Abstract
Background and Objectives
Neural antibodies are detected by tissue-based indirect immunofluorescence assay (IFA) in Mayo Clinic's Neuroimmunology Laboratory practice, but the process of characterizing and validating novel antibodies is lengthy. We report our assessment of human protein arrays.
Methods
Assessment of arrays (81% human proteome coverage) was undertaken using diverse known positive samples (17 serum and 14 CSF). Samples from patients with novel neural antibodies were reflexed from IFA to arrays. Confirmatory assays were cell-based (CBA) or line blot. Epitope mapping was undertaken using phage display immunoprecipitation sequencing (PhiPSeq).
Results
Control positive samples known to be reactive with linear epitopes of intracellular antigens (e.g., ANNA-1 [anti-Hu]) were readily identified by arrays in 20 of 21 samples. By contrast, 10 positive controls known to be enriched with antibodies against cell surface protein conformational epitopes (e.g., GluN1 subunit of NMDA-R) were indistinguishable from background signal. Three antibodies, previously characterized by other investigators (but unclassified in our laboratory), were unmasked in 4 patients using arrays (July-December 2022): Neurexin-3α, 1 patient; regulator of gene protein signaling (RGS)8, 1 patient; and seizure-related homolog like 2 (SEZ6L2), 2 patients. All were accompanied by previously reported phenotypes (encephalitis, 1; cerebellar ataxia, 3). Patient 1 had subacute onset of seizures and encephalopathy. Neurexin-3α ranked high in CSF (second ranked neural protein) but low in serum (660th overall). Neurexin-3α CBA was positive in both samples. Patient 2 presented with rapidly progressive cerebellar ataxia. RGS8 ranked the highest neural protein in available CSF sample by array (third overall). RGS8-specific line blot was positive. Patients 3 and 4 had rapidly progressive cerebellar ataxia. SEZ6L2 was the highest ranked neural antigen by arrays in all samples (CSF, 1, serum, 2; Patient 3, ranked 9th overall in CSF, 11th in serum; Patient 4, 6th overall in serum]). By PhIPSeq, diverse neurexin-3α epitopes (including cell surface) were detected in CSF from patient 1, but no SEZ6L2 peptides were detected for serum or CSF samples from Patient 3.
Discussion
Individualized autoimmune neurologic diagnoses may be accelerated using protein arrays. They are optimal for detection of intracellular antigen-reactive antibodies, though certain cell surface-directed antibodies (neurexin-3α and SEZ6L2) may also be detected.
Introduction
Neural IgG autoantibodies have proven essential for evaluation of patients with diverse neurologic disorders of subacute onset where autoimmunity is a suspected cause. Antibody positivity in serum, CSF, or both is not only informative for neurologic diagnosis but may provide data pertaining to the likelihood of paraneoplastic causation, immune treatment response, and prognosis.1 In the Mayo Clinic Neuroimmunology Laboratory, antibodies are initially detected using a composite of cryosectioned mouse tissues (including brain and also kidney and gut as controls) interpretable by indirect immunofluorescence assay (IFA). Individual characterized antibodies (e.g., Purkinje cytoplasmic antibody type 1 [or anti-Yo]) are identifiable with high accuracy by applying pattern-specific IFA diagnostic criteria.2 Antibodies detected by IFA are then confirmed for antigen specificity by reflex to a protein-specific assay (such as transfected cell-based assay, immune blot or ELISA).1 Antibodies of unknown antigen specificity are also routinely identified (known as unclassified neural antibodies). Some of these unknown antibodies are subsequently characterized in our own laboratory using western blot and immunoprecipitation assays, followed by mass spectrometry.3-5 Other antibodies, characterized elsewhere, but routinely encountered in our own laboratory by IFA, have been relatively easy to validate using commercially available cell-based assays. Examples include those targeting gamma aminobutyric acid (GABA) receptors, metabotropic glutamate receptors (mGluR), and dipeptidyl peptidase 6 (DPPX).6-13 Some recently reported (and seemingly rarer) antibodies are more challenging to identify from among a multitude of diverse and rarely encountered unclassified staining patterns, which collectively account for the 5th most common serologic entity in our laboratory (after MOG, NMDA-R, high-titer GAD65, and Lgi1). In some circumstances, that antibody may coexist with a readily identifiable IgG (e.g., KCTD16 coexisting with GABABR antibody).14
In 2018, we reported the utility of protein microarrays containing 48% of human canonical proteins for validation of our discovery of septin-5-IgG.15 Herein, we report our assessment of contemporary protein microarrays (81% coverage), using diverse patient serum and CSF samples with known neural antibodies. We also report our early experience in 4 patients with seemingly unclassified antibodies detected by IFA, but in whom recently reported IgGs (neurexin-3α, regulator of gene protein signaling [RGS]8 and seizure-related 6 homolog like [SEZ6L]2) were detected using arrays and then confirmed by individual protein-specific assays. For neurexin-3α and SEZ6L2 antibodies, we also cross-checked for cell surface reactivity using whole-human proteome phage display immunoprecipitation sequencing (PhIPSeq).
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
This retrospective study was approved by the Mayo Clinic Institutional Review Board (IRB, 21-001297). Medical records of patients who consented to research review were included.
