers, hereafter called 'challenging behaviours' for the sake of brevity (e.g. wandering, agitation, general restlessness), cognitive functioning, levels of depression and quality of life. Examples of tools for assessing each outcome are included below. We planned to accept all behavioural and psychological tools reported by the authors of the identified primary studies.
Challenging behaviours, which could be assessed with standardised instruments (e.g. Cohen Mansfield Agitation Inventory, CMAI (Cohen‐Mansfield 1989)) or with quantitative observational tools specifically designed for an individual study to measure frequency of occurrence of wandering, agitation, general restlessness, etc.
Cognitive functioning, assessed with standardised instruments, e.g. the Mini‐Mental State Exam (MMSE) (Folstein 1975) or the Alzheimer's Disease Assessment Scale (ADAS‐cog; Rosen 1984).
Depression, assessed with standardised instruments, e.g. the Cornell Scale for Depression in Dementia (Alexopoulos 1988).
Quality of life, assessed with standardised instruments, e.g. Quality of Life‐Alzheimer's Disease (QOL‐AD, Logsdon 1999).
If other similar outcome measures were found in the included studies, we would consider them.
Secondary outcomes
The secondary outcomes were mobility and balance, fatigue, anxiety, social and occupational functioning, economic outcomes (cost‐effectiveness of treatment), treatment or research discontinuation/dropout (as measures of acceptability). We also aimed to consider adverse events, including falls and injuries associated with the intervention. Outcomes were to be included as used within the primary studies.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Dementia and Cognitive Improvement Group’s Specialised Register until 8 December 2022. The Register is maintained by the Information Specialists of the Cochrane Dementia and Cognitive Improvement Group and contains studies in the areas of dementia (prevention and treatment), mild cognitive impairment and cognitive improvement. The studies are identified from:
Monthly searches of a number of major healthcare databases: MEDLINE, Embase, CINAHL, PsycINFO and LILACS
Monthly searches of the trial registers: the WHO International Clinical Trials Registry Platform (which covers ClinicalTrials.gov, ISRCTN, the Chinese Clinical Trials Register, the German Clinical Trials Register, the Iranian Registry of Clinical Trials, and the Netherlands National Trials Register, plus others)
Quarterly search of the Cochrane Library’s Central Register of Controlled Trials (CENTRAL)
Six‐monthly searches of a number of grey literature sources from Web of Science Core Collection
Details of the search strategies used for the retrieval of reports of trials from the healthcare databases, CENTRAL and conference proceedings can be viewed in the ‘Methods used in reviews’ section within the editorial information about the Dementia and Cognitive Improvement Group https://dementia.cochrane.org/our-trials-register. We performed additional searches in many of the sources listed above, to cover the timeframe from the last searches performed for ALOIS to ensure that the search for the review was as up‐to‐date and as comprehensive as possible.
The search strategies used are described in Appendix 1.
Searching other resources
We also took the following actions in order to identify published, unpublished and ongoing trials that may not have appeared in the electronic searches listed above.
We searched the bibliographies of relevant studies and reviews.
We contacted professional associations and educational programmes in DMT from around the world using a standard request form to inform us of Masters‐degree, PhD or independent work that is published or unpublished, completed, or ongoing that might meet the inclusion criteria.
We contacted DMT researchers, theoreticians and practitioners who specialise in this area of work, whom we regard as experts in the field.
Data collection and analysis
The full methods section of the review protocol is attached in Appendix 2.
Selection of studies
There were no studies to carry over from the older version (Karkou 2017) to this review update. From the new search, we imported records to Covidence software (www.covidence.org) for screening purposes. In the first instance, two of the authors (MR and EH) independently screened titles and abstracts for studies that may have met the inclusion criteria. These studies were then obtained in full‐text form and assessed for eligibility by the same two review authors with another review author (SA) acting as a referee when needed.
Data extraction and management
We carried out data extraction using the 1.0 extraction template on Covidence. Two authors (SA and MR) independently extracted relevant data from the included study (see Appendix 3). When extracting results, we preferred endpoint data to change scores.
Assessment of risk of bias in included studies
In order to identify any risk of bias, we used the Cochrane Risk of bias tool as presented in Appendix 4 (Higgins 2011a) to make a judgement of 'low risk’, ’high risk’ or ’unclear risk’ of bias in each of the following domains:
random sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
other sources of bias.
We did not exclude studies that were based on a high risk of bias.
Measures of treatment effect
For dichotomous outcomes, we expressed the treatment effect using the risk ratio (RR). For continuous outcomes, such as scores from a scale, we used the mean difference (MD).
Unit of analysis issues
There were no eligible cross‐over or cluster‐randomised trials. The only included trial had two comparator groups. We presented separate comparisons of DMT with each control group (see Differences between protocol and review).
Dealing with missing data
We extracted intention‐to‐treat data and reported the method used by the study authors to deal with missing values.
Assessment of heterogeneity
There was only a single included study and hence no between‐study heterogeneity to assess.
Assessment of reporting biases
No graphical or statistical assessment for reporting biases was possible because we identified only one eligible study.
Data synthesis
There was only a single included study and hence we did not perform any meta‐analyses.
Our main analysis was based on the end‐of‐intervention time point. We also reported outcomes at the following time points after the end of the intervention: short‐term follow‐up (up to 14 weeks), medium‐term follow‐up (15 to 27 weeks, not applicable to the included study) and long‐term follow‐up (28 weeks and over).
Subgroup analysis and investigation of heterogeneity
With only one included study, no subgroup analyses were possible.
Sensitivity analysis
We did not perform any sensitivity analyses.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE Working Group’s software, GRADEpro GDT (www.gradepro.org), to assess the quality of the body of evidence in the review and report a summary of the key findings in relation to each outcome. One review author (SA) initially conducted the evaluation using the GRADE system and then discussed it with VK before reaching the final decision.
We graded the certainty of the evidence, considering the following five domains: risk of bias, inconsistency, indirectness, imprecision, and publication bias. Our review included only one RCT. Therefore, we started the assessment at high quality and downgraded the evidence for each outcome from high quality by one level if we identified a serious limitation in relation to a specific domain or by two levels if we considered there was a very serious limitation.
Results
Description of studies
Results of the search
See PRISMA flow chart in Figure 1. We found 752 records from electronic searches and none from personal communication. Two studies that were identified as ongoing in the previous version of this review were included for screening. After de‐duplication, we screened titles and abstracts of 719 records. We retrieved 12 full texts and assessed them for eligibility against the pre‐stated inclusion and exclusion criteria relating to the type of study, population, and intervention. At the full‐text eligibility checking stage, we excluded nine studies. One study (four references) met the criteria to be included in this review.
1.
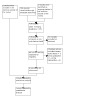
Study flow diagram
Included studies
We summarise the characteristics of the one included study (Ho 2020) here.
Design
Ho 2020 was a single‐blinded, three‐arm, parallel‐group RCT with a DMT intervention group, a physical exercise group, and a waiting‐list control group.
Sample size
A total of 204 older adults participated in the included study. Sixty‐nine people were allocated to the DMT intervention, while 67 and 68 people were allocated to the exercise group and the waiting‐list control group, respectively.
Setting
The study was based in Hong Kong and the participants were recruited from psychogeriatric outpatient departments of a local hospital and from older adults in community centres through referrals from the treating psychiatrists or facility staff.
Participants
Ho 2020 included older adults (mean age 79 years, SD 8) with a clinical diagnosis of dementia or mild neurocognitive disorder according to the DSM‐5 criteria. The severity of dementia of the participants was mostly at the lower end, as around two‐thirds (68.6%) of them were diagnosed with very mild dementia with a Clinical Dementia Rating of 0.5 and the remaining (31.4%) had a Clinical Dementia Rating of 1 indicating very mild to mild dementia.
Most of the participants in all three arms were females (81.9%.) who were single/widowed (62.7%) with at most 6 years of education (67.5%) and performed regular exercise (78.4%).
Interventions
In the Ho 2020 study, the participants were randomly allocated to either DMT, exercise, or the waiting‐list control group.
Sixty‐nine participants were allocated to six DMT intervention groups of 10 to 12 participants. The DMT intervention was delivered by two therapists in one‐hour sessions held twice a week for 12 weeks (24 hours of DMT). One therapist was a registered dance movement therapist and one was in training, monitored by the principal investigator of the study. The intervention protocol was modified from an established DMT programme that had been previously found useful to the Chinese population. The key differences were that the Ho 2020 study placed emphasis on easy rhythmic contralateral movements on both sides of the body to enhance co‐ordination and stimulation between the left and right cerebral hemispheres. The therapists tailored the movements to the needs of the population by identifying movements that could be performed in both standing and sitting positions. The intervention was informed by humanistic principles and offered a semi‐structured approach to adhere to the protocol with some room for flexibility based on the groups' needs. Each session concluded with verbal sharing and group discussion where the participants were invited to use one or two words or a gesture or movement to express their feelings about the movement experience or to say something supportive or caring to each other, with an intent to facilitate articulation and mutual support amongst the participants.
Sixty‐seven participants in exercise groups received 24 sessions that consisted of a warm‐up (15 min), stretching and joint movements (15 min), exercising with towels (15 min), and a cool‐down (15 min). These sessions were offered by trained and qualified fitness instructors.
Primary outcomes
1. Neuropsychiatric symptoms: There was no specific measure of challenging behaviours as we proposed in the protocol of this review. Ho 2020 assessed neuropsychiatric symptoms using the 12‐item Neuropsychiatric Inventory (Lam 2006) from the primary caregivers' perspective. The severity of the symptoms such as apathy, hallucination, and agitation are marked on a 4‐point scale (0 = none, 3 = severe).
2. Cognitive functioning: Ho 2020 used the Fuld Object Memory Evaluation (Ho 2018) to assess two measures of episodic retrieval, total immediate retrieval, and delayed recall. Participants were asked to identify ten easy‐to‐find objects from one's daily life that were placed in a bag; they were invited to touch, look at, and name them. After a one‐minute distraction exercise, participants were invited to recall these items in 60 seconds (immediate recall) and repeat this exercise four times and 20 minutes later (delayed recall). The distraction exercise was a semantic retrieval task that also evaluated verbal fluency. All the tasks associated with the Fuld Object Memory Evaluation (i.e. total immediate retrieval and delayed retrieval tasks) including the distraction exercise were repeated five times at each measurement time.
Ho 2020 completed two more cognitive tests; the Digit Span Test of the Wechsler Adult Intelligence Scale (Yao 2007) measures the short‐term and working memory of the respondents involving immediate memory of numbers and retaining and processing this information in one's mind, while the Trail‐Making Test (Lu 2002) was used to measure complex attention and executive function. The latter involved visuospatial construction with participants invited to draw a line between alternative numbers.
In all cases, people who were trained in the tests administered the tests, revealing acceptable inter‐rater reliability (intraclass correlation coefficients > 0.70).
3. Depression: In the Ho 2020 study, depression was measured using the 4‐item Geriatric Depression Scale (Cheng 2005) focusing on the sum of four dichotomous items.
