Abstract
The global crisis of opioid overdose fatalities has led to an urgent search to discover the neurobiological mechanisms of opioid use disorder (OUD). A driving force for OUD is the dysphoric and emotionally painful state (hyperkatifeia) that is produced during acute and protracted opioid withdrawal. Here, we explored a mechanistic role for extrahypothalamic stress systems in driving opioid addiction. We found that glucocorticoid receptor (GR) antagonism with mifepristone reduced opioid addiction-like behaviors in rats and zebrafish of both sexes and decreased the firing of corticotropin-releasing factor neurons in the rat amygdala (i.e., a marker of brain stress system activation). In support of the hypothesized role of glucocorticoid transcriptional regulation of extrahypothalamic GRs in addiction-like behavior, an intra-amygdala infusion of an antisense oligonucleotide that block GR transcriptional activity reduced addiction-like behaviors. Finally, we identified transcriptional adaptations of GR signaling in the amygdala of humans with OUD. Thus, GRs, their coregulators and downstream systems may represent viable therapeutic targets to treat the “stress side” of OUD.
Introduction
Opioid overdose fatalities have risen substantially in the United States and are considered a public health emergency [1, 2]. These opioid-related overdose deaths and the association between episodes of overdose and “deaths of despair” [3] have led to an urgent search to reveal the neurobiological mechanisms that link opioids to dysphoria, negative emotional states, and heightened stress sensitivity [4–6].
Opioid withdrawal is a severely dysphoric and emotionally painful state [4, 5, 7] that is characterized by the intense activation of specific stress-engaged brain areas and the hypothalamic-pituitary-adrenal (HPA) axis [8, 9]. These evolutionarily conserved systems are engaged during the adaptive response to stress to increase an individual’s chances of survival [10–13]. High circulating levels of corticosteroids, such as those that are produced by intense stress (e.g., opioid withdrawal), activate glucocorticoid receptors (GRs) in the brain. Opioid withdrawal also activates other extrahypothalamic brain stress systems, such as the corticotropin-releasing factor (CRF) system in the central nucleus of the amygdala (CeA) [14, 15]. Based on correlative studies, dysregulation of brain stress systems is hypothesized to drive hyperkatifeia, allostasis, and drug intake [9, 12, 16], but mechanistic studies are lacking. Here, we tested the hypothesis that opioid addiction-like behaviors are driven by the GR-dependent sensitization of extrahypothalamic brain stress circuits and that the blockade of GR or its transcriptional activity would reduce opioid addiction-like behaviors.
Glucocorticoid receptors (encoded by the NR3C1 gene in humans) are transcription factors that activate or repress gene transcription [13]. With chronic activation of the HPA axis, the CRF system in the CeA is sensitized [9, 12, 17, 18]. Glucocorticoids control both the release of CRF in the CeA [19] and the transcription of CRF in the CeA [9, 12, 17, 18] via GRs. Region-specific adaptations of CRF expression are hypothesized to involve the recruitment of different GR coregulators, such as steroid receptor coactivator 1 (SRC) isoforms [9, 13, 17, 18]. Two functionally characterized splice variants of SRC-1, SRC-1a and SCR-1e (both encoded by Ncoa1) have been identified. The recruitment of SRC-1a inhibits GR-mediated CRF transcription, whereas the recruitment of SRC-1e facilitates CRF transcription [13, 17, 18]. Thus, we also hypothesized that elevating the SRC-1a isoform and lowering SRC-1e would decrease opioid addiction-like behavior.
Material and Methods
Animals
Adult male and female Wistar rats and Long Evans rats were purchased from Charles River (Kingston, New York, NY, USA). Male CRH-Cre rats (Wistar background) were obtained from the National Institute on Drug Abuse (NIDA) breeding facility. The male rats weighed 250–350 g, and the female rats weighed 150–250 g at the beginning of the study. The rats were group-housed (2–3/cage) and maintained at 21°C ± 2°C under a reverse 12 h/12 h light/dark cycle (lights off at 8:00 AM, lights on at 8:00 PM) for short-access (ShA; 1 h) conditions and under a regular 12 h/12 h light/dark cycle (lights off at 7:00 PM, lights on at 7:00 AM) for long-access (LgA; 12 h) conditions. Self-administration sessions were conducted during the dark cycle (LgA rats were tested in overnight sessions). The animals had ad libitum access to food and water in their home cages. The LgA rats also had ad libitum access to food and water during their 12 h self-administration sessions. All the animal procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of the NIDA Intramural Research Program. The experimenters were not blinded to group allocation or drug treatment, but the majority of the outcomes were recorded by computers and not manually scored by the experimenters.
Drugs
We obtained heroin hydrochloride and (±)methadone hydrochloride from the NIDA drug supply program (Research Triangle Institute, Research Triangle Park, NC, USA). The drugs were dispensed by the NIDA Intramural Research Program pharmacy. Heroin and methadone were dissolved in 0.9% sterile saline. For LgA intravenous heroin self-administration, we used doses of 60 μg/kg/0.1 ml for males and 180 μg/kg/0.1 ml for females. We used a higher heroin dose for females because they are more resistant to heroin-induced antinociception and hyperalgesia during withdrawal than males [20]. For intravenous methadone self-administration, we used a unit dose of 300 μg/kg/0.1 ml based on studies that reported that rats dose-dependently self-administered methadone and that methadone substitutes for morphine [21–23]. We used the 300 μg/kg/0.1 ml dose for both males and females based on their similar response to methadone-induced antinociception and hyperalgesia during withdrawal [24]. We purchased naloxone hydrochloride from Hospira (Lake Forest, IL, USA). We dissolved naloxone in 0.9% sterile saline for subcutaneous injections in a volume of 1 ml/kg and used concentrations of 30 μg/kg for operant heroin self-administration or 1 mg/kg [25] for precipitating somatic signs of methadone withdrawal. We purchased mifepristone (RU486) from Cayman Chemical (Ann Harbor, MI, USA). We used mifepristone for pellet implants for chronic release (described below) and for acute intraperitoneal injections. For intraperitoneal injections, we prepared mifepristone with 10% dimethylsulfoxide, 10% Kolliphor EL (Sigma-Aldrich, St. Louis, MO, USA), and 80% saline. We injected mifepristone at 0, 30, 60, and 90 mg/kg in a within-subjects Latin-square design in an injection volume of 3 ml/kg 90 min prior to drug self-administration. The doses, pretreatment time, volume of injection, and vehicle were based on our published studies [26–28].
Intravenous catheter implant surgery
We surgically implanted intravenous catheters (Dow, Midland, MI, USA) into the right jugular vein in rats to access the vascular system painlessly and chronically. All surgical procedures were performed under isoflurane (1.5–2.5%) general anesthesia. Surgery was performed in a dedicated surgery room using sterile instruments, surgical gloves, and aseptic procedures. The surgical site (back and neck) was shaved and disinfected with Betadine and an alcohol scrub. A 3 cm incision was made on the back, and a 1 cm incision was made on the neck to insert the catheter. We secured the catheter to the vein with suture thread and passed the catheter subcutaneously to exit the animal’s back. The incisions were sutured or glued with veterinary adhesive (Vetbond, 3M, St. Paul, MN, USA). A plastic stopper was placed over the open end of the intravenous connector, and a metal cap was screwed onto the back mount to cover the plastic stopper. After surgery, we administered the analgesic meloxicam (1 mg/kg/ml, subcutaneous; Covetrus North America, Dublin, OH, USA) and the antibiotic gentamicin (3 mg/kg/ml, subcutaneous; Fresenius Kabi, Lake Zurich, IL, USA). Before each self-administration session, we flushed the catheters with 0.3 ml of a sterile solution that contained heparinized (30 USP units/ml; Sagent Pharmaceuticals, Schaumburg, IL, USA) saline and gentamicin (3.2 mg/ml). The rats were allowed to recover for 7 days before drug self-administration.
Self-administration training
We conducted self-administration sessions in standard operant conditioning chambers (Med Associates, St. Albans, VT, USA). The operant chambers (30.5 cm × 24.1 cm × 21.0 cm) were housed inside light-attenuating and sound-attenuating wood chambers. The rats were trained in 1-h sessions to press one of two levers (the active lever) on a fixed-ratio 1 (FR1) schedule of reinforcement, in which each lever press resulted in drug delivery through the catheter from a syringe that was connected to a syringe pump outside the wood chamber. A 20-s timeout period followed reinforced responses, during which a cue light (above the active lever) was turned on and lever presses did not result in additional injections. In 1-h self-administration sessions, the rats did not have access to food or water while in the test chambers. Responses on the inactive lever had no programmed consequences.
