Abstract
Aims
The microvascular resistance reserve (MRR) was introduced as a means to characterize the vasodilator reserve capacity of the coronary microcirculation while accounting for the influence of concomitant epicardial disease and the impact of administration of potent vasodilators on aortic pressure. This study aimed to evaluate the diagnostic and prognostic performance of MRR.
Methods and results
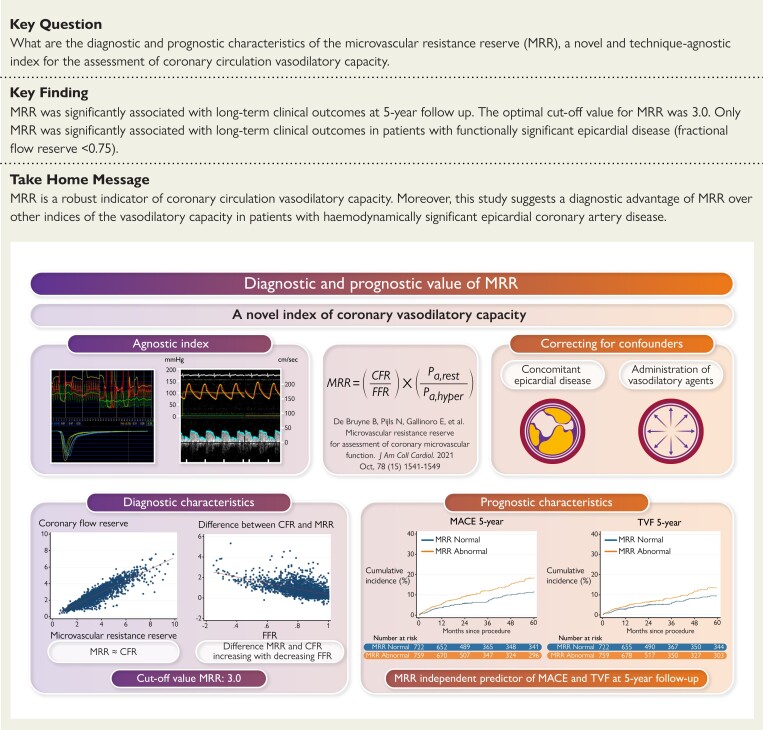
A total of 1481 patients with stable symptoms and a clinical indication for coronary angiography were included from the global ILIAS Registry. MRR was derived as a function of the coronary flow reserve (CFR) divided by the fractional flow reserve (FFR) and corrected for driving pressure. The median MRR was 2.97 [Q1–Q3: 2.32–3.86] and the overall relationship between MRR and CFR was good [correlation coefficient (Rs) = 0.88, P < 0.005]. The difference between CFR and MRR increased with decreasing FFR [coefficient of determination (R2) = 0.34; Coef.—2.88, 95% confidence interval (CI): -3.05–−2.73; P < 0.005]. MRR was independently associated with major adverse cardiac events (MACE) at 5-year follow-up [hazard ratio (HR) 0.78; 95% CI 0.63–0.95; P = 0.024] and with target vessel failure (TVF) at 5-year follow-up (HR 0.83; 95% CI 0.76–0.97; P = 0.047). The optimal cut-off value of MRR was 3.0. Based on this cut-off value, only abnormal MRR was significantly associated with MACE and TVF at 5-year follow-up in vessels with functionally significant epicardial disease (FFR <0.75).
Conclusion
MRR seems a robust indicator of the microvascular vasodilator reserve capacity. Moreover, in line with its theoretical background, this study suggests a diagnostic advantage of MRR over other indices of vasodilatory capacity in patients with hemodynamically significant epicardial coronary artery disease.
Keywords: Coronary microvascular dysfunction, Coronary physiology, Microvascular resistance reserve, Coroanry artery disease
Structured Graphical Abstract
Structured Graphical Abstract.
Diagnostic and prognostic value of the microvascular resistance reserve (MRR).The MRR is a novel index of the coronary vasodilatory capacity. The MMR is an index that corrects the assessment of the vasodilatory capacity for concomitant epicardial disease and the administration of vasodilatory indices. The MRR shows an excellent correlation with the coronary flow reserve (CFR), which decreases with decreasing fractional flow reserve (FFR) values. The MRR is an independent and significant predictor of both major adverse cardiac events (MACE) and target vessel failure (TVF) at 5-year follow-up.
See the editorial comment for this article ‘Microvascular resistance reserve: a reference test of the coronary microcirculation?’, by S. Rigattieri et al., https://doi.org10.1093/eurheartj/ehad291.
Introduction
Coronary microvascular dysfunction (CMD) is increasingly recognized as an important contributor to, or even the sole origin of, chronic coronary syndromes (CCS).1 In patients with CMD, the coronary circulation is unable to accommodate an increase in myocardial demand due to impairment of the vasodilator reserve capacity of the coronary circulation.1,2 This fundamental issue of impaired vasodilator reserve may originate from the inability of the microvasculature to fully dilate, or from adaptive vasodilatation already in resting conditions reducing the available reserve capacity despite a normal ability to fully dilate.3 Hereby, CMD considerably impacts the clinical course of CCS, and studies on targeted therapy to improve the functional status of the coronary microcirculation are emerging.4
Importantly, the diagnostic criteria of CMD remain ill-defined. The coronary microcirculation cannot be visualized directly and there are no animal models emulating human coronary microvascular pathology.5 Moreover, the compartments of the coronary arterial circulation communicate in a continuum, and previously described indices of CMD—derived from distal coronary pressure and/or flow measurements—do not optimally distinguish between the epicardial and microvascular compartments of the coronary circulation.6 Furthermore, hemodynamic alterations due to pharmacologically-induced maximal vasodilatation, a requisite to study the vasodilator capacity of the coronary circulation, may impact contemporary physiological indices used to diagnose CMD.7 As a result, studies investigating CMD report different techniques, indices, and cut-off values to define the presence or absence of CMD, yet their validity in the presence of concomitant obstructive disease is uncertain.
