Objective:
To investigate whether pancreatic resections (PR) for pancreatic ductal adenocarcinoma (PDAC) is associated with worse survival when resection of the superior mesenteric vein/portal vein (SMV/PV) is required.
Background:
PR for PDAC with resection of the superior mesenteric vein/portal vein (SMV/PV, PR+V resection) may be associated with inferior overall survival (OS) compared with PR without the need for SMV/PV resection (PR–V). We hypothesized that PR+V results in lower OS compared with PR–V.
Method:
Retrospective study using data from the nationwide Danish Pancreatic Cancer Database from 2011 to 2020. Data on patients who underwent PR for PDAC were extracted. A group of PR patients found nonresectable on exploratory laparotomy (EXP) was also included. OS was assessed using Kaplan-Meier and Cox proportional hazards models adjusting for confounders (age, sex, R-resection level, chemotherapy, comorbidities, histology T and N classification, procedure subtype as well as tumor distance to the SMV/PV).
Results:
Overall, 2403 patients were identified. Six hundred two underwent exploration only (EXP group), whereas 412 underwent pancreatic resection with (PR+V group) and 1389 (PR–V) without SMV/PV resection. Five-year OS for the PR+V group was lower (20% vs 30%) compared with PR–V, although multivariate Cox proportional hazards modeling could not associate PR+V status with OS (Hazard ratio 1.11, P = 0.408).
Conclusion:
When correcting for confounders, PR+V was not associated with lower OS compared with PR–V.
Keywords: pancreatic cancer, venous resection, survival
Mini-abstract: The study investigates the association between overall survival (OS) following pancreatic resection (PR) for pancreatic adenocarcinoma (PDAC) with (PR+V) and without (PR–V) concomitant resection of the superior mesenteric/portal vein (SMV/PV). We find that PR+V is not associated with differences in OS when compared with PR–V if confounders are controlled for.
Supplemental Digital Content is available in the text.
INTRODUCTION
Despite treatment advances, pancreatic ductal adenocarcinoma (PDAC) continues to have dismal outcomes with reported 5-year survival rates of 10%,1 rising to 25% in successfully resected patients1 and only exhibiting a slight increase in survival rates over the last decade.2
Currently, surgery presents the only potentially curable treatment option. Pivotal preoperative workup includes classification of tumors as upfront resectable (UR), borderline resectable (BR), or locally advanced (LA), which includes an assessment of tumor invasion of the venous mesentericoportal axis, defined by the National Comprehensive Cancer Network (NCCN) as solid tumor contact of 180° or more with the superior mesenteric or portal vein (SMV/PV) or contact of less than 180° with vein irregularity or thrombosis but with suitable vessels for anastomoses distally.3 The classification is, however, complicated by the fact that the MD Anderson Cancer Center,4 the Americas Hepato-Pancreatobiliary Association5 and Intergroup Alliance6 all have other definitions of BR with varied acceptance in the literature, although NCCN criteria remain the most widely adopted.
Although SMV/PV resections are technically feasible7 and associated with acceptable short-term outcomes,8 reports have indicated that patients undergoing combined pancreatic and venous resection (PR+V) may have shorter overall survival (OS) than those without venous resection (PR–V).9,10 Conversely, other reports have found similar long-term outcomes.11 Collectively, these discrepant findings thus complicate decisions on whether to proceed with upfront surgery or neoadjuvant therapy for BR patients with SMV/PV involvement, a decision that is further complicated by the unknown factor of whether the tumor will respond to chemotherapy. Recent recommendations, however, favor the latter approach when venous invasion is identified preoperatively.12
To a creatine degree, the above-mentioned discrepancies could be attributed to differences in BR definitions as well as small sample sizes from single-center cohorts, where both institutional protocols and surgeon-related factors may impact on results.
The aim of this study was to use a large, nationwide Danish cohort spanning almost 10 years and including more than 2400 patients operated for PDAC, to investigate long-term outcomes of patients undergoing PR+V versus PR–V.
We hypothesize that patients with radical combined pancreatic and venous resections for PDAC have inferior OS compared with patients where venous resection was not indicated.
METHODS
The study utilized data from the Danish Pancreatic Cancer Database (DPCD).
The DPCD was established in 2011 and collects Danish nationwide data on all patients diagnosed with pancreatic and periampullary cancers. From this registry, data on surgically treated PDAC patients from May 1, 2011, to December 31, 2020, including patients scheduled for surgery but with nonresectable tumors found perioperatively, were extracted and used for analyses. All patients were operated in one of the 4 dedicated PDAC centers in Denmark.
