Abstract
Background
Breast cancer is one of the main causes of death in women. Uncaria gambir is an Indonesian herbal plant that can be used as an anti-cancer. However, herbal medicines have low bioavailability, which affects their bioactivity. Nanoencapsulation can increase bioavailability and stability of bioactive compounds in herbal medicines.
Purpose
This recent finding tried to unravel anti-cancer and chemopreventive of U. gambir nano-encapsulated by Na-alginate.
Study Design
U. gambir bioactive compounds were isolated and characterized using UV–Vis spectrometer, FTIR, NMR and HR-MS. U. gambir extract was nanoencapsulated using Na-alginate. Anti-cancer effect was assessed by MTT assay towards T47D cell. Meanwhile, a chemopreventive analysis was carried out in breast cancer mice-induced benzo[α]pyrene. The healthy mice were divided into 8 groups comprising control and treatment.
Results
Elucidation of U. gambir ethyl acetate extract confirmed high catechin content, 89.34% (w/w). Successful nanoencapsulation of U. gambir (G-NPs) was indicated. The particle size of G-NPs was 78.40 ± 12.25 nm. Loading efficiency (LE) and loading amount (LA) of G-NPs were 97.56 ± 0.04% and 32.52 ± 0.01%, respectively. G-NPs had an EC50 value of 10.39 ± 3.50 µg/mL, which was more toxic than the EC50 value of extract towards the T47D cell line. Administration of 200 mg/kg BW G-NPs to mice induced by benzo[α]pyrene exhibited SOD and GSH levels of 13.69 ng/mL and 455.6 ng/mL. In addition, the lowest TNF-α level was 27.96 ng/mL. A dose of 100 mg/kg BW G-NPs could best increase CAT levels by 7.18 ng/mL. There was no damage or histological abnormalities found in histological analysis of the breast tissue in the group given 200 mg/kg BW G-NPs.
Keywords: breast cancer, chemopreventive, cytotoxicity, nanoencapsulation, U. gambir
Introduction
Cancer is the uncontrolled and abnormal growth of various body cells. Nowadays, 100 different types of cancer pose diversity in the mechanism of action and response to the related treatment.1 Based on global burden cancer (GLOBOCAN) data reported in 2020, global incidents of new-diagnosed cancer and death-associated cancer death reached about 19.3 million and 10.0 million cases, respectively. With an estimated 2.3 million new cases, female breast cancer is the most frequently diagnosed cancer, followed by lung (11.4%), colorectal (10.0%), prostate (7.3%), and stomach (5.6%) cancers. However, lung cancer is still the most common cause of cancer death.2 According to RISKESDAS data, Indonesia ranks 8th for cancer cases, increasing from 1.4 cases per 1000 people in 2013 to 1.79 cases per 1000 in 2018.3
The interplay between genetic risk and environmental factors causes breast cancer. Both lead to a series of development processes starting from genetically altered epithelial cells, hyperplasia, dysplasia, and in situ cancer till the end with malignant tumor (cancer).4–6 This ailment has three major subtypes: luminal, HER2+, and Triple-negative.6 Oxidative stress is essential in early cancer initiation, including breast cancer. Overexposure to ROS (reactive oxygen species) promotes tumor progression and causes an imbalance of antioxidant enzymes. Level of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx and paraoxonases (PONs) decrease in bladder cancer compared to control. Moreover, oxidative stress also triggers the production of pro-inflammatory cytokines, TNF-α (tumor necrosis alpha factor), which are involved in cancer development.7 Therefore, a proper medication that targets oxidative stress, inflammation, and carcinogenesis axis is vital to prevent further cancer development.
Radiotherapy and chemotherapy have so far been the mainstays of breast cancer treatment. Because both methods can harm normal cells while killing cancer cells, their adverse effects are frequently devastating.8 Several drawbacks of chemotherapy, such as hair loss, bone marrow suppression, drug resistance, gastrointestinal lesions, neurological dysfunctions, and cardiac toxicity, are also inevitable.9,10 Hence, the need to develop effective cancer treatment has become the main focus of many cancer researches.11 The use of herbal-based medicines is known to reduce cancer cell proliferation while perceived to have fewer side effects than chemical drugs.12,13 In respect of abundant herb with anti-cancer potency, Uncaria gambir, a member of the Rubiaceae family commonly found in West Sumatra, contains catechin that already proves cytotoxicity against breast cancer cell lines.14 Evacuasiany et al reported that catechin better exhibits antioxidant and cytotoxicity effects in T47D than in MCF7 cells.15 The anti-cancer mechanism of catechin is associated with the ability to induce cell apoptosis and inhibit angiogenesis and metastasis of malignant cells.16 In particular, hydrated catechin suppress MCF7 cell proliferation as well as induces apoptosis by improving the expression of caspase-3, −8, −9, and tumor suppressor p53.17 Nowadays, successful nanoparticle research as anticancer is reported on epigallocatechin-3-gallate,18 biosynthesized ZnO NPs, CuO NPs derived from pumpkin seeds extract,19–21 MnONPs,22 AuNPs,23 and AgNPs.24
Cancer-nanomedicine (cancer treatment that applied nanotechnology) has been broadly studied and even applied clinically in recent decades.25 Among numerous nanotechnology tools, biopolymeric nano-capsules offer an up-and-coming alternative design of targeted cancer therapy. Nanocapsules (made from alginate, pullulan, cellulose, polylactic acid, chitosan, and other biopolymers) gain much attention as anti-cancer drug delivery system since nano-size increase the surface area of an active material, exhibiting a high stability and bioavailability but lowering drug toxicity.26,27 Moreover, biopolymeric nano-capsules are acknowledged for their cost-effective and environmentally friendly drug preparation.28 There are many technologies to produce nanocapsule, including ultrasound-assisted nanoencapsulation. Bioactive-loaded biopolymeric nanocarriers prepared with sonication are reported to exhibit better colloidal dispersion, gastrointestinal fate and safety in many studies.29 In addition, ultrasonication owns broad technically benefits comprising simplicity, versatility, environmentally friendly and by-product free, making it preferred in the nanoparticle fabrication.30 However, excessive immune response as well as production of inflammatory mediators still become common limitation of polymeric-based nanoparticle administration because toxicity of polymeric nanoparticle is influenced by quantum size.31 Hence proper nanoencapsulation technique must be established to achieve better nanocapsules.
