Abstract
Objective
To assess the outcome of extubation in COVID-19 patients and the use of noninvasive ventilation in the weaning process.
Methods
This retrospective, observational, single-center study was conducted in COVID-19 patients aged 18 years or older who were admitted to an intensive care unit between April 2020 and December 2021, placed under mechanical ventilation for more than 48 hours and progressed to weaning. Early extubation was defined as extubation without a spontaneous breathing trial and immediate use of noninvasive ventilation after extubation. In patients who underwent a spontaneous breathing trial, noninvasive ventilation could be used as prophylactic ventilatory assistance when started immediately after extubation (prophylactic noninvasive ventilation) or as rescue therapy in cases of postextubation respiratory failure (therapeutic noninvasive ventilation). The primary outcome was extubation failure during the intensive care unit stay.
Results
Three hundred eighty-four extubated patients were included. Extubation failure was observed in 107 (27.9%) patients. Forty-seven (12.2%) patients received prophylactic noninvasive ventilation. In 26 (6.8%) patients, early extubation was performed with immediate use of noninvasive ventilation. Noninvasive ventilation for the management of postextubation respiratory failure was administered to 64 (16.7%) patients.
Conclusion
We found that COVID-19 patients had a high rate of extubation failure. Despite the high risk of extubation failure, we observed low use of prophylactic noninvasive ventilation in these patients.
Keywords: COVID-19, Coronavirus infections, SARS-CoV-2, Respiration, artificial, Ventilator weaning, Noninvasive ventilation, Airway extubation
INTRODUCTION
A large proportion of coronavirus disease 2019 (COVID-19) patients progress to a more severe form of the disease and require hospitalization in an intensive care unit (ICU).(1,2) Most of these patients require mechanical ventilation (MV) and have a protracted clinical course marked by difficulty in ventilator liberation.(3,4) Data on successfully weaning COVID-19 patients from MV are limited.
The decision to extubate a patient can be quite difficult. Very early extubation can increase the risk of reintubation, prolong the ICU length of stay and increase mortality.(5,6) On the other hand, an unnecessary delay in extubation can also lead to complications associated with a longer MV duration and inefficient use of intensive care resources.(7,8)
The use of noninvasive ventilation (NIV) during weaning has been extensively investigated in non-COVID-19 patients. Early extubation followed by immediate NIV, prophylactic NIV after extubating a patient who tolerated a spontaneous breathing trial (SBT), and NIV as rescue therapy for postextubation respiratory failure are applied during weaning from MV.(9-11) To date, there have been very few investigations on the role of NIV in weaning COVID-19 patients from MV.
This study aimed to assess the outcome of extubation in COVID-19 patients and the use of NIV in the weaning process.
METHODS
This retrospective, observational, single-center study was conducted in the ICU of Hospital Nossa Senhora da Conceição, located in Porto Alegre, Brazil, from April 2020 to December 2021. During the COVID-19 pandemic, the hospital increased the number of ICU beds and allocated 50 ICU beds exclusively for COVID-19 patients at the peak of the pandemic. This study was approved by the Research Ethics Committee of the hospital (no. 4164341). Due to the retrospective nature of the study, the need for informed consent was waived.
Patients aged 18 years or older who were admitted to the ICU with COVID-19 confirmed by reverse transcriptase polymerase chain reaction (RT‒PCR) or antigen testing for SARS-CoV-2, placed under MV for a period of at least 48 hours, and progressed to weaning were included. Patients who were self-extubated or accidentally extubated, who underwent tracheostomy before an extubation attempt, or who died before weaning were excluded.
