Abstract
Surgeons use different medical devices in the surgery, such as patient-specific anatomical models, cutting and positioning guides, or implants. These devices must be sterilized before being used in the operation room. There are many sterilization processes available, with autoclave, hydrogen peroxide, and ethylene oxide being the most common in hospital settings. Each method has both advantages and disadvantages in terms of mechanics, chemical interaction, and post-treatment accuracy. The aim of the present study is to evaluate the dimensional and mechanical effect of the most commonly used sterilization techniques available in clinical settings, i.e., Autoclave 121, Autoclave 134, and hydrogen peroxide (HPO), on 11 of the most used 3D-printed materials fabricated using additive manufacturing technologies. The results showed that the temperature (depending on the sterilization method) and the exposure time to that temperature influence not only the mechanical behavior but also the original dimensioning planned on the 3D model. Therefore, HPO is a better overall option for most of the materials evaluated. Finally, based on the results of the study, a recommendation guide on sterilization methods per material, technology, and clinical application is presented.
Keywords: Additive manufacturing, Sterilization, Materials, Surgical planning, 3D printing accuracy
1. Introduction
145146Additive manufacturing (AM) and three-dimensional (3D) printing technologies are revolutionizing manufacturing industries by enabling the development of devices and products at the point of demand in a unique way. There are seven categories of AM technologies according to ISO/ ASTM 52900[1]: (i) vat photopolymerization (VP), which includes stereolithography (SLA), digital light processing (DLP), and volumetric 3D printing (3DVP); (ii) material extrusion (ME), which includes fused filament fabrication (FFF) or fused deposition modeling (FDM) and direct ink writing (DIW); (iii) material jetting (MJ); (iv) binder jetting (BJ); (v) powder bed fusion (PBF), which includes selective laser sintering (SLS) and selective laser melting (SLM); (vi) directed energy deposition (DED); and (vii) sheet lamination.
This revolution has been accelerated due to the COVID-19 pandemic and the supply shortages in the medical field[2-6], further popularizing the manufacturing of patient-specific point-of-care medical device. AM and 3D printing in healthcare refer mainly to technologies focused on generating 3D physical objects to produce personalized medical devices (from anatomical models to personalized splints, advanced medicines, or implants)[7]. The generation of personalized tools for surgical planning and medical training models have become the main applications of 3D printing technologies[8]. In most cases, the process is based on acquired images from a human body, typically taken from both computed tomography (CT) and magnetic resonance imaging (MRI). An identical copy, obtained either from volume rendering (VR) or 3D computer-aided design (CAD) models, of the clinical case is an advantage for customizing the surgical approach[2,4,9]. In this process, customized surgical tools and implants can be designed and produced[10-14]. These tools printed with AM technologies are being rapidly adopted, but most of the materials used for printing the tools were originally designed for applications in other (nonmedical) industries.
In medical applications, functional products are subject to application-specific mechanical loads, pressure, erosion and stress, and are exposed to chemicals and environmental factors limited to specific working and storage conditions. Additionally, the materials and manufacturing processes and the design of parts depend not only on their indication of use and the time of usage, but also on the performance needed and the physical and chemical conditions they will work in. Since May 2017, depending on their indication of use and risk, AM medical applications are classified by the European Union as Medical Devices regulated under the Medical Device Regulation (MDR)[15]. Thus, each application is classified according to its risk and time in contact with the patient. For example, an anatomical model for surgical planning and training is classified as Class I; a cutting guide or a positioning guide (that will be in short contact with the patient’s mucosa/body) is classified as Class IIa, the same as a patient-specific tracheotomy tube. An implantable plate will be a Class IIb, and a functional implant, such as a knee implant, is classified as Class III.
Although the first 3D printing or AM materials tested and validated for medical use have appeared in recent years, most of the existing materials are not designed and validated to follow the hospital standards and MDR compliance, nor are their mechanical properties analyzed for the main sterilization processes used in hospital settings, taking into account their indications of use[9,16-19]. Thus, it is important to understand the effects of these chemical and pressure processes and how the mechanical properties of 3D-printed parts are affected. All surgical instruments are cleaned and sterilized before they are used. In some applications, certain sets of materials containing surgical aid tools and implants are cleaned and sterilized several times per day[20]. The effect of sterilization on mechanical behavior and dimensional changes and distortion of 3D-printed parts is key to understanding its potential applications and is the underlying cause of failures[21].
Sterilization process can be performed by two different types of known processes[22]: (i) thermal sterilization by dry heat or steam, also known as moist heat sterilization or autoclave; (ii) low-temperature sterilization, such as chemical (with ethylene oxide or hydrogen peroxide) or radiation (ionizing or ultraviolet [UV] radiation). Most common sterilization methods available in hospitals are steam heat sterilization (also known as autoclave [AU]), gas plasma (also known as hydrogen peroxide [HPO] autoclave), and ethylene oxide[23]. Other sterilization techniques have significant disadvantages for their use in hospitals. For instance, thermal sterilization by dry heat is at this moment banned from hospitals of the European Union due to the inactivity on prions[24]. Then, radiation sterilization, which is mainly used in the food industry as well as in the medical device industry, is not suitable for hospitals[24]. Ethylene oxide should be avoided for several reasons: (i) it changes the polymer structures; (ii) it causes molecular weight loss; and (iii) it generates toxicity on the surface of the sample, for example, in polylactic acid (PLA) or polyethylene terephthalate glycol (PETG)[25]. Unlike the HPO low-temperature sterilization, no toxic residues remain on the items that have been sterilized. Additionally, this technique is not only effective and safe but also does not require any aeration time compared to ethylene oxide[24].
The materials that have been studied so far include: (i) PLA[24,26], acrylonitrile butadiene styrene (ABS)[27-30] or 147 thermoplastic polyurethane (TPU)[31] using FDM (a ME technology); (ii) DentaGuide (Asiga)[32], Dental Surgical Guide Material (Formlabs)[32-34] or Clear V02 (Formlabs) using SLA (a VP technology)[35]; and (iii) MED610 using MJ technology[33,34]. No data on SLS (a PBF technology) have been obtained regarding the effect of sterilization methods in terms of mechanical properties, limited to only 3D printing accuracy[36]. At present, no study has been performed to analyze the impact of sterilization in a wide range of materials for making surgical planning models and surgical guides.
Therefore, the purpose of this study is to evaluate the dimensional and mechanical effect of three of the most commonly used sterilization techniques in clinical settings, i.e., Autoclave 121, Autoclave 134, and HPO, on 11 of the most used 3D-printed materials using 3D printing technologies, such as FDM, SLA, SLS, and MJ. The goal of the present study is to contribute a practical guide regarding the materials which may or may not be used for each medical application.
2. Materials and methods
This study evaluates the effects of different sterilization methods (Autoclave 121, Autoclave 134 and HPO) on four different 3Dprinting technologies and 11 common AM materials used for the manufacture of 3D-printed surgical guides, anatomical models, and other customized medical devices. To do so, a literature review and a mechanical testing study was performed. Figure 1 shows the schematic of the process in this study.
Figure 1.

Scheme of the present study.
2.1. Materials
All materials used in this study, including the manufacturer’s name, city and country of origin, are listed in Table 1.
Table 1. Information about the material and the 3D printing technology.
| Printing technology | Material | Vendor | City and country of origin | Sample | Flexible/Elastic | Institute responsible for printing |
|---|---|---|---|---|---|---|
| SLA | Elastic 50 | Formlabs | Massachusetts, USA | Cylindrical | Yes | HSJD |
| SLA | Flexible 80 | Formlabs | Massachusetts, USA | Cylindrical | Yes | HSJD |
| SLA | Durable | Formlabs | Massachusetts, USA | Type 1A ISO 527 | No | HSJD |
| SLA | Surgical Guide | Formlabs | Massachusetts, USA | Type 1A ISO 527 | No | HSJD |
| MJ | Elastic Clear | Stratasys | Stratasys, Minnesota, USA | Cylindrical | Yes | HSJD |
| MJ | MED610 | Stratasys | Stratasys, Minnesota, USA | Type 1A ISO 527 | No | HSJD |
| MJ | VERO | Stratasys | Stratasys, Minnesota, USA | Type 1A ISO 527 | No | HSJD |
| FDM | ABS | Kimya | Nantes, France | Type 1A ISO 527 | No | HSJD |
| FDM | PLA | Kimya | Nantes, France | Type 1A ISO 527 | No | HSJD |
| FDM | TPU/TPE | Recreus | Alicante, Spain | Cylindrical | Yes | CIM UPC |
| SLS | PA12 | 3D Systems | Hemel Hempstead, UK | Type 1A ISO 527 | No | CIM UPC |
Abbreviations: ABS, acrylonitrile butadiene styrene; CIM UPC, Centre CIM of Universitat Politècnica de Catalunya; FDM, fused deposition modeling; HSJD, Barcelona Children’s Hospital Sant Joan de Déu; MJ, material jetting; PLA, polylactic acid; SLA, stereolithography; SLS, selective laser sintering; TPE, thermoplastic elastomer; TPU, thermoplastic polyurethane
2.2. 3D printing
In this section, the different AM technologies used are described with summaries of the printing parameters needed for the manufacture of the 3D-printed samples. Two types of samples were manufactured, one for hard materials and the other for soft materials, with different specimens depending on the mechanical tests to be performed. 3D-printed tensile samples following the ISO 527 type IA were produced for all rigid materials. For elastic materials, cylindrical elastic samples (16 mm diameter × 8 mm height) were printed for Shore hardness test.
