Abstract
Background and Objectives
Readmissions in heart failure (HF), historically reported as 20%, contribute to significant patient morbidity and high financial cost to the healthcare system. The changing population landscape and risk factor dynamics mandate periodic epidemiologic reassessment of HF readmissions.
Methods
National Readmission Database (NRD, 2019) was used to identify HF-related hospitalizations and evaluated for demographic, admission characteristics, and comorbidity differences between patients readmitted vs. those not readmitted at 30-days. Causes of readmission and predictors of all-cause, HF-specific, and non-HF-related readmissions were analyzed.
Results
Of 48,971 HF patients, the readmitted cohort was younger (mean 67.4 vs. 68.9 years, p≤0.001), had higher proportion of males (56.3% vs. 53.7%), lowest income quartiles (33.3% vs. 28.9%), Charlson comorbidity index (CCI) ≥3 (61.7% vs. 52.8%), resource utilization including large bed-size hospitalizations, Medicaid enrollees, mean length of stay (6.2 vs. 5.4 days), and disposition to other facilities (23.9% vs. 20%) than non-readmitted. Readmission (30-day) rate was 21.2% (10,370) with cardiovascular causes in 50.3% (HF being the most common: 39%), and non-cardiac in 49.7%. Independent predictors for readmission were male sex, lower socioeconomic status, nonelective admissions, atrial fibrillation, chronic obstructive pulmonary disease, chronic kidney disease, anemia, and CCI ≥3. HF-specific readmissions were significantly associated with prior coronary artery disease and Medicaid enrollment.
Conclusions
Our analysis revealed cardiac and noncardiac causes of readmission were equally common for 30-day readmissions in HF patients with HF itself being the most common etiology highlighting the importance of addressing the comorbidities, both cardiac and non-cardiac, to mitigate the risk of readmission.
Keywords: Heart failure; Thirty day readmission; 30 day readmission; United States Agency for Healthcare Research and Quality, Etiology; Outcome assessment, health care
INTRODUCTION
Heart failure (HF) is a well-known public health problem and a major financial burden on our healthcare system. The prevalence of HF continues to rise due to the aging United States (US) population and is projected to increase by 46% between 2012 and 2030.1) In recent years, novel medical and device therapies have led to improved HF prognosis. These include the use of a combination of angiotensin receptor–neprilysin inhibitor,2) SGLT2 inhibitors,3) a novel algorithm for guideline-directed medical therapy,4,5) biventricular pacemakers, remote pulmonary artery pressure monitors, mechanical circulatory assist devices, and the use of disease management programs in the outpatient setting.6,7,8) However, the risk of readmissions in HF patients remains high resulting in patient morbidity and high healthcare resource utilization.1) Historically studies have reported readmission rates of nearly 20% in this patient population warranting additional research to identify aspects of care to improve patient outcomes.9,10,11) Using the 2019 National Readmission Database (NRD) dataset, we investigated the epidemiologic profile of patients readmitted with HF patients to identify high-risk predictors in this patient population.
METHODS
We used the dataset of 2019 from the NRD for this analysis. Agency for Healthcare Research and Quality sponsors NRD under the Healthcare Cost and Utilization Projects.12) NRD provides publicly available large de-identified all-payer datasets of hospitalized patients in the US containing 18 million unweighted and 35 million weighted discharges per year that are nationally representative of the US, thereby providing sufficient data for analysis across hospital types and the study of readmissions for various disorders and procedures. Patients are tracked across the time for readmissions using the variable “NRD_visitlink”, while the time between readmissions is calculated using already existing variables “NRD_daystoevent” and length of stay available as “LOS” in the NRD. Owing to the de-identified nature of the dataset, we did not require Institutional Review Board approval to conduct our study.
