Abstract
Envelope glycoproteins gH and gL, which form a complex, are conserved throughout the family Herpesviridae. The gH-gL complex is essential for the fusion between the virion envelope and the cellular cytoplasmic membrane during penetration and is also required for direct viral cell-to-cell spread from infected to adjacent noninfected cells. It has been proposed for several herpesviruses that gL is required for proper folding, intracellular transport, and virion localization of gH. In pseudorabies virus (PrV), glycoprotein gL is necessary for infectivity but is dispensable for virion localization of gH. A virus mutant lacking gL, PrV-ΔgLβ, is defective in entry into target cells, and direct cell-to-cell spread is drastically reduced, resulting in only single or small foci of infected cells (B. G. Klupp, W. Fuchs, E. Weiland, and T. C. Mettenleiter, J. Virol. 71:7687–7695, 1997). We used this limited cell-to-cell spreading ability of PrV-ΔgLβ for serial passaging of cells infected with transcomplemented virus by coseeding with noninfected cells. After repeated passaging, plaque formation was restored and infectivity in the supernatant was observed. One single-plaque isolate, designated PrV-ΔgLPass, was further characterized. To identify the mutation leading to this gL-independent infectious phenotype, Southern and Western blot analyses, radioimmunoprecipitations, and DNA sequencing were performed. The results showed that rearrangement of a genomic region comprising part of the gH gene into a duplicated copy of part of the unique short region resulted in a fusion fragment predicted to encode a protein consisting of the N-terminal 271 amino acids of gD fused to the C-terminal 590 residues of gH. Western blotting and radioimmunoprecipitation with gD- and gH-specific antibodies verified the presence of a gDH fusion protein. To prove that this fusion protein mediates infectivity of PrV-ΔgLPass, cotransfection of PrV-ΔgLβ DNA with the cloned fusion fragment was performed, and a cell line, Nde-67, carrying the fusion gene was established. After cotransfection, infectious gL-negative PrV was recovered, and propagation of PrV-ΔgLβ on Nde-67 cells produced infectious virions. Thus, a gDH fusion polypeptide can compensate for function of the essential gL in entry and cell-to-cell spread of PrV.
Initiation of herpesvirus infection is thought to require a cascade of interactions between different viral and cellular components. In the alphaherpesviruses at least four envelope glycoproteins, gB, gD, gH, and gL, are essential for infectious entry of virions into target cells, a process which involves fusion between the virion envelope and the cellular cytoplasmic membrane at neutral pH (18, 33, 34). These proteins are generally also necessary for direct spread of infectivity from infected to adjacent noninfected cells. Whereas gB, gH, and gL are conserved throughout the Herpesviridae, which might be indicative of a common herpesvirus-specific entry mechanism, gD is present in only several alphaherpesviruses. Interestingly, gD is required for direct cell-to-cell spread of, e.g., herpes simplex virus type 1 (HSV-1) (15) or bovine herpesvirus 1 (BHV-1) (3) but is dispensable for this process in pseudorabies virus (PrV) (23, 24). Thus, gD-negative PrV mutants are able to spread by direct cell-to-cell transmission. Based on this property, we recently isolated, by repeated copassaging of infected and noninfected cells, a PrV mutant which is infectious in the absence of gD (29). A similar finding has been reported for BHV-1 (31). Thus, during passaging a compensatory mutation(s) which rendered gD nonessential for virion infectivity occurred. For BHV-1, these mutations have been shown to include a point mutation in the gH gene (31).
The gH-gL complex is conserved throughout the herpesviruses, although the nature of the linkage between the two components may differ. In various systems it has been shown that gH requires gL for proper folding and intracellular transport (2, 6, 32, 36, 37). In HSV-1, virion localization of gH requires the presence of gL and vice versa (25). Thus, gH-negative virions invariably also lack gL, and gL-negative virions fail to contain gH. Therefore, the contributions of the individual complex partners to function is difficult to analyze. We recently reported the isolation of a gL-negative PrV mutant, PrV-ΔgLβ, which lacks gL and requires transcomplementation by gL-expressing cells for productive infection (13). Contrary to the situation in HSV-1, PrV-ΔgLβ virions contain gH despite the absence of gL. Virion gH of PrV-ΔgLβ carries N-linked carbohydrates which are processed to a greater extent than in the presence of gL. Despite its location in the virion, gH of PrV-ΔgLβ is apparently not able to mediate infectivity. Polyethylene glycol-induced fusion partially overcomes the defect in PrV-ΔgLβ, indicating a deficiency in penetration. Correlating with the entry defect, PrV-ΔgLβ is drastically impaired in direct cell-to-cell spread, resulting in only single or small foci of infected cells when noncomplementing cells were inoculated with gL-transcomplemented PrV-ΔgLβ virions. Based on our results with gD− PrV, we used this (albeit very limited) cell-to-cell spreading ability for passaging of PrV-ΔgLβ-infected cells by coseeding with noninfected cells. We report here the isolation by this procedure of a PrV mutant which is infectious even in the absence of gL. We also demonstrate that a fusion between gD and gH is responsible for mediating PrV infection in the absence of gL.
MATERIALS AND METHODS
Viruses and cells.
Virus mutants are based on the Kaplan strain of PrV (PrV-Ka) (7). PrV-1112 carries a lacZ expression cassette within the nonessential gG locus (19) (Table 1). PrV-ΔgLβ carries a deletion of most of the gL gene and concomitant insertion of a gG-lacZ expression cassette (13, 19). PrV-ΔgLPassgC+ was isolated after cotransfection of PrV-ΔgLPass DNA with a plasmid containing the gC-coding region (30). gC-positive recombinants were identified by black-plaque assay with a gC-specific monoclonal antibody (MAb). PrV-ΔgLBsp was isolated after cotransfection (5) of PrV-ΔgLβ DNA with a cloned copy of the BspEI fragment comprising the gDH fusion gene as well as the gG, gD, gI, and gE genes (see Fig. 6). African green monkey (Vero), bovine (MDBK), rabbit (RK-13), and porcine (PK15 and PSEK) kidney cells were used. VnB6 cells are Vero cells stably carrying genomic BamHI fragment 6 of PrV. This fragment encompasses the gL gene, and VnB6 cells complement the defect in PrV-ΔgLβ (13). Nde-67 cells are RK-13 cells stably transfected with an NdeI fragment originating from PrV-ΔgLPass DNA (see Fig. 6).
TABLE 1.
