Abstract
Autonomic neural control of the cardiovascular system is formed of complex and dynamic processes able to adjust rapidly to mitigate perturbations in hemodynamics and maintain homeostasis. Alterations in autonomic control feature in the development or progression of a multitude of diseases with wide-ranging physiological implications given the neural system’s responsibility for controlling inotropy, chronotropy, lusitropy and dromotropy. Imbalances in sympathetic and parasympathetic neural control are also implicated in the development of arrhythmia in several cardiovascular conditions sparking interest in autonomic modulation as a form of treatment.
A number of measures of autonomic function have shown prognostic significance in health and in pathological states and have undergone varying degrees of refinement, yet adoption into clinical practice remains extremely limited. The focus of this contemporary narrative review is to summarize the anatomy, physiology and pathophysiology of the cardiovascular autonomic nervous system and describe the merits and shortfalls of testing modalities available.
The autonomic nervous system - an overview
The autonomic nervous system is complex and wide-ranging with innervation extending to nearly every organ in the body. It is responsible for regulating involuntary physiologic processes with three distinct divisions: sympathetic, parasympathetic and enteric. Knowledge of the anatomy, function, modulation and testing of the autonomic nervous system is often lacking despite autonomic dysregulation serving as the primary basis of, or at least a secondary component of, a great multitude of diseases. The goal of this review is to broadly cover the historical context of the autonomic nervous system, its anatomy and its physiology, before focusing on its role in control of the cardiovascular system and some of the available methods of assessment of its status.
Historical context
John Newport Langley first proposed the term ‘autonomic nervous system’ in 1898, suggesting differences and opposing actions of the sympathetic and parasympathetic components (Fig. 1). (1) Walter Holbrook Gaskell suggested that the lateral horn of the spinal cord was the source of sympathetic fibers leading to white rami. (2) Claude Bernard demonstrated the action of vagus nerve activity on heart rate and suggested the importance of maintenance of constancy within the internal environment which was later termed ‘homeostasis’ by Walter Bradford Cannon who also explored the impact and role of emotional input into autonomic nervous control. (3) Cannon also helped define the role of adrenaline (epinephrine) in maintenance of homeostasis. Sir Henry Hallett Dale (a student of John Langley, who later worked in Ernest Starling’s laboratory in University College in London and went on to receive the 1936 Nobel Prize in Physiology alongside Otto Loewi) advanced the concept of chemical transmission of nerve impulses and Loewi discovered the effects of acetylcholine released from the vagus nerve reducing heart rate, and the effects of acetylcholinesterase. During the 20th century, use of histochemical techniques and pharmacological investigation provided more detailed understanding of the cellularity and connectivity of the sympathetic and parasympathetic systems. Retrograde labelling studies using application of horseradish peroxidase (HRP) on target tissues led to a greater understanding of autonomic innervation at the cellular level. Using this technique, Kalia was able to label vagal preganglionic neurons within the brainstem in cats. (4) Immunohistochemical techniques used in the 1970s provided further understanding of catecholamine biosynthesis, particularly the role of tyrosine hydroxylase. Techniques such as histofluorescence bolstered knowledge of sympathetic neuronal development. (5) In 1948 Raymond Ahlquist made the crucial distinction between α- and β-adrenoceptors. (6) Sir James Black, whose own father died of myocardial infarction, hypothesized that a form of treatment for angina pectoris would be to reduce oxygen demand of the heart rather than increase oxygen supply to it, ultimately leading to his development of propranolol, later earning him the Nobel Prize for his discoveries in 1988. (7)
Fig. 1.
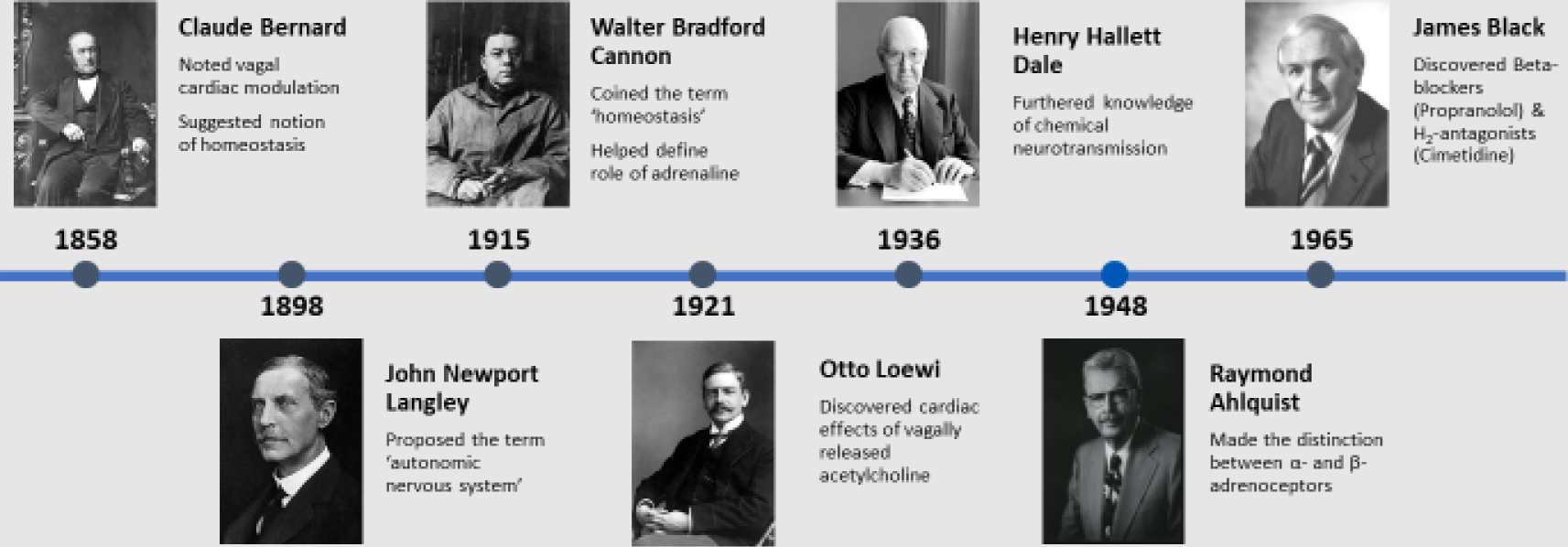
A broad timeline of major discoveries of the autonomic nervous system
Whilst difficult to assess directly, function is usually assessed by measuring complex and overlapping reflex loops after controlled perturbations and is usually tied to an organ of interest. The autonomic nervous system is formed from highly migratory neural crest cells arising from neuroepithelial precursors within the dorsal neural tube. (8) Controlled by membrane bound and secreted guidance factors, the neural crest cells migrate vast distances to form their derivatives. Cranial neural crest cells go on to form sensory, sympathetic, and parasympathetic ganglia, bone, connective tissue, cartilage and muscle tendons of the head and truncal neural crest cells form adrenal chromaffin cells, melanocytes and sympathetic and sensory neurons. (8, 9) (10) Testing of the autonomic nervous system can potentially be helpful in distinguishing between static or progressive disorders or in gauging treatment response. The focus of this paper is to discuss the normal workings of the autonomic nervous system with a particular emphasis on neural circulatory control, and to explore the various modalities of assessment of this neural control.
Broad anatomy of the ANS
An understanding of the anatomical distribution of the autonomic nervous system is required to be able to fully appreciate its function and role. Cell bodies of sympathetic preganglionic neurons are found bilaterally within spinal gray matter, predominantly within the intermediolateral cell column (previously referred to as the lateral horn). Sympathetic preganglionic neurons are limited to the thoracic spine and the first few lumbar spinal segments. (11) Conversely, parasympathetic preganglionic neurons are located within the intermediolateral cell column of the sacral spinal segments, running through the lumbosacral plexus and pelvic nerve to synapse on pelvic ganglia in the pelvic plexus. Parasympathetic preganglionic neurons also arise from the parasympathetic nuclei of the vagus, oculomotor, facial and glossopharyngeal nerves. (12) Cardiopulmonary reflexes of the autonomic nervous system consist of sensory receptors present in the heart and lungs, afferent sensory parasympathetic fibers which include the vagus nodose ganglia whose sensory information is relayed via polysynaptic pathways to medullary control centers and higher centers such as the hypothalamus preganglionic neurons synapsing within thoracic ganglia in the case of sympathetic innervation, and at intravisceral ganglia for parasympathetic control. Small, unmyelinated C fibers and lightly myelinated Aδ fibers provide visceral sensory afferent input, allowing adaptive responses to physiological stimuli and maintenance of homeostasis. (13) Their cell bodies are found within spinal dorsal root ganglia and cranial nodose ganglia. Their sensory inputs are conveyed to the spinal cord dorsal horn or the nucleus of the tractus solitarius within the brainstem. Both the sympathetic and parasympathetic nervous systems can respond to input from visceral fibers, independently, antagonistically, or synergistically.
Sympathetic anatomy
The sympathetic chain is a structure formed of paravertebral ganglia, axons of preganglionic neurons terminating on postganglionic neurons in ganglia located further away and of axons of postganglionic neurons travelling towards their effector targets. Axons of sympathetic preganglionic neurons tend to be short given that the majority synapse with postganglionic neurons within the sympathetic chain close to the spinal cord. Axons of sympathetic postganglionic neurons are much longer by comparison as they lead on to the effector organ itself.
Sympathetic preganglionic neurons are small in diameter when compared to α-motor neurons, most are lightly myelinated and some are unmyelinated. (14) Their axons lead ventrally, along the lateral margin of the ventral horn, leaving the spinal cord and forming the white rami (white due to the presence of myelin). Preganglionic axons can then take several routes (Fig. 2). Most will project and terminate on postganglionic sympathetic neuron cell bodies within paravertebral ganglia. Some will pass through paravertebral ganglia to terminate on postganglionic neurons within the abdomen and pelvis, such as the aorticorenal ganglion, which is composed of the renal as well as superior and inferior mesenteric ganglia.(15) The other route taken by sympathetic preganglionic axons is to project and terminate directly on the effector organ, as is the case with preganglionic neurons extending directly to catecholamine producing cells (chromaffin cells) found in the medullary portion of the adrenal gland. In this regard, chromaffin cells act as sympathetic postganglionic neurons, particularly noting that they are also derived from neural crest cells, depositing their transmitter (both norepinephrine and epinephrine in this case) directly into the blood. (16)
Fig. 2.
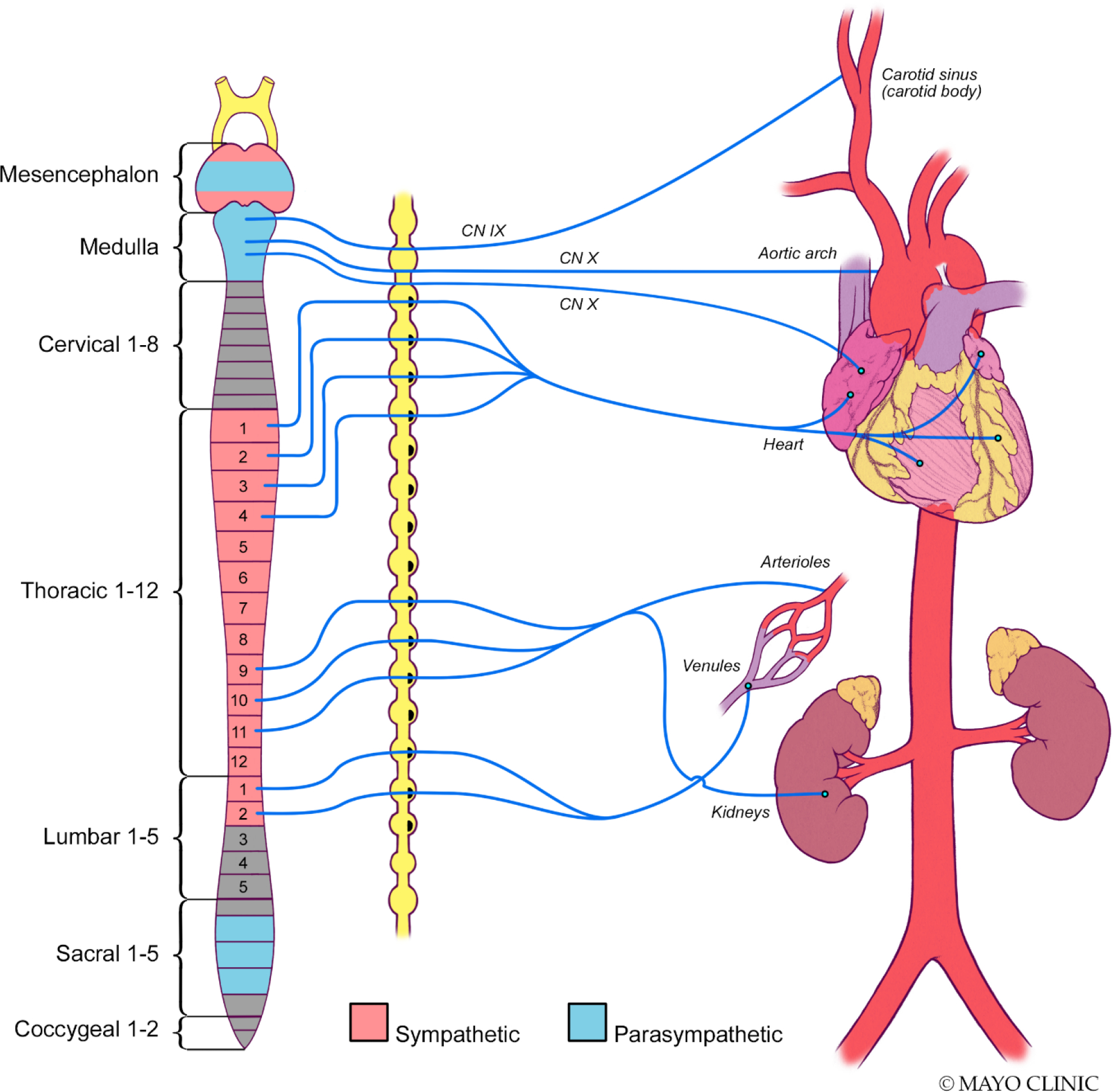
Autonomic afferent nerve scheme of the baroreceptors governing blood pressure
Some sympathetic postganglionic neurons exit paravertebral ganglia to then reenter spinal nerves via the gray rami before leading to and terminating on their effectors. The appearance is gray due to the absence of myelin, typical of almost all axons of sympathetic postganglionic neurons. Other sympathetic postganglionic neurons exit paravertebral ganglia to project to and terminate on the heart and bronchi. Each of the neurons running along smooth muscle membranes within blood vessels, sweat glands and hair follicles for example are beaded along their length with vesicles containing norepinephrine, up to 20,000 vesicles per neuron, allowing a single neuron to make multiple contacts with their target, known as synapse en passant.
