Abstract
Transsylvian selective amygdalohippocampectomy (TSA) is one of the predominant surgical options for drug-resistant mesial temporal lobe epilepsy. The purpose of this article is to highlight the unique features of TSA and determine the setting to perform safe and secure TSA with special reference to the optimal head position. TSA should be performed via a small surgical corridor in the temporal stem that contains functionally important fiber tracts, including the uncinate fasciculus, the inferior fronto-occipital fasciculus, and the optic radiation. Graphical simulations proposed that low-degree (<30°) head rotation had the advantage of sufficiently opening the surgical field in TSA and may help surgical procedures within the limited exposure of the medial temporal structures. Inspection of the surgical videos implied that the collapse of the inferior horn was prevented in low-degree rotation, probably because the deformation due to the brain shift was minimized in the medial temporal structures. A simulation also implied that chin-up position had the advantage of resecting the tail of the hippocampus in a straightforward manner. We suggest that the setting is optimized in TSA with low-degree rotation and chin-up head position.
Keywords: amygdalohippocampectomy, anterior temporal lobectomy, head position, lateral ventricle, mesial temporal lobe epilepsy
Introduction
The transsylvian approach is a standard neurosurgical procedure that allows accessibility to deep-seated lesions in the basal part of the frontotemporal regions and in the brain stem. Because of its high usability, the transsylvian approach is indicated in a wide range of neurosurgical procedures, such as vascular, tumor, and epilepsy surgeries. Transsylvian selective amygdalohippocampectomy (TSA) is a standard surgical technique for drug-resistant mesial temporal lobe epilepsy (mTLE).1,2) TSA requires advanced surgical skills3) because the surgical exposure of the medial temporal structures is limited (Fig. 1). Complications due to surgical procedures may lead to severe neurological sequelae because of the vital neural tissue and vessels surrounding the limited surgical field.
Fig. 1.
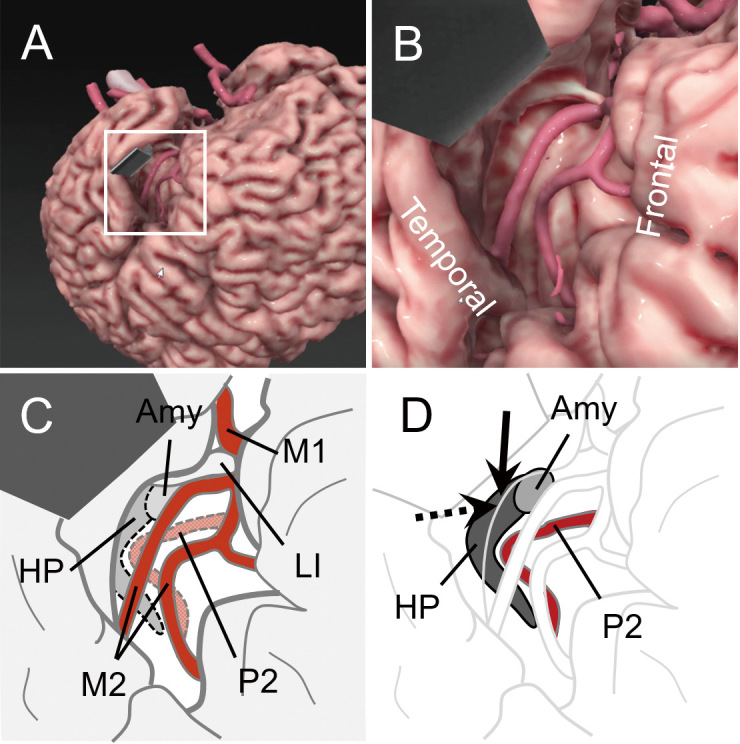
Graphical computer simulation and schematic diagrams of the surgical field in transsylvian selective amygdalohippocampectomy.
A. A macroscopic view after opening the left Sylvian fissure. The temporal lobe is retracted using a spatula. The boxed area is enlarged in B. The image was generated using software specialized for surgical simulation (GRID, Kompath Inc., Tokyo). B. An enlarged view of A. In the center, two branches of the middle cerebral artery (MCA) are exposed on the insular cortex. C. A schematic diagram of C. The M1 and M2 portions of the MCA are indicated. Dotted lines delineate the locations assumed for the hippocampus (HP), amygdala (Amy), and P2 portion of the posterior cerebral artery in the deeper layer. LI, the limen insulae. D. The same view as C, in which HP, Amy, and P2 are illuminated. An arrow indicates the cortical incision on the inferior periinsular sulcus to reach the inferior horn of the lateral ventricle and the HP. A dotted arrow indicates the trajectory to resect the amygdala.