Pellet implantation surgery
After five 1-h heroin self-administration sessions, the rats were subcutaneously implanted with mifepristone (Mifep; 200 mg total dose delivered over 21 days) or vehicle pellets (Innovative Research of America, Sarasota, FL, USA) for chronic (21-day) release [27, 28]. During surgery under isoflurane anesthesia, a 1 cm incision was made on the rat’s back. The pellet was implanted 2 cm above the incision site toward the neck. The incision was closed with veterinary adhesive (Vetbond, 3M, St. Paul, MN, USA).
Heroin self-administration under ShA and LgA conditions in rats that received vehicle or mifepristone pellet implantation
Twenty-four hours after pellet implantation, the rats were split into ShA and LgA groups. The rats were allowed ShA or LgA to heroin self-administration on an FR1 schedule of reinforcement for nine sessions. The same rats were tested for the effects of naloxone on heroin self-administration. This was a 2-day naloxone (30 μg/kg) vs. saline (0 μg/kg) challenge test (FR1). After 30 min of heroin self-administration, we injected saline or naloxone in random order and collected data for 30 min after naloxone because of its short half-life, as previously reported [14]. We also tested the same rats on a progressive-ratio (PR) schedule, in which the number of lever presses that were necessary to obtain the next drug infusion progressively increased according to the following progression: 1, 1, 2, 2, 3, 3, 4, 4, 5, 5, 6, 6, 7, 7, 8, 8, 9, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, etc. The PR session lasted 6 h. Self-administration sessions occurred every other day, so that the rats were in spontaneous withdrawal before each self-administration session. The following numbers of rats were tested: vehicle (n = 17 [10 males, seven females]), Mifep (n = 22 [14 males, eight females]).
Intracranial surgery
We used aseptic surgical techniques for the infusion of a Cre-dependent viral vector (AAV1-CAG-FLEX-enhanced green fluorescent protein [eGFP]; Vector Core, Pennsylvania State University) to allow CRF+ cells to produce GFP in the CeA in CRH-Cre male Wistar rats29. The rats received bilateral infusions (5 min infusion plus 10 min diffusion with injectors in place) of AAV1-CAG-FLEX-eGFP in the CeA (anterior/posterior: −2.06; medial/lateral: ±4.55; dorsal/ventral: −8.45). We started electrophysiological recordings 4 weeks after intracranial surgery. Green-fluorescent neurons (i.e., CRF+) were patched for whole-cell recordings.
Whole-cell recordings
Two to 6 h after the last self-administration session (i.e., during acute withdrawal), the rats were anesthetized with 1–5% isoflurane and euthanized by decapitation. Brains were quickly harvested and placed in ice-cold artificial cerebrospinal fluid (aCSF; 92 mM NMDG, 20 mM 4-[2-hydroxyethyl]-1-piperazineethanesulfonic acid [HEPES], 25 mM glucose, 30 mM NaHCO3, 1.2 mM NaH2PO4, 2.5 mM KCl, 5 mM sodium ascorbate, 3 mM sodium pyruvate, 2 mM thiourea, 10 mM MgSO4, and 0.5 mM CaCl2 saturated with 95% O2 and 5% CO2). Coronal slices (200 μm thick) that contained the CeA were obtained using a VT-1200 vibratome (Leica, Nussloch, Germany) and placed in a holding chamber that was filled with the same solution but held at 32°C. After 15 min, the slices were transferred to a holding chamber at room temperature that contained aCSF (same composition as above). For recordings, the slices were transferred to a chamber at 32°C that was superfused with aCSF (125 mM NaCl, 2.5 mM KCl, 1.25 mM NaH2PO4, 1 mM MgCl2, 2.4 mM CaCl2, 26 mM NaHCO3, and 11 mM glucose. Electrodes (4–6 MΩ) were backfilled with an internal solution that contained the following: 130 mM potassium gluconate, 5 mM NaCl, 1 mM MgCl2, 10 mM HEPES, 2 mM Mg-ATP, 0.5 mM Na2-GTP, 10 mM Na-phosphocreatine, 0.1 mM ethylene-bis[oxyethylenenitrilo]tetraacetic acid tetrasodium [EGTA], and 0.2% mM biocytin (pH 7.2; 280–290 mOsm). Neurons were visualized on an upright microscope using infrared differential interference contrast video microscopy and fluorescence microscopy. CRF+ neurons were identified by the expression of eGFP. Whole-cell current-clamp recordings were made using a MultiClamp 700B amplifier (2 kHz low-pass Bessel filter and 10 kHz digitization) with pClamp 10.3 software (Molecular Devices, Sunnyvale, CA, USA). Membrane potential and membrane resistance were determined after a 10-min adaptation period after patching a neuron. To determine the excitability of CeA CRF+ neurons, an input/output curve was generated that consisted of depolarizing current steps (1000 ms duration) from 0 to 300 pA. The number of action potentials that fired in each current step was measured using p-clamp 10 software. The following numbers of cells were used: Mifep (n = 9), no treatment (n = 10).
Dose-response effect of acute mifepristone treatment on methadone or heroin self-administration
The rats were prepared with an intravenous catheter and trained for heroin or methadone self-administration as described above. After 5 days of operant training, the rats were split into ShA and LgA groups. The rats were allowed differential ShA or LgA to heroin (FR1) or methadone for 8–10 sessions. Before testing mifepristone, we confirmed that the rats that self-administered methadone were opioid dependent by evaluating mechanical hyperalgesia and somatic signs of withdrawal (see Supplemental Material and Table S1) [20, 25].
The rats were intraperitoneally administered with mifepristone at 0, 30, 60, and 90 mg/kg 90 min before heroin or methadone self-administration. Self-administration sessions occurred every other day, so that the rats were in spontaneous withdrawal before each self-administration session. The following numbers of rats were tested: ShA (n = 18 [10 males, eight females]), LgA (n = 18 [nine males, nine females]).
Measurement of phosphorylated GRs by Western blot
All rats received an injection of naloxone (1 mg/kg) 4 h after the ShA or LgA methadone self-administration session without mifepristone treatment. One hour later, the rats were euthanized by decapitation under anesthesia. Brains were collected, snap-frozen in isopentane (-40°C) and stored at −80°C until microdissection. We collected the CeA from frozen coronal brain sections (300 μm thickness; Figure S1).
For homogenization, lysis buffer that contained 320 mM sucrose, 5 mM HEPES, 1 mM EGTA, 1 mM ethylenediaminetetraacetic acid (EDTA), 1% sodium dodecyl sulphate (SDS), protease inhibitor cocktail (1:100), and phosphatase inhibitor cocktails II and III (1:100; Sigma-Aldrich) was added to the tissue samples. The samples were then briefly sonicated and heated at 100°C for 5 min. Protein concentrations were determined using the DC protein assay (Bio-Rad, Hercules, CA, USA). For Western blot, samples that contained 20 mg of total protein underwent SDS–polyacrylamide gel electrophoresis on 8% acrylamide gels using a Tris/glycine/SDS buffer system (Bio-Rad, Hercules, CA, USA), followed by overnight electrophoretic transfer to polyvinylidene difluoride membranes (ThermoFisher, Rockford, IL, USA). Membranes were blocked in 5% nonfat milk for 1 h at 23°C and then incubated in primary antibody in 2.5% milk overnight at 4°C. The primary antibodies were phosphorylated GR (Ser211) antibody (1:500, catalog no. 4161, Cell Signaling, Danvers, MA, USA), GR monoclonal antibody (1:500, catalog no. MA1–510, Invitrogen, Waltham, MA, USA), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1:2500, catalog no. AB9485, Abcam, Cambridge, MA, USA). Membranes were washed in TTBS (Tris-buffered saline with 0.1% Tween 20 Detergent, Bio-Rad, Hercules, CA, USA) three times for 10 min each at room temperature, labeled with species-specific peroxidase-conjugated secondary antibody (1:10,000, Bio-Rad, Hercules, CA, USA) for 1 h at room temperature, washed, and then detected by chemiluminescence (SuperSignal West Pico, ThermoFisher, Rockford, IL, USA) using a myECL imager (ThermoFisher, Rockford, IL, USA). Phosphorylated-GR blots were stripped for 30 min at room temperature (Restore, ThermoFisher, Rockford, IL, USA) and reprobed for total GR protein levels. Immunoreactivity was quantified by densitometry using ImageJ software (National Institutes of Health, Bethesda, MD, USA). No significant changes in total GRs were observed when normalized to GAPDH. Accordingly, phosphorylated-GR densitometry values were normalized to total GR densitometry values to generate phosphorylation:total ratio values for statistical comparison. Density values are expressed as a percentage of the mean of ShA rat values. The following numbers of rats were tested: ShA: (n = 14 [seven males, seven females]), LgA (n = 12 [six males, six females]).