The microvascular resistance reserve (MRR) was introduced as a means to characterize the vasodilator reserve capacity of the coronary microcirculation while accounting for the influence of concomitant epicardial disease and the impact of administration of potent vasodilators on aortic pressure.8 Although initially derived from absolute coronary flow measurements using the continuous thermodilution technique, MRR can theoretically be applied to all modalities as long as accurate coronary flow and pressure measurements are obtained.
The present study aims to evaluate the diagnostic and prognostic performance of MRR, derived by either Doppler flow velocity- or bolus thermodilution-based flow measurements, both in patients with and without functionally significant epicardial coronary artery disease (CAD).
Methods
Study population
The ILIAS (Inclusive Invasive Physiological Assessment in Angina Syndromes) registry is a retrospective global, multi-center initiative pooling vessel-level coronary pressure and flow data, as well as vessel-level clinical outcome data. All studies included were approved by local medical ethics committees. The registry is composed of 20 expert medical institutes from the Netherlands, Korea, Japan, Spain, Denmark, Italy, and the United States of America. All data were gathered in local study protocols between 1998 and 2018. Patients who underwent clinically indicated invasive coronary angiography and comprehensive invasive physiological assessment of at least one native coronary artery were enrolled in the registry. Patients with hemodynamic instability, significant valvular pathology, and prior coronary artery bypass graft surgery, as well as patients with a clinical presentation of acute coronary syndromes upon the index procedure, were excluded. Individual vessel-level data for pooled analysis were collected using standardized spreadsheets and a fully compliant cloud-based clinical data platform (Castor EDC, Amsterdam, The Netherlands). Standardized definitions were used for all variables. ILIAS Registry was registered at Clinicaltrials.gov (ClinicalTrials.gov Identifier: NCT04485234).
Coronary angiography and physiological assessment
Coronary angiography and intracoronary physiological assessments were performed in all institutions using standard techniques. After diagnostic coronary angiography, invasive physiological indices were measured using either separate pressure- (PressureWire, RADI medical—now Abbott Vascular, St Paul, MN) and Doppler velocity sensor-equipped coronary guidewires (FloWire, Endosonics—now Philips-Volcano, San Diego, CA), dual pressure- and Doppler flow velocity-equipped guide wire (ComboWire, Volcano Corp.—now Philips-Volcano, San Diego, CA), or a temperature-sensitive pressure sensor-equipped guide wire (PressureWire, St Jude Medical—now Abbott Vascular, St. Paul, MN) using routine techniques. Intracoronary nitrate (100 or 200 μg) was administered before physiologic measurements. Using the Doppler velocity technique, baseline and hyperemic average peak flow velocities were labeled baseline and hyperemic flow, respectively. Using the bolus coronary thermodilution technique, resting and hyperemic thermodilution curves were obtained in triplicate using three injections (4 mL each) of room-temperature saline, and the inverse of the average basal and hyperemic mean transit times was labeled baseline and hyperemic flow, respectively. Hyperemia was induced by intravenous infusion of adenosine (140 μg/kg/min) or adenosine triphosphate (150 μg/kg/min) through a peripheral or central vein, intracoronary bolus injection of adenosine (20–200 μg), or intracoronary bolus injection of nicorandil (3 mg), according to local standards.9,10
Derivation of MRR
MRR was derived based on the theoretic framework by De Bruyne et al. The final formula used in the current analysis is a product of coronary flow reserve (CFR) and fractional flow reserve (FFR) with correction for the impact of changes in hemodynamics from non-hyperemic to hyperemic conditions, as follows:
where CFR indicates the ratio of coronary flow (velocity) at maximal hyperemia to coronary flow (velocity) at non-hyperemic conditions, FFR indicates the ratio of distal coronary pressure to aortic pressure at maximal hyperemia, and Parest and Pahyper indicate aortic pressure during non-hyperemic conditions and maximal hyperemia, respectively. Herewith, MRR corrects the vasodilator reserve capacity of the coronary circulation (expressed by CFR) for the impact of epicardial CAD severity (expressed by FFR), and the impact of pharmacological vasodilatation on perfusion pressure (expressed by the ratio of resting to hyperemic aortic pressure).
Treatment and clinical follow-up
Percutaneous coronary intervention (PCI) was performed according to clinical practice guidelines at the time of the procedure. However, final decisions regarding revascularization were at the discretion of the operator. Clinical follow-up was obtained at outpatient clinic visits or by telephone contact to ascertain the occurrence of major adverse cardiac events (MACE) or target vessel failure (TVF). MACE was defined as the composite of all-cause death, acute myocardial infarction of the target vessel, and clinically driven (urgent) revascularization through coronary artery bypass or PCI. TVF was defined as the composite of cardiac death, acute myocardial infarction of the target vessel, and clinically driven revascularization of the target vessel through coronary artery bypass graft surgery or PCI. All patient-reported events were verified by evaluating hospital records or contacting the treating cardiologist or general practitioner.