Recent findings elucidate the nanoencapsulation of U. gambir using sodium alginate to enhance bioactive compounds’ activity with anti-cancer potency. Anti-cancer activity of nano-gambir formulation against breast cancer is assessed in vitro and in vivo. Furthermore, the cytotoxic effect of nano-gambir is evaluated in vitro in the T47D cell line. Meanwhile, model mice suffering from breast cancer are used to evaluate in vivo chemopreventive activity of nano-gambir.
Materials and Methods
Material
The Uncaria gambir was taken from Bima, Sumbawa Island, Nusa Tenggara Barat Province, Indonesia. The plant was identified by staff at the Indonesian Biology Generation Foundation (“Yayasan Generasi Biologi Indonesia [YGBI]”) with the certificate No. 232/02.Genbinesia/2022, and a voucher specimen had been deposited at the herbarium of YGBI. Methanol, ethanol, n-hexane, ethyl acetate, sodium alginate, distilled water, phosphate buffer, phosphate buffer saline (PBS); benzo[α]pyrene (Merck), Na-CMC, SOD kit (BT-Lab), CAT kit (BT-Lab), GSH kit (BT-Lab), TNF-α kit (BT-Lab), Neutral Buffered Formalin (NBF) 10%. The T47D cell line used has been approved by the health research ethical clearance commission, Faculty of dental medicine, Airlangga University with ethical clearance certificate number: 028/HRECC.FODM/I/2020.
Extraction and Isolation of U. gambir
The powder of U. gambir sap (± 1 kg) was macerated by methanol for 1×24 h with 1:2 ratio and repeated 3 times. A rotary vacuum evaporator then concentrated the methanolic extract at 50 oC. The thick extract already obtained was subsequently partitioned by n-hexane and ethyl acetate at the same ratio. Next, the ethyl acetate extract of U. gambir was concentrated by rotary vacuum evaporator until yielded U. gambir ethyl acetate extract powder. The ethyl acetate extract of U. gambir sap was isolated using vacuum column chromatography followed by gravity column chromatography. The pure isolates were then characterized using a UV-Vis spectrometer, FTIR, NMR, and HR-MS.
Characterization of G-NPs
The technique reported by Kristanti et al was used to nanoencapsulation an ethyl acetate extract of U. gambir with Na-alginate.32 The nano-capsule product is then stated as G-NPs. The physicochemical properties of G-NPs were evaluated by a polydispersity index (PDI), zeta potential (ζ), and particle size (Dynamic Light Scattering, Zetasizer Nano ZS, Malvern). Analysis of the functional groups of NPs was assessed by FTIR (Shimadzu IRTracer-100), while analysis decomposition of G-NPs was performed by TGA (Perkin Elmer TGA 4000). AFM characterized the topography of G-NPs.
Stability Analysis of G-NPs
The stability of G-NPs in protecting bioactive compounds was assessed against several parameters, comprising temperature, pH, and salt concentration (NaCl). In addition, UV-Vis absorption spectra were performed in each parameter evaluation. Moreover, the degree of turbidity of nanoparticles was evaluated too.33
Loading and Release of G-NPs Calculations
The loading amount (LA) and loading efficiency (LE) of bioactive components were, respectively, calculated by Equations 1 and 2. The release of the G-NPs bioactive compound was determined by Equation 3.33,34
 |
(1) |
 |
(2) |
 |
(3) |
Where Ct’: concentration correction at t time
Ct: measured concentration at t time
V: total volume of buffer used
v: volume of aliquots
Cytotoxicity Assay
Research using the T47D cell line has been ethically certified by the Health Research Ethical Clearance Commission, Faculty of Dentistry, Universitas Airlangga, Indonesia (Ethical Clearance Certificate, No. 028/HRECC.FODM/I/2020). The research was conducted at the Cancer Chemoprevention Research Center, Faculty of Pharmacy, Universitas Gadjah Mada. T47D cell lines were cultured in RPMI 1640 media and further seeded at 96-well plates with a density of 10×104 cells / well upon reaching 80% confluence. The culture was further incubated for 24 h in an incubator (37°C; 5% CO2). About 100 μL of culture media containing samples (U. gambir extract and G-NPs) were added to each well, and incubation was carried out for 24 h. After incubation, all medium was discarded and then washed with PBS. Cytotoxicity assay was done by adding 100 μL of MTT (5 mg/mL) reagent to each well and then incubated for 4 hours at 37°C in 5% CO2. The MTT assay was stopped by giving 100 μL of 10% SDS within 0.01 N HCl. The absorbance was measured using an ELISA reader (Bio-Rad) at λ 550 nm. The EC50 value of each sample was obtained by using a dose-response calculation.
Chemopreventive Potency
Current animal research has been ethically certified by the Health Research Ethical Clearance Commission, Faculty of Dental Medicine, Universitas Airlangga, Indonesia (Ethical Clearance Certificate, No. 351/HRECC.FODM/VI/2021). The healthy mice (Mus musculus) Balb/c at the age of 6–8 weeks with 20–25 g of body weight and never experienced pregnancy were placed in the cage with lighting conditions 12 hours per day, temperature 25 °C, and humidity ± 50–60%. All mice were then grouped into 8 cohorts comprising:
C0: Control, mice were treated with water only.
C1: Cancer control, mice were injected with benzo[α]pyrene (0.2 mL / 2 days for 5 times) in the 2nd week and observed for 8 weeks.
T1: Treatment 1, mice were given U. gambir extract (50 mg/kg BW) orally every day for 8 weeks. In the 2nd week of treatment, an injection of benzo[α]pyrene was carried out.
T2: Treatment 2, mice were given U. gambir extract (100 mg/kg BW) orally every day for 8 weeks. In the 2nd week of treatment, an injection of benzo[α]pyrene was carried out.
T3: Treatment 3, mice were given U. gambir extract (200 mg/kg BW) orally every day for 8 weeks. In the 2nd week of treatment, an injection of benzo[α]pyrene was carried out.