Methods for SCR-1 rat antisense oligonucleotide
Synthesis and purification of the antisense oligonucleotides (ASOs) were performed as described previously [29–31]. The ASOs were uniformly modified with 2’-O-(2-methoxy)ethyl sugars, a phosphorothioate backbone, and 5’-methyl cytosine as previously described [31]. Lyophilized ASOs were dissolved in sterile phosphate-buffered saline without calcium or magnesium and sterilized through a 0.2 μm filter. The ASO sequences are presented in supplemental Table S2.
Cell culture and transfection
Rat PC-12 cells were grown in HyClone Dulbecco’s Modified Eagle Medium/high-glucose media that were supplemented with 10% fetal bovine serum. The ASOs (80 nM final concentration) were transfected into rat PC-12 cells using Invitrogen Lipofectamine 2000 Reagent (Life Technologies, Carlsbad, CA, USA). RNA was collected 48 h post-transfection.
RNA isolation and analysis
Tissues were homogenized in TRIzol reagent (Life Technologies, Carlsbad, CA, USA) using a power homogenizer, and RNA isolation was performed on both rat brain tissue and cells in culture according to the manufacturer’s protocol. RNA was reverse transcribed using the GoScript reverse transcription system (Promega, Madison, WI, USA). Radiolabeled polymerase chain reaction (PCR) was performed using complementary DNA (cDNA) with primers, α−32P-dCTP, and GoTaq Green (Promega, Madison, WI, USA). The primer sequences were ATGAATGATCCAGCACTGAG for NCOA1ex21F and CAGGTTCACTGTACACTGG for NCOA1ex23R. Products were separated on a 6% non-denaturing polyacrylamide gel and quantified using a Typhoon FLA 7000 phosphorimager (GE Healthcare, Uppsala, Sweden). Polymerase chain reaction amplicons were sequenced to confirm isoform identity.
Antisense oligonucleotide and intravenous heroin self-administration
The rats were prepared with intravenous catheters and trained for heroin self-administration as described above. We used aseptic techniques for the infusion of ASO in the CeA. We anesthetized the rats with isoflurane (1.5–2.5% in oxygen). The skin on top of the skull was shaved and scrubbed with 70% alcohol and Betadine. Using blunt ear bars, we placed the rats in the stereotaxic instrument. The rats received bilateral infusions (5 min infusion plus 5 min diffusion with injectors in place) of the SRC-1-targetted ASO (16.66 μg in 0.5 μl/side) or control ASO in the CeA (anterior/posterior: −2.06; medial/lateral: ±4.55; dorsal/ventral: −8.45). The incision was sutured, and the rats received the analgesic meloxicam (1 mg/kg/ml, subcutaneous; Covetrus North America, Dublin, OH, USA) and the antibiotic gentamicin (3 mg/kg/ml, subcutaneous; Fresenius Kabi, Lake Zurich, IL, USA). Gentamicin was administered for two more days after surgery. After recovery, the rats resumed heroin self-administration. The following numbers of rats were tested: control ASO (n = 18 [11 males, seven females]), rat SRC-1 ASO (n = 18 [11 males, seven females]).
Statistical analyses
The data are expressed as mean and SEM. The data were analyzed using analysis of variance (ANOVA) with or without repeated measures or using Student’s t-test (two-sided). When appropriate, post hoc comparisons were performed using Sidak’s test, Dunnett’s test, or the Benjamini, Krieger, and Yekutieli test that controlled for multiple comparisons by controlling the False Discovery Rate (FDR). The accepted level of significance for all tests was p < 0.05. The number of animals that were necessary for the experiments was calculated based on the effects of mifepristone that were observed in our previous published studies [26, 27]. Some differences in sample sizes that are presented in the figures were attributable to the loss of catheter patency over time (e.g., intravenous self-administration), technical practicality (e.g., Western blot), or the removal of outlier values based on Grubb’s test. Data, separated by sex (replicates), are reported in the Supplemental Materials.
RNA sequencing of human CeA
Demographic data for the human CeA samples are presented in Table S5. Amygdala samples from humans with OUD and unaffected controls were processed for total RNA isolation using the mirVana miRNA Isolation Kit (ThermoFisher Scientific, San Diego, CA, USA) and Zymo purification kit (Zymo Research, Irvine, CA, USA). Libraries were prepared with the KAPA RNA HyperPrep Kit with RiboErase (Roche, Basel, Switzerland), which removes both cytoplasmic and mitochondrial ribosomal RNA (rRNA) species and uses random priming in cDNA synthesis, according to the manufacturer’s protocol. The rRNA-depleted libraries were subsequently sequenced on an Illumina Novaseq sequencer at 100-million-read target coverage (100 bp paired-end reads) at the Yale Center for Genome Analysis facility. The data have been deposited under Gene Expression Omnibus (GEO), accession number GSE194368.
Gene expression profiling and pathway enrichment analysis
After performing a quality check using FastQC v0.11.8, raw reads were trimmed for quality with fastp v0.19.6 using default parameters. Paired-end RNA-sequencing trimmed data were subsequently aligned to the human genome assembly (build hg38) with HISAT2 v2.1.0 and reads counted to genomic features with featureCounts v2.0.1 and the following arguments: -s 2 -t transcript -g gene_name, using Ensembl Homo_sapiens.GRCh38.103.gtf annotation as the reference. The raw count matrix was imported into R 4.0.4 software. Differential expression analysis was performed using the DESeq2 v1.30.1 package [32]. Genes with an absolute log2 fold change > 0.5 and adjusted p ≤ 0.05 were considered differentially expressed genes. Gene Set Enrichment Analysis (GSEA) [33] was performed using the corto v1.1.10 package [34] and gene sets from the C2 category derived from Broad Institute’s MSigDB collection, downloaded through msigdbr v7.4.1 [33, 35]. The FDR was calculated using the Benjamini-Hochberg procedure for multiple-testing correction. Significantly enriched GR-related pathways were then identified by searching for the string pattern “GLUCOCORTICOID” in gene set names. Quantitative RT-PCR for validation was performed with a standard curve method with normalization to β-actin.
Results
Chronic GR antagonism blunts the development of heroin addiction-like behaviors
We used a rat model of salient components of opioid addiction that has construct validity. In this model, rats are allowed either limited access to opioid, termed short-access (ShA; 1 h), or extended access to opioid, termed long-access (LgA; 12 h). For the rats in the LgA condition (dependent), when exposed to 12 h sessions of opioid self-administration, multiple signs of opioid dependence that reflect those that are observed in humans with OUD were observed, including both somatic signs (e.g., “wet-dog shakes”) and motivational signs (e.g., compulsive-like opioid seeking and taking) [7, 14, 25, 36–39]. Importantly, rats that are allowed ShA (nondependent) sessions to opioid self-administration show very few of these signs.
We found that chronic, systemic GR antagonism with mifepristone blunted the escalation of heroin self-administration in rats that were allowed LgA sessions compared with vehicle-treated rats (Figure 1b, Figure S2; two-way repeated-measures ANOVA, session × treatment interaction: F8,296 = 6.9, p < 0.0001). Mifepristone-treated rats self-administered less heroin in sessions 5–9 compared with vehicle-treated rats (adjusted p < 0.05; controlled for multiple comparisons by controlling the FDR). Mifepristone-treated rats also exhibited lower motivation to work for heroin infusions in a progressive-ratio test (Figure 1c, Figure S2; unpaired Student’s t-test: t37 = 3.1, p < 0.01). We also examined the effect of mifepristone on naloxone-induced heroin self-administration [14]. At low doses, naloxone precipitates a motivational withdrawal syndrome in opioid-dependent rats and increases heroin self-administration, hypothetically to reverse this withdrawal [7, 14]. Mifepristone-treated rats exhibited less naloxone-induced heroin self-administration than vehicle-treated rats (Figure 1d, Figure S2; two-way repeated-measures ANOVA, mifepristone × naloxone interaction: F1,37 = 6.4, p < 0.05; Sidak’s post hoc test: p < 0.001).
Figure 1. Chronic glucocorticoid receptor antagonism blunts the development of heroin addiction-like behaviors.

(a) Male and female rats were surgically implanted with intravenous (IV) catheters. After recovery from surgery, the rats were trained to self-administer heroin (unit dose: 60 μg/kg/0.1 ml for males; 180 μg/kg/0.1 ml for females) in 1 h short-access (ShA) self-administration (SA) sessions under a fixed-ratio 1 (FR1) schedule of reinforcement (each active lever press was reinforced with heroin). The rats were subcutaneously implanted with pellets that released the GR antagonist mifepristone (Mifep; 200 mg for continuous 21-day delivery) or vehicle pellets. Twenty-four hours later, the rats were allowed ShA or long-access (LgA; 12 h) to heroin self-administration (FR1) for 9 sessions. This was followed by a two-session naloxone (vs. saline) challenge test (FR1) that lasted 30 min because of the short-acting effect of naloxone [14] and by a progressive-ratio (PR) test, in which the number of lever presses that was required to receive a heroin infusion progressively increased. (b) Heroin infusions (± SEM) in rats under LgA FR1 heroin self-administration sessions. (c) Heroin infusions (± SEM) in rats in the LgA group in a PR test. (d) Heroin infusions (± SEM) in rats in the LgA group in 30 min sessions (FR1) following vehicle (saline) administration (0 μg/kg naloxone, subcutaneous) or naloxone administration (30 μg/kg naloxone, subcutaneous). * p < 0.05, **p < 0.01, ***p < 0.001, difference from Mifep. Vehicle: n = 17 (10 males, 7 females). Mifep: n = 22 (14 males, 8 females).