4. Quality of life: a specific measure for quality of life was not included in the study.
Secondary outcomes
Ho 2020 reported results from a daily living (IADL) scale (Tong 2002) to assess social and occupational functioning. This included skills in cooking, doing housework, and taking medications. This involved a combination of self‐reported and caregiver‐reported responses and addressed social and occupational functioning, a secondary outcome in our protocol. Ho 2020 also reported dropout rates along with reasons. However, they did not report mobility and balance, fatigue, anxiety, and cost‐effectiveness. Similarly, there was no systematic collection of data on adverse events. Instead, they evaluated neuroendocrine functioning by monitoring cortisol levels at different time points. However, given that biomarkers, as an outcome measure was not included in the review protocol, we did not report this finding here.
Excluded studies
As indicated in Characteristics of excluded studies of the original review, we excluded 19 records on the basis of: the type of study design employed (n = 11), the population (n = 3), or the intervention used (n = 3), while we excluded two studies because they were ongoing studies (n = 2).
We identified new records through recent searches for screening and assessing eligibility (Figure 1). We assessed 13 full‐text records for eligibility and excluded nine records. The remaining four records were all related to the same study that met our inclusion criteria (Ho 2020) and this study was one of the two records that were identified as ongoing in the original review. As described in Characteristics of excluded studies, eight excluded studies were considered carefully but did not fully qualify to be included. The most common reason for exclusion was the type of intervention (Cheung 2018; Choo 2019; Merom 2016; Merom 2016a). A number of studies investigated training in dance or movement, and participating in social dancing. It was apparent that DMT, as per our stated criteria, was not used as the intervention in those studies. For example, three studies (Cheung 2018; Merom 2016; Merom 2016a) used social dancing as an intervention where dancing was applied as a complex sensorimotor rhythmic activity or exercise without a psychotherapeutic framework. Choo 2019 used a creative dance programme called intuitive movement re‐embodiment which appeared to have some influence from DMT. However, upon close inspection, it did not qualify as DMT as the intervention taught a series of dance exercises using reminiscent music and natural gestures of people with dementia. Three studies were excluded based on the study design employed. Methodologies used in these excluded studies were: (i) systematic review or literature review (e.g. Kressig 2015; Ruiz‐Muelle 2019); and (ii) case study design (e.g. Lyons 2019). During the time of the original review, the Lyons 2019 doctoral proposal was registered to be undertaken in the UK as a small mixed‐methods trial. However, this was excluded as the final thesis did not report the implementation of randomised and controlled groups. Esmail 2020 was excluded because of the wrong population. The participants were older adults without a diagnosis of dementia or cognitive impairment.
Risk of bias in included studies
Our risk of bias assessment is shown in Figure 2 and Figure 3.
2.

Risk of bias graph
3.

Risk of bias summary
Allocation
We judged that Ho 2020 had a low risk of bias for random sequence generation. Randomisation took place on a 1:1:1 basis using a simple randomisation technique with computer‐generated random numbers. There was no information on allocation concealment and we judged there to be an unclear risk related to this element of the design.
Blinding
Ho 2020 described their study as single‐blinded. The assessments were conducted by research coordinators who were blinded to the allocation and had received training in using the instruments. However, the participants could not be blinded to the intervention (Juul 2021). We had some concerns about the lack of clarity on whether participants were blinded when they were allocated to each of the study arms. For this reason, when we evaluated the outcome‐specific risk of bias using the GRADE tool, we downgraded this criterion by one level.
Incomplete outcome data
We evaluated the study by Ho 2020 to have a low risk of bias for this criterion since the authors reported that only 38 participants out of 204 (18.6%) dropped out of the study over the year. Reasons for dropout were reported, i.e. refusal to participate, hospitalisation, no longer living in Hong Kong, or had passed away. The authors used a full information maximum likelihood method to interpolate missing data, which assumes that the data were missing due to random chance (Hu 1999). As a result, all randomised participants were included in the analyses at each time point.
Selective reporting
We evaluated this criterion as being at low risk for Ho 2020 since the trial was analysed in accordance with a prespecified protocol that was finalised before unblinded outcome data were available for analysis. Therefore, the numerical results reported were less likely to have been selected on the basis of the results from multiple outcome measurements within the outcome domain.
Other potential sources of bias
No other potential bias were identified.
Effects of interventions
Primary outcomes
1. Neuropsychiatric symptoms
DMT versus waiting‐list control
Ho 2020 included 137 participants (69 DMT, 68 waiting list) in this comparison, and examined the effect of DMT compared to a waiting list on the 12‐item Neuropsychiatric Inventory immediately after the three‐month intervention period (range 0 to 36, higher scores indicating more symptoms). Baseline scores were very low, indicating few, mild symptoms at the beginning of the study. There was no evidence of a clinically important difference in scores between groups (mean difference (MD) 0.30, 95% confidence interval (CI) ‐0.96 to 1.56, low‐certainty evidence). Thus, DMT may result in little to no difference in this outcome. Likewise, there was no evidence of an effect at three months (MD 0.20, 95% CI 0.10 to 2.03) or nine months (MD 1.10, 95% CI ‐0.14 to 2.34) after the end of the intervention.
DMT versus exercise
There were 136 participants in this comparison. Again, there was no evidence of any clinically important difference between groups (MD ‐0.30, 95% CI ‐1.83 to 1.23, low‐certainty evidence). DMT may result in little to no difference in this outcome. Likewise, there was no evidence of an effect three months (MD ‐0.10, 95% CI ‐1.38 to 1.18) or nine months (MD 0.3, 95% CI ‐1.21 to 1.81) after the end of the intervention.
2. Cognitive functioning
DMT versus waiting‐list control
Ho 2020 reported data for several aspects of cognitive functioning, i.e. Fuld Object Memory Evaluation (total retrieval and delayed recall); semantic retrieval (verbal fluency); Digit Span Test (forward and backward digit span); and Trail‐making Test. In all cases, there were no statistically significant differences between the DMT and waiting‐list control groups, either immediately after the intervention or at follow‐up three or nine months later. For our primary end‐of‐intervention time point, we considered the certainty of the evidence to be low.
DMT versus exercise
Similarly, there were no statistically significant differences between the DMT and exercise groups in any aspect of cognitive functioning immediately after the intervention or at follow‐up three or nine months later. We considered the evidence for the end‐of‐intervention result to be of low certainty.
3. Depression
DMT versus waiting‐list control
Depression was assessed with the 4‐item Geriatric Depression Scale (range 0 to 4, higher scores indicating more depression). Immediately after the end of the intervention, there was a small difference in depression scores in favour of DMT when compared with the waiting‐list control group (MD ‐0.60, 95% CI ‐0.96 to ‐0.24, low‐certainty evidence). A small positive effect was maintained three months after the end of the intervention (MD ‐0.40, 95% CI ‐0.75 to ‐0.05) and after nine months (MD ‐0.30, 95% CI ‐0.63 to 0.03). Therefore, there may be a small benefit of DMT on depressive symptoms. However, the observed difference at all time points is of uncertain clinical importance.
DMT versus exercise
There was a small difference in depression scores in favour of the DMT group compared with the exercise group immediately after the intervention (MD ‐0.40. 95% CI ‐0.76 to ‐0.04, low‐certainty evidence). Again, this was maintained three months (MD ‐0.60, 95% CI ‐0.99 to ‐0.21) and nine months after the end of the intervention (MD ‐0.50, 95% CI ‐0.91 to ‐0.09). There may be a slight benefit of DMT over exercise for depressive symptoms in dementia, but the clinical importance of the observed difference is uncertain.
Secondary outcomes
1. Social and occupational functioning
DMT versus waiting‐list control
Social and occupational functioning was assessed using the Instrumental Activities of Daily Living (IADL) scale (range 0 to 18, higher scores indicate better functioning). There was no evidence of an important difference between DMT and waiting‐list control for social and occupational functioning in the immediate term (MD 0.60, 95% CI ‐1.22 to 2.42, very low‐certainty evidence) or after three months (MD 1.10, 95% CI ‐0.79 to 2.99) and nine months (MD 0.40, 95% CI ‐1.64 to 2.44). Our confidence in the result was very low due to serious concern about indirectness and very serious concern about imprecision.
DMT versus exercise
When DMT was compared with exercise, there was also no clear evidence of an important difference in social and occupational functioning at the end of the intervention (MD 1.40, 95% CI ‐0.33 to 3.13, very low‐certainty evidence) or at three months (MD 1.70, 95% CI ‐0.18 to 3.58) and nine months (MD 1.40, 95% CI ‐0.62 to 3.42) after the end of the intervention. Our confidence in this result is also very low.
2. Dropouts
From the 204 adults that Ho 2020 initially randomised, 38 participants dropped out within the year of the delivery of the interventions. The reasons reported included: not being willing to complete the follow‐up assessments, hospitalisation, re‐location away from Hong Kong, or death.
DMT versus waiting‐list control
Immediately after the end of the intervention, one out of 69 had dropped out from the DMT group (1.44%) and six out of 68 (8.82%) had dropped out from the waiting‐list control group (odds ratio (OR) 0.15, 95% CI 0.02 to 1.30, very low‐certainty evidence). We cannot be certain of any difference in acceptability of the interventions.
DMT versus exercise
Immediately after the end of the intervention, one out of the 69 people who were assigned to DMT intervention had dropped out (1.44%) and three of the 67 who received exercise as the intervention had dropped out (4.48%) (OR 0.31, 95% CI 0.03 to 3.09, very low‐certainty evidence). We cannot be certain of any difference in acceptability between the DMT and exercise interventions.
Other secondary outcomes specified in our protocol (mobility and balance, fatigue, anxiety, economic outcomes, and cost‐effectiveness of treatment) were not reported in the study included in the review. Systematic collection of data relating to adverse events did not take place either.
Discussion
Summary of main results
Only one study (Ho 2020) was included in this review. It evaluated the effects of DMT for older adults (mean age 79 years) with mild dementia or mild neurocognitive disorder. Outcomes were assessed immediately after the end of the intervention and again three months and nine months after the end of therapy. Short‐term (12 weeks) group DMT was compared with a waiting‐list control group and an exercise group in an attempt to control for all nonspecific elements of DMT such as the use of movement, the therapeutic relationship, or the attention of a therapist.
We found low‐certainty evidence that DMT may have little or no effect on neuropsychiatric symptoms or cognitive function in comparison to either waiting list or exercise.
We found low‐certainty evidence that DMT may have a small positive effect on depression when compared with exercise and waiting list. This was observed at the end of the intervention and at three‐ and nine‐months follow‐up. Depression was assessed using a short version of the Geriatric Depression Scale, a four‐item tool that has been normally used to screen for major depression (Lafont 2021) rather than assess changes in depressive symptoms. The version of the Geriatric Depression Scale used was in Chinese and Brañez‐Condorena 2021 suggests that the several available Geriatric Depression Scale versions are very different in their sensitivity and specificity. Therefore, we are very unsure of the clinical importance of the difference detected.
Social and occupational functioning, a secondary outcome in this review, was measured using the Instrumental Activities of Daily Living (IADL) scale. We are very uncertain about any effect of DMT on functioning when compared to either exercise or waiting list. There was no clear evidence of between‐group differences, but the evidence was of very low certainty.
We used dropouts as a proxy for assessing the acceptability of the interventions. Due to very low‐certainty evidence, we could not be sure of any difference in acceptability between DMT and exercise or between DMT and waiting list.
