Abstract
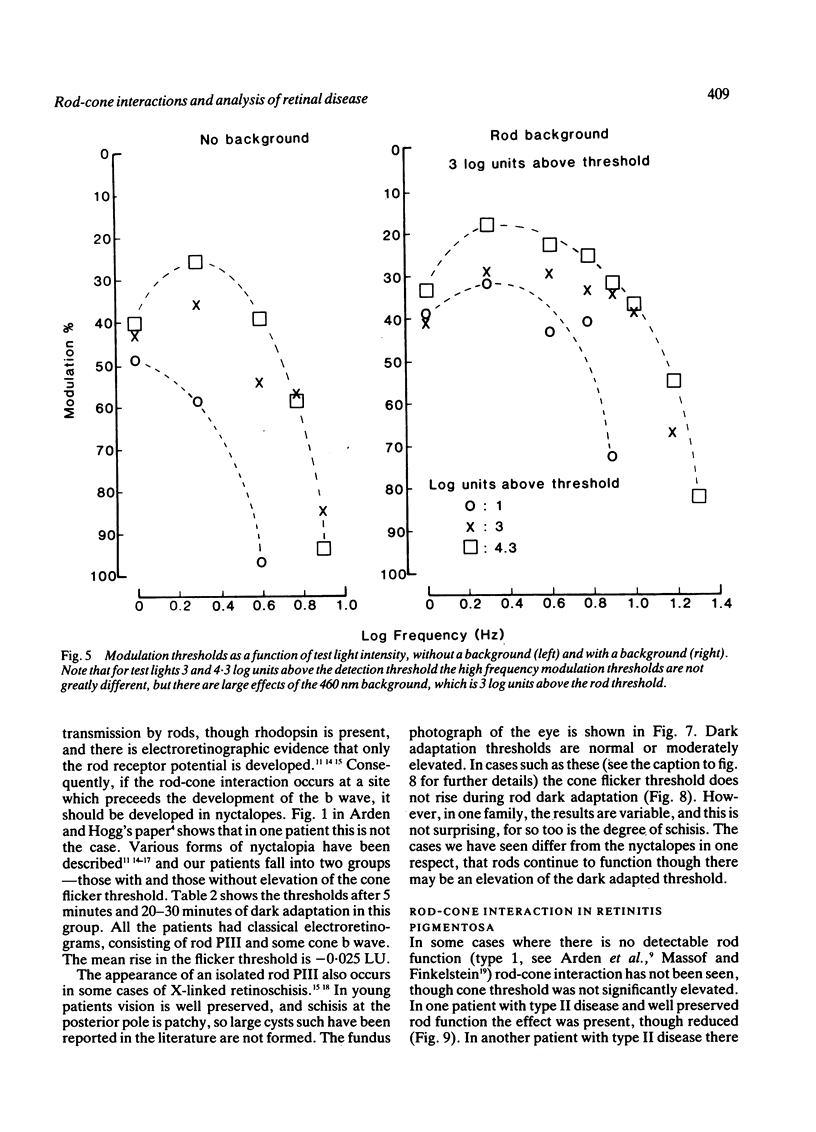
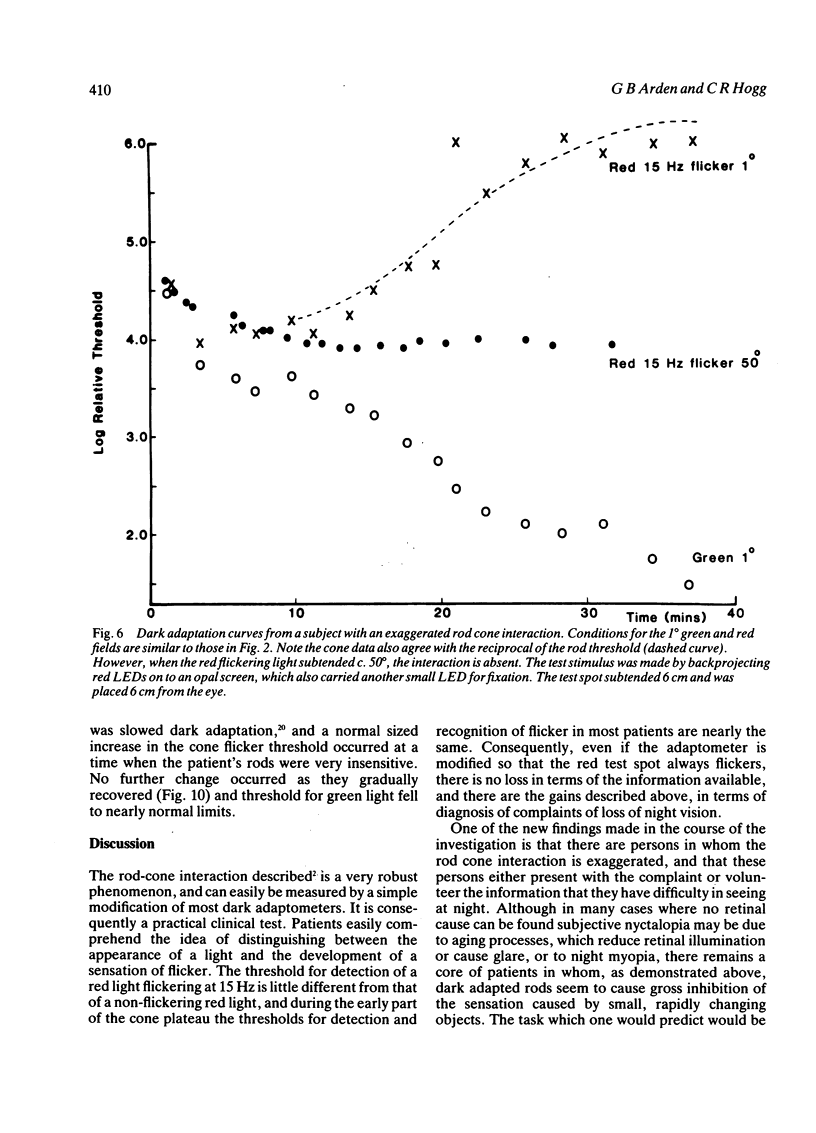
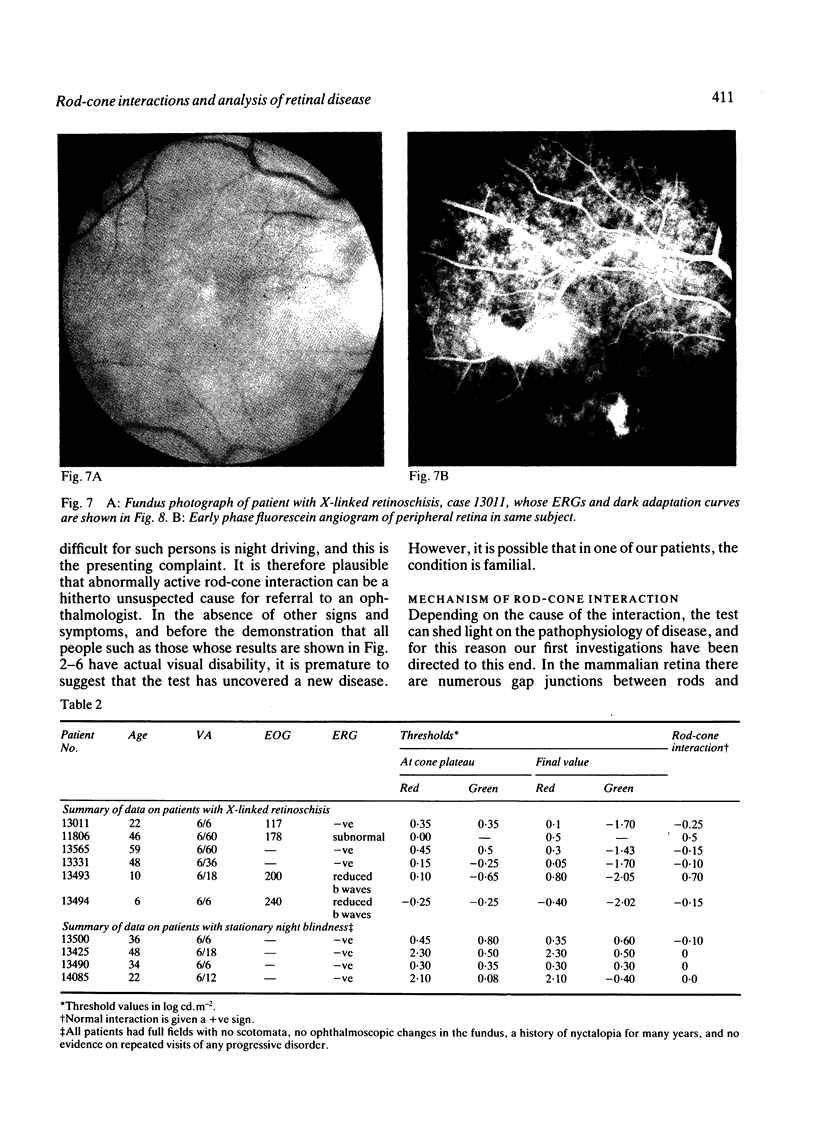
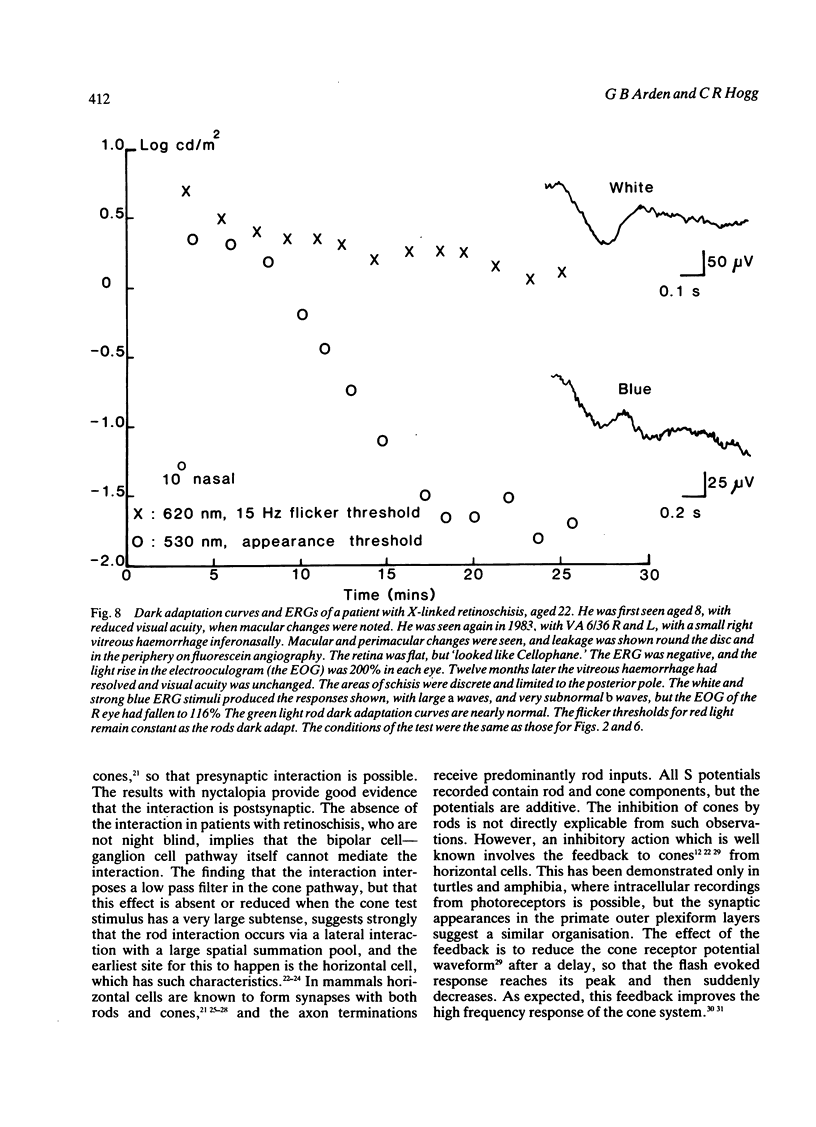
Cone flicker threshold rises as the rods dark adapt, though the cone threshold to continuous light remains constant. The rise is normally about 1 log unit, but in certain patients who complain of night blindness it may be as great as 2.5 log units. In these persons the kinetics of the rod-cone interaction are those of the recovery of rod sensitivity. The rods impose a low-pass filter on the cones. This effect is absent in congenital nyctalopia and X-linked retinoschisis. We suggest that cone flicker is maintained through a feedback system involving horizontal cells, and when the rod dark current returns in dark adaptation this feedback is altered. Rod cone