Statistical analysis
Data were analyzed on a per-patient basis by selecting a single vessel per patient. Only the vessel with the lowest MRR value was considered. Normality and homogeneity of the variances were tested using Shapiro–Wilk and Levene tests. Continuous variables are presented as mean ± standard deviation or median (first, third quartile [Q1, Q3]) and were compared with the Student’s t-test or Mann–Whitney U test. Categorical variables are presented as counts and percentages and were compared using Fisher exact test. The relationship between MRR and CFR was evaluated using linear regression and Bland–Altman analysis. In the absence of a validated cut-off value for normal vs. abnormal MRR, receiver operating characteristic (ROC) curve analysis was performed to determine an independent cut-off value for MRR. For this analysis, the presence of reversible perfusion abnormalities during non-invasive stress testing before coronary angiography was used as the standard of reference. Additionally, time-dependent ROC analysis was performed to derive the optimal MRR cut-off value for MACE and TVF. The optimal cut-off value was determined using the Liu method.11
We subsequently evaluated the association of MRR and CFR as continuous variables with TVF and MACE at 5-year follow-up. Survival analyses were performed based on time-to-first-event analyses. The hazard ratio (HR) for MACE per unit increase of MRR and CFR was calculated with the use of a Cox proportional hazards model. The HRs for TVF and the individual components of the composite endpoint (except death) per unit increase of MRR and CFR were calculated with cause-specific proportional hazards models using the Fine and Gray method to account for the competing risk of death. All models were adjusted for the effect of relevant clinical and angiographic characteristics (P < 0.1 for inclusion). All clinical and angiographic characteristics (Table 1) were considered covariates. For normal vs. abnormal MRR, cumulative incidence rates were visualized using the Kaplan–Meier method for the MACE endpoint, and by means of the cumulative incidence function of Fine and Gray for the TVF endpoint to account for the competing risk of death. Subsequently, the prognostic value of MRR was compared to established indices of CMD, such as CFR (cut-off: < 2.5) and the presence of abnormal minimal microvascular resistance (MR), using the Doppler-derived hyperemic microvascular resistance index (HMR; cut-off: ≤ 2.5 mmHg/cm/s) or bolus coronary thermodilution-derived index of microcirculatory resistance (IMR; cut-off: ≥ 25 U). All proportional hazard models were preceded by verification of the proportional hazard assumption using Schoenfeld’s residuals. The 95% confidence intervals (CI) have not been adjusted for multiple comparisons, and therefore inferences drawn from these intervals may not be reproducible. A P < 0.05 (2-sided) was considered statistically significant. The STATA version 14.0 (StataCorp, College Station, TX) software package was used for calculations.
Table 1.
Baseline characteristics
| Patients, n | 1481 |
|---|---|
| Demographics Age, years Male sex | 63 ± 10 1091 (74) |
| Coronary risk factors Hypertension Diabetes Hyperlipidemia Positive family history Current smoking Prior myocardial infarction Prior coronary intervention | 854 (58) 384 (26) 996 (67) 517 (35) 303 (20) 260 (18) 334 (23) |
| Quantitative analysis Diameter stenosis, % Lesion length, mm Minimal lumen diameter, mm | 50.9 ± 17.9 15.3 ± 10.8 1.58 ± 0.65 |
| Physiological indices FFR CFR MRR HMRa IMRb | 0.83 ± 0.12 2.44 ± 0.99 3.22 ± 1.27 2.24 ± 0.89 21.0 ± 14.1 |
Values are given as mean ± standard deviation, or n (%).
MRR (microvascular resistance reserve); CFR (coronary flow reserve); FFR (fractional flow reserve); HMR (hyperemic microvascular resistance); IMR (index of microcirculatory resistance).
N = 708 (48%) with Doppler.
N = 773 (52%) with bolus thermodilution.
Results
Characteristics of the study population
A total of 2322 patients were included in the registry and underwent invasive physiological assessment in a total of 3046 vessels. Among these, 2209 patients had angiographic apparent obstructive CAD (≥50% diameter stenosis) in 2808 vessels. The other vessels were marked as non-obstructive reference vessels based on angiography. Patients with acute coronary syndrome (=217) as clinical diagnosis upon the index procedure were excluded. In the remaining stable CAD population, complete physiological data and patient and vessel follow-up data were available in 1481 patients [4 patients (0.2%) with incomplete physiological data, 62 patients (3%) lost to follow-up].
The key baseline and vessel characteristics are shown in Table 1. A total of 708 patients (48%) were evaluated with Doppler-derived metrics and 773 patients (52%) with bolus coronary thermodilution-derived metrics. The population consisted of 1091 men (74%) and the mean age was 63 ± 10 years. The left anterior descending artery (LAD) was the most evaluated vessel (63%) and the mean % diameter stenosis by quantitative analysis was 50.9 ± 17.5.
Diagnostic characteristics of MRR and comparison with CFR
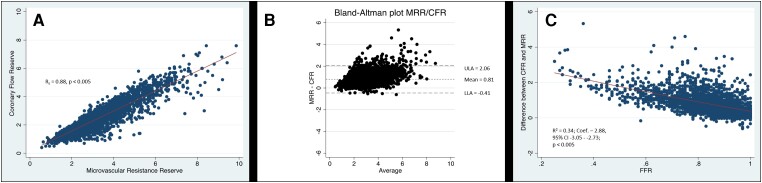
The distributions were significantly non-normal for both MRR (W = 0.93, P < 0.005) and CFR (W = 0.91, P < 0.005) according to Shapiro–Wilk tests. In the overall study population, the median MRR was 2.97 [Q1–Q3: 2.32–3.86] and the median CFR was 2.30 [Q1–Q3: 1.70–2.90] (P < 0.005 vs. MRR). Figure 1 displays the scatter plot of individual CFR and MRR values, the corresponding linear correlation between CFR and MRR (Figure 1A), the corresponding Bland–Altman plot (Figure 1B), and a modified Bland–Altman plot with the effect of FFR on the difference between CFR and MRR (Figure 1C). The overall agreement between MRR and CFR was excellent (rs = 0.88, P < 0.005). The Bland–Altman analysis documented a mean bias of 0.79 and limits of agreement of −0.41 to 2.06. The difference between CFR and MRR increased with decreasing FFR (R2 = 0.34; Coef.—2.88, 95% CI: -3.05–2.73; P < 0.005).