Arrays
Microarrays contained >21,000 human proteins (HuProt v4.0, CDI Laboratories, Puerto Rico).16 The expression library was created by inserting full-length human open reading frames (ORFs) into a yeast high-copy expression vector that produces fusion proteins when induced in yeast.16,17 Per the manufacturer, the ORFs were obtained from public ORF libraries or independently synthesized by Zhu and Blackshaw (Johns Hopkins University).16 Each microarray contains 15,889 of total 19,613 canonical human proteins (81%) in recombinant form (N-terminal GST and RGS-His6–tagged), preprinted in duplicate on nitrocellulose-coated glass slides. After blocking, 4 mL of total specimen volume (serum [1:1000] dilution or CSF [1:50]) was added to microarray surfaces. After incubation and washing, probing was done with goat-anti-human IgG secondary (AlexaFluor 647). After washing and drying, secondary anti-GST staining was applied. Fluorescence intensity was evaluated (Genepix 4000B scanner). Using Excel, gene names for proteins were arranged in descending order of AF647 signal. Control proteins and nonspecific reactivities with immunoglobulins and Ig fragments were removed by filtering (except for IgLON molecules), with a remaining list in each case of 10,000–25000 hits (which included data for duplicate protein spots). Gene names were interrogated for protein and RNA expression data (organ, brain regional enrichment, and cellular location) using the Human Protein Atlas (proteinatlas.org). Those documented in that atlas as having brain-enriched tissue RNA were considered neural protein hits.
Samples Tested
Array validation samples were 17 serums and 14 CSFs (all high titer) from patients with known neural IgG positivities (Tables 1 and 2). These were contemporary samples from the clinical laboratory mostly without clinical information available. The relevant proteins were confirmed to be included in the arrays by referring to manufacturer's manual. Patient samples (July to December 2022) were 87 specimens (44 CSF and 43 serums) with unclassified neural antibodies detected by tissue-based IFA. Of those, 4 patients harbored antibodies detected as high-ranking hits recently characterized in other laboratories yet to be identified in our own. Available samples were CSF (3 patients) and serum (3 patients). Confirmatory protein-specific assays, cell-based (CBA) or line blot, were performed, as previously described (EUROIMMUN, AG).8,18 Two patients were clinically evaluated in the Department of Neurology, Mayo Clinic, Rochester, MN; one at the University of Virginia, Charlottesville, VA, and one elsewhere.
Table 1.
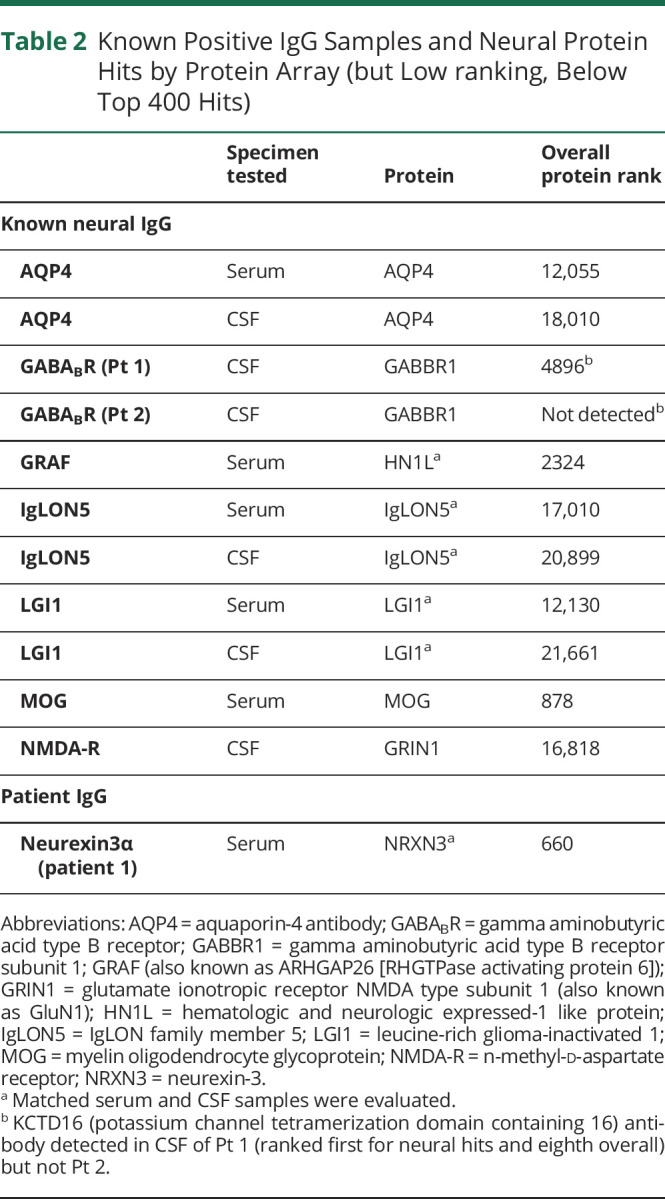
Known Positive IgG Samples and Neural Protein Hits by Protein Array (and High Ranking, Within Top 400 Hits)
Table 2.
Known Positive IgG Samples and Neural Protein Hits by Protein Array (but Low ranking, Below Top 400 Hits)
Epitope Mapping
Phage Immunoprecipitation Sequencing
Ten microliters of CSF were incubated with 4 × 1010 plaque-forming units of the whole-human proteome phage display library, and antibody-bound phage particles were isolated by protein A/G immunoprecipitation. Patient antibody-bound phage particles were eluted, and next-generation sequencing libraries were prepared using the Illlumina TruSeq Nano DNA library preparation kit with unique barcodes for each sample. Prepared libraries were sequenced on the Illumina Next Seq 1000 platform to a depth of approximately 1 million reads per sample. Sequenced reads were processed using an in-house developed bioinformatics pipeline to identify putative autoantigens.