Parasympathetic anatomy
Preganglionic parasympathetic neurons are similarly largely unmyelinated with slow conduction velocities. However, unlike most sympathetic preganglionic neurons, they are longer given that they synapse with (comparatively shorter) postganglionic neurons present at or close to the effector organ. Parasympathetic preganglionic neurons are located within cranial nerve nuclei with their anatomical and functional targets clearly evident. Vagal preganglionic neurons are found in the nucleus ambiguus and dorsal motor vagal nucleus within the medulla. Lightly myelinated neurons are present within the nucleus ambiguus that go on to synapse with postganglionic neurons within the sinoatrial and atrioventricular nodes. Unmyelinated neurons leaving the dorsal motor vagal nucleus are responsible for innervating postganglionic neurons within the gastrointestinal tract. Finally, parasympathetic innervation originating in the sacral spinal cord is responsible for neural control over the terminal gastrointestinal tract as well as sex organs. (12)
ANS Neurotransmission
Parasympathetic neurotransmission
Initial neurotransmission between preganglionic and postganglionic neurons of both the parasympathetic and sympathetic nervous systems uses acetylcholine acting on nicotinic receptors within autonomic ganglia. Choline is transported into nerve terminals by a Na+-dependent transporter, a rate-limiting step which may be inhibited by hemicholinium. The enzyme choline acetyltransferase is responsible for catalyzing acetylation of choline with acetyl coenzyme A, before acetylcholine is then transported into nerve terminal vesicles. Post release into the synapse and effect, acetylcholinesterase rapidly breaks acetylcholine into choline and acetate. Neurotransmission between parasympathetic postganglionic neurons and effector sites also utilizes acetylcholine acting on muscarinic receptors (whose responses are blocked by atropine). (17)
Sympathetic Neurotransmission
As in the parasympathetic nervous system, acetylcholine is the neurotransmitter utilized by preganglionic neurons synapsing with postganglionic neurons. Thereafter, neurotransmission between postganglionic neurons and effector sites in sweat glands continue to use acetylcholine but the majority of postganglionic sympathetic neurons release norepinephrine at their postsynaptic effector target, acting primarily on α- and β-adrenoceptors. Given this is the case, plasma levels of catecholamines are often used as a surrogate measure of global sympathetic nervous system activity. Norepinephrine, epinephrine and dopamine are found within plasma and may be directly measured as opposed to acetylcholine which is not. The vast majority of norepinephrine found in plasma originates from sympathetic nerve endings with the rest coming from the adrenal medulla, which is also the source of the majority of circulating epinephrine. Knowledge of catecholamine synthesis is important as each step is a potential target for pharmacotherapy or dysfunction. A study by Lefroy et al found a significantly lower density of beta-adrenoceptors in patients with hypertrophic cardiomyopathy vs. controls when assessed by labelled PET scan with similar peripheral circulating plasma levels of epinephrine and norepinephrine in both groups likely indicating downregulation of beta-adrenoceptors secondary to elevated myocardial norepinephrine concentrations in keeping with elevated sympathetic drive. (18) Similarly, Brush et al reported reduced cardiac neuronal uptake of labelled norepinephrine in patients with hypertrophic cardiomyopathy (HCM) upon infusion but with similar circulating plasma levels of norepinephrine between hypertrophic cardiomyopathy patients and controls again suggesting potential downregulation of receptors or diffuse abnormality of neuronal uptake. (19)
Catecholamines are classified as such because of the presence of an amine and a catechol within their molecular structure. Both the amine and the catechol act as substrates for termination via their corresponding enzymes; monoamine oxidase (MAO) acting on the amine and catechol-O-methyltransferase (COMT) acting on the catechol. Synthesis of catecholamines begins with the essential amino acid tyrosine. Depending on the presence or absence of converting enzymes, tyrosine is ultimately converted, in a stepwise manner to dopamine, norepinephrine or epinephrine (Fig. 3).
Fig. 3.
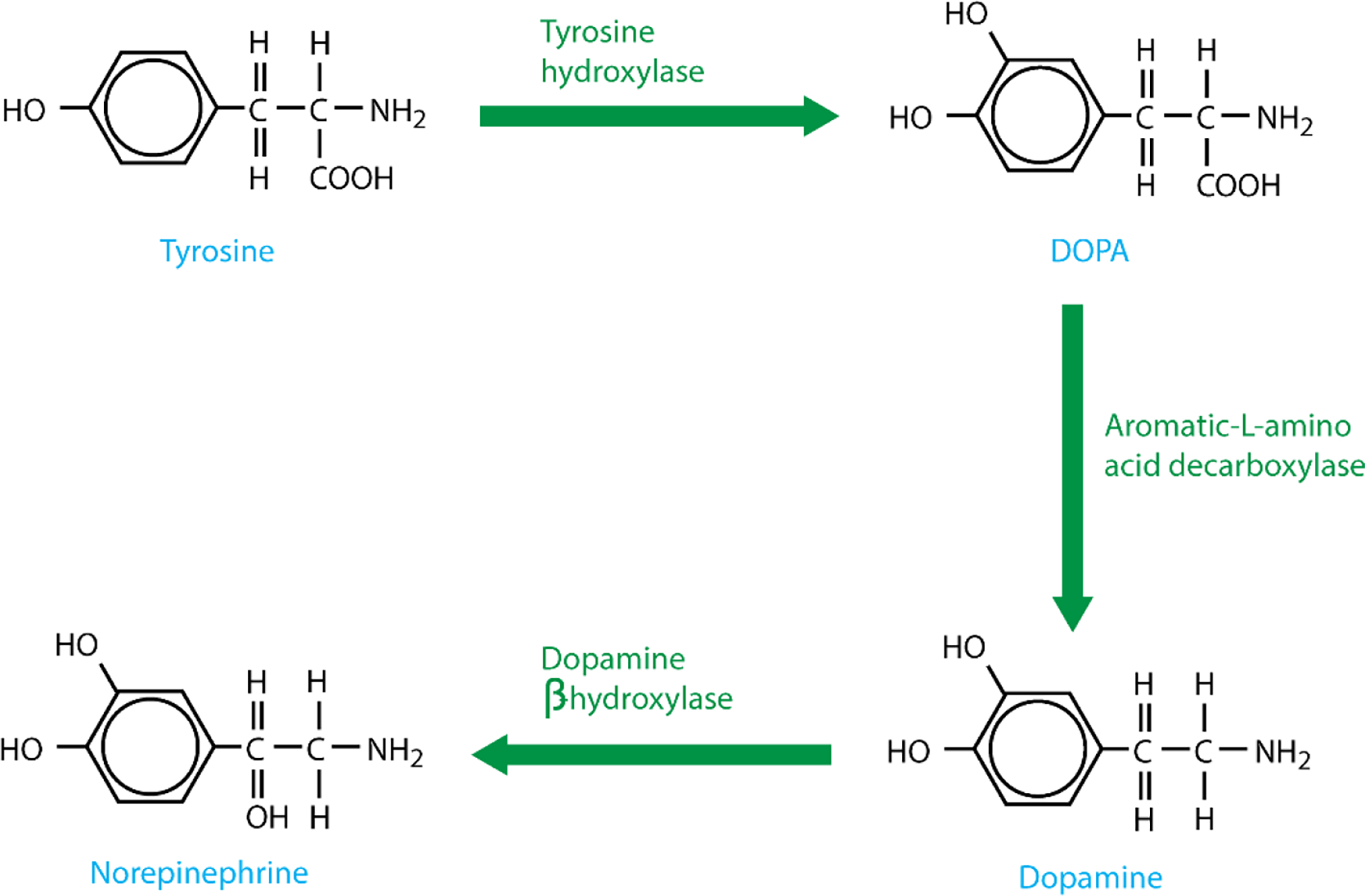
Pathway of catecholamine synthesis
Tyrosine is transported into catecholaminergic neurons via a Na+ dependent channel and converted to DOPA via the actions of tyrosine hydroxylase (Fig. 3). Negative feedback from both dopamine and norepinephrine regulates levels of tyrosine hydroxylase, offering control over catecholamine production. DOPA decarboxylase then converts DOPA into dopamine which is transported into vesicles by vesicular monoamine transporter (VMAT). At this stage, dopamine is converted into norepinephrine via the actions of dopamine β-hydroxylase. The enzyme phenylethanolamine-N-methyltransferase (PNMT), present within chromaffin cells in the adrenal medulla, converts norepinephrine to epinephrine (Fig. 4). Once an action potential arrives at the nerve ending, voltage-gated Ca2+ channels allow an influx of Ca2+, causing fusion of vesicles with the synaptic membrane and subsequent release of contained catecholamines, ATP and neuropeptide Y.
Fig. 4.
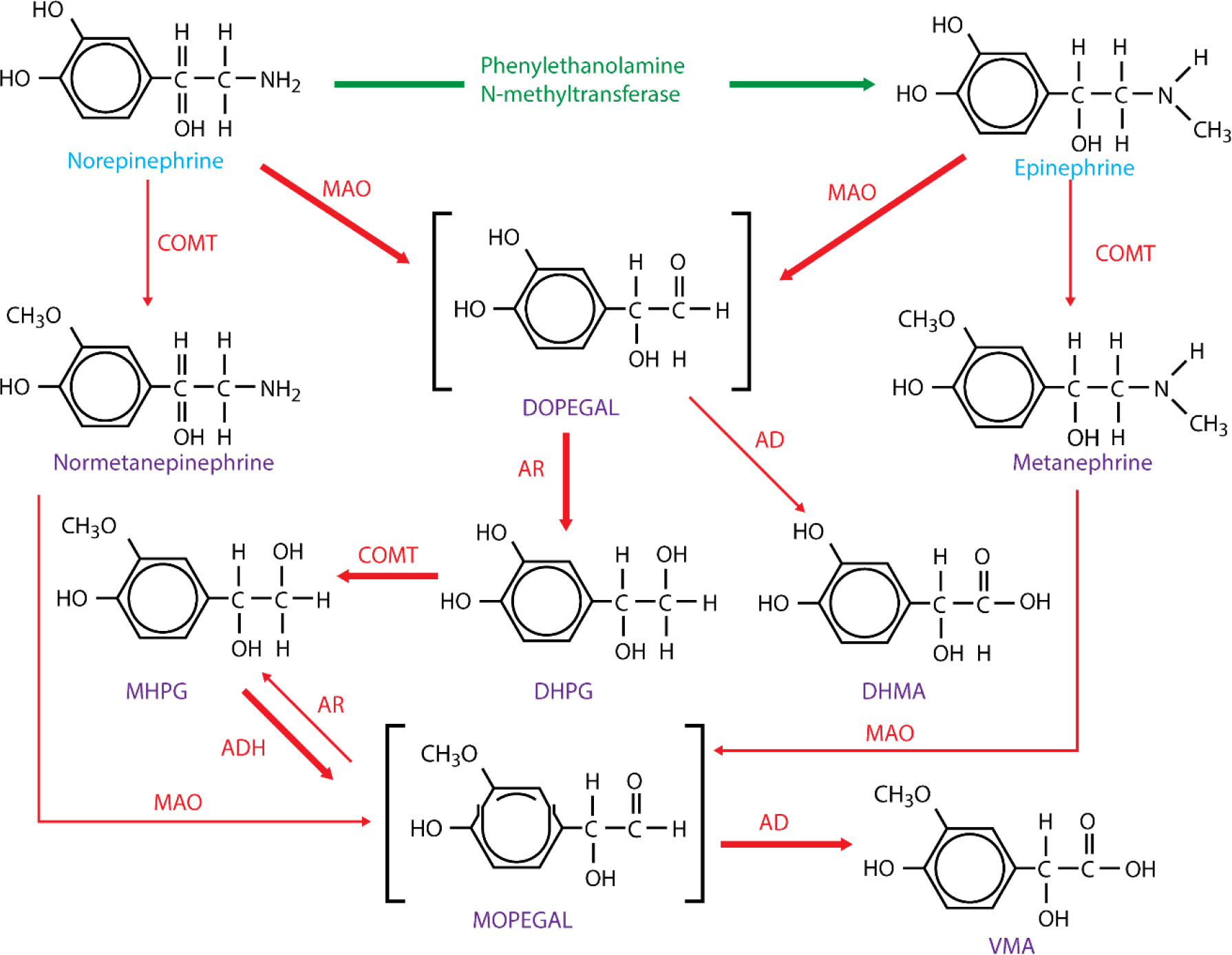
Catecholamine metabolism
Autonomic regulation of the cardiovascular system
In his seminal work ‘De Motu Cordis et Sanguinis’ (‘Motion of the heart and blood’) published in 1628, the physician William Harvey suggested that movement of blood within the body was in circulation rather than oscillation and went on to describe the relationship between the heart and nervous system. (20) Autonomic control of the cardiovascular system is dynamic with multiple levels of control and feedback loops and is comprised of receptors, afferent and efferent nerves, and effectors. A complex network of ganglia is found within fat pads on the posterior atrial surfaces and base of the great vessels, allowing extensive connections with the brainstem and cortex ultimately providing physiological stability in rhythm and circulation (Fig. 5). Bulbopontine control of circulation, respiration and gastrointestinal function is coordinated via the spinal cord with the upper brainstem integrating control of autonomic function alongside pain and behavioral responses to stress. Sensation and emotion-based responses to stimuli are integrated into the autonomic nervous system by the forebrain, in particular the anterior limbic circuits (insula, anterior cingulate cortex and the amygdala) and the hypothalamus. (21) Widespread connections pass from the anterior cingulate gyrus, through the insula, to the prefrontal cortex, amygdala, hypothalamus and brainstem allowing control over sympathetic and parasympathetic function. (22)
Fig. 5.