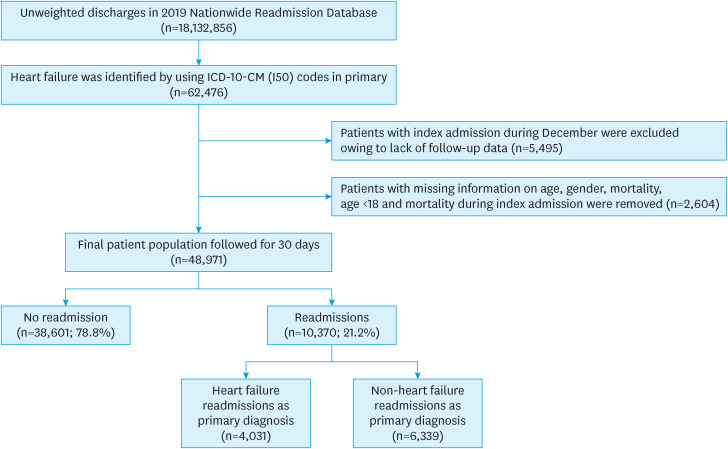
The details of sample selection and cohort division are shown in Figure 1. HF-related hospitalizations (index admissions) were identified using the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) code of “I50” in the primary discharge diagnosis. Hospitalizations for less than 18 years, missing information on sex, mortality during this index admission, and discharges in December 2019 (due to lack of follow-up) were excluded from this analysis. The primary outcome was 30-day readmissions in the HF study population. Combining similar codes, we identified causes of readmission using ICD-10-CM codes in the primary discharge diagnosis field of these readmissions. Readmissions were either HF-specific readmissions, i.e., with HF being the primary reason for readmission or non-HF readmissions, i.e., primary reason other than HF. Multivariate logistic regression analysis was used for the predictors of HF-specific and non-HF readmissions, and for all-cause readmissions (combined HF-specific and non-HF readmissions).
Figure 1. Patient selection and study design.
ICD-10-CM = International Classification of Diseases, Tenth Revision, Clinical Modification.
Demographics and admission-specific, including hospital-related characteristics, provided as in-built variables (Table 1, Supplementary Table 1), were compared between HF patients who were readmitted (herein, readmitted cohort) and not readmitted (herein, non-readmitted cohort) within 30-days. Comorbidities were generated as binary variables using ICD-10-CM codes in the secondary discharge diagnosis fields (Supplementary Table 2). Charlson comorbidity index (CCI) assessed the severity of comorbid conditions and was employed to compare the 2 cohorts with Deyo’s modification and translation to ICD-10-CM codes.13,14) Conventional CCI gives the weighted score to the comorbidities based on the relative risk of 1-year mortality. To apply the index to the administrative databases, like in our study, Deyo’s modification, a simplified version of the conventional CCI, is commonly used in clinical practice where using ICD-10-CM system medical conditions are coded as present on admission.
Table 1. Baseline characteristics of heart failure patients from National Readmission Dataset 2019.
| Variables | Readmissions | Overall | p value | |||
|---|---|---|---|---|---|---|
| No | Yes | |||||
| Index admissions, No. (%) | 38,601 (78.8) | 10,370 (21.2) | 48,971 | |||
| Demographic characteristics | ||||||
| Age (mean years) | 68.9 | 67.4 | 68.6 | <0.001 | ||
| Sex | <0.001 | |||||
| Male | 53.7 | 56.3 | 54.2 | |||
| Female | 46.3 | 43.7 | 45.8 | |||
| Median household income (percentile)* | <0.001 | |||||
| 0–25 | 28.9 | 33.3 | 29.8 | |||
| 26–50 | 27.6 | 26.0 | 27.3 | |||
| 51–75 | 24.8 | 23.4 | 24.5 | |||
| 76–100 | 18.7 | 17.3 | 18.4 | |||
| Admission characteristics | ||||||
| Admission type | 0.003 | |||||
| Elective | 5.6 | 4.8 | 5.4 | |||
| Non-elective | 94.4 | 95.2 | 94.6 | |||
| Day of admission | 0.205 | |||||
| Weekday | 77.3 | 76.7 | 77.1 | |||
| Weekend | 22.7 | 23.3 | 22.9 | |||
| Bed size of hospital† | 0.006 | |||||
| Small | 24.1 | 22.9 | 23.9 | |||
| Medium | 25.5 | 25.1 | 25.4 | |||
| Large | 50.4 | 52.1 | 50.8 | |||
| Teaching status of hospital‡ | <0.001 | |||||
