Abstract
Although postoperative neurological events due to brain compression by the swollen temporal muscle are a rare complication, the chronological volume changes of the temporal muscle pedicle and their clinical impact have not yet been documented. This prospective observational study aimed to investigate the chronological volume changes in the temporal muscle pedicle in Moyamoya disease (MMD). Eighteen consecutive combined revascularization procedures using the temporal muscle were performed for symptomatic MMD in 2021. The postoperative pedicle volume was quantified using repeated computed tomography images on postoperative days (PODs) 0, 1, 7, 14, and 30. Postoperative neurological events with radiological evaluations and collateral development evaluated using magnetic resonance angiography obtained 6 months after surgery were studied. On average, the postoperative temporal muscle pedicle volume was most significantly increased by as much as 112% ± 9.6% on POD 7 (P < 0.001) and decreased by as little as 52% ± 21% on POD 30 (P < 0.0001) relative to POD 0. One exceptional patient (overall incidence, 5.6%) demonstrated postoperative transient neurological events due to brain compression by the swollen temporal muscle with decreased focal cerebral blood flow in the adjacent cortical area. The postoperative collateral development via direct and indirect revascularizations was confirmed in 16 (89%) and 12 (67%) hemispheres, respectively. All patients, except for one rebleeding case, showed independent outcomes at the mean latest follow-up period on 290 ± 96 days after surgery. Our observations confirmed the temporal profile of muscle pedicle volume changes after combined revascularization. Through routine attempts to avoid the unfavorable effects of temporal muscle swelling, combined revascularization can provide favorable outcomes in symptomatic MMD.
Keywords: encephalo-myo-synangiosis, Moyamoya disease, swollen temporal muscle, time-course, vascular disorder
Introduction
Moyamoya disease (MMD) is a rare, chronic cerebrovascular disease characterized by progressive stenosis of the terminal portion of the internal carotid arteries and the formation of a fragile vascular network at the base of the brain.1,2) MMD is associated with a high risk of ischemic or hemorrhagic stroke, leading to functional and cognitive disabilities.3,4) Surgical revascularization, including direct superficial temporal artery (STA) to middle cerebral artery (MCA) anastomosis, indirect procedures such as encephalo-myo-duro-synangiosis (EDMS), and combined direct and indirect procedures, is a widely accepted therapeutic option for symptomatic MMD in all age populations.5-7) Although direct STA-MCA bypass offers the advantage of immediate blood flow augmentation in the selected area, indirect bypass using the temporal muscle as a vascularized donor (i.e., EDMS) covers a larger area of the brain and widely improves impaired cerebral hemodynamics over time.3,8-10) A recent meta-analysis of the surgical outcomes of symptomatic MMD in adults indicated that direct revascularization provides better risk reduction with respect to stroke than indirect revascularization;11) thus, direct or combined direct and indirect revascularization seems to be the most standard revascularization procedure for symptomatic MMD if technically feasible.6,11) To achieve favorable outcomes after combined revascularization procedures, understanding the potential complications specific to each procedure (i.e., focal cerebral hyperperfusion after direct bypass and cerebral ischemia after indirect bypass) is essential.9)
Indirect revascularization using the temporal muscle is an effective revascularization procedure that requires the insertion of the muscle pedicle under the cranial bone flap. Postoperative neurological deterioration due to compression of the brain by a swollen temporal muscle pedicle is a rare but potential complication,12-14) but the time course of intracranial temporal muscle pedicle volume changes has not yet been demonstrated in the literature. This prospective observational study, therefore, aimed to investigate the chronological volume changes in the temporal muscle pedicle after combined revascularization using the temporal muscle (i.e., EDMS) in patients with MMD. We further studied the incidence of neurological events with decreased focal cerebral blood flow due to compression of the brain by the swollen muscle and postoperative collateral development as the clinical impact of the temporal muscle pedicle volume changes. Our hypothesis is that combined revascularization using temporal muscle requires attempts such as inner layer drilling of the cranial bone flap, making a wide cranial bone window, and splitting temporal muscle to avoid the unfavorable effects of temporal muscle swelling and thus provide favorable outcomes in symptomatic MMD by postoperative collateral development via direct and indirect bypass.
Materials and Methods
Ethics statements
This prospective observational study was approved by an institutional review board (number: 014-053). All patients provided written informed consent before participating in this study.