The cohort was divided into 2 groups comprising patients undergoing pancreatic resection with (PR+V group) or without (PR–V group) venous resection. For comparative purposes, patients undergoing surgical exploration for PDAC with curative intent but with tumors found to be nonresectable (EXP group) were included as a separate group. Patients undergoing surgical exploration without resection, downstaging and then a subsequent surgical resection attempt, were included in the relevant group only for the last surgical procedure(PR+V, PR–V, or EXP, respectively). TNM staging followed the American Joint Committee on Cancer, eighth edition.
The study was approved by the DPCD board of governors as well as the Capital Region Data Protection Authority (Videncenter for dataanmeldelser, Approval no. P-2020-180). Under Danish law, registry-based research does not require patient consent nor ethics board approval. The study was prepared and reported in accordance with the strengthening the reporting of observational studies in epidemiology (STROBE) guidelines.13
Statistical Analyses
The study was planned as a survival analysis using OS defined as time to event (all-cause mortality or follow-up censoring) as the primary outcome parameter. Censoring date was set to November 1, 2021.
For survival analyses, Kaplan-Meier (KM) estimates were calculated for the EXP, PR–V, and PR+V groups. Survival curves for resectable patients were compared using the Log-Rank test.
A Cox proportional hazards model was calculated for the PR–V and PR+V groups, associating OS with PR+V and PR–V status in both a univariate and a multivariate model. For the latter, correction for age, sex, occurrence of preoperative or postoperative chemotherapy, Charlson Comorbidity Index (CCI), histological T and N classification, surgical procedure (pancreaticoduodenectomy, total pancreatectomy, or distal pancreatectomy), histological assessment of the distance in mm of tumor to the SMV/PV as well as resection margin outcome (R0, R1, or above) was also performed. According to local protocols, R0 margins were defined as minimum 1.5 mm distance from the resection margin to the tumor, which of note is wider than the internationally adopted 1 mm margin.14
Mann-Whitney U test was used for comparisons between continuous variables, whereas a chi-square test was used to compare discrete variables.
Data are presented as medians with interquartile range (IQR) or percentages where appropriate. KM survival estimates are presented with 95% confidence intervals.
The R statistical suite15 was used for the analyses. The Package “survival” was used for Cox regression models.
A P value of <0.05 was considered significant.
Missing Data
Missing data were considered missing at random (MAR). To assess the impact of the missing data, the dataset was subjected to multiple imputation using the R “MICE” package using predictive mean matching. A sensitivity analyses comparing Cox regression results of the original versus imputed dataset was performed. Missing data are listed as “missing” in the demographics table (Table 1). Supplementary Table 1 (http://links.lww.com/AOSO/A183) holds information on Cox regression results for the imputed dataset for the multivariate model.
TABLE 1.
Overview of Demographic and Treatment-related Variables for Patients Undergoing Surgical Exploration Only (EXP group), Pancreatic Resection With (PR+V), or Without (PR–V) Venous Resection
| Surgical Exploration Only (n = 602) | Pancreatic Resection With Venous Resection (PR+V, n = 412) | Pancreatic Resection Without Venous Resection (PR–V, n = 1389) | P* | ||
|---|---|---|---|---|---|