Phenotypes of virus mutants used in this study
| Virus mutant | Parental strain | Phenotype
|
||||
|---|---|---|---|---|---|---|
| gL | gC | gH | gD | gDH | ||
| PrV-1112 | PrV-Ka | + | + | + | + | − |
| PrV-ΔgLβ | PrV-Ka | − | + | + | + | − |
| PrV-ΔgLPass | PrV-ΔgLβ | − | − | − | + | + |
| PrV-ΔgLPassgC+ | PrV-ΔgLPass | − | + | − | + | + |
| PrV-ΔgLBsp | PrV-ΔgLβ | − | + | + | + | + |
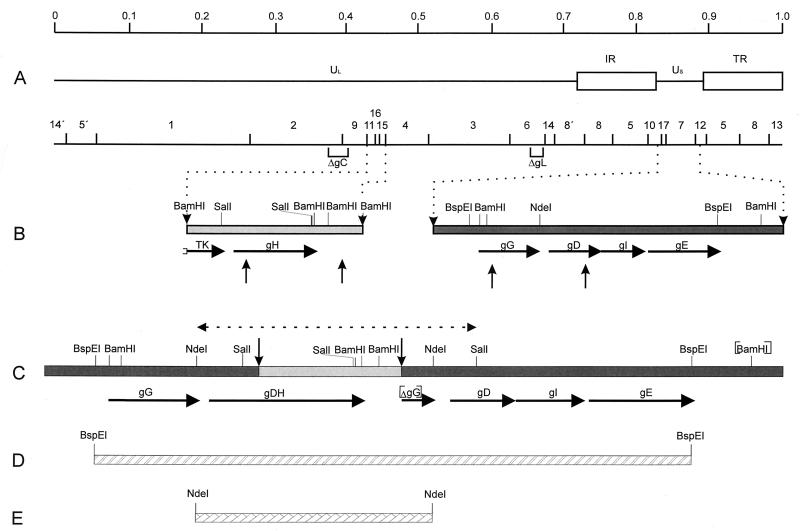
FIG. 6.
Genomic arrangement in PrV-ΔgLPass. (A) BamHI restriction fragment map below a schematic diagram of the wild-type PrV genome. UL, unique long region; US, unique short region; IR, internal repeat; TR, terminal repeat. In the BamHI restriction fragment map, deletions in the gC and gL genes within PrV-ΔgLPass are indicated. (B) Enlargement of the gH gene region and the US region. Vertical arrows point to sites of recombination. (C) Arrangement of the US region in PrV-ΔgLPass, containing the translocation of part of the gH gene region fused to a duplicated copy of the gD gene. [BamHI] indicates absence of BamHI cleavage site separating fragments 7 and 12, and [ΔgG] denotes the presence of a second, truncated copy of the gG gene. The region verified by sequencing is indicated by a dashed line bounded by arrowheads. (D) Location of the BspEI fragment used for rescue of infectivity in PrV-ΔgLβ. (E) Location of the NdeI fragment contained in cell line Nde-67.
Passaging of gL− PrV.
Vero cells were infected at a multiplicity of infection (MOI) of 0.01 with PrV-ΔgLβ which had been phenotypically complemented by propagation on VnB6 cells. Cells were repeatedly split 1:3 until a complete cytopathic effect (CPE) was observed. After development of CPE, cells were trypsinized and reseeded at a ratio of approximately 1:10 with noninfected Vero cells in 75-cm2 tissue culture flasks in 10 ml of medium. After complete CPE developed, the remaining adherent cells were again trypsinized and reseeded. Supernatant from every passage was cleared from cells and cellular debris by centrifugation and titrated on Vero cells.
Antibodies and antisera.
MAbs A13-d6-3 (anti-gH) and c14-c27 (anti-gD) were used for radioimmunoprecipitation and neutralization assays. For Western blotting, anti-gB MAb b43-b5, anti-gC MAb B16-c8, and anti-gE MAb A9-b15-26 were used. Preparation of polyclonal gL-specific serum directed against a glutathione S-transferase (GST)–gL fusion protein (13) and of polyclonal gD-specific serum directed against vaccinia virus-expressed gD (12) has been described previously. To obtain a polyclonal antiserum specific for gH, a GST-gH fusion protein containing amino acids (aa) 88 to 632 of PrV gH was used for immunization of a rabbit.
Southern blot analysis and sequencing.
DNA sequencing by the dideoxy chain termination method (28) with double-stranded plasmid DNA was performed as described in detail before (10). The sequenced portion of 4.6 kbp is indicated in Fig. 6 and extended from the NdeI site within the intact gG gene to the SalI site within the intact gD gene. Southern blot analysis of BamHI-restricted viral DNA was by standard procedures (14, 27).
Western blotting and radioimmunoprecipitation.
Western blotting (13) and radioimmunoprecipitation (16) were performed as described previously.
One-step growth analysis and penetration kinetics.
One-step growth analysis was performed essentially as described previously (13). Assay of penetration kinetics by using low-pH inactivation of extracellular virus has been described previously (17). The input virus amount was ca. 500 PFU per well of a six-well tissue culture plate.
Neutralization assays.
Approximately 200 PFU of each virus mutant was incubated with the appropriate dilution of antiserum or hybridoma supernatant in a 200-μl volume for 1 h at 37°C without addition of complement. Thereafter, assays were used for inoculation of Vero cells in 24-well tissue culture plates. After 1 h at 37°C, the inoculum was replaced by semisolid methylcellulose medium. After 2 days, the monolayers were fixed and stained with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside), and plaques were counted. The percentage of plaques compared to that for controls with the same amount of preimmune serum or nonneutralizing hybridoma supernatant is reported.
RESULTS
Isolation of infectious gL− PrV.
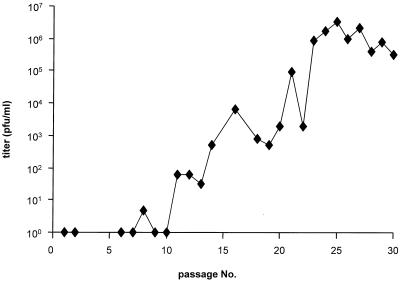
PrV gL has been found to be required for virion infectivity. Virions lacking gL are unable to penetrate into target cells and initiate infection. Correlating with the entry defect, cell-to-cell spreading ability is also drastically reduced in the absence of gL (13). Upon infection of noncomplementing cells with phenotypically gL-complemented PrV-ΔgLβ, only single infected cells or small foci of infection arise (13) (see also Fig. 2). Based on our results after serial passaging of a gD-negative PrV mutant which resulted in the isolation of an infectious gD− PrV (29), we reasoned that the limited cell-to-cell spread capability of gL− PrV might be sufficient for performing serial passages by repeatedly coseeding infected and noninfected cells. Cleared supernatant from every passage was analyzed for the presence of extracellular infectious virions. As shown in Fig. 1, within the first 10 passages no or only very few extracellular infectious units were detected. Interestingly, plaque formation appeared to be restored as early as passage 5 (data not shown). Beginning at passage 10, infectivity in the supernatant started to appear, and it subsequently rose, reaching titers of between 106 and 107 PFU/ml after passage 25. From the infectious supernatant of passage 25, six single-plaque isolates were picked, and viral DNA was analyzed after cleavage with BamHI by Southern blotting. Since all six isolates exhibited similar restriction profiles, one isolate was randomly chosen for further analysis and was designated PrV-ΔgLPass.
