Abstract
Immune escape mechanisms in non-small cell lung cancer (NSCLC) can disrupt every step of the anti-cancer immune response. In recent years, an increased understanding of the specific mechanisms fueling immune escape has allowed for the development of numerous immunotherapeutic treatments that have been introduced into the clinical practice. The advent of immunotherapy has dramatically changed the current treatment landscape of advanced or metastatic NSCLC because of its durable efficacy and manageable toxicity. In this review, we will first present a brief overview of recent evidence on immune escape mechanisms in NSCLC. We will then discuss the current promising immunotherapeutic strategies in advanced or metastatic NSCLC tumors.
Keywords: Advanced or metastatic, Immune escape mechanisms, Immunotherapy, Non-small cell lung cancer
Introduction
Lung cancer remains a major public health concern, as it is the most frequent cause of cancer-related deaths worldwide.[1] Non-small cell lung cancer (NSCLC) is the predominant type, representing >85% of all cases. According to the data, about 50% of the NSCLC cases were diagnosed with an advanced disease that has already metastasized, which resulted in a 5-year overall survival (OS) rate <5% before the application of immune-checkpoint inhibitors (ICIs).[2–4] In recent years, immunotherapies have provided long-term survival benefits to a proportion of patients, which may be helpful for advanced or metastatic NSCLC cases. Therefore, it is time to review the mechanisms and current immunotherapy strategies of NSCLC.
A Brief Overview of Immune Escape Mechanisms in NSCLC
NSCLC tumor cells (TC) are surviving in a complex and highly organized immune microenvironment that includes neoplastic cells, vasculature, extracellular matrix, and immune cells (IC), as well as various signaling molecules such as cytokines and chemokines.[5] The dynamic interactions between TC and the immune microenvironment have been described as immunoediting, which also applies to NSCLC.[6] TC can better adapt to the immune microenvironment to survive under the immune selection pressures. The immunoediting includes three phases: elimination, equilibrium, and escape. In the elimination phase, the adaptive and innate IC are able to recognize the neoantigens and eradicate TC before the cancer becomes clinically apparent. In the equilibrium phase, the surviving or new tumor variants can persist in a dormant state, and the immune system can suppress the development of the tumor. In the escape phase, the TC acquire the ability to avoid being recognized and attacked by the immune system.[7]
The anti-tumor immune response is often summarized as three phases: (1) the activation of effector T cells, (2) migration and penetration of effector T cells into the tumor site, and (3) activation of the cytotoxic T-cell response. The lung cancer cells cannot be detected and eliminated by the immune system when any of the above steps are disrupted [Figure 1].
Figure 1.
Mechanisms of immune escape in NSCLC. The gray boxes represent the immune escape mechanisms of NSCLC. Drawing tools: ScienceSlides (http://www.scienceslides.com/) and SMART (https://smart.servier.com/). CTLA-4: Cytotoxic T-lymphocyte-associated protein 4; DC: Dendritic cells; IDO: Indoleamine-2,3-dioxygenase; KRAS: Kirsten rat sarcoma 2 viral oncogene homolog; MHC: Major histocompatibility complex; NSCLC: Non-small cell lung cancer; PD-1: Programed cell death protein 1; PD-L1: Programed cell death ligand 1; STK11/LKB1: Serine/threonine kinase 11/liver kinase B1; TMB: Tumor mutational burden.
In the effector T-cell activation phase, dendritic cells (DCs) capture neoantigens released by NSCLC cells and present them to effector T cells. Downregulation of NSCLC cell immunogenicity, such as neoantigen loss[8,9] or low tumor mutational burden (TMB),[10–12] interrupts that ability of DCs to prime the immune response. Overexpression of immunosuppressive signals, such as cytotoxic-T-lymphocyte-associated antigen 4 (CTLA-4) on the surface of activated T cells, can also prevent DCs from presenting neoantigens to T cells and activating effector T cells.[13]
In the effector T cells migration phase, T cells migrate via blood vessels and lymphoid capillaries and penetrate into tumor. Abnormal vessel formation[14] and matrix fibers[15] create a barrier that can block this penetration. The immunosuppressive cells in the tumor microenvironment (TME) such as tumor-associated macrophages,[16] myeloid-derived suppressor cells,[17] and regulatory T (Treg) cells[18] have been demonstrated to limit the trafficking of T cells to tumor sites.[19]
In the cytotoxic phase, the effector T cells recognize the TC through the T cell receptor (TCR) and its cognate antigenic peptide/major histocompatibility complex I (MHC I) complex on the TC. In NSCLC cells, downregulation of MHC I leads to defective recognition by T cells[20–22] and genetic changes, such as an inactivating serine-threonine kinase 11/liver kinase B1 mutation or Kirsten rat sarcoma 2 viral oncogene homolog (KRAS) mutation, which can suppress the immune response.[23] For T cells, overexpression of immune checkpoints can interrupt the cytotoxic T-cell response through downstream immunosuppressive transduction pathways.[24] Additionally, the expression of major rate-limiting enzymes involved in the immunosuppressive metabolic pathways in the TME, such as ectonucleotidase CD73/CD39[25] and indoleamine-2,3-dioxygenase 1 (IDO 1),[26] can suppress the anticancer response of cytotoxic T-cells.
Various strategies have been developed to overcome immune escape mechanisms: (1) For impaired activation of effector T cells, therapeutic cancer vaccines and oncolytic viruses (OV) can provide exogenous or endogenous tumor antigens to enhance the immune response. (2) Anti-angiogenic (AA) therapies that lead to normalization of the vasculature can induce the infiltration of effector T cells into the tumor. These are frequently combined with other immunotherapies. (3) Several methods are currently being explored to overcome the inhibited activation of the cytotoxic T-cell response. For example, adoptive cell therapy (ACT) can improve the non-inflamed TME by supplementing lymphocytes or antigen presenting cells. Immune checkpoint monoclonal antibodies can help restore the functions of IC. Targeting rate-limiting enzymes involved in the immunosuppressive metabolic pathways can also activate the immune response.
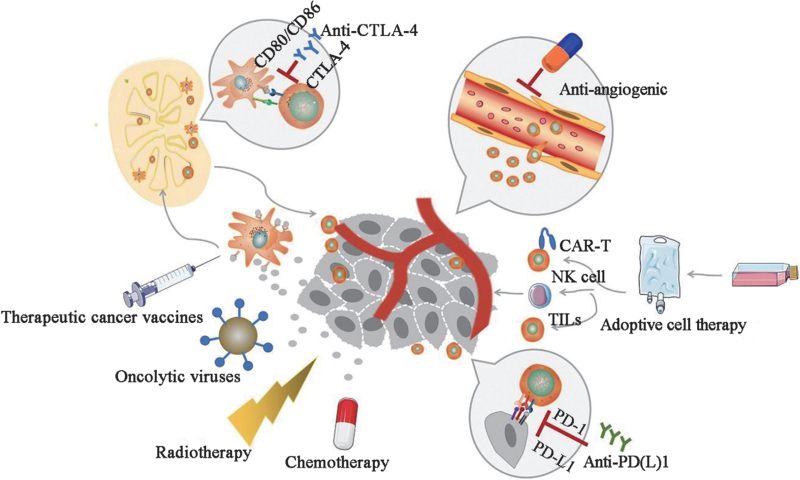
Throughout this review, we will further discuss these promising immunotherapeutic strategies in the treatment of advanced or metastatic NSCLC with respect to the above three aspects of immune escape mechanisms [Figure 2].
Figure 2.
Current immunotherapy in NSCLC. Drawing tools: ScienceSlides (http://www.scienceslides.com/) and SMART (https://smart.servier.com/). CAR-T: Chimeric antigen receptor-modified T; CTLA-4: Cytotoxic T-lymphocyte-associated protein 4; NK: Natural killer; NSCLC: Non-small cell lung cancer; PD-1: Programed cell death protein 1; PD-L1: Programed cell death 1 ligand 1; TILs: Tumor-infiltrating lymphocytes.
Promising Strategies of Immunotherapy in Advanced or Metastatic NSCLC
Strategies of activation of effector T cells
Therapeutic cancer vaccines
Therapeutic cancer vaccines comprise the category of active immunotherapies that can stimulate and expand the immune response against tumors by inducing selected tumor antigens. Historically, therapeutic cancer vaccines have often failed to improve the outcome of advanced NSCLC patients. There are two promising vaccines that have shown good performances in phase III clinical trials.
OSE2101 (Tedopi) is a therapeutic cancer vaccine with combination of 10 neoepitopes restricted to human leucocyte antigen (HLA)-A2. In the phase III Atalante-1 study, OSE2101 monotherapy was associated with improved OS (11.1 months vs. 7.5 months; hazard ratio [HR] = 0.59; 95% confidence interval [CI]: 0.38–0.91) and fewer severe adverse events (AEs) (38% vs. 68%) compared with single-agent chemotherapy (docetaxel or pemetrexed) in patients with HLA-A2-positive advanced or metastatic NSCLC whose disease progressed after platinum-based chemotherapy and ICIs. From these results, the Food and Drug Administration (FDA) granted orphan-drug designation to OSE2101.[27] There are ongoing clinical trials to evaluate the combination of OSE2101 and docetaxel or nivolumab as a second-line therapy in patients with advanced or metastatic NSCLC after apparent resistance to first-line chemotherapy plus ICI.
The CIMAvax-EGF vaccine developed in Cuba could stimulate the immune system and induce anti-epidermal growth factor (anti-EGF) antibodies to interrupt binding of endogenous EGF to epidermal growth factor receptor (EGFR). This therefore inhibits the EGFR pathway, which plays an important role in promoting the proliferation and survival of TC. The vaccine was approved by the Cuban Regulatory Agency in 2008 as a switch maintenance treatment for patients with advanced or metastatic NSCLC following first-line chemotherapy. In a phase III clinical trial (RPCEC00000161) of this vaccine as a switch maintenance treatment, there was no statistically significant improvement to OS in the treatment group compared with the control group with best supportive care (10.83 vs. 8.86 months; HR = 0.82; P = 0.100) in the safety population. However, a significant OS benefit was observed in a post hoc analysis using Harrington–Fleming test (a weighted log-rank test) in the safety population and was also exhibited among the subgroup of patients who received at least four vaccine doses.[28] An exploratory analysis suggested that the quantity and quality of anti-EGF antibodies were potential biomarkers to predict the efficacy of the CIMAvax-EGF vaccine.[29] No comparison was made between using the CIMAvax-EGF vaccine as a switch maintenance treatment and as a standard maintenance therapy, which made this study less convincing.
The extent and breadth of the immune response induced by a vaccine are affected by the individual immune status of each patient. Unfortunately, there are currently no objective indicators for assessing a person's immune status to identify those who would benefit from such treatments. Advanced or metastatic NSCLC tumors have complicated immunosuppressive microenvironments and well-established immune escape mechanisms, suggesting that using a vaccine as a monotherapy could only have a moderate effect. Combining this with other treatment methods is a reasonable strategy for advanced or metastatic disease cases. There are now about 20 ongoing clinical trials being conducted to investigate therapeutic cancer vaccines in NSCLC, according to National Institute of Health (NIH) ClinicalTrials.gov. Some of these focus on personalized cancer vaccines targeting neoantigens with high tumor specificity and combining these vaccines with other treatment methods.
OVs
OVs are viruses selected from nature or genetically engineered that can specifically target TC with little effect on normal cells. OVs have complicated working mechanisms. They kill TC and act as an “in situ vaccination” by releasing a large number of tumor antigens to stimulate and expand the immune response.[30] Additionally, OVs can disrupt the tumor stroma, resulting in disruption of the tumor vasculature.[31] Furthermore, OVs transfected with a therapeutic gene can directly target the TC.[32] Currently, there are two OVs on the market, including Oncorine (H101) for nasopharyngeal carcinoma approved by National Medical Products Administration (NMPA) and IMLYGIC™ (talimogene laherparepvec) for melanoma by the FDA.
Reolysin, a type 3 Dearing strain reovirus, combined with chemotherapy (paclitaxel plus carboplatin) was shown to be well tolerated and active (objective response rate [ORR]: 31%; 1-year OS rate: 57%) in 37 patients with metastatic/recurrent KRAS-mutated or EGFR-mutated/amplified NSCLC as a first-line treatment (National Clinical Trial [NCT] 00861627).[33] However, in a subsequent phase II study (IND211study, NCT01708993), a second-line therapy of intravenously injected Reolysin combined with chemotherapy (pemetrexed or docetaxel) failed to demonstrate superior efficacy over chemotherapy alone, even among the patients with a KRAS or EGFR mutation.[34] According to the preclinical data, reovirus may have enhanced infection efficiency in RAS-activated cells such as KRAS-mutated or EGFR-mutated/amplified NSCLC. The original intention of developing Reolysin was to overcome the lack of effective therapies targeting the abovementioned genetic alterations by activating host immunity and inducing cell cycle arrest together with chemotherapy. However, no progression-free survival (PFS) or OS benefit was observed in patients who had received platinum-based chemotherapy in the IND211 study, which suggested that proper timing and therapeutic combination sequence may have a positive effect on the outcome.
The current difficulties of this method include ensuring the effective transportation of OVs to the tumor site and determining the optimal combination partner. Currently, OVs injected intratumorally and in combination with ICIs are being investigated in clinical trials.
CTLA-4 inhibitors
The checkpoint cytotoxic-T-lymphocyte-associated antigen 4 (CTLA-4) expressed on the surface of activated T cells or regulatory T cells is a critical inhibitory receptor of the B7 family on the surface of antigen-presenting cell (APC). Combination of CTLA-4 with B7 can induce immunosuppressive signals to inhibit the priming and activation of effector T cells.[13] The main mechanism of anti-CTLA-4 monoclonal antibody is the activation of immune response of T cells rather than enhancing the cytotoxicity of T cells to attack TC, which limits the efficacy of CTLA-4 inhibitors as a single agent in solid tumor including NSCLC.[35] Therefore, the CTLA-4 inhibitors are mainly applied in combination with other ICIs to perform different biological functions at different time and sites during T cell activation and regulation. Thus, the combinations often have a synergistic effect.[36] Now, anti-CTLA-4 monoclonal antibodies, ipilimumab and tremelimumab, in combination with programed cell death protein 1 (PD-1) or programed death-ligand 1 (PD-L1) inhibitors have made great progress in recent phase III clinical trials of advanced or metastatic NSCLC [Table 1].
Table 1.