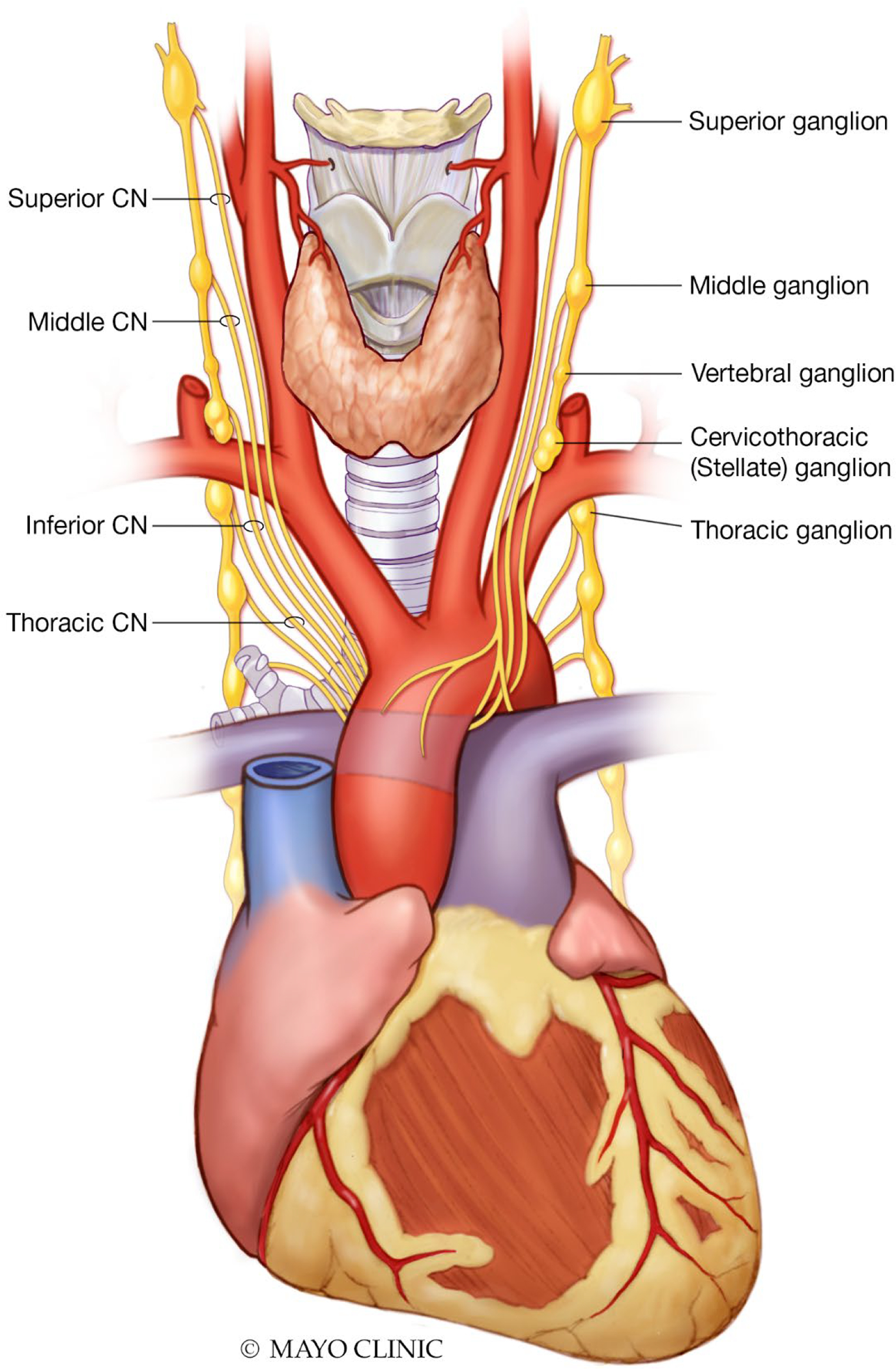
Cardiac autonomic nervous system
Given sympathetic and parasympathetic activity is continuous, the autonomic nervous system most often utilizes a double antagonistic system of innervation allowing greater regulatory power through rhythmical activity with mutual feedbacks. Preganglionic neurons of both the sympathetic and parasympathetic systems are cholinergic fibers whereas postganglionic fibers of the sympathetic system are noradrenergic and parasympathetic postganglionic fibers are cholinergic. The nucleus solitarius regulates respiratory circuits and acts as a relay for cardiac and baroreflex function. (23) The rostral ventrolateral medulla (RVLM) exerts control over cardiac output and peripheral resistance via connections to preganglionic sympathetic neurons and mediates the baroreflex and chemoreflex. In inherited cardiovascular conditions such as HCM, sympathetic stimulation may precipitate ventricular arrhythmia and predispose to sudden death. (24)
Most afferent nerves (primary cardiac afferent neurons) reach the nucleus tractus solitarius via the vagus nerve, providing beat by beat sensory information. A small proportion of cardiac afferent nerves (spinal cardiac afferent neurons) instead have cell bodies within the thoracic root ganglion and relay information to thalamic, hypothalamic and brainstem centers. (25) The periaqueductal gray coordinates autonomic and somatic cardiac responses to pain and stress. (26)
Sympathetic efferent transmission primarily originates in the brainstem with preganglionic neurons exiting the spinal cord between T1-T4, synapsing in the stellate ganglia, thoracic and middle cervical ganglia with postganglionic neurons extending epicardially along the routes of the coronary vasculature (namely the left and right coronary cardiac nerves and the left lateral cardiac nerve) synapsing either directly onto myocardium or with atrial or ventricular plexi (Fig. 6). (27, 28) Postganglionic sympathetic neurons extend from the right stellate ganglion and innervate the sinus node, right atrium and anterior left ventricle. Similar neurons originating from the left stellate ganglion innervate the atrioventricular node, left atrium and posterior left ventricle. (29) Norepinephrine released from the presynaptic nerve terminal into the synaptic cleft binds with post-synaptic α- and β-adrenergic receptors.
Fig. 6.
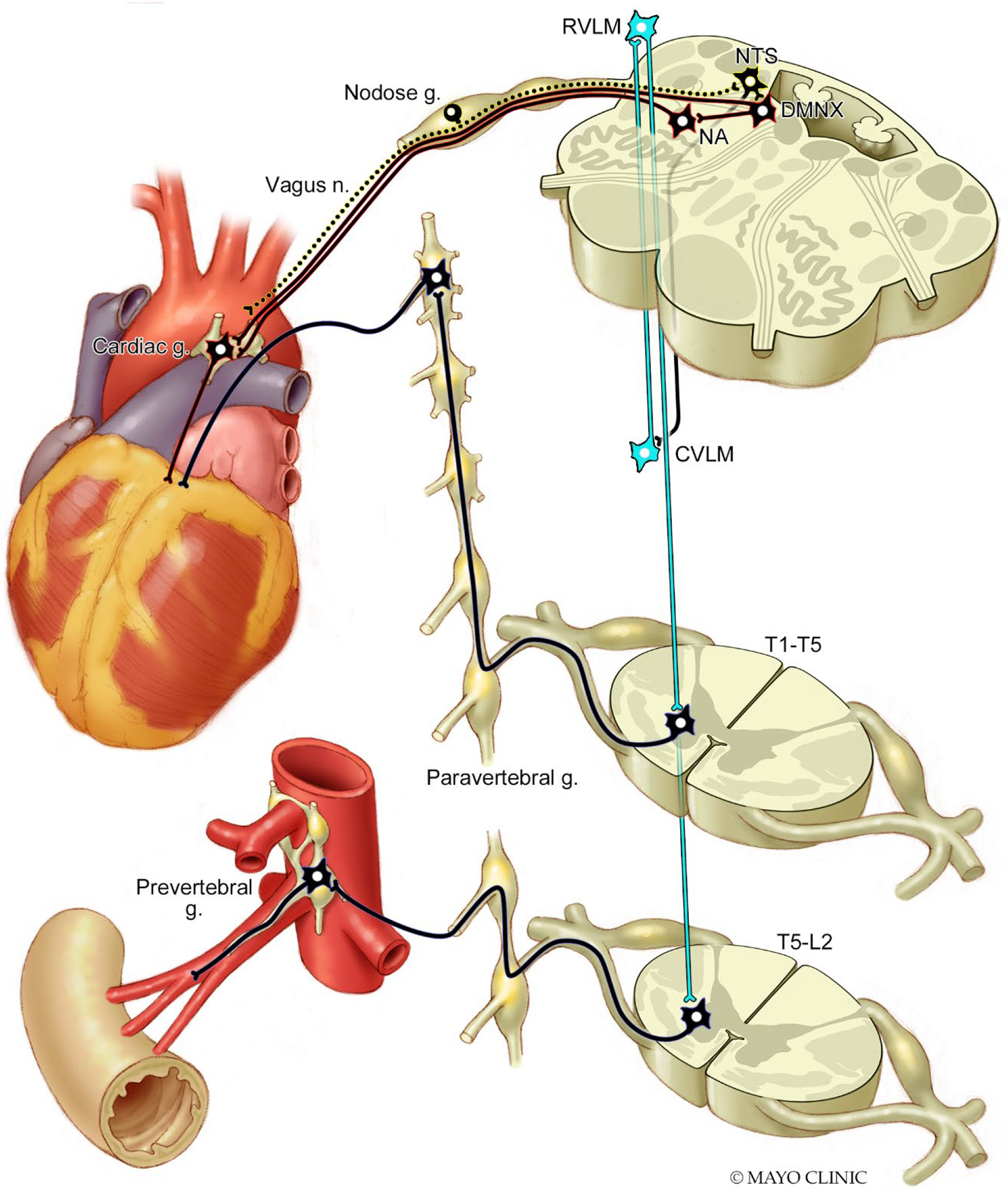
Sympathetic and parasympathetic innervation of the heart and splanchnic arterial bed
β-1 receptors are the primary β receptor within the heart and increase ventricular contractility with stimulation. β-1 and β-2 receptor stimulation also increases heart rate by accelerating depolarization of the sinus node and enhancing atrioventricular node conduction. β-2 stimulation also induces coronary artery dilation and a reduction in peripheral vascular resistance through vasodilation of splanchic and skeletal muscle beds. (31) Stimulation of β-1 receptors within renal juxtaglomerular cells leads to increased release of renin which catalyzes the conversion of angiotensinogen to angiotensin which is converted via the actions of angiotensin converting enzyme to angiotensinogen II which goes on to act on angiotensin type 1 receptors and elevate blood pressure through mechanisms including vasoconstriction, aldosterone and vasopressin release, inflammation, oxidative stress, sympathetic activation and baroreflex dysfunction. (32)
Parasympathetic efferent transmission originates in the nucleus ambiguus, travelling bilaterally within the vagosympathetic trunk and synapsing thereafter within cardiac ganglia in atrial fat pads. Thereafter, parasympathetic postganglionic neurons synapse directly with the sinus and atrioventricular nodes as well as directly with atria and ventricles. While both sympathetic and parasympathetic activity influence the sinus node, under resting conditions vagal tone presides; however, fluctuations in the balance of both systems’ influences lead to variations in heart rate which are a target for external measurement. Inspiration leads to transient suppression of vagal tone, causing an increase in heart rate and vice versa, known as respiratory sinus arrhythmia. (33)
As well as the neural control described above, there exist a number of reflex loops such as the baroreflex and chemoreflex that are responsible for providing immediate counteractions to stimuli, maintaining homeostasis. These reflex loops may also be targets for assessment of autonomic neural control of the cardiovascular system.
The Bezold-Jarisch reflex
Chemoreceptors and mechanoreceptors present within the left ventricle detect noxious stimuli and may be activated by ischemia or revascularization and result in this reflex characterized by a triad of bradycardia, hypotension and coronary artery dilation. The reflex may also be activated by significant changes in venous return and has been noted subsequent to administration of regional anesthesia. (34) First reported in 1867 by von Bezold and Hirt and later confirmed by Jarisch in 1940, the reflex described the effects of intravenous injection of an alakaloidal extract of Veratrum Viride although the reflex may be elicited by snake and insect venom as well as potassium chloride, histamine, serotonin, synthetic organic compounds and halogenated anesthetic agents. (35, 36) The reflex is considered to confer innate defense mechanisms against chemical inhalants with bradycardia/asystole considered to offer protection from further distribution of the irritant. (37) A variant of the reflex may also serve to protect against orthostatic hypotension with prolonged standing (see section that follows), by the reflex tachycardia, consequent reduced left ventricular filling time, and hence reduced left ventricular end diastolic volume, causing mechanical distortion of the infero-posterior wall of the left ventricle, activating mechanoreceptors that induce bradycardia and vasodilation, resulting in assumption of supine posture by eliciting presyncope or syncope (as exemplified by passing out of soldiers on parade). This variant may also potentially contribute to syncopal events such as that induced by the sight blood or other phobias. (38)
The diving reflex
The diving reflex serves purpose in birds and mammals by simultaneously stimulating sympathetic and parasympathetic outflow in order to conserve oxygen and so allow enhanced underwater exploration. (39, 40) Its role in humans is less well understood. Activation of the reflex induces vagally-mediated bradycardia alongside marked peripheral vasoconstriction through sympathetic activation in order to maintain cerebral and cardiac circulation. (41, 42) Activation of the diving reflex has been seen during apneic episodes in those with obstructive sleep apnea and noted to be a potential trigger for arrhythmia in susceptible patients, such as those with a history of long QT syndrome. (43, 44) Apneic stimuli and facial cold exposure in the laboratory setting have been successfully used to simulate the diving reflex. (45)
The baroreflex
Arterial baroreceptors, present in the wall of the aortic arch and carotid sinuses, detect dynamic changes in arterial pressure by the degrees that these specialized receptors are stretched and maintain homeostasis through modulation of cardiac output and peripheral vascular resistance. (46, 47) When arterial pressure rises, this leads to an increase in vagal output to the heart causing lengthening of the R-R interval which is accompanied by inhibition of sympathetic tone leading to reduced peripheral vascular α-adrenoceptor stimulation inducing a reduction in peripheral resistance as well as a reduction in stimulation of myocardial β-adrenoceptors leading to reduced contraction and subsequently, stroke volume. (46, 47) Transient reductions in blood pressure occur with changes in posture and the effects of gravity inducing blood pooling and ‘third spacing’ due to increased intravascular hydrostatic pressure in the lower extremities. Between 500–1000mL of blood from within the thorax and splanchnic vasculature transfer to the lower extremities upon standing. (48) The subsequent reduction in venous return, stroke volume and arterial pressure thereafter is detected by baroreceptors which activate the baroreflex. Baroreceptors are mediated by ion channels PIEZO1 and PIEZO2 which are activated mechanically and genetic deactivation of them has induced hypertensive lability and impairment of the baroreflex. (49) The mechanoreceptors are present in the adventitia and receive innervation from the vagus (aortic arch baroreceptors) and glossopharyngeal nerves (carotid sinus baroreceptors) with aortic arch afferents found in the nodose ganglion and carotid sinus afferents in the petrosal ganglion. (50) Changes in the degree of wall stretch following variation in blood pressure generate signals from baroreceptors which are transmitted to the nucleus solitarius via monosynaptic, excitatory glutaminergic pathways. (51)While the arterial baroreceptors remain the most sensitive, cardiopulmonary receptors present in the venae cavae and heart respond to lower pressure changes due to changes in volume. (52) Stretching of these receptors inhibits vasopressin release and induces vasodilation as well as suppression of renal sympathetic nerve activity. (53) Interventions such as exercise (54) and endurance training (55) have been shown to increase baroreflex gain and lower BP, with endurance training also increasing variability of the RR interval.