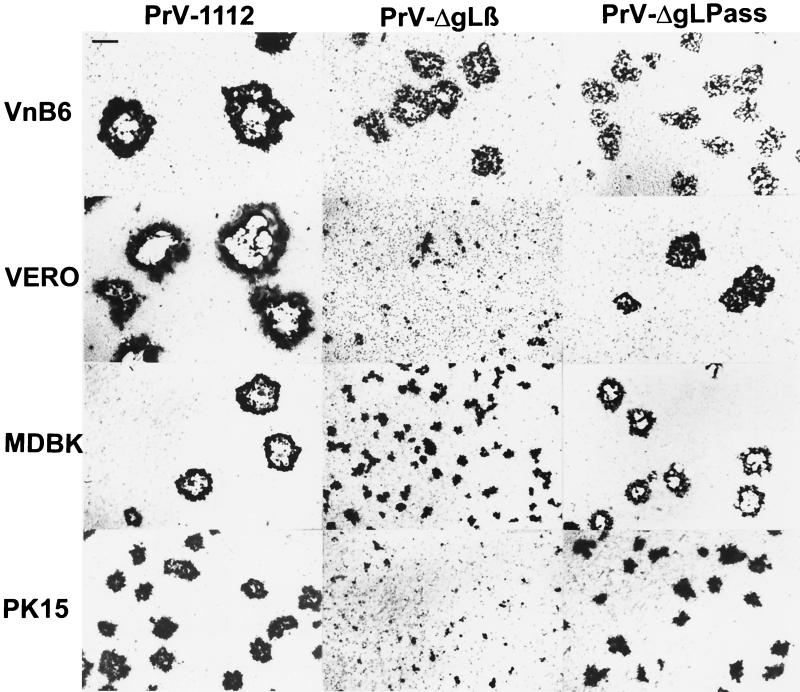
FIG. 2.
Plaque formation by PrV-ΔgLPass. Plaque formation by phenotypically complemented PrV-ΔgLβ and of PrV-ΔgLPass propagated on Vero cells was assayed on gL-complementing VnB6 cells or noncomplementing Vero, MDBK, and PK15 cells. At 2 days postinfection, cells were stained with X-Gal. As a control, PrV-1112, which carries a gG-lacZ expression cassette in the nonessential gG locus, was used. Bar, 500 μm.
FIG. 1.
Selection of infectious gL-negative PrV by passaging in cell culture. Vero cells were infected with phenotypically complemented PrV-ΔgLβ. After development of CPE, cells were trypsinized and reseeded with uninfected cells. The cleared supernatant of each passage was checked for the presence of extracellular infectious virus by titration on Vero cells.
Plaque formation of PrV-ΔgLPass.
To assay the ability of PrV-ΔgLPass to form plaques on different noncomplementing cells, Vero, MDBK, and PK15 cells were infected with PrV-ΔgLβ grown on VnB6 cells or with PrV-ΔgLPass propagated on Vero cells. Two days after infection under plaque assay conditions, monolayers were fixed and stained with X-Gal. As a control, wild-type-like β-galactosidase-expressing PrV-1112 was included. As shown in Fig. 2, PrV-ΔgLβ was able to form plaques only on complementing VnB6 cells, whereas only single infected cells or small foci of infection were observed on noncomplementing cells. In contrast, PrV-ΔgLPass was able to form plaques on Vero, MDBK, and PK15 cells, although they were smaller than those formed by PrV-1112. Plaques formed by PrV-ΔgLPass on VnB6 cells were similar in size to those on noncomplementing Vero cells, which indicates that the presence of gL is neither required nor even beneficial for plaque formation of PrV-ΔgLPass. Thus, PrV-ΔgLPass gained the capacity to form plaques in the absence of gL.
Genome analysis of PrV-ΔgLPass.
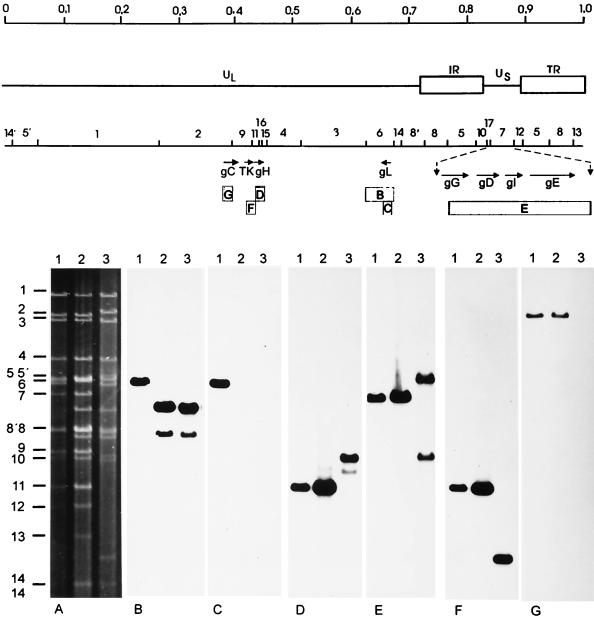
To analyze the genotype of PrV-ΔgLPass, DNAs of wild-type PrV-Ka (Fig. 3, lanes 1), noninfectious PrV-ΔgLβ (Fig. 3, lanes 2), and infectious PrV-ΔgLPass (Fig. 3, lanes 3) were isolated, cleaved with BamHI, and separated on a 0.8% agarose gel. Hybridization with radiolabelled fragment BamHI-6, which encompasses the gL gene (Fig. 3B), showed identical profiles for PrV-ΔgLβ and PrV-ΔgLPass, indicating that the gL mutation is still present in PrV-ΔgLPass. This was confirmed after hybridization with a gL-specific probe (Fig. 3C), which failed to hybridize to DNA of PrV-ΔgLβ and PrV-ΔgLPass but correctly recognized BamHI fragment 6 in PrV-Ka DNA. Hybridization with a gH-specific probe (Fig. 3D) resulted in the detection of BamHI fragment 11, which encompasses the gH gene, in wild-type PrV and in PrV-ΔgLβ. Interestingly, a fragment of 3.9 kb which nearly comigrates with BamHI-10 was recognized in DNA of PrV-ΔgLPass, whereas BamHI-11 was absent. Labelled BamHI fragment 7, which contains most of the US region of PrV (Fig. 3E), correctly detected the homologous 6.6-kb BamHI-7 in PrV-Ka and PrV-ΔgLβ, whereas two fragments of 8.2 and 3.9 kb were observed in PrV-ΔgLPass. The smaller of these fragments was identical in size to that detected by the gH-specific probe. The larger fragment results from fusion of BamHI-12 to BamHI-7 due to loss of a BamHI cleavage site (see Fig. 6). A probe specific for the thymidine kinase gene, which is situated immediately upstream from the gH gene (Fig. 3F), recognized BamHI-11 in PrV-Ka and PrV-ΔgLβ, as anticipated. In PrV-ΔgLPass, a significantly smaller fragment of 1.7 kb was detected. When assayed with a gC-specific probe, correct hybridization of BamHI-2 containing the gC gene was observed in PrV-Ka and PrV-ΔgLβ, whereas no signal could be detected in DNA of PrV-ΔgLPass. In summary, these data show that in PrV-ΔgLPass (i) a rearrangement occurred in the gH and US regions, (ii) gH and US sequences could have been fused, and (iii) gC sequences were lost.