Figure 1.
Correlation between CFR and MRR in the whole study population. Scatter plot of individual coronary flow reserve (CFR) and microvascular resistance reserve (MRR) values, and the corresponding linear correlation between CFR and MRR (A), as well as the corresponding Bland–Altman plot (B) and a modified Bland–Altman plot with the effect of fractional flow reserve (FFR) on the difference between CFR and MRR (C). Upper limit of agreement (ULA), lower limit of agreement (LLA).
The threshold for abnormal MRR, and classification agreement with CFR
A total of 503 patients (31%) had non-invasive stress testing performed before undergoing invasive coronary angiography. Table 2 shows the specific test involved. Using an abnormal non-invasive stress test as the standard of reference, the area under the curve of MRR was 0.51 (95% CI 0.47–0.54; P < 0.005) and the optimal cut-off value was 3.0 with a sensitivity of 0.49 (95% CI 0.43–0.56; P < 0.005) and a specificity of 0.52 (95% CI 0.44–0.60; P < 0.005). Additional sensitivity analysis including only patients with single photon emission computed tomography (n = 275) provided an optimal cut-off value of 3.0. Including only patients with functionally non-obstructive CAD (FFR >0.80; n = 311), or those tested with Doppler flow velocity (n = 489) identified an optimal cut-off value of 3.0. Similarly, the optimal cut-off value of MRR for MACE and TVF at 5-year follow-up in the overall study population was 3.1.
Table 2.
Non-invasive stress test modalities
| Non-invasive stress test | Patients, n (%) |
|---|---|
| MRI | 8 (2) |
| SPECT/MIBI | 275 (55) |
| PET | 115 (23) |
| Stress echocardiography | 1 (0) |
| Other/not-specified | 104 (20) |
MRI (magnetic resonance imaging); SPECT (single-photon emission computed tomography); PET (positron emission tomography).
Using a 3.0 cut-off value for MRR, diagnostic agreement with CFR <2.5 was 85%, whereas disagreement occurred in 226 patients (15%). A normal MRR with abnormal CFR occurred in 87% (196 out of 226), of which 67% (131 out of 196) had concomitant functionally significant epicardial disease (FFR <0.80). Supplementary data online, Table S1 shows the clinical characteristics across patients divided by normal and abnormal MRR based on the cut-off value of 3.0.
Microvascular resistance reserve: survival analysis
Median follow-up was 3.6 years (Q1–Q3: 2.0–5.1 years). Table 3 shows the absolute MACE and TVF rates, as well as their components at a 5-year follow-up. Supplementary data online, Tables S1 and S2 show the detailed outline of the proportional hazards analyses for MACE and TVF, respectively. From the clinical and angiographic characteristics, age, diabetes, familial predisposition for CAD, previous myocardial infarction, coronary lesion length, stenosis diameter, minimal lumen diameter, flow method, and target vessel intervention were associated with 5-year MACE and TVF (P < 0.1 for all). After correction for these confounders, MRR was independently associated with MACE at 5-year follow-up (HR 0.78; 95% CI 0.63–0.95; P = 0.024) and with TVF at 5-year follow-up (HR 0.83; 95% CI 0.76–0.97; P = 0.047). There was no interaction between the flow method used and the association of MRR with either MACE (P = 0.749) or TVF (0.653) at 5-year follow-up. Likewise, CFR in the same population was independently and significantly associated with MACE and TVF at 5-year follow-up (HR 0.77; 95% CI 0.62–0.95; P = 0.034 and HR 0.81; 95% CI 0.63–0.99; P = 0.048, respectively). A sensitivity analysis excluding patients who underwent revascularization during the index procedure led to the same results and conclusions (data not shown).
Table 3.
MACE and TVF rates and their components across the whole study population
| Total, n (%) | Hazard ratio (95% CI)* | P-value | |
|---|---|---|---|
| MACE at 5 years All-cause death Acute myocardial infarction (target vessel) Urgent revascularization | 163 (11) 61 (4) 23 (1) 149 (10) | 0.95 (0.71–1.27) 1.03 (0.67–1.57) 0.89 (0.75–1.05) | 0.727 0.891 0.193 |
| TVF at 5 years Cardiac death Acute myocardial infarction (target vessel) Urgent revascularization (target vessel) | 122 (8) 46 (3) 23 (1) 81 (5) | 0.88 (0.78–1.01) 0.95 (0.68–1.34) 0.94 (0.83–1.08) | 0.081 0.782 0.433 |
CI (confidence interval); MACE (Major Adverse Cardiac Events); TVF (Target Vessel Failure). *The point estimate represents a hazard ratio, since analyses taking into consideration the competing risk of death using the fine-gray model were performed.
