Abstract
Objective:
Cancer-related fatigue (CRF) affects a substantial number of cancer patients and survivors. Recommendations for CRF treatments are largely based on results of randomized controlled trials. The interpretability of such results is limited to patients eligible and willing to participate in these trials. We aimed to address this limitation in a retrospective study of patients seen at a CRF clinic in a comprehensive cancer center. The objectives were to 1) determine the effectiveness of clinician-initiated interventions for CRF and identify their mediators and 2) describe the frequency and effectiveness of patient-initiated physical activity (PA) behavior for alleviating CRF and identify determinants of this PA.
Methods:
Data (patient-reported somatic and mood symptoms; clinical data; clinician-documented changes in medication and behavior) from n=213 patients collected as part of the clinic’s standard of care at initial clinical consult and follow-up 4-11 weeks later were included. Effects of clinician-initiated interventions and patient-initiated PA on change in fatigue were analyzed using linear models.
Results:
Of all clinician-initiated interventions, only psychostimulant start was recorded frequent enough for further investigation and was associated with reduced fatigue; this association was mediated by a reduction in apathy. PA was also associated with reduced fatigue severity. PA initiation/increase after consult was associated with lower apathy at consult.
Conclusions:
These results demonstrate a major role for patient apathy in the effectiveness and initiation of CRF-targeting interventions. Behavioral therapies focusing on reduction in apathy should be considered as initial treatment of CRF in those with substantial apathy.
BACKGROUND
Cancer-related fatigue (CRF) affects almost all cancer patients undergoing treatment and persists in up to 39% of survivors months to years after treatment completion.(1, 2) The U.S. Food and Drug Administration has not approved any interventions for CRF. Furthermore, intervention recommendations by the National Comprehensive Cancer Network (NCCN) are largely based on low levels of scientific evidence - with the exception of physical activity and psychosocial interventions (e.g., cognitive behavioral therapy, psychoeducational therapy).(3) Thus, much remains to be learned regarding the effective management of CRF.
The gold standard for assessing the effect of an intervention is a randomized controlled trial whereby eligible and willing patients are randomly allocated to either an intervention or placebo-control arm. Such studies are necessary to identify effective treatments, but they also have downsides. Eligibility criteria are by definition restrictive and eligible patients can refuse to be part of a study, so study results have limited generalizability. The limitation of eligibility criteria is solved by using varying criteria across studies, ultimately leading to a consensus regarding the general effectiveness of an intervention. However, patients’ reasons to enroll or not enroll in a given study arguably remain the same across studies. Patient recruitment for clinical trials has many barriers, including lack of motivation and interest among the patients.(4-6) Patients encountering these barriers will more likely decline enrollment than will those not facing such barriers. Thus, whether CRF interventions would also be effective for refusing patients and if so, how to get these patients to adhere to interventions remain unclear.
The problem of lack of patient interest or self-motivation in study recruitment may be even more apparent when studying psychostimulants in treating CRF. The level of evidence for the effectiveness of psychostimulants in reducing CRF is still low.(3) Several studies failed to find an effect of psychostimulants greater than a placebo effect,(7) and although pooled effect sizes point to a possible benefit, even these are currently inconclusive.(8) Given evidence that psychostimulants increase motivation and interest,(9-11) the effects of psychostimulants on fatigue may be mediated by increasing motivation. Failure to enroll patients with low motivation would mean excluding patients who would potentially experience the greatest benefit of psychostimulants. With lack of motivation being a prominent barrier for recruitment, most psychostimulant studies very likely suffer from inherently biased samples, interfering with results.
