Abstract
Background
Massage and aromatherapy massage are used to relieve cancer‐related symptoms. A number of claims have been made for these treatments including reduction of pain, anxiety, depression, and stress. Other studies have not shown these benefits.
Objectives
To evaluate the effects of massage with or without aromatherapy on pain and other symptoms associated with cancer.
Search methods
We searched the following databases and trials registries up to August 2015: the Cochrane Central Register of Controlled Trials (CENTRAL, 2015, Issue 7), MEDLINE (Ovid), EMBASE (Ovid), PsycINFO (Ovid), CINAHL (EBSCO), PubMed Cancer Subset, SADCCT, and the World Health Organization (WHO) ICTRP. We also searched clinical trial registries for ongoing studies.
Selection criteria
Randomised controlled studies (RCTs) reporting the effects of aromatherapy or massage therapy, or both, in people with cancer of any age. We applied no language restrictions. Comparators were massage (using carrier oil only) versus no massage, massage with aromatherapy (using carrier oil plus essential oils) versus no massage, and massage with aromatherapy (using carrier oil plus essential oils) versus massage without aromatherapy (using carrier oil only).
Data collection and analysis
At least two review authors selected studies, assessed the risk of bias, and extracted data relating to pain and other symptoms associated with cancer, using standardised forms. We assessed the evidence using GRADE (Grading of Recommendations Assessment, Development and Evaluation) and created two 'Summary of findings' tables.
Main results
We included 19 studies (21 reports) of very low quality evidence with a total of 1274 participants. We included 14 studies (16 reports) in a qualitative synthesis and five studies in a quantitative synthesis (meta‐analysis). Thirteen studies (14 reports, 596 participants) compared massage with no massage. Six studies (seven reports, 561 participants) compared aromatherapy massage with no massage. Two studies (117 participants) compared massage with aromatherapy and massage without aromatherapy. Fourteen studies had a high risk of bias related to sample size and 15 studies had a low risk of bias for blinding the outcome assessment. We judged the studies to be at unclear risk of bias overall. Our primary outcomes were pain and psychological symptoms. Two studies reported physical distress, rash, and general malaise as adverse events. The remaining 17 studies did not report adverse events. We downgraded the GRADE quality of evidence for all outcomes to very low because of observed imprecision, indirectness, imbalance between groups in many studies, and limitations of study design.
Massage versus no‐massage groups
We analysed results for pain and anxiety but the quality of evidence was very low as most studies were small and considered at an unclear or high risk of bias due to poor reporting. Short‐term pain (Present Pain Intensity‐Visual Analogue Scale) was greater for the massage group compared with the no‐massage group (one RCT, n = 72, mean difference (MD) ‐1.60, 95% confidence interval (CI) ‐2.67 to ‐0.53). Data for anxiety (State‐Trait Anxiety Inventory‐state) relief showed no significant difference in anxiety between the groups (three RCTs, n = 98, combined MD ‐5.36, 95% CI ‐16.06 to 5.34). The subgroup analysis for anxiety revealed that the anxiety relief for children was greater for the massage group compared with the no‐massage group (one RCT, n = 30, MD ‐14.70, 95% CI ‐19.33 to ‐10.07), but the size of this effect was considered not clinically significant. Furthermore, this review demonstrated no differences in effects of massage on depression, mood disturbance, psychological distress, nausea, fatigue, physical symptom distress, or quality of life when compared with no massage.
Massage with aromatherapy versus no‐massage groups
We analysed results for pain, anxiety, symptoms relating to the breast, and quality of life but the quality of evidence was very low as studies were generally at a high risk of bias. There was some indication of benefit in the aromatherapy‐massage group but this benefit is unlikely to translate into clinical benefit. The relief of medium‐ and long‐term pain (medium‐term: one RCT, n = 86, MD 5.30, 95% CI 1.52 to 9.08; long‐term: one RCT, n = 86, MD 3.80, 95% CI 0.19 to 7.41), anxiety (two RCTs, n = 253, combined MD ‐4.50, 95% CI ‐7.70 to ‐1.30), and long‐term symptoms relating to the breast in people with breast cancer (one RCT, n = 86, MD ‐9.80, 95% CI ‐19.13 to ‐0.47) was greater for the aromatherapy‐massage group, but the results were considered not clinically significant. The medium‐term quality of life score was lower (better) for the aromatherapy‐massage group compared with the no‐massage group (one RCT, n = 30, MD ‐2.00, 95% CI ‐3.46 to ‐0.54).
Massage with aromatherapy versus massage without aromatherapy groups
From the limited evidence available, we were unable to assess the effect of adding aromatherapy to massage on the relief of pain, psychological symptoms including anxiety and depression, physical symptom distress, or quality of life.
Authors' conclusions
There was a lack of evidence on the clinical effectiveness of massage for symptom relief in people with cancer. Most studies were too small to be reliable and key outcomes were not reported. Any further studies of aromatherapy and massage will need to address these concerns.
Plain language summary
Aromatherapy and massage for symptom relief in people with cancer
Background
People with cancer may experience symptoms such as pain, anxiety, or distress. Massage with or without aromatherapy (using essential oils, which are natural oils that may have the odour of the plant from which it was extracted) may help relieve these symptoms. Massage involves working and acting on the body with pressure. Massage is given using a carrier oil (base oil or vegetable oil) with or without essential oils. Massage with essential oils such as rose or lavender oil is known as aromatherapy massage.
Key results and quality of evidence
In August 2015, we searched for clinical trials looking at massage with or without aromatherapy for symptom relief in people with cancer. We found 19 small studies (1274 participants) of very low quality. Some small studies suggested that massage without aromatherapy may help relieve short‐term pain and anxiety in people with cancer. Other small studies suggested that aromatherapy massage may provide medium‐ or long‐term relief for these symptoms. However, the quality of evidence was very low and the results were not consistent. We cannot be sure that these treatments will bring any benefit.
Summary of findings
Summary of findings for the main comparison. Massage versus no massage for symptom relief in people with cancer.
| Massage versus no massage for symptom relief in people with cancer | ||||||
|
Patient or population: people with cancer Settings: oncology unit, cancer centre, hospice Intervention: massage | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) |
Quality of the evidence (GRADE) |
Comments | |
| Assumed risk | Corresponding risk (95% CI) | |||||
| No massage | Massage | |||||
| Pain (PPI‐VAS) | The mean pain (PPI‐VAS) in the control group was 4.2 points | The mean pain (PPI‐VAS) in the intervention group was 1.6 lower (2.67 to 0.53 lower) |
Continuous data | 72 (1 study) | ⊕⊝⊝⊝ Very low1,2,3 | Lower score indicates less pain |
| Anxiety (STAI‐state) | The mean anxiety (STAI‐state) ranged across control groups from 30.0 to 37.7 points | The mean anxiety (STAI‐state) in the intervention groups was 5.36 lower (16.06 lower to 5.34 higher) |
Continuous data | 98 (3 studies) | ⊕⊝⊝⊝ Very low1,3,4 | Not statistically significant by random‐effects model |
|
Anxiety (STAI‐state) subgroup 1: children vs. adults ‐ children |
The mean anxiety (STAI‐state) for children in the control group was 37.7 points | The mean anxiety (STAI‐state) for children in the intervention group was 14.70 lower (19.33 to 10.07 lower) |
Continuous data | 30 (1 study) | ⊕⊝⊝⊝ Very low1,2,3 | Lower score indicates less severity in anxiety |
|
Anxiety (STAI‐state) subgroup 1: children vs. adults ‐ adults |
The mean anxiety (STAI‐state) for adults ranged across control groups from 30.0 to 30.3 points | The mean anxiety (STAI‐state) for adults in the intervention groups was 0.74 lower (5.99 lower to 4.51 higher) |
Continuous data | 68 (2 studies) | ⊕⊝⊝⊝ Very low3 | Not statistically significant |
|
Anxiety (STAI‐state) subgroup 2: short‐term vs. medium‐term ‐ short‐term (≤ 4 weeks) |
The short‐term mean anxiety (STAI‐state) ranged across control groups from 30.3 to 37.7 points |
The short‐term mean anxiety (STAI‐state) in the intervention groups was 10.66 lower (14.72 to 6.6 lower) |
Continuous data | 64 (2 studies) | ⊕⊝⊝⊝ Very low1,3,4 | Lower score indicates less severity in anxiety |
|
Anxiety (STAI‐state) subgroup 2: short‐term vs. medium‐term ‐ medium‐term (> 4 weeks to < 8 weeks) |
The medium‐term mean anxiety (STAI‐state) in the control group was 30.0 points | The medium‐term mean anxiety (STAI‐state) in the intervention group was 3.00 lower (9.69 lower to 3.69 higher) |
Continuous data | 34 (1 study) | ⊕⊝⊝⊝ Very low1,2,3 | Not statistically significant |
| * The assumed risk (e.g. the mean control group risk across studies) is provided. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group. CI: confidence interval; PPI‐VAS: Present Pain Intensity‐Visual Analogue Scale; STAI: State‐Trait Anxiety Inventory. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Study with high risk of bias. 2 Only one trial, unknown heterogeneity. 3 Small study. 4 Only one or two trials, unknown publication bias.
All downgraded by three levels due to very serious imprecision.
Summary of findings 2. Aromatherapy massage versus no massage for symptom relief in people with cancer.
| Aromatherapy massage versus no massage for symptom relief in people with cancer | ||||||
|
Patient or population: people with cancer Settings: oncology unit, cancer centre, hospice Intervention: aromatherapy massage | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) |
Relative effect (95% CI) |
No of participants (studies) |
Quality of the evidence (GRADE) |
Comments | |
| Assumed risk | Corresponding risk | |||||
| No massage | Aromatherapy massage | |||||
| Bodily pain (SF‐8) ‐ medium‐term (> 4 weeks to < 8 weeks) | The medium‐term mean bodily pain (SF‐8) in the control group was 44.4 points | The medium‐term mean bodily pain (SF‐8) in the intervention group was 5.30 higher (1.52 to 9.08 higher) |
Continuous data | 86 (1 study) | ⊕⊝⊝⊝ Very low1,3,4 | Higher score indicates less pain |
| Bodily pain (SF‐8) ‐ long‐term (≥ 8 weeks) | The long‐term mean bodily pain (SF‐8) in the control group was 45.4 points | The long‐term mean bodily pain (SF‐8) in the intervention group was 3.80 higher (0.19 to 7.41 higher) |
Continuous data | 86 (1 study) | ⊕⊝⊝⊝ Very low1,3,4 | Higher score indicates less pain |
| Anxiety (STAI‐state) | The mean anxiety (STAI‐state) ranged across control groups from 24.7 to 47.3 points | The mean anxiety (STAI‐state) in the intervention groups was 4.50 lower (7.70 to 1.30 lower) |
Continuous data | 253 (2 studies) | ⊕⊝⊝⊝ Very low1,4 | Lower score indicates less severity in anxiety |
| Anxiety (STAI‐state) subgroup: short‐term vs. medium‐term ‐ short‐term (≤ 4 weeks) | The short‐term mean anxiety (STAI‐state) in the control groups was 24.7 points | The short‐term mean anxiety (STAI‐state) in the intervention groups was 1.1 lower (9.35 lower to 7.15 higher) |
Continuous data | 32 (1 study) | ⊕⊝⊝⊝ Very low1,3,4 | Not statistically significant |
| Anxiety (STAI‐state) subgroup: short‐term vs. medium‐term ‐ medium‐term (> 4 weeks to < 8 weeks) | The medium‐term mean anxiety (STAI‐state) in the control group was 47.3 points | The medium‐term mean anxiety (STAI‐state) in the intervention group was 5.1 lower (8.57 to 1.63 lower) |
Continuous data | 221 (1 study) | ⊕⊕⊕⊝ Moderate4 | Lower score indicates less severity in anxiety |
| Symptoms relating to the breast (EORTC QLQ‐BR23): long‐term (≥ 8 weeks) | The long‐term mean symptoms relating to the breast (EORTC QLQ‐BR23) in the control group was 31.9 points | The long‐term mean symptoms relating to the breast (EORTC QLQ‐BR23) in the intervention group was 9.80 lower (19.13 to 0.47 lower) |
Continuous data | 86 (1 study) | ⊕⊝⊝⊝ Very low1,3,4 | Lower scores indicate fewer symptoms |
|
Quality of life (MYMOP): medium‐term (> 4 weeks to < 8 weeks) |
The medium‐term mean quality of life (MYMOP) in the control group was 3.9 points | The medium‐term mean quality of life (MYMOP) in the intervention group was 2.00 lower (3.46 to 0.54 lower) |
Continuous data | 29 (1 study) | ⊕⊝⊝⊝ Very low2,3,4 | Lower score indicates greater quality of life |
| * The assumed risk (e.g. the mean control group risk across studies) is provided. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group. CI: confidence interval; EORTC QLQ‐BR23: European Organization of Research and Treatment of Cancer Quality of Life Questionnaire Breast Module; MYMOP: Measure Yourself Medical Outcome Profile; SF‐8: Short‐Form Health Survey‐8; STAI: State‐Trait Anxiety Inventory. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Study with high risk of bias. 2 Only one trial, unknown heterogeneity. 3 Small study. 4 Only one or two trials, unknown publication bias (evident asymmetry).
Most except anxiety (four to eight weeks) downgraded by three levels due to very serious imprecision.
Background
Description of the condition
Cancer significantly affects a person's quality of life (Alacacioglu 2010), and is associated with a variety of psychological symptoms, such as anxiety (Jackson 2004) and depression (Akechi 2008), and physiological symptoms, such as pain (Jackson 2007), fatigue (Bennett 2009), constipation (Yu 2010), and nausea and vomiting (Hines 2009). Some palliative care reports state that between 14% and 25% of all people with cancer show signs of anxiety disorders (Mantovan 2009), and that 50% of people with cancer are diagnosed with a psychiatric disorder; with the most common diagnosis being depressive disorders (Derogatis 1983).
Description of the intervention
This review focused on massage interventions using a blended carrier oil with essential oils (aromatherapy) and a carrier oil without essential oils.
Massage intervention
Massage is defined as the manipulation of the soft tissues of the body, performed by the hands, for the purpose of producing effects on the vascular, muscular, and nervous systems (Fellowes 2004a). Massage interventions are increasingly being considered as a means of achieving cancer‐related symptom relief (Ernst 2009; Wilkinson 2008), and are often used to address people with cancer's need for human contact (Russell 2008). The main reported effects of massage treatment, in studies with or without randomisation, include pain relief (Gorman 2008); improved immune function (Hernandez‐Reif 2004); reduced levels of anxiety (Campeau 2007) and depression (Krohn 2010); reduced fatigue (Listing 2009), nausea (Billhult 2007), and stress (Listing 2010); and an improved quality of life (Keir 2010). In massage therapy, the pressure should not be applied on the affected area and massage should not be given to people with contraindications such as acute thrombosis, or inflamed skin in the area of therapy (Listing 2009; Listing 2010). Toth 2013 and Hernandez‐Reif 2004 reported that no adverse effects were associated with massage. However, some people reported experiencing physical distress and feeling stressed at follow‐up (Jane 2011). Ernst 2003 also concluded that massage therapies are not totally devoid of risk, though the incidence is rare.