| Metropolitan non-teaching | 20.3 | 19.8 | 20.2 | |||
| Metropolitan teaching | 64.7 | 67.1 | 65.2 | |||
| Non-metropolitan hospital | 14.9 | 13.1 | 14.5 | |||
| Expected primary payer | <0.001 | |||||
| Medicare | 65.1 | 65.5 | 65.2 | |||
| Medicaid | 13.6 | 17.9 | 14.5 | |||
| Private insurance | 15.3 | 11.6 | 14.5 | |||
| Self-pay | 3.7 | 2.7 | 3.5 | |||
| No charge | 0.2 | 0.3 | 0.2 | |||
| Other | 2.1 | 2.0 | 2.1 | |||
| Comorbidities§ | ||||||
| Hypertension | 22.5 | 23.8 | 22.8 | 0.005 | ||
| Diabetes | 28.4 | 32.1 | 29.2 | <0.001 | ||
| Dyslipidemia | 30.4 | 30.4 | 30.4 | 0.95 | ||
| Atrial fibrillation or flutter | 43.8 | 45.6 | 44.2 | 0.001 | ||
| PVD | 5.9 | 7.0 | 6.1 | <0.001 | ||
| Prior CAD | 37.6 | 39.9 | 38.1 | <0.001 | ||
| Prior TIA/Stroke | 8.7 | 9.2 | 8.8 | 0.141 | ||
| Prior CABG | 9.0 | 9.8 | 9.2 | 0.021 | ||
| Prior PCI | 0.8 | 1.0 | 0.8 | 0.184 | ||
| FH of CAD | 6.6 | 6.4 | 6.6 | 0.552 | ||
| COPD | 30.5 | 34.9 | 31.5 | <0.001 | ||
| CKD | 22.6 | 28.5 | 23.8 | <0.001 | ||
| Obesity | 20.4 | 19.3 | 20.2 | 0.016 | ||
| OSA | 12.8 | 12.7 | 12.8 | 0.831 | ||
| Anemia | 25.3 | 31.5 | 26.6 | <0.001 | ||
| Tobacco | 44.1 | 46.2 | 44.6 | <0.001 | ||
| Alcohol | 6.2 | 6.4 | 6.2 | 0.36 | ||
| CCI∥ | <0.001 | |||||
| 1 | 20.8 | 16.1 | 19.8 | |||
| 2 | 26.4 | 22.2 | 25.6 | |||
| ≥3 | 52.8 | 61.7 | 54.7 | |||
| Healthcare infrastructure utilization | ||||||
| Disposition | <0.001 | |||||
| Home | 80.0 | 76.1 | 79.2 | |||
| Facility/Others | 20.0 | 23.9 | 20.8 | |||
| Length of stay (days, mean±SE) | 5.4±0.03 | 6.2±0.08 | 5.6±0.03 | <0.001 | ||
Values are percentages.
PVD = peripheral vascular disease; CAD = coronary artery disease; TIA = transient ischemic attack; CABG = coronary artery bypass graft; PCI = percutaneous coronary intervention; FH = family history; COPD = chronic obstructive pulmonary disease; CKD = chronic kidney disease; OSA = obstructive sleep apnea; CCI = Charlson comorbidity index; SE = standard error.
*Represents a quartile classification of the estimated median household income of residents within the patient’s zip code, https://www.hcup-us.ahrq.gov/db/vars/zipinc_qrtl/nrdnote.jsp.
†The bed size cutoff points divided into small, medium, and large have been done so that approximately one-third of the hospitals in a given region, location, and teaching status combination would fall within each bed size category, https://www.hcup-us.ahrq.gov/db/vars/hosp_bedsize/nrdnote.jsp.
‡A hospital is considered to be a teaching hospital if it has an American Medical Association-approved residency program, https://www.hcup-us.ahrq.gov/db/vars/hosp_ur_teach/nrdnote.jsp.
§International Classification of Diseases, Tenth Revision codes were utilized to identify respective co-morbidities as per Supplementary Table 1.
∥CCI/Deyo comorbidity index was calculated as per Deyo classification.
SAS 9.4 (SAS Institute Inc., Cary, NC, USA) was used for this analysis. Pearson’s χ2 and Student’s t-test were used for qualitative and quantitative variables, respectively. In our multivariate model for predictors of all-cause, HF-specific and non-HF readmissions, we included demographics, admission characteristics, comorbidities, categorized CCI (CCI with 1,2 or ≥3), disposition pattern, and length of stay variables shown in Table 1.