The bioinformatics method started by quality assessing the raw read data of each sample using FASTQC software.19 Reads were then mapped against a custom-built reference containing the 150bp-length oligo sequences, each representing a peptide, using bwa-mem software configured with default parameters.20 An in-house developed program written in AWK programming language processed the aligned data and enumerated the total number of reads that perfectly mapped (i.e., full length match with no sequence modifications) to each peptide. Sample-wise peptides and their respective read counts were loaded into R programming environment (version 4.0.1) to generate enrichment scores, as previously described.21 This process started by generating the mean read count observed for each peptide in control CSF samples. A peptide enrichment score was computed for each (sample, peptide) pair by taking the ratio of counts observed in that pair to the mean count observed in control CSF.
Using available residual CSF sample from patient 1 (neurexin-3α antibody positive) and serum and CSF from patient 3 (SEZ6L2 antibody positive), specific peptides were mapped to their canonical proteins (UniProt: Q9Y4C0 and Q6UXD5). The phage library contained peptides spanning the entire extracellular region of neurexin-3α and SEZ6L2 and portions of intracellular protein regions. For neurexin-3α and SEZ6L2, respectively, enrichment scores for each peptide were plotted as a function of the first amino acid position of each peptide on the canonical protein.
Data Availability
Anonymized data used for this study are available on request.
Results
Protein Array Assessment
Control IgGs known to be reactive with linear epitopes of intracellular antigens (e.g., antineuronal nuclear antibody type 1 [also known as anti-Hu]) ranked highly by protein array in all but 1 of 21 samples (13 serums, 8 CSFs) Table 1. The exception was a GRAF-IgG positive serum (ranked 2324 overall), although was readily identifiable in CSF (10th overall), Tables. Control IgGs known to be reactive with 3D confirmation-dependent epitopes of cell surface antigens (e.g., GluN1 subunit of NMDA-R) ranked very low in all 10 samples (4 serums and 6 CSFs), Table 2. Although the cell surface antigen GABABR was not a high ranking hit in 2 known GABABR-positive CSF controls (Pt 1 and Pt 2), cytoplasmic potassium channel tetramerization domain containing 16 (KCTD16) was the first ranked neural hit, and eighth overall in the CSF of Pt1, an elderly patient with a background of chronic obstructive pulmonary disease, who presented with limbic encephalitis with prominent seizures, had hyponatremia, and was found to have radiologic evidence of metastatic carcinoma (lung and adrenal gland lesions) and received palliative management. KCTD16 was not detected in CSF of a teenage patient, without cancer, who had refractory status epilepticus and responded to immunotherapy.
Unclassified Antibody Cohort
Of the 87 samples tested, 6 had antibodies recently characterized in other laboratories detected by protein array (7%: serum, 3; CSF, 3) in a total of 4 patients (median age at symptom onset was 62 years; 3 were men). Serologic diagnoses for the 4 patients were neurexin-3α, 1 patient; RGS8, 1 patient; and SEZ6L2, 2 patients, confirmed by protein-specific assay in each sample.
Patient 1
The patient presented with subacute onset of seizures, emotional lability, and cognitive impairment. Brain MRI was unremarkable. EEG recorded myoclonic seizures. CSF revealed 27 white blood cells/µL (normal, ≤5), lymphocyte predominant (89%), and protein of 53 mg/dL (normal, ≤35 mg/dL). Oligoclonal bands and CSF cytology were negative. Positive antinuclear, double-stranded DNA and extractable nuclear antigen serologies prompted ‘neuropsychiatric SLE’ as diagnosis. Cancer screening with abdominal ultrasonography and abdominal MRI did not reveal an underlying tumor. Tissue IFA of both serum and CSF revealed a synaptic/neural pattern of IgG staining (Figure 1), resembling glutamic acid decarboxylase 65 kilodalton isoform (GAD65) antibody, but GAD65 immunoprecipitation assay was negative. Protein microarray testing of CSF disclosed reactivity with protein encoded by NRXN3 (neurexin-3α, second highest ranked neural antigen), although it was a low ranking hit in serum (>600th). The 1st ranked neural hit in CSF was OLIG2 (oligodendrocyte transcription factor 2; this ranked 258th in serum). OLIG2 is enriched in oligodendrocytes, rather than neuropil. Neurexin-3α CBA was positive in both serum and CSF (Figure 1). The patient was started on levetiracetam with no improvement, but a course of IV methylprednisolone followed by oral prednisone taper resulted in complete resolution of seizures and encephalopathy. The patient also received azathioprine and hydroxychloroquine for long-term management, given the SLE diagnosis.