Aromatherapy massage intervention
Aromatherapy massage involves the use of essential oils that are combined with a carrier oil or cream to manipulate the soft tissues of the body (Fellowes 2004a). Lavender essential oil is used as a traditional therapy for pain and relaxation (Denner 2009). Bergamot is a well‐known essential oil used to minimise the symptoms of stress‐induced anxiety, mild mood disorders, and cancer pain (Bagetta 2010). Of all the uses of essential oils in aromatherapy, massage for 30 minutes (Listing 2009;Wilcock 2004), by trained therapists (Listing 2009; Wilcock 2004; Wilkinson 2007), is the most common (Holt 2009), followed by aromatic baths. Vapourisation is also a very effective way of using essential oils, whereby a small amount of oil is vaporised into the air (Oh 2000). Essential oils such as lavender (Soden 2004; Wilcock 2004), rose (Listing 2009), and chamomile (Wilcock 2004) were used for aromatherapy‐massage intervention for people with cancer. The effects of aromatherapy interventions in relieving cancer‐related symptoms have been reported (Wilkinson 2007). However, the evidence supporting a clear benefit of aromatherapy for people with cancer has yet to be established. There are a few studies that appear to exhibit a benefit. For example, Wilkinson 1999 performed a randomised controlled trial (RCT) that suggested that massage with or without essential oils was an effective therapy for reducing anxiety levels. It is believed that the addition of an essential oil can enhance the effect of massage and improve the psychological symptoms and overall quality of life among people with cancer. Soden 2004 compared the effects of massage with an essential oil (aromatherapy massage) to massage without an essential oil, and noted the changes in physical and psychological symptoms in people with advanced cancer. The results appeared to show that the addition of lavender essential oil to the massage did not increase the beneficial effects of massage. Wilcock 2004 reported there were no serious adverse events except a rash following aromatherapy massage. Miller 2012 reported that topical application of limonene containing massage oil to the breast was possibly or probably related to adverse events such as itching, rash, dry skin, lightening skin colour, burning sensation, acne, and headaches.
How the intervention might work
Massage is thought to have an effect by stimulating the skin, blood, and lymphatic system, which boosts blood circulation, aids muscle relaxation, and soothes nerves (McGilvery 1994). However, the mechanisms underlying these effects remain unknown. Aromatherapy massage using essential oils is considered a therapeutic treatment for both the mind and body, and it works mainly on the nervous system, but may also stimulate the immune system and affect emotions. Essential oils are composed of small organic molecules that penetrate the outer skin, work their way into the body and accumulate in the fatty tissue. In addition, the highly volatile oils evaporate and can also be inhaled through the nasal passages. These olfactory cells send messages straight to higher centres of the brain, including the limbic system, which controls the arousal functions of the body and emotional states. Thus, during an aromatherapy treatment, essential oils may enhance both physical and psychological well‐being at the same time (McGilvery 1994).
Why it is important to do this review
This review is an update of a Cochrane review first published in 2004 (Fellowes 2004a). The earlier review found some indication of the usefulness of this alternative therapy, yet with little evidence. As this former review was significantly out of date, Cochrane withdrew it. Nevertheless, this topic needs to be updated due to more recently published RCTs and methodological updates. Physicians and people with cancer need access to the best available and up‐to‐date evidence to make informed treatment decisions. Alternative therapies continue to generate substantial interest.
Objectives
To evaluate the effects of massage with or without aromatherapy on pain and other symptoms associated with cancer.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) that evaluated the effect of massage with or without aromatherapy. We excluded studies if they were quasi‐randomised trials, non‐randomised trials, case reports, abstracts, or letters. We applied no language restrictions.
Types of participants
We included adults and children diagnosed with cancer. We included both inpatients and outpatients who received care in any healthcare setting (e.g. hospital, hospice, oncology centre, or community).
Types of interventions
We compared the following interventions:
massage (using carrier oil only) versus no massage;
massage with aromatherapy (using carrier oil plus essential oils) versus no massage;
massage with aromatherapy (using carrier oil plus essential oils) versus massage without aromatherapy (using carrier oil only).
Massage was required to include tissue manipulation using a carrier oil, thereby excluding touch therapies such as therapeutic touch, acupressure, and reflexology. We defined aromatherapy as the use of a blended carrier oil with essential oils and will include only aromatherapy administered with massage. Thus, we excluded inhalations and humidification methods.
Types of outcome measures
Primary outcomes
Pain (using validated standard subjective scales (numerical rating scale (NRS), verbal rating scale (VRS), or visual analogue scale (VAS)) for pain intensity or pain relief, or both).
Psychological symptoms (including anxiety and depression assessed using validated scales).
Secondary outcomes
Other physical symptoms (including fatigue and nausea, etc.).
Quality of life (assessed by a valid and reliable assessment instrument).
Adverse events.
Search methods for identification of studies
Electronic searches
We searched the following databases.
The Cochrane Central Register of Controlled Trials (CENTRAL), 2015, Issue 7 of 12.
MEDLINE (Ovid) 1946 to week one of August 2015.
EMBASE (Ovid) 1974 to week one of August 2015.
PsycINFO (Ovid) 1806 to week one of August 2015.
CINAHL (EBSCO) to week one of August 2015.
PubMed Cancer Subset to week one of August 2015.
South Asian Database of Controlled Clinical Trials (SADCCT) to week one of August 2015.
World Health Organization (WHO) ICTRP to week one of August 2015.
See Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5; and Appendix 6 for the search strategies used. We applied no date or language restrictions.
Searching other resources
We expanded the search strategy to include regional databases such as the Korean databases (KISS, KMbase, KoreaMed, and RISS).
Data collection and analysis
Selection of studies
One review author (SHL) screened the titles and abstracts of the studies identified from the search to eliminate those studies that were not relevant to this review. When the title and abstract did not have sufficient information for screening purposes, we retrieved a full‐text copy to review. Two review authors (ESS, MJK) independently examined each full‐text report for the remaining studies to determine their eligibility for inclusion using a pre‐developed checklist. We excluded articles if they were not randomised. We did not consider for inclusion participants who were not diagnosed with cancer, did not receive a massage or aromatherapy‐massage intervention, or were not evaluated for any of the primary and secondary outcomes. An expert in massage and aromatherapy (ESS) and the research methodologist (SHL) made final decisions about which studies should be included in this review. We resolved disagreements about inclusions by discussion or via a formal consensus method.
Data extraction and management
Two review authors (YMJ, JEJ) independently extracted data from the included studies using a data collection form, which included the following information: source, eligibility, methods, participants, interventions, outcomes, results, and miscellaneous data. We completed a data extraction sheet for every study included in the review. We did not consider extracting individual participant data. Krohn 2010 only presented the results graphically and did not provide mean and standard deviation (SD) values for depression and mood. Soden 2004 reported the mean change value instead of mean and SD for the outcomes including pain, psychological distress such as anxiety and depression, other physical symptoms, and quality of life. We contacted five study authors to request missing data, but they did not respond (Batalha 2013; Khiewkhern 2013; Krohn 2010; Soden 2004; Wang 2015).
Assessment of risk of bias in included studies
Two review authors (KHS, JYY) independently assessed the risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and adapted from those used by the Cochrane Pregnancy and Childbirth Group, with any disagreements resolved by discussion. We assessed the following for each study.
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (any truly random process, e.g. random number table; computer random number generator); unclear risk of bias (method used to generate sequence not clearly stated). We excluded studies using a non‐random process (e.g. odd or even date of birth; hospital or clinic record number).
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions prior to assignment determines whether intervention allocation could have been foreseen in advance of or during recruitment, or changed after assignment. We assessed the methods as: low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes); unclear risk of bias (method not clearly stated). We excluded studies that did not conceal allocation (e.g. open list).
Blinding of outcome assessment (checking for possible detection bias). We assessed the methods used to blind study outcome assessors from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias when this was clearly described, unclear risk of bias when not stated, and high risk of bias if an inappropriate method was described.
Incomplete outcome data (checking for possible attrition bias due to the amount, nature, and handling of incomplete outcome data). We assessed the methods used to deal with incomplete data as: low risk (less than 10% of participants did not complete the study or used 'baseline observation carried forward' analysis, or both); unclear risk of bias (used 'last observation carried forward' analysis); high risk of bias (used 'completer' analysis).
Selective outcome reporting. We considered studies at low risk of bias if all adverse events were reported. Where there was clear evidence of partial reporting (e.g. most common or more than a given rate), then we considered these studies at high risk of bias. Anything else was unclear risk of bias.
Size of study (checking for possible biases confounded by size). We assessed studies as being at low risk of bias (200 participants or greater); unclear risk of bias (50 to 199 participants); high risk of bias (fewer than 50 participants).
Other bias. Any other bias noted at the data extraction phase.
We used the Review Manager 5 'Risk of bias' tool (RevMan 2014).
Measures of treatment effect
For dichotomous data, we intended to describe the treatment effect as risk ratios (RRs) with 95% confidence intervals (CIs). For continuous data, we established the mean difference (MD) or the standardised mean difference (SMD) and calculated the 95% CI. If studies did not report SDs, we calculated them using software developed by the UK Cochrane Group (UK Cochrane Centre 2010) based on the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We planned to calculate outputs such as the number needed to treat for an additional beneficial outcome (NNTB), number needed to treat for an additional harmful outcome (NNTH), or number needed to treat to prevent an event (NNTp), with 95% CIs from dichotomous data (McQuay 1997); however, this was not possible because no outcomes were reported in this way.
Unit of analysis issues
The analysis aimed to take into account the level at which randomisation occurred in the clinical trials. Review authors considered the unit of analysis issues in each study as follows.
Groups of participants randomised together with the same intervention, such as cluster‐randomised trials.
Participants receiving more than one intervention, such as a cross‐over trial, or undergoing simultaneous treatment of multiple study sites.
Multiple observations for the same outcome, such as repeated measurements, recurring events, and measurements of different body parts.
Dealing with missing data
We contacted the relevant study authors to provide the appropriate data for the meta‐analysis, but they did not respond to our requests. Where necessary, we imputed SDs from the standard error (SE) (Ahles 1999, Wilkinson 2007).
Assessment of heterogeneity
Where there was substantial heterogeneity, we checked whether the data were incorrectly extracted or entered into Review Manager 5 (RevMan 2014). We reviewed inconsistencies in the data that could cause misleading effects. We performed analyses both with and without outlying studies as part of a sensitivity analysis. We conducted subgroup analyses to explore heterogeneity. We assessed statistical heterogeneity visually and by using the I2 statistic. We considered studies with an I2 greater than 60% as having substantial heterogeneity.
Assessment of reporting biases
For assessing publication bias, we intended to assess how much data (e.g. studies and participants) would be required both to be unpublished and to have no treatment effect; however, this was not possible due to the lack of reliable data.
Data synthesis
Where data were available, we combined data from studies in a meta‐analysis. We used a fixed‐effect model if the studies were homogenous; otherwise, we used a random‐effects model. We combined continuous data only where means and SDs were available or calculable and there was no clear evidence of a skewed distribution.
Subgroup analysis and investigation of heterogeneity
When data were available, we undertook subgroup analyses for comparing the magnitudes of effect within Review Manager 5 (RevMan 2014). We compared effect estimates in different subgroups by considering the meta‐analysis results from each subgroup separately.
Children versus adults.
Short‐term (four weeks or less) versus medium‐term (greater than weeks and less than eight weeks) versus long‐term (eight weeks or greater).
We were unable to perform subgroup analysis for full‐body massage versus partial massage (hand, foot, shoulder, neck, back, abdomen, and scalp etc.) as planned due to lack of data.
Sensitivity analysis
We undertook a sensitivity analysis to explore the effects of risk of bias and other sources of heterogeneity, where data were available. In subgroup analyses, we estimated the effect of the intervention for each subgroup. We created two 'Summary of findings' tables with pre‐specified outcomes (Higgins 2011).
'Summary of findings' tables
We assessed the overall quality of the evidence using the GRADE system (GRADEpro GDT 2015), and presented in the 'Summary of findings' tables, to present the main findings of the review in a transparent and simple tabular format. In particular, we included key information concerning the quality of evidence, the magnitude of effect of the interventions examined, and the sum of available data on the main outcomes of pain, anxiety, symptoms relating to the breast, and quality of life.
The GRADE system uses the following criteria for assigning grade of evidence.
High quality: further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: we are very uncertain about the estimate.
We decreased grade if:
serious (‐1) or very serious (‐2) limitation to study quality;
important inconsistency (‐1);
some (‐1) or major (‐2) uncertainty about directness;
imprecise or sparse data (‐1);
high probability of reporting bias (‐1).
Results
Description of studies
See: Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
The literature search identified 21,376 studies (to week 1 of August 2015), which was reduced to 17,512 after we removed duplicates. We assessed 55 full‐text articles as potentially eligible and excluded 34 of them. A total of 19 studies (21 reports) met the inclusion criteria for this review; we included 14 studies (16 reports) in a qualitative synthesis and five studies in a quantitative synthesis (meta‐analysis). There were two reports each for two studies (Fernandez‐Lao 2012; Listing 2009). The results of the search and screening processes are illustrated in Figure 1.
1.
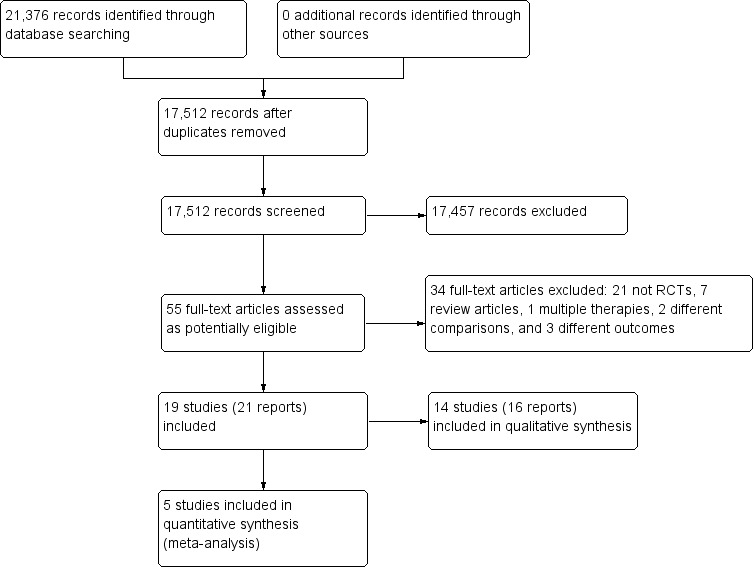
Study flow diagram.
Included studies
We included 19 studies (21 reports) with 1274 participants (700 interventions and 574 controls). Thirteen studies (14 reports) compared massage with no massage; these studies included 596 participants (302 interventions and 294 controls). Six studies (seven reports) compared aromatherapy massage with no massage; these studies included 561 participants (281 interventions and 280 controls). Two studies compared massage with aromatherapy with massage without aromatherapy; these studies included 117 participants (54 interventions and 63 controls). See Characteristics of included studies table. Table 3 provides additional details of the evaluated trials.