Clinical trials of the combination of CTLA-4 inhibitor and PD-1/PD-L1 inhibitor.
| Trial | PD-L1 assay | Phase | Histology | Design | PD-L1 status | ORR (%) | Median PFS (months) (HR, 95% CI) | Median OS (months) (HR, 95% CI) | ≥3 TRAE |
| CheckMate 227 | Dako28-8 | III | nsq/sq | Nivo/IPI (583) Chemo (583) |
PD-L1 ≥1% and <1% | 33.1% vs. 27.8% | 5.1 vs. 5.5 (HR 0.79; 0.69–0.91) |
17.1 vs. 13.9 (HR 0.73; 0.64–0.84) |
32.8% vs. 36.0% (grades 3–4) |
| PD-L1 <1% (subgroup) | 27.3% vs. 23.1% | 5.1 vs. 4.7 (HR 0.75; 0.59–0.96) |
17.2 vs. 12.2 (HR 0.62; 0.49–0.79) |
||||||
| PD-L1 ≥1% (subgroup) | 35.9% vs. 30.0% | 5.1 vs. 5.6 (HR 0.82; 0.69–0.97) |
17.1 vs. 14.9 (HR 0.79; 0.65–0.96) |
||||||
| CheckMate 9LA | Dako28–8 | III | nsq/sq | Nivo/IPI + chemo (361) Chemo (358) |
PD-L1 ≥1% and <1% | 38.2% vs. 24.9% | 6.7 vs. 5.0 (HR 0.68; 0.57–0.82) |
15.6 vs. 10.9 (HR 0.66; 0.55–0.80) |
47% vs. 38% (grades 3–4) |
| PD-L1 <1% (subgroup) | 31.1% vs. 20.2% | 5.8 vs. 4.6 (HR 0.71; 0.53–0.94) |
16.8 vs. 9.8 (HR 0.62; 0.45–0.85) |
||||||
| PD-L1 ≥1% (subgroup) | 43.3% vs. 27.5% | 7.1 vs. 4.7 (HR 0.67; 0.53–0.84) |
15.8 vs. 10.9 (HR 0.64; 0.50–0.82) |
||||||
| MYSTIC | SP263 | III | nsq/sq | Durva + treme (163) Chemo (162) |
TC ≥25% | 34.4% vs. 37.7% | 3.9 vs. 5.4 (HR 1.05; 99.5% CI, 0.72–1.53) |
11.9 vs. 12.9 (HR 0.85; 98.77% CI, 0.61–1.17) |
22.9% vs. 33.8% |
| TC ≥50% | − | − | 15.2 vs. 12.7 (HR 0.77; 0.56–1.07) |
||||||
| POSEIDON | SP263 | III | nsq/sq | Durva + treme + chemo (338) Chemo (337) |
TC <1% or TC ≥1% | 38.8% vs. 24.4% | 6.2 vs. 4.8 (HR 0.72; 0.60–0.86) |
14.0 vs. 11.7 (HR 0.77; 0.65–0.92) |
51.8% vs. 44.4% |
Chemo: Chemotherapy; CTLA-4: CI: Confidence interval; Cytotoxic T-lymphocyte-associated protein 4; Durva: Durvalumab; HR: Hazard ratio; IPI: Ipilimumab; Nivo: Nivolumab; NSCLC: Non-small cell lung cancer; nsq: Non-squamous NSCLC; ORR: Objective response rate; OS: Overall survival; PD-1: Programed cell death protein 1; PD-L1: Programed cell death ligand 1; PFS: Progression-free survival; sq: Squamous NSCLC; TC: Tumor cell; TRAE: Treatment related adverse events; Treme: Tremelimumab. “–” represents unavailability of data.
In the CheckMate 227 clinical trial,[37] the combination of ipilimumab and nivolumab (anti-PD-1 therapy) demonstrated longer OS than chemotherapy alone as a first-line treatment in metastatic NSCLC patients, regardless of PD-L1 expression and histology. The 4-year update outcomes confirmed the long-term OS benefit, with 4-year OS rates of 29% (nivolumab plus ipilimumab) vs. 18% (chemotherapy) in patients with PD-L1 ≥1% and 24% (nivolumab plus ipilimumab) vs. 10% (chemotherapy) in patients with PD-L1 <1%.[38] The FDA has approved the use of ipilimumab plus nivolumab as a first-line treatment in metastatic NSCLC patients with PD-L1 positive expression.
The advantage of ICIs is their durable response, even though they could exhibit a poorer efficacy in a subset of patients after the start of therapy because of its slow-acting feature or hyperprogressive disease.[39,40] So, in CheckMate 9LA study,[41] two cycles of platinum-doublet chemotherapy were added to the first-line nivolumab plus ipilimumab treatment for disease control in the early stages of immunotherapy. The results showed a significant improvement in OS regardless of PD-L1 expression level and histology. More grade 3 to 4 treatment related adverse events (TRAEs) were reported for the nivolumab + ipilimumab + chemotherapy regimen vs. chemotherapy alone (25% vs. 15%). The combination of ipilimumab plus nivolumab and two cycles of chemotherapy has been approved by the FDA as a first-line treatment for patients with metastatic NSCLC irrespective of PD-L1 expression status.
Another combination of tremelimumab plus durvalumab did not significantly improve OS or PFS compared with chemotherapy alone in previously untreated patients with PD-L1 TC ≥25% in the MYSTIC study. Exploratory analyses found that TMB was a possible good biomarker associated with optimal OS benefit in durvalumab plus tremelimumab group with a threshold of ≥20 mutations/megabase (21.9 vs. 10.0 months; HR 0.49; 95% CI: 0.32–0.74).[42] In the phase III POSEIDON study, 4 cycles of chemotherapy were added to first-line durvalumab/tremelimumab treatment.[43] The results showed durvalumab+tremelimumab+chemotherapy significantly prolonged both PFS and OS compared with chemotherapy alone, and these outcomes were presented at the 2021 World Conference on Lung Cancer.
Recently, the development of modified CTLA-4 inhibitors with better efficiency and lower toxicity has been on the way. BMS-986218 and BMS-986249 are a new generation of CTLA-4 inhibitors that enhance the effect of antibody-dependent cellular cytotoxicity by the structural changes based on ipilimumab. Additionally, the bispecific antibodies, such as AK104 (PD-1/CTLA-4), MEDI5752 (PD-1/CTLA-4), ATOR-1015 (CTLA-4/tumor necrosis factor receptor superfamily member 4 [OX40]), and XmAb20717 (CTLA-4/lymphocyte-activation gene 3 [LAG-3]) are being assessed in early phase clinical trials of solid tumors including NSCLC (NCT05215067, NCT03530397, NCT03782467, NCT03517488).
Strategies to increase infiltration of effector T cells
Preclinical studies have provided evidence that AA treatment can induce a transient normalization of the vasculature and then increase cytotoxic T lymphocyte (CTL) infiltration and activity.[44] The combination of AA with ICIs has presented synergistic antitumor responses in clinical trials [Table 2].
Table 2.
Clinical trials of combination of AA and other therapies.
| Trial | PD-L1 assay | Phase | Histology | Design | PD-L1 status | ORR (%) | Median PFS (months) (HR, 95% CI) | Median OS (months) (HR, 95% CI) | ≥3 TRAE |
| IMpower150 | SP142 | III | Nsq | ABCP (WT 356) BCP (WT 336) |
TC 0/1/2/3 IC 0/1/2/3 |
63.5% vs. 48.0% | 8.3 vs. 6.8 (HR 0.62; 0.52–0.74) |
19.5 vs. 14.7 (HR 0.80; 0.67–0.95) |
58.5% vs. 50.0% |
| TC3 or IC3 (subgroup) | – | 15.2 vs. 6.8 (HR 0.34; 0.23–0.50) |
30.0 vs. 15.0 (HR 0.70; 0.46–1.08) |
||||||
| TC1/2/3 or IC1/2/3 (subgroup) | – | 11.1 vs. 6.8 (HR 0.47; 0.38–0.60) |
22.5 vs. 16.0 (HR 0.73; 0.57–0.94) |
||||||
| TC0 and IC0 (subgroup) | – | 7.2 vs. 6.9 (HR 0.71; 0.57–0.89) |
16.9 vs. 14.1 (HR 0.90; 0.71–1.14) |
||||||
| LEAP 007 | Dako22C3 | III | Nsq/sq | Pembro + lenva (309) Pembro (314) |
TPS ≥1% | 40.5% vs. 27.7% | 6.6 vs. 4.2 (HR 0.78; 0.64–0.95) |
14.1 vs. 16.4 (HR 1.10; 0.87–1.39) |
57.9% vs. 24.4% |
AA: Anti-angiogenic; ABCP: Atezolizumab plus BCP; BCP: Bevacizumab plus carboplatin plus paclitaxel; CI: Confidence interval; EGFR: Epidermal growth factor receptor; HR: Hazard ratio; IC: Immune cells; lenva: Lenvatinib; NR: Not reached; NSCLC: Non-small cell lung cancer; nsq: Non-squamous NSCLC; ORR: Objective response rate; OS: Overall survival; PD-1: Programed cell death protein 1; PD-L1: Programed cell death ligand 1; pembro: Pembrolizumab; PFS: Progression-free survival; sq: Squamous NSCLC; TC: Tumor cell; TPS: Tumor proportion score; TRAE: Treatment related adverse events; WT: EGFR or ALK wild type. “–” represents unavailability of data. TC3 or IC3: PD-L1 expression on ≥50% of TC or on ≥10% of IC; TC2/3 or IC2/3: PD-L1 expression on ≥5% of TC or IC; TC1/2/3 or IC1/2/3: PD-L1 expression on ≥1% of TC or IC. TC0 and IC0: PD-L1 expression on <1% of TC and IC.
The phase III IMpower150 study assessed the efficacy and safety of atezolizumab plus bevacizumab combined with chemotherapy in chemotherapy-naïve patients with metastatic non-squamous NSCLC. Three treatment groups were established, including the atezolizumab plus carboplatin plus paclitaxel group, bevacizumab plus carboplatin plus paclitaxel (BCP) group, and atezolizumab plus BCP (ABCP) group.[45] For patients without EGFR or ALK genetic alterations, the ABCP regimen significantly improved PFS (8.4 vs. 6.8 months; HR = 0.57; 95% CI: 0.48–0.67) and OS (19.5 vs. 14.7 months; HR = 0.80; 95% CI: 0.67–0.95)[46] compared with the BCP regimen. The FDA approved this first-line ABCP regimen for metastatic non-squamous NSCLC without EGFR or ALK genomic tumor aberrations based on these results. However, this strong combination model is associated with more toxicities and may impose a financial burden on patients. From the results of the subgroup analysis, the improvements to OS and PFS with ABCP were also observed among patients with low or negative PD-L1 expression, sensitizing EGFR mutations, baseline liver metastases, or KRAS mutations. Therefore, ABCP regimen is more likely to be given priority to rapidly control the disease for patients with good performance status and high TMB such as liver metastases. This regimen provides a new perspective for treating patients with EGFR-tyrosine kinase inhibitors resistance and KRAS mutations.
However, in another phase III LEAP 007 study,[47] the combination of pembrolizumab with lenvatinib failed to show improved OS compared with pembrolizumab alone in previously untreated metastatic NSCLC cases with PD-L1 tumor proportion score (TPS) ≥1%. This combination was associated with more grade 3 to 5 TRAEs. Although the combination showed clear advantages in PFS and ORR, this short-term anti-tumor effect did not translate into long-term survival benefits. OS remains the gold standard for reflecting patient benefit from trials. The LEAP 007 study set up two primary endpoints, OS and PFS, which made the statistical significance standard stricter and increased the difficulty of reaching the endpoint. The increased toxicity, reduced synergy in immunomodulatory effects, and lack of included chemotherapy in this combination require careful analysis. Compared with bevacizumab, lenvatinib is a multi-kinase inhibitor that may more strongly contribute to antitumor immunity. We believe pembrolizumab and lenvatinib are a potential combination, and more clinical trials including pembrolizumab plus lenvatinib with chemotherapy (LEAP 006, LEAP 008) in NSCLC are ongoing.
In addition, other combinations such as pembrolizumab plus ramucirumab,[48] sintilimab plus anlotinib,[49] and sitravatinib plus nivolumab[50] showed high antitumor activity in early clinical trials and may be potential regimens. Notably, a novel bispecific antibody targeting anti-PD-1 and VEGF-A (AK112) showed encouraging antitumor activity and safety in a phase I study.[51] The agent is assessed in the ongoing clinical trials.
Strategies to enhance cytotoxic T cells response
ACT
ACT belongs to passive immunotherapy. Autogenic or allogenic IC were prepared ex vivo through stimulation, expansion, and activation, and finally infused into cancer patients.
The use of tumor-infiltrating lymphocytes (TILs) is a promising therapeutic strategy in NSCLC patients according to early clinical trials. In a phase I pilot trial (NCT03215810), the efficacy and safety of nivolumab followed by TILs were assessed in ICI-naïve patients with metastatic NSCLC. The therapy was demonstrated to be safe and active with 23% (3 of 13) confirmed responses.[52] LN-145, a centrally manufactured autologous TIL cell product, showed activity in previously treated patients with advanced or metastatic NSCLC in the phase II IOV-COM-202 (NCT03645928) study. Although patients had received at least one prior line of therapy, 6 (21%) of them achieved responses and one patient experienced complete remission lasting beyond 20 months.[53] Currently, the efficacy and safety of LN-145 is being evaluated in the phase II IOV-LUN-202 study (NCT04614103) in metastatic NSCLC patients with one prior line of ICI and chemotherapy and without actionable driver mutations. However, a basic premise of TIL therapy is the availability of tumor tissues from patients, which limits its clinical application.
Another potential ACT strategy is a genetically engineered tumor-specific T cell therapy generated through the introduction of TCRs or transduction of synthetic chimeric antigen receptors (CARs). However, the lack of tumor-specific antigens is a significant barrier to the development of this type of therapy in solid tumors. Recently, a preliminary report from a phase I study (NCT02457650) showed that TCR-T cells targeting New York esophageal squamous cell carcinoma-1 (NY-ESO-1) were well tolerated and active in advanced NSCLC patients with HLA-A2 and NY-ESO-1 expression.[54] Additionally, CAR-T cells with new targets such as EGFR, mucin 1, mesothelin, carcinoembryonic antigen, and prostate stem cell antigen are being investigated in ongoing clinical trials and may help improve clinical outcomes for patients with advanced or metastatic NSCLC.[55]
There are also some adoptive cells that are developed non-specific to tumor-associated antigens. The efficacy and safety of adoptive autologous/allogeneic natural killer (NK) cells used alone or in combination with other therapies are being assessed in various trials. In a phase I/II clinical trial (NCT02843815), cryoablation followed by adoptive allogenic NK cell therapy improved ORR vs. cryoablation alone (63.3% vs. 43.3%) and had manageable safety profiles in advanced NSCLC.[56] Adoptive NK cell therapy along with ICIs can also be beneficial to advanced NSCLC patients. In the phase II study (NCT02843204), pembrolizumab plus allogeneic NK cells prolonged PFS (median PFS: 6.5 vs. 4.3 months; HR = 0.58; 95% CI: 0.38–0.89) and OS (median OS: 15.5 vs. 13.3 months; HR = 0.60; 95% CI: 0.39–0.92) compared with pembrolizumab alone in patients with previously treated advanced NSCLC and with PD-L1 TPS ≥1%.[57]
ACT using DC-cytokine-induced killer (CIK) cells is also a promising strategy. DCs present tumor antigens and activate early T cells. CIK cells represent a group of mixed cells (T cells, NK T cells, and NK cells) that are generated ex vivo. When co-cultured with CIKs, DCs can specifically present the antigen to the T cells and promote their proliferation. A meta-analysis of 28 randomized controlled trials revealed that DC-CIK cell therapy in advanced NSCLC cases could reconstruct immunity by increasing the levels of T cells, NK cells, and CIK cells in peripheral blood.[58] Adoptive DC-CIK cell therapy combined with chemotherapy is a promising strategy in patients with advanced NSCLC, and is associated with superior PFS and OS compared with chemotherapy or DC-CIK therapy alone.[59,60]
Many uncertainties remain regarding ACT, such as the number of activated IC, production process, safety, and support from government policy. Additionally, the means to avoid T cell exhaustion and ensure that the effector IC can infiltrate into the tumor site are difficulties that need to be addressed. Combination with other therapies is the likely future direction of ACT.