FIG. 3.
Southern blot analysis of mutant viruses. Southern blot analysis was performed with DNAs of wild-type PrV-Ka (lanes 1), PrV-ΔgLβ (lanes 2), and PrV-ΔgLPass (lanes 3) after cleavage with BamHI. The upper part of the figure shows a genomic and BamHI restriction map of the PrV genome, with part of the unique short region (US) enlarged and genes of interest indicated by arrows. Locations of hybridization probes B to F are shown by boxes. In the lower part of the figure, the ethidium bromide stained gel is shown in panel A. The other panels depict hybridization with BamHI-fragment 6 (B), a gL-specific probe (C), a gH-specific probe (D), BamHI fragment 7 (E), or a gC-specific probe (G).
Analysis of glycoproteins of PrV-ΔgLPass.
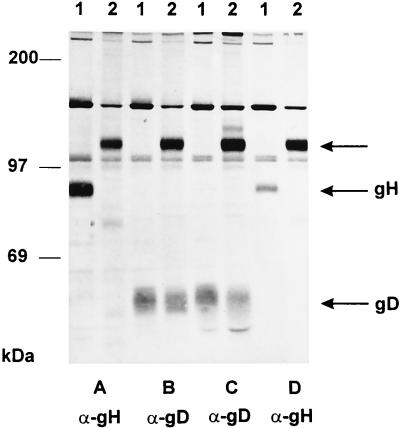
Southern blot analysis indicated a genomic rearrangement which affected at least the gH gene. To analyze the presence of glycoproteins in virions, immunoprecipitation experiments were first performed (Fig. 4). An approximately 120-kDa protein was precipitated from PrV-ΔgLPass virions by a gH-specific MAb (Fig. 4A), a gD-specific MAb (Fig. 4B), an anti-gD polyclonal serum (Fig. 4C), and an anti-gH polyclonal serum (Fig. 4D). The anti-gD antibodies also precipitated wild-type-sized gD from PrV-ΔgLPass virions, whereas no other gH-specific product could be detected. From PrV-Ka virions wild-type gH and gD were precipitated by the respective antibodies, as expected. Thus, the 120-kDa protein of PrV-ΔgLPass virions is recognized by both gH- and gD-specific antibodies. This either could be indicative of complex formation between a 120-kDa gH-derived protein and gD or could suggest the presence of a protein containing gH- and gD-specific epitopes.
FIG. 4.
Radioimmunoprecipitation. [35S]methionine-cysteine-labelled purified virions of wild-type PrV (lanes 1) or PrV-ΔgLPass (lanes 2) were precipitated with a MAb against gH (A) or gD (B) or with polyclonal serum against gD (C) or gH (D). Indicated by arrows are the locations of the 120-kDa protein, gH, and gD. Positions of molecular mass markers are shown on the left.
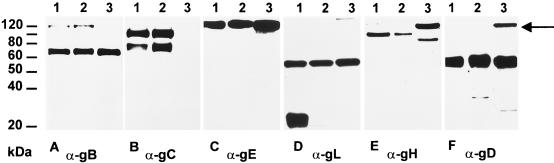
To differentiate between these possibilities and to check for the appearance of other glycoproteins, purified PrV-Ka (Fig. 5, lanes 1), PrV-ΔgLβ (Fig. 5, lanes 2), and PrV-ΔgLPass (Fig. 5, lanes 3) virions were analyzed by Western blotting with antibodies specific for gB (Fig. 5A), gC (Fig. 5B), gE (Fig. 5C), gL (Fig. 5D), gH (Fig. 5E), or gD (Fig. 5F). The 69-kDa subunit of the gB complex and the 130-kDa gE were recognized in all three virion preparations to similar extents. Mature gC and a commonly observed degradation product were detected in PrV-Ka and PrV-ΔgLβ. As anticipated from the Southern blotting results, gC was absent in PrV-ΔgLPass. Mature 20-kDa gL was detected only in PrV-Ka and not in PrV-ΔgLβ and PrV-ΔgLPass, proving the continuing absence of gL in these virus mutants. The approximately 56-kDa protein reacting with the anti-gL serum in all three virion preparations nonspecifically binds the polyclonal serum (12) and serves as an internal control for loading of equivalent amounts of protein. The anti-gH serum reacted with the 95-kDa gH in PrV-Ka and PrV-ΔgLβ virions. However, in PrV-ΔgLPass a prominent protein of ca. 120 kDa reacted with the gH-specific antibodies in addition to a minor 80-kDa band. A 120-kDa protein was also recognized by the gD-specific serum in PrV-ΔgLPass, in addition to wild-type-sized gD. The 80-kDa protein may represent a degradation product of the 120-kDa polypeptide. These data suggested that in PrV-ΔgLPass a protein of 120 kDa which contains gH- and gD-specific epitopes is expressed.
FIG. 5.
Western blot analysis. Purified virions of PrV-Ka (lanes 1), PrV-ΔgLβ (lanes 2), and PrV-ΔgLPass (lanes 3) were lysed, and proteins were separated in a sodium dodecyl sulfate–10% polyacrylamide gel. After electrophoretic transfer, nitrocellulose membranes were probed with antibodies against gB (A), gC (B), gE (C), gL (D), gH (E), and gD (F). After incubation with peroxidase-conjugated secondary antibody, bound antibody was visualized by chemiluminescence recorded on X-ray films.
