Abstract
Background and Objectives
In women with highly active multiple sclerosis (MS), suspending rituximab (RTX) for planning pregnancy is associated with low disease reactivation. Whether this strategy reduces the risk of disease reactivity as compared with suspending natalizumab (NTZ) 3 months after conception is unclear.
Methods
We retrospectively included women with MS followed in our department during pregnancy and 1 year after birth who suspended NTZ at the end of the first trimester (option mostly proposed before 2016) or suspended RTX/ocrelizumab (RTX/OCR) in the year before conception (option proposed since 2016).
Results
In women who suspended NTZ, 45 pregnancies resulted in 3 miscarriages and 42 live births, including 1 newborn with major malformations. In women who suspended RTX/OCR, 37 pregnancies resulted in 3 miscarriages and 33 live births; 1 pregnancy was terminated for malformation. During pregnancy, relapse occurred in 3/42 (7.1%) patients of the NTZ group and 1/33 (3%) of the RTX/OCR group (p = 0.6). After delivery, relapse occurred in 9/42 (21.4%) patients of the NTZ group and 0/33 of the RTX/OCR group (p < 0.01). In the NTZ group, 8/9 relapses occurred in patients who restarted NTZ less than 4 weeks after delivery. The proportion of patients with gadolinium-enhanced and/or new T2 lesions on brain or spinal cord MRI performed after delivery was higher in the NTZ than RTX/OCR group (14/40 [35%] vs 1/31 [3%] patients, p = 0.001), the proportion with EDSS score progression during the period including pregnancy and the year after delivery was higher (7/42 [17%] vs 0/33 patients, p = 0.01), and the proportion fulfilling NEDA-3 during this period was lower (21/40 [53%] vs 30/31 [97%] patients, p < 0.001).
Discussion
Suspending RTX/OCR in the year before conception in women with highly active MS was associated with no disease reactivation during and after pregnancy. As previously reported, stopping NTZ at the end of the first trimester was associated with disease reactivation. In women receiving NTZ who are planning pregnancy, a bridge to RTX/OCR for pregnancy or continuing NTZ until week 34 are both reasonable clinical decisions. The RTX/OCR option is more comfortable for women and reduces the exposure of infants to monoclonal antibodies.
Introduction
In the past years, the development of disease-modifying therapies in multiple sclerosis (MS) has greatly improved the disease prognosis.1 Of note, the emergence of high-efficacy therapies has allowed for controlling inflammatory activity in most patients with relapsing remitting MS. Thus, planning a pregnancy has become increasingly conceivable for women with MS, even those with highly active disease.2 However, the best therapeutic strategy that should be used for planning pregnancy in women with highly active MS remains not fully determined.
Several studies suggested that natalizumab (NTZ) has a good benefit/risk balance for planning pregnancy in women with highly active MS when infusions are continued after conception.3-6 Especially, maintenance of NTZ during the first trimester of pregnancy is associated with low disease reactivity during pregnancy and no complications in the newborns, but disease activity can recur after delivery.4 Maintenance of NTZ during pregnancy is associated with a 24.5% reduction in odds of relapse during pregnancy per month continued.6 In that way, maintaining NTZ until 30–34 weeks of gestation has been recommended in patients with highly active MS.7-9 This strategy seems safer for newborns because an exposure throughout the entire third trimester is associated with frequent hematologic complications but not totally safe because anemia was reported in several newborns exposed at this term.8,10,11 Moreover, the risk of disease activity in women treated until this term remains significant.6,8 Indeed, postpartum relapse occurred in patients receiving NTZ beyond the first trimester, and MRI analysis demonstrated that 20% of women treated during the second trimester presented at least 1 new T2 lesion and 13% at least 1 enhancing lesion after delivery.6,8
Recent studies suggested that anti-CD20 therapies, mainly rituximab (RTX), may be safe and associated with no return of disease activity when they are suspended for planning pregnancy.12-16 Of note, women who suspended RTX vs NTZ before pregnancy showed lower disease activity.14 However, to the best of our knowledge, no study has compared disease activity between women who suspended NTZ into pregnancy and women who suspended anti-CD20 therapies before conception.
