Abstract
To describe the impact of the US varicella vaccination program on severe varicella outcomes, we analyzed varicella hospitalizations using the National Inpatient Sample 1993–2019 and varicella deaths using the National Center for Health Statistics data 1990–2019. Over 25 years of vaccination program (1995–2019), varicella hospitalizations, and deaths declined 94% and 97%, respectively, among persons aged <50 years. Most of the decline (~90%) occurred during the 1-dose period (through 2006/2007) by attaining and maintaining high vaccination coverage; additional declines occurred during the 2-dose period, especially in the age groups covered by the 2-dose recommendation. The greatest decline for both hospitalizations and deaths (97% and >99%, respectively) was among persons aged <20 years, born during the varicella vaccination program. In the <20 age group, varicella hospitalization has become a rare event, and varicella deaths have been practically eliminated in the United States. A total of >10 500 varicella hospitalizations and 100 varicella deaths are now prevented annually in the United States as a direct result of vaccination and reduction in varicella-zoster virus circulation.
Keywords: Varicella, hospitalization, death, mortality, VZV, vaccine, program, impact
Varicella is an acute, highly contagious viral disease characterized by a generalized pruritic maculo-papulovesicular rash that appears in successive crops and rapidly develops crusts. Although most persons usually recover without serious complications, varicella can cause significant morbidity and mortality in otherwise healthy persons [1–3]. Complications include secondary bacterial infection of skin lesions sometimes resulting in bacteremia/sepsis; pneumonia; cerebellar ataxia; encephalitis; hemorrhagic conditions; and rarely (approximately 1 in 40 000 cases), varicella may result in death. Morbidity and mortality are higher in immunocompetent infants and adults, as well as in immunocompromised persons, compared with healthy children [2, 3]. Before the United States initiated its varicella vaccination program, varicella resulted in approximately 10 500–13 500 hospitalizations and 100–150 deaths annually; two thirds of hospitalizations and approximately half the deaths occurred in children [2–4].
The United States was the first country to introduce varicella vaccine into the routine childhood vaccination program, with 1 dose recommended at age 12–18 months in 1995 and 2 doses, at age 12–15 months and 4–6 years, recommended in 2007 [5]. The impact of the vaccine on severe varicella disease was evident within 5 years of program implementation: declines in varicella hospitalizations and deaths were either similar to or exceeded declines in incidence in all age groups, including infants and adults who were not vaccinated [6–8].
In this paper, we review trends in varicella hospitalizations and deaths over the 25 years of the US varicella vaccination program and provide new data from the mature 2-dose vaccination period.
METHODS
Hospitalizations: National (Nationwide) Inpatient Sample, 1993–2019
National Inpatient Sample (NIS) is the largest nationwide inpatient care data set publicly available in the United States [9]; it is a nationally representative sample of approximately 20% of annual discharges from all community hospitals (short-term, nonfederal, general, and specialty) in the United States. Discharge-level weights were provided to calculate national estimates of hospital discharges.
We defined varicella hospitalizations using the International Classification of Diseases, 9th Revision (ICD-9), Clinical Modification codes for 1993 through September 2015, and ICD 10th Revision (ICD-10) for October 2015 through 2019, as previously described [2, 10]: (1) varicella as the principal discharge code (052.xx; B01.xx); (2) postvaricella encephalitis (052.0; B01.11) or varicella pneumonitis (052.1; B01.2) in any diagnostic position; (3) varicella in any diagnostic position in a person with a severe immunocompromising condition (human immunodeficiency virus [HIV] infection, malignancy, severe defect of T-cell immunity, organ transplant recipient, or chemotherapy recipient); or (4) a well described potential complication of varicella as the principal discharge diagnosis and a varicella code in any subsequent position.
We excluded records with a varicella code that did not meet the above criteria that were considered incidental varicella hospitalizations (ie, the hospitalization was for a reason other than varicella) and records with coexistent herpes zoster (HZ) codes (053.xx; B02.xx). Analyses were limited to the first 7 diagnostic positions for consistency with previous analyses; during 1993–2019, varicella codes in these positions accounted for 97% of all varicella codes in NIS. We report trends and characteristics of hospitalizations comparing prevaccine years (1993–1995) with the end of the 1-dose period (2005–2006) and mature 2-dose period (2018–2019). We calculated overall and age-specific varicella hospitalization rates using US Census population estimates; the overall hospitalization rate was age-adjusted using the 2000 Census population. To calculate 95% confidence intervals (CIs) and to compare rates over time, a Z-test was used to account for survey standard errors. Two-sided P < .05 were considered statistically significant. SAS survey procedures in SAS 9.4 (SAS Institute, Inc., Cary, NC) were used for analysis.
Deaths: National Center for Health Statistics, 1990–2019
Data on varicella deaths for 1990–2019 were obtained from National Center for Health Statistics (NCHS)’s Mortality Multiple Cause-of Death public use records. A death from varicella was defined as one for which a varicella code (052.xx; B01.xx) was listed on the death certificate. Deaths were classified by NCHS with varicella as the underlying (the disease or injury that initiated the events that directly led to death), or a contributing (diseases/conditions that did not directly cause death but unfavorably influenced the course of disease or injury) cause of death [11].
