Abstract
Chicken domestication began at least 3,500 years ago for purposes of divination, cockfighting, and food. Prior to industrial scale chicken production, domestication selected larger birds with increased egg production. In the mid-20th century companies began intensive selection with the broiler (meat) industry focusing on improved feed conversion, rapid growth, and breast muscle yield. Here we present proteomic analysis comparing the modern broiler line, Ross 708, with the UIUC legacy line which is not selected for growth traits. Breast muscle proteome analysis identifies cellular processes that have responded to human directed artificial selection. Mass spectrometry was used to identify protein level differences in the breast muscle of 6-day old chicks from Modern and Legacy lines. Our results indicate elevated levels of stress proteins, ribosomal proteins and proteins that participate in the innate immune pathway in the Modern chickens. Furthermore, the comparative analyses indicated expression differences for proteins involved in multiple biochemical pathways. In particular, the Modern line had elevated levels of proteins affecting the pentose phosphate pathway, TCA cycle and fatty acid oxidation while proteins involved in the first phase of glycolysis were reduced compared to the Legacy line. These analyses provide hypotheses linking the morphometric changes driven by human directed selection to biochemical pathways. These results also have implications for the poultry industry, specifically Wooden Breast disease which is linked to rapid breast muscle growth.
Introduction
The chicken has played an important role in human culture and nutrition since its domestication [1,2]. The majority of the modern chicken’s genome was derived from the red junglefowl with documented introgression of alleles from the grey, Ceylon, and green junglefowl [3]. Early domestication likely led to larger birds that matured quicker and increased egg production in comparison with their wild progenitors [4,5]. The advent of modern agriculture in the early 20th century led to intensive genetic selection for either meat (broiler) or egg (layer) production traits in the chicken. Initial efforts selecting for increased broiler mass led to adipose tissue accumulation, probably because the selection did not channel the increased level of nutrients to a particular tissue [6]. Ultimately, improving broiler production traits led to selection for a combination of larger breast muscle mass, improved feed efficiency and rapid growth.
Comparative studies describing differences between selected and unselected (legacy) lines provide insight into the impact of artificial selection of species. Several studies have characterized the differences between modern broilers and legacy lines that have not been subjected to production level human directed selection [7–13]. In our work [14–16] we compared the modern Ross 708 broiler line and the legacy University of Illinois, Urbana Campus (UIUC) lines (Fig 1) [17,18]. In the legacy line the breast muscle comprises approximately 9% of the body mass, while in the modern broiler this tissue constitutes up to 22% of the body mass [14]. Evolving in the tropics, the red junglefowl had little need for long distance flying. The wild chicken is an episodic flier, only needing the ability to fly up into a tree to escape predators and to roost. Like the legacy line, the breast muscle of the red junglefowl constitutes approximately 9% of its body mass [19]. For comparison, the breast muscle of birds capable of more sustained flight averages 17% while that of hummingbirds varies between 25–30% [20].
Fig 1. Results of artificial selection for increased feed efficiency and breast muscle yield.
Comparison of 35-day old male UIUC legacy chicken (left) with a 35-day old male Ross 708 chicken (right). The UIUC bird is typical in size and shape for a chicken from the 1950s while the Ross 708 bird is from a modern commercial flock grown for human consumption.
The increase in the modern broiler’s breast muscle mass, which almost reaches the hummingbird level, came at the expense of other tissues. For example, the normalized masses of the heart, spleen and brain are larger in legacy lines compared with modern broilers [13,14,21]. In modern broilers, this likely is responsible for increased incidence of cardiomyopathy [22,23] and immune deficiencies [10]. The reduced brain mass in broilers could contribute to the behavioral differences seen in modern broilers compared with other lines. These include a reduction in movement, along with reduced fear and risk aversion in comparison with legacy or layer lines [24]. In addition, a myopathy called Wooden Breast disease has arisen during the selection process [25–27]. This myopathy is apparently due to selection for rapid growth and is characterized by necrosis, fibrosis, and immune cells infiltration that results in a product that is unappealing to consumers [28–31]. This disease results in significant losses to the poultry industry as the meat is condemned at processing [32].
Our previous research involved comparing the breast muscle transcriptome of 6-day old (D6) modern and legacy lines [16]. That comparison identified differently expressed genes affecting growth factors, lipid metabolism and the pentose phosphate pathway. In particular, the modern transcriptome indicated elevated expression of insulin like growth factor 1 (IGF1) which stimulates muscle hypertrophy combined with reduced expression of myostatin (MSTN), an inhibitor of skeletal muscle hyperplasia [33]. These studies suggest that human directed selection increased growth factor stimulation in the modern breast muscle. Furthermore, elevated expression of genes involved in the pentose phosphate pathway and lipid metabolism would provide necessary intermediates and energy required for rapid growth.
Here we report a comparative study between modern Ross 708 and legacy UIUC breast muscle proteomes. The Ross 708 modern line is a commercial breed that is produced for human consumption. The breeding process for this line is proprietary information owned by Aviagen. As a result, a detailed description of the breeding process and the specific genetic background of Ross 708 is not publicly available. The UIUC legacy line birds were obtained from the University of Illinois, Urbana Campus. They are the progeny of a cross between males from the inbred New Hampshire and females from the inbred Columbian lines [14]. The results support and extend the conclusions of our prior studies, providing further insight into the impact of selection that yielded modern broilers [14,16]. The data also have implications for the negative effects of intense selection on the broiler chicken along with the emergence of Wooden Breast disease [25,28,29,34,35].
Materials and methods
Animal care and sample collection
Animal raising, handling and sample collection methods were approved by the University of Delaware Institutional Animal Care and Use Committee (Permit Number: 2703-12-10). Six male UIUC and six male Ross 708 breast muscle samples were collected at day 6 (D6) post-hatch, frozen in liquid nitrogen and stored at -80˚C until processed for proteome analysis. All samples were taken from the posterior region of the left pectoralis major muscle [16]. We chose D6 because this is the earliest day we detect difference in the normalized breast muscle mass between the two lines [14].
Proteomic analysis
For each of the muscle samples, six technical replicates were analyzed by mass spectrometry. In each case 50 mg of each muscle sample was subjected to differential detergent fractionation and 20 μg of each fraction was trypsin digested as previously described [36,37]. Following digestion, each fraction was desalted using a peptide macrotrap (Michrom BioResources) according to the manufacturer’s instructions. After desalting, each fraction was further purified using a strong cation exchange macrotrap to remove any residual detergent, which could interfere with the mass spectrometry. Fractions were dried and resuspended in 10 μl of 2% acetonitrile, 0.1% formic acid and transferred to low retention vials in preparation for separation using reverse phase liquid chromatography.
An Ultimate 3000 (Dionex) high performance liquid chromatography system coupled with an LTQ Velos Pro (Thermo) mass spectrometer were used for peptide separation and mass spectrum acquisition. The U3000 was operated at a flow rate of 333 nl per minute and equipped with a 75 μm x 10 cm fused silica column packed with Halo C18 reverse phase material (Mac-Mod Analytical). Each peptide sample was separated using a 4 h gradient from 2% to 50% acetonitrile with 0.1% formic acid as a proton source. The column was located on a Nanospray Flex Ion Source (Thermo) and connected directly to a silica Nanospray emitter to minimize peak broadening. High voltage was applied using a stainless-steel junction between the column and the emitter. Scan parameters for the LTQ Velos Pro were one MS scan followed by 10 MS/MS scans of the 5 most intense peaks. MS/MS scans were performed in pairs, one using collision induced dissociation (CID) and the other using higher-energy collisional dissociation (HCD). Dynamic exclusion was enabled with a mass exclusion time of 3 min and a repeat count of 1 within 30 sec of initial m/z measurement.
