Abstract
Aims
An observational nationwide all-comers prospective register study to analyse outcomes after coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI) in unprotected left main coronary artery (LMCA) disease.
Methods and results
All patients undergoing coronary angiography in Sweden are registered in the Swedish Web-system for Enhancement and Development of Evidence-based care in Heart disease Evaluated According to Recommended Therapies registry. Between 01/01/2005 and 12/31/2015, 11 137 patients with LMCA disease underwent CABG (n = 9364) or PCI (n = 1773). Patients with previous CABG, ST-elevation myocardial infarction (MI) or cardiac shock were excluded. Death, MI, stroke, and new revascularization during follow-up until 12/31/2015 were identified using national registries. Cox regression with inverse probability weighting (IPW) and an instrumental variable (IV), administrative region, were used. Patients undergoing PCI were older, had higher prevalence of comorbidity but lower prevalence of three-vessel disease. PCI patients had higher mortality than CABG patients after adjustments for known cofounders with IPW analysis (hazard ratio [HR] 2.0 [95% confidence interval (CI) 1.5–2.7]) and known/unknown confounders with IV analysis (HR 1.5 [95% CI 1.1–2.0]). PCI was associated with higher incidence of major adverse cardiovascular and cerebrovascular events (MACCE; death, MI, stroke, or new revascularization) than CABG, with IV analysis (HR 2.8 [95% CI 1.8–4.5]). There was a quantitative interaction for diabetic status regarding mortality (P = 0.014) translating into 3.6 years (95% CI 3.3–4.0) longer median survival time favouring CABG in patients with diabetes.
Conclusion
In this non-randomized study, CABG in patients with LMCA disease was associated with lower mortality and fewer MACCE compared to PCI after multivariable adjustment for known and unknown confounders.
Keywords: Left main coronary artery disease, Percutaneous coronary intervention, Coronary artery bypass grafting, Mortality, Instrumental variable analysis, Cox regression
Structured Graphical Abstract
Structured graphical abstract.
CABG = coronary artery bypass grafting, CAD = coronary artery disease, CI = confidence interval, PCI = percutaneous coronary intervention, IPW adj. = inverse probability weighting adjustment, IV adj. = instrumental variable adjusted; MACCE = major adverse cardiovascular and cerebrovascular events.
See the editorial comment for this article ‘How to treat left main coronary artery disease: the complementary lessons from trials and registries’, by M. McEntegart and T. Gori, https://doi.org10.1093/eurheartj/ehad287.
Introduction
Patients with untreated significant left main coronary artery (LMCA) disease have a poor prognosis and revascularization with coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI) is indicated.1–3 The 2018 ESC/EACTS Guidelines on myocardial revascularization, which relies on evidence from randomized clinical trials (RCTs) and meta-analyses, suggest equivalent results for the composite of death, myocardial infarction (MI), and stroke up to 5 years of follow-up when comparing CABG with PCI.4 There is a significant and beneficial interaction between time and PCI with regard to MI and peri-interventional stroke, however this is offset by risk of spontaneous MI and new revascularization during follow-up.4
The recommendations for choosing mode of revascularization of unprotected LMCA disease is based on coronary anatomical complexity assessed by the Synergy between PCI with Taxus and Cardiac Surgery (SYNTAX) score,5 individual cardiac and extracardiac characteristics, and patient preference. For patients with a low SYNTAX score (≤22), PCI and CABG have the same class and level of recommendation (I A), but for patients with intermediate SYNTAX score (23–32) the recommendation for CABG is I A and for PCI IIa A. Only CABG is recommended (I A) for patients with very complex coronary anatomy (SYNTAX score ≥33) and PCI is not recommended (III B) for such patients.4
The recommendations are, to a large extent, based on RCTs that have been conducted in highly selected cohorts. Consequently, the guidelines and the RCTs may not be fully applicable to the diversity of real-life patients and circumstances associated with revascularization of LMCA disease in clinical practice. Thus, we analysed the outcome of all subjects with LMCA disease undergoing revascularization with PCI or CABG in Sweden between 1 January 2005 and 31 December 2015.
Methods
Study population
All patients undergoing coronary angiography, PCI and CABG in Sweden are registered in the Swedish Web-system for Enhancement and Development of Evidence-based care in Heart disease Evaluated According to Recommended Therapies (SWEDEHEART) registry which includes the Swedish Coronary Angiography and Angioplasty Registry and the Swedish Cardiac Surgery Registry. All subjects that underwent coronary angiography in 28 PCI centres in Sweden during the 11 years from 1 January 2005, to 31 December 2015 were screened. We included 11 137 patients who had undergone revascularization with either PCI or CABG due to LMCA disease within 3 months after the index angiography. Exclusion criteria were previous CABG (excluding protected LMCA disease), ST-elevation MI and/or cardiogenic shock, subjects deemed and registered not being eligible for CABG, patients with data error or age <18 years, no revascularization within 90 days after coronary angiography, and PCI in left main with another device than first-, second-, or third-generation drug-eluting stent (DES) (Figure 1). The study was reviewed and approved by the local ethics committee in Stockholm, Sweden (2015/1258–31).
Figure 1.
