Abstract
Mycoplasma hyopneumoniae causes enzootic pneumonia, a highly contagious respiratory disease in swine that causes significant economic losses worldwide. It is unknown whether the nucleotide oligomerization domain-like receptor (NLR) family pyrin domain containing 3 (NLRP3) inflammasome regulates the immune response in swine during M. hyopneumoniae infection. The current study utilized an in vivo swine model of M. hyopneumoniae infection to investigate the regulatory functional role of the NLRP3 inflammasome during M. hyopneumoniae infection. Notable histopathological alterations were observed in M. hyopneumoniae-infected swine tissues, which were associated with an inflammatory response and disease progression. Swine M. hyopneumoniae infection was associated with an increase in the expression of the NLRP3 inflammasome, which stimulated pro-inflammatory cytokines such as tumor necrosis factor-alpha, interleukin 18, and interleukin 1 beta (IL-1β). The impact of the NLRP3 inhibitor, MCC950 on NLRP3 and pro-inflammatory cytokines in M. hyopneumoniae-infected swine was examined to investigate the relationship between the NLRP3 inflammasome and M. hyopneumoniae infection. Taken together, our findings provide strong evidence that the NLRP3 inflammasome plays a critical regulatory functional role in M. hyopneumoniae infection in swine.
Keywords: Mycoplasma hyopneumoniae, NLR family pyrin domain containing 3 (NLRP3) inflammasome, pro-inflammatory cytokines, Swine
Our study uncovered the crucial regulatory role of the NLR family pyrin domain containing 3 (NLRP3) inflammasome in managing the invasion of respiratory mycoplasma in swine. The findings of our research suggest that the levels of NLRP3 expression can serve as a reliable diagnostic indicator for identifying Mycoplasma hyopneumoniae infection. Furthermore, inhibiting NLRP3 could offer therapeutic advantages in treating NLRP3-related disorders, such as porcine enzootic pneumonia.
Introduction
Mycoplasma hyopneumoniae is a type of respiratory pathogen that causes a chronic respiratory infectious disease in swine known as enzootic pneumonia. This disease is highly prevalent worldwide and can lead to significant financial losses for the swine industry (Maes et al., 2008; Tao et al., 2019; Yeske et al., 2020) due to decreased performance and increased medication use (Thacker, 2004). The economic impact of M. hyopneumoniae is significant, with losses in China alone exceeding 1.4 billion US dollars per year (Zhang et al., 2019). Enzootic pneumonia in swine has a slower spread than other respiratory diseases but has a high progression rate over several months, resulting in high morbidity rates, with some herds experiencing morbidity levels of up to 70% to 100% (Sibila et al., 2009; Yu et al., 2018). Common symptoms of swine infected with M. hyopneumoniae include dry coughing, as well as a reduction in feed conversion and a decrease in productivity, highlighting the significant economic impact (Thacker et al., 1999; Ni et al., 2019). Mycoplasma hyopneumoniae invades the swine airway and attaches to the respiratory epithelium using virulent adhesion factors such as P97, P102, and P146 (Sibila et al., 2009). These adhesion factors have been found to trigger the production of reactive oxygen species and potentially harmful metabolites and induce apoptosis through lipid-associated transmembrane proteins (Li et al., 2019).
Several previous studies conducted in murine models have shown that the nucleotide oligomerization domain-like receptor (NLR) family pyrin domain containing 3 (NLRP3) inflammasome regulates the inflammatory response and affects the progression of M. hyopneumoniae infection (McNeela et al., 2010; Segovia et al., 2018). Tang et al. (2019) emphasized the critical role of NLRP3 inflammasome activation in the development of several chronic diseases, including porcine enzootic pneumonia. Upregulation of NLRP3 inflammasome expression during M. hyopneumoniae infection leads to the release of pro-inflammatory cytokines, such as TNF-α, IL-18, and IL-1β (Segovia et al., 2018). NLRP3 knockout mice exhibited lower IL-1β cytokine release and bacterial clearance than infected wild-type mice (Segovia et al., 2018). The mRNA and protein expression of NLRP3 are critical factors in the activation of the inflammasome during multiple diseases, including porcine enzootic pneumonia. Therefore, strict control of NLRP3 expression is necessary to manage the severity of clinical symptoms after M. hyopneumoniae infection and maintain immunological homeostasis (Huai et al., 2014). Inhibiting the NLRP3 inflammasome may be beneficial in treating inflammatory diseases (Mangan et al., 2018). However, very few NLRP3 inflammasome inhibitors have been identified and validated to downregulate mRNA and protein expression of NLRP3 using in vitro and in vivo models of NLRP3-driven diseases (Chuang et al., 2013; Huai et al., 2014). MCC950 is a commonly used NLRP3 inhibitor that inhibits NLRP3 assembly and has potential therapeutic implications (Coll et al., 2015).
To the best of our knowledge, it is not clear if NLRP3 inflammasome regulates inflammation and affects M. hyopneumoniae infection in swine. Therefore, we utilized swine as an in vivo model to investigate the expression of NLRP3 inflammasome at different stages of M. hyopneumoniae infection (Borjigin et al., 2016). Using MCC950, an NLRP3 inflammasome inhibitor, we validated its effect in downregulating mRNA and protein expression of NLRP3, as well as tumor necrosis factor-alpha (TNF-α), interleukin 18 (IL-18), and interleukin 1 beta (IL-1β) pro-inflammatory cytokines in an in vivo swine model. Our findings suggest that the NLRP3 inflammasome plays an important regulatory role in regulating the inflammatory response and maintaining immune homeostasis in swine infected with M. hyopneumoniae.
Materials and Methods
In accordance with animal ethics guidelines and approved protocols from a Committee of the Academy of Animal Science and Veterinary Medicine of Hainan Agricultural Sciences (Haikou, Hainan, China), animal care procedures and experiments were performed. The Animal Ethics Committee approval number was SYXK (LU) 20210008.
Bacterial strain and DNA extraction
Mycoplasma hyopneumoniae (strain JS, ATCC 27715) was used in this study and cultivated at 37 °C overnight in mycoplasma medium obtained from Mycoplasma Experience Ltd, Bletchingley, Surrey, UK. The growing culture of mycoplasma was pelleted at 3,500 g for 15 min and re-suspended in phosphate-buffered saline (PBS). Serial dilutions of suspended bacteria were prepared and each dilution was spotted on mycoplasma agar plates. The plates were incubated at 37 °C and 5% CO2 for 5 d before determining the number of colony-forming units. Mycoplasmal genomic DNA was extracted using the TIANamp Bacteria DNA kit (Tiangen, Beijing, China) according to the manufacturer’s instructions. Total DNA of clinical samples was extracted and diluted in 50 μL of nuclease-free water. DNA samples were quantified with an ND-2000C spectrophotometer (Nanodrop, Wilmington, USA) and stored at −80 °C until use in further analysis.
Animals
We randomly collected Landrace piglets aged 20-30 days from ten pig farms. Conventional polymerase chain reaction (PCR) was used to screen M. hyopneumoniae (Mhp-P97), porcine respiratory and reproductive syndrome (PRRSV), porcine circovirus type-2 (PCV2), and Actinobacillus pleuropneumoniae (APP-A). Target genes were amplified using specific oligonucleotide primers listed in Table 1. The piglets were housed in isolation rooms at Shandong Binzhou Academy of Animal Science and Veterinary Medicine, Binzhou, Shandong, China, and all animal care procedures followed state laws governing animal welfare under the guidance of an ethics committee.
Table 1.