Protein identification
Spectrum matching programs X!tandem [38] and OMSSA [39] were used via the University of Arizona High Throughput Computing Center. Raw spectra were converted to MGF format for analysis using the MSConvert tool from the ProteoWizard software suite [40]. X!tandem was run with 12 threads, precursor and fragment tolerance of 0.2 Da, and up to two missed tryptic cleavages. Variable modifications used in the searches were: single and double oxidation of Methionine, carbamidomethylation of Cysteine, and phosphorylation of Tyrosine, Threonine, and Serine. OMSSA was run with 12 threads, precursor and fragment tolerance of 0.2 Da, up to two missed tryptic cleavages, and set to XML output format. A custom Perl script was used to parse XML search results from both X!tandem and OMSSA. Peptides with e-values ≤ 0.05 were accepted and single spectrum identifications were rejected unless they were identified by both search engines. To verify data set quality, decoy searches were performed in the exact manner as before, but with a randomized version of the protein databases. False discovery rates ranged from 0.9–2.3% with an average of 1.7%.
Identifying differentially expressed proteins
Differential expression of proteins between lines was performed pairwise using peptide elution profiles as described previously [41]. Precursor mass spectra were extracted from the raw data in MS1 format using the MSConvert software from the ProteoWizard toolset. Peptide precursor m/z values were extracted from the previously compiled protein identifications using Perl. Peptide intensities were summed for each protein on a per-replicate basis. Data were normalized based on the mode of each replicate rather than the mean to minimize the effect of extreme values. A resampling analysis was performed for each pairwise comparison. Proteins were considered to be differentially expressed if the difference in means between conditions resulted in a P-value ≤ 0.05.
Functional analysis
Gene Ontology (GO) [42] enrichment analysis was done using the KOBAS [43] and gProfiler [44] enrichment tools with the hypergeometric test and the Benjamini and Hochberg FDR correction method. Enriched GO terms with a corrected P-value < 0.05 were used in downstream analyses. Network diagrams were generated using Cytoscape [44,45].
Data deposition
Transcript data discussed in this publication have been deposited in NCBI’s Gene Expression Omnibus (GEO) and are accessible through GEO Series accession number GSE65217. Proteomics data is available from ProteomeXchange (PXD005288).
Results
Proteomic results
In the comparison between D6 modern Ross 708 and legacy UIUC breast muscle, a total of 222 differentially expressed proteins were detected (P-value <0.05), with 173 enriched in modern samples and 49 enriched in legacy samples. Among the 222 differentially expressed proteins, 130 (57%) exhibited the same direction of enrichment as shown in an earlier transcriptome study [16] (S1 Table).
Gene Ontology [GO) [42]
Muscle from legacy birds were enriched for GO terms relevant to myofibers and energy production in striated muscle, along with vesicle transport (Fig 2). Muscle from modern birds were enriched for terms including stress response, myofibers, translation, energy production, metabolism, vesicle transport and innate immunity.
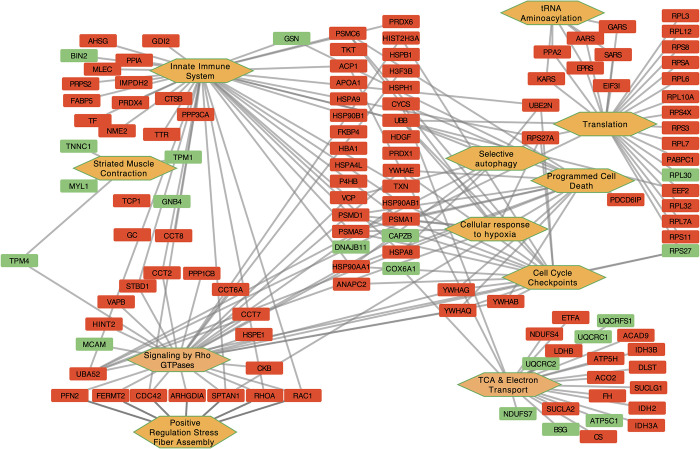
Fig 2. Gene ontology results.
Orange hexagons refer to GO terms and rectangles refer to proteins differentially enriched between modern (Red) and legacy (Green) chicken muscle.
Stress response
In the comparison between modern and legacy muscle, 49 differentially regulated proteins were part of the stress response. Five stress response proteins were elevated in the legacy breast muscle including cytochrome c oxidase subunit 6A1 (COX6A1), capping actin protein of muscle Z-line subunit beta (CAPZB), ribosomal protein S27 (RPS27), G protein subunit beta 4 (GNB4) and DnaJ heat shock protein family (Hsp40) member B11 (DNAJB11). 44 stress-associated proteins were elevated in the modern chicken muscle compared to the legacy samples. These function in a variety of stress related processes including acting as chaperones and cochaperones, responding to genotoxic stress, redox regulation, and protein degradation. Among these were four proteasome subunits that likely offset the increased burden of misfolded proteins due to the large amount of protein synthesis required for muscle hypertrophy. Nine heat shock proteins (HSPs) were also elevated in muscle from the modern line, all of which function as chaperones controlling the proper folding of client proteins. Five members of the TCP complex that acts as an ATP dependent chaperone controlling actin folding were enriched in the modern samples [5,6]. Four isomerases responsible for controlling disulfide or proline conformation and three proteins that control redox stress were also elevated in the modern line.
Myofibers
Gene expression in legacy muscle showed enrichment for several myofibril proteins including gelsolin (GSN), troponin C (TNCC), tropomyosins (TMP1 and TMP4), myosin light chain 1 (MYL1), myomesin 2 (MYOM2), cytoskeletal keratins (KRT5 and KRT7), and FMR1 autosomal homolog 1 (FXR1). CAPZB, GSN, TNCC, TPM1 and TPM4 regulate the dynamics of actin filament assembly. MYL1 is a non-regulatory light chain that interacts with actin in generating contraction. MYOM2 is a structural component that stabilizes the M band of muscle and KRT5 and KRT7 are intermediate filaments components. FXR1 controls mRNA transport and translation [46]. Knockdown mutations of FXR1 in mice reduce limb musculature and result in early mortality [47] and recessive mutations in humans results in multi-minicore myopathy [48]. The transcript encoding bridging integrator 1 (BIN1) is enriched in the modern line. BIN1 protein localizes to T-tubules [49] where it controls Ca2+ signaling [50]. Mutations in BIN1 cause centronuclear myopathy, which causes muscle weakness and atrophy [51–53].
Translation
The most striking contrast between modern broilers such as Ross 708 chickens and earlier breeds is the difference in both normalized and total breast muscle yield. Some of the increase in normalized breast muscle of modern broilers can be attributed to hypertrophy due to increased protein synthesis [54,55]. This is consistent with the enrichment in the Ross 708 birds for protein translation initiation factors and ribosomal structural proteins (S1 Table and Fig 2). In addition to ribosomal proteins, five enzymes encoding tRNA ligases were enriched in Ross 708 breast muscle including: lysyl-tRNA synthetase 1 (KARS1), seryl-tRNA synthetase (SARS), glycyl-tRNA synthetase 1 (GARS), alanyl-tRNA synthetase (AARS) and glutamyl-prolyl-tRNA synthetase 1 (EPRS). These would support the elevated translation required for the muscle hypertrophy seen in the modern line.