According to these recent data on anti-CD20 therapies, in our department, we progressively changed clinical practice during the past years for women with MS who planned pregnancy. The strategy to maintain NTZ during the first trimester was progressively replaced by an extension of the anti-CD20 dosing interval for planning pregnancy. In the present observational study, we included all consecutive women with highly active MS followed in our department who received NTZ during the first trimester of pregnancy (option mostly proposed before 2016) or at least 1 anti-CD20 infusion (RTX/ocrelizumab [OCR]) in the year before conception (option proposed since 2016) and who were followed at least 1 year after birth. We compared the efficacy and safety of these 2 distinct therapeutic strategies.
Methods
Protocol and Participants
Over the past decade in the MS center of Marseille, we used 2 main standardized therapeutic strategies for women with highly active MS who were planning pregnancy. Between 2014 and 2016, we proposed only 1 strategy based on the use of NTZ. We advised all women with highly active MS and anti-JC virus antibody index <1.5 who were planning pregnancy to continue NTZ up to the end of the first trimester. The therapeutic strategy was not standardized concerning the time to restart NTZ after birth, but most physicians restarted NTZ in the first 4 weeks after birth. Since 2016, a second strategy based on RTX was also proposed. In that case, we advised women to maintain contraception at least 3 months after the last infusion of RTX. If no pregnancy occurred during the year after the last infusion, a new infusion of RTX was given. In case of pregnancy, no infusion was administered during pregnancy, and most physicians restarted RTX within the first 24 weeks after birth. After 2018, we applied the same strategy for OCR.
Patients were seen in the MS center for a clinical evaluation at the beginning of pregnancy (within 1 month after conception), at 6 months of pregnancy, 1 month after delivery, 3–6 months after birth, and 6–12 months after birth. Brain and spinal cord MRI were performed within 2 months after birth and 6–12 months after birth. All examinations for a given patient were performed by the same experienced neurologists (S.D., A.R., C.B., A.M., J.P., or B.A.). The Expanded Disability Status Scale (EDSS) score was collected at each visit. All patients were given the phone number of our neuroinflammatory unit that was open 24 h/d and 7 d/wk. We informed each patient about the need to call the center in case of new neurologic signs. Relapse was considered the occurrence of neurologic signs persisting for more than 24 hours in the absence of fever, infection, or other intercurrent phenomena. In case of relapse, the patient was admitted to hospital and corticosteroids were administered if necessary.
The NTZ group included patients who stopped NTZ at the end of the first trimester (3 or 4 infusions after conception depending on the interval between conception and the first infusion during pregnancy). The anti-CD20 group included patients who received an infusion of RTX or OCR in the year before conception. We extracted data on demographics and disease characteristics before pregnancy. All relapses occurring during pregnancy and postpartum were prospectively recorded.
Morphologic outcomes of newborns (weight and size) and gestational age were prospectively recorded.
Ethical Approval
The authors obtained ethical approval from the Institutional Review Board of the University Hospital of Marseille, France, for this study (Approval No. PADS-21-60).
Statistical Analyses
Analyses were conducted with JMP 16.1.0 (SAS Institute Inc). Baseline demographics and disease characteristics were compared between the NTZ and RTX/OCR groups by the Mann-Whitney U test. Kaplan-Meier survival analysis was used to compare time to first relapse between the NTZ and RTX/OCR groups during the period including pregnancy and the year after delivery. Disability progression was determined by comparing the EDSS scores at 12 months after birth and within 1 month after conception. Disability progression was defined as a worsening of at least 1.5 points of the EDSS score with baseline EDSS score 0, at least 1 point with baseline EDSS score 1 to 4, and at least 0.5 points with baseline EDSS score >4. The proportion of patients with confirmed disability progression was compared by the Fisher exact test. MRI activity in the year after delivery was defined as the presence of at least 1 enhancing and/or new T2 lesion in at least 1 brain or spinal cord MRI performed during the year after delivery compared with an MRI performed before conception after the onset of NTZ or anti-CD20 therapy. The proportion of patients with MRI activity in the year after delivery was compared by the Fisher exact test. Finally, we determined the proportion of patients fulfilling no evidence of disease activity 3 (NEDA-3) between conception and 1 year after delivery.
Multivariable logistic regression models were used to assess possible predictors of maintenance of NEDA-3 in the period including pregnancy and the year after birth, estimating odds ratios (ORs), and 95% confidence intervals (CIs). The model contained the following covariates: age at conception, EDSS score at conception, annual relapse before highly active therapy, disease duration at conception, and therapeutic strategies (NTZ vs anti-CD20 suspension).