Flowchart of patient selection. *Bare metal stent, bioresorbable vascular scaffold or self-expandable stent.
Outcomes
All subjects were followed through the Swedish Population Register for death, the Swedish National Patient Registry (NPR) for MI and stroke, and the SWEDEHEART registry for MI and repeat revascularization after the index angiography until the onset of outcome or until 31 December 2015. New revascularization was defined as new PCI or new CABG during follow-up. Major adverse cardiovascular and cerebrovascular events (MACCE) were defined as death, MI, stroke, or new revascularization (whichever occurred first) within the follow-up period.
Co-variates
Variables associated with mortality available in the SWEDEHEART and the NPR registers, as well as administrative region and year of treatment were used for adjustments. Multivariable analysis included the year of treatment, administrative region, body mass index, diabetes mellitus (diabetes mellitus diagnosis known to the patient, independently of treatment), insulin treatment (yes/no), estimated creatinine clearance, indication for revascularization [non-ST-elevation MI (NSTEMI)/unstable angina, stable angina], number of diseased coronary arteries (left main, left main + one-vessel, left main + two-vessel, left main + three vessel disease), smoking status (no, former smoker, current smoker), age at diagnosis, gender, previous MI, previous PCI, chronic obstructive pulmonary disease (COPD), peripheral artery disease, history of cancer, and dialysis. Hypertension was defined as treatment with anti-hypertensive drug and hyperlipidaemia as treatment with statins. COPD was defined as medication with bronchodilators or steroids for lung disease. Administrative region was categorized (Stockholm, Uppsala-Örebro, South-east, South, West, North). The risk of surgery was assessed using the European System for Cardiac Operative Risk Evaluation Score (EuroSCORE) II6 in CABG patients only. Creatinine clearance was calculated using the Cockcroft and Gault equation.7
Statistics
Baseline characteristics of patients in the PCI and CABG groups were described as frequencies for categorical variables and the mean value with standard deviations for continuous variables. The chi-square test and t-test were applied separately to compare the distribution of characteristics in the two groups at baseline. Due to an imbalance between the groups, we applied the inverse probability weighting (IPW) method to create a weighted population after which the distribution of characteristics was similar in the two groups.8 Propensity scores and standard mean differences prior and following IPW adjustments are presented in Supplementary data online, Figures S1 and S2.
A standard Cox regression model reporting hazard ratios (HRs) together with Laplace regression to compare the median survival time9 was used as the primary analysis to compare the PCI and CABG groups. The models were built on weighted population to calculate HR with 95% confidence interval (CI) and median of event free time between PCI and CABG patients as two comparable groups.
We performed instrumental variable (IV) analyses10,11 to adjust for unknown confounders. For an IV analysis, one must identify a naturally varying variable in the observed data which predicts the treatment that will be assigned to the individual patient. The IV variable must fulfil the following criteria: (i) it must be associated with the received treatment and (ii) it must not be associated directly or indirectly with the outcome, except through the effect of the treatment itself. We identified the variable administrative region (Stockholm, Uppsala-Örebro, South-east, South, West, North) to be associated with the received treatment, PCI or CABG (see Supplementary data online, Table S1). The variable administrative region is not expected to be associated with the outcome, except through the effect of the treatment itself. To test for the strength of the instrument variable, we examined the partial F-test, which predicts treatment as a function of the instrument and covariates. The partial F-test has the null hypothesis that the coefficient for the effect of the instrument in the first-stage regression model is zero.12 An F-statistic >10 indicates that the instrument is not weak. The F-value for the association of the IV, administrative region, with treatment (PCI or CABG) was 153 and the IV was considered valid. The IV might be imperfect, thus the following variables were entered in the IV regression to also adjust for known confounders; age, gender, number of diseased coronary arteries, hypertension, hyperlipidaemia, year of treatment, body mass index, diabetes mellitus, insulin treatment, estimated creatinine clearance, indication for revascularization, smoking status, previous MI, previous PCI, COPD, peripheral artery disease, history of cancer, and dialysis. All the analyses were performed using Stata MP 17.1. The significance level of the statistic tests was set to 0.05.
Results
A total of 11 137 patients were included of which 9364 (84%) patients had undergone CABG and 1773 (16%) PCI. The proportion of patients treated with PCI increased from 7% in 2005 to 34% in 2015 (see Supplementary data online, Table S1). There was a large regional variation in the choice of revascularization method ranging from 8% PCI in the South and West regions to 34% in the Uppsala-Örebro regions (see Supplementary data online, Table S1). Subjects undergoing PCI were three years older than CABG patients. Hyperlipidaemia, previous MI, previous PCI, reduced renal function and isolated left main stenosis were more common in the PCI than in the CABG group (Table 1). A history of smoking, diabetes mellitus, peripheral artery disease and LMCA disease combined with three vessel disease were more common in the CABG group. Most of the patients were revascularized due to NSTEMI and/or unstable angina, but chronic coronary syndrome (CCS) was significantly more common in the CABG group (Table 1).
Table 1.