| Male gender (n) | 359 (59.6%) | 201 (48.8%) | 755 (54.4%) | 0.058 | |
| Missing | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Age (years) | 70 (63–76) | 70 (62–75) | 71 (64–76) | 1.15 × 10 –4 | |
| Missing | 0 (0%) | 0 (0%) | 1 (0.1%) | ||
| CCI | 1 (0–2) | 1 (0–2) | 1 (0–2) | 0.928 | |
| Missing | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Preoperative chemotherapy (n, %) | 131 (21.8%) | 69 (16.7%) | 68 (4.9%) | 3.90 × 10 –15 | |
| Missing | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Postoperative chemotherapy (n,%) | 400 (66.4%) | 324 (78.6%) | 1051 (75.7%) | 0.235 | |
| Missing | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Surgical procedure | Distal pancreatectomy | NA | 17 (4.1%) | 267 (19.2%) | 0.797 |
| Total pancreatectomy | NA | 136 (33.0%) | 178 (12.8%) | – | |
| Pancreaticoduodenectomy | NA | 253 (61.4%) | 919 (66.2%) | – | |
| Other | NA | 7 (1.7%) | 43 (2.4%) | – | |
| Missing | NA | 0 (0%) | 0 (0%) | ||
| Histology T grade | T1 | NA | 10 (2.4%) | 110 (7.9%) | 8.65 × 10–5 |
| T2 | NA | 100 (24.3%) | 281 (20.2%) | - | |
| T3 | NA | 251 (60.9%) | 790 (56.9%) | - | |
| T4 | NA | 6 (1.5%) | 46 (3.3%) | - | |
| Missing | NA | 45 (10.9%) | 162 (11.7%) | - | |
| Histology N grade | N0 | NA | 82 (19.9%) | 409 (29.4%) | 2.22 × 10–5 |
| N1 | NA | 230 (55.8%) | 677 (48.7%) | ||
| N2 | NA | 59 (14.3%) | 119 (8.6%) | ||
| Missing | 41 (9.9%) | 184 (13.2%) | |||
| Resection result† | R0 | NA | 183 (44.0%) | 512 (36.9%) | 0.636 |
| R1 | NA | 73 (17.7%) | 195 (14.0%) | ||
| R2 | NA | 8 (1.9%) | 6 (0.4%) | ||
| Missing | NA | 148 (35.9%) | 676 (48.7%) | ||
| Superior Mesenteric Vein Margin | 0 mm | NA | 70 (17.0%) | 70 (5.0%) | 3.46 × 10–14 |
| 0.5 mm | NA | 61 (14.8%) | 87 (6.3) | ||
| 1.0 mm | NA | 42 (10.2%) | 55 (4.0%) | ||
| >1.0 mm | NA | 96 (23.3%) | 330 (23.8%) | ||
| Missing/unknown | NA | 143 (34.7%) | 847 (61.0%) | ||
| Status at end of follow-up | Alive | 60 (10.0%) | 120 (29.1%) | 467 (33.6%) | 0.098 |
| Dead | 542 (90.0%) | 292 (70.9%) | 922 (66.4%) | ||
| Missing | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Follow-up time (months) | 12 (6-20) | 21 (12-36) | 25(14-47) | 3.10 × 10–5 | |
| Missing | 0 (0%) | 0 (0%) | 0 (0%) |
Continuous variables are shown as medians (IQR). Frequency of missing data is also shown for all variables.
Missing/unknown indicates missing data.
Bold indicates p<0.05.
*Comparison between PR+V and PR–V groups. For multilevel variables (eg, T and N stages, P value indicates differences between frequency distributions between groups).
†According to local reporting criteria, R0 was defined as a 1.5-mm resection margin or greater.
CCI indicates Charlson Comorbidity Index; IQR, interquartile range; NA, not applicable.
RESULTS
In total, 2403 patients with PDAC were included. Of these, 602 underwent exploration only (EXP group), whereas 412 underwent pancreatic resection with (PR+V group) and 1389 (PR–V) without SMV/PV resection. Table 1 provides an overview of the demographic, treatment related as well as outcome parameters of the EXP, PR+V, and PR–V groups.
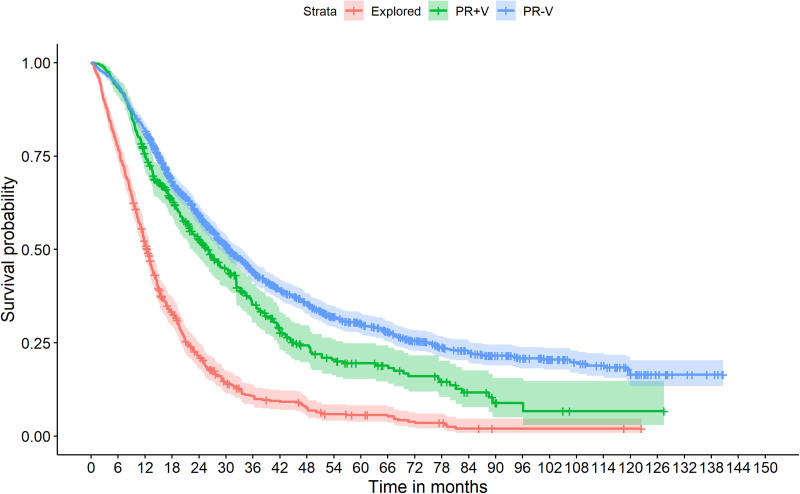
KM survival estimates are shown in Table 2, as well as graphically depicted in Figure 1. Log-Rank test identified a significant survival difference between PR–V and PR+V groups (P = 1 × 10–6). For comparative purposes KM curves were also calculated for PR+V and PR–V patients sub stratified on pathology N-stage (Supplementary Figure 1, http://links.lww.com/AOSO/A183), tumor distance to the SMV/PV (Supplementary Figure 2, http://links.lww.com/AOSO/A183) and adjuvant chemotherapy (Supplementary Figure 3, http://links.lww.com/AOSO/A183).