To address these issues, we conducted a retrospective study of patient-reported and clinical data collected as part of the standard of care at a CRF clinic in a comprehensive cancer center. The clinic uses a standardized approach to patients with CRF presenting for initial evaluation(12) consisting of a thorough assessment of the nature of fatigue (likely related to cancer or its treatment or predominantly related to other factors), the presence of comorbidities known or likely to be associated with fatigue (e.g., depression, pain, anemia, hypothyroidism), and the patient’s current health behaviors, including physical activity. When indicated, patients are referred to other supportive clinics, such as pain, sleep, and psychiatric oncology clinics. Based on the physician’s judgement, medications are adjusted or added, and the possibility of starting psychostimulants is discussed when no contra-indications for these medication exist. In all cases, patients receive psychoeducation on the phenomenon of CRF, both verbally and in a booklet. In addition, the importance of physical activity in alleviating fatigue is emphasized and a plan to increase physical activity is discussed. The clinic has been run by the same two physicians throughout most of its tenure, thereby reducing variability in CRF outcomes due to individual physicians’ approaches. The objectives of this study were to 1) determine the frequency and effectiveness of clinician-initiated interventions for alleviating CRF and identify their mediators and 2) describe the frequency and effectiveness of patient-initiated physical activity and identify determinants for engaging in or increasing this behavior.
METHODS
Study design
This is a retrospective study using patient-reported data collected at a CRF clinic as part of the clinic’s standard practice. This protocol was approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center (protocol PA16-0436), and a consent waiver was granted.
Patients who presented for an initial consult and at least one follow-up examination 4-11 weeks afterward from July 27, 2008, to December 15, 2017, were included in the study. Prior to every clinic visit, patients were asked to complete several questionnaires. The majority of the data from these questionnaires were collected using paper-and-pencil forms, which were either scanned or manually entered into a database. All scanned data were checked by trained research personnel. Manually entered data were entered twice by different people, and inconsistencies between the resulting data sets were checked and corrected by one of the authors (T.E.L.). When completed forms were unusable due to errors in the administrative system, questionnaire scores were obtained from the patients’ medical records. Patients seen from January 2011 to August 2012 completed all questionnaires except for those concerning pain and apathy on iPads. These digital data were used when available, and pain and apathy sum scores were obtained from the medical records. All other data were abstracted from the institution’s electronic medical record system.
Fatigue
Fatigue was assessed using the Brief Fatigue Inventory (BFI), a 10-item instrument designed for rapid assessment of fatigue levels in cancer patients. The first three items ask the respondent to rate fatigue severity on a 0-10 scale (0, no fatigue; 10, fatigue as bad as you can imagine) for the previous 24 hours at its “worst” and “usual” levels and also their current (“now”) fatigue severity. Using the same type of response scale, patients are also asked to rate how their fatigue interferes with several quality-of-life domains (from 0, “does not interfere” to 10, “interferes completely”). The BFI was found to have one underlying factor in a sample of cancer patients, suggesting that an average score for the nine items should be used as indication of fatigue severity (13). The BFI was shown to have good concurrent and discriminant validity and excellent reliability in previous studies (Cronbach’s alpha, 0.96) (13) as well as the present study (Cronbach’s alpha, 0.92 in consults and 0.94 in follow-up examinations).
Other physical and emotional symptoms
Pain intensity was assessed as the average score for the four intensity items of the Brief Pain Inventory instrument: pain over the past 24 hours at its worst and least, pain on average, and pain right now.(14, 15)
Mood symptoms were assessed with the Depression, Anxiety, and Stress Scale (DASS-21), a 21-item scale with three subscales. The DASS-21 uses a four-point severity/frequency scale for rating the extent to which each state was experienced over the past week. Sum scores for the separate subscales were computed to assess the severity of depression, anxiety, and stress.(16)
The Epworth Sleepiness Scale was used for assessment of sleepiness. This is an eight-item questionnaire, with higher scores indicating greater daytime sleepiness.(17, 18)
Apathy was assessed using the Apathy Evaluation Scale (AES).(19) This 18-item scale was developed for multiple rater sources: clinicians, informants (e.g., significant other), and patients. In the present study, only the self-rated form was used. The self-rated AES has excellent psychometric properties, with Cronbach’s alpha of 0.86 for internal consistency and 0.76 for test-retest reliability. (19)
Interventions
Physicians at the CRF clinic record detailed notes on any behavioral, medical, or environmental changes that may affect fatigue. These data were abstracted from medical records by trained individuals and verified by one of the authors (T.E.L.). The following behavioral and medical changes between the initial consults and follow-up examinations were summarized from the clinical notes: start or increase in frequency of physical activity (patient-initiated intervention), any clinic-initiated intervention: start or adjustment of stimulating medications (i.e., methylphenidate, armodafinil, modafinil, or dextroamphetamine/amphetamine), sleep interventions (including sleep medications and Continuous Positive Airway Pressure for sleep apnea), pain interventions (including pain medications, acupuncture, and massage), mental health interventions (including psychotropic medications and psychotherapy), physical therapy, thyroid medications, or other (any change in medication or behavior that was recorded by the physician as relevant for fatigue).