RESULTS
NRD dataset from 2019 included 48,971 index admissions for HF hospitalizations as the primary admitting diagnosis. During index admission, differences in demographics and comorbidities were observed between readmitted and non-readmitted cohorts. The readmitted cohort was younger (67.4 vs. 68.9 years, p<0.001). Readmitted patients had a higher prevalence of males (56.3% vs. 53.7%, p<0.001), lowest quartile of median household income patients (33.3% vs. 28.9%, p<0.001), admissions of nonelective (95.2% vs. 94.4%, p=0.003), large bed-size hospitals (52.1% vs. 50.4%, p=0.006), metropolitan teaching hospitals (67.1% vs. 64.7%, p<0.001), Medicaid enrollees (17.9% vs. 13.6%, p<0.001). Comorbidities most prevalent in the readmitted cohort were hypertension (HTN, 23.8% vs. 22.5%, p=0.005), diabetes mellitus (DM, 32.1% vs. 28.4%, p<0.001), atrial fibrillation or flutter (AFF, 45.6% vs. 43.8%, p=0.001), peripheral vascular disease (PVD, 7% vs. 5.9%, p<0.001), prior coronary artery disease (CAD, 39.9% vs. 37.6%, p<0.001), prior coronary artery bypass graft (CABG, 9.8% vs. 9%, p=0.021), chronic obstructive lung disease (COPD, 34.9% vs. 30.5%, p<0.001), chronic kidney disease (CKD, 28.5% vs. 22.6%, p<0.001), anemia (31.5% vs. 25.3%, p<0.001), tobacco abuse (46.2% vs. 44.1%, p<0.001) and higher CCI, i.e., CCI ≥3 (61.7% vs. 52.8%, p<0.001) vs. the non-readmitted cohort. The average length of hospital stay was longer for readmitted cohort (mean 6.2±0.08 vs. 5.4±0.03 days, p<0.001) and a greater percentage of patients required transfer to other facilities (23.9% vs. 20%, p<0.001) during index admissions.
Of the 48,971 hospitalizations, 10,370 (21.2%) were readmitted within a 30-day period. Amongst these, 85.26% (n=8,841) were readmitted once, 12.93% (n=1,341) twice, 1.58% (n=164) 3 times, 0.17% (n=18) 4 times, 0.05% (n=5) 5 times, and 0.01% (n=1), and 6 times (Table 2). Regarding etiologies of admissions, HF-specific readmissions constituted 39%, followed by CAD/ischemic heart disease 2.7%, AFF 1%, HTN 0.18%, congenital heart disease 0.1%, and valvular heart disease 0.8%. Pulmonary causes of readmission occurred in 12.4%, which included acute respiratory failure in 4.2%, pneumonia and influenza-related hospitalizations in 3.0%, pulmonary embolism in 0.8%, and obstructive lung disease in 0.14%. Infections constituted 9.3%, followed by gastrointestinal causes in 4.6% and renal in 4.1% of the readmissions (Table 3, Figure 2).
Table 2. Readmission frequencies in heart failure patients during 30-day follow-up period.
| Readmission frequencies | Number of patients | Percentage of patients |
|---|---|---|
| 1 | 8,841 | 85.26 |
| 2 | 1,341 | 12.93 |
| 3 | 164 | 1.58 |
| 4 | 18 | 0.17 |
| 5 | 5 | 0.05 |
| 6 | 1 | 0.01 |
Table 3. Causes of 30-day readmissions in heart failure patients (n=10,370).
| System classification | Specific diagnosis | Percentage (%) | |
|---|---|---|---|
| Cardiac | 50.34 | ||
| Valvular | 0.8 | ||
| Hypertension | 0.18 | ||
| Coronary artery disease | 2.74 | ||
| Heart failure | 39.01 | ||
| Systolic | 8.67 | ||
| Diastolic | 3.21 | ||
| Combined | 3.04 | ||
| HF due to hypertension disorder +/− CKD | 22.78 | ||
| Unspecified | 1.31 | ||
| Atrial fibrillation or flutter | 1.05 | ||
| Congenital | 0.11 | ||
| Others | 6.45 | ||
| Pulmonary | 12.44 | ||
| Pneumonia & Influenza | 3.05 | ||
| Pulmonary embolism | 0.8 | ||
| Respiratory failure | 4.18 | ||
| Emphysema & Bronchitis | 0.14 | ||
| Asthma | 0.07 | ||
| Bronchiectasis | 0.02 | ||
| Others | 4.15 | ||
| Infections | 9.35 | ||
| Gastrointestinal | 4.65 | ||
| Renal | 4.14 | ||
| Endocrine | 2.79 | ||
| Thyroid | 0.02 | ||
| DM | 0.89 | ||
| Metabolic | 1.69 | ||
| Others | 0.18 | ||
| Central nervous system | 2.16 | ||
| Ischemic | 1.29 | ||
| Others | 0.87 | ||
| Vascular | 1.97 | ||
| Neoplasms | 1.11 | ||
| Hematological | 1.05 | ||
| Musculoskeletal | 0.89 | ||
| Psychiatric | 0.86 | ||
| Dermatology | 0.17 | ||
| Others | 8.05 | ||
HF = heart failure; CKD = chronic kidney disease; DM = diabetes mellitus.