Importantly, mifepristone did not change any of these behaviors in rats in the ShA group (Figure S3), which models a more nondependent, recreational pattern of opioid intake that is observed in rodents [14, 25, 36–39] and humans [40]. These findings suggest that GRs are recruited only under conditions of an opioid-dependent state and not under conditions of a nondependent state in rats, supporting the hypothesis that GRs contribute to the progression and maintenance of opioid addiction-like behaviors.
Chronic GR antagonism decreases the excitability of CeA CRF cells
Although evidence demonstrates activation of the extrahypothalamic CRF system in opioid addiction [8, 16], the role of GRs in this action has not been elucidated. To test the hypothesis that GR activation mediated the increase in the activity of CRF neurons in the CeA, we tested the effect of chronic mifepristone treatment on CeA CRF cell excitability. The application of currents from 0 to 300 pA increased the number of action potential in CeA CRF neurons. This increase was blunted in rats that received chronic mifepristone treatment and were tested for heroin self-administration in LgA conditions compared with rats that were not treated with mifepristone and were tested under LgA conditions (two-way repeated-measures ANOVA: session × treatment interaction: F30,527 = 1.5, p < 0.05). We detected significant differences between mifepristone and control groups from 260 to 300 pA (p < 0.05, controlled for multiple comparisons by controlling the FDR; Figure 2b, c). However, we did not detect differences in current-evoked CRF cell action potentials between opioid-naive and rats tested in ShA conditions (Figure S4). Although we did not design the experiments to compare rats in the LgA and ShA conditions and naive rats directly, the number of current-evoked action potentials in CeA neurons in rats in the LgA condition (18.1 at 300 pA) was more than two-fold higher than rats in the ShA condition (8.27 at 300 pA) and four-fold higher than opioid-naive rats (4.25 at 300 pA). In the CeA, we detected CRF in dense core vesicles in cell bodies and in axon terminals that established symmetric synapses with dendrites (Figure S5). These findings support our hypothesis of a gain in GR-mediated CRF function in the CeA as a key component in opioid dependence.
Figure 2. Chronic glucocorticoid receptor antagonism decreases the excitability of CeA CRF cells.

(a) Male Wistar CRH-Cre rats were prepared with intravenous (IV) catheters and trained (1 h ShA sessions; FR1) for intravenous (IV) heroin self-administration. Via intracerebral surgery (IC), the rats received bilateral infusions of AAV1-CAG-FLEX-eGFP (0.5 μl/side; AAV) in the CeA (anterior/posterior: −2.06; medial/lateral: ±4.55; dorsal/ventral: −8.45). The rats were implanted with Mifep pellets and allowed LgA (12 h) to heroin self-administration. Eight hours after their last self-administration session (i.e., acute opioid withdrawal), the rats were euthanized, and coronal slices were prepared for electrophysiology (Ephys). Neurons that expressed green fluorescent protein (CRF+) were patched. (b) Input-output curves of the number of action potentials of CRF neurons in rats in the LgA group that received chronic treatment with Mifep (n = 9 cells) or no treatment (n = 10 cells). * p < 0.05, difference from Mifep. (c) Representative current-clamp traces of action potential firing in CeA CRF neurons induced by 0, 200, and 300 pA stimulus currents.
Acute GR antagonism decreases opioid self-administration in opioid-dependent rats
The results described above demonstrate a role for GRs in the development of opioid addiction-like behavior. To test the hypothesis that GRs also participate in the maintenance of opioid addiction-like behavior, we tested the acute effects of mifepristone in already opioid-dependent rats to mimic the treatment of opioid-dependent humans. For this experiment, we used both intravenous heroin and methadone self-administration in rats in ShA and LgA conditions.
Female Wistar rats that self-administered heroin (180 μg/kg/0.1 ml) in LgA sessions (FR1) escalated their heroin intake. Acute treatment with 60 and 90 mg/kg mifepristone significantly reduced heroin self-administration in the LgA group compared with vehicle (one-way ANOVA, main effect of treatment: F3,15 = 6.0, p < 0.01, followed by Dunnett’s post hoc test, p < 0.05), whereas the same treatment did not significantly alter heroin self-administration in the ShA group (Figure S6).
We replicated these results using methadone self-administration in male and female Wistar rats (Figure 3, Figure S7, S9). Rats that were tested under LgA conditions escalated their methadone self-administration, an effect that was significant from sessions 6 to 10 compared with session 1 (one-way repeated-measures ANOVA, main effect of session, F9,153 = 18.1, p < 0.0001, followed by Dunnett’s post hoc test, p < 0.0001; Figure 3b). Although the one-way repeated-measures ANOVA also revealed a significant effect of session in rats that were tested under ShA conditions (F9,153 = 6.9, p < 0.05), the Dunnett’s post hoc test did not indicate differences between ShA sessions 2–10 and session 1 (Figure 3b).
Figure 3. Acute glucocorticoid receptor antagonism reverses escalated IV methadone self-administration.
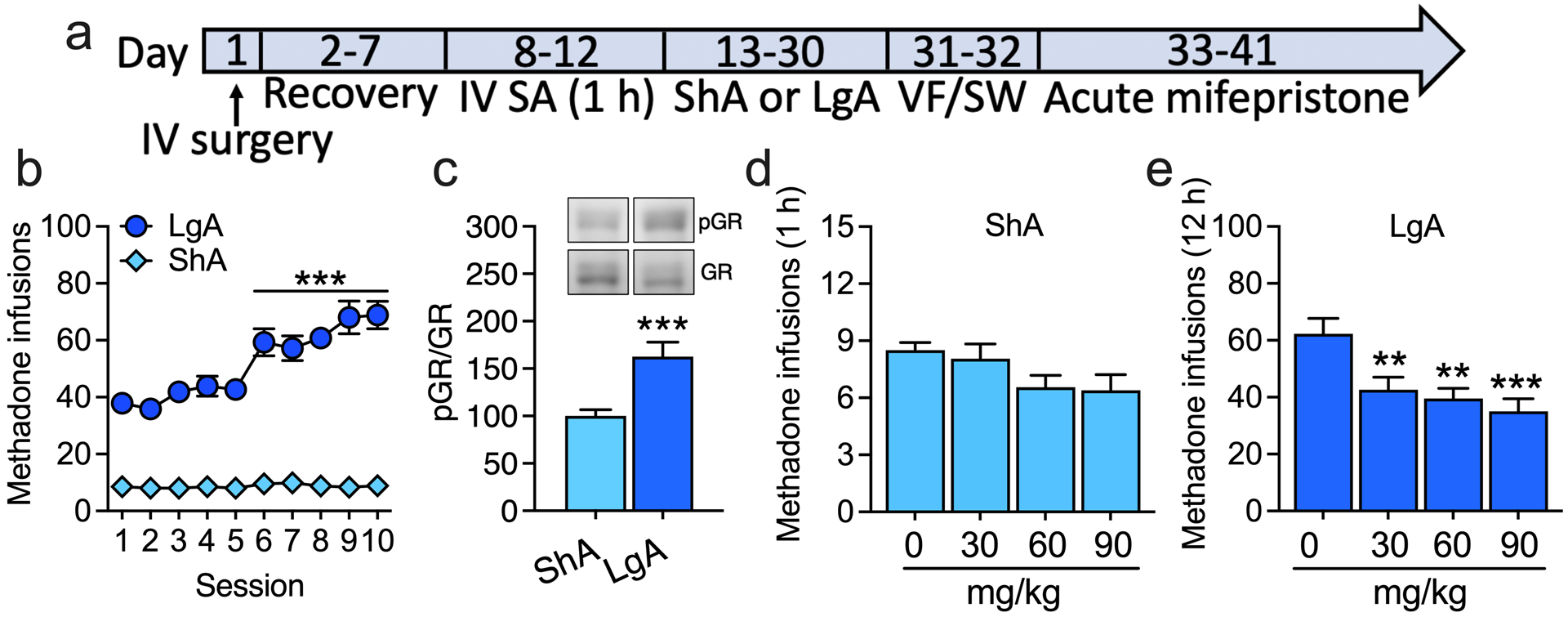
(a) Male and female rats were prepared with intravenous (IV) catheters and trained to self-administer (SA) methadone (unit dose: 300 μg/kg/0.1 ml) in 1 h ShA sessions (FR1) and split into ShA and LgA (12 h) groups. We used the unit dose of 300 μg/kg/0.1 ml based on studies that reported that rats dose-dependently self-administered methadone and that methadone substitutes for morphine [21–23]. We used the 300 μg/kg/0.1 ml dose for both sexes based on their similar response to methadone-induced antinociception and hyperalgesia during withdrawal [24]. Once rats in the LgA group significantly escalated their methadone self-administration, rats in both the ShA and LgA groups were acutely administered with mifepristone (0, 30, 60, and 90 mg/kg; intraperitoneal, 90 min before testing) in a within-subjects Latin-square design. (b) Methadone infusions (± SEM) in rats in the ShA and LgA groups. ***p < 0.0001, difference from session 1. ShA: n = 18 (10 males, 8 females). LgA: n = 18 (9 males, 9 females). To confirm that the rats in the LgA group exhibited greater signs of opioid dependence than rats in the ShA group, we tested the rats in the von Frey (VF) test to measure hyperalgesia during spontaneous withdrawal and naloxone-precipitated signs of somatic withdrawal (STW). (c) Phosphorylation of GRs at Ser232 in the CeA in rats in the LgA group relative to rats in the ShA group. The optical densities of total GR bands were normalized to the housekeeping protein GAPDH. Because there was no significant difference in total GR levels between the ShA and LgA groups, we normalized the optical densities of phosphorylated GR bands to the optical densities of total GR bands. ***p < 0.001, difference from ShA. ShA: n = 14 (7 males, 7 females). LgA: n = 12 (6 males, 6 females). (d, e) Methadone infusions (± SEM) in (d) rats in the ShA group and (e) rats in the LgA group. **p < 0.01, ***p = 0.0002, difference from 0 mg/kg (Dunnett’s post hoc comparisons). ShA: n = 18. LgA: n = 18.