Oligonucleotide primers used in this study
| Primer | Primer sequence (5ʹ-3ʹ) | GenBank accession No. |
|---|---|---|
| Conventional PCR | ||
| Mhp-P97-F | TGACTGCCGGAATTGTTGGT | NC_007295 |
| Mhp-P97-R | AATCTTTCGTGGACTTTCTGATCTG | |
| PRRSV-F | CGTAGAACTGTGACAACAAC | EF112446 |
| PRRSV-R | TGAGTATTTTGGGCGTGTGAT | |
| PCV2-F | CGGTGGACATGATGAGATTC | AY735451.1 |
| PCV2-R | TAAAGTAGCGGGAGTGGTAG | |
| APP-A-F | AACCGGACGGAAATAATGGG | FJ807045.1 |
| APP-A-R | TAAAGCAGCCAACTCCTCAG | |
| qRT-PCR | ||
| NLRP3-F | AGCCTTGAAGAGGAATGGATGG | AB292177.1 |
| NLRP3-R | GCCTGGTGGAAGGGTTTGTTGAG | |
| IL-β1-F | ACCTGGACCTTGGTTCTC | NM_214055.1 |
| IL-β1-R | GGATTCTTCATCGGCTTC | |
| IL-18-F | ATGGACCAGAGCCAGAGAGA | AY450287.1 |
| IL-18-R | GGTGGAAAGGTGTGGAATGC | |
| TNF-α-F | GGCGTGAAGCTGAAAGACA | JF831365.1 |
| TNF-α-F | CTGGTAGGAGACGGCGATG | |
Experimental design
To investigate the effects of NLRP3-inflammasome inhibition on M. hyopneumoniae infection, 24 healthy piglets were divided randomly into four groups of six. Landrace piglets were specifically chosen based on preliminary investigations and consultations with experts in the field (Borjigin et al., 2016). The availability of a larger number of pigs meeting our specific criteria was limited, leading us to work with a sample size of 24. The negative control group, group I received a tracheal inoculation of 5 mL of sterile saline. Group II piglets were infected with 5 mL of M. hyopneumoniae strain JS via the intratracheal route at a final dose of 5 × 108 CFU/mL (Lorenzo et al., 2006). Piglets in group III were infected with M. hyopneumoniae, and 10 d post-infection (dpi), the piglets were treated with MCC950, a NLRP3-inflammasome inhibitor, intramuscularly at a dose of 6 mg/kg with a volume of 5 mL (van Hout et al., 2017). Piglets in group IV were infected with M. hyopneumoniae and administrated with dimethyl sulfoxide (DMSO) as a vehicle control group. The challenge dose was estimated to be similar to natural mycoplasma exposure during an outbreak. The animals were sacrificed at the end of the experiment to assess the treatment effects.
Clinical records and sampling
Clinical symptoms, including lethargy, loss of appetite, diarrhea, and dyspnea, as well as body temperatures, were monitored daily for at least 30 min starting from the first day of infection until the end of the experiment. Additionally, to evaluate cough severity, we utilized the Simplified Cough Score (SCS), which is recommended by the Respiratory Branch of the Chinese Medical Association as a tool for evaluating cough severity (Wang et al., 2019). The SCS rates cough symptoms from 0 to 3 based on the frequency and intensity of cough during the monitoring period: 0 for no cough, 1 for transient cough, 2 for mild cough, and 3 for severe cough. Animals scoring 3 exhibited a significant difference compared to the control animals. Two milliliters of blood samples were collected from the ear and then incubated at 37 °C for 2 h, followed by centrifugation at 8,000 rpm for 20 min. Serum samples were collected from all groups at different time points (0, 7, 14, 21, 28, and 35 dpi). These serum samples were heat-inactivated at 56 °C for 30 min and tested using commercially available enzyme-linked immunosorbent assay (ELISA) kits, as listed below, to measure protein concentration levels of NLRP3, IL-1β, IL-18, and TNF-α.
Nasal swab samples were collected from each group between days 0 and 35 post-infection, and then suspended in 2 mL Eagle’s minimum essential medium (Sigma-Aldrich, Shanghai, China) containing 5% FBS and 10% antibiotics. The specimens were stored at −80 °C until further analysis by conventional PCR or quantitative real-time RT-PCR (qRT-PCR).
To euthanize the animals, a vein channel was established after the administration of a sedative, and then an overdose of pentobarbital sodium (>150mg/kg) was injected intravenously. Tissue samples, including lung tissues, were collected from the euthanized animals on days 0, 7, 14, 21, 28, and 35 post-infections and stored at −80 °C until further use. During post-mortem inspection, gross lesions in the lungs were monitored and scored using Madec and Kobisch’s 28-point scoring method (Ostanello et al., 2007).
Hematoxylin & eosin staining
Hematoxylin and eosin (H&E) staining was performed using a procedure previously reported (Shi et al., 2019). Fresh lung tissues were fixed in 10% formaldehyde, embedded in paraffin, sliced into sections with a thickness of 4 µm using a Leica microtome (Leica, Germany), and placed on glass slides before being baked in an oven at 60 °C. Longitudinal sections with a thickness of 4 µm were stained with hematoxylin solution for 5 min, followed by hematoxylin differentiation solution and rinsed with tap water. The sections were then stained with eosin solution for 5 min, followed by dehydration with graded alcohol. The mounted slides were examined and photographed using an Olympus BX53 fluorescence microscope (Tokyo, Japan). The staining intensity was analyzed by Image-Pro Plus 6.0 software and expressed as an Integrated Optical Density value.
Immunohistochemistry
Immunohistochemistry (IHC) was performed as previously described (Zhou et al., 2021). Briefly, tissue samples from the lung, lymph nodes, large intestine, liver, spleen, trachea, kidney, small intestine, and heart were fixed with 4% paraformaldehyde. Tissue sections were dehydrated using a serial dilution of ethanol and washed with PBS. Antigen retrieval was performed for 30 min, followed by blocking of tissue sections with 4% hydrogen peroxide for 5 min. After washing with PBS, the tissue sections were incubated with a homemade rabbit anti-NLRP3 polyclonal antiserum (Zhang et al., 2019) at room temperature for 60 min. A corresponding anti-rabbit secondary antibody labeled with HRP (Sigma-Aldrich) was used. Cell nuclei were counterstained with Hematoxylin staining reagent (Bioss, China). The stained tissue sections were inspected and imaged using a Nikon Eclipse 80i microscope.
Enzyme-linked immunosorbent assay
The protein concentrations of NLRP3, IL-1β, IL-18, and TNF-α were measured in the serum or supernatant of the tested specimens. To prepare the supernatant, 10 mg of frozen piglet lung tissue was ground in 900 μL of PBS, and the resulting supernatant was collected and stored at −20 °C. Commercially available ELISA kits were used, including the porcine NLRP3, IL-1β, IL-18, and TNF-α kits (MyBioSource, #MBS7261140, #MBS260684, #MBS2504974, and #MBS262753, respectively) to measure the protein concentrations according to the manufacturer’s instructions. The reaction was stopped by adding 1 M H2SO4, and the absorbance was read at 490 nm using a Bio-Rad model 680 microplate reader (Bio-Rad, Hercules, CA).
qRT-PCR
Total RNA was extracted from tissues or nasal swab specimens using the RNeasy Mini kit (Qiagen, USA), following the manufacturer’s protocol. The concentration and purity of the RNA were evaluated using a Nanodrop (Biotech, Beijing, China). RNA samples with an A260/280 ratio of 1.8 to 2.0 were further evaluated for integrity using a TapeStation 4200 (Agilent, California, USA). RNA samples with an integrity number above 5 were used to synthesize complementary DNA using kits purchased from Biotech. Specific oligonucleotide primers targeting the NLRP3, IL-1β, IL-18, and TNF-α genes were designed based on Clone Manager 8.0 and are listed in Table 1. qRT-PCR was performed using the ABI 7500 system (Applied Biosystems, USA), as previously described (Strait et al., 2008), with Premix Ex Taq (Biotech). The total volume of the PCR reaction was 20 μL, and the PCR conditions consisted of a hold stage at 50 °C for 2 min, followed by 95 °C for 3 min, and then 30 cycles of 95 °C for 30 s, 58 °C for 30 s, and 72 °C for 30 s. β-Actin was used as an internal control for normalization. The experiment was performed in three technical replicates.
Western blotting
Western blot analysis was performed to assess the protein expression of NLRP3 as previously described (Liu et al., 2022). Briefly, 50 mg of lung tissues were homogenized in liquid nitrogen and lysed in a radioimmunoprecipitation assay lysis buffer with a protease inhibitor (Sigma, USA). The supernatant was collected and concentrated 30-fold with a 30-kD Amicon filter (Millipore, Billerica, MA), and protein concentration was determined using the Enhanced BCA Protein Assay Kit (Biotech) according to the manufacturer’s instructions. Proteins were separated on 10% SDS-PAGE and transferred to a PVDF membrane (Bio-Rad). The membrane was then blocked with 5% skimmed milk in 1X-PBS (pH 7.4) at room temperature for 2 h. After blocking, the membrane was incubated with rabbit anti-NLRP3 polyclonal antiserum (1:100 dilution) followed by goat anti-rabbit IgG (1:3000) (Biotech) conjugated with horseradish peroxidase as the secondary antibody. Protein bands were visualized by incubating the membrane in diaminobenzidine solution (Bio-Rad) at 37 °C for 50 min, and band intensity was measured using Image Lab 4.0 (Bio-Rad).