TABLE 2.
Kaplan-Meier Survival Estimates at 1, 2, and 5 Years Following Surgical Exploration Only as well as Pancreatic Resection With or Without Venous Resection
| Group | Time (years) | Survival Estimate (%) | 95% Confidence Interval |
|---|---|---|---|
| Surgically explored only (n = 709) | 1 | 52 | 48%–56% |
| 2 | 21 | 18%–25% | |
| 5 | 6 | 4%–8% | |
| Pancreatic resection with venous resection (n=423) | 1 | 75 | 71%–80% |
| 2 | 53 | 48%–58% | |
| 5 | 20 | 15%–25% | |
| Pancreatic resection without venous resection (n=1758) | 1 | 82 | 80%–84% |
| 2 | 59 | 57%–62% | |
| 5 | 30 | 27%–33% |
FIGURE 1.
Kaplan-Meier survival curves for patients undergoing surgical exploration only (n = 602), pancreatic resection with venous resection (n = 412), and pancreatic resection without venous resection (n = 1389). Log-Rank test comparing survival curves for pancreatic resections with (PR+V) versus without (PR–V), identified a significant survival difference associated with PR–V (P = 1 × 10–6).
Results from the univariate and multivariate Cox proportional hazard models are shown in Table 3 (univariate) and Table 4 (multivariate).
TABLE 3.
Results of the Univariate Cox Proportional Hazard Ratio Models
| Variable | Subtype | Hazard Ratio | P | |
|---|---|---|---|---|
| Univariate | Resection type* | Pancreatic resection with venous resection | 1.31 | 4.79 × 10–5 |
| Procedure type† | Total pancreatectomy | 1.32 | 0.004 | |
| Distal pancreatectomy | 0.85 | 0.043 | ||
| Demographic | Age | 1.01 | 1.96 × 10–5 | |
| Female gender | 0.85 | 0.005 | ||
| Charlson comorbidity index | 1.08 | 9.53 × 10–8 | ||
| Treatment related | Preoperative Chemotherapy | 0.72 | 0.006 | |
| Postoperative Chemotherapy | 0.79 | 6.00 × 10–4 | ||
| Tumor histology‡ | T2 tumor | 2.36 | 3.49 × 10–6 | |
| T3 tumor | 3.70 | 7.93 × 10–14 | ||
| T4 tumor | 2.89 | 6.41 × 10–6 | ||
| N1 tumor | 2.12 | 2.00 × 10–16 | ||
| N2 tumor | 1.94 | 1.33 × 10–07 | ||
| Resection margins | R1 resection$ | 1.62 | 6.76 × 10–8 | |
| R2 resection$ | 2.95 | 2.30 × 10–4 | ||
| Superior mesenteric Vein Resection Margin in mm | 0.99 | 1.26 × 10–13 |
*Bold indicates p<0.05.
*Compared with resections without venous resection.
†Compared with pancreaticoduodenectomy.
‡Compared with T1 stage (T stages) and N0 (N stages).
$Compared with R0 resection.
TABLE 4.
Results of the Multivariate Cox Proportional Hazard Ratio Models
| Multivariate | Resection Type* | Pancreatic Resection With Venous Resection | 1.11 | 0.408 |
|---|---|---|---|---|
| Procedure type† | Total pancreatectomy | 1.06 | 0.966 | |
| Distal pancreatectomy | 0.84 | 0.851 | ||
| Demographic | Age | 0.99 | 0.841 | |
| Female gender | 1.10 | 0.3823 | ||
| Charlson comorbidity index | 1.07 | 0.011 | ||
| Treatment related | Preoperative Chemotherapy | 0.73 | 0.289 | |
| Postoperative Chemotherapy | 0.49 | 3.80 × 10–7 | ||
| Tumor histology‡ | T2 tumor | 4.96 | 0.001 | |
| T3 tumor | 5.67 | 0.001 | ||
| T4 tumor | 4.12 | 0.027 | ||
| N1 tumor | 2.03 | 9.34 × 10–6 | ||
| N2 tumor | 2.24 | 2.56 × 10–6 | ||
| Resection margins | R1 resection$ | 1.46 | 0.002 | |
| R2 resection$ | 1.92 | 0.156 | ||
| Superior Mesenteric Vein Resection Margin in mm | 0.99 | 0.046 |
*Bold indicates p<0.05.
