Abstract
Some phthalate esters alter male rat reproductive development during sexual differentiation by interfering with fetal testis maturation resulting in reduced Leydig Cell synthesis of testosterone and insulin-like 3 (Insl3) hormones. Gene transcripts associated with steroid hormone and cholesterol transport, and cholesterol synthesis and lipid metabolism also are reduced. These alterations cause permanent malformations of hormone-dependent tissues, sperm production and fertility in male offspring; effects known as the “Phthalate Syndrome.” We have shown that administration of a high dose of 750 mg diisononyl phthalate (750 mg/kg/d DINP) during sex differentiation reduced fetal testis testosterone production (T Prod), testis gene expression and induced a low incidence of reproductive malformations in male rat offspring.
In the current study we administered DINP at even higher dose levels (1.0 and 1.5 g/kg/d) from gestational day ( GD) 14 to postnatal (PND) 3 to determine if these effects were dose related and if the magnitude of the effects could be predicted from a statistical model of fetal testosterone production (T Prod) and Insl3 mRNA levels. These models were previously developed using dipentyl phthalate (DPeP) data from fetal T Prod and postnatal studies. We found that the severity of the demasculinizing effects on the androgen-dependent organs and gubernaculum by DINP were accurately predicted from the statistical models of fetal T prod and Insl3 mRNA, respectively. Taken together, our results indicate that reductions fetal T prod and Insl3 predict the severity of demasculinizing effects in utero exposure to the phthalates DINP and DPeP regardless of potency.
Keywords: Phthalates, New Approach Methodologies, Reproductive Tract Malformations, DINP, Altered Sexual Differentiation, Biologically Relevant Reductions in Fetal Testosterone
Introduction
Many of the phthalate esters (PEs) with alkyl groups of three to seven carbons (C3 to C7) alter male rat reproductive development during sexual differentiation by interfering with fetal Leydig cell synthesis of testosterone and Insl3 hormones. Gene transcripts associated with steroid hormone and cholesterol transport and cholesterol synthesis and lipid metabolism also are reduced. Consequently, male rat offspring display effects that are part of the “Phthalate Syndrome” including permanent alterations of hormone-dependent tissues, reduced sperm production and fertility (Foster and Gray 2003). Dose response studies of fetal testis endocrine function reveal a wide-range of potencies among these PEs with di-isononyl phthalate (DINP) being one of the least potent, only reducing fetal androgen production and testis gene expression at high dosage levels (Gray et al. 2021; Hannas et al. 2011b) as compared to the other active PEs. In contrast, dipentyl phthalate (DPeP) is one of the most post potent PEs (Gray et al. 2016; Hannas et al. 2011a).
Several PEs, including DINP, are currently the focus of assessments by several regulatory agencies in the US and abroad (e.g., Consumer Products Safety Improvement Act 2008; USEPA IRIS; ECHA Final Report on DIDP and DINP, 2013). In 2019 EPA received a “Manufacturer-Requested Risk Evaluation of Diisononyl Phthalate (DINP)” under TSCA (https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/manufacturer-requested-risk-evaluation-diisononyl-0, last accessed 8/1/2022). EPA granted the request in Dec 2019 and posted a draft scope of the risk evaluation of DINP in 2020 (docket EPA-HQ-OPPT-2018–0436). In addition,, on 8/8/2022 EPA issued a supplemental proposed rule that would add a DINP category to the list of toxic chemicals subject to the Toxics Release Inventory (TRI) reporting requirements under the Emergency Planning and Community Right-to-Know Act and the Pollution Prevention Act (https://www.epa.gov/chemicals-under-tsca/epa-releases-proposed-rule-requiring-tri-reporting-dinp, last accessed on 8/9/2022). In the 2022 document, EPA “ released an updated hazard assessment which proposes that the DINP category can reasonably be anticipated to cause cancer and serious or irreversible chronic health effects in humans; specifically, developmental effects, kidney toxicity, and liver toxicity.” The data from the current study will fill key data gaps on the reproductive toxicity of this phthalate.
Our laboratory previously reported that oral administration of a high dose of DINP, (750 mg/kg/d orally to the dam from gestational day (GD) 14 to postnatal day (PND) 3, induced a low incidence of reproductive abnormalities in androgen dependent tissues in male rat offspring (Gray et al. 2000). Twenty-two percent of DINP-exposed males displayed areolae/nipple retention at 13 days of age (22% incidence), and 4 of 52 males displayed permanent reproductive alterations. The reproductive malformations included permanent nipples in two males (see Figure 2 in (Gray et al. 2000)), bilateral testicular hypoplasia and epididymal atrophy in another male (see Figure 7 in (Gray et al. 2000)), and unilateral epididymal agenesis with hypospermatogenesis and scrotal fluid-filled testis devoid of spermatids in the fourth male. Furthermore, one male displayed hemorrhagic testis at 9 days of age. Given the low incidence of reproductive malformations seen in the 750 mg DINP/kg/d group, the current study was executed using higher dose levels to determine if the adverse postnatal effects of in utero DINP exposure increased with increasing dose levels.
Figure 2.
Maternal oral exposure to DINP from gestational day (GD) 14 to postnatal day (PND) 3 reduced anogenital distance at two days of age (AGD2) in male but not female rat pups. Body weight was reduced at two days of age, but the effect only attained statistical significance in female pups.
Figure 7.
Comparison of the effects of perinatal DINP and DPeP on testosterone production T Prod with reductions in anogenital distance (AGD) at two days of age and increases in the number of female-like retained areolae/nipples in 13 day old F1 male rats
In comparison to DINP, in utero DPeP is a much more potent endocrine disrupting chemical (EDC) inducing multiple reproductive tract malformations in 100% of the male rat offspring at 300 mg/kg/d whereas exposure to 750 mg DINP/kg/d only induced malformations in 7.7% of the offspring. In the current study, we compared the incidence of reproductive abnormalities induced by in utero exposure to DPeP and DINP by using the reductions of fetal T Prod and Insl3 as the dose-metrics. This approach addresses the hypothesis that the low rate of DINP-induced effects on the differentiating male reproductive tract can be predicted by the levels of T Prod by the fetal testis during sexual differentiation. We have found that reductions in fetal testis T Prod and associated gene expression are sensitive, and reproducible biomarkers of phthalate-induced male reproductive tract malformations (Furr et al. 2014; Gray et al. 2021; Hannas et al. 2012; Hannas et al. 2011b). We are developing quantitative statistical models using the data from DPeP and DINP and several other PEs to predict the adverse reproductive effects of exposure to phthalates in utero on reproductive abnormalities in the male offspring from reductions in fetal T Prod and testis Insl3 mRNA expression. The statistical models of using fetal testis testosterone production and Insl3 mRNA levels as the dose-metric accurately predict the postnatal effects of in utero exposure to phthalates on hormone-dependent tissues in the male rat.