1. Characteristics of the outcomes and measurement scales used in the evaluated trials.
| Study ID | Primary outcome | Secondary outcome | |||
| Pain | Psychological symptoms | Other physical symptoms | Quality of life | Adverse events | |
| Comparison 1. Massage vs. no massage | |||||
| Ahles 1999 | ‐ | Anxiety‐STAI‐state Depression‐BDI |
‐ | ‐ | ‐ |
| Batalha 2013 | Pain‐VAS | ‐ | ‐ | ‐ | ‐ |
| Billhult 2007 | ‐ | Anxiety‐VAS Anxiety‐HAD Depression‐HAD | Nausea‐VAS | ‐ | ‐ |
| Campeau 2007 | ‐ | Anxiety‐VAS Anxiety‐STAI‐state |
‐ | ‐ | ‐ |
| Fernandez‐Lao 2012 | ‐ | ‐ | Fatigue‐POMS | ‐ | ‐ |
| Haun 2009 | ‐ | Anxiety‐STAI‐state | ‐ | ‐ | ‐ |
| Hernandez‐Reif 2004 | ‐ | Anxiety‐STAI‐state Anxiety‐SCL‐90‐R Depression‐POMS Depression‐SCL‐90‐R |
‐ | ‐ | ‐ |
| Jane 2011 | Pain‐PPI‐VAS | Mood‐VAS | ‐ | ‐ | Having physical distress Progress of disease |
| Krohn 2010 | ‐ | Depression‐PHQ Mood‐BSF |
‐ | ‐ | ‐ |
| Soden 2004 | Pain‐VAS | Anxiety‐HAD Depression‐HAD Psychological distress‐RSCL |
Physical symptom distress‐RSCL | Quality of Life‐RSCL | ‐ |
| Toth 2013 | Pain‐VAS | Anxiety‐VAS | ‐ | Quality of Life‐McGill | ‐ |
| Wang 2015 | Pain‐ESAS:AM | Anxiety‐ESAS:AM Depression‐ESAS:AM |
Nausea‐ESAS:AM | ‐ | ‐ |
| Wilkie 2000 | Pain‐PAT (or SNVR) | ‐ | ‐ | Quality of Life‐Graham | ‐ |
| Comparison 2. Aromatherapy with massage vs. no massage | |||||
| Khiewkhern 2013 | Pain‐VAS | Anxiety‐VAS | Fatigue‐VAS | ‐ | ‐ |
| Listing 2009 | Limb pain‐GBB Bodily pain‐SF‐8 | ‐ | Arm symptoms‐EORTC QLQ‐BR23 Breast symptoms‐EORTC QLQ‐BR23 | ‐ | ‐ |
| Soden 2004 | Pain‐VAS | Anxiety‐HAD Depression‐HAD Psychological distress‐RSCL |
Physical symptom distress‐RSCL | Quality of Life‐RSCL | ‐ |
| Sohn 2005 | Pain‐VAS | Anxiety‐STAI‐state Depression‐BDI | ‐ | ‐ | ‐ |
| Wilcock 2004 | ‐ | Mood disturbance scale‐POMS | ‐ | Quality of life‐MYMOP | Rash |
| Wilkinson 2007 | Pain‐EORTC | Anxiety‐STAI‐state Depression‐CES‐D | Fatigue‐EORTC | Quality of life‐EORTC | ‐ |
| Comparison 3. Aromatherapy with massage vs. massage without aromatherapy | |||||
| Soden 2004 | Pain‐VAS | Anxiety‐HAD Depression‐HAD Psychological distress‐RSCL |
Physical symptom distress‐RSCL | Quality of Life‐RSCL | ‐ |
| Wilkinson 1999 | ‐ | Anxiety‐STAI‐state (SAI) Anxiety‐STAI‐trait (TAI) Psychological distress‐RSCL |
Physical symptom distress‐RSCL | Quality of life‐RSCL | ‐ |
| Abbreviations: BDI: Beck Depression Inventory; BSF: Berlin Mood Questionnaire; CES‐D: Center for Epidemiological Studies Depression; EORTC QLQ‐BR23: European Organization of Research and Treatment of Cancer Quality of Life Questionnaire Breast Module (lower scores of the arm and breast symptoms indicate fewer symptoms); ESAS:AM: Edmonton Symptom Assessment System‐Ascites Modification; GBB: Giessen Complaints Inventory; HAD: Hospital Anxiety Depression Scale; MYMOP: Measure Yourself Medical Outcome Profile (lower score indicates greater quality of life, 7 = 'as bad as it could be' and 0 = 'as good as it could be'); PAT: Pain Assessment Tool; PHQ: Patient Health Questionnaire; POMS: Brief Profile of Mood States; PPI‐VAS: Present Pain Intensity‐Visual Analogue Scale (lower score indicates less pain, 10 = 'pain as bad as it could be' and 0 = 'no pain'); RSCL: Rotterdam Symptom Checklist; SCL‐90‐R: Symptom Checklist‐90‐R; SF‐8: Short‐Form Health Survey‐8 (higher score on bodily pain indicates less pain); SNVR: Skilled Nursing Visit Report form; STAI: State‐Trait Anxiety Inventory (lower score indicates less severity in anxiety); VAS: Visual Analogue Scale. | |||||
Excluded studies
We excluded 34 studies because they were not RCTs (21 studies); they were review articles (seven articles); or assessed multiple therapies (one study), different comparison (two studies), or different outcomes (three studies). See Characteristics of excluded studies table.
Risk of bias in included studies
We created a risk of bias table and graph to summarise our judgements on random sequence generation, allocation concealment, blinding of outcome assessment, incomplete outcome data, selective reporting, size, and other potential sources of bias in the analysed studies (Figure 2; Figure 3). We assessed and classified the methodological components of the trials as low, high, or unclear according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
2.
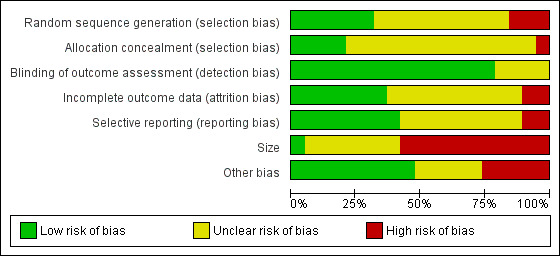
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
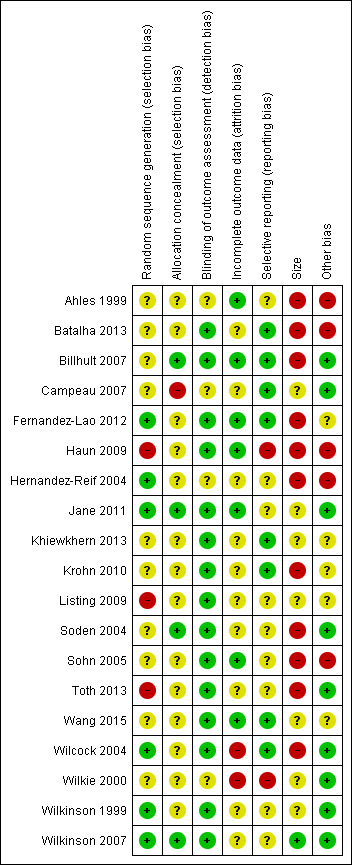
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Three of the 19 studies were potentially at high risk of bias due to the inadequate random sequence generation (Haun 2009; Listing 2009; Toth 2013). Only six studies were at low risk of bias for selection bias (Fernandez‐Lao 2012; Hernandez‐Reif 2004; Jane 2011; Wilcock 2004; Wilkinson 1999; Wilkinson 2007). One of the 19 studies had a high risk of bias in allocation concealment (Campeau 2007), and 14 studies showed an unclear risk of bias in allocation concealment. Numbered, sealed, opaque envelopes, and central allocation by computer were used for the proper allocation of concealment.
Blinding
Fifteen of the included studies had a low risk of bias for blinding of outcome assessment, whereas four studies showed an unclear risk of bias for this assessment (Ahles 1999; Campeau 2007; Hernandez‐Reif 2004; Wilkie 2000).
Incomplete outcome data
Of the 19 studies, seven were at low risk of attrition bias having allocated the same number of participants to both groups (Ahles 1999; Billhult 2007; Fernandez‐Lao 2012; Haun 2009; Jane 2011; Sohn 2005; Wang 2015). Ten studies were at unclear risk of attrition bias, and the remaining two studies showed a high risk of bias (Wilcock 2004; Wilkie 2000).
Selective reporting
Of the 19 studies, eight were at low risk of bias due to selective reporting (Batalha 2013; Billhult 2007; Campeau 2007; Fernandez‐Lao 2012; Khiewkhern 2013; Krohn 2010; Wang 2015; Wilcock 2004). Nine studies were at unclear risk of reporting bias, and the remaining two studies showed a high risk of bias (Haun 2009; Wilkie 2000).
Size
Eleven studies had fewer than 50 participants in total and we judged them at high risk of bias. One study was at low risk of bias with more than 200 participants (Wilkinson 2007). We judged the remaining studies to be at unclear risk of bias (see: Figure 3).
Other potential sources of bias
We found some imbalanced baseline measurements between the intervention and control groups, which caused potential bias. However, some trials reported that there were no significant between‐group differences in terms of participant characteristics (Hernandez‐Reif 2004), or measurement of symptoms relating to the breast in people with breast cancer or the use of medication (Ahles 1999), or sociodemographic variables at baseline (Krohn 2010; Listing 2009) between the massage and no‐massage groups. There were no imbalances in the baseline measurement of quality of life (Wilkinson 1999), pain intensity, and anxiety (Sohn 2005) between the aromatherapy‐massage and no‐massage groups. Of the 19 studies, five had a high risk of other bias, and five studies had an unclear risk of other bias. We judged the remaining studies at low risk of bias.
Effects of interventions
See Table 1 and Table 2 for the main comparisons.
Studies used a variety of scales to measure symptoms. These included:
BDI: Beck Depression Inventory (measurement of depression);
BSF: Berlin Mood Questionnaire (measurement of mood);
CES‐D: Center for Epidemiological Studies Depression (measurement of depression);
EORTC QLQ: European Organization of Research and Treatment of Cancer Quality of Life Questionnaire (measurement of pain, fatigue, and quality of life);
EORTC QLQ‐BR23: European Organization of Research and Treatment of Cancer Quality of Life Questionnaire Breast Module (measurement of symptoms relating to the breast and arm; low scale scores of the arm and breast symptoms indicate fewer symptoms);
ESAS:AM: Edmonton Symptom Assessment System‐Ascites Modification (measurement of pain, anxiety, depression, and nausea);
GBB: Giessen Complaints Inventory (measurement of limb pain);
the Graham scale (measurement of quality of life);
HAD: Hospital Anxiety Depression Scale (measurement of anxiety and depression);
the McGill scale (measurement of quality of life);
MYMOP: Measure Yourself Medical Outcome Profile (measurement of quality of life; lower score indicates greater quality of life, 7 = 'as bad as it could be' and 0 = 'as good as it could be');
PAT: Pain Assessment Tool (measurement of pain);
PHQ: Patient Health Questionnaire (measurement of depression);
POMS: Brief Profile of Mood States (measurement of depression, mood disturbance, and fatigue);
PPI‐VAS: Present Pain Intensity‐Visual Analogue Scale (measurement of pain; lower score indicates less pain, 10 = 'pain as bad as it could be' and 0 = 'no pain');
RSCL: Rotterdam Symptom Checklist (measurement of psychological distress, physical symptom distress, and quality of life);
SCL‐90‐R: Symptom Checklist‐90‐R (measurement of anxiety and depression);
SF‐8: Short‐Form Health Survey‐8 (measurement of bodily pain; higher score on bodily pain indicates less pain);
SNVR: Skilled Nursing Visit Report form (measurement of pain);
STAI: State‐Trait Anxiety Inventory; low score indicating less severity in anxiety (measurement of anxiety);
VAS: Visual Analogue Scale (measurement of pain, anxiety, mood, nausea and fatigue).
Comparison 1: massage versus no massage
For the comparison of massage versus no massage, we found primary outcome data for pain and psychological symptoms and secondary outcome data for other physical symptoms and quality of life.
Primary outcomes
Pain
One trial with a short‐term follow‐up provided data on pain using PPI‐VAS (n = 72, Jane 2011). Both the massage and no‐massage groups exhibited improvements over the baseline values and short‐term pain relief was greater for the massage group compared with the no‐massage group (one RCT, n = 72, MD ‐1.60, 95% CI ‐2.67 to ‐0.53). One trial reported on pain using PAT or SNVR at short‐term follow‐up and there was no statistically significant difference between groups (n = 56, Wilkie 2000). Four trials with no appropriate data for meta‐analysis reported no effect on pain using the PPI‐VAS (Batalha 2013; Soden 2004; Toth 2013) and ESAS:AM (Wang 2015).
We downgraded the GRADE quality of the evidence for this outcome to very low because of observed imprecision, indirectness, and limitation of study design.
Psychological symptoms
There were data on psychological symptoms including anxiety, depression, mood, and psychological distress.
Anxiety
Three included studies assessed anxiety using STAI‐state (Ahles 1999; Haun 2009; Hernandez‐Reif 2004). There was no significant difference in anxiety between the groups (three RCTs, n = 98, combined MD ‐5.36, 95% CI ‐16.06 to 5.34) (Analysis 1.1; Figure 4). The pooled results showed very high heterogeneity (I2 = 88%). Subgroup analysis revealed that anxiety relief for children was greater for the massage group compared with the no‐massage group (one RCT, n = 30, MD ‐14.70, 95% CI ‐19.33 to ‐10.07; lower score indicated less severity in anxiety) (Haun 2009). However, we considered the size of the effect to be not clinically significant. Data from adults showed no significant difference in anxiety relief between the groups (Ahles 1999; Hernandez‐Reif 2004) (Analysis 2.1). The subgroup analysis revealed that short‐term anxiety relief was greater for the massage group compared with the no‐massage group (two RCTs, n = 64, combined MD ‐10.66, 95% CI ‐14.72 to ‐6.60; lower score indicated less severity in anxiety) (Ahles 1999; Haun 2009). However, we considered the size of the effect to be not clinically significant. Data from medium‐term anxiety relief showed no significant difference between the groups (Hernandez‐Reif 2004) (Analysis 2.2). Three trials assessed anxiety using a VAS. Two trials with no usable data claimed massage therapy had no major impact on intermediate‐term anxiety (n = 100, Campeau 2007; n = 30, Toth 2013). Another trial reported that differences in anxiety between the two treatment regimens were not statistically significant (n = 39, Billhult 2007). Two further trials reported anxiety using a HAD tool and there were no statistically significant differences between the groups (n = 39, Billhult 2007; n = 42, Soden 2004). One trial reported reduced anxiety for an immediate massage therapy effect using the SCL‐90‐R scale (n = 34, Hernandez‐Reif 2004). One trial reported on anxiety at short‐term follow‐up using the ESAS:AM scale and there were significant reductions in anxiety scores in the massage group, but it did not provide evaluable data (n = 80, Wang 2015).
1.1. Analysis.
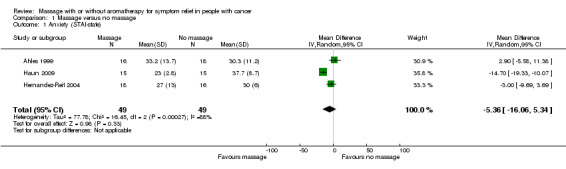
Comparison 1 Massage versus no massage, Outcome 1 Anxiety (STAI‐state).
4.
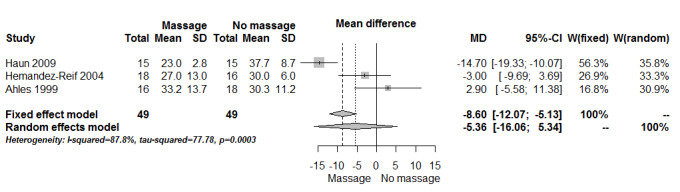
Forest plot of comparison: massage versus no massage, outcome anxiety (State‐Trait Anxiety Inventory (STAI)‐state).
2.1. Analysis.
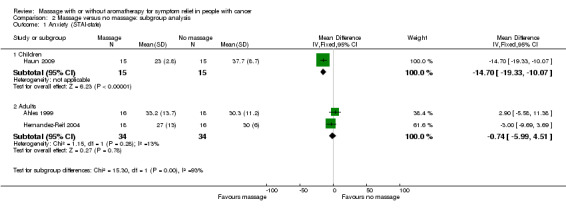
Comparison 2 Massage versus no massage: subgroup analysis, Outcome 1 Anxiety (STAI‐state).