Overall completeness and applicability of evidence
Wide searching of the literature revealed only one study to include. Although this was a well‐conducted randomised controlled trial, with results reported for a total of 204 participants, the evidence is limited by being based on only one study. Several studies were excluded because they focused on social dancing and exercise to music rather than DMT. It is likely that the limited number of randomised controlled trials of DMT is due to limited funding in this discipline (often with only female participants). As they are often unable to form well‐funded research teams that can conduct large studies in this area, practitioners often report on their own work through clinical observations and small case studies; see for example studies from Hill 2017 and Hayes 2011. Although rich information about current practice is shared through these studies, claims of effectiveness are not possible. It is, therefore, apparent that there is a need to pay attention to this area, further highlighted by the impact from the recent pandemic and its consequences on high‐risk older adults. Further funding allocated to conduct randomised controlled trials in this area can offer more evidence‐based options to the growing number of older people with dementia.
Although the included study described its participants as having mild dementia, it used an inclusion criterion of mild neurocognitive disorder according to DSM‐5. Mild neurocognitive disorder is usually considered to be broadly synonymous with mild cognitive impairment (MCI), which may – or may not – progress to dementia. A CDR of 0.5 to 1 as in inclusion criterion is also compatible with many participants having MCI, not dementia. It seems that an unknown proportion of participants in this study did not meet diagnostic criteria for dementia, reducing the applicability of the results of this study to the population with dementia. Additionally, since the participants in this study had mild or very mild symptoms at baseline, there is no evidence on whether DMT can be effective for people with moderate or severe dementia or with clinically significant neuropsychiatric symptoms.
All the participants were based in Hong Kong, adding a particular cultural dimension to these results. Culture and associated ways in which therapists are accredited around the world is an important consideration in this area. Since there are no agreed ways in which dance movement therapists receive accreditation around the world, it is likely that DMT is also practised differently from country to country. There are certainly differences in the length of time people are trained, and the type of training they receive in both DMT and the arts therapies professions as a whole (Karkou 2017a). In order to bypass these differences, a clear description of the intervention becomes important. When such a description is not offered, as is the case with non‐manualised treatments like DMT, it becomes very difficult to monitor the therapist's adherence to expected DMT practice and to argue that the intervention is delivered consistently for all participants. Other possible variations in the intervention could be found in the inclusion or exclusion of caregivers and in the delivery of the intervention as a one‐to‐one or a group option.
One of our primary outcomes (quality of life) and many of our secondary outcomes, including mobility and balance, fatigue, anxiety, economic outcomes (cost‐effectiveness of treatment), and adverse events, including falls and injuries associated with the intervention, were not reported.
We did not include changes in positive and negative mood, nor loneliness in this review, although they were included in the Ho 2020 study. It may be useful in future updates to include a range of different measurements to supplement measures of depression and depressive symptoms, including mood scales and scales for loneliness. We also did not include biomarkers such as cortisol levels as outcomes in this review. It is possible that cortisol levels or other neuroendocrine factors (HPA axis functioning) are mediating factors in changes observed in psychological outcomes (depressed mood and emotional distress).
Quality of the evidence
We judged that the study we reviewed was well‐conducted. Following the guidance from Juul 2021, we did not make a judgement of high risk of bias due to lack of blinding of participants and personnel, which is not feasible in interventions of this nature. The protocol for the review trial was published and all results appeared to have been reported.
We considered the evidence for all outcomes to be of low or very low certainty when we applied GRADE criteria. We downgraded all results due to serious concern about indirectness due to the inclusion of people with mild cognitive impairment in the sample. We also downgraded all results due to imprecision because, although the study was large for an arts therapy study, it is unlikely that there were enough participants to meet the optimum information size. For two outcomes, social and occupational functioning and acceptability, we downgraded the evidence an additional level. For functioning, our concern about imprecision was very serious due to wide confidence intervals, while for acceptability, we considered that the use of dropouts as a proxy measure was another reason to downgrade for indirectness.
The work reported in Ho 2020 was supported by the General Research Fund, Hong Kong Research Grants Council (GRF/HKU17402714) which is not industry‐funded.
Potential biases in the review process
Several exhaustive electronic searches were conducted. In addition, we contacted professional associations and other leading researchers in DMT to identify relevant published studies and ongoing trials. DMT is a relatively small field and it is unlikely that we have missed any study or eligible studies did not come to our attention, albeit we cannot be entirely certain. Since studies in arts therapies tend to receive funding from charitable organisations, if we did miss relevant studies, it is likely that they were not trials and, thus, they would not have met our eligibility criteria (Geretsegger 2022).
Two independent healthcare professionals who were not dance therapists screened the studies and, thus, we mitigated the potential bias of reviewers with a DMT background having to evaluate and assess the eligibility of the studies.
Agreements and disagreements with other studies or reviews
Because we assessed the evidence to be of low and very low certainty, we are unsure if DMT has a clinically important impact on dementia for outcomes such as depression. This poses a challenge in comparing the results of this review with other systematic reviews in the field such as Karkou 2019 and Koch 2019 which suggest that DMT can have an important impact on depression for this client group. Furthermore, the study included in the review (Ho 2020) suggested that loneliness and negative mood were also positively impacted. The systematic review by Lyons 2018 highlights the potential impact of DMT on the mood of people with dementia in DMT groups. However, since these outcomes were not included in the protocol, we did not consider them as part of this review. Considering a wider range of mood‐related outcomes may be important in future studies and future updates of this review.
It is interesting that the reviewed study compares DMT with exercise. Reviews on exercise (e.g. Forbes 2015) have concluded that there is promising evidence that exercise programmes can have a significant impact in improving the ability to perform daily activities and possibly improving cognition in people with dementia. Further research is needed to establish whether DMT, a psychological intervention with active physical engagement, is more, less or as impactful as exercise for this client population.
The positive effect on depression in dementia is also a finding for the music‐based interventions reviewed by Van der Steen 2018, in which the authors were "moderately confident that music‐based treatments improve symptoms of depression and overall behavioural problems" (p. 1). Future updates of the current review on DMT for this client population may also indicate whether DMT, an intervention that also uses music (De Witte 2021; Karkou 2006; Karkou 2012), may produce similar results.
Authors' conclusions
Implications for practice.
Despite the methodological rigour of the one included study, we are not able to draw firm conclusions about the impact of DMT on people with dementia. Because of the small quantity of evidence and the fact that the study included an unspecified number of participants with mild neurocognitive disorder rather than dementia, our confidence in the results is low. The most promising outcome was the one relating to depression, but the clinical importance of the small benefit detected for DMT on this outcome was unclear. Further high‐quality research is needed to establish if DMT has a positive effect on this or any other outcome measure.
On the basis of reviewing one study only, it is not possible to draw any conclusions about the best type of DMT to use with people with dementia either. However, it may be useful to examine the intervention used in the reviewed study and to consider its relevance for the different cultural contexts in which DMT can be practised.
Implications for research.
Although there is a growing research literature on DMT for people with dementia, there was only one outcome study meeting the inclusion criteria for this review.
Further research in this area is needed that pays attention to who is included, what type of intervention is used, appropriate comparators and sensitive outcome measures, i.e. the PICOS criteria. Use of recognised screening tools to identify participants is important. Conducting studies with participants with a different severity of dementia will also be important. In all cases, clear descriptions of interventions can create a bridge between research and practice and provide opportunities to compare and contrast different approaches to DMT, moving closer to understanding the important components responsible for change. Identification of relevant primary outcomes will also benefit future studies, avoiding potentially misplaced attention to a primary emphasis on either improving or reducing deterioration of cognitive skills. As a form of psychotherapy, it is more likely that this intervention will have a higher impact on depression and mood, as suggested in the reviewed study and in other reviews in the field (Karkou 2019; Karkou 2022; Koch 2019; Lyons 2018). Defining appropriate and relevant primary outcomes and identifying robust measures that assess these outcomes should be an important consideration.
We make the above suggestions with an awareness that the area poses methodological challenges as discussed in relevant literature about complex interventions (Campbell 2007; Craig 2008). In the case of DMT, the holistic and person‐centred nature of the intervention, the context within which studies often take place (e.g. care homes for example), and certainly the responses of participants, who may find it difficult to either remember or articulate their experiences, all add to the complexity of research in this area. Guidance offered in the framework for developing and evaluating randomised controlled trials for complex interventions by the Medical Research Council (Skivington 2021) should be considered carefully in future research in this area.
We also recommend that future research engages with the development of logic models that can support process evaluations, and considers important contextual factors such as settings of delivery, culture and so on. The type of design adopted also needs to be considered and decisions need to be made on whether cluster versus individual trial designs are preferable. Finally, future studies will have to give careful attention to the type of control group/s used, including the presence of usual care versus active control groups.
What's new
| Date | Event | Description |
|---|---|---|
| 7 August 2023 | New citation required but conclusions have not changed | A new search was performed. One study eligible for inclusion. |
| 7 August 2023 | New search has been performed | A new search was performed. One study has been included |
History
Protocol first published: Issue 3, 2014 Review first published: Issue 2, 2017
Acknowledgements
We wish to acknowledge the support by Edge Hill University for releasing staff to undertake this review.
We would like to thank peer reviewers Richard Coaten and Robyn Cruz and a consumer reviewer who wishes to remain anonymous for their comments and feedback.