The objective of the current study was to determine if the statistical associations between DINP-induced reduction in fetal testis T Prod and Insl3 mRNA expression with the postnatal reproductive tract malformations were like those seen with DPeP. If so, PE-induced reduction in fetal T Prod might provide a useful, quantitative new approach method (NAM) to predict the postnatal effects of in utero exposure to phthalates. We used statistical models of the relationship between reductions in fetal T Prod and Insl3 mRNA expression to predict the postnatal effects of in utero phthalate exposure. These models address the question “How much of a reduction in fetal T Prod and Insl3 mRNA are required to produce reproductive tract alterations in the F1 male rat?” This approach uses far fewer resources than a one-generation study and provides data useful for regulatory decision makers to identify points of departure for hazard identification of phthalates that induce the “Phthalate Syndrome” in utero. In the current study, DINP was administered orally to pregnant Sprague-Dawley rats at 0, 1 and 1.5 g/kg from GD 14 - PND 3. The male offspring were monitored through adulthood and the results were combined, for statistical analysis, with an earlier study with that used 0.75 g DINP/kg/d during the same dosing window (Gray et al. 2000).
Methods
Animals
Timed-pregnant Sprague-Dawley (Crl:CD(SD)) rats (approximately 90 days of age) were purchased from Charles River Laboratories (Raleigh, NC). Dams were delivered to EPA facilities on GD2 and housed individually; the day sperm plug positive was considered GD1. Animals were housed in 20 × 25 × 47 cm clear polycarbonate cages with laboratory-grade heat-treated pine shavings (heat-treated to remove resins; Northeastern Products, Warrensburg NY). Animals were maintained on a 14:10 light/dark photoperiod (lights off at 11:00 h) at 20–24°C and 40–50% relative humidity. Dams were fed ad libitum with Purina Rat Chow 5008, and weanling and adult rats were fed Purina Rat Chow 5001. Animals had 24 hour access to filtered (5 micron) Durham, NC municipal drinking water. Water was tested monthly for Pseudomonas and every 4 months for a suite of chemicals including pesticides and heavy metals. The current study was conducted under a protocol approved by the National Health and Environmental Effects Research Laboratories Institutional Animal Care and Use Committee (IRP-NHEERL-RTP/RTD/EB/LEG/01–01-00, LAPR # - 01–02-006UD).
Dosing
Twenty pregnant rats were randomly assigned to treatment groups on gestational day 3 (GD3) in a manner that provided similar mean (± SE) body weight per group prior to dosing. Dams were dosed by oral gavage with 0 (n=6), 1 (n=7) or 1.5 (n=7) g DINP/kg/d (technical grade obtained from Aldrich Chemical; CAS No. 68515–48-0; Lot 03005TR), in 2.5 ml vehicle/kg/d (corn oil) from gestational day 14 (GD 14, GD1 = sperm positive) through PND 3. Maternal weight was measured each day prior to dosing and the dose adjusted accordingly and the general condition of the dams was monitored throughout gestation and lactation.
Postnatal Data Collection on F1 male rats.
At 2 days of age (PND2), pups were sexed, weighed and anogenital distance (AGD) was measured using a Leica MZ6 dissecting scope with an optical reticle. Skin between the phallus and tail-base was extended maximally and AGD measurements were made to the nearest 0.1 mm. Scope magnification ( 15 X (1.5×10)) was calibrated using a 1 mm stage micrometer with 0.01mm divisions in the optical reticle. At 13 days of age (PND 13), the offspring were weighed, and the number of areolas/nipples per male were recorded for each male. They were not examined under a dissecting scope so one cannot reliably discriminate between areolae versus nipple buds. Data for AGD and the number of areolae/male were collected with the observer blind to treatment.
At 21 days of age (PND 21), the male offspring were weighed, weaned, and housed in littermate groups with 2–3 males/cage. At 22 days of age, dams and female offspring were weighed, euthanized and the number of implantation scars were recorded in the dams and post implantation fetal and pup losses (PIL) were calculated (PIL = total implantation scars minus the total number of live pups in the litter).
Males were checked for complete preputial separation (PPS) as an index of puberty on 41, 43, 45, 48 and 50 days of age and the males were weighed on the day that PPS was attained. Males were euthanized via decapitation and necropsied from 220 to 240 days of age. The ventral surface was shaved, and the males were examined for permanent nipples and malformations of the external genitalia. The males also were examined for internal malformations including agenesis or hypoplasia of the epididymides, vas deferens, seminal vesicles, ventral prostate, testes, or gubernaculum and the length of the gubernaculum was measured with calipers. The testes were examined to determine if they were fluid -filled, flaccid, atrophic, hypoplastic, absent or undescended (with or without an attached gubernaculum). In addition, the glans penis, ventral prostate, seminal vesicles, right and left testis, right cauda, right caput plus corpus epididymis, left whole epididymis, Cowper’s glands, levator ani/bulbocavernosus muscle (LABC), liver, adrenal glands and right and left kidneys were weighed. Fluid-filled testes and hypoplastic epididymides were not weighed.
The left testis and epididymis were fixed in Bouin’s solution for 24 hours and then stored in 70 % ethanol for histological examination by Experimental Pathology Laboratories, Inc (Durham, NC). Tissues were embedded in paraffin, sectioned at 4–6 μm, stained using hematoxylin and eosin and examined a board-certified pathologist for histological alterations (Supplemental File).
Statistical Analyses
Maternal and offspring data were analyzed using PROC GLM and PROC MIXED (to confirm the results of PROC GLM) available in SAS 9.4 software. AGD, the PND 13 number of areolae/nipple/male (ranging from 0 to 12 maximum), the percentage of males with any retained areolae/nipples (none = 0 %, 1–12 areolae = 100%), and body and organ weight data were analyzed in PROC GLM using litter means and in PROC MIXED with litter as a random factor for confirmation of the litter means analyses. AGD data were analyzed with and without the cube root of body weight as a covariate (as recommend (Gray et al. 1994) (Gray et al. 1999)) and litter-mean organ weights were analyzed with and without body weight as a covariate. In addition, data from this prenatal-postnatal study using 1.0 and 1.5 g DINP/kg/d and our previous study (Gray et al. 2000) which used 0.75 g DINP/kg/d were pooled and reanalyzed to compare the rates of reproductive alterations with the reductions in testis T Prod and gene expression from Gray et al. (2021).
Statistical four parameter nonlinear regression models were developed using GraphPad Prism 8 software comparing the effects of DINP with the administered dose and with the reductions of T Prod and Insl3 mRNA. The incidence of permanent reproductive tract malformations and testis and epididymal histopathology were analyzed using SAS 9.4 PROC FREQ with the option for the Cochran-Armitage Trend Test. Statistical power calculations and optimal sample sizes were done with SAS 9.4 PROC POWER.
Results
Maternal Effects
Total maternal body weight was not significantly reduced but weight gain during dosing was significantly reduced. Body weight gain was significantly reduced but total body weight was not because weight gain is less variable than is total body weight. The reduction in weight gain was not large and did not exceed 5% of total maternal body weight. (Figure 1). The maximum reduction in weight between the control and high dose was 17.7 g at PND1. In addition, none of the dams displayed any signs of overt toxicity or distress at any time.
Figure 1.
Effects of gestational oral administration of DINP on maternal body weight and body weight change during dosing. Values are means ± standard errors.