Baseline characteristics in 11 137 patients with left main coronary artery disease who had percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) from January 1st 2005 to December 31st 2015
| PCI (n = 1773, 16%) | CABG (n = 9364, 84%) | P-value | |
|---|---|---|---|
| Age (years), mean (SD) | 72.8 (10.7) | 69.6 (8.8) | <0.001 |
| BMI (kg/m2), median (IQR) | 26.2 (24.0–28.9) | 26.6 (24.4–29.3) | 0.001 |
| Creatinine clearance (mL/min/1.73 m2), median (IQR) | 69.9 (52.1–92.3) | 79.2 (62.0–99.2) | <0.001 |
| Female sex, n (%) | 500 (28.2) | 1963 (21.0) | <0.001 |
| Smoking, n (%) | <0.003 | ||
| Former | 639 (37.6) | 3649 (42.4) | |
| Present | 226 (13.3) | 1340 (15.6) | |
| Hypertension, n (%) | 1124 (64.3) | 5648 (63.8) | 0.70 |
| Hyperlipidaemia, n (%) | 922 (52.9) | 5403 (61.2) | <0.001 |
| Diabetes, n (%) | 0.027 | ||
| Medical treated | 187 (10.6) | 1124 (12.6) | |
| Insulin treated | 151 (8.6) | 833 (9.4) | |
| Previous myocardial infarction, n (%) | 332 (19.1) | 1428 (16.3) | 0.004 |
| Previous PCI, n (%) | 133 (7.5) | 491 (5.2) | <0.001 |
| COPD, n (%) | 91 (5.1) | 301 (3.2) | <0.001 |
| Peripheral vascular disease, n (%) | 7 (0.4) | 62 (0.7) | 0.19 |
| History of cancer, n (%) | 147 (8.3) | 413 (4.4) | <0.001 |
| Indication, n (%) | <0.001 | ||
| CCS | 533 (30.1) | 3574 (38.2) | |
| NSTEMI/Unstable angina | 1240 (69.9) | 5790 (61.8) | |
| Dialysis, n (%) | 17 (1.0) | 65 (0.7) | 0.22 |
| Number of diseased vessels, n (%) | <0.001 | ||
| Isolated left main stenosis | 183 (10.3) | 414 (4.4) | |
| Left main + one vessel | 500 (28.2) | 1043 (11.1) | |
| Left main + two vessel | 589 (33.2) | 2498 (26.7) | |
| Left main + three vessel | 501 (28.3) | 5409 (57.8) | |
| Number of stents, n (%) | NA | ||
| 1 | 565 (31.9) | NA | |
| 2 | 538 (30.3) | NA | |
| 3 | 300 (16.9) | NA | |
| 4 | 205 (11.6) | NA | |
| 5 | 83 (4.7) | NA | |
| ≥6 | 82 (4.6) | NA | |
| Distal anastomoses, n (%) | NA | ||
| 1 | NA | 83 (1.0) | |
| 2 | NA | 1439 (18.0) | |
| 3 | NA | 3216 (40.3) | |
| 4 | NA | 2219 (27.8) | |
| ≥5 | NA | 702 (8.8) | |
| Unknown | NA | 315 (4.0) | |
| Left internal mammary artery, n (%) | NA | 7201 (90.3) | NA |
| Right internal mammary artery, n (%) | NA | 207 (2.6) | NA |
| Bilateral internal mammary artery, n (%) | NA | 194 (2.1) | NA |
BMI: body mass index; COPD: chronic obstructive pulmonary disease; CCS: chronic coronary syndrome; NSTEMI: non-ST-elevation myocardial infarction.
The CABG patients had a median EuroSCORE II risk of 4% (interquartile range 3%–7%), and 73% of the patients had normal left ventricular function. The left internal thoracic artery (ITA) was used in more than 90% of the cases, whereas both ITA in 2% of the cases. Three or more distal anastomoses were performed in 77% of the patients (Table 1).
Two-hundred and eighty-five patients (16%) had first generation, 1324 (75%) had second generation and 164 (9%) patients had third-generation DES in the LMCA (see Supplementary data online, Figure S3). One thousand five-hundred fifty-seven patients (88%) had one stent in the left main, 207 patients (11.5%) had two stents, and nine patients (0.5%) had three stents positioned in the left main.
Outcome
The median follow-up for the whole cohort was 4.7 years (interquartile range 2.1–7.6). The crude incidence rates for death, MI, revascularization, and MACCE were higher in the PCI group compared to the CABG group (Figure 2). IPW-adjusted HR (aHR) comparing PCI with CABG were significant for mortality (aHR 2.0, 95% CI 1.5–2.7), MI (aHR 4.0, 95% CI 2.9–5.5), new revascularization (aHR 5.1, 95% CI 3.8–7.0), and MACCE (aHR 2.5, 95% CI 1.9–3.2). After adjustment for unknown confounders in the IV analysis, the differences between groups were still evident for mortality (IV-HR 1.5, 95% CI 1.1–2.0), MI (IV-HR 6.1, 95% CI 1.4–26.3), new revascularization (IV-HR 14.0, 95% CI 5.8–33.6), and MACCE (IV-HR 2.8, 95% CI 1.8–4.5). There were no significant differences in HRs for stroke before (crude HR 1.2, 95% CI 0.95–1.2) or after adjustments (aHR 1.2, 95% CI 0.77–1.9 and IV-HR 5.1, 95% CI 0.72–35.8) (Figure 2).
