Abstract
Objective
To analyze the effect of CytoSorb® on mortality, interleukin levels, vasopressor use and adverse events in patients with sepsis.
Methods
We searched MEDLINE®, Embase and the Cochrane Library for randomized controlled trials and cohort studies that reported the use of CytoSorb® among septic patients. The primary outcome was mortality, and secondary outcomes included the use of vasopressors, levels of inflammatory markers, predicted versus observed mortality, length of stay in the intensive care unit, and adverse events.
Results
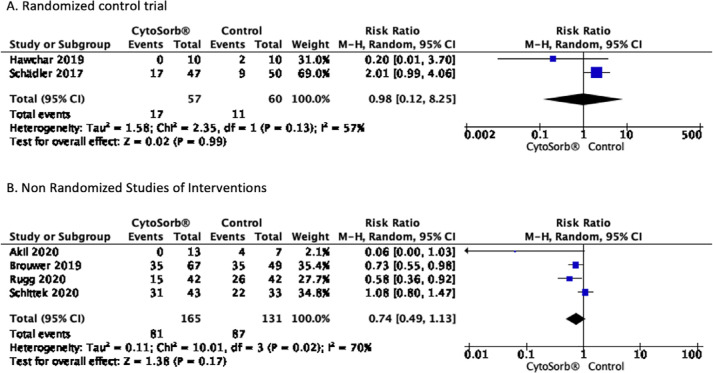
We included 6 studies enrolling 413 patients, and assessment for risk of bias indicated variations in study quality from high to moderate. The overall mortality rate was 45%, and no significant effect on mortality was found at 28 - 30 days (RR 0.98 [0.12 - 8.25] for the randomized clinical trial and RR 0.74 [0.49 - 1.13] for cohort studies). We did not perform a metanalysis for other outcomes due to the small number of studies found or the lack of data.
Conclusion
Our study found very low certainty evidence, due to imprecision, risk of bias, and heterogeneity, thereby showing no benefit of CytoSorb® use in terms of mortality at 28 - 30 days. We cannot recommend the use of CytoSorb® in septic or septic shock patients outside clinical trials. Further high-quality randomized trials with a common intervention arm are needed to evaluate the influence of CytoSorb® in this population.
PROSPERO register
Keywords: Cytokine, Hemoperfusion, Mortality, Sepsis, Septic shock
INTRODUCTION
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection,(1) and its most severe state, septic shock, represents a highly lethal condition that causes substantial morbidity and mortality among critically ill patients.(2) The pathophysiology of sepsis is very complex, involving many factors, such as proinflammatory and anti-inflammatory cytokines, pathogen-associated molecular patterns (PAMPs), bacterial exotoxins and endotoxins, mycotoxins, damage-associated molecular patterns (DAMPs) released by injured cells and host-specific factors such as activated complement and procalcitonin.(3) Inflammation can lead to severe immune system dysfunction ranging from destructive maladaptive systemic inflammatory response syndrome (SIRS) to advanced immunosuppression, which could lead to multisystem organ dysfunction and death.(3,4)
Despite early treatment and multiple efforts to reduce mortality in sepsis and septic shock, such as the surviving sepsis campaign, which provides treatment guidelines,(5) mortality is still high, approximately 20 - 40% for severe sepsis and 40 - 60% in septic shock,(1,6,7) without significant variations in this figure in recent years. This is why adjuvant therapies, such as blood purification techniques including extracorporeal removal of cytokines by hemoadsorption, have been described.(2,8-10)
There are currently multiple blood purification techniques, with different results, such as cytokine removal, decrease in vasopressors and even decrease in mortality; such techniques include high-volume dialysis, high-cut membranes, adsorption by filtration coupled plasma and special adsorption filters (such as Oxiris, CytoSorb®, HA 330 and Polymyxin B filters).(8,11) Blood purification therapies have been used in different acute inflammatory scenarios, such as sepsis, cardiac surgery, and autoimmune diseases; however, their use is controversial, and despite a theoretical justification, the use of blood purification methods cannot yet be recommended for patients with sepsis due to a lack of evidence.(12)
CytoSorb® is a cartridge composed of polystyrene-divinyl-benzene polymer beads with a highly porous and biocompatible polyvinylpyrrolidone cover. Its estimated size is 300 to 800µm with a total surface area of more than 40,000m2. The elimination of substances from the blood is based on the capture of substances in the pores and surface adsorption. The typical duration of therapy is up to 24 hours per session, daily for 2 to 7 consecutive days.(8,13) The physiological reason for using CytoSorb® in the setting of sepsis is to restore a balanced response of pro-inflammatory and anti-inflammatory mediators. Elevated circulating concentrations of several cytokines, including TNF-α, IL-1β, IL-6, IL-8, and IL-10, have been reported to be associated with morbidity and mortality in patients with sepsis, so their removal would be useful for treatment.(3,11)
We performed a systematic review and meta-analysis with the aim of analyzing the use of CytoSorb® in terms of mortality, interleukin levels, the use of vasopressors and adverse events in patients with sepsis and septic shock since the available evidence is still controversial.