2.2. Analysis.
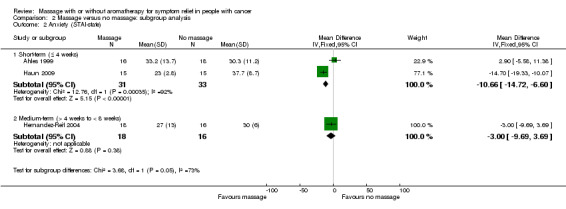
Comparison 2 Massage versus no massage: subgroup analysis, Outcome 2 Anxiety (STAI‐state).
We downgraded the GRADE quality of the evidence for anxiety to very low because of observed imprecision, indirectness, and limitation of study design.
Depression
One trial measured depression using the BDI scale and reported no effects of massage on depression (n = 34, Ahles 1999). Two trials reported no effect of massage therapy on depression using the HAD scale (n = 39, Billhult 2007; n = 42, Soden 2004). Soden 2004 reported statistically significant reductions in depression scores in the massage group; however, this trial did not provide evaluable data. One trial reported that the long‐term massage effects reduced depression using the POMS scale, and also that the immediate massage therapy effects reduced depression using the SCL‐90‐R scale (n = 34, Hernandez‐Reif 2004). One trial reported depression using the PHQ scale, which was significantly reduced immediately after massage compared to the control group (n = 34, Krohn 2010). One trial measured depression using the ESAS:AM scale at short‐term follow‐up and reported significant reductions in depression scores in the massage group; however, this trial did not provide evaluable data (n = 80, Wang 2015).
Mood
One trial reported that massage therapy showed beneficial effects on mood using a VAS (n = 72, Jane 2011). Another trial reported no significant alterations in mood after massage therapy using BSF (n = 34, Krohn 2010).
Psychological symptoms
One trial reported no significant differences between massage and no‐massage groups, and this trial provided no appropriate data for meta‐analysis (n = 42, Soden 2004).
Secondary outcomes
Other physical symptoms
Data were available for nausea, fatigue, and physical symptom distress.
Nausea
Two trials reported no significant differences in short‐term nausea between groups (VAS, ESAS:AM) (Billhult 2007; Wang 2015).
Fatigue
One trial reported no significant differences in short‐term fatigue between groups (POMS) (Fernandez‐Lao 2012).
Physical symptom distress
One trial reported no significant differences between groups (n = 42, Soden 2004).
Quality of life
One trial reported on quality of life at short‐term follow‐up using the Graham scale, and there were no statistically significant differences between groups (n = 29, Wilkie 2000). One trial reported that they were unable to demonstrate any significant long‐term benefit on quality of life using the RSCL scale but this trial provided no usable data (n = 42, Soden 2004). In contrast, one small trial reported that providing massage improved the quality of life at the end of life using the McGill scale (n = 30, Toth 2013). We considered these data to be too unreliable to include in the 'Summary of findings' table.
Adverse events
One trial reported physical distress in one participant who received the massage intervention (n = 36, Jane 2011).
Comparison 2: massage with aromatherapy versus no massage
For the comparison of massage with aromatherapy versus no massage, we found primary outcome data for pain and psychological symptoms and secondary outcome data for other physical symptoms, quality of life, and adverse events.
Primary outcomes
Pain
Three trials reported pain intensity using a VAS. Soden 2004 reported that the effects of aromatherapy demonstrated no significant long‐term benefit on pain intensity (n = 42). In contrast, Sohn 2005 reported that pain intensity was significantly decreased in the aromatherapy group compared with control group (n = 32). Khiewkhern 2013 reported pain at short‐term follow‐up was significantly decreased in the aromatherapy‐massage group than control group (n = 66). One study measured pain using EORTC at short‐, medium‐, and long‐term follow‐ups and reported no significant difference in pain relief between the aromatherapy‐massage and no‐massage groups (n = 221, Wilkinson 2007). One trial reported no significant difference in limb pain relief between groups at medium‐ and long‐term follow‐ups (n = 86, Listing 2009). The same study reported bodily pain using SF‐8 at medium‐ and long‐term follow‐ups. The relief of bodily pain was greater for the aromatherapy‐massage group compared with the no‐massage group for medium‐term (one RCT, n = 86, MD 5.30, 95% CI 1.52 to 9.08) and for long‐term (one RCT, n = 86, MD 3.80, 95% CI 0.19 to 7.41).
We downgraded the GRADE quality of the evidence for this outcome to very low because of observed imprecision, indirectness, and limitation of study design.
Psychological symptoms
We found data for anxiety, depression, mood disturbances, and psychological distress.
Anxiety
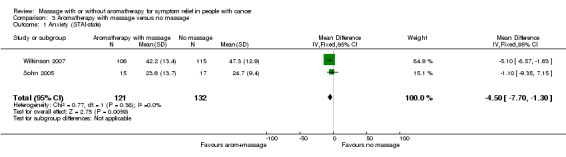
Two studies reported anxiety using STAI‐state at short‐, medium‐, and long‐term follow‐ups (Sohn 2005; Wilkinson 2007). The relief of anxiety was greater for the aromatherapy with massage group compared with the no‐massage group (two RCTs, n = 253, combined MD ‐4.50, 95% CI ‐7.70 to ‐1.30) (Analysis 3.1). Subgroup analysis revealed that medium‐term anxiety relief was greater for the aromatherapy‐massage group compared with the no‐massage group (one RCT, n = 221, MD ‐5.10, 95% CI ‐8.57 to ‐1.63) (Wilkinson 2007) (see: Analysis 4.1). Data from short‐term anxiety relief showed no significant difference between the groups (Sohn 2005). One trial using HAD reported no significant long‐term benefit of aromatherapy massage, but did not provide evaluable data (n = 42, Soden 2004). Khiewkhern 2013 (n = 66) reported that anxiety at short‐term follow‐up was significantly decreased in the aromatherapy‐massage group than no‐massage group using VAS, but did not provide appropriate data for meta‐analysis.
3.1. Analysis.
Comparison 3 Aromatherapy with massage versus no massage, Outcome 1 Anxiety (STAI‐state).
4.1. Analysis.
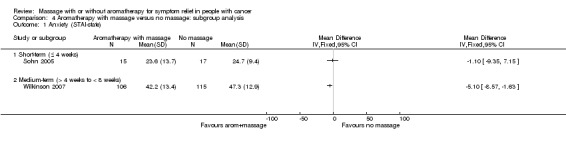
Comparison 4 Aromatherapy with massage versus no massage: subgroup analysis, Outcome 1 Anxiety (STAI‐state).
We downgraded the GRADE quality of the evidence for this outcome to very low because of observed imprecision, indirectness, and limitation of study design.
Depression
There were no significant differences using BDI and VAS in short‐term depression between the aromatherapy‐massage group and the no‐massage group (n = 32, Sohn 2005; n = 66, Khiewkhern 2013). One trial reported on depression using HAD and found no significant long‐term benefit of aromatherapy massage, but this trial did not provide evaluable data (n = 42, Soden 2004). There were no significant differences using CES‐D in short‐, medium‐, or long‐term depression between the aromatherapy‐massage group and the no‐massage group (n = 221, Wilkinson 2007).
Mood
One trial reported no significant differences in medium‐term mood disturbances between the aromatherapy‐massage group and the no‐massage group using POMS (n = 29, Wilcock 2004).
Psychological symptoms
One trial reported that there were no significant differences in psychological distress between the aromatherapy‐massage group and the no‐massage group using RSCL (n = 42, Soden 2004). This trial did not provide any appropriate data for meta‐analysis.
Secondary outcomes
Other physical symptoms
We found data for fatigue, physical symptom distress, and symptoms relating to the arm in people with cancer and to the breast in people with breast cancer.
Fatigue
One trial reported no significant difference between the aromatherapy‐massage group and the usual care only (no massage) group at six or 10 weeks post‐randomisation using EORTC (n = 221, Wilkinson 2007). Khiewkhern 2013 reported fatigue using VAS at short‐term follow‐up was significantly decreased in the aromatherapy‐massage group than the no‐massage group, but this trial did not provide appropriate data for meta‐analysis (n = 66).
Physical symptom distress
One trial reported that there was no significant difference between the aromatherapy‐massage group and no‐massage group using RSCL, but this trial did not provide any appropriate data for meta‐analysis (n = 42, Soden 2004).
Symptoms relating to the arm and breast
One study measured symptoms relating to the arm in people with cancer using EORTC QLQ‐BR23 (n = 86, Listing 2009). There were no significant differences between the groups in "arm symptoms" at medium‐ or long‐term follow‐ups. The same study measured "breast symptoms" in people with breast cancer at medium‐ and long‐term follow‐ups using the EORTC QLQ‐BR23 (Listing 2009). Long‐term relief of symptoms relating to the breast in people with breast cancer was greater for the aromatherapy‐massage group compared with the no‐massage group (one RCT, n = 86, MD ‐9.80, 95% CI ‐19.13 to ‐0.47).
Quality of life
One study used MYMOP to measure quality of life at medium‐term follow‐up (40 participants randomised but only 29 reported, Wilcock 2004). The medium‐term quality of life score was lower for the aromatherapy‐massage group compared with no‐massage group (one RCT, n = 29, MD ‐2.00, 95% CI ‐3.46 to ‐0.54; lower score indicated good quality of life). In contrast, in two other studies using RSCL and EORTC, the level of quality of life showed no significant difference between the aromatherapy‐massage group and the no‐massage group (n = 42, Soden 2004; n = 221, Wilkinson 2007).
Adverse events
One study reported rash (n = 1) and general malaise (n = 5) among the participants who received aromatherapy massage (n = 29, Wilcock 2004).
We downgraded the GRADE quality of the evidence for the secondary outcomes to very low because of observed imprecision, indirectness, and limitation of study design.
Comparison 3: massage with aromatherapy versus massage without aromatherapy
For the comparison of massage with aromatherapy versus massage without aromatherapy, we found primary outcome data for pain and psychological symptoms, and secondary outcome data for physical symptoms and quality of life.
Primary outcomes
Pain
One trial reported that there was no long‐term benefit of aromatherapy massage on improving pain control using VAS, but this trial did not provide any evaluable data for meta‐analysis (n = 42, Soden 2004).
Psychological symptoms
We found data for anxiety, depression, and psychological distress.
Anxiety
There were no significant differences in anxiety using STAI‐state or HAD at medium‐term follow‐up between the massage with aromatherapy group and the massage without aromatherapy group (n = 42, Soden 2004; n = 103, Wilkinson 1999). One trial did not provide any evaluable data for meta‐analysis (Soden 2004).
Depression
One trial reported that there were statistically significant reductions in depression scores using HAD in the massage without aromatherapy group, but this trial did not provide any evaluable data for meta‐analysis (n = 42, Soden 2004).
Psychological symptoms
Two trials reported no significant differences in psychological symptoms using RSCL at medium‐term follow‐up between the massage with aromatherapy group and the massage without aromatherapy group (n = 42, Soden 2004; n = 103, Wilkinson 1999). One trial provided no evaluable data for meta‐analysis (Soden 2004).
Secondary outcomes
Other physical symptoms
One trial reported on physical symptom distress using RSCL, and found no significant difference between the massage with aromatherapy group and the massage without aromatherapy group (Soden 2004). However, this trial did not provide any evaluable data for meta‐analysis.
Quality of life
Two trials reported no significant difference in quality of life using RSCL at medium‐term follow‐up between the massage with aromatherapy group and the massage without aromatherapy group (n = 42, Soden 2004; n = 103, Wilkinson 1999). We considered these data to be too unreliable to include in the 'Summary of findings' table.
Adverse events
We found no trials reporting adverse events of massage with aromatherapy or massage without aromatherapy.
Discussion
Summary of main results
We included 19 studies (21 reports) with 1274 participants. Thirteen studies (14 reports, 596 participants) compared massage with no massage. Six studies (seven reports 561 participants) compared aromatherapy massage with no massage. Two studies (117 participants) compared massage with aromatherapy with massage without aromatherapy. There was a lack of clear evidence to either support or not support the use of massage for symptom relief in people with cancer. Massage with or without aromatherapy may reduce pain or anxiety, or both. However, most studies were too small to be reliable. One study reported physical distress as an adverse event, another study reported one rash and five general malaises; the remaining 17 studies did not report adverse events.
Overall completeness and applicability of evidence
The objective of this review was to evaluate the effects of massage with or without aromatherapy on symptom relief in people with cancer. We included 19 studies (21 reports) with 1274 participants. The qualitative report included 14 studies (16 reports) and the quantitative synthesis (meta‐analysis) included five studies (Ahles 1999; Haun 2009; Hernandez‐Reif 2004; Sohn 2005; Wilkinson 2007). We included adults and children diagnosed with cancer. Two trials investigated the effectiveness of massage therapy for children with cancer with limited outcome measurement (Batalha 2013; Haun 2009). Batalha 2013 reported pain relief and Haun 2009 only demonstrated the effect on anxiety at short‐term, although both studies had small sample sizes. We included both inpatients and outpatients who received care in any healthcare setting (e.g. hospital, hospice, oncology centre, or community). However, we were unable to examine the differences between inpatients and outpatients because some of the studies did not provide information about the settings. There were just three comparisons: massage (using carrier oil only) versus no massage; massage with aromatherapy (using carrier oil plus essential oils) versus no massage; and massage with aromatherapy (using carrier oil plus essential oils) versus massage without aromatherapy (using carrier oil only). We excluded touch therapies such as therapeutic touch, acupressure, and reflexology, and inhalations and humidification methods since they were not administered using the massage technique. Five small studies reported quality of life (Soden 2004; Toth 2013; Wilcock 2004; Wilkie 2000; Wilkinson 1999). The primary outcomes for this review were pain and psychological symptoms including anxiety, depression, and mood disturbance assessed using validated scales. Ten studies reported pain and 13 studies reported psychological symptoms. We considered other physical symptoms including fatigue and nausea, quality of life, and adverse events as secondary outcomes. Eight studies reported other physical symptoms and six studies reported quality of life. Only two trials reported adverse events: having physical distress due to the progression of the disease during massage treatment (Jane 2011), and rash and general malaise with aromatherapy massage (Wilcock 2004).
Quality of the evidence
Ten studies (52.6%) had an unclear risk of bias for random sequence generation, and three studies (15.8%) had a high risk of bias for random sequence generation. Fourteen studies (73.7%) had an unclear risk of bias for allocation concealment. Fourteen studies (73.7%) had a high risk of bias related to sample size and only one study met our criteria of low risk of bias for size. However, 15 studies (78.9%) had a low risk of bias for blinding of outcome assessment. Two studies (10.55) had a high risk of bias related to incomplete outcome data, and nine studies (47.4%) had an unclear risk of bias related to selective reporting. We evaluated overall quality of the evidence using GRADE (see: Table 1; Table 2). Domains of the quality of evidence assessment included study design limitations, inconsistency, indirectness, imprecision, and publication bias. We downgraded the GRADE quality of the evidence for all outcomes to very low because of observed imprecision, indirectness, imbalance between groups in many studies, and limitations of study design.