PD-1/PD-L1 inhibitors
PD-1 is mainly expressed on activated T cells. PD-L1 and PD-L2 expressed on TC are the ligands of PD-1. The interactions of PD-1 and its ligand can interrupt the activated immune system through activating the immunosuppressive downstream transduction pathway and thus resulting in the impaired function of T cells.[61] The emergence of PD-1/PD-L1 inhibitors has dramatically altered the landscape of NSCLC therapies. From the results of multiple clinical trials, PD-1/PD-L1 inhibitors have been recommended as a single agent or in combination with chemotherapy for advanced or metastatic driver gene-negative NSCLC patients.
Monotherapy
Single agent pembrolizumab has been approved by both the FDA and NMPA for use as a first-line therapy in PD-L1 positive (TPS ≥1%) advanced NSCLC patients without EGFR, ALK, or ROS1 aberrations. Approval is based on the results of KEYNOTE-024 and KEYNOTE-042 trials. Both were phase III randomized trials that compared pembrolizumab monotherapy with platinum-based chemotherapy in a first-line setting in patients with advanced NSCLC without EGFR/ALK aberrations. In the KEYNOTE-024 study,[62] enrolled patients had a PD-L1 TPS of ≥50%. The updated 5-year data showed that pembrolizumab illustrated superior outcomes compared with platinum-based chemotherapy (5-year OS rate of 31.9% vs. 16.3%). In addition, there were fewer TRAEs in patients receiving pembrolizumab monotherapy than chemotherapy.[63] The KEYNOTE-042 study further demonstrated the benefit of pembrolizumab monotherapy in patients with PD-L1 TPS ≥1%, and Chinese patients were also included in this study.[64,65] OS was significantly increased in the pembrolizumab group compared with the chemotherapy group (16.7 vs. 12.1 months, HR = 0.81, 95% CI: 0.71–0.93, P = 0.0018). However, the survival advantage of pembrolizumab was driven primarily by patients with PD-L1 TPS ≥50% (20.0 vs. 12.2 months, HR = 0.69, 95% CI: 0.56–0.85, P = 0.0003). In an analysis of a subgroup of patients with PD-L1 TPS of 1% to 49%, OS rates were similar between the two groups (13.4 vs. 12.1 months, HR = 0.92, 95% CI: 0.77–1.11). The safety profile was better than chemotherapy [Table 3].
Table 3.
Clinical trials of anti-PD-1/PD-L1 monotherapy in the first-line setting.
| Trial | PD-L1 assay | Phase | Histology | Design | PD-L1 status | ORR (%) | Median PFS (months) (HR, 95% CI) | Median OS (months) (HR, 95% CI) | ≥3 TRAE |
| Keynote-024 | Dako 22C3 | III | nsq/sq | Pembro (154) Chemo (151) | TPS ≥50% | 46.1% vs. 31.1% | 7.7 vs. 5.5 (HR 0.50; 0.39–0.65) |
26.3 vs. 13.4 (HR 0.62; 0.48–0.81) |
31.2% vs. 53.3% |
| Keynote-042 | Dako 22C3 | III | nsq/sq | Pembro (637) Chemo (637) |
TPS ≥1% | 27.3% vs. 26.5% | 5.4 vs. 6.5 (HR 1.07; 0.94–1.21) |
16.7 vs. 12.1 (HR 0.81; 0.71–0.93) |
18.0% vs. 41.0% |
| TPS ≥50% (subgroup) | 39.5% vs. 32.0% | 7.1 vs. 6.4 (HR 0.81; 0.67–0.99) |
20.0 vs. 12.2 (HR 0.69; 0.56–0.85) |
||||||
| TPS = 1%–49% (subgroup) | – | – | 13.4 vs. 12.1 (HR 0.92; 0.77–1.11) |
||||||
| IMpower110 | SP142 | III | nsq/sq | Atezo (277) Chemo (277) |
TC ≥1% or IC ≥1% | 29.2 vs. 31.8% | 5.7 vs. 5.5 (HR 0.77; 0.53–0.94) |
17.5 vs. 14.1 (HR 0.83; 0.65–1.07) |
33.9% vs. 56.7% |
| TC ≥50% or IC ≥10% (subgroup) | 38.3% vs. 28.6% | 8.1 vs. 5.0 (HR 0.63; 0.45–0.88) |
20.2 vs. 13.1 (HR 0.59; 0.40–0.89) |
||||||
| TC ≥5% or IC ≥5% (subgroup) | 30.7% vs. 32.1% | 7.2 vs. 5.5 (HR 0.67; 0.52–0.88) |
18.2 vs. 14.9 (HR 0.72; 0.52–0.99) |
||||||
| EMPOWER-Lung 1 | Dako22C3 | III | nsq/sq | Cemi (283) Chemo (280) |
TPS ≥50% | 39.2% vs. 20.4% | 8.2 vs. 5.7 (HR 0.54; 0.43–0.68) |
NR vs. 14.2 (HR 0.57; 0.42–0.77) |
14.1% vs. 39.2% |
| CheckMate-026 | Dako28–8 | III | nsq/sq | Nivo (211) Chemo (212) |
PD-L1 ≥5% | 26.1% vs. 33.5% | 4.2 vs. 5.9 (HR 1.15; 0.91–1.45) |
14.4 vs. 13.2 (HR 1.02; 0.80–1.30) |
17.6% vs. 50.6% (grade 3–4) |
| PD-L1 ≥50% (subgroup) | 34% vs. 39% | 5.4 vs. 5.8 (HR 1.07; 0.77–1.49) |
15.9 vs. 13.9 (HR 0.90; 0.63–1.29) |
||||||
| MYSTIC | SP263 | III | nsq/sq | Durva (163) Durva + treme (163) Chemo (162) |
TC ≥25% | 35.6% vs. 34.4% vs. 37.7% | 4.7 vs. 3.9 vs. 5.4 (HR 0.87; 99.5% CI, 0.59–1.29) (HR 1.05; 99.5% CI, 0.72–1.53) |
16.3 vs. 11.9 vs. 12.9 (HR 0.76; 97.54% CI, 0.56–1.02) (HR 0.85; 98.77% CI, 0.61–1.17) |
14.9% vs. 22.9% vs. 33.8% |
| TC ≥50% | – | – | 18.3 vs. 15.2 vs. 12.7 (HR 0.76; 0.55–1.04) (HR 0.77; 0.56–1.07) |
Atezo: Atezolizumab; cemi: Cemiplimab; chemo: Chemotherapy; CI: Confidence interval; durva: Durvalumab; HR: Hazard ratio; IC: Immune cells; nivo: Nivolumab; NR: Not reached; NSCLC: Non-small cell lung cancer; nsq: Non-squamous NSCLC; ORR: Objective response rate; OS: Overall survival; PD1: Programed cell death protein 1; PD-L1: Programed cell death 1 ligand 1; pembro: Pembrolizumab; PFS: Progression-free survival; sq: Squamous NSCLC; TC: Tumor cell; TPS: Tumor proportion score; TRAE: Treatment related adverse events; treme: Tremelimumab. “–” represents unavailability of data. TC3 or IC3: PD-L1 expression on ≥50% of TC or on ≥10% of IC; TC2/3 or IC2/3: PD-L1 expression on ≥5% of TC or IC; TC1/2/3 or IC1/2/3: PD-L1 expression on ≥1% of TC or IC. TC0 and IC0: PD-L1 expression on <1% of TC and IC.
Single-agent atezolizumab is another first-line ICI approved by the FDA and NMPA for metastatic NSCLC patients with high PD-L1 expression and without EGFR mutations or ALK fusions. In the phase III IMpower110 trial,[66] atezolizumab showed significantly longer OS compared with platinum-based chemotherapy (20.2 months vs. 13.1 months; HR = 0.59; 95% CI: 0.40–0.89; P = 0.01) in previously untreated metastatic NSCLC patients who had PD-L1 positive staining in >50% of TC (TC ≥50%) or in ≥10% of tumor-infiltrating IC (IC ≥10%). Another anti-PD-1 antibody monotherapy, cemiplimab, was approved by the FDA for advanced NSCLC patients with high PD-L1 expression (TPS ≥50%) based on the results of the EMPOWER-Lung 1 study.[67] This drug has not entered the Chinese market yet [Table 3].
However, two phase III clinical trials, CheckMate 026 and MYSTIC, failed to show improved PFS and OS in the nivolumab or durvalumab monotherapy groups compared with the chemotherapy group, even among the patients with high PD-L1 expression.[68,69] But in the CheckMate 026 study, the exploratory analysis showed that nivolumab was associated with a higher ORR (47% vs. 28%) and longer PFS (9.7 vs. 5.8 months; HR = 0.62; 95% CI: 0.38–1.00) than chemotherapy in patients with high TMB, which indicated that further excavation of predictive markers would allow for more suitable patients to be selected for immunotherapy [Table 3].
Despite different detection and scoring algorithms of PD-L1 expression among the above mentioned clinical trials, these results suggest that single-agent PD-1/PD-L1 inhibitors as first-line treatments have superior survival benefits and lower toxicity compared with platinum-based chemotherapy among certain groups. Now, National Comprehensive Cancer Network (NCCN) guidelines recommend single agent pembrolizumab, atezolizumab, or cemiplimab as a first-line treatment in eligible advanced NSCLC patients with high PD-L1 expression (≥50%). For patients with a TPS of 1% to 49% who refuse or cannot tolerate chemotherapy, pembrolizumab monotherapy is an alternative strategy for its superior safety and similar efficacy.
In previously treated NSCLC cases, evidence suggests that ICI monotherapies are associated with significant activity. The CheckMate-017[70] and CheckMate-057[71] are the first two phase III clinical trials showing an improved survival and favorable safety of nivolumab over docetaxel in Caucasians. The pooled analysis of these trials reported that[72] a considerable proportion of the patients had long-term OS benefits: nivolumab showed 5-fold increase in OS rate (13.4% vs. 2.6%) regardless of the PD-L1 expression status or histological type of the tumor. The CheckMate-078[73] study mainly focused on a Chinese patient population, and the results were consistent with the data from the global CheckMate-017/057 studies. Nivolumab significantly prolonged OS compared with docetaxel (11.9 vs. 9.5 months; HR = 0.75; 95% CI: 0.61–0.93), and the estimated 3-year OS rates were 19% with nivolumab and 12% with docetaxel.[74] From these studies, both the FDA and NMPA approved nivolumab as a second-line treatment for advanced NSCLC cases that are negative for EGFR and ALK variants. [Table 4].
Table 4.
Clinical trials of anti-PD-1/PD-L1 monotherapy in second-line.
| Trial | PD-L1 assay | Phase | Histology | Design | PD-L1 status | ORR (%) | Median PFS (months) (HR, 95% CI) | Median OS (months) (HR, 95% CI) | ≥3 TRAE |
| Keynote-017 | Dako28-8 | III | sq | Nivo (135) DOC (137) |
PD-L1 ≥1% and <1% | 20% vs. 9% | 3.5 vs. 2.8 (HR 0.62; 0.47–0.81) |
9.2 vs. 6.0 (HR 0.59; 0.44–0.79) |
7% vs. 57% |
| PD-L1 <1% (subgroup) | 9% vs. 5% | 3.1 vs. 3.0 (HR 0.66; 0.43–1.00) |
8.7 vs. 5.9 (HR 0.58; 0.37–0.92) |
||||||
| PD-L1 ≥1% (subgroup) | 11% vs. 6% | 3.3 vs. 2.8 (HR 0.67; 0.44–1.00) |
9.3 vs. 7.2 (HR 0.69; 0.45–1.10) |
||||||
| Keynote-057 | Dako28-8 | III | nsq | Nivo (292) DOC (290) |
PD-L1 ≥1% and <1% | 19% vs. 12% | 2.3 vs. 4.2 (HR 0.92; 0.77–1.11) |
12.2 vs. 9.4 (HR 0.73, 0.59–0.89) |
10% vs. 54% |
| PD-L1 <1% (subgroup) | 10% vs. 15% | 2.1 vs. 3.6 (HR 1.19; 0.88–1.61) |
10.5 vs. 10.1 (HR 0.87; 0.63–1.19) |
||||||
| PD-L1 ≥1% (subgroup) | 38% vs. 15% | 4.2 vs. 4.5 (HR 0.70; 0.53–0.94) |
17.7 vs. 9.0 (HR 0.58; 0.43–0.79) |
||||||
| Keynote-078 | Dako28-8 | III | nsq/sq | Nivo (338) DOC (166) |
PD-L1 ≥1% and <1% | 18% vs. 4% | 2.8 vs. 2.8 (HR 0.79; 0.65–0.98); |
11.9 vs. 9.5 (HR 0.75; 0.61–0.93); |
12% vs. 47% (grade 3–4) |
| PD-L1 <1% (subgroup) | 18.1% vs. 3.0% | 2.9 vs. 2.8 (HR 0.78; 0.56–1.07) |
11.4 vs. 10.2 (HR 0.76; 0.55–1.02) |
||||||
| PD-L1 ≥1% (subgroup) | 17.3% vs. 6.0% | 2.8 vs. 2.6 (HR 0.74; 0.55–0.98) |
12.0 vs. 7.9 (HR 0.70; 0.53–0.92) |
||||||
| OAK | SP142 | III | nsq/sq | Atezo (613) DOC (612) |
TC 0/1/2/3 IC 0/1/2/3 |
14% vs. 13% | 2.8 vs. 4.0 (HR 0.95; 0.82–1.10) |
13.3 vs. 9.8 (HR 0.78; 0.68–0.89) |
15.3% vs. 42.6% |
| TC 3 or IC 3 (subgroup) | 30.6% vs. 10.8% | 4.2 vs. 3.3 (HR 0.63; 0.43–0.91) |
20.5 vs. 9.7 (HR 0.50; 0.36–0.71) |
||||||
| TC 0 and IC 0 (subgroup) | 7.8% vs. 10.6% | 2.6 vs. 4.0 (HR 1.00; 0.80–1.25) |
11.8 vs. 8.9 (HR 0.78; 0.65–0.94) |
||||||
| KEYNOTE-010 | Dako22C3 | II/III | nsq/sq | Pembro (691) DOC (343) |
TPS ≥1% | 21.2% vs. 9.6% | 4.0 vs. 4.1 (HR 0.84; 0.73–0.96) |
11.8 vs. 8.4 (HR 0.70; 0.61–0.80) |
16.1% vs. 36.6% |
| TPS ≥50% | 33.1% vs. 9.2% | 5.3 vs. 4.2 (HR 0.57; 0.46–0.71) |
16.9 vs. 8.2 (HR 0.55; 0.44–0.69) |
||||||
| JAVELIN Lung 200 | 73–10 | III | nsq/sq | Avelu (264) DOC (265) |
PD-L1 ≥1% | 18.9% vs. 11.7% | 3.4 vs. 4.1 (HR 1.01; 0.80–1.28) |
11.4 vs. 10.3 (HR 0.90; 0.72–1.12) |
9.9% vs. 49.3% |
| RATIONALE 303 | SP263 | III | nsq/sq | TIS (535) DOC (270) |
PD-L1 ≥1% and <1% | 21.9% vs. 7.1% | 4.1 vs. 2.6 (HR 0.64; 0.53–0.76) |
17.2 vs. 11.9 (HR 0.64; 0.53–0.78) |
14.4% vs. 66.3% |
Atezo: Atezolizumab; Avelu: Avelumab; CI: Confidence interval; cemi: Cemiplimab; DOC: Docetaxel; HR: Hazard ratio; IC: Immune cells; Nivo: Nivolumab; NR: Not reached; nsq: Non-squamous NSCLC; ORR: Objective response rate; OS: Overall survival; PD1: Programed cell death protein 1; PD-L1: Programed cell death 1 ligand 1; pembro: Pembrolizumab; PFS: Progression-free survival; sq: Squamous NSCLC; TC: Tumor cell; TIS: Tislelizumab; TPS: Tumor proportion score; TRAE: Treatment related adverse events. TC3 or IC3: PD-L1 expression on ≥50% of TC or on ≥10% of IC; TC2/3 or IC2/3: PD-L1 expression on ≥5% of TC or IC; TC1/2/3 or IC1/2/3: PD-L1 expression on ≥1% of TC or IC. TC0 and IC0: PD-L1 expression on <1% of TC and IC.