Statistical analyses
All analyses were performed with IBM SPSS version 24.(20) Data distributions were checked for normality by inspecting histograms and Q-Q plots. Presence of multivariate outliers was checked by calculating Mahalanobis distance for the predictors in the regression models. One multivariate outlier was detected and all analyses were run with and without this outlier - with similar results. Change in fatigue (Δ-fatigue) was calculated as [fatiguefollow-up –fatigueconsult]. For descriptive purposes, Pearson’s r correlations were computed for the associations between Δ-fatigue and demographic, clinical, and change in other symptom-variables.
For the first study objective, linear models were calculated including Δ-fatigue as dependent and each clinic-initiated intervention (dichotomous: yes (1), no (0)) as independent variable. Age, sex, race, and body mass index were included as covariates in all linear models. In case of a significant association between intervention and Δ-fatigue, possible mediation by change in other symptoms was investigated by a) assessing association between intervention and Δ-[symptom] and b) assessing change in the association intervention - Δ-fatigue after adding Δ-[symptom] to the model using Sobel’s test. (21)
For objective 2, the association between physical activity initiation/increase with Δ-fatigue was assessed in a linear model including the above specified covariates. Potential predictors of physical activity initiation or increase were assessed with chi-square tests for categorical predictors and student’s t-test for continuous predictors. For all analyses, results with a p-value <0.05 and 95% confidence interval not including zero were considered significant.
Missing data
No BFI data were missing, and less than 3% of the data from the other symptom questionnaires was missing. Missing data were imputed using an iterative Markov chain Monte Carlo method in which for each iteration and for each variable the fully conditional specification method fits a univariate model using other specified models as predictors. A maximum of 10 iterations, 50 case draws, and 5 parameter draws were used and questionnaire data (except BFI data) from consults and follow-up were used as predictors.
RESULTS
Sample characteristics
We included 213 patients in this study, the majority of whom were female, married, and white non-Hispanic (Table 1). The most common cancer diagnosis was breast cancer followed by hematological cancers. About one-third of the patients was undergoing primary treatment of their cancer at the time of their consult at the CRF clinic. The majority of patients (54%) reported that their fatigue started during treatment. Based on physicians’ recorded assessments, about 13% of patients had fatigue that probably was not related to their cancer or its treatment, as the fatigue started either years before the cancer diagnosis or years after completion of cancer treatment. The average time from consult to follow-up examination was 7.25 weeks (standard deviation [SD] = 1.99 weeks, range: 4.00-11.00 weeks).
TABLE 1.
Patient sample characteristics - n (%) unless otherwise indicated.
| N=213 with baseline and follow-up data | |
|---|---|
| Demographics | |
| Sex (female) | 136 (64) |
| age (M SD) | 57.59 (28.67) |
| Relationship status | |
| Married | 147 (69) |
| Divorced/separated | 35 (16) |
| Single | 21 (10) |
| Widowed | 10 (5) |
| Race/ethnicity | |
| White non-Hispanic | 163 (77) |
| Hispanic | 19 (9) |
| Black non-Hispanic | 18 (8) |
| Asian/Pacific Islander | 10 (5) |
| Other/unknown | 3 (1) |
| Clinical | |
| Cancer diagnosis | |
| Breast | 70 (32) |
| Hematological | 55 (26) |
| Head and neck | 21 (10) |
| GU | 16 (8) |
| Lung | 9 (4) |
| Brain | 9 (4) |
| GI | 9 (4) |
| Other | 24 (12) |
| Cancer treatment status at consult | |
| No active treatment | 100 (47) |
| Chemo/radiation/immune | 71 (33) |
| Endocrine/other treatment | 42 (20) |
| Fatigue | |
| Fatigue onset | |
| During treatment | 116 (54) |
| After treatment | 36 (17) |
| Before diagnosis | 32 (15) |
| During diagnosis | 23 (11) |
| Other/unknown | 6 (3) |
| Fatigue (partly) cancer-related? | |
| Yes, probably | 185 (87) |
| No, probably not | 28 (13) |
Fatigue
Fatigue severity decreased from 6.24 (SD = 1.94) at consult to 5.04 (SD = 2.36) at follow-up. Change in fatigue was not associated with time from consult to follow-up examination (r = 0.10; p = .15), treatment status at consult (F(2, 210) = 0.610, p = .55), time of onset of fatigue (F(5, 207) = 1.402, p = .23), or whether fatigue was assessed as cancer-related (F(1, 211) = 2.277, p = .13).