Disclaimer
The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Sources searched and search strategies
| Source | Search strategy | Hits retrieved |
| 1. CENTRAL (The Cochrane Library) http://crso.cochrane.org/SearchSimple.php (date of most recent search: 8 December 2022) |
#1 MESH DESCRIPTOR Dancing EXPLODE ALL TREES 173 #2 MESH DESCRIPTOR Dance Therapy EXPLODE ALL TREES 82 #3 danc*:TI,AB,KY 1060 #4 DTM:TI,AB,KY 48 #5 ("authentic movement*"):TI,AB,KY 1 #6 ("movement therap*"):TI,AB,KY 845 #7 ("movement psychot*"):TI,AB,KY 7 #8 ("body psychot*"):TI,AB,KY 28 #9 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 1926 #10 MESH DESCRIPTOR Dementia EXPLODE ALL TREES 5892 #11 MESH DESCRIPTOR Delirium EXPLODE ALL TREES 780 #12 MESH DESCRIPTOR Wernicke Encephalopathy EXPLODE ALL TREES 4 #13 MESH DESCRIPTOR Neurocognitive Disorders EXPLODE ALL TREES 11163 #14 dement*:TI,AB,KY 12889 #15 alzheimer*:TI,AB,KY 11127 #16 (lewy* adj2 bod*):TI,AB,KY 395 #17 (chronic adj2 cerebrovascular):TI,AB,KY 113 #18 ("organic brain disease" or "organic brain syndrome"):TI,AB,KY 137 #19 ("benign senescent forgetfulness"):TI,AB,KY 2 #20 (cerebr* adj2 deteriorat*):TI,AB,KY 10 #21 (cerebral* adj2 insufficient*):TI,AB,KY 1 #22 ("major neurocognitive disorder*"):TI,AB,KY 33 #23 deliri*:TI,AB,KY 3475 #24 ("normal pressure hydrocephalus" and "shunt*"):TI,AB,KY 75 #25 (pick* adj2 disease):TI,AB,KY 73 #26 (creutzfeldt or jcd or cjd):TI,AB,KY 54 #27 huntington*:TI,AB,KY 666 #28 binswanger*:TI,AB,KY 6 #29 korsako*:TI,AB,KY 68 #30 #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 27503 #31 #9 AND #30 85 #32 01/01/2016 TO 11/03/2021:CD 867486 #33 #31 AND #32 71 |
March 2016: 2 March 2021: 71 Feb 2022: 10 Dec 2022: |
| 2. MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950‐present (Ovid SP) (date of most recent search: 8 December 2022) |
1 exp Dancing/ 2 exp Dance Therapy/ 3 danc*.ti,ab. 4 DTM.ti,ab. 5 "authentic movement*".ti,ab. 6 "movement therap*".ti,ab. 7 "movement psychot*".ti,ab. 8 "body psychot*".ti,ab. 9 or/1‐8 10 exp Dementia/ 11 Delirium/ 12 Wernicke Encephalopathy/ 13 Delirium, Dementia, Amnestic, Cognitive Disorders/ 14 dement*.mp. 15 alzheimer*.mp. 16 (lewy* adj2 bod*).mp. 17 (chronic adj2 cerebrovascular).mp. 18 ("organic brain disease" or "organic brain syndrome").mp. 19 "benign senescent forgetfulness".mp. 20 (cerebr* adj2 deteriorat*).mp. 21 (cerebral* adj2 insufficient*).mp. 22 "major neurocognitive disorder*".ti,ab. 23 deliri*.mp. 24 ("normal pressure hydrocephalus" and "shunt*").mp. 25 (pick* adj2 disease).mp. 26 (creutzfeldt or jcd or cjd).mp. 27 huntington*.mp. 28 binswanger*.mp. 29 korsako*.mp. 30 or/10‐29 31 9 and 30 |
March 2016: 7 March 2021: 66 Feb 2022: 25 Dec 2022: 33 |
| 3. Embase (OVID) 1974 to present (date of most recent search: 8 December 2022) |
1 exp dancing/ 2 exp dance therapy/ 3 danc*.ti,ab. 4 DTM.ti,ab. 5 "authentic movement*".ti,ab. 6 "movement therap*".ti,ab. 7 "movement psychot*".ti,ab. 8 "body psychot*".ti,ab. 9 or/1‐8 10 exp dementia/ 11 dement*.ti,ab. 12 alzheimer*.ti,ab. 13 (lewy* adj2 bod*).ti,ab. 14 deliri*.ti,ab. 15 (cerebr* adj2 deteriorat*).ti,ab. 16 (cerebral* adj2 insufficient*).ti,ab. 17 (creutzfeldt or jcd or cjd).ti,ab. 18 huntington*.mp. 19 "cognit* impair*".ti,ab. 20 or/10‐19 21 9 and 20 |
March 2016: 28 March 2021: 219 Feb 2022: 73 Dec 2022: 122 |
| 4. PsycINFO (OVID) (date of most recent search: 8 December 2022) |
1 exp Dance/ 2 exp Dance Therapy/ 3 danc*.ti,ab. 4 DTM.ti,ab. 5 "authentic movement*".ti,ab. 6 "movement therap*".ti,ab. 7 "movement psychot*".ti,ab. 8 "body psychot*".ti,ab. 9 or/1‐8 10 exp Vascular Dementia/ or exp Dementia/ or exp Semantic Dementia/ or exp Presenile Dementia/ or exp Dementia with Lewy Bodies/ or exp Senile Dementia/ 11 exp Cognition/ 12 dement*.ti,ab. 13 alzheimer*.ti,ab. 14 "cognit* impair*".ti,ab. 15 or/10‐14 16 9 and 15 |
March 2016: 21 March 2021: 99 Feb 2022: 29 Dec 2022: 27 |
| 5. CINAHL (EBSCOhost) (date of most recent search: 8 December 2022) |
S17 S10 AND S16 S16 S11 OR S12 OR S13 OR S14 OR S15 S15 MH “Cognition Disorders” S14 TX “cognit* impair*” S13 TX Alzheimer* S12 TX dement* S11 (MH “Dementia+”) OR (MH “Delirium, Dementia, Amnestic, Cognitive Disorders”) S10 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 S9 MH "Aerobic Dancing" S8 TX "body psychot*" S7 TX "movement psychot*" S6 TX "movement therap*" S5 TX "authentic movement*" S4 TX DTM S3 TX danc* S2 (MH "Dance Therapy") S1 (MH "Dancing+") |
March 2016: 13 March 2021: 113 Feb 2022: 34 Dec 2022: 23 |
| 6. Web of Science – core collection (Clarivate) (date of most recent search: 8 December 2022) |
TOPIC: (danc* OR "movement therapy" OR salsa OR jive OR tango OR ballroom OR "ball room") AND TOPIC: (dement* OR alzheimer* OR FTLD OR FTD OR "primary progressive aphasia" OR "progressive non‐fluent aphasia" OR "frontotemporal lobar degeneration" OR "frontolobar degeneration" OR "frontal lobar degeneration" OR "pick* disease" OR "lewy bod*" OR "mild cognit* impair*" OR MCI) AND TOPIC: (randomly OR randomised OR randomized OR RCT OR "controlled trial" OR "double blind" OR "single blind") | March 2016: 9 March 2021: 46 Feb 2022: 18 Dec 2022: 20 |
| 7. LILACS (BIREME) (date of most recent search: 8 December 2022) |
dance OR dancing OR "movement therapy" OR baile OR dança [Words] and dementia OR demencia OR demência OR alzheimer OR alzheimers OR alzheimer's [Words] | March 2016: 4 March 2021: 13 Feb 2022: 1 Dec 2022: 0 |
| 8. ClinicalTrials.gov (www.clinicaltrials.gov) (date of most recent search: 8 December 2022) |
alzheimer OR alzheimers OR dementia OR (major neurocognitive disorder) | dance OR dancing OR movement therapy | March 2016: 0 March 2021: 0 Feb 2022: 3 Dec 2022: 4 |
| 9. ICTRP (date of most recent search: 8 December 2022) |
[Condition:] alzheimer OR alzheimers OR dementia OR (major neurocognitive disorder) [AND Intervention:] dance OR dancing OR movement therapy |
March 2016: 5 March 2021: 0 Feb 2022: 0 Dec 2022: 3 |
| 10. CDCIG specialised register (CRS web) (date of most recent search: 8 December 2022) |
Dance OR Dancing OR “movement therapy” | March 2016: 4 March 2021: 46 Feb 2022: 7 Dec 2022: 15 |
| TOTAL before de‐duplication | March 2016: 89 March 2021: 673 Feb 2022: 200 Dec 2022: 288 |
|
| TOTAL after de‐duplication | March 2016: March 2021: 421 Feb 2022: 126 Dec 2022: 204 |
|
Appendix 2. Protocol of methods
Methods
Criteria for considering studies for this review
Types of studies
Our review will include published or unpublished randomised controlled trials (RCTs) in any language. We will also consider cross‐over designs and cluster‐RCTs.
Types of participants
The review will include studies of people who, according to the DSM‐IV, ICD‐10 or other comparable diagnostic criteria, have been formally diagnosed as having any type of dementia of any type of severity. Women and men in all age groups and in all settings (e.g. statutory and voluntary organisations, inpatient or outpatient) will be considered for inclusion in the review.
We will identify the inclusion criteria that determined the selection of participants for each trial. When this information is not available in the source material, we will contact the trial authors to determine how they screened participants, and how many people declined to participate.
Types of interventions
Experimental interventions
We will consider interventions delivered by a DMT practitioner who: has received formal training; is a dance movement therapist in training; or is otherwise recognised as a dance movement therapist in the country in which the study was conducted.
We will include both group, individual and family/couple DMT with any number and duration of sessions.
Sessions must include active involvement in dance/movement in the presence of a dance movement therapist, or dance/movement interaction with a dance movement therapist with or without other group members, or both. Dance/movement can be improvisatory or structured. Sessions will have clear therapeutic intent and a clear description of the intervention will be reported or available on request. All approaches to DMT will be considered.
The presence or absence of verbal interaction and reflection will be considered as a factor to be examined in subgroup analysis. In all cases, however, creative movement work may be the main means of working through cognitive, behavioural, emotional or social issues faced by the participants.
Comparators
No treatment or standard care.
Psychological therapies: e.g. counselling, verbal psychotherapies including psychodynamic psychotherapy, interpersonal therapy or cognitive behavioural therapy.
Pharmacological interventions: medications such as cholinesterase inhibitors (CgEIs) or memantine.
Other interventions: e.g. exercise, dance or music.
Different types of DMT: different types of DMT, as defined above.
Types of outcome measures
Primary outcomes
Changes in challenging behaviours (e.g. wandering, agitation, general restlessness), cognitive functioning, levels of depression and quality of life; examples of tools for assessing each outcome are listed below. We will accept all behavioural and psychological tools reported by the authors of the identified primary studies in the same way that the author, Vink, did for the Vink 2011 review of music therapy for dementia.
-
Challenging behaviours:
quantitative observational tools specifically designed for an individual study to measure frequency of occurrence of wandering, agitation and general restlessness;
standardised tests such as: Cohen‐Mansfield Agitation Inventory Clifton (CMAI; Cohen‐Mansfield 1989) scoring from 29 to 203; the higher the total score the more frequent the agitated behaviours.
-
Cognitive functioning:
Mini‐Mental State Exam (MMSE) (Folstein 1975); scoring from 0 to 30 with lower scores indicating poorer cognitive function;
Alzheimers Disease Assessment Scale (ADAS‐cog; Rosen 1984); scores on the cognitive subscale range from 0 to 70 with higher scores indicating poorer cognitive function.
-
Depression:
Cornell Scale for Depression in Dementia (Alexopoulos 1988); scoring from 0 to 38 with higher scores indicating more severe depression.
-
Quality of life:
Quality of Life‐Alzheimer's Disease (QOL‐AD, Logsdson 1999): scoring from 13 to 52; higher scores suggest better quality of life.
If other similar outcome measures are found in the included studies, they will be considered.
Secondary outcomes
Mobility and balance, fatigue, anxiety, social and occupational functioning, economic outcomes (cost‐effectiveness of treatment), and treatment or research discontinuation/dropout (as measures of acceptability). Adverse events will also be considered, including falls and injuries associated with the intervention. Outcomes will be included as used within the primary studies.
Search methods for identification of studies
Electronic searches
We will search ALOIS (www.medicine.ox.ac.uk/alois) ‐ the Cochrane Dementia and Cognitive Improvement Group’s Specialized Register. The search terms used will be: "dance*" or "authentic movement" or "movement therap*" or "movement psychot*" or "body psychot*".
ALOIS is maintained by the Trials Search Co‐ordinator of the Cochrane Dementia Group and contains dementia and cognitive improvement studies identified from:
monthly searches of a number of major healthcare databases: MEDLINE, Embase, CINAHL, PsycINFO and LILACS;
monthly searches of a number of trial registers: meta‐Register of Controlled Trials; Umin Japan Trial Register; WHO portal (which covers ClinicalTrials.gov; ISRCTN; Chinese Clinical trials Register; German Clinical trials register; Iranian Registry of Clinical trials and the Netherlands National Trials Register, plus others);
quarterly search of The Cochrane Library’s Central register of Controlled trials (CENTRAL);
monthly searches of a number of grey literature sources: ISI Web of Knowledge Conference Proceedings; Index to Theses; Australasian Digital Theses.