Likewise, second-line atezolizumab was approved by the FDA based on the phase III OAK study.[75] The study demonstrated that second-line atezolizumab therapy was associated with improved OS compared with docetaxel across histological type and PD-L1 expression subgroups (13.3 months vs. 9.8 months; HR = 0.78; 95% CI: 0.68–0.89). The 4-year OS rates were 15.5% and 8.7%, respectively[76] [Table 4].
However, the KEYNOTE-010[77] study assessed the efficacy of second-line pembrolizumab treatment only in patients with positive PD-L1 expression. The OS of the two pembrolizumab dose group (2 and 10 mg/kg) was significantly longer than that of the docetaxel group, especially in the subgroup with PD-L1 TPS ≥50%. The 5-year OS rates of the pooled analysis of two pembrolizumab doses were 15.6% vs. 6.5% in patients with PD-L1 TPS ≥1% and 25.0% vs. 8.2% in those with PD-L1 TPS ≥50%.[78] These data led to FDA approval of pembrolizumab in a second-line setting for PD-L1 positive NSCLC patients [Table 4].
Another anti-PD-1 antibody tislelizumab (made in China) was assessed in a phase III clinical trial (RATIONALE 303), which met its primary endpoint of OS (17.2 months with tislelizumab vs. 11.9 months with docetaxel; HR = 0.52; 95% CI: 0.38–0.71) regardless of baseline PD-L1 expression and histology, which was reported at the American Association of Cancer Research Annual Meeting in 2021.[79] However, the phase III study JAVELIN Lung 200 did not show any consistent OS improvement with avelumab compared with docetaxel in previously treated NSCLC patients with PD-L1 positive expression.[80] In addition, durvalumab[81] and camrelizumab (made in China)[82] exhibited significant activity in previously treated NSCLC patients in early clinical studies (NCT01693562 and NCT03085069). The effects were enhanced in NSCLC cases with higher levels of PD-L1 expression [Table 4].
From the above data and FDA approvals, nivolumab and atezolizumab are recommended by both NCCN guidelines as subsequent treatment options for patients with metastatic NSCLC irrespective of histology and PD-L1 expression status. Pembrolizumab is recommended to patients with PD-L1 expression levels of ≥1%. In addition to these recommendations, tislelizumab is also recommended by Chinese Society of Clinical Oncology (CSCO) guidelines as a second-line therapy.
Combination of PD-1/PD-L1 inhibitors and chemotherapy
Research has shown that chemotherapy can induce immunogenic cell death to initiate the anti-tumor immune response,[83] and increase the proportion of immunologic effector cells and immunosuppressive IC in the TME,[84] as well as upregulate the immune checkpoints.[85] Therefore, strategies for combining PD-1/PD-L1 inhibitors with chemotherapies can lead to enhanced efficacy.
The phase III Keynote-189 and Keynote-407 trials assessed the efficacy and safety, respectively, of pembrolizumab combined with chemotherapy in NSCLC regardless of PD-L1 expression levels. The data revealed that the addition of pembrolizumab to platinum-based chemotherapy could prolong OS and PFS compared with chemotherapy alone and was generally well tolerated. The updated 3-year outcome of the two studies[86,87] showed durable survival benefits of this combined therapy compared with chemotherapy (3-year OS rate was 31.3% vs. 17.4% in non-squamous NSCLC; 29.7% vs. 18.2% in squamous NSCLC). Pembrolizumab plus chemotherapy has now been approved by the FDA and NMPA as the first-line treatment in patients with non-squamous or squamous NSCLC [Table 5].
Table 5.
Clinical trials of the combination of anti-PD-1/PD-L1 with chemotherapy in the first-line setting.
| Trial | PD-L1 assay | Phase | Histology | Design | PD-L1 status | ORR (%) | Median PFS (months) (HR, 95% CI) | Median OS (months) (HR, 95% CI) | ≥3 TRAE |
| Keynote-189 | Dako22C3 | III | nsq | Pembro + PEM + platin (410) PEM + platin (206) |
TPS ≥1% and <1% | 48.3% vs. 19.9% | 9.0 vs. 4.9 (HR 0.50; 0.41–0.59) |
22.0 vs. 10.6 (HR 0.60; 0.50–0.72) |
52.1% vs. 42.1% |
| TPS <1% (subgroup) | 33.1% vs. 14.3% | 6.2 vs. 5.1 (HR 0.68; 0.49–0.93) |
17.2 vs. 10.2 (HR 0.52; 0.37–0.72) |
||||||
| TPS 1–49% (subgroup) | 50.0% vs. 20.7% | 9.4 vs. 4.9 (HR 0.54; 0.39–0.76) |
21.8 vs. 12.1 (HR 0.66; 0.47–0.93) |
||||||
| TPS ≥50% (subgroup) | 62.1% vs. 25.7% | 11.1 vs. 4.8 (HR 0.50; 0.41–0.59) |
27.7 vs. 10.1 (HR 0.71; 0.50–1.00) |
||||||
| Keynote-407 | Dako22C3 | III | sq | Pembro + TAX + CBP (278) TAX + CBP (281) |
TPS ≥1% and <1% | 62.6% vs. 38.8% | 8.0 vs. 5.1 (HR 0.59; 0.49–0.71) |
17.2 vs. 11.6 (HR 0.71; 0.59–0.86) |
74.8% vs. 70.0% |
| TPS <1% (subgroup) | 67.4% vs. 41.4% | 6.3 vs. 5.9 (HR 0.68; 0.50–0.93) |
15.0 vs. 11.0 (HR 0.78; 0.57–1.07) |
||||||
| TPS ≥1% (subgroup) | 59.1% vs. 37.3% | 8.3 vs. 4.6 (HR 0.53; 0.42–0.67) |
18.7 vs. 12.8 (HR 0.68; 0.53–0.87) |
||||||
| IMpower 130 | SP142 | III | nsq | Atezo + nP + CBP (483; WT 451) nP + CBP (240; WT 228) |
TC 0/1/2/3 IC 0/1/2/3 |
49.2% vs. 31.9% | 7.0 vs. 5.5 (HR 0.64; 0.54–0.77) |
18.6 vs. 13.9 (HR 0.79; 0.64–0.98) |
74.8% vs. 60.8% |
| TC3 or IC3 (subgroup) | – | 6.4 vs. 4.6 (HR 0.51; 0.34–0.77) |
17.3 vs. 16.9 (HR 0.84; 0.51–1.39) |
||||||
| TC1/2 or IC1/2 (subgroup) | – | 8.3 vs. 6.0 (HR 0.61; 0.43–0.85) |
23.7 vs. 15.9 (HR 0.70; 0.45–1.08) |
||||||
| TC0 and IC0 (subgroup) | – | 6.2 vs. 4.7 (HR 0.72; 0.56–0.91) |
15.2 vs. 12.0 (HR 0.81; 0.61–1.08) |
||||||
| IMpower 132 | SP142 | III | nsq | Atezo + PEM + CBP (292) | TC 0/1/2/3 IC 0/1/2/3 |
47% vs. 32% | 7.6 vs. 5.2 (HR 0.60; 0.47–0.72) |
17.5 vs. 13.6 (HR 0.86; 0.71–1.06) |
58.4% vs. 43.1% |
| PEM + CBP (286) | TC3 or IC3 (subgroup) | 72% vs. 55% | 10.8 vs. 6.5 (HR 0.46; 0.22–0.96) |
NR vs. 26.9 (HR 0.73; 0.31–1.73) |
|||||
| TC1/2 or IC1/2 (subgroup) | 38% vs. 38% | 6.2 vs. 6.7 (HR 0.80; 0.56–1.16) |
12.7 vs. 16.2 (HR 1.18; 0.80–1.76) |
||||||
| TC0 and IC0 (subgroup) | 44% vs. 27% | 8.5 vs. 4.9 (HR 0.45; 0.31–0.64) |
15.9 vs. 10.5 (HR 0.67; 0.46–0.96) |
||||||
| IMpower 131 | SP142 | III | sq | Atezo + nP + CBP (343) nP + CBP (340) |
TC 0/1/2/3 IC 0/1/2/3 |
49.7% vs. 41.0% | 6.3 vs. 5.6 (HR 0.71; 0.60–0.85) |
14.2 vs. 13.5 (HR 0.88; 0.73–1.05) |
69.2% vs. 58.4% |
| TC3 or IC3 (subgroup) | 61.7% vs. 31.8% | 10.1 vs. 5.1 (HR 0.41; 0.25–0.68) |
23.4 vs. 10.2 (HR 0.48; 0.29–0.81) |
||||||
| TC1/2 or IC1/2 (subgroup) | 52.6% vs. 43.5% | 6.5 vs. 5.6 (HR 0.70; 0.54–0.91) |
12.8 vs. 15.5 (HR 1.08; 0.81–1.45) |
||||||
| TC0 and IC0 (subgroup) | 43.8% vs. 41.5% | 5.7 vs. 5.6 (HR 0.82; 0.65–1.04) |
14.0 vs. 12.5 (HR 0.87; 0.67–1.03) |
||||||
| CameL | Dako22C3 | III | nsq | Camre + PEM + CBP (205) PEM + CBP (207) |
TPS ≥1% and <1% | 60.5% vs. 38.6% | 11.3 vs. 8.3 (HR 0.60; 0.45–0.79) |
NR vs. 20.9 (HR 0.73; 0.53–1.02) |
69% vs. 47% |
| CameL-sq | Dako22C3 | III | sq | Camre + TAX + CBP (193) TAX + CBP (196) |
TPS ≥1% and <1% | 64.8% vs. 36.7% | 8.5 vs. 4.9 (HR 0.37; 0.29–0.47) |
NR vs. 14.5 (HR 0.55; 0.40–0.75) |
73.6% vs. 71.9% |
| RATIONALE 304 | SP263 | III | nsq | TIS + PEM + platin (223) PEM + platin (111) |
PD-L1 ≥1% and <1% | 57.4% vs. 36.9% | 9.7 vs. 7.6 (HR 0.65; 0.46–0.90) |
– | 67.6% vs. 53.6% |
| RATIONALE 307 | SP263 | III | sq | A: TIS + TAX + CBP (120) B: TIS + nP + CBP (118) C: nP + CBP (117) |
PD-L1 ≥1% and <1% | 73% vs. 75% vs. 50% | A vs. C: 7.6 vs. 5.5 (HR 0.52; 0.37–0.74) B vs. C: 7.6 vs. 5.5 (HR 0.48; 0.34–0.68) |
– | 85.8% vs. 83.9% vs. 80.3% |
| ORIENT-11 | Dako22C3 | III | nsq | Sinti + PEM + platin (266) PEM + platin (131) |
TPS ≥1% and <1% | 51.9% vs. 29.8% | 8.9 vs. 5.0 (HR 0.48; 0.36–0.64) |
NR vs. NR (HR 0.61; 0.40–0.93) |
61.7% vs. 58.8% |
| ORIENT-12 | Dako22C3 | III | sq | Sinti + GEM + platin (179) GEM + platin (178) |
TPS ≥1% and <1% | 44.7% vs. 35.4% | 5.5 vs. 4.9 (HR 0.54; 0.42–0.68) |
NR vs. NR (HR 0.57; 0.35–0.91) |
86.6% vs. 83.1% |
| CHOICE-01 | JS311 | III | nsq/sq | Tori + chemo (309) Chemo (156) |
TC ≥1% and <1% | 65.7% vs. 46.2% | 8.4 vs. 5.6 (HR 0.49; 0.39–0.61) |
NR vs. 17.1 (HR 0.69; 0.52–0.92) |
78.6% vs. 82.1% |
| GEMSTONE-302 | SP263 | III | nsq | Suge + PEM + CBP (191) PEM + CBP (96) |
PD-L1 ≥1% and <1% | 58.6% vs. 36.5% | 9.6 vs. 5.8 (HR 0.59; 0.45–0.79) |
22.8 vs. 17.7 (HR 0.67; 0.50–0.90) |
56.9% vs. 57.2% |
| sq | Suge + TAX + CBP (129) TAX + CBP (63) |
70.5% vs. 46.0% | 8.3 vs. 4.8 (HR 0.34; 0.24–0.48) |
Atezo: Atezolizumab; Camre: Camrelizumab; CBP: Carboplatin; CI: Confidence interval; EGFR: Epidermal growth factor receptor; GEM: Gemcitabine; HR: Hazard ratio; IC: Immune cells; nivo: Nivolumab; nP: Albumin-bound paclitaxel; NR: Not reached; NSCLC: Non-small cell lung cancer; nsq: Non-squamous NSCLC; ORR: Objective response rate; OS: Overall survival; PD1: Programed cell death protein 1; PD-L1: Programed cell death ligand 1; PEM: Pemetrexed; pembro: Pembrolizumab; PFS: Progression-free survival; platin: Platinum; Sinti: Sintilimab; sq: Squamous NSCLC; Suge: Sugemalimab; TAX: Paclitaxel; TC: Tumor cell; TIS: Tislelizumab; Tori: Toripalimab; TPS: Tumor proportion score; TRAE: Treatment related adverse events; WT: EGFR or ALK wild type. “–” represents unavailability of data. TC3 or IC3: PD-L1 expression on ≥50% of TC or on ≥10% of IC; TC2/3 or IC2/3: PD-L1 expression on ≥5% of TC or IC; TC1/2/3 or IC1/2/3: PD-L1 expression on ≥1% of TC or IC. TC0 and IC0: PD-L1 expression on <1% of TC and IC.