We used US Census population estimates to calculate mortality rates overall (age-adjusted for the 2000 Census population) and age-specific. Previous analyses summarized varicella mortality during 1990–2016 [3, 7, 12, 13], and we updated trends through 2019. As in past analyses, persons at high risk for severe varicella were considered those with a severe immunocompromising condition (defined in the hospitalization section), and complications were grouped as secondary bacterial infection, pneumonia, complications affecting the central nervous system (CNS), and hemorrhagic conditions [3, 7]. Likewise, we used previously defined periods for prevaccine years (1990–1994) and end of the 1-dose period (2005–2007) [12, 13]; we defined the mature 2-dose period as 2017–2019. Because of the small number of deaths in the postvaccine period, for rate comparison we used the <20-, 20- to 49-, and ≥50-year age groups. We used Poisson regression to evaluate trends in rates and the Cochrane Armitage test to compare the trend in age distribution of deaths by study period. Two-sided P < .05 were considered statistically significant.
RESULTS
Hospitalizations: National Inpatient Sample, 1993–2019
Among hospital discharges with a varicella code in NIS during 1993–2019, after excluding 5.9% with a coexistent HZ code and 27% considered incidental varicella, there were an estimated total of 111 065 US varicella hospitalizations; 74 674 (67%) had varicella as the principal discharge code.
Trends in Varicella Hospitalizations
In the prevaccine period, 1993–1995, an average of 12 189 (95% CI, 7150–17 229) varicella hospitalizations occurred annually in the United States (Table 1). Persons aged <20 years accounted for 70% of hospitalizations, with children aged 1–4 years representing half of these. By 2005–2006, the annual average had declined to 2510 (95% CI, 743–5763) hospitalizations; persons aged <20 years represented 48% and children aged 1–4 years represented 12% of all hospitalizations. Additional declines occurred over the next decade; by 2018–2019, an average of 1390 (95% CI, 818‒1962) varicella hospitalizations occurred annually with persons aged <20 years representing one fifth (21%) and children 1–4 years represented 6% of all hospitalizations. The number of hospitalizations among persons aged ≥50 years remained relatively stable over time; however, this group increased from 5% to 22% to 44% of all varicella hospitalizations in 1993–1995, 2005–2006, and 2018–2019, respectively (P < .001).
Table 1.
Average Annual Number, Rates of Varicella Hospitalizations, and Percentage Decline of Hospitalization Rates by Age Group for Selected Years: 1993–1995 (Prevaccine), 2005–2006 (Mature 1-Dose Period), and 2018–2019 (Mature 2-Dose Period)—United States, 1993–2019a
| Age Group | Prevaccine 1993–1995 | Mature 1-Dose 2005–2006 | Mature 2-Dose 2018–2019 | % Decline in Hospitalization Ratesb | |||||
|---|---|---|---|---|---|---|---|---|---|
| Annual Average No. (%) | Rate/100000 | Annual Average No. (%) | Rate/100000 | Annual Average No. (%) | Rate/100000 | 2005–2006 vs 1993–1995 | 2018–2019 vs 1993–1995 | 2018–2019 vs 2005–2006 | |
| <1 | 1338 (11.0) | 34.8 | 291 (11.5) | 7.2 | 55 (3.9) | 1.9 | 79.2 | 95.9 | 80.1 |
| 1–4 | 4309 (35.4) | 27.2 | 308 (12.1) | 1.9 | 80 (5.8) | 0.5 | 92.9 | 98.1 | 74.0 |
| 5–9 | 2110 (17.3) | 11.1 | 292 (11.6) | 1.5 | 33 (2.4) | 0.1 | 86.4 | 98.6 | 89.3 |
| 10–19 | 816 (6.7) | 2.2 | 312 (12.4) | 0.7 | 118 (8.4) | 0.2 | 67.0 | 87.3 | 61.6 |
| <20 | 8574 (70.4) | 11.3 | 1204 (47.7) | 1.5 | 285 (20.5) | 0.3 | 87.1 | 96.9 | 76.2 |
| 20–49 | 3000 (24.6) | 2.5 | 758 (30.3) | 0.6 | 498 (35.8) | 0.4 | 76.1 | 84.7 | 36.0 |
| <50 | 11 573 (95.1) | 5.9 | 1961 (77.9) | 0.9 | 783 (56.3) | 0.4 | 84.1 | 93.8 | 60.7 |
| ≥50 | 598 (4.9) | 0.9 | 541(21.8) | 0.6 | 608 (43.7) | 0.5 | 30.6 | 40.7 | 14.5NS |
| Totalc | 12 189 (100) | 4.4 | 2510 (100) | 0.9 | 1390 (100) | 0.4 | 80.4 | 90.4 | 51.3 |
Abbreviations: NS, not statistically significant.
Numbers reported are averages of the annual weighted numbers; because of that, there may be some slight differences between the total number of cases and the sum of cases for all age groups.
Decline statistically significant unless otherwise indicated, P < .05.
Rates are age-adjusted for 2000 US Census population.
Rates of Varicella Hospitalizations
Overall, compared with the prevaccine period, varicella hospitalization rates declined 80% by 2005–2006 and 90% by 2018–2019, from an average rate of 4.4/100 000 population during 1993–1995 to 0.9 and 0.4/100 000 population, respectively (P < .001) (Table 1). During the 2-dose period, hospitalizations declined 51% compared with the 1-dose period (P < .001).
Hospitalization rates declined for all age groups (Table 1, Figure 1). By 2005–2006, the greatest decline (93%) was seen in children aged 1–4 years followed by children aged 5–9 years (86%); however, declines in infants and adults aged 20‒49 were also substantial (76%–79%). By 2018–2019, the declines had increased to 98% and 99% for children aged 1–4 and 5–9 years, respectively; infants and adults also experienced additional declines. Among persons aged <50 years, the declines were 84% and 94%, by 2005–2006 and 2018–2019, respectively. During the 2-dose period, the greatest relative decline occurred among the 5- to 9-year-old age group, 89% in 2018–2019 vs 2005–2006.