Glycolysis
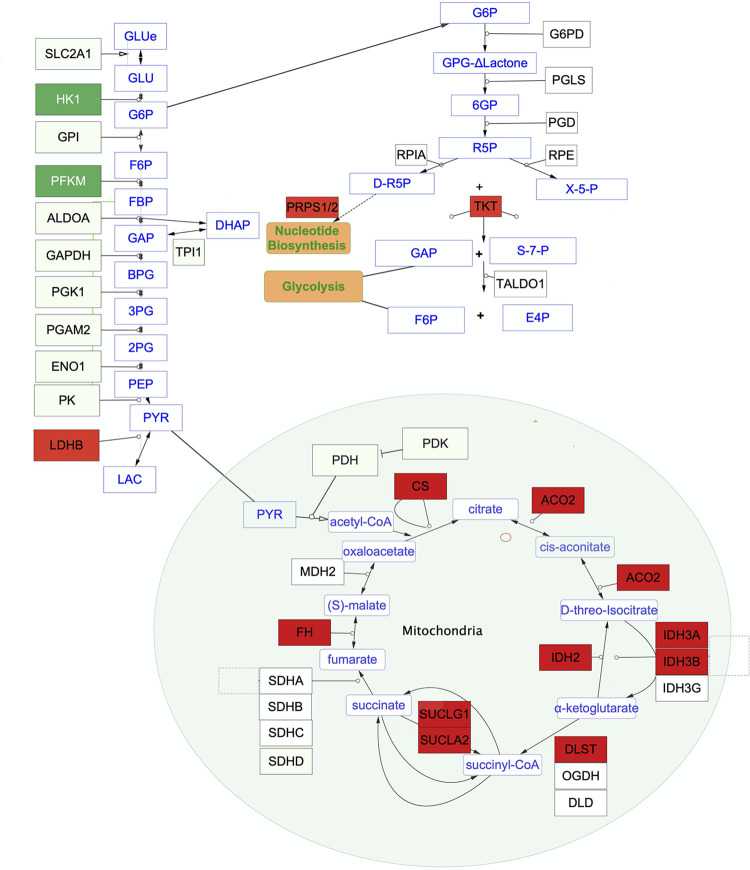
Muscle from the UIUC legacy line is enriched for two proteins involved in glycolysis: hexose kinase 1 (HK) and muscle phosphofructokinase (PFKM) (Fig 3). HK1 catalyzes the first reaction of glycolysis by phosphorylating glucose while PFKM drives the commitment step to glycolysis. Elevated levels of these glycolytic enzymes are consistent with the fast twitch nature of breast muscle from wild chickens, which is driven by glycolysis.
Fig 3. Glycolysis, pentose phosphate pathway and TCA cycle.
Red rectangles indicate enzymes enriched in the modern line while dark green indicates the enzymes enriched in the legacy line.
Pentose Phosphate Pathway (PPP)
The modern line breast muscle contained higher levels of the PPP enzymes transketolase (TKT) and phosphoribosyl pyrophosphate synthetase (PRPS1 and PRPS2). These enzymes function in the nonoxidative phase of PPP returning fructose 6 phosphate and glyceraldehyde 3 phosphate to glycolysis and providing ribose 5 phosphate as a precursor for purine and pyrimidine synthesis (Fig 3).
TCA cycle
The main pathway affecting energy production enriched in the modern line breast muscle was the TCA cycle (Fig 3). Most enzymes directly involved in the TCA cycle were elevated in the modern line muscle, including citrate synthase (CS), aconitase 2 (ACO2), isocitrate dehydrogenases (IDH, IDH3A, IDH3B), dihydrolipoamide S-succinyltransferase (DLST, a component of α-Ketoglutarate dehydrogenase), succinyl-CoA ligases (SUCLA2 and SUCLG1) and fumarate hydratase (FH). The components not elevated were succinate dehydrogenase complex flavoprotein subunit A (SDHA) and malate dehydrogenase (MDH). The enrichment for components of the TCA cycle indicates the central role of this pathway in the energy metabolism of the modern Ross 708 chickens compared with the legacy UIUC birds. In addition to providing coenzymes for oxidative phosphorylation, the TCA cycle provides intermediates for cataplerosis to be used as precursors for nucleotides, amino acids, and lipids. Anaplerosis could be supported by elevated levels of glutamic-oxaloacetic transaminase 2 (GOT2) that converts glutamate to α-ketoglutarate thus allowing for replenishing TCA cycle metabolites.
Beta-oxidation
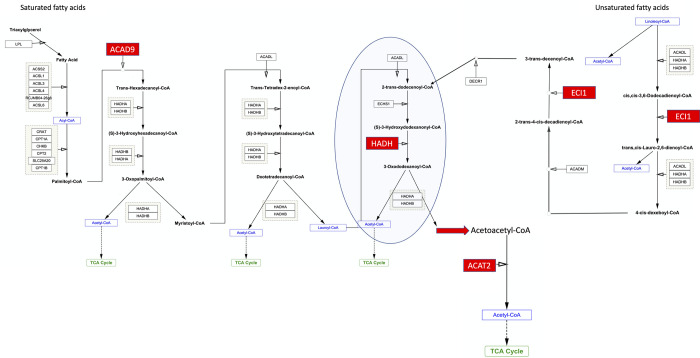
Enzymes involved in fatty acid beta oxidation are enriched in the modern birds compared with the legacy line (Fig 4). One enriched enzyme is acyl-CoA dehydrogenase family member 9 (ACAD9), the rate limiting enzyme in the oxidation of fatty acyl CoA. ACAD9 is responsible for introducing trans double bonds into palmitoyl-CoA and initiating the beta-oxidation of this common lipid. Also enriched is enoyl-CoA delta isomerase 1 (ECI1) which is necessary for beta oxidation of unsaturated fatty acids. The transcript encoding hydroxyacyl-CoA dehydrogenase (HADH) is also elevated in the modern Ross 708 muscle. This enzyme acts repeatedly on lipids, sequentially removing two carbon units by oxidizing a 12-carbon fatty acid to acetoacetyl-CoA. Acetyl-CoA acyltransferase 2 (ACAT2), also enriched in Ross 708 breast muscle, oxidizes acetoacetyl-CoA to acetyl-CoA. The product of beta oxidation, acetyl-CoA, can then be metabolized via the TCA cycle to generate energy, or used in anabolic reactions to support rapid breast muscle growth.
Fig 4. Beta-oxidation of Saturated Fatty Acids (SFA) and Unsaturated Fatty acids (USFA).
The step indicated by the oval is repeated, sequentially removing acetyl-CoA, until the 12 carbon Lauroyl-CoA is oxidized to the 4 carbon acetoacetyl-CoA which is oxidized to Acetyl-CoA by ACAT2. Red rectangles indicate enzymes enriched in the modern line.