Genomic rearrangement in PrV-ΔgLPass results in a gD-gH fusion gene.
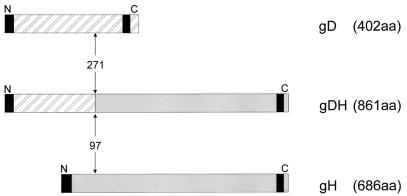
Sequence analysis of the region encompassing the gH gene as well as sequence analysis and restriction fragment mapping of the US region of PrV-ΔgLPass revealed the nature of the genomic rearrangement present in PrV-ΔgLPass. Part of the gH gene, including downstream sequences extending into BamHI-fragment 15, was found to be translocated into a partially duplicated part of the US region, resulting in the presence of wild-type gG, gD, gI, and gE genes, as well as creation of a fusion gene consisting of the first 271 codons of one copy of the gD gene fused to the 3′-terminal 590 codons of the gH gene (Fig. 6). The 3′ part of the gG gene has also been duplicated and is present in front of the wild-type gD gene (ΔgG in Fig. 6). Thus, PrV-ΔgLPass expresses wild-type gD as well as a gDH fusion protein but lacks wild-type gH (see also Fig. 4 and 5). Figure 7 diagramatically depicts the gDH fusion protein. The first 271 aa of the 402-aa PrV gD are fused to the C-terminal 590 aa of the 686-aa gH. Thus, the 861-aa gDH fusion protein contains the predicted N-terminal signal sequence of gD and the C-terminal transmembrane anchor of gH.
FIG. 7.
Diagram of the gDH fusion protein. gD, gH, and the gDH fusion protein are drawn to scale. Vertical arrows mark the site of fusion. Black areas denote hydrophobic predicted signal and transmembrane sequences. Numbers indicate the amino acid position at the fusion site.
A gD-gH fusion protein mediates infectivity of PrV-ΔgLPass.
To verify that the fusion gene is indeed responsible for infectivity of PrV-ΔgLPass, two independent sets of experiments were performed. First, a BspEI fragment encompassing the rearranged portion of the US region (Fig. 6) was cotransfected with DNA of noninfectious PrV-ΔgLβ into Vero cells. As a control, PrV-ΔgLβ DNA was transfected into Vero cells on its own. Whereas neither plaques nor infectivity in the supernatant was observed after transfection of PrV-ΔgLβ DNA alone, plaque formation ensued and infectivity in the supernatant appeared after cotransfection of PrV-ΔgLβ DNA with the BspEI fragment. The resulting virus progeny was designated PrV-ΔgLBsp and was included in further experiments. Due to its origin from PrV-ΔgLβ, PrV-ΔgLBsp differs from PrV-ΔgLPass in that it expresses, in addition to the gDH fusion gene, wild-type gH as well as wild-type gC (Table 1). Second, an RK-13 cell line, designated Nde-67, that stably carries a genomic NdeI fragment comprising the fusion gene was established (Fig. 6). Propagation of PrV-ΔgLβ on Nde-67 cells resulted in infectious progeny (data not shown). These results prove that the gDH fusion protein compensates for the absence of gL in PrV-ΔgLPass.
One-step growth kinetics of PrV mutants.
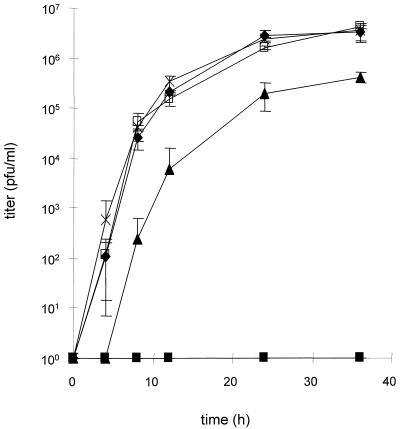
To assay the replicative abilities of the different mutant viruses, Vero cells were infected at an MOI of 10 with wild-type-like PrV-1112, phenotypically gL-complemented PrV-ΔgLβ, PrV-ΔgLPass, and PrV-ΔgLBsp. At various time points, virus titers were determined on Vero cells. The results are shown in Fig. 8. Compared to PrV-1112, PrV-ΔgLPass replicated with a delay and to approximately 10-fold-lower titers. This deficiency was not observed in PrV-ΔgLBsp. Since one of the differences between these two viruses is the absence of gC in PrV-ΔgLPass, gC expression was restored by rescue of the gC deletion, leading to virus mutant PrV-ΔgLPassgC+. Restoration of gC expression fully restores wild-type-like one-step growth kinetics to PrV-ΔgLPass, indicating that the impairment in one-step growth is solely due to the lack of gC and is not correlated with the mutation resulting in gL-independent infectivity. As expected, PrV-ΔgLβ was not able to productively replicate on noncomplementing Vero cells.
FIG. 8.
One-step growth kinetics of PrV-ΔgLPass. Vero cells were infected at an MOI of 10 with PrV-1112 (⧫), phenotypically complemented PrV-ΔgLβ (■), PrV-ΔgLPass (▴), PrV-ΔgLPassgC+ (□), and PrV-ΔgLBsp (×). Cells and supernatants were harvested at the time points indicated and titrated on Vero cells. Averages and standard deviations from three independent experiments are shown.
Penetration kinetics.
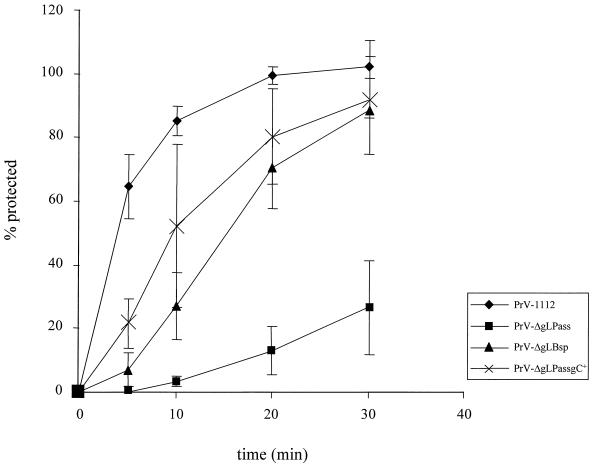
PrV gL has been shown to be required for fusion between the virion envelope and the cellular cytoplasmic membrane (13). To analyze penetration of the different mutant viruses, entry kinetics were determined by using low-pH inactivation of extracellularly remaining virus. As shown in Fig. 9, PrV-ΔgLPass entered cells significantly slower than PrV-1112, with a half time of penetration of >30 min. In contrast, PrV-ΔgLBsp entered cells with a half time of penetration of approximately 20 min. Since the absence of gC has been shown to delay entry of PrV (17), penetration of PrV-ΔgLPassgC+ was also analyzed. Restoration of gC expression resulted in an increase in the rate of entry of PrV-ΔgLPass. Thus, the infectious gL-negative mutants PrV-ΔgLPassgC+ and PrV-ΔgLBsp exhibited an only slight delay in entry compared to PrV-1112.