IMpower 130[88] and IMpower 132[89] trials evaluated the efficacy and safety of atezolizumab in combination with platinum-based chemotherapy (carboplatin plus nab-paclitaxel in IMpower130; pemetrexed plus a platinum-based drug in IMpower 132) in patients with treatment-naïve metastatic non-squamous NSCLC. Both studies showed that the addition of atezolizumab to chemotherapy resulted in improved OS and PFS. The subgroup analysis of IMpower130 indicated that the majority of clinical subgroups could benefit from the combination therapy, except the subgroup with liver metastases and EGFR or ALK genomic alterations. The IMpower131[90] study involved patients with squamous NSCLC. The results showed longer PFS in the atezolizumab + carboplatin + nab-paclitaxel group compared with the chemotherapy group. OS changes were not statistically significant, except in the PD-L1 high expression subgroup. From the results of this study, the FDA has approved atezolizumab in combination with chemotherapy (nab-paclitaxel + carboplatin) as a first-line treatment for metastatic non-squamous NSCLC with wild-type EGFR/ALK [Table 5].
According to the data from these clinical trials and FDA approvals, both the NCCN and CSCO guidelines recommend the combination of pembrolizumab with pemetrexed plus platinum (cisplatin or carboplatin) and atezolizumab with nab-paclitaxel plus carboplatin as first-line therapies for metastatic non-squamous NSCLC. For squamous NSCLC, the guidelines recommend the combination of pembrolizumab with paclitaxel or albumin-bound paclitaxel plus carboplatin.
Moreover, phase III clinical trials have suggested that an increasing number of PD-1/PD-L1 inhibitors made in China, including camrelizumab,[91,92] tislelizumab,[93,94] sintilimab,[95,96] toripalimab,[97] and sugemalimab,[98] showed superior efficacy when combined with chemotherapy compared with chemotherapy alone [Table 5]. Until now, the former four agents combined with chemotherapy have been approved by the NMPA for first-line treatment in patients with non-squamous and squamous metastatic NSCLC, independent of PD-L1 expression levels.
Determining the best regimen among the numerous PD-1/PD-L1 inhibitors requires further study. (1) Anti-PD-1 vs. anti-PD-L1: A recent large meta-analysis that included 19 randomized clinical trials showed that anti-PD-1 is associated with a statistically significant improved OS and comparable toxicities compared with anti-PD-L1 agents in patients with solid tumors, including NSCLC.[99] However, there have been no head-to-head studies comparing the differences between anti-PD-1 and anti-PD-L1 agents. (2) Comparisons of anti-PD-1 agents: There are subtle differences in the structural designs of PD-1 monoclonal antibodies, which may lead to different antitumor activities and toxicity profiles. The abovementioned clinical trials indicated similar clinical efficacies and toxicity profiles between anti-PD-1 agents. Yet, only head-to-head clinical studies will be able to answer this question accurately. Recently, a head-to-head phase II study (CTONG1901) with a small sample size compared the efficacy and safety profiles between sintilimab ± chemotherapy (n = 34) and pembrolizumab ± chemotherapy (n = 34). The results indicated a similar response (ORR: 51.6% vs. 30.3%) and safety profile between the two agents, but the survival data were not yet complete.[100] (3) Whether chemotherapy is necessary in patients with PD-L1 score ≥50%: Patients with high PD-L1 expression levels are a special group that highly benefit from anti-PD-(L)1 therapies and account for 24% to 30% of advanced NSCLC patients. The necessity of chemotherapy is unclear in this population. A 2022 FDA pooled analysis showed that combination chemotherapy with ICIs had survival advantages over ICIs in patients aged ≤65 years, but might not improve survival in patients aged ≥75 years. These results indicate that an adequate assessment of risks and benefits and establishing a risk assessment model based on clinical characteristics could help facilitate better treatment decisions.[101] (4) Which combination is better: Although anti-PD-(L)1 strategy has already reached a consensus in advanced or metastatic NSCLC patients without driver gene alterations, a proportion of patients still cannot benefit from the therapy. The purpose of combination therapy is to further expand the beneficiary population. The current combinations, however, have not achieved the effect of 1 + 1 > 2, which may be attributed to the complicated interactions of immune regulatory networks in the TME. Understanding the relationship between immune pathways may help clinicians choose the best treatment combination.
Other ICIs or immune co-stimulator (ICOS) agonist
Currently, multiple ICIs or ICOS agonists have been developed in addition to PD-(L)1 and CTLA-4 inhibitors [Table 6]. They are promising therapeutic targets for immunotherapy of advanced or metastatic NSCLC. The preclinical researches showed that they work synergistically with PD-1 to suppress antitumor immunity,[102,103] and thus the addition of these ICIs to PD-1/PD-L1 inhibitors was supposed to improve the anti-tumor activity in patients lacking the response to PD-1/PD-L1 inhibitors.
Table 6.
Immune checkpoints and costimulatory receptors in IC.
| Target | Location | Ligand | Inhibitor |
| CTLA-4 | Activated T cells, Treg cells, NK cells, and DCs | CD80/CD86 | Ipilimumab (Yervoy), tremelimumab, BMS-986218, BMS-986249, AGEN1181, AGEN 1184, etc. |
| PD-1 | T cells, B cells, NK cells, and NKT cells | PD-L1 and PD-L2 | Nivolumab (Opdivo), pembrolizumab (Keytruda), cemiplimab (Libtayo), toripalimab, sintilimab, camrelizumab, tislelizumab, penpulimab, zimberelimab, etc. |
| PD-L1 | TC, antigen presenting cells, T lymphocytes, endothelial cells, and fibroblasts | PD-1 | Atezolizumab (Tecentriq), durvalumab (Imfinzi), avelumab (Bavencio), envafolimab, sugemalimab, etc. |
| LAG-3 | Activated T cells, B cells, NK cells, Treg cells, and DCs | MHC II, FGL1, LSECtin, and Galectin-3 | Relatilimab, eftilagimod alpha, MK-4280 (favezelimab), LAG525, REGN3767 (fianlima), BI 754111, LBL-007, IBI110, etc. |
| TIGIT | T cells, NK cells, and Treg cells | CD155 and CD112 | RG6058 (tiragolumab), MK-7684 (vibostolimab), ociperlimab, BMS-986207, OMP-313M32, AB154, ASP8374, etc. |
| TIM-3 | Activated T cells, B cells, NK cells, Treg cells, monocytes, macrophages, and mast cells | Galectin 9, HMGB1, phosphatidyl serine, and ceacam-1 | TSR-022, MBG453, sym023, RO7121661, LY3321367, INCAGN2390, SHR-1702, BMS-986258, etc. |
| VISTA | T cells, neutrophils, and macrophages | VSIG-3 | JNJ-61610588, CA-170, etc. |
| B7H3 | Activated T cells, NK cells, DCs, monocytes, macrophages, and TC | Unknown | Omburtamab, MGC018, MGA271, MGD009, etc. |
| BTLA | T cells, B cells, macrophages, NK cells, and DCs | HVEM | TAB 004 |
| NKG2A | NK cells, NKT cells, and CD8+ T cells | HLA-E | Monalizumab |
| CD47 | Macrophages and DCs | SIRPα | Hu5F9-G4, CC-90002, ALX148, etc. |
| OX40 (CD134) | Activated T-cells, neutrophils, and NK cells | OX40L (CD252) | PF-04518600, KHK4083, GBR830, BMS-986178, MEDI-6469, ABBV-368, etc. |
| 4-1BB (CD137) | Activated T-cells, NK cells, | 4-1BBL (CD137L) | Urelumab (BMS-663513), utomilumab (PF-05082566), etc. |
| CD40 | DC, B cells, and macrophages, granulocytes, endothelial cells, smooth muscle cells, fibroblasts, and epithelial cells | CD40L (CD154) | Dacetuzumab, selicrelumab, APX005M, mitazalimab, etc. |
| GITR | T cells, Treg cells, and NK cells | GITRL | INCAGN01876, BMS 986156, AMG228, MK-1248, GWN-323, TRX518, IBI102, etc. |
BTLA: B and T lymphocyte attenuator; CTLA-4: Cytotoxic T-lymphocyte-associated protein 4; DC: Dendritic cells; FGL1: Fibrinogen-like protein 1; GITR: Glucocorticoid-induced TNF receptor; HLA-E: Human leukocyte antigen E; HMGB1: High-mobility group protein B1; HVEM: Herpesvirus entry mediator; IC: Immune cells; LAG3: Lymphocyte-activation gene 3; MHC II: Major histocompatibility complex II; NK: Natural killer; NKG2A: Natural Killer group protein 2A; OX40L: Tumor necrosis factor receptor superfamily member 4 ligand; PD-1: Programed cell death protein 1; PD-L1: Programed cell death ligand 1; SIRPα: Signal regulatory protein-α; TC: Tumor cell; TIGIT: T cell immunoreceptor with Ig and ITIM domain; TIM-3: T cell immunoglobulin domain and mucin domain-3; Treg: Regulatory T; VISTA: V-domain immunoglobulin suppressor of T-cell activation; VSIG-3: V-set and immunoglobulin domain containing 3.
Lymphocyte activation gene-3 (LAG-3) is mainly expressed on exhausted tumor-infiltrating T cells including CD4+ T cells and CD8+ T cells[104] and can transduce inhibitory signals on activated CD8+ T cells.[105] Eftilagimod alpha, a soluble LAG-3 protein, was demonstrated to be safe when combined with pembrolizumab in first-line metastatic NSCLC regardless of PD-L1 expression in a recent phase II study (TACTI-002 study, NCT03625323). And this combination exhibited encouraging antitumor activity in the evaluable patients (ORR 41.8%),[106] and is thus worthy of further evaluation. Other inhibitors targeting LAG-3, such as monoclonal antibody BI754111 and PD-1-LAG3 bispecific antibody RO7247669, are being assessed in ongoing clinical trials (NCT 03156114 and NCT 04140500).
Like LAG-3, T-cell immunoglobulin and ITIM domain (TIGIT) can also be detected on CD8+ exhausted tumor-infiltrating T cells. The immunosuppressive effects of TIGIT are complicated through interruption of TCR expression and blocking binding of its co-stimulatory counterpart, thus resulting in inhibition of proliferation and activation of CD8+ T cells.[107] In a phase 2 study (CITYSCAPE, NCT 03563716), tiragolumab (an anti-TIGIT antibody) plus atezolizumab showed a significant improvement in ORR (31.3% vs. 16.2%) and PFS (5.4 vs. 3.6 months; HR 0.57; 95% CI: 0.37–0.90) compared with atezolizumab monotherapy in first-line treatment in PD-L1 positive recurrent or metastatic NSCLC patients.[108] But the Roche revealed the latest news that this combination failed to meet its co-primary endpoint of PFS in metastatic NSCLC patients with high PD-L1 expression. Another TIGIT inhibitor, vibostolimab, was demonstrated to be well tolerated and active with a 26% confirmed response when combined with pembrolizumab in anti-PD-1/PD-L1 naïve NSCLC patients with advanced or metastatic disease in a phase I study (NCT 02964013).[109] The combination of vibostolimab and pembrolizumab is investigated in metastatic NSCLC patients in an ongoing phase III clinical trial (NCT 05226598). Ociperlimab (made in China) plus tislelizumab is also a promising combination in advanced or metastatic NSCLC, and this is assessed in another ongoing clinical trial (NCT 04746924).
Co-stimulatory receptors (OX40, 4-1BB, CD40, glucocorticoid-induced tumor necrosis factor receptor [GITR], and others) play important roles in enhancing anti-tumor immunity in multiple ways including T-cell priming and activation, T-cell differentiation and survival, and effector function of T-cells.[110–113] Preclinical studies showed that co-stimulatory pathways could improve the antitumor responses induced by anti-CTLA-4 or anti-PD-1 therapies.[114,115] In addition, an interesting preclinical study found that sequentially administering anti-OX40 followed by anti-PD-1 was associated with significant anti-tumor activity compared with the reverse order or concurrent combination.[116] This result might suggest the rational sequence should be considered in clinical trials. Currently, some ICOS agonists, such as PF-04518600 (target OX40), INBRX-105 (target 4-1BB + PD-L1), APX005M (target CD40), and INCAGN01876 (target GITR), are being investigated in solid tumor including NSCLC in early phase trials.
Targeting these molecules presents promising strategies [Table 6]. But the role of these molecules in tumor immune escape has remained obscure, thus warranting further study.
Targeting rate-limiting enzyme in TME
CD73, a nucleotidase, is able to catalyze dephosphorylation of adenosine monophosphate to generate adenosine, which acts as an immunosuppressive mediator in the TME.[117] Oleclumab can block CD73 to reduce the production of extracellular adenosine, resulting in an enhanced antitumor response. In the phase II COAST study,[118] which had a similar research setting to the PACIFIC study,[119] oleclumab was combined with durvalumab to bring clinical benefit with increased ORR (30.0% vs. 17.9%) and prolonged PFS (not reached vs. 6.3 months; HR = 0.44; 95% CI: 0.26–0.75) compared with durvalumab monotherapy in patients with unresectable, stage III NSCLC who have not progressed after concurrent chemoradiotherapy. The safety profiles of both were similar.
Indoleamine 2,3-dioxygenase 1 (IDO1) is a rate-limiting enzyme that catalyzes the first step of tryptophan catabolism. This process leads to the depletion of l-tryptophan and then impaired T cell functions.[120] IDO inhibitors, including indoximod and epacadostat, combined with chemotherapy or ICI are being assessed in ongoing clinical trials.