Figure 2.
Outcome for PCI and CABG in left main coronary artery disease. The number of deaths, MI, stroke, revascularization, MACCE, and corresponding incidence rates for CABG and PCI, and crude and adjusted hazard ratios for PCI compared to CABG. IPW = inverse probability weighting, IV = instrumental variable.
Crude and IPW-adjusted one-minus survival curves for mortality, MI and stroke are presented in Supplementary data online, Figures S4–S9. Thirty-day event rates are presented in Supplementary data online, Table S2.
Of the 580 new revascularizations in the CABG group, 26 (4.5%) were done by CABG and 128 (22.0%) included revascularizations of one or more grafts. There were 235 new revascularizations in the PCI group of which 41(17%) included left main revascularization and 18 (7.7%) were done by CABG.
The incidence of hospitalizations for serious non-cardiac events (Alzheimer/Parkinson, bleeding, infection, chronic pulmonary obstructive disease, malignant tumor, peripheral artery disease, renal insufficiency, any trauma) was higher in the PCI group than in the CABG group (Supplementary data online, Figure S10).
Outcome in subgroups
IPW- aHRs for mortality (Figure 3) and MACCE (Figure 4) for PCI vs. CABG are presented for (i) left main and left main plus one vessel and left main plus two or three vessels, (ii) diabetes and no diabetes, (iii) age >70 years and age ≤70 years, (iv) women and men, and (v) for patients undergoing angiography at hospital with and without thoracic surgery. There was a significant quantitative interaction for diabetes status with a larger benefit for CABG vs. PCI in patients with diabetes with regard to mortality compared to non-diabetic patients (Figure 3). There was also an interaction for MACCE with regard to number of diseased vessels showing a larger benefit for CABG vs. PCI in patients with left main plus two or three vessels disease compared to left main only or left main plus one vessel disease (Figure 4). Patients ≤70 years had a larger benefit, with regard to MACCE for CABG vs. PCI, than patients >70 years (Figure 4).
Figure 3.
Inverse probability weighting adjusted hazard ratios for mortality for PCI vs. CABG in subgroups.
Figure 4.
Inverse probability weighting adjusted hazard ratios for MACCE for PCI vs. CABG in subgroups.
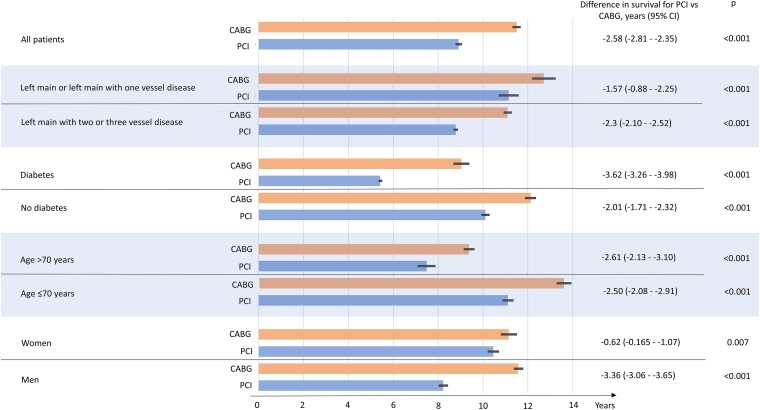
To illustrate the difference in expected survival between CABG and PCI, IPW-adjusted differences in median survival time for mortality in the whole cohort and subgroups are presented in Figure 5. There was a benefit in median survival time for CABG vs. PCI in the whole cohort as well as in the subgroups. Furthermore, there was a notable difference in IPW-adjusted median survival time of 3.6 years (95% CI 3.3–4.0) in favour of CABG compared to PCI in patients with diabetes.
Figure 5.
Inverse probability weighting adjusted median survival for mortality for PCI vs. CABG in the whole cohort and subgroups. Thin bar at point estimate = 95% confidence interval.
Discussion
The main finding in this observational non-randomized study of all-comers with LMCA disease was that CABG was associated with lower mortality and fewer cardiovascular or cerebrovascular events compared to PCI before and after multivariable adjustment for known and unknown confounders (Structured Graphical Abstract).