Only a few studies have investigated intravenous methadone self-administration in rats. Thus, we confirmed that rats in the LgA group exhibited more signs of methadone dependence compared with rats in the ShA group prior to mifepristone testing. Indeed, rats in the LgA group exhibited hyperalgesia during spontaneous methadone withdrawal and an increase in somatic signs of naloxone-precipitated withdrawal compared with rats in the ShA group (Figure S8). We found an increase in GR activity, reflected by an increase in GR phosphorylation at Ser232, in the CeA in rats in the LgA group during methadone withdrawal compared with rats in the ShA group (unpaired two-sided Student’s t-test: t24 = 3.9, p < 0.001; Figure 3c, Figure S9). Acute mifepristone treatment dose-dependently decreased methadone self-administration in rats in the LgA group (main effect of treatment: F3,68 = 7.1, p < 0.001, followed by Dunnett’s post hoc test, p < 0.01) but not in rats in the ShA group (Figure 3d, e, Figure S9).
Additionally, the function and distribution of CRF and regulation of CRF by GRs are remarkably similar across both mammalian and non-mammalian species [12, 41], suggesting positive evolutionary selection for this stress system. Therefore, we assessed whether the regulation of opioid self-administration by GRs occurs in zebrafish (Danio rerio), a non-mammalian species that has an analog of the HPA axis (the hypothalamus-pituitary-intrarenal axis) and self-administers opioids [42]. We found that acute mifepristone treatment dose-dependently decreased hydrocodone self-administration in zebrafish (Figure S10). This finding suggests evolutionary conservation of the function of GR and opioid systems and supports the concept that opioid dependence is linked to dysregulations of ancient biological systems that are critical for adaptive responses to stress. These findings suggest the participation of GRs in several aspects of opioid dependence once dependence has already been established.
Increasing SRC-1a isoform levels in the CeA reduces heroin self-administration
An unresolved question is how chronic glucocorticoids sensitize CRF in the extended amygdala. One hypothesis is that there is region-specific recruitment of GR coregulators. More abundant expression levels of the SRC-1e isoform in the CeA putatively contributes to the increase in CRF expression in the CeA, and this may contribute to addiction-like behavior. Thus, we altered the splicing of endogenous rat Src-1 RNA using an ASO designed to induce skipping of the SRC-1e-specific exon, which encodes a stop codon, to alter the splicing of endogenous rat Src-1 RNA (Figure 4a, b). The skipping of this exon gives rise to an mRNA that encodes Src-1a, which in turn encodes a SRC-1 protein with a C-terminal domain that has a putative repressive effect on the Crh promoter [17]. We screened 27 ASOs in rat PC12 cells and identified several that effectively induced SRC-1e-specific exon skipping, as indicated by an increase in Src-1a abundance relative to SRC-1e (Figure 4c, d). The five ASOs with the highest activity in vitro were tested in rats and ASO-16 was selected for further study based on both its activity and the absence of apparent toxicity (Figure S11, Figure S12, Table S3).
Figure 4. Increasing the SRC-1a isoform in the CeA reduces heroin self-administration in rats under LgA conditions.
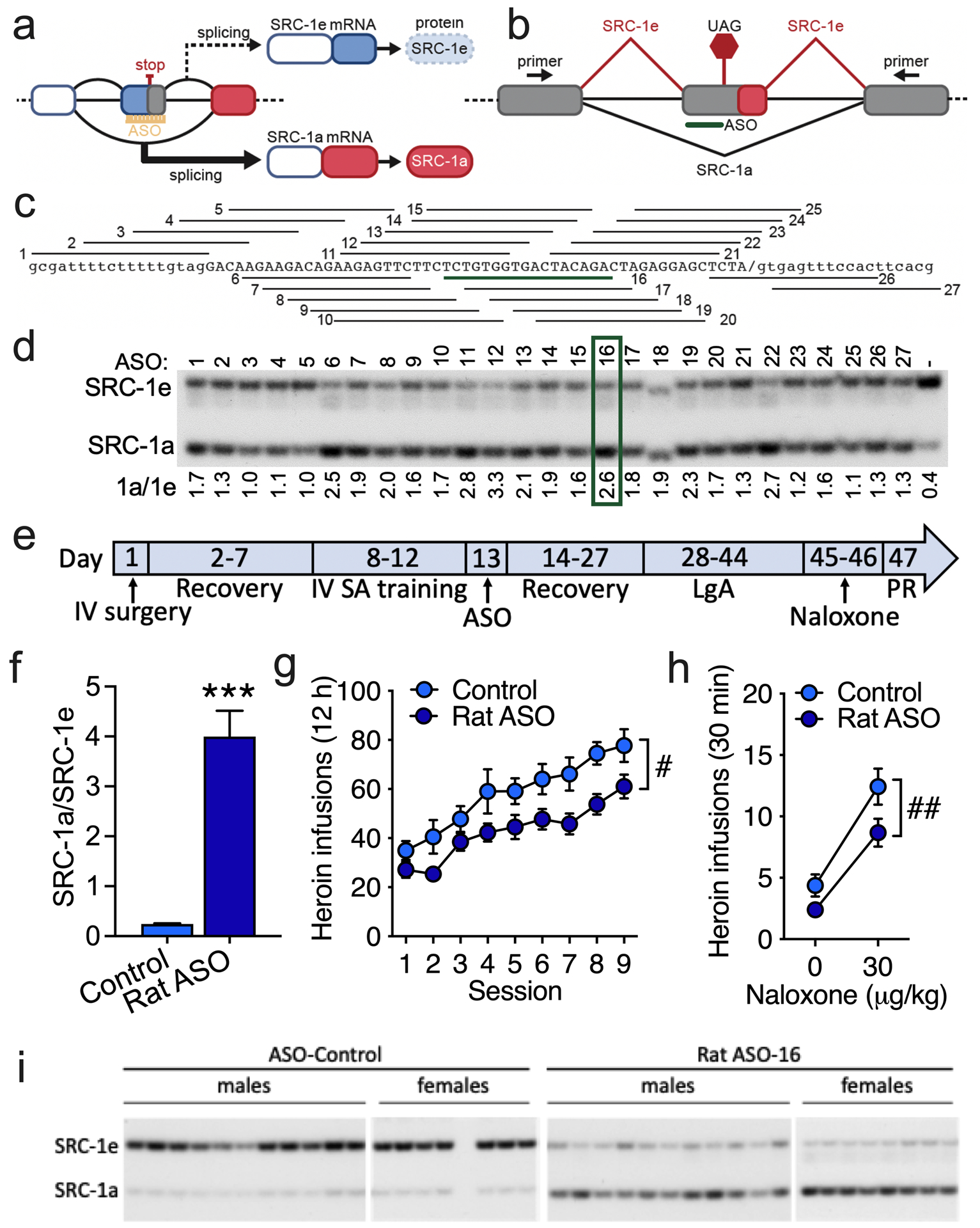
(a) Schematic representation of the divergent activities of SRC-1 isoforms in gene transcription. (b) Schematic diagram of the alternative splicing pattern of SRC-1. Exons are boxes, and introns are lines. SRC-1 splicing is shown as red diagonal lines, and SRC-1a is shown in black. The SRC-1e termination codon is shown (UAG). A representative antisense oligonucleotide (ASO) that is aligned to the targeted region is shown in green. The location of the primers that were used to detect SRC-1 spliced isoforms in the polymerase chain reaction (PCR) are shown in exons that flank the alternatively spliced exon. (c) General alignment of the 27 ASOs that were individually tested for activity in blocking SRC-1e splicing. ASO 16, which was selected for further testing, is highlighted in green. (d) Analysis of individual ASO activity in rat PC-12 cells. SRC-1e-specific exon skipping assessed by the separation of radioactive RT-PCR products by polyacrylamide gel electrophoresis. The quantification of products by phosphorimaging analysis (SRC-1a/SRC-1e) is shown below the gel image. The green box indicates the ASO selected for further testing. The sequence of the most active ASOs and their alignment to the SRC-1 sequences with which they base pair, the SRC-1 amplicons from the CeA in rats that were treated with the SRC-1-targetted or control ASO, and the quantification of RT-PCR products are shown in Figure S12. (e) Male rats were prepared with intravenous (IV) catheters and trained to self-administer (SA) heroin (60 μg/kg/0.1 ml) in 1 h ShA sessions (FR1). The rats received bilateral infusions (16.66 μg in 0.5 μl/side) of the SRC-1-targeted antisense oligonucleotide (ASO) or control ASO in the CeA (anterior/posterior: −2.06; medial/lateral: ±4.55; dorsal/ventral: −8.45) and were allowed LgA heroin self-administration (FR1). This regimen was followed by a PR test (Figure S14) and a naloxone challenge test. (f) Ratio of SRC-1a and SRC-1e splice variants in the CeA in rats that were treated with the SRC-1-specific ASO or non-targeted control ASO. ***p < 0.001, difference from controls. Control ASO: n = 18 (11 males, 7 females). Rat SRC-1 ASO: n = 18 (11 males, 7 females). (g) Heroin infusions (± SEM; FR1) in rats that received intra-CeA infusions of SRC-1-specific ASO (Rat SRC-1 ASO) or non-targeted ASO (Control) and were tested in LgA self-administration sessions. #p < 0.05, overall treatment effect. Control ASO: n = 19 (11 males, 8 females). Rat SRC-1 ASO: n = 19 (11 males, 8 females). (h) Heroin self-administration (± SEM) following saline administration (0 μg/kg naloxone, subcutaneous) or naloxone-precipitated withdrawal (30 μg/kg, subcutaneous) in rats. ##p < 0.01, overall treatment effect. Control ASO: n = 18 (11 males, 7 females). Rat SRC-1 ASO: n = 18 (11 males, 7 females). (i) Polyacrylamide gel of Src-1a/1e RT-PCR products quantified in (f).