The criteria to start the weaning process were as follows: improvement or resolution of the patient’s condition by MV; body temperature below 38.5°C; hemoglobin ≥ 8g/dL; no or minimal doses of vasoactive drugs and sedatives; arterial oxygen pressure (PaO2) > 60mmHg or peripheral oxygen saturation (SpO2) > 90%; fraction of inspired oxygen (FiO2) < 0.4; and positive end-expiratory pressure (PEEP) ≤ 8cmH2O. Patients who tolerated pressure support ventilation (PSV) mode with a PEEP of 5 - 8cmH2O and pressure above PEEP of 7 - 14cmH2O underwent an SBT, which was performed in PSV mode with pressure over PEEP ≤ 8cmH2O and PEEP ≤ 5cmH2O or with a T-piece for 30 minutes. The criteria for SBT intolerance were agitation, anxiety, low level of consciousness (Glasgow coma scale score < 13), respiratory rate > 35/minute and/or use of accessory muscles, SpO2 < 90%, heart rate > 140 beats/minute or > 20% of the baseline, systolic blood pressure < 90mmHg, or the development of arrhythmia. Patients who tolerated the SBT were extubated. For patients who failed the SBT, the assisted ventilation mode was reapplied, and a new SBT was performed after 24 hours.
The primary outcome was extubation failure, which was defined as the need for reintubation during the ICU stay. Secondary outcomes were extubation failure within 48 hours and 96 hours of extubation, ICU mortality, and in-hospital mortality.
Early extubation was defined as extubation without an SBT and immediate use of NIV after extubation.(9) NIV applied in this way has been proposed as an alternative to invasive MV in patients who are not yet ready to be extubated (i.e., NIV to facilitate weaning).(9) These two criteria were necessary to define early extubation, i.e., not performing an SBT and using NIV immediately after extubation. These patients had a pressure over PEEP ≤ 14cmH2O (criterion to initiate weaning) and > 8cmH2O (≤ 8cmH2O was considered to indicate an SBT). In the patients who underwent an SBT, NIV could be used as prophylactic ventilatory assistance when started immediately after extubation (prophylactic NIV) or as rescue therapy in cases of postextubation respiratory failure (therapeutic NIV).
The clinical and demographic data collected were as follows: age; sex; Simplified Acute Physiology Score 3 (SAPS 3); use of NIV as preintubation support; preintubation PaO2/FiO2; ventilatory parameters on the first day of MV (PEEP and plateau pressure); use of NIV (prophylactic, therapeutic, or associated with early extubation) after extubation; fluid balance in the 24 hours before extubation; weaning time (time between the first SBT and extubation); duration of invasive MV; and ICU or hospital mortality.
Continuous variables are described as means and standard deviations or medians and interquartile ranges, and categorical variables are described as absolute numbers and percentages. Student’s t or Wilcoxon Mann-Whitney tests were used for continuous variables, and Fisher’s exact test was used for categorical variables. A Cox proportional hazards model was constructed to evaluate whether prophylactic NIV was associated with extubation failure. Prophylactic NIV was maintained as a variable of interest in the model. Other variables defined post hoc were those plausibly associated with the primary outcome (age, fluid balance, SAPS 3, and duration of MV). The assumption of linearity of independent variables with log-odds was assessed by Box-Tidwell transformation. A logarithmic transformation was performed on nonlinear independent variables. The transformed variables were then included in the Cox proportional hazards model as independent variables. For all comparisons, a p value < 0.05 was considered statistically significant. Statistical analyses were performed using IBM Statistical Package for the Social Sciences (SPSS), version 20.0 (IBM Corp., Armonk, NY, USA) and R 3.6.2 (The R Foundation).
RESULTS
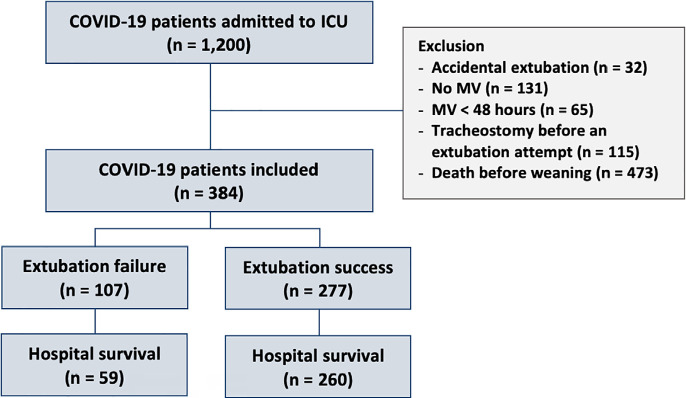
During the study period, 1200 patients with a confirmed diagnosis of COVID-19 were admitted to the ICU. Of these, 816 were excluded from the study. Thus, a total of 384 patients were included in the final analysis (Figure 1).