There are several factors that make the comparison between CABG and PCI challenging: (i) the unequal number of patients in the groups; (ii) substantial differences in baseline characteristics; (iii) unknown selection mechanisms for choice of the revascularization method; and (iv) the PCI group, consisting of more frail patients, had more hospitalisations due to non-cardiac events. Despite these challenges, it is important to analyse revascularization mode in relation to outcomes in observational registry studies to complement RCTs. In RCTs, the external validity is restrained by the selection of patients, omitting older, more frail patients with a large number of comorbidities that are treated in the cardiology department in everyday clinical practice.13–15
Adjustments for baseline characteristics
The treatment decision, CABG or PCI, for patients with left main coronary stenosis is based on a patient's pre-treatment characteristics and personal preference.4,16,17 If these are imbalanced and associated with the study outcome, the assessment of the treatment effect from an observational study suffers from bias caused by confounding by indication. We have thus adjusted for known confounders with the IPW method.8
Adjustments for unknown confounders
In our study, important confounders such as SYNTAX score, EuroSCORE (in the PCI group), left ventricular ejection fraction (in the PCI group), site of LMCA lesion (shaft vs. bifurcation), proportion of calcified lesions or chronic total occlusions and heart team assessment (yes vs. no) are missing. The selection of revascularization mode is to a large extent associated with administrative region (see Supplementary data online, Table S1). We used statistical modelling based on the IV to reduce bias due to unmeasured confounders. To use IV analysis, one must identify a naturally varying phenomenon in the observed data, which, like randomization in an RCT, predicts the treatment assigned to the individual patient. A valid instrument must fulfil some necessary criteria. First, the variable has to be strongly associated (F-test >10) with the treatment received. Second, it must not be directly or indirectly associated with the outcome except through the effect of the treatment itself. The variable with these statistical qualities is called an IV. We used the administrative region as the treatment-preference instrument. The administrative region is frequently employed as an instrument because this variable type usually fulfils the theoretical criteria for a valid instrument.10,11 Because administrative region may be an imperfect instrument, the following variables were entered into IV regression: age, gender, number of diseased coronary arteries, hypertension, hyperlipidemia, year of treatment, body mass index, diabetes mellitus, insulin treatment, estimated creatinine clearance, indication for revascularization, smoking status, previous MI, previous PCI, COPD, peripheral artery disease, history of cancer, and dialysis.10,11
Comparison to other studies on revascularisation strategies for LMCA disease
The two large landmark trials, the Nordic-Baltic-British Left Main Revascularization Study (NOBLE) and Evaluation of XIENCE Everolimus Eluting Stent Versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization (EXCEL) trial, evaluating PCI vs. CABG for LMCA disease have reported conflicting results.18–21 The NOBLE trial reported that CABG was superior to PCI in 1201 subjects up to 5 years of follow-up. The primary endpoint, a combination of all-cause mortality, non-procedural MI, any repeat coronary revascularization, and stroke, was more frequent in subjects assigned to PCI.
In the EXCEL trial, PCI and CABG showed comparable results with regard to the primary endpoint, a composite of death, stroke, or MI at 3 and 5 years of follow-up. Of note, total mortality was significantly higher in the PCI group than in the CABG group and CABG proved to be beneficial over PCI when adding revascularization to outcome at 5 years.19 The European Association for Cardio-Thoracic Surgery has withdrawn its support from the current recommendation on treatment of left main disease in the 2018 ESC/EACTS myocardial revascularization guidelines. They state that there is a significant survival advantage of CABG over PCI in the EXCEL trial and that the EXCEL investigators adopted a new definition for MI leading to results appearing to favour the PCI option.22
Patients in our observational study differ from the patients included in RCTs. The patients in our cohort were approximately 4 years older compared to subjects in the NOBLE and EXCEL trials. CCS was the predominant indication for index revascularization in the NOBLE and EXCEL trials (82% and 53%, respectively) whereas the rate of CCS was 30.9% in our cohort. In the NOBLE trial, complex lesions (chronic total occlusions, non-left main bifurcation lesions requiring two stent techniques or lesions with calcified or tortuous vessel morphology) were excluded and there was a high proportion of isolated left main disease.18 In the EXCEL trial, patients with high anatomical complexity of coronary artery disease as defined by a SYNTAX score of ≥33, were excluded. In our analysis, we have not excluded subjects based on the complexity of the coronary artery disease. In fact, almost half of the PCI patients had four or more stents. Furthermore, we have included patients that were stented with first-, second- and third-generation DES.
In a meta-analysis of individual patient data from four clinical randomised trials comparing 5-year outcomes for PCI with DES (n = 2197) with CABG (n = 2197) for LMCA disease, there was no significant difference in 5-year mortality between groups.18,19,23–26 However, complementary Bayesian analysis suggested a probable mortality difference (more likely than not <0.2% per year) in favour of CABG.26 This low, yet probable, excess risk for death within five years with PCI compared to CABG in patients eligible for RCTs could result in larger differences between groups in our study of consecutive all-comers including older, frailer patients with more comorbidities than patients in RCTs.
In a recent study from Canada, revascularization strategy in LMCA disease has been compared using clinical and administrative databases in Ontario.27 Like our study, Tam et al. reported that CABG was the preferred method for revascularization and propensity score matched analysis showed that PCI was associated with higher mortality (HR 1.63 [95% CI 1.42–1.87]) and higher MACCE rates (HR 1.77 [95% CI 1.57–2.00]). Additionally, differences in patient characteristics between the PCI and CABG groups were similar to our study. Patients who underwent PCI were older, more often women, more likely presented with an acute coronary syndrome event, but had fewer diseased coronary vessels.27
Possible mechanisms for differences in mortality
Invasive treatment with PCI or CABG restores flow in obstructive lesions in fundamentally different ways. With PCI, a DES is positioned in flow-limiting lesions after balloon dilatation, leaving non-flow-limiting lesions untreated. With CABG, a blood conduit (arterial or venous graft) is sewn distal to the flow-limiting lesion, creating a collateral flow. Through distal surgical collateralization, the graft prevents non-flow-limiting lesions from causing MIs. Establishing distal collateral blood flow is an important feature of CABG since new MIs frequently develop in non-flow-limiting coronary segments.28,29 Most deaths in high-risk patients with proximal and extensive coronary artery disease are caused by MIs.30 RCTs in patients with left main and three-vessel disease have shown that CABG decreased the occurrence of new MIs more than PCI.21,31,32 Our study showed that CABG was associated with fewer MIs and supports evidence from RCTs, thus adding weight to the hypothesis that surgical collateralization and a larger proportion of complete revascularization33 are possible mechanisms responsible for the mortality benefit for CABG vs. PCI.