Patients and surgical procedure
We included 15 consecutive adult patients with MMD, except for 1 adolescent, with ischemic or hemorrhagic symptoms who underwent a total of 18 combined direct and indirect revascularizations at our hospital in 2021. Thus, all patients who underwent combined revascularization for MMD in our hospital in 2021 were included in this study without exception. The diagnosis of MMD and surgical indications were determined according to current Japanese guidelines.2,5)
The surgical procedure for combined revascularization was standardized as direct STA-MCA (M4) single anastomosis combined with indirect pial synangiosis by EDMS, with proper perioperative hydration, prompt blood pressure control, hemoglobin concentration maintenance, and single antiplatelet agent administration, as described elsewhere.9) In brief, an L-shaped skin incision was made along the parietal branch of the STA, followed by an inverted triangle-shaped craniotomy, approximately 7 cm in diameter around the Sylvian fissure end. The stump of the distal STA was prepared as a semi-fish mouth shape, and end-to-side anastomosis was performed by suturing a distal branch of the STA to a cortical branch of the MCA (M4 segment) with a 10-0 nylon monofilament suture (Fig. 1A). After patency of the STA-MCA bypass was confirmed using intraoperative indocyanine green videoangiography (ICG-VAG, Fig. 1B) and Doppler ultrasonography, we performed indirect EDMS (Fig. 1C). To avoid compression of the brain by the swollen temporal muscle pedicle, we routinely attempted to 1) drill the inner layer of the bone flap for insurance against transient temporal muscle swelling (Fig. 1D, E), 2) create a wide bone window on the side of the temporal muscle pedicle insertion (Fig. 1F), 3) split the temporal muscle into two layers by the coronal split technique15) to reduce the thickness of the muscle pedicle, 4) perform gentle dissection and manipulation of temporal muscle tissue using the monopolar electrode, and 5) preserve the deep temporal artery and the middle temporal veins of the muscle pedicle. Thus, the temporal muscle was detached from the cranial bone by preserving the deep temporal fascia and periosteum, cutting along the muscle fiber, coagulate bleeding using a monopolar electrode, and splitting the coronal plane where less intramuscular vessel density was seen. However, the main arterial supply and venous drainage route of the muscle pedicle was always preserved. In all operated hemispheres in this series, patency of the STA-MCA bypass was confirmed during and after surgery by ICG-VAG and magnetic resonance (MR) angiography, respectively, on postoperative day (POD) 2.
Fig. 1.
Surgical procedure of combined revascularization using the temporal muscle with routine attempts to avoid unfavorable effects caused by the temporal muscle swelling.
Superficial temporal artery to middle cerebral artery anastomosis was performed using a 10-0 nylon monofilament suture (A), and patency was confirmed by intraoperative indocyanine green videoangiography (B). An intraoperative photograph demonstrating the temporal muscle pedicle, split into two layers to reduce the thickness of the pedicle, was used for encephalo-duro-myo-synangiosis after direct bypass (C). Photographs of the cranial bone flap (outer (D) and inner (E) surfaces) depict the inner layer (arrows) of the bone flap to avoid compression of the brain by the swollen temporal muscle pedicle. Intraoperative photograph (inset: postoperative 3-dimensional cranial bone image) demonstrating a wide bone window (black arrows) on the side of the temporal muscle pedicle insertion to avoid strangulation (F). Repeat computed tomography images demonstrate the chronological volume changes of the intracranially inserted temporal muscle pedicle on pre- and postoperative days 0, 1, 7, 14, and 30 (G).
Quantification and time-course analysis of the temporal muscle pedicle volume
Postoperative temporal muscle pedicle volume was quantified using repeated computed tomography (CT) images obtained on PODs 0, 1, 7, 14, and 30 by a simple estimation method known as ABC/2, which was validated by computer-assisted volumetric analysis.16) Thus, using the picture archiving and communication system (PACS), the maximum thickness, length, and height of the intracranially inserted temporal muscle pedicle were determined on an axial CT image on which the temporal muscle pedicle was visible for the ABC/2 formula. For computer-assisted volumetric analysis, the intracranial temporal muscle pedicle margins were hand-traced on each axial image using the PACS program, and the area of the hand-traced muscle pedicle was calculated in square centimeters. The product of the area by CT slice thickness (5 mm) gave the volume of the temporal muscle pedicle on that particular slice in cubed centimeters, and the sum of the volumes on each slice provided the total volume of the temporal muscle pedicle. Representative CT images showing chronological volume changes in the temporal muscle pedicle after STA-MCA single anastomosis combined with EDMS are shown in Fig. 1G.