2.2.1. Fused deposition modeling
FDM is defined as the continuous deposition of a filament over a heat plate layer-by-layer. The PLA, ABS and TPU148 samples were manufactured using Epsilon W50 (BCN3D, Barcelona, Spain). The printing parameters were the same for all materials, i.e., a nozzle diameter of 0.4 mm, a layer height of 0.1 mm, an infill of 80%, an infill overlap of 15%, and a wall thickness of 0.8 mm. The samples were manufactured horizontally.
2.2.2. Stereolithography
This process is based on photopolymerization of resins using UV laser to create the layers. Each layer is solidified in x–y directions and the building platform rises in z direction to create the different layers. For the manufacture of Surgical Guide, Durable samples, Elastic 50, and Flexible 80 samples, a Form 3BL (Formlabs, Massachusetts, USA) was used at Barcelona Children’s Hospital Sant Joan de Déu (HSJD). The samples were manufactured with an angle of 20° from the building platform to increase product resistance and facilitate postprocessing.
2.2.3. Selective laser sintering
SLS is a process in which the 3D printer uses a laser as both the power and heat source to sinter powdered material layer-bylayer until the 3D object is manufactured. For the manufacture of the PA12 samples, a Ricoh AM S5500P was used at CIM UPC facilities, which has a layer thickness between 0.08 and 0.1 mm. The samples were manufactured horizontally.
2.2.4. Material jetting
MJ is based on photopolymerization of material jetted onto the printing platform, where it is solidified by UV light and the model is built layer-by-layer. For the manufacture of the MED610, VERO and Elastic Clear samples, a J5 printer was used at HSJD. The samples were manufactured horizontally.
2.3. Sterilization method
To evaluate the critical effect of sterilization methods in 3D-printed, custom-made medical devices used in hospitals, three of the most used sterilization processes available in clinical settings were selected following clinically validated protocols. To compare the effect of the different processes, the produced specimens were divided into control and study groups. For each material, three specimens were printed for each sterilization method, and three more specimens were printed as controls. The sample size is considered appropriate since the objective is to demonstrate the effect of sterilization on each material, instead of demonstrating the exact mechanical property value of each material (since the mechanical property values of each material are already given by the manufacturers [Table 2] and several studies have already investigated in this regard for each material). Mechanical results between studies and manufacturers may vary due to different testing methods used.
Table 2. Mechanical properties and methods used according to manufacturer for each material and 3D printing technology used.
| Parameters | Manufacturer | Mechanical properties according to manufacturer | |||||
|---|---|---|---|---|---|---|---|
| Tensile strength (MPa) | Young’s modulus (MPa) | Elongation at break (%) | Glass transition temperature (Tg) (°C) | Shore hardness | Methods | ||
| PLA (Manufacturer) | Kimya | 22.9 | 2.097 | 4.2 | 107 | 76.8D | ISO 527-2/5A/50, ISO 178, ISO 868 |
| ABS | Kimya | 35.3 | 1443 | 9.8 | 107 | 70.0D | ISO 527-2/5A/50, ISO 178, ISO 868 |
| TPU/TPE (Filaflex 60A Pro) | Recreus | 26 | 2.5 | 950 | – | 63A | DIN ISO 7619-1, DIN 53504-S2 |
| PA12 | 3D Systems | 43 | 1387 | 14 | 192 | 73D | ASTM D638, ASTM D790, ASTM D2240 |
| Elastic 50 | Formlabs | 3.23 | 1.59 | 160 | – | 50A | ASTM D 412-06 (A), ASTM 2240 |
| Flexible 80 | Formlabs | 8.9 | 6.3 | 120 | 27 | 80A | ASTM D 412-06 (A), ASTM 2240 |
| Durable | Formlabs | 28 | 1000 | 55 | – | – | ASTM D638-14, ASTM D 790-15 |
| Surgical Guide | Formlabs | 73 | 2900 | 12.3 | – | 67D | ASTM D790, ASTM D638 |
| Elastic Clear | Stratasys | 3–5 | – | 360–400 | – | 45A | ASTM D-412, ASTM D-395, ASTM D-2240 |
| MED610 | Stratasys | 50–65 | 2000–3000 | 10–20 | 54 | 86D | ASTM D-638-03-04-05, D-790-04, DMA E |
| VERO | Stratasys | 40–55 | 2000–2500 | 15–20 | 54 | 86D | ASTM D-638-03-04-05, D-790-04, DMA E |
Abbreviations: ABS, acrylonitrile butadiene styrene; PLA, polylactic acid; TPE, thermoplastic elastomer; TPU, thermoplastic polyurethane
No sterilization or disinfection process was applied to the control sample. The study group samples were subjected to three different sterilization procedures, i.e., HPO, Autoclave 121, and Autoclave 134, available at a sterilization-certified facility at HSJD. All of them were performed using machines from Matachana (Italy). Those methods are among the most used for the sterilization of medical devices. Not all material samples were subjected to sterilization methods. The melting limit of each material,149 which depends on its glass transition temperature (Tg), can be known from the manufacturing technical file. The sterilization methods performed to each material samples can be found in Table S1 (4.9MB, pdf) (Supplementary File).
2.3.1. Hydrogen peroxide
HPO sterilization is a low-temperature chemical sterilization process that uses HPO as the sterilant. The process involves the following steps:
-
(1)
Prevacuum phase: Air is removed from the sterilization chamber to prepare for the introduction of HPO. This phase lasts for 3–5 min.
-
(2)
Pulse phase: A measured amount of HPO is introduced into the sterilization chamber. The HPO vaporizes and begins to penetrate and sterilize the equipment and contents. This phase lasts for 3–5 min.
-
(3)
Pressure holding phase: The pressure inside the sterilization chamber is maintained for a specified period of time to allow the HPO to penetrate and sterilize the equipment and contents. This phase lasts for approximately 30 min.
-
(4)
Decontamination phase: The HPO is then neutralized and removed from the sterilization chamber. This phase lasts for 10 min.
During the HPO sterilization cycle, the temperature reaches 60°C, while the maximum pressure reached is around 69 kPa.
2.3.2. Autoclave 121
Autoclave 121 (AU121) is a process that uses a temperature of 121°C for sterilization. The sterilization process involves the following steps:
-
(1)
Preheating:
Air removal: The air inside the autoclave is removed through a vacuum cycle, which helps to improve steam penetration. This stage lasts for 2–5 min.
Steam injection: Steam is introduced into the autoclave and the pressure and temperature begin to rise. This stage lasts for 5 min.
-
(2)
Holding time: The temperature and pressure are maintained around 121°C and 2.5–3 atm, respectively, for 20 min. This is the time required for the steam to penetrate and kill any microbial organisms.
-
(3)
Depressurization: The pressure inside the autoclave is reduced back to atmospheric pressure. This step lasts for 10 min.
-
(4)
Drying: The items inside the autoclave are dried. This stage lasts for 15 min.
The maximum pressure reached is 2.5–3 atm, and the temperature reached during the cycle is 121°C.
2.3.3. Autoclave 134
Autoclave 134 (AU134) is a process that uses a higher temperature for sterilization. The sterilization process150 involves the same steps as AU121 but with different duration:
-
(1)
Preheating:
Air removal: the air inside the autoclave is removed through a vacuum cycle, which helps to improve steam penetration. This stage lasts for 2–5 min.
Steam injection: Steam is injected into the autoclave and the pressure and temperature begin to rise. This stage lasts for 5 min.
-
(2)
Holding time: The temperature and pressure are maintained around 134°C and 2.5–3 atm, respectively, for 4–5 min. This is the time required for the steam to penetrate and kill any microbial organisms.
-
(3)
Depressurization: The pressure inside the autoclave is reduced back to atmospheric pressure. This step lasts for 10 min.
-
(4)
Drying: The items inside the autoclave are dried. This stage lasts for 15 min.
The maximum pressure reached is 2.5–3 atm and the temperature reached during the cycle is 134°C.
2.4. Tensile testing
The tensile tests were performed for the rigid materials with Instron 4507 at the EEBE-UPC (School of Engineering of Barcelona East, a UPC facility) using 3D-printed samples following the ISO 527 type IA. Three control tensile tests and three tensile tests for each sterilization process and each material were performed. Deformation measurements were made by Digital Imaging Correlation (DIC) with the Vic-Gauge 2D/3D software. It uses optimized 2D and 3D correlation algorithms for providing the real-time displacement and deformation data for mechanical testing. This can be seen as a set of virtual strain gauges in which data can be obtained for various points and plotted in live versus analog load inputs. Then, results were saved for each point examined, and complete images stored for analysis in both Vic-2D and Vic-3D (Figure S1 (4.9MB, pdf) ).