T4: Treatment 4, mice were given G-NPs (50 mg/kg BW) orally every day for 8 weeks. In the 2nd week of treatment, an injection of benzo[α]pyrene was carried out.
T5: Treatment 5, mice were given G-NPs (100 mg/kg BW) orally every day for 8 weeks. In the 2nd week of treatment, an injection of benzo[α]pyrene was carried out.
T6: Treatment 6, mice were given G-NPs (200 mg/kg BW) orally every day for 8 weeks. In the 2nd week of treatment, an injection of benzo[α]pyrene was carried out.
All mice cohorts were sacrificed at week 8, and their blood was taken for clinical blood analysis to measure levels of SOD, GSH, CAT, and TNF-α. In addition, each mice group was also taken breast tissue, liver, kidney, spleen, and pancreas for histopathological analysis.
Statistical Analysis
The software GraphPad Prism 8 was utilized for the statistical analysis. The anti-cancer effects of G-NPs and U. gambir extract were examined using one-way ANOVA. Tukey’s test was also used to conduct post-hoc analysis to identify group differences. In this instance, p < 0.05 was deemed significant.
Results
The Elucidation of U. gambir Bioactive Compound
Methanol was used to extract the U. gambir sap, divided using n-hexane and ethyl acetate. U. gambir extract was further determined its main bioactive compound by chromatographic separation and elucidation of the bioactive compound structure was also carried out. Based on the elucidation result, the main bioactive compound of U. gambir was (+)-catechin (Figure 1A). This compound was found to be a brownish-white solid with spectra analysis showed as followed, UV-Vis (MeOH) spectra: λmax 281 nm; IR spectra vmax (cm−1): 3650 (OH stretching), 2962 (C-H sp3 stretching), dan 1598 (C=C in ring); 1H-NMR (Bruker 600 MHz, DMSO-d6) δH (ppm): 4.59 (d, 1H, J = 5.0, H-2), 3.80 (m, 1H, H-3), 2.65 dan 2.34 (dd, 1H, J = 5.0 dan 16.0, H-4), 5.78 (d, 1H, J = 2.0, H-6), 5.66 (d, 1H, J = 2.0, H-8), 6.70 (d, 1H, J = 2.0, H-2’), 6.66 (d, 1H, J = 8.0, H-5’), 6.58 (dd, 1H, J = 2.0 dan 8.0, H-6’), 4.83 (s, 1H, OH-3), 9.15 (s, 1H, OH-7), 8.91 (s, 1H, OH-5), 8.83 (s, 1H, OH-3’), 8.87 (s, 1H, OH-4’); 13C-NMR (Bruker 150 MHz, DMSO-d6) δC (ppm): 80.7 (C-H, C-2), 67.5 (C-H, C-3), 28.1 (C-H2, C-4), 157.3 (C, C-5), 95.5 (C-H, C-6), 157.8 (C, C-7), 94.8 (C-H, C-8), 157.2 (C, C-9), 99.0 (C, C-10), 131.5 (C, C-1’), 115.2 (C-H, C-2’), 145.8 (C, C-3’), 144.6 (C, C-4’), 116.1 (C-H, C-5’), 121.0 (C-H, C-6’); ESI-MS: m/z 291.2 [M+H+]; optical rotation +17°. The highest catechin content, 89.34% (w/w), was found in the U. gambir ethyl acetate extract (Figure 1B), which was then processed with Na-alginate for nano encapsulation.
Figure 1.
(A) Structure of (+)-catechin; (B) Catechin content of U. gambir extract.
Abbreviations: ME, methanol extract; EAE, ethyl acetate extract; HE, n-hexane extract.
Characterization of G-NPs
The physicochemical properties of G-NPs are shown in Table 1. G-NPs were nano-capsule of U. gambir extract encapsulated by Na-alginate biopolymer. G-NPs had a particle size of 78.40 ± 12.25 nm. The polydispersity index (PDI) measured size heterogeneity of G-NPs indicating size distribution or agglomeration/aggregation of sample where PDI > 0.7 was considered broad particle size distribution.35,36 PDI value of G-NPs was 0.55 ± 0.01, indicating that particle size distribution tended to be homogeneous since the smaller polydispersity index meant more uniform particle size.37
Table 1.
Physicochemical of G-NPs
| Type | Size ± SD (nm) | PDI ± SD | ζ ± SD (mV) |
|---|---|---|---|
| Na-Alginate | 600.20 ± 105.02 | 1.00 ± 0.00 | −37.20 ± 3.06 |
| U. gambir extract | 586.00 ± 13.86 | 0.47 ± 0.05 | −44.47 ± 0.47 |
| G-NPs | 452.63 ± 5.29 | 0.55 ± 0.01 | −40.87 ± 0.90 |
Notes: Each data presented as mean ± SD (n=3).
Zeta potential (ζ) analysis validated the successful encapsulation of G-NPs. Based on the result shown in Table 1, Na-alginate −37.20 ± 3.06 mV, U. gambir extract −44.47 ± 0.47 mV, and G-NPs −40.87 ± 0.90 mV had a negative value which was indicated as a low ζ value. A large number of electronegative hydroxy groups were to blame for the phenomenon. The Zeta potential value for G-NPs was comparable to that of U. gambir extract and Na-alginate. This is presumably because an electropositive group of Na-alginate partially compromised the electronegative group within U. gambir extract. Since a Zeta potential value more significant than ± 30 mV was considered stable because surface charge prevented aggregation,38 this recent nanoencapsulation Zeta potential result of −40.87 ± 0.90 mV demonstrated stable suspension. Due to the increased electrostatic repulsion between particles, a more significant Zeta potential indicated improved stability. Based on AFM analysis, the size of G-NPs was ± 70 nm. This supported result of the DLS measurement (Figure 2).
Figure 2.
AFM 2D topography images of G-NPs.