FIG. 9.
Penetration kinetics of PrV-ΔgLPass. The kinetics of penetration of PrV-1112, PrV-ΔgLPass, PrV-ΔgLBsp, and PrV-ΔgLPassgC+ into Vero cells were analyzed by low-pH inactivation. Indicated is the percentage of PFU which was resistant against treatment with pH 3.0 citrate buffer compared to a phosphate-buffered saline-treated control at different times after the temperature shift. Averages and standard deviations from three independent experiments are indicated.
Neutralization of PrV mutants by anti-gD and anti-gH antibodies.
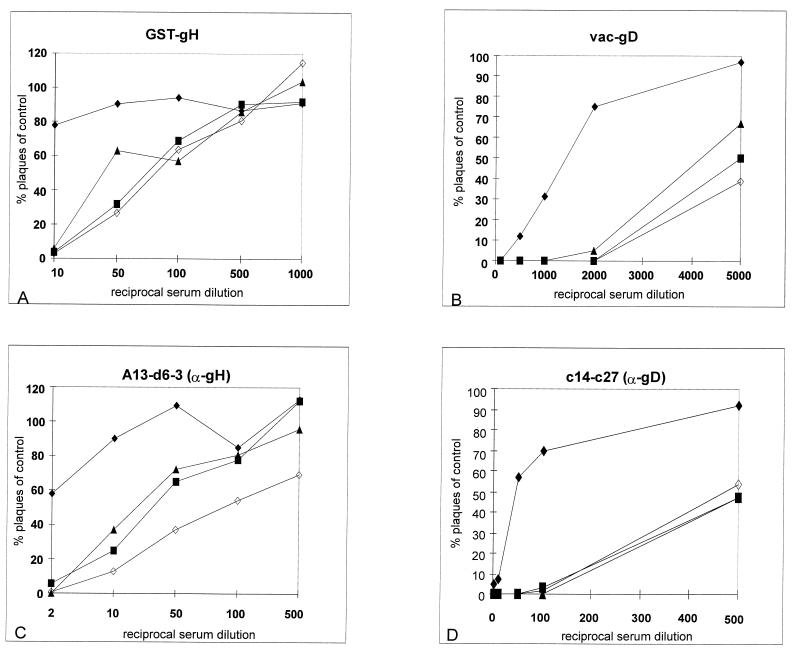
To test for sensitivity of the obtained infectious gL-negative PrV mutants to neutralization by anti-gD and anti-gH antibodies, serial dilutions of a polyclonal serum directed against a GST-gH fusion protein, a polyclonal serum directed against vaccinia virus-expressed gD (11), a MAb against gH, and a MAb against gD were incubated with PrV-1112, PrV-ΔgLPass, PrV-ΔgLBsp, and PrV-ΔgLPassgC+ in the absence of complement. Data were plotted as the percentage of plaques compared to that on a control plate incubated with negative serum or negative control MAb (Fig. 10). It is evident that all three infectious gL-negative virus mutants were significantly more sensitive to neutralization by the anti-gH antibodies and even more so to neutralization by the anti-gD antibodies.
FIG. 10.
Sensitivity of PrV-ΔgLPass to neutralization with anti-gH and anti-gD antibodies. Approximately 200 PFU of PrV-1112 (⧫), PrV-ΔgLPass (■), PrV-ΔgLBsp (▴), and PrV-ΔgLPassgC+ (◊) was incubated with different dilutions of a gH-specific polyclonal serum (A), a gD-specific polyclonal serum (B), an anti-gH MAb (C), or an anti-gD MAb (D) in the absence of complement. Indicated is the percentage of plaques counted in comparison to a control treated with a negative serum.
DISCUSSION
Glycoproteins gB, gH, and gL are conserved throughout the herpesviruses and have been found to be essential for infectivity in every herpesvirus so far analyzed in this respect (33). In several alphaherpesviruses, including PrV, another glycoprotein, gD, is also required for infectious entry of virions into target cells (23, 24). Since gD is dispensable for direct cell-to-cell spread of PrV, copassaging of cells infected with gD− PrV with noninfected cells is possible. After several passages, a PrV mutant which was infectious even in the absence of gD could be isolated (29). Obviously, the function that gD normally executes in infectious entry of PrV was compensated for by another, still unknown mutation(s). This result demonstrated that an essential herpesvirus glycoprotein could become nonessential under selective evolutionary pressure, in this case passaging in cell culture.
We applied the same procedure to a noninfectious gL-negative PrV mutant. PrV gL is required for infectivity of free virions, and gL-negative PrV mutants exhibit a defect in penetration which is correlated with a drastically reduced capacity for direct cell-to-cell spread (13) (Fig. 2). Surprisingly, this rather limited ability to spread directly from cell to cell was sufficient for performing the copassaging experiment. Plaque formation as a reflection of the ability for direct cell-to-cell spread was observed as early as passage 5, whereas infectivity in the cleared supernatant started to rise after passage 10. So far, it is unclear whether different mutational events are responsible for restoration of cell-to-cell spread and infectivity of free virions and whether one is a prerequisite for the other. However, our data clearly show that the gDH fusion protein alone confers both properties to gL-negative PrV-ΔgLβ. After 25 passages, DNAs of six single-plaque isolates were analyzed by restriction endonuclease digestion and Southern blotting and were found to be similar. This indicates that once this mutation occurred, the mutant virus progeny dominated the resulting virus population. Thus, we randomly chose one isolate, PrV-ΔgLPass, for further analysis. With the isolation of an infectious gL-negative PrV mutant, we show for the second time the power of in vitro evolution for overcoming otherwise lethal defects by compensatory mutations. gL is the second glycoprotein which is required for virion infectivity under normal circumstances but can be dispensed with after selection.