Neonatal, Infantile and Pubertal Effects
DINP treatment did not significantly increase pup mortality at birth or reduce litter sizes at 2 days of age. Male, but not female AGD was reduced significantly at 1.5 g DINP/kg/d reduced male (significant, but not with cube root of body weight as the covariate) (Figure 2). Also, at 2 days of age, 1.5 g DINP/kg reduced body weight in F1 male and female pups but this effect did not persist and was no longer apparent at or after 13 days of age. Pup loss as measured by the number of implantation scars minus the number of pups surviving to weaning was not affected by treatment. When the two studies were pooled for analysis of AGD in male pups in the 0, 0.75, 1 and 1.5 g DINP/kg/d dose groups, (n (litter (male pups)) = 25(161), 14(84), 7(41) and 7(39), respectively), AGD was significantly shorter in the 1.5 g DINP group when analyzed with (p<0.03) and without (p<0.009) adjustment for the cube root of body weight by Analysis of Covariance (Table 1; Figure 2). The reduction in the 1.5 g group is about 17% of control (female value is baseline).
Table 1.
Neonatal and pubertal effects of perinatal DINP exposure on male pups
| STUDY 1 AND 2 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dinp g/kg/d GD 14 - PND 3 | 0 | 1 | 1.5 | DOSES 0, 1 AND 1.5 | POOLED | |||||||
| PUPS | PUPS | PUPS | PUPS | PUPS | PUPS | p VALUE from Litter Means | LITTER MEANS | |||||
| MEAN | STDERR | N PUPS | MEAN | STDERR | N PUPS | MEAN | STDERR | N PUPS | 0 VERSUS 1g | 0 VERSUS 1.5g | LOEL p <0.05 | |
| Male AGD PND 2 | 3.41 | 0.07 | 41 | 3.32 | 0.056 | 39 | 3.06 ** | 0.053 | 39 | NS | p<0.03 | 1.5 |
| MALE BWT PND 2 | 7.99 | 0.119 | 41 | 7.61 | 0.1448 | 39 | 7.24 ** | 0.138 | 39 | NS | p<0.04 | 1.5 |
| FEMALE AGD PND 2 | 1.39 | 0.035 | 36 | 1.35 | 0.018 | 41 | 1.41 | 0.029 | 43 | NS | NS | NS |
| FEMALE BwT PND 2 | 7.81 | 0.08 | 36 | 7.39 * | 0.118 | 41 | 6.8 * | 0.098 | 43 | NS | p<0.02 | 1.5 |
| MALE NIPS PND13 | 0.74 | 0.33 | 34 | 2.65 ** | 0.5 | 40 | 4.11 ** | 0.6 | 34 | p<0.053 | p<0.008 | 1 |
| % NIPS OF 12 | 6.1 | 2.7 | 34 | 22.1 ** | 4.2 | 40 | 34.3 ** | 5.1 | 34 | p<0.053 | p<0.008 | 1 |
| % WITH ANY NIPS PND13 17.6 | 6.6 | 34 | 50 ** | 8 | 40 | 73.5 ** | 7.7 | 34 | p<0.04 | p<0.004 | 0.75 | |
| AGE PPS | 47 | 0.31 | 33 | 47.3 | 0.3 | 38 | 45.8 | 0.33 | 35 | NS | NS | ND |
| WT PPS | 261 | 4 | 33 | 258 | 3.7 | 38 | 259 | 4.7 | 35 | NS | NS | ND |
| WEAN WT MALES | 50.6 | 0.72 | 33 | 46.4 | 1 | 38 | 49.7 | 0.95 | 35 | NS | NS | ND |
Shaded cells differ significantly from Control;
p < 0.05;
p < 0.01 using individual values
In the current study, the number of areolae/nipples per male and the percent of males with any areolae/nipples were significantly increased in a dose related manner ((litter means analysis, Table 1, Table 1 also shows the means and sample sizes of individual males). Examination of the litter means data from (Gray et al. 2000) combined with litter means data from the current study indicate that DINP significantly increased the number of nipples/areolae per male in the 1 and 1.5 g DINP/kg groups (0 DINP - 0.14, 0.75 DINP - 1.07, 1.0 DINP - 2.9 and 1.5 DINP - 3.6 areolae/male), and significantly increased the percent of males with any areolae/nipples in all DINP treated groups (0 DINP – 3.4% of males with areolae, 0.75 DINP – 22.4% of males with areolae, 1.0 DINP – 54.9% of males with areolae and 1.5 DINP – 70.2% of males with areolae).
DINP treatment did not affect male, female, or average pup body weight at PND 13 or reduce male or female body weight at weaning. DINP treatment did not delay puberty as measured by preputial separation or alter male body weight at puberty (Table 1, Figure 3).
Figure 3.
Photographs of 14 day old rat pups showing a control male with no nipples on the left, and control female in the middle panel (with nipple locations numbered 1–12) and a DINP exposed male from the 0.75 g/kg/d group with black arrows highlighting areolae/nipples at positions 8 and 10 from (Gray et al. 2000).
F1 Male Adult Necropsy Data
Organ weights
When necropsied at about 210 days of age male offspring body weights and weights of the liver, adrenal and kidney weights were not significantly affected by in utero/perinatal exposure to DINP (Table 2). In contrast, three of eight reproductive organs and the number of areolae/nipples per male were significantly reduced increased in this study. Four of eight androgen-dependent organ weights were reduced significantly and the areolae/nipple numbers were increased when the data from our 2000 DINP data set using 0.75 g/kg/d DINP (Gray et al. 2000) were pooled with the data from this study. The five significantly affected tissues included the seminal vesicles, glans penis, LABC, and ventral prostate weights and areolae/nipple retention. As compared to the effects of more potent PEs, however, these effects are not large, ranging from about 5 to 12% (analysis of data from both studies combined with 0, 0.75, 1 and 1.5 DINP g/kg/d).
Table 2.