Head positioning is an essential initial step for safe and secure neurosurgical procedures. The degree to which the patient's head is rotated to the unoperated side should be optimized, depending on the direction of the surgical trajectory and location of the target lesion. An optimal head position helps in achieving the necessary and sufficient opening of the surgical field with minimum brain retraction and subsequent uneventful surgical procedures.
Here, we highlight a unique feature of TSA and present a consideration of the setting to perform safe and secure TSA with special reference to optimal head position.
Unique Features in Transsylvian Selective Amygdalohippocampectomy
After opening the Sylvian fissure, the insular cortex is exposed in the surgical field (Fig. 1). The question is, which part is appropriate for the cortical incision to be made on the inferior periinsular sulcus (arrow in Fig. 1D). The underlined white matter of the temporal stem consists of functionally important pathways such as the uncinate fasciculus (UF), inferior fronto-occipital fasciculus (IFOF), and optic radiation (OR). The UF is a fiber tract that connects the temporal lobe structures with the inferior portions of the frontal lobe and plays a putative role in episodic memory, language, and emotional processing.4) Previous reports have described that the disruption of the UF in epilepsy surgery on the language-dominant hemisphere deteriorated verbal learning and memory.5,6) The IFOF is a fiber tract that connects the frontal lobe (orbitofrontal cortex and prefrontal region) with the posterolateral temporal and occipital lobes7) and plays an important role in the semantic component of language processing.8) Disruption of the temporal stem can cause deficits in the visual field because the OR passes through it. A view of the distributions of three fiber tracts in a section of the temporal stem indicates a putative junction between the UF and IFOF/OR (arrow in Fig. 2), which is normally located 10 mm posterior to the limen insulae.9) This point approximates the safe entry point to the inferior horn of the lateral ventricle described by Morino et al.6) to preserve verbal memory after TSA on the language-dominant side.
Fig. 2.
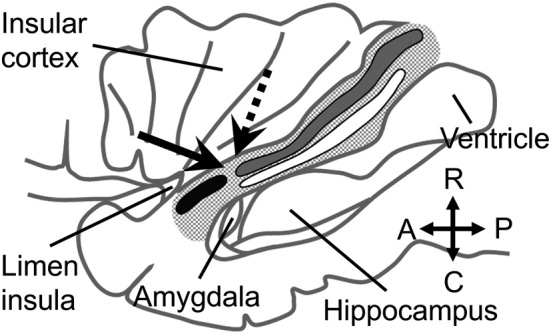
Fiber tracts in the temporal stem.
A lateral view of the left temporal stem (tiny dotted area) after removing the frontal and temporal operculi. The black, gray, and white bands indicate the uncinate fasciculus (UF), inferior fronto-occipital fasciculus (IFOF), and optic radiation (OR), respectively. The hippocampus is seen through a window opened on the inferior horn of the lateral ventricle (ventricle). The arrow indicates the surgical corridor in the temporal stem made to reach the inferior horn and hippocampus via a putative junction between the UF and IFOF/OR. A dotted arrow indicates the trajectory to resect the amygdala. The diagram was drawn according to references from Kier et al.19) and Ribas et al.9)
Identifying the inferior horn of the lateral ventricle in the white matter of the temporal stem is the important initial step to safely perform TSA. Dissections that go too far in depth may injure the basal ganglia and may disrupt the internal capsule. An original procedure by Yaşargil reported that the amygdala should be removed first and the inferior horn should be entered in the posterolateral position.1) The correct angle of the approach route is reportedly parallel to the most medial surface of the transverse temporal lobe or the sphenoid ridge.10) The deep medullary vein is a milestone in the white matter to reach the inferior horn.11) Slight changes in color or tension of the white matter indicate the proximity to the ependium of the inferior horn.2) A number of options to find the inferior horn as described above are helpful in avoiding loss of orientation in the white matter due to individual variations. In this situation, intraoperative navigation is unreliable in most cases because of the brain shift after opening the Sylvian fissure.10)
After entering the inferior horn of the lateral ventricle, the initial part of the hippocampal dissection proceeds on the lateral side of the hippocampus. The final part of the surgical field is on the medial surface of the hippocampus, where the arteries and veins in the perimesencephalic cistern are resected to devascularize the hippocampus. All of these procedures are performed via a small surgical corridor in the temporal stem. In addition, the unique morphology of the hippocampal body should be considered in the case of en bloc resection for the postoperative histopathological diagnosis of hippocampal sclerosis. The shape of the hippocampus is curbed around the midbrain in the axial plane (Fig. 3). The axis of the hippocampus is tilted with the tail upward in the sagittal plane (Fig. 4). A randomized trial reported that the rate of seizure freedom following a 3.5 cm resection of the hippocampus was greater than that following a 2.5 cm resection, although the difference was not statistically significant.12) This report indicates the possibility that sufficient resection of the hippocampal tail is necessary for favorable seizure outcomes. Thus, it is pivotal to set the optimal surgical trajectory to preserve the vital structures around the hippocampus and dissect a sufficient length of the hippocampus in TSA.