Na-alginate and U. gambir extract shared a peak in the G-NPs’ FTIR spectra. U. gambir extract was responsible for absorptions at 1033 cm−1 (C-O-C stretching vibration) and 1519 cm−1 (C-C in ring). In contrast, Na-alginate was absorbed in 1419 cm−1 (vibrations of the carboxylate salt ion) and 1122 cm−1 (deformation of -OH in -COOH) (Figure 3). The composition of the active compounds in the U. gambir extract and their interactions with the sodium alginate coating material were determined through TGA analysis of G-NPs (Figure 4). G-NPs and U. gambir extract deteriorated at a temperature of 70° C, which showed a decreasing sample weight of 10.5% and 12.1%, respectively. At 178 °C, G-NPs and extracts of U. gambir decomposed with a decrease in sample weight of 16.2% and 33.1%, respectively, while Na-alginate decomposed at a temperature of 290 °C with a decrease in sample weight of 35.3%. G-NPs also decomposed with a decrease in sample weight of 25.2% at 290 °C. FTIR and TGA analysis indicated there was no chemical interaction between the constituent of U. gambir extract and Na-alginate in the G-NPs but only physical interactions.
Figure 3.
FTIR spectra of (A) Na-alginate, (B) G-NPs, and (C) U. gambir extract.
Figure 4.
TGA analysis of (A) Na-alginate, (B) G-NPs, and (C) U. gambir extract.
Stability of G-NPs
Previously, (+)-catechin was confirmed in the U. gambir extract. Nanoencapsulation was opposed as a means of safeguarding this bioactive compound against degradation. G-NPs’ stability could be maintained without causing aggregation or changes to their bioactive components. Absorption band of G-NPs did not change at 383–386 nm after exposure to different pH 3 to 11 (Figure 5). At pH 12, first absorption band experience bathochromic shift that appeared in wave length 410 nm. A hyperchromic effect was usually observed in the second absorption band when pH was changed from 3 to 11. In the meantime, it caused a shift between hypochromic and hyperchromic at pH 12. G-NPs typically had a turbidity level higher in an acidic (pH 3–5) environment and lower in a neutral to a basic one (pH 6–12). G-NPs tended to be less stable and precipitated at acidic pH because of the stabilizing effect of Na-alginate. Below pH 5, the free –COO− ions would form protonated –COOH. As a result, the electrostatic repulsion between the chains decreased enabling hydrogen bonds formation which further caused viscosity increment. However, depolymerization retardation occurred in an alkaline environment, resulting in a decrease of viscosity.39,40
Figure 5.
Stability of G-NPs against pH. (A) UV-Vis spectra, and (B) Turbidity.
G-NPs’ turbidity level and UV-Vis absorption pattern were largely unaffected by temperature changes between 30°C and 100°C. The viscosity of Na-alginate will decrease as the temperature rises (Figure 6).41 G-NPs’ ionic stability indicated that the UV-Vis absorption band of G-NPs was unaffected by NaCl at concentrations between 0 and 0.3 M. However, G-NP turbidity was affected by an increased NaCl concentration. This phenomenon is probably because Na-alginate is stable in NaCl solution. The monovalent salt NaCl could affect the precipitation rate of Na-alginate (Figure 7).42
Figure 6.
Stability of G-NPs against temperature. (A) UV-Vis spectra, and (B) Turbidity.
Figure 7.
Stability of G-NPs against NaCl concentration. (A) UV-Vis spectra, and (B) Turbidity.
Loading and Release of G-NPs
Both loading efficiency (LE) and loading amount (LA) analysis indicated successful adsorption of U. gambir bioactive compound in Na-alginate micelles with percentages 97.56 ± 0.04% and 32.52 ± 0.01%, respectively. Release of bioactive compound tended to slow at pH 4 since the electrostatic repulsion of Na-alginate decreased, which was further ended by G-NPs aggregation (Figure 8). Besides that, the formation of hydrogen bonds caused the shrinking of G-NPs pores. This is exemplified by the percentage release of 12.10 ± 0.03% after 24 h exposure to acidic pH (Figure 8). As for pH 7 and 9, the release got to be faster, with percentages of 27.65 ± 0.03% and 31.37 ± 0.00% after 24 h, respectively. Repulsion between particles at neutral or alkaline pH caused G-NPs pores enlargement.
Figure 8.
Effect of pH on the release of bioactive components of U. gambir from G-NPs.
Cytotoxic Activity of G-NPs
The results of an evaluation of the anti-cancer activity of U. gambir extract and G-NPs against T47D cell lines were presented as EC50 ± SE. The cytotoxicity effect of both extract and G-NPs was followed in dose-dependent manner. However, a decrease in % cell viability of G-NPs was better than extract (Figure 9). G-NPs formulation had EC50 10.39 ± 3.50 µg/mL, which was better toxic than the EC50 value of extract 297.15 ± 15.41 µg/mL. This result demonstrated that nanoencapsulation increased U. gambir extract’s growth inhibitory activity against the T47D cell line. Measurable examination affirmed that nanoencapsulation of U. gambir removed essentially expanded inhibitory development impact with p-value = 0.003.
Figure 9.
Cell viability vs concentration of U. gambir extract and G-NPs towards T47D cell line. (A) U. gambir extract and (B) G-NPs.