*Compared with resections without venous resection.
†Compared with pancreaticoduodenectomy.
‡Compared with T1 stage (T stages) and N0 (N stages).
$Compared with R0 resection.
Overall, venous resection (PR+V) was associated with higher Hazard Ratios (HRs) in the univariate model (HR 1.31, P = 4.79 × 10–5), but not in the multivariate model (HR 1.11, P = 0.408).
In the multivariate model, significantly altered risks could be associated with tumor T and N histology classification levels when compared with T1 and N0, respectively (HR ranging from 4.11 to 6.57, P < 0.01), comorbidities as measured by the CCI (HR 1.07, P = 0.011), postoperative chemotherapy (HR 0.49, P = 4.09 × 10–7), and R-resection margins when compared with R0 (R1 HR 1.46, P = 0.002). Free margin in mm from tumor to the SMV/PV was also significantly associated with OS (HR 0.99, P = 0.046).
When the analysis was performed on the imputed dataset (Supplementary Table 1, http://links.lww.com/AOSO/A183), comparable results were obtained, although a significant association with OS and female sex (HR 0.88, P = 0.025) and age (HR 1.01, P = 6.74 × 10–4) could be identified. Of note, no association between OS and PR+V status could be identified (HR 1.05, P = 0.488).
DISCUSSION
When assessed through univariate modeling, PR+V was significantly associated with shorter OS, findings that were confirmed by KM survival estimates (5-year survival rates of 20% in PR+V vs 30% in PR–V). However, 2-year OS estimates were comparable between groups (53% vs 59%).
Findings from the univariate modeling could, however, not be confirmed in multivariate models corrected for relevant confounders, neither in the original nor in the imputed datasets. These findings contrast with previous reports indicating shorter OS for PR+V patients, regardless of resection margin status (R0, R1 etc.),9,10,12 although they are in line with other reports indicating comparable outcomes.11 A recent systematic review furthermore supports the use of venous resection (grade B evidence).12
As expected, results indicate that factors such as comorbidities (as measured by the CCI), postoperative chemotherapy, histology T and N classification and achievable resection margins (R0, R1) status and margin in mm to the SMV/PV all impact on survival rates and should be included in the risk assessment. Overall, these results are in line with other studies indicating worse outcomes associated with advanced T16 and N17 stages, CCI classification18 and non-R0 resection rates19 as well as tumor involvement of the SMV/PV.20 Although venous resection in distal pancreatectomies is rare,21 comprising only 4% of venous resection procedures in this dataset, our analyses could not identify a significant survival differences associated with this procedure type, nor total pancreatectomy. These findings are in line with other reports indicating comparable outcomes for vein patency following PR+V irrespective of procedure type.22
Collectively, the univariate and multivariate modeling suggest that while PR+V is associated with inferior outcomes, this effect may not be due to the venous resection per se. Rather, comorbidity, and tumor-related parameters (T and N-stage, R0-resection rates, and tumor distance to the SMV/PV) may be driving factors. In line with this, KM survival estimates of 2-year survival of 55% in the PR+V group versus 22% in the EXP group supports that the surgeon should not defer from proceeding with PR+V if venous involvement is unexpectedly encountered perioperatively and acceptable resection margins can be achieved, as opposed to aborted surgery and chemotherapy alone.