Scanning electron microscopic
The specimens for scanning electron microscopy were prepared and examined following the methodology previously described (Feng et al., 2013). The isolated lung was washed with cold PBS (0.1 M, pH 7.4) and fixed in 2.5% glutaraldehyde in PBS (pH 7.4) for 2 h at 4 °C. After rinsing the specimens in PBS, the tissues were post-fixed in 1% osmic acid tetroxide in PBS for 1 h at room temperature. The fixed tissues were then rinsed twice in PBS and immediately dehydrated in a series of ethanol dilutions (30%, 50%, 70%, 80%, 90%, 95%, and 100%) for 15 min each. Following 15-min incubations in isoamyl acetate, the specimens were air-dried using a Critical Point Dryer, attached to metallic stubs using carbon stickers, and then sputter-coated with gold for 30 s. All specimens were inspected using a scanning electron microscope (S-3000N, HITACHI) at an accelerated voltage of 1.5 kV.
Statistical analysis
The severity of cough in infected animals was evaluated using the SCS, which assigns a rating from 0 to 3 based on the frequency and intensity of cough observed during the monitoring period. Animals that scored 3 showed a significant difference compared to the control group.
To assess the differences in protein concentration, mRNA, and protein expression of NLRP3 between the control and mhp-infection groups at different time points post-infection, we conducted statistical analysis using one-way analysis of variance (ANOVA) with Tukey’s multiple comparison test in GraphPad Prism 5.0 software. Results with P-values < 0.05 were deemed statistically significant (*P < 0.05; **P < 0.01).
To analyze the impact of the NLRP3 inhibitor on protein concentration, mRNA, and protein expression of NLRP3, TNF-α, IL-18, and IL-1β, four groups of animals were utilized. These groups included a Saline group as the negative control, an infected group with M. hyopneumoniae, an infect/DMSO group as a vehicle control, and an infected/NLRP3 inhibitor group. Statistical analysis was performed using ANOVA with Tukey’s multiple comparison test in GraphPad Prism 5.0 software. Results with P-values < 0.05 were considered statistically significant (*P < 0.05; **P < 0.01).
The experiments were conducted in three technical replicates, and the data are presented as mean ± standard error of the mean (SEM).
Results
Clinical findings
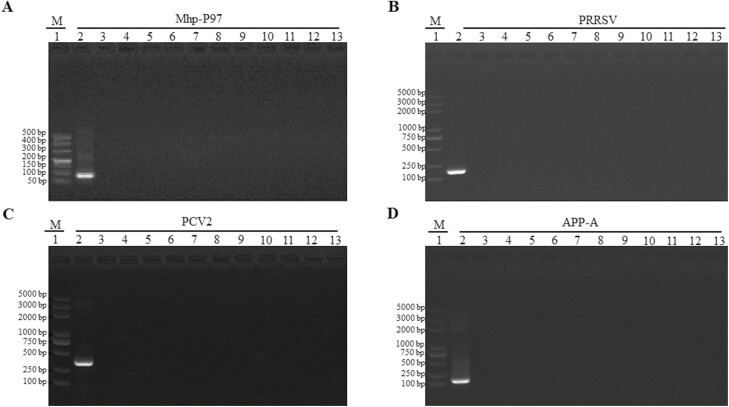
To screen piglets prior to challenge, nasal swabs were randomly collected from the piglets and subjected to conventional PCR, using positive controls of Mhp-P97, PRRSV, PCV2, and APP-A samples. The results showed that, except for the positive control samples (Figure 1A to D), all of the animals tested negative for M. hyopneumoniae, blue ear disease virus, circovirus-2, and APP-A. This confirmed that all selected piglets met the criteria for inclusion in experimentation. The piglets were monitored daily for clinical signs of M. hyopneumoniae both before and after infection by the same monitor to ensure consistency. Prior to the challenge, all piglets were found to be clinically healthy. However, after infection, they exhibited common symptoms such as wheezing, shortness of breath, and depressed-like behavior, along with poor appetite, viscous liquid discharge from their eyes and nose, abdominal breathing, and a dog-sitting posture from day 7 onwards. In addition, the piglets also showed a decrease in aerobic respiration and weight gain, as well as experiencing a dry cough in the early stages of infection. The SCS was utilized to assess the intensity of cough, and it was observed that the infected animals had a score of 3, indicating a significant difference compared to the control group.
Figure 1.
Animals screening prior to challenge using conventional PCR. PCR amplifications were performed for mhp-P97 gene (A), PRRSV gene (B), PCV2 gene (C), and APP-A gene (D) with amplicon size 92, 282, 252, and 752, respectively. Lane 1; DL500 DNA Marker (M; TaKaRa), lane 2; positive control and lanes 3 to 12; tested porcine nasal swabs and lane 13; blank control.
Lung gross lesions of infected piglets with M. hyopneumoniae
The necropsy results showed pathological changes such as cilia damage, lung infections, and pneumonia, which led to fleshy or shrimp fleshy consolidation in various areas of the lung septum, such as the tip, heart, middle, and front (Figure 2). During the early stage of infection (7 to 14 d), only a few small fleshy consolidations were observed in the lung apical lobe septum, and no abnormalities were found in the lungs or other organs (Figure 2). However, in the middle stage of infection (14 to 28 d), the lungs of infected piglets showed varying degrees of edema. The lung tissue mostly appeared grayish-red or dark purple with shrimp fleshy consolidation, and seemed translucent, but was less elastic when pressed (Figure 2). In the late stage of infection (day 35), the infected lungs showed a deep grayish-white color, a wet and dense cutting surface, and bronchi filled with a cloudy white foamy viscous fluid. Furthermore, infected piglets exhibited connective tissue hypertrophy and sclerosis in the hilar lymph nodes and lung lobules, while the mediastinal lymph nodes were significantly enlarged with greyish edema.
Figure 2.
Gross pathological changes in swine lungs over time post-infection with mhp. Gross lung lesion of piglets infected with mhp was examined at different time points (0, 7, 14, 21, 28, and 35 dpi). Gross examination showed no gross lesion at 0 dpi, however, a purple colored with consolidated area in the front of lung septum was developed starting from days 7 to 35 post-infection.
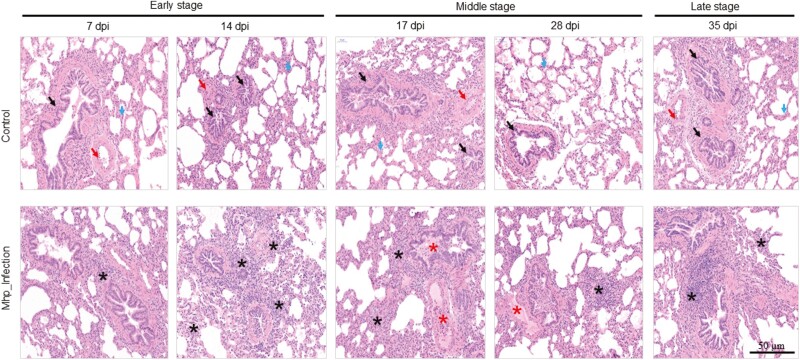
Histopathological changes of piglet lung tissues infected with M. hyopneumoniae
To characterize histopathological abnormalities in lung tissue sections from both control and infected groups of piglets at various stages of infection, H&E staining was performed. The histopathological analysis of lung tissues from the control group revealed normal lung tissue morphology, including a normal alveolar septum, bronchi, and blood vessels (Figure 3). In contrast, comparison of lung tissues between the group infected with M. hyopneumoniae and the control group at different time points of infection showed varying degrees of inflammatory changes, such as proliferation, necrosis, widening of the alveolar septum, destruction of alveolar structures, abscission of bronchial epithelial cells, and infiltration of inflammatory cells dominated by lymphocytes in the septum, parabronchial, and perivascular areas (Figure 3). In the early stage of infection (7 to 14 dpi), the lesions were relatively limited scope, with a slight dilatation of the alveolar septum, a low degree of lymphocyte infiltration in the septum, a regular arrangement of the bronchiolar epithelium, and a low degree of inflammatory cell infiltration around the lumen (Figure 3). However, in the middle and late stages of infection, histopathological alterations became widespread, with widened alveolar septum and increased lymphocyte infiltration. During the middle stage (17 to 28 dpi), there was partial damage to the alveolar structure, bronchiolar epithelial growth and destruction, and lymphocyte infiltration surrounding tiny arteries. In the late stage of infection (35 dpi), the bronchiolar epithelium was destroyed and exfoliated, with obvious exudation in the lumen, pulmonary interstitial capillaries, and parabronchial venules, resulting in hemophilia (Figure 3).