The SRC-1-targeted ASO significantly induced SRC-1a expression in vivo (Figure 4f, i, Figure S12, Table S4; unpaired Student’s t-test: t34 = 7.3, p < 0.001). Rats in the LgA group that were injected in the CeA with the SRC-1-targeted ASO self-administered significantly less heroin (Figure 4g, Figure S13) during the escalation phase (two-way repeated-measures ANOVA, main effect of treatment: F1,36 = 6.0, p < 0.05) and following naloxone treatment (two-way repeated-measures ANOVA, main effect of treatment: F1,35 = 8.4, p < 0.01; Figure 4h) but not during the PR test (Figure S13) compared with rats in the LgA condition that received a non-targeted ASO control. Notably, the SRC-1-targeted ASO did not reduce heroin self-administration in rats in the ShA group (Figure S14). These findings are consistent with a role for GR-driven transcriptional activity in the CeA in promoting heroin self-administration under LgA conditions. The less pronounced behavioral effects of the ASO compared with mifepristone suggests the involvement of other coregulators in addiction-like behaviors.
Transcriptional evidence of activation of GR signaling in individuals with a history of opioid dependence
Finally, we tested the hypothesis that the results from the animal studies translate to a role for glucocorticoid-dependent adaptations in OUD in humans. We conducted RNA sequencing of the postmortem CeA from humans with OUD and unaffected controls (Table S5, Figure S15). Differential expression analysis revealed a total of 194 differentially expressed genes in OUD vs. control CeA samples, of which 180 genes were upregulated and 14 were downregulated (Figure S16). The complete differential expression (DE) analysis results are shown in Supplemental File 2. We then performed a GSEA to identify significantly enriched pathways that characterized OUD samples. We found significant positive enrichment of the GR pathway (Figure 5), indicating that a history of opioid use was associated with the adaptation of GR signaling in the CeA. Several genes that were included in the leading edge (i.e., the subset of a gene set that accounted for the gene set enrichment; Table S6) of this analysis are involved in cell function. More specifically, the top differentially regulated pathways, indicated by GSEA, indicated that the main processes in the gene signature of OUD vs. control samples are representative of inflammatory and neurodegenerative processes (Figure S16). Cell type analysis of the OUD gene signature with the Enrichr gene set search engine [43] and interrogation of the Azimuth and Descartes Cell types database [44] indicated that the primary cell types that were involved were astrocytes and microglia cells (Figure S16).
Figure 5. Transcriptional evidence of the activation of GR signaling in individuals with histories of opioid dependence.

Gene expression was profiled from the CeA in opioid-dependent individuals and unaffected controls by RNA sequencing. Demographic data are shown in Table S5. A representative coronal brain section of the human CeA is shown in Figure S15. We used the Gene Set Enrichment Analysis (GSEA) algorithm [33], a computational method that determines whether a gene set shows significant concordant differential expression between two conditions. The gene expression signature in humans with a history of opioid dependence vs. unaffected control individuals showed significant enrichement of the WP_GLUCOCORTICOID_RECEPTOR_PATHWAY gene set from the Broad Institute’s MSigDB database [58], indicating transcriptional adaptations of GR signaling in the amygdala in humans with OUD.
Discussion
In the present study, we provided key support for the hypothesis that glucocorticoids play an etiological role in activating the brain stress system to drive key elements of opioid addiction. We found GR-dependent plasticity in stress systems (Figure 6) in opioid dependence across three species (i.e., humans, rats, and zebrafish) and both sexes.
Figure 6. Glucocorticoid receptor-dependent plasticity in the CeA mediates opioid addiction-like behaviors.

The collective data suggest that excessive HPA axis activation during repeated episodes of opioid withdrawal leads to long-lasting neuroadaptations in extrahypothalamic brain regions. In CeA neurons, corticosteroids bind to GRs. Bound GRs dimerize and translocate to the nucleus where the coregulator SRC-1e is preferentially recruited. The GR/SRC-1e complex causes gene transactivation, including the expression of stress-related neuropeptides, such as CRF. Increases in the expression and release of CRF during opioid withdrawal may drive excessive drug seeking and taking. Blocking GRs and increasing the SRC-1a isoform constitute viable approaches to reverse allostatic changes in stress-related brain regions in opioid addiction.
Chronic GR antagonism with mifepristone prevented the development of opioid addiction-like behavior in rats under LgA (opioid dependent) but not ShA (nondependent) conditions and that the same treatment blunted current-evoked action potentials of CRF neurons in the CeA in rats with a history of LgA heroin self-administration. Acute mifepristone treatment reduced opioid addiction-like behavior in rats under LgA conditions that were already dependent on heroin or methadone and decreased hydrocodone self-administration in zebrafish. Acute mifepristone treatment did not alter behavior in rats under ShA conditions.
Mifepristone is not selective to GRs and also blocks progesterone receptors. We previously reported that mifepristone and the selective GR antagonist CORT113176 similarly decreased alcohol self-administration in dependent rats [26]. In a preliminary study in female rats that were tested under LgA conditions, we found that CORT113176 decreased heroin self-administration (data not shown). The more selective GR antagonist PT150 decreased the stress-induced reinstatement of fentanyl seeking [45]. These findings support a role for GR in opioid taking and seeking, independent of the participation of progesterone receptors.
Previous studies showed that metyrapone, a corticosteroid synthesis inhibitor, in combination with diazepam blocked psychostimulant seeking and psychostimulant self-administration [46]. Ketoconazole, another corticosteroid synthesis inhibitor, decreased the stress-induced reinstatement of cocaine seeking [47]. However, adrenalectomy or metyrapone (a corticosteroid synthesis inhibitor) did not reduce stress-induced heroin seeking in male rats, whereas a CRF receptor antagonist did [48]. The differential effects of corticosteroid synthesis inhibition and GR antagonism on drug seeking and drug self-administration may involve the recruitment of different cofactors/modulators [49].