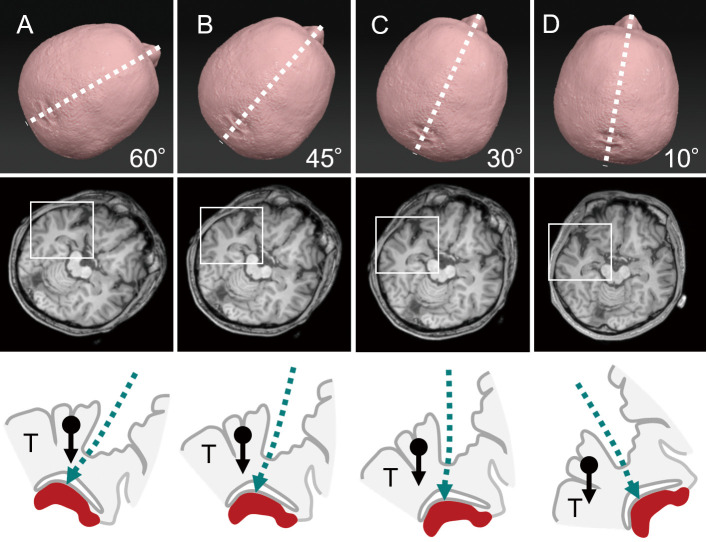
Fig. 3.
Degree of head rotation and surgical fields on the axial plain.
Schematic diagrams showing cases with 60° (A), 45° (B), 30° (C), and 10° (D) head rotations to the unoperated side (upper row). The boxed area in the axial magnetic resonance image, including the Sylvian fissure and hippocampus (middle row), is enlarged and schematized (bottom row). An arrow with a dot indicates the gravity to the temporal lobe (T). The hippocampus is drawn as a (red) solid object at the bottom. The direction of the surgical trajectory is indicated by the (green) dashed lines. Note that the direction of gravity to the temporal lobe is more overlapped with the surgical trajectory at higher degrees of head rotation.
Fig. 4.