Figure 1.
Flowchart for inclusion of patients in the study.
MV - mechanical ventilation; ICU - intensive care unit.
Demographic characteristics and clinical parameters are described in table 1. One hundred thirty-seven (35.7%) patients received noninvasive support (prophylactic, therapeutic, or associated with early extubation) after extubation. Extubation failure was observed in 107 (27.9%) patients. The failure rate was 19.5% and 24.0% at 48 and 96 hours after extubation, respectively. The ICU mortality rate was 39.4% in patients who required reintubation during their ICU stay. Intensive care unit mortality did not differ between patients requiring reintubation within less than 48 hours (34.2%) or after more than 48 hours (47.2%) (p = 0.214).
Table 1.
Characteristics of mechanically ventilated COVID-19 patients
| Patients who received noninvasive support after
extubation (n = 137) |
Patients who did not receive noninvasive support
after extubation (n = 247) |
p value | |
|---|---|---|---|
| Age (years) | 53.8 ± 13.1 | 53.9 ± 14.9 | 0.988 |
| Sex, male | 79 (57.7) | 133 (53.8) | 0.471 |
| SAPS 3 | 64.3 ± 13.2 | 61.2 ± 15.6 | 0.091 |
| PEEP in the first day (cmH2O) | 12.4 ± 3.4 | 11.2 ± 3.0 | < 0.001 |
| Plateau pressure in the first day (cmH2O) | 27.0 (24.0 - 30.0) | 26.0 (24.0 - 29.0) | 0.062 |
| PaO2/FiO2 preintubation | 84.0 (69.0 - 118.0) | 115.5 (82.0 - 209.0) | < 0.001 |
| NIV preintubation | 58 (42.3) | 60 (24.3) | < 0.001 |
| Fluid balance - last 24 hours (mL) | -4.0 (-544.0 - 900.0) | 0.0 (-666.3 - 499.3) | 0.412 |
| Duration of weaning (days) | 0.0 (0.0 - 0.0) | 0.0 (0.0 - 1.0) | 0.261 |
| Duration of MV (days) | 12.6 ± 7.0 | 10.0 ± 6.4 | < 0.001 |
| Reintubation at 48 hours | 34 (24.8) | 41 (16.6) | 0.052 |
| Reintubation at 96 hours | 44 (32.1) | 48 (19.4) | 0.005 |
| Reintubation in ICU | 56 (40.9) | 51 (20.6) | < 0.001 |
| ICU mortality | 23 (16.8) | 28 (11.3) | 0.132 |
| Hospital mortality | 30 (21.9) | 35 (14.2) | 0.053 |
SAPS 3 - Simplified Acute Physiology Score 3; PEEP - positive end-expiratory pressure; PaO2 - partial pressure of oxygen; FiO2 - fraction of inspired oxygen; NIV - noninvasive ventilation; MV - mechanical ventilation; ICU - intensive care unit. The results are expressed as the mean ± standard deviation, n (%) or median (interquartile range).
Regarding the use of NIV, 47 (12.2%) patients received prophylactic NIV. Of these, 16 (34.0%) experienced extubation failure. The extubation failure rate in the 337 patients who did not receive prophylactic NIV was 27.0% (n = 91; p = 0.304) (Figure 2). The Cox regression analysis showed that prophylactic NIV was not associated with extubation failure after multivariable adjustment (hazard ratio - HR 1.27; 95%CI 0.75 - 2.17; adjustment hazard ratio - aHR 0.57; 95%CI 0.16 - 1.98). In 26 (6.8%) patients, early extubation was performed with the immediate use of NIV. There was no difference in the mean duration of MV between the patients who underwent early extubation (11.6 ± 6.1 days) and patients who underwent an SBT (10.9 ± 6.8 days) (p = 0.586). Noninvasive ventilation for the management of postextubation respiratory failure was provided to 64 (16.7%) patients; 32 (50.0%) required reintubation.