Chemopreventive Potency
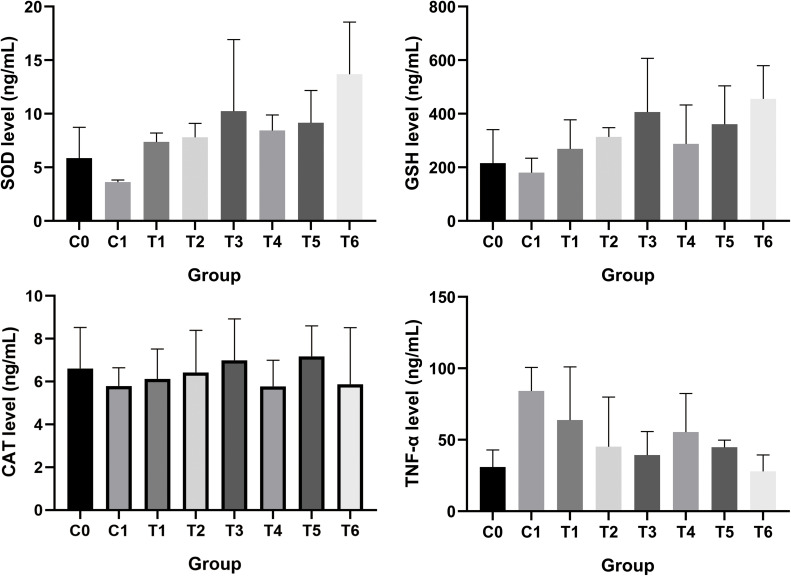
The chemopreventive potency of both U. gambir extract and G-NPs was assessed in the mice-induced breast cancer by benzo[α]pyrene. Benzo[α]pyrene was known to induce oxidative stress and affect the level of antioxidant enzyme (SOD and CAT), GST metabolizing enzyme, and inflammatory mediator TNF-α.43,44 The enzyme superoxide dismutase (SOD) played an essential role in mitigating cancer progression by catalyzing the breakdown of toxic components, superoxide radicals, into harmless components consisting of oxygen and hydrogen peroxide.45 Based on Figure 10, the lowest level of SOD was found in the cancer control group, 3.62 ng/mL, while the highest level of SOD was found in 200 mg/kg BW G-NPs treatment, 13.69 ng/mL. Catalase (CAT) enzyme was needed to detoxify hydrogen peroxide into water and oxygen. This enzyme was aberrantly regulated in cancer.46 The highest level of CAT was owned by a group of 100 mg/kg BW G-NPs treatment at 7.18 ng/mL, as cancer control had the lowest CAT level, 5.79 ng/mL. Like other antioxidant enzymes, glutathione (GSH) was indispensable to scavenging excessive radicals and detoxifying xenobiotics, further preventing oxidative stress in cells.47 Here, treating both U. gambir extract and G-NPs could maintain a high level of GSH but 200 mg/kg BW. G-NPs showed the best one, 455.6 ng/mL. The cancer control cohort had the lowest level of GSH, 180.0 ng/mL. Tumor necrosis factor alpha (TNF-α) was broadly known as a pro-inflammatory cytokine that was up-regulated in breast cancer. Exposure to a carcinogenic agent like benzo[α]pyrene could promote TNF- α.44 It was proved in the result that the cancer control group had a high level of TNF-α 84.24 ng/mL while treatment of U. gambir and G-NPs could alleviate modulation of TNF-α. Treatment of G-NPs maintained a high level of antioxidant enzyme and suppressed pro-inflammatory mediators better than extract. This indicated that nanoencapsulation could enhance the chemopreventive activity of U. gambir.
Figure 10.
Level of SOD, GSH, CAT, and TNF-α in each treatment group.
Abbreviations: C0, control; C1, cancer control; T1, U. gambir extract (50 mg/kg BW); T2, U. gambir extract (100 mg/kg BW); T3, U. gambir extract (200 mg/kg BW); T4, G-NPs (50 mg/kg BW), T5, G-NPs (100 mg/kg BW); T6, G-NPs (200 mg/kg BW).
Histological analysis of breast tissue confirmed the carcinogenicity of benzo[α]pyrene exposure and the ability of both U. gambir extract and G-NPs to prevent alteration of breast cells caused by benzo[α]pyrene. According to Figure 11, there was no histopathological changes in C0, but the ductal mammary gland in C1 experience hyperplasia (red arrow). Inflammation was indicated in all treatment groups except T6. Inflammatory cell infiltration was shown within adipocytes in T1 and T3. The T3 treatment group suffered an abscess where inflammatory cells infiltrated, mainly composed of neutrophils.48 However, hyperplasia was not indicated in both cohort. Fibrosis presented in T2 was indicated by increasing in dense connective tissue,49 and cell debris and apocrine metaplasia also indicated in T2. Ductal hyperplasia and apocrine metaplasia were indicated in T4 and T5. Meanwhile, T6 was indicated normal with no histopathological changes.
Figure 11.
Breast histology imaging (100 × magnification).
Abbreviations: C0, control; C1, cancer control; T1, U. gambir extract (50 mg/kg BW); T2, U. gambir extract (100 mg/kg BW); T3, U. gambir extract (200 mg/kg BW); T4, G-NPs (50 mg/kg BW), T5, G-NPs (100 mg/kg BW); T6, G-NPs (200 mg/kg BW); yellow arrow (→), ductus mammary; red arrow (→), hyperplasia.
Discussion
U. gambir was one of the plants with many catechins. Recent research revealed that U. gambir ethyl acetate extract contained 89.34% catechins. It was known that catechin had anti-cancer properties. Antioxidants,50 regulation of drug-metabolizing enzymes, induction of apoptosis,51 inhibition of cell proliferation52 and metastasis,53 anti-inflammatory,54 and regulation of the microbiota in the gut may all play a role in catechins’ anti-cancer activity.55,56 However, the preparation of natural ingredients such as catechin with no modification showed several drawbacks comprising low solubility and off-target. Such factors affected overall bioactivity.57
By increasing the bioavailability of the extract’s bioactive compound, the U. gambir nanoencapsulation process may enhance its anti-cancer activity. By effectively participating in a paracellular pathway and entering systemic channel,46 the small G-NPs could increase surface area. In addition, Na-alginate was widely regarded as a coating material that was biodegradable, biocompatible, and non-mutagenic, making it suitable for drug delivery.58–60 As indicated in this research, G-NPs could prevent the degradation of the bioactive component against pH, temperature, and salt concentrations. Additionally, the G-NPs could control release, increase dissolution rate and permeability, prolong plasma half-life, and improve the pharmacokinetic profile of the bioactive compound of U. gambir compared to extract alone.51 Previously, alginate in curcumin–casein–alginate– chitosan nanocomplexes was also proven to improve pharmacokinetics (enhanced bioavailability and cancer therapeutic efficacy against Ehrlich carcinoma) in per-oral treatment.61
Regarding cytotoxicity towards the T47D cell line, G-NPs showed high toxicity with low EC50 value compared to U. gambir extract. This result is supported by Syarifah et al, by whom the IC50 of U. gambir extract with no modification against the T47D cell line was 1000 µg/mL.62 Moreover, G-NPs also exhibited effective chemoprevention in a model of breast cancer mice-induced benzo[α]pyrene (B[a]P). Internalization of B[a]P would attract cytochrome P450 to detoxify. However, the metabolism of B[a]P resulted in the production of a lot of reactive oxygen species (ROS), a variety of unstable and reactive intermediates of B[a]P that have the potential to harm DNA and cause cell transformation and toxicity.63 Treatment of G-NPs maintained a high level of antioxidant enzyme (SOD, CAT, and GSH) and suppressed pro-inflammatory (TNF-α) mediators better than extract. This further reduced prolonged oxidative stress and inflammation, which are known to contribute to malignancy, including breast cancer.64,65
The chemopreventive ability of G-NPs is also proven in the histological analysis. No histological changes, such as hyperplasia, were reported in treating G-NPs containing 200 mg/kg BW of U. gambir extract. Conversely, treatment of T3 (200 mg/kg BW of U. gambir extract only) causes an abscess. Another G-NPs group, T4, experienced ductal hyperplasia, while T1 and T2 also experienced inflammation disorders. This presumably extracts concentration was not enough to compromise the toxicity of B[a]P. Hyperplasia was defined as increasing cell proliferation.57 These were a form of adaptation in responding to injury caused by B[a]P.