Effects of perinatal DINP exposure on adult F1 male offspring
| dinp DINP g/kg/d GD 14 - PND 3 | STUDY 1 AND 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 1.5 | DOSES 0, 1 AND 1.5 | POOLED | ||||||||
| p VALUE using litter means | PROC MIXED | |||||||||||
| MEAN | STDERR | N PUPS | MEAN | STDERR | N PUPS | MEAN | STDERR | N PUPS | 0 VERSUS 1g | 0 VERSUS 1.5g | LOEL p <0.05 | |
| Body weight g | 704.87 | 12.59 | 33 | 676.41 | 12.74 | 38 | 736.03 | 17.55 | 34 | NS | NS | NS |
| Cowper’s Glands mg | 238.08 | 11.98 | 33 | 228.62 | 7.82 | 38 | 241.75 | 12.57 | 34 | NS | NS | NS |
| Epididymides mg | 1357.97 | 17.21 | 31 | 1371.65 | 14.64 | 37 | 1389.11 | 17.18 | 32 | NS | NS | NS |
| Glans Penis mg | 105.07 | 1.16 | 33 | 105.08 | 1.19 | 38 | 99.74 | 1.48 | 34 | NS | p<0.04 | 1.5 |
| LABC mg | 1.31 | 0.02 | 33 | 1.33 | 0.02 | 38 | 1.24 | 0.03 | 34 | NS | NS | 1.5 |
| Seminal Vesicles g | 2.21 | 0.05 | 33 | 2.12 | 0.05 | 38 | 2.03 | 0.04 | 34 | NS | p<0.02 | 1.5 |
| Ventral Prostate mg | 811.55 | 24.24 | 33 | 812.79 | 34.19 | 38 | 744.61 | 31.45 | 34 | NS | NS | 1.5 |
| Aerolae/nipples/male | 0.00 | 0.00 | 33 | 0.71 | 0.22 | 38 | 1.38 | 0.31 | 34 | p<0.04 | p<0.003 | 1 |
| Caput Epididymis mg | 335.59 | 5.19 | 32 | 340.25 | 4.76 | 37 | 343.50 | 5.36 | 33 | NS | NS | NS |
| Cauda Epididymis mg | 338.10 | 4.81 | 32 | 346.90 | 4.46 | 37 | 353.92 | 6.04 | 33 | NS | NS | NS |
| Testes g | 3.76 | 0.05 | 31 | 3.91 | 0.05 | 38 | 3.87 | 0.07 | 33 | NS | NS | NS |
| Liver g | 22.18 | 0.57 | 33 | 21.08 | 0.64 | 38 | 23.36 | 0.79 | 34 | NS | NS | NS |
| Adrenals mg | 54.40 | 0.97 | 33 | 52.82 | 1.13 | 38 | 52.58 | 1.24 | 34 | NS | NS | NS |
| Kidneys g | 4.28 | 0.07 | 33 | 4.20 | 0.10 | 38 | 4.25 | 0.08 | 34 | NS | NS | NS |
Shaded cells differ significantly from Control;
p < 0.05;
p < 0.01
Gross and Histological Abnormalities
Administration of DINP at 0.75, 1.0 and 1.5 g/kg/d induced a low, but significant, dose related incidence of severe testicular and epididymal malformations and/or histopathological alterations (Table 3) (Cochran-Armitage Trend Test Exact Test p < 0.0006, one sided Z). When the data were analyzed separately without the data from the previously published study with 0.75 g/kg/d, the trend was also statistically significant (p < 0.05). Testis seminiferous tubular hypospermatogenesis/atrophy was seen in 1, 3 and 3 males in the 0, 1 and 1.5 g/kg/d groups, respectively. In addition, in the 1.5 g/kg/d group one male displayed bilateral fluid filled testis hypoplasia and epididymal atrophy (Figure 4a) and another male displayed complete unilateral testis and gubernacular testis agenesis with and undescended atrophic epididymis (Figure 4b). Figure 5 (from (Gray et al. 2000)) displays the testicular and epididymal gross and histopathological abnormalities in a male exposed to 0.75 g DINP/kg/d treatment group. The malformation data in the Table 3 includes 2 adult males in the 0.75 g DINP/kg/d that displayed permanent nipples. When the malformation data were reanalyzed including only testicular and epididymal agenesis or hypoplasia and histopathological alterations in these tissues, and not the two males with permanent nipples, the trend remained highly significant (p < 0.0007) (one sided Z). It is important to note that the testicular, epididymal and gubernacular agenesis and hypoplasia seen in several DINP treated males are effects that have not been displayed by any control male rat in our studies, dating back to the late 1980s.
Table 3.

|
Figure 4.
Perinatal maternal administration of 1.5 g DINP/kg/d from gestational day 14 to postnatal day 3 induces testicular and epididymal malformations in the male offspring.
Figure 5.
Effect of perinatal maternal treatment with 750 mg/kg/d alters testis and epididymal morphology and histology in one malformed male rat (previously shown in (Gray et al. 2000))
The effects of DINP and DPeP on T Prod and testis mRNA expression from (Gray et al. 2021) are shown in Table 4. Values highlighted in yellow are significantly lower than control values.
Table 4.
Exposure to DINP and DPeP from gestational days 14 to 18 significantly reduces testis testosterone production (TPROD) and Insl3mRNA, measured on gestational day 18. These data were used to produce statistical models that compared the effects of the two phthalates on TPROD and Insl3mRNA with the rate of postnatal alterations in male rat offspring.
| Dose Response Data | % Control | ||
|---|---|---|---|
| DOSE | TPROD | Insl3 | |
| DINP | 500 | 70.5 | 73 |
| 750 | 63.1 | 79 | |
| 1000 | 43.1 | 56 | |
| 1500 | 31.6 | 51 | |
| DOSE | TPROD | Insl3 | |
| DPeP | 11 | 91.4 | 86 |
| 33 | 53.1 | 65 | |
| 100 | 24.3 | 47 | |
| 300 | 10.9 | 18 | |
| 750 | 12.5 | 21 | |
Table 4. Exposure to DINP and DPeP from gestational days 14 to 18 significantly reduces testis testosterone production (TPROD) and Insl3 mRNA, measured on gestational day 18. These data were used to produce statistical models that compared the effects of the two phthalates on TPROD and Insl3 mRNA with the rate of postnatal alterations in male rat offspring.
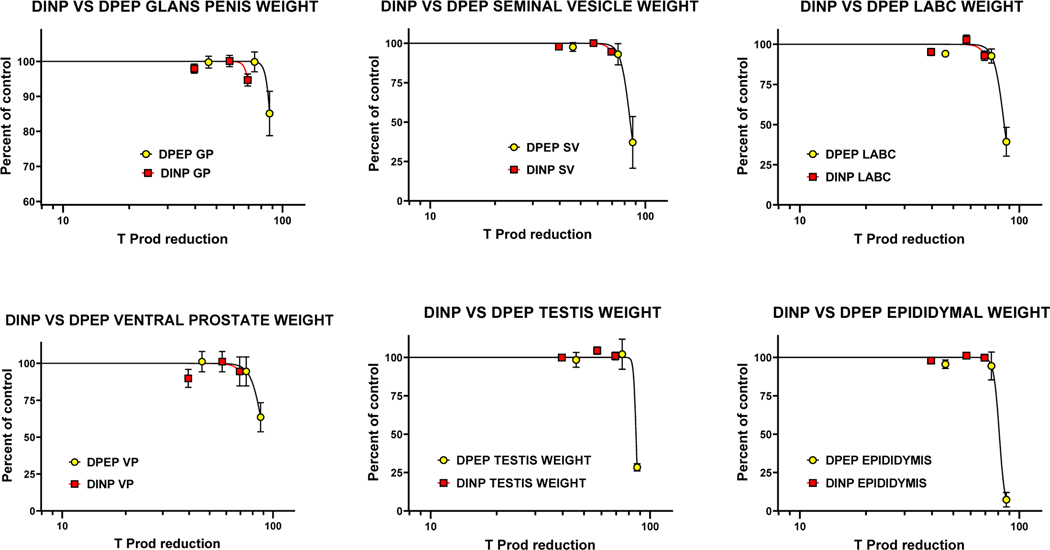
As indicated earlier, the main objective of this study was to determine the levels of fetal testis T Prod and Insl3 mRNA transcripts that were associated with alternations in male offspring reproductive endpoints from in utero DINP exposure, a relatively weak PE, and to compare the DINP results with the in utero effects of DPeP, one of the most potent PEs, on T Prod and Insl3 with male reproductive abnormalities. Rather than only display the effects versus the dose of DINP from the current study and DPeP (Gray et al. 2016), we display the effects versus the percent reduction in T Prod (Figures 6, 7, 8, 9).