METHODS
A systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement,(14) and a research protocol was developed and registered in the International Prospective Register of Systematic Reviews (PROSPERO) database (CRD42021262219).(15)
Information sources and search strategy
We conducted a systematic search in the PubMed, Embase, and Cochrane Library databases. Medical Subject Headings (MeSH) or equivalent terms were used. Articles in English and Spanish were included. A preliminary search strategy was created for MEDLINE®/PubMed®, the other searches were tailored to individual databases (Table 1S - Supplementary Material), and the overall search was performed from inception to November 2022. Additionally, the bibliographic references of the selected articles were reviewed to identify other references relevant to the topic. The MEDLINE search strategy was developed as follows: (“CytoSorb”[tiab] OR “Cytokine adsor*”[tiab]) AND (Sepsis [Mesh] OR Sepsis [tiab] OR sept* [tiab]).
For the Embase and Cochrane Library databases, the search strategy was developed with the terms “CytoSorb”, “Cytokine adsorption”, “hemadsorption”, “Septic shock” and “Sepsis”.
Study selection
Relevant studies were identified by 2 reviewers, who independently assessed them using the research objectives and question (PICO). When an agreement was not reached, a third reviewer member of the investigator group was included and resolved any discrepancies. The articles selected in each database were exported to Zotero software, where the elimination of duplicates was carried out.
We included studies that met the following criteria: adult patients with sepsis or septic shock; randomized clinical trial (RCT) studies, propensity score-matched cohort studies (prospective or retrospective), or studies with historical control; patients who received at least one hemoadsorption therapy with CytoSorb®; and studies that reported on mortality at 28-30 days, requirement for the use of vasopressors, inflammatory marker levels and adverse effects of CytoSorb® treatment. The exclusion criteria were as follows: use of CytoSorb® in contexts other than sepsis and septic shock (such as pancreatitis, cardiac surgery, endocarditis, transplant, trauma or coronavirus disease 2019 - COVID-19); type of study or publication of type reports of cases or letters to the editor. We also did not include abstracts from conferences or before and after studies without a comparator group; studies in neonates or pediatric patients; and studies that did not report mortality data. It was deemed appropriate to include nonrandomized studies of interventions (NRSI) due to the low number of clinical trials found according to the research question.
Data extraction and risk of bias assessment
A standardized data extraction sheet was used. Two independent reviewers extracted the data, and disagreements were resolved by discussion and consensus in case no agreement was reached. A third reviewer was included to resolve discrepancies.
The following information was extracted: name of the main author, year of publication, journal of publication, place of study, inclusion and exclusion criteria, patient population, time of initiation of intervention use, CytoSorb® dose used, mean age, number of patients, general mortality rate and predicted mortality for the groups. Additionally, data on preand posttreatment changes in inflammatory markers and vasopressor levels were collected, if available.
Two authors performed the risk of bias assessment. We used the risk of bias tool (ROB) for the RCTs(16) and the Review Manager 5.4 program (Review Manager; The Nordic Cochrane Centre, Copenhagen, Denmark). The risk of bias assessment tool for nonrandomized interventions (ROBINS I)(17) was used for the cohort studies, as recommended by the Cochrane collaboration. Importantly, ROBINS-I bias assessments were made based on the comparison between a given study and a theoretical randomized controlled trial with an ideal design for the study question, which represented the standard for a “low risk study” (Tables 2 and 3 - Supplementary Material)
Data synthesis and analysis
The outcomes were analyzed using the Mantel-Hansel statistical method and the Der Simonian-Laird random effects models, in relation to the high heterogeneity between the studies. The studies were not equivalent, they differed in the starting time, the duration of therapy, type of administration, and the source of sepsis, among other characteristics, which could have affected the results; therefore, a common effect size could not be assumed. Relative risks (RRs) for overall mortality, with 95% confidence intervals (95%CIs), were calculated for the conventional treatment and CytoSorb® treatment groups. Quantitative synthesis was not performed when only one study per outcome was identified or the studies were of a different type of design, or when the studies did not report the necessary statistics, which in that scenario were limited to a qualitative description.
The Review Manager 5.4 program was used for the analysis, and a p value < 0.05 was considered to indicate statistical significance.
Publication bias was not assessed due to the number of included studies.