Figure 2.
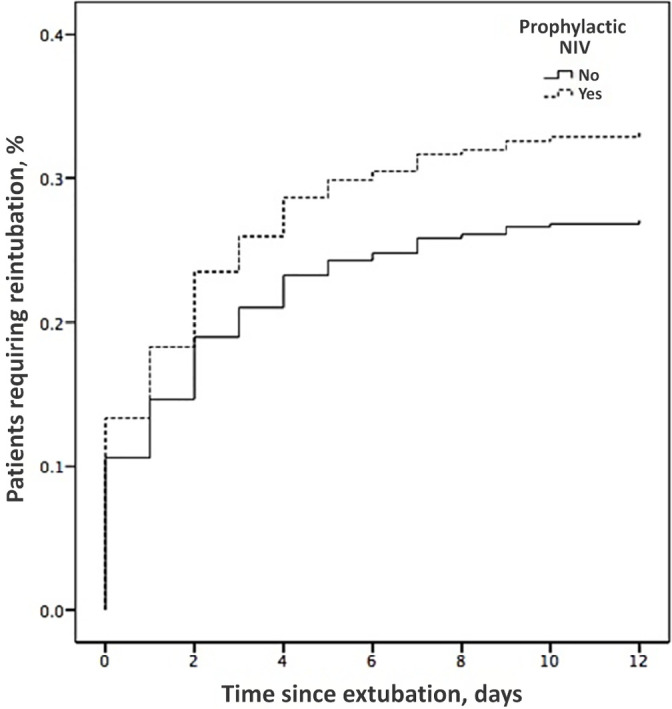
Kaplan-Meier analysis of the time from extubation to reintubation, including all patients and dividing according to prophylactic noninvasive ventilation use.
NIV - noninvasive ventilation.
DISCUSSION
We observed an extubation failure rate of 27.9% in patients with COVID-19. Interestingly, despite the high risk of extubation failure, we observed low use of prophylactic NIV in these patients.
The extubation failure rate found in our study was similar to the previously described rate described in COVID-19 patients when considering reintubation during the ICU stay as a criterion (22.1% to 33.1%).(12-14) Guzatti et al. found that the extubation failure rate was approximately three times higher during the ICU stay (22.1%) than during the 48-hour period (7.8%) after extubation and hypothesized that COVID-19 patients experience late extubation failure.(13) In our study, we did not find this trend of late extubation failure because 81% of the patients who required reintubation were reintubated within the first three days after extubation.
A longer MV duration is a characteristic that differs from non-COVID-19 patients and may be associated with a higher rate of extubation failure. The mean duration of MV in this study was 10.9 ± 6.7 days, which is similar to that in other studies of COVID-19 patients(12-14) and is more than double that in non-COVID-19 patients.(15)
Little is known about the use of NIV in weaning COVID-19 patients. The strategy of early extubation with immediate NIV was associated with a reduced invasive MV duration in a meta-analysis of studies of non-COVID-19 patients.(9) Thille et al. found a reduction in the extubation failure rate with prophylactic NIV use in non-COVID-19 patients at high risk of extubation failure,(10) and a recent network meta-analysis confirmed this finding.(16) Finally, NIV to treat postextubation respiratory failure in non-COVID-19 patients also reduced the need for reintubation in a recent randomized clinical trial.(11) There are limited data on these three modes of NIV use during weaning in COVID-19 patients. In an observational study, Cammarota et al. found that the strategy of early extubation followed by immediate NIV was chosen in 54.5% of patients and reduced both the duration of invasive MV and the need for reintubation.(12) In this study, prophylactic NIV was used in 60% of patients undergoing standard weaning, and 29% of these patients received NIV as a salvage treatment for postextubation respiratory failure. In our study, the strategy of early extubation followed by immediate NIV was chosen infrequently and was not associated with a shorter invasive MV duration. Furthermore, the rates of prophylactic NIV (12.2%) and NIV as rescue therapy for postextubation respiratory failure (16.7%) were much lower in our study. The low frequency of patients supported with NIV after extubation is probably related to the uncertainty of the benefits of this strategy in this group of patients. Only one study(12) evaluated the use of early extubation followed by immediate NIV, and no studies have evaluated the use of prophylactic NIV after extubation. It remains unclear whether prophylactic NIV after extubation should be used more frequently in COVID-19 patients. However, the rationale for increasing its use is based not only on the fact that these patients could be considered at high risk for extubation failure but also on the evidence of benefits from this strategy in high-risk non-COVID-19 patients.