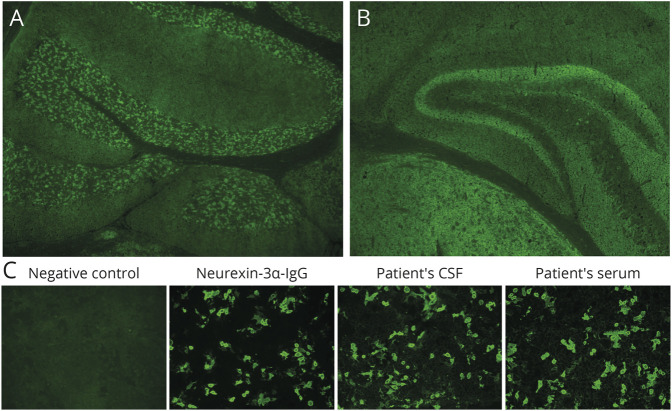
Figure 1. Neurexin-3α Antibody by Tissue IFA and Cell-Based Assay.
Top, murine tissue-based indirect IFA. Patient CSF produced a synaptic pattern of IgG staining of the cerebellum (A) and hippocampus and thalamus (B). (C) NRXN3-transfected CBA. Neurexin3α-specific antibody, patient CSF, and patient serum (but not serum from a healthy donor [negative control]) were reactive. Nontransfected cells were nonreactive (not shown). IFA = immunofluorescence assay.
Patient 2
The patient presented with a rapidly progressive cerebellar syndrome and reported subacute onset of diplopia, vertigo, and ataxia. There was rapid progression to use of a cane and by 2 months, a walker. Neurologic examination revealed a severe pancerebellar syndrome. Brain MRI revealed mild cerebellar atrophy. CSF analysis revealed white cell count of 104/µL (lymphocyte predominant), protein of 85 mg/dL, and 4 CSF-exclusive oligoclonal bands (normal, <2). CSF cytology was negative. Cancer screening with CT scans of the chest, abdomen, and pelvis; testicular ultrasonography; esophagogastroduodenoscopy; and colonoscopy did not reveal an underlying neoplasm. Whole-body FDG-PET showed FDG avid lymph nodes although histology showed no evidence of lymphoma or other neoplasia. Tissue IFA of CSF revealed a cloudy pattern of cerebellar molecular layer synaptic staining and Purkinje cell cytoplasmic staining (Figure 2).22 Protein microarray testing of CSF disclosed reactivity with RGS8 (highest ranked neural-predominant protein, third overall highest ranked antigen, Figure 2). RGS8-specific line blot was positive (Figure 2). Corticosteroids, rituximab, and cyclophosphamide were followed by neurologic stabilization.
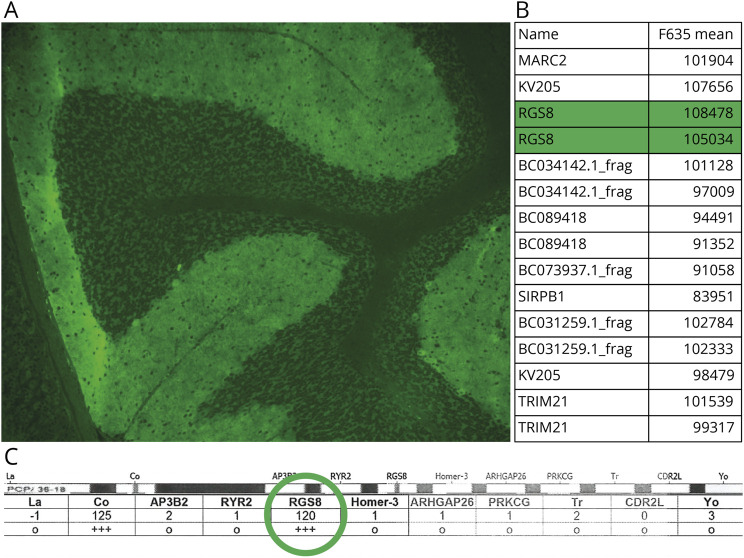
Figure 2. RGS8 Antibody by Tissue IFA, Protein Array, and Cell-Based Assay.
(A) Tissue indirect IFA revealed ‘smudgy’ synaptic staining of cerebellar molecular layer and Purkinje cells. (B) RGS8 ranked 3rd highest protein overall by protein array. (C) RGS8 was strongly positive by a line blot for ataxia-pertinent antigens. IFA = immunofluorescence assay.
Patients 3 and 4
Patient 3 presented with rapid-onset cerebellar ataxia and autonomic dysfunction. CSF revealed lymphocytic pleocytosis of 26/µL (95% lymphocytes) and an elevated protein of 103 mg/dL. MRI of the brain revealed generalized atrophy and nonspecific white matter T2 hyperintensities. CT of the chest abdomen and pelvis and FDG-PET scan were negative for malignancy. The patient was treated with IV methylprednisolone with taper, IVIg, and cyclophosphamide, which was followed by neurologic stabilization but no improvement. Patient 4 had a rapidly progressive cerebellar syndrome over 15 months and became walker dependent. CSF white cell count, protein, glucose, and oligoclonal band tests were normal or negative. Brain MRI demonstrated mild diffuse cerebellar volume loss. Treatment and outcome data were not available. Neither patient had a family history of ataxia. Tissue IFA of serum (both patients) and CSF (available in patient 3) revealed identical patterns of cytoplasmic staining on a synaptic background, most prominent in the cerebellar granular layer and Purkinje cells and the cerebral cortex (Figure 3). Protein microarray testing of serum and CSF disclosed reactivity with SEZ6L2. This was the highest ranked neural antigen in all samples, (9th overall in CSF and 11th in serum for patient 3 and 6th overall for patient 4). SEZ6L2 CBA was positive in all specimens.