Figure 1.
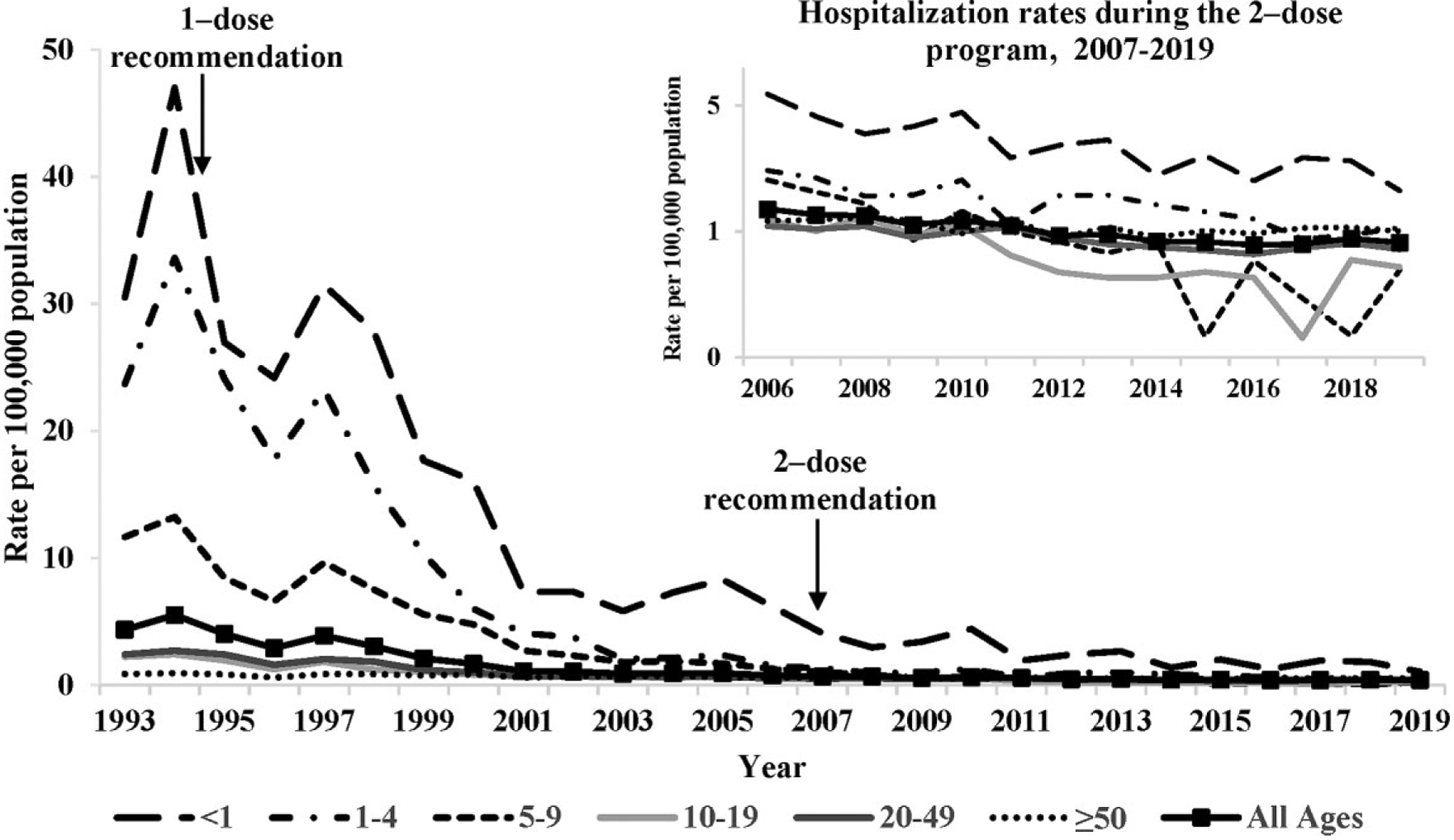
Varicella hospitalization rates by age group—United States, 1993–2019. The inset presents decline in hospitalization rates during the 2-dose program using the logarithmic scale.
Characteristics of Persons Hospitalized With Varicella
The median age of persons hospitalized with varicella increased from 5 years (interquartile range [IQR], 1–24) in the prevaccine period to 16 years (IQR, 3–41) by the end of the 1-dose period, to 44 years (IQR, 26–64) by 2018–19 (P < .001). For all years combined, there was a slight male and White race preponderance among those hospitalized, which was similar over the vaccination periods; the proportion of Blacks declined from 24% in prevaccine to 16% during the vaccine period (Supplementary Table 1).
An increase in the proportion of persons hospitalized with varicella who had severe underlying immunocompromising conditions was observed over time, from 16% in the prevaccine period to 30% by 2005–2006 and 26% by 2018–2019 (P < .001); malignancy was the main condition both prevaccine (47%) and during the vaccine period (58%) and was followed by HIV infection (35%) in the prevaccine and organ transplant (27%) during the vaccine period. Hospitalizations involving varicella and pregnancy averaged an annual number of 191 during 1993–1995, 14 in 2005–2006, and <10—(Actual numbers not shown. Per NIS policy, number of observations ≤10 can increase the risk for identification of persons and publication of values of 1–10 should be avoided.)—in 2018–2019 (P < .001).