Conclusion
U. gambir contained 89.34% brownish-white solid (+)-catechin. Nanoencapsulation of U. gambir extract using Na-alginate was successfully established and has spherical form with the size of 78.40 ± 12.25 nm. It was proven to prevent bioactive compound deterioration against pH, temperature, and salinity as well as control drug release. Moreover, nanoencapsulation of U. gambir method increased bioactive compound effectiveness against breast cancer at in vitro level as well as improve its chemopreventive effect at in vivo level compared to free extracts.
Acknowledgments
This research was supported by the Universitas Airlangga “Penelitian Unggulan Airlangga 2023 (PUA)” with contract number: 311/UN3.15/PT/2023.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Cooper GM, Hausman R. A molecular approach. In: The Cell. 2nd ed. Sunderland, MA: Sinauer Associates; 2000. [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 3.Kemenkes. Hari Kanker Sedunia 2019. 2019. Available from: http://www.depkes.go.id/article/view/19020100003/hari-kanker-sedunia-2019.html. Accessed July 05, 2019.
- 4.Putti TC, Abd El-Rehim DM, Rakha EA, et al. Estrogen receptor-negative breast carcinomas: a review of morphology and immunophenotypical analysis. Mod Pathol. 2005;18(1):26–35. doi: 10.1038/modpathol.3800255 [DOI] [PubMed] [Google Scholar]
- 5.Ataollahi M, Sharifi J, Paknahad M, Paknahad A. Breast cancer and associated factors: a review. J Med Life. 2015;8(Spec Iss 4):6. [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou L, Wang D, Sheng D, et al. NOTCH4 maintains quiescent mesenchymal-like breast cancer stem cells via transcriptionally activating SLUG and GAS1 in triple-negative breast cancer. Theranostics. 2020;10(5):2405. doi: 10.7150/thno.38875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wigner P, Grębowski R, Bijak M, Saluk-Bijak J, Szemraj J. The interplay between oxidative stress, inflammation and angiogenesis in bladder cancer development. Int J Mol Sci. 2021;22(9):4483. doi: 10.3390/ijms22094483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu H, Lv L, Yang K. Chemotherapy targeting cancer stem cells. Am J Cancer Res. 2015;5(3):880. [PMC free article] [PubMed] [Google Scholar]
- 9.Hosseini A, Ghorbani A. Cancer therapy with phytochemicals: evidence from clinical studies. Avicenna J Phytomedicine. 2015;5(2):84–97. [PMC free article] [PubMed] [Google Scholar]
- 10.Gowd V, Ahmad A, Tarique M, et al. Advancement of cancer immunotherapy using nanoparticles-based nanomedicine. In: Seminars in Cancer Biology. Academic Press; 2022. [DOI] [PubMed] [Google Scholar]
- 11.Greenwell M, Rahman P. Medicinal plants: their use in anticancer treatment. Int J Pharm Sci Res. 2015;6(10):4103–4112. doi: 10.13040/IJPSR.0975-8232.6(10).4103-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du G-J, Zhang Z, Wen X-D, et al. Epigallocatechin Gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients. 2012;4(11):1679–1691. doi: 10.3390/nu4111679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phillipson J, R CE. Alkaloids of Uncaria. V. their occurrence and chemotaxonomy; 1978.