Quantification of the cranial bone window for the temporal muscle pedicle insertion
To quantify the cranial bone window for the temporal muscle pedicle insertion (please see Fig. 1F), postoperative CT images obtained on POD 0 were utilized. The maximal length of the aforementioned cranial bone window was measured on each axial image using the PACS program. The cranial window area on each axial image was calculated in square centimeters by multiplying CT slice thickness (5 mm), and the sum of the areas on each slice provided the total area of the cranial bone window.
Postoperative neurological events and surgical outcome
Postoperative neurological events can occur after a combined revascularization procedure due to various factors, including intraoperative hypotension, anemia, inadequate general anesthesia, and surgical procedures.9) In this study, both transient and permanent postoperative neurological events occurring within a month after surgery were categorized based on radiological evaluations, including CT imaging obtained at the aforementioned 5 postoperative time points (for intracranial bleeding and temporal muscle swelling), MR imaging obtained on POD 2 (for cerebral infarct or other parenchymal pathology), and N-isopropyl-p-[123I] iodoamphetamine single-photon emission computed tomography (123I-IMP-SPECT) imaging obtained on PODs 1 and 7 (for radiological focal hyper- or hypoperfusion), as follows: 1) thromboembolic ischemia; 2) watershed-shift ischemia; 3) compression of the brain surface by the swollen temporal muscle pedicle; 4) cerebral hyperperfusion syndrome (with or without intracranial bleeding); 5) subdural hematoma; and 6) other miscellaneous mechanisms. Focal cerebral blood flow changes (hyper- or hypoperfusion) on 123I-IMP-SPECT were determined by qualitative evaluation based on previously reported criteria.17,18) To evaluate the efficacy of combined revascularization, postoperative collateral development was visually evaluated as an outcome measure using time-of-flight MR angiography obtained 6 months after surgery, as described previously.19,20) Briefly, the postoperative development of each direct and indirect surgical collaterals was evaluated by the development of STA (for direct collateral) and middle meningeal artery and deep temporal artery (for indirect collateral), respectively. Thus, the postoperative development of each collateral was dichotomized as excellent or not based on the visibility of each donor artery on MR angiography. The surgical outcome was also evaluated with regard to the performance status at the latest follow-up as of 2022.
Statistical analysis
To address potential sources of bias, we included all 18 consecutive procedures performed in 2021 in our hospital; thus, the study size was determined. There were no missing subjects. Continuous variables (i.e., age and absolute and relative temporal muscle volume) are expressed as mean and standard deviation. Pearson correlation analysis of the volumes obtained using ABC/2 and volumetric analysis was performed. For multiple comparisons of the temporal muscle volume at each postoperative time point with that on POD 0, fitting a mixed effects model to analyze repeated measures data with missing values was used, followed by a post-hoc test. Seven instances (CT on POD 7 for 4 patients and that on POD 14 for 3 patients) were missing because MR scans were performed instead of CT scans for multiple random reasons. The level of significance was set at P < 0.05. GraphPad Prism (version 9.5.0, GraphPad Software, San Diego, CA, USA) was used to conduct all the analyses.
Results
Patient characteristics
The demographic and baseline characteristics of the study population are summarized in Table 1.
Table 1.