Figure 3.
H&E staining stained sections of lungs. Histological changes were determined at different time points of infection (7 and 14 dpi; early stage, 17 and 28 dpi; middle stage and 35 dpi; late stage). The lung tissue samples from control and infection groups were stained with H&E staining (amplification: 50 μm) and examined by light microscopy. Black arrow; bronchi, red arrow; blood vessels, blue arrow; alveolar septum, black star; lymphocyte and monocytes infiltration, red star; exudate containing inflammatory cells and necrotic cells.
Effect of M. hyopneumoniae infection on mRNA and protein expression levels of NLRP3
Protein concentrations of NLRP3 in serum from M. hyopneumoniae-infected piglets were measured using an ELISA assay. Two groups were included: a control group of healthy piglets, and a group infected with M. hyopneumoniae. Serum samples were collected at different time points post infection (0, 7, 14, 21, 28, and 35 dpi) from the two groups of animals. Our findings revealed that the levels of NLRP3 protein gradually increased in serum samples of M. hyopneumoniae-infected piglets, with the highest levels observed on day 28 post-infection (** P < 0.01 vs. control group) (Figure 4A). In addition, we performed qRT-PCR and immunoblotting analyses to evaluate the effect of M. hyopneumoniae infection on NLRP3 mRNA and protein expression levels. The results showed that both NLRP3 mRNA and protein expression levels in the infected group gradually increased from day 7, peaked on day 28, and then slightly declined on day 35, but remained significantly higher than on day 0 (* P < 0.05; ** P < 0.01 vs. control group) (Figure 4B and C). Furthermore, of interest, significant differences in expression levels of NLRP3 were observed in different tissues of infected piglets using immunohistochemical staining (Figure 4D), with higher expression levels noted in tissues of lung, lymph, trachea, and kidney and lower expression levels observed in tissues of spleen, liver, large intestine, small intestine, and heart. As expected, NLRP3 mRNA and protein levels were significantly higher (P < 0.05) in the infected group compared to the healthy control group, implying a potential linkage between NLRP3 and M. hyopneumoniae infection in swine.
Figure 4.
NLRP3 expression in swine over time post-infection with mhp. Protein concentration, mRNA and protein expression levels of NLRP3 were assessed in control and mhp-infection groups at different time points post-infection. (A) protein concentration of NLRP3 in serum was assayed by ELISA. (B) NLRP3-mRNA expression was measured by qRT-PCR. (C) Lung tissues were prepared at different times after infection (0, 7, 14, 28, and 35 dpi) and subjected to Western blot analysis. Rabbit anti-NLRP3 polyclonal antiserum was used to detect NLRP3 expression, and β-actin was determined as the loading control. Data are expressed as mean ± SD (n = 8). *: P < 0.05; **: P < 0.01. (D) Histopathological findings of lung, lymphatic, trachea, kidney, spleen, liver, large intestine, small intestine, and heart tissues from mycoplasma-infected swine. Rabbit anti-NLRP3 polyclonal antiserum and anti-Rabbit secondary antibody labeled with HRP were used for staining as a primary and secondary antibody, respectively. The Nikon Eclipse 80i microscope was used for inspection and imaging stained tissue sections. The black bar represents the scale: 50 μm. dpi; day post-infection.
Effect of NLRP3 inhibition on mRNA and protein expression
To investigate the impact of NLRP3 inhibition on mRNA and protein expression levels of NLRP3, four groups of piglets were used. The first group served as a healthy control and was injected with normal saline, while the second group was infected with M. hyopneumoniae JN strain. One-week post-infection, a group of mycoplasma-infected animals was randomly selected to receive the NLRP3 inhibitor (MCC950), while another group received DMSO as a vehicle control. Our findings at day 28 post-infection showed a significant increase (** P < 0.01 vs. control group) in the protein concentration of NLRP3 in the supernatant of ground lung tissues (Figure 5A) and the absolute mRNA expression levels in lung tissues (Figure 5B) of both groups of mycoplasma-infected pigs, with or without DMSO treatment, compared to the healthy control group and the infected group treated with an NLRP3 inhibitor. To further validate the effect of the NLRP3 inhibitor on protein expression, we performed immunoblotting and IHC analyses. Interestingly, the protein expression level of NLRP3 assessed by WB or IHC was significantly downregulated (** P < 0.01 vs. control group) in pig lung tissues of NLRP3-inhibitor treated animals at day 28 post-infection compared to the infected and control animal groups (Figure 5C to E).
Figure 5.
Effect of NLRP3 inhibitor on expression of NLRP3 during M. hyopneumoniae infection. To assess the effect of the NLRP3 inhibitor on NLRP3 expression, four groups of swine were tested: saline group as a negative control, an infected group with M. hyopneumoniae, an infected/DMSO group as a vehicle control, and an infected/NLRP3 inhibitor group. (A) Protein concentration in the collected serum from the four groups was determined by ELISA. (B) Absolute mRNA expression of NLRP3 was measured using qRT-PCR. (C) Immunoblotting analysis was used to validate the protein expression level of NLRP3, and β-actin was used as the loading control. (D and E) Immunohistochemistry was performed to inspect histopathological changes in lung tissues collected from the four indicated groups. Perinuclear staining for NLRP3 was brown, and nuclear staining was blue in representative immunohistochemistry images. The Nikon Eclipse 80i microscope was used for inspection, and the black bar represents the scale: 50 μm. Data are expressed as mean ± SD (n = 8). **P < 0.01 indicates statistically significant differences among different groups.
Taken together, our results suggest that the NLRP3 inhibitor was able to significantly reduce the mRNA and protein expression level of NLRP3 associated with M. hyopneumoniae infection in swine.
Effects of NLRP3 inhibition on bronchial cilia distribution after infection of piglets with M. hyopneumoniae
The scanning electron microscope was used to reveal ultrastructural changes in the distribution of bronchial cilia in the lung under the influence of NLRP3 inhibition, in comparison to infected and control animal groups (the negative control group, receiving saline, and the vehicle control group, receiving infected/DMSO). The bronchial cilia of the negative control group did not show any visible abnormalities, and there were no mycoplasma-like particles or aggregations observed in any samples with correctly positioned cilia clusters (Figure 6). In contrast, the M. hyopneumoniae-infected group displayed mycoplasma-like particles, and many sections of epithelial cells lacked cilia or had clumped cilia that had lost their parallel arrangement and gentle curvature, as anticipated. In contrast, the vehicle control group did not exhibit these characteristics (Figure 6). Interestingly, we noted that NLRP3 inhibitor-treated infected pigs’ lungs predominantly had ciliated epithelial cells that were similar in length and diameter to those of the negative control group’s lungs.
Figure 6.
Scanning electron microscope observation of bronchial ciliary ultrastructure. Bronchial cilia were observed in lungs from four groups: a negative control group injected with saline, an infected group with M. hyopneumoniae, an infected/DMSO group as a vehicle control, and an infected/NLRP3 inhibitor group. The scale bars represent 10, 20, and 100 μm.
Secretion of pro-inflammatory cytokines following M. pneumoniae infection and NLRP3 inhibitor.