Data Availability
All data analyzed during this study will be shared anonymized by reasonable request of a qualified investigator to the corresponding author.
Results
Study Population
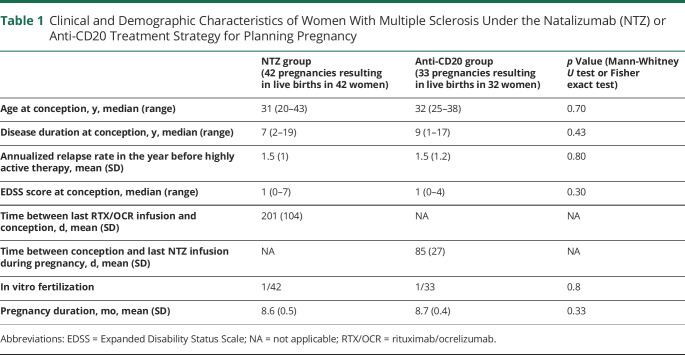
Overall, 45 pregnancies in 45 women matched the inclusion criteria for NTZ suspension and 37 pregnancies in 36 women the criteria for anti-CD20 suspension. In women who suspended NTZ, 45 pregnancies resulted in 42 live births. In women who suspended RTX/OCR, 37 pregnancies resulted in 33 live births. No women were lost to follow-up. The demographic and clinical characteristics of the cohort are presented in Table 1. The NTZ and RTX/OCR groups did not differ in clinical and demographic characteristics before pregnancy (Table 1). The mean (SD) time from suspension of anti-CD20 therapy to conception was 201 days (104). The mean (SD) time from conception to last NTZ infusion was 86 (26) days. The mean (SD) time from birth to restarting therapy was 23 (18) days for the NTZ group and 123 (130) days for the RTX/OCR group. The mean (SD) time of free intervals was 180 (29) days for the NTZ group and 471 (106) days for the RTX/OCR group. In the NTZ group, one patient switched to RTX and another to OCR after delivery. The mean (SD) time from birth to first MRI was 83 (61) days.
Table 1.
Clinical and Demographic Characteristics of Women With Multiple Sclerosis Under the Natalizumab (NTZ) or Anti-CD20 Treatment Strategy for Planning Pregnancy
Effect of Therapeutic Strategies on Disease Activity During the Period Including Pregnancy and the Year After Delivery
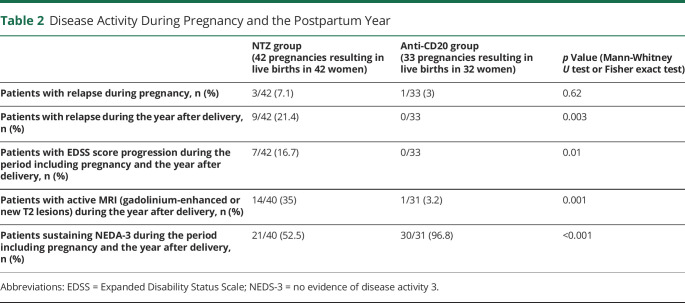
During the entire period including pregnancy and the year after delivery, the relapse rate was significantly higher in the NTZ than RTX/OCR group (p = 0.01, log-rank, Kaplan-Meier survival curves) (Figure). During pregnancy, relapse occurred in 3/42 (7.1%) patients of the NTZ group and 1/33 (3%) of the RTX/OCR group (p = 0.6). During the year after delivery, relapse occurred in 9/42 (21.4%) patients of the NTZ group and 0/33 of the RTX/OCR group (p < 0.01). In the NTZ group, the median (range) time of occurrence of the relapse after delivery was 4 weeks (2–8), and 5/9 (55.5%) relapses occurred after restarting treatment. In the NTZ group, 8/9 relapses occurred in patients who restarted NTZ less than 4 weeks after delivery. The proportion of patients with gadolinium-enhanced and/or new T2 lesions on brain or spinal cord MRI performed during the year after delivery was higher in the NTZ than the RTX/OCR group (14/40 [35%] vs 1/31 [3%] patients, p = 0.001), the proportion with EDSS score progression during the period including pregnancy and the year after delivery was higher (7/42 [17%] vs 0/33 patients, p = 0.01), and the proportion fulfilling NEDA-3 during the period including pregnancy and the year after delivery was lower (21/40 [53%] vs 30/31 [97%] patients, p < 0.001) (Table 2).