Summary of baseline characteristics of the study subjects
| Number of Patients | 15 |
| Male:Female | 1:14 |
| Number of Hemispheres | 18 |
| Right:Left | 9:9 |
| Age at Surgery | 38 ± 13 |
| Clinical Presentation | |
| TIA | 12 |
| Infarct | 2 |
| ICH | 3 |
| Headache | 1 |
| Suzuki’s Angiographical Stage | |
| 3 | 8 |
| 4 | 9 |
| 5 | 1 |
| Comorbidities | |
| none | 8 |
| HT | 6 |
| DLp | 2 |
| pAf | 1 |
| Sjögren’s syndrome | 1 |
TIA, transient ischemic attack; ICH, intracranial hemorrhage; HT, hypertension; DLp, dyslipidemia; pAf, paroxysmal atrial fibrillation
Time-course analysis of the temporal muscle pedicle volume
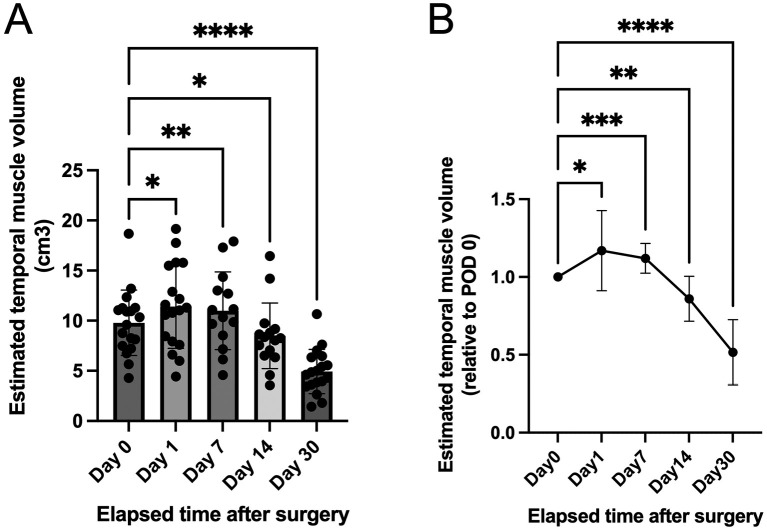
Postoperatively, the temporal muscle pedicle inserted intracranially for EDMS was visualized by CT in all 18 hemispheres at all time points (PODs 0, 1, 7, 14, and 30), except for 7 instances (CT on POD 7 for 4 patients and that on POD 14 for 3 patients) for the abovementioned random reasons. The mean area of the cranial bone window for the pedicle insertion was 740 ± 75.9 mm2. A simple estimation of the temporal muscle pedicle volume by the ABC/2 method revealed the following mean pedicle volumes: 9.8 ± 3.3 cm3 on POD 0, 11 ± 4.2 cm3 on POD 1, 11 ± 3.9 cm3 on POD 7, 8.5 ± 3.3 cm3 on POD 14, and 4.9 ± 2.2 cm3 on POD 30 (Fig. 2A). The correlation coefficients for each temporal muscle pedicle volume obtained using the ABC/2 formula validated by computer-assisted volumetric analysis were as follows: 0.96 on POD 0 (n = 18 pairs), 0.73 on POD 1 (n = 18 pairs), 0.94 on POD 7 (n = 14 pairs), 0.96 on POD 14 (n = 15 pairs), and 0.77 on POD 30 (n = 18 pairs). Each of these coefficients was significant at P < 0.001; therefore, the time course of the temporal muscle pedicle volume was analyzed using the estimated volumes calculated using the ABC/2 formula validated by computer-assisted volumetric analysis. As a result, the relative volume of the temporal muscle pedicle was most significantly increased by as much as 112% ± 9.6% on POD 7 (P < 0.001) and decreased by as little as 52% ± 21% on POD 30 (P < 0.0001) when compared with that on POD 0 (Fig. 2B).
Fig. 2.
Time-course analysis of the temporal muscle pedicle volume.
(A) Interleaved scatterplot with bars shows the temporal muscle pedicle volume estimated by the ABC/2 method on postoperative days (PODs) 0, 1, 7, 14, and 30 after combined revascularization, including encephalo-duro-myo-synangiosis. (B) Graph shows changes in the temporal muscle volume ratio relative to that on POD 0 at each postoperative time point. The relative temporal muscle pedicle volume is most significantly increased (swelled) on POD 7 and most significantly decreased (shrinked) on POD 30 compared with that on POD 0. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, fitting a mixed effects model to analyze repeated measures data with missing values followed by post-hoc multiple comparison tests.
Clinical impact of the postoperative chronological volume changes of the temporal muscle pedicle
Surgical revascularization was successfully completed in all 18 hemispheres in this series, with a patent STA-MCA single anastomosis and indirect EDMS procedure confirmed during and after surgery by ICG-VAG and MR angiography, respectively. The postoperative course was predominantly uneventful in all 18 procedures with a variability of maximal relative temporal muscle volume between 0.88 and 1.80 observed on POD 1 or 7 (Table 2). Thus, one exceptional case demonstrated a postoperative transient neurological event due to brain compression by the swollen temporal muscle, with a maximal temporal muscle volume of 163% on POD 1 relative to POD 0 and decreased focal cerebral blood flow in the adjacent cortical area (Fig. 3A). Interestingly, an adolescent patient also demonstrated postoperative brain compression by the swollen muscle (relative volume of 180% on POD 1) with decreased cerebral blood flow, but she did not show transient or permanent neurological symptoms (Fig. 3B). Collectively, the incidence of symptomatic brain compression by the swollen muscle was 5.6%, while radiologically confirmed brain compression by the swollen temporal muscle with focal hypoperfusion in adjacent cortical areas was observed when the maximal relative muscle volume was >160%, regardless of neurological symptoms, as summarized in Table 2.