- 14.Anggraini T, Tai A, Yoshino T, Itani T. Antioxidative activity and catechin content of four kinds of Uncaria gambir extracts from West Sumatra, Indonesia. Afr J Biochem Res. 2011;5(1):33–38. [Google Scholar]
- 15.Evacuasiany E, Ratnawati H, Liana LK, et al. Cytotoxic and antioxidant activities of catechins in inhibiting the malignancy of breast cancer. Oxid Antioxid Med Sci. 2014;3(2):141–146. doi: 10.5455/oams.240614.or.066 [DOI] [Google Scholar]
- 16.Yu Y, Deng Y, Lu B-M, Liu Y-X, Li J, Bao J-K. Green tea catechins: a fresh flavor to anticancer therapy. Apoptosis. 2014;19(1):1–18. doi: 10.1007/s10495-013-0908-5 [DOI] [PubMed] [Google Scholar]
- 17.Alshatwi AA. Catechin hydrate suppresses MCF-7 proliferation through TP53/Caspase-mediated apoptosis. J Exp Clin Cancer Res. 2010;29(1):1–9. doi: 10.1186/1756-9966-29-167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alserihi RF, Mohammed MRS, Kaleem M, et al. Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment. Nanotechnol Rev. 2021;11(1):298–311. doi: 10.1515/ntrev-2022-0013 [DOI] [Google Scholar]
- 19.Tabrez S, Khan AU, Hoque M, et al. Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer. Nanotechnol Rev. 2022;11(1):2714–2725. doi: 10.1515/ntrev-2022-0154 [DOI] [Google Scholar]
- 20.Zughaibi TA, Mirza AA, Suhail M, et al. Evaluation of anticancer potential of biogenic copper oxide nanoparticles (CuO NPs) against breast cancer. J Nanomater. 2022;2022:1–7. doi: 10.1155/2022/5326355 [DOI] [Google Scholar]
- 21.Tabrez S, Khan AU, Mirza AA, et al. Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer. Nanotechnol Rev. 2022;11(1):1322–1331. doi: 10.1515/ntrev-2022-0081 [DOI] [Google Scholar]
- 22.Tabrez S, Khan AU, Hoque M, et al. Investigating the anticancer efficacy of biogenic synthesized MgONPs: an in vitro analysis. Front Chem. 2022;10. doi: 10.3389/fchem.2022.970193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alafaleq NO, Alomari A, Khan MS, et al. Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests. Nanotechnol Rev. 2022;11(1):3292–3304. doi: 10.1515/ntrev-2022-0502 [DOI] [Google Scholar]
- 24.Khan MS, Alomari A, Tabrez S, et al. Anticancer potential of biogenic silver nanoparticles: a mechanistic study. Pharmaceutics. 2021;13(5):707. doi: 10.3390/pharmaceutics13050707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bharali DJ, Mousa SA. Emerging nanomedicines for early cancer detection and improved treatment: current perspective and future promise. Pharmacol Ther. 2010;128(2):324–335. doi: 10.1016/j.pharmthera.2010.07.007 [DOI] [PubMed] [Google Scholar]
- 26.Shamsara O, Muhidinov ZK, Jafari SM, et al. Effect of ultrasonication, pH and heating on stability of apricot gum–lactoglobuline two layer nanoemulsions. Int J Biol Macromol. 2015;81:1019–1025. doi: 10.1016/j.ijbiomac.2015.09.056 [DOI] [PubMed] [Google Scholar]
- 27.Mehrnia M-A, Jafari S-M, Makhmal-Zadeh BS, Maghsoudlou Y. Crocin loaded nano-emulsions: factors affecting emulsion properties in spontaneous emulsification. Int J Biol Macromol. 2016;84:261–267. doi: 10.1016/j.ijbiomac.2015.12.029 [DOI] [PubMed] [Google Scholar]
- 28.Pathak N, Singh P, Singh PK, et al. Biopolymeric nanoparticles based effective delivery of bioactive compounds toward the sustainable development of anticancerous therapeutics. Front Nutr. 2022;2022:1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koshani R, Jafari SM. Ultrasound-assisted preparation of different nanocarriers loaded with food bioactive ingredients. Adv Colloid Interface Sci. 2019;270:123–146. doi: 10.1016/j.cis.2019.06.005 [DOI] [PubMed] [Google Scholar]
- 30.Tsai ML, Bai SW, Chen RH. Cavitation effects versus stretch effects resulted in different size and polydispersity of ionotropic gelation chitosan–sodium tripolyphosphate nanoparticle. Carbohydr Polym. 2008;71(3):448–457. doi: 10.1016/j.carbpol.2007.06.015 [DOI] [Google Scholar]
- 31.Elmowafy M, Shalaby K, Elkomy MH, et al. Polymeric nanoparticles for delivery of natural bioactive agents: recent advances and challenges. Polymers. 2023;15(5):1123. doi: 10.3390/polym15051123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kristanti AN, Zahra HI, Yuliati A, Aminah NS, Wardana AP, Inventors. nanoenkapsulasi gambir (Uncaria gambir) dengan penyalut natrium alginate menggunakan metode ultrasonikasi; 2020.
- 33.Wardana AP, Aminah NS, Fahmi MZ, et al. Nanoencapsulation of syzygium polycephalum extract using folate modified κ-carrageenan as vehicles for pronounced anticancer activity. Trop J Nat Prod Res. 2020;4(11):945–952. [Google Scholar]
- 34.Fahmi MZ, Haris A, Permana AJ, et al. Bamboo leaf-based carbon dots for efficient tumor imaging and therapy. RSC Adv. 2018;8(67):38376–38383. doi: 10.1039/C8RA07944G [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Worldwide MI. Dynamic light scattering, Common terms defined. Inform White Paper Malvern Inst Limited. 2011;2011:1–6. [Google Scholar]
- 36.Mudalige T, Qu H, Van Haute D, Ansar SM, Paredes A, Ingle T. Characterization of nanomaterials: tools and challenges. Nanomat Food Appl. 2019;2019:313–353. [Google Scholar]
- 37.Manmode AS, Sakarkar DM, Mahajan NM. Nanoparticles-tremendous therapeutic potential: a review. Int J Pharmtech Res. 2009;1(4):1020–1027. [Google Scholar]
- 38.Mohanraj V, Chen Y. Nanoparticles-a review. Trop J Pharm Res. 2006;5(1):561–573. [Google Scholar]
- 39.Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37(1):106–126. doi: 10.1016/j.progpolymsci.2011.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qin Y. Seaweed hydrocolloids as thickening, gelling, and emulsifying agents in functional food products. In: Bioactive Seaweeds for Food Applications. Elsevier; 2018:135–152. [Google Scholar]
- 41.McHugh DJ. Production, properties and uses of alginates. Production and utilization of products from commercial seaweeds. FAO Fish Tech Pap. 1987;288:58–115. [Google Scholar]