Peripheral vascular resistance is dependent on tonically active vasopressor neurons within the RVLM and is modulated by inhibition received from the caudal ventrolateral medulla via noradrenergic neurons. The sympathetic neurons extending from the RVLM to nerve fibers present within the adventitia of peripheral vessels controls the release of norepinephrine, neuropeptide Y and ATP resulting (within 1–3 seconds) in constriction in smooth muscle, mesenteric and renal blood vessels. Neuropeptide Y independently induces a pressor response and modulates the release of norepinephrine and ATP. (56) The nucleus solitarius also regulates heart rate via vagal cardioinhibitory pathways as well as vasopressin secretion via inhibitory pathways to the hypothalamus. (46, 57, 58) Parasympathetic cardioinhibitory control utilizes cholinergic pathways from vagal preganglionic neurons within the nucleus ambiguus to cardiac ganglion neurons altering heart rate within 1–2 beats. (46)
The chemoreflex
The chemoreflex holds an important role in modulating sympathetic activation and serves to regulate tissue perfusion by modulating cardio-vagal and sympathetic control in coordination with control of breathing and the baroreflex. Peripheral chemoreceptors present in carotid bodies primarily respond to hypoxemia but are also activated by hypercapnia, hypo-perfusion, hypoglycemia, changes in temperature and the pH of arterial blood. (59–61)Carotid bodies are extremely vascular organs which is often considered a functional characteristic of the chemoreceptor. (62) The functional role of the carotid body is by far primarily to respond to hypoxia. Upon reductions in arterial Po2, the carotid body creates a graded chemoafferent discharge via the carotid sinus nerve. (63) Central chemoreceptors present in the brainstem primarily respond to hypercapnia, also enabling them to act as buffers for the effects of carotid chemoreceptors. For example, hypoxia will stimulate peripheral chemoreceptors to increase ventilatory drive but will also increase cerebral blood flow, decreasing the partial pressure of carbon dioxide at central chemoreceptors, withdrawing their ventilatory stimulus and countering the hypoxic drive to ventilation. (64) The baroreflex exerts significant negative feedback on the chemoreflexes; (increased blood pressure will attenuate the ventilatory response to hypoxia) as do pulmonary afferents during ventilation whereby the sympathetic cardiovascular effects of the chemoreflex are attenuated by increases in ventilation. (65–67)
Activation of either form of chemoreflex increases ventilatory drive and sympathetic activation, the degree of which is influenced by co-activation from pulmonary stretch afferents, the effect of changes in lung inflation, and the sensitivity of the reflex. (68) The hyperventilation that occurs due to chemoreflex activation also serves to inhibit sympathetic activity with peripheral chemoreceptors being most affected by this inhibition, potentially as peripheral chemoreceptor afferents and thoracic afferents synapsing close to the nucleus tractus solitarii within the brainstem. (65–67, 69–71) In times of crisis, where marked hypotension may occur alongside hypoxia, activation of the chemoreflex alongside withdrawal of the baroreflex together, further enhances sympathetic activation and ventilation; however in chronic pathological states such as heart failure, this extended dysautonomia and disproportionate sympathetic excitation/reduced parasympathetic activation is strongly correlated with increased mortality. (72) Cohn et al’s study of 106 hospitalized heart failure patients reported a strong association between elevated plasma norepinephrine levels and mortality with <10% 2-year survival in those with levels >800 pg/ml and ~60% 5-year survival in those with levels <400 pg/ml. (72) In conditions such as sleep apnea, where apnea induced hypoxia and/or hypercapnia activate the chemoreflex and cause subsequent sympathetic activation, the lack of accompanying increases in ventilation (and its associated sympathetic activity inhibition) leads to excessive hypoxia-induced reflex sympathetic drive alongside increased vagal tone resulting in vasoconstriction and bradycardia potentially explaining the increased risk of cardiovascular morbidity, arrhythmias and hypertension. (67, 73) Patients with a history of obstructive sleep apnea demonstrate increased sympathetic nerve activity during periods of apnea, particularly during REM sleep and also demonstrate increased sympathetic nerve activity during the daytime. (74, 75) Obstructive sleep apnea is often present in patients with a history of resistant hypertension and studies examining the effects of continuous positive airway pressure treatment on blood pressure in such patients have yielded differing results with some demonstrating effectiveness in blood pressure reduction and others showing no significant improvement. (76–78)
Autonomic dysfunction and clinical implications
Dysfunction of the autonomic nervous system may be intrinsic, caused by diseases affecting nerves directly such as diabetes, or extrinsic, with secondary changes in function induced by cardiac or other diseases. Cardiac autonomic neuropathy is associated with a significant increase in mortality. The relative risk of mortality conferred from cardiac autonomic neuropathy was 3.45 (95% CI 2.7–4.5; p<0.001) in one meta-analysis which also noted the magnitude of association was strongly dependent on how many modalities by which cardiac autonomic neuropathy was defined. (80) Disorders of autonomic function tend primarily to affect the sympathetic nervous system - however in circumstances such as with endurance training or during sleep, increased parasympathetic tone may be clinically relevant. Disorders of sympathetic function may be characterized by either a state of increased sympathetic drive, where hypertension and/or tachycardia would be expected vs. states of decreased sympathetic drive where orthostatic intolerance would preside. Failure of the autonomic nervous system may be acute or chronic, primary, or secondary to another disease process, which is by far most common. In cases of secondary autonomic failure, treatment should predominantly be aimed at the underlying cause. Assessment of autonomic dysfunction should always include a thorough history of the development of symptoms, family history of autonomic dysfunction and particularly of drug use, as well as chemical and toxic material exposure.
Primary autonomic failure:
Pure primary autonomic failure is rare and is characterized by orthostatic intolerance. Pathophysiologically it is an α-synucleinopathy characterized by deposition of α-synuclein within autonomic ganglia and nerves. (81) The subsequent dysfunction of peripheral sympathetic nerves results in reduced catecholamine production and an inability to sufficiently raise norepinephrine levels on standing. (82) Receptor hypersensitivity accompanies peripheral denervation leading to marked BP responses with administration of pressor agents. (83, 84) In cases of multiple system atrophy, progressive autonomic failure is accompanied by progressive neurodegenerative changes and is characterized by dysfunction of both the sympathetic and parasympathetic nervous systems with symptoms including incontinence, impotence, reduced intraocular pressure, anhidrosis, abnormal pupillary responses, and orthostatic hypotension, which may be marked. (85) Distinction between multiple system atrophy and Parkinson’s disease with autonomic failure can be challenging and response to levodopa-carbidopa as a means of distinction is imperfect, particularly as some patients with multi system atrophy do show degrees of improvement with administration of levodopa. (82) Neuroimaging techniques, particularly PET scans may be utilized to visually assess cardiac innervation and make the distinction where sympathetic innervation of the heart would be intact in multiple system atrophy and reduced in Parkinson disease. (86, 87) The focus of treatment is in expanding circulating volume either through increased ingestion of fluids, higher salt intake or through use of fludrocortisone. Use of alpha-adrenoceptor agonists such as midodrine may be useful in cases where cardiac sympathetic denervation occurs, such as in Parkinson’s disease with autonomic failure.
Secondary autonomic failure:
Of the secondary causes of autonomic failure, diabetes is certainly the most common. The exact pathophysiological mechanism by which diabetes induces autonomic neuropathy is not fully understood but mechanisms such as vagal axonal myelin damage and neuroaxonal dystrophy due to production of advanced glycosylation products or neurovascular ischemia have been suggested. (88) (89, 90) Approximately 15–20% of diabetic patients without symptoms of autonomic dysfunction do indeed have signs of abnormal cardiovascular autonomic function and some have been noted to demonstrate autonomic dysfunction before the diagnosis of diabetes is reached. Dysfunction in the cardiac actions of the vagus nerve are usually the first detectable signs of diabetes related autonomic dysfunction. (91–93) Enhanced control of blood sugar and blood pressure offer protection against the development and progression of autonomic dysfunction and from microvascular complications.
Renal failure, vitamin B12 deficiency, amyloidosis, paraneoplastic syndromes, and autoimmune conditions are also common causes of secondary autonomic failure. Postural orthostatic tachycardia syndrome is characterized by reduced actions of the baroreflex and sympathetic effects on peripheral vasculature but with maintained heart rate responses leading to orthostatic symptoms, the lack of significant hypotension, but accompanying tachycardia. The precise mechanisms behind neurally mediated syncope are unclear. Patients often show normal autonomic function between syncopal episodes. Peripheral venous pooling of blood is suspected to induce cardiac hypercontractility which in turn is suspected to activate cardiac mechanoreceptors, leading to a reflex bradycardia and further reduction in peripheral vascular resistance, as occurs in the Bezold-Jarisch reflex, ultimately causing syncope.
Myocardial infarction or other cardiac conditions such as cardiomyopathy may result in direct ischemic damage to autonomic nerves. A reduction in cardiac output and subsequent aortic filling leads to restraint of the baroreflex and activation of the sympathetic noradrenergic system with increased delivery of norepinephrine to myocardial cells in an effort to maintain cardiac function. Norepinephrine increases cardiac contractility but also stimulates myocardial hypertrophy, eventually leading to increased oxygen demand. (94–97) This increased oxygen demand may struggle to be met by the hypertrophied heart, particularly in the setting of established coronary artery disease. Norepinephrine which is continuously leaked from sympathetic nerve vesicles into the cytosol requires energy for reuptake. If this energy demand is not met, vesicular stores of norepinephrine will begin to decrease, leading to reduced ability for its release from sympathetic nerves at times of need which in turn will require further activation of the sympathetic noradrenergic system at times of stress. Oxidation of built up cytosolic catecholamines into quinones and chromes as well as enzymatic oxidation to aldehydes causes direct damage to sympathetic nerves further reducing releasable norepinephrine. (98–101) Ultimately, this progression leads to heart failure - a state of myocardial norepinephrine depletion, increased norepinephrine release and reduced neuronal reuptake of norepinephrine.
Excessive adrenergic activity following myocardial infarction or in heart failure confers a worse prognosis and is further confirmed by the efficacy of beta blocker administration in these patients. Sympathetic over activity may also precipitate ventricular arrhythmia due to the presence of heterogeneous effective refractory periods formed between infarcted and non-infarcted bordering myocardium for example. (105) This, alongside damaged or secondarily hyper-innervated areas of myocardium in infarcted zones or infarct-peripheral zones respectively, forms further substrate for ventricular arrhythmia. (106) Excessive sympathetic activation also promotes negative myocardial remodeling secondary to the neurally mediated pro-inflammatory state. (107) Similarly, the development of atrial fibrillation may be mediated by imbalance of sympathetic or parasympathetic activity. This is supported by the fact that atrial fibrillation is often seen during non-REM sleep periods where parasympathetic tone presides and sinoatrial nodal activity is most suppressed. (108)
Pharmacological, surgical, and other forms of modulation of cardiac sympathetic activity have been used to reduce the burden of arrhythmia and show varying degrees of promise.
Autonomic function testing
Sympathetic and parasympathetic nervous control over organs may act antagonistically, in concert or independently. Functional assessment of cardiac reflex activity may be performed via standardized stressor tests designed to induce perturbations in blood pressure. Measurement of systemic concentrations of catecholamines may be considered as a marker of global sympathetic nervous activity. The ‘norepinephrine spillover’ technique was developed by Esler et. al and utilized an intravenous infusion of titrated norepinephrine with an assay taken of titrated and unlabeled norepinephrine at steady state to assess the rate of entry of it into plasma however it should be borne in mind that serum levels of norepinephrine will be affected by other factors such as circulatory issues such as reductions in cardiac output or regional blood flow as well as effectiveness of synaptic junctional clearance of norepinephrine such as in situations of pharmacological neuronal uptake blockade or sympathetic nerve degeneration. (109, 110) Another approach to measuring sympathetic nervous activity is to directly assess electrical activity through a sympathetic nerve using microneurography.
Baroreflex sensitivity testing
Acute regulation of blood pressure is dependent primarily on arterial baroreceptor reflexes. Assessment of the baroreflex response can be particularly helpful in risk stratification and in the clinical management of patients with cardiovascular disease. (111) Baroreflex sensitivity (BRS) is a parameter for quantification of changes in arterial blood pressure based on corresponding changes in heart rate. Patients are usually fasted and asked to avoid caffeine, nicotine, exercise, or any ‘autonomically active’ agents. A number of methods for assessment of the baroreflex exist. The ‘Modified Oxford technique’ is considered the gold standard method of evaluation of sympathetic baroreflex sensitivity using sequential doses of nitroprusside and phenylephrine to determine baroreflex sensitivity through effects on blood pressure and/or muscle sympathetic nerve activity (MSNA) as measured with electrode placement, usually in the peroneal nerve. (112–114) Pharmacological testing modalities using vasoconstrictor medications such as angiotensin or phenylephrine (pure α-adrenoceptor agonist) have largely been replaced by non-invasive methods which are discussed below. In this method of testing, bolus injections of phenylephrine are given until a rise in arterial pressure of 20–30mmHg occurs. (115) Consequent systolic pressure values, usually from the middle to the end of the induced rise, are corresponded with RR intervals (with a 1-beat delay) and a linear regression line is applied, the slope of which is taken as the measure of baroreflex sensitivity (expressed in ms/mmHg). Using this method, values of ~15 ms/mmHg have been reported in normal subjects and ~7ms/mmHg in patients with heart failure. (116, 117) Similarly, cardiac mortality post myocardial infarction in those with severe left ventricular dysfunction was reported to be 18% in those with baroreceptor sensitivity <3 ms/mmHg vs 8% in those with baroreceptor sensitivity >3 ms/mmHg by La Rovere et al. (117) Studies assessing interventions to modulate baroreflex sensitivity have also shown promise in reducing mortality risk. (117–119)
The Valsalva maneuver voluntarily raises intrathoracic and intraabdominal pressures, leading to 4 distinct phase changes within the blood pressure waveform. (121) 15 seconds of strained expiration against a partially obstructed tube takes place, generating ~40mmHg of expiratory force, usually measured with a manometer. An initial rise in pressure occurs due to great vessel compression (stage I). A short-lived decline in pressure then occurs due to reduced venous return which is counteracted by vagal release and peripheral sympathetic vasoconstriction leading to recovery of pressure and an increase in heart rate (stage II early, then late phase). When straining ends, there is a transient (1–3 second) reduction in pressure as mechanical compression has ceased (stage III). Finally, overshoot of pressure remains in place as sympathetic action continues and is followed by normalization of cardiac output (stage IV). Linear regression analysis of the changes between systolic pressure and RR intervals is usually performed during stage IV. (122)
Use of a negative pressure neck chamber device (neck suction technique) allows selective deactivation of carotid body baroreceptors. (124) Negative pressure of between −7 to −40mmHg is applied whilst the maximum lengthening of the RR interval is recorded over the next three beats.
More recently, noninvasive, and relatively simple assessment of baroreflex sensitivity is performed by analyzing spontaneous oscillations in blood pressure and heart rate, usually during spontaneous and/or paced breathing tests. Blood pressure is measured beat-to-beat noninvasively with the Finapres device (Finapres, Ohmeda 2003, Englewood, Florida). The technique uses servo-plethysmography to detect finger volume and continuously counterbalances intra-arterial pressure to keep transmural pressure at zero. (125, 126) Two approaches exist, the ‘time domain/sequence’ and ‘frequency domain’ methods.
The sequence method identifies 3 or more consecutive beats with progressive increase or decrease in systolic pressure and their associated changes in RR interval with minimum recorded changes of 1mmHg for systolic pressure and 4ms for RR interval. The linear sequence is described as: RR = aP + b where the variable RR (RR interval) is dependent on the independent variable P (systolic pressure) and a is baroreflex sensitivity in ms/mmHg and b represents the theoretical RR interval for a systolic pressure of 0 mmHg. (127) The sequence method has the advantage of providing baroreflex sensitivity (BRS) as well as the baroreflex effectiveness index (BEI) which characterizes the percentage of beat-to-beat arterial pressure changes that are translated into reflex changes of heart rate. This index is the ratio of the number of systolic pressure changes that are translated into changes in RR interval, independent of the magnitude of change. Delays between changes in systolic pressure and RR changes are apparent in the oscillatory pattern in the baroreflex effectiveness index, theorized to be due to the influence of respiration. The sequence method requires a minimum threshold of change in RR interval (usually 4 or 5ms) and systolic pressure (usually 1mmHg) to be determined as above as well as a minimum length of sequence (usually 3 beats). A delay between ramps of changes in systolic pressure and RR interval (usually 0–3 beats) is also selected as is a minimum correlation coefficient between systolic pressure and RR ramps (we use 0.85).