In PrV, gH is transported to the virion in the absence of gL (13), as opposed to the situation in HSV-1, where virion localization of gH requires the presence of gL (25). We do not know whether this particular phenotype in PrV was a prerequisite for the success of the copassaging experiment. It could be hypothesized that virion localization of gH on its own is required for the compensating mutation to function. Alternatively, it could be speculated that the gD portion of the gDH fusion protein affects transport and virion localization of gH in the absence of gL. It will be interesting to analyze a similar fusion protein in an HSV-1 background to test whether the different properties of the gH proteins influence the outcome of this experiment.
gD has been assigned two different functions during initiation of herpesvirus infection (33). HSV-1 gD binds cellular receptors which have recently been identified as members of the tumor necrosis factor receptor or immunoglobulin superfamily (4, 20, 35). PrV gD is required for a secondary stable binding of virions to target cells (8). In particular, PrV gD binds to human poliovirus receptor and to poliovirus receptor-related proteins 1 and 2 (4, 35). Moreover, gD is normally required for penetration, and gD-deficient noninfectious mutants of PrV, HSV-1, and BHV-1 can be forced to enter target cells by polyethylene glycol-induced fusion (3, 15, 24). Thus, gD appears to have a function in attachment and in penetration. Currently it is unclear whether the gD portion in the gDH fusion protein is able to execute both normal gD functions. Interestingly, upon sequence alignment, aa 271 of PrV gD correlates with aa 295 of HSV-1 gD (data not shown). The most carboxy-terminal functional region of HSV-1 gD, region IV, is thought to extend to aa 300 (26). Thus, it is striking that the remaining part of gD in gDH matches nearly exactly the proposed functional part of HSV-1 gD (1, 21, 22, 26). We are currently analyzing in detail properties of gDH in mediating receptor binding and initiation of fusion.
Rescue of the infectious phenotype by cotransfection of PrV-ΔgLβ DNA and a fragment comprising the gDH fusion protein gene, as well as successful propagation of normally noninfectious PrV-ΔgLβ on a cell line carrying only the gDH fusion gene, clearly shows that the gDH fusion protein is responsible for mediating infectivity of PrV in the absence of gL. We hypothesize that in the fusion protein the functionally important parts of gD and gH are linked so that the resulting chimeric protein executes the normal roles of both gD and gH in herpesvirus infection. So far it is unclear whether the gDH fusion protein will complement a virus mutant simultaneously lacking gD, gH, and gL. However, it is clear from our data that gDH is able to complement the absence of gH and gL in PrV-ΔgLPass. It has to be emphasized, however, that PrV-ΔgLPass, PrV-ΔgLBsp, and PrV-ΔgLPassgC+ still express normal wild-type gD, whose contribution, if any, to the observed phenotype is unclear at present.
Given our results on gD- and gL-negative PrV mutants, the question arises which glycoproteins ultimately are indispensable for the fusion process. So far, repeated attempts to isolate infectious gH- and gB-negative virus mutants by the same protocol have failed (9). This seems not to be surprising, since neither of these mutant viruses is able to perform cell-to-cell spread even at the limited scale seen in PrV-ΔgLβ. However, it is impossible to predict whether appropriate mutations could not also compensate for the absence of gH or gB. Alternatively, these two glycoproteins may turn out to be truly essential components of the fusion machinery.
In PrV-ΔgLPass a fortuitous deletion of gC also occurred. As shown after repair of this defect, the slight impairment in one-step growth and penetration of PrV-ΔgLPass is, at least partially, attributable to the absence of gC. Thus, the gDH fusion protein nearly fully complements the absence of gL in PrV-ΔgLPassgC+, resulting in a virus mutant with wild-type-like one-step growth characteristics.
The demonstration of a gDH fusion protein which could execute both gD and gH functions has fascinating implications. By altering the gD part, novel receptor binding specificities might be introduced into a recombinant herpesvirus, leading to viruses with altered tropism. If it turns out that the gDH fusion protein indeed complements both gD and gH/gL function simultaneously, it would be a prime candidate for a factor which not only binds virion to target cells but also initiates fusion. Theoretically, the number of glycoproteins required for herpesvirus fusion could then be reduced from four (gB, gD, and gH/L) to only two (gDH and gB), which resembles a level of complexity found in many simpler viruses. We are currently testing whether this is indeed the case.
In summary, we have shown by reversion analysis that a protein consisting of the amino-terminal 271 aa of gD fused to the carboxy-terminal 590 aa of gH, which originates from a genomic translocation of parts of the gH gene into the gD locus, can compensate for gL function, resulting in a herpesvirus mutant which is fully infectious in the absence of gL.
REFERENCES
- 1.Chiang H-Y, Cohen G, Eisenberg R. Identification of functional regions of herpes simplex virus glycoprotein gD by using linker-insertion mutagenesis. J Virol. 1994;68:2529–2543. doi: 10.1128/jvi.68.4.2529-2543.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duus K M, Grose C. Multiple regulatory effects of varicella-zoster virus (VZV) gL on trafficking patterns and fusogenic properties of VZV gH. J Virol. 1996;70:8961–8971. doi: 10.1128/jvi.70.12.8961-8971.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fehler F, Herrmann J, Saalmüller A, Mettenleiter T C, Keil G M. Glycoprotein IV of bovine herpesvirus 1-expressing cell line complements and rescues a conditionally lethal viral mutant. J Virol. 1992;66:6372–6390. doi: 10.1128/jvi.66.2.831-839.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geraghty R J, Krummenacher C, Cohen G, Eisenberg R, Spear P G. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science. 1998;280:1618–1620. doi: 10.1126/science.280.5369.1618. [DOI] [PubMed] [Google Scholar]
- 5.Graham F, van der Eb A. A new technique for the assay of infectivity of human adenovirus. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 6.Hutchinson L, Browne H, Wargent V, Davis-Poynter N, Primorac S, Goldsmith K, Minson A C, Johnson D C. A novel herpes simplex virus glycoprotein, gL, forms a complex with glycoprotein H (gH) and affects normal folding and surface expression of gH. J Virol. 1992;66:2240–2250. doi: 10.1128/jvi.66.4.2240-2250.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaplan A, Vatter A. A comparison of herpes simplex and pseudorabies viruses. Virology. 1959;7:394–407. doi: 10.1016/0042-6822(59)90068-6. [DOI] [PubMed] [Google Scholar]
- 8.Karger A, Mettenleiter T C. Glycoproteins gIII and gp50 play dominant roles in the biphasic attachment of pseudorabies virus. Virology. 1993;194:654–664. doi: 10.1006/viro.1993.1305. [DOI] [PubMed] [Google Scholar]
- 9.Klupp, B. G., and T. C. Mettenleiter. Unpublished observations.