RESULTS
Search results and study characteristics
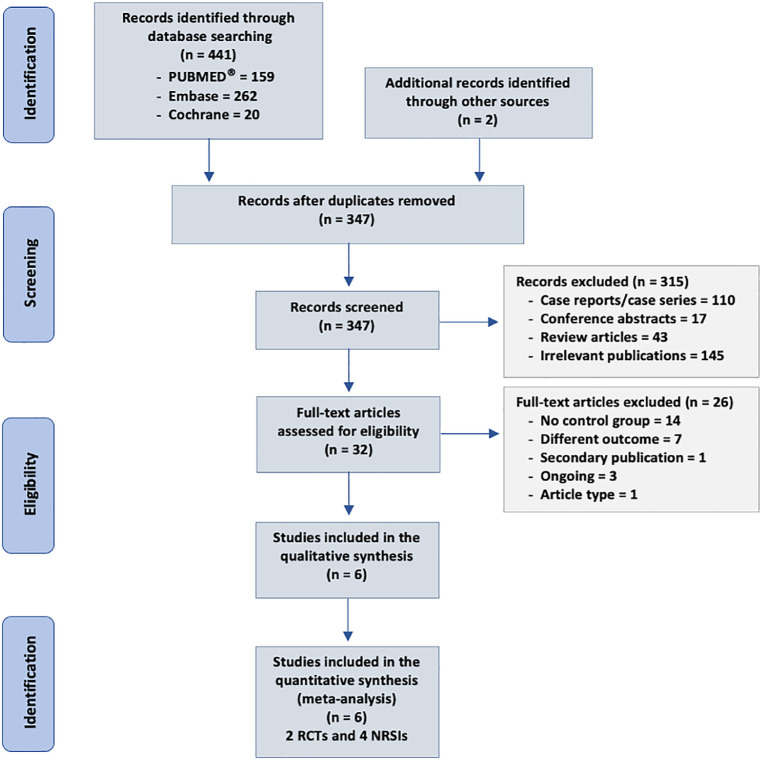
Our search strategy identified 443 citations, of which 32 were judged to be potentially eligible based on titles or abstracts, or both, and the full texts were obtained. We excluded 26 articles after reviewing the full text: 14 for not having a comparator group, 7 for having a different outcome or mixed population, 3 for reporting studies in progress, 1 for being a different type of article, and 1 for being a secondary publication. Finally, 6 studies were included (2 RCTs and 4 cohort studies) including 413 patients.(9-11,18-20)Figure 1 shows our flow chart of study selection.
Figure 1.
Flowchart of study selection.
RCT - randomized controlled trial; NRSI - nonrandomized studies of interventions.
Patients in the included studies had different causes of sepsis and septic shock. In addition, they differed in the mode, starting time and number of CytoSorb® treatment sessions. Table 1 summarizes the characteristics of the included studies.
Table 1.
Characteristics of the included studies
| Study/country | Study design | Specific population | Control group | Time of first CytoSorb® initiation | Duration of CytoSorb® therapy | Mortality in CytoSorb® group (%) |
|---|---|---|---|---|---|---|
| Schittek et al.,(9) Germany | Retrospective control group and prospective intervention group | Patients in severe septic shock with sepsis-associated acute kidney injury | Retrospective controls with septic shock (rising noradrenaline dose above 20µg/minute) with sepsis associated acute kidney injury in CVVHDF | No information | No information overall. Survivors, approximately one cartridge per patient was utilized as the median (IQR 1 - 2) for 35.5 hours (17 - 47) | 76.70 |
| Hawchar et al.,(10), Hungary | RCT | Septic shock | Patients with septic shock of medical origin, on mechanical ventilation, norepinephrine > 10µg/minute, procalcitonin > 3ng/mL without the need for renal replacement therapy | Started within 24 hours after ICU admission | 24 hours | 0 |
| Rugg et al.,(11) Austria | Propensity-score-weighted retrospective study | Primary or secondary sepsis | Matched controls were treated for septic shock and required RRT but did not receive CytoSorb® therapy. A generalized propensity score and Mahalanobis distance matching method (‘genetic’ matching) was applied | Initiation of CytoSorb therapy varied from 0.5 to 719 hours after ICU admission, but most patients received treatment within the first days | 1 - 6 x 24 hours without interruption | 21.40 |
| Akil et al.,(18) Germany | Cohort historic control | Pneumogenic sepsis and ECMO | Historical cohort. Patients with pneumogenic septic shock accompanying acute respiratory failure, invasive hemodynamic monitoring, and demand for norepinephrine 0.3µg/minute; elevated lactate concentrations 2.0mmol/L; and procalcitonin serum level 1ng/mL were eligible | Within 6 hours after admission to our ICU | Minimum 2 x 24 hours without interruption | 0 |
| Brouwer et al.,(19) The Netherlands | Propensity-score weighted retrospective study | Septic shock | Patients with septic shock treated with CRRT without CytoSorb®. Stabilized inverse probability treatment weight was applied | CytoSorb® was initiated at the discretion of the treating intensive care physician | 24 hours, mean duration of 2.34 ± 0.16 days | 52.20 |
| Schädler et al.,(20) Germany | RCT | Severe sepsis or septic shock within 72 hours of ARDS or acute lung injury | Mechanically ventilated patients with severe sepsis or septic shock in the setting of acute lung injury or acute respiratory distress syndrome established within the last 72 hours | Enrollment within 72 hours of diagnosis of sepsis with ARDS/ALI | Maximum 7 x 6 hours 24 hours apart | 36 |
CVVHDF - continuous venovenous hemodiafiltration; IQR - interquartile range; RCT - randomized controlled trial; ICU - intensive care unit; RRT - renal replacement therapy; ECMO - extracorporeal membrane oxygenation; CRRT - continuous renal replacement therapy; ARDS - acute respiratory distress syndrome; ALI - acute lung injury.