Figure. Survival Analysis With Time to First Relapse in the NTZ and RTX/OCR Groups During the Period Including Pregnancy and the Year After Delivery.
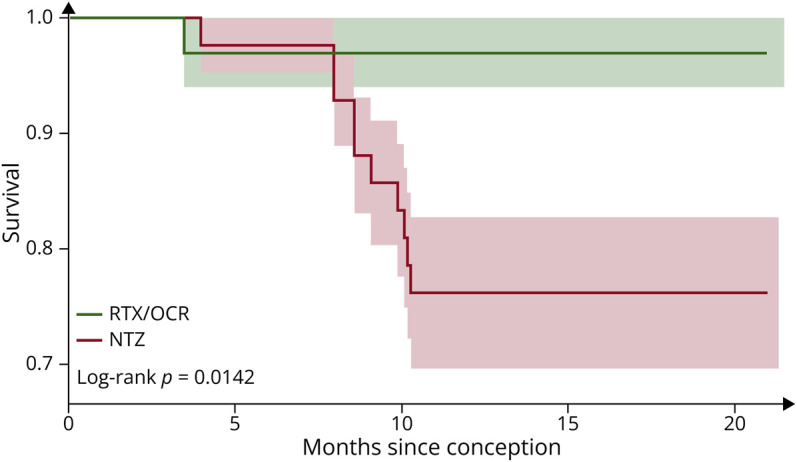
NTZ = natalizumab; RTX/OCR = rituximab/ocrelizumab.
Table 2.
Disease Activity During Pregnancy and the Postpartum Year
On multivariable regression analysis, the probability of maintaining NEDA-3 during the entire period including pregnancy and the year after delivery was associated with the therapeutic strategy (RTX/OCR vs NTZ) (OR = 27, 95% CI: 3–219, p = 0.002). This association remained significant (OR = 20, 95% CI: 2–175, p = 0.006) after excluding from the NTZ group women who restarted treatment more than 4 weeks after delivery (n = 8).
In the NTZ group, multivariable logistic regression analysis revealed no predictor of loss of NEDA-3 in the period including pregnancy and the year after delivery. Covariates included age at conception, EDSS score at conception, annual relapse before highly active therapy, and time without treatment after the last NTZ treatment during pregnancy. In the NTZ group, multivariable logistic regression analysis revealed no predictor of occurrence of relapse in the year after delivery. Covariates included age at conception, EDSS score at conception, annual relapse before highly active therapy, breastfeeding, and time to restarting treatment after delivery.
Mother, Fetal, and Newborn Safety
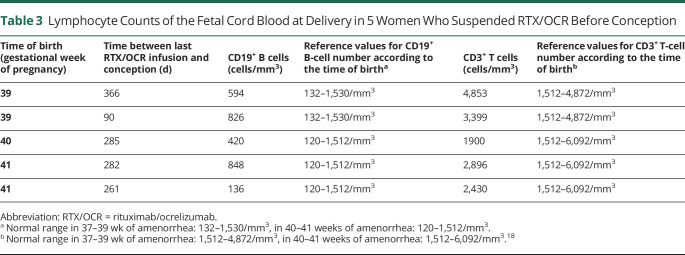
Among women who suspended NTZ, there were 3 miscarriages, and 1 newborn had a cardiac abnormality. Among women who suspended RTX/OCR, there were 3 miscarriages, and 1 pregnancy was terminated for cardiac malformation. The mean (SD) pregnancy duration did not differ between the 2 groups (8.6 [0.5] months for the NTZ group and 8.7 [0.7] months for the RTX/OCR group, p = 0.33). A case of febrile bacteremia of Klebsiella oxytoca occurred in a woman who suspended RTX; she had been hospitalized for threatened preterm labor with shortening of the cervix, which resulted in premature birth (31 weeks of amenorrhea). Bacteriologic analysis of the placenta ruled out chorioamnionitis. The outcome was favorable for the newborn and the mother, with a probabilistic treatment of urinary tract infection. All other newborns of women who suspended RTX/OCR before conception had morphologic measures (weights and length) between 3 and 97 percentiles of the national normative values.17 Lymphocyte counts measured on fetal cord blood were available for 5 newborns of women who suspended RTX/OCR before conception and did not show any abnormalities as compared with normal values18 (Table 3).