To view a list of all sources searched for ALOIS, see About ALOIS on the ALOIS website.
Additional separate searches will be run in many of the above sources to ensure that the most up‐to‐date results are retrieved.
Searching other resources
We will also do the following in order to identify published, unpublished and ongoing trials that may not appear in the electronic searches listed above.
We will search the bibliographies of relevant studies and reviews.
We will contact professional associations and educational programmes in DMT from around the world using a standard request form to inform us of Masters degree, PhD or independent work that is published or unpublished, completed or ongoing that might meet the inclusion criteria.
We will contact DMT researchers, theoreticians and practitioners who specialise in this area of work, whom we will regard as experts in the field.
Data collection and analysis
Selection of studies
In the first instance, the two authors (VK and BM) will screen titles and abstracts for inclusion of studies that meet the inclusion criteria as discussed in Criteria for considering studies for this review and further specified in Data extraction and management. The two review authors will act independently and will assess different studies' eligibility using the relevant proforma.
All potentially relevant titles and abstracts will be recorded and assessed to decide whether they should be included, excluded or whether additional information is required regarding:
general information;
-
inclusion criteria:
study design;
participants;
interventions;
outcomes.
A final decision will be made only after additional information has been sought, where required. If there are disagreements, the two authors will meet to discuss them and, if required, will seek a third opinion from the Cochrane Dementia and Cognitive Improvement Group. A record will be kept of all studies, charting the decisions made, and the stage at which these decisions were made (i.e. title and abstract, on further enquiry from the authors, or on reading the full paper), together with the reason for this decision.
Data extraction and management
Full reports will be obtained of all included studies. A proforma specifically designed for this purpose will be used to extract relevant data from all included studies. The two review authors will extract data independently using this data extraction proforma. Again, any disagreements will be resolved by discussion; where this does not result in agreement, a third opinion will be sought from the Cochrane Dementia and Cognitive Improvement Group.
The following details will be extracted:
study characteristics;
interventions;
outcome measures used in the study;
study results;
additional notes.
Assessment of risk of bias in included studies
In order to identify any risk of bias in the included studies, we will use the Cochrane Risk of Bias (Higgins 2011). With the aid of this tool, we will make a judgement of 'low risk’, ’high risk’ or ’unclear risk’ of bias for each trial of the following areas:
-
selection bias:
random sequence generation,
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective reporting;
other sources of bias.
Measures of treatment effect
When changes in challenging behaviours (e.g. wandering, agitation, general restlessness), cognitive functioning, depression and quality of life are measured using rating scales presented as dichotomous data, we will summarise data using risk ratios (RR). For continuous outcomes, such as scores from a scale, we will use mean differences (MD).
When different scales are used to measure the same outcome, the standardised MD will be calculated in Review Manager (RevMan 2012) to summarise outcomes across scales.
In all cases, endpoint data will be regarded as a superior method over change scores. This is preferred since data can be skewed in favour of the treatment or the control group where randomisation is inadequate. It is possible that change scores could appear as different when in fact the two end points (control and treatment group) are similar (see, for example, Meekums 2012b).
Unit of analysis issues
We are not expecting to find large‐scale studies taking place in this area but, depending on the type of studies available, we will make the following choices:
in the case of cross‐over trials, we will use only the first active treatment period;
in studies with multiple arms we will include only the arm with DMT and the control;
in the case of two DMT arms with one single control group, we will consult the Cochrane Handbook (Higgins 2011, chapter 16.5.4).
If there is a cluster‐randomisation, depending on the data available, analysis will follow the Cochrane Handbook (Higgins 2011, chapters 16.3.3 to 16.3.7).
Dealing with missing data
If there are missing data in terms of end of treatment scores, we will attempt either to obtain the missing data or find out why data are missing through contacting trialists. Data that remain unavailable will be treated as 'missing at random’ (Higgins 2011, 16.1.2), and, if it is end of treatment scores that are missing, we will assume that participants either dropped out of the intervention, died, or became too ill to continue. The percentage dropout will be calculated, and cautiously interpreted as a measure of acceptability.
If standard deviations (SD) are missing, we will consider the values of other reported measures such as P values, t values, confidence intervals and standard errors. If a sufficient number of studies become available, we will try to impute a SD. We will, however, make a note of this, and we will perform a sensitivity analysis, as imputed measures may bias towards lack of effect.
For continuous data that remain missing, we plan to report completers' data only. For binary data, we will assume that dropouts had 'no change' and we will analyse using intention‐to‐treat analysis.
In general terms, we will report all missing data in the risk of bias tables.
Assessment of heterogeneity
Initially, sources of clinical heterogeneity will be examined; studies will be pooled only where review authors judge that there is sufficient clinical homogeneity. In order to assist our judgement regarding potential sources of clinical heterogeneity, we will summarise studies in terms of participants, settings, method of delivery (that is, group or individual, number of sessions), type of DMT, and reported outcomes.
We will assess heterogeneity in the results of the trials by inspection of graphical presentations and by the I2 and Chi2 statistics (Higgins 2011).
We will pool studies with I2 values of up to 80% heterogeneity, which may be an acceptable level of heterogeneity for psychological assessments. We will then perform a sensitivity analysis to establish the effects of including studies of lower quality (see Sensitivity analysis).
Assessment of reporting biases
Where there are more than ten studies that address the same outcome, we will use a funnel plot analysis to examine for publication bias.
Data synthesis
Data from all trials included in the review will be entered into Review Manager software (RevMan 2012). A meta‐analysis will be performed on extracted data, if:
there is more than one study with an estimated treatment effect;
the included studies appear to differ minimally in characteristics and can be investigated for heterogeneity through subgroup analyses;
studies use the same outcome measures;
each study has available data (Higgins 2011; Meekums 2012b).
Finally, as it is expected that studies will use different time points for measurement, an initial main analysis will be based on the final time point. Following this, subgroup analysis will also be considered as follows: short follow‐up (up to 14 weeks); moderate follow‐up (15 to 27 weeks) and long follow‐up (28 weeks and over). In cases where the type of intervention, the populations studied, or the outcome measures used are very different from one study to another, only narrative descriptions of the findings will be included in the review.
Subgroup analysis and investigation of heterogeneity
If possible, we will perform subgroup analyses as follows:
mode of delivery including group or individual;
actively moving or not actively moving therapist;
presence or absence of verbal interaction;
length of treatment including number of sessions (fewer than 12 sessions versus 12 or more sessions);
intensity of intervention, to include frequency (weekly or less frequent, bi‐weekly or more frequent) and duration of sessions (one hour or less, more than one hour);
type of dementia;
severity of dementia (mild, moderate or severe as defined by the trialist);
participant characteristics including gender (men, women or other) and age (under 65 years old; 65 years old and over);
setting (statutory and non‐statutory).
Sensitivity analysis
If it is relevant, the following types of sensitivity analysis will be performed relating to:
different types of risk of bias;
imputed measures vs available case analysis.
Appendix 3. Sample of data extraction form
Information/data extracted for all relevant studies
Identification
Study details
Sponsorship Source
Country
Setting
Comments
Author's contact details
Author's name
Institution
Address
Methods
1. Design
Select an option
Randomised controlled trial
Case‐control study
Cluster‐randomised controlled trial
Controlled interrupted time series
Historically controlled trial
Prospective cohort study
Retrospective cohort study
Other
2. Design
Select an option
Parallel‐group
Cross‐over
Not applicable
Additional Methods data
Aims and Objectives
Data collection period
Number of data collection centres
Type of randomisation
Population
Inclusion criteria
Exclusion criteria
Group differences
Additional population data
Education and/or occupation
Ethnicity
Gender
Medication
Previous depression episodes
Severity of depression
Socioeconomic status
Dance Movement Therapy
Control/waiting list/standard care
Description of interventions
| Characteristic | Dance Movement Therapy | Active control/ Waiting list/ Standard care |
| Theoretical rationale | ||
| Materials and activities | ||
| Mode of delivery, configuration and client therapist ratio | ||
| Dosage (frequency, duration and intensity) | ||
| Tailoring and modifications | ||
| Fidelity assessment | ||
| Reference or link to the intervention protocol if it is available |
Outcomes
-
Primary outcome measures that provide scores for changes in:
challenging behaviours;
cognitive functioning;
depression;
quality of life.
-
Secondary outcome measures that provide scores for:
mobility and balance;
fatigue;
anxiety;
social and occupational functioning;
economic outcomes;
treatment or research discontinuation/dropouts;
adverse events, including falls and injuries associated with the intervention.
| Pre‐Intervention | Post‐Intervention | 6 months follow‐up | |||||||
| Mean | SD | N | Mean | SD | N | Mean | SD | N | |
| DANCE MOVEMENT THERAPY | |||||||||
| ACTIVE CONTROL/ WAITING LIST/ STANDARD CARE | |||||||||
Additional notes
Record of details regarding correspondence with author/s for additional information or clarification of queries .......................................................................................................................................................
Ethics of stated conflict of interest ....................................................................................................
Details of other studies cited in the references ................................................................................
Duplicate publications .....................................................................................................................
Translation required .......................................................................................................................
Appendix 4. Risk of Bias
Risk of bias assessment tool (Higgins 2011)
| Domain | Support for judgement | Review authors’ judgement |
| Selection bias | ||
| Random sequence generation | Describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups | Selection bias (biassed allocation to interventions) due to inadequate generation of a randomised sequence |
| Allocation concealment | Describe the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment | Selection bias (biassed allocation to interventions) due to inadequate concealment of allocations prior to assignment |
| Performance bias | ||
| Blinding of participants and personnel: assessments should be made for each main outcome (or class of outcomes) | Describe all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Provide any information relating to whether the intended blinding was effective | Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
| Detection bias | ||
| Blinding of outcome assessment: assessments should be made for each main outcome (or class of outcomes) | Describe all measures used, if any, to blind outcome assessors from knowledge of which intervention a participant received. Provide any information relating to whether the intended blinding was effective | Detection bias due to knowledge of the allocated interventions by outcome assessors |
| Attrition bias | ||
| Incomplete outcome data: assessments should be made for each main outcome (or class of outcomes) | Describe the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. State whether attrition and exclusions were reported, the numbers in each intervention group (compared with total randomised participants), reasons for attrition/exclusions where reported, and any re‐inclusions in analyses performed by the review authors | Attrition bias due to amount, nature or handling of incomplete outcome data |
| Reporting bias | ||
| Selective reporting | State how the possibility of selective outcome reporting was examined by the review authors, and what was found | Reporting bias due to selective outcome reporting |
| Other bias | ||
| Other sources of bias | State any important concerns about bias not addressed in the other domains in the tool. If particular questions/entries were prespecified in the review’s protocol, responses should be provided for each question/entry |
Bias due to problems not covered elsewhere in the table |
Checklist into which the tool outlined above will be translated
1. Selection bias
a. Random sequence generation
Was the trial reported as randomised? YES/NO/UNCLEAR Was the method of randomisation appropriate? YES/NO/UNCLEAR YES: randomisation will be rated as appropriate if every participant had an equal chance to be selected for either intervention and if the investigator was unable to predict to which treatment the participant would be assigned. Examples of appropriate randomisation methods include use of: random number table; computer random‐number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing lots; minimisation NO: inappropriate methods include: use of date of birth; sequence generated by a rule e.g. date of admission; patient preference; clinician's judgement; availability of the intervention UNCLEAR: insufficient information provided on which to base judgement
b. Allocation concealment
Was allocation concealment adequate? YES/NO/UNCLEAR YES: allocation concealment will be rated as adequate when the following methods are used: central allocation (e.g. telephone, web‐ or pharmacy‐based randomisation); serially‐numbered, opaque, sealed envelopes; other descriptions with convincing concealment. NO: allocation concealment will be rated as inadequate when the following methods are used: open random allocation schedule, i.e. a list of random numbers; envelopes without safeguards, i.e. unsealed, non‐opaque or not sequentially‐numbered; alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. UNCLEAR: authors did not report adequately on method of concealment.