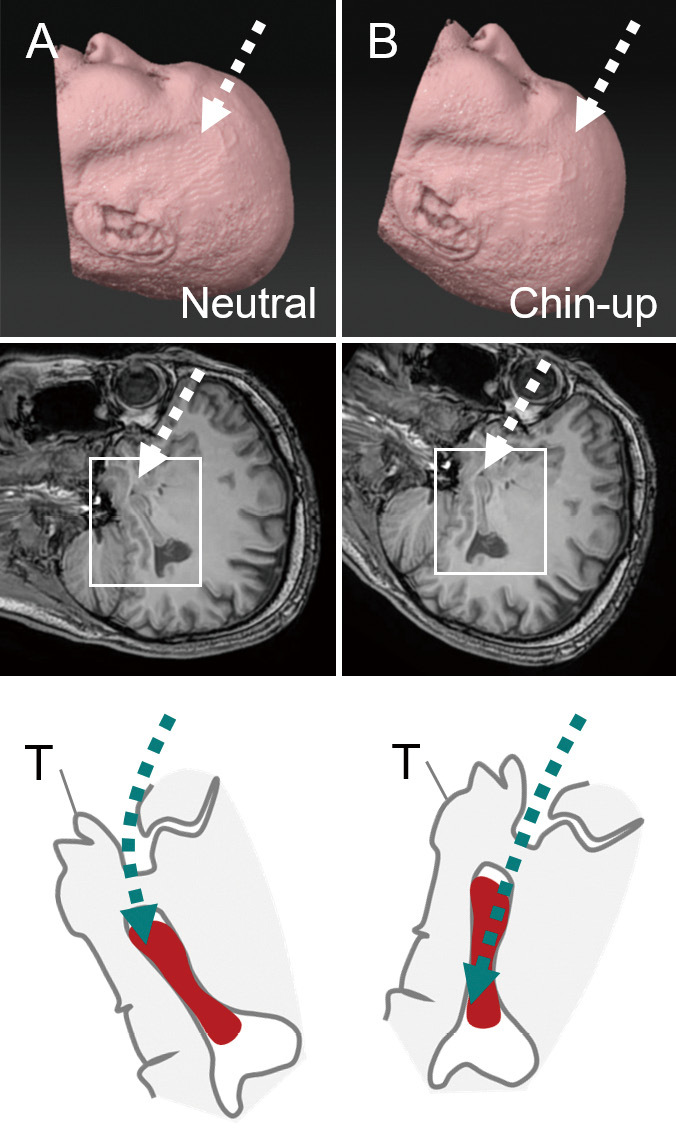
Chin-up position and surgical fields on the sagittal plain.
Schematic diagrams showing cases in the neutral (A) and chin-up (B) positions (upper row). The boxed area in the sagittal MR image, including the Sylvian fissure and hippocampus (middle row), is enlarged and schematized (bottom row). The hippocampus is drawn as a (red) solid object. The direction of the surgical trajectory is indicated by the (green) dashed lines. Note that the long axis of the hippocampus is on the straight surgical trajectory.
Optimal Head Position in TSA
As per the standard procedure mentioned in the textbook, the optimal head rotation for TSA is approximately 30°.2,13) In vascular or tumor surgeries, head rotation at 60° is optimal when the surgical field is directed to the frontal side, for example, in cases with anterior communicating artery aneurysms or suprasellar tumors. Head rotation at 45° is optimal for middle exposure, for example, in cases with internal carotid artery and those deeper into basilar artery aneurysms. Thirty degrees is optimal when the surgical field is directed to the temporal side, for example, in cases with middle cerebral artery aneurysm.14) Is less than 30° optimal for TSA because the surgical field is located on a more temporal side? We simulated the relationship between the degree of head rotation and surgical field of the TSA using an axial slice of a magnetic resonance image (MRI) (Fig. 3). Our results demonstrated that the direction of gravity to the temporal lobe was more overlapped with the surgical trajectory at higher degrees of head rotation. Consequently, a stronger force of brain retraction was needed to open the surgical field. A simulation using the sagittal plane demonstrated that chin-up position had the advantage of reaching the long axis of the hippocampus (Fig. 4).
Collapse of the Inferior Horn of the Lateral Ventricle at High-Degree Rotation
We often encounter collapse of the lateral ventricle after the cerebrospinal fluid has been drained from the Sylvian fissure. We reviewed surgical videos to evaluate whether there was a difference in the inferior horn of the lateral ventricle depending on the degree of head rotation. Between April 2013 and September 2021, 51 surgeries for drug-resistant temporal lobe epilepsy were performed at the Department of Neurosurgery, Kumamoto University Hospital. A total of 24 TSAs were enrolled after excluding 27 surgeries without TSA procedures, such as an ATL. The mean age was 30.3 years (8-58), and the right-left ratio was 1:2. Twelve patients (50.0%) presented with hippocampal sclerosis. We bisected the surgeries using head rotation at 30° as the boundary in reference to the operative records of each patient. Thirteen and eleven surgeries were in the high-degree (≥30°) and low-degree (<30°) groups, respectively. There was an impression that the tension of the translucent ependymal layer was readily recognizable in the white matter of the temporal stem in the low-degree group. By contrast, the inferior horn often collapsed in the high-degree group because of the brain shift during the opening of the Sylvian fissure. We rated the degree of collapse of the inferior horn of the lateral ventricle and found that the collapse was more often encountered in the high-degree group (Fig. 5).