In the prevaccine period, 80% of hospitalized patients with varicella had ≥1 complication; this proportion declined by 2005–2006 (67%) and 2018–2019 (68%) (P < .001). The trend differed by age group; by 2018–2019, the proportion of hospitalized patients with complications was 42% and 11% lower than in the prevaccine period among children aged <10 years and 10–19 years, respectively, whereas among adults it remained relatively constant over time. The average rate of complications in hospitalized patients declined from 3.7/100 000 population in the prevaccine period to 0.6 in 2005–2006 and 0.3/100 000 population in 2017–2018, a 92% decline from 1993–1995 to 2018–2019 (P < .001) (Supplementary Table 2). Fluid/electrolyte disturbance, soft/skin tissue infection, and lower respiratory infection were the most common complications of hospitalized varicella patients; their rates declined 93%–96% during the vaccination program. Sepsis was the second most common complication in 2018–2019 but still with a 67% decline from the prevaccine period.
Overall, the median length of stay was 3 days (IQR, 2–6), with no changes over time. Persons with immunocompromising conditions had a median length of stay 2 days longer, 5 days (IQR, 3–8).
Deaths: National Center for Health Statistics, 1990–2019
Trends in Varicella Deaths
Varicella-related deaths averaged 145 (range 129–160) per year in the prevaccine period (1990–1994) considering both underlying (n = 105) and contributing (n = 40) causes of death. The deaths started to decline within 5 years of program implementation (Figure 1, Table 2); by 2005–2007, the annual average was 35 deaths (combined underlying and contributing cause) and by 2017–2019, the annual average was 29 (Table 2). For the underlying cause of death, persons aged <20 years represented 45% of all deaths in the prevaccine period. This proportion declined to 11% by the end of the 1-dose period and to 2% in 2017–2019 (P < .001). Since 1999–2001, between 54% and 85% of varicella deaths have been reported among persons aged ≥50 years. A similar trend was seen for the contributing cause of death.
Table 2.
Average Annual Number and Rates of Varicella-Related Deaths, by Age Group (Selected Years) and Percentage Decline of Mortality Rates in 2005–2007 (Mature 1-Dose Period) and 2017–2019 (Mature 2-Dose Period) Compared with 1990–1995 (Prevaccine)—United States, 1990–2019
| Varicella Listed as Underlying Cause of Death | Varicella Listed as Contributing Cause of Death | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1990–1994a | 1999–2001b | 2005–2007c | 2012–2016d | 2017–2019e | 1990–1994a | 1999–2001b | 2005–2007c | 2012–2016d | 2017–2019 | |
| Total Average No. of Deaths per Year | 105 | 39.3 | 15 | 9.6 | 18.0 | 39.6 | 26.7 | 20.3 | 10.0 | 11.3 |
| Average No. (%) of Deaths per Year | ||||||||||
| Age Group (Years) | ||||||||||
| <1 | 8.8 (8.4) | 1.7 (4.3) | 0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 3 (7.6) | 0.7 (2.6) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
| 1–4 | 13.2 (12.6) | 1.3 (3.3) | 0.7 (4.4) | 0.2 (2.1) | 0.3 (1.9) | 5.2 (13.1) | 0.7 (2.6) | 0.7 (3.3) | 0.2 (2.0) | 0.0 (0.0) |
| 5–9 | 15.6 (14.9) | 2 (5.1) | 0.3 (2.2) | 0.4 (4.2) | 0.0 (0.0) | 3.2 (8.1) | 2.3 (8.6) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
| 10–19 | 10.4 (9.9) | 3.3 (8.4) | 0.7 (4.4) | 0 (0.0) | 0.0 (0.0) | 2.2 (5.6) | 1 (3.7) | 0.3 (1.6) | 0.0 (0.0) | 0.0 (0.0) |
| <20 | 48 (45.2) | 8.3 (21.6) | 1.7 (11.1) | 0.6 (6.3) | 0.3 (1.9) | 13.6 (34.4) | 4.7 (17.5) | 1 (4.9) | 0.2 (2.0) | 0.0 (0.0) |
| 20–49 | 35.6 (33.9) | 9.7 (24.7) | 3.3 (22.2) | 1.8 (18.8) | 2.3 (13.0) | 11.6 (29.3) | 7 (26.3) | 3.0 (14.8) | 2.4 (24.0) | 1.7 (14.7) |
| <50 | 83.6 (79.6) | 18 (45.8) | 5 (33.3) | 2.4 (25.0) | 2.7 (14.8) | 25.2 (63.7) | 11.7 (43.8) | 4 (19.7) | 2.6 (26.0) | 1.7 (14.7) |
| ≥50 | 21.4 (20.4) | 21.3 (54.2) | 10.0 (66.7) | 7.2 (75.0) | 15.3 (85.2) | 14.4 (36.4) | 15 (56.2) | 16.3 (80.3) | 7.4 (74.0) | 9.6 (85.3) |
| Mortality Rates/1 Million Population | ||||||||||
| <20 | 0.65 | 0.10 | 0.02 | 0.007 | 0.004 | 0.18 | 0.06 | 0.01 | 0.002 | 0.00 |
| 20–49 | 0.30 | 0.08 | 0.03 | 0.014 | 0.02 | 0.1 | 0.06 | 0.02 | 0.02 | 0.01 |
| <50 | 0.44 | 0.09 | 0.02 | 0.01 | 0.01 | 0.13 | 0.06 | 0.02 | 0.01 | 0.01 |
| ≥50 | 0.33 | 0.28 | 0.11 | 0.07 | 0.13 | 0.22 | 0.2 | 0.18 | 0.07 | 0.08 |
| Overallf | 0.41 | 0.14 | 0.05 | 0.03 | 0.05 | 0.16 | 0.10 | 0.06 | 0.03 | 0.03 |
| %Decline in Mortality Ratesg | ||||||||||
| 2005–2007 vs. | 2017–2019 vs. | 2005–2007 vs. | 2017–2019 vs. | |||||||
| 1990–1994 | 1990–1994 | 1990–1994 | 1990–1994 | |||||||
| <20 | 96.9 | 99.4 | 94.4 | 100.0 | ||||||
| 20–49 | 90.0 | 94.0 | 80.0 | 87.1 | ||||||
| <50 | 95.5 | 97.1 | 84.6 | 93.9 | ||||||
| ≥50 | 66.7 | 59.8 | 18.2NS | 61.9 | ||||||
| Overall | 87.8 | 88.9 | 62.5 | 82.1 | ||||||
Abbreviations: NS, not statistically significant.