Four digital gauges (rosette gauges) were used for the tests, with varying distances between the gauges according to the material deformation, and were placed in the test zone of the specimen. To take the images and measure the deformation, a Basler camera was used. For that, Fujifilm lenses of 50 or 35 mm were used and varied according to the deformation of the material (because if it deforms too much, it comes out of the camera). The samples were prepared as per the steps in the following: (i) a visual inspection was made; (ii) with a micrometer, the measurements of the specimens were taken (see Table S1 (4.9MB, pdf) in Supplementary File); and (iii) to place the gauges and ensure high accuracy in the measurement, the specimens were painted in white color and the reference point markers (“little black dots”) were placed on the specimens to ensure contrast and accurate references for scanning. In this way, the digital gauges take the initial pattern where they are placed, and the spatial reference on the specimen is even if there is a lot of deformation.
The painting did not affect the results of the tests since the painting was finished before the test was performed; therefore, no chemicals from the paint could influence the samples. Samples were manually placed on the testing machine, and the tests were performed at 3 mm/min speed for all the materials.
2.5. Shore hardness
Hardness tests were only performed on soft materials with cylindrical specimens because the hardness of these materials could vary due to sterilization. The durometer always produced the highest value when the hardness of rigid materials was being measured. In terms of the Shore hardness test, the ATSM D2240—Durometer Hardness method was carried out. For that, the Shore durometer type A (Baxlo, Instrumentos de medida y precisión S.L., Spain) was used for measuring the hardness of the different samples. To obtain more accurate results, a stand arm was used, and a durometer support was designed and fabricated. The hardness value was always measured at the same level of the stand arm, and three measurements were taken from each sample.
2.6. 3D printing accuracy
For the rigid materials, surface comparison of tensiles between the different groups (sterilized and control) was performed to analyze the dimensional changes since in some tensiles; potential dimensional and geometrical deformations were detected once they were subjected to sterilization at high temperatures or pressures.
A CT scan of all tensiles was performed using a 1.5 T System MR-Philips in HSJD to obtain the 3D digitalized model of each printed tensile. Once the CT scan was acquired, the resulting DICOM (Digital Imaging and Communications in Medicine) images were segmented to obtain the STL (Standard Tessellation Language) model for each tensile. Using 3-Matic from Materialise®, every 3D mesh of each sterilized tensile was aligned to a control tensile mesh (Figure 2) and the point cloud were compared by a point cloud-based analysis.
Figure 2.

Prealignment between a MED610 control tensile (gray) and a MED610 tensile that was sterilized with AU134 method (blue).
The point-based evaluation of the 3D cloud meshes involved analyzing individual points within the mesh in the x, y, and z coordinates. The following steps outline the process:
-
(1)
Obtain the 3D point cloud mesh data which includes the x, y, and z coordinate values for each point.
-
(2)
151Transform the point cloud data into the desired reference frame (e.g., World Coordinate System [WCS]) by applying a transformation matrix.
-
(3)
Compute the distances between the points along the x, y, and z axes.
-
(4)
Determine the average distance between the points.
-
(5)
Each point in the cloud had an RGB color value assigned.
The obtained file in .txt format of each analysis was then analyzed to obtain the average distance (see Figure S36 (4.9MB, pdf) ).
3. Results
3.1. Mechanical testing of the 3D-printed materials
3.1.1. Polylactic acid
Figure 3 shows the mechanics testing performed on the 3D-printed PLA samples, with the group of samples sterilized by HPO and the control samples (not sterilized) being compared. Overall, it seems that the HPO sterilization does not significantly change the behavior of the mechanical properties of PLA. Table 3 shows different mechanical properties about PLA with different methods. Other sterilization methods were not tested with PLA as its Tg is lower (Table 2) than the temperature reached in AU121 and AU134 sterilization methods.
Figure 3.

PLA sterilization comparison. Data are represented as mean values. N = 3 PLA Control/group; N = 3 PLA HPO/group.
Table 3. Mechanical properties of the 3D-printed PLA.
| Parameters | Mechanical properties | |||
|---|---|---|---|---|
| Tensile strength (MPa) | Young’s modulus (MPa) | Elongation at yield (%) | Elongation at break (%) | |
| Control | 21.85 ± 0.37 | 1568 ± 45 | 0.44 ± 0.74 | 4.18 ± 1.96 |
| HPO | 21.63 ± 1.2204 | 1408 ± 40 | 1.37 ± 0.10 | 4.11 ± 0.58 |
Data are represented as mean ± SD. N = 3 PLA Control/group; N = 3 PLA HPO/group.
3.1.2. Acrylonitrile butadiene styrene
Figure 4 shows the mechanics of the 3D-printed ABS samples sterilized by HPO and the control samples. The effect of HPO sterilization on the ABS samples was not significantly different when compared to the control samples. The results showed differences below 10% in elongation at break (8%) and tensile strength (2.4%) between HPO samples and control samples. This means that the use of this method is effective for its use in surgical planning. Table 4 shows different mechanical properties of ABS with different methods.
Figure 4.

ABS sterilization comparison. Data are represented as mean values. N = 3 ABS Control/group; N = 3 ABS HPO/group.
Table 4. Mechanical properties of the 3D-printed ABS.
| Parameters | Mechanical properties | |||
|---|---|---|---|---|
| Tensile strength (MPa) | Young’s modulus (MPa) | Elongation at yield (%) | Elongation at break (%) | |
| Control | 23.01 ± 1.32 | 1352 ± 53 | 1.73 ± 0.08 | 4.35 ± 0.85 |
| HPO | 23.57 ± 1.42 | 1271 ± 23 | 1.79 ± 0.12 | 4.73 ± 0.76 |
Data are represented as mean ± SD. N = 3 ABS Control/group; N = 3 ABS HPO/group.
3.1.3. MED610
Figure 5 shows the mechanics of the control samples and 3D-printed MED610 samples sterilized by HPO, AU121, and AU134. Among the three different sterilization techniques, autoclave has a bigger influence on the mechanical properties in comparison to HPO. A similar tensile strength was found between control samples and HPO-sterilized samples (with a difference of 0.64%), although there was a major difference when compared to both AU121 (17.40%) and AU134 (14.57%), showing the AU134 results in higher tensile strength in samples compared to the control samples. Table 5 shows different mechanical properties of MED610 with different methods. It can be noticed that one specimen of AU134 has a significant difference with respect to others specimens, which can be attributed to a printing defect.
Figure 5.

MED610 sterilization comparison. Data are represented as mean values. N = 3 MED610 Control/group; N = 3 MED610 HPO/group; N = 3 MED610 AU121/group; N = 3 MED610 AU134/group.
Table 5. Mechanical properties of the 3D-printed MED610.
| Parameters | Mechanical properties | |||
|---|---|---|---|---|
| Tensile strength (MPa) | Young’s modulus (MPa) | Elongation at yield (%) | Elongation at break (%) | |
| Control | 26.26 ± 4.30 | 1375 ± 168 | 1.92 ± 0.15 | 8.82 ± 1.90 |
| HPO | 26.43 ± 0.82 | 1341 ± 43 | 1.92 ± 0.16 | 12.95 ± 3.55 |
| AU121 | 21.69 ± 2.71 | 1201 ± 76 | 1.93 ± 0.19 | 12.14 ± 2.10 |
| AU134 | 30.74 ± 2.75 | 1380 ± 145 | 1.33 ± 0.67 | 6.04 ± 5.55 |
Data are represented as mean ± SD. N = 3 MED610 Control/group; N = 3 MED610 HPO/group; N = 3 MED610 AU121/group; N = 3 MED610 AU134/group.
3.1.4. VERO
152153154155Figure 6 shows the mechanics of the control sample and 3D-printed VERO samples sterilized by HPO, AU121, and AU134. Both AU121 and AU134 slightly decreased the mechanical properties of the tested VERO samples, which had a tensile strength of 29.16 ± 1.49 MPa and 30.26 ± 3.02 MPa respectively, as compared to the 31.32 ± 2.25 MPa of control samples tested. The HPO method increased the tensile strength by 15% (36.07 ± 4.73 MPa). There were no significant differences in tensile strength impact between AU121 and AU134; however, AU134 showed a bigger impact. Table 6 shows different mechanical properties of VERO with different methods.
Figure 6.

VERO sterilization comparison. Data are represented as mean values. N = 3 VERO Control/group; N = 3 VERO HPO/group; N = 3 VERO AU121/group; N = 3 VERO AU134/group.