Risk of bias in the included studies
Randomized clinical trials presented a high risk of bias; in none of them was it possible to blind the intervention for the outcome assessors. Cohort studies presented a moderate to severe risk. The risk of bias assessment is shown in figure 2.
Figure 2.
Risk of bias assessment of eligible studies. (A) Randomized controlled trial; (B) Nonrandomized studies of interventions.
Effect on mortality at 28 - 30 days
Overall mortality was 45% (42% intervention group and 48% control group),(9-11,18-20) and only one study showed mortality greater than 70%.(9) A quantitative review was carried out, finding no significant effect on mortality at 28 - 30 days RR 0.98 [0.12 - 8.25] for the RCT and RR 0.74 [0.49 - 1.13] for NRSI. The results are shown in figure 3, and a summary of the findings is shown in figure 4.
Figure 3.
Forest plot effect of CytoSorb® on mortality at 28 - 30 days. (A) Randomized controlled trial; (B) Nonrandomized studies of interventions.
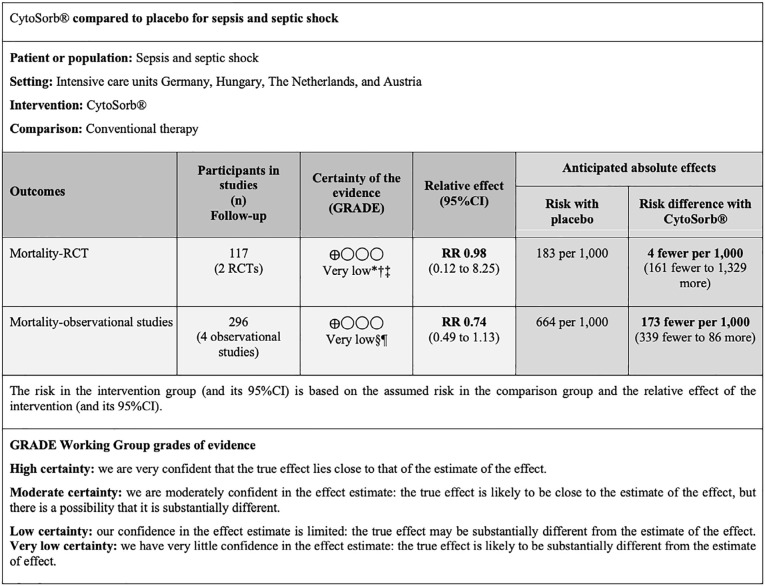
Figure 4.
Summary of findings for the main comparison.
* It was decided to decrease two levels due to the high risk of bias; † it was decided to decrease one level due to the different results; ‡ it was decided to decrease two levels due to the wide confidence interval; § it was decided to decrease two levels due to the different results; ¶ it was decided to decrease two levels due to the wide confidence interval. GRADE - Grading of Recommendations Assessment, Development and Evaluation; 95%CI - 95% confidence interval; RCT - randomized controlled trial; RR - risk ratio.
Effect on the use of vasopressors
Two studies, one RCT(10) and one cohort study,(18) reported the use of vasopressors as an outcome; both reported a significant decrease in vasopressor levels in the intervention group; however, this reduction was also shown in the control group (Table 2).
Table 2.