To evaluate the role of NLRP3 in activating pro-inflammatory cytokines such as IL-1β, IL-18, and TNF-α during in vivo M. hyopneumoniae infection, we divided animals into groups: those infected with M. hyopneumoniae, those infected and treated with either an NLRP3 inhibitor or DMSO as a vehicle control, and a negative control group injected with saline. Protein concentration in serum (Figure 7A), as well as mRNA (Figure 7B) and protein (Figure 7C and D) expression levels in lung tissue using qRT-PCR and WB, respectively, showed significantly (* P < 0.05; ** P < 0.01 vs. control group; Saline) higher NLRP3 protein and mRNA expression levels in the infected and infected/DMSO-treated groups than in the healthy and infected/NLRP3 inhibitor-treated groups (Figure 7A to D), as expected. Our findings indicate that active expression of the NLRP3 gene during M. hyopneumoniae infection dramatically increases the release of pro-inflammatory cytokines (IL-1β, IL-18, and TNF-α). Strikingly, we also observed a significant reduction in the secretion of IL-1β, IL-18, and TNF-α in the infected/NLRP3 inhibitor-treated group compared to the infected group (* P < 0.05; ** P < 0.01 vs. control group; Saline) (Figure 7A to D). These results suggest that NLRP3 is necessary for the activation of IL-1β, IL-18, and TNF-α during M. hyopneumoniae infection and thus can serve as an essential marker for detecting M. hyopneumoniae infection.
Figure 7.
Activation or inhibition of the NLRP3 inflammasome during M. hyopneumoniae infection affects the secretion of pro-inflammatory cytokines. The protein concentration (A), mRNA expression levels (B) of NLRP3, IL-1β, IL-18, and TNF-α were measured in the saline group as a negative control, the infected group with M. hyopneumoniae, infected/DMSO group as a vehicle control and infected/NLRP3 inhibitor group. (C and D) Immunoblotting analysis was used to validate the protein expression levels of NLRP3, IL-1β, IL-18, and TNF-α. β-Actin was used as the loading control. Protein quantification and relative protein expression of the immunoblot signal from IL-1β, IL-18, and TNF-α were normalized to the average protein expression of the immunoblot signal of β-actin using ImageJ software (http://rsb.info.nih.gov/ij/). Statistical analysis was performed using ANOVA with Tukey’s multiple comparison test in GraphPad Prism 5.0 software on data from three independent experiments. The data are expressed as mean ± SD (n = 8). Differences were considered statistically significant at *: P < 0.05 and **: P < 0.01.
Discussion
The development of M. hyopneumoniae is significantly impacted by both mycoplasma virulence factors and the pro-inflammatory response of the host (Fonseca-Aten et al., 2005; Shimizu et al., 2008, 2011; Hardy et al., 2009). Therefore, it is crucial to control the innate immune defense against respiratory mycoplasma invasion to suppress mycoplasma growth and minimize lung tissue damage caused by mycoplasma virulence factors and hyperinflammation (Wei et al., 2009). However, the stimulation of pro-inflammatory cytokine production, such as IL-1β, IL-18, and TNF-α, via the NLRP3 inflammasome during M. hyopneumoniae infection in swine and its role in inducing the immune response remains unclear. To address these uncertainties, we investigated the regulatory functional role of the NLRP3 inflammasome during acute M. hyopneumoniae infection using an in vivo swine model. In this study, we examined various parameters, including clinical evaluation, immunostaining, microscopic analysis, variations in the protein expression of the NLRP3 inflammasome, and its effect on the release of pro-inflammatory cytokines.
PCR screening was performed on all animals used in the study, and the results showed that none of them had porcine enzootic pneumonia or other respiratory diseases, indicating they were suitable for further experimentation. The piglets were then closely monitored on a daily basis for clinical signs of M. hyopneumoniae infection. Prior to the infection, the piglets were in a healthy state, but after infection, they exhibited symptoms related to mycoplasma infection. Notably, the intensity of cough, assessed using the SCS, showed a significant difference with a score of 3 at the late stage of infection compared to the control group. It was important to determine whether the histological changes observed in piglet lung tissues infected with M. hyopneumoniae were consistent with previous studies that reported severe lung lesions in infected swine (Kwon et al., 2002; Sarradell et al., 2003; Choi et al., 2006; Lorenzo et al., 2006; Redondo et al., 2009). The necropsy results of this study demonstrated the development of acute necrotizing inflammatory lesions that began with excessive inflammation and caused damage to the cilia of epithelial cells in the lung and tracheal tissues of M. hyopneumoniae-infected animals compared to control animals. Histological examination provided additional information and was a vital tool for identifying histopathological alterations in lung piglet tissues infected with M. hyopneumoniae (Pallares et al., 2021; Zong et al., 2022). The lesions in the infected lung tissues were significant and began around the bronchi and bronchioles, expanding into the lungs of infected piglets during the middle and severe stages of infection. The development of lymphoid follicles, irregular thickening of the bronchiolar and alveolar walls, and the widening of the alveolar septa caused by the infiltration of lymphocytes, macrophages, and plasma cells were the hallmark histopathological abnormalities.
The NLRP3 inflammasome is a key sensor of tissue damage and a critical initiator of various autoimmune disorders (Grebe et al., 2018). Therefore, it is essential to understand the regulation and function of the NLRP3 inflammasome and its impact on diverse disorders (Zhong et al., 2016). By monitoring NLRP3 inflammasome expression, it was observed that M. hyopneumoniae infection led to an increase in the levels of both NLRP3 mRNA and protein. This finding suggests that NLRP3 expression levels have the potential to serve as a novel diagnostic marker for detecting M. hyopneumoniae infection in the respiratory tract of pigs. Strict control of NLRP3 expression is critical for maintaining immune homeostasis during mycoplasma infection since NLRP3 mRNA and protein levels are indicators of inflammasome activation (Wang et al., 2021). However, it is still unknown which molecular processes regulate NLRP3 expression levels during M. hyopneumoniae infection in swine. Therefore, in this study, mycoplasma-infected swine were treated with an NLRP3 inhibitor, MCC950 to investigate how NLRP3 inhibition affects the expression of inflammatory cytokines. NLRP3-driven diseases have been associated with a few NLRP3 inhibitors previously validated in in vitro and in vivo models (Coll et al., 2015; Jiang et al., 2017; Gordon et al., 2018; He et al., 2018; Huang et al., 2018). Since it can directly target NLRP3, the NLRP3 inhibitor, MCC950 has demonstrated good therapeutic properties (Coll et al., 2015; Gordon et al., 2018). Our results confirmed that the NLRP3 inhibitor, MCC950 significantly reduced the mRNA and protein expression of NLRP3, indicating its specific targeting of the NLRP3 inflammasome during M. hyopneumoniae infection in swine. As such, MCC950 is a promising therapeutic option for treating NLRP3-related disorders, including porcine enzootic pneumonia. Our findings are in agreement with previous studies (Vigano et al., 2015; Perera et al., 2018; Jiao et al., 2020), which reported that some molecules have been used to downregulate NLRP3 expression at both mRNA and protein expression levels. These findings prompted us to investigate the potential regulatory role of the NLRP3 inflammasome in inflammation during M. hyopneumoniae infection in swine. It has been previously known that M. hyopneumoniae colonization of swine respiratory epithelial cells requires primary cilia to adhere to the host cell cytomembrane, causing inflammation (Rottem, 2003; Parrott et al., 2016; Wang et al., 2020). The cilia located on the bronchial epithelial cells of the lung and trachea have been identified as the primary location of numerous mycoplasmas in previous scanning electron microscope studies conducted on the bronchial tissues of M. hyopneumoniae-infected pigs (Blanchard et al., 1992; Ruiz et al., 2002; Raymond et al., 2018). Our scanning electron microscope findings from this study revealed that in the group of piglets that were either infected or infected and treated with DMSO as a vehicle control, many regions of cilia were damaged, disconnected, and had lost their normal architecture. These findings were in agreement with previous research, which indicated that swine infected with M. hyopneumoniae experienced serious lesions in bronchial cilia of the lung and trachea (Maes et al., 2008). Strikingly, we noticed that bronchial lung cilia were neatly distributed in clusters under the microscope in infected piglets treated with the NLRP3 inhibitor, MCC950, similar to the negative control group. Overall, the collected data suggest that the activation of the NLRP3 inflammasome can explain the regulatory and inflammatory role of NLRP3 in mycoplasma colonization of ciliated host epithelial cells, by promoting migration and invasion into these cells (Xu et al., 2013).