Table 2.
Relationship between postoperative swollen temporal muscle and brain compression with decreased focal cerebral blood flow, neurological events, and collateral development
| Patient Demographics | Postoperative Max. temporal muscle swelling | Postoperative Neurological Event | Postoperative Collateral Development | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case
No. |
Hemisphere
No. |
Age
(yrs) |
Sex | Clinical Presentation | Brain compression with focal hypoperfusion | Neurological Event | STA | DTA | MMA | |
| 1 | 1 | 35 | F | TIA | 0.88 | No | No | Yes | No | Yes |
| 2 | 2 | 41 | F | TIA | 1.13 | No | No | Yes | No | Yes |
| 3 | 41 | F | TIA | 1.09 | No | No | No | No | Yes | |
| 3 | 4 | 39 | F | TIA | 0.99 | No | No | Yes | Yes | No |
| 5 | 39 | F | TIA | 1.63 | Yes | Transient | Yes | No | No | |
| 4 | 6 | 19 | F | ICH | 1.00 | No | No | Yes | No | Yes |
| 5 | 7 | 47 | F | TIA | 0.99 | No | No | Yes | No | No |
| 6 | 8 | 17 | F | Infarct | 1.12 | No | No | Yes | Yes | No |
| 9 | 17 | F | TIA | 1.14 | No | No | No | Yes | Yes | |
| 7 | 10 | 43 | F | TIA | 1.07 | No | No | Yes | No | No |
| 8 | 11 | 45 | F | ICH | 1.17 | No | No | Yes | No | No |
| 9 | 12 | 54 | F | ICH | 1.47 | No | Transient | Yes | No | Yes |
| 10 | 13 | 47 | F | TIA | 1.30 | No | No | Yes | Yes | Yes |
| 11 | 14 | 45 | M | TIA | 1.02 | No | No | Yes | No | No |
| 12 | 15 | 49 | F | Infarct | 1.31 | No | No | Yes | Yes | Yes |
| 13 | 16 | 38 | F | TIA | 1.16 | No | No | Yes | No | No |
| 14 | 17 | 14 | F | Headache | 1.80 | Yes | No | Yes | Yes | Yes |
| 15 | 18 | 60 | F | TIA | 1.40 | No | No | Yes | No | Yes |
No., number; yrs, years; TIA, transient ischemic attack; ICH, intracranial hemorrhage; STA, superficial temporal artery; DTA, deep temporal artery; MMA, middle meningeal artery.
Age indicates age at surgery, Brain compression with focal hypoperfusion was due to temporal muscle swelling, Max.temporal muscle swelling indicates maximal relative temporal muscle volume compared with that on postoperative day 0.
Fig. 3.
Representative cases with postoperative temporal muscle pedicle swelling after combined revascularization with encephalo-duro-myo-synangiosis.
(A) A 39-year-old woman with ischemic-onset Moyamoya disease (MMD) who developed transient postoperative aphasia between postoperative days (PODs) 1 and 7. Computed tomography (CT) image and N-isopropyl-p-[123I] iodoamphetamine single-photon emission computed tomography (123I-IMP SPECT) on POD 1 (right panels in the middle) depicting compression of the brain by the swollen temporal muscle pedicle accompanied with decreased cerebral blood flow at the compression site (arrowhead). Magnetic resonance (MR) angiography on POD 2 (left panel in the middle) showing a patent superficial temporal artery to middle cerebral artery (STA-MCA) bypass (white arrow). On POD 7, the temporal muscle pedicle volume is decreased, and cerebral blood flow is restored (right panels on the bottom) compared with before (right panels on the top) and a day after surgery (right panels in the middle). Postoperative collateral development via direct bypass is evident on MR angiography 6 months after surgery (left panel on the bottom). (B) A 14-year-old female patient with headache-onset MMD who showed asymptomatic brain compression by the temporal muscle pedicle. CT image and 123I-IMP SPECT on POD 1 (right panels in the middle) depict compression of the brain by the swollen temporal muscle pedicle accompanied with decreased cerebral blood flow at the compression site (arrowhead), which is comparable with the findings of the aforementioned 39-year-old woman. MR angiography on POD 2 (left panel in the middle) shows a patent STA-MCA bypass (white arrow). On POD 7, the swollen pedicle and decreased cerebral blood flow are restored (right panels on the bottom). Postoperative collateral development via direct (white arrow) and indirect bypass (arrowheads) is evident on MR angiography 6 months after surgery (left panel on the bottom).