As tumor distance to the SMV/PV was found to be inversely associated with inferior outcomes, it would be tempting to use this data to advocate for the use of neoadjuvant therapy as opposed to upfront surgery in BR patients with suspected SMV/PV involvement. Although it could be argued that SMV/PV invasion and venous resection are two sides of the same coin, it is important to note that margins of 0.5 mm or less from tumor to the SMV/PV were only identified in 29.8% of the PR+V pathology specimens, thus indicating that only about 1/3 of patients had actual tumor involvement of the SMV/PV, with the remainder likely undergoing venous resection due to inflammation perceived to be tumor ingrowth. Surgical evaluation of tumor ingrowth into the SMV/PV may thus be suboptimal to histological evaluation when assessing outcomes, a notion that is supported by reports indicating that both CT and ultrasound (including endoscopic) imaging also have suboptimal sensitivity for detecting SMV/PV involvement in borderline cases.23,24 Therefore, opting for neoadjuvant therapy due to perceived SMV/PV involvement based on imaging findings only, may lead to an overuse of the downstaging regimen in patients who are candidates for upfront resection.
Previously published results have recommended neoadjuvant chemotherapy in BR patients,3,12,25 which also includes those with SMV/PV involvement as per NCCN criteria, although considerable debate still surrounds this issue. Current NCCN and the American Society of Clinical Oncology (ASCO) guidelines suggests the use of neoadjuvant chemotherapy in BR patients,3,26 whereas the European Society of Medical Oncology (ESMO) recommends neoadjuvant therapy only for patients included in clinical trials, with the option to consider this modality in nontrial patients.27 To a large extent, the lack of consensus stems from data coming from heterogeneous studies utilizing different neoadjuvant strategies sometimes also combined with radiotherapy approaches. Reports from these studies suggest that increased rates of R0 resections can be achieved through chemoradiotherapy compared with upfront surgery28 in BR patients as well as for those with locally advanced disease, although it remains debatable whether the increased R0 resection rate could be translated into increased survival. Furthermore, in a study of 48 BR patients selected for a neoadjuvant chemoradiotherapy approach, only 67% of patients proceeded to surgical exploration, thus indicating a substantial loss compared with upfront surgery where feasible.28 Studies have, however, indicated that for patients achieving R0 resection rates following neoadjuvant approaches, a survival benefit could be demonstrated,29 although the majority of this evidence is based on small, nonrandomized studies.
This study was not designed for assessing the value of preoperative chemotherapy and care should be taken when drawing the conclusion of whether these data support this recommendation for BR patients. First, neoadjuvant therapy was not the nationwide standard for BR patients in Denmark during the study period and was only used for downstaging locally advanced PDAC. As such, this is reflected in the incidence difference of patients receiving preoperative chemotherapy with subsequent PR+V (16%) versus PR–V (4%).
Furthermore, we were not able to retrieve information on chemotherapeutic agents used, doses given nor the duration of treatment, an issue that was also mirrored when assessing the effect of adjuvant chemotherapy. While the use adjuvant chemotherapy is thus supported by these results, limitations in the underlying data should be acknowledged.
While lymph node involvement (N-stage), is likely the key factor regulating OS, achieving higher R0 resection rates either assisted by neoadjuvant therapy or through upfront surgery and mesopancreatic resection approaches, appears to be another important factor impacting on survival rates. When interpreting these data in comparison with other studies, it is, however, important to note the lack of consistency in R0 definition and thus reporting across studies. As mentioned, national pathology protocols used by all 4 centers in Denmark with standardized training, defines R0 as a >1.0 mm margin, as opposed to the internationally adopted margin of ≥1 mm.14 As margins are reported in 0.5 mm increments, a ≥1.5 mm reported margin will thus be reported for R0, although margins between 1 mm and 1.5 mm will also be classified as R0.
As such, reports have indicated that R0 rates are rarely achieved in PR+V (4%) versus PR–V (46%)30 patients, which is supported by comparable numbers extracted from a meta analysis.14 These findings could not be replicated in this study where R0 resection rates were comparable between PR+V and PR–V (38% vs 44%) groups. Differences can only to a minor degree be attributed to the surgical approach, including the artery first and mesopancreatic resection techniques, although these have been reported to result in higher R0 rates.31,32 In contrast, it is important to note that R0 rates may be dependent on both definitions as well as the pathological examination technique and reporting standards (bivalving or axial slicing),33 which would influence the reported R0 rates.
This study has several limitations that should be acknowledged. First, as is the case for any retrospective study, we can only observe associations and thus not draw conclusions on causality. Conclusions are furthermore dependent on the underlying data integrity and completeness, and fluctuations in data completeness and quality could impact on results. We have sought to address the latter issue by including a sensitivity analysis based on imputed data, with overall comparable findings, albeit with results suggesting that the study may suffer from statistical power issues. Most notably, the degree of missing data for R0 resection rates and SMV/PV margins, could likely have impacted on results.