Fig. 5.
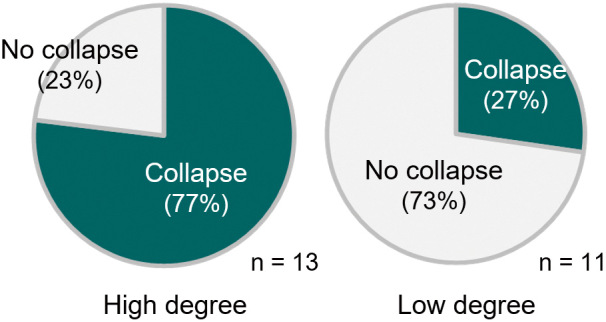
Collapse of the inferior horn of the lateral ventricle.
Collapse of the inferior horn is evaluated in video recordings of 24 transsylvian selective amygdalohippocampectomy surgeries. The rates of collapse are 77% (10/13) and 27% (3/11) in high (30° or more) and low (less than 30°) degrees of head rotation, respectively. The difference is statistically significant at p < 0.05 (chi-square test).
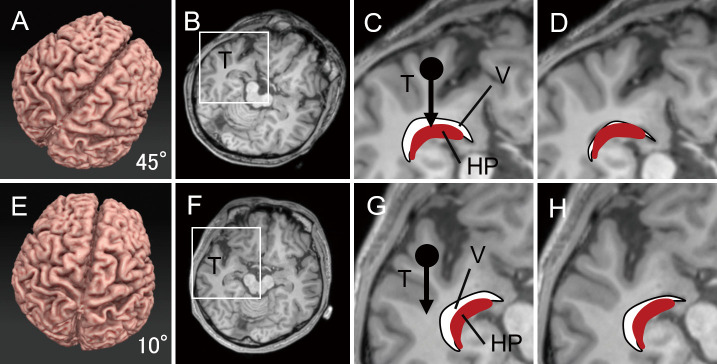
A simple simulation using axial MRI slices confirmed our findings (Fig. 6). In the case of high-degree head rotation, the inferior horn and hippocampus resided directly beneath the temporal lobe (Fig. 6A-D). The temporal lobe sank because of gravity to flatten the inferior horn before reaching it (Fig. 6D). By contrast, at low-degree head rotation, the shift of the temporal lobe due to gravity occurred outside the inferior horn and hippocampus (Fig. 6E-H). The collapse of the inferior horn remained at a minimum before reaching it (Fig. 6H). A graphical computer simulation was performed to reinforce this finding, and it is shown in Supplementary Video 1. These simulations implied that the degree of collapse of the inferior horn of the lateral ventricle depended, at least in part, on the degree of head rotation due to the effect of the brain shift of the temporal lobe. We suggest that low-degree head rotation has the advantage of preserving the inferior horn in its original shape and helps in successfully opening the inferior horn in TSA.
Fig. 6.
Degree of rotation, brain shift, and collapse of the ventricle.
A-D. A simulation of 45° head rotation. A boxed area in an axial slice of MRI (B) is enlarged in C. An arrow with a dot in C indicates the gravity to the temporal lobe. A curbed white area and (red) solid object indicate the inferior horn of the lateral ventricle (V) and the hippocampus (HP), respectively. Note that the V and HP reside directly beneath the temporal lobe (T). In D, the space of the ventricle collapses because the temporal lobe has sunken toward the ventricle because of the brain shift during Sylvian fissure opening.
E-H. A simulation of 10° head rotation. The collapse of the ventricle is minimum in H because the direction of the brain shift (an arrow with a dot) of the temporal lobe (T) is outside the area containing the ventricle (V) and the hippocampus (HP).