Amino acid metabolism
In addition to TCA enzymes affecting amino acid anaplerosis, modern skeletal muscle was enriched for enzymes affecting glycine, lysine, and methionine metabolism. Enzymes affecting glycine impact choline and creatine levels include aldehyde dehydrogenase 7 family member A1 (ALDH7A1), betaine—homocysteine S-methyltransferase (BHMT), dimethylglycine dehydrogenase (DMGDH) and glycine amidinotransferase (GATM). ALDH7A1, BHMT and DMGDH form a pathway in the conversion of choline to sarcosine that is found in high concentrations in skeletal muscle. Choline is a precursor to phosphatidylcholine, an important component of cellular membranes. GATM is part of the pathway that mediates the interconversion between creatine and glycine. Creatine is also found at high concentration in muscle as it is important for energy storage as creatine phosphate. Further supporting creatine phosphorylation is creatine kinase B (CKB), which is elevated in modern breast muscle compared with the legacy line tissue. Three enzymes enriched in the modern line are involved in S-adenosylmethionine (SAM) production from methionine. These include methionine adenosyltransferase (MAT1A), aldehyde dehydrogenase 7 family member A1 (ALDH7A1), betaine—homocysteine S-methyltransferase (BHMT), and adenosylhomocysteinase (AHCY). SAM functions as a universal methyl donor in biological systems.
Electron transport chain
Enzymes associated with the mitochondrial respirasome showed enrichment in the legacy line breast muscle. These enzymes include NADH:ubiquinone oxidoreductase core subunit S7 (NDUFS7), cytochrome c reductases (UQCRFS1, UQCRC1, UQCRC2), cytochrome c oxidase subunit 6A1 (COX6A1) and ATP synthase F1 subunit gamma (ATP5C1), a component of the ATP synthase central stalk. Four mitochondrial components were found enriched in modern Ross 708 muscle: one subunit of the electron-transfer-flavoprotein (ETFA) along with inorganic pyrophosphatase 2 (PPA2). One NADH dehydrogenase (NDUFS4) and one component of the ATPase stalk, ATP synthase peripheral stalk subunit d (ATP5H) were also elevated in the modern muscle.
Vesicle and protein transport
The legacy line breast muscle was enriched for proteins affecting vesicle transport including: COPI coat complex subunit beta 2 (COPB2), transmembrane p24 trafficking protein 2 (TMED2), and amphiphysin (AMPH). COPB2 functions in retrograde transport from the Golgi to the ER and is involved in recognition of specific vesicle cargo proteins [56]. The TMED proteins function in anterograde transport of vesicles from the ER to the Golgi apparatus and play roles in cargo selection [9]. AMPH is enriched in neural tissue where it is involved in endocytosis through interaction with clathrin [57,58]. Some instances of the paraneoplastic Stiff-person Syndrome (SPS) [59,60] are due to AMPH autoantibodies produced in breast and lung cancer patients. SPS is characterized by muscle spasms, rigidity and hypertrophy that arise from the effect of the autoantibody on nerve cells that control muscles [61]. Modern Ross 708 breast muscle was enriched for vesicle regulatory components including: cell division cycle 42 (CDC42), GDP dissociation inhibitor 2 (GDI2), Rac family small GTPase 1 (RAC1) and ras homolog family member A (RHOA).
Innate immunity
Gene Ontology analysis identified 39 proteins that function in innate immunity. Two, gelsolin (GSN) and bridging integrator 2 (BIN2) were enriched in the legacy line breast muscle. Elevated expression of GSN is associated with a decreased response to a variety of inflammatory stimuli [62–67] and elevated BIN2 levels are associated with decreased phagocytic activity [68]. The 26 proteins enriched in the modern Ross 708 breast muscle are associated with neutrophil degranulation. For example, cathepsin B CTSB is secreted during neutrophil degranulation and degrades collagen upon release into the extracellular space [69,70]. Peroxiredoxin 6 PRDX6 and RAC1 associate with and activate NADPH oxidase, a major source of reactive oxygen species [71–73]. RAC1 [74], RHOA [75] and WD repeat domain 1 (WDR1) [76] participate in the polarization that drives neutrophil migration. Birds do not have cells named neutrophils, but neutrophil function is carried out by heterophils in avian species [77]. It is reasonable to hypothesize that the innate immunity proteins are present in heterophils within the breast muscle.
Discussion
The differences between modern broilers (Ross 708) and legacy lines (UIUC), arose from human directed selection leading to broiler’s rapid growth, improved feed efficiency and increased breast muscle mass. Rapid growth is evidenced in the time from hatch to market. Typically, for the legacy line it takes 16 weeks for birds to reach market weight, while birds from the modern line take seven weeks (see Fig 1 for growth comparison). The improved feed efficiency arose in part from selection lengthening the absorptive segments of the small intestine combined with earlier maturation of the liver [14]. Selection for increased breast muscle has generated modern birds with more than twice the breast muscle mass of legacy lines [14]. The proteomic results presented here indicate that selection caused metabolic reprogramming that supports the excessive breast muscle growth seen in the modern lines.
In chickens, skeletal muscle hyperplasia is thought to occur prior to hatch, and the increased size of the post-hatch muscle is largely due to hypertrophy [78]. Hypertrophy is driven by controlling the balance between protein synthesis and degradation [54,55]. The enrichment of 12 ribosomal subunit proteins, eukaryotic translation initiation factors EIF3I and EIF4A4, eukaryotic translation elongation factor 2EEF2, along with five tRNA ligases likely play an important role in the increased level of muscle hypertrophy seen in modern lines (Fig 2). Elevated protein synthesis could also cause the increase in stress response evidenced in the modern birds. The stress proteins function in a variety of processes including serving as chaperones or cochaperones, or in modulating genotoxic stress, redox regulation, and protein degradation (Fig 2).
The legacy line birds exhibited enrichment for HK1 and PFKM enzymes, which drive the first phase of glycolysis (Fig 3). PFKM catalyzes the rate limiting step of glycolysis, and its enrichment would direct Glucose 6-Phosphate down the glycolytic pathway. This is consistent with the typical fast-twitch breast muscle fibers that are seen in birds that exhibit brief episodes of flight. In contrast, modern birds are enriched in one protein (TKT) that supports the nonoxidative part of the pentose phosphate pathway (PPP). The non-oxidative portion of the PPP provides precursors for nucleotide synthesis and feeds glucose metabolites back into glycolysis. Modern Ross 708 birds also express higher levels of phosphoribosyl pyrophosphate synthetases, which direct Ribose-5-Phosphate to nucleotide production. The elevated level of LDHB in the Ross 708 birds is expected to drive the lactate:pyruvate equilibrium towards pyruvate. This would retain pyruvate for further metabolism by the TCA cycle and limit the release of lactate for energy production by other tissues [79]. Furthermore, glucose consumption supporting the modern broiler’s breast muscle has ramifications for brain development. As glucose is the main energy source for the brain, diversion of this nutrient to the breast muscle may cause the reduced brain growth seen in modern broilers compared with legacy chicken lines or the red junglefowl [13].
The elevated levels of multiple enzymes of the TCA cycle in the Ross 708 breast muscle allows this pathway to meet demands of breast muscle hypertrophy (Fig 3). This is supported by studies in other species implicating elevated TCA cycle activity in hypertrophy. For example, enrichment of TCA metabolites was noted in a Klf10 mouse knockout model of soleus muscle hypertrophy [80] with similar results seen in aerobic exercise induced hypertrophy in humans [81,82]. Furthermore, resistance training induced hypertrophy in humans increases the activity of citrate synthase, the gateway enzyme to the TCA cycle [83].
Several enzymes involved in lipid beta-oxidation are also elevated in birds from the modern line (Fig 4]. Elevated expression of ACAD9 is particularly informative as this is the rate-limiting enzyme controlling lipid oxidation and elevated HADH activity also plays a role in skeletal muscle hypertrophy [83]. Increased ACAD9, HADH, and other enzymes involved in lipid oxidation indicates that modern Ross 708 birds are using lipid metabolism, in addition to the pentose phosphate and TCA pathways, to provide resources supporting expansion of the breast muscle.