Potential biases in the review process
We needed to make some variations from the original protocol, which are reported in the Differences between protocol and review section. In this review, we applied no language restrictions; however, only one trial included in the quantitative synthesis (meta‐analysis) was published in languages other than English (Korean) (Sohn 2005). Overlooking some published trials in languages other than English can be the source of potential bias. We found imbalance in the baseline measurement for the following: nausea and distress between the massage and no‐massage groups (Ahles 1999); anxiety (STAI) between the massage and no‐massage groups (Hernandez‐Reif 2004); symptoms relating to the breast between the massage and no‐massage groups (Listing 2009); and quality of life (RSCL) between the massage with aromatherapy and massage without aromatherapy groups (Wilkinson 1999). Batalha 2013 reported differences between the massage and no‐massage groups at the beginning of the trial. One trial had a potential problem due to carryover effects in crossover design (Fernandez‐Lao 2012). One trial applied a different intervention: inpatients received daily session and outpatients received weekly sessions (Haun 2009), which could have presented a source of potential bias. Due to high heterogeneity (I2 = 88%), we performed analyses using both the fixed‐effect model and random‐effects model (see Figure 4), and conducted subgroup analyses by duration (short‐term versus medium‐term) and population group (children versus adults). There were statistically different results according to the effect models. When applying the fixed‐effect model, anxiety relief was greater for the massage group compared with the no‐massage group (three RCTs, n = 98, combined MD ‐8.60, 95% CI ‐12.07 to ‐5.13) (Analysis 1.1; Figure 4). However, the overall size was small so we accepted the finding from analyses applying a random‐effects model that showed no significant difference in anxiety relief between the massage and no‐massage groups (three RCTs, n = 98, combined MD ‐5.36, 95% CI ‐16.06 to 5.34) (Analysis 1.1; Figure 4).
Agreements and disagreements with other studies or reviews
The results of this review of 19 RCTs (1274 participants) were generally in agreement with the original version, which included eight RCTs (357 participants) (Fellowes 2004a), and a previously updated version, which included 10 trials (Wilkinson 2008). While our findings showed that massage without aromatherapy may help relieve short‐term pain and anxiety in people with cancer and aromatherapy massage may provide medium‐ or long‐term relief for pain, anxiety, symptoms relating to the breast, and quality of life, the quality of the studies was poor and not reliable. There remains a lack of reliable evidence for the use of aromatherapy and massage to improve clinical outcomes.
Authors' conclusions
Implications for practice.
While we accept that aromatherapy and massage may be a positive experience for some people, we found no evidence to support the use of this intervention for clinical benefit.
Implications for research.
Alternative therapies are popular with people with cancer, carers, and practitioners. Research is required to determine if interventions such as those described in this review are effective for reducing pain as well as being cost effective. To increase the compatibility of massage with or without aromatherapy‐massage interventions, intervention protocols or guidelines would benefit from being standardised in terms of the number and duration of massage treatments; the optimal massage techniques; the body parts to be massaged; and which essential oils should be blended into the carrier oil (Walters 2010). We were surprised by the large number of assessment tools used in these studies and, in our opinion, some research needs to be undertaken to identify reliable, validated tools. We consider that more large, well‐designed studies are required to give some definitive answers to the question of effectiveness. Well‐designed studies focusing on children would be a valuable addition to our knowledge.
What's new
| Date | Event | Description |
|---|---|---|
| 2 June 2016 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 6, 2012 Review first published: Issue 6, 2016
| Date | Event | Description |
|---|---|---|
| 15 July 2014 | New citation required and minor changes | Amendments to title, outcomes, risk of bias assessment and subgroup analyses. For more details see Published notes. |
Notes
In July 2014, we republished the protocol because we amended the title to clarify the comparison.
Upon publication in June 2016, this review has been stabilised following discussion with the authors and editors. A new search within two years is not likely to identify any potentially relevant studies likely to change the conclusions. The review will be re‐assessed for updating in five years. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Acknowledgements
The authors wish to acknowledge Prof. Phil Wiffen for expert advice and support during the preparation of this review. We thank the previous authors, Fellowes D, Barnes K, and Wilkinson S. We appreciate the support of the Pain, Palliative and Supportive Care (PaPaS) Review Group editorial team, especially Anna Erskine, Managing Editor.
Cochrane Review Group funding acknowledgement: The National Institute for Health Research (NIHR) is the largest single founder of the Cochrane PaPaS Group. Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS), or the Department of Health.
Appendices
Appendix 1. The Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
1 MeSH descriptor: [Aromatherapy] this term only
2 MeSH descriptor: [Oils, Volatile] explode all trees
3 MeSH descriptor: [Massage] this term only
4 aromatherap*:ti,ab,kw (Word variations have been searched)
5 ((volatile or essential) next oil*):ti,ab,kw (Word variations have been searched)
6 massag*:ti,ab,kw (Word variations have been searched)
7 #1 or #2 or #3 or #4 or #5 or #6
8 MeSH descriptor: [Neoplasms] explode all trees
9 (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or oncolog*):ti,ab,kw (Word variations have been searched)
10 #8 or #9
11 #7 and #10
Appendix 2. MEDLINE search strategy
1 aromatherapy/
2 exp Oils, Volatile/tu [Therapeutic Use]
3 massage/
4 aromatherap*.ti,ab.
5 ((volatile or essential) adj oil*).ti,ab.
6 massag*.ti,ab.
7 1 or 2 or 3 or 4 or 5 or 6
8 exp Neoplasms/
9 (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or oncolog*).ti,ab.
10 8 or 9
11 7 and 10
12 randomized controlled trial.pt.
13 controlled clinical trial.pt.
14 randomized.ab.
15 placebo.ab.
16 drug therapy.fs.
17 randomly.ab.
18 trial.ab.
19 or/12‐18
20 exp animals/ not humans.sh.
21 19 not 20
22 11 and 21
Appendix 3. EMBASE Ovid search strategy
1 aromatherapy/
2 massage/
3 aromatherap*.ti,ab.
4 ((volatile or essential) adj oil*).ti,ab.
5 massag*.ti,ab.
6 essential oil/
7 or/1‐6
8 exp neoplasm/
9 (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or oncolog*).ti,ab.
10 8 or 9
11 7 and 10
12 random$.tw.
13 factorial$.tw.
14 crossover$.tw.
15 cross over$.tw.
16 cross‐over$.tw.
17 placebo$.tw.
18 (doubl$ adj blind$).tw.
19 (singl$ adj blind$).tw.
20 assign$.tw.
21 allocat$.tw.
22 volunteer$.tw.
23 Crossover Procedure/
24 double‐blind procedure.tw.
25 Randomized Controlled Trial/
26 Single Blind Procedure/
27 or/12‐26
28 (animal/ or nonhuman/) not human/
29 27 not 28
30 11 and 29
Appendix 4. PsycINFO Ovid search strategy
1 aromatherapy/
2 massage/
3 aromatherap*.ti,ab.
4 ((volatile or essential) adj oil*).ti,ab.
5 massag*.ti,ab.
6 exp neoplasm/
7 (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or oncolog*).ti,ab.
8 6 or 7
9 or/1‐5
10 8 and 9
Appendix 5. CINAHL (EBSCO) search strategy
S21 S11 AND S20
S20 S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19
S19 (allocat* random*)
S18 (MH "Quantitative Studies")
S17 (MH "Placebos")
S16 placebo*
S15 (random* allocat*)
S14 (MH "Random Assignment")
S13 (Randomi?ed control* trial*)
S12 (singl* blind* ) or (doubl* blind* ) or (tripl* blind* ) or (trebl* blind* ) or (trebl* mask* ) or (tripl* mask* ) or (doubl* mask* ) or (singl* mask* )
S11 S7 AND S10
S10 S8 OR S9
S9 (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or oncolog*)
S8 (MH "Neoplasms")
S7 S1 OR S2 OR S3 OR S4 OR S5 OR S6
S6 TI massag* OR AB massag*
S5 TI ( ((volatile or essential) N1 oil*) ) OR AB ( ((volatile or essential) N1 oil*) )
S4 TI aromatherap* OR AB aromatherap*
S3 (MH "Massage")
S2 (MH "Essential Oils+/TU")
S1 (MH "Aromatherapy")
Appendix 6. PubMed Cancer Subset search strategy
Search: (((aromatherapy[MeSH Terms] AND (cancer[sb])) OR (Oils, Volatile[MeSH Terms] AND (cancer[sb])) OR (massage[MeSH Terms] AND (cancer[sb])) OR (aromatherap* AND (cancer[sb])) OR ((volatile oil* OR essential oil*) AND (cancer[sb])) OR (massag* AND (cancer[sb]))) AND (cancer[sb])) AND (((randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR randomised[tiab] OR random*[tiab] OR placebo[tiab] OR drug therapy[sh] OR randomly[tiab] OR trial[tiab] OR groups[tiab])) AND (cancer[sb]))
Data and analyses
Comparison 1. Massage versus no massage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Anxiety (STAI‐state) | 3 | 98 | Mean Difference (IV, Random, 95% CI) | ‐5.36 [‐16.06, 5.34] |
Comparison 2. Massage versus no massage: subgroup analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Anxiety (STAI‐state) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Children | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐14.70 [‐19.33, ‐10.07] |
| 1.2 Adults | 2 | 68 | Mean Difference (IV, Fixed, 95% CI) | ‐0.74 [‐5.99, 4.51] |
| 2 Anxiety (STAI‐state) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Short‐term (≤ 4 weeks) | 2 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐10.66 [‐14.72, ‐6.60] |
| 2.2 Medium‐term (> 4 weeks to < 8 weeks) | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐9.69, 3.69] |
Comparison 3. Aromatherapy with massage versus no massage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Anxiety (STAI‐state) | 2 | 253 | Mean Difference (IV, Fixed, 95% CI) | ‐4.50 [‐7.70, ‐1.30] |
Comparison 4. Aromatherapy with massage versus no massage: subgroup analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Anxiety (STAI‐state) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Short‐term (≤ 4 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Medium‐term (> 4 weeks to < 8 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahles 1999.
| Methods | Study design: RCT Country: USA Total: 35 randomised (massage group n = 16, no‐massage group n = 19) |
|
| Participants | Population: people undergoing autologous BMT; diagnosis ‐ breast cancer, Non‐Hodgkin's lymphoma, acute myelogenous lymphoma, Hodgkin's disease, acute myelogenous leukaemia, or ovarian cancer Setting: Dartmouth‐Hitchcock Medical Center after providing informed consent for participation in the study Mean age: 41 years |
|
| Interventions | Intervention: 9 x 20‐minute massages during hospital stay. Mean length of hospital stay was 3 weeks at the time that the study was conducted Comparison: usual care Country of training: not reported Years in practice: not reported |
|
| Outcomes | Outcome measures at pretreatment (day ‐7), mid‐treatment (range = day ‐1 to day +7), and pre‐discharge
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly assigned", but provided no further information |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Performed the same outcome assessment in both groups Quote: "the overall effects of massage therapy on anxiety, depression, and mood were assessed pre treatment, mid treatment, and pre discharge by having patients in both groups complete the following measures" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "one patient in the control group did not complete all of the assessment because of significant medical conditions" |
| Selective reporting (reporting bias) | Unclear risk | No selective outcome reporting Quote: "the POMS is an 11‐item adjective checklist which provides a summary measure of general distress or mood. The brief POMS has been shown to be highly correlated with the original 65‐item POMS, a reliable and valid measure of mood states" |
| Size | High risk | n = 35 |
| Other bias | High risk | Imbalance found in the baseline measurement of nausea and distress between the massage and standard care group; however, no significant between‐group differences in participant characteristics and any class of medication |
Batalha 2013.
| Methods | Study design: RCT Country: Portugal Total number: 52 Sample size: 52 |
|
| Participants | Population: children aged 10‐18 years who were hospitalised in a paediatric cancer ward Setting: not described Median age: 13.5 years intervention group and 12 years control group |
|
| Interventions | Intervention: massage protocol with 3 sessions of 20‐30 minutes on alternate days over a 1‐week period Comparison: usual care for management of pain or other symptoms Country of training: not described Years in practice: not described |
|
| Outcomes | Outcome measures at baseline and then follow‐up measurement after: to evaluate pain and interference with the child's activities on days 1 and 6
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the selection of participants was randomized into two groups (intervention and control)", but provided no further information |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Performed the same outcome assessment in both groups Quote: "pain assessment was performed in all children upon admission at the service (day 1) and on the last day of the protocol (day 6)" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | No selective outcome reporting. Reported pain intensity at the previous week before starting the protocol and at the end of the protocol in both groups |
| Size | High risk | n = 52 |
| Other bias | High risk | Reported differences between groups at the beginning Quote: "there was a predominance of the male gender (20 patients; 76.9%) in the IG [intervention group], and of the female gender (14 patients; 53.8%) in the CG [control group]. Most children reported pain that was different from the usual in the previous week before starting the protocol (day 1), in both groups: 19 patients (73.1%) in the IG and 13 patients (50.0%) in CG" |
Billhult 2007.
| Methods | Study design: RCT Country: Sweden Total: 39 randomised (massage group n = 19, no‐massage group n = 20) |
|
| Participants | Population: women with breast cancer undergoing chemotherapy Setting: oncology clinic at a hospital in the southwest of Sweden Mean age: 51.8 years |
|
| Interventions | Intervention: 20 minutes of massage on 5 occasions Comparison: 5 x 20‐minute visits by hospital staff Country of training: not reported Years in practice: not reported |
|
| Outcomes | Outcome measures before and immediately after each of 5 interventions:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "if consenting, they were randomized", but provided no further information |
| Allocation concealment (selection bias) | Low risk | Used sealed, opaque envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Assessments: before and immediately after each of the five interventions, the patients scored nausea and anxiety on the VAS. This was done equally for both the massage and the visit group', and 'all patients recorded nausea and anxiety on the VAS before and after each intervention. They also completed the HADS" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysed the same number of participants in both groups (see Fig. 1. Study participant flow chart: 19 participants allocated to massage group and analysed 19, 20 participants allocated to visit and analysed 20) |
| Selective reporting (reporting bias) | Low risk | No selective outcome reporting Quote: "the VAS was chosen because it is designed to gather information about internal, subjective feelings such as nausea and anxiety", and "the HAD, a 14‐item instrument comprising 7 items for anxiety and 7 items for depression, was used" |
| Size | High risk | n = 39 |
| Other bias | Low risk | No other significant bias found Quote: "there were no statistically significant differences between groups in demographics or baseline values" |
Campeau 2007.
| Methods | Study design: RCT Country: Canada Total: 100 randomised (massage group n = 52, no‐massage group n = 48) |
|
| Participants | Population: people undergoing radiotherapy Setting: Department of Radiation Oncology at Notre‐Dame Hospital (CHUM) in Montreal, Quebec Mean age: 60 years (massage group), 58 years (control group) |
|
| Interventions | Intervention: 10 massage sessions. All sessions took place before the radiation treatment over 10 consecutive days Comparison: meeting with massage therapist every day before their radiation treatment to assess their anxiety Country of training: not reported Years in practice: not reported |
|
| Outcomes | 2 validated tests used to assess participant's anxiety levels
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomized between the massage therapy and control groups" |
| Allocation concealment (selection bias) | High risk | Used a random number table allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Performed the same outcome assessment in both groups; VAS, STAI |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | No selective outcome reporting Quote: "every day during the 10 consecutive days of treatment, a VAS score was obtained for all patients. The STAI questionnaire was completed before the massage session and radiation treatment by the patients in both groups at the first, fifth, and last session" |
| Size | Unclear risk | n = 100 |
| Other bias | Low risk | No other significant bias found |
Fernandez‐Lao 2012.