NOTE: Percentage decline 2017–2019 vs 2005–2007: for varicella as the underlying cause of death: 9.2% overall, by age group: 79.6% in <20 years, 39.9% in 20–49 years, 36.9% in <50 years, −20.7% in ≥50 years; for varicella as the contributing cause of death: 52.3% overall, by age group: 100% in <20 years, 35.6% in 20–49 years, 60.5% in <50 years, 53.5 in ≥50 years.
Data from Meyer et al [3].
Data from Nguyen et al [7].
Data from Marin et al [12].
Data from Leung et al [13].
During 2017–2019, the annual numbers of varicella deaths with varicella as the underlying cause of death were 16, 20, and 18 in 2017, 2018, and 2019, respectively. During this period, 1 death occurred among persons aged <20 years, in a foreign-born child in the 1- to 4-year-old group (2019). Among 20- to 49-year-olds, the annual numbers of varicella deaths were 4, 2, and 1 in 2017, 2018, and 2019, respectively.
Rates are age-adjusted for 2000 US Census population.
Decline statistically significant unless otherwise indicated, P < .05.
Including underlying and contributing causes of death, varicella deaths among children and adolescents aged 1–19 years declined to 10–11/year early in the 1-dose period (1999–2001) and have been rare during 2007–2019 (<1 death/year), compared with an average of 50/year in the prevaccine period. Among children aged <1 year, by 1999–2001 the number of deaths was 2/year, and no deaths occurred during 2004–2019 compared with an average of 12/year in the prevaccine period.
Varicella Mortality Rates
Overall, compared with the prevaccine period, the age-adjusted mortality rate attributed to varicella as the underlying cause of death declined 88% by 2005–2007 and 89% by 2017–2019, from an average rate of 0.41/million population to 0.05/million population (Table 2, Supplementary Figure 1). Most of the decline occurred during the 1-dose period; during the 2-dose period, mortality rates declined 9% compared with the 1-dose period (P=.203).
Mortality rates declined for all age groups (Table 2). By 2017–2019, compared with the prevaccine period, the age-specific mortality rates attributed to varicella as the underlying cause of death declined >99% for persons aged <20 years, 94% for persons aged 20–49 years and 60% for those aged ≥50 years (Figure 2). As with the overall mortality rate, most of the decline occurred during the 1-dose period when the rates declined 90%–97% among those <50 years. Substantial declines (82% overall) occurred in the rates attributed to varicella as a contributing cause of death, with 100% decline in persons aged <20 years (2017–2019 vs. 1990–1994).
Figure 2.
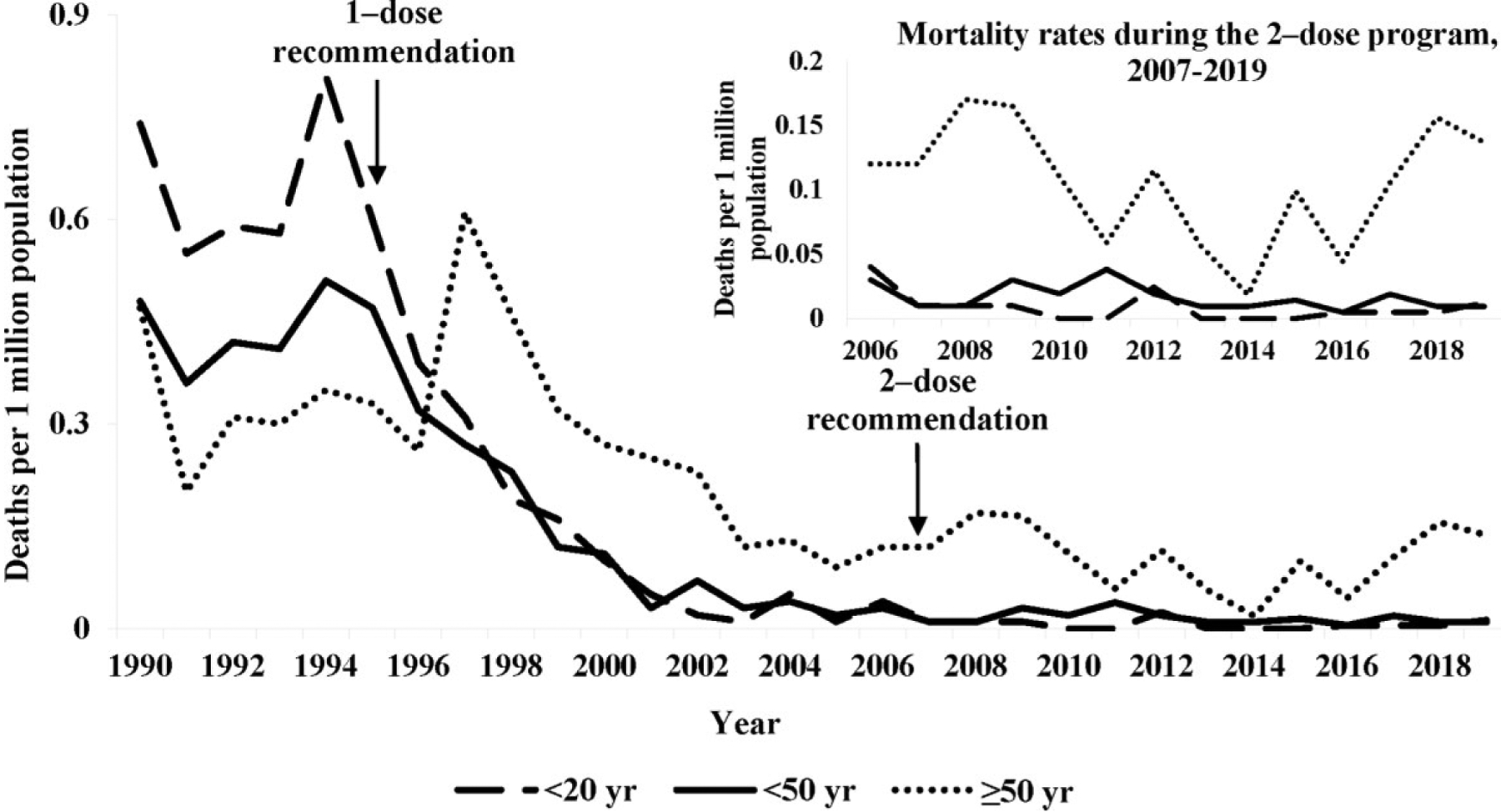
Varicella-related mortality rates for varicella as underlying cause by age group—United States, 1990–2019. The mortality rate for <20 years age group was zero during 2010, 2011, 2013, 2014, and 2015.
Characteristics of Persons Who Died With Varicella
Among decedents for which varicella was the underlying cause of death, throughout the study periods, more than half were males and more than three quarters were Whites; the proportion of Blacks declined from 18% in prevaccine to approximately 7% during the vaccine period (Supplementary Table 3). For persons with varicella as the underlying cause of death, high-risk pre-existing medical conditions declined from being listed on 19% (99 of 525) of varicella death certificates in 1990–1994 to 5.6% (3 of 54) in 2017–2019. All 3 high-risk conditions in 2017–2019 were