Elevated lipid beta-oxidation in the breast muscle may have ramifications for morphometric changes seen in the growth of modern broilers. For example, the reduced normalized heart mass in the modern Ross 708 line compared with legacy UIUC birds could cause the cardiomyopathy seen in modern broilers. Also, if normalized spleen mass is viewed as a proxy for immune functions, the morphometric data indicates that immune function is significantly lower in modern lines compared with birds from the legacy line. Metabolically this may arise from the elevated lipid use in modern Ross 708 skeletal muscle. Cardiac muscle and the immune system use lipids as a major source of energy. Consequently, increased competition with skeletal muscle for lipids might inhibit heart growth and immune function seen in modern broilers. In addition to glucose the brain also readily uses ketone bodies, such as acetoacetate, to function. The elevated levels of ACAT2 in modern breast muscle may reduce the availability of ketone bodies for use by the brain.
These data support prior studies comparing the transcriptomes of 6-day old modern Ross 708 and legacy UIUC birds which concluded that the legacy line breast muscle was enriched for transcripts associated with glycolysis, while the transcriptome of the modern birds favored beta-oxidation [16]. Additionally, TKT transcripts were also elevated in a study of birds with high feed efficiency compared with low feed efficiency chickens [26]. Elevated levels of this enzyme seen in this proteome analysis provide further support for increased expression of these proteins improving feed efficiency.
Although these samples were obtained at day 6 post-hatch, there are already differences between the modern and legacy lines that have implications for the development of Wooden Breast Disease [28]. The elevated levels of stress proteins seen in breast muscle from the modern line provide compelling evidence that this tissue is undergoing a variety of stresses, likely due to its rapid growth. Oxidative stress is thought to be a major contributor to the development of this disease [28,84,85] and five of the stress responsive proteins detected in this study, PIT54 protein (PIT54) [86,87], peroxiredoxins (PRDX1, PRDX4, PRDX6) and thioredoxin (TXN) play important roles in regulating oxidative stress. Gene Ontology analysis of our data also detected proteins associated with neutrophils in the mammalian immune system. Neutrophils are one of the earliest responders to inflammation and in birds the role of these phagocytic cells is filled by heterophils [77]. Histological examination of birds prone to Wooden Breast Myopathy revealed heterophilic infiltration in the pectoral muscle of D14 chickens and this is thought to be an early sign of disease development [25]. Furthermore, lipid metabolism has been shown to be altered in rapidly growing broilers that develop Wooden Breast Disease [88]. Taken together, the proteomic data suggests that Wooden Breast Disease starts to develop well before the disease is visually or palpably evident.
Conclusions
In support of our earlier transcriptome studies [16], these data indicate specific responses to artificial selection for improved feed efficiency and breast muscle yield in the modern broiler. The breast muscles of Ross 708 birds exhibit increased ribosomal protein synthesis, along with changes in the glycolytic, pentose phosphate, TCA, and beta-oxidation pathways when compared with the UIUC chickens. A reasonable hypothesis is that these adaptations are the direct result of artificial selection as they would support the improved feed efficiency and rapid breast muscle growth seen in the Ross 708 chickens. The selection possibly drove some of the negative outcomes seen in modern broilers. The diversion of nutrients to support the excessive breast muscle growth could cause the reduced growth of the brain and heart along with reduced immune function. In addition, the increased levels of proteins controlling oxidative damage and heterophil function suggest that the Wooden Breast myopathy seen at market age may begin very early post-hatch. Finally, the identity of these differentially regulated proteins generates testable hypotheses regarding the regulatory mechanisms that orchestrate the changes that have been introduced to the broiler chicken by human selection.
Supporting information
Included are proteins and gene accessions from NCBI and expression data supported by transcriptome expression [16] is indicated (RNASeq Concordance).
(DOCX)
Data Availability
Transcript data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus (GEO) with accession number GSE65217 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE65217). Proteomics data is available from ProteomeXchange with accession number PXD005288 (https://proteomecentral.proteomexchange.org/cgi/GetDataset?ID=PXD005288).
Funding Statement
CJS was supported by the Agriculture and Food Research Institute competitive grant (2011-67003-30228) from the United States Department of Agriculture National Institute of Food and Agriculture The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Peters J, Lebrasseur O, Irving-Pease EK, Paxinos PD, Best J, Smallman R, et al. The biocultural origins and dispersal of domestic chickens. Proc Natl Acad Sci U S A. 2022;119(24):e2121978119. doi: 10.1073/pnas.2121978119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wood-Gush DGM. A History of the Domestic Chicken from Antiquity to the 19th Century. Poultry Science. 1959;38(2):321–6. [Google Scholar]
- 3.Lawal RA, Martin SH, Vanmechelen K, Vereijken A, Silva P, Al-Atiyat RM, et al. The wild species genome ancestry of domestic chickens. BMC Biol. 2020;18(1):13. doi: 10.1186/s12915-020-0738-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atikuzzaman M, Sanz L, Pla D, Alvarez-Rodriguez M, Ruber M, Wright D, et al. Selection for higher fertility reflects in the seminal fluid proteome of modern domestic chicken. Comp Biochem Phys D. 2017;21:27–40. doi: 10.1016/j.cbd.2016.10.006 [DOI] [PubMed] [Google Scholar]
- 5.Schutz K, Kerje S, Carlborg O, Jacobsson L, Andersson L, Jensen P. QTL analysis of a red junglefowl x white leghorn intercross reveals trade-off in resource allocation between behavior and production traits. Behav Genet. 2002;32(6):423–33. doi: 10.1023/a:1020880211144 [DOI] [PubMed] [Google Scholar]
- 6.Zerjal T, Härtle S, Gourichon D, Guillory V, Bruneau N, Laloë D, et al. Assessment of trade-offs between feed efficiency, growth-related traits, and immune activity in experimental lines of layer chickens. Genetics Selection Evolution. 2021;53(1). doi: 10.1186/s12711-021-00636-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Havenstein GB, Ferket PR, Scheideler SE, Larson BT. Growth, livability, and feed conversion of 1957 vs 1991 broilers when fed "typical" 1957 and 1991 broiler diets. Poult Sci. 1994;73(12):1785–94. doi: 10.3382/ps.0731785 [DOI] [PubMed] [Google Scholar]