Effect of CytoSorb® on secondary outcomes
| Control group | CytoSorb® group | |||||
|---|---|---|---|---|---|---|
| Norepinephrine levels (ug/kg/min) | ||||||
| Study | NE T0 | NE 48 hours | p value | NE T0 | NE 48 hours | p value |
| Hawchar et al.(10) | 0.43 [0.19 - 0.64] | 0.25 [0.08 - 0.65] | NR | 0.54 [0.20 - 1.22] | 0.16 [0.07 - 0.48] | 0.016 |
| Akil et al.(18) | 0.83 ± 0.16 | 0.38 ± 0.11 | 0.05 | 0.603 ± 0.08 | 0.009 ± 0.005 | 0.0001 |
| C-reactive protein levels (mg/L) | ||||||
| Study | CRP T0 | CRP 48 hours | p value | PCR T0 | PCR 48 hours | p value |
| Hawchar et al.(10) | 307.4 ± 116.7 | 189.9 ± 48.5 | NS | 238.1 ± 95.5 | 169.54 ± 86.4 | NS |
| Akil et al.(18) | 27.2 ± 2.9 | 22.6 ± 3.1 | 0.31 | 35 ± 5 | 12 ± 3 | 0.002 |
| Procalcitonin levels (ng/mL) | ||||||
| Study | PCT T0 | PCT 48 hours | p value | PCT T0 | PCT 48 hours | p value |
| Hawchar et al.(10) | 13.2 [7.6 - 47.8] | 9.2 [3.8 - 44.2] | NR | 20.6 [6.5 - 144.5] | 5.6 [1.9 - 54.4] | 0.004 |
| Akil et al.(18) | 13.14 ± 9.7 | 8.14 ± 5.9 | 0.68 | 15.6 ± 5.4 | 2.71 ± 1.5 | 0.03 |
| Predicted versus observed mortality | ||||||
| Study | Observed mortality | Predicted mortality | p value | Observed mortality | Predicted mortality | p value |
| Brouwer et al.(19) | 51% | 67,9% | 0,035 | 47,8% | 74,5% | < 0,001 |
| Rugg et al.(11) | 47,6% | 65,7% | NR | 21,4% | 85,7% | NR |
NE - norepinephrine; CRP - C-reactive protein; PCT - procalcitonin.
Effect on levels of inflammatory markers
Only one study reported a 5 - 18% decrease in interleukin-6 (IL-6) levels;(20) however, no statistical significance was found.
Two studies, one RCT(10) and one cohort study,(18) reported results for C-reactive protein (CRP). In the RCT, CRP levels did not show a significant difference; however, in the other study, a significant difference was found in the CytoSorb® group. These results are shown in table 2.
Regarding the PCT (procalcitonin) levels, two studies, one RCT(10) and one cohort study,(18) reported a significant decrease in PCT levels compared to the baseline level; nevertheless, this significant reduction was not found in the control group. These results are shown in table 2.
Effect on predicted versus observed mortality
Two NRSI-type(11,19) studies reported a decrease in observed mortality overpredicted mortality. Brouwer et al.(19) 75% versus 52.2%, and Rugg et al.(11) 85.1% versus 21.4%. In both studies, predicted mortality was calculated by the Sequential Sepsis-related Organ Failure Assessment (SOFA) score; nevertheless, a reduction in predicted versus observed mortality was also found in the control group. Table 2 summarizes the findings.
Effect on length of stay in the intensive care unit
Five studies reported the effect on length of stay in the intensive care unit (ICU);(9-11,18,19) however, only two of them(10,18) (one RCT and one NRSI) reported standard deviation, so a quantitative synthesis was not performed. Only one NRSI(18) found significant differences that favored the use of CytoSorb®.
Effect on adverse events
Three studies reported adverse events,(10,18,20) two reported no adverse effects,(10,18) and the other reported one serious adverse event,(20) namely a decreased platelet count, which was identified as probably related to the use of CytoSorb®. In this same study, 3 treatment discontinuations were reported in 3 patients during the study due to adverse events likely related to therapy.
DISCUSSION
To our knowledge, this is the first meta-analysis evaluating the use of CytoSorb®, a hemadsorption device, in the setting of sepsis and septic shock, including clinical trials and cohort studies. Our study did not demonstrate a benefit of the use of CytoSorb® on mortality; however, it should be noted that the studies were heterogeneous, that the evidence for the RCTs was of high risk of bias, and that for the NRSIs, it was of moderate-to-severe risk of bias. Therefore, future research, of higher quality, could change or modify the direction of the effect.
Moderate heterogeneity was found in the RCTs (I2 = 57%), and high heterogeneity was found among the NRSIs (I2 = 70%), which could be explained by the different etiologies of sepsis, the severity of the disease, different kinds of interventions such as ECMO or continuous renal replacement therapy (CRRT) and the mode of use of the therapy. This heterogeneity makes it difficult to interpret a meta-analysis of these studies.
Two previous meta-analyses have evaluated the use of extracorporeal blood purification in sepsis;(21,22) unlike our study, they did not focus on the use of CytoSorb® hemadsorption and did not include cohort studies; however, they included the same RCT,(10,20) finding similar results. There is a recently published meta-analysis that evaluated the use of CytoSorb® in critically ill patients. This study found low-certainty evidence showing that the use of CytoSorb® might increase mortality; however, it did not find differences in adverse events.(23)
We found a significant decrease in the use of vasopressors in two studies,(10,18) but we did not carry out a quantitative synthesis because they were of a different type. These findings are consistent with multiple quasiexperimental before-and-after studies that indicated that the use of CytoSorb® therapy resulted in decreased doses of vasopressors, hemodynamic stabilization, and improvement in metabolic parameters.(2,24-27) Some studies reported that early use (within the first 24 - 48 hours), filtered blood volume, and prolonged duration of CytoSorb® therapy were associated with lower mortality;(2,20,26,28-30) unfortunately, not all studies reported these variables.