Figure 6.
Fetal Testis Testosterone Production versus the phenotypic effects of DINP in F1 Male Rat Offspring.
Figure 8.
Comparison of the effects of perinatal DINP and DPeP on testosterone production T Prod with reductions in anogenital distance (AGD) at two days of age and increases in the number of female-like retained areolae/nipples in 13 day old F1 male rats.
Figure 9.
Comparison of the effects of perinatal DINP and DPeP on testosterone production T Prod with reductions in androgen-dependent organ weights in adult F1 male rats
Figures 7, 8 and 9 clearly demonstrate that the effects of DINP on male rat AGD (PND2), and areolae/nipple retention (PND13). The effects of DINP are statistically significant effects on some of the androgen-dependent organ weights and testis and epididymal malformations (≥PND210) are statistically significant and they occur at levels that can be accurately predicted using fetal T Prod or Insl3 as the quantitative dose metric. Logistic regression models of the relationship between DPeP-induced male reproductive tract malformations and reductions in fetal male rat testosterone production (T Prod) and Insl3 during sexual differentiation were nonlinear with a steep increase in the rate of malformations when T Prod was reduced by ≥75% with a low incidence of effects in a dose group that reduced T Prod by ≈ 46%.
Figure 10 similarly demonstrates the low incidence of DINP induced testis nondescent and agenesis of the gubernaculum testis is consistent with the measured levels of Insl3 mRNA when compared to DPeP Insl3 mRNA (Furr et al. 2014; Gray et al. 2016; Gray et al. 2021; Hannas et al. 2011b).
Figure 10.
Comparison of the effects of perinatal DINP and DPeP on testis Insl3 mRNA levels with increases in testis nondescent and agenesis of the gubernaculum in adult F1 male rats
Discussion
The results of these studies demonstrate that in utero DINP caused dose-related reductions of male rat neonatal AGD, increased areolae/nipple retention and induced a dose related increase in the percentage of male offspring displaying the Phthalate Syndrome. DINP treatment affected about ≈ 4, 10 and 15% of the males when fetal testosterone levels were reduced by about ≈ 35%, 57% and 70% of control T Prod (at 750, 1000 and 1500 mg DINP/kg/d, respectively). Furthermore, the DINP logistic regression models for the relationship between fetal T Prod and Insl3 reductions and with reproductive abnormalities did not differ from the DPeP models, which indicate that reductions in fetal T Prod and Insl3 mRNA can be used to predict the incidence of reproductive tract alterations in male rat offspring from in utero PE exposure during sexual differentiation.
Many of the phthalate esters with ester chain lengths of 3 to 7 carbons are metabolized in the gut to monoesters that disrupt male reproductive development by targeting the developing testis in utero and during pubertal life stages. The critical period in utero when phthalates (Carruthers and Foster 2005; Ema et al. 2000) and androgen receptor antagonists (Welsh et al. 2008; Wolf et al. 2000) disrupt sexual differentiation is between gestational days 14 to 19 in the rat. Phthalate exposure during this stage of life reduces the expression of mRNA for a multiplicity of key genes in the fetal testis including those involved with sterol transport into cells and steroid entry into mitochondria, the synthesis of the hormones Insl3, inhibin α and testosterone and cholesterol synthesis and lipid metabolism (Gray et al. 2021; Hannas et al. 2012).
Androgens are critical for development of several tissues in males during sexual differentiation including the genitalia, epididymides, seminal vesicles, ventral prostate and LABC muscles, for example. Other tissues like the brain and spinal cord, for example, also can be affected significantly by EDCs. Sexual differentiation of the nervous system occurs later than the reproductive tract differentiation and includes the first few days after birth in rodents (reviewed by (Macleod et al. 2010; Scott et al. 2008)). The indifferent rat brain and behavior can be demasculinized by perinatal exposure to antiandrogens (Hotchkiss et al. 2003) and masculinized by exposure to androgens (Hotchkiss et al. 2002) and estrogens (Gray et al. 1985). Androgens are also are responsible in male rodents for inducing agenesis of the nipples, the lower vagina and the cranial suspensory ligament of the testes which prevents testis descent if they do not regress (Kassim et al. 2010). In addition, the unique differentiation of the testis vasculature versus the ovary is dependent upon complex paracrine interactions between the fetal Sertoli cells, androgen receptors on the arteriole smooth muscle cells and Leydig cell androgens (Coveney et al. 2008; Welsh et al. 2010).
In contrast to these tissues, development of the gubernaculum testis and testis descent are also critically dependent upon the hormone Insl3 (Nef and Parada 1999; Zimmermann et al. 1999). Several laboratories have shown that phthalates like DBP, DEHP and DPeP, which reduce fetal testosterone (Gray et al. 2016; Mylchreest et al. 2002; Parks et al. 2000) and also reduce Leydig cell Insl3 mRNA levels (Gray et al. 2021; Wilson et al. 2004). Administration of high dose levels of DINP (both CAS 68515–48-0 and 28553–12-0 ) reduces fetal testis T Prod and mRNA expression for many of the testis genes reduced by DPeP, including Insl3. However, the DINP doses that reduce T Prod and mRNA expression are higher than other phthalates and the reduction in T Prod also plateaus at a higher level (≈30% of control) than other active phthalates (≈0 to 12% of control), including DPeP. The results of this study demonstrate that even though some males are severely affected at high dose levels (mg DINP/kg/d) levels, the reductions in T Prod or Insl3 were insufficient to induce high levels of malformations, large reductions in male AGD, or a high percent of nipple/areolae retention in infant males. A summary of the high dose effect of DINP on the male rat reproductive tract is shown in the following adverse outcome pathway (AOP) figure (Figure 11).
Figure 11.

In the current study, we hypothesized that the dose related in utero effects of DINP on the male rat reproductive tract would occur at similar T Prod and Insl3 levels as we previously reported for DPeP. The results shown in Figures 7 to 10 support this hypothesis. Currently, we are compiling similar information (T Prod versus postnatal male reproductive tract alterations) from published studies on several other phthalates to continue to evaluate this hypothesis and to use the statistical model to predict the effects of phthalate mixtures (Gray, et al, in preparation). For example, the statistical model for DBP (our T Prod data versus the epididymal and testicular malformations reported by Mylchreest (Mylchreest et al. 1998; Mylchreest et al. 1999; Mylchreest et al. 2000) is almost identical to the DPeP model) (Supplemental Figure 1). The (ED50s for reductions in T Prod causing a 50% incidence of reproductive tract malformations are DPeP = 77.1, DBP = 76.1%; p value that the models differ =0.946).
While it is unknown why such large reductions in fetal testis T Prod are required to disrupt differentiation of male AGD, to cause areolae/nipple retention in infant males, and to permanently reduce reproductive organ weights and induce malformations and histopathological changes in the testis and epididymis, this phenomenon is not unique. It has been demonstrated that complete spermatogenesis can be quantitatively maintained (Zirkin et al. 1989) or restored (Awoniyi et al. 1989) when seminiferous tubular testosterone levels are only 20% of control, indicating that there is far more testosterone in the testis than is needed to maintain full spermatogenesis.