Improvement in fatigue was significantly associated with improvement in pain, sleepiness, stress, anxiety, depression, and apathy (r’s between 0.28 -for sleepiness- and −0.44 -for depression, all: p < .001; see supplemental Figure S1).
Clinic-initiated interventions
The most frequent clinic-initiated intervention was prescription of psychostimulants or wakefulness agents (from here on referred to as psychostimulants). Fifty-six patients (26%) started psychostimulants following their consult: methylphenidate, n=43; armodafinil, n=8; modafinil, n=3; and dextroamphetamine/amphetamine, n=2. Twenty-eight patients (13%) began mental health-targeting interventions. Every other intervention was initiated for <10% of patients. For 42%, no intervention was started following consult; for 48% one intervention was started and for 10%, more than one intervention was started.
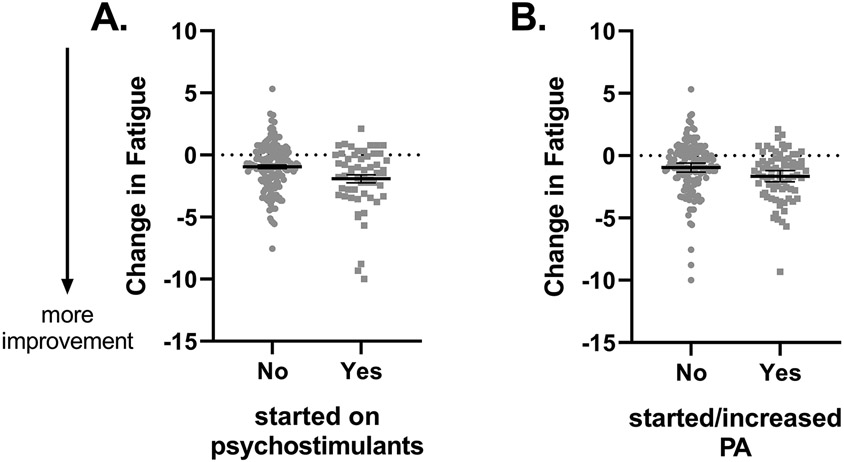
Results of a linear model including age, sex, race, and body mass index at consult showed a significant effect of psychostimulant-based treatment on Δ-fatigue (Table 2 – Model 1). As illustrated in Figure 1 – panel A, patients who started psychostimulants following consult exhibited greater reductions in fatigue than did patients who did not start this treatment. Psychostimulant use was also associated with Δ-apathy (B = −3.15, standard error [SE] = 1.25, 95% confidence interval [CI]: [−5.60 – −0.69], p = .012) but not with Δ-pain, Δ-sleepiness, Δ-depression, Δ-anxiety, or Δ-stress (see supplemental Table S1). Adding Δ-apathy to the models for Δ-fatigue reduced the effects of psychostimulant use on fatigue (Table 2 – Model 2), suggesting that these effects were mediated by a reduction in apathy. The presence of this mediation effect was confirmed with the Sobel test (Z = −2.10, SE = 0.0004, p = .036). Further exploration of the role of apathy showed an interaction effect of initial apathy x psychostimulant start on change in fatigue (B = −0.045, SE 0.012, 95% CI [−0.069 - −0.021], p < 0.001). Exclusion of one multivariate outlier did not change the results.
TABLE 2.