The spectral method is based on the principle that 2 major oscillations can be used for quantification of short and long-term changes in cardiovascular neural control. The high frequency (HF, 0.25Hz) respiratory component, reflecting predominantly vagal modulation and the low frequency (LF, 0.1Hz) oscillation which predominantly reflects sympathetic modulation. The square root of the ratio between RR interval and systolic pressure components are used to compute two BRS measurements, labelled α–LF and α–HF. Measurements are retained if coherence between both signals is >0.5. (128, 129) BRS is calculated as the average value of gain between systolic pressure and RR interval in the frequency range 0.07–0.14Hz. BRS calculated through the sequence method correlates highly with BRS estimated by the spectral method in the high frequency band but correlates poorly with the low frequency band suggesting the sequence method is unable to account for slower components of the baroreflex including changes due to slower, adrenergic transmission and therefore is felt unable to reflect the sympathetic-modulated portion of the baroreflex. (130) (131) Measurement of the average of transfer function over the LF band has been shown to give the highest degree of measurability and accuracy however the presence of ectopic activity can potentially reduce measurability but given the advantage in accuracy, it is our preferred method. (132, 133) The aforementioned methods analyze baroreflex gain, the relation between amplitudes of input – systolic blood pressure and output – RR interval. Another attribute of the feedback system that can be analyzed is its stability. This method specifically measures the delay between input and output. Assessment of the coherence of oscillations in systolic blood pressure and RR intervals during 0.1Hz breathing most readily identifies differences in baroreflex gain and stability between individuals. A study by Halamek et. al found no significant differences in baroreflex gain nor stability between young or old healthy individuals, those with established coronary heart disease or those considered at high risk of sudden death (ICD recipients) when assessed during free breathing or during 0.33Hz breathing. However, when assessed during 0.1Hz breathing, ICD recipients demonstrated a significant difference in baroreflex stability suggesting that this measure could have a role in determining the risk of sudden death in at risk individuals. (134)
Handgrip, cold pressor, mental stress and sudomotor testing
A number of other methods to test adrenergic responses exist including the sustained handgrip test, cold pressor and mental stress tests. These ‘pressor’ tests lead to stimulation of sympathetic afference “independent” of baroreceptor afferents. (135, 136) The sustained handgrip test uses a handgrip dynamometer to assess maximum handgrip with the mean of three readings taken. The subject will then be asked to maintain 30% of the mean maximum grip for 3 minutes. During this time, blood pressure, heart rate and heart rate variability are measured while the need for a normal breathing pattern during assessment is stressed, so as to avoid the possibility of a Valsalva effect occurring. Greater changes in systolic and diastolic blood pressure in response to isometric handgrip exercise testing have been demonstrated in pre-hypertensive patients. (137)
Dimitrow et al assessed coronary blood flow velocity responses in patients with HCM during rest and handgrip exercises. The increase in coronary blood flow velocity was significantly reduced in HCM patients versus HCM patients who were taking Verapamil (16.2 +/− 5% versus 6.8 +/− 3.8%, p<0.001). Coronary blood flow velocity was similar in HCM patients taking Verapamil and healthy controls (17.4 +/− 5.7 versus 16.2 +/− 5%, p>0.05) suggesting abnormal endothelium-dependent coronary artery vasodilation in HCM. (138)
In the cold pressor test, a hand is fully immersed in ice water for 2-minutes during a period of monitoring, similar to during the handgrip test. Sudden immersion of the hand into ice water induces a significant release of norepinephrine, increasing cardiac contractility as well as vasoconstriction and a resultant change in blood pressure. The cold pressor test has been shown to induce dilation of the coronary arteries in healthy subjects as assessed angiographically and via Doppler measurements of coronary flow during the test when compared to hypertensive patients in whom constriction occurs. (139) Interestingly, the cold pressor test has also been used as a form of mental stress test with associations reported between perceived pain ratings during the test and blood pressure reactivity, more so even when subjects are forewarned by investigators of the expected pain from the test. (140, 141)
Similarly, Dimitrow et al found coronary blood flow velocity was reduced in patients with HCM undergoing assessment during cold pressor testing and that this response was reversed following 1 month of treatment with verapamil (from −4.1 +/− 6.4% to +11 +/− 10.9%, P<0.01). Verapamil was also seen to reverse the increase seen in coronary vascular resistance index on cold pressor testing in HCM patients from a +12 +/− 9.8% increase to a −5.2 +/− 10.2% decrease (P<0.01) suggesting that verapamil improves endothelium-dependent vasodilation which is abnormal in HCM. (142)
In the mental stress test, subjects are asked to answer concurrent arithmetic questions over a 2 or 3 minute interval or may undergo the Stroop test in which the names of colors are written in colors different to them (the word ‘Red’ may be written in a blue font for example) and the subject’s attention capacity and processing speed are tested whilst assessment of changes in heart rate and blood pressure take place. (143) Similar to handgrip testing, measures of blood pressure and heart rate are taken during testing with analysis focusing on low frequency oscillations in heart rate and systolic blood pressure. Due to the lack of standardized protocols for undertaking the test or its analysis, it remains a tool mostly used in the research setting despite evidence of greater reactivity and poor recovery from stress being associated with cardiovascular morbidity, in particular with hypertension and carotid intima-media thickness. (144) The sudomotor autonomic system works alongside cardiovascular autonomic control in maintaining stable thermoregulation of the body, the two major mechanisms of which are via control of constriction or dilation of cutaneous vessels and via sweat production. Input from visceral and peripheral thermoreceptors is processed by the hypothalamus before travelling via the pons and lateral reticular medulla and intermediolateral column. Preganglionic cholinergic neurons leave the intermediolateral column and synapse with postganglionic sympathetic cholinergic neurons, the axons of which are unmyelinated C-fibers which innervate the skin and control sweat production.
The thermoregulatory sweat test allows qualitative assessment of pre- and postganglionic control of sweating on the ventral aspect of the body using color indicators to highlight sweating. The test requires a temperature and humidity-controlled environment, and patients are tested in a preheated room, usually to 45°C. The indicator dye Quinizarin is spread on the ventral surface of the body and once the surface skin temperature is 39°C, images of the pattern of sweating are taken and a percentage of the surface changing color due to sweating is calculated. The lack of dedicated testing facilities and equipment mean this is generally reserved for the research space.
The quantitative sudomotor axon reflex sweat test (QSART) assesses the sweat response to acetylcholine application on an area of skin, usually the forearm. Once local ‘direct’ sweating is produced due to direct binding of acetylcholine to nicotinic and muscarinic receptors, an antidromically conducted action potential reaches adjacent nerve fibers producing an ‘indirect’ sweat response in nearby sweat glands, the degree of which act as a surrogate marker for the sympathetic C fiber mediated axon reflex. When assessing the sweat response in a patient with hypertrophic cardiomyopathy, it should be noted that patients with Fabry’s disease, a phenocopy of HCM, suffer from abnormal sweating, usually hypohidrosis although hyperhidrosis has also been reported. It is therefore important that any HCM patient being studied have Fabry’s disease excluded first, usually through testing of alpha-galactosidase activity levels, followed by GLA gene testing. In female patients, direct gene testing is preferable given the GLA gene mutation may not always result in decreased levels of alpha-galactosidase.
Heart rate variability testing
Heart rate variability (HRV) describes the measurement of R-R interval means and variance allowing evaluation of autonomic control of the sinus node with higher levels of HRV being associated with efficient autonomic control in health and lower levels suggesting dysfunction of autonomic control. (146, 147) Given the variety of ways available to collect heart rate data, measurement of HRV tends to be cost effective and relatively straight forward. Average heart rate and standard deviation of R-R intervals over a specific time period, most commonly 24-hours, have conventionally been used to assess HRV. This approach, however, is primarily a reflection of very low frequency variations in heart rate and faster, short-term fluctuations are not as easily seen. (148) Shorter durations of 5-minutes, and even as low as 2-minutes have also been used. Similar to assessment of baroreflex sensitivity, the use of spectral analysis methods allows more accurate estimation of oscillations in heart rate at multiple frequencies. (149)
A number of observational studies have demonstrated an association between reduced HRV and significantly increased risk of sudden cardiac death post myocardial infarction. (150) In other observational studies analyzing heart rate variability data gathered from cardiac defibrillators, a decline in HRV prior to episodes of VT and VF has been noted. (151, 152) These studies have focused on high-risk subjects making generalizability of their findings difficult. Sample sizes and event rates however are relatively low, making definitive answers on HRV and its use as a predictor of arrhythmic death challenging.
Accurate assessment of HRV requires consideration of factors that may influence levels such as alcohol, tobacco or illicit drug use as well as medication in use. The environment of testing should aim to be uniform in terms of noise, humidity and temperature and the time of testing should be standardized where possible. Stimulants such as nicotine or caffeine should be avoided for 24-hours prior to testing and strenuous exertion should also be refrained from in this time. The sampling rate of devices used to measure R-R intervals during the period of recording should be higher where possible to increase the number of signal measurements and 250–500Hz has been suggested as the ideal frequency range. (148) A period of 5 minutes of recording time is considered the minimum to calculate low and high frequency components of spectral analysis and at least 512 points of data are recommended. Very-low (VLF) and ultra-low (ULF) frequency component analysis is best performed with longer term recordings such as 24-hour Holter recording with at least 18 hours of sinus rhythm suggested for the most reliable assessment. (148) Very-low and ultra-low frequencies are considered to reflect changes in heart rate secondary to thermoregulatory controls, peripheral vasomotor tone and neuro-hormonal control which includes the renin-angiotensin-aldosterone system. Time-domain analysis is preferred for longer recordings while frequency-domain analysis is recommended for short-term recordings such as those performed in the outpatient setting. (147, 148)
Similar to assessment of the baroreflex, simultaneous recording of respiratory effort allows synchronization with the high-frequency (HF, 0.15–0.4 Hz) component during spectral analysis and allows more selective assessment of parasympathetic modulation with low-frequency (LF, 0.04–0.15Hz) oscillations during spectral analysis reflecting sympathetic and parasympathetic modulation with sympathetic predominance. (148, 153) Given HRV is based on analysis of sinus rhythm variability, the presence of ectopy, artifact or arrhythmia will introduce error in HRV spectral analysis and should be removed from analysis where possible. (154) Analysis of heart rate variability during deep breathing is a core component of Ewing’s battery of autonomic tests, considered a gold standard for assessment and diagnosis of autonomic disorders. (155)
A number of statistical indices are reached through each of the methods of analysis. Using the time domain, SDNN represents the standard deviation of all sinus R-R intervals recorded. SDANN represents the standard deviation of the means of sinus R-R intervals every 5 minutes in a time interval and SDNNi is the average of standard deviations every 5 minutes. These indices are gained from long-term recordings and reflect both parasympathetic and sympathetic modulation but may not reflect when changes are due to sympathetic overdrive or parasympathetic withdrawal. (156) rMSSD is the square root of the mean of the square of differences between adjacent R-R intervals and pNN50 is the percentage of adjacent R-R intervals with differences in duration greater than 50ms. These two indices reflect parasympathetic activity. (157, 158) HRV measures derived from 2-minute electrocardiographic recordings of 12,543 patients demonstrated an association between low heart rate variability and sudden cardiac death. Each standard deviation decrement in SDNN, LF and HF were associated with an increased risk of sudden cardiac death of 24%, 27% and 16% respectively. (159)
Conclusions
This review is intended to give the reader a general overview of the main components of the autonomic nervous system and their relation to cardiovascular autonomic control in the hope of providing a greater appreciation for the very important role of the ANS in health and in disease states. The ANS is responsible for maintaining a plethora of functions including rapid adjustments to heart rate, blood pressure, peripheral vascular reactivity, bowel, bladder and sexual organ function as well as thermoregulation and pupillary reaction. Modulation of the autonomic nervous system’s function is gaining increasing interest, particularly in the domain of arrhythmia which is particularly relevant in conditions such as heart failure and hypertrophic cardiomyopathy.
Fig. 7.
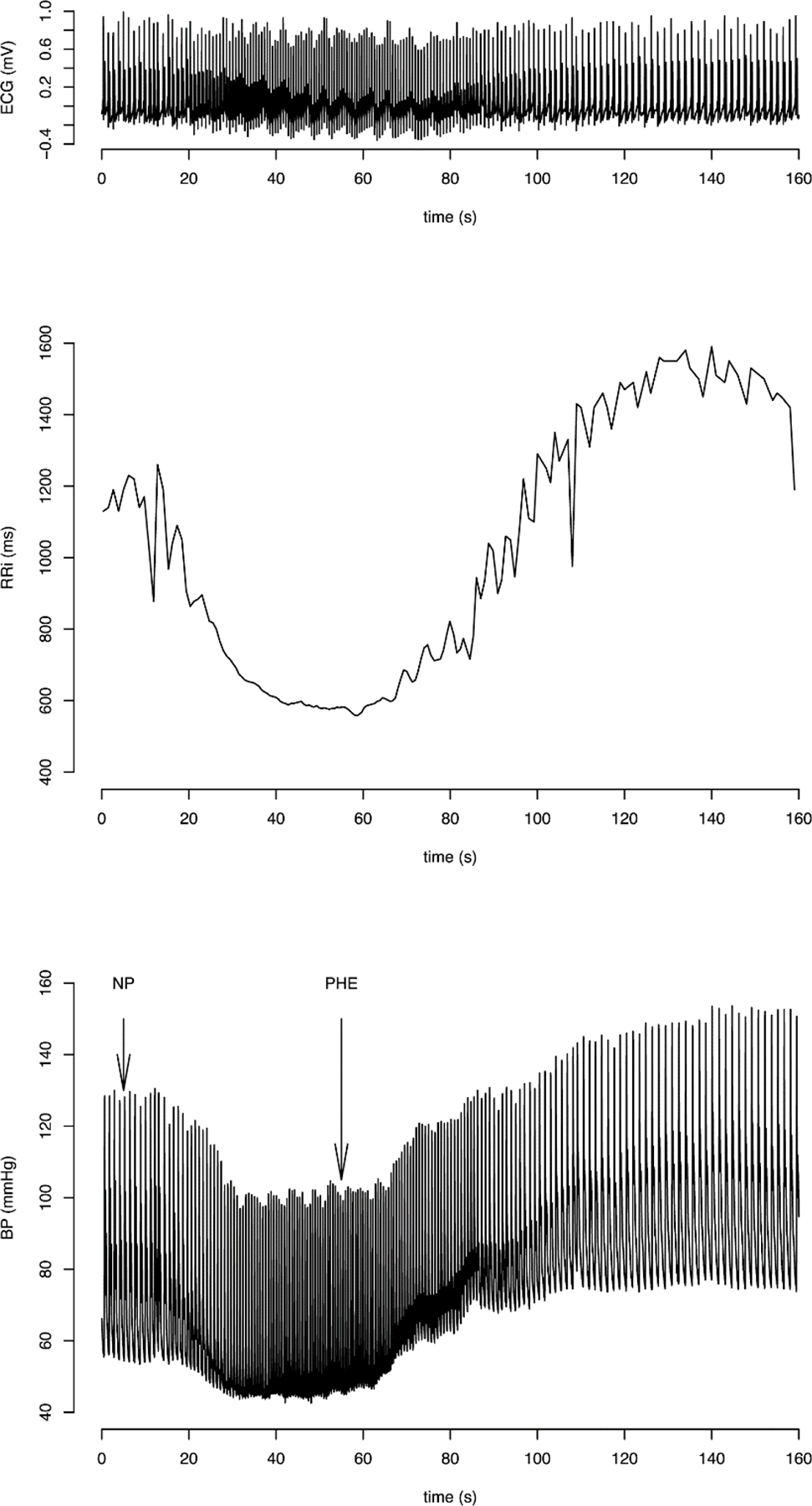
Modified Oxford baroreflex test. ECG, RR interval and blood pressure recordings following sodium nitroprusside (NP) and phenylephrine (PHE) bolus injections. Blood pressure fall is followed by a rise in response to the bolus injections. Reproduced with permission from PLoS One. (120)
Fig. 8.