Head Position, Surgical Field, and Surgical Results
We considered that low-degree rotation had the advantage of entering the inferior horn of the lateral ventricle (Fig. 5) and chin-up position had the advantage of resecting the tail of the hippocampus in a straightforward manner (Fig. 4). This is also the case when the hippocampus is resected in a piecemeal fashion. What about resecting the amygdala, which is on the “ceiling” of the anterior end of the surgical field? A part of the amygdala should be out of vision through the incision posterior to the UF (an arrow in Fig. 2). As is the case with other deep-seated lesions, we need to optimize the angle of the microscope, change the position of the surgeon, and adjust the height and rotation of the surgical table. To sufficiently put the amygdala in the field of microscopic view, we lift up the surgical table and make the microscope look up (dotted arrows in Figs. 1 and 2). Sufficient resection of the amygdala is important for seizure control in mTLE surgery.15,16) Our postoperative imaging showed that the lower two-thirds of the amygdala was resected (Supplementary Figure 1C). Seizure outcome by the partial resection of the lower three quarters of the amygdala in TSA2) was reportedly comparable to that in ATL.17)
We evaluated the degree of resection using three indices: the length of the hippocampal resection in the anteroposterior axis (A-P length), the length between the posterior end of the hippocampal resection and the quadrigeminal plate (QP length, Supplementary Figure 2),18) and the resection ratio of the amygdala (Supplementary Figure 1A-C). The seizure outcome was also evaluated using Engel classification (Supplementary Figure 1D). We found no statistical difference in these indices between cases with high- and low-degree head rotations. There was no death, intracranial hemorrhage, infection, hemiparesis, or hemianopsia in the confrontation test in this series.
Limitation
There are several limitations in this paper. The evidence to support the advantage of our head position setting was no more than the subjective evaluation of the surgical field. The graphical computer simulation was not designed to simulate brain shift due to gravity but to simulate brain retraction (“brain deformation function” in GRID, Kompath Inc., Tokyo, Japan). We found no statistical differences in the surgical outcome depending on the degrees of head rotation (Supplementary Figure 1). Postoperative tractography to evaluate a collateral damage on functionally important fiber tracts through the temporal stem (Fig. 2) was lacking. Finally, we could not evaluate postoperative neuropsychological outcome; the number of patients was too small for statistics after we discriminated left- and right-side surgeries.
Conclusions
TSA is the predominant surgical option for drug-resistant mTLE. Setting the optimal head position is a prerequisite for secure and safe TSA because the exposure of the mesial temporal structures is limited via a small surgical corridor in the temporal stem that includes functionally important fiber tracts. Using graphical simulations, we propose that low-degree (<30°) head rotation has the advantage of sufficiently opening the surgical field. Inspection of surgical videos revealed that the collapse of the inferior horn was prevented in low-grade rotation, probably because the deformation due to the brain shift was minimized in the area of the medial temporal structures. In addition, chin-up position had the advantage of resecting the tail of the hippocampus in a straightforward manner.
Some of the clinical data in Fig. 5, Supplementary Video 1, and related discussions were presented at the 45th Annual Meeting of the Epilepsy Surgery Society of JAPAN held on January 27-28, 2022, in Osaka.
Ethics Approval
This clinical study was approved by the Institutional Review Board for Scientific Research at Kumamoto University Hospital (approval number: 1760). The requirement for informed consent was waived because the study was retrospective and noninvasive.
Abbreviations
ATL, anterior temporal lobectomy;
AMTL, anteromesial temporal lobectomy;
IFOF, inferior fronto-occipital fasciculus;
mTLE, mesial temporal lobe epilepsy;
MRI, magnetic resonance image;
OR, optic radiation;
TSA, transsylvian selective amygdalohippocampectomy;
UF, uncinate fasciculus.
Conflicts of Interest Disclosure
The authors declare no conflict of interest related to this work.
Supplementary Material
A. A graph showing the length of the hippocampal resection in the anteroposterior axis (A–P length). The A–P length was defined as the distance between the anterior and posterior ends of the surgical cavity in coronal slices of postoperative MRI. The difference in A–P length between cases with high-degree (27.3 ± 1.6 mm) and low-degree (28.0 ± 1.1 mm) rotations was not statistically significant (p = 0.701, Student’s t-test). The data were expressed as the mean length and standard errors.
B. A graph showing the length between the posterior end of the hippocampal resection and the quadrigeminal plate (QP length, Supplementary Figure 2).18) The difference in QP length between cases with high-degree (6.7 ± 2.1 mm) and low-degree (5.7 ± 1.1 mm) rotations was not statistically significant (p = 0.678, Student’s t-test).
C. A graph showing the degree of amygdala resection, which was expressed as the ratio in area of surgical defect in the amygdala on a postoperative coronal section through the center of the amygdala. The difference in amygdala resection ratio between cases with high-degree (67.7% ± 3.9%) and low-degree (63.5% ± 4.6%) rotations was not statistically significant (p = 0.498, Student’s t-test).