Table 3.
Lymphocyte Counts of the Fetal Cord Blood at Delivery in 5 Women Who Suspended RTX/OCR Before Conception
Discussion
This study revealed that in women with highly active MS, the therapeutic strategy consisting of suspending RTX/OCR in the year before conception was associated with lower risk of disease reactivity and disability progression in pregnancy and postpartum than a strategy of maintaining NTZ up to the end of the first trimester. Crucially, we found no increased incidence of miscarriage or birth defect in women who suspended OCR/RTX before conception, in accordance with previous studies (see references 19 and 20 for review).
A recent study performed in a large sample warned about the potential severe disease reactivation in patients stopping NTZ for pregnancy.4 Importantly, this study found that continuing NTZ during the first trimester and restarting NTZ within the first 4 weeks after delivery did not fully prevent the risk of severe disease reactivation. Accordingly, we found that a short delay in restarting treatment after birth in the NTZ group did not decrease the risk of disease evolution during the year after birth. In that way, patients with highly active MS treated with NTZ who are planning pregnancy are recommended to maintain NTZ until the end of the second trimester and to restart NTZ as soon as possible after delivery.7-9 However, this strategy does not prevent return of disease activity in all patients because 20% of women treated during the second trimester had at least 1 new T2 lesion and 13% at least 1 enhancing lesion after delivery.8 By contrast, only 1/31 (3%) patients of our RTX/OCR group had at least 1 new T2 lesion after delivery.
Recent studies argued about the potential value of the strategy of suspending RTX before conception to prevent disease reactivation in pregnancy and postpartum.6,12-14,16,21 This study supports the relevancy of this strategy in women with MS who are planning pregnancy, comparing for the first time this strategy to maintaining NTZ up to the end of the first trimester in 2 groups of patients with the same demographic and clinical characteristics. The probability of maintaining NEDA-3 between conception and 1 year after birth was 20-fold higher in patients suspending anti-CD20 in the year before conception than those continuing NTZ up to the end of the first trimester and restarting NTZ within the 4 weeks after birth.
Numerous studies demonstrated that the efficacy of RTX/OCR outlasts 6 months in MS, suggesting that renewing the immune system contributes to the efficacy of these therapies.12,22-27 This long-lasting efficacy enables planning for pregnancy in patients with highly active MS without the need to infuse treatment after conception. This strategy prevents the risk of B-cell depletion in the newborn because of transfer of RTX/OCR across the placenta. Indeed, we did not find abnormal B-cell counts in the fetal cord blood analysis available for 5 newborns. Of note, the long-lasting efficacy of RTX/OCR allows for not restarting treatment immediately after birth. In this study, the mean time to restarting treatment after birth in the anti-CD20 group was 123 days, and no patient exhibited disease reactivity during the year after birth in this group. However, the number of patients included in this study is too small to conclude on whether we can safely wait a long time postpartum to resume anti-CD20 therapy.
This study has several limitations. First, the allocation of the specific strategy (NTZ vs RTX/OCR) was not randomized and was mainly driven by the evolution of clinical practice in our MS center. Of note, the demographics and the level of disease activity in the 2 groups were similar before pregnancy, which enabled comparison of the efficacy of the 2 therapeutic strategies after conception. Second, the relatively small number of patients prevents any definitive conclusion about the safety profile of these 2 strategies including the risk of miscarriage or malformations. Of note, the existence of infection in a woman who previously received RTX should be interpreted considering the previous studies that clearly found increased risk of infection in patients with MS receiving anti-CD20 therapies.28 Moreover, because of the lack of follow-up of infants after birth, we cannot rule out the potential increased risk of infection or reduced vaccine efficacy. Third, the lack of inclusion of patients who maintained NTZ until the end of the second trimester prevents a definitive conclusion about the potential difference of these 2 strategies for control of disease activity. Finally, the very large CI of the OR of the probability of not maintaining NEDA-3 with the NTZ strategy prevents a definitive conclusion about the extent of this risk.
This study reveals that the strategy of suspending RTX/OCR in the year before conception in women with highly active MS who are planning pregnancy is associated with very low disease reactivity during pregnancy and the year after birth. At the time of therapy choice, this option should be considered in women with highly active MS who are planning pregnancy whatever their anti-JCV antibody serostatus. In women already receiving NTZ who are planning pregnancy, the possibility to switch to RTX/OCR for planning pregnancy instead of maintaining NTZ until the end of the second trimester should be discussed.