- 10.Klupp B G, Mettenleiter T C. Sequence and expression of the glycoprotein gH gene of pseudorabies virus. Virology. 1991;182:732–741. doi: 10.1016/0042-6822(91)90614-h. [DOI] [PubMed] [Google Scholar]
- 11.Klupp B G, Visser N, Mettenleiter T C. Identification and characterization of pseudorabies virus glycoprotein H. J Virol. 1992;66:3048–3055. doi: 10.1128/jvi.66.5.3048-3055.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klupp B G, Baumeister J, Karger A, Visser N, Mettenleiter T C. Identification and characterization of a novel structural glycoprotein in pseudorabies virus, gL. J Virol. 1994;68:3868–3878. doi: 10.1128/jvi.68.6.3868-3878.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klupp B G, Fuchs W, Weiland E, Mettenleiter T C. Pseudorabies virus glycoprotein L is necessary for virus infectivity but dispensable for virion localization of glycoprotein H. J Virol. 1997;71:7687–7695. doi: 10.1128/jvi.71.10.7687-7695.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kopp A, Mettenleiter T C. Stable rescue of a glycoprotein gII deletion mutant of pseudorabies virus by glycoprotein gI of bovine herpesvirus 1. J Virol. 1992;66:2754–2762. doi: 10.1128/jvi.66.5.2754-2762.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ligas M, Johnson D. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by β-galactosidase sequences binds to but is unable to penetrate into cells. J Virol. 1988;62:1486–1494. doi: 10.1128/jvi.62.5.1486-1494.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lukács N, Thiel H-J, Mettenleiter T C, Rziha H-J. Demonstration of three major species of pseudorabies virus glycoproteins and identification of a disulfide-linked glycoprotein complex. J Virol. 1985;53:166–173. doi: 10.1128/jvi.53.1.166-173.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mettenleiter T C. Glycoprotein gIII deletion mutants of pseudorabies virus are impaired in virus entry. Virology. 1989;171:623–625. doi: 10.1016/0042-6822(89)90635-1. [DOI] [PubMed] [Google Scholar]
- 18.Mettenleiter T C. Initiation and spread of α-herpesvirus infections. Trends Microbiol. 1994;2:2–4. doi: 10.1016/0966-842x(94)90335-2. [DOI] [PubMed] [Google Scholar]
- 19.Mettenleiter T C, Rauh I. A glycoprotein gX-β-galactosidase fusion gene as insertional marker for rapid identification of pseudorabies virus mutants. J Virol Methods. 1990;30:55–66. doi: 10.1016/0166-0934(90)90043-f. [DOI] [PubMed] [Google Scholar]
- 20.Montgomery R, Warner M S, Lum B J, Spear P G. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 21.Nicola A, Ponce de Leon M, Xu R, Hou W, Whitbeck J C, Krummenacher C, Montgomery R, Spear P G, Eisenberg R, Cohen G. Monoclonal antibodies to distinct sites on herpes simplex virus (HSV) glycoprotein D block HSV binding to HVEM. J Virol. 1998;72:3595–3601. doi: 10.1128/jvi.72.5.3595-3601.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicola A, Willis S, Naidoo N, Eisenberg R, Cohen G. Structure-function analysis of soluble forms of herpes simplex virus glycoprotein D. J Virol. 1996;70:3815–3822. doi: 10.1128/jvi.70.6.3815-3822.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peeters B, de Wind N, Hooisma M, Wagenaar F, Gielkens A, Moormann R. Pseudorabies virus envelope glycoproteins gp50 and gII are essential for virus penetration, but only gII is involved in membrane fusion. J Virol. 1992;66:894–905. doi: 10.1128/jvi.66.2.894-905.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rauh I, Mettenleiter T C. Pseudorabies virus glycoproteins gII and gp50 are essential for virus penetration. J Virol. 1991;65:5348–5356. doi: 10.1128/jvi.65.10.5348-5356.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roop C, Hutchinson L, Johnson D C. A mutant herpes simplex virus type 1 unable to express glycoprotein L cannot enter cells, and its particles lack glycoprotein H. J Virol. 1993;67:2285–2297. doi: 10.1128/jvi.67.4.2285-2297.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rux A H, Willis S, Nicola A, Hou W, Peng C, Lou H, Cohen G H, Eisenberg R J. Functional region IV of glycoprotein D from herpes simplex virus modulates glycoprotein binding to the herpesvirus entry mediator. J Virol. 1998;72:7091–7098. doi: 10.1128/jvi.72.9.7091-7098.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 28.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt J, Klupp B G, Karger A, Mettenleiter T C. Adaptability in herpesviruses: glycoprotein D-independent infectivity of pseudorabies virus. J Virol. 1997;71:17–24. doi: 10.1128/jvi.71.1.17-24.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schreurs C, Mettenleiter T C, Zuckermann F, Sugg N, Ben-Porat T. Glycoprotein gIII of pseudorabies virus is multifunctional. J Virol. 1988;62:2251–2257. doi: 10.1128/jvi.62.7.2251-2257.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schröder C, Linde G, Fehler F, Keil G M. From essential to beneficial: glycoprotein D loses importance for replication of bovine herpesvirus 1 in cell culture. J Virol. 1997;71:25–33. doi: 10.1128/jvi.71.1.25-33.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spaete R, Perot K, Scott P I, Nelson J A, Stinski M F, Pachl C. Coexpression of human cytomegalovirus gH with the UL115 gene product or the truncated human fibroblast growth factor receptor results in transport of gH to the cell surface. Virology. 1993;193:853–861. doi: 10.1006/viro.1993.1194. [DOI] [PubMed] [Google Scholar]
- 33.Spear P G. Entry of alphaherpesviruses into cells. Semin Virol. 1993;4:167–180. [Google Scholar]
- 34.Turner A, Bruun B, Minson T, Browne H. Glycoproteins gB, gD, and gHL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a COS cell transfection system. J Virol. 1998;72:873–875. doi: 10.1128/jvi.72.1.873-875.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Warner M S, Geraghty R J, Martinez W, Montgomery R, Whitbeck J C, Xu R, Eisenberg R, Cohen G, Spear P G. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2, and pseudorabies virus. Virology. 1998;246:179–189. doi: 10.1006/viro.1998.9218. [DOI] [PubMed] [Google Scholar]
- 36.Westra D F, Glazenburg K, Harmsen M, Tiran A, Scheffer A, Welling G, The T, Welling-Wester S. Glycoprotein H of herpes simplex virus type 1 requires glycoprotein L for transport to the surfaces of insect cells. J Virol. 1997;71:2285–2291. doi: 10.1128/jvi.71.3.2285-2291.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yaswen L, Stephens E, Davenport L, Hutt-Flettcher L M. Epstein-Barr virus glycoprotein gp85 associates with the BKRF2 gene product and is incompletely processed as a recombinant protein. Virology. 1993;195:387–396. doi: 10.1006/viro.1993.1388. [DOI] [PubMed] [Google Scholar]