interaction thus tests rod dark current, and cases of abnormal interaction in patients with retinitis pigmentosa occur, which indicate that the transduction mechanism and the membrane dark current may be differentially affected.
Full text
PDF
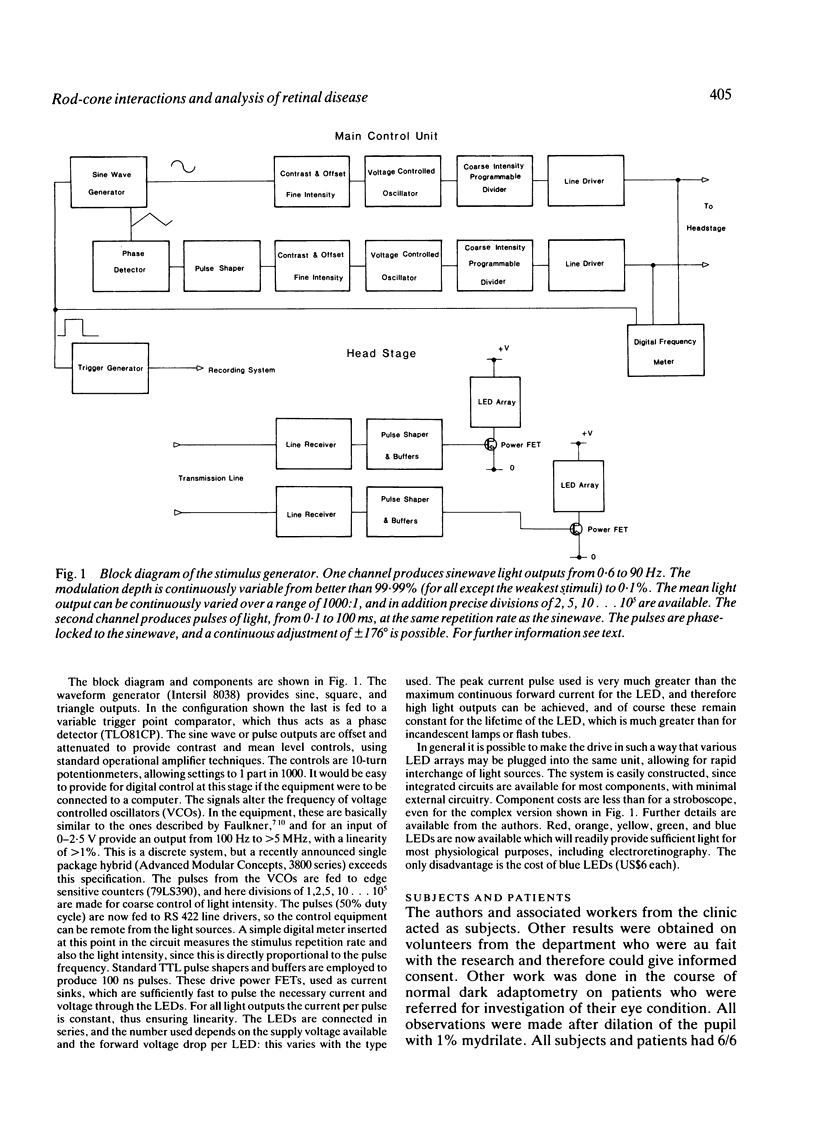
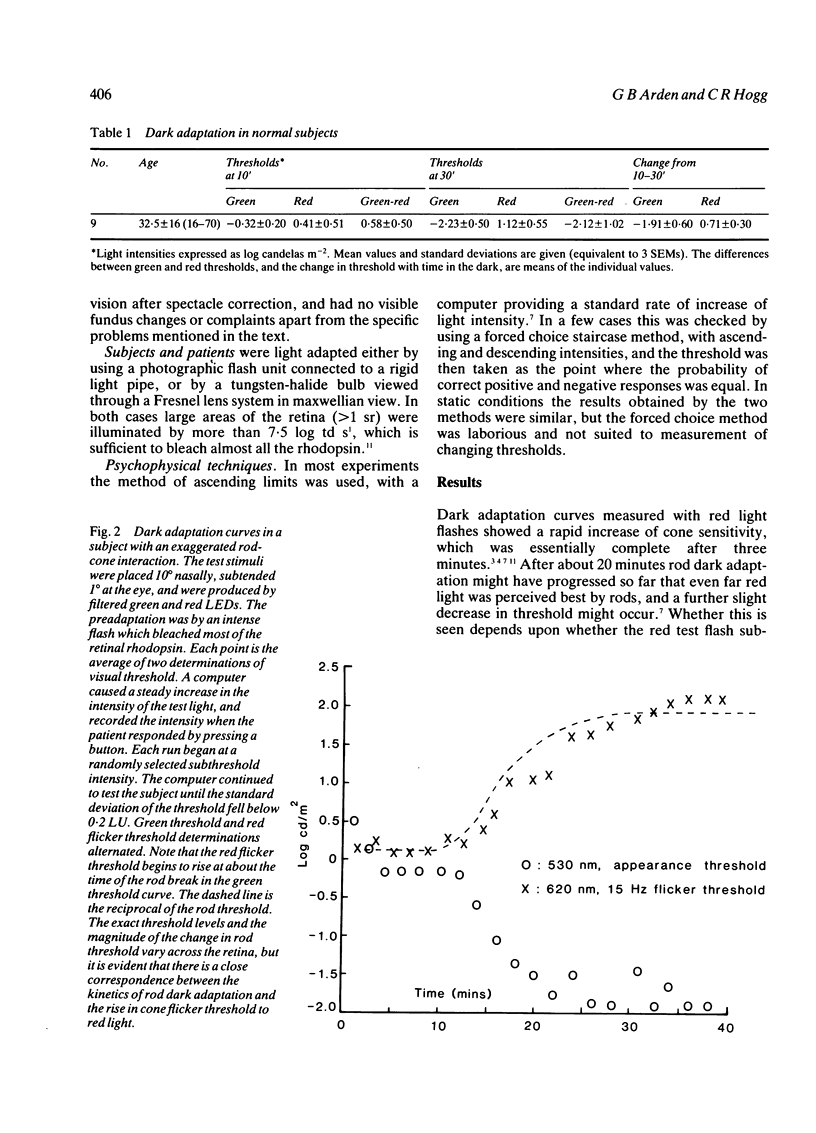
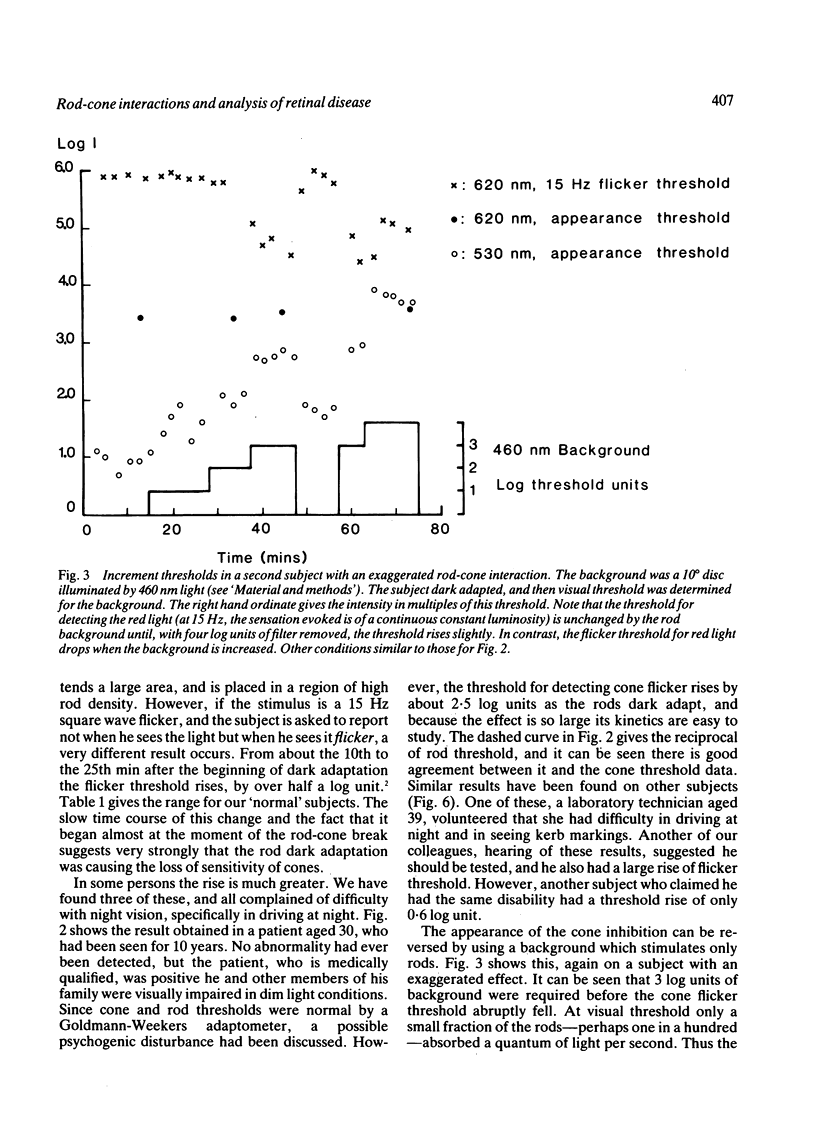
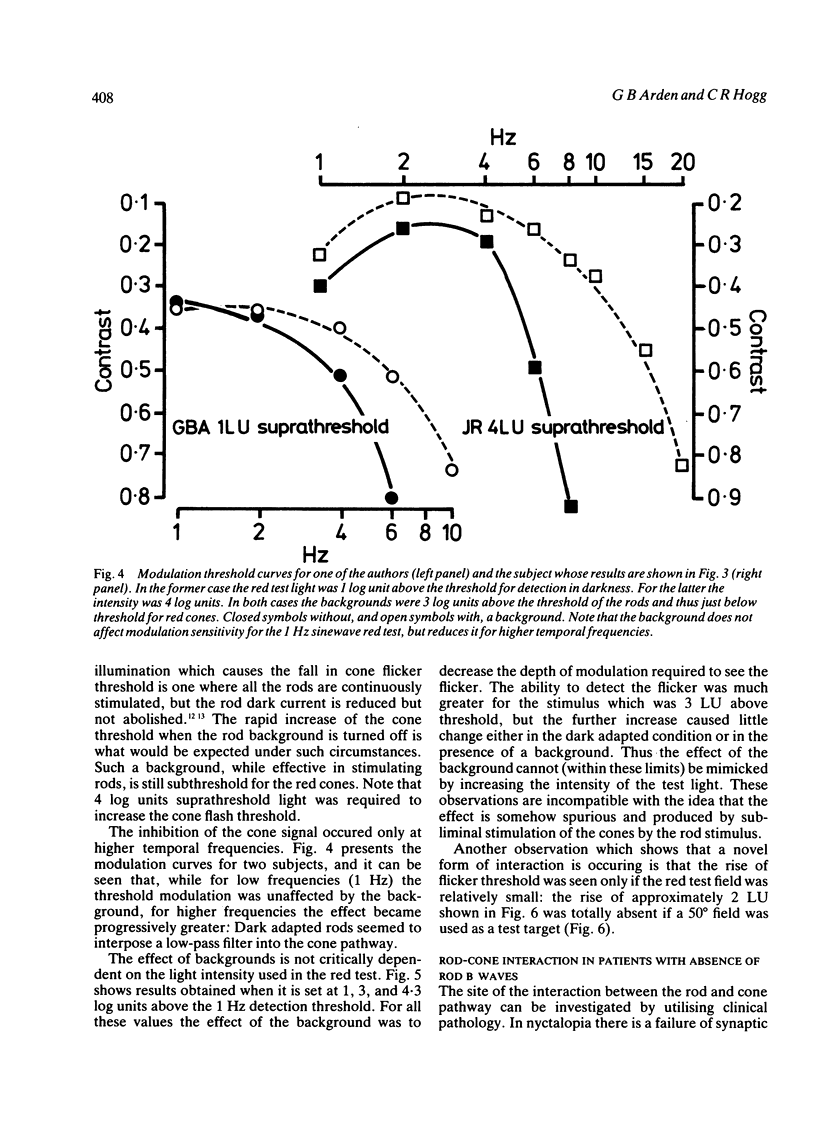


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander K. R., Fishman G. A. Rod-cone interaction in flicker perimetry. Br J Ophthalmol. 1984 May;68(5):303–309. doi: 10.1136/bjo.68.5.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arden G. B., Carter R. M., Hogg C. R., Powell D. J., Ernst W. J., Clover G. M., Lyness A. L., Quinlan M. P. Rod and cone activity in patients with dominantly inherited retinitis pigmentosa: comparisons between psychophysical and electroretinographic measurements. Br J Ophthalmol. 