Glossary
- EDSS
Expanded Disability Status Scale
- NTZ
natalizumab
- OCR
ocrelizumab
- OR
odds ratio
- RTX
rituximab
Appendix. Authors
Study Funding
The authors report no targeted funding.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/NN for full disclosures.
References
- 1.Tintore M, Vidal-Jordana A, Sastre-Garriga J. Treatment of multiple sclerosis—success from bench to bedside. Nat Rev Neurol. 2019;15(1):53-58. doi: 10.1038/s41582-018-0082-z [DOI] [PubMed] [Google Scholar]
- 2.Krysko KM, Bove R, Dobson R, Jokubaitis V, Hellwig K. Treatment of women with multiple sclerosis planning pregnancy. Curr Treat Options Neurol. 2021;23(4):11. doi: 10.1007/s11940-021-00666-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demortiere S, Rico A, Maarouf A, Boutiere C, Pelletier J, Audoin B. Maintenance of natalizumab during the first trimester of pregnancy in active multiple sclerosis. Mult Scler. 2021;27(5):712-718. doi: 10.1177/1352458520912637 [DOI] [PubMed] [Google Scholar]
- 4.Hellwig K, Tokic M, Thiel S, et al. Multiple sclerosis disease activity and disability following discontinuation of natalizumab for pregnancy. JAMA Netw Open. 2022;5(1):e2144750. doi: 10.1001/jamanetworkopen.2021.44750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Portaccio E, Moiola L, Martinelli V, et al. Pregnancy decision-making in women with multiple sclerosis treated with natalizumab: II: maternal risks. Neurology. 2018;90(10):e832-e839. doi: 10.1212/WNL.0000000000005068 [DOI] [PubMed] [Google Scholar]
- 6.Yeh WZ, Widyastuti PA, Van der Walt A, et al. Natalizumab, fingolimod, and dimethyl fumarate use and pregnancy-related relapse and disability in women with multiple sclerosis. Neurology. 2021;96(24):e2989-e3002. doi: 10.1212/WNL.0000000000012084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krysko KM, Dobson R, Alroughani R, et al. Family planning considerations in people with multiple sclerosis. Lancet Neurol. 2023;22(4):350-366. doi: 10.1016/S1474-4422(22)00426-4 [DOI] [PubMed] [Google Scholar]
- 8.Landi D, Bovis F, Grimaldi A, et al. Exposure to natalizumab throughout pregnancy: effectiveness and safety in an Italian cohort of women with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2022;93:1306-1316. doi: 10.1136/jnnp-2022-329657 [DOI] [PubMed] [Google Scholar]
- 9.Vukusic S, Carra-Dalliere C, Ciron J, et al. Pregnancy and multiple sclerosis: 2022 recommendations from the French multiple sclerosis society. Mult Scler. 2023;29(1):11-36. doi: 10.1177/13524585221129472 [DOI] [PubMed] [Google Scholar]
- 10.Proschmann U, Thomas K, Thiel S, Hellwig K, Ziemssen T. Natalizumab during pregnancy and lactation. Mult Scler. 2018;24(12):1627-1634. doi: 10.1177/1352458517728813 [DOI] [PubMed] [Google Scholar]
- 11.Valero-López G, Millán-Pascual J, Iniesta-Martínez F, et al. Treatment with natalizumab during pregnancy in multiple sclerosis: the experience of implementing a clinical practice protocol (NAP-30). Mult Scler Relat Disord. 2022;66:104038. doi: 10.1016/j.msard.2022.104038 [DOI] [PubMed] [Google Scholar]
- 12.Juto A, Fink K, Al Nimer F, Piehl F. Interrupting rituximab treatment in relapsing-remitting multiple sclerosis; no evidence of rebound disease activity. Mult Scler Relat Disord. 2020;37:101468. doi: 10.1016/j.msard.2019.101468 [DOI] [PubMed] [Google Scholar]
- 13.Kümpfel T, Thiel S, Meinl I, et al. Anti-CD20 therapies and pregnancy in neuroimmunologic disorders: a cohort study from Germany. Neurol Neuroimmunol Neuroinflamm. 2021;8(1):e913. doi: 10.1212/NXI.0000000000000913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Razaz N, Piehl F, Frisell T, Langer-Gould AM, McKay KA, Fink K. Disease activity in pregnancy and postpartum in women with MS who suspended rituximab and natalizumab. Neurol Neuroimmunol Neuroinflamm. 2020;7(6):e903. doi: 10.1212/NXI.0000000000000903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seyed Ahadi M, Sahraian MA, Baghbanian SM, et al. Pregnancy outcome in patients with multiple sclerosis treated with rituximab: a case-series study. Mult Scler Relat Disord. 2021;47:102667. doi: 10.1016/j.msard.2020.102667 [DOI] [PubMed] [Google Scholar]