Regarding the levels of inflammatory markers, it has been reported that CytoSorb® is effective in vitro for the elimination of both inflammatory and proinflammatory cytokines, as well as for a decrease in CRP and procalcitonin levels,(3,31) and that the levels of cytokines correlate with both the severity of the disease and mortality.(32,33) In this revision, only one study reported a nonsignificant decrease in IL-6,(20) and two studies reported a decrease in the levels of CRP and procalcitonin;(10,18) these findings coincide with before-and-after studies, not included in this review, where CytoSorb® was shown to reduce the levels of inflammatory markers.(26,27,34,35)
The predicted mortality based on the SOFA score was calculated in two studies; unfortunately, these findings could not be meta-analyzed due to a lack of data in one of the studies.(11) A reduction between the observed versus predicted mortality was found in both studies. These findings are similar to those reported in other studies not included.(30,36)
CytoSorb® is considered to be a biocompatible and hemocompatible device,(37) and studies in cardiac surgery and sepsis suggest that CytoSorb® does not induce coagulopathy, hemolysis, or clinically relevant side effects,(26,38,39) which seems to coincide with the findings of the present review, where only one serious adverse event related to therapy was reported. However, it is worth mentioning that the use of CytoSorb® in the setting of sepsis is generally longer and that CytoSorb® may influence the elimination of or decrease in serum concentrations of some drugs; most of the time, CytoSorb® application requires interventions including extracorporeal membrane oxygenation (ECMO) and renal replacement therapy to be carried out. Therefore, as in other studies, we suspect that the adverse effects were underreported and not systematically evaluated.(23)
An important limitation is that meta-analysis was only conducted for the main outcome, and we did not perform it for secondary outcomes due to the small number of studies found or the lack of data. Studies were at moderate-to-high risk of bias, mainly due to confounding and study participant selection bias. It should be noted that the number of studies evaluating the use of CytoSorb® in sepsis and septic shock is limited.
Our review has other limitations. First, we did not include unpublished studies, conduct a search of the gray literature, or include conference abstracts or nonoriginal articles. Second, studies without a control group were not included since our main objective was to assess mortality. Third, the starting time, the duration of therapy, the volume of blood filtered, and the number of cartridges used were different or were not described in some studies, which could have affected the results. Fourth, only one study did not use renal replacement therapy (RRT), and two of them used it according to the patient’s needs, as it is known that acute renal failure can amplify the septic cascade induced by endotoxins, so the use of RRT could have affected the result. Fifth, our study focused only on short-term mortality.
CONCLUSION
Our study found very low certainty evidence that shows no benefit of CytoSorb® use in terms of mortality at 28 - 30 days. We cannot recommend the use of CytoSorb® in septic or septic shock patients outside clinical trials. Further high-quality randomized trials with a common intervention arm are needed to evaluate the influence of CytoSorb® in this population.
REFERENCES
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kogelmann K, Jarczak D, Scheller M, Drüner M. Hemoadsorption by CytoSorb in septic patients: a case series. Crit Care. 2017;21(1):74. doi: 10.1186/s13054-017-1662-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gruda MC, Ruggeberg KG, O’Sullivan P, Guliashvili T, Scheirer AR, Golobish TD, et al. Broad adsorption of sepsis-related PAMP and DAMP molecules, mycotoxins, and cytokines from whole blood using CytoSorb® sorbent porous polymer beads. PLoS One. 2018;13(1):e0191676. doi: 10.1371/journal.pone.0191676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ito T. PAMPs and DAMPs as triggers for DIC. J Intensive Care. 2014;2(1):67. doi: 10.1186/s40560-014-0065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit Care Med. 2021;49(11):e1063–143. doi: 10.1097/CCM.0000000000005337. [DOI] [PubMed] [Google Scholar]