Surgical outcome
Transient neurological events due to focal cerebral hyperperfusion syndrome were observed in one case (hemisphere number 12 in case 9) and the abovementioned brain compression due to the swollen temporal muscle (hemisphere number 5 in case 3), but no other postoperative complications with neurological sequelae were observed in this study. We further confirmed the excellent development of postoperative collateral vessels via direct and indirect revascularizations in 16 (89%) and 12 (67%) hemispheres, respectively. The postoperative collateral development was observed regardless of the time course of the pedicle volume changes. All patients, except for case 9 with recurrent intraventricular hemorrhage from a peripheral artery aneurysm along with choroidal periventricular anastomosis, showed independent outcomes at the latest follow-up on POD 290 ± 96.
Representative cases
A 39-year-old woman with ischemic-onset MMD developed postoperative transient aphasia after STA-MCA single anastomosis combined with EDMS in her left hemisphere due to compression of the brain by the slightly swollen temporal muscle pedicle (163% in volume relative to that on POD 0) with decreased focal cerebral blood flow on POD 1. The cranial bone window for the temporal muscle pedicle insertion was 857 mm2. On POD 2, MR angiography demonstrated a patent STA-MCA bypass. She was treated conservatively with daily oral aspirin and herbal medicine (JIDABOKUIPPO); subsequently, spontaneous resolution of the slight pedicle swelling was observed with increased cerebral blood flow, and no further ischemic symptoms were observed on POD 7. Her postoperative course was uneventful, with an independent outcome (modified Rankin scale score of 0) (Fig. 3A).
In contrast, a 14-year-old female patient with headache-onset MMD demonstrated compression of the brain by the swollen temporal muscle pedicle (180% in volume relative to that on POD 0) with decreased focal cerebral blood flow on POD 1 after STA-MCA single anastomosis combined with EDMS in her left hemisphere. The cranial bone window for the temporal muscle pedicle insertion was 695 mm2. Since she did not develop transient or permanent postoperative neurological symptoms, she was treated conservatively, and pedicle swelling with radiological focal hypoperfusion was restored by POD 7. Her postoperative course was also uneventful, with a modified Rankin scale score of 0 (Fig. 3B).
Discussion
Herein, we documented the chronological volume changes in the temporal muscle pedicle used for combined revascularization in adult patients with MMD treated in our hospital. When compared with the volume on POD 0 (i.e., immediately after surgery), the intracranially inserted temporal muscle pedicle was swollen by as much as 112% on day 7 and decreased by as little as 52% on POD 30 on average; this is the first report of such data in the literature.
In terms of the clinical impact of temporal muscle swelling, symptomatic brain compression accompanied by focal hypoperfusion was observed in 5.6% of our consecutive adult MMD series. In 2009, Fujimura et al. first documented a case of symptomatic brain compression by a swollen temporal muscle as a rare but potential complication of revascularization surgery for MMD.14) Recently, the incidences were reported to be 16.2% in a pediatric case series13) and 6.7% in an adult case series12) of patients that underwent combined STA-MCA and indirect revascularization using the temporal muscle. In our adult series, brain compression by the swollen temporal muscle was depicted in two hemispheres by CT, and focal hypoperfusion was shown by 123I-IMP-SPECT. Although both hemispheres showed preoperative impaired cerebral hemodynamics, swollen temporal muscle by as much as 163% and 180% on POD 1 relative to that on POD 0, and patent direct STA-MCA bypasses, transient neurological symptoms were observed in only one hemisphere (5.6%). Because of the small number of study patients, it was difficult to document the risk factors for symptomatic brain compression by the swollen temporal muscle pedicle in this study. The differences between these two instances were age (i.e., the symptomatic case was an adult, and the other was an adolescent) and clinical presentation before surgery (ischemic onset for the symptomatic case and headache for the non-symptomatic one), which might be responsible for the clinical impact of the brain compression by the swollen muscle. Although indirect collateral development was observed postoperatively in 67% of our adult cases, which is comparable to the incidence of successful indirect bypass development in adult MMD,19-22) the time course of the pedicle volume changes was not associated with postoperative indirect collateral development.