malignancies and were listed among those aged ≥50 years; prevaccine and throughout the program, persons aged ≥20 years represented the majority of decedents with high-risk conditions. In the prevaccine and early vaccine years, malignancy represented 61%–63% of all high-risk conditions; since 1999, malignancies have accounted for 81%–100% of high-risk conditions. Human immunodeficiency virus infection was the second most common high-risk condition in the prevaccine and early vaccine program years (32%–33%), with no reports since 1999.
Among deaths for which varicella was the underlying cause, at least 1 varicella-associated complication was listed for 89% of deaths in 2017–2019 compared with 39% in the prevaccine period and 73% in 2005–2007. During 2017–2019, the most common complications were CNS-associated (67% [84% in persons aged ≥50 years, none in persons aged <20 years], mostly encephalitis), secondary bacterial infection (29%, mostly sepsis), and pneumonia (25%). This pattern of complications differed from the prevaccine period when pneumonia represented 45% of all complications, followed by secondary bacterial infection (33%) and hemorrhagic complications (20%).
DISCUSSION
During the 25 years of the US varicella vaccination program implementation, severe disease was dramatically reduced in the United States, with near elimination of varicella deaths among persons aged <50 years. In this age group, hospitalizations declined 94% and deaths declined 97%. The greatest decline for both hospitalizations and deaths occurred among the cohort of persons born during the varicella vaccination program, with 97% and >99% declines, respectively, among those aged <20 years. Consistent with the high effectiveness of 1 dose of varicella vaccine in preventing severe varicella [14], most of the decline occurred during the 1-dose period concurrently with achieving and sustaining high 1-dose varicella vaccination coverage among young children [15, 16]. The second dose was introduced in 2007 to further decrease varicella disease and outbreaks that, although less frequent and much smaller, continued to occur in highly vaccinated school children [17–19]. Nevertheless, additional declines in severe disease, especially in the age groups covered by the 2-dose recommendation, occurred during the 2-dose program.
Although rates inform on the frequency of an event and allow comparisons among populations, absolute numbers are also instructive to define burden in a population. Varicella hospitalizations were not uncommon in the prevaccine era in the United States, with >12 000 hospitalizations each year estimated in our study, more than two thirds (approximately 8500) of which occurred among persons aged <20 years. The declines in hospitalizations have been dramatic: 25 years into the program, <1400 hospitalizations occurred annually, with persons aged <20 years representing one fifth of all hospitalizations (<300).
A dramatic decline also was documented for varicella deaths, such that by the end of the 1-dose period, varicella deaths had declined 96% among persons aged <50 years. Among persons aged <20 years, varicella deaths were essentially eliminated with a decline of 97% by 2005–2007 and >99% by 2017–2019. Starting with 2001, there were years in which consistently no varicella deaths were reported in 1 or more of the age groups <20 years; during the 2-dose program period, 2008–2019, only a total of 6 deaths occurred in this age group.