2. Blinding of participants and personnel
Was participant and personnel blinding adequate? YES/NO/UNCLEAR YES: adequate blinding will involve blinding of participants and personnel to the allocated interventions during the study. NO: inadequate blinding is implied in studies in which blinding of participants and personnel has not taken place. Please note that for DMT studies, it is highly likely that it will not be possible to blind participants or those providing the DMT interventions. Therefore, it is likely that for this domain DMT studies will be assessed as having inadequate blinding and thus being at a high risk of bias. UNCLEAR: unclear blinding is implied when insufficient information is reported to determine who was blinded, or whether blinding occurred at all.
3. Blinding of outcome assessment
Was discussion of blinding of outcome assessment adequate? YES/NO/UNCLEAR YES: adequate blinding achieved when outcome assessors are unaware of the intervention allocated. NO: inadequate blinding means that outcome assessors are aware of the intervention allocated. UNCLEAR: a judgement of unclear will be used if a study does not address this point.
4. Incomplete outcome data
Were incomplete outcome data adequately considered? YES/NO/UNCLEAR YES: incomplete outcome data will have been adequately considered when: there are no missing outcome data; reasons for missing data are unlikely to be related to the true outcome; missing outcome data are balanced across comparison groups, with similar reasons; plausible effect size (difference in means or standardised difference in means) amongst missing outcomes is insufficient to have clinically relevant impact on observed effect size; missing data are imputed using appropriate methods. NO: incomplete outcome data will have been inadequately considered when: reasons for missing outcome data are likely to be related to the true outcome, with either imbalance of numbers or reasons for missing data across groups; plausible effect size amongst missing outcomes is sufficient to induce clinically relevant bias in observed effect size; ’as treated’ analysis performed with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. UNCLEAR: insufficient reporting of attrition or exclusions to permit judgement, e.g. number randomised not stated or no reasons provided for missing data; or the study did not address this outcome.
For continuous data, an intention‐to‐treat (ITT) analysis will be calculated if not presented by the authors of the study.
5. Selective reporting
Was the reporting bias acceptable? YES/NO/UNCLEAR YES: reporting bias will be assessed as acceptable if: the study protocol is available and all prespecified outcomes of interest in the review have been reported in the prespecified way; or the protocol is not available but it is clear that the published reports include all expected outcomes. NO: reporting bias will be assessed as unacceptable if: not all the prespecified primary outcomes are reported; one or more of these is reported using measurements or analytic methods or subsets of data that were not prespecified; one or more of the primary outcomes were not prespecified (unless clearly justified, e.g. an unexpected adverse effect); one or more outcomes of interest is reported incompletely and so cannot be entered into meta‐analysis; failure to report results for a key outcome that would be expected from such a study (including adverse outcomes). UNCLEAR: insufficient information provided to make a judgement.
6. Other sources of bias
Were other sources of bias eliminated: YES/NO/UNCLEAR Examples of other risk of bias include: those related to study design; claimed to be fraudulent. Examples of unclear risk of bias include: insufficient rationale or evidence that an identified problem would introduce bias.
The above criteria will be used to give each article an overall quality rating of A to C, as follows:
A: low risk of bias ‐ all criteria met. B: moderate risk of bias ‐ one or more of the criteria only partly met. C: high risk of bias ‐ one or more criteria not met.
Studies will not be excluded on the basis of a low‐quality score.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ho 2020.
| Study characteristics | ||
| Methods | Single‐blinded 3‐arm parallel‐group RCT with DMT intervention group, physical exercise group and a waiting‐list control | |
| Participants | Older adults (mean = 79.0 years; SD = 8.0) with a clinical diagnosis of dementia or mild neurocognitive disorder (DSM‐V) Screening: Clinical Dementia Rating Scale (Hughes 1982). Also semi‐structured interviews at baseline with the participants and their caregivers to determine impairment in memory, orientation, judgement and problem‐solving, community affairs, hobbies and habits, and personal care Inclusion criteria The inclusion criteria of the study were a Clinical Dementia Rating Scale rating of 0.5 and 1 (very mild to mild dementia), aged 65 or older, mobility of at least the upper limbs and body, sufficient visual and auditory abilities to complete assessments, and stable doses of medication for at least 30 days before screening. Exclusion criteria The exclusion criteria included the clinical diagnosis of major psychiatric disorder or abuses of drug or alcohol that could cause cognitive impairment, history of stroke, or other severe illnesses that led to neurological deficits that limit participation in the interventions. |
|
| Interventions |
Dance Movement Therapy (DMT) Six DMT groups received DMT interventions spanning 12 weeks, with 1‐h sessions held twice a week in groups of about 10–12 participants each. In total 24 h of DMT intervention was offered by qualified therapists. Materials, such as scarves, elastic bands, ribbons, and small musical instruments were used more often to enhance the sense of working with objects, elasticity, strength, and co‐ordination. The intervention emphasised rhythmic and contralateral movements on both sides of the body to enhance co‐ordination and stimulation between the left and right cerebral hemispheres (Phillips‐Silver 2007). Finally, verbal sharing was done as a group discussion following the end of each session. All participants were invited to use one or two words or a gesture or movement to express their feelings about the movement experience or to say something supportive or caring to each other, which facilitated articulation and mutual support amongst the participants. Exercise The exercise intervention spanned 12 weeks, with 1‐h sessions held twice a week in a total of 6 groups of about 10–12 participants each. Participants in both groups received in total 24 h of intervention offered by trained and qualified fitness instructors. The exercise group contained a mild‐to‐moderate exercise programme of comparable length and intensity as the DMT group. Each exercise session consisted of a warm‐up (15 min), stretching and joint movements (15 min), exercising with towels (15 min) and a cool‐down (15 min). Heart rates of the participants were monitored throughout the entire intervention sessions using portable heart rate monitors to maintain a similar level of exercise exertion in both groups (40%–60% of the VO2 max value). Waiting list The waiting‐list control group received regular medication and routine care during the study and was offered DMT or exercise interventions upon study completion. |
|
| Outcomes | Neuropsychiatric symptoms: 12‐item Neuropsychiatric Inventory (Lam 2006). Cognitive functioning:
Depression: Geriatric Depression Scale to measure scores on depression (Cheng 2005). Social and Occupational Functioning: instrumental activities of daily living (IADL) scale (Tong 2002) Finally, the study included measures for loneliness, positive and negative mood, and cortisol level, but these were not extracted in this review. Timing of outcome measurements: Time 1: baseline; Time 2: end of intervention; Time 3: three months after end of intervention; Time 4: nine months after end of intervention |
|
| Notes | The study was funded by the General Research Fund, Hong Kong Research Grants Council (GRF/HKU17402714). All the authors have no financial or other conflicts of interests to disclose. Note that the first author is a dance movement therapist. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | On a 1:1:1 basis using simple randomisation with computer‐generated random numbers and no blocking |
| Allocation concealment (selection bias) | Unclear risk | Description of allocation concealment was not clear on the published paper. Following communication with the authors, no further clarity was obtained. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for this outcome were available for nearly all participants randomised. Missing outcome data from participants were balanced across comparison groups, with similar reasons. |
| Selective reporting (reporting bias) | Low risk | The trial was analysed in accordance with a prespecified plan that was finalised before unblinded outcome data were available for analysis; the numerical result being assessed was likely to have been selected, on the basis of the results, from multiple outcome measurements within the outcome domain. |
| Other bias | Low risk | There were no other biases identified. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The research coordinators were blinded but not the participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Therapists and researchers separately functioned as two different teams. An observer not directly involved in the intervention collected the data. |
DMT: dance movement therapy DSM: Diagnostic and Statistical Manual of Mental Disorders IADL: instrumental activities of daily living RCT: randomised controlled trial SD: standard deviation VO2 max: maximal oxygen consumption
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cheung 2018 | Ineligible intervention (music and movement intervention) |
| Choo 2019 | Ineligible intervention (creative dance movement programme) |
| Esmail 2020 | Ineligible intervention (dance movement training and aerobic exercises) |
| Kressig 2015 | Ineligible study design |
| Lyons 2019 | Ineligible study design (systematic review and case study) |
| Merom 2016 | Ineligible intervention (social dancing) |
| Merom 2016a | Ineligible intervention (social dancing) |
| Ruiz‐Muelle 2019 | Ineligible study design (review) |
Differences between protocol and review
Our protocol stated that we would include only clients with dementia. However, we decided to include a study in which some clients had DSM‐5 diagnoses of mild neurocognitive disorder.
One of our primary outcomes was termed 'challenging behaviour' in the protocol, with examples of wandering, agitation and general restlessness. We included scores on the Neuropsychiatric Inventory as a primary outcome in this category. However, the participants in the study had very mild cognitive syndromes and the Neuropsychiatric Inventory scores measured in the study were very low. We recognised that the symptoms and behaviours being measured are not likely to be 'challenging behaviours' and we amended the description of the outcome to the broader 'neuropsychiatric symptoms'.
The protocol stated that, in studies with more than one comparator intervention, we would compare dance therapy with a single control group. We decided to include comparisons with both control groups (exercise and waiting list) from the only included study because we considered that both comparisons were interesting for readers.
The original protocol stated that the authors planned to decide on an appropriate model for synthesis by looking at the results from the statistical test for homogeneity. This statement has been deleted from the Data synthesis section as this is no longer considered appropriate. The original protocol also stated that sensitivity analysis would be performed by comparing the results from high versus low‐risk studies. This statement has been deleted in this update, as this method of sensitivity analysis is not recommended.
Minor edits on the text for clarity and updates on background literature were also made that allowed us to keep this review current.