1983 Jul;67(7):405–418. doi: 10.1136/bjo.67.7.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G., O'Bryan P. M. Receptive fields of cones in the retina of the turtle. J Physiol. 1971 Apr;214(2):265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Detection and resolution of visual stimuli by turtle photoreceptors. J Physiol. 1973 Oct;234(1):163–198. doi: 10.1113/jphysiol.1973.sp010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch J. M. Quantitative layer-by-layer perimetry. Invest Ophthalmol Vis Sci. 1978 Mar;17(3):208–257. [PubMed] [Google Scholar]
- Ernst W., Faulkner D. J., Hogg C. R., Powell D. J., Arden G. B., Vaegan An automated statis perimeter/adaptometer using light emitting diodes. Br J Ophthalmol. 1983 Jul;67(7):431–442. doi: 10.1136/bjo.67.7.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst W., Kemp C. M., Lake N. Studies on the effects of bleaching amphibian rod pigments. IV. Photoresponses recorded intracellularly from axolotl red rods following bleaching flashes. Exp Eye Res. 1978 Jul;27(1):117–127. doi: 10.1016/0014-4835(78)90058-1. [DOI] [PubMed] [Google Scholar]
- Fuortes M. G., Schwartz E. A., Simon E. J. Colour-dependence of cone responses in the turtle retina. J Physiol. 1973 Oct;234(1):199–216. doi: 10.1113/jphysiol.1973.sp010341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg S. H., Frumkes T. E., Nygaard R. W. Inhibitory influence of unstimulated rods in the human retina: evidence provided by examining cone flicker. Science. 1983 Jul 8;221(4606):180–182. doi: 10.1126/science.6857279. [DOI] [PubMed] [Google Scholar]