Other potential immunotherapies
Gut microbiome
Some researchers have found that the diversity of the gut microbiome can influence patient responses to ICIs.[121,122] The diversity of the gut microbiome is a potential biomarker for predicting the efficacy of ICIs. Intervening with the gut microbiome, for example with oral probiotic supplements, is a potential strategy of immunotherapy. The relationship between the use of antibiotics and efficacy of ICIs in NSCLC remains controversial, as revealed by two recent pooled analyses of clinical trials.[123,124] The regulatory mechanisms of how the microbiome can affect the host's immune system require further investigation.
Cytokine-based therapy
Cytokines are important mediators of cell communication that can promote or inhibit immune responses against cancer.[125] Early cytokine therapies are believed to be ineffective because of their short half-life and severe toxicity.[126] To overcome these limitations, improved cytokines have been combined with polyethylene glycol or another fusion protein. The combination of cytokine therapy with other therapy types is now a mainstream strategy.[127] To date, the combinations of modified IL-2 (bempegaldesleukin) and IL-15 (ALT-803) have been shown to be safe with synergistic effects in metastatic NSCLC in early stage clinical trials,[128,129] but the efficacy has not been fully evaluated. Anti-cytokine therapies are also noteworthy. Canakinumab (anti-IL-1β antibody) and M7824 a (bifunctional fusion protein targeting PD-L1 and transforming growth factor-β) are two promising agents, although both of their phase III clinical trials did not meet primary endpoints (NCT03626545 and NCT03631706). Further studies are ongoing with these agents.
Discussion
In this review, we only focused on immunotherapy in advanced or metastatic NSCLC cases, while peri-operative (adjuvant/neoadjuvant) immunotherapy in resectable NSCLC and immunotherapy after radical chemoradiotherapy in unresectable stage III NSCLC were not discussed here. Immunotherapy is a very highly studied research area. A large number of immunotherapy drugs have entered into clinical application, bringing more treatment options to NSCLC patients with advanced or metastatic disease. However, there are still many challenges.
The first challenge is the design of clinical trials and selection of surrogate endpoints. The survival time of patients with advanced NSCLC has increased with improved healthcare, which makes the OS in immunotherapy clinical studies susceptible to various factors of subsequent treatment. OS, which has been considered the gold standard in oncology clinical trials, is more difficult to reach in these circumstances. Therefore, some clinical trials have established PFS or ORR as the surrogate primary endpoint, which shortened the time needed to market and reduced the development costs of agents. However, these surrogate endpoints could not objectively reflect the efficacy of the immunotherapy drugs. Unlike chemotherapy and targeted drugs, immunotherapy agents function slowly but persistently, leading to long plateau tails in survival curves. Thus, surrogate endpoints like the milestone survival rate, which can better reflect the characteristics of the immunotherapy agents, should be selected. Future clinical trials call for more elaborate designs and improved endpoints.
Another persistent challenge is the means to select patients that could potentially benefit from the therapy. Only a small, specific proportion of patients benefit from current immunotherapies. PD-L1 expression and TMB are the two most important markers for efficacy prediction. In the current study of advanced or metastatic NSCLC, TMB may be associated with the response to combination therapies containing anti-CTLA-4 agents. PD-L1 expression is used to predict the patients who may benefit from anti-PD-(L)1 therapy. However, none of these are ideal biomarkers for predicting the efficacy of immunotherapy. A single marker cannot fully reflect the complex immune response network. The current view is that an integrated biomarker that includes different components of the TME should be the focus of future research.[130,131] The progress that has been made in high-throughput omics technology has facilitated the comprehensive and dynamic analysis of complicated immune regulatory networks in the TME. This technology can also contribute to the selection of immunotherapy-related biomarkers. In the future, the predictive models based on integrated biomarkers combined with clinical characteristics of patients may help identify individual patients who are most likely to benefit from immunotherapy.
The next challenge is to find the optimal therapeutic combination. An ideal combination can cover multiple immune response pathways to achieve maximum synergistic effects with minimum cumulative toxicity. Currently, anti-PD-(L)1 therapy combined with chemotherapy, radiotherapy, anti-CTLA-4 therapy, or AA therapy has been demonstrated to have synergistic antitumor activity in clinical studies and has been approved for advanced or metastatic NSCLC. The indications for these combinations almost cover all the advanced or metastatic NSCLC cases without driver gene mutations. Unfortunately, over half of these patients are “also-rans” who suffer the poor efficacy and potential risk of toxicity. The means to select the optimal combination therapy and arrange the scheme of the drugs remain difficult issues. To address these problems, further studies on the immunomodulatory mechanism of each drug and synergistic mechanism of the combination in preclinical models are necessary. Additionally, more clinical trials are needed to further explore the dosage, sequence, and timing of each drug and subdivide different groups to identify markers that guide combination drug selection. Furthermore, more efficient and low-toxicity drugs should be designed to create more possibilities for combination therapy.
Another important challenge is the management of immune-related adverse events (irAEs), which have their own characteristics compared with AEs of chemotherapy or targeted therapy: there is little association between dosage and degree of AE; the timing of the AE is difficult to predict; the AE is often longer in duration and all organ systems can be involved.[39,132] These bring difficulties to the management of irAE. Early detection and timely intervention are the two most important aspects in the management of irAEs.[133] However, there is no warning system for early recognition, the adverse effect of steroids on efficacy of immunotherapy is controversial, and the restart of immunotherapy after termination remains to be further studied. Several guidelines on the management of irAEs have been established. However, the increasing application of the immunotherapies leads to emergence of various irAEs under real-world scenarios. Large amounts of real-world data should be managed properly to enrich the experience in practice and optimize the guidelines. Additionally, biomarkers associated with early recognition and real-time monitoring warrant further development.
Conclusions
In this review, we discussed possible immune escape mechanisms and current promising immune therapeutic strategies of NSCLC from three aspects of immune escape mechanisms. Anti-PD-(L)1 monotherapy and combinations with other therapy types have become the standard treatment for eligible advanced or metastatic NSCLC patients without driver gene aberrations. Other novel immunotherapy agents and combination regimens are being actively assessed in clinical trials. We believe the future direction of immunotherapy will be combination therapy because of the complexity of immune regulatory networks. The predictive model integrating multiple elements of the immune response may help in the selection of patients who could benefit and guide the optimal combinations. More in-depth research on immune escape mechanisms, the accumulation of real-world experience, development of new drugs, and continuous optimization of clinical trial design will promote immunotherapy. This will benefit more patients with advanced or metastatic NSCLC by enabling them to achieve long-term survival.
Acknowledgements
Authors thank J. Iacona, Ph.D., from Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing the English text of a draft of this manuscript.
Funding
This work was supported by the CAMS Innovation Fund for Medical Sciences (CIFMS) (No. 2021-I2M-1-050) and the Beijing Hospital Project (No. BJ-2019-145).
Conflicts of interest
None.
Footnotes
How to cite this article: Xu J, Liu C, Wu X, Ma J. Current immune therapeutic strategies in advanced or metastatic non-small cell lung cancer. Chin Med J 2023;136:1765–1782. doi: 10.1097/CM9.0000000000002536
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Mattiuzzi C, Lippi G. Current cancer epidemiology. J Epidemiol Glob Health 2019; 9:217–222. doi: 10.2991/jegh.k.191008.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brahmer JR, Govindan R, Anders RA, Antonia SJ, Sagorsky S, Davies MJ, et al. The society for immunotherapy of cancer consensus statement on immunotherapy for the treatment of non-small cell lung cancer (NSCLC). J Immunother Cancer 2018; 6:75.doi: 10.1186/s40425-018-0382-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin 2022; 72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 5.Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 2013; 501:346–354. doi: 10.1038/nature12626. [DOI] [PubMed] [Google Scholar]
- 6.Fridman W, Remark R, Goc J, Giraldo N, Becht E, Hammond SA, et al. The immune microenvironment: a major player in human cancers. Int Arch Allergy Immunol 2014; 164:13–26. doi: 10.1159/000362332. [DOI] [PubMed] [Google Scholar]
- 7.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011; 331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 8.Anagnostou V, Smith KN, Forde PM, Niknafs N, Bhattacharya R, White J, et al. Evolution of neoantigen landscape during immune checkpoint blockade in non-small cell lung cancer. Cancer Discov 2017; 7:264–276. doi: 10.1158/2159-8290.CD-16-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenthal R, Cadieux EL, Salgado R, Bakir MA, Moore DA, Hiley CT, et al. Neoantigen-directed immune escape in lung cancer evolution. Nature 2019; 567:479–485. doi: 10.1158/2159-8290.CD-16-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hellmann MD, Nathanson T, Rizvi H, Creelan BC, Sanchez-Vega F, Ahuja A, et al. Genomic features of response to combination immunotherapy in patients with advanced non-small-cell lung cancer. Cancer Cell 2018; 33:843–852. e4. doi: 10.1016/j.ccell.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gainor JF, Shaw AT, Sequist LV, Fu X, Azzoli CG, Piotrowska Z, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clin Cancer Res 2016; 22:4585–4593. doi: 10.1158/1078-0432.CCR-15-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su S, Dong ZY, Xie Z, Yan LX, Li YF, Su J, et al. Strong programmed death ligand 1 expression predicts poor response and de novo resistance to EGFR tyrosine kinase inhibitors among NSCLC patients with EGFR mutation. J Thorac Oncol 2018; 13:1668–1675. doi: 10.1016/j.jtho.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 13.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med 2009; 206:1717–1725. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bourhis M, Palle J, Galy-Fauroux I, Terme M. Direct and indirect modulation of T cells by VEGF-a counteracted by anti-angiogenic treatment. Front Immunol 2021; 12:616837.doi: 10.3389/fimmu.2021.616837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salmon H, Franciszkiewicz K, Damotte D, Dieu-Nosjean MC, Validire P, Trautmann A, et al. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J Clin Invest 2012; 122:899–910. doi: 10.1172/JCI45817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larionova I, Tuguzbaeva G, Ponomaryova A, Stakheyeva M, Cherdyntseva N, Pavlov V, et al. Tumor-associated macrophages in human breast, colorectal, lung, ovarian and prostate cancers. Front Oncol 2020; 10:566511.doi: 10.3389/fonc.2020.566511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Z, Guo J, Weng L, Tang W, Jin S, Ma W. Myeloid-derived suppressor cells-new and exciting players in lung cancer. J Hematol Oncol 2020; 13:10.doi: 10.1186/s13045-020-0843-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie M, Wei J, Xu J. Inducers, attractors and modulators of CD4(+) treg cells in non-small-cell lung cancer. Front Immunol 2020; 11:676.doi: 10.3389/fimmu.2020.00676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science 2015; 348:74–80. doi: 10.1126/science.aaa6204. [DOI] [PubMed] [Google Scholar]
- 20.Perea F, Bernal M, Sánchez-Palencia A, Carretero J, Torres C, Bayarri C, et al. The absence of HLA class I expression in non-small cell lung cancer correlates with the tumor tissue structure and the pattern of T cell infiltration. Int J Cancer 2016; 140:888–899. doi: 10.1002/ijc.30489. [DOI] [PubMed] [Google Scholar]
- 21.McGranahan N, Rosenthal R, Hiley CT, Rowan AJ, Watkins TBK, Wilson GA, et al. Allele-specific HLA loss and immune escape in lung cancer evolution. Cell 2017; 171:1259–1271. e11. doi: 10.1016/j.cell.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ichinokawa K, Nakanishi Y, Hida Y, Tsuchikawa T, Kato T, Itoh T, et al. Downregulated expression of human leukocyte antigen class I heavy chain is associated with poor prognosis in non-small-cell lung cancer. Oncol Lett 2019; 18:117–126. doi: 10.3892/ol.2019.10293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pons-Tostivint E, Lugat A, Fontenau JF, Denis MG, Bennouna J. STK11/LKB1 modulation of the immune response in lung cancer: from biology to therapeutic impact. Cells 2021; 10:3129.doi: 10.3390/cells10113129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 2015; 15:486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giatromanolaki A, Kouroupi M, Pouliliou S, Mitrakas A, Hasan F, Pappa A, et al. Ectonucleotidase CD73 and CD39 expression in non-small cell lung cancer relates to hypoxia and immunosuppressive pathways. Life Sci 2020; 259:118389.doi: 10.1016/j.lfs.2020.118389. [DOI] [PubMed] [Google Scholar]