Table 6. Mechanical properties of the 3D-printed VERO.
| Parameters | Mechanical properties | |||
|---|---|---|---|---|
| Tensile strength (MPa) | Young’s modulus (MPa) | Elongation at yield (%) | Elongation at break (%) | |
| Control | 31.32 ± 2.25 | 1492 ± 175 | 1.92 ± 0.14 | 14.16 ± 4.55 |
| HPO | 36.07 ± 4.73 | 1818 ± 224 | 1.87 ± 0.05 | 15.38 ± 2.72 |
| AU121 | 29.16 ± 1.49 | 1502 ± 254 | 1.31 ± 1.11 | 12.29 ± 4.96 |
| AU134 | 30.26 ± 3.02 | 1380 ± 137 | 1.97 ± 0.07 | 11.69 ± 2.90 |
Data are represented as mean ± SD. N = 3 VERO Control/group; N = 3 VERO HPO/group; N = 3 VERO AU121/group; N = 3 VERO AU134/group.
3.1.5. Surgical Guide resin
Figure 7 shows the mechanics of the control sample and 3D-printed Surgical Guide resin samples sterilized by HPO, AU121, and AU134. Table 7 shows different mechanical properties of Surgical Guide resin with different methods. HPO sterilization imparted a very small effect on the tensile strength of this material, with a difference of 0.64% as compared to the control group. The AU121 and the AU134 methods resulted in a tensile strength difference of 14% and 36.04%, respectively. Interestingly, tensile strength was increased in the case of AU121 sterilization.
Figure 7.
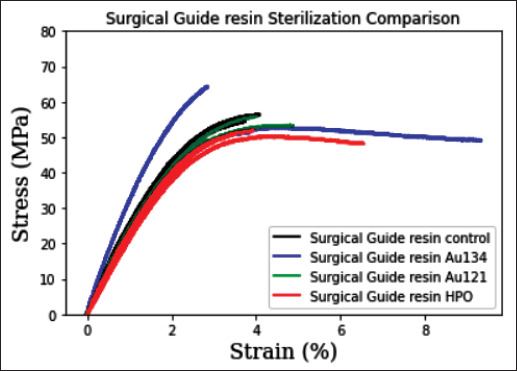
Surgical Guide resin sterilization comparison. Data are represented as mean values. N = 3 Surgical Guide resin Control/group; N = 3 Surgical Guide resin HPO/group; N = 3 Surgical Guide resin AU121/group; N = 3 Surgical Guide resin AU134/group.
Table 7. Mechanical properties of the 3D-printed Surgical Guide resin.
| Parameters | Mechanical properties | |||
|---|---|---|---|---|
| Tensile strength (MPa) | Young’s modulus (MPa) | Elongation at yield (%) | Elongation at break (%) | |
| Control | 57.60 ± 6.32 | 2154 ± 278 | 1.89 ± 0.93 | 6.23 ± 0.90 |
| HPO | 57.23 ± 4.31 | 2248 ± 726 | 1.46 ± 0.65 | 5.45 ± 2.69 |
| AU121 | 67.01 ± 11.28 | 2303 ± 140 | 1.31 ± 0.48 | 5.48 ± 1.13 |
| AU134 | 42.34 ± 12.06 | 3834 ± 952 | 0.39 ± 0.62 | 2.04 ± 0.65 |
Data are represented as mean ± SD. N = 3 Surgical Guide resin Control/group; N = 3 Surgical Guide resin HPO/group; N = 3 Surgical Guide resin AU121/group; N = 3 Surgical Guide resinAU134/group.
3.1.6. Durable
Figure 8 shows the mechanics of the control sample and 3D-printed Durable samples sterilized by HPO, AU121, and AU134. Table 8 shows different mechanical properties of Durable material with different methods. It was found that a higher temperature to which the sample is exposed caused a bigger drop in the tensile strength. HPO resulted in a tensile strength difference of 3.53%, as compared to the tensile strength of control sample. The AU121 method resulted in a tensile strength difference of 4.47%, which is quite close to that of the HPO method. The impact of heat was noticed in AU134 method with a bigger difference in156 all mechanical properties tested, having a mean decrease of 17.35% in tensile strength.
Figure 8.

Durable sterilization comparison. Data are represented as mean values. N = 3 Durable Control/group; N = 3 Durable HPO/group; N = 3 Durable AU121/group; N = 3 Durable AU134/group.
Table 8. Mechanical properties of the 3D-printed Durable.
| Parameters | Mechanical properties | |||
|---|---|---|---|---|
| Tensile strength (MPa) | Young’s modulus (MPa) | Elongation at yield (%) | Elongation at break (%) | |
| Control | 18.46 ± 0.36 | 673 ± 37 | 15.39 ± 13.27 | 23.92 ± 0.60 |
| HPO | 17.83 ± 0.24 | 665 ± 54 | 16.27 ± 14.02 | 20.16 ± 7.47 |
| AU121 | 17.67 ± 0.55 | 685 ± 65 | 15.98 ± 13.92 | 25.38 ± 2.86 |
| AU134 | 15.73 ± 0.09 | 659 ± 36 | 20.83 ± 5.43 | 21.15 ± 5.37 |
Data are represented as mean ± SD. N = 3 Durable Control/group; N = 3 Durable HPO/group; N = 3 Durable AU121/group; N = 3 Durable AU134/group.
3.1.7. PA12
Figure 9 shows the mechanics of the control sample and 3D-printed PA12 samples sterilized by HPO, AU121, and AU134. The HPO, AU121, and AU134 methods directly influenced the mechanical properties of the material by decreasing the tensile strength of every sample measured. Table 9 shows different mechanical properties of PA12 with different methods. The HPO method resulted in a mean decrease of tensile strength by 8.64%, as compared to the control group. However, there was no significant difference in tensile strength between the samples sterilized by AU121 (9.10%), AU134 (10.78%), and HPO (8.64%) methods; the difference was less than 2% among the three methods.
Figure 9.
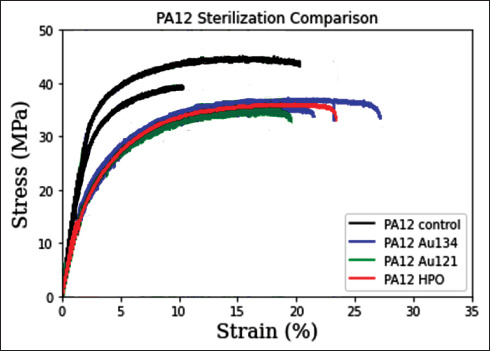
PA12 sterilization comparison. Data are represented as mean values. N = 3 PA12 Control/group; N = 3 PA12 HPO/group; N = 3 PA12 AU121/ group; N = 3 PA12 AU134/group.
Table 9. Mechanical properties of the 3D-printed PA12.
| Parameters | Mechanical properties | |||
|---|---|---|---|---|
| Tensile strength (MPa) | Young’s modulus (MPa) | Elongation at yield (%) | Elongation at break (%) | |
| Control | 41.10 ± 2.63 | 1487 ± 48 | 1.94 ± 0.23 | 13.58 ± 5.80 |
| HPO | 37.83 ± 2.57 | 1170 ± 246 | 2.24 ± 0.02 | 16.48 ± 2.19 |
| AU121 | 37.67 ± 1.28 | 990 ± 54 | 2.11 ± 0.19 | 16.44 ± 2.96 |
| AU134 | 37.10 ± 3.17 | 1021 ± 65 | 1.92 ± 0.21 | 10.80 ± 3.51 |
Data are represented as mean ± SD. N = 3 PA12 Control/group; N = 3 PA12 HPO/group; N = 3 PA12 AU121/group; N = 3 PA12 AU134/group.
3.2. Shore hardness
Table 10 shows the Shore hardness of the 3D-printed cylindrical elastic samples. In all cases, sterilization increased the hardness of materials, although the effect could vary, depending on the material. The Shore hardness of Elastic 50 sterilized by HPO or AU121 was not significantly different when compared to that of the control sample, despite the increases of 2.38% and 3.59% for samples sterilized by HPO and AU121. The results also showed almost similar effect on TPU sample sterilized by HPO, which had an increase of Shore hardness by 1.58%. Elastic Clear samples sterilized by HPO and AU121 had a similar increase of Shore hardness by 7.35%. The Shore hardness of Flexible 80 appeared to be the most affected, evidenced by an increase of 15.36% and 7.23% in samples sterilized by HPO and AU121, respectively.
Table 10. Shore hardness of the different elastic materials.
| Material | Shore hardness A | |||||
|---|---|---|---|---|---|---|
| Control | HPO | AU 121 | ||||
| Mean | SD | Mean | SD | Mean | SD | |
| Elastic 50 | 55.67 | 1.15 | 57.00 | 1.00 | 57.67 | 1.15 |
| Elastic Clear | 25.67 | 0.58 | 30.33 | 1.53 | 27.67 | 1.53 |
| Flexible 80 | 67.33 | 2.52 | 72.67 | 1.15 | 72.67 | 1.53 |
| TPU | 63.00 | 1.73 | 62.00 | 2.00 | – | – |
Abbreviation: SD, standard deviation; TPU, thermoplastic polyurethane.