Linear models for associations of psychostimulant use with Δ-fatigue (model 1) and for associations of psychostimulant use while controlling for Δ-apathy (model 2). Model 2 included all covariates shown for model 1.
| Δ-Fatigue | |||||
|---|---|---|---|---|---|
| B | SE | 95%CI | P | ||
| Model 1 | Intercept | −1.485 | 0.699 | −2.855 – −0.116 | 0.034 |
| Age | −0.003 | 0.005 | −0.012 – 0.007 | 0.62 | |
| Sex1 | 0.479 | 0.296 | −0.101 – 1.059 | 0.11 | |
| Race2 | −0.107 | 0.335 | −0.763 – 0.549 | 0.75 | |
| BMI | 0.018 | 0.021 | −0.024 – 0.060 | 0.41 | |
| Stimulant start 3 | −0.945 | 0.323 | −1.578 – −0.313 | 0.003 | |
| Model 2 | Δ-Apathy | 0.094 | 0.016 | 0.062 – 0.126 | <0.001 |
| Stimulant start 3 | −0.606 | 0.306 | −1.205 – −0.007 | 0.047 | |
Female is reference
Caucasian is reference
No change in stimulant use is reference.
FIGURE 1.
Psychostimulant use (A) and patient-initiated initiation or increase in physical activity (PA) (B) lead to greater reductions in fatigue severity. Bars represent raw mean + standard error of the mean.
Because the low frequency of clinic-initiated interventions other than psychostimulants did not allow for analysis per intervention, we assessed the effect of starting any of these interventions on fatigue. Starting any intervention other than psychostimulants was not associated with fatigue reduction (B = −0.115, SE = 0.287, 95% CI: [−0.677 – 0.447], p = .69).
Patient-initiated physical activity
Seventy-five patients (35%) started exercising or increased their physical activity frequency and/or intensity following their consult. Starting or increasing physical activity was related to a greater reduction in fatigue severity (Table 3 – Model 1; Figure 1 – panel B). Entering start of physical activity and psychostimulants in one model (to ascertain effects of physical activity were not a reflection of psychostimulant use or vice versa) did not change their association with Δ-fatigue (Table 3 – Model 2). Physical activity was not related to changes in severity of the symptoms associated with fatigue (see supplemental Table S2).
TABLE 3.
Linear model for associations of physical activity (PA) with Δ-fatigue (model 1) and for associations of PA and psychostimulant use (model 2). Model 2 included all covariates shown for Model 1.
| Δ-Fatigue | |||||
|---|---|---|---|---|---|
| B | 95%CI | P | |||
| Model 1 | Intercept | −1.506 | 0.703 | −2.885 – −0.128 | 0.032 |
| Age | −0.003 | 0.005 | −0.013 – 0.007 | 0.59 | |
| Sex1 | 0.654 | 0.300 | 0.066 – 1.241 | 0.029 | |
| Race2 | −0.034 | 0.334 | −0.689 – 0.621 | 0.92 | |
| BMI | 0.017 | 0.022 | −0.025 – 0.059 | 0.42 | |
| PA | −0.785 | 0.300 | −1.374 - −0.197 | 0.009 | |
| Model 2 | Stimulant start 3 | −0.858 | 0.321 | −1.487 – −0.229 | 0.008 |
| PA | −0.692 | 0.298 | −1.275 – −0.109 | 0.020 | |
female is reference
Caucasian is reference
No change in stimulant use is reference.
Patients who started or increased physical activity were more likely than those who did not to be male (p = .04) and reported less pain (p = .024) and lower apathy (p = .04) at their consult (supplemental Table S3).
DISCUSSION
In this retrospective study of patients seen at a CRF clinic, psychostimulant use and patients’ initiation or increase in physical activity both led to a decrease in fatigue, wherein apathy seems to play a central role. Specifically, the effects of psychostimulants on change in fatigue were mediated by a decrease in apathy and interacted with initial apathy severity, suggesting that psychostimulants are particularly effective at alleviating fatigue in patients reporting high initial apathy – by reducing these high levels. Additionally, patients with lower apathy at consult were more likely to start or increase physical activity. Taken together, these findings suggest that high apathy is a barrier for self-initiation of or increasing physical activity and that psychostimulant use reduces this barrier by reducing apathy.