We also demonstrated the surgical attempts with intraoperative photographs to avoid the unfavorable effects of temporal muscle pedicle swelling, which were collectively demonstrated by a recent review paper on flow-augmentation bypass for MMD.9) These attempts include the following: 1) drilling the inner layer of the cranial bone flap for insurance against transient temporal muscle swelling; 2) creating a cranial bone window wide enough for pedicle insertion to avoid strangulation of the swollen temporal muscle pedicle; 3) splitting the temporal muscle pedicle by the coronal split technique15) to reduce its thickness; 4) performing gentle dissection using the monopolar electrode and manipulation of the temporal muscle tissue; and 5) preserving the deep temporal artery and middle temporal vein of the pedicle. In the latter three attempts, we sought to reduce muscle tissue damage and preserve the integrity of the muscle vascular system. According to our standards, we routinely performed the aforementioned attempts, in addition to other technical tips for revascularization in MMD.9) As a result, the short-term surgical outcome (mean follow-up period, 290 ± 96 days) was uneventful, except in one case, and excellent postoperative collateral development via direct and indirect revascularization was achieved. Although a single experienced neurosurgeon (M. F., last author) performed fairly standardized surgical attempts to avoid this complication, there was still variability in the time course of temporal muscle volume changes. The underlying mechanism for this difference might be unknown patient factors, which should be clarified in future studies.
The limitations of this study include its small sample size and short follow-up duration; thus, these findings should be validated in a larger and longer-term cohort. Another limitation was that this study predominantly included adult cases with combined and indirect revascularization. Since indirect revascularization using the temporal muscle is believed to be effective for 100% of pediatric MMD,8) the chronological change in temporal muscle volume and its clinical impact should be clarified in this population.
Conclusions
This study demonstrated the time course of temporal muscle pedicle volume changes after combined revascularization, including EDMS. It is worth knowing the potential timing of temporal muscle swelling. Through routine attempts to avoid the unfavorable effects of temporal muscle swelling, combined revascularization can provide favorable outcomes in symptomatic MMD through direct and indirect collateral development.
Abbreviations
MMD, Moyamoya disease; STA, superficial temporal artery; MCA, middle cerebral artery; EDMS, encephalo-myo-duro-synangiosis; ICG-VAG, intraoperative indocyanine green videoangiography; MR, magnetic resonance; POD, postoperative day; CT, computed tomography; PACS, picture archiving and communication system; 123I-IMP-SPECT, N-isopropyl-p-[123I] iodoamphetamine single-photon emission computed tomography
Availability of Data and Materials
Data supporting the findings of this study are available from the corresponding author on reasonable request.
Conflicts of Interest Disclosure
All authors have no conflict of interest.
Acknowledgments
This work was partially supported by JSPS KAKENHI grant number 19H03765 (M.I.) and 20K09362 (M.F.). The authors would like to thank Editage for the English language review.
References
- 1). Suzuki J, Takaku A: Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol 20: 288-299, 1969 [DOI] [PubMed] [Google Scholar]
- 2). Kuroda S, Fujimura M, Takahashi J, et al. : Diagnostic criteria for Moyamoya disease - 2021 revised version. Neurol Med Chir (Tokyo) 62: 307-312, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Scott RM, Smith ER: Moyamoya disease and Moyamoya syndrome. N Engl J Med 360: 1226-1237, 2009 [DOI] [PubMed] [Google Scholar]
- 4). Fujimura M, Bang OY, Kim JS: Moyamoya disease. Front Neurol Neurosci 40: 204-220, 2016 [DOI] [PubMed] [Google Scholar]
- 5). Fujimura M, Tominaga T, Kuroda S, et al. : 2021 Japanese guidelines for the management of Moyamoya disease: guidelines from the research committee on Moyamoya disease and Japan stroke society. Neurol Med Chir (Tokyo) 62: 165-170, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Acker G, Fekonja L, Vajkoczy P: Surgical management of moyamoya disease. Stroke 49: 476-482, 2018 [DOI] [PubMed] [Google Scholar]