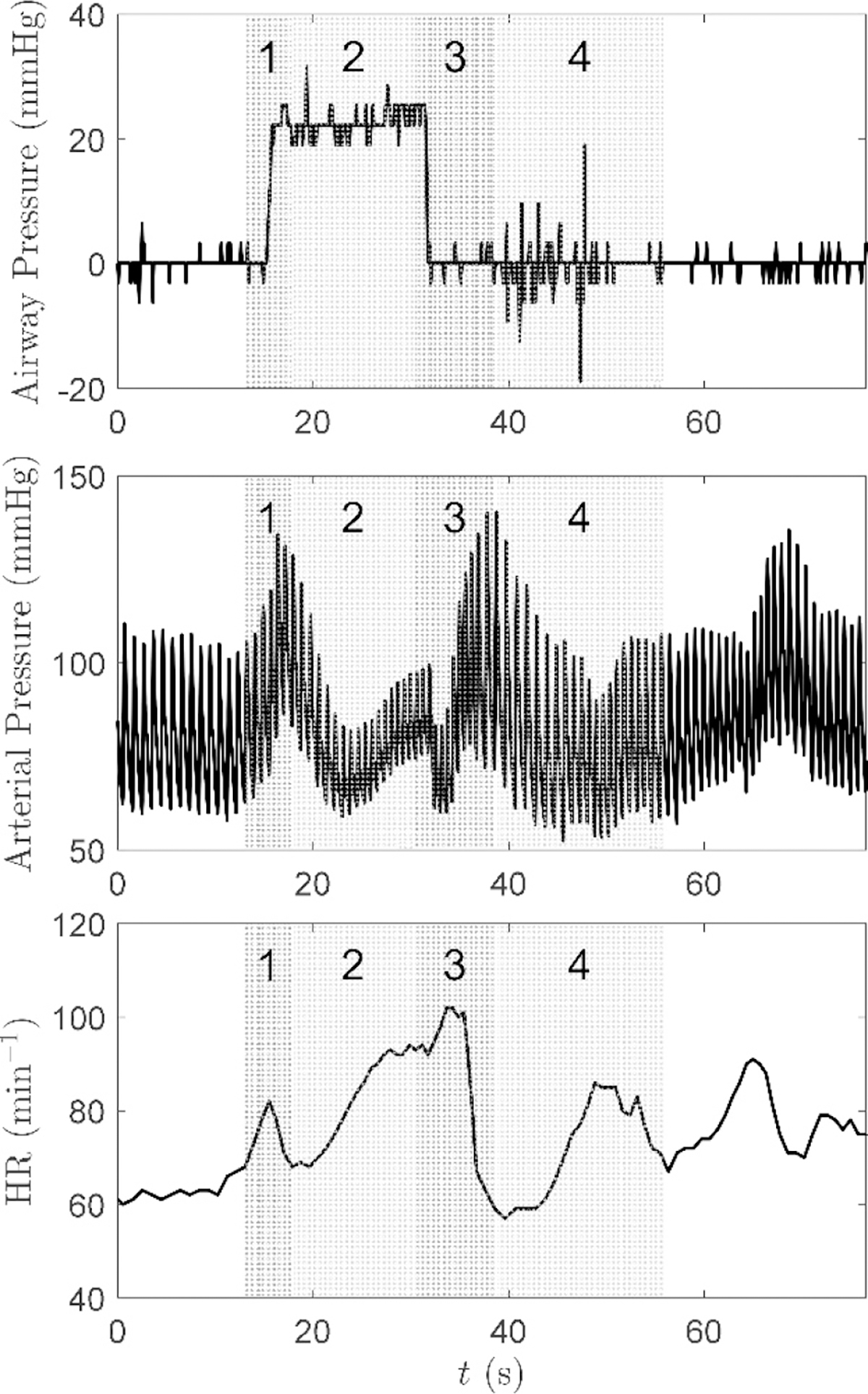
Arterial pressure and heart rate response to the Valsalva maneuver. The four described phases of the baroreflex are indicated on the graphs – Reproduced with permission from Journal of Applied Physiology. (123).
Fig. 9.
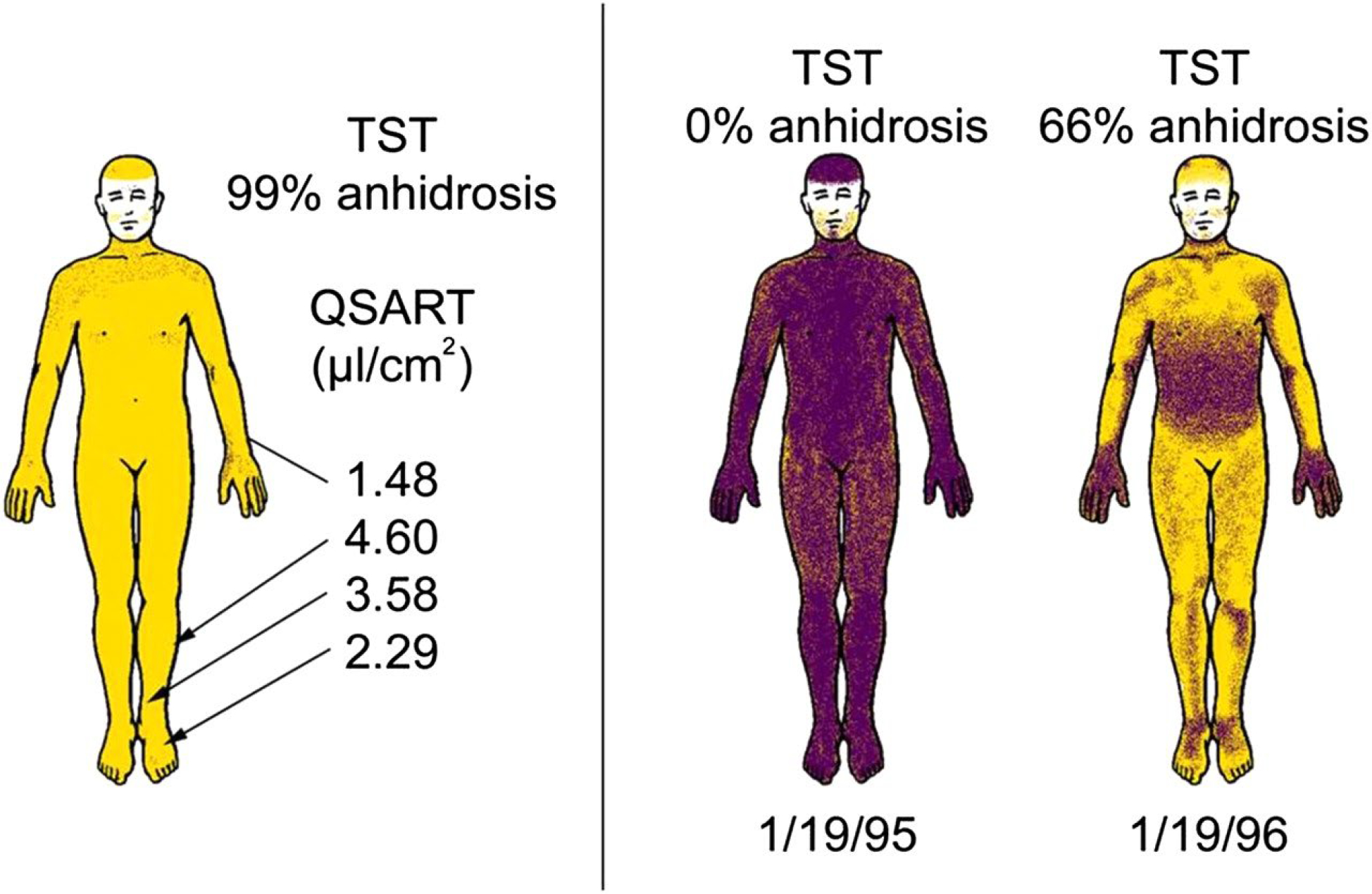
Characteristics of sudomotor autonomic failure in multiple system atrophy. Left: Preserved postganglionic sweat responses (Quantitative Sudomotor Axon Reflex Test (QSART)) with complete anhidrosis on the Thermoregulatory Sweat Test (TST), suggesting a central or preganglionic lesion. Right: Rapid progression (from 0% to 66% body surface anhidrosis) in only 1 year (sweating in shaded areas). Reproduced with permission from Journal of Neurology, Neurosurgery & Psychiatry. (145)
Didactic Synopsis.
Major teaching points:
Autonomic control of the cardiovascular system is dynamic with multiple levels of control and feedback loops controlling parameters such as heart rate, cardiac contractility and stroke volume as well as peripheral vasoconstriction and vasodilation amongst many others
Reflex loops such as the baroreflex and chemoreflex may act as targets for assessment of autonomic neural control
The ANS works in conjunction with other systems such as the Renin-Angiotensin-Aldosterone system in regulating blood pressure
The baroreflex maintains homeostasis through modulation of cardiac output and peripheral vascular resistance
The chemoreflex modulates sympathetic activation to regulate tissue perfusion in combination with control of breathing and the baroreflex
Cardiac autonomic dysfunction is associated with increased mortality and may be intrinsic or extrinsic
The sequence method has the advantage of providing baroreflex sensitivity (BRS) and the baroreflex effectiveness index (BEI)
The spectral method has a high frequency component reflecting vagal modulation and a low frequency component reflecting sympathetic modulation
In controlled conditions, heart rate variability may be a cost-effective method of assessing autonomic control over the sinus node
Interventions such as exercise and physical training can lower BP, increase BRS and increase heart rate variability.
Low heart rate variability is associated with an increased risk of sudden cardiac death
Footnotes
Disclosures
All authors report no conflicts of interest
References
- 1.Maehle AH. “Receptive substances”: John Newport Langley (1852–1925) and his path to a receptor theory of drug action. Med Hist 48: 153–174, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langley JN. On the Union of Cranial Autonomic (Visceral) Fibres with the Nerve Cells of the Superior Cervical Ganglion. J Physiol 23: 240–270, 1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernard C De I’influence de système nerveaux grand sympathetique sur la chaleur animal. C R Acad Sci 34: 472–475, 1852. [Google Scholar]
- 4.Kalia M Brain stem localization of vagal preganglionic neurons. J Auton Nerv Syst 3: 451–481, 1981. [DOI] [PubMed] [Google Scholar]
- 5.Kirby ML, and Gilmore SA. A correlative histofluorescence and light microscopic study of the formation of the sympathetic trunks in chick embryos. Anat Rec 186: 437–449, 1976. [DOI] [PubMed] [Google Scholar]
- 6.Ahlquist RP. A study of the adrenotropic receptors. American Journal of Physiology-Legacy Content 153: 586–600, 1948. [DOI] [PubMed] [Google Scholar]
- 7.Black JW. Ahlquist and the development of beta-adrenoceptor antagonists. Postgrad Med J 52 Suppl 4: 11–13, 1976. [PubMed] [Google Scholar]
- 8.Le Douarin NM, Creuzet S, Couly G, and Dupin E. Neural crest cell plasticity and its limits. Development 131: 4637–4650, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Cordero DR, Brugmann S, Chu Y, Bajpai R, Jame M, and Helms JA. Cranial neural crest cells on the move: their roles in craniofacial development. Am J Med Genet A 155a: 270–279, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loewy AD. Anatomy of the autonomic nervous system : an overview. Central Regulation of Autonomic Functions 1990. [Google Scholar]
- 11.Deuchars SA, and Lall VK. Sympathetic preganglionic neurons: properties and inputs. Compr Physiol 5: 829–869, 2015. [DOI] [PubMed] [Google Scholar]
- 12.Hislop HJ. The Autonomic Nervous System: Morphological, Comparative, Clinical and Surgical Aspects. Physical Therapy 52: 119–120, 1972. [Google Scholar]
- 13.Saper CB. The Central Autonomic Nervous System: Conscious Visceral Perception and Autonomic Pattern Generation. Annual Review of Neuroscience 25: 433–469, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Krassioukov AV, Bygrave MA, Puckett WR, Bunge RP, and Rogers KA. Human sympathetic preganglionic neurons and motoneurons retrogradely labelled with DiI. J Auton Nerv Syst 70: 123–128, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Dolezel S Monoaminergic innervation of the kidney. Aorticorenal ganglion--A sympathetic, monoaminergic ganglion supplying the renal vessels. Experientia 23: 109–111, 1967. [DOI] [PubMed] [Google Scholar]
- 16.McCorry LK. Physiology of the autonomic nervous system. Am J Pharm Educ 71: 78, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becker DE. Basic and clinical pharmacology of autonomic drugs. Anesth Prog 59: 159–168; quiz 169, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lefroy DC, de Silva R, Choudhury L, Uren NG, Crake T, Rhodes CG, Lammertsma AA, Boyd H, Patsalos PN, Nihoyannopoulos P, and et al. Diffuse reduction of myocardial beta-adrenoceptors in hypertrophic cardiomyopathy: a study with positron emission tomography. J Am Coll Cardiol 22: 1653–1660, 1993. [DOI] [PubMed] [Google Scholar]
- 19.Brush JE Jr., Eisenhofer G, Garty M, Stull R, Maron BJ, Cannon RO 3rd, Panza JA, Epstein SE, and Goldstein DS . Cardiac norepinephrine kinetics in hypertrophic cardiomyopathy. Circulation 79: 836–844, 1989. [DOI] [PubMed] [Google Scholar]
- 20.Harvey W Exercitatio anatomica de motucordis et sanguinis in animalibus. 1628. [Google Scholar]
- 21.Shields RW Jr. Functional anatomy of the autonomic nervous system. J Clin Neurophysiol 10: 2–13, 1993. [DOI] [PubMed] [Google Scholar]
- 22.LeDoux J The amygdala. Curr Biol 17: R868–874, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Dampney RA, and Horiuchi J. Functional organisation of central cardiovascular pathways: studies using c-fos gene expression. Prog Neurobiol 71: 359–384, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Shen MJ, and Zipes DP. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ Res 114: 1004–1021, 2014. [DOI] [PubMed] [Google Scholar]
- 25.Palma JA, and Benarroch EE. Neural control of the heart: recent concepts and clinical correlations. Neurology 83: 261–271, 2014. [DOI] [PubMed] [Google Scholar]
- 26.Bandler R, Keay KA, Floyd N, and Price J. Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain Res Bull 53: 95–104, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Manousiouthakis E, Mendez M, Garner MC, Exertier P, and Makita T. Venous endothelin guides sympathetic innervation of the developing mouse heart. Nat Commun 5: 3918, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janes RD, Brandys JC, Hopkins DA, Johnstone DE, Murphy DA, and Armour JA. Anatomy of human extrinsic cardiac nerves and ganglia. Am J Cardiol 57: 299–309, 1986. [DOI] [PubMed] [Google Scholar]
- 29.Dae MW, O’Connell JW, Botvinick EH, Ahearn T, Yee E, Huberty JP, Mori H, Chin MC, Hattner RS, Herre JM, and et al. Scintigraphic assessment of regional cardiac adrenergic innervation. Circulation 79: 634–644, 1989. [DOI] [PubMed] [Google Scholar]
- 30.O’Connell TD, Swigart PM, Rodrigo MC, Ishizaka S, Joho S, Turnbull L, Tecott LH, Baker AJ, Foster E, Grossman W, and Simpson PC. Alpha1-adrenergic receptors prevent a maladaptive cardiac response to pressure overload. J Clin Invest 116: 1005–1015, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng GA, Mantravadi R, Walker WH, Ortin WG, Choi BR, de Groat W, and Salama G. Sympathetic nerve stimulation produces spatial heterogeneities of action potential restitution. Heart Rhythm 6: 696–706, 2009. [DOI] [PubMed] [Google Scholar]
- 32.Lavoie JL, and Sigmund CD. Minireview: overview of the renin-angiotensin system--an endocrine and paracrine system. Endocrinology 144: 2179–2183, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Eckberg DL. Point:counterpoint: respiratory sinus arrhythmia is due to a central mechanism vs. respiratory sinus arrhythmia is due to the baroreflex mechanism. Journal of applied physiology (Bethesda, Md : 1985) 106: 1740–1742; discussion 1744, 2009. [DOI] [PubMed] [Google Scholar]
- 34.Mark AL. The Bezold-Jarisch reflex revisited: clinical implications of inhibitory reflexes originating in the heart. J Am Coll Cardiol 1: 90–102, 1983. [DOI] [PubMed] [Google Scholar]
- 35.von Bezold AV HL. Uber die physiologischen wirkungen des essigsauren veratrins. Untersuchungen aus dem Physiologischen Laboratorium Wurzburg 1: 75–156, 1867. [Google Scholar]
- 36.Jarisch A RH. Die kreislauf des veratrins. Arch Exp Pathol Pharmacol 193: 347–354, 1939. [Google Scholar]
- 37.Aviado DM, and Guevara Aviado D. The Bezold-Jarisch reflex. A historical perspective of cardiopulmonary reflexes. Ann N Y Acad Sci 940: 48–58, 2001. [PubMed] [Google Scholar]
- 38.Accurso V, Winnicki M, Shamsuzzaman AS, Wenzel A, Johnson AK, and Somers VK. Predisposition to vasovagal syncope in subjects with blood/injury phobia. Circulation 104: 903–907, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Davis RW, Polasek L, Watson R, Fuson A, Williams TM, and Kanatous SB. The diving paradox: new insights into the role of the dive response in air-breathing vertebrates. Comp Biochem Physiol A Mol Integr Physiol 138: 263–268, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Butler PJ, and Jones DR. Physiology of diving of birds and mammals. Physiological reviews 77: 837–899, 1997. [DOI] [PubMed] [Google Scholar]
- 41.Asmussen E, and Kristiansson NG. The “diving bradycardia” in exercising man. Acta physiologica Scandinavica 73: 527–535, 1968. [DOI] [PubMed] [Google Scholar]
- 42.Leuenberger UA, Hardy JC, Herr MD, Gray KS, and Sinoway LI. Hypoxia augments apnea-induced peripheral vasoconstriction in humans. Journal of applied physiology (Bethesda, Md : 1985) 90: 1516–1522, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Ackerman MJ, Tester DJ, and Porter CJ. Swimming, a gene-specific arrhythmogenic trigger for inherited long QT syndrome. Mayo Clin Proc 74: 1088–1094, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Paton JF, Nalivaiko E, Boscan P, and Pickering AE. Reflexly evoked coactivation of cardiac vagal and sympathetic motor outflows: observations and functional implications. Clin Exp Pharmacol Physiol 33: 1245–1250, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Shamsuzzaman A, Ackerman MJ, Kuniyoshi FS, Accurso V, Davison D, Amin RS, and Somers VK. Sympathetic nerve activity and simulated diving in healthy humans. Auton Neurosci 181: 74–78, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benarroch EE. The arterial baroreflex: functional organization and involvement in neurologic disease. Neurology 71: 1733–1738, 2008. [DOI] [PubMed] [Google Scholar]
- 47.Suarez-Roca H, Mamoun N, Sigurdson MI, and Maixner W. Baroreceptor Modulation of the Cardiovascular System, Pain, Consciousness, and Cognition. Compr Physiol 11: 1373–1423, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jacob G, Ertl AC, Shannon JR, Furlan R, Robertson RM, and Robertson D. Effect of standing on neurohumoral responses and plasma volume in healthy subjects. J Appl Physiol 84: 914–921, 1998. [DOI] [PubMed] [Google Scholar]
- 49.Zeng WZ, Marshall KL, Min S, Daou I, Chapleau MW, Abboud FM, Liberles SD, and Patapoutian A. PIEZOs mediate neuronal sensing of blood pressure and the baroreceptor reflex. Science 362: 464–467, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mancia G, Ferrari A, Gregorini L, Parati G, Pomidossi G, Bertinieri G, Grassi G, and Zanchetti A. Blood pressure variability in man: its relation to high blood pressure, age and baroreflex sensitivity. Clin Sci (Lond) 59 Suppl 6: 401s–404s, 1980. [DOI] [PubMed] [Google Scholar]