- 26.Kocher F, Amann A, Zimmer K, Geisler S, Fuchs D, Pichler R, et al. High indoleamine-2,3-dioxygenase 1 (IDO) activity is linked to primary resistance to immunotherapy in non-small cell lung cancer (NSCLC). Transl Lung Cancer Res 2021; 10:304–313. doi: 10.21037/tlcr-20-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Besse B, Campelo MG, Dols MC, Quoix E, Madroszyk A, Felip E, et al. LBA47 Activity of OSE-2101 in HLA-A2+ non-small cell lung cancer (NSCLC) patients after failure to immune checkpoint inhibitors (IO): final results of phase III Atalante-1 randomised trial. Ann Oncol 2021; 32:S1325.doi: 10.1016/j.annonc.2021.08.2126. [Google Scholar]
- 28.Rodriguez PC, Popa X, Martinez O, Mendoza S, Santiesteban E, Crespo T, et al. A phase III clinical trial of the epidermal growth factor vaccine CIMAvax-EGF as switch maintenance therapy in advanced non-small cell lung cancer patients. Clin Cancer Res 2016; 22:3782–3790. doi: 10.1158/1078-0432.CCR-15-0855. [DOI] [PubMed] [Google Scholar]
- 29.Popa X, Garcia B, Fuentes KP, Huerta V, Alvarez K, Viada CE, et al. Anti-EGF antibodies as surrogate biomarkers of clinical efficacy in stage IIIB/IV non-small-cell lung cancer patients treated with an optimized CIMAvax-EGF vaccination schedule. Oncoimmunology 2020; 9:1762465.doi: 10.1080/2162402X.2020.1762465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alvanegh AG, Ganji SM, Kamel A, Tavallaie M, Rafati A, Arpanaei A, et al. Comparison of oncolytic virotherapy and nanotherapy as two new miRNA delivery approaches in lung cancer. Biomed Pharmacother 2021; 140:111755.doi: 10.1016/j.biopha.2021.111755. [DOI] [PubMed] [Google Scholar]
- 31.Breitbach CJ, Arulanandam R, De Silva N, Thorne SH, Patt R, Daneshmand M, et al. Oncolytic vaccinia virus disrupts tumor-associated vasculature in humans. Cancer Res 2013; 73:1265–1275. doi: 10.1158/0008-5472.CAN-12-2687. [DOI] [PubMed] [Google Scholar]
- 32.Ungerechts G, Bossow S, Leuchs B, Holm PS, Rommelaere J, Coffey M, et al. Moving oncolytic viruses into the clinic: clinical-grade production, purification, and characterization of diverse oncolytic viruses. Mol Ther Methods Clin Dev 2016; 3:16018.doi: 10.1038/mtm.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Villalona-Calero MA, Lam E, Otterson GA, Zhao W, Timmons M, Subramaniam D, et al. Oncolytic reovirus in combination with chemotherapy in metastatic or recurrent non-small cell lung cancer patients with KRAS-activated tumors. Cancer 2016; 122:875–883. doi: 10.1002/cncr.29856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bradbury PA, Morris DG, Nicholas G, Tu D, Tehfe M, Goffin JR, et al. Canadian Cancer Trials Group (CCTG) IND211: a randomized trial of pelareorep (Reolysin) in patients with previously treated advanced or metastatic non-small cell lung cancer receiving standard salvage therapy. Lung Cancer 2018; 120:142–148. doi: 10.1016/j.lungcan.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 35.Lingel H, Brunner-Weinzierl MC. CTLA-4 (CD152): a versatile receptor for immune-based therapy. Semin Immunol 2019; 42:101298.doi: 10.1016/j.smim.2019.101298. [DOI] [PubMed] [Google Scholar]
- 36.Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol 2020; 20:651–668. doi: 10.1038/s41577-020-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med 2019; 381:2020–2031. doi: 10.1056/NEJMoa1910231. [DOI] [PubMed] [Google Scholar]
- 38.Paz-Ares LG, Ciuleanu TE, Lee JS, Urban L, Reck M. Nivolumab (NIVO) plus ipilimumab (IPI) versus chemotherapy (chemo) as first-line (1L) treatment for advanced non-small cell lung cancer (NSCLC): 4-year update from CheckMate 227. J Clin Oncol 2021; 39: (15 Suppl): 9016–9016. doi: 10.1200/JCO.2021.39.15_suppl.9016. [Google Scholar]
- 39.de Miguel M, Calvo E. Clinical challenges of immune checkpoint inhibitors. Cancer Cell 2020; 38:326–333. doi: 10.1016/j.ccell.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 40.Champiat S, Ferrara R, Massard C, Besse B, Marabelle A, Soria JC, et al. Hyperprogressive disease: recognizing a novel pattern to improve patient management. Nat Rev Clin Oncol 2018; 15:748–762. doi: 10.1038/s41571-018-0111-2. [DOI] [PubMed] [Google Scholar]
- 41.Paz-Ares L, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol 2021; 22:198–211. doi: 10.1016/s1470-2045(20)30641-0. [DOI] [PubMed] [Google Scholar]
- 42.Rizvi NA, Cho BC, Reinmuth N, Lee KH, Luft A, Ahn MJ, et al. Durvalumab with or without Tremelimumab vs Standard Chemotherapy in first-line treatment of metastatic nonsmall cell lung cancer: The MYSTIC phase 3 randomized clinical trial. JAMA Oncol 2020; 6:661–674. doi:10.1001/jamaoncol.2020.0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson M, Cho BC, Luft A, Alatorre-Alexander J, Geater SL, Laktionov K, et al. PL02.01 durvalumab ± tremelimumab + chemotherapy as first-line treatment for mNSCLC: results from the phase 3 POSEIDON study. J Thorac Oncol 2021; 16:S844–S943. doi: 10.1016/j.jtho.2021.08.029. [Google Scholar]
- 44.Allen E, Jabouille A, Rivera LB, Lodewijckx I, Missiaen R, Steri V, et al. Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci Transl Med 2017;9:eaak9679. doi: 10.1126/scitranslmed.aak9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018; 378:2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 46.Socinski MA, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, et al. IMpower150 final overall survival analyses for atezolizumab plus bevacizumab and chemotherapy in first-line metastatic nonsquamous NSCLC. J Thorac Oncol 2021; 16:1909–1924. doi: 10.1016/j.jtho.2021.07.009. [DOI] [PubMed] [Google Scholar]
- 47.Yang JH, Luft A, Jiménez EDLM, Lee J, Koralewski P, Karadurmus N, et al. 120O pembrolizumab (Pembro) with or without lenvatinib (Lenva) in first-line metastatic NSCLC with PD-L1 TPS≥ 1%(LEAP-007): a phase III, randomized, double-blind study. Ann Oncol 2021; 32:S1429–S1430. doi: 10.1016/j.annonc.2021.10.139. [Google Scholar]
- 48.Herbst RS, Arkenau HT, Santana-Davila R, Calvo E, Paz-Ares L, Cassier PA, et al. Ramucirumab plus pembrolizumab in patients with previously treated advanced non-small-cell lung cancer, gastro-oesophageal cancer, or urothelial carcinomas (JVDF): a multicohort, non-randomised, open-label, phase 1a/b trial. Lancet Oncol 2019; 20:1109–1123. doi: 10.1016/s1470-2045(19)30458-9. [DOI] [PubMed] [Google Scholar]
- 49.Chu T, Zhong R, Zhong H, Zhang B, Zhang W, Shi C, et al. Phase 1b study of sintilimab plus anlotinib as first-line therapy in patients with advanced NSCLC. J Thorac Oncol 2021; 16:643–652. doi: 10.1016/j.jtho.2020.11.026. [DOI] [PubMed] [Google Scholar]
- 50.Leal TA, Berz D, Rybkin I, Iams WT, Bruno D, Blakely C, et al. 43P MRTX-500: phase II trial of sitravatinib (sitra) + nivolumab (nivo) in patients (pts) with non-squamous (NSQ) non-small cell lung cancer (NSCLC) progressing on or after prior checkpoint inhibitor (CPI) therapy. Ann Oncol 2022; 33: (Suppl 1): S19–S20. doi: 10.1016/j.annonc.2022.01.052. [Google Scholar]
- 51.Coward J, Mislang ARA, Frentzas S, Lemech CR, Nagrial A, Jin X, et al. Safety and efficacy of AK112, an anti-PD-1/VEGF-A bispecific antibody, in patients with advanced solid tumors in a phase I dose escalation study. J Clin Oncol 2021; 39: (15 Suppl): 2515.doi: 10.1200/JCO.2021.39.15_suppl.2515. [Google Scholar]
- 52.Creelan BC, Wang C, Teer JK, Toloza EM, Yao J, Kim S, et al. Tumor-infiltrating lymphocyte treatment for anti-PD-1-resistant metastatic lung cancer: a phase 1 trial. Nat Med 2021; 27:1410–1418. doi: 10.1038/s41591-021-01462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schoenfeld A, Lee S, Paz-Ares L, Doger B, Gettinger S, Haefliger S, et al. 458 First phase 2 results of autologous tumor-infiltrating lymphocyte (TIL; LN-145) monotherapy in patients with advanced, immune checkpoint inhibitor-treated, non-small cell lung cancer (NSCLC). J Immunother Cancer 2021; 9: (Suppl 2): doi: 10.1136/jitc-2021-SITC2021.458. [Google Scholar]
- 54.Xia Y, Tian X, Wang J, Qiao D, Liu X, Xiao L, et al. Treatment of metastatic non-small cell lung cancer with NY-ESO-1 specific TCR engineered-T cells in a phase I clinical trial: a case report. Oncol Lett 2018; 16:6998–7007. doi: 10.3892/ol.2018.9534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qu J, Mei Q, Chen L, Zhou J. Chimeric antigen receptor (CAR)-T-cell therapy in non-small-cell lung cancer (NSCLC): current status and future perspectives. Cancer Immunol Immunother 2021; 70:619–631. doi: 10.1007/s00262-020-02735-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin M, Liang SZ, Wang XH, Liang YQ, Zhang MJ, Niu LZ, et al. Clinical efficacy of percutaneous cryoablation combined with allogenic NK cell immunotherapy for advanced non-small cell lung cancer. Immunol Res 2017; 65:880–887. doi: 10.1007/s12026-017-8927-x. [DOI] [PubMed] [Google Scholar]
- 57.Lin M, Luo H, Liang S, Chen J, Liu A, Niu L, et al. Pembrolizumab plus allogeneic NK cells in advanced non-small cell lung cancer patients. J Clin Invest 2020; 130:2560–2569. doi: 10.1172/JCI132712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiao Z, Wang C, Zhou M, Li N, Sun Y, Wang Y, et al. The antitumor immunity and tumor responses of chemotherapy with or without DC-CIK for non-small-cell lung cancer in China: a meta-analysis of 28 randomized controlled trials. J Immunol Res 2018; 2018:9081938.doi: 10.1155/2018/9081938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao Y, Qiao G, Wang X, Song Y, Zhou X, Jiang N, et al. Combination of DC/CIK adoptive T cell immunotherapy with chemotherapy in advanced non-small-cell lung cancer (NSCLC) patients: a prospective patients’ preference-based study (PPPS). Clin Transl Oncol 2019; 21:721–728. doi: 10.1007/s12094-018-1968-3. [DOI] [PubMed] [Google Scholar]
- 60.Wang L, Dai Y, Zhu F, Qiu Z, Wang Y, Hu Y. Efficacy of DC-CIK-based immunotherapy combined with chemotherapy in the treatment of intermediate to advanced non-small cell lung cancer. Am J Transl Res 2021; 13:13076. [PMC free article] [PubMed] [Google Scholar]
- 61.Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res 2005; 65:1089–1096. doi: 10.1158/0008-5472.1089.65.3. [PubMed] [Google Scholar]
- 62.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csöszi T, Fulop A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016; 375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 63.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non–small-cell lung cancer with PD-L1 tumor proportion score≥ 50%. J Clin Oncol 2021; 39:2339–2349. doi: 10.1200/JCO.21.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019; 393:1819–1830. doi: 10.1016/s0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 65.Wu YL, Zhang L, Fan Y, Zhou J, Zhang L, Zhou Q, et al. 389P Updated analysis from the KEYNOTE-042 China study: 1L pembrolizumab (pembro) vs chemotherapy (chemo) in Chinese patients (pts) with advanced NSCLC with PD-L1 TPS ≥1%. Ann Oncol 2020; 31: (Suppl 6): S1394.doi: 10.1016/j.annonc.2020.10.383. [Google Scholar]
- 66.Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med 2020; 383:1328–1339. doi: 10.1056/NEJMoa1917346. [DOI] [PubMed] [Google Scholar]
- 67.Sezer A, Kilickap S, Gümüş M, Bondarenko I, Özgüroğlu M, Gogishvili M, et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet 2021; 397:592–604. doi: 10.1016/s0140-6736(21)00228-2. [DOI] [PubMed] [Google Scholar]
- 68.Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med 2017; 376:2415–2426. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rizvi NA, Cho BC, Reinmuth N, Lee KH, Luft A, Ahn MJ, et al. Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic non-small cell lung cancer: the MYSTIC phase 3 randomized clinical trial. JAMA Oncol 2020; 6:661–674. doi: 10.1001/jamaoncol.2020.0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015; 373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung Cancer. N Engl J Med 2015; 373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Borghaei H, Gettinger S, Vokes EE, Chow LQ, Burgio MA, de Castro Carpeno J, et al. Five-year outcomes from the randomized, phase III trials checkmate 017 and 057: nivolumab versus docetaxel in previously treated non–small-cell lung cancer. J Clin Oncol 2021; 39:723.doi: 10.1200/JCO.20.01605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu YL, Lu S, Cheng Y, Zhou C, Wang J, Mok T, et al. Nivolumab versus docetaxel in a predominantly chinese patient population with previously treated advanced NSCLC: CheckMate 078 randomized phase III clinical trial. J Thorac Oncol 2019; 14:867–875. doi: 10.1016/j.jtho.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 74.Chang J, Wu YL, Lu S, Wang J, Mok T, Zhang L, et al. Three-year follow-up and patient-reported outcomes from CheckMate 078: Nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced non-small cell lung cancer. Lung Cancer 2021; 165:71–81. doi: 10.1016/j.lungcan.2021.12.009. [DOI] [PubMed] [Google Scholar]
- 75.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017; 389:255–265. doi: 10.1016/s0140-6736(16)32517-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mazieres J, Rittmeyer A, Gadgeel S, Hida T, Gandara DR, Cortinovis DL, et al. Atezolizumab versus docetaxel in pretreated patients with NSCLC: final results from the randomized phase 2 POPLAR and phase 3 OAK clinical trials. J Thorac Oncol 2021; 16:140–150. doi: 10.1016/j.jtho.2020.09.022. [DOI] [PubMed] [Google Scholar]
- 77.Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387:1540–1550. doi: 10.1016/s0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 78.Herbst RS, Garon EB, Kim DW, Cho BC, Gervais R, Perez-Gracia JL, et al. Five year survival update from KEYNOTE-010: pembrolizumab versus docetaxel for previously treated, programmed death-ligand 1-positive advanced NSCLC. J Thorac Oncol 2021; 16:1718–1732. doi: 10.1016/j.jtho.2021.05.001. [DOI] [PubMed] [Google Scholar]
- 79.Zhou C, Huang D, Yu X, Liu Y, Fan Y, Shu Y, et al. Abstract CT039: results from RATIONALE 303: a global phase 3 study of tislelizumab (TIS) vs docetaxel (TAX) as second- or third-line therapy for patients with locally advanced or metastatic NSCLC. Cancer Res 2021; 81: (13 Suppl): CT039–CT139. doi: 10.1158/1538-7445.Am2021-ct039. [Google Scholar]
- 80.Barlesi F, Vansteenkiste J, Spigel D, Ishii H, Garassino M, de Marinis F, et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol 2018; 19:1468–1479. doi: 10.1016/s1470-2045(18)30673-9. [DOI] [PubMed] [Google Scholar]