This study has some limitations. First, the observational and retrospective design does not allow for the establishment of a cause‒effect association between the various factors evaluated and the outcome of extubation. Second, it is a single-center study with a small number of patients, which limits the generalizability of the results. Third, data for some variables, such as ICU-acquired weakness and need for aspiration, could not be collected. Finally, the experience gained over time and the availability of resources may have influenced the use of NIV.
CONCLUSION
We found that COVID-19 patients had a high rate of extubation failure. This highlights the need for careful monitoring of these patients after extubation and the importance of identifying and addressing risk factors for extubation failure in this population. Interestingly, despite the high risk of extubation failure, we observed low use of prophylactic noninvasive ventilation in these patients. Future studies are needed to investigate the reasons behind this underutilization and to determine whether prophylactic noninvasive ventilation can reduce the risk of extubation failure in patients with COVID-19.
REFERENCES
- 1.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Botta M, Tsonas AM, Pillay J, Boers LS, Algera AG, Bos LD, Dongelmans DA, Hollmann MW, Horn J, Vlaar AP, Schultz MJ, Neto AS, Paulus F, PRoVENT-COVID Collaborative Group Ventilation management and clinical outcomes in invasively ventilated patients with COVID-19 (PRoVENT-COVID): a national, multicentre, observational cohort study. Lancet Respir Med. 2021;9(2):139–148. doi: 10.1016/S2213-2600(20)30459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, et al. Covid-19 in critically ill patients in the Seattle Region - Case series. N Engl J Med. 2020;382(21):2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, Iotti G, Latronico N, Lorini L, Merler S, Natalini G, Piatti A, Ranieri MV, Scandroglio AM, Storti E, Cecconi M, Pesenti A, COVID-19 Lombardy ICU. Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thille AW, Richard JC, Brochard L. The decision to extubate in the intensive care unit. Am J Respir Crit Care Med. 2013;187(12):1294–1302. doi: 10.1164/rccm.201208-1523CI. [DOI] [PubMed] [Google Scholar]
- 6.Baptistella AR, Mantelli LM, Matte L, Carvalho ME, Fortunatti JA, Costa IZ, et al. Prediction of extubation outcome in mechanically ventilated patients: Development and validation of the Extubation Predictive Score (ExPreS) PLoS One. 2021;16(3):e0248868. doi: 10.1371/journal.pone.0248868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleuren LM, Dam TA, Tonutti M, de Bruin DP, Lalisang RC, Gommers D, Cremer OL, Bosman RJ, Rigter S, Wils EJ, Frenzel T, Dongelmans DA, de Jong R, Peters M, Kamps MJ, Ramnarain D, Nowitzky R, Nooteboom FG, de Ruijter W, Urlings-Strop LC, Smit EG, Mehagnoul-Schipper DJ, Dormans T, de Jager CP, Hendriks SH, Achterberg S, Oostdijk E, Reidinga AC, Festen-Spanjer B, Brunnekreef GB, Cornet AD, van den Tempel W, Boelens AD, Koetsier P, Lens J, Faber HJ, Karakus A, Entjes R, de Jong P, Rettig TC, Arbous S, Vonk SJ, Fornasa M, Machado T, Houwert T, Hovenkamp H, Noorduijn Londono R, Quintarelli D, Scholtemeijer MG, de Beer AA, Cinà G, Kantorik A, de Ruijter T, Herter WE, Beudel M, Girbes AR, Hoogendoorn M, Thoral PJ, Elbers PW, Dutch ICU. Data Sharing Against Covid-19 Collaborators. Predictors for extubation failure in COVID-19 