- 7). Miyamoto S, Yoshimoto T, Hashimoto N, et al. : Effects of extracranial-intracranial bypass for patients with hemorrhagic moyamoya disease: results of the Japan Adult Moyamoya Trial. Stroke 45: 1415-1421, 2014 [DOI] [PubMed] [Google Scholar]
- 8). Kuroda S, Houkin K: Moyamoya disease: current concepts and future perspectives. Lancet Neurol 7: 1056-1066, 2008 [DOI] [PubMed] [Google Scholar]
- 9). Fujimura M, Tominaga T: Flow-augmentation bypass for moyamoya disease. J Neurosurg Sci 65: 277-286, 2021 [DOI] [PubMed] [Google Scholar]
- 10). Fujimura M, Tominaga T: Lessons learned from Moyamoya disease: Outcome of direct/indirect revascularization surgery for 150 affected hemispheres. Neurol Med Chir (Tokyo) 52: 327-332, 2012 [DOI] [PubMed] [Google Scholar]
- 11). Jeon JP, Kim JE, Cho WS, Bang JS, Son YJ, Oh CW: Meta-analysis of the surgical outcomes of symptomatic Moyamoya disease in adults. J Neurosurg 128: 793-799, 2018 [DOI] [PubMed] [Google Scholar]
- 12). Machida T, Higuchi Y, Nakano S, et al. : Sagittal splitting of the temporalis muscle for encephalo-myo-synangiosis to prevent ischemic complications due to a swollen temporalis muscle without inhibiting collateral developments in patients with Moyamoya disease. J Neurosurg 130: 1957-1964, 2018 [DOI] [PubMed] [Google Scholar]
- 13). Kanamori F, Araki Y, Yokoyama K, et al. : Brain compression by encephalo-myo-synangiosis is a risk factor for transient neurological deficits after surgical revascularization in pediatric patients with Moyamoya disease. World Neurosurg 133: e558-e566, 2020 [DOI] [PubMed] [Google Scholar]
- 14). Fujimura M, Kaneta T, Shimizu H, Tominaga T: Cerebral ischemia owing to compression of the brain by swollen temporal muscle used for encephalo-myo-synangiosis in Moyamoya disease. Neurosurg Rev 32: 245-249, 2009 [DOI] [PubMed] [Google Scholar]
- 15). Cheung LK: The vascular anatomy of the human temporalis muscle: implications for surgical splitting techniques. Int J Oral Maxillofac Surg 25: 414-421, 1996 [DOI] [PubMed] [Google Scholar]
- 16). Sucu HK, Gokmen M, Gelal F: The value of XYZ/2 technique compared with computer-assisted volumetric analysis to estimate the volume of chronic subdural hematoma. Stroke 36: 998-1000, 2005 [DOI] [PubMed] [Google Scholar]
- 17). Kanoke A, Fujimura M, Tashiro R, Ozaki D, Tominaga T: Transient global cerebral hypoperfusion as a characteristic cerebral hemodynamic pattern in the acute stage after combined revascularization surgery for pediatric Moyamoya disease: N-isopropyl-p-[123I] iodoamphetamine single-photon emission computed tomography study. Cerebrovasc Dis 51: 453-460, 2022 [DOI] [PubMed] [Google Scholar]
- 18). Fujimura M, Shimizu H, Inoue T, Mugikura S, Saito A, Tominaga T: Significance of focal cerebral hyperperfusion as a cause of transient neurologic deterioration after extracranial-intracranial bypass for moyamoya disease: comparative study with non-moyamoya patients using N-isopropyl-p-[(123)I]iodoamphetamine single-photon emission computed tomography. Neurosurgery 68: 957-965, 2011 [DOI] [PubMed] [Google Scholar]
- 19). Ito M, Kawabori M, Sugiyama T, et al. : Impact of RNF213 founder polymorphism (p.R4810K) on the postoperative development of indirect pial synangiosis after direct/indirect combined revascularization surgery for adult Moyamoya disease. Neurosurg Rev 45: 2305-2313, 2022 [DOI] [PubMed] [Google Scholar]
- 20). Kawabori M, Ito M, Kazumata K, et al. : Impact of RNF213 c.14576G>A variant on the development of direct and indirect revascularization in pediatric moyamoya disease. Cerebrovasc Dis in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Mizoi K, Kayama T, Yoshimoto T, Nagamine Y: Indirect revascularization for moyamoya disease: Is there a beneficial effect for adult patients? Surg Neurol 45: 541-548, 1996 [DOI] [PubMed] [Google Scholar]
- 22). Czabanka M, Vajkoczy P, Schmiedek P, Horn P: Age-dependent revascularization patterns in the treatment of moyamoya disease in a European patient population. Neurosurg Focus 26: E9, 2009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author on reasonable request.