Special mention is warranted for decreasing trends in severe varicella outcomes among infants and persons aged ≥20 years. Infants are not eligible for vaccination; therefore, the >1250 infant hospitalizations that are prevented each year result from high population immunity and reduced transmission, which is attributable to the varicella vaccination program. For age groups beyond childhood, concern was raised before vaccine implementation that a universal childhood varicella vaccination program could shift the varicella burden to older ages and result in more severe disease [20, 21]. This has not occurred. Hospitalization data showed a continuous decline of numbers and rates among adults: 76% by the end of the 1-dose period and 85% by 2018–2019 for those aged 20–49 years, with smaller declines among those aged ≥50 years (31%–41%). The increase seen in the median age at hospitalization is due to the larger relative decrease in hospitalizations among the younger age groups who are recommended for routine vaccination and does not indicate an increase in the burden among older ages. Likewise, deaths declined by 94% among persons aged 20–49 years and 60% among those aged ≥50 years by 2017–2019. The decline in varicella hospitalizations during pregnancy, from several hundred to <10 per year, is of great benefit because of the additional risk of congenital varicella syndrome if varicella is acquired during the first 20 weeks of pregnancy [22]. The recommendation for antenatal screening and postpartum vaccination of susceptible women [5] is an important adjunct to the pediatric vaccination program to prevent congenital varicella.
Data suggest that the severity of presentations among hospitalized persons might be declining. Approximately one third of records in NIS did not include a complication for persons hospitalized for varicella during the vaccination program, compared with one fifth in the prevaccine period. In a review of the reason for hospitalization in a convenience sample of hospitalizations from 27 states (Supplementary Appendix), we found that 29% of patients aged <50 years were hospitalized for observation, suggesting that those hospitalizations might not necessarily reflect severe disease. We observed an increase in the proportion of persons with high-risk immunocompromising conditions among persons hospitalized with varicella; from our experience reviewing the hospitalization data, some patients with varicella, and especially vaccinated patients, were hospitalized as a precaution because of their increased risk for developing severe disease. On the other hand, our data showing the decline in high-risk medical conditions (eg, malignancy) noted on varicella death certificates support the fact that with the increases in vaccination coverage, children and adolescents in particular are being vaccinated before acquiring the condition that would put them at risk for severe disease, and therefore they have added protection against severe disease.
The impressive declines in hospitalizations and deaths are the direct consequence of the vaccination program, either through direct protection of those vaccinated or through indirect effects due to high immunity in the population and decreased transmission. The 1-dose program focused initially on children aged 12–18 months; nationwide, coverage among children aged 19–35 months increased progressively from 26% in 1997 to 85% in 2003 and has remained ≥90% since 2007 [15, 16]. With the aging of the child cohorts and implementation of elementary, middle, and high school requirements for varicella vaccine, high 1-dose vaccine coverage has also been attained among adolescents aged 13–17 years: 76% in 2007 and approximately 95% since 2013 [16, 23]. Additional control of the disease and in the circulation of the varicella-zoster virus (VZV) was achieved with the implementation of the second dose. Among adolescents aged 13–17 years, 2-dose coverage increased from 34% in 2008 to 81% in 2014 and ≥90% in 2018–2020 [16, 23].
Postlicensure experience has demonstrated that 1 dose of varicella vaccine is approximately 82% effective for preventing varicella of any severity but has a much higher effectiveness (97–100%) for prevention of severe varicella, defined as either ≥500 lesions or presence of complications and hospitalizations [14]. The second dose adds improved protection against varicella of any severity (92%). We documented additional declines in hospitalizations and deaths during the second dose period.
Our study has several limitations. Vaccination status is not included in the available databases. Analyses relied on diagnostic coding, which are subject to coding errors. For example, misclassification or miscoding of HZ as varicella was possible. Considering the epidemiology of varicella and HZ, cases of VZV infection among persons aged ≥50 years are more likely to represent HZ and some complications or immunocompromising conditions among hospitalized patients suggest HZ. A small study that validated death certificates for varicella diagnoses reported a positive predictive value of a varicella code among persons aged ≥50 years of 43% [24]. For hospitalizations, we detected seasonal distribution (characteristic for varicella) among persons aged ≥50 years through the early 2000s, although it was less pronounced than among those aged <50 years. Because a proportion of reported varicella hospitalizations and deaths in persons aged ≥50 years may be due to varicella, we included all ages for comprehensiveness but focused our discussion on persons aged <50 years. The reasons for hospitalization by vaccination status were described in persons aged <50 years and using a convenience sample. Different baseline periods were used for hospitalizations and deaths based on previous publications and availability of data.
CONCLUSIONS
We documented the impressive impact of 25 years of the US varicella vaccination program on varicella hospitalizations and deaths, with 94% and 97% declines, respectively, among persons aged <50 years. Most of this decline occurred during the 1-dose period by attaining and maintaining high vaccination coverage, but improved implementation of the 1-dose and the addition of the second dose led to additional declines during the 2-dose period. The most dramatic declines occurred among persons aged <20 years who were born during the vaccination program and were therefore routinely vaccinated; deaths in this age group have been practically eliminated in the United States. Hospitalization occurs at very low rates, but a sizable proportion may represent conservative practice rather than severe disease. Not only did we not observe the feared shift of varicella into adulthood, we observed significant declines in hospitalizations and deaths among adults via indirect protection from the varicella vaccination program. More than 10 500 varicella hospital admissions and 100 deaths are now being prevented every year in the United States; this is an underestimate considering the population growth over 25 years. Most of the hospitalizations and deaths continue to occur in persons without apparent contraindications to vaccination and are therefore potentially preventable.