Figure 2. Causes of 30-day readmissions in heart failure patients.
Multivariable analysis of 30-day readmission in HF patients was performed to identify significant predictors by adjusting the odds ratio (aOR). Significant predictors of all-cause readmissions (Table 4, Figure 3) were male sex (aOR, 1.05; 95% confidence interval [CI], 1.01–1.10; p=0.026), lowest quartile of median household income (aOR, 1.21; 95% CI, 1.13–1.30; p<0.001), index admissions with nonelective (aOR, 1.12; 95% CI, 1.01–1.24; p=0.031), metropolitan nonteaching (aOR, 1.14; 95% CI, 1.05–1.23; p=0.002) and teaching (aOR, 1.16; 95% CI, 1.08–1.25, p<0.001) hospitalizations, AFF (aOR, 1.15; 95% CI, 1.10–1.21; p<0.001), COPD (aOR, 1.16; 95% CI, 1.10–1.22; p<0.001), CKD (aOR, 1.17; 95% CI, 1.10–1.24; p<0.001), anemia (aOR, 1.25; 95% CI, 1.19–1.32; p<0.001), CCI ≥3 (aOR, 1.29; 95% CI, 1.19–1.39; p<0.001), discharged to other facilities (aOR, 1.27; 95% CI, 1.20–1.34; p<0.001), and length of stay (aOR, 1.006; 95% CI, 1.003–1.008; p<0.001). Both prior CAD and Medicaid status were significant independent predictors in HF-specific readmissions (Table 5). On the other hand, PVD and CCI 2 or greater were significant predictors in the non-HF readmission cohort (Table 5).
Table 4. Multivariate predictors of readmissions of heart failure patients.
| Variables | 30-day readmission | ||||
|---|---|---|---|---|---|
| aOR | 95% CI | p value | |||
| LL | UL | ||||
| Age | 0.987 | 0.985 | 0.989 | <0.001 | |
| Sex | |||||
| Female | Referent | ||||
| Male* | 1.05 | 1.01 | 1.10 | 0.026 | |
| Median household income (percentile)† | |||||
| 0–25* | 1.21 | 1.13 | 1.30 | <0.001 | |
| 26–50 | 1.010 | 0.943 | 1.083 | 0.771 | |
| 51–75 | 1.009 | 0.941 | 1.082 | 0.794 | |
| 76–100 | Referent | ||||
| Admission type | |||||
| Elective | Referent | ||||
| Non-elective* | 1.12 | 1.01 | 1.24 | 0.031 | |
| Day of admission | |||||
| Weekday | Referent | ||||
| Weekend | 1.03 | 0.98 | 1.09 | 0.275 | |
| Bed size of hospital‡ | |||||
| Small | Referent | ||||
| Medium | 0.995 | 0.933 | 1.061 | 0.879 | |
| Large | 0.995 | 0.940 | 1.054 | 0.875 | |
| Teaching status of hospital§ | |||||
| Non-metropolitan hospital | Referent | ||||
| Metropolitan non-teaching* | 1.14 | 1.05 | 1.23 | 0.002 | |
| Metropolitan teaching* | 1.16 | 1.08 | 1.25 | <0.001 | |
| Expected primary payer | |||||
| Medicare | Referent | ||||
| Medicaid | 1.08 | 1.00 | 1.16 | 0.054 | |
| Private insurance | 0.71 | 0.65 | 0.76 | <0.001 | |
| Self-pay | 0.68 | 0.59 | 0.78 | <0.001 | |
| No charge | 1.21 | 0.78 | 1.86 | 0.392 | |
| Other | 0.88 | 0.75 | 1.03 | 0.114 | |
| Comorbidities∥ | |||||
| Hypertension | 1.00 | 0.95 | 1.06 | 0.899 | |
| Diabetes | 1.04 | 0.98 | 1.10 | 0.165 | |
| Dyslipidemia | 0.98 | 0.93 | 1.04 | 0.551 | |
| Atrial fibrillation or flutter* | 1.15 | 1.10