Subgroup analyses
There was a significant quantitative interaction for diabetes with a large difference in aHR for mortality favouring CABG over PCI. The interaction translates into a median survival benefit of 3.6 years for CABG compared to PCI. In the EXCEL20 and NOBLE18 trials, there were no interactions for diabetes for the primary outcomes. However, we know from the randomized Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease trial31 that patients with diabetes and multivessel disease (excluding left main coronary stenosis) have a better outcome with CABG in comparison with PCI, including death from any cause. Our data reinforce diabetes as an important clinical factor when deciding on revascularization mode in LMCA disease. Effect modification for MACCE between concomitant coronary artery disease and type of revascularization has shown higher benefit for CABG in patients with more severe disease. This finding is line with the results from RCTs showing less repeat revascularization for CABG.18,20 This interaction could be mediated by more complete revascularization achieved with CABG.33 There was also a quantitative interaction for age group with a larger benefit in MACCE for CABG compared to PCI in patients ≤70 years. The relatively larger procedural risks with CABG compared to PCI might lessen the beneficial effects compared to PCI in older patients.
IPW-adjusted median survival time
Since HRs are measurements of relative effects and not absolute treatment effects, they may be difficult to communicate to patients and physicians when discussing revascularization options for LMCA disease. In a study of patients’ preferences, only 38% of the subjects with LMCA disease or three-vessel disease consented to go through CABG, although CABG was the recommended option over PCI (n = 763).17 Thus, we analysed the IPW-adjusted median survival time for PCI and CABG for the whole cohort and subgroups. The median survival time might be helpful when discussing revascularization modes with the patients. The benefits of CABG with a longer median survival time must be scrutinized against the disadvantages with open-heart surgery. Longer hospital stays and recovery time, and higher risk of perioperative complications can all be discussed between the patient and the heart team. The differences in median survival time favour CABG in all subgroups and there is a large difference in survival for CABG vs. PCI in diabetic patients.
Limitations
The data in the SWEDEHEART registry are prospectively collected and the analysis is a post hoc non-randomized comparison of patients undergoing PCI or CABG for LMCA disease. The data are observational, and we have tried to adjust for known imbalances in patient characteristics with IPW adjustment. We do not have data in all patients about the SYNTAX score, EuroSCORE, LVEF, site of LMCA lesion, heart team assessment, or completeness of revascularization. However, IV adjustment allows estimation of treatment effects in the presence of unmeasured confounding. A disadvantage with the IV analyses is a somewhat higher statistical uncertainty than with IPW adjustments, which manifests itself in larger CIs with less precise estimates and enhanced probability of type 2 error. Although IV adjustment models are designed to adjust for unknown confounders, residual confounding cannot be ruled out. The cohort of studied patients were entered into the registry between January 1st 2005 and December 31st 2015 and the selection of revascularization mode for patients with LMCA disease might differ in our study from today’s clinical practice.
Conclusion
In this non-randomized study, CABG in patients with LMCA disease was associated with lower mortality and fewer MACCE compared to PCI after multivariable adjustment for known and unknown confounders.
Supplementary Material
Acknowledgements
We would like to acknowledge the work collecting data from the procedures performed by the staff at each PCI- and Thoracic surgery centre in Sweden.
Contributor Information
Jonas Persson, Division of Cardiovascular Medicine, Department of Clinical Sciences, Karolinska Institutet, Danderyd University Hospital, Entrevägen 2, 182 88 Stockholm, Sweden.
Jacinth Yan, Division of Biostatistics, Institute of Environmental Medicine, Karolinska Institutet, Nobels väg 13, 17177 Stockholm, Sweden.
Oskar Angerås, Department of Cardiology, Sahlgrenska University Hospital, Blå stråket 5, 413 45 Gothenburg, Sweden.
Dimitrios Venetsanos, Division of Cardiology, Department of Medicine, Karolinska Institutet Solna and Karolinska University Hospital, Eugeniavägen 3, 171 76 Stockholm, Sweden.
Anders Jeppsson, Department of Cardiothoracic Surgery, Sahlgrenska University Hospital, Blå stråket 5, 413 46 Gothenburg, Sweden; Department of Molecular and Clinical Medicine, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Blå stråket 5B, 413 45 Gothenburg, Sweden.
Iwar Sjögren, Department of Cardiology, Falu Hospital, Lasarettsvägen 10, 791 82 Falun, Sweden.
Rikard Linder, Division of Cardiovascular Medicine, Department of Clinical Sciences, Karolinska Institutet, Danderyd University Hospital, Entrevägen 2, 182 88 Stockholm, Sweden.