One of the important goals of this study is to evaluate the regulatory inflammatory role of NLRP3 in the activation of host immune cells during M. hyopneumoniae infection. Activation of the NLRP3 inflammasome leads to the release of several pro-inflammatory cytokines such as IL-1β, IL-18, and TNF-α. According to several studies (Kanneganti et al., 2006; Allen et al., 2009; Gross et al., 2009; Thomas et al., 2009; Kelley et al., 2019), this mechanism has been identified as a crucial component of the host’s immune defenses against bacterial, viral, and fungal infections. As such, it may present a novel approach to developing new medications targeting the NLRP3 inflammasome. The role of NLRP3 in activating the host immune defense and causing inflammatory diseases has been extensively studied in vitro and in vivo mice models (Fonseca-Aten et al., 2005; Segovia et al., 2018). However, to determine the specific mechanism by which NLRP3 regulates inflammation and triggers the release of pro-inflammatory cytokines during M. hyopneumoniae infection, we utilized swine as an in vivo model. At different times post-infection, the mRNA and protein expression levels of NLRP3 inflammasome were found to be increased in the lung tissues of M. hyopneumoniae-infected animals, leading to the activation and release of IL-1β, IL-18, and TNF-α. It is noteworthy that the administration of an NLRP3 inhibitor to infected piglets significantly reduced the production of IL-1β, IL-18, and TNF-α in the lungs. These results support the notion that NLRP3 plays a regulatory role in augmenting the host’s immune response to mycoplasma infection. Together, our findings highlight the critical role of NLRP3 inflammasome in controlling the release of IL-1β, IL-18, and TNF-α during M. hyopneumoniae infection in swine.
Conclusion
To summarize, our findings will help to clarify how NLRP3 impacts M. hyopneumoniae infection by examining its effects on host innate immune cells. Furthermore, our study sheds light on the downstream effects of NLRP3, which could provide valuable insights for developing novel therapeutic approaches and detection indicators to control and prevent mycoplasmal infection and mitigate inflammatory disease progression in swine.
Acknowledgments
This work was supported by the Special project of technological innovation of Hainan Provincial Research Institute (KYYS-2021-29). Hainan Academy of Agricultural Sciences Key Laboratory Open Project (HLP202107), the open project of Hainan Province Livestock and Poultry Engineering Technology Research Center (HLP201801), Shandong Provincial Double Hundred Plan (WST2018014), the Shandong Provincial Pig Industry Technology System (SDAIT-08-13) and the National Natural Science Foundation Program of China (31560696).
Glossary
Abbreviations:
- ANOVA
one-way analysis of variance
- APP-A
Actinobacillus pleuropneumoniae
- dpi
days post-infection
- ELISA
enzyme-linked immunosorbent assay
- H&E
hematoxylin and eosin
- IHC
Immunohistochemistry
- IL-1β
interleukin 1 beta
- IL-18
interleukin 18
- NLRP3
NLR family pyrin domain containing 3
- PBS
phosphate-buffered saline
- PCR
conventional polymerase chain reaction
- PCV2
porcine circovirus type-2
- PRRSV
porcine respiratory and reproductive syndrome
- qRT-PCR
quantitative real-time RT-PCR
- SCS
simplified Cough Score
- SEM
mean ± Standard error of the mean
- TNF-α
tumor necrosis factor-alpha
Contributor Information
Yan Zhang, Institute of Animal Science and Veterinary Medicine, Hainan Academy of Agricultural Sciences, Haikou 571100, China; Key Laboratory of Tropical Animal Breeding and Disease Research, Haikou 571100, China.
Bo Liu, Shandong Binzhou Animal Science and Veterinary Medicine Academy, Binzhou 256600, China; Lvdu Bio-Sciences &Technology Co. Ltd., Binzhou 256600, Shandong, China.
Abdelrahman Said, Genetics and Genome Biology Program, The Hospital for Sick Children, Toronto, ON, Canada; Parasitology and Animal Diseases Department, National Research Center, Dokki, Giza, Egypt.
Jinwen Xie, Shandong Binzhou Animal Science and Veterinary Medicine Academy, Binzhou 256600, China.
Fengrong Tian, Shandong Binzhou Animal Science and Veterinary Medicine Academy, Binzhou 256600, China.
Zongxi Cao, Institute of Animal Science and Veterinary Medicine, Hainan Academy of Agricultural Sciences, Haikou 571100, China; Key Laboratory of Tropical Animal Breeding and Disease Research, Haikou 571100, China.
Zhe Chao, Institute of Animal Science and Veterinary Medicine, Hainan Academy of Agricultural Sciences, Haikou 571100, China; Key Laboratory of Tropical Animal Breeding and Disease Research, Haikou 571100, China.
Feng Li, Shandong Binzhou Animal Science and Veterinary Medicine Academy, Binzhou 256600, China; Shandong Academician Workstation, Binzhou 256600, Shandong, China.
Xin Li, Xinjiang Agricultural University, Wulumuqi, Xinjiang, China.
Shuguang Li, Shandong Binzhou Animal Science and Veterinary Medicine Academy, Binzhou 256600, China.
Hailong Liu, Institute of Animal Science and Veterinary Medicine, Hainan Academy of Agricultural Sciences, Haikou 571100, China; Key Laboratory of Tropical Animal Breeding and Disease Research, Haikou 571100, China.
Wenxiu Wang, Shandong Binzhou Animal Science and Veterinary Medicine Academy, Binzhou 256600, China; Shandong Academician Workstation, Binzhou 256600, Shandong, China.
Author Contributions
BL, JX, SL, FT, FL, and XL carried out experiments, analyzing and interpreting the data. HL and YZ contributed to project administration and resources. HL and WW contributed to funding acquisition. AS and WW contributed to analyze, interpret the data of this work, additionally, contributed to the drafting of the manuscript, revising it critically, and giving final approval of the version to be published. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that they have no competing interests.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Literature Cited
- Allen, I. C., Scull M. A., Moore C. B., Holl E. K., McElvania-TeKippe E., Taxman D. J., Guthrie E. H., Pickles R. J., and Ting J. P.. . 2009. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard, B., Vena M. M., Cavalier A., Le Lannic J., Gouranton J., and Kobisch M.. . 1992. Electron microscopic observation of the respiratory tract of SPF piglets inoculated with Mycoplasma hyopneumoniae. Vet. Microbiol. 30:329–341. doi: 10.1016/0378-1135(92)90020-t. [DOI] [PubMed] [Google Scholar]
- Borjigin, L., Shimazu T., Katayama Y., Li M., Satoh T., Watanabe K., Kitazawa H., Roh S. G., Aso H., Katoh K., . et al. 2016. Immunogenic properties of Landrace pigs selected for resistance to mycoplasma pneumonia of swine. Anim. Sci. J. 87:321–329. doi: 10.1111/asj.12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, C., Kwon D., Jung K., Ha Y., Lee Y. H., Kim O., Park H. K., Kim S. H., Hwang K. K., and Chae C.. . 2006. Expression of inflammatory cytokines in pigs experimentally infected with Mycoplasma hyopneumoniae. J. Comp. Pathol. 134:40–46. doi: 10.1016/j.jcpa.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Chuang, S. Y., Yang C. H., Chou C. C., Chiang Y. P., Chuang T. H., and Hsu L. C.. . 2013. TLR-induced PAI-2 expression suppresses IL-1beta processing via increasing autophagy and NLRP3 degradation. Proc. Natl. Acad. Sci. U.S.A. 110:16079–16084. doi: 10.1073/pnas.1306556110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll, R. C., Robertson A. A., Chae J. J., Higgins S. C., Munoz-Planillo R., Inserra M. C., Vetter I., Dungan L. S., Monks B. G., Stutz A., . et al. 2015. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 21:248–255. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, Z. X., Wei Y. N., Li G. L., Lu X. M., Wan X. F., Pharr G. T., Wang Z. W., Kong M., Gan Y., Bai F. F., . et al. 2013. Development and validation of an attenuated Mycoplasma hyopneumoniae aerosol vaccine. Vet. Microbiol. 167:417–424. doi: 10.1016/j.vetmic.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Fonseca-Aten, M., Rios A. M., Mejias A., Chavez-Bueno S., Katz K., Gomez A. M., G. H.McCracken, Jr., and Hardy R. D.. . 2005. Mycoplasma pneumoniae induces host-dependent pulmonary inflammation and airway obstruction in mice. Am. J. Respir. Cell Mol. Biol. 32:201–210. doi: 10.1165/rcmb.2004-0197OC. [DOI] [PubMed] [Google Scholar]