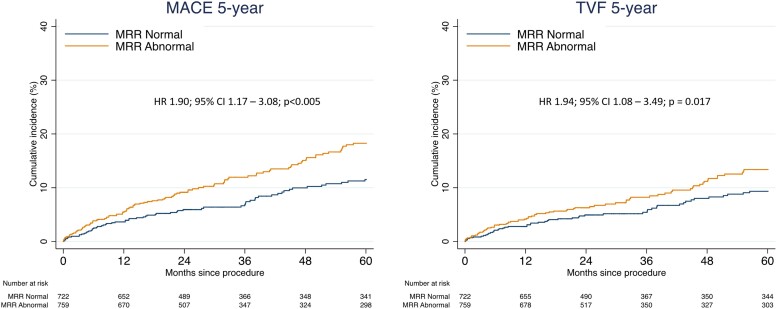
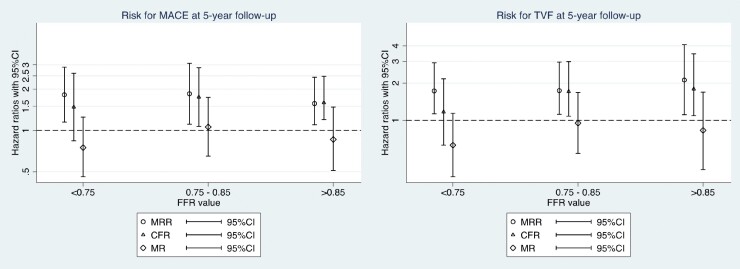
Figure 2 depicts the cumulative incidence curves for MACE (A) and TVF (B) at 5-year follow-up stratified by normal vs. abnormal MRR (≤3.0). The clinical characteristics across the subgroups divided by normal and abnormal MRR are shown in Table S3. In the whole study population, abnormal MRR was significantly and independently associated with an increased risk for MACE at 5-year follow-up (HR 1.90; 95% CI 1.17–3.08; P < 0.005) and with TVF at 5-year follow-up (HR 1.94; 95% CI 1.08–3.49; P = 0.017). Figure 3 shows the HRs and 95% CIs for MACE and TVF at 5-year follow-up of normal vs. abnormal MRR (cut-off value 3.0) and other currently applied indices of CMD [abnormal CFR or abnormal MR (either abnormal HMR or IMR)], stratified by the presence of functionally important epicardial disease (FFR <0.75), intermediate (FFR 0.75–0.85) epicardial disease, or functionally non-significant epicardial disease (FFR >0.85). In vessels with an intermediate or functionally non-significant epicardial disease, both MRR and CFR were significantly and independently associated with MACE at 5-year follow-up. In vessels with a functionally significant epicardial disease, only MRR was significantly associated with MACE and TVF at 5-year follow-up. Abnormal MR did not show an association with MACE or TVF at 5-year follow-up across any of the FFR subgroups (Figure 3).
Figure 2.
Cumulative incidence curves for MACE (A) and TVF (B) according to normal and abnormal MRR. Cumulative incidence curves for major adverse cardiac events (MACE) (A) and target vessel failure (TVF) (B) up to 5-year follow-up according to normal or abnormal microvascular resistance reserve (MRR) (cut-off value: 3.0). Hazard ratio (HR) with 95% confidence interval (CI) presented based on proportional hazards analysis corrected for confounders and accounting for the competing risk of death using the Fine-Gray model for TVF.
Figure 3.
Hazard ratios for the risk of MACE (A) and TVF (B) at 5-year follow-up according to different indices of CMD. Plot showing hazard ratios with 95% confidence intervals associated with major adverse cardiac events (MACE) and target vessel failure (TVF) at 5-year follow-up based on multivariate proportional hazards analysis for indices of CMD (as binary variables). HR was adjusted for the competing risk of death for TVF using the Fine and Gray method. MR [minimal resistance (HMR >2.5; IMR >25)].
A sensitivity analysis with the use of alternative CFR cut-off values (CFR ≤2.0 as used in obstructive coronary artery disease, CFR ≤2.6 as identified as the optimal threshold for abnormal non-invasive stress testing in the present study, or CFR ≤3.0 using the same threshold as applied for MRR) did not alter the conclusions concerning MACE and TVF at 5 years (data not shown). Likewise, a sensitivity analysis using corrected microvascular resistance indices to adjust for the impact of collateral flow by Yong’s formula12 did not alter the conclusions concerning the association of abnormal MR and MACE (HR 1.01; 95% CI 0.98–1.02; P = 0.893) or TVF at 5 years (HR 0.99; 95% CI 0.98–1.01; P = 0.478).
Discussion
This study is the first to describe the diagnostic and prognostic characteristics of MRR, an index of microvascular vasodilator reserve capacity that conceptually accounts for epicardial CAD severity and the impact of administration of potent vasodilators on aortic pressure in the assessment of microvascular vasodilator function. Most importantly, we document that MRR may provide a more accurate tool than CFR for the assessment of microvascular vasodilator function across the spectrum of epicardial CAD. Although both MRR and CFR were independently associated with MACE and TVF at 5-year follow-up in vessels with functionally intermediate or non-significant epicardial CAD, only abnormal MRR was independently associated with MACE and TVF in patients with hemodynamically significant epicardial disease (FFR <0.75) (Structured Graphical Abstract). These findings relate directly to the diagnostic characteristics of MRR confirmed in this study, where, as expected, the difference between MRR and CFR is closely related to the functional severity of concomitant epicardial disease expressed by FFR.
MRR: theoretical framework and diagnostic characteristics
The MRR provides a specific and robust index of the vasodilator capacity of coronary microcirculation.8 MRR represents the extent to which resting resistance (R) would decrease upon induction of maximal coronary vasodilatation in the hypothetical case the epicardial artery were to be completely normal. Based on its theoretical framework, MRR is independent of the epicardial resistance to coronary flow and thereby specific for the microvasculature. In our population, the median MRR was 2.97 (Q1–Q3: 2.32–3.86), comparable to the results of the index paper by De Bruyne et al.8 Furthermore, in line with the findings of De Bruyne et al., MRR differed from CFR as a function of FFR (Figure 1C), showing the fundamental difference in both indices. Overall, MRR and CFR showed an excellent agreement (rs = 0.88; P < 0.005), and the difference between both indices is closely related to the functional severity of concomitant epicardial disease expressed by FFR (R2 = 0.34; Coef.—2.88, 95% CI: −3.05–2.73; P < 0.005) (Figure 1C). These data reinforce the conceptual background of MRR described by De Bruyne et al. and serve as the basis for the documented differences in prognostic value between the two indices.