- 8.Havenstein GB, Ferket PR, Scheideler SE, Rives DV. Carcass composition and yield of 1991 vs 1957 broilers when fed "typical" 1957 and 1991 broiler diets. Poult Sci. 1994;73(12):1795–804. doi: 10.3382/ps.0731795 [DOI] [PubMed] [Google Scholar]
- 9.Qureshi MA, Havenstein GB. A comparison of the immune performance of a 1991 commercial broiler with a 1957 randombred strain when fed "typical" 1957 and 1991 broiler diets. Poult Sci. 1994;73(12):1805–12. doi: 10.3382/ps.0731805 [DOI] [PubMed] [Google Scholar]
- 10.Cheema MA, Qureshi MA, Havenstein GB. A comparison of the immune response of a 2001 commercial broiler with a 1957 randombred broiler strain when fed representative 1957 and 2001 broiler diets. Poult Sci. 2003;82(10):1519–29. doi: 10.1093/ps/82.10.1519 [DOI] [PubMed] [Google Scholar]
- 11.Havenstein GB, Ferket PR, Qureshi MA. Carcass composition and yield of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult Sci. 2003;82(10):1509–18. doi: 10.1093/ps/82.10.1509 [DOI] [PubMed] [Google Scholar]
- 12.Havenstein GB, Ferket PR, Qureshi MA. Growth, livability, and feed conversion of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult Sci. 2003;82(10):1500–8. doi: 10.1093/ps/82.10.1500 [DOI] [PubMed] [Google Scholar]
- 13.Jackson S, Diamond J. Metabolic and Digestive Responses to Artificial Selection in Chickens. Evolution. 1996;50(4):1638–50. doi: 10.1111/j.1558-5646.1996.tb03936.x [DOI] [PubMed] [Google Scholar]
- 14.Schmidt CJ, Persia ME, Feierstein E, Kingham B, Saylor WW. Comparison of a modern broiler line and a heritage line unselected since the 1950s. Poult Sci. 2009;88(12):2610–9. doi: 10.3382/ps.2009-00055 [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Schmidt CJ, Lamont SJ. Transcriptome analysis reveals potential mechanisms underlying differential heart development in fast- and slow-growing broilers under heat stress. BMC Genomics. 2017;18(1):295. doi: 10.1186/s12864-017-3675-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis RV, Lamont SJ, Rothschild MF, Persia ME, Ashwell CM, Schmidt CJ. Transcriptome analysis of post-hatch breast muscle in legacy and modern broiler chickens reveals enrichment of several regulators of myogenic growth. PLoS One. 2015;10(3):e0122525. doi: 10.1371/journal.pone.0122525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schoettle CER, EF, Norton H, Alberts J. A study of New Hampshire X Barred Columbian chicks from two days of age to ten weeks of age: Effects of coccidiostats. Poultry Science. 1956;35 596–9. [Google Scholar]
- 18.Schoettle R, E.F., Alberts J.O., Scott H.M. A Study of New Hampshire X Barred columbina chicks from two days of age to ten weeks of age growth, organ weights. Poultry Science. 1956;35:95–8. [Google Scholar]
- 19.Endo H, Tsunekawa N, Kudo K, Oshida T, Motokawa M, Sonoe M, et al. Comparative morphological study of skeletal muscle weight among the red jungle fowl (Gallus gallus) and various fowl breeds (Gallus domesticus). J Exp Zool B Mol Dev Evol. 2022;338(8):542–51. doi: 10.1002/jez.b.23111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenewalt CH. Dimensional relationships for flying animals. Smithsonian miscellaneous collections. 1962;144:1–46. [Google Scholar]
- 21.Kawabe S, Tsunekawa N, Kudo K, Tirawattanawanich C, Akishinonomiya F, Endo H. Morphological variation in brain through domestication of fowl. J Anat. 2017;231(2):287–97. doi: 10.1111/joa.12623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olkowski AA, Wojnarowicz C, Laarveld B. Pathophysiology and pathological remodelling associated with dilated cardiomyopathy in broiler chickens predisposed to heart pump failure. Avian Pathol. 2020;49(5):428–39. doi: 10.1080/03079457.2020.1757620 [DOI] [PubMed] [Google Scholar]
- 23.Olkowski AA. Pathophysiology of heart failure in broiler chickens: structural, biochemical, and molecular characteristics. Poult Sci. 2007;86(5):999–1005. doi: 10.1093/ps/86.5.999 [DOI] [PubMed] [Google Scholar]
- 24.Chen S, Yan C, Xiang H, Xiao J, Liu J, Zhang H, et al. Transcriptome changes underlie alterations in behavioral traits in different types of chicken. Journal of Animal Science. 2019;98(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papah MB, Brannick EM, Schmidt CJ, Abasht B. Evidence and role of phlebitis and lipid infiltration in the onset and pathogenesis of Wooden Breast Disease in modern broiler chickens. Avian Pathol. 2017;46(6):623–43. doi: 10.1080/03079457.2017.1339346 [DOI] [PubMed] [Google Scholar]
- 26.Abasht B, Zhou N, Lee WR, Zhuo Z, Peripolli E. The metabolic characteristics of susceptibility to wooden breast disease in chickens with high feed efficiency. Poult Sci. 2019;98(8):3246–56. doi: 10.3382/ps/pez183 [DOI] [PubMed] [Google Scholar]
- 27.Abasht B, Papah MB, Qiu J. Evidence of vascular endothelial dysfunction in Wooden Breast disorder in chickens: Insights through gene expression analysis, ultra-structural evaluation and supervised machine learning methods. PLoS One. 2021;16(1):e0243983. doi: 10.1371/journal.pone.0243983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abasht B, Mutryn MF, Michalek RD, Lee WR. Oxidative Stress and Metabolic Perturbations in Wooden Breast Disorder in Chickens. PLoS One. 2016;11(4):e0153750. doi: 10.1371/journal.pone.0153750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark DL, Velleman SG. Spatial influence on breast muscle morphological structure, myofiber size, and gene expression associated with the wooden breast myopathy in broilers. Poult Sci. 2016;95(12):2930–45. doi: 10.3382/ps/pew243 [DOI] [PubMed] [Google Scholar]
- 30.Velleman SG, Clark DL, Tonniges JR. The Effect of the Wooden Breast Myopathy on Sarcomere Structure and Organization. Avian Dis. 2018;62(1):28–35. doi: 10.1637/11766-110217-Reg.1 [DOI] [PubMed] [Google Scholar]
- 31.Chen LR, Suyemoto MM, Sarsour AH, Cordova HA, Oviedo-Rondon EO, Wineland M, et al. Temporal characterization of wooden breast myopathy ("woody breast") severity and correlation with growth rate and lymphocytic phlebitis in three commercial broiler strains and a random-bred broiler strain. Avian Pathol. 2019;48(4):319–28. doi: 10.1080/03079457.2019.1598541 [DOI] [PubMed] [Google Scholar]
- 32.Zanetti MA, Tedesco DC, Schneider T, Teixeira STF, Daroit L, Pilotto F, et al. Economic losses associated with Wooden Breast and White Striping in broilers. Semina: Ciências Agrárias. 2018;39(2). [Google Scholar]
- 33.Hennebry A, Oldham J, Shavlakadze T, Grounds MD, Sheard P, Fiorotto ML, et al. IGF1 stimulates greater muscle hypertrophy in the absence of myostatin in male mice. J Endocrinol. 2017;234(2):187–200. doi: 10.1530/JOE-17-0032 [DOI] [PubMed] [Google Scholar]
- 34.Velleman SG, Clark DL. Histopathologic and Myogenic Gene Expression Changes Associated with Wooden Breast in Broiler Breast Muscles. Avian Dis. 2015;59(3):410–8. doi: 10.1637/11097-042015-Reg.1 [DOI] [PubMed] [Google Scholar]