- Gordon, R., Albornoz E. A., Christie D. C., Langley M. R., Kumar V., Mantovani S., Robertson A. A. B., Butler M. S., Rowe D. B., O’Neill L. A., . et al. 2018. Inflammasome inhibition prevents alpha-synuclein pathology and dopaminergic neurodegeneration in mice. Sci. Transl. Med. 10. doi: 10.1126/scitranslmed.aah4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebe, A., Hoss F., and Latz E.. . 2018. NLRP3 inflammasome and the IL-1 pathway in atherosclerosis. Circ. Res. 122:1722–1740. doi: 10.1161/CIRCRESAHA.118.311362. [DOI] [PubMed] [Google Scholar]
- Gross, O., Poeck H., Bscheider M., Dostert C., Hannesschlager N., Endres S., Hartmann G., Tardivel A., Schweighoffer E., Tybulewicz V., . et al. 2009. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 459:433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- Hardy, R. D., Coalson J. J., Peters J., Chaparro A., Techasaensiri C., Cantwell A. M., Kannan T. R., Baseman J. B., and Dube P. H.. . 2009. Analysis of pulmonary inflammation and function in the mouse and baboon after exposure to Mycoplasma pneumoniae CARDS toxin. PLoS One. 4:e7562. doi: 10.1371/journal.pone.0007562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, H., Jiang H., Chen Y., Ye J., Wang A., Wang C., Liu Q., Liang G., Deng X., Jiang W., . et al. 2018. Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity. Nat. Commun. 9:2550. doi: 10.1038/s41467-018-04947-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huai, W., Zhao R., Song H., Zhao J., Zhang L., Zhang L., Gao C., Han L., and Zhao W.. . 2014. Aryl hydrocarbon receptor negatively regulates NLRP3 inflammasome activity by inhibiting NLRP3 transcription. Nat. Commun. 5:4738. doi: 10.1038/ncomms5738. [DOI] [PubMed] [Google Scholar]
- Huang, Y., Jiang H., Chen Y., Wang X., Yang Y., Tao J., Deng X., Liang G., Zhang H., Jiang W., . et al. 2018. Tranilast directly targets NLRP3 to treat inflammasome-driven diseases. EMBO Mol. Med. 10. doi: 10.15252/emmm.201708689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, H., He H., Chen Y., Huang W., Cheng J., Ye J., Wang A., Tao J., Wang C., Liu Q., . et al. 2017. Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J. Exp. Med. 214:3219–3238. doi: 10.1084/jem.20171419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao, J., Zhao G., Wang Y., Ren P., and Wu M.. . 2020. MCC950, a selective inhibitor of NLRP3 inflammasome, reduces the inflammatory response and improves neurological outcomes in mice model of spinal cord injury. Front. Mol. Biosci. 7:37. doi: 10.3389/fmolb.2020.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanneganti, T. D., Body-Malapel M., Amer A., Park J. H., Whitfield J., Franchi L., Taraporewala Z. F., Miller D., Patton J. T., Inohara N., . et al. 2006. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J. Biol. Chem. 281:36560–36568. doi: 10.1074/jbc.M607594200. [DOI] [PubMed] [Google Scholar]
- Kelley, N., Jeltema D., Duan Y., and He Y.. . 2019. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int. J. Mol. Sci. 20. doi: 10.3390/ijms20133328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon, D., Choi C., and Chae C.. . 2002. Chronologic localization of mycoplasma hyopneumoniae in experimentally infected pigs. Vet. Pathol. 39:584–587. doi: 10.1354/vp.39-5-584. [DOI] [PubMed] [Google Scholar]
- Li, X., Zhang Y. K., Yin B., Liang J. B., Jiang F., and Wu W. X.. . 2019. Toll-like receptor 2 (TLR2) and TLR4 mediate the IgA immune response induced by Mycoplasma hyopneumoniae. Infect. Immun. 88. doi: 10.1128/IAI.00697-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, W., Jiang P., Yang K., Song Q., Yuan F., Liu Z., Gao T., Zhou D., Guo R., Li C., . et al. 2022. Mycoplasma hyopneumoniae infection activates the NOD1 signaling pathway to modulate inflammation. Front. Cell. Infect. Microbiol. 12:927840. doi: 10.3389/fcimb.2022.927840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo, H., Quesada O., Assuncao P., Castro A., and Rodriguez F.. . 2006. Cytokine expression in porcine lungs experimentally infected with Mycoplasma hyopneumoniae. Vet. Immunol. Immunopathol. 109:199–207. doi: 10.1016/j.vetimm.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Maes, D., Segales J., Meyns T., Sibila M., Pieters M., and Haesebrouck F.. . 2008. Control of Mycoplasma hyopneumoniae infections in pigs. Vet. Microbiol. 126:297–309. doi: 10.1016/j.vetmic.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan, M. S. J., Olhava E. J., Roush W. R., Seidel H. M., Glick G. D., and Latz E.. . 2018. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Discov. 17:688. doi: 10.1038/nrd.2018.149. [DOI] [PubMed] [Google Scholar]
- McNeela, E. A., Burke A., Neill D. R., Baxter C., Fernandes V. E., Ferreira D., Smeaton S., El-Rachkidy R., McLoughlin R. M., Mori A., . et al. 2010. Pneumolysin activates the NLRP3 inflammasome and promotes proinflammatory cytokines independently of TLR4. PLoS Pathog. 6:e1001191. doi: 10.1371/journal.ppat.1001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni, L., Song C., Wu X., Zhao X., Wang X., Li B., and Gan Y.. . 2019. RNA-seq transcriptome profiling of porcine lung from two pig breeds in response to Mycoplasma hyopneumoniae infection. PeerJ. 7:e7900. doi: 10.7717/peerj.7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostanello, F., Dottori M., Gusmara C., Leotti G., and Sala V.. . 2007. Pneumonia disease assessment using a slaughterhouse lung-scoring method. J. Vet. Med. A Physiol. Pathol. Clin. Med. 54:70–75. doi: 10.1111/j.1439-0442.2007.00920.x. [DOI] [PubMed] [Google Scholar]
- Pallares, F. J., Anon J. A., Rodriguez-Gomez I. M., Gomez-Laguna J., Fabre R., Sanchez-Carvajal J. M., Ruedas-Torres I., and Carrasco L.. . 2021. Prevalence of mycoplasma-like lung lesions in pigs from commercial farms from Spain and Portugal. Porcine Health Manag. 7:26. doi: 10.1186/s40813-021-00204-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott, G. L., Kinjo T., and Fujita J.. . 2016. A compendium for Mycoplasma pneumoniae. Front. Microbiol. 7:513. doi: 10.3389/fmicb.2016.00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera, A. P., Fernando R., Shinde T., Gundamaraju R., Southam B., Sohal S. S., Robertson A. A. B., Schroder K., Kunde D., and Eri R.. . 2018. MCC950, a specific small molecule inhibitor of NLRP3 inflammasome attenuates colonic inflammation in spontaneous colitis mice. Sci. Rep. 8:8618. doi: 10.1038/s41598-018-26775-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond, B. B. A., Turnbull L., Jenkins C., Madhkoor R., Schleicher I., Uphoff C. C., Whitchurch C. B., Rohde M., and Djordjevic S. P.. . 2018. Mycoplasma hyopneumoniae resides intracellularly within porcine epithelial cells. Sci. Rep. 8:17697. doi: 10.1038/s41598-018-36054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo, E., Masot A. J., Fernandez A., and Gazquez A.. . 2009. Histopathological and immunohistochemical findings in the lungs of pigs infected experimentally with Mycoplasma hyopneumoniae. J. Comp. Pathol. 140:260–270. doi: 10.1016/j.jcpa.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Rottem, S. 2003. Interaction of mycoplasmas with host cells. Physiol. Rev. 83:417–432. doi: 10.1152/physrev.00030.2002. [DOI] [PubMed] [Google Scholar]