MRR: prognostic characteristics
We document that MRR as a continuous variable is independently associated with MACE at 5-year follow-up (HR 0.78; 95% CI 0.63–0.95, P = 0.024) and TVF at 5-year follow-up (HR 0.83; 95% CI 0.76–0.97; P = 0.047). Although there are no studies to compare these results with, they are in line with multiple reports regarding the increased risk for MACE and TVF in patients with CMD.13,14 CMD has been proposed to contribute to the development and prognosis of epicardial disease via the reduction of coronary blood flow and alteration of wall shear stress.15,16 As mentioned before, MRR is theoretically a microvasculature-specific index as it accounts for epicardial disease severity and the impact of the administration of potent vasodilators on aortic pressure. Hereby, it is arguably a more robust indicator of CMD than CFR and indices describing microvascular resistance such as HMR and IMR.
In addition, we found that in patients with intermediate or functionally non-significant epicardial disease, both CFR and MRR were equally and independently associated with clinical outcomes. Hence, it can be argued whether MRR has advantages over CFR in these cases. However, in patients with functionally significant epicardial disease (FFR <0.75), only an abnormal MRR was found to be an independent predictor of MACE and TVF. In contrast to these findings, abnormal microvascular resistance alone was not associated with either 5-year MACE or TVF, which is in line with other reports on the prognostic value of microvascular resistance indices.17
These findings suggest that MRR is a more reliable indicator of CMD particularly in the presence of functionally significant epicardial disease (FFR <0.75). Prospective investigations to assess the MRR approach are warranted to confirm these findings.
Limitations
The results from the present study should be interpreted in consideration of some limitations. First, this is an observational study and patients were treated according to clinical guidelines applicable at the time of the coronary angiography, but decisions in treatment were ultimately at the discretion of the treating physician. Second, not all studies employed central adjudication of adverse events, which may lead to heterogeneity in results across studies. Nonetheless, event rates in the ILIAS registry are similar to those documented in prospective studies with central adverse event adjudication,18 strengthening the findings of the present study. Moreover, a variety of vasodilators were used in our study and, although all agents are documented to provide similar hyperemic responses, future studies should be carried out with a more homogeneous protocol. In addition, although the MRR theoretically can be applied to all modalities that provide robust coronary flow measurements, the results should be interpreted considering the potential overestimation of flow metrics through bolus thermodilution described previously.19,20 Nonetheless, no interaction between flow measurement technique and diagnostic or prognostic efficacy of MRR was documented in the present study. Uniform application of continuous flow thermodilution, as used in the initial derivation of MRR, may further enhance its diagnostic and prognostic value considering the operator-independent nature of this absolute flow assessment.21 Further, no detailed information on the medication profiles of enrolled patients was available during the follow-up period, nor specifics regarding angina burden. It is important to note that our study population largely consists of male patients. Hereby, there might be an underrepresentation of female patients, and the results of this study should be interpreted with this consideration. In order to determine an independent cut-off value of MRR for this analysis, we assessed the optimal cut-off value to determine the presence of ischemia on a non-invasive stress test prior to invasive coronary angiography. Although a large group of patients (n = 503) was included in this analysis, further validation studies should confirm this MRR threshold for clinical decision-making. Finally, despite the length of follow-up and the large number of included patients and vessels, this study is subject to the basic limitations of a retrospective registry.
Conclusion
MRR is a robust indicator of the microvascular vasodilator reserve capacity. In line with its theoretical background, our data suggest a diagnostic advantage of MRR over CFR in patients with hemodynamically significant CAD. Further studies are required to further elucidate the potential of MRR for the diagnosis of CMD.
Clinical Trial Registration—Inclusive Invasive Physiological Assessment in Angina Syndromes Registry (ILIAS Registry), NCT04485234.
Supplementary data
Supplementary data are available at European Heart Journal online.
Declarations
Disclosure of Interest
T.v.d.H. has received speaker fees and institutional research grants from Abbott and Philips. J.M.L. has received research grants from Abbott and Philips. M.E.P. has received speaker fees from Abbott and Philips. B.K.K. has received institutional research grants from Abbott Vascular and Philips Volcano. J.J.P. has received support as consultant for Philips/Volcano, and has received institutional research grants from Philips. The other authors report no relationship with industry related to this work.
Supplementary Material
Contributor Information
Coen K M Boerhout, Heart Center, Amsterdam UMC, Meibergdreef 9, 1105 AZ, Amsterdam, The Netherlands.
Joo Myung Lee, Samsung Medical Center, Division of Cardiology, Department of Medicine, Sungkyunkwan University School of Medicine, Heart Vascular Stroke Institute, 81 Irwon-ro, Gangnam-gu, Seoul 06351, Republic of Korea.
Guus A de Waard, Heart Center, Amsterdam UMC, Meibergdreef 9, 1105 AZ, Amsterdam, The Netherlands.
Hernan Mejia-Renteria, Hospital Clínico San Carlos, IDISSC, and Universidad Complutense de Madrid, Calle del Prof Martín Lagos, S/N, 28040 Madrid, Spain.
Seung Hun Lee, Division of Cardiology, Department of Internal Medicine, Chonnam National University Hospital, 42 Jebong-ro, Dong-gu, Gwangju, South Korea.
Ji-Hyun Jung, Sejong General Hospital, Sejong Heart Institute, 20 Gyeyangmunhwa-ro, Gyeyang-gu, Incheon, South Korea.
Masahiro Hoshino, Department of Cardiovascular Medicine, Gifu Heart Center, 4 Chome-14-4 Yabutaminami, Gifu, 500-8384, Japan.