Contributions of authors
The screening was completed by MR and EH with SA acting as a referee. | Data were extracted by SA, VK, and MR. Assessment of quality: VK and SA Analysis and results: SA and VK Narrative synthesis: VK and SA Drafting and revising: VK, SA, and BM
Sources of support
Internal sources
-
University of Leeds, UK and Edge Hill University, UK
The two institutions offered support in the form of accessing learning resources
External sources
-
NIHR, UK
This updated review was supported by the National Institute for Health and Care Research, via Cochrane Infrastructure funding to Cochrane Dementia and Cognitive Improvement. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
VK and BM are both dance movement psychotherapists registered with the Association for Dance Movement Psychotherapy UK and active researchers in the field with relevant research activities. SA is also a registered dance movement therapist with the Indian Association of Dance Movement Therapy and an active researcher in this area.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Ho 2020 {published data only}
- Ho RT, Fong TC, Chan WC, Kwan JS, Chiu PK, Yau JC, et al. Psychophysiological effects of dance movement therapy and physical exercise on older adults with mild dementia: a randomized controlled trial. Journals of Gerontology: Series B 2020;75(3):560-70. [DOI] [PubMed] [Google Scholar]
- Ho RTH, Cheung JKK, Chan WC, Cheung IKM, Lam LCW. A 3‐arm randomized controlled trial on the effects of dance movement intervention and exercises on elderly with early dementia. BMC Geriatrics 2015;15(127):1-8. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho RTH, Hon T, Chan WC, Joseph SK, Chun Chiu PK, Lam LCW. Effects of dance movement intervention on elderly with early dementia: preliminary results of a randomized controlled trial. Annals of Behavioral Medicine (SBM 39th Annual Meeting Abstract Supplement) April 2018;52(Suppl 1):S1–S838. [DOI: ] [Google Scholar]
- Ho RTH, Hon T, Chan WC, Kwan JSK, Chiu PKC, Lam LCW. Effects of dance movement intervention on elderly with early dementia: preliminary results of a randomized controlled trial. Innovation in Aging 2017;1 (Suppl 1)(S1):345. [DOI: 10.1093/geroni/igx004.1264] [DOI] [Google Scholar]
References to studies excluded from this review
Cheung 2018 {published data only}
- Cheung Daphne Sze Ki, Lai Claudia Kam Yuk, Wong Frances Kam Yuet, Leung Mason Chin Pang. The effects of the music-with-movement intervention on the cognitive functions of people with moderate dementia: a randomized controlled trial. Aging and Mental health 2018;22(3):306-315. [DOI] [PubMed] [Google Scholar]
Choo 2019 {published data only}
- Choo, T, Barak, Y, & East, A. The Effects of Intuitive Movement Reembodiment on the Quality of Life of Older Adults With Dementia: A Pilot Study. American Journal of Alzheimer's disease & Other Dementias 2019;January 1:1. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Esmail 2020 {published data only}
- Esmail A, Vrinceanu T, Lussier M, Predovan D, Berryman N, Houle J, Karelis A, Grenier S, Vu T T M, Villalpando J M, Bherer L. Effects of Dance/Movement Training vs. Aerobic Exercise Training on cognition, physical fitness and quality of life in older adults: A randomized controlled trial. Journal of Bodywork and Movement Therapies 2020;24(1):212-220. [DOI] [PubMed] [Google Scholar]
Kressig 2015 {published data only}
- Kressig R W. Non-pharmacological interventions in dementia care. [German] [Demenz vom Alzheimer-Typ: Nicht-medikamentöse und medikamentöse Therapie]. Therapeutische Umschau 2015;72:233-238. [DOI] [PubMed] [Google Scholar]
Lyons 2019 {published data only}
- Lyons S. Arts therapies for dementia: a systematic review and community-based case study on the value of music therapy and dance movement therapy. Student thesis: Doctoral Thesis, Edge Hill University 2019. [https://research.edgehill.ac.uk/ws/portalfiles/portal/20738903/Lyons_Steven_Final_Viva_Final_Thesis_Pure_2019.06.17.pdf]
Merom 2016 {published data only}
- Merom D, Grunseit A, Eramudugolla R, Jefferis B, McNeill J, Anstey K J. Cognitive benefits of social dancing and walking in old age: the dancing mind randomized controlled trial. Frontiers in Aging Neuroscience 2016;8:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Merom 2016a {published data only}
- Merom Dafna, Mathieu Erin, Cerin Ester, Morton Rachael L, Simpson Judy M, Rissel Chris, Anstey Kaarin J, Sherrington Catherine, Lord Stephen R, Cumming Robert G. Social Dancing and Incidence of Falls in Older Adults: A Cluster Randomised Controlled Trial. PLoS medicine 2016;13(8):1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruiz‐Muelle 2019 {published data only}
- Ruiz-Muelle A, Lopez-Rodriguez M M. Dance for People with Alzheimer's Disease: A Systematic Review. Current Alzheimer Research 2019;16(10):919-933. [DOI] [PubMed] [Google Scholar]
Additional references
ADMP UK 2021
- Association of Dance Movement Psychotherapy UK (ADMP UK). What is Dance Movement Psychotherapy? www.admt.org.uk/whatis.html 2021.
ADTA 2020
- American Dance Therapy Association. About the American Dance Therapy Association. http://www.adta.org/about 2020.
Alexopoulos 1988
- Alexopoulos GS, Abrams RC, Young RC, Shamoian CA. Cornell scale for depression in dementia. Biological Psychology 1988;23(3):271-84. [DOI] [PubMed] [Google Scholar]
Alzheimers Society 2022
- Alzheimers Society. The progression and stages of dementia. https://www.alzheimers.org.uk/ 2022.
American Psychiatric Association 2022
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed., text rev.). https://doi.org/10.1176/appi.books.9780890425787 2022.
Banovic 2018
- Banovic S, Zunic LJ, Sinanovic O. Communication Difficulties as a Result of Dementia. Mater Sociomed 2018;30(3):221-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bek 2020
- Bek J, Arakaki AI, Lawrence A, Sullivan M, Ganapathy G, Poliakoff E. Dance and Parkinson’s: A review and exploration of the role of cognitive representations of action. Neuroscience & Biobehavioral Reviews 2020;109:16-28. [DOI] [PubMed] [Google Scholar]
Berrol 2006
- Berrol C. Neuroscience meets dance/movement therapy: mirror neurons, the therapeutic process and empathy. The Arts in Psychotherapy 2006;33:302-15. [Google Scholar]
Bradt 2015
- Bradt J, Shim M, Goodill SW. Dance/movement therapy for improving psychological and physical outcomes in cancer patients. Cochrane Database Systematic Review 2015;1(1):CD007103. [DOI: 10.1002/14651858.CD007103.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brañez‐Condorena 2021
- Brañez-Condorena A, Soriano-Moreno DR, Navarro-Flores A, Solis-Chimoy B, Diaz-Barrera ME, Taype-Rondan A. Accuracy of the Geriatric Depression Scale (GDS)-4 and GDS-5 for the screening of depression among older adults: A systematic review and meta-analysis.. PLoS One 2021 ;16(7):0. [DOI: doi: 10.1371/journal.pone.0253899] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brooks 1989
- Brook D, Stark A. The effects of dance/movement therapy on affect: a pilot study. American Journal of Dance Therapy 1989;11:101-12. [Google Scholar]
Campbell 2007
- Campbell NC, Murray E, Darbyshire J, Emery J, Farmer A, Griffiths F, et al. Designing and evaluating complex interventions to improve health care. BMJ 2007;334:455-10.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaiklin 1986
- Chaiklin S, Schmais D. The Chace approach to dance therapy. In: Lewis P, editors(s). Theoretical Approaches in Dance/Movement Therapy. Vol. 1. Iowa: KIendall/Hunt, 1986:17-36. [Google Scholar]
Chen 2020
- Chen FT, Etnier JL, Chan KH, Chiu PK, Hung TM, Chang YK. Effects of exercise training interventions on executive function in older adults: a systematic review and meta-analysis. Sports Medicine 2020;50(8):1451-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheng 2005
- Cheng ST, Chan AC. Comparative performance of long and short forms of the geriatric depression scale in mildly demented Chinese. International Journal of Geriatric Psychiatry 2005;20:1131-1137. [DOI: 10.1002/gps.1405] [DOI] [PubMed] [Google Scholar]
Cohen‐Mansfield 1989
- Cohen-Mansfield J, Marx MS, Rosenthal AS. A description of agitation in a nursing home. Journal of Gerontology: Medical Sciences 1989;44(3):M77-M84. [DOI] [PubMed] [Google Scholar]
Craig 2008
- Craig P, Dieppe P, Macintyre S, Ritchie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337:a1655-10.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Witte 2021
- De Witte M, Orkibi H, Zarate R, Karkou V, Sajnani N, Malhotra B, et al. From therapeutic factors to mechanisms of change in the creative arts therapies: A scoping review. Frontiers in Psychology 2021;12:1-27. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deshmukh 2018
- Deshmukh SR, Holmes J, Cardno A. Art therapy for people with dementia. Cochrane Database of Systematic Reviews 2018, Issue 9. Art. No: CD011073. [DOI: 10.1002/14651858.CD011073] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dosamantes‐Alperson 1981
- Dosamantes-Alperson E. Experiencing in movement therapy. American Journal of Dance Therapy 1981;4(2):33-44. [Google Scholar]
EADMT 2021
- The European Association of Dance Movement Therapy. What is Dance Movement Therapy (DMT)? http://eadmt.com/eadmt/what-is 2021.