- Ruiz, A., Galina L., and Pijoan C.. . 2002. Mycoplasma hyopneumoniae colonization of pigs sired by different boars. Can. J. Vet. Res. 66:79–85. [PMC free article] [PubMed] [Google Scholar]
- Sarradell, J., Andrada M., Ramirez A. S., Fernandez A., Gomez-Villamandos J. C., Jover A., Lorenzo H., Herraez P., and Rodriguez F.. . 2003. A morphologic and immunohistochemical study of the bronchus-associated lymphoid tissue of pigs naturally infected with Mycoplasma hyopneumoniae. Vet. Pathol. 40:395–404. doi: 10.1354/vp.40-4-395. [DOI] [PubMed] [Google Scholar]
- Segovia, J. A., Chang T. H., Winter V. T., Coalson J. J., Cagle M. P., Pandranki L., Bose S., Baseman J. B., and Kannan T. R.. . 2018. NLRP3 is a critical regulator of inflammation and innate immune cell response during Mycoplasma pneumoniae infection. Infect. Immun. 86. doi: 10.1128/IAI.00548-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, S., Zhang X., Zhou Y., Tang H., Zhao D., and Liu F.. . 2019. Immunosuppression reduces lung injury caused by Mycoplasma pneumoniae infection. Sci. Rep. 9:7147. doi: 10.1038/s41598-019-43451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu, T., Kida Y., and Kuwano K.. . 2008. Mycoplasma pneumoniae-derived lipopeptides induce acute inflammatory responses in the lungs of mice. Infect. Immun. 76:270–277. doi: 10.1128/IAI.00955-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu, T., Kida Y., and Kuwano K.. . 2011. Cytoadherence-dependent induction of inflammatory responses by Mycoplasma pneumoniae. Immunology. 133:51–61. doi: 10.1111/j.1365-2567.2011.03408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibila, M., Pieters M., Molitor T., Maes D., Haesebrouck F., and Segales J.. . 2009. Current perspectives on the diagnosis and epidemiology of Mycoplasma hyopneumoniae infection. Vet. J. 181:221–231. doi: 10.1016/j.tvjl.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strait, E. L., Madsen M. L., Minion F. C., Christopher-Hennings J., Dammen M., Jones K. R., and Thacker E. L.. 2008. Real-time PCR assays to address genetic diversity among strains of Mycoplasma hyopneumoniae. J. Clin. Microbiol. 46(8):2491–2498. doi: 10.1128/JCM.02366-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, J., Li Y., Wang J., Wu Q., and Yan H.. . 2019. Polydatin suppresses the development of lung inflammation and fibrosis by inhibiting activation of the NACHT domain-, leucine-rich repeat-, and pyd-containing protein 3 inflammasome and the nuclear factor-kappaB pathway after Mycoplasma pneumoniae infection. J. Cell. Biochem. 120:10137–10144. doi: 10.1002/jcb.28297. [DOI] [PubMed] [Google Scholar]
- Tao, Y., Shu J., Chen J., Wu Y., and He Y.. . 2019. A concise review of vaccines against Mycoplasma hyopneumoniae. Res. Vet. Sci. 123:144–152. doi: 10.1016/j.rvsc.2019.01.007. [DOI] [PubMed] [Google Scholar]
- Thacker, E. L. 2004. Diagnosis of Mycoplasma hyopneumoniae. Anim. Health Res. Rev. 5:317–320. doi: 10.1079/ahr200491. [DOI] [PubMed] [Google Scholar]
- Thacker, E. L., Halbur P. G., Ross R. F., Thanawongnuwech R., and Thacker B. J.. . 1999. Mycoplasma hyopneumoniae potentiation of porcine reproductive and respiratory syndrome virus-induced pneumonia. J. Clin. Microbiol. 37:620–627. doi: 10.1128/JCM.37.3.620-627.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, P. G., Dash P., J. R.Aldridge, Jr, Ellebedy A. H., Reynolds C., Funk A. J., Martin W. J., Lamkanfi M., Webby R. J., Boyd K. L., . et al. 2009. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. 30:566–575. doi: 10.1016/j.immuni.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hout, G. P., Bosch L., Ellenbroek G. H., de Haan J. J., van Solinge W. W., Cooper M. A., Arslan F., de Jager S. C., Robertson A. A., Pasterkamp G., . et al. 2017. The selective NLRP3-inflammasome inhibitor MCC950 reduces infarct size and preserves cardiac function in a pig model of myocardial infarction. Eur. Heart J. 38:828–836. doi: 10.1093/eurheartj/ehw247. [DOI] [PubMed] [Google Scholar]
- Vigano, E., Diamond C. E., Spreafico R., Balachander A., Sobota R. M., and Mortellaro A.. . 2015. Human caspase-4 and caspase-5 regulate the one-step non-canonical inflammasome activation in monocytes. Nat. Commun. 6:8761. doi: 10.1038/ncomms9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W., Qin Y., Song H., Wang L., Jia M., Zhao C., Gong M., and Zhao W.. . 2021. Galectin-9 targets NLRP3 for autophagic degradation to limit inflammation. J. Immunol. 206:2692–2699. doi: 10.4049/jimmunol.2001404. [DOI] [PubMed] [Google Scholar]
- Wang, Z., Wang M., Wen S., Yu L., and Xu X.. . 2019. Types and applications of cough-related questionnaires. J. Thorac. Dis. 11:4379–4388. doi: 10.21037/jtd.2019.09.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H., Zhang Z., Xie X., Liu B., Wei Y., Gan Y., Yuan T., Ni B., Wang J., Zhang L., . et al. 2020. Paracellular pathway-mediated Mycoplasma hyopneumoniae migration across porcine airway epithelial barrier under air-liquid interface conditions. Infect. Immun. 88. doi: 10.1128/IAI.00470-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, H., Myungsoo J., Brett E. M., Christman J. W., Yull F. E., and Blackwell T. S.. . 2009. Myeloid cells control termination of lung inflammation through the NF-κB pathway. Am. J. Physiol. Lung Cell. Mol. Physiol. 296:L320–L327. doi: 10.1152/ajplung.90485.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Y., Li H., Chen W., Yao X., Xing Y., Wang X., Zhong J., and Meng G.. . 2013. Mycoplasma hyorhinis activates the NLRP3 inflammasome and promotes migration and invasion of gastric cancer cells. PLoS One. 8:e77955. doi: 10.1371/journal.pone.0077955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeske, P., Valeris-Chacin R., Singer R. S., and Pieters M.. . 2020. Survival analysis of two Mycoplasma hyopneumoniae eradication methods. Prev. Vet. Med. 174:104811. doi: 10.1016/j.prevetmed.2019.104811. [DOI] [PubMed] [Google Scholar]
- Yu, Y., Liu M., Hua L., Qiu M., Zhang W., Wei Y., Gan Y., Feng Z., Shao G., and Xiong Q.. . 2018. Fructose-1,6-bisphosphate aldolase encoded by a core gene of Mycoplasma hyopneumoniae contributes to host cell adhesion. Vet. Res. 49:114. doi: 10.1186/s13567-018-0610-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y., Wang W., Liu H., Cao Z., and Tan S.. . 2019. Prokaryotic expression of porcine NLRP3 protein protein and preparation of polyclonal antibody. Chin. J. Vet. Sci. 39:1075–1079. [Google Scholar]
- Zhong, Z., Sanchez-Lopez E., and Karin M.. . 2016. Autophagy, NLRP3 inflammasome and auto-inflammatory/immune diseases. Clin. Exp. Rheumatol. 34:12–16. [PubMed] [Google Scholar]
- Zhou, L., Wang J., Lyu S. C., Pan L. C., Shi X. J., Du G. S., and He Q.. . 2021. PD-L1(+)NEUT, Foxp3(+)Treg, and NLR as new prognostic marker with low survival benefits value in hepatocellular carcinoma. Technol. Cancer Res. Treat. 20:15330338211045820. doi: 10.1177/15330338211045820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong, B., Zhu Y., Liu M., Wang X., Chen H., Zhang Y., and Tan C.. . 2022. Characteristics of Mycoplasma hyopneumoniae strain ES-2 isolated from Chinese Native Black Pig Lungs. Front. Vet. Sci. 9:883416. doi: 10.3389/fvets.2022.883416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.