- 51.Gordon FJ, and Leone C. Non-NMDA receptors in the nucleus of the tractus solitarius play the predominant role in mediating aortic baroreceptor reflexes. Brain Res 568: 319–322, 1991. [DOI] [PubMed] [Google Scholar]
- 52.Bainbridge FA. The influence of venous filling upon the rate of the heart. J Physiol 50: 65–84, 1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Waxman MB, Asta JA, and Cameron DA. Vasodepressor reaction induced by inferior vena cava occlusion and isoproterenol in the rat. Role of beta 1- and beta 2-adrenergic receptors. Circulation 89: 2401–2411, 1994. [DOI] [PubMed] [Google Scholar]
- 54.Somers VK, Conway J, LeWinter M, and Sleight P. The role of baroreflex sensitivity in post-exercise hypotension. J Hypertens Suppl 3: S129–130, 1985. [PubMed] [Google Scholar]
- 55.Somers VK, Conway J, Johnston J, and Sleight P. Effects of endurance training on baroreflex sensitivity and blood pressure in borderline hypertension. Lancet (London, England) 337: 1363–1368, 1991. [DOI] [PubMed] [Google Scholar]
- 56.Kennedy B, Shen GH, and Ziegler MG. Neuropeptide Y-mediated pressor responses following high-frequency stimulation of the rat sympathetic nervous system. J Pharmacol Exp Ther 281: 291–296, 1997. [PubMed] [Google Scholar]
- 57.Thomas GD. Neural control of the circulation. Adv Physiol Educ 35: 28–32, 2011. [DOI] [PubMed] [Google Scholar]
- 58.Burnstock G Autonomic Neurotransmission: 60 Years Since Sir Henry Dale. Annual Review of Pharmacology and Toxicology 49: 1–30, 2009. [DOI] [PubMed] [Google Scholar]
- 59.Nurse CA. Neurotransmission and neuromodulation in the chemosensory carotid body. Auton Neurosci 120: 1–9, 2005. [DOI] [PubMed] [Google Scholar]
- 60.Iturriaga R, and Alcayaga J. Neurotransmission in the carotid body: transmitters and modulators between glomus cells and petrosal ganglion nerve terminals. Brain Res Brain Res Rev 47: 46–53, 2004. [DOI] [PubMed] [Google Scholar]
- 61.Pardal R, and López-Barneo J. Low glucose-sensing cells in the carotid body. Nat Neurosci 5: 197–198, 2002. [DOI] [PubMed] [Google Scholar]
- 62.Kumar P, and Prabhakar NR. Peripheral chemoreceptors: function and plasticity of the carotid body. Compr Physiol 2: 141–219, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Biscoe TJ, Purves MJ, and Sampson SR. The frequency of nerve impulses in single carotid body chemoreceptor afferent fibres recorded in vivo with intact circulation. J Physiol 208: 121–131, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Santiago TV EN. Brain Blood Flow and Control of Breathing. In: Comprehensive Physiology 2011, p. 163–179. [Google Scholar]
- 65.Somers VK, Mark AL, and Abboud FM. Interaction of baroreceptor and chemoreceptor reflex control of sympathetic nerve activity in normal humans. The Journal of clinical investigation 87: 1953–1957, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Somers VK, Mark AL, Zavala DC, and Abboud FM. Contrasting effects of hypoxia and hypercapnia on ventilation and sympathetic activity in humans. Journal of applied physiology (Bethesda, Md : 1985) 67: 2101–2106, 1989. [DOI] [PubMed] [Google Scholar]
- 67.Somers VK, Mark AL, Zavala DC, and Abboud FM. Influence of ventilation and hypocapnia on sympathetic nerve responses to hypoxia in normal humans. J Appl Physiol 67: 2095–2100, 1989. [DOI] [PubMed] [Google Scholar]
- 68.Guyenet PG. Regulation of breathing and autonomic outflows by chemoreceptors. Compr Physiol 4: 1511–1562, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kline DD. Chronic intermittent hypoxia affects integration of sensory input by neurons in the nucleus tractus solitarii. Respiratory physiology & neurobiology 174: 29–36, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kara T, Narkiewicz K, and Somers VK. Chemoreflexes--physiology and clinical implications. Acta physiologica Scandinavica 177: 377–384, 2003. [DOI] [PubMed] [Google Scholar]
- 71.Wehrwein EA, Orer HS, and Barman SM. Overview of the Anatomy, Physiology, and Pharmacology of the Autonomic Nervous System. Compr Physiol 6: 1239–1278, 2016. [DOI] [PubMed] [Google Scholar]
- 72.Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, and Rector T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med 311: 819–823, 1984. [DOI] [PubMed] [Google Scholar]
- 73.Angell-James JE, and Daly MB. Some aspects of upper respiratory tract reflexes. Acta Otolaryngol 79: 242–252, 1975. [DOI] [PubMed] [Google Scholar]
- 74.Prabhakar NR, Peng YJ, Kumar GK, and Nanduri J. Peripheral chemoreception and arterial pressure responses to intermittent hypoxia. Compr Physiol 5: 561–577, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Narkiewicz K, and Somers VK. The sympathetic nervous system and obstructive sleep apnea: implications for hypertension. J Hypertens 15: 1613–1619, 1997. [DOI] [PubMed] [Google Scholar]
- 76.Dudenbostel T, and Calhoun DA. Resistant hypertension, obstructive sleep apnoea and aldosterone. J Hum Hypertens 26: 281–287, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Becker HF, Jerrentrup A, Ploch T, Grote L, Penzel T, Sullivan CE, and Peter JH. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation 107: 68–73, 2003. [DOI] [PubMed] [Google Scholar]
- 78.Imadojemu VA, Mawji Z, Kunselman A, Gray KS, Hogeman CS, and Leuenberger UA. Sympathetic chemoreflex responses in obstructive sleep apnea and effects of continuous positive airway pressure therapy. Chest 131: 1406–1413, 2007. [DOI] [PubMed] [Google Scholar]
- 79.Daly MdB, and Ungar A. Comparison of the reflex responses elicited by stimulation of the separately perfused carotid and aortic body chemoreceptors in the dog. The Journal of Physiology 182: 379–403, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maser RE, Mitchell BD, Vinik AI, and Freeman R. The Association Between Cardiovascular Autonomic Neuropathy and Mortality in Individuals With Diabetes. A meta-analysis 26: 1895–1901, 2003. [DOI] [PubMed] [Google Scholar]
- 81.Peelaerts W, Bousset L, Van der Perren A, Moskalyuk A, Pulizzi R, Giugliano M, Van den Haute C, Melki R, and Baekelandt V. α-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature 522: 340–344, 2015. [DOI] [PubMed] [Google Scholar]
- 82.Goldstein DS. Dysautonomia in Parkinson’s disease: neurocardiological abnormalities. Lancet Neurol 2: 669–676, 2003. [DOI] [PubMed] [Google Scholar]
- 83.Biaggioni I, Onrot J, Stewart CK, and Robertson D. The potent pressor effect of phenylpropanolamine in patients with autonomic impairment. Jama 258: 236–239, 1987. [PubMed] [Google Scholar]
- 84.Polinsky RJ, Kopin IJ, Ebert MH, and Weise V. Pharmacologic distinction of different orthostatic hypotension syndromes. Neurology 31: 1–7, 1981. [DOI] [PubMed] [Google Scholar]
- 85.Singer W, Berini SE, Sandroni P, Fealey RD, Coon EA, Suarez MD, Benarroch EE, and Low PA. Pure autonomic failure: Predictors of conversion to clinical CNS involvement. Neurology 88: 1129–1136, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tipre DN, and Goldstein DS. Cardiac and extracardiac sympathetic denervation in Parkinson’s disease with orthostatic hypotension and in pure autonomic failure. J Nucl Med 46: 1775–1781, 2005. [PubMed] [Google Scholar]
- 87.Yoshida M, Fukumoto Y, Kuroda Y, and Ohkoshi N. Sympathetic denervation of myocardium demonstrated by 123I-MIBG scintigraphy in pure progressive autonomic failure. Eur Neurol 38: 291–296, 1997. [DOI] [PubMed] [Google Scholar]
- 88.Guo YP, McLeod JG, and Baverstock J. Pathological changes in the vagus nerve in diabetes and chronic alcoholism. Journal of Neurology, Neurosurgery & Psychiatry 50: 1449–1453, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kristensson K, Nordborg C, Olsson Y, and Sourander P. Changes in the vagus nerve in diabetes mellitus. Acta Pathol Microbiol Scand A 79: 684–685, 1971. [DOI] [PubMed] [Google Scholar]
- 90.Schmidt RE. Neuropathology and pathogenesis of diabetic autonomic neuropathy. Int Rev Neurobiol 50: 257–292, 2002. [DOI] [PubMed] [Google Scholar]
- 91.Pop-Busui R Cardiac Autonomic Neuropathy in Diabetes. A clinical perspective 33: 434–441, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vinik AI, Maser RE, Mitchell BD, and Freeman R. Diabetic Autonomic Neuropathy. Diabetes Care 26: 1553–1579, 2003. [DOI] [PubMed] [Google Scholar]
- 93.O’Brien IA, O’Hare JP, Lewin IG, and Corrall RJ. The prevalence of autonomic neuropathy in insulin-dependent diabetes mellitus: A controlled study based on heart rate variability. QJM 61: 957–967, 1986. [PubMed] [Google Scholar]
- 94.Goldstein DS. Concepts of scientific integrative medicine applied to the physiology and pathophysiology of catecholamine systems. Compr Physiol 3: 1569–1610, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Patel MB, Loud AV, King BD, Anversa P, Sack D, and Hintze TH. Global myocardial hypertrophy in conscious dogs with chronic elevation of plasma norepinephrine levels. J Mol Cell Cardiol 21 Suppl 5: 49–61, 1989. [DOI] [PubMed] [Google Scholar]
- 96.Imrich R, Eldadah BA, Bentho O, Pechnik S, Sharabi Y, Holmes C, and Goldstein DS. Attenuated pre-ejection period response to tyramine in patients with cardiac sympathetic denervation. Ann N Y Acad Sci 1148: 486–489, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kawada T, Yamazaki T, Akiyama T, Shishido T, Miyano H, Sato T, Sugimachi M, Alexander J Jr., and Sunagawa K. Interstitial norepinephrine level by cardiac microdialysis correlates with ventricular contractility. Am J Physiol 273: H1107–1112, 1997. [DOI] [PubMed] [Google Scholar]
- 98.Graham DG. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol Pharmacol 14: 633–643, 1978. [PubMed] [Google Scholar]
- 99.Singal PK, Dhillon KS, Beamish RE, Kapur N, and Dhalla NS. Myocardial cell damage and cardiovascular changes due to i.v. infusion of adrenochrome in rats. Br J Exp Pathol 63: 167–176, 1982. [PMC free article] [PubMed] [Google Scholar]
- 100.Burke WJ, Li SW, Chung HD, Ruggiero DA, Kristal BS, Johnson EM, Lampe P, Kumar VB, Franko M, Williams EA, and Zahm DS. Neurotoxicity of MAO metabolites of catecholamine neurotransmitters: role in neurodegenerative diseases. Neurotoxicology 25: 101–115, 2004. [DOI] [PubMed] [Google Scholar]
- 101.Tank AW, and Lee Wong D. Peripheral and central effects of circulating catecholamines. Compr Physiol 5: 1–15, 2015. [DOI] [PubMed] [Google Scholar]
- 102.Inoue H, and Zipes DP. Time course of denervation of efferent sympathetic and vagal nerves after occlusion of the coronary artery in the canine heart. Circulation Research 62: 1111–1120, 1988. [DOI] [PubMed] [Google Scholar]
- 103.Martins JB, Kerber RE, Marcus ML, Laughlin DL, and Levy DM. Inhibition of adrenergic neurotransmission in ischaemic regions of the canine left ventricle. Cardiovasc Res 14: 116–124, 1980. [DOI] [PubMed] [Google Scholar]
- 104.Fukuda K, Kanazawa H, Aizawa Y, Ardell JL, and Shivkumar K. Cardiac Innervation and Sudden Cardiac Death. Circulation Research 116: 2005–2019, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zipes DP. Influence of myocardial ischemia and infarction on autonomic innervation of heart. Circulation 82: 1095–1105, 1990. [DOI] [PubMed] [Google Scholar]
- 106.Cao JM, Fishbein MC, Han JB, Lai WW, Lai AC, Wu TJ, Czer L, Wolf PL, Denton TA, Shintaku IP, Chen PS, and Chen LS. Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation 101: 1960–1969, 2000. [DOI] [PubMed] [Google Scholar]
- 107.Jänig W Sympathetic nervous system and inflammation: a conceptual view. Auton Neurosci 182: 4–14, 2014. [DOI] [PubMed] [Google Scholar]
- 108.Bettoni M, and Zimmermann M. Autonomic tone variations before the onset of paroxysmal atrial fibrillation. Circulation 105: 2753–2759, 2002. [DOI] [PubMed] [Google Scholar]
- 109.Esler M, Jackman G, Bobik A, Kelleher D, Jennings G, Leonard P, Skews H, and Korner P. Determination of norepinephrine apparent release rate and clearance in humans. Life Sci 25: 1461–1470, 1979. [DOI] [PubMed] [Google Scholar]