3.3. 3D printing accuracy
The result of the comparison of the meshes (between the 3D-printed samples with and without sterilization) is represented by a color map and a histogram showing the distance differences in millimeters in all axes between the points of each surface mesh (see Figure 11). The mean with a confidence interval of 95% and standard deviation of all distances was computed. The rest of the images can be seen in Supplementary File (Figures S2 (4.9MB, pdf) –S35 (4.9MB, pdf) ).
Figure 11.

Surface comparison showing the differences in the distances between the points of each of the tensiles.
The obtained results are summarized in Table 11. The results showed that MED610 tensile samples that were sterilized by AU134 were the ones that suffered more deformation when compared to the control samples. PA12 is the most stable and resistant to deformation among the tested materials sterilized by all three methods. In general, there were more surface and dimensional differences between samples sterilized by AU134 and control samples, possibly due to the high temperatures and pressures used in this sterilization method, despite the shorter duration of sterilization. When compared to the control samples, the HPO-sterilized samples showed smaller differences in the distances.
Table 11. Results of the surface comparison between each group of sterilized tensiles with the control group.
| Material | Comparison | Mean with 95% CI (mm) | Standard deviation (mm) |
|---|---|---|---|
| ABS | Control vs HPO | −0.045 | 0.198 |
| Durable | Control vs HPO | 0.160 | 1.044 |
| Control vs AU121 | −0.092 | 0.625 | |
| Control vs AU134 | 0.273 | 0.779 | |
| MED610 | Control vs HPO | −0.055 | 0.379 |
| Control vs AU121 | 0.342 | 1.254 | |
| Control vs AU134 | 1.684 | 2.090 | |
| PA12 | Control vs HPO | −0.028 | 0.192 |
| Control vs AU121 | 0.042 | 0.336 | |
| Control vs AU134 | 0.024 | 0.215 | |
| PLA | Control vs HPO | −0.007 | 0.165 |
| Surgical Guide | Control vs HPO | −0.059 | 0.511 |
| Control vs AU121 | −0.126 | 0.529 | |
| Control vs AU134 | 0.258 | 0.741 | |
| VERO | Control vs HPO | −0.092 | 0.306 |
| Control vs AU121 | −0.036 | 0.721 | |
| Control vs AU134 | 0.509 | 0.905 |
Abbreviations: ABS, acrylonitrile butadiene styrene; AU, autoclave; PLA, polylactic acid.
Finally, a summary of potential applications for each material and AM method is provided as a guide (Table 12).
Table 12. Guide to potential applications in accordance with mechanical performance needed and sterilization method recommended.
| Printing technology | Material | Vendor | Recommended sterilization method (based on the mechanicala and dimensionalb results of this study) | Manufacturer’s recommended sterilization method (if any) | Flexible/ Elastic | Applicationc | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| HPO | AU121 | AU134 | HPO | AU121 | AU134 | |||||
| SLA | Elastic 50 | Formlabs | Yes | Yes | No | Yes | Yes | Yes | Yes | AM for visualization |
| AM for training | ||||||||||
| SLA | Flexible 80 | Formlabs | No | Yes | No | – | – | – | Yes | AM for visualization |
| AM for training | ||||||||||
| SLA | Durable | Formlabs | Yes | Yes | No | – | – | – | No | AM for visualization |
| AM for training | ||||||||||
| SLA | Surgical Guide | Formlabs | Yes | Yes | No | Yes | Yes | Yes | No | AM for visualization |
| AM for training | ||||||||||
| Cutting guides | ||||||||||
| Positioning guides | ||||||||||
| MD mucous contact | ||||||||||
| MD skin contact | ||||||||||
| MJ | Elastic Clear | Stratasys | Yes | Yes | No | – | – | – | Yes | AM for visualization |
| AM for training | ||||||||||
| MJ | MED610 | Stratasys | Yes | Yes | No | Yes | Yes | Yes | No | AM for visualization |
| AM for training | ||||||||||
| Cutting guides | ||||||||||
| Positioning guides | ||||||||||
| MD mucous contact | ||||||||||
| MD skin contact | ||||||||||
| MJ | VERO | Stratasys | Yes | Yes | Yes | Yes | Yes | Yes | No | AM for visualization |
| AM for training | ||||||||||
| MD skin contact | ||||||||||
| FDM | ABS | Kimya | Yes | No | No | – | – | – | No | AM for visualization |
| AM for training | ||||||||||
| MD skin contact | ||||||||||
| FDM | PLA | Kimya | Yes | No | No | – | – | – | No | AM for visualization |
| AM for training | ||||||||||
| MD skin contact | ||||||||||
| FDM | TPU/TPE | Recreus | Yes | No | No | – | – | – | Yes | AM for visualization |
| AM for training | ||||||||||
| SLS | PA12 | 3D Systems | Yes | Yes | Yes | Yes | Yes | Yes | No | AM for visualization |
| AM for training | ||||||||||
| Cutting guides | ||||||||||
| Positioning guides | ||||||||||
| MD mucous contact | ||||||||||
| MD skin contact | ||||||||||
Recommended sterilization depending on the mechanical properties of the present study.
Recommended sterilization depending on the dimensional changes of the present study.
Detailed definitions for applications: AM for visualization, family–doctor communication in education and surgical planning; AM for training, education and surgical planning simulation; MD mucous contact, medical device prototyping or development for any applications (only materials that can be used in contact with mucous are considered); MD skin contact, medical device prototyping or development for any applications (only materials that can be used in contact with skin are considered); Cutting guides, materials used in patient-specific products for surgical planning or in surgery setting to guide the surgeon in the cutting; Positioning guides, materials used in patient-specific products for surgical planning or in surgery setting to guide the surgeon in the positioning of plates, screws, distractors and other implanted or temporary material.
Abbreviations: ABS, acrylonitrile butadiene styrene; AM, additive manufacturing; FDM, fused deposition modeling; MD, medical device; MJ, material jetting; PLA, polylactic acid; SLA, stereolithography; SLS, selective laser sintering; TPE, thermoplastic elastomer; TPU, thermoplastic polyurethane.
4. Discussion
4.1. Materials and sterilization processes
4.1.1. Thermoplastic materials
According to the experiment results, it can be concluded that hard thermoplastic materials such as PLA, ABS, TPU, or thermoplastic elastomer (TPE) should be sterilized using non–heat-based sterilization methods such as HPO. Other studies reached the same conclusion, finding deformation when autoclave or dry heat processes were used[27,37,38]. For example, in our results, PLA showed similar tensile strength and elongation at break in samples sterilized by HPO and control samples. Therefore, the use of HPO sterilization is157acceptable for its use in surgical planning prototypes. This was also confirmed by Oth et al.[24] that HPO is regarded as a better alternative than the conventional heat-based sterilization processes such as autoclave sterilization. This is also confirmed by Told et al.[37] who demonstrated the impact of heat-based sterilization and thus recommended low-temperature sterilization.
Popescu et al.[39] applied HPO vapor and low-temperature gas plasma sterilization techniques on the 3D-printed ABS samples. Based on the obtained results, there was no negative effect on the 3D-printed ABS samples after the above-mentioned sterilization methods have been used. These results are in line with the work of Bosc et al.[40] who used Sterrad sterilization process (a combination158 of hydrogen peroxide [H2O2] and low-temperature gas plasma) to sterilize ABS surgical guides and analyzed the impact of sterilization on mechanical properties. Based on their results, it is recommended to use low-temperature sterilization for ABS as an alternative to AU121.
It is important to take special care in hollow or thin models while defining the printing parameters such as infill. With the obtained results, we consider that ABS and PLA can be used for the development of custom-made medical products, such as anatomical models for training, visualization and enhanced patient–clinician communication, and for the production of certain medical devices. It is important to note that biocompatibility is an important aspect in the production of medical devices; therefore, biocompatibility documentation and testing is required.
Despite the evidence on the good performance of TPU/TPE materials sterilized by HPO and the studies on personal protective equipment used during the COVID pandemic[40,41], relevant data on the performance of TPU/ TPE materials in clinical applications remain scarce[42,43]; therefore, more studies are needed to decipher the behavior of these materials in clinical applications.
4.1.2. Hard liquid resin materials (SLA, MJ)
In general, hard liquid resin materials produced by MJ such as MED610 and VERO, or produced by SLA such as Durable, Surgical Guide resin, have better behavior than thermoplastic materials in heat-based sterilization processes. According to our findings, all four materials could be sterilized using HPO and AU121 methods. This is in line with findings by Török et al.[44] However, MJ materials have better heat resistance and could be sterilized by AU134 method, as recommended by the manufacturer[45], although they may have potential effects on tensile strength and elastic modulus. In this work, the tensile strength of the MED610 samples sterilized by AU121 decreased by around 18%, leading to a decrease in Young’s modulus and an increase in elongation at break. This differs from the findings of Chan et al.[34], who found that MED610 had a tensile strength of about 46.550 MPa, but it decreased by 6.5% after sterilization by AU134.