Figure 3. SEZ6L2 Antibody by Tissue IFA.

Murine tissue-based indirect IFA from serum of patient 4 demonstrates neuronal cytoplasmic staining on a synaptic background, most prominent in the cerebellar granular layer and Purkinje cells (A) but also in the striatum (B), hippocampus (C), cerebral cortex (D) with adjacent myenteric nerves in the composite (arrow). HuProt array disclosed SEZ6L2 as high-ranking hits in serum (both patients) and CSF (available in patient 3), and SEZ6L2-transfected CBAs were positive in all samples (not shown). IFA = immunofluorescence assay.
Peptide Epitope Mapping for Neurexin-3α and SEZ6L2
Enrichment peptide scores were ranked from highest to lowest in the patient's samples, and rankings were compared with controls, eFigure 1 (links.lww.com/NXI/A882). For neurexin-3α, of the 20 most highly enriched IgGs in the patient's CSF, neurexin-3α–targeted peptides accounted for 7 (rank: 1, 2, 4, 9, 10, 17) but none in the CSF of controls. Highly enriched neurexin-3α peptides mapped to 2 distinct regions, the N-terminal EGF-1 like domain and a lamin-neurexin-sex hormone-binding globulin domain (LNS6), near the C-terminus. Both domains are extracellular. For all SEZ6L2-positive samples, serum and CSF, peptide scores ranked too low to provide an enrichment profile.
Discussion
Microarrays containing a large representation of the human proteome have potential to aid in rapid detection of certain neural antibodies. In the validation component of our study, all the classical neuronal nuclear, cytoplasmic, and nucleolar antibodies (Hu, Yo, Ri, CRMP-5, Ma2, amphiphysin, and GAD65) and some more recently described (TRIM46, KLHL11, neurochondrin, and DACH1) were readily identifiable, as was KCTD16 (a cytoplasm-located auxiliary subunit of the GABAB receptor reported as a biomarker of high prediction for small cell carcinoma among patients with GABAB receptor encephalitis).14,23 By contrast, the arrays were insensitive for detection of antibodies against well-characterized cell surface antigens (NMDA-R, GABA-B-R, Lgi1, MOG, IglON5, and AQP4). Despite this, 2 of our 3 patients prospectively characterized had antibodies directed against transmembrane proteins (neurexin-3α and SEZ6L2) for which extracellular antibody binding and downstream pathogenic effects have been reported.24,25 Other potential drawbacks of the array-based approach include the lack of complete proteome coverage (e.g., contactin-1 is not included in the current iteration, but is an important biomarker26,27) and current cost of arrays (approximately $650 each). Future iterations of arrays, if developed for clinical use, may permit rapid multiplexed assessment of mostly neuronal nuclear and cytoplasmic antibodies.
Protein arrays may also provide the means to rapidly identify certain high likelihood novel targets more rapidly than was possible heretofore, whereby extensive experiments were required to fully characterize antigens of interest.28,29 Some ‘novel’ staining patterns we had observed on tissue and subsequently evaluated in detail using western blot, immunoprecipitation, and mass spectrometry ultimately proved to be antibodies already discovered by other investigators.30-35 By contrast, with protein arrays, we were able to rapidly identify in ‘real-time’ neurexin-3α, RGS8, and SEZ6L2 antibodies. Each specificity was confirmed by other protein-specific assays (cell-based or line blot). Both clinical phenotypes and review of tissue-staining characteristics were consistent with data previously published.18,24,25,36,37 Similar to our patient 1, Gresa-arribas et al. reported 5 patients with neurexin-3α autoimmunity and an encephalitic phenotype.25 Their patients presented with prodromal fever, headache, or gastrointestinal symptoms, followed by confusion, seizures, and decreased level of consciousness. Mild orofacial dyskinesias developed in 2 patients. CSF was abnormal in all patients (4 pleocytosis, 1 elevated immunoglobulin G [IgG] index). Like patient 2, Miske et al.18 reported 4 patients with RGS8 autoimmunity and a cerebellar phenotype, accompanied by B-cell lymphoma of the stomach or Hodgkin lymphoma in 2 patients. Lymphoma was suspected in our patient but not detected. Like our patient 2, CSF analysis showed lymphocytic pleocytosis, slightly elevated protein level, and the presence of CSF-specific oligoclonal bands. Similar to our patients 3 and 4, prior reported patients with SEZ6L2 autoimmunity have had cerebellar ataxia.36-38 Although patients with autoimmune cerebellar ataxia rarely have significant improvements with immune therapy, stabilization is common.39