Our ASO approach that was designed to favor the expression of SRC-1a, which putatively decreases CRF expression in the CeA [17], reduced opioid addiction-like behavior. However, other receptors also interact with SRC-1 [50]. Thus, the posibility that these other receptors regulate CRF expression by interacting with SRC-1 cannot be excluded. Additionally, different brain SRC-1 expression levels have been reported between male and female mice [51]. Thus, future research is required to investigate potential sex differences in the interaction between GRs, SRC-1, and opioid addiction-like behavior. We measured SRC-1a and SRC-1e levels in the CeA in humans with OUD and unaffected controls and did not find group differences (Figure S17), suggesting that opioid dependence is associated with alterations of upstream GR signaling rather than SRC-1 isoform levels, which are known to interact with receptors other than GRs [50].
The mechanism of action of GR involves both fast nongenomic and slow genomic actions [13]. Although the rapid GR-mediated transcription and translation of genes is possible, the synthesis, transport, and storage of peptides (e.g., CRF) may take longer than the 90-min pretreatment time in the present acute mifepristone experiments. Future studies are required to determine the exact mechanisms by which GR antagonism reduces addiction-like behavior.
Using RNA sequencing, followed by GSEA [33], we observed changes in glucocorticoid-induced gene expression that were indicative of an increase in inflammation, as well as astrocyte and microglial transcriptional activity in the CeA in humans with OUD compared with unaffected controls. These results suggest that the activation of glucocorticoid-induced gene expression affects brain function and contributes to the perpetuation of compulsive opioid seeking and taking. Consistent with our hypothesis, these findings provide important initial evidence that opioid dependence is associated with adaptations that are caused by an increase in GR activity, which warrants further investigation.
The implications of these results are that targeting GRs with mifepristone and ASOs may have a unique dual action in “resetting” a dysfunctional HPA axis [52] and normalizing GR signaling in extrahypothalamic stress circuits. Glucocorticoid receptors may also regulate several other neuropeptide and neurotransmitter systems to contribute to addiction-like behaviors. For example, understanding the potential role of GRs in driving pro-stress systems (e.g., norepinephrine, dynorphin, hypocretin, vasopressin, and substance P) and inhibiting anti-stress systems (e.g., oxytocin, neuropeptide Y, nociceptin, and endocannabinoids) may provide new insights into OUD.
The present study also emphasized the importance of stress-related neuroadaptive mechanisms in opioid dependence and suggests adding stress systems to the “most wanted” list of targets for drug development for OUD [53]. This strategy may also be valuable for managing the problematic use of substances that are frequently co-misused with opioids, such as alcohol. Recent studies reported changes in the epigenetic regulation of NR3C1 (the gene that encodes the GR) in brain samples from humans with alcohol use disorder [54], and we previously reported that mifepristone decreased alcohol craving and drinking in alcohol dependent rats and in individuals with alcohol use disorder [26]. Another recent study reported the safety of the combined use of PT150 and alcohol in health volunteers [55].
Mifepristone is safe, well-tolerated, and currently used for the treatment of hyperglycemia in Cushing’s syndrome. Mifepristone has shown promising results in hundreds of patients with psychotic depression [56]. The research and development of new drugs for specific indications are both costly and time-consuming. Thus, the repurposing of mifepristone, combined with harm-reduction and psychosocial support programs, may be a viable, rapid, and cost-effective strategy for the treatment of OUD, especially in individuals who use opioids to cope with stress. Potential scenarios for the use of mifepristone could be to assist individuals with tapering down methadone to minimize its side effects or facilitate the transition from methadone to buprenorphine or the transition from opioids to abstinence. Likewise, ASOs have proven to be highly specific and effective therapeutics for the treatment of neurological disease [57] and may be another promising platform for the future development of treatments for OUD.
Supplementary Material
Acknowledgments:
This study was supported by the NIDA Intramural Research Program. We thank the NIDA drug supply program and NIDA Intramural Research Program pharmacy for providing and dispensing heroin and methadone. Human tissue was obtained from the National Institutes of Health NeuroBioBank at the University of Miami Brain Endowment Bank and University of Pittsburgh Brain Tissue Donation Program. The authors thank Shiliang Zhang and Rong Ye from the NIDA Confocal and Electron Microscopy Core for their expert technical assistance, Lauren Brick from NIDA media for figure preparation, Brandon Harvey for providing PC12 cells, and Michael Arends for proofreading the manuscript. SAC, JCMV, MAM, JMB, CR, MM, GFK, and LFV were supported by the NIDA Intramural Research Program. ROM was supported by a grant from the National Institute on Alcohol Abuse and Alcoholism (AA026075). VRC received support from NIDA (DA048882). PPS received support from NIDA (DA043268). GDB received a Canadian Institutes of Health Research fellowship. Crh-Cre rats can be obtained from the Rat Resource and Research.
Footnotes
Competing interests: The authors declare no competing interests.
Supplementary information is available on Molecular Psychiatry’s website
References
- 1.Strang J, Volkow ND, Degenhardt L, Hickman M, Johnson K, Koob GF, et al. Opioid use disorder. Nat Rev Dis Primers. 2020;6:3. [DOI] [PubMed] [Google Scholar]
- 2.Understanding the Epidemic | Drug Overdose | CDC Injury Center. 2019. https://www.cdc.gov/drugoverdose/epidemic/index.html. Accessed 7 January 2020.
- 3.Case A, Deaton A. Rising morbidity and mortality in midlife among white non-Hispanic Americans in the 21st century. Proc Natl Acad Sci USA. 2015;112:15078–15083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koob GF. Neurobiology of Opioid Addiction: Opponent Process, Hyperkatifeia, and Negative Reinforcement. Biological Psychiatry. 2020;87:44–53. [DOI] [PubMed] [Google Scholar]
- 5.Evans CJ, Cahill CM. Neurobiology of opioid dependence in creating addiction vulnerability. F1000Res. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mechling AE, Arefin T, Lee H-L, Bienert T, Reisert M, Ben Hamida S, et al. Deletion of the mu opioid receptor gene in mice reshapes the reward-aversion connectome. Proc Natl Acad Sci USA. 2016;113:11603–11608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pantazis CB, Gonzalez LA, Tunstall BJ, Carmack SA, Koob GF, Vendruscolo LF. Cues conditioned to withdrawal and negative reinforcement: Neglected but key motivational elements driving opioid addiction. Science Advances. 2021;7:eabf0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koob GF, Schulkin J. Addiction and stress: An allostatic view. Neurosci Biobehav Rev. 2019;106:245–262. [DOI] [PubMed] [Google Scholar]
- 10.McEwen BS, Akil H. Revisiting the Stress Concept: Implications for Affective Disorders. J Neurosci. 2020;40:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selye H Stress and the general adaptation syndrome. Br Med J. 1950;1:1383–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulkin J The CRF Signal: Uncovering an Information Molecule. Oxford University Press; 2017. [Google Scholar]
- 13.de Kloet ER, Meijer OC, de Nicola AF, de Rijk RH, Joëls M. Importance of the brain corticosteroid receptor balance in metaplasticity, cognitive performance and neuro-inflammation. Front Neuroendocrinol. 2018;49:124–145. [DOI] [PubMed] [Google Scholar]
- 14.Carmack SA, Keeley RJ, Vendruscolo JCM, Lowery-Gionta EG, Lu H, Koob GF, et al. Heroin addiction engages negative emotional learning brain circuits in rats. J Clin Invest. 2019;129:2480–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Upadhyay J, Maleki N, Potter J, Elman I, Rudrauf D, Knudsen J, et al. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain. 2010;133:2098–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberto M, Spierling SR, Kirson D, Zorrilla EP. Chapter Two - Corticotropin-Releasing Factor (CRF) and Addictive Behaviors. In: Thiele TE, editor. International Review of Neurobiology, vol. 136, Academic Press; 2017. p. 5–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zalachoras I, Verhoeve SL, Toonen LJ, van Weert LTCM, van Vlodrop AM, Mol IM, et al. Isoform switching of steroid receptor co-activator-1 attenuates glucocorticoid-induced anxiogenic amygdala CRH expression. Mol Psychiatry. 2016;21:1733–1739. [DOI] [PubMed] [Google Scholar]