- Kelsey J. H., Arden G. B. Acquired unilateral loss of dark adaptation. Br J Ophthalmol. 1971 Jan;55(1):38–43. doi: 10.1136/bjo.55.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D. Spatial properties of horizontal cell responses in the turtle retina. J Physiol. 1976 Dec;263(2):239–255. doi: 10.1113/jphysiol.1976.sp011630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasater E. M., Lam D. M. The identification and some functions of GABAergic neurons in the distal catfish retina. Vision Res. 1984;24(5):497–506. doi: 10.1016/0042-6989(84)90047-6. [DOI] [PubMed] [Google Scholar]
- Manschot W. A. Pathology of hereditary juvenile retinoschisis. Arch Ophthalmol. 1972 Aug;88(2):131–138. doi: 10.1001/archopht.1972.01000030133002. [DOI] [PubMed] [Google Scholar]
- Massof R. W., Finkelstein D. Rod sensitivity relative to cone sensitivity in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1979 Mar;18(3):263–272. [PubMed] [Google Scholar]
- Nelson R. Cat cones have rod input: a comparison of the response properties of cones and horizontal cell bodies in the retina of the cat. J Comp Neurol. 1977 Mar 1;172(1):109–135. doi: 10.1002/cne.901720106. [DOI] [PubMed] [Google Scholar]
- Niemeyer G., Gouras P. Rod and cone signals in S-potentials of the isolated perfused cat eye. Vision Res. 1973 Aug;13(8):1603–1612. doi: 10.1016/0042-6989(73)90017-5. [DOI] [PubMed] [Google Scholar]
- Nygaard R. W., Frumkes T. E. LEDs: convenient, inexpensive sources for visual experimentation. Vision Res. 1982;22(4):435–440. doi: 10.1016/0042-6989(82)90190-0. [DOI] [PubMed] [Google Scholar]