Folstein 1975
- Folstein MF, Folstein SE, McHigh PR. 'Mini-Mental State': a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12:189-98. [DOI] [PubMed] [Google Scholar]
Forbes 2015
- Forbes D, Forbes SC, Blake CM, Thiessen EJ, Forbes S. Exercise programs for people with dementia. Cochrane Database of Systematic Reviews 2015, Issue 4. Art. No: CD006489. [DOI: 10.1002/14651858.CD006489.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gauthier 2021
- Gauthier S, Rosa-Neto P, Morais JA, Webster C. Journey through the diagnosis of dementia. Alzheimers Disease International 2021. [https://www.alzint.org/u/World-Alzheimer-Report-2021.pdf]
Gazzola 2006
- Gazzola V, Aziz-Zadeh L, Keysers C. Empathy and the somatotopic auditory mirror system in humans. Current Biology 2006;16:1824-9. [DOI] [PubMed] [Google Scholar]
Geretsegger 2022
- Geretsegger, M, Fusar-Poli L, Elefant C, Mössler KA, Vitale G, Gold C. Music therapy for autistic people. Cochrane Database of Systematic Reviews 2022, Issue 5. Art. No: CD004381. [DOI: 10.1002/14651858.CD004381.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldstein‐Levitas 2016
- Goldstein-Levitas N. Dance/movement therapy and sensory stimulation: A holistic approach to dementia care. American Journal of Dance Therapy 2016;38(2):429-436. [Google Scholar]
Groot 2016
- Groot C, Hooghiemstra AM, Raijmakers PG, Berckel BN, Scheltens P, Scherder EJ, et al. The effect of physical activity on cognitive function in patients with dementia: a meta-analysis of randomized control trials. Ageing research reviews 2016;25:13-23. [DOI] [PubMed] [Google Scholar]
Hackney 2007
- Hackney ME, Kantorovich S, Levin R, Earhart GA. Effects of tango on functional mobility in Parkinson's disease: a preliminary study. Journal of Neurologic Physical Therapy 2007;31(4):173-9. [DOI] [PubMed] [Google Scholar]
Hackney 2010
- Hackney ME, Earhart GA. Effects of dance on balance and gait in severe Parkinson disease: a case study. Disability and Rehabilitation 2010;32(8):679-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hayes 2011
- Hayes J, Povey S. The Creative Arts in Dementia Care: Practical Person-Centred Approaches and Ideas. London: Jessica Kingsley, 2011. [Google Scholar]
Hill 2017
- Hill H. Dance movement therapy and the possibility of wellbeing for people with dementia. In: The Oxford Handbook of Dance and Wellbeing . New York: Oxford University Press, 2017:821-838. [Google Scholar]
Ho 2018
- Ho RTH, Fong TCT, Hon T, Chan WC, Kwan JSK, Chiu PKC, et al. Psychometric validation of Fuld Object Memory Evaluation in older adults with cognitive impairments. Aging & Mental Health 2018;23(6):711-717. [DOI: 10.1080/13607863.2018.1442414] [DOI] [PubMed] [Google Scholar]
Hu 1999
- Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling 1999;6:1-55. [DOI: 10.1080/10705519909540118] [DOI] [Google Scholar]
Hughes 1982
- Hughes CP, Berg L, Danziger W, Coben LA, Martin RL. A new clinical scale for the staging of dementia. British Journal of Psychiatry 1982;140(6):566-72. [DOI] [PubMed] [Google Scholar]
Juul 2021
- Juul S, Gluud C, Simonsen S, Frandsen FW, Kirsch I, Jakobsen JC. Blinding in randomised clinical trials of psychological interventions: a retrospective study of published trial reports. BMJ Evidence-Based Medicine 2021;26 (3):109. [DOI: ] [DOI] [PubMed] [Google Scholar]
Karkou 2006
- Karkou V, Sanderson P. Arts Therapies: A Research-Based Map of the Field. Edinburgh: Elsevier, 2006. [Google Scholar]
Karkou 2012
- Karkou V. Aspects of theory and practice in dance movement psychotherapy in the UK: Similarities and differences from music therapy. In: R MacDonald, G Kreutz, L Mitchell, editors(s). Music, health and wellbeing. New York: Oxford University Press, 2012:213-229. [Google Scholar]
Karkou 2017a
- Karkou V, Tsiris G, Kayafa D. Training and professionalisation in the arts therapies: some examples and perspectives from Europe. International Art Therapy Conference Proceedings 2017;8:1. [Google Scholar]
Karkou 2019
- Karkou V, Aithal S, Zubala A, Meekums B. Effectiveness of dance movement therapy in the treatment of adults with depression: a systematic review with meta-analyses. Frontiers in Psychology 2019;0:936. [DOI: 10.3389/fpsyg.2019.00936] [DOI] [PMC free article] [PubMed] [Google Scholar]
Koch 2019
- Koch SC, Riege RF, Tisborn K, Biondo J, Martin L, Beelmann A. Effects of dance movement therapy and dance on health-related psychological outcomes. A meta-analysis update. Frontiers in Psychology 2019;0:1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
Laban 1975
- Laban R. Modern Educational Dance. London: MacDonald Evans, 1975. [Google Scholar]
Lafont 2021
- Lafont C, Chah Wakilian A, Lemogne C, Gouraud C, Fossey-Diaz V, Orvoen G, et al. Diagnostic Performance of the 4-Item Geriatric Depression Scale for Depression Screening in Older Patients with Cancer: The ELCAPA Cohort Study. The Oncologist 2021;26(6):e983-e991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lam 2006
- Lam C, Leung T, Lui VW, Leung VP, Chiu HF. Association between cognitive function, behavioral syndromes and two-year clinical outcome in Chinese subjects with late-onset Alzheimer's disease. International psychogeriatrics. International Psychogeriatrics 2006;18(3):517-526. [DOI] [PubMed] [Google Scholar]
Livingston 2020
- Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020;396(10248):413-446. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Logsdon 1999
- Logsdon RG, Gibbons LE, McCurry SM, Teri L. Quality of life in Alzheimer's disease: patient and caregiver reports. Journal of Mental Health and Aging 1999;5:21-32. [Google Scholar]
Lu 2002
- Lu L, Bigler ED. Normative data on trail making test for neurologically normal, Chinese-speaking adults. Applied Neuropsychology 2002;9:219-225. [DOI: 10.1207/S15324826AN0904_4] [DOI] [PubMed] [Google Scholar]
Lyons 2018
- Lyons S, Karkou V, Roe B, Meekums B, Richards M. What research evidence is there that dance movement therapy improves the health and wellbeing of older adults with dementia? A systematic reviewand descriptive narrative summary. The Arts in Psychotherapy 2018;60:32-40. [Google Scholar]
Macaskie 2012
- Macaskie J, Meekums B, Nolan G. Transformational education for psychotherapy and counselling: a relational dynamic approach. British Journal of Guidance & Counselling 2012;41(4):351-62. [DOI: 10.1080/03069885.2012.726348] [DOI] [Google Scholar]
Meekums 1991
- Meekums B. Dance/movement therapy with mothers and young children at risk of abuse. Arts in Psychotherapy 1991;18(3):223-30. [Google Scholar]
Meekums 2002
- Meekums B. Dance Movement Therapy. London: Sage, 2002. [Google Scholar]
Meekums 2008
- Meekums B. Pioneering dance movement therapy in Britain: results of narrative research. The Arts in Psychotherapy 2008;35:99-106. [Google Scholar]
Meekums 2012
- Meekums B. Kinesthetic Empathy and Movement Metaphor in Dance Movement Psychotherapy. In: Reynolds D, Reason M, editors(s). Kinaesthetic Empathy in Creative and Cultural Practices. Bristol: Intellect Publishing, 2012. [Google Scholar]
Millman 2021
- Millman LM, Terhune DB, Hunter EC, Orgs G. Towards a neurocognitive approach to dance movement therapy for mental health: A systematic review. Clinical Psychology & Psychotherapy 2021;28(1):24-38. [DOI] [PubMed] [Google Scholar]
Norcross 2011
- Norcross JC, Wampold BE. What works for whom: tailoring psychotherapy to the person. Journal of Clinical Psychology: In Session 2011;67:127-32. [DOI: 10.1002/jclp.20764] [DOI] [PubMed] [Google Scholar]
Payne 1992
- Payne H. Dance Movement Therapy: Theory and Practice. 1st edition. London: Routledge, 1992. [Google Scholar]
Phillips‐Silver 2007
- Phillips-Silver J, Trainor LJ. Hearing what the body feels: auditory encoding of rhythmic movement. Cognition 2007;105(3):533-46. [DOI] [PubMed] [Google Scholar]
Ren 2013
- Ren J, Xia J. Dance therapy for schizophrenia. Cochrane Database of Systematic Reviews 2013, Issue 10. Art. No: CD006868. [DOI: 10.1002/14651858.CD006868.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rizzolatti 1996
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L. Premotor cortex and the recognition of motor actions. Cognitive Brain Research 1996;3:131-41. [DOI] [PubMed] [Google Scholar]
Rosen 1984
- Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. American Journal of Psychiatry 1984;141:1356-64. [DOI] [PubMed] [Google Scholar]
Sikkes 2020
- Sikkes SA, Tang Y, Jutten RJ, Wesselman LM, Turkstra LS, Brodaty H, et al. Toward a theory-based specification of non-pharmacological treatments in aging and dementia: Focused reviews and methodological recommendations. Alzheimers and Dementia The Journal of the Alzheimers Association 2020;17(2):255-270. [doi.org/10.1002/alz.12188] [DOI] [PMC free article] [PubMed] [Google Scholar]
Skivington 2021
- Skivington K, Matthews L, Simpson SA, Craig P, Baird J, Blazeby JM, et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. bmj, .. British Medical Journal 2021;374:2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Terada 2019
- Terada S, Nakashima M, Wakutani Y, Nakata K, Kutoku Y, Sunada Y, et al. Social problems in daily life of patients with dementia. Geriatrics Gerontology International 2019;2:113-118. [DOI] [PubMed] [Google Scholar]
Tong 2002
- Tong AYC, Man DWK. The validation of the Hong Kong Chinese version of the Lawton instrumental activities of daily living scale for institutionalized elderly persons. Otjr-Occupation Participation and Health 2002;22:132–142. [DOI: 10.1177/153944920202200402] [DOI] [Google Scholar]
UKCP 2021
- UK Council for Psychotherapy. What is psychotherapy? http://www.psychotherapy.org,uk 2021.
Van der Steen 2018
- Van der Steen JT, Smaling HJ, Van der Wouden JC, Bruinsma MS, Scholten RJ, Vink AC. Music‐based therapeutic interventions for people with dementia. Cochrane Database of Systematic Reviews 2018. Art. No: CD003477. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vaverniece 2012
- Vaverniece I, Majore-Dusele I, Meekums B, Rasnacs O. Dance movement therapy for obese women with emotional eating: a controlled pilot study. The Arts in Psychotherapy 2012;39:126-33. [DOI: 10.1016/j.aip.2012.02.004] [DOI] [Google Scholar]
Vilkin 2022
- Vilkin E, Sullivan TJ, Goldfried MR. Conceptualizing the therapeutic relationship: Mediator or moderator of change? Journal of Psychotherapy Integration 2022;0:0. [Google Scholar]
Whitehouse 1979
- Whitehouse M. CG Jung and Dance-Therapy: Two Major Principles. In: Bernstein P L, editors(s). Eight Theoretical Approaches in Dance/Movement Therapy. Vol. 1. Iowa: Kendall/Hunt, 1979. [Google Scholar]
WHO 2019
- World Health Organization. International statistical classification of diseases and related health problems (11th ed.). https://icd.who.int/ 2021.
Woods 2018
- Woods B, O'Philbin L, Farrell EM, Spector AE, Orrell M. Reminiscence therapy for dementia. Cochrane Database of Systematic Reviews 2018, Issue 3. Art. No: CD001120. [DOI: 10.1002/14651858.CD001120.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2021
- Wu VX, Chi Y, Lee JK, Goh HS, Chen DYM, Haugan G, et al. The effect of dance interventions on cognition, neuroplasticity, physical function, depression, and quality of life for older adults with mild cognitive impairment: a systematic review and meta-analysis.. International Journal of Nursing Studies 2021;122:104025. [DOI] [PubMed] [Google Scholar]
Yao 2007
- Yao S, Chen H, Jiang L, Tam WC. Replication of factor structure of Wechsler Adult Intelligence Scale-III Chinese version in Chinese mainland non-clinical and schizophrenia samples. Psychiatry and Clinical Neurosciences 2007;61:379–384. [DOI: 10.1111/j.1440-1819.2007.01672.x] [DOI] [PubMed] [Google Scholar]
Yates 2019
- Yates L, Csipke E, Moniz-Cook E, Leung P, Walton H, Charlesworth G, et al. The development of the Promoting Independence in Dementia (PRIDE) intervention to enhance independence in dementia. Clinical Interventions in aging 2019;14:1615-1630. [PMID: doi: 10.2147/CIA.S214367. PMID: 31571842; PMCID: PMC6748161.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Young 2011
- Young TJ, Manthrop C, Howells D, Tullo E. Developing a career communication intervention to support personhood and quality of life in dementia. Ageing and Society 2011;23(07):1003-25. [Google Scholar]
References to other published versions of this review
Karkou 2014
- Karkou V, Meekums B. Dance movement therapy for dementia. Cochrane Database of Systematic Reviews 2014, Issue 3. Art. No: CD011022. [DOI: 10.1002/14651858.CD011022] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karkou 2017
- Karkou V, Meekums B. Dance movement therapy for dementia. Cochrane Database of Systematic Reviews 2017, Issue 2. Art. No: CD011022. [DOI: 10.1002/14651858.CD011022] [DOI] [PMC free article] [PubMed] [Google Scholar]