| Methods | Study design: RCT Country: Spain Total: 20 randomised (cross‐over trial) |
|
| Participants | Population: breast cancer survivors: diagnosis of breast cancer (stage Ⅰ‐ⅢA); aged 25‐65 years; had completed co‐adjuvant treatment, except hormone therapy; active cancer; an interest in improving lifestyle; the presence of moderate‐to‐high fatigue Setting: Breast Oncology Unit, Hospital Virgen de las Nieves, Granada, Spain Mean age: 49 years |
|
| Interventions | Intervention: myofascial intervention (neck‐shoulder area of approximately 40 minutes (duration adapted to the participant's tissue response) using the Barnes approach Comparison: usual care plus special attention to the participant for 40 minutes Country of training: not described Years in practice: physiotherapist with > 5 years of clinical experience in manual therapy and > 2 years of treating breast cancer survivors before beginning training in massage therapy |
|
| Outcomes | Outcome measures before (pre‐intervention) and after intervention (post‐intervention):
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned by a coin flip" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Performed the same outcome assessment for control group and myofascial massage group: 7 outcomes (tension‐anxiety, depression, fatigue, vigour, anger‐hostility, confusion, and mood disturbance) assessed for both groups (see table 3) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysed the same number of participants in a cross‐over trial: 20 participants for control session and 20 participants for myofascial massage session (see table 2 and table 3) |
| Selective reporting (reporting bias) | Low risk | No selective outcome reporting Quote: "the Spanish version of Profile of Mood States questionnaire was used. The Profile of Mood States of 63 items grouped into six sub scales" |
| Size | High risk | n = 20 |
| Other bias | Unclear risk | Potential problem due to carryover effects in cross‐over trial; authors explained as a need to accommodate the high inter‐individual variability in the outcome measures |
Haun 2009.
| Methods | Study design: RCT, non‐blinded Country: USA Total: 30 randomised (massage group n = 15, no‐massage group n = 15) |
|
| Participants | Population: children with cancer and blood diseases with diverse ages, diseases, and inpatient or outpatient status Setting: paediatric haematology and oncology division Mean age: 10.7 years (massage group), 9.3 years (no‐massage group) |
|
| Interventions | Intervention: regularly scheduled appointment sessions and 20 minutes of Swedish massage therapy on the hands, feet, arms, neck, back, and shoulders Comparison: regularly scheduled appointments in a treatment room or the participant's room within the clinical setting Country of training: nationally certified and licensed in the State of Florida Years in practice: 5 years of experience in the field of massage therapy |
|
| Outcomes | Outcome measures before (pre‐session) and after each session (post‐session)
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "using convenience sampling, resident physicians choose participants from a patient population" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Performed the same outcome assessment: 7 physiological outcomes, 4 psychological outcomes (see table 2), and general clinical progress scale assessed for treatment and control group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysed the same number of participants in both groups (see table 2; n = 15 for each group) |
| Selective reporting (reporting bias) | High risk | Quote: "if participants were unable to respond because of age or illness, parent reports were collected in lieu of the self‐reports, consistent with common practice in clinical settings" |
| Size | High risk | n = 30 |
| Other bias | High risk | Applied different sessions; inpatients received daily sessions, and outpatients received weekly sessions |
Hernandez‐Reif 2004.
| Methods | Study design: RCT Country: USA Total: 34 randomised (massage group n = 18, no‐massage group n = 16) |
|
| Participants | Population: women diagnosed with Stage 1 or 2 breast cancer within the past 3 years who were at least 3 months post‐surgery, chemotherapy, radiotherapy, or a combination of these Setting: not described Mean age: 53 years |
|
| Interventions | Intervention: 30‐minute massages 3 times per week for 5 weeks Comparison: standard medical care alone. At the 5‐week study period, the women in the control group were offered massage therapy Country of training: not described Years in practice: not described |
|
| Outcomes | Outcome measures at before and after sessions (immediate/short‐term effects ‐ STAI, POMS) and on the first/last day of measurements (longer‐term effects ‐ SCL‐90‐R, LEQ, urinary biochemistry, immunological measures):
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned using a flip of a coin at times of screening" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "of the 34 women comprising the final sample, 27 women provided immune measure data" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 7 women did not have their blood drawn; reasons provided |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information provided |
| Size | High risk | n = 34 |
| Other bias | High risk | Imbalance found in the baseline measurement of anxiety (STAI) between the massage therapy and control group; however, no significant between‐group differences in participant demographic data |
Jane 2011.
| Methods | Study design: RCT Country: Taiwan Total: 72 randomised (massage group n = 36, no‐massage group n = 36) |
|
| Participants | Population: participants with metastatic bone pain aged ≥ 18 years; oriented to person, place, and time; able to speak and read Chinese; radiologically diagnosed with evident bone metastases via a bone scan; reportedly experiencing at least moderate metastatic bone pain, with an intensity of P4 on a 0‐10 scale Setting: oncology unit in Chang Gung Memorial Hospital (CGMH), a 3500‐bed‐capacity teaching medical centre in northern Taiwan Mean age: 49.9 years |
|
| Interventions | Intervention: massage therapy lasting 37‐50 minutes, mean 40 minutes Comparison: 45‐minute social attention intervention designed to provide the same amount of time and attention as the message therapy Country of training: not described Years in practice: first author was trained in massage therapy during a 4‐month period at the University of Washington in 2003 and had previous experience providing massage for 30 people with cancer |
|
| Outcomes | Outcome measures on day 2 (T1) to day 4 (T3):
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used computer random number generator |
| Allocation concealment (selection bias) | Low risk | Central allocation by computer |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Performed the same outcome assessment in both groups (see tables 1, 2, 3). 4 outcomes (PPI‐VAS, Mood‐VAS, Relaxation‐VAS, Sleep‐VAS) assessed for both the massage therapy and social attention groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysed the same number of participants in each group (see Figure 1; 36 allocated to massage therapy and 36 analysed, 36 allocated to social attention and 36 analysed) |
| Selective reporting (reporting bias) | Unclear risk | Quote: "the Mood‐VAS was adapted from the Linear Analog Self‐Assessment‐Profile of mood States, a 6‐item vertical form of VAS" |
| Size | Unclear risk | n = 72 |
| Other bias | Low risk | No significant bias found Quote: "no significant differences between MT [massage therapy] and SA [social attention] groups on demographic and medical characteristics, nor on any of the 4 outcome measures gathered at baseline" |
Khiewkhern 2013.
| Methods | Study design: RCT Country: Thailand Total number: 94 Sample size: 66 |
|
| Participants | Population: people with colorectal cancer who have received chemotherapy Setting: Phichit Hospital, Phichit, Thailand Mean age: 59 years (massage group) and 58 years (no‐massage group) |
|
| Interventions | Intervention: 3 massage sessions with ginger and coconut oil over 1‐week period Comparison: standard supportive care only Country of training: not described Years in practice: not described |
|
| Outcomes | Outcome measures at baseline and then follow‐up measurement after: pre‐assessment (5‐15 minutes before first massage in treatment group) and at the end of 1 week of massage or standard care (1‐2 days after last massage in treatment group)
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the remaining 66 patients were assigned in equal numbers into either the treatment or control group by block randomization" |
| Allocation concealment (selection bias) | Unclear risk | Used a block size of 4 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Performed the same outcome assessment in both groups Quote: "at the pre‐ and post‐assessment, patients were given a card with numerical rating scales for common symptoms (pain, fatigue, stress or anxiety, nausea, and depression) and were asked to rate the severity of each symptom on a 0 ('not at all bothersome') to 10 ('extremely bothersome') scale" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "three patients in the treatment group discontinued massage after the second session due to fatigue, but they were able to complete the post‐assessment. Another patient declined to continue the massage sessions due to lack of interest. In the control group, four patients ceased participating: three decided to receive treatment at another hospital, and one received radiotherapy" |
| Selective reporting (reporting bias) | Low risk | Common symptoms (pain, fatigue, stress or anxiety, nausea, and depression) reported in both groups |
| Size | Unclear risk | n = 66 |
| Other bias | Unclear risk | Analysed the data from all participants in both groups, even though some participants lost to follow‐up |
Krohn 2010.
| Methods | Study design: RCT Country: Germany Total: 34 randomised (massage group n = 17, no‐massage group n = 17) |
|
| Participants | Population: people with breast cancer; tumour size ≤ T2, nodal state ≤ N2, disease onset ≤ 4 years prior. Surgery, chemotherapy, or radiotherapy (or a combination) had to be completed ≥ 3 months prior to the beginning of the study Setting: not described Mean age: 59.7 years |
|
| Interventions | Intervention: 30‐minute classical massage twice per week Comparison: standard medical care Country of training: not described Years in practice: not described |
|
| Outcomes | Outcome measures before intervention (T1), at the end of the 5‐week intervention period (T2), and 6 weeks after the end of intervention (T3)
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized into two groups by simple randomisation" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "all participants completed the PHQ, BSF, and PSQ, and a blood sample was taken" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "one patient had to be excluded from the analysis of immunological data because of highly elevated Th1 cytokine concentrations", and 5 participants lost during 5‐week intervention; reasons provided |
| Selective reporting (reporting bias) | Low risk | Quote: "the German 20‐item version of the PSQ was used", "the depression score is derived from nine items of the depression module", "the 30‐item BSF measures six different mood states" |
| Size | High risk | n = 34 |
| Other bias | Unclear risk | Imbalance in the baseline measurement of the cytokine IL‐4 between the massage and no‐massage groups; however, no significant between‐group differences in sociodemographic or clinical variables at baseline |
Listing 2009.
| Methods | Study design: RCT Country: Germany Total: 115 randomised (aromatherapy with massage group n = 58, no‐massage group n = 57) |
|
| Participants | Population: people with breast cancer, tumour size ≤ T2 (5 cm), nodal state ≤ N2 (≤ 9 tumour positive axillary nodes), no distant metastases, disease onset ≤ 4 years and time since last chemotherapy or radiotherapy (or both) > 3 months Setting: Breast Cancer Center of the Charité University Hospital, Berlin Mean age: 59 years |
|
| Interventions | Intervention: bi‐weekly 30‐minute classical aromatherapy massages to the back and head‐neck areas for 5 weeks Comparison: no treatment in addition to routine healthcare Country of training: not described Years in practice: not described |
|
| Outcomes | Outcome measures at baseline (T1) at the end of the 5‐week intervention period (T2) and follow‐up measurements 11 weeks later
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | 'Used simple unrestricted randomisation' |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Performed the same outcome assessment in both groups. Physical discomforts (4 outcomes) and mood states (7 outcomes) assessed for both the aromatherapy‐massage and no‐massage group |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 14 participants dropped out during the intervention period. |
| Selective reporting (reporting bias) | Unclear risk | Quote: "the DF‐8TM contains one item for each of the eight concepts of the SF‐36. Only the dimension of 'bodily pain' was evaluated in this study" and "the GBB questionnaire consists of 57 items, the scales of 'fatigue' and 'pain of limbs' (six items of each) were included in our analysis" |
| Size | Unclear risk | n = 115 |
| Other bias | Unclear risk | Imbalance found in the baseline measurement of breast symptoms between the aromatherapy‐massage and no‐massage groups; however, no significant between‐group differences in sociodemographic or clinical variables at baseline |
Soden 2004.
| Methods | Study design: RCT Country: UK Total: 42 randomised |
|
| Participants | Population: people with cancer Breast cancer 36%, lung cancer 19% Setting: hospice Median age: 73 years (range 44‐85 years) |
|
| Interventions | Intervention 1: weekly 30 minutes back massage with lavender essential oil and an inert carrier oil (aromatherapy group)
Intervention 2: weekly 30 minutes back massage with an inert carrier oil only (massage group) Comparison: no massage Country of training: not reported Years in practice: not reported |
|
| Outcomes | Outcome measurement: all participants completed the following scales at a baseline assessment during the week before the first treatment and at a final assessment in the week after the last massage
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly allocated to one of three group" |
| Allocation concealment (selection bias) | Low risk | Used a numbered opaque envelope |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Performed the same outcome assessment in each group; VAS pain intensity, VSH sleep scale, HAD anxiety and depression, and RSCL symptom checklist |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 6 participants did not complete the study, reasons provided (n = 42) |
| Selective reporting (reporting bias) | Unclear risk | Used the Modified Tursky pain Descriptors Scale |
| Size | High risk | n = 42 |
| Other bias | Low risk | No other significant bias found |
Sohn 2005.
| Methods | Study design: RCT, non‐blinded Country: Korea Total: 32 randomised (aromatherapy with massage group n = 15, no‐massage group n = 17) |
|
| Participants | Population: women with breast cancer aged > 20 years Setting: not described Mean age: 48.6 years |
|
| Interventions | Intervention: participants massaged their own hands using aromatic oils at home twice per day for 2 weeks Comparison: no intervention Country of training: not described Years in practice: not described |
|
| Outcomes | Outcome measures at 0, 1, and 3 weeks:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized‐controlled clinical trial", but provided no further information |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessed the same outcomes (Pain intensity‐VAS, BDIS, BEPSI revised edition, STAI, physiological measures) for the aromatherapy and control group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysed the same number of participants in each groups (15 allocated to aromatherapy‐massage group and 15 analysed, 17 allocated to control group and 17 analysed) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information provided |
| Size | High risk | n = 32 |
| Other bias | High risk | Imbalance found in the baseline measurements of pain intensity (VAS) and anxiety (STAI) between the aromatherapy and control groups |
Toth 2013.
| Methods | Study design: RCT Country: USA Total: 39 randomised (massage group n = 20, no touch n = 10, usual care n = 9) |
|
| Participants | Population: people with metastatic cancer Setting: home Mean age: 54.9 years (massage group), 55.6 years (usual care) |
|
| Interventions | Intervention: massage therapy (touch intervention) Comparison no touch intervention with no therapeutic intention or usual care Country of training: not reported Years in practice: minimum 3 years full‐time or 5 years part‐time practice |
|
| Outcomes | Primary outcomes:
Secondary outcome:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "patients were randomized" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessed the same outcomes (Pain‐VAS, Anxiety‐VAS, Alertness‐VAS) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information provided |
| Size | High risk | n = 39 |
| Other bias | Low risk | No other significant bias found |
Wang 2015.
| Methods | Study design: RCT Country: Taiwan Total number: 80 |
|
| Participants | Population: people with malignant ascites from gastroenterology and oncology units, aged ≥ 18 years; clinically diagnosed stage Ⅳ cancer; clinically diagnosed malignant ascites; able to speak Mandarin or Taiwanese; obtained medical clearance from an attending physician to participate in the study; consent to participate, which was witnessed by a family member Setting: medical centre in northern Taiwan Mean age: 59.1 years |
|
| Interventions | Intervention: 15‐minute gentle abdominal massage, using straight rubbing, point rubbing, and kneading, administered twice daily for 3 days Comparison: twice‐daily 15‐minute social interaction contact with the same nurse Country of training: not described Years in practice: 8 hours (nurse practitioner) |
|
| Outcomes | Outcome measures at baseline and then follow‐up measurement after: in the morning for 4 consecutive days from pre‐ to post‐test
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned using random allocation software. However, participants at the same room were assigned to the same study group to avoid interactions between the intervention and control group" |
| Allocation concealment (selection bias) | Unclear risk | Used random allocation software. However, participants at the same room assigned to the same study group to avoid interactions between the intervention and control group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Performed the same outcome assessment in both groups Quote: "A blinded outcome assessor measured body weight and collected self‐report data on malignant ascites symptoms from both groups each morning before breakfast (between 7 and 8 a.m.) over the 4 consecutive days from pre‐ to post‐test" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "all 80 participants completed the study" |
| Selective reporting (reporting bias) | Low risk | Quote: "the Edmonton Symptom Assessment System‐Ascites Modification (ESAS:AM) (Easson et al., 2007) was used to measure the severity of malignant ascites symptoms. The ESAS:AM has 11 items, each of which targets a specific ascites‐associated symptom. The participants were asked to indicate the severity of each symptom during the preceding 24 hours using an 11‐point (0 ‐ 10) numeric rating scale (NRS)" |
| Size | Unclear risk | n = 80 |
| Other bias | Unclear risk | Level of nausea was different at the baseline between groups (P value = 0.041) |
Wilcock 2004.