David Erlinge, Clinical Sciences, Lund University, Sölvegatan 19, BMC I12, 221 84 Lund, Sweden.
Torbjörn Ivert, Department of Cardiothoracic Surgery, Karolinska University Hospital and Department of Molecular Medicine and Surgery, Karolinska Institutet, Eugeniavägen 3, 171 76 Stockholm, Sweden.
Elmir Omerovic, Department of Cardiology, Sahlgrenska University Hospital, Blå stråket 5, 413 45 Gothenburg, Sweden.
Supplementary data
Supplementary data is available at European Heart Journal online.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Funding
This work has been funded by the regional cooperation for medical research and healthcare development in Stockholm (ALF; 20140076; 20150422; 20130339).
References
- 1. Conley MJ, Ely RL, Kisslo J, Lee KL, McNeer JF, Rosati RA. The prognostic spectrum of left main stenosis. Circulation 1978;57:947–952. 10.1161/01.cir.57.5.947 [DOI] [PubMed] [Google Scholar]
- 2. Bittl JA, He Y, Jacobs AK, Yancy CW, Normand SL. Bayesian methods affirm the use of percutaneous coronary intervention to improve survival in patients with unprotected left main coronary artery disease. Circulation 2013;127:2177–2185. 10.1161/circulationaha.112.000646 [DOI] [PubMed] [Google Scholar]
- 3. Yusuf S, Zucker D, Peduzzi P, Fisher LD, Takaro T, Kennedy JW, et al. Effect of coronary artery bypass graft surgery on survival: overview of 10-year results from randomised trials by the coronary artery bypass graft surgery trialists collaboration. Lancet 1994;344:563–570. 10.1016/s0140-6736(94)91963-1 [DOI] [PubMed] [Google Scholar]
- 4. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 2019;40:87–165. 10.1093/eurheartj/ehy394 [DOI] [PubMed] [Google Scholar]
- 5. Sianos G, Morel MA, Kappetein AP, Morice MC, Colombo A, Dawkins K, et al. The SYNTAX score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention 2005;1:219–227. [PubMed] [Google Scholar]
- 6. Nashef SA, Sharples LD, Roques F, Lockowandt U. EuroSCORE II and the art and science of risk modelling. Eur J Cardiothorac Surg 2013;43:695–696. 10.1093/ejcts/ezs468 [DOI] [PubMed] [Google Scholar]
- 7. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976;16:31–41. 10.1159/000180580 [DOI] [PubMed] [Google Scholar]
- 8. Heinze G, Jüni P. An overview of the objectives of and the approaches to propensity score analyses. Eur Heart J 2011;32:1704–1708. 10.1093/eurheartj/ehr031 [DOI] [PubMed] [Google Scholar]
- 9. Bottai M, Zhang J. Laplace regression with censored data. Biom J 2010;52:487–503. 10.1002/bimj.200900310 [DOI] [PubMed] [Google Scholar]
- 10. Rassen JA, Schneeweiss S, Glynn RJ, Mittleman MA, Brookhart MA. Instrumental variable analysis for estimation of treatment effects with dichotomous outcomes. Am J Epidemiol 2009;169:273–284. 10.1093/aje/kwn299 [DOI] [PubMed] [Google Scholar]
- 11. Li J, Fine J, Brookhart A. Instrumental variable additive hazards models. Biometrics 2015;71:122–130. 10.1111/biom.12244 [DOI] [PubMed] [Google Scholar]
- 12. Bound J, Jaeger DA, Baker RM. Problems with instrumental variables estimation when the correlation between the instruments and the endogeneous explanatory Variable is weak. J Am Stat Assoc 1995;90:443–450. 10.2307/2291055 [DOI] [Google Scholar]
- 13. Frieden TR. Evidence for health decision making—beyond randomized, controlled trials. N Engl J Med 2017;377:465–475. 10.1056/NEJMra1614394 [DOI] [PubMed] [Google Scholar]
- 14. Rothwell PM. External validity of randomised controlled trials: “to whom do the results of this trial apply?”. Lancet 2005;365:82–93. 10.1016/s0140-6736(04)17670-8 [DOI] [PubMed] [Google Scholar]
- 15. Megaly M, Buda K, Mashayekhi K, Werner GS, Grantham JA, Rinfret S, et al. Comparative analysis of patient characteristics in chronic total occlusion revascularization studies: trials vs real-world registries. JACC Cardiovasc Interv 2022;15:1441–1449. 10.1016/j.jcin.2022.05.023 [DOI] [PubMed] [Google Scholar]
- 16. Gripenberg T, Jokhaji F, Östlund-Papadogeorgos N, Ekenbäck C, Linder R, Samad B, et al. Outcome and selection of revascularization strategy in left main coronary artery stenosis. Scand Cardiovasc J 2018;52:100–107. 10.1080/14017431.2018.1429648 [DOI] [PubMed] [Google Scholar]
- 17. Kim C, Hong SJ, Ahn CM, Kim JS, Kim BK, Ko YG, et al. Patient-centered decision-making of revascularization strategy for left main or multivessel coronary artery disease. Am J Cardiol 2018;122:2005–2013. 10.1016/j.amjcard.2018.08.064 [DOI] [PubMed] [Google Scholar]