Interestingly, it has been found that sterilizing Surgical Guide resin tends to improve the mechanical properties of the tensile samples. This is in concordance with Chan et al.[34]. It has been shown that the tensile strength slightly improved with an amplitude between 2.35% and 11.4%. On the other hand, Pop et al.[32] showed a decrease of tensile strength in samples sterilized by AU121 and AU134 methods compared to control samples. However, the elastic modulus was higher. The samples were autoclaved for around 20 min at 121°C, and 10 min at 134°C. These results are also in line with a study by Sharma et al.[33].
159160Given the biocompatibility and heat-resistance, both MED610 and Surgical Guide resins stand as good options to produce surgical guides and positioning guides in contact with mucous for less than 24 h (following biocompatibility testing). MED610 has a good mechanical resistance after being sterilized by HPO and AU121, and thus, it is a more solid option. This result is in line with Gielisch et al.[46] who compared the behavior of polylactide/ polyhydroxyalkanoate (PLA/PHA) surgical guides printed by FFF and MED610 guides printed by MJ in fully guided dental implant placement before and after steam sterilization, and the study found significant deviations in angles and accuracy in the PLA/PHA guide as compared to the MED610 guide. MED610 and Surgical Guide resin can also be used for the production of custom-made medical devices to support treatments with materials needing skin or mucous contact for less than 24 h[47,48].
VERO and Durable, although do not have biocompatibility tested for mucous contact, are good alternatives to produce material that do not have to be in contact with patients, such as anatomical models and material for education and simulation purposes. Durable is normally used for low-friction assemblies and impactresistant applications; however, very few information regarding its biocompatibility and sterilization resistance is provided by the manufacturer[49]. VERO is used for the production of custom-made bone and tissue simulators, such as the case presented by Lioufas et al.[50].
4.1.3. Liquid resin flexible/elastic materials (SLA, MJ)
SLA and MJ flexible materials such as Elastic Clear (MJ) or Elastic 50 and Flexible 80 (SLA) are normally used for the production of anatomical models mimicking vessels or soft tissues[51]. According to the presented results, overall, the elastic materials become harder after different sterilization methods are applied. This is consistent with Told et al.[37] and Fuentes et al.[53]. Although the main application of elastic materials is the production of anatomical models for surgical training and education, most of them fail in mechanically mimicking the behavior of real human tissue (Figure 10). Moreover, there is still a lack of mucous-biocompatible soft materials, which could have an important impact on the improvement of patient-specific temporary implants, such as stents.
Figure 10.

Tissue-material comparison in terms of the elastic modulus.
4.1.4. Powder polymeric material (SLS)
Powder polymeric materials printed using SLS technology tend to have good resistance to heat. According to this work, PA12 could be sterilized following any of the studied sterilization processes (HPO, AU121, and AU134); although few mechanical properties were affected, their minor changes were not found to be significant from a practical point of view. This is in accordance with previous works[54]. For instance, Msallem et al.[55] found that SLS PA12 is the most accurate material and has better heat resistance when they compared the mechanical performance of a 3D-printed dry human bony mandible, made of polyamide (PA) (SLS), White V4 resin (SLA), VERO (MJ), PLA (FFF) and four other binder jetting materials, sterilized by different methods.
Additionally, it is important to highlight that PA12 is commonly used in surgical guides because according to EN ISO 10993-1, PA12 is a material that is chemically and physically durable and biocompatible[56]. However, it has a main drawback, which is the dust formed at where mechanical friction forces are applied.
PA12 represents a good candidate of hard and resistant material for the production of patient-specific cutting and positioning guides, as well as custom-made medical devices.
4.2. Tissue-material-mimicking comparison
Producing anatomical models is a common application of 3D printing in healthcare sector. These models are usually used for training, simulation or enhancing the comprehension and communication between patients and clinicians. However, most of the present 3D-printable materials are far from being mechanically comparable to the behavior of human tissues and therefore lack a certain tactile realism. Figure 10 shows the comparison of different data obtained from different research papers[57-62] with the mechanical properties of the 3D-printed materials used in this work. The Surgical Guide resin material is the best material for mimicking hard tissues such as the bone. However, the analyzed materials were unable to mimic the softness of tissues such as those in liver or heart. This means that for these tissues, it is necessary to find softer materials which have been previously analyzed by Tejo-Otero et al.[62]. Figure 12 shows a comparison of Shore hardness between the elastic materials shown in the present paper and those in other studies, in which the Shore hardness of soft tissue has been investigated[62-65]. The Shore hardness values of the elastic materials fall within the range of Shore A, while those of the soft tissues fall within the range of Shore 00. This implies that, even if the mentioned materials are closer to other materials in terms of hardness/softness, they are not the best materials used for mimicking soft tissues. This shows that in tissue-mimicking applications, other softer materials, such as hydrogels or silicones, must be used.
Figure 12.

Tissue–matevrial comparison in terms of the Shore hardness (ranges 00 and A).
4.3. Contribution of the current work
This current work presents practical testing complemented with a literature review, bringing in new insights into161 the use of the most clinically used sterilization processes for the disinfection of the most used AM materials and technologies in the hospital settings. Some of the data provided herein are not available from the material providers at the time of the study, nor published by previous research, as can be seen in Table 12. This work also provides a practical proposal of potential applications of custom-made medical products for each material, according to the research conducted on sterilization processes and mechanical properties.
As for the limitations of the study, it must be stated that the potential effect on mechanical properties and thermal behavior of different printing parameters (infill, printing direction, etc.) were not investigated in detail, nor a thorough study of the mechanical properties of each material was conducted. This work intended to demonstrate the practical feasibility of the proper use of 3D-printed materials in common healthcare applications. Most of the mechanical properties of each material can be found in data sheets provided by each provider.
5. Conclusion
The growing adoption of 3D technologies in medical applications necessitates the precise delineation of the properties and effects of sterilization and working processes in the clinical and hospital settings on each material, as well as the limitations in each of their applications. It is important to know when and for what purpose we can make use of each material and AM technology, and to know the effects of temperature, sterilization, chemicals162 and other agents on the final medical product to decide how we should treat them.
This paper can be used as a guide for future studies and as a guide for doctors who are starting to use AM technologies as well as sterilization methods. There are several points that must be highlighted:
-
(1)
The temperature (depending on the sterilization method) and the exposure time influence the mechanical behavior of materials. The higher the temperature and the longer the exposure time, the higher the risk of the mechanical and geometrical properties to be affected and the bigger the changes from its original form.
-
(2)
The 3D printing accuracy showed that AU134 and AU121 methods have a greater influence on the samples compared to HPO method. Therefore, HPO method is a better option, depending on the selected material.
-
(3)
In general, hard liquid resin materials produced by MJ such as MED610, or produced by SLA such as Surgical Guide resin, and powder polymeric materials printed using SLS technology such as PA12 have better behavior than thermoplastic materials produced by ME in heat-based sterilization processes; therefore, it is a better option for the production of surgical guides. Among these hard liquid resin materials, MED610 and specially PA12 are the strongest candidates.
-
(4)
The selection of materials, technology, and sterilization process to be used depends on the final application and its own mechanical and dimensional requirements.
-
(5)
The materials analyzed in this study can mostly mimic hard tissues, owing to their comparable elastic modulus. However, other materials such as silicones or hydrogels are needed for mimicking soft tissues.
For materials whose surface and geometry could be potentially affected by the sterilization process, design and dimensions of the final parts may play a role in manipulating the desired mechanical properties. For standardization purposes, the analysis of the present study was based on the ISO tensile testing. Nevertheless, future work should be focused on the analysis of the impact of the design and the sample dimensions of each material to be subjected to a sterilization process. For future studies, softer materials such as silicones or hydrogels could also be included for analysis.
Acknowledgments
None.
Funding
The research described in this paper was partially funded by the project named QuirofAM (Exp. COMRDI16-1-0011) and funded by ACCIÓ from the Catalan government and ERDF from European Union.
Conflict of interest
The authors declare no conflict of interests.
Author contributions
Conceptualization: Arnau Valls-Esteve, Pamela Lustig-Gainza, Aitor Tejo-Otero, Nuria Adell-Gomez
Investigation: All authors
Methodology: Arnau Valls-Esteve, Pamela Lustig-Gainza, Nuria Adell-Gomez, Aitor Tejo-Otero, Felip Fenollosa-Artés, Estibaliz Julian-Alvarez, Osmeli Navarro-Sureda, Josep Rubio-Palau, Lucas Krauel, Josep Munuera
Formal analysis: Arnau Valls-Esteve, Aitor Tejo-Otero, Felip Fenollosa-Artés, Josep Rubio-Palau, Lucas Krauel, Josep Munuera
Writing – original draft: Arnau Valls-Esteve, Aitor Tejo-Otero
Writing – review & editing: All authors
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data
Data can be available for readers upon reasonable request.