Furthermore, results can only be corrected for known and registered confounding factors, and we thus cannot say whether the inclusion of other covariates would have altered findings.
Most notably, the data did not allow for a stratification of the chosen pre or postoperative chemotherapeutic regimen nor patient adherence to these. Also, whether patients were evaluated as resectable, BR, or locally advanced preoperatively, could not be determined. PR+V patients are more likely to also have undergone arterial resections, but this event is only inconsistently recorded in the dataset and thus not included in the present analyses.
Finally, as data spans treatment outcomes over almost a decade, advances in both surgical and oncological treatment would likely impact on results, as would differences in center volumes. As such, these factors should be considered when interpreting the presented results.
In conclusion, this study suggests that patients undergoing pancreatic resection requiring venous resection for PDAC, may have inferior survival outcomes compared with patients not requiring venous resection, although these differences are likely due to advanced tumor stages rather than the need for SMV/PV resection per se. Collectively, these results thus support the PR+V approach when required, and furthermore suggest that the procedure should be considered when vascular involvement necessitating vascular resection is encountered perioperatively, regardless of whether there is actual tumor invasion of the SMV/PV or just inflammatory changes in the perivascular tissue.
ACKNOWLEDGEMNTS
We would like to acknowledge the work of Jens Hillingsø and Frank Mortensen in providing critical review of the article.
Supplementary Material
Funding Statement
Disclosure: The authors declare that they have nothing to disclose.
Footnotes
Published online 2 November 2022
Supported by a grant (#NNF19OC0055183) to M.S. Other authors did not receive funding support.
Conflicts of interest: The authors declare no conflicts of interest or financial disclosures.
The study was supported by a grant (#NNF19OC0055183) to MS.
Disclosure: The authors declare that they have nothing to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.annalsofsurgery.com).
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Bengtsson A, Andersson R, Ansari D. The actual 5-year survivors of pancreatic ductal adenocarcinoma based on real-world data. Sci Rep. 2020;10:16425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tempero MA, Arnoletti JP, Behrman SW, et al. Pancreatic adenocarcinoma, version 2.2012: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2012;10:703–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopez NE, Prendergast C, Lowy AM. Borderline resectable pancreatic cancer: definitions and management. World J Gastroenterol. 2014;20:10740–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vauthey JN, Dixon E. AHPBA/SSO/SSAT consensus conference on resectable and borderline resectable pancreatic cancer: rationale and overview of the conference. Ann Surg Oncol. 2009;16:1725–6. [DOI] [PubMed] [Google Scholar]
- 6.Katz MHG, Marsh R, Herman JM, et al. Borderline resectable pancreatic cancer: need for standardization and methods for optimal clinical trial design. Ann Surg Oncol. 2013;20:2787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fortner JG, Kim DK, Cubilla A, et al. Regional pancreatectomy: en bloc pancreatic, portal vein and lymph node resection. Ann Surg. 1977;186:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Storkholm JH, Hansen CP. Mesenterico-portal vein resection in patients with pancreatico-duodenal cancer is safe and may increase survival. Dan Med J. 2014;61:A4757. [PubMed] [Google Scholar]
- 9.Anger F, Döring A, Schützler J, et al. Prognostic impact of simultaneous venous resections during surgery for resectable pancreatic cancer. HPB. 2020;22:1384–93. [DOI] [PubMed] [Google Scholar]
- 10.Groen J, Michiels N, van Roessel S, et al. Venous wedge and segment resection during pancreatoduodenectomy for pancreatic cancer: impact on short- and long-term outcomes in a nationwide cohort analysis. Br J Surg. 2021;109:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feo CF, Deiana G, Ninniri C, et al. Vascular resection for locally advanced pancreatic ductal adenocarcinoma: analysis of long-term outcomes from a single-centre series. World J Surg Oncol. 2021;19:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delpero JR, Sauvanet A. Vascular resection for pancreatic cancer: 2019 french recommendations based on a literature review from 2008 to 6-2019. Front Oncol. 2020;10:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019;13(Suppl 1):S31–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leonhardt CS, Niesen W, Kalkum E, et al. Prognostic relevance of the revised R status definition in pancreatic cancer: meta-analysis. BJS Open. 2022;6:zrac010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; 2014. http://www.R-project.org/. [Google Scholar]