- 6.Tian HC, Zhou JF, Weng L, Hu XY, Peng JM, Wang CY, Jiang W, Du XP, Xi XM, An YZ, Duan ML, Du B, for China Critical Care Clinical Trials Group (CCCCTG) Epidemiology of Sepsis-3 in a sub-district of Beijing: secondary analysis of a population-based database. Chin Med J (Engl) 2019;132(17):2039–2045. doi: 10.1097/CM9.0000000000000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiu C, Legrand M. Epidemiology of sepsis and septic shock. Curr Opin Anaesthesiol. 2021;34(2):71–76. doi: 10.1097/ACO.0000000000000958. [DOI] [PubMed] [Google Scholar]
- 8.Ankawi G, Xie Y, Yang B, Xie Y, Xie P, Ronco C. What have we learned about the use of Cytosorb adsorption columns? Blood Purif. 2019;48(3):196–202. doi: 10.1159/000500013. [DOI] [PubMed] [Google Scholar]
- 9.Schittek GA, Zoidl P, Eichinger M, Orlob S, Simonis H, Rief M, et al. Adsorption therapy in critically ill with septic shock and acute kidney injury: a retrospective and prospective cohort study. Ann Intensive Care. 2020;10(1):154. doi: 10.1186/s13613-020-00772-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hawchar F, László I, Öveges N, Trásy D, Ondrik Z, Molnar Z. Extracorporeal cytokine adsorption in septic shock: a proof of concept randomized, controlled pilot study. J Crit Care. 2019;49:172–178. doi: 10.1016/j.jcrc.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Rugg C, Klose R, Hornung R, Innerhofer N, Bachler M, Schmid S, et al. Hemoadsorption with CytoSorb in septic shock reduces catecholamine requirements and in-hospital mortality: a single-center retrospective ‘Genetic’ Matched Analysis. Biomedicines. 2020;8(12):539. doi: 10.3390/biomedicines8120539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seeliger B, Stahl K, David S. [Extracorporeal techniques for blood purification in sepsis: an update] Internist (Berl) 2020;61(10):1010–1016. doi: 10.1007/s00108-020-00862-5. German. [DOI] [PubMed] [Google Scholar]
- 13.Houschyar KS, Pyles MN, Rein S, Nietzschmann I, Duscher D, Maan ZN, et al. Continuous hemoadsorption with a cytokine adsorber during sepsis - a review of the literature. Int J Artif Organs. 2017;40(5):205–211. doi: 10.5301/ijao.5000591. [DOI] [PubMed] [Google Scholar]
- 14.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, PRISMA-P Group Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 15.Schiavo JH. PROSPERO: An International Register of Systematic Review Protocols. Med Ref Serv Q. 2019;38(2):171–180. doi: 10.1080/02763869.2019.1588072. [DOI] [PubMed] [Google Scholar]
- 16.Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 17.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akil A, Ziegeler S, Reichelt J, Rehers S, Abdalla O, Semik M, et al. Combined use of CytoSorb and ECMO in patients with severe pneumogenic sepsis. Thorac Cardiovasc Surg. 2021;69(3):246–251. doi: 10.1055/s-0040-1708479. [DOI] [PubMed] [Google Scholar]
- 19.Brouwer WP, Duran S, Kuijper M, Ince C. Hemoadsorption with CytoSorb shows a decreased observed versus expected 28-day all-cause mortality in ICU patients with septic shock: a propensity-score-weighted retrospective study. Crit Care. 2019;23(1):317. doi: 10.1186/s13054-019-2588-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schädler D, Pausch C, Heise D, Meier-Hellmann A, Brederlau J, Weiler N, et al. The effect of a novel extracorporeal cytokine hemoadsorption device on IL-6 elimination in septic patients: A randomized controlled trial. PLoS One. 2017;12(10):e0187015. doi: 10.1371/journal.pone.0187015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Putzu A, Schorer R, Lopez-Delgado JC, Cassina T, Landoni G. Blood purification and mortality in sepsis and septic shock: a systematic review and meta-analysis of randomized trials. Anesthesiology. 2019;131(3):580–593. doi: 10.1097/ALN.0000000000002820. [DOI] [PubMed] [Google Scholar]
- 22.Snow TAC, Littlewood S, Corredor C, Singer M, Arulkumaran N. Effect of extracorporeal blood purification on mortality in sepsis: a meta-analysis and trial sequential analysis. Blood Purif. 2021;50(4-5):462–472. doi: 10.1159/000510982. [DOI] [PubMed] [Google Scholar]
- 23.Heymann M, Schorer R, Putzu A. Mortality and adverse events of hemoadsorption with CytoSorb® in critically ill patients: a systematic review and meta-analysis of randomized controlled trials. Acta Anaesthesiol Scand. 2022;66(9):1037–1050. doi: 10.1111/aas.14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calabrò MG, Febres D, Recca G, Lembo R, Fominskiy E, Scandroglio AM, et al. Blood purification with CytoSorb in critically ill patients: single-center preliminary experience. Artif Organs. 2019;43(2):189–194. doi: 10.1111/aor.13327. [DOI] [PubMed] [Google Scholar]
- 25.Friesecke S, Stecher SS, Gross S, Felix SB, Nierhaus A. Extracorporeal cytokine elimination as rescue therapy in refractory septic shock: a prospective single-center study. J Artif Organs. 2017;20(3):252–259. doi: 10.1007/s10047-017-0967-4. [DOI] [PubMed] [Google Scholar]