In our studies we typically exposure pregnant rats from GD 14 to 18, or longer and measure ex vivo fetal testis T Prod and gene expression at the end of the critical period two to four hours after the last dose to the dam. Individual intact testes are incubated in media for 3 hrs. testosterone is measured in the media. In the current study, the DINP-induced reduction in T Prod plateaued at about 30% of control. Several other laboratories also have shown that DINP reduces fetal T Prod during sexual differentiation (Boberg et al. 2011; Borch et al. 2004; Clewell et al. 2013) and the reductions in ex vivo testis T Prod were similar to the maximal reduction seen in the current study (Boberg et al. (Boberg et al. 2011) at 900 mg DINP/kg/d (25% of control), and Borch et al., (Borch et al. 2004) at 750 mg DINP/kg/d (30% of control)).
Studies also have shown that the effects of DINP and other PEs on fetal T Prod are not permanent. For example, Clewell et al. (Clewell et al. 2013) found that the effect of 750 mg DINP/kg/d from GD 12 to 19 reduced fetal testis testosterone by 65% when measured 2h after the last dose but there was no effect on testosterone levels 24h later. Similarly, Thompson et al., (Thompson et al. 2004) demonstrated that the effect of DBP (500 mg/kg/d from GD 12 to 17 or 18 and necropsied on GD19) on fetal testosterone and mRNA expression was relatively rapid and reversible, the reduction of testosterone was much less at 48 than at 24 hours and there were no longer any effects on mRNA expression in the testis. This likely explains why Adamsson et al. (Adamsson et al. 2009), who examined extracted testosterone from testicular homogenates on GD 19.5 two days after the last dose of DINP did not detect any effect of DINP on testosterone levels or mRNA expression of genes involved in steroid transport, testosterone or Insl3 synthesis.
In addition, shortening the duration of exposure to a phthalate reduces the magnitude of the effect. For example, five days of treatment with DPeP was about 15 fold more effective in reducing T Prod than one day ED50_one_day = 667 mg/kg versus ED50_five_day = 47 mg/kg/d) (Hannas et al. 2011a).
Other studies have shown that extracting testosterone from the testis or plasma provides less consistent results than does determination of testosterone in media following ex vivo incubation of the testes. For example, Boberg et al. (Boberg et al. 2011) reported that intratesticular testosterone (reduced by 30%) was much less effected than was ex vivo T Prod (reduced by 70%), while plasma testosterone levels were slightly increased versus control (105%), rather than decreased from exposure to DINP.
Thus, the shortened exposure period, method of testosterone determination, and uncertain timing of necropsy may explain the negative effects of 125 and 750 mg DINP/kg on testosterone reported by van den Driesche et al. (van den Driesche et al. 2020). In their study 750 mg DBP/kg only reduced testosterone extracted from the testis by about 30% whereas we find that this dose of DBP reduces ex vivo T Prod by about 80% (estimated their ED50 ≈1200 versus our ED50 ≈ 400 mg DBP/kg, respectively).
In the current study, we extended the dose range of DINP upwards and observed statistically significant dose related reductions of male AGD, increases in retained PND 13 areolae/nipples, permanent reductions in several androgen-dependent organ weights and an increase in gross malformations and histological alterations of testicular and epididymal tissues. The gross malformations included complete unilateral testis agenesis in one male and bilateral malformed testes and hypoplastic epididymides in another male (Fig 4a,b and 5), severe effects that we have never seen in control males. Similarly, a dose response 300 to 900 mg DINP/kg/d study (GD 7 to PND 17) (Boberg et al. 2011) also reduced male AGD, increased retained nipples on PND 13, and induced permanent malformations in 4 of 75, 90 day old male offspring (2 with small testes and epididymides and 2 with permanent nipples) in the top 3 dose groups combined. In contrast, several other studies have not observed statistically significant alterations on these endpoints (Clewell et al. 2013; van den Driesche et al. 2020; Waterman et al. 2000). As discussed below, there are several possible explanations for the absence of effects on the male tract in these studies.
In addition, while some have concluded that in utero DINP does not induce malformations in male rat because it did not induce hypospadias or undescended testis (Waterman et al. 2000), it is important to realize that these are not the most sensitive malformations in the Phthalate Syndrome in the SD rat strain (Wilson et al. 2007). For example, the ED50s for the effects of DBP on epididymal agenesis and hypoplasia and testis abnormalities are about half that for hypospadias (Mylchreest et al. 1998; Mylchreest and Foster 2000; Mylchreest et al. 1999) and when administered for only two days during sexual differentiation DBP induced epididymal and testis abnormalities but did not cause testis nondescent or hypospadias (Carruthers and Foster 2005). Taken together, these studies indicate that studies evaluating endocrine disrupting chemicals like phthalates should include a thorough evaluation of malformations of the epididymis and testis and not just the incidence of hypospadias and testis nondescent. In addition, it is unclear why so few studies include an assessment of gubernacular agenesis or elongation since this is occasionally the only malformation noted in the phthalate treated male offspring. Gubernacular abnormalities are pathognomonic of the Phthalate Syndrome due to the relatively unique effects of phthalates on the testis peptide hormone Insl3.
Methodological basis for the absence of permanent effects of DINP on the male rat tract
Study did not include dose levels of 750 mg/kg/d or higher.
Exposure duration did not include the entire critical period (GD 14 to 18 at a minimum)
Observation of immature males when some effects cannot be detected accurately.
Incomplete/limited observation of all possible male tract tissues known to be part of the Phthalate Syndrome (“the absence of evidence is not evidence of absence”)
Sample Size examined is insufficient to detect the effects when they occur at a low rate
Among the above experimental design considerations that is critical in the detection of low rates of reproductive tract abnormalities is the sample size and the associated statistical power. Power is the likelihood of detecting a “true” effect in a study. For example, using SAS Proc Power we determined that a study using a sample size of 38 pups per group in the control and 750 mg DINP/kg/d group, as appears to be the design used in one study (van den Driesche et al. 2020), the likelihood of detecting a “ true” effect that occurs 7.7% of the time is only 35% . This indicates that if you run the study 10 times 6 – 7 of the studies will falsely appear negative. Masutomi (Masutomi et al. 2003) also only reported minimal effects of DINP (estimated high dose during sex differentiation of ≈ 1,200 mg/kg/d) on Sertoli cells and did not observe any “developmental alterations”. However, the likelihood of detecting a 15% incidence of testis and epididymal malformations with five males per dose group is infinitesimally low, too low to calculate with SAS 9.4 PROC POWER. In our two DINP studies combined we evaluated 110–161 control males (varied with endpoint) and 34 to 52 males per dose group in the DINP exposed groups. The power to detect the increase of about 14.7% in malformations in the 1.5 g/kg dose group versus the control group with such a sample sizes is about 77%. Taken together, statistical power analyses indicate that many studies of in utero exposure to DINP on the male rat reproductive tract are severely under powered and have a high likelihood of being falsely negative and, as a result, are likely to add inaccurate conclusions to the literature.