| Methods | Study design: RCT Country: UK Total: 46 randomised 1:1 (aromatherapy with massage group n = 23, no‐massage group n = 23) |
|
| Participants | Population: people with any type of cancer attending day care approached on their third visit and invited to participate in the study Setting: not reported Mean age: 71.5 years |
|
| Interventions | Intervention: day care plus weekly aromatherapy massage for 4 weeks Comparison: day care alone for 4 weeks Country of training: not described Years in practice: not described |
|
| Outcomes | Outcome measures at baseline and at the end of the intervention (4 weeks):
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used block randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessed the same outcomes (MYMOP, POMS) Quote: "all questionnaires were completed by the patients with the help of a research nurse, who had no involvement in their clinical care" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 17 lost after 4 weeks (37.0%); reasons provided for drop‐out (n = 46) |
| Selective reporting (reporting bias) | Low risk | Quote: "completed the Profile of Mood State (POMS) questionnaire. This consists of six sub scales: tension, depression, anger, fatigue, confusion and vigour that combine to give an overall score of total mood of disturbance of between 0 and 76, a lower score indication less disturbance" |
| Size | High risk | n = 46 |
| Other bias | Low risk | The age, sex, performance status, and prior experience of complementary therapy of those completing the study did not differ significantly between the 2 groups |
Wilkie 2000.
| Methods | Study design: RCT Country: USA Total: 56 randomised (massage group n = 26, no‐massage group n = 30) |
|
| Participants | Population: people had pain associated with primary cancers Setting: person's homes except for 4 participants who resided in 3 different nursing homes Mean age: 63 years |
|
| Interventions | Intervention: 30‐50 minutes of massage on twice‐weekly for 2 weeks Comparison: usual hospice care Country of training: not reported Years in practice: not reported |
|
| Outcomes | Outcome measures before the first and immediately after the fourth massages:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned to groups" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "the attrition rate was high (48%) including 16 control group patients and 11 massage group patients. The 14 control group and 15 massage group subjects who completed the study. The most frequent reasons for withdrawal were death (n=15) or rapid mental or physical deterioration with inability to complete the study questionnaire (n = 6) |
| Selective reporting (reporting bias) | High risk | Did not report the QoL as an immediate outcome. Did not report the emotional distress as a long‐term outcome |
| Size | Unclear risk | n = 56 |
| Other bias | Low risk | Quote: "subjects who completed or did not complete the study were not statistically different in age or baseline mean scores for any of the outcomes variables" |
Wilkinson 1999.
| Methods | Study design: RCT Country: UK Total: 103 randomised (aromatherapy with massage group n = 46, massage without aromatherapy group n = 57) |
|
| Participants | Population: people attending a palliative care centre as inpatients or outpatients, who were referred to the aromatherapy co‐ordinator on the written referral form for massage Setting: not reported Mean age: 53.5 years |
|
| Interventions | Intervention: full‐body massage with a carrier oil and Roman chamomile essential oil Comparison: full‐body massage with carrier oil only Country of training: not described Years in practice: not described |
|
| Outcomes | Outcome measures at pre‐test (1 week) and post‐test (4 weeks):
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a computer‐generated randomised table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessed the same outcomes (7 RSCL sub‐scales and STAI) for the aromatherapy group and massage group Quote: "each patient completed RSCL, SAI, TAI, a semi‐structured questionnaire" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 16 participants did not complete the study; 13 died before completion and 3 were too ill to complete |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information provided |
| Size | Unclear risk | n = 103 |
| Other bias | Low risk | Imbalance found in the baseline measurement of RSCL‐QoL between the aromatherapy and massage groups; provided descriptive statistics of the 87 participants |
Wilkinson 2007.
| Methods | Study design: RCT Country: UK Total: 288 randomised (aromatherapy with massage group n = 144, no‐massage group n = 144) |
|
| Participants | Population: people with cancer, referred to complementary therapy services with clinical anxiety or depression, or both Setting: recruited from 4 cancer centres and 1 hospice in England between September 1998 and May 2002 Mean age: 52.1 years |
|
| Interventions | Intervention: 4‐week course of weekly, 1‐hour sessions of aromatherapy massage Comparison: psychological support services as part of cancer care (usual supportive care) Country of training: not reported Years in practice: not reported |
|
| Outcomes | Outcome measures at randomisation, 6 weeks, and 10 weeks post‐randomisation Primary outcome
Secondary outcomes
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned using random number sequence, and balanced in randomly sized blocks" |
| Allocation concealment (selection bias) | Low risk | Used numbered sealed opaque envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessed 6 same outcomes (SAI, CES‐D, EORTC‐pain, fatigue, nausea/vomiting, global QoL) at 6 weeks and 10 weeks post‐randomisation in both groups |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 67/288 lost to follow‐up Quote: "the data available at 6 and 10 weeks post randomisation were not representative of the complete sample of randomly assigned patients" |
| Selective reporting (reporting bias) | Unclear risk | Quote: "along with missing questionnaires, the continuous secondary outcome measures were considered missing if fewer than half of the items of a factor were completed" |
| Size | Low risk | n = 288 |
| Other bias | Low risk | No other significant bias found |
BMT: bone marrow transplantation; DBP: diastolic blood pressure; ms: millisecond; n: number of participants; QoL: quality of life;
RCT: randomised controlled trial; SBP: systolic blood pressure.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Beider 2007 | Review |
| Benney 2013 | Review |
| Billhult 2008 | Different outcomes, only assessed immune function |
| Billhult 2009 | Different outcomes, assessed immune function. No data on stress |
| Calenda 2006 | Review |
| Cassileth 2004 | Not RCT, no control group |
| Chang 2008 | Non‐randomised, non‐blinded, non‐equivalent control group pre‐test and post‐test design |
| Chun 2010 | Non‐randomised, non‐blinded non‐equivalent control group pre‐test and post‐test design |
| Corner 1995 | Not RCT, quasi‐experimental study |
| Currin 2008 | Not RCT, no control group |
| Dion 2015 | Mixed intervention, guided meditation and massage therapy |
| Dyer 2013 | Different comparison vs. aromatherapy massage |
| Ernst 2009 | Review |
| Han 2005 | Non‐randomised, non‐blinded non‐equivalent control group pre‐test and post‐test design |
| Han 2012 | Non‐randomised, non‐blinded non‐equivalent control group pre‐test and post‐test design |
| Hernandez‐Reif 2005 | Not RCT, non‐randomised and non‐blinded |
| Hughes 2008 | Review |
| Imanishi 2009 | Not RCT, quasi‐experimental study |
| Jane 2009 | Not RCT, a pilot study |
| Karagozoglu 2013 | Not RCT, quasi‐experimental and cross‐sectional study |
| Keir 2011 | Not RCT, a pilot study |
| Lai 2011 | Not RCT, a pilot study |
| Lopez‐Sendin 2012 | Mixed intervention, physiotherapy including massage and exercise |
| Moyer 2004 | Review |
| Oh 2008 | Non‐randomised, non‐blinded, non‐equivalent control group pre‐test and post‐test design |
| Osaka 2009 | Not RCT, no control group |
| PostWhite 2009 | Not RCT, a pilot study |
| Serfaty 2012 | Different comparison vs. aromatherapy massage |
| Smith 2002 | Not RCT, quasi‐experimental study |
| Song 2009 | Non‐randomised, non‐blinded, non‐equivalent control group pre‐test and post‐test design |
| Stringer 2008 | Different outcomes, only assessed serum cortisol and prolactin |
| Sturgeon 2009 | Not RCT, a pilot study |
| Tarhan 2005 | Not RCT, no control group |
| Wilkinson 2008 | Review |
RCT: randomised controlled trial.
Differences between protocol and review
There were some variations from the protocol, as follows.
We added a 'Summary of findings' table using GRADEpro; we searched the PubMed Cancer Subset database instead of the CANCERLIT database, which is no longer available. To increase quality of evidence, we excluded quasi‐randomised trials and controlled clinical trials from the review. Adverse events were originally a primary outcome, but are now a secondary outcome. We could not perform subgroup analysis for full body massage versus partial massage (hand, foot, shoulder, neck, back, abdomen, and scalp, etc.) due to a lack of data. We could not calculate the number needed to treat for an additional beneficial outcome because there were no dichotomous data for pre‐defined outcome measurements (Moore 2002). We changed the definitions of short‐, medium‐, and long‐term interventions. Instead, we employed a duration of treatment concept (four weeks or less was short‐term, and eight weeks or greater was long‐term).
Contributions of authors
Writing the full review: ESS.
Search databases: ESS, KHS.
Study selection: ESS, SHL, MJK.
Assessment of methodological quality: ESS, KHS, JYY.
Data extraction: ESS, YMJ, JEJ.
Assessment of risk of bias: KHS, JYY.
Statistical analysis: ESS, KHS.
Updating the review: ESS, KHS.
Sources of support
Internal sources
KAMS Research Center, Korean Academy of Medical Sciences (KAMS), Korea, South.
External sources
The South Asian Cochrane Network and Centre, Christian Medical College, Vellore, India.
Declarations of interest
Shin, Seo, Lee, Jang, Jung, Kim, and Yeon are free of any real or perceived bias introduced by receipt of any benefit in cash or kind, any hospitality, or any subsidy derived from any source that may have or be perceived to have an interest in the outcome of the review. In particular, there are no conflicts of interest that relate to the pharmaceutical industry.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Ahles 1999 {published data only}
- Ahles TA, Tope DM, Pinkson B, Walch S, Hann D, Whedon M, et al. Massage therapy for patients undergoing autologous bone marrow transplantation. Journal of Pain and Symptom Management 1999;18(3):157‐63. [DOI] [PubMed] [Google Scholar]
Batalha 2013 {published data only}
- Batalha LM, Mota AA. Massage in children with cancer: effectiveness of a protocol. Journal of Pediatics 2013;89(6):595‐600. [DOI] [PubMed] [Google Scholar]
Billhult 2007 {published data only}
- Billhult A, Bergbom I, Stener‐Victorin E. Massage relieves nausea in women with breast cancer who are undergoing chemotherapy. Journal of Alternative and Complementary Medicine 2007;13(1):53‐7. [DOI] [PubMed] [Google Scholar]
Campeau 2007 {published data only}
- Campeau MP, Gaboriault R, Drapeau M, Nguyen TV, Roy I, Fortin B, et al. Impact of massage therapy on anxiety levels in patients undergoing radiation therapy: randomized controlled trial. Journal of the Society for Integrative Oncology 2007;5(4):133‐8. [DOI] [PubMed] [Google Scholar]
Fernandez‐Lao 2012 {published data only}
- Fernandez‐Lao C, Cantarero‐Villanueva I, Diaz‐Rodriguez L, Cuesta‐Vargas AI, Fernandezdelas‐Penas C, Arroyo‐Morales M. Attitudes towards massage modify effects of manual therapy in breast cancer survivors: a randomised clinical trial with crossover design. European Journal of Cancer care 2012;21(2):233‐41. [DOI] [PubMed] [Google Scholar]
- Fernandez‐Lao C, Cantarero‐Villanueva I, Diaz‐Rodriguez L, Fernandez‐De‐Las‐Penas C, Sanchez‐Salado C, Arroyo‐Morales M. The influence of patient attitude toward massage on pressure pain sensitivity and immune system after application of myofascial release in breast cancer survivors: a randomized, controlled crossover study. Journal of Manipulative and Physiological Therapeutics 2012;35(2):94‐100. [DOI] [PubMed] [Google Scholar]
Haun 2009 {published data only}
- Haun JN, Graham‐Pole J, Shortley B. Children with cancer and blood diseases experience positive physical and psychological effects from massage therapy. International Journal of Therapeutic Massage and Bodywork 2009;2(2):7‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hernandez‐Reif 2004 {published data only}
- Hernandez‐Reif M, Ironson G, Field T, Hurley J, Katz G, Diego M, et al. Breast cancer patients have improved immune and neuroendocrine functions following massage therapy. Journal of Psychosomatic Research 2004;57(1):45‐52. [DOI] [PubMed] [Google Scholar]
Jane 2011 {published data only}
- Jane SW, Chen SH, Wilkie DJ, Lin YC, Foreman SW, Beaton RD, et al. Effects of massage on pain, mood status, relaxation, and sleep in Taiwanese patients with metastatic bone pain: a randomized clinical trial. Pain 2011;152(10):2432‐42. [DOI] [PubMed] [Google Scholar]
Khiewkhern 2013 {published data only}
- Khiewkhern S, Promthet S, Sukprasert A, Eunhpinitpong W, Bradshaw P. Effectiveness of aromatherapy with light Thai massage for cellular immunity improvement in colorectal cancer patients receiving chemotherapy. Asian Pacific Journal of Cancer Prevention 2013;14:3903‐7. [DOI] [PubMed] [Google Scholar]
Krohn 2010 {published data only}
- Krohn M, Listing M, Tjahjono G, Reisshauer A, Peters E, Klapp BF, et al. Depression, mood, stress, and Th1/Th2 immune balance in primary breast cancer patients undergoing classical massage therapy. Supportive Care in Cancer 2011;19(9):1303‐11. [DOI] [PubMed] [Google Scholar]
Listing 2009 {published data only}
- Listing M, Krohn M, Liezmann C, Kim I, Reisshauer A, Peters E. The efficacy of classical massage on stress perception and cortisol following primary treatment of breast cancer. Archive of Women's Mental Health 2010;13(2):165‐73. [DOI] [PubMed] [Google Scholar]
- Listing M, Reisshauer A, Krohn M, Voigt B, Tjahono G, Becker J, et al. Massage therapy reduces physical discomfort and improves mood disturbances in women with breast cancer. Psycho‐Oncology 2009;18(12):1290‐9. [DOI] [PubMed] [Google Scholar]
Soden 2004 {published data only}
- Soden K, Vincent K, Craske S, Lucas C, Asley S. A randomized controlled trial of aromatherapy massage in a hospice setting. Palliative Medicine 2004;18(2):87‐92. [DOI] [PubMed] [Google Scholar]
Sohn 2005 {published data only}
- Sohn KJ. The Effects of Aroma Hand Massage on Pain. Depressive Mood and Anxiety in Breast Cancer Patients [Master's thesis]. Seoul, South Korea: Korea University, 2005. [Google Scholar]
Toth 2013 {published data only}
- Toth M, Marcantonio ER, Davis RB, Walton T, Kahn JR, Phillips RS. Massage therapy for patients with metastatic cancer: a pilot randomized controlled trial. Journal of Alternative and Complementary Medicine 2013;19(7):650‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2015 {published data only}
- Wang TJ, Wang HM, Yang TS, Jane SW, Huang TH, Wang CH, et al. The effect of abdominal massage in reducing malignant ascites symptoms. Research in Nursing & Health 2015;38:51‐9. [DOI] [PubMed] [Google Scholar]
Wilcock 2004 {published data only}