- 35.Velleman SG, Clark DL, Tonniges JR. Fibrillar Collagen Organization Associated with Broiler Wooden Breast Fibrotic Myopathy. Avian Dis. 2017;61(4):481–90. doi: 10.1637/11738-080217-Reg.1 [DOI] [PubMed] [Google Scholar]
- 36.McCarthy FM, Cooksey AM, Burgess SC. Sequential detergent extraction prior to mass spectrometry analysis. Methods Mol Biol. 2009;528:110–8. doi: 10.1007/978-1-60327-310-7_7 [DOI] [PubMed] [Google Scholar]
- 37.McCarthy FM, Cooksey AM, Wang N, Bridges SM, Pharr GT, Burgess SC. Modeling a whole organ using proteomics: the avian bursa of Fabricius. Proteomics. 2006;6(9):2759–71. doi: 10.1002/pmic.200500648 [DOI] [PubMed] [Google Scholar]
- 38.Craig R, Beavis RC. A method for reducing the time required to match protein sequences with tandem mass spectra. Rapid Commun Mass Spectrom. 2003;17(20):2310–6. doi: 10.1002/rcm.1198 [DOI] [PubMed] [Google Scholar]
- 39.Geer LY, Markey SP, Kowalak JA, Wagner L, Xu M, Maynard DM, et al. Open mass spectrometry search algorithm. J Proteome Res. 2004;3(5):958–64. doi: 10.1021/pr0499491 [DOI] [PubMed] [Google Scholar]
- 40.Kessner D, Chambers M, Burke R, Agus D, Mallick P. ProteoWizard: open source software for rapid proteomics tools development. Bioinformatics. 2008;24(21):2534–6. doi: 10.1093/bioinformatics/btn323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wright ML, Pendarvis K, Nanduri B, Edelmann MJ, Jenkins HN, Reddy JS, et al. The Effect of Oxygen on Bile Resistance in Listeria monocytogenes. J Proteomics Bioinform. 2016;9(4):107–19. doi: 10.4172/jpb.1000396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25(1):25–9. doi: 10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bu D, Luo H, Huo P, Wang Z, Zhang S, He Z, et al. KOBAS-i: intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021;49(W1):W317–W25. doi: 10.1093/nar/gkab447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reimand J, Isserlin R, Voisin V, Kucera M, Tannus-Lopes C, Rostamianfar A, et al. Pathway enrichment analysis and visualization of omics data using g:Profiler, GSEA, Cytoscape and EnrichmentMap. Nature Protocols. 2019;14(2):482–517. doi: 10.1038/s41596-018-0103-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–504. doi: 10.1101/gr.1239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith JA, Curry EG, Blue RE, Roden C, Dundon SER, Rodriguez-Vargas A, et al. FXR1 splicing is important for muscle development and biomolecular condensates in muscle cells. J Cell Biol. 2020;219(4). doi: 10.1083/jcb.201911129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mientjes EJ, Willemsen R, Kirkpatrick LL, Nieuwenhuizen IM, Hoogeveen-Westerveld M, Verweij M, et al. Fxr1 knockout mice show a striated muscle phenotype: implications for Fxr1p function in vivo. Hum Mol Genet. 2004;13(13):1291–302. doi: 10.1093/hmg/ddh150 [DOI] [PubMed] [Google Scholar]
- 48.Estan MC, Fernandez-Nunez E, Zaki MS, Esteban MI, Donkervoort S, Hawkins C, et al. Recessive mutations in muscle-specific isoforms of FXR1 cause congenital multi-minicore myopathy. Nat Commun. 2019;10(1):797. doi: 10.1038/s41467-019-08548-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Butler MH, David C, Ochoa GC, Freyberg Z, Daniell L, Grabs D, et al. Amphiphysin II (SH3P9; BIN1), a member of the amphiphysin/Rvs family, is concentrated in the cortical cytomatrix of axon initial segments and nodes of ranvier in brain and around T tubules in skeletal muscle. J Cell Biol. 1997;137(6):1355–67. doi: 10.1083/jcb.137.6.1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tjondrokoesoemo A, Park KH, Ferrante C, Komazaki S, Lesniak S, Brotto M, et al. Disrupted membrane structure and intracellular Ca(2)(+) signaling in adult skeletal muscle with acute knockdown of Bin1. PLoS One. 2011;6(9):e25740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nicot AS, Toussaint A, Tosch V, Kretz C, Wallgren-Pettersson C, Iwarsson E, et al. Mutations in amphiphysin 2 (BIN1) disrupt interaction with dynamin 2 and cause autosomal recessive centronuclear myopathy. Nat Genet. 2007;39(9):1134–9. doi: 10.1038/ng2086 [DOI] [PubMed] [Google Scholar]
- 52.Bohm J, Vasli N, Maurer M, Cowling BS, Shelton GD, Kress W, et al. Altered splicing of the BIN1 muscle-specific exon in humans and dogs with highly progressive centronuclear myopathy. PLoS Genet. 2013;9(6):e1003430. doi: 10.1371/journal.pgen.1003430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Claeys KG, Maisonobe T, Bohm J, Laporte J, Hezode M, Romero NB, et al. Phenotype of a patient with recessive centronuclear myopathy and a novel BIN1 mutation. Neurology. 2010;74(6):519–21. doi: 10.1212/WNL.0b013e3181cef7f9 [DOI] [PubMed] [Google Scholar]
- 54.Figueiredo VC, McCarthy JJ. Regulation of Ribosome Biogenesis in Skeletal Muscle Hypertrophy. Physiology (Bethesda). 2019;34(1):30–42. doi: 10.1152/physiol.00034.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.West DW, Burd NA, Staples AW, Phillips SM. Human exercise-mediated skeletal muscle hypertrophy is an intrinsic process. Int J Biochem Cell Biol. 2010;42(9):1371–5. doi: 10.1016/j.biocel.2010.05.012 [DOI] [PubMed] [Google Scholar]
- 56.Eugster A, Frigerio G, Dale M, Duden R. The alpha- and beta’-COP WD40 domains mediate cargo-selective interactions with distinct di-lysine motifs. Mol Biol Cell. 2004;15(3):1011–23. doi: 10.1091/mbc.e03-10-0724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lichte B, Veh RW, Meyer HE, Kilimann MW. Amphiphysin, a novel protein associated with synaptic vesicles. EMBO J. 1992;11(7):2521–30. doi: 10.1002/j.1460-2075.1992.tb05317.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang LH, Sudhof TC, Anderson RG. The appendage domain of alpha-adaptin is a high affinity binding site for dynamin. J Biol Chem. 1995;270(17):10079–83. doi: 10.1074/jbc.270.17.10079 [DOI] [PubMed] [Google Scholar]
- 59.Moersch FP, Woltman HW. Progressive fluctuating muscular rigidity and spasm ("stiff-man" syndrome); report of a case and some observations in 13 other cases. Proc Staff Meet Mayo Clin. 1956;31(15):421–7. [PubMed] [Google Scholar]
- 60.Murinson BB, Guarnaccia JB. Stiff-person syndrome with amphiphysin antibodies: distinctive features of a rare disease. Neurology. 2008;71(24):1955–8. doi: 10.1212/01.wnl.0000327342.58936.e0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baizabal-Carvallo JF, Jankovic J. Stiff-person syndrome: insights into a complex autoimmune disorder. J Neurol Neurosurg Psychiatry. 2015;86(8):840–8. doi: 10.1136/jnnp-2014-309201 [DOI] [PubMed] [Google Scholar]
- 62.Bucki R, Georges PC, Espinassous Q, Funaki M, Pastore JJ, Chaby R, et al. Inactivation of endotoxin by human plasma gelsolin. Biochemistry. 2005;44(28):9590–7. doi: 10.1021/bi0503504 [DOI] [PubMed] [Google Scholar]