- 18. Mäkikallio T, Holm NR, Lindsay M, Spence MS, Erglis A, Menown IBA, et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in treatment of unprotected left main stenosis (NOBLE): a prospective, randomised, open-label, non-inferiority trial. Lancet 2016;388:2743–2752. 10.1016/S0140-6736(16)32052-9 [DOI] [PubMed] [Google Scholar]
- 19. Stone GW, Kappetein AP, Sabik JF, Pocock SJ, Morice MC, Puskas J, et al. Five-year outcomes after PCI or CABG for left main coronary disease. N Engl J Med 2019;381:1820–1830. 10.1056/NEJMoa1909406 [DOI] [PubMed] [Google Scholar]
- 20. Stone GW, Sabik JF, Serruys PW, Simonton CA, Généreux P, Puskas J, et al. Everolimus-eluting stents or bypass surgery for left main coronary artery disease. N Engl J Med 2016;375:2223–2235. 10.1056/NEJMoa1610227 [DOI] [PubMed] [Google Scholar]
- 21. Holm NR, Mäkikallio T, Lindsay MM, Spence MS, Erglis A, Menown IBA, et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in the treatment of unprotected left main stenosis: updated 5-year outcomes from the randomised, non-inferiority NOBLE trial. Lancet 2020;395:191–199. 10.1016/s0140-6736(19)32972-1 [DOI] [PubMed] [Google Scholar]
- 22. European Association for Cardio-Thoracic Surgery . Changing Evidence, Changing Practice. https://www.eacts.org/changing-evidence-changing-practice/. 19 December 2019.
- 23. Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009;360:961–972. 10.1056/NEJMoa0804626 [DOI] [PubMed] [Google Scholar]
- 24. Morice M-C, Serruys PW, Kappetein AP, Feldman TE, Ståhle E, Colombo A, et al. Outcomes in patients with de novo left main disease treated with either percutaneous coronary intervention using paclitaxel-eluting stents or coronary artery bypass graft treatment in the synergy between percutaneous coronary intervention with TAXUS and cardiac surgery (SYNTAX) trial. Circulation 2010;121:2645–2653. 10.1161/CIRCULATIONAHA.109.899211 [DOI] [PubMed] [Google Scholar]
- 25. Park S-J, Kim Y-H, Park D-W, Yun S-C, Ahn J-M, Song HG, et al. Randomized trial of stents versus bypass surgery for left main coronary artery disease. N Engl J Med 2011;364:1718–1727. 10.1056/NEJMoa1100452 [DOI] [PubMed] [Google Scholar]
- 26. Sabatine MS, Bergmark BA, Murphy SA, O'Gara PT, Smith PK, Serruys PW, et al. Percutaneous coronary intervention with drug-eluting stents versus coronary artery bypass grafting in left main coronary artery disease: an individual patient data meta-analysis. Lancet 2021;398:2247–2257. 10.1016/S0140-6736(21)02334-5 [DOI] [PubMed] [Google Scholar]
- 27. Tam DY, Fang J, Rocha RV, Rao SV, Dzavik V, Lawton J, et al. Real-World examination of revascularization strategies for left main coronary disease in Ontario, Canada. JACC Cardiovasc Interv 2023;16:277–288. 10.1016/j.jcin.2022.10.016 [DOI] [PubMed] [Google Scholar]
- 28. Ambrose JA, Tannenbaum MA, Alexopoulos D, Hjemdahl-Monsen CE, Leavy J, Weiss M, et al. Angiographic progression of coronary artery disease and the development of myocardial infarction. J Am Coll Cardiol 1988;12:56–62. 10.1016/0735-1097(88)90356-7 [DOI] [PubMed] [Google Scholar]
- 29. Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation 1995;92:657–671. 10.1161/01.cir.92.3.657 [DOI] [PubMed] [Google Scholar]
- 30. Milojevic M, Head SJ, Parasca CA, Serruys PW, Mohr FW, Morice MC, et al. Causes of death following PCI versus CABG in Complex CAD: 5-year follow-up of SYNTAX. J Am Coll Cardiol 2016;67:42–55. 10.1016/j.jacc.2015.10.043 [DOI] [PubMed] [Google Scholar]
- 31. Farkouh ME, Domanski M, Sleeper LA, Siami FS, Dangas G, Mack M, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012;367:2375–2384. 10.1056/NEJMoa1211585 [DOI] [PubMed] [Google Scholar]
- 32. Head SJ, Davierwala PM, Serruys PW, Redwood SR, Colombo A, Mack MJ, et al. Coronary artery bypass grafting vs. percutaneous coronary intervention for patients with three-vessel disease: final five-year follow-up of the SYNTAX trial. Eur Heart J 2014;35:2821–2830. 10.1093/eurheartj/ehu213 [DOI] [PubMed] [Google Scholar]
- 33. Takahashi K, Serruys PW, Gao C, Ono M, Wang R, Thuijs D, et al. Ten-year all-cause death according to completeness of revascularization in patients with three-vessel disease or left main coronary artery disease: insights from the SYNTAX extended survival study. Circulation 2021;144:96–109. 10.1161/circulationaha.120.046289 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.