- Wilcock A, Manderson C, Weller R, Walker G, Carr D, Carey AM, et al. Does aromatherapy massage benefit patients with cancer attending a specialist palliative care day centre?. Palliative Medicine 2004;18(4):287‐90. [DOI] [PubMed] [Google Scholar]
Wilkie 2000 {published data only}
- Wilkie DJ, Kampbell J, Cutshall S, Halabisky H, Harmon H, Johnson LP, et al. Effects of massage on pain intensity, analgesics and quality of life in patients with cancer pain: a pilot study of a randomized clinical trial conducted within hospice care delivery. Hospice Journal‐Physical, Psychosocial & Pastoral Care of the Dying 2000;15(3):31‐53. [PubMed] [Google Scholar]
Wilkinson 1999 {published data only}
- Wilkinson S, Aldridge J, Salmon I, Cain E, Wilson B. An evaluation of aromatherapy massage in palliative care. Palliative Medicine 1999;13(5):409‐17. [DOI] [PubMed] [Google Scholar]
Wilkinson 2007 {published data only}
- Wilkinson SM, Love SB, Westcombe AM, Gambles MA, Burgess CC, Cargill A, et al. Effectiveness of aromatherapy massage in the management of anxiety and depression in patients with cancer: a multicenter randomized controlled trial. Journal of Clinical Oncology 2007;25(5):532‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Beider 2007 {published data only}
- Beider S, Moyer CA. Randomized controlled trials of pediatric massage: a review. Evidence‐based Complementary & Alternative Medicine 2007;4(1):23‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Benney 2013 {published data only}
- Benney S, Gibbs V. A literature review evaluating the role of Swedish massage and aromatherapy massage to alleviate the anxiety of oncology patients. Radiology 2013;19:35‐41. [Google Scholar]
Billhult 2008 {published data only}
- Billhult A, Lindholm C, Gunnarsson R, Stener‐Victorin E. The effect of massage on cellular immunity, endocrine and psychological factors in women with breast cancer ‐ a randomized controlled clinical trial. Automatic Neuroscience: Basic and Clinical 2008;140(1‐2):88‐95. [DOI] [PubMed] [Google Scholar]
Billhult 2009 {published data only}
- Billhult A, Lindholm C, Gunnarsson R, Stener‐Victorin E. The effect of massage on immune function and stress in women with breast cancer ‐ a randomized controlled trial. Automatic Neuroscience: Basic and Clinical 2009;150(1.2):111‐5. [DOI] [PubMed] [Google Scholar]
Calenda 2006 {published data only}
- Calenda E. Massage therapy for cancer pain. Current Pain and Headache Reports 2006;10(4):270‐4. [DOI] [PubMed] [Google Scholar]
Cassileth 2004 {published data only}
- Cassileth BR, Vickers AJ. Massage therapy for symptom control: outcome study at a major cancer centre. Journal of Pain and Symptom Management 2004;28(3):244‐9. [DOI] [PubMed] [Google Scholar]
Chang 2008 {published data only}
- Chang SY. Effects of aroma hand massage on pain, state anxiety and depression in hospice patients with terminal cancer. Journal of Korean Academy Nursing 2008;38(4):493‐502. [DOI] [PubMed] [Google Scholar]
Chun 2010 {published data only}
- Chun N, Kim SH. The effects of hand massage on comfort in women with gynecologic cancer undergoing chemotherapy. Journal of Korean Oncology Nursing 2010;10(1):88‐94. [Google Scholar]
Corner 1995 {published data only}
- Corner JCN, Hildebrand S. An evaluation of the use of massage and essential oils on the well being of cancer patients. International Journal of Palliative Nursing 1995;24(6):827‐31. [DOI] [PubMed] [Google Scholar]
Currin 2008 {published data only}
- Currin J, Meister EA. A hospital‐based intervention using massage to reduce distress among oncology patients. Cancer Nursing 2008;31(3):214‐21. [DOI] [PubMed] [Google Scholar]
Dion 2015 {published data only}
- Dion LJ, Engen DJ, Lemaine V, Lawson DK, Brock CG, Thomley BS, et al. Massage therapy alone and in combination with meditation for breast cancer patients undergoing autologous tissue reconstruction: a randomized pilot study. Complementary Therapies in Clinical Practice 2015;23:1‐6. [DOI] [PubMed] [Google Scholar]
Dyer 2013 {published data only}
- Dyer J, Thomas K, Sandsund C, Shaw C. Is reflexology as effective as aromatherapy massage for symptom relief in an adult outpatient oncology population?. Complementary Therapies in Clinical Practice 2013;19:139‐46. [DOI] [PubMed] [Google Scholar]
Ernst 2009 {published data only}
- Ernst E. Massage therapy for cancer palliation and supportive care: a systematic review of randomized clinical trials. Supportive Care in Cancer 2009;17(4):333‐7. [DOI] [PubMed] [Google Scholar]
Han 2005 {published data only}
- Han JE, Moon YI, Park HR. Effect of hand massage on nausea, vomiting and anxiety in childhood with leukemia on chemotherapy. Korean Journal of Child Health Nursing 2005;11(4):456‐64. [Google Scholar]
Han 2012 {published data only}
- Han MS, Lee KY. The effect of back massage on degree of pain, state anxiety and quality of sleep of postoperative patients with gastrectomy. Asian Oncology Nursing 2012;12(1):69‐76. [DOI: 10.5388/aom.2012.12.1.69] [DOI] [Google Scholar]
Hernandez‐Reif 2005 {published data only}
- Hernandez‐Reif M, Field T, Ironson G, Beutler J, Vera Y, Hurley J, et al. Natural killer cells and lymphocytes increase in women with breast cancer following massage therapy. International Journal of Neuroscience 2005;115(4):495‐510. [DOI] [PubMed] [Google Scholar]
Hughes 2008 {published data only}
- Hughes D, Ladas E, Rooney D, Kelly K. Massage therapy as a supportive care intervention for children with cancer. Oncology Nursing Forum 2008;35(3):431‐42. [DOI] [PubMed] [Google Scholar]
Imanishi 2009 {published data only}
- Imanishi J, Kuriyama H, Shigemori I, Watanabe S, Aihara Y, Kita M, et al. Anxiolytic effect of aromatherapy massage in patients with breast cancer. Evidence‐based Complement and Alternative Medicine 2009;6(1):123‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jane 2009 {published data only}
- Jane S, Wilkie DJ, Gallucci BB, Beaton RD, Huang H. Effects of a full‐body massage on pain intensity, anxiety, and physiological relaxation in Taiwanese patients with metastatic bone pain: a pilot study. Journal of Pain and Symptom Management 2009;37(4):754‐63. [DOI] [PubMed] [Google Scholar]
Karagozoglu 2013 {published data only}
- Karagozoglu S, Kahve E. Effects of back massage on chemotherapy‐related fatigue and anxiety: supportive care and therapeutic touch in cancer nursing. Applied Nursing Research 2013;26:210‐7. [DOI] [PubMed] [Google Scholar]
Keir 2011 {published data only}
- Keir ST. Effect of massage therapy on stress levels and quality of life in brain tumor patients ‐ observations from a pilot study. Supportive Care in Cancer 2011;19(5):711‐5. [DOI] [PubMed] [Google Scholar]
Lai 2011 {published data only}
- Lai TK, Cheung MC, Lo CK, Ng KL, Fung YH, Tong M, et al. Effectiveness of aroma massage on advanced cancer patients with constipation: a pilot study. Complementary Therapies in Clinical Practice 2011;17(1):37‐43. [DOI] [PubMed] [Google Scholar]
Lopez‐Sendin 2012 {published data only}
- Lopez‐Sendin N, Alburquerque‐Sendin F, Cleland JA, Femandez‐De‐Las‐Penas C. Effects of physical therapy on pain and mood in patients with terminal cancer: a pilot randomized clinical trial. Journal of Alternative and Complementary Medicine 2012;18(5):480‐6. [DOI] [PubMed] [Google Scholar]
Moyer 2004 {published data only}
- Moyer CA, Rounds J, Hannum JW. A meta‐analysis of massage therapy research. Psychological Bulletin 2004;130(1):3‐18. [DOI] [PubMed] [Google Scholar]
Oh 2008 {published data only}
- Oh HS. The Effects of Aroma Hand Massage on the Stress, Sleep Satisfaction and Death Anxiety in Terminal Cancer Patients [Master's thesis]. Gyeongsangbuk‐do, South Korea: Catholic University of Daegu, 2008. [Google Scholar]
Osaka 2009 {published data only}
- Osaka I, Kurihara Y, Tanaka K, Nishizaki H, Aoki S, Adachi I. Endocrinological evaluations of brief hand massages in palliative care. Journal of Alternative and Complementary Medicine 2009;15(9):981‐5. [DOI] [PubMed] [Google Scholar]
PostWhite 2009 {published data only}
- PostWhite J, Fitzgerald M, Savik K, Hooke MC, Hannahan AB, Sencer SF. Massage therapy for children with cancer. Journal of Pediatric Oncology Nursing 2009;26(1):16‐28. [DOI] [PubMed] [Google Scholar]
Serfaty 2012 {published data only}
- Serfaty M, Wilkinson S, Freeman C, Mannix K, King M. The ToT study: helping with Touch or Talk (ToT): A pilot randomized controlled trial to examine the clinical effectiveness of aromatherapy massage versus cognitive behaviour therapy for emotional distress in patients in cancer palliative care. Psycho‐Oncology 2012;21(5):563‐9. [DOI] [PubMed] [Google Scholar]
Smith 2002 {published data only}
- Smith MC, Kemp J, Hemphill L, Vojir CP. Outcomes of therapeutic massage for hospitalised cancer patients. Journal of Nursing Scholarship 2002;34(3):257‐62. [DOI] [PubMed] [Google Scholar]
Song 2009 {published data only}
- Song BE, Yoo YS, Cho OH. Effects of back massage on immune response, symptom distress and mood state of patients undergoing allogeneic hematopoietic stem cell transplantation. Journal of Korean Academy Adult Nursing 2009;21(3):269‐80. [Google Scholar]
Stringer 2008 {published data only}
- Stringer J, Swindell F, Dennis M. Massage in patients undergoing intensive chemotherapy reduces serum cortisol and prolactin. Psycho‐Oncology 2008;17(10):1024‐31. [DOI] [PubMed] [Google Scholar]
Sturgeon 2009 {published data only}
- Sturgeon M, Wetta‐Hall R, Hart T, Good M, Dakhil S. Effects of therapeutic massage on the quality of life among patients with breast cancer during treatment. Journal of Alternative and Complementary Medicine 2009;15(4):373‐80. [DOI] [PubMed] [Google Scholar]
Tarhan 2005 {published data only}
- Tarhan F, Orcun A, Kucukercan I, Camursoy N, Kuyumcuoglu U. Effect of prostatic massage on serum complexed prostate‐specific antigen levels. Urology 2005;66(6):1234‐8. [DOI] [PubMed] [Google Scholar]
Wilkinson 2008 {published data only}
- Wilkinson S, Barnes K, Storey L. Massage for symptom relief in patients with cancer: systematic review. Journal of Advanced Nursing 2008;63(5):430‐9. [DOI] [PubMed] [Google Scholar]
Additional references
Akechi 2008
- Akechi T, Akuyama T, Onishi J, Morita T, Furukawa TA. Psychotherapy for depression among incurable cancer patients. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD005537.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alacacioglu 2010
- Alacacioglu A, Binicier O, Gungor O, Oztop I, Dirioz M, Yilmaz U. Quality of life, anxiety, and depression in Turkish colorectal cancer patients. Supportive Care in Cancer 2010;18(4):417‐21. [DOI] [PubMed] [Google Scholar]
Bagetta 2010
- Bagetta G, Morrone LA, Rombola L, Amantea D, Russo R, Berliocchi L, et al. Neuropharmacology of the essential oil of bergamot. Fitoterapia 2010;81(6):453‐61. [DOI] [PubMed] [Google Scholar]
Bennett 2009
- Bennett S, Purcell A, Meredith P, Beller E, Haines T, Fleming J. Educational interventions for the management of cancer‐related fatigue in adults. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD008144] [DOI] [PMC free article] [PubMed] [Google Scholar]
Denner 2009
- Denner SS. Lavandula angustifolia miller: English lavender. Holistic Nursing Practice 2009;23(1):57‐64. [DOI] [PubMed] [Google Scholar]
Derogatis 1983
- Derogatis LR, Morrow GR, Fetting J, Penman D, Piasetsky S, Schmale AM, et al. The prevalence of psychiatric disorders among cancer patients. JAMA 1983;249(6):751‐7. [DOI] [PubMed] [Google Scholar]
Ernst 2003
- Ernst E. The safety of massage therapy. Rheumatology 2003;42:1101‐6. [DOI] [PubMed] [Google Scholar]
Fellowes 2004a
- Fellowes D, Barnes K, Wilkinson S. Aromatherapy and massage for symptom relief in patients with cancer. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD002287.pub2] [DOI] [PubMed] [Google Scholar]
Gorman 2008
- Gorman G, Forest J, Stapleton SJ, Hoenig NA, Marschke M, Durham J, et al. Massage for cancer pain: a study with university and hospice collaboration. Journal of Hospice and Palliative Nursing 2008;10(4):191‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University. GRADEpro. McMaster University, 2015.
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hines 2009
- Hines S, Steels E, Chang A, Gibbons K. Aromatherapy for treatment of postoperative nausea and vomiting. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD007598.pub2] [DOI] [Google Scholar]
Holt 2009
- Holt FE, Birks TPH, Thorgrimsen LM, Spector AE, Wiles A, Orrell M. Aroma therapy for dementia. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD003150] [DOI] [PubMed] [Google Scholar]
Jackson 2004
- Jackson KC, Lipman AG. Drug therapy for anxiety in adult palliative care patients. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD004596.pub2] [DOI] [PubMed] [Google Scholar]
Jackson 2007
- Jackson KC, Wiffen PJ. Codeine, alone and with paracetamol (acetaminophen), for cancer pain. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD006601.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Keir 2010
Listing 2010
- Listing M, Krohn M, Liezmann C, Kim I, Reisshauer A, Peters E, et al. The efficacy of classical massage on stress perception and cortisol following primary treatment of breast cancer. Archives of Women's Mental Health 2010;13(2):165‐73. [DOI] [PubMed] [Google Scholar]
Mantovan 2009
- Mantovan F, Rauter E, Muller I. Massage and music therapy for relief of anxiety of cancer patients in palliative care. Pflege Zeitschrift 2009;62(3):164‐9. [PubMed] [Google Scholar]
McGilvery 1994
- McGilvery C, Read J, Mehta M, Mehta S. The Encyclopedia of Aromatherapy Massage and Yoga. London: Anness Publishing Limited, 1994. [Google Scholar]
McQuay 1997
- McQuay HJ, Moore RA. Using numerical results from systematic reviews in clinical practice. Annals of Internal Medicine 1997;126(9):712‐20. [DOI] [PubMed] [Google Scholar]
Miller 2012
- Miller JA, Thompson PA, Hakim IA, Lopez AM, Thomson CA, Chew W, et al. Safety and feasibility of topical application of Limonene as a massage oil to the breast. Journal of Cancer Therapy 2012;3(5A):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2002
- Moore RA, Gavaghan DJ, Edwards JE, Wiffen P, McQuay HJ. Pooling data for number needed to treat: no problems for apples. BMC Medical Research Methodology 2002;2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oh 2000
- Oh HK. Aromatherapy Handbook. Seoul: Korean Aromatherapy Association, 2000. [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Russell 2008
- Russell NC, Sumler SS, Beinhorn CM, Frenkel MA. Role of massage therapy in cancer care. Journal of Alternative and Complementary Medicine 2008;14(2):209‐14. [DOI] [PubMed] [Google Scholar]
UK Cochrane Centre 2010
- UK Cochrane Centre. Workshop series for review authors. Workshop RA4: advanced topics in the analysis and reporting of systematic reviews. Programme: continuous data‐calculating SDs from P values, t values and confidence intervals, using the standardised mean difference. The Cochrane Collaboration 2010.
Walters 2010
- Walters SJ. Massage and cancer: practice guidelines. Journal of the Traditional‐Medicine Society 2010;16(3):141‐3. [Google Scholar]
Yu 2010
- Yu CT, Ko NY. Evidence‐based nursing care for cancer patients with opioid‐induced constipation. Hu Li Za Zhi the Journal of Nursing 2010;57(4):100‐5. [PubMed] [Google Scholar]
References to other published versions of this review
Fellowes 2004b
- Fellowes D, Barnes K, Wilkinson S. Aromatherapy and massage for symptom relief in patients with cancer. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD002287.pub2] [DOI] [PubMed] [Google Scholar]