- 16.Smith JB, Hellwig K, Fink K, Lyell DJ, Piehl F, Langer-Gould A. Rituximab, MS, and pregnancy. Neurol Neuroimmunol Neuroinflamm. 2020;7(4):e734. doi: 10.1212/NXI.0000000000000734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vendittelli F, Rivière O, Crenn-Hébert C, et al. [Audipog perinatal network. Part 1: principal perinatal health indicators, 2004-2005]. Gynecol Obstet Fertil. 2008;36(11):1091-1100. doi: 10.1016/j.gyobfe.2008.08.010 [DOI] [PubMed] [Google Scholar]
- 18.Amatuni GS, Sciortino S, Currier RJ, Naides SJ, Church JA, Puck JM. Reference intervals for lymphocyte subsets in preterm and term neonates without immune defects. J Allergy Clin Immunol. 2019;144(6):1674-1683. doi: 10.1016/j.jaci.2019.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dobson R, Rog D, Ovadia C, et al. Anti-CD20 therapies in pregnancy and breast feeding: a review and ABN guidelines. Pract Neurol. 2023;23(1):6-14. doi: 10.1136/pn-2022-003426 [DOI] [PubMed] [Google Scholar]
- 20.Das G, Damotte V, Gelfand JM, et al. Rituximab before and during pregnancy: a systematic review, and a case series in MS and NMOSD. Neurol Neuroimmunol Neuroinflamm. 2018;5(3):e453. doi: 10.1212/NXI.0000000000000453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galati A, McElrath T, Bove R. Use of B-cell-depleting therapy in women of childbearing potential with multiple sclerosis and neuromyelitis optica spectrum disorder. Neurol Clin Pract. 2022;12(2):154-163. doi: 10.1212/CPJ.0000000000001147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boremalm M, Sundström P, Salzer J. Discontinuation and dose reduction of rituximab in relapsing–remitting multiple sclerosis. J Neurol. 2021;268(6):2161-2168. doi: 10.1007/s00415-021-10399-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Flon P, Gunnarsson M, Laurell K, et al. Reduced inflammation in relapsing-remitting multiple sclerosis after therapy switch to rituximab. Neurology. 2016;87(2):141-147. doi: 10.1212/WNL.0000000000002832 [DOI] [PubMed] [Google Scholar]
- 24.Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing–remitting multiple sclerosis. N Engl J Med. 2008;358(7):676-688. doi: 10.1056/NEJMoa0706383 [DOI] [PubMed] [Google Scholar]
- 25.Maarouf A, Rico A, Boutiere C, et al. Extending rituximab dosing intervals in patients with MS during the COVID-19 pandemic and beyond? Neurol Neuroimmunol Neuroinflamm. 2020;7(5):e825. doi: 10.1212/NXI.0000000000000825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rolfes L, Pawlitzki M, Pfeuffer S, et al. Ocrelizumab extended interval dosing in multiple sclerosis in times of COVID-19. Neurol Neuroimmunol Neuroinflamm. 2021;8(5):e1035. doi: 10.1212/NXI.0000000000001035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Starvaggi Cucuzza C, Longinetti E, Ruffin N, et al. Sustained low relapse rate with highly variable B-cell repopulation dynamics with extended rituximab dosing intervals in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2023;10(1):e200056. doi: 10.1212/NXI.0000000000200056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luna G, Alping P, Burman J, et al. Infection risks among patients with multiple sclerosis treated with fingolimod, natalizumab, rituximab, and injectable therapies. JAMA Neurol. 2020;77(2):184. doi: 10.1001/jamaneurol.2019.3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data analyzed during this study will be shared anonymized by reasonable request of a qualified investigator to the corresponding author.