- 42.González A, Espinoza D, Vidal C, Moenne A. Benzopyrene induces oxidative stress and increases expression and activities of antioxidant enzymes, and CYP450 and GST metabolizing enzymes in Ulva lactuca (Chlorophyta). Planta. 2020;252(6):1–13. doi: 10.1007/s00425-020-03508-w [DOI] [PubMed] [Google Scholar]
- 43.David RM, Gooderham NJ, Gooderham NJ. Mechanistic evidence that benzo [a] pyrene promotes an inflammatory microenvironment that drives the metastatic potential of human mammary cells. Arch Toxicol. 2018;92(10):3223–3239. doi: 10.1007/s00204-018-2291-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Griess B, Tom E, Domann F, Teoh-Fitzgerald M. Extracellular superoxide dismutase and its role in cancer. Free Radic Biol Med. 2017;112:464–479. doi: 10.1016/j.freeradbiomed.2017.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doskey CM, Buranasudja V, Wagner BA, et al. Tumor cells have decreased ability to metabolize H2O2: implications for pharmacological ascorbate in cancer therapy. Redox Biol. 2016;10:274–284. doi: 10.1016/j.redox.2016.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kwon DH, Cha H-J, Lee H, et al. Protective effect of glutathione against oxidative stress-induced cytotoxicity in RAW 264.7 macrophages through activating the nuclear factor erythroid 2-related factor-2/heme oxygenase-1 pathway. Antioxidants. 2019;8(4):82. doi: 10.3390/antiox8040082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.D’Alfonso TM, Ginter PS, Shin SJ. A review of inflammatory processes of the breast with a focus on diagnosis in core biopsy samples. J Pathol Transl Med. 2015;49(4):279–287. doi: 10.4132/jptm.2015.06.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guinebretiere J, Menet E, Tardivon A, Cherel P, Vanel D. Normal and pathological breast, the histological basis. Eur J Radiol. 2005;54(1):6–14. doi: 10.1016/j.ejrad.2004.11.020 [DOI] [PubMed] [Google Scholar]
- 49.Khan N, Afaq F, Saleem M, Ahmad N, Mukhtar H. Targeting multiple signaling pathways by green tea polyphenol (−)-epigallocatechin-3-gallate. Cancer Res. 2006;66(5):2500–2505. doi: 10.1158/0008-5472.CAN-05-3636 [DOI] [PubMed] [Google Scholar]
- 50.Pan M-H, Chiou Y-S, Wang Y-J, C-T H, Lin J-K. Multistage carcinogenesis process as molecular targets in cancer chemoprevention by epicatechin-3-gallate. Food Funct. 2011;2(2):101–110. doi: 10.1039/c0fo00174k [DOI] [PubMed] [Google Scholar]
- 51.Crozier A, Jaganath IB, Clifford MN. Dietary phenolics: chemistry, bioavailability and effects on health. Nat Prod Rep. 2009;26(8):1001–1043. doi: 10.1039/b802662a [DOI] [PubMed] [Google Scholar]
- 52.Ko H, So Y, Jeon H, et al. TGF-β1-induced epithelial–mesenchymal transition and acetylation of Smad2 and Smad3 are negatively regulated by EGCG in human A549 lung cancer cells. Cancer Lett. 2013;335(1):205–213. doi: 10.1016/j.canlet.2013.02.018 [DOI] [PubMed] [Google Scholar]
- 53.Mackenzie GG, Carrasquedo F, Delfino JM, Keen CL, Fraga CG, Oteiza PI. Epicatechin, catechin, and dimeric procyanidins inhibit PMA‐induced NF‐κB activation at multiple steps in Jurkat T cells. FASEB J. 2004;18(1):167–169. doi: 10.1096/fj.03-0402fje [DOI] [PubMed] [Google Scholar]
- 54.Chen H, Sang S. Biotransformation of tea polyphenols by gut microbiota. J Funct Foods. 2014;7:26–42. doi: 10.1016/j.jff.2014.01.013 [DOI] [Google Scholar]
- 55.Jiang Y, Jiang Z, Ma L, Huang Q. Advances in nanodelivery of green tea catechins to enhance the anticancer activity. Molecules. 2021;26(11):3301. doi: 10.3390/molecules26113301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yao H, Liu J, Xu S, Zhu Z, Xu J. The structural modification of natural products for novel drug discovery. Expert Opin Drug Discov. 2017;12(2):121–140. doi: 10.1080/17460441.2016.1272757 [DOI] [PubMed] [Google Scholar]
- 57.Chaturvedi K, Ganguly K, More UA, et al. Sodium alginate in drug delivery and biomedical areas. In Natural Polysaccharides in Drug Delivery and Biomedical Applications. Elsevier; 2019:59–100. [Google Scholar]
- 58.Lei H, Xie M, Zhao Y, Zhang F, Xu Y, Xie J. Chitosan/sodium alginate modificated graphene oxide-based nanocomposite as a carrier for drug delivery. Ceram Int. 2016;42(15):17798–17805. doi: 10.1016/j.ceramint.2016.08.108 [DOI] [Google Scholar]
- 59.Xie M, Zhang F, Liu L, et al. Surface modification of graphene oxide nanosheets by protamine sulfate/sodium alginate for anti-cancer drug delivery application. Appl Surf Sci. 2018;440:853–860. doi: 10.1016/j.apsusc.2018.01.175 [DOI] [Google Scholar]
- 60.Elbialy NS, Mohamed N. Fabrication of the quaternary nanocomplex curcumin-casein-alginate-chitosan as a potential oral delivery system for cancer nutraceutical therapy. J Drug Deliv Sci Technol. 2022;70:103226. doi: 10.1016/j.jddst.2022.103226 [DOI] [Google Scholar]
- 61.Shimada T. Xenobiotic-metabolizing enzymes involved in activation and detoxification of carcinogenic polycyclic aromatic hydrocarbons. Drug Metab Pharmacokinet. 2006;21(4):257–276. doi: 10.2133/dmpk.21.257 [DOI] [PubMed] [Google Scholar]
- 62.Syarifah S, Widyawati T, Anggraini DR. Anticancer activity of uncaria gambir roxb on T47D breast cancer cells. J Phys Conf Ser. 2019;1317(1):012106. [Google Scholar]
- 63.Hecht F, Pessoa CF, Gentile LB, Rosenthal D, Carvalho DP, Fortunato RS. The role of oxidative stress on breast cancer development and therapy. Tumor Biol. 2016;37(4):4281–4291. doi: 10.1007/s13277-016-4873-9 [DOI] [PubMed] [Google Scholar]
- 64.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49(11):1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hartmann LC, Degnim AC, Santen RJ, Dupont WD, Ghosh K. Atypical hyperplasia of the breast—risk assessment and management options. N Engl J Med. 2015;372(1):78–89. doi: 10.1056/NEJMsr1407164 [DOI] [PMC free article] [PubMed] [Google Scholar]