References
- 1.ISO/ASTM 2015. INTERNATIONAL STANDARD ISO / ASTM 52900 Additive manufacturing—General principles—Terminology. Int Organ Stand. https://doi.org/10.1520/ISOASTM52900-15
- 2.M. T. P. MATTHEW FOX 2016. 3-D printing: Revolutionizing preoperative planning, resident training, and the future of surgical care. Bull Am Coll Surg 101 7 9 18 https://bulletin.facs.org/2016/07/3-d-printing-revolutionizing-preoperative-planning-resident-training-and-the-future-of-surgical-care/ (accessed Nov. 30, 2022). [PubMed] [Google Scholar]
- 3.Krauel L, Fenollosa F, Riaza L, et al. Use of 3D prototypes for complex surgical oncologic cases. World J Surg. 2016;40(4):889–894. doi: 10.1007/s00268-015-3295-y. https://doi.org/10.1007/s00268-015-3295-y. [DOI] [PubMed] [Google Scholar]
- 4.163Tack P, Victor J, Gemmel P, et al. 3D-printing techniques in a medical setting: A systematic literature review. Biomed Eng Online. 2016:1–21. doi: 10.1186/s12938-016-0236-4. https://doi.org/10.1186/s12938-016-0236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dodziuk H. Applications of 3D printing in healthcare. Kardiochirurgia i Torakochirurgia Polska/Polish. J Thorac Cardiovasc Surg. 2016;13(3):283–293. doi: 10.5114/kitp.2016.62625. https://doi.org/10.5114/kitp.2016.62625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kunovjanek M, Wankm C. An analysis of the global additive manufacturing response to the COVID-19 pandemic. J Manuf Technol Manag. 2021;32(9):75–100. https://doi.org/10.1108/JMTM-07-2020-0263. [Google Scholar]
- 7.Kalaskar DM. 3D Printing in Medicine. Woodhead Publishing; 2022. Woodhead P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tejo-Otero A, Buj-Corral I, Fenollosa-Artés F. 3D printing in medicine for preoperative surgical planning: A review. Ann Biomed Eng. 2020;48(2):536–555. doi: 10.1007/s10439-019-02411-0. https://doi.org/10.1007/s10439-019-02411-0. [DOI] [PubMed] [Google Scholar]
- 9.Marconi S, Pugliese L, Botti M, et al. Value of 3D printing for the comprehension of surgical anatomy. Surg Endosc. 2017;31(10):4102–4110. doi: 10.1007/s00464-017-5457-5. https://doi.org/10.1007/s00464-017-5457-5. [DOI] [PubMed] [Google Scholar]
- 10.Lee K. Accuracy of three-dimensional printing for manufacturing replica teeth. Korean J Orthod. 2015;45(5):217–225. doi: 10.4041/kjod.2015.45.5.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rozema FR, Bos RRM, Boering G, et al. The effects of different steam-sterilization programs on material properties of poly (L-lactide). J Appl Biomater. 1991;2(1):23–28. doi: 10.1002/jab.770020104. https://doi.org/10.1002/jab.770020104. [DOI] [PubMed] [Google Scholar]
- 12.Cappello IA, Candelari M, Panone L, et al. 3D printed surgical guide for coronary artery bypass graft: Workflow from computed tomography to prototype. Bioengineering. 2022;9(5):179. doi: 10.3390/bioengineering9050179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costanzo R, Ferini G, Brunasso L, et al. The role of 3D-printed custom-made vertebral body implants in the treatment of spinal tumors: A systematic review. Life. 2022;12(4):489. doi: 10.3390/life12040489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornejo J, Cornejo-Aguilar JA, Vargas M, et al. Anatomical engineering and 3D printing for surgery and medical devices: International review and future exponential innovations. Biomed Res Int. 2022;2022:6797745. doi: 10.1155/2022/6797745. https://doi.org/10.1155/2022/6797745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ISO 13485:2016—Medical devices—Quality management systems—Requirements for regulatory purposes. www.iso.org (accessed Nov. 30, 2022)
- 16.Neches RY, Flynn KJ, Zaman L, et al. On the intrinsic sterility of 3D printing. PeerJ. 2016;4:e2661. doi: 10.7717/peerj.2661. https://doi.org/10.7717/peerj.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ISO 11357-2. Plastics—Differential scanning calorimetry—Part 2: Determination of glass transition temperature. 1999.
- 18.Kubyshkina G, Zupančič B, Štukelj M, et al. 2011. Sterilization effect on structure, thermal and time-dependent properties of polyamides In: Proulx T. (ed.) Mechanics of Time-Dependent Materials and Processes in Conventio. [Google Scholar]
- 19.Sicard GK, Hayashi K, Manley PA. 2002. Evaluation of 5 types of fishing ,aterial, 2 sterilization methods, and a crimp-clamp system for extra-articular stabilization of the canine stifle joint. Vet Surg 31, 78 84 https://doi.org/10.1053/jvet.2002.30539 [DOI] [PubMed] [Google Scholar]
- 20.De Melo D, Kelly de Oliveira L, Vickery K, et al. Reprocessing safety issues associated with complex-design orthopaedic loaned surgical instruments and implants. Injury. 2018;49(11):2005–2012. doi: 10.1016/j.injury.2018.09.006. https://doi.org/10.1016/j.injury.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 21.George M, Aroom KR, Hawes HG, et al. 3D printed surgical instruments: The design and fabrication process. World J Surg. 2017;41(1):314–319. doi: 10.1007/s00268-016-3814-5. https://doi.org/10.1007/s00268-016-3814-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wallace CA, Words K. New developments in disinfection and sterilization. Am J Infect Control. 2016;44(5):e23–e27. doi: 10.1016/j.ajic.2016.02.022. https://doi.org/10.1016/j.ajic.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 23.Perez MA, Block M, Espalin D, et al. Sterilization of FDM-manufactured parts. In 2012 international solid freeform fabrication symposium. University of Texas at Austin. 2012:285–296. http://dx.doi.org/10.26153/tsw/15350. [Google Scholar]
- 24.Oth O, Dauchot C, Orellana M, et al. How to sterilize 3D printed objects for surgical use? An evaluation of the volumetric deformation of 3D-printed genioplasty guide in PLA and PETG after sterilization by low-temperature hydrogen peroxide gas plasma abstract. Open Dent J. 2019;13(1):410–417. https://doi.org/10.2174/1874210601913010410. [Google Scholar]
- 25.Modjarrad K, Ebnesajjad S, editors. Handbook of Polymer Applications in Medicine and Medical Devices. 2014. p. 354. Eds., [Google Scholar]
- 26.Chen JV, Tanaka K S, Dang ABC, et al. 2020. Identifying a commercially-available 3D printing process that minimizes model distortion after annealing and autoclaving and the effect of steam sterilization on mechanical strength. 3D Print Med 6, 1 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Popescu D, Baciu F, Vl D, et al. 2020. Effects of multiple sterilizations and natural aging on the mechanical behavior of 3D-printed ABS. Mech Mater 148(December 2019): 2020. https://doi.org/10.1016/j.mechmat.2020.103423 [Google Scholar]
- 28.164Aboamer MA, Elgohary DH, Almukil AA, et al. A comparative study of mechanical behavior of ABS material based on UVC sterilization for medical usage. J Mech Sci Technol. 2022;36(7):3373–3385. https://doi.org/10.1007/s12206-022-0616-6. [Google Scholar]
- 29.Bosc R, Tortolano L, Hersant B, et al. Bacteriological and mechanical impact of the Sterrad sterilization method on personalized 3D printed guides for mandibular reconstruction. Sci Rep. 2021;11(1):1–10. doi: 10.1038/s41598-020-79752-7. https://doi.org/10.1038/s41598-020-79752-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong JY, Pfahnl AC. 2014. 3D printing of surgical instruments for long-duration space missions. Aviat Sp Env Med 85, 758 763 https://doi.org/10.3357/ASEM.3898.2014 [DOI] [PubMed] [Google Scholar]
- 31.Haryńska A, Gubanska I, Kucinska-Lipka J, et al. Fabrication and characterization of flexible medicalgrade TPU filament for Fused Deposition Modeling 3DP technology. Polymers (Basel) 2018;10(12):1304. doi: 10.3390/polym10121304. https://doi.org/10.3390/polym10121304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pop SI, Dudescu M, Mihali SG, et al. Effects of disinfection and steam sterilization on the mechanical properties of 3D SLA- and DLP-printed surgical guides for orthodontic implant placement. Polymers (Basel) 2022;14(10):2107. doi: 10.3390/polym14102107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma N, Cao S, Msallem B, et al. Effects of steam sterilization on 3D printed biocompatible resin materials for surgical guides—An accuracy assessment study. J Clin Med. 2020;9(5):1506. doi: 10.3390/jcm9051506. https://doi.org/10.3390/jcm9051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan M, Huang LG, Wu P, et al. Study the design parameters of 3D printing surgical guide deformation quantity by disinfection and sterilization. J Ind Prod Eng. 2021;38(7):499–507. https://doi.org/10.1080/21681015.2021.1943719. [Google Scholar]