At present, we are evaluating data from about ten different phthalate esters including DPeP and DINP discussed herein to develop a statistical model of the relationship between T Prod and phthalate induced male reproductive abnormalities. Such a statistical model could then be used to predict the postnatal effects of unstudied phthalates or mixtures on the F1 male reproductive tract by measuring the effects of that chemical on T Prod after administration during sexual differentiation. This would enable risk assessors to determine a point of departure for an adverse effect from a short-term in utero study using a fewer animals and resources than a one generation reproduction test (i.e., OECD Test Guideline 443,(2018. Extended One-Generation Reproductive Toxicity Study, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris, https://doi.org/10.1787/9789264185371-en).
In summary, the results of our DINP fetal and postnatal studies demonstrate that DINP disrupts fetal testis hormone and gene expression via the same Adverse Outcome Pathway (Figure 11) as the more potent phthalates like DPeP, DEHP, DBP, BBP, DCHP, and DiBP (Gray et al. 2021), for example. Because of the effects of phthalates on fetal testis development, male rat offspring display permanent alterations of androgen and Insl3 hormone-dependent tissues including AGD, nipple retention, some androgen-dependent organ weights, and testis and epididymal morphology and histology (Figures 2–5). Although the frequency of severe effects of DINP are lower than other phthalates at similar dose levels, this phthalate induced dose-related increases in testis and epididymal malformations (Table 3, Figures 4 and 5 ) with 14.7% of the males displaying a malformation in the top dose group. When we compare the effects of DINP to those of DPeP using T Prod and Insl3 reductions as the dose-metric rather than by oral dose levels (mg/kg/d) the dose-response curves for DINP and DPeP are nearly identical (Figures 6–9). In conclusion, these results are consistent with our hypothesis that quantifiable alterations in fetal T Prod and Insl3 mRNA expression can be used to predict the in utero effects of phthalates on the reproductive tract of male rats later in life.
Supplementary Material
Acknowledgements.
I would like to acknowledge the excellent assistance of Joseph Ostby, Andrew Hotchkiss, and Johnathan Furr for their skillful execution of the DINP postnatal and fetal rat studies and Christy Lambright for determination of fetal testosterone production and testis Insl3 mRNA transcript levels in the DINP fetal studies. In addition, I thank Justin Conley, Kembra Howdeshell, Bethany Hannas, Andrew Hotchkiss, and Phillip Hartig for their technical manuscript reviews prior to EPA clearance for publication and the TAAP editor and reviewers for their constructive comments on the draft manuscript.
Funding:
The USEPA, ORD entirely funded this work,
Disclaimer:
The research described in this article has been reviewed by U.S. Environmental Protection Agency and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
REFERENCES
- Adamsson A, Salonen V, Paranko J, and Toppari J. (2009). Effects of maternal exposure to di-isononylphthalate (DINP) and 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene (p,p’-DDE) on steroidogenesis in the fetal rat testis and adrenal gland. Reprod Toxicol 28, 66–74. [DOI] [PubMed] [Google Scholar]
- Awoniyi CA, Santulli R, Chandrashekar V, Schanbacher BD, and Zirkin BR (1989). Quantitative restoration of advanced spermatogenic cells in adult male rats made azoospermic by active immunization against luteinizing hormone or gonadotropin-releasing hormone. Endocrinology 125, 1303–9. [DOI] [PubMed] [Google Scholar]
- Boberg J, Christiansen S, Axelstad M, Kledal TS, Vinggaard AM, Dalgaard M, Nellemann C, and Hass U. (2011). Reproductive and behavioral effects of diisononyl phthalate (DINP) in perinatally exposed rats. Reprod Toxicol 31, 200–9. [DOI] [PubMed] [Google Scholar]
- Borch J, Ladefoged O, Hass U, and Vinggaard AM (2004). Steroidogenesis in fetal male rats is reduced by DEHP and DINP, but endocrine effects of DEHP are not modulated by DEHA in fetal, prepubertal and adult male rats. Reprod Toxicol 18, 53–61. [DOI] [PubMed] [Google Scholar]
- Carruthers CM, and Foster PM (2005). Critical window of male reproductive tract development in rats following gestational exposure to di-n-butyl phthalate. Birth Defects Res B Dev Reprod Toxicol 74, 277–85. [DOI] [PubMed] [Google Scholar]
- Clewell RA, Sochaski M, Edwards K, Creasy DM, Willson G, and Andersen ME (2013). Disposition of diiosononyl phthalate and its effects on sexual development of the male fetus following repeated dosing in pregnant rats. Reprod Toxicol 35, 56–69. [DOI] [PubMed] [Google Scholar]
- Coveney D, Cool J, Oliver T, and Capel B. (2008). Four-dimensional analysis of vascularization during primary development of an organ, the gonad. Proc Natl Acad Sci U S A 105, 7212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ema M, Miyawaki E, and Kawashima K. (2000). Critical period for adverse effects on development of reproductive system in male offspring of rats given di-n-butyl phthalate during late pregnancy. Toxicol Lett 111, 271–8. [DOI] [PubMed] [Google Scholar]
- Furr JR, Lambright CS, Wilson VS, Foster PM, and Gray LE Jr. (2014). A short-term in vivo screen using fetal testosterone production, a key event in the phthalate adverse outcome pathway, to predict disruption of sexual differentiation. Toxicol Sci 140, 403–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray LE Jr., Ferrell JM, and Ostby JS (1985). Alteration of behavioral sex differentiation by exposure to estrogenic compounds during a critical neonatal period: effects of zearalenone, methoxychlor, and estradiol in hamsters. Toxicol Appl Pharmacol 80, 127–36. [DOI] [PubMed] [Google Scholar]
- Gray LE Jr., Furr J, Tatum-Gibbs KR, Lambright C, Sampson H, Hannas BR, Wilson VS, Hotchkiss A, and Foster PM (2016). Establishing the “Biological Relevance” of Dipentyl Phthalate Reductions in Fetal Rat Testosterone Production and Plasma and Testis Testosterone Levels. Toxicol Sci 149, 178–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray LE Jr., Ostby J, Furr J, Price M, Veeramachaneni DN, and Parks L. (2000). Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol Sci 58, 350–65. [DOI] [PubMed] [Google Scholar]
- Gray LE Jr., Ostby J, Monosson E, and Kelce WR (1999). Environmental antiandrogens: low doses of the fungicide vinclozolin alter sexual differentiation of the male rat. Toxicol Ind Health 15, 48–64. [DOI] [PubMed] [Google Scholar]
- Gray LE, Lambright CS, Conley JM, Evans N, Furr JR, Hannas BR, Wilson VS, Sampson H, and Foster PMD (2021). Genomic and Hormonal Biomarkers of Phthalate-Induced Male Rat Reproductive Developmental Toxicity Part II: A Targeted RT-qPCR Array Approach That Defines a Unique Adverse Outcome Pathway. Toxicol Sci 182, 195–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray LE, Ostby J, Monosson E, and Kelce WR (1994). Alterations of Sex-Differentiation in Male-Rats Following Perinatal Exposure to Low-Doses of the Antiandrogenic Pesticide Vinclozolin (V). In Biology of Reproduction, Vol. 50, pp. 101-101. SOC STUDY REPRODUCTION 1603 MONROE ST, MADISON, WI 53711–2021. [Google Scholar]