- 110.Esler M, Jennings G, Lambert G, Meredith I, Horne M, and Eisenhofer G. Overflow of catecholamine neurotransmitters to the circulation: source, fate, and functions. Physiological reviews 70: 963–985, 1990. [DOI] [PubMed] [Google Scholar]
- 111.Dampney RA, Horiuchi J, Killinger S, Sheriff MJ, Tan PS, and McDowall LM. Long-term regulation of arterial blood pressure by hypothalamic nuclei: some critical questions. Clin Exp Pharmacol Physiol 32: 419–425, 2005. [DOI] [PubMed] [Google Scholar]
- 112.Horsman HM, Peebles KC, Galletly DC, and Tzeng YC. Cardiac baroreflex gain is frequency dependent: insights from repeated sit-to-stand maneuvers and the modified Oxford method. Appl Physiol Nutr Metab 38: 753–759, 2013. [DOI] [PubMed] [Google Scholar]
- 113.Charkoudian N, and Wallin BG. Sympathetic neural activity to the cardiovascular system: integrator of systemic physiology and interindividual characteristics. Compr Physiol 4: 825–850, 2014. [DOI] [PubMed] [Google Scholar]
- 114.Shoemaker JK, Badrov MB, Al-Khazraji BK, and Jackson DN. Neural Control of Vascular Function in Skeletal Muscle. Compr Physiol 6: 303–329, 2015. [DOI] [PubMed] [Google Scholar]
- 115.Milic M, Sun P, Liu F, Fainman C, Dimsdale J, Mills PJ, and Ziegler MG. A comparison of pharmacologic and spontaneous baroreflex methods in aging and hypertension. J Hypertens 27: 1243–1251, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Davies L, Francis D, Jurak P, Kara T, Piepoli M, and Coats A. Reproducibility of methods for assessing baroreflex sensitivity in normal controls and in patients with chronic heart failure. Clinical science (London, England : 1979) 97: 515–522, 1999. [PubMed] [Google Scholar]
- 117.La Rovere MT, Bigger JT, Jr., Marcus FI, Mortara A, and Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet (London, England) 351: 478–484, 1998. [DOI] [PubMed] [Google Scholar]
- 118.Hull SS, Jr., Vanoli E, Adamson PB, Verrier RL, Foreman RD, and Schwartz PJ . Exercise training confers anticipatory protection from sudden death during acute myocardial ischemia. Circulation 89: 548–552, 1994. [DOI] [PubMed] [Google Scholar]
- 119.Parati G, Mutti E, Frattola A, Castiglioni P, di Rienzo M, and Mancia G. Beta-adrenergic blocking treatment and 24-hour baroreflex sensitivity in essential hypertensive patients. Hypertension (Dallas, Tex : 1979) 23: 992–996, 1994. [DOI] [PubMed] [Google Scholar]
- 120.Bonyhay I, Risk M, and Freeman R. High-pass filter characteristics of the baroreflex--a comparison of frequency domain and pharmacological methods. PLoS One 8: e79513, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Trimarco B, Volpe M, Ricciardelli B, Vigorito C, De Luca N, Saccà L, and Condorelli M. Valsalva maneuver in the assessment of baroreflex responsiveness in borderline hypertensives. Cardiology 70: 6–14, 1983. [DOI] [PubMed] [Google Scholar]
- 122.Convertino VA, Ratliff DA, Doerr DF, Ludwig DA, Muniz GW, Benedetti E, Chavarria J, Koreen S, Nguyen C, and Wang J. Effects of repeated Valsalva maneuver straining on cardiac and vasoconstrictive baroreflex responses. Aviat Space Environ Med 74: 212–219, 2003. [PubMed] [Google Scholar]
- 123.Kosinski SA, Carlson BE, Hummel SL, Brook RD, and Beard DA. Computational model-based assessment of baroreflex function from response to Valsalva maneuver. Journal of applied physiology (Bethesda, Md : 1985) 125: 1944–1967, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Eckberg DL, Cavanaugh MS, Mark AL, and Abboud FM. A simplified neck suction device for activation of carotid baroreceptors. J Lab Clin Med 85: 167–173, 1975. [PubMed] [Google Scholar]
- 125.Penaz. Photoelectric measurement of blood pressure, volume and flow in the finger. Digest of the International Conference on Medicine and Biological Engineering 104, 1973. [Google Scholar]
- 126.Wesseling KH, Settels JJ, and de Wit B. The Measurement of Continuous Finger Arterial Pressure Noninvasively in Stationary Subjects. In: Biological and Psychological Factors in Cardiovascular Disease, edited by Schmidt TH, Dembroski TM, and Blümchen G. Berlin, Heidelberg: Springer Berlin Heidelberg, 1986, p. 355–375. [Google Scholar]
- 127.Bertinieri G, Di Rienzo M, Cavallazzi A, Ferrari AU, Pedotti A, and Mancia G. Evaluation of baroreceptor reflex by blood pressure monitoring in unanesthetized cats. Am J Physiol 254: H377–383, 1988. [DOI] [PubMed] [Google Scholar]
- 128.Pagani M, Somers V, Furlan R, Dell’Orto S, Conway J, Baselli G, Cerutti S, Sleight P, and Malliani A. Changes in autonomic regulation induced by physical training in mild hypertension. Hypertension (Dallas, Tex : 1979) 12: 600–610, 1988. [DOI] [PubMed] [Google Scholar]
- 129.Robbe HW, Mulder LJ, Rüddel H, Langewitz WA, Veldman JB, and Mulder G. Assessment of baroreceptor reflex sensitivity by means of spectral analysis. Hypertension (Dallas, Tex : 1979) 10: 538–543, 1987. [DOI] [PubMed] [Google Scholar]
- 130.Oosting J, Struijker-Boudier HA, and Janssen BJ. Validation of a continuous baroreceptor reflex sensitivity index calculated from spontaneous fluctuations of blood pressure and pulse interval in rats. J Hypertens 15: 391–399, 1997. [DOI] [PubMed] [Google Scholar]
- 131.Herring N, Paterson DJ, Paterson DJ, and Levick JR. Levick’s introduction to cardiovascular physiology. 2018. [Google Scholar]
- 132.Pinna GD, Maestri R, Capomolla S, Febo O, Robbi E, Cobelli F, and La Rovere MT. Applicability and clinical relevance of the transfer function method in the assessment of baroreflex sensitivity in heart failure patients. J Am Coll Cardiol 46: 1314–1321, 2005. [DOI] [PubMed] [Google Scholar]
- 133.Pinna GD, and Maestri R. New criteria for estimating baroreflex sensitivity using the transfer function method. Med Biol Eng Comput 40: 79–84, 2002. [DOI] [PubMed] [Google Scholar]
- 134.Halámek J, Kára T, Jurák P, Souček M, Francis DP, Davies LC, Shen WK, Coats AJS, Novák M, Nováková Z, Panovský R, Toman J, Šumbera J, and Somers VK. Variability of Phase Shift Between Blood Pressure and Heart Rate Fluctuations. Circulation 108: 292–297, 2003. [DOI] [PubMed] [Google Scholar]
- 135.Li K, Lindauer C, Haase R, Rüdiger H, Reichmann H, Reuner U, and Ziemssen T. Autonomic Dysfunction in Wilson’s Disease: A Comprehensive Evaluation during a 3-Year Follow Up. Front Physiol 8: 778, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hilz MJ, Axelrod FB, Braeske K, and Stemper B. Cold pressor test demonstrates residual sympathetic cardiovascular activation in familial dysautonomia. J Neurol Sci 196: 81–89, 2002. [DOI] [PubMed] [Google Scholar]
- 137.Bond V, Curry BH, Adams RG, Obisesan T, Pemminati S, Gorantla VR, Kadur K, and Millis RM. Cardiovascular Responses to an Isometric Handgrip Exercise in Females with Prehypertension. N Am J Med Sci 8: 243–249, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dimitrow PP, Krzanowski M, Nitankowski R, Szczeklik A, and Dubiel JS. The effect of verapamil on response of coronary vasomotion to handgrip exercise in symptomatic patients with hypertrophic cardiomyopathy. Cardiovasc Drugs Ther 15: 331–337, 2001. [DOI] [PubMed] [Google Scholar]
- 139.Antony I, Aptecar E, Lerebours G, and Nitenberg A. Coronary artery constriction caused by the cold pressor test in human hypertension. Hypertension (Dallas, Tex : 1979) 24: 212–219, 1994. [DOI] [PubMed] [Google Scholar]
- 140.Kamarck TW, and Lovallo WR. Cardiovascular reactivity to psychological challenge: conceptual and measurement considerations. Psychosom Med 65: 9–21, 2003. [DOI] [PubMed] [Google Scholar]
- 141.Carter JR, and Goldstein DS. Sympathoneural and adrenomedullary responses to mental stress. Compr Physiol 5: 119–146, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dimitrow PP, Krzanowski M, Nizankowski R, Szczeklik A, and Dubiel JS. Verapamil improves the response of coronary vasomotion to cold pressor test in asymptomatic and mildly symptomatic patients with hypertrophic cardiomyopathy. Cardiovasc Drugs Ther 13: 259–264, 1999. [DOI] [PubMed] [Google Scholar]
- 143.Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology 18: 643–662, 1935. [Google Scholar]
- 144.Chida Y, and Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension (Dallas, Tex : 1979) 55: 1026–1032, 2010. [DOI] [PubMed] [Google Scholar]
- 145.Iodice V, Lipp A, Ahlskog JE, Sandroni P, Fealey RD, Parisi JE, Matsumoto JY, Benarroch EE, Kimpinski K, Singer W, Gehrking TL, Gehrking JA, Sletten DM, Schmeichel AM, Bower JH, Gilman S, Figueroa J, and Low PA. Autopsy confirmed multiple system atrophy cases: Mayo experience and role of autonomic function tests. J Neurol Neurosurg Psychiatry 83: 453–459, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sassi R, Cerutti S, Lombardi F, Malik M, Huikuri HV, Peng C-K, Schmidt G, Yamamoto Y, Reviewers D, Gorenek B, Lip GYH, Grassi G, Kudaiberdieva G, Fisher JP, Zabel M, and Macfadyen R . Advances in heart rate variability signal analysis: joint position statement by the e-Cardiology ESC Working Group and the European Heart Rhythm Association co-endorsed by the Asia Pacific Heart Rhythm Society. EP Europace 17: 1341–1353, 2015. [DOI] [PubMed] [Google Scholar]
- 147.Shaffer F, and Ginsberg JP. An Overview of Heart Rate Variability Metrics and Norms. Front Public Health 5: 258, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.ESC/NASPE. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 93: 1043–1065, 1996. [PubMed] [Google Scholar]
- 149.Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, and Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science 213: 220–222, 1981. [DOI] [PubMed] [Google Scholar]
- 150.Buccelletti F, Gilardi E, Scaini E, Galiuto L, Persiani R, Biondi A, Basile F, and Gentiloni Silveri N. Heart rate variability and myocardial infarction: Systematic literature review and metanalysis. European Review for Medical and Pharmacological Sciences 13: 299–307, 2009. [PubMed] [Google Scholar]
- 151.Pruvot E, Thonet G, Vesin JM, van-Melle G, Seidl K, Schmidinger H, Brachmann J, Jung W, Hoffmann E, Tavernier R, Block M, Podczeck A, and Fromer M. Heart rate dynamics at the onset of ventricular tachyarrhythmias as retrieved from implantable cardioverter-defibrillators in patients with coronary artery disease. Circulation 101: 2398–2404, 2000. [DOI] [PubMed] [Google Scholar]
- 152.Lombardi F, Porta A, Marzegalli M, Favale S, Santini M, Vincenti A, and De Rosa A. Heart rate variability patterns before ventricular tachycardia onset in patients with an implantable cardioverter defibrillator. Participating Investigators of ICD-HRV Italian Study Group. Am J Cardiol 86: 959–963, 2000. [DOI] [PubMed] [Google Scholar]
- 153.Malliani A, Pagani M, Lombardi F, and Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation 84: 482–492, 1991. [DOI] [PubMed] [Google Scholar]
- 154.Montano N, Porta A, Cogliati C, Costantino G, Tobaldini E, Casali KR, and Iellamo F. Heart rate variability explored in the frequency domain: A tool to investigate the link between heart and behavior. Neuroscience & Biobehavioral Reviews 33: 71–80, 2009. [DOI] [PubMed] [Google Scholar]
- 155.Ewing DJ, and Clarke BF. Diagnosis and management of diabetic autonomic neuropathy. Br Med J (Clin Res Ed) 285: 916–918, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Niskanen JP, Tarvainen MP, Ranta-Aho PO, and Karjalainen PA. Software for advanced HRV analysis. Comput Methods Programs Biomed 76: 73–81, 2004. [DOI] [PubMed] [Google Scholar]
- 157.Aubert AE, Seps B, and Beckers F. Heart rate variability in athletes. Sports Med 33: 889–919, 2003. [DOI] [PubMed] [Google Scholar]
- 158.Pumprla J, Howorka K, Groves D, Chester M, and Nolan J. Functional assessment of heart rate variability: physiological basis and practical applications. Int J Cardiol 84: 1–14, 2002. [DOI] [PubMed] [Google Scholar]
- 159.Maheshwari A, Norby FL, Soliman EZ, Adabag S, Whitsel EA, Alonso A, and Chen LY. Low Heart Rate Variability in a 2-Minute Electrocardiogram Recording Is Associated with an Increased Risk of Sudden Cardiac Death in the General Population: The Atherosclerosis Risk in Communities Study. PLoS One 11: e0161648, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]