Apathy can be broadly defined as a state of decreased motivation(22) and as such shows theoretical overlap with the anhedonic component of depression and with reduced motivation as hallmark symptom of fatigue.(23, 24) Nevertheless, several studies have shown apathy to be distinct from depression and fatigue in clinical populations.(25, 26) Although apathy is mostly studied as a treatment outcome, there are some indications that high initial apathy can interfere with treatment of other symptoms.(27, 28) Our finding that a high level of apathy is a barrier to initiating physical activity in cancer patients and survivors is in line with previous reports.(29) Although researchers have shown that psychostimulants can reduce apathy in patients with Alzheimer’s disease or dementia,(30-32) to the best of our knowledge, we are the first to show a similar effect of psychostimulants in fatigued cancer patients. Our finding that the effects of psychostimulants on CRF are at least partially mediated by reducing apathy is a potential explanation for previously reported inconsistent findings regarding the efficacy of psychostimulants in reducing CRF. Use of psychostimulants for treatment of fatigue may be particularly beneficial in patients with high initial apathy levels. Quite possibly, such patients often do not enroll in clinical trials due to the inherent amotivational nature of apathy. Thus, in previous clinical trials, researchers may have inadvertently and unknowingly included mostly subjects who may benefit less from psychostimulant use.
The beneficial effects of physical activity on CRF have been shown frequently and physical activity is indeed recommended by the NCCN for cancer patients and survivors with fatigue.(3, 33, 34) As discussed above, most studies of the effects of physical activity on CRF have been randomized controlled trials with inherent selection of patients with the motivation, willingness, and time to start a prescribed physical activity program. Here, we report that any patient-initiated improvement in physical activity is beneficial. We further showed that pain and apathy form a barrier to the initiation of physical activity. Thus, patient-initiated physical activity may be promoted by addressing the presence of pain and apathy first. The effects of pain on physical activity have been reported before,(35) but our finding regarding apathy is novel and deserves more attention in future studies.
We did not observe any effects of other clinic-initiated interventions. A possible explanation is the low frequency of most interventions, resulting in low statistical power to observe effects. It is also that these interventions did not have the intended primary effect such as improving sleep or alleviating pain. This notion is supported by our observation that reductions in all somatic and mood symptoms were related to reductions in fatigue, whereas the interventions targeting these symptoms were not.
Limitations
Inherent to the retrospective, observational nature of the present study, we lacked a control group. Therefore, we cannot rule out that our findings may reflect placebo effects. However, our finding of a mediatory effect of change in apathy on the effect of psychostimulants on fatigue means that this association likely is real. The expectation for a beneficial effect of psychostimulants on fatigue was raised during the initial consult, no such expectation was mentioned for apathy. Regarding the role of apathy in physical activity, the observed association was significant only when not adjusting for multiple tests, suggesting that it needs to be replicated before firm conclusions can be drawn. We suggest that these observational findings need confirmation from experimental studies that specifically test high and low apathetic CRF patients in various treatment groups (i.e., psycho-stimulant/physical activity vs. placebo).
Clinical implications
The results of this study suggest that clinicians treating patients with CRF should be mindful of the patient’s apathy levels, which are distinct from depression. If apathy is high, it may pose a barrier for behavioral changes including initiating exercise – one of the few interventions shown to reduce fatigue. Psychostimulants can effectively treat apathy and our results suggest that these medications might treat CRF particularly in patients with high apathy.
Conclusions
Both patient-initiated physical activity and physician-prescribed psychostimulant use were associated with a reduction in fatigue in patients seen at a CRF clinic. We identified a central role for apathy in these two interventions: apathy was identified as a barrier for initiating physical activity and psychostimulant use reduced apathy. We further showed that a reduction in apathy mediated the effects of psychostimulants on fatigue. These novel findings point to the importance of recruiting patients with high levels of apathy in any clinical intervention-study targeting CRF and suggest that patients with high apathy might benefit from interventions lowering apathy before targeting CRF.
Supplementary Material
Acknowledgements:
Supported by the NIH/NCI under award number P30CA016672.