- Penn R. D., Hagins W. A. Kinetics of the photocurrent of retinal rods. Biophys J. 1972 Aug;12(8):1073–1094. doi: 10.1016/S0006-3495(72)86145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripps H. Night blindness revisited: from man to molecules. Proctor lecture. Invest Ophthalmol Vis Sci. 1982 Nov;23(5):588–609. [PubMed] [Google Scholar]
- Simon E. J. Two types of luminosity horizontal cells in the retina of the turtle. J Physiol. 1973 Apr;230(1):199–211. doi: 10.1113/jphysiol.1973.sp010183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg R. H. Rod and cone contributions to S-potentials from the cat retina. Vision Res. 1969 Nov;9(11):1319–1329. doi: 10.1016/0042-6989(69)90069-8. [DOI] [PubMed] [Google Scholar]
- Steinberg R. H. Rod-cone interaction in S-potentials from the cat retina. Vision Res. 1969 Nov;9(11):1331–1344. doi: 10.1016/0042-6989(69)90070-4. [DOI] [PubMed] [Google Scholar]
- Steinberg R. H. The rod after-effect in S-potentials from the cat retina. Vision Res. 1969 Nov;9(11):1345–1355. doi: 10.1016/0042-6989(69)90071-6. [DOI] [PubMed] [Google Scholar]
- Thaler A., Heilig P., Slezak H. Elektoretinogramm und Elektrookulogramm bei juveniler Retinoschisis. Klin Monbl Augenheilkd. 1973 Dec;163(6):699–703. [PubMed] [Google Scholar]
- Yanoff M., Kertesz Rahn E., Zimmerman L. E. Histopathology of juvenile retinoschisis. Arch Ophthalmol. 1968 Jan;79(1):49–53. doi: 10.1001/archopht.1968.03850040051014. [DOI] [PubMed] [Google Scholar]
- ZIMMERMAN L. E., SPENCER W. H. The pathologic anatomy of retinoschisis with a report of two cases diagnosed clinically as malignant melanoma. Arch Ophthalmol. 1960 Jan;63:10–19. doi: 10.1001/archopht.1960.00950020012002. [DOI] [PubMed] [Google Scholar]