Studies evaluating the 3D crystallographic structure of individual array protein are not available, although it seems likely that tagged proteins would be impeded from folding into tertiary structural conformation. This contention is supported by the insensitivity of the protein arrays for potentially pathogenic cell surface–directed antibodies that may recognize specific protein conformations or discontinuous epitopes in our assessment. For example, the protein array was insensitive for aquaporin-4 (AQP4) antibody, in contrast to our laboratory's prior experience with western blot using M1 or M23 AQP4 isoforms, solubilized in native form from transfected cell lines, whereby 68% of NMO patients but no controls were positive.40 Nonetheless it is possible that for certain other autoimmune diseases, antibody populations (owing to polyclonality) may consist of IgGs reactive with both linear and conformation dependent epitopes. For example, we detected antibody directed against neurexin-3α (a synaptic cell-cell adhesion molecule) as a high ranking hit in CSF of 1 patient and antibody targeting SEZ6L2 (a transmembrane protein binding partner of GluA1 subunit of AMPA receptors) in another. Neurexin-3α antibody is reported to produce live cell binding and pathogenic effects in vitro.25 SEZ6L2 antibody is reported to inhibit the interaction between SEZ6L2 and GluA1 and impair the AMPA receptor signaling pathway.24 In addition, our patient's CSF was enriched with antibodies for neurexin-3α peptides mapped to 2 distinct regions, the N-terminal EGF-1–like domain and a more C-terminally located lamin-neurexin-sex hormone-binding globulin domain (LNS6), consistent with the importance of live cell binding to extracellular regions. Both domains are extracellular and are present in most neurexin 3 transcripts expressed in the brain.41 Multiple distinct peptides from the LNS6 domain were found to be enriched in the patient. Interestingly, in the homologous protein neurexin-1, the LNS6 domain plays an important role in ligand binding where it essential for neurexin interaction with dystroglycans.42 Future studies should determine whether the LNS6 domain of neurexin-3α is a common target and whether antibodies binding this region may have a pathogenic consequence. We did not detect an enrichment profile for SEZ6L2 in serum or CSF. We surmise that, much like protein arrays, the sensitivity of peptide-based techniques, such as PhIPSeq, is variable for cell surface antibodies. For sensitivity for some intracellular targets, full-length proteins may have advantages over peptides, which were only 38% sensitive for ANNA-1 (anti-Hu).43
Protein microarrays are useful for novel antibody screening and may expedite individualized serologic diagnoses. For the remaining 81 of 87 samples in which we did not detect known antigens, future studies will focus on integrating the protein array technology into our existing efforts to characterize unclassified neural antibody biomarkers of autoimmune neurologic disease.
Glossary
- AQP4
aquaporin-4
- GABA
gamma aminobutyric acid
- GAD65
glutamic acid decarboxylase 65 kilodalton isoform
- IFA
immunofluorescence assay
- KCTD16
potassium channel tetramerization domain containing 16
- PCA-1
Purkinje cytoplasmic antibody type 1
Appendix. Authors
Study Funding
NIH (RO1NS126227).
Disclosure
A. McKeon reports research funding from NIH (NIH: RO1NS126227, U01NS120901), patents issued for GFAP and MAP1B-IgGs and patents pending for PDE10A, Septins-5 and -7, and KLCHL11-IgGs, and has consulted for Janssen and Roche pharmaceuticals, without personal compensation. C. Lesnick reports no disclosures relevant to the manuscript. N. Vorasoot reports no disclosures relevant to the manuscript. M.W. Buckley reports no disclosures relevant to the manuscript. S. Dasari reports no disclosures relevant to the manuscript. E.P. Flanagan has funding from NIH (R01NS113828), has served on advisory boards for Alexion, Genentech, Horizon Therapeutics and UCB, has received honoraria from Pharmacy Times and UpToDate, and has a patent pending for DACH1-IgG as a biomarker of paraneoplastic autoimmunity; M. Gilligan reports no disclosures relevant to the manuscript. R. Lafrance Corey reports no disclosures relevant to the manuscript. R. Miske is employed by Euroimmun. S.J. Pittock is a named inventor on filed patents that relate to functional AQP4/NMO-IgG assays and NMO-IgG as a cancer marker, has patents pending for KLHL11-IgG and Septin-5-IgG, and issued for MAP1B-IgG as markers of neurologic autoimmunity and paraneoplastic disorders, has consulted for Alexion and Medimmune; and has received research support from Genentech, Grifols, Medimmune, and Alexion; M Scharf is employed by Euroimmun. B. Yang reports no disclosures relevant to this manuscript. A. Zekeridou has patent applications pending on PDE10A-IgG and DACH1-IgG as biomarkers of paraneoplastic neurologic autoimmunity and has received research funding from Genentech; D. Dubey has research support from Department of Defence (CA210208), Centers of Multiple Sclerosis and Autoimmune Neurology, and Clinical and Translational Science, Mayo Clinic, and Grifols pharmaceuticals, has consulted for UCB, Immunovant, Argenx and Astellas pharmaceuticals (compensation for consulting activities paid directly to Mayo Clinic), and has patents pending for KLHL11-IgG, LUZP4-IgG, and cavin-4-IgG as markers of neurologic autoimmunity; J Mills reports no disclosures relevant to the manuscript. Go to Neurology.org/NN for full disclosures.