Data Availability Statement:
The data that support the findings of this study are available on reasonable request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1.Servaes P, Verhagen C, Bleijenberg G. Fatigue in cancer patients during and after treatment: Prevalence, correlates and interventions. Eur J Cancer. 2002;38(1):27–43. [DOI] [PubMed] [Google Scholar]
- 2.Goedendorp MM, Gielissen MFM, Verhagen CAHHVM, Bleijenberg G. Development of fatigue in cancer survivors: A prospective follow-up study from diagnosis into the year after treatment. J Pain Symptom Manage. 2013;45(2):213–22. [DOI] [PubMed] [Google Scholar]
- 3.NCCN Clinical Practice Guidelines in Oncology Cancer-Related Fatigue. Version 2.2019 ed 2019. [Google Scholar]
- 4.Ford JG, Howerton MW, Lai GY, Gary TL, Bolen S, Gibbons MC, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: A systematic review. Cancer. 2008;112(2):228–42. [DOI] [PubMed] [Google Scholar]
- 5.Guidon M, McGee H. Recruitment to clinical trials of exercise: challenges in the peripheral arterial disease population. Physiotherapy. 2013;99(4):305–10. [DOI] [PubMed] [Google Scholar]
- 6.Aitken L, Gallagher R, Madronio C. Principles of recruitment and retention in clinical trials. Int J Nurs Pract. 2003;9(6):338–46. [DOI] [PubMed] [Google Scholar]
- 7.Breitbart W, Alici Y. Psychostimulants for cancer-related fatigue. JNCCN Journal of the National Comprehensive Cancer Network. 2010;8(8):933–42. [DOI] [PubMed] [Google Scholar]
- 8.Gong S, Sheng P, Jin H, He H, Qi EB, Chen W, et al. Effect of Methylphenidate in Patients with Cancer-Related Fatigue: A Systematic Review and Meta-Analysis. PLoS One. 2014;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chelonis JJ, Johnson TA, Ferguson SA, Berry KJ, Kubacak B, Edwards MC, et al. Effect of methylphenidate on motivation in children with attention-deficit/hyperactivity disorder. Exp Clin Psychopharmacol. 2011;19(2):145–53. [DOI] [PubMed] [Google Scholar]
- 10.Oemisch M, Johnston K, Pare M. Methylphenidate does not enhance visual working memory but benefits motivation in macaque monkeys. Neuropharmacology. 2016. [DOI] [PubMed] [Google Scholar]
- 11.Volkow ND, Wang GJ, Fowler JS, Telang F, Maynard L, Logan J, et al. Evidence that methylphenidate enhances the saliency of a mathematical task by increasing dopamine in the human brain. Am J Psychiatry. 2004;161(7):1173–80. [DOI] [PubMed] [Google Scholar]
- 12.Escalante CP, Manzullo EF. Cancer-Related Fatigue: The Approach and Treatment. J Gen Intern Med. 2009;24(2):412–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85(5):1186–96. [DOI] [PubMed] [Google Scholar]
- 14.Atkinson TM, Rosenfeld BD, Sit L, Mendoza TR, Fruscione M, Lavene D, et al. Using confirmatory factor analysis to evaluate construct validity of the Brief Pain Inventory (BPI). J Pain Symptom Manage. 2011;41(3):558–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cleeland C, ed. The Brief Pain Inventory User Guide. Houston, Texas: Cleeland Charles S., The University of Texas M.D. Anderson Cancer Center; 2009 [Google Scholar]
- 16.Lovibond S, Lovibond P. Manual for the Depression Anxiey Stress Scales. 2nd. Ed. ed. Sydney: Psychology Foundation; 1995. [Google Scholar]
- 17.Johns MW. A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep. 1991;14(6):540–5. [DOI] [PubMed] [Google Scholar]
- 18.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15(4):376–81. [DOI] [PubMed] [Google Scholar]
- 19.Marin RS, Biedrzycki RC, Firinciogullary S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res. 1991;38(2):143–62. [DOI] [PubMed] [Google Scholar]
- 20.IBM SPSS Statistics. 24.0.0.0 ed1989, 2016.