Mauro Echavarria-Pinto, Hospital General ISSSTE Querétaro—Facultad de Medicina, Universidad Autónoma de Querétaro, Av Tecnológico 101, Las Campanas, 76000 Santiago de Querétaro, México.
Martijn Meuwissen, Department of Cardiology, Amphia Hospital, Molengracht 21, 4818 CK Breda, The Netherlands.
Hitoshi Matsuo, Department of Cardiovascular Medicine, Gifu Heart Center, 4 Chome-14-4 Yabutaminami, Gifu, 500-8384, Japan.
Maribel Madera-Cambero, Department of Cardiology, Tergooi Hospital, Laan van Tergooi 2, 1212 VG Hilversum, The Netherlands.
Ashkan Eftekhari, Department of Cardiology, Aarhus University Hospital, Palle Juul-Jensens Blvd. 161, 8200 Aarhus, Denmark.
Mohamed A Effat, Division of Cardiovascular Health and Diseases, Department of Internal Medicine, University of Cincinnati, 231 Albert Sabin Way, Cincinnati, OH 45229, USA.
Tadashi Murai, Department of Cardiology, Tsuchiura Kyodo General Hospital, 4 Chome-1-1 Otsuno, Tsuchiura, Ibaraki 300-0028, Tsuchiura city, Japan.
Koen Marques, Heart Center, Amsterdam UMC, Meibergdreef 9, 1105 AZ, Amsterdam, The Netherlands.
Joon-Hyung Doh, Department of Medicine, Inje University Ilsan Paik Hospital, 170 Juhwa-ro, Ilsanseo-gu, Goyangsi, Gyeonggi-do, Goyang, South Korea.
Evald H Christiansen, Department of Cardiology, Aarhus University Hospital, Palle Juul-Jensens Blvd. 161, 8200 Aarhus, Denmark.
Rupak Banerjee, Mechanical and Materials Engineering Department, University of Cincinnati, 2901 Woodside Drive, Cincinnati, OH 45219, USA; Research Services, Veteran Affairs Medical Center, 3200 Vine St, Cincinnati, OH 45220, USA.
Chang-Wook Nam, Department of Medicine, Keimyung University, 1095 Dalgubeol-daero, Sindang-dong, Dalseo-gu, Daegu, South Korea.
Giampaolo Niccoli, Department of Cardiovascular Medicine, Catholic University of the Sacred Heart, Institute of Cardiology, 296-12 Changgyeonggung-ro, Jongno-gu, Seoul, Rome, Italy.
Masafumi Nakayama, Department of Cardiovascular Medicine, Gifu Heart Center, 4 Chome-14-4 Yabutaminami, Gifu, 500-8384, Japan; Cardiovascular Center, Toda Central General Hospital, 1 Chome-19-3 Honcho, Toda, Saitama 335-0023, Toda, Japan.
Nobuhiro Tanaka, Department of Cardiology, Tokyo Medical University Hachioji Medical Center, 1163 Tatemachi, Hachioji, Tokyo 193-0998, Japan.
Eun-Seok Shin, Department of Cardiology, Ulsan University Hospital, University of Ulsan College of Medicine, Zuid-Korea, Ulsan, Dong-gu 25, South Korea.
Yolande Appelman, Heart Center, Amsterdam UMC, Meibergdreef 9, 1105 AZ, Amsterdam, The Netherlands.
Marcel A M Beijk, Heart Center, Amsterdam UMC, Meibergdreef 9, 1105 AZ, Amsterdam, The Netherlands.
Niels van Royen, Department of Cardiology, Radboud University Medical Centre, Geert Grooteplein Zuid 10, 6525 GA Nijmegen, The Netherlands.
Paul Knaapen, Heart Center, Amsterdam UMC, Meibergdreef 9, 1105 AZ, Amsterdam, The Netherlands.
Javier Escaned, Hospital Clínico San Carlos, IDISSC, and Universidad Complutense de Madrid, Calle del Prof Martín Lagos, S/N, 28040 Madrid, Spain.
Tsunekazu Kakuta, Department of Cardiology, Tsuchiura Kyodo General Hospital, 4 Chome-1-1 Otsuno, Tsuchiura, Ibaraki 300-0028, Tsuchiura city, Japan.
Bon Kwon Koo, Department of Internal Medicine, Cardiovascular Center, Seoul National University Hospital, 101 Daehak-ro, Yeongeon-dong, Jongno-gu, Seoul, South Korea.
Jan J Piek, Heart Center, Amsterdam UMC, Meibergdreef 9, 1105 AZ, Amsterdam, The Netherlands.
Tim P van de Hoef, Heart Center, Amsterdam UMC, Meibergdreef 9, 1105 AZ, Amsterdam, The Netherlands; Department of Cardiology, University Medical Centre Utrecht, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Funding
All authors declare no funding for this contribution.
Ethical Approval
All data were gathered in local study protocols. All studies included were approved by local medical ethics committees. The registry is composed of 20 expert medical institutes from the Netherlands, Korea, Japan, Spain, Denmark, Italy, and the United States of America.
Pre-registered Clinical Trial Number
The pre-registered clinical trial number is NCT0448523.