References
- 1.Budhram A, Dubey D, Sechi E, et al. Neural antibody testing in patients with suspected autoimmune encephalitis. Clin Chem. 2020;66(12):1496-1509. doi: 10.1093/clinchem/hvaa254 [DOI] [PubMed] [Google Scholar]
- 2.Altermatt HJ, Rodriguez M, Scheithauer BW, Lennon VA. Paraneoplastic anti-Purkinje and type I anti-neuronal nuclear autoantibodies bind selectively to central, peripheral, and autonomic nervous system cells. Lab Invest. 1991;65(4):412-420. [PubMed] [Google Scholar]
- 3.Hinson SR, Honorat JA, Grund EM, et al. Septin-5 and -7-IgGs: neurologic, serologic, and pathophysiologic characteristics. Ann Neurol. 2022;92(6):1090-1101. doi: 10.1002/ana.26482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zekeridou A, Kryzer T, Guo Y, et al. Phosphodiesterase 10A IgG: a novel biomarker of paraneoplastic neurologic autoimmunity. Neurology. 2019;93(8):e815-e822. doi: 10.1212/wnl.0000000000007971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mandel-Brehm C, Dubey D, Kryzer TJ, et al. Kelch-like protein 11 antibodies in seminoma-associated paraneoplastic encephalitis. N Engl J Med. 2019;381(1):47-54. doi: 10.1056/nejmoa1816721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tobin WO, Lennon VA, Komorowski L, et al. DPPX potassium channel antibody: frequency, clinical accompaniments, and outcomes in 20 patients. Neurology. 2014;83:1797-1803. doi: 10.1212/wnl.0000000000000991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeffery OJ, Lennon VA, Pittock SJ, Gregory JK, Britton JW, McKeon A. GABAB receptor autoantibody frequency in service serologic evaluation. Neurology. 2013;81(10):882-887. doi: 10.1212/wnl.0b013e3182a35271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Connor K, Waters P, Komorowski L, et al. GABAA receptor autoimmunity: a multicenter experience. Neurol Neuroimmunol Neuroinflamm. 2019;6(3):e552. doi: 10.1212/nxi.0000000000000552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez-Chiriboga AS, Komorowski L, Kumpfel T, et al. Metabotropic glutamate receptor type 1 autoimmunity: clinical features and treatment outcomes. Neurology. 2016;86(11):1009-1013. doi: 10.1212/wnl.0000000000002476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boronat A, Sabater L, Saiz A, Dalmau J, Graus F. GABA(B) receptor antibodies in limbic encephalitis and anti-GAD-associated neurologic disorders. Neurology. 2011;76(9):795-800. doi: 10.1212/wnl.0b013e31820e7b8d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petit-Pedrol M, Armangue T, Peng X, et al. Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol. 2014;13(3):276-286. doi: 10.1016/s1474-4422(13)70299-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boronat A, Gelfand JM, Gresa-Arribas N, et al. Encephalitis and antibodies to dipeptidyl-peptidase-like protein-6, a subunit of Kv4.2 potassium channels. Ann Neurol. 2013;73(1):120-128. doi: 10.1002/ana.23756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smitt PS, Kinoshita A, De Leeuw B, et al. Paraneoplastic cerebellar ataxia due to autoantibodies against a glutamate receptor. N Engl J Med. 2000;342(1):21-27. doi: 10.1056/nejm200001063420104 [DOI] [PubMed] [Google Scholar]
- 14.van Coevorden-Hameete MH, de Bruijn M, de Graaff E, et al. The expanded clinical spectrum of anti-GABABR encephalitis and added value of KCTD16 autoantibodies. Brain. 2019;142(6):1631-1643. doi: 10.1093/brain/awz094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Honorat JA, Lopez-Chiriboga AS, Kryzer TJ, Fryer JP, Devine M, Flores A, Lennon VA, Pittock SJ, McKeon A. Autoimmune septin-5 cerebellar ataxia. Neurol Neuroimmunol Neuroinflamm. 2018. Jul 9;5(5):e474. doi: 10.1212/NXI.0000000000000474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeong JS, Jiang L, Albino E, et al. Rapid identification of monospecific monoclonal antibodies using a human proteome microarray. Mol Cell Proteomics 2012;11(6):O111.016253. doi: 10.1074/mcp.o111.016253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu S, Xie Z, Onishi A, et al. Profiling the human protein-DNA interactome reveals ERK2 as a transcriptional repressor of interferon signaling. Cell 2009;139(3):610-622. doi: 10.1016/j.cell.2009.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miske R, Scharf M, Stark P, et al. Autoantibodies against the purkinje cell protein RGS8 in paraneoplastic cerebellar syndrome. Neurol Neuroimmunol Neuroinflamm. 2021;8(3):e987. doi: 10.1212/nxi.0000000000000987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wingett SW, Andrews S. FastQ screen: a tool for multi-genome mapping and quality control. F1000Res. 2018;7:1338. doi: 10.12688/f1000research.15931.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754-1760. doi: 10.1093/bioinformatics/btp324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dubey D, Beecher G, Hammami MB, et al. Identification of Caveolae-associated protein 4 autoantibodies as a biomarker of immune-mediated rippling muscle disease in adults. JAMA Neurol 2022;79(8):808-816. doi: 10.1001/jamaneurol.2022.1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jarius S, Wildemann B. Medusa head ataxia': the expanding spectrum of Purkinje cell antibodies in autoimmune cerebellar ataxia. Part 3: anti-Yo/CDR2, anti-Nb/AP3B2, PCA-2, anti-Tr/DNER, other antibodies, diagnostic pitfalls, summary and outlook. J Neuroinflammation. 2015;12(1):168. doi: 10.1186/s12974-015-0358-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teng X, Aouacheria A, Lionnard L, et al. KCTD: a new gene family involved in neurodevelopmental and neuropsychiatric disorders. CNS Neurosci Ther. 2019;25(7):887-902. doi: 10.1111/cns.13156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yaguchi H, Yabe I, Takahashi H, et al. Anti-Sez6l2 antibody detected in a patient with immune-mediated cerebellar ataxia inhibits complex formation of GluR1 and Sez6l2. J Neurol. 2018;265(4):962-965. doi: 10.1007/s00415-018-8785-z [DOI] [PubMed] [Google Scholar]