- 63.Bucki R, Byfield FJ, Kulakowska A, McCormick ME, Drozdowski W, Namiot Z, et al. Extracellular gelsolin binds lipoteichoic acid and modulates cellular response to proinflammatory bacterial wall components. J Immunol. 2008;181(7):4936–44. doi: 10.4049/jimmunol.181.7.4936 [DOI] [PubMed] [Google Scholar]
- 64.Cheng Y, Hu X, Liu C, Chen M, Wang J, Wang M, et al. Gelsolin Inhibits the Inflammatory Process Induced by LPS. Cell Physiol Biochem. 2017;41(1):205–12. doi: 10.1159/000456043 [DOI] [PubMed] [Google Scholar]
- 65.Lee WM, Galbraith RM. The extracellular actin-scavenger system and actin toxicity. N Engl J Med. 1992;326(20):1335–41. doi: 10.1056/NEJM199205143262006 [DOI] [PubMed] [Google Scholar]
- 66.Osborn TM, Dahlgren C, Hartwig JH, Stossel TP. Modifications of cellular responses to lysophosphatidic acid and platelet-activating factor by plasma gelsolin. Am J Physiol Cell Physiol. 2007;292(4):C1323–30. doi: 10.1152/ajpcell.00510.2006 [DOI] [PubMed] [Google Scholar]
- 67.Sezen D, Bongiovanni AM, Gelber S, Perni U, Hutson JM, Skupski D, et al. Gelsolin down-regulates lipopolysaccharide-induced intraamniotic tumor necrosis factor-alpha production in the midtrimester of pregnancy. Am J Obstet Gynecol. 2009;200(2):191 e1–4. doi: 10.1016/j.ajog.2008.09.011 [DOI] [PubMed] [Google Scholar]
- 68.Sanchez-Barrena MJ, Vallis Y, Clatworthy MR, Doherty GJ, Veprintsev DB, Evans PR, et al. Bin2 is a membrane sculpting N-BAR protein that influences leucocyte podosomes, motility and phagocytosis. PLoS One. 2012;7(12):e52401. doi: 10.1371/journal.pone.0052401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mohamed MM, Sloane BF. Cysteine cathepsins: multifunctional enzymes in cancer. Nat Rev Cancer. 2006;6(10):764–75. doi: 10.1038/nrc1949 [DOI] [PubMed] [Google Scholar]
- 70.Ricaño-Ponce I, Peeters T, Matzaraki V, Houben B, Achten R, Cools P, et al. Impact of Human Genetic Variation on C-Reactive Protein Concentrations and Acute Appendicitis. Front Immunol. 2022;13:862742. doi: 10.3389/fimmu.2022.862742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ambruso DR. Peroxiredoxin-6 and NADPH oxidase activity. Methods Enzymol. 2013;527:145–67. doi: 10.1016/B978-0-12-405882-8.00008-8 [DOI] [PubMed] [Google Scholar]
- 72.Filippi MD, Szczur K, Harris CE, Berclaz PY. Rho GTPase Rac1 is critical for neutrophil migration into the lung. Blood. 2007;109(3):1257–64. doi: 10.1182/blood-2006-04-017731 [DOI] [PubMed] [Google Scholar]
- 73.Dang PM, Cross AR, Babior BM. Assembly of the neutrophil respiratory burst oxidase: a direct interaction between p67PHOX and cytochrome b558. Proc Natl Acad Sci U S A. 2001;98(6):3001–5. doi: 10.1073/pnas.061029698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wong K, Pertz O, Hahn K, Bourne H. Neutrophil polarization: spatiotemporal dynamics of RhoA activity support a self-organizing mechanism. Proc Natl Acad Sci U S A. 2006;103(10):3639–44. doi: 10.1073/pnas.0600092103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kunisaki Y, Nishikimi A, Tanaka Y, Takii R, Noda M, Inayoshi A, et al. DOCK2 is a Rac activator that regulates motility and polarity during neutrophil chemotaxis. J Cell Biol. 2006;174(5):647–52. doi: 10.1083/jcb.200602142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kuhns DB, Fink DL, Choi U, Sweeney C, Lau K, Priel DL, et al. Cytoskeletal abnormalities and neutrophil dysfunction in WDR1 deficiency. Blood. 2016;128(17):2135–43. doi: 10.1182/blood-2016-03-706028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Genovese KJ, He H, Swaggerty CL, Kogut MH. The avian heterophil. Dev Comp Immunol. 2013;41(3):334–40. doi: 10.1016/j.dci.2013.03.021 [DOI] [PubMed] [Google Scholar]
- 78.Smith JH. Relation of Body Size to Muscle Cell Size and Number in the Chicken. Poultry Science. 1963;42(2):283–90. [Google Scholar]
- 79.Hui S, Ghergurovich JM, Morscher RJ, Jang C, Teng X, Lu W, et al. Glucose feeds the TCA cycle via circulating lactate. Nature. 2017;551(7678):115–8. doi: 10.1038/nature24057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baroukh N, Canteleux N, Lefevre A, Dupuy C, Martias C, Presset A, et al. Serum and Soleus Metabolomics Signature of Klf10 Knockout Mice to Identify Potential Biomarkers. Metabolites. 2022;12(6). doi: 10.3390/metabo12060556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huffman KM, Koves TR, Hubal MJ, Abouassi H, Beri N, Bateman LA, et al. Metabolite signatures of exercise training in human skeletal muscle relate to mitochondrial remodelling and cardiometabolic fitness. Diabetologia. 2014;57(11):2282–95. doi: 10.1007/s00125-014-3343-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Konopka AR, Harber MP. Skeletal muscle hypertrophy after aerobic exercise training. Exerc Sport Sci Rev. 2014;42(2):53–61. doi: 10.1249/JES.0000000000000007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tang JE, Hartman JW, Phillips SM. Increased muscle oxidative potential following resistance training induced fibre hypertrophy in young men. Appl Physiol Nutr Metab. 2006;31(5):495–501. doi: 10.1139/h06-026 [DOI] [PubMed] [Google Scholar]
- 84.Mutryn MF, Brannick EM, Fu W, Lee WR, Abasht B. Characterization of a novel chicken muscle disorder through differential gene expression and pathway analysis using RNA-sequencing. BMC Genomics. 2015;16:399. doi: 10.1186/s12864-015-1623-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sihvo HK, Immonen K, Puolanne E. Myodegeneration with fibrosis and regeneration in the pectoralis major muscle of broilers. Vet Pathol. 2014;51(3):619–23. doi: 10.1177/0300985813497488 [DOI] [PubMed] [Google Scholar]
- 86.Iwasaki K, Morimatsu M, Inanami O, Uchida E, Syuto B, Kuwabara M, et al. Isolation, characterization, and cDNA cloning of chicken turpentine-induced protein, a new member of the scavenger receptor cysteine-rich (SRCR) family of proteins. J Biol Chem. 2001;276(12):9400–5. doi: 10.1074/jbc.M011713200 [DOI] [PubMed] [Google Scholar]
- 87.Wicher KB, Fries E. Haptoglobin, a hemoglobin-binding plasma protein, is present in bony fish and mammals but not in frog and chicken. Proc Natl Acad Sci U S A. 2006;103(11):4168–73. doi: 10.1073/pnas.0508723103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Papah MB, Abasht B. Dysregulation of lipid metabolism and appearance of slow myofiber-specific isoforms accompany the development of Wooden Breast myopathy in modern broiler chickens. Sci Rep. 2019;9(1):17170. doi: 10.1038/s41598-019-53728-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Included are proteins and gene accessions from NCBI and expression data supported by transcriptome expression [16] is indicated (RNASeq Concordance).
(DOCX)
Data Availability Statement
Transcript data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus (GEO) with accession number GSE65217 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE65217). Proteomics data is available from ProteomeXchange with accession number PXD005288 (https://proteomecentral.proteomexchange.org/cgi/GetDataset?ID=PXD005288).