- 21.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic and statistical considerations. J Pers Soc Psychol. 1986;51:1173–82. [DOI] [PubMed] [Google Scholar]
- 22.Thant T, Yager J. Updating Apathy: Using Research Domain Criteria to Inform Clinical Assessment and Diagnosis of Disorders of Motivation. The Journal of Nervous and Mental Disease. 2019;207(9):707–14. [DOI] [PubMed] [Google Scholar]
- 23.Kuhnt S, Friedrich M, Schulte T, Cella D, Hinz A. Screening Properties of the Diagnostic Criteria for Cancer-Related Fatigue. Oncology Research and Treatment. 2019;42(9):440–5. [DOI] [PubMed] [Google Scholar]
- 24.Gledhill J. A qualitative study of the characteristics and representation of fatigue in a French speaking population of cancer patients and healthy subjects. Eur J Oncol Nurs. 2005;9(4):294–312. [DOI] [PubMed] [Google Scholar]
- 25.Douven E, Kohler S, Schievink SHJ, van Oostenbrugge RJ, Staals J, Verhey FRJ, et al. Temporal Associations between Fatigue, Depression, and Apathy after Stroke: Results of the Cognition and Affect after Stroke, a Prospective Evaluation of Risks Study. Cerebrovasc Dis. 2017;44(5-6):330–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skorvanek M, Gdovinova Z, Rosenberger J, Saeedian RG, Nagyova I, Groothoff JW, et al. The associations between fatigue, apathy, and depression in Parkinson's disease. Acta Neurol Scand. 2015;131(2):80–7. [DOI] [PubMed] [Google Scholar]
- 27.Siddarth P, Funes CM, Laird KT, Ercoli L, Lavretsky H. Predictors of Cognitive Improvement Following Treatment for Late-Life Depression. J Geriatr Psychiatry Neurol. 2020:891988720915515. [DOI] [PubMed] [Google Scholar]
- 28.Faerden A, Barrett EA, Nesvåg R, Friis S, Finset A, Marder SR, et al. Apathy, poor verbal memory and male gender predict lower psychosocial functioning one year after the first treatment of psychosis. Psychiatry Res. 2013;210(1):55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eng L, Pringle D, Su J, Shen XW, Mahler M, Niu C, et al. Patterns, perceptions, and perceived barriers to physical activity in adult cancer survivors. Support Care Cancer. 2018;26(11):3755–63. [DOI] [PubMed] [Google Scholar]
- 30.Padala PR, Padala PP, Lensing SY, Ramirez D, Monga V, Bopp MM, et al. Methylphenidate for Apathy in Community-Dwelling Older Veterans With Mild Alzheimer’s Disease: A Double-Blind, Randomized, Placebo-Controlled Trial. Am J Psychiatry. 2018;175(2):159–68. [DOI] [PubMed] [Google Scholar]
- 31.Herrmann N, Rothenburg LS, Black SE, Ryan M, Liu BA, Busto UE, et al. Methylphenidate for the treatment of apathy in Alzheimer disease: prediction of response using dextroamphetamine challenge. J Clin Psychopharmacol. 2008;28(3):296–301. [DOI] [PubMed] [Google Scholar]
- 32.Dolder CR, Nicole Davis L, McKinsey J. Use of Psychostimulants in Patients with Dementia. Ann Pharmacother. 2010;44(10):1624–32. [DOI] [PubMed] [Google Scholar]
- 33.Hilfiker R, Meichtry A, Eicher M, Nilsson Balfe L, Knols RH, Verra ML, et al. Exercise and other non-pharmaceutical interventions for cancer-related fatigue in patients during or after cancer treatment: A systematic review incorporating an indirect-comparisons meta-analysis. Br J Sports Med. 2018;52(10):651–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Vulpen JK, Peeters PHM, Velthuis MJ, Van Der Wall E, May AM. Effects of physical exercise during adjuvant breast cancer treatment on physical and psychosocial dimensions of cancer-related fatigue: A meta-analysis. Maturitas. 2016;85:104–11. [DOI] [PubMed] [Google Scholar]
- 35.Romero SAD, Brown JC, Bauml JM, Hay JL, Li QS, Cohen RB, et al. Barriers to physical activity: a study of academic and community cancer survivors with pain. J Cancer Surviv. 2018;12(6):744–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on reasonable request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.