- 25.Gresa-Arribas N, Planaguma J, Petit-Pedrol M, et al. Human neurexin-3α antibodies associate with encephalitis and alter synapse development. Neurology. 2016;86(24):2235-2242. doi: 10.1212/wnl.0000000000002775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carrera-Garcia L, Natera-de Benito D, Lleixa C, et al. Chronic inflammatory demyelinating polyneuropathy associated with contactin-1 antibodies in a child. Neurol Neuroimmunol Neuroinflamm. 2019;6(5):e602. doi: 10.1212/nxi.0000000000000602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dubey D, Honorat JA, Shelly S, et al. Contactin-1 autoimmunity: serologic, neurologic, and pathologic correlates. Neurol Neuroimmunol Neuroinflamm. 2020;7(4):e771. doi: 10.1212/nxi.0000000000000771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu Z, Kryzer TJ, Griesmann GE, Kim K, Benarroch EE, Lennon VA. CRMP-5 neuronal autoantibody: marker of lung cancer and thymoma-related autoimmunity. Ann Neurol. 2001;49(2):146-154. [PubMed] [Google Scholar]
- 29.Zekeridou A, Yang B, Lennon VA, et al. Anti-neuronal nuclear antibody 3 autoimmunity targets dachshund homolog 1. Neurology 2022;99(23 Supplement 2):S7–S8. doi: 10.1212/01.wnl.0000903092.20137.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miske R, Gross CC, Scharf M, et al. Neurochondrin is a neuronal target antigen in autoimmune cerebellar degeneration. Neurol Neuroimmunol Neuroinflamm. 2017;4(1):e307. doi: 10.1212/nxi.0000000000000307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shelly S, Kryzer TJ, Komorowski L, et al. Neurochondrin neurological autoimmunity. Neurol Neuroimmunol Neuroinflamm. 2019;6:e612. doi: 10.1212/nxi.0000000000000612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Honorat JA, Lopez-Chiriboga AS, Kryzer TJ, et al. Autoimmune gait disturbance accompanying adaptor protein-3B2-IgG. Neurology 2019;93(10):e954-e963. doi: 10.1212/wnl.0000000000008061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Darnell RB, Furneaux HM, Posner JB. Antiserum from a patient with cerebellar degeneration identifies a novel protein in Purkinje cells, cortical neurons, and neuroectodermal tumors. J Neurosci. 1991;11(5):1224-1230. doi: 10.1523/jneurosci.11-05-01224.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dubey D, Honorat JA, Shelly S, et al. Contactin-1 autoimmunity serologic, neurologic, and pathologic correlates. Neurol Neuroimmunol Neuroinflamm. 2020;7(4):e771. doi: 10.1212/nxi.0000000000000771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Querol L, Nogales-Gadea G, Rojas-Garcia R, et al. Antibodies to contactin-1 in chronic inflammatory demyelinating polyneuropathy. Ann Neurol. 2013;73(3):370-380. doi: 10.1002/ana.23794 [DOI] [PubMed] [Google Scholar]
- 36.Landa J, Guasp M, Petit-Pedrol M, et al. Seizure-related 6 homolog like 2 autoimmunity: neurologic syndrome and antibody effects. Neurol Neuroimmunol Neuroinflamm. 2021;8(1):e916. doi: 10.1212/nxi.0000000000000916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehdiyeva A, Hietaharju A, Sipila J. SEZ6L2 antibody-associated cerebellar ataxia responsive to sequential immunotherapy. Neurol Neuroimmunol Neuroinflamm. 2022;9(2):e1131. doi: 10.1212/nxi.0000000000001131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yaguchi H, Yabe I, Takahashi H, et al. Identification of anti-Sez6l2 antibody in a patient with cerebellar ataxia and retinopathy. J Neurol 2014;261(1):224-226. doi: 10.1007/s00415-013-7134-5 [DOI] [PubMed] [Google Scholar]
- 39.Jones AL, Flanagan EP, Pittock SJ, et al. Responses to and outcomes of treatment of autoimmune cerebellar ataxia in adults. JAMA Neurol. 2015;72(11):1304-1312. doi: 10.1001/jamaneurol.2015.2378 [DOI] [PubMed] [Google Scholar]
- 40.Iorio R, Fryer JP, Hinson SR, et al. Astrocytic autoantibody of neuromyelitis optica (NMO-IgG) binds to aquaporin-4 extracellular loops, monomers, tetramers and high order arrays. J Autoimmun. 2013;40:21-27. doi: 10.1016/j.jaut.2012.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Treutlein B, Gokce O, Quake SR, Sudhof TC. Cartography of neurexin alternative splicing mapped by single-molecule long-read mRNA sequencing. Proc Natl Acad Sci USA. 2014;111(13):E1291-E1299. doi: 10.1073/pnas.1403244111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sugita S, Saito F, Tang J, Satz J, Campbell K, Südhof TC. A stoichiometric complex of neurexins and dystroglycan in brain. J Cell Biol. 2001;154(2):435-446. doi: 10.1083/jcb.200105003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Donovan B, Mandel-Brehm C, Vazquez SE, et al. High-resolution epitope mapping of anti-Hu and anti-Yo autoimmunity by programmable phage display. Brain Commun. 2020;2:fcaa059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data used for this study are available on request.