- 81.Antonia SJ, Balmanoukian A, Brahmer J, Ou SI, Hellmann MD, Kim SW, et al. Clinical activity, tolerability, and long-term follow-up of durvalumab in patients with advanced NSCLC. J Thorac Oncol 2019; 14:1794–1806. doi: 10.1016/j.jtho.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 82.Yang JJ, Huang C, Fan Y, Pan H, Feng J, Jiang L, et al. Camrelizumab in different PD-L1 expression cohorts of pre-treated advanced or metastatic non-small cell lung cancer: a phase II study. Cancer Immunol Immunother 2022; 71:1393–1402. doi: 10.1007/s00262-021-03091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vanmeerbeek I, Sprooten J, De Ruysscher D, Tejpar S, Vandenberghe P, Fucikova J, et al. Trial watch: chemotherapy-induced immunogenic cell death in immuno-oncology. Oncoimmunology 2020; 9:1703449.doi: 10.1080/2162402X.2019.1703449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liang Y, Lu W, Zhang X, Lu B. Tumor-infiltrating CD8+ and FOXP3+ lymphocytes before and after neoadjuvant chemotherapy in cervical cancer. Diagn Pathol 2018; 13:93.doi: 10.1186/s13000-018-0770-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang P, Ma Y, Lv C, Huang M, Li M, Dong B, et al. Upregulation of programmed cell death ligand 1 promotes resistance response in non-small-cell lung cancer patients treated with neo-adjuvant chemotherapy. Cancer Sci 2016; 107:1563–1571. doi: 10.1111/cas.13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Robinson A, Vicente D, Tafreshi A, Parra HS, Mazieres J, Cicin I, et al. 97O First-line pembrolizumab plus chemotherapy for patients with advanced squamous NSCLC: 3-year follow-up from KEYNOTE-407. J Thorac Oncol 2021; 16:S748–S749. doi: 10.1016/S1556-0864(21)01939-0. [Google Scholar]
- 87.Gray J, Rodríguez-Abreu D, Powell S, Hochmair M, Gadgeel S, Esteban E, et al. FP13. 02 pembrolizumab+ pemetrexed-platinum vs pemetrexed-platinum for metastatic NSCLC: 4-year follow-up from KEYNOTE-189. J Thorac Oncol 2021; 16:S224.doi: 10.1016/j.jtho.2021.01.141. [Google Scholar]
- 88.West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019; 20:924–937. doi: 10.1016/s1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
- 89.Nishio M, Barlesi F, West H, Ball S, Bordoni R, Cobo M, et al. Atezolizumab plus chemotherapy for first-line treatment of nonsquamous NSCLC: results from the randomized phase 3 IMpower132 trial. J Thorac Oncol 2021; 16:653–664. doi: 10.1016/j.jtho.2020.11.025. [DOI] [PubMed] [Google Scholar]
- 90.Jotte R, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Rodriguez-Abreu D, Hussein M, et al. Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous NSCLC (IMpower131): results from a randomized phase III trial. J Thorac Oncol 2020; 15:1351–1360. doi: 10.1016/j.jtho.2020.03.028. [DOI] [PubMed] [Google Scholar]
- 91.Zhou C, Chen G, Huang Y, Zhou J, Lin L, Feng J, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med 2021; 9:305–314. doi: 10.1016/s2213-2600(20)30365-9. [DOI] [PubMed] [Google Scholar]
- 92.Ren S, Chen J, Xu X, Jiang T, Cheng Y, Chen G, et al. Camrelizumab plus carboplatin and paclitaxel as first-line treatment for advanced squamous NSCLC (CameL-Sq): a phase 3 trial. J Thorac Oncol 2022; 17:544–557. doi: 10.1016/j.jtho.2021.11.018. [DOI] [PubMed] [Google Scholar]
- 93.Lu S, Wang J, Yu Y, Yu X, Hu Y, Ai X, et al. Tislelizumab plus chemotherapy as first-line treatment for locally advanced or metastatic nonsquamous NSCLC (RATIONALE 304): a randomized phase 3 trial. J Thorac Oncol 2021; 16:1512–1522. doi: 10.1016/j.jtho.2021.05.005. [DOI] [PubMed] [Google Scholar]
- 94.Wang J, Lu S, Yu X, Hu Y, Sun Y, Wang Z, et al. Tislelizumab plus chemotherapy vs chemotherapy alone as first-line treatment for advanced squamous non-small-cell lung cancer: a phase 3 randomized clinical trial. JAMA Oncol 2021; 7:709–717. doi: 10.1001/jamaoncol.2021.0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang Y, Wang Z, Fang J, Yu Q, Han B, Cang S, et al. Efficacy and safety of sintilimab plus pemetrexed and platinum as first-line treatment for locally advanced or metastatic nonsquamous NSCLC: a randomized, double-blind, phase 3 study (Oncology pRogram by InnovENT anti-PD-1-11). J Thorac Oncol 2020; 15:1636–1646. doi: 10.1016/j.jtho.2020.07.014. [DOI] [PubMed] [Google Scholar]
- 96.Zhou C, Wu L, Fan Y, Wang Z, Liu L, Chen G, et al. Sintilimab plus platinum and gemcitabine as first-line treatment for advanced or metastatic squamous NSCLC: results from a randomized, double-blind, phase 3 trial (ORIENT-12). J Thorac Oncol 2021; 16:1501–1511. doi: 10.1016/j.jtho.2021.04.011. [DOI] [PubMed] [Google Scholar]
- 97.Wang J, Wang Z, Wu L, Li B, Cheng Y, Li X, et al. Final progression-free survival, interim overall survival, and biomarker analyses of CHOICE-01: a phase III study of toripalimab versus placebo in combination with first-line chemotherapy for advanced NSCLC without EGFR/ALK mutations. J Clin Oncol 2022; 40: (36 Suppl): 362936–362936. doi: 10.1200/JCO.2022.40.36_suppl.362936. [Google Scholar]
- 98.Zhou C, Wang Z, Sun Y, Cao L, Ma Z, Wu R, et al. Sugemalimab versus placebo, in combination with platinum-based chemotherapy, as first-line treatment of metastatic non-small-cell lung cancer (GEMSTONE-302): interim and final analyses of a double-blind, randomised, phase 3 clinical trial. Lancet Oncol 2022; 23:220–233. doi: 10.1016/s1470-2045(21)00650-1. [DOI] [PubMed] [Google Scholar]
- 99.Duan J, Cui L, Zhao X, Bai H, Cai S, Wang G, et al. Use of immunotherapy with programmed cell death 1 vs programmed cell death ligand 1 inhibitors in patients with cancer: a systematic review and meta-analysis. JAMA Oncol 2020; 6:375–384. doi: 10.1001/jamaoncol.2019.5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu SYM, Zhou Q, Yan HH, Bin G, Yang MY, Deng JY, et al. Sintilimab versus pembrolizumab in monotherapy or combination with chemotherapy as first-line therapy for advanced non–small cell lung cancer: results from phase 2, randomized clinical trial (CTONG1901). J Clin Oncol 2022; 40: (16 Suppl): 9032.doi: 10.1200/JCO.2022.40.16_suppl.9032. [Google Scholar]
- 101.Akinboro O, Vallejo JJ, Nakajima EC, Ren Y, Mishra-Kalyani PS, Larkins EA, et al. Outcomes of anti–PD-(L)1 therapy with or without chemotherapy (chemo) for first-line (1L) treatment of advanced non–small cell lung cancer (NSCLC) with PD-L1 score ≥ 50%: FDA pooled analysis. J Clin Oncol 2022; 40: (16 Suppl): 9000.doi: 10.1200/JCO.2022.40.16_suppl.9000. [Google Scholar]
- 102.Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res 2012; 72:917–927. doi: 10.1158/0008-5472.Can-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Banta KL, Xu X, Chitre AS, Au-Yeung A, Takahashi C, O’Gorman WE, et al. Mechanistic convergence of the TIGIT and PD-1 inhibitory pathways necessitates co-blockade to optimize anti-tumor CD8(+) T cell responses. Immunity 2022; 55:512.e–526.e. doi: 10.1016/j.immuni.2022.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maruhashi T, Sugiura D, Okazaki IM, Okazaki T. LAG-3: from molecular functions to clinical applications. J ImmunoTher Cancer 2020; 8:e001014.doi: 10.1136/jitc-2020-001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lecocq Q, Keyaerts M, Devoogdt N, Breckpot K. The next-generation immune checkpoint LAG-3 and its therapeutic potential in oncology: third time's a charm. Int J Mol Sci 2020; 22:75.doi: 10.3390/ijms22010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Felip E, Majem M, Doger B, Clay TD, Carcereny E, Bondarenko I, et al. A phase II study (TACTI-002) in first-line metastatic non–small cell lung carcinoma investigating eftilagimod alpha (soluble LAG-3 protein) and pembrolizumab: updated results from a PD-L1 unselected population. J Clin Oncol 2022; 40: (16 Suppl): 9003.doi: 10.1200/JCO.2022.40.16_suppl.9003. [Google Scholar]
- 107.Ge Z, Peppelenbosch MP, Sprengers D, Kwekkeboom J. TIGIT, the next step towards successful combination immune checkpoint therapy in cancer. Front Immunol 2021; 12:699895.doi: 10.3389/fimmu.2021.699895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cho BC, Abreu DR, Hussein M, Cobo M, Patel AJ, Secen N, et al. Tiragolumab plus atezolizumab versus placebo plus atezolizumab as a first-line treatment for PD-L1-selected non-small-cell lung cancer (CITYSCAPE): primary and follow-up analyses of a randomised, double-blind, phase 2 study. Lancet Oncol 2022; 23:781–792. doi: 10.1016/s1470-2045(22)00226-1. [DOI] [PubMed] [Google Scholar]
- 109.Niu J, Maurice-Dror C, Lee DH, Kim DW, Nagrial A, Voskoboynik M, et al. First-in-human phase 1 study of the anti-TIGIT antibody vibostolimab as monotherapy or with pembrolizumab for advanced solid tumors, including non-small-cell lung cancer(☆). Ann Oncol 2022; 33:169–180. doi: 10.1016/j.annonc.2021.11.002. [DOI] [PubMed] [Google Scholar]
- 110.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol 2013; 13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kraehenbuehl L, Weng CH, Eghbali S, Wolchok JD, Merghoub T. Enhancing immunotherapy in cancer by targeting emerging immunomodulatory pathways. Nat Rev Clin Oncol 2022; 19:37–50. doi: 10.1038/s41571-021-00552-7. [DOI] [PubMed] [Google Scholar]
- 112.Thommen DS, Koelzer VH, Herzig P, Roller A, Trefny M, Dimeloe S, et al. A transcriptionally and functionally distinct PD-1(+) CD8(+) T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat Med 2018; 24:994–1004. doi: 10.1038/s41591-018-0057-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Feng LL, Wang X. Targeting Foxp3+ regulatory T cells-related immunosuppression for cancer immunotherapy. Chin Med J 2010; 123:3334–3342. doi: 10.3760/cma.j.issn.0366-6999.2010.22.030. [PubMed] [Google Scholar]
- 114.Houot R, Goldstein MJ, Kohrt HE, Myklebust JH, Alizadeh AA, Lin JT, et al. Therapeutic effect of CD137 immunomodulation in lymphoma and its enhancement by Treg depletion. Blood 2009; 114:3431–3438. doi: 10.1182/blood-2009-05-223958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Guo Z, Wang X, Cheng D, Xia Z, Luan M, Zhang S. PD-1 blockade and OX40 triggering synergistically protects against tumor growth in a murine model of ovarian cancer. PLoS One 2014; 9:e89350.doi: 10.1371/journal.pone.0089350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Messenheimer DJ, Jensen SM, Afentoulis ME, Wegmann KW, Feng Z, Friedman DJ, et al. Timing of PD-1 blockade is critical to effective combination immunotherapy with anti-OX40. Clin Cancer Res 2017; 23:6165–6177. doi: 10.1158/1078-0432.Ccr-16-2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vigano S, Alatzoglou D, Irving M, Ménétrier-Caux C, Caux C, Romero P, et al. Targeting adenosine in cancer immunotherapy to enhance T-cell function. Front Immunol 2019; 10:925.doi: 10.3389/fimmu.2019.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Herbst RS, Majem M, Barlesi F, Carcereny E, Chu Q, Monnet I, et al. COAST: an open-label, phase II, multidrug platform study of durvalumab alone or in combination with oleclumab or monalizumab in patients with unresectable, stage III non-small-cell lung cancer. J Clin Oncol 2022; 40:3383–3393. doi: 10.1200/jco.22.00227. [DOI] [PubMed] [Google Scholar]
- 119.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 2017; 377:1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 120.Schalper KA, Carvajal-Hausdorf D, McLaughlin J, Altan M, Velcheti V, Gaule P, et al. Differential expression and significance of PD-L1, IDO-1, and B7-H4 in human lung cancer. Clin Cancer Res 2017; 23:370–378. doi: 10.1158/1078-0432.CCR-16-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jin Y, Dong H, Xia L, Yang Y, Zhu Y, Shen Y, et al. The diversity of gut microbiome is associated with favorable responses to anti-programmed death 1 immunotherapy in Chinese patients with NSCLC. J Thorac Oncol 2019; 14:1378–1389. doi: 10.1016/j.jtho.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 122.Tomita Y, Ikeda T, Sakata S, Saruwatari K, Sato R, Iyama S, et al. Association of probiotic Clostridium butyricum therapy with survival and response to immune checkpoint blockade in patients with lung cancer. Cancer Immunol Res 2020; 8:1236–1242. doi: 10.1158/2326-6066.Cir-20-0051. [DOI] [PubMed] [Google Scholar]
- 123.Hopkins AM, Badaoui S, Kichenadasse G, Karapetis CS, McKinnon RA, Rowland A, et al. Efficacy of atezolizumab in patients with advanced NSCLC receiving concomitant antibiotic or proton pump inhibitor treatment: pooled analysis of five randomized control trials. J Thorac Oncol 2022; 17:758–767. doi: 10.1016/j.jtho.2022.02.003. [DOI] [PubMed] [Google Scholar]
- 124.Chalabi M, Cardona A, Nagarkar DR, Dhawahir Scala A, Gandara DR, Rittmeyer A, et al. Efficacy of chemotherapy and atezolizumab in patients with non-small-cell lung cancer receiving antibiotics and proton pump inhibitors: pooled post hoc analyses of the OAK and POPLAR trials. Ann Oncol 2020; 31:525–531. doi: 10.1016/j.annonc.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 125.Propper DJ, Balkwill FR. Harnessing cytokines and chemokines for cancer therapy. Nat Rev Clin Oncol 2022; 19:237–253. doi: 10.1038/s41571-021-00588-9. [DOI] [PubMed] [Google Scholar]
- 126.Conlon KC, Miljkovic MD, Waldmann TA. Cytokines in the treatment of cancer. J Interferon Cytokine Res 2019; 39:6–21. doi: 10.1089/jir.2018.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Berraondo P, Sanmamed MF, Ochoa MC, Etxeberria I, Aznar MA, Perez-Gracia JL, et al. Cytokines in clinical cancer immunotherapy. Br J Cancer 2019; 120:6–15. doi: 10.1038/s41416-018-0328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Diab A, Tannir NM, Bentebibel SE, Hwu P, Papadimitrakopoulou V, Haymaker C, et al. Bempegaldesleukin (NKTR-214) plus nivolumab in patients with advanced solid tumors: phase I dose-escalation study of safety, efficacy, and immune activation (PIVOT-02). Cancer Discov 2020; 10:1158–1173. doi: 10.1158/2159-8290.CD-19-1510. [DOI] [PubMed] [Google Scholar]
- 129.Wrangle JM, Velcheti V, Patel MR, Garrett-Mayer E, Hill EG, Ravenel JG, et al. ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: a non-randomised, open-label, phase 1b trial. Lancet Oncol 2018; 19:694–704. doi: 10.1016/s1470-2045(18)30148-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer 2019; 19:133–150. doi: 10.1038/s41568-019-0116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol 2016; 17:e542–e551. doi: 10.1016/s1470-2045(16)30406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Puzanov I, Diab A, Abdallah K, Bingham CO, 3rd, Brogdon C, Dadu R, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the society for immunotherapy of cancer (SITC) toxicity management working group. J Immunother Cancer 2017; 5:95.doi: 10.1186/s40425-017-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018; 36:1714–1768. doi: 10.1200/jco.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]