D. A graph showing the seizure outcome in Engel classification. The rate of Engel class I between cases with high-degree (9/13; 69.2%) and low-degree (9/11, 81.8%) rotations was not statistically significant (p = 0.478, chi-square test) at the latest follow-up (mean, 56 and 25 months, respectively).
A. An axial section of T1-weighted MR imaging through the surgical cavity after left hippocampal resection. The boxed area is enlarged and schematized in B.
B. The QP length is defined as the length along the anteroposterior axis (a two-headed arrow) between the posterior end of the hippocampal resection (a line with a dot) and the quadrigeminal plate (a dotted line with an open dot) according to the method by Helmstaedter et al.18)
A video of graphical computer simulation showing the difference in brain shift between cases with high and low degrees of head rotation. We used GRID (Kompath Inc., Tokyo, Japan), a commercially available 3D medical image viewer driven by a deep-learning algorithm. We utilized the “brain deformation function” that was originally designed to simulate brain retraction in neurosurgical procedures.
Acknowledgments
We thank Editage (https://www.editage.jp/) for the English language editing.
References
- 1). Yaşargil MG, Teddy PJ, Roth P: Selective Amygdalo-hippocampectomy: operative anatomy and surgical technique. In: Advances and Technical Standards in Neurosurgery. Vol 12. Symon L, Brihaye J, Guidetti B, et al. , Eds. Springer-Verlag, Wien, New York, 1985: 93-123 [DOI] [PubMed] [Google Scholar]
- 2). Morino M: Selective amygdalohippocampectomy. In: Microsurgery of Intractable Epilepsy. Morino M, Ed. Medical View Co., Ltd., Tokyo, 2013: 16-26 [Google Scholar]
- 3). Yonekawa Y: [Operative neurosurgery: personal view and historical backgrounds (4). Selective amygdalohippocampectomy SAHE]. No Shinkei Geka 35: 1183-1196, 2007(Japanese) [PubMed] [Google Scholar]
- 4). Von Der Heide RJ, Skipper LM, Klobusicky E, Olson IR: Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain 136: 1692-1707, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Helmstaedter C, Richter S, Roske S, Oltmanns F, Schramm J, Lehmann TN: Differential effects of temporal pole resection with amygdalohippocampectomy versus selective amygdalohippocampectomy on material-specific memory in patients with mesial temporal lobe epilepsy. Epilepsia 49: 88-97, 2008 [DOI] [PubMed] [Google Scholar]
- 6). Morino M, Ichinose T, Uda T, Kondo K, Ohfuji S, Ohata K: Memory outcome following transsylvian selective amygdalohippocampectomy in 62 patients with hippocampal sclerosis. J Neurosurg 110: 1164-1169, 2009 [DOI] [PubMed] [Google Scholar]
- 7). Martino J, Vergani F, Robles SG, Duffau H: New insights into the anatomic dissection of the temporal stem with special emphasis on the inferior fronto-occipital fasciculus: implications in surgical approach to left mesiotemporal and temporoinsular structures. Neurosurgery 66: 4-12, 2010 [DOI] [PubMed] [Google Scholar]
- 8). Duffau H, Gatignol P, Mandonnet E, Peruzzi P, Tzourio-Mazoyer N, Capelle L: New insights into the anatomo-functional connectivity of the semantic system: a study using cortico-subcortical electrostimulations. Brain 128: 797-810, 2005 [DOI] [PubMed] [Google Scholar]
- 9). Ribas EC, Yagmurlu K, Wen HT, Rhoton AL, Jr: Microsurgical anatomy of the inferior limiting insular sulcus and the temporal stem. J Neurosurg 122: 1263-1273, 2015 [DOI] [PubMed] [Google Scholar]
- 10). Hamasaki T, Hirai T, Yamada K, Kuratsu J: An in vivo morphometry study on the standard transsylvian trajectory for mesial temporal lobe epilepsy surgery. Springerplus 4: 406, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Ibayashi K, Kunii N, Kawai K, Saito N: A secure approach to the inferior horn using the deep medullary vein as an anatomic guide. World Neurosurg 108: 325-327, 2017 [DOI] [PubMed] [Google Scholar]
- 12). Helmstaedter C, Roeske S, Kaaden S, Elger CE, Schramm J: Hippocampal resection length and memory outcome in selective epilepsy surgery. J Neurol Neurosurg Psychiatry 82: 1375-1381, 2011 [DOI] [PubMed] [Google Scholar]