- 26.Mehta Y, Mehta C, Kumar A, George JV, Gupta A, Nanda S, et al. Experience with hemoadsorption (CytoSorb®) in the management of septic shock patients. World J Crit Care Med. 2020;9(1):1–12. doi: 10.5492/wjccm.v9.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hakemi MS, Nassiri AA, Nobakht A, Mardani M, Darazam IA, Parsa M, et al. Benefit of hemoadsorption therapy in patients suffering sepsis-associated acute kidney injury: a case series. Blood Purif. 2022;51(10):823–830. doi: 10.1159/000521228. [DOI] [PubMed] [Google Scholar]
- 28.Kogelmann K, Hübner T, Schwameis F, Drüner M, Scheller M, Jarczak D. First Evaluation of a new dynamic scoring system intended to support prescription of adjuvant CytoSorb hemoadsorption therapy in patients with septic shock. J Clin Med. 2021;10(13):2939. doi: 10.3390/jcm10132939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berlot G, Samola V, Barbaresco I, Tomasini A, di Maso V, Bianco F, et al. Effects of the timing and intensity of treatment on septic shock patients treated with CytoSorb®: clinical experience. Int J Artif Organs. 2022;45(3):249–253. doi: 10.1177/03913988211073812. [DOI] [PubMed] [Google Scholar]
- 30.Schultz P, Schwier E, Eickmeyer C, Henzler D, Köhler T. High-dose CytoSorb hemoadsorption is associated with improved survival in patients with septic shock: a retrospective cohort study. J Crit Care. 2021;64:184–192. doi: 10.1016/j.jcrc.2021.04.011. [DOI] [PubMed] [Google Scholar]
- 31.Malard B, Lambert C, Kellum JA. In vitro comparison of the adsorption of inflammatory mediators by blood purification devices. Intensive Care Med Exp. 2018;6(1):12. doi: 10.1186/s40635-018-0177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murugan R, Wen X, Shah N, Lee M, Kong L, Pike F, Keener C, Unruh M, Finkel K, Vijayan A, Palevsky PM, Paganini E, Carter M, Elder M, Kellum JA, Biological Markers for Recovery of Kidney (BioMaRK) Study Investigators Plasma inflammatory and apoptosis markers are associated with dialysis dependence and death among critically ill patients receiving renal replacement therapy. Nephrol Dial Transplant. 2014;29(10):1854–1864. doi: 10.1093/ndt/gfu051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frencken JF, van Vught LA, Peelen LM, Ong DS, Klein Klouwenberg PM, Horn J, Bonten MJ, van der Poll T, Cremer OL, MARS Consortium An unbalanced inflammatory cytokine response is not associated with mortality following sepsis: a prospective cohort study. Crit Care Med. 2017;45(5):e493–9. doi: 10.1097/CCM.0000000000002292. [DOI] [PubMed] [Google Scholar]
- 34.Kaya Uğur B, Çiçek H, Kul S, Mete Ö, Yılmaz M. Effect of a novel extracorporeal cytokine apheresis method on endocan, copeptin and interleukin-6 levels in sepsis: an observational prospective study. Transfus Apher Sci. 2020;59(6):102919. doi: 10.1016/j.transci.2020.102919. [DOI] [PubMed] [Google Scholar]
- 35.Singh YP, Chhabra SC, Lashkari K, Taneja A, Garg A, Chandra A, et al. Hemoadsorption by extracorporeal cytokine adsorption therapy (CytoSorb®) in the management of septic shock: a retrospective observational study. Int J Artif Organs. 2020;43(6):372–378. doi: 10.1177/0391398819891739. [DOI] [PubMed] [Google Scholar]
- 36.Paul R, Sathe P, Kumar S, Prasad S, Aleem M, Sakhalvalkar P. Multicentered prospective investigator initiated study to evaluate the clinical outcomes with extracorporeal cytokine adsorption device (CytoSorb®) in patients with sepsis and septic shock. World J Crit Care Med. 2021;10(1):22–34. doi: 10.5492/wjccm.v10.i1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Köhler T, Schwier E, Praxenthaler J, Kirchner C, Henzler D, Eickmeyer C. Therapeutic modulation of the host defense by hemoadsorption with CytoSorb®-Basics, indications and perspectives-A scoping review. Int J Mol Sci. 2021;22(23):12786. doi: 10.3390/ijms222312786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Träger K, Skrabal C, Fischer G, Datzmann T, Schroeder J, Fritzler D, et al. Hemoadsorption treatment of patients with acute infective endocarditis during surgery with cardiopulmonary bypass - a case series. Int J Artif Organs. 2017;40(5):240–249. doi: 10.5301/ijao.5000583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wisgrill L, Lamm C, Hell L, Thaler J, Berger A, Weiss R, et al. Influence of hemoadsorption during cardiopulmonary bypass on blood vesicle count and function. J Transl Med. 2020;18(1):202. doi: 10.1186/s12967-020-02369-x. [DOI] [PMC free article] [PubMed] [Google Scholar]