patients using a machine learning approach. Crit Care. 2021;25(1):448. doi: 10.1186/s13054-021-03864-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013;369(22):2126–2136. doi: 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- 9.Vaschetto R, Pecere A, Perkins GD, Mistry D, Cammarota G, Longhini F, et al. Effects of early extubation followed by noninvasive ventilation versus standard extubation on the duration of invasive mechanical ventilation in hypoxemic non-hypercapnic patients: a systematic review and individual patient data meta-analysis of randomized controlled trials. Crit Care. 2021;25(1):189. doi: 10.1186/s13054-021-03595-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thille AW, Muller G, Gacouin A, Coudroy R, Decavèle M, Sonneville R, Beloncle F, Girault C, Dangers L, Lautrette A, Cabasson S, Rouzé A, Vivier E, Le Meur A, Ricard JD, Razazi K, Barberet G, Lebert C, Ehrmann S, Sabatier C, Bourenne J, Pradel G, Bailly P, Terzi N, Dellamonica J, Lacave G, Danin PÉ, Nanadoumgar H, Gibelin A, Zanre L, Deye N, Demoule A, Maamar A, Nay MA, Robert R, Ragot S, Frat JP, HIGH-WEAN Study Group and the REVA Research Network Effect of postextubation high-flow nasal oxygen with noninvasive ventilation vs high-flow nasal oxygen alone on reintubation among patients at high risk of extubation failure: a randomized clinical trial. JAMA. 2019;322(15):1465–1475. doi: 10.1001/jama.2019.14901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belenguer-Muncharaz A, Mateu-Campos ML, Vidal-Tegedor B, Ferrándiz-Sellés MD, Micó-Gómez ML, Altaba-Tena S, et al. Noninvasive ventilation versus conventional oxygen therapy after extubation failure in high-risk patients in an intensive care unit: a pragmatic clinical trial. Rev Bras Ter Intensiva. 2021;33(3):362–373. doi: 10.5935/0103-507X.20210059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cammarota G, Vaschetto R, Azzolina D, De Vita N, Olivieri C, Ronco C, et al. Early extubation with immediate non-invasive ventilation versus standard weaning in intubated patients for coronavirus disease 2019: a retrospective multicenter study. Sci Rep. 2021;11(1):13418. doi: 10.1038/s41598-021-92960-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guzatti NG, Klein F, Oliveira JA, Rático GB, Cordeiro MF, Marmitt LP, et al. Predictive factors of extubation failure in COVID-19 mechanically ventilated patients. J Intensive Care Med. 2022;37(9):1250–1255. doi: 10.1177/08850666221093946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ionescu F, Zimmer MS, Petrescu I, Castillo E, Bozyk P, Abbas A, et al. Extubation failure in critically ill COVID-19 patients: risk factors and impact on in-hospital mortality. J Intensive Care Med. 2021;36(9):1018–1024. doi: 10.1177/08850666211020281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaber S, Quintard H, Cinotti R, Asehnoune K, Arnal JM, Guitton C, et al. Risk factors and outcomes for airway failure versus non-airway failure in the intensive care unit: a multicenter observational study of 1514 extubation procedures. Crit Care. 2018;22(1):236. doi: 10.1186/s13054-018-2150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boscolo A, Pettenuzzo T, Sella N, Zatta M, Salvagno M, Tassone M, et al. Noninvasive respiratory support after extubation: a systematic review and network meta-analysis. Eur Respir Rev. 2023;32(168):220196. doi: 10.1183/16000617.0196-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]