References
- 1. Crea F, Camici PG, Bairey Merz CN. Coronary microvascular dysfunction: an update. Eur Heart J 2014;35:1101–1111. 10.1093/eurheartj/eht513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Camici PG, d’Amati G, Rimoldi O. Coronary microvascular dysfunction: mechanisms and functional assessment. Nat Rev Cardiol 2015;12:48–62. 10.1038/nrcardio.2014.160 [DOI] [PubMed] [Google Scholar]
- 3. Rahman H, Ryan M, Lumley M, Modi B, McConkey H, Ellis H, et al. Coronary microvascular dysfunction is associated with myocardial ischemia and abnormal coronary perfusion during exercise. Circulation 2019;140:1805–1816. 10.1161/CIRCULATIONAHA.119.041595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ford TJ, Stanley B, Good R, Rocchiccioli P, McEntegart M, Watkins S, et al. Stratified medical therapy using invasive coronary function testing in angina. J Am Coll Cardiol 2018;72:2841–2855. 10.1016/j.jacc.2018.09.006 [DOI] [PubMed] [Google Scholar]
- 5. Sorop O, van de Wouw J, Chandler S, Ohanyan V, Tune JD, Chilian WM, et al. Experimental animal models of coronary microvascular dysfunction. Cardiovasc Res 2020;116:756–770. 10.1093/cvr/cvaa002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hoffman JIE. Problems of coronary flow reserve. Ann Biomed Eng 2000;28:884–896. 10.1114/1.1308503 [DOI] [PubMed] [Google Scholar]
- 7. Kodeboina M, Nagumo S, Munhoz D, Sonck J, Mileva N, Gallinoro E, et al. Simplified assessment of the index of microvascular resistance. J Interv Cardiol 2021;2021:9971874. 10.1155/2021/9971874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Bruyne B, Pijls NHJ, Gallinoro E, Candreva A, Fournier S, Keulards DCJ, et al. Microvascular resistance reserve for assessment of coronary microvascular function. J Am Coll Cardiol 2021;78:1541–1549. 10.1016/j.jacc.2021.08.017 [DOI] [PubMed] [Google Scholar]
- 9. Adjedj J, Toth GG, Johnson NP, Pellicano M, Ferrara A, Floré V, et al. Intracoronary adenosine: dose-response relationship with hyperemia. JACC Cardiovasc Interv 2015;8:1422–1430. 10.1016/j.jcin.2015.04.028 [DOI] [PubMed] [Google Scholar]
- 10. Jang HJ, Koo BK, Lee HS, Park JB, Kim JH, Seo MK, et al. Safety and efficacy of a novel hyperaemic agent, intracoronary nicorandil, for invasive physiological assessments in the cardiac catheterization laboratory. Eur Heart J 2013;34:2055––2062.. 10.1093/eurheartj/eht040 [DOI] [PubMed] [Google Scholar]
- 11. Liu X. Classification accuracy and cut point selection. Stat Med 2012;31:2676–2686. 10.1002/sim.4509 [DOI] [PubMed] [Google Scholar]
- 12. Yong AS, Layland J, Fearon WF, Ho M, Shah MG, Daniels D, et al. Calculation of the index of microcirculatory resistance without coronary wedge pressure measurement in the presence of epicardial stenosis. JACC Cardiovasc Interv 2013;6:53–58. 10.1016/j.jcin.2012.08.019 [DOI] [PubMed] [Google Scholar]
- 13. Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia. J Am Coll Cardiol 2010;55:2825–2832. 10.1016/j.jacc.2010.01.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou W, Lee JCY, Leung ST, Lai A, Lee TF, Chiang JB, et al. Long-term prognosis of patients with coronary microvascular disease using stress perfusion cardiac magnetic resonance. JACC Cardiovasc Imaging 2021;14:602–611. 10.1016/j.jcmg.2020.09.034 [DOI] [PubMed] [Google Scholar]
- 15. Patel MB, Bui LP, Kirkeeide RL, Gould KL. Imaging microvascular dysfunction and mechanisms for female-male differences in CAD. JACC Cardiovasc Imaging 2016;9:465–482. 10.1016/j.jcmg.2016.02.003 [DOI] [PubMed] [Google Scholar]
- 16. Dhawan SS, Corban MT, Nanjundappa RA, Eshtehardi P, McDaniel MC, Kwarteng CA, et al. Coronary microvascular dysfunction is associated with higher frequency of thin-cap fibroatheroma. Atherosclerosis 2012;223:384–388. 10.1016/j.atherosclerosis.2012.05.034 [DOI] [PubMed] [Google Scholar]
- 17. Boerhout CKM, de Waard GA, Lee JM, Mejia-Renteria H, Lee SH, Jung JH, et al. Prognostic value of structural and functional coronary microvascular dysfunction in patients with non-obstructive coronary artery disease; from the multicentre international ILIAS registry. EuroIntervention 2022;18:719–728. 10.4244/EIJ-D-22-00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van de Hoef TP, Lee JM, Boerhout CKM, de Waard GA, Jung JH, Lee SH, et al. Combined assessment of FFR and CFR for decision making in coronary revascularization. JACC Cardiovasc Interv 2022;15:1047–1056. 10.1016/j.jcin.2022.03.016 [DOI] [PubMed] [Google Scholar]
- 19. Everaars H, de Waard GA, Driessen RS, Danad I, van de Ven PM, Raijmakers PG, et al. Doppler flow velocity and thermodilution to assess coronary flow reserve: a head-to-head comparison with [15O]H2O PET. JACC Cardiovasc Interv 2018;20:2044–2054. 10.1016/j.jcin.2018.07.011 [DOI] [PubMed] [Google Scholar]
- 20. Demir OM, Boerhout CKM, de Waard GA, van de Hoef TP, Patel N, Beijk MAM, et al. Comparison of Doppler flow velocity and thermodilution derived indexes of coronary physiology. JACC Cardiovasc Interv 2022;15:1060–1070. 10.1016/j.jcin.2022.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Candreva A, Gallinoro E, van’t Veer M, Sonck J, Collet C, Di Gioia G, et al. Basics of coronary thermodilution. JACC Cardiovasc Interv 2021;14:595–605. 10.1016/j.jcin.2020.12.037 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.