- Hannas BR, Furr J, Lambright CS, Wilson VS, Foster PM, and Gray LE Jr. (2011a). Dipentyl phthalate dosing during sexual differentiation disrupts fetal testis function and postnatal development of the male Sprague-Dawley rat with greater relative potency than other phthalates. Toxicol Sci 120, 184–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannas BR, Lambright CS, Furr J, Evans N, Foster PM, Gray EL, and Wilson VS (2012). Genomic biomarkers of phthalate-induced male reproductive developmental toxicity: a targeted RT-PCR array approach for defining relative potency. Toxicol Sci 125, 544–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannas BR, Lambright CS, Furr J, Howdeshell KL, Wilson VS, and Gray LE Jr. (2011b). Dose-response assessment of fetal testosterone production and gene expression levels in rat testes following in utero exposure to diethylhexyl phthalate, diisobutyl phthalate, diisoheptyl phthalate, and diisononyl phthalate. Toxicol Sci 123, 206–16. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AK, Ostby JS, Vandenbergh JG, and Gray LE Jr. (2003). An environmental antiandrogen, vinclozolin, alters the organization of play behavior. Physiol Behav 79, 151–6. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AK, Ostby JS, Vandenburgh JG, and Gray LE Jr. (2002). Androgens and environmental antiandrogens affect reproductive development and play behavior in the Sprague-Dawley rat. Environ Health Perspect 110 Suppl 3, 435–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassim NM, Russell DA, and Payne AP (2010). Does the cranial suspensory ligament have a role in cryptorchidism? Cells Tissues Organs 191, 307–15. [DOI] [PubMed] [Google Scholar]
- Macleod DJ, Sharpe RM, Welsh M, Fisken M, Scott HM, Hutchison GR, Drake AJ, and van den Driesche S. (2010). Androgen action in the masculinization programming window and development of male reproductive organs. Int J Androl 33, 279–87. [DOI] [PubMed] [Google Scholar]
- Masutomi N, Shibutani M, Takagi H, Uneyama C, Takahashi N, and Hirose M. (2003). Impact of dietary exposure to methoxychlor, genistein, or diisononyl phthalate during the perinatal period on the development of the rat endocrine/reproductive systems in later life. Toxicology 192, 149–170. [DOI] [PubMed] [Google Scholar]
- Mylchreest E, Cattley RC, and Foster PMD (1998). Male reproductive tract malformations in rats following gestational and lactational exposure to di(n-butyl) phthalate: An antiandrogenic mechanism? Toxicological Sciences 43, 47–60. [DOI] [PubMed] [Google Scholar]
- Mylchreest E, and Foster PM (2000). DBP exerts its antiandrogenic activity by indirectly interfering with androgen signaling pathways. Toxicol Appl Pharmacol 168, 174–5. [DOI] [PubMed] [Google Scholar]
- Mylchreest E, Sar M, Cattley RC, and Foster PMD (1999). Disruption of androgen-regulated male reproductive development by di(n-butyl) phthalate during late gestation in rats is different from flutamide. Toxicology and Applied Pharmacology 156, 81–95. [DOI] [PubMed] [Google Scholar]
- Mylchreest E, Sar M, Wallace DG, and Foster PMD (2002). Fetal testosterone insufficiency and abnormal proliferation of Leydig cells and gonocytes in rats exposed to di(n-butyl) phthalate. Reproductive Toxicology 16, 19–28. [DOI] [PubMed] [Google Scholar]
- Mylchreest E, Wallace DG, Cattley RC, and Foster PM (2000). Dose-dependent alterations in androgen-regulated male reproductive development in rats exposed to Di(n-butyl) phthalate during late gestation. Toxicol Sci 55, 143–51. [DOI] [PubMed] [Google Scholar]
- Nef S, and Parada LF (1999). Cryptorchidism in mice mutant for Insl3. Nat Genet 22, 295–9. [DOI] [PubMed] [Google Scholar]
- Parks LG, Ostby JS, Lambright CR, Abbott BD, Klinefelter GR, Barlow NJ, and Gray LE (2000). The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicological Sciences 58, 339–349. [DOI] [PubMed] [Google Scholar]
- Scott HM, Hutchison GR, Jobling MS, McKinnell C, Drake AJ, and Sharpe RM (2008). Relationship between androgen action in the “male programming window,” fetal sertoli cell number, and adult testis size in the rat. Endocrinology 149, 5280–7. [DOI] [PubMed] [Google Scholar]
- Thompson CJ, Ross SM, and Gaido KW (2004). Di(n-butyl) phthalate impairs cholesterol transport and steroidogenesis in the fetal rat testis through a rapid and reversible mechanism. Endocrinology 145, 1227–37. [DOI] [PubMed] [Google Scholar]
- van den Driesche S, Shoker S, Inglis F, Palermo C, Langsch A, and Otter R. (2020). Systematic comparison of the male reproductive tract in fetal and adult Wistar rats exposed to DBP and DINP in utero during the masculinisation programming window. Toxicol Lett 335, 37–50. [DOI] [PubMed] [Google Scholar]
- Waterman SJ, Keller LH, Trimmer GW, Freeman JJ, Nikiforov AI, Harris SB, Nicolich MJ, and McKee RH (2000). Two-generation reproduction study in rats given di-isononyl phthalate in the diet. Reprod Toxicol 14, 21–36. [DOI] [PubMed] [Google Scholar]
- Welsh M, Saunders PT, Fisken M, Scott HM, Hutchison GR, Smith LB, and Sharpe RM (2008). Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. The Journal of clinical investigation 118, 1479–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M, Sharpe RM, Moffat L, Atanassova N, Saunders PT, Kilter S, Bergh A, and Smith LB (2010). Androgen action via testicular arteriole smooth muscle cells is important for Leydig cell function, vasomotion and testicular fluid dynamics. PLoS ONE 5, e13632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson VS, Howdeshell KL, Lambright CS, Furr J, and Earl Gray L Jr. (2007). Differential expression of the phthalate syndrome in male Sprague-Dawley and Wistar rats after in utero DEHP exposure. Toxicol Lett 170, 177–84. [DOI] [PubMed] [Google Scholar]
- Wilson VS, Lambright C, Furr J, Ostby J, Wood C, Held G, and Gray LE (2004). Phthalate ester-induced gubernacular lesions are associated with reduced insl3 gene expression in the fetal rat testis. Toxicology Letters 146, 207–215. [DOI] [PubMed] [Google Scholar]
- Wolf CJ, LeBlanc GA, Ostby JS, and Gray LE Jr. (2000). Characterization of the period of sensitivity of fetal male sexual development to vinclozolin. Toxicol Sci 55, 152–61. [DOI] [PubMed] [Google Scholar]
- Zimmermann S, Steding G, Emmen JM, Brinkmann AO, Nayernia K, Holstein AF, Engel W, and Adham IM (1999). Targeted disruption of the Insl3 gene causes bilateral cryptorchidism. Mol Endocrinol 13, 681–91. [DOI] [PubMed] [Google Scholar]
- Zirkin BR, Santulli R, Awoniyi CA, and Ewing LL (1989). Maintenance of advanced spermatogenic cells in the adult rat testis: quantitative relationship to testosterone concentration within the testis. Endocrinology 124, 3043–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.