- 35.Robles Linares-Alvelais HRS José A, Obedt Figueroa-Cavazos J, Chuck-Hernandez C, et al. Hydrostatic high-pressure post-processing of specimens fabricated by DLP, SLA, and FDM: An alternative for the sterilization of polymer-based biomedical devices. Materials (Basel) 2018;11:2540. doi: 10.3390/ma11122540. https://doi.org/10.3390/ma11122540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rouz F, Cade R, Naveau A, et al. Accuracy of commercial 3D printers for the fabrication of surgical guides in dental implantology. J Dent. 2022;117:103909. doi: 10.1016/j.jdent.2021.103909. https://doi.org/10.1016/j.jdent.2021.103909. [DOI] [PubMed] [Google Scholar]
- 37.Told R, Ujfalusi Z, Pentek M, et al. A state-of-the-art guide to the sterilization of thermoplastic polymers and resin materials used in the additive manufacturing of medical devices. Mater Des. 2022;223:111119. https://doi.org/10.1016/j.matdes.2022.111119. [Google Scholar]
- 38.Rynio P, Galant K, Łukasz W, et al. 2022. Effects of sterilization methods on different 3D printable materials for templates of physician-modified aortic stent grafts used in vascular surgery—A preliminary study. Int J Mol Sci 23, 1 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Popescu D, Baciu F, Vl D, et al. Mechanics of materials effects of multiple sterilizations and natural aging on the mechanical behavior of 3D-printed ABS. Mech Mater. 2020;148:103423. https://doi.org/10.1016/j.mechmat.2020.103423. [Google Scholar]
- 40.Luchini K, Sloan SNB, Mauro R, et al. Sterilization and sanitizing of 3D-printed personal protective equipment using polypropylene and a single wall design. 3D Print Med. 2021;7(1):1–10. doi: 10.1186/s41205-021-00106-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coté J J, Haggstrom J, Vivekanandan R, et al. COVID-19 and a novel initiative to improve safety by 3D printing personal protective equipment parts from computed tomography. 3D Print Med. 2020;6(1):1–12. doi: 10.1186/s41205-020-00073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spörk M. 2018. Optimisation of the mechanical properties and processing of polypropylene and poly (lactic acid) parts produced by extrusion-based additive manufacturing, Ghent University. [Google Scholar]
- 43.Głowacki M, Mazurkiewicz A, Słomion M, et al. Resistance of 3D-printed components , test specimens and products to work under environmental conditions—Review. Materials (Basel) 2022;15(17):1–19. doi: 10.3390/ma15176162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Török G, Gombocz P, Bognár E, et al. Effects of disinfection and sterilization on the dimensional changes and mechanical properties of 3D printed surgical guides for implant therapy—Pilot study. BMC Oral Health. 2020;20(1):1–12. doi: 10.1186/s12903-020-1005-0. https://doi.org/10.1186/s12903-020-1005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.PolyJet materials. 2023. https://www.stratasys.com/en/materials/materials-catalog/polyjet-materials/?filter=MT_PolyJet Stratasys,
- 46.Gielisch M, Heimes D, Thiemet DGE, et al. Steam-sterilized and degradable fused filament fabrication-printed polylactide/polyhydroxyalkanoate surgical guides for dental implants: Are they accurate enough for static navigation? Int J Bioprint. 2022;9(2) doi: 10.18063/ijb.v9i2.655. http://dx.doi.org/10.18063/ijb.v9i2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu WY, Su YX, Pow EHN, et al. ‘Three-in-one’ patient-specific surgical guides for simultaneous dental implants in fibula flap jaw reconstruction: A prospective case series. Clin Implant Dent Relat Res. 2021;(23):43–53. doi: 10.1111/cid.12954. https://doi.org/10.1111/cid.12954. [DOI] [PubMed] [Google Scholar]
- 48.Schwarzkopf R, Fernandez E, Buckland A, et al. Feasibility of single-use 3D-printed instruments for total knee arthroplasty. Bone Jt J. 2019;(101):115–120. doi: 10.1302/0301-620X.101B7.BJJ-2018-1506.R1. https://doi.org/10.1302/0301-620X.101B7.BJJ-2018-1506. R1. [DOI] [PubMed] [Google Scholar]
- 49.https://formlabs-media.formlabs.com/datasheets/1801084-TDS-ENUS-0P.pdf Formlabs, Resin for pliable prototyping.
- 50.Lioufas PA, Hons M, Quayle MR, et al. 3D printed models of cleft palate pathology for surgical education. Plast Reconstr Surg Glob Open. 2016;4(9):1–6. doi: 10.1097/GOX.0000000000001029. https://doi.org/10.1097/GOX.0000000000001029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aimar A, Palermo A, Innocenti B. The role of 3D printing in medical applications: A state of the art. J Healthc Eng. 2019:5340616. doi: 10.1155/2019/5340616. https://doi.org/10.1155/2019/5340616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bosc R, Tortolano L, Hersant B, et al. Bacteriological and mechanical impact of the Sterrad sterilization method on personalized 3D printed guides for mandibular reconstruction. Sci Rep. 2021;11:581. doi: 10.1038/s41598-020-79752-7. https://doi.org/10.1038/s41598-020-79752-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fuentes JM, Arrieta MP, Boronat T. Effects of steam heat and dry heat sterilization processes on 3D printed commercial polymers printed by fused deposition modeling. Polymers (Basel) 2022;14(855):855. doi: 10.3390/polym14050855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zunita M, Makertiharta IGBN, Irawanti R. 2022. 3D printed polyether ether ketone (PEEK), polyamide (PA) and its evaluation of mechanical properties and its uses in healthcare applications. IOP Conf Ser Mater Sci Eng 1224, 0 8 https://doi.org/10.1088/1757-899X/1224/1/012005 [Google Scholar]
- 55.Msallem B, Sharma N, Cao S, et al. Evaluation of the dimensional accuracy of 3D-printed anatomical mandibular models using. J Clin Med. 2020;9(3):817. doi: 10.3390/jcm9030817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Razaviye MK, Tafti RA, Khajehmohammadi M. 2022. SLS 3D printer: An experimental approach. CIRP J Manuf Sci Technol 38, 760 768 https://doi.org/10.1016/j.cirpj.2022.06.016 [Google Scholar]
- 57.Chen YW, Moussi J, Drury JL, et al. Zirconia in biomedical applications. Expert Rev Med Devices. 2016;13(10):945–963. doi: 10.1080/17434440.2016.1230017. https://doi.org/10.1080/17434440.2016.1230017. [DOI] [PubMed] [Google Scholar]
- 58.Xing H, Zou B, Li S, et al. Study on surface quality, precision and mechanical properties of 3D printed ZrO2 ceramic components by laser scanning stereolithography. Ceram Int. 2017;43(18):16340–16347. https://doi.org/10.1016/j.ceramint.2017.09.007. [Google Scholar]
- 59.Kassab GS, Sacks MS. 2016. Structure-based mechanics of tissues and organs. [Google Scholar]
- 60.Abbott RD, Kaplan DL. Strategies for improving the physiological relevance of human engineered tissues. Trends Biotechnol. 2015;33(7):401–407. doi: 10.1016/j.tibtech.2015.04.003. https://doi.org/10.1016/j.tibtech.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gu Q, Tomaskovic-Crook E, Lozano R. Functional 3D neural mini‐tissues from printed gel‐based bioink and human neural stem cells. Adv Healthc Mater. 2016;5(12):1429–1438. doi: 10.1002/adhm.201600095. [DOI] [PubMed] [Google Scholar]
- 62.Tejo-Otero A, Fenollosa-Artés F, Achaerandio I, et al. Soft-tissue-mimicking using hydrogels for the development of phantoms. Gels, 2022;8(1):40. doi: 10.3390/gels8010040. https://doi.org/10.3390/gels8010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoon YC, Lee JS, Park SU, et al. Quantitative assessment of liver fibrosis using shore durometer. Ann Surg Treat Res. 2017;93(6):300–304. doi: 10.4174/astr.2017.93.6.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Estermann SJ, Pahr DH, Reisinger A. Quantifying tactile properties of liver tissue, silicone elastomers, and a 3D printed polymer for manufacturing realistic organ models. J Mech Behav Biomed Mater. 2020;104:103630. doi: 10.1016/j.jmbbm.2020.103630. [DOI] [PubMed] [Google Scholar]
- 65.Tejo-Otero A, Lustig-Gainza P, Fenollosa-Artés F, et al. 3D printed soft surgical planning prototype for a biliary tract rhabdomyosarcoma. J Mech Behav Biomed Mater. 2020;109:103844. doi: 10.1016/j.jmbbm.2020.103844. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be available for readers upon reasonable request.