- 16.Schlitter AM, Jesinghaus M, Jäger C, et al. pT but not pN stage of the 8th TNM classification significantly improves prognostication in pancreatic ductal adenocarcinoma. Eur J Cancer. 2017;84:121–9. [DOI] [PubMed] [Google Scholar]
- 17.Lu TP, Wu CH, Chang CC, et al. Distinct survival outcomes in subgroups of stage iii pancreatic cancer patients: Taiwan cancer registry and surveillance, epidemiology and end results registry. Ann Surg Oncol. 2022;29:1608–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bagni K, Chen IM, Johansen AZ, et al. Prognostic impact of Charlson’s Age-Comorbidity Index and other risk factors in patients with pancreatic cancer. Eur J Cancer Care (Engl). 2020;29:e13219. [DOI] [PubMed] [Google Scholar]
- 19.Tummers WS, Groen JV, Sibinga Mulder BG, et al. Impact of resection margin status on recurrence and survival in pancreatic cancer surgery. Br J Surg. 2019;106:1055–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Navez J, Bouchart C, Lorenzo D, et al. What should guide the performance of venous resection during pancreaticoduodenectomy for pancreatic ductal adenocarcinoma with venous contact? Ann Surg Oncol. 2021;28:6211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu D, Wu P, Zhang K, et al. The short-term outcomes of distal pancreatectomy with portal vein/superior mesenteric vein resection. Langenbeck’s Arch Surg. 2022;407:2161–2168. [DOI] [PubMed] [Google Scholar]
- 22.Dua MM, Tran TB, Klausner J, et al. Pancreatectomy with vein reconstruction: technique matters. HPB. 2015;17:824–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clanton J, Oh S, Kaplan SJ, et al. Does mesenteric venous imaging assessment accurately predict pathologic invasion in localized pancreatic ductal adenocarcinoma? HPB (Oxford). 2018;20:925–31. [DOI] [PubMed] [Google Scholar]
- 24.Yamada K, Kawashima H, Ohno E, et al. Diagnosis of vascular invasion in pancreatic ductal adenocarcinoma using endoscopic ultrasound elastography. BMC Gastroenterol. 2020;20:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Versteijne E, van Dam JL, Suker M, et al. Neoadjuvant chemoradiotherapy versus upfront surgery for resectable and borderline resectable pancreatic cancer: long-term results of the Dutch randomized PREOPANC trial. J Clin Oncol. 2022;40:1220–30. [DOI] [PubMed] [Google Scholar]
- 26.Khorana AA, McKernin SE, Berlin J, et al. Potentially curable pancreatic adenocarcinoma: ASCO clinical practice guideline update. J Clin Oncol. 2019;37:2082–8. [DOI] [PubMed] [Google Scholar]
- 27.Ducreux M, Cuhna AS, Caramella C, et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26:v56–68. [DOI] [PubMed] [Google Scholar]
- 28.Murphy JE, Wo JY, Ryan DP, et al. Total neoadjuvant therapy with FOLFIRINOX followed by individualized chemoradiotherapy for borderline resectable pancreatic adenocarcinoma: a phase 2 clinical trial. JAMA Oncol. 2018;4:963–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giovinazzo F, Soggiu F, Jang J-Y, et al. Gemcitabine-based neoadjuvant treatment in borderline resectable pancreatic ductal adenocarcinoma: a meta-analysis of individual patient data. Front Oncol. 2020;10:1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleive D, Labori KJ, Line P-D, et al. Pancreatoduodenectomy with venous resection for ductal adenocarcinoma rarely achieves complete (R0) resection. HPB. 2020;22:50–7. [DOI] [PubMed] [Google Scholar]
- 31.Jiang X, Yu Z, Ma Z, et al. Superior mesenteric artery first approach can improve the clinical outcomes of pancreaticoduodenectomy: a meta-analysis. Int J Surg. 2020;73:14–24. [DOI] [PubMed] [Google Scholar]
- 32.Safi SA, Haeberle L, Fluegen G, et al. Mesopancreatic excision for pancreatic ductal adenocarcinoma improves local disease control and survival. Pancreatology. 2021;21:787–95. [DOI] [PubMed] [Google Scholar]
- 33.Wang H, Chetty R, Hosseini M, et al. Pathologic examination of pancreatic specimens resected for treated pancreatic ductal adenocarcinoma: recommendations from the pancreatobiliary pathology society. Am J Surg Pathol. 2022;46:754–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.