Supplementary Material
Acknowledgments.
We thank Dr. Jane Seward for scientific review and Mary Ann Kirkconnell Hall of the Centers for Disease Control and Prevention for the editorial review of the manuscript.
Financial support.
No financial support was received for this work.
Footnotes
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Supplement sponsorship. This supplement is sponsored by the Centers for Disease Control and Prevention, Atlanta, GA, USA.
References
- 1.LaRussa PS, Marin M, Gershon AA. Varicella-zoster virus (chapter 280). In Kliegman RM, St. Geme JW, eds. Nelson’s Textbook of Pediatrics, 21th ed. Philadelphia: Elsevier, 2020: pp 374–81. [Google Scholar]
- 2.Galil K, Brown C, Lin F, et al. Hospitalizations for varicella in the United States, 1988 to 1999. Pediatr Infect Dis J 2002; 21:931–5. [DOI] [PubMed] [Google Scholar]
- 3.Meyer PA, Seward JF, Jumaan AO, et al. Varicella mortality: trends before vaccine licensure in the United States, 1970–1994. J Infect Dis 2000; 182:383–90. [DOI] [PubMed] [Google Scholar]
- 4.Davis MM, Patel MS, Gebremariam A. Decline in varicella-related hospitalizations and expenditures for children and adults after introduction of varicella vaccine in the United States. Pediatrics 2004; 114:786–92. [DOI] [PubMed] [Google Scholar]
- 5.Marin M, Guris D, Chaves SS, Schmid S, Seward JF. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56:1–40. [PubMed] [Google Scholar]
- 6.Zhou F, Harpaz R, Jumaan AO, Winston CA, Shefer A. Impact of varicella vaccination on health care utilization. JAMA 2005; 294:797–802. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen HQ, Jumaan AO, Seward JF. Decline in mortality due to varicella after implementation of varicella vaccination in the United States. N Engl J Med 2005; 352:450–8. [DOI] [PubMed] [Google Scholar]
- 8.Seward JF, Watson BM, Peterson CL, et al. Varicella disease after introduction of varicella vaccine in the United States, 1995–2000. JAMA 2002; 287:606–11. [DOI] [PubMed] [Google Scholar]
- 9.Healthcare Cost and Utilization Project (HCUP). Overview of the National (Nationwide) Inpatient Sample (NIS). Available at: https://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed 12 March 2021.
- 10.Lopez AS, Zhang J, Brown C, et al. Varicella-related hospitalizations in the United States, 2000–2006: the 1-dose varicella vaccination era. Pediatrics 2011; 127:238–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Center for Health Statistics. NCHS instruction manuals for classifying underlying and multiple, part 2. Available at: https://www.cdc.gov/nchs/nvss/instruction-manuals.htm. Accessed 12 March 2021.
- 12.Marin M, Zhang JX, Seward JF. Near elimination of varicella deaths in the US after implementation of the vaccination program. Pediatrics 2011; 128:214–20. [DOI] [PubMed] [Google Scholar]
- 13.Leung J, Marin M. Update on trends in varicella mortality during the varicella vaccine era-United States, 1990–2016. Hum Vaccin Immunother 2018; 14:2460–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marin M, Marti M, Kambhampati A, Jeram SM, Seward JF. Global varicella vaccine effectiveness: a meta-analysis. Pediatrics 2016; 137:e20153741. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Varicella vaccination coverage among children 19–35 months by State, HHS Region, and the United States, National Immunization Survey-Child (NIS-Child), 1996 through 2017. Available at: https://www.cdc.gov/vaccines/imz-managers/coverage/childvaxview/data-reports/varicella/trend/index.html. Accessed 15 March 2021.
- 16.Elam-Evans LD, Valer MR, Fredua B, et al. Celebrating 25 years of varicella vaccination coverage for children and adolescents in the United States: a success story. J Infect Dis 2022; 226(Suppl 4):S416–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Civen R, Lopez AS, Zhang J, et al. Varicella outbreak epidemiology in an active surveillance site, 1995–2005. J Infect Dis 2008; 197(Suppl 2):S114–9. [DOI] [PubMed] [Google Scholar]
- 18.Lopez AS, Guris D, Zimmerman L, et al. One dose of varicella vaccine does not prevent school outbreaks: is it time for a second dose? Pediatrics 2006; 117:e1070–7. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC). Outbreak of varicella among vaccinated children–Michigan, 2003. MMWR Morb Mortal Wkly Rep 2004; 53:389–92. [PubMed] [Google Scholar]
- 20.Ross LF, Lantos JD. Immunisation against chickenpox. BMJ 1995; 310:2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brisson M, Edmunds WJ. Economic evaluation of vaccination programs: the impact of herd-immunity. Med Decis Making 2003; 23:76–82. [DOI] [PubMed] [Google Scholar]
- 22.Enders G, Miller E, Cradock-Watson J, et al. Consequences of varicella and herpes zoster in pregnancy: prospective study of 1739 cases. Lancet 1994; 343:1548–51. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. Varicella vaccination coverage (with and without history disease) among adolescents 13–17 years by State, HHS Region, and the United States, National Immunization Survey-Teen (NIS-Teen), 2008 through 2019. Available at: https://www.cdc.gov/vaccines/imz-managers/coverage/teenvaxview/data-reports/varicella/trend/index.html. Accessed March 16 2021.
- 24.Galil K, Pletcher MJ, Wallace BJ, et al. Tracking varicella deaths: accuracy and completeness of death certificates and hospital discharge records, New York State, 1989–1995. Am J Public Health 2002; 92:1248–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