- 18.Edwards S, Little HJ, Richardson HN, Vendruscolo LF. Divergent regulation of distinct glucocorticoid systems in alcohol dependence. Alcohol. 2015;49:811–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cook CJ. Glucocorticoid feedback increases the sensitivity of the limbic system to stress. Physiol Behav. 2002;75:455–464. [DOI] [PubMed] [Google Scholar]
- 20.Marchette RCN, Gregory-Flores A, Tunstall BJ, Carlson ER, Jackson SN, Sulima A, et al. κ-Opioid receptor antagonism reverses heroin withdrawal-induced hyperalgesia in male and female rats. Neurobiology of Stress. 2021;14:100325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreton JE, Roehrs T, Khazan N. Drug self-administration and sleep-awake activity in rats dependent on morphine, methadone, or l -alpha-acetylmethadol. Psychopharmacology. 1976;47:237–241. [DOI] [PubMed] [Google Scholar]
- 22.Werner TE, Smith SG, Davis WM. A dose-response comparison between methadone and morphine self-administration. Psychopharmacology. 1976;47:209–211. [DOI] [PubMed] [Google Scholar]
- 23.Collins RJ, Weeks JR. Relative potency of codeine, methadone and dihydromorphinone to morphine in self-maintained addict rats. Naunyn - Schmiedebergs Arch. 1965;249:509–514. [DOI] [PubMed] [Google Scholar]
- 24.Holtman JR, Wala EP. Characterization of the Antinociceptive and Pronociceptive Effects of Methadone in Rats. Anesthesiology. 2007;106:563–571. [DOI] [PubMed] [Google Scholar]
- 25.Vendruscolo JCM, Tunstall BJ, Carmack SA, Schmeichel BE, Lowery-Gionta EG, Cole M, et al. Compulsive-Like Sufentanil Vapor Self-Administration in Rats. Neuropsychopharmacology. 2018;43:801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vendruscolo LF, Estey D, Goodell V, Macshane LG, Logrip ML, Schlosburg JE, et al. Glucocorticoid receptor antagonism decreases alcohol seeking in alcohol-dependent individuals. J Clin Invest. 2015;125:3193–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Logrip ML, et al. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci. 2012;32:7563–7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Somkuwar SS, Vendruscolo LF, Fannon MJ, Schmeichel BE, Nguyen TB, Guevara J, et al. Abstinence from prolonged ethanol exposure affects plasma corticosterone, glucocorticoid receptor signaling and stress-related behaviors. Psychoneuroendocrinology. 2017;84:17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rigo F, Chun SJ, Norris DA, Hung G, Lee S, Matson J, et al. Pharmacology of a Central Nervous System Delivered 2′-O-Methoxyethyl–Modified Survival of Motor Neuron Splicing Oligonucleotide in Mice and Nonhuman Primates. J Pharmacol Exp Ther. 2014;350:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swayze EE, Siwkowski AM, Wancewicz EV, Migawa MT, Wyrzykiewicz TK, Hung G, et al. Antisense oligonucleotides containing locked nucleic acid improve potency but cause significant hepatotoxicity in animals. Nucleic Acids Res. 2007;35:687–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baker BF, Lot SS, Condon TP, Cheng-Flournoy S, Lesnik EA, Sasmor HM, et al. 2′-O-(2-Methoxy)ethyl-modified Anti-intercellular Adhesion Molecule 1 (ICAM-1) Oligonucleotides Selectively Increase the ICAM-1 mRNA Level and Inhibit Formation of the ICAM-1 Translation Initiation Complex in Human Umbilical Vein Endothelial Cells. J Biol Chem. 1997;272:11994–12000. [DOI] [PubMed] [Google Scholar]
- 32.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology. 2014;15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mercatelli D, Lopez-Garcia G, Giorgi FM. corto: a lightweight R package for gene network inference and master regulator analysis. Bioinformatics. 2020;36:3916–3917. [DOI] [PubMed] [Google Scholar]
- 35.Liberzon A, Subramanian A, Pinchback R, Thorvaldsdóttir H, Tamayo P, Mesirov JP. Molecular signatures database (MSigDB) 3.0. Bioinformatics. 2011;27:1739–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22:413–421. [DOI] [PubMed] [Google Scholar]
- 37.Moussawi K, Ortiz MM, Gantz SC, Tunstall BJ, Marchette RCN, Bonci A, et al. Fentanyl vapor self-administration model in mice to study opioid addiction. Science Advances. 2020;6:eabc0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McConnell SA, Brandner AJ, Blank BA, Kearns DN, Koob GF, Vendruscolo LF, et al. Demand for fentanyl becomes inelastic following extended access to fentanyl vapor self-administration. Neuropharmacology. 2021;182:108355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lenoir M, Cantin L, Vanhille N, Serre F, Ahmed SH. Extended Heroin Access Increases Heroin Choices Over a Potent Nondrug Alternative. Neuropsychopharmacology. 2013;38:1209–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zinberg NE, Jacobson RC. The natural history of ‘chipping.’ The American Journal of Psychiatry. 1976;133:37–40. [DOI] [PubMed] [Google Scholar]
- 41.Yao M, Schulkin J, Denver RJ. Evolutionarily conserved glucocorticoid regulation of corticotropin-releasing factor expression. Endocrinology. 2008;149:2352–2360. [DOI] [PubMed] [Google Scholar]
- 42.Bossé GD, Peterson RT. Development of an opioid self-administration assay to study drug seeking in zebrafish. Behav Brain Res. 2017;335:158–166. [DOI] [PubMed] [Google Scholar]
- 43.Xie Z, Bailey A, Kuleshov MV, Clarke DJB, Evangelista JE, Jenkins SL, et al. Gene Set Knowledge Discovery with Enrichr. Curr Protoc. 2021;1:e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hao Y, Hao S, Andersen-Nissen E, Mauck WM, Zheng S, Butler A, et al. Integrated analysis of multimodal single-cell data. Cell. 2021;184:3573–3587.e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hammerslag LR, Denehy ED, Carper B, Nolen TL, Prendergast MA, Bardo MT. Effects of the glucocorticoid receptor antagonist PT150 on stress-induced fentanyl seeking in male and female rats. Psychopharmacology. 2021. 18 May 2021. 10.1007/s00213-021-05865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goeders NE, Guerin GF, Schmoutz CD. The combination of metyrapone and oxazepam for the treatment of cocaine and other drug addictions. Adv Pharmacol. 2014;69:419–479. [DOI] [PubMed] [Google Scholar]
- 47.Mantsch JR, Goeders NE. Ketoconazole blocks the stress-induced reinstatement of cocaine-seeking behavior in rats: relationship to the discriminative stimulus effects of cocaine. Psychopharmacology (Berl). 1999;142:399–407. [DOI] [PubMed] [Google Scholar]
- 48.Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, Stewart J. Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J Neurosci. 1997;17:2605–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McGinn MA, Tunstall BJ, Schlosburg JE, Gregory-Flores A, George O, de Guglielmo G, et al. Glucocorticoid receptor modulators decrease alcohol self-administration in male rats. Neuropharmacology. 2021;188:108510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stashi E, York B, O’Malley BW. Steroid receptor coactivators: servants and masters for control of systems metabolism. Trends Endocrinol Metab. 2014;25:337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bian C, Zhang D, Guo Q, Cai W, Zhang J. Localization and sex-difference of steroid receptor coactivator-1 immunoreactivities in the brain of adult female and male mice. Steroids. 2011;76:269–279. [DOI] [PubMed] [Google Scholar]
- 52.Dalm S, Karssen AM, Meijer OC, Belanoff JK, de Kloet ER. Resetting the Stress System with a Mifepristone Challenge. Cell Mol Neurobiol. 2019;39:503–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rasmussen K, White DA, Acri JB. NIDA’s medication development priorities in response to the Opioid Crisis: ten most wanted. Neuropsychopharmacol. 2019;44:657–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gatta E, Grayson DR, Auta J, Saudagar V, Dong E, Chen Y, et al. Genome-wide methylation in alcohol use disorder subjects: implications for an epigenetic regulation of the cortico-limbic glucocorticoid receptors (NR3C1). Mol Psychiatry. 2019:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morice C, Baker DG, Patel MM, Nolen TL, Nowak K, Hirsch S, et al. A randomized trial of safety and pharmacodynamic interactions between a selective glucocorticoid receptor antagonist, PT150, and ethanol in healthy volunteers. Scientific Reports. 2021;11:9876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Block TS, Kushner H, Kalin N, Nelson C, Belanoff J, Schatzberg A. Combined Analysis of Mifepristone for Psychotic Depression: Plasma Levels Associated With Clinical Response. Biological Psychiatry. 2018;84:46–54. [DOI] [PubMed] [Google Scholar]
- 57.Scharner J, Aznarez I. Clinical Applications of Single-Stranded Oligonucleotides: Current Landscape of Approved and In-Development Therapeutics. Mol Ther. 2021;29:540–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martens M, Ammar A, Riutta A, Waagmeester A, Slenter DN, Hanspers K, et al. WikiPathways: connecting communities. Nucleic Acids Res. 2021;49:D613–D621. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