- 13). Yasargil MG, Teddy PJ, Roth P: Selective amygdalo-hippocampectomy. Operative anatomy and surgical technique. Adv Tech Stand Neurosurg 12: 93-123, 1985 [DOI] [PubMed] [Google Scholar]
- 14). Kamiyama H, Houkin K: Craniotomy, basic techniques of cerebral aneurysm surgery. In: Microsurgery of Cerebral Aneurysms. Kamiyama H, Houkin K, Eds. Nankodo Co., Ltd., Tokyo, 2010: 26-28 [Google Scholar]
- 15). Usui N, Kondo A, Nitta N, Tottori T, Inoue Y: Surgical resection of amygdala and uncus. Neurol Med Chir (Tokyo) 58: 377-383, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Gotman J, Levtova V: Amygdala-hippocampus relationships in temporal lobe seizures: a phase-coherence study. Epilepsy Res 25: 51-57, 1996 [DOI] [PubMed] [Google Scholar]
- 17). Morino M, Uda T, Naito K, et al. : Comparison of neuropsychological outcomes after selective amygdalohippocampectomy versus anterior temporal lobectomy. Epilepsy & Behavior: E&B 9: 95-100, 2006 [DOI] [PubMed] [Google Scholar]
- 18). Helmstaedter C, Van Roost D, Clusmann H, Urbach H, Elger CE, Schramm J: Collateral brain damage, a potential source of cognitive impairment after selective surgery for control of mesial temporal lobe epilepsy. J Neurol Neurosurg Psychiatry 75: 323-326, 2004 [PMC free article] [PubMed] [Google Scholar]
- 19). Kier EL, Staib LH, Davis LM, Bronen RA: MR imaging of the temporal stem: anatomic dissection tractography of the uncinate fasciculus, inferior occipitofrontal fasciculus, and Meyer's loop of the optic radiation. AJNR Am J Neuroradiol 25: 677-691, 2004 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. A graph showing the length of the hippocampal resection in the anteroposterior axis (A–P length). The A–P length was defined as the distance between the anterior and posterior ends of the surgical cavity in coronal slices of postoperative MRI. The difference in A–P length between cases with high-degree (27.3 ± 1.6 mm) and low-degree (28.0 ± 1.1 mm) rotations was not statistically significant (p = 0.701, Student’s t-test). The data were expressed as the mean length and standard errors.
B. A graph showing the length between the posterior end of the hippocampal resection and the quadrigeminal plate (QP length, Supplementary Figure 2).18) The difference in QP length between cases with high-degree (6.7 ± 2.1 mm) and low-degree (5.7 ± 1.1 mm) rotations was not statistically significant (p = 0.678, Student’s t-test).
C. A graph showing the degree of amygdala resection, which was expressed as the ratio in area of surgical defect in the amygdala on a postoperative coronal section through the center of the amygdala. The difference in amygdala resection ratio between cases with high-degree (67.7% ± 3.9%) and low-degree (63.5% ± 4.6%) rotations was not statistically significant (p = 0.498, Student’s t-test).
D. A graph showing the seizure outcome in Engel classification. The rate of Engel class I between cases with high-degree (9/13; 69.2%) and low-degree (9/11, 81.8%) rotations was not statistically significant (p = 0.478, chi-square test) at the latest follow-up (mean, 56 and 25 months, respectively).
A. An axial section of T1-weighted MR imaging through the surgical cavity after left hippocampal resection. The boxed area is enlarged and schematized in B.
B. The QP length is defined as the length along the anteroposterior axis (a two-headed arrow) between the posterior end of the hippocampal resection (a line with a dot) and the quadrigeminal plate (a dotted line with an open dot) according to the method by Helmstaedter et al.18)
A video of graphical computer simulation showing the difference in brain shift between cases with high and low degrees of head rotation. We used GRID (Kompath Inc., Tokyo, Japan), a commercially available 3D medical image viewer driven by a deep-learning algorithm. We utilized the “brain deformation function” that was originally designed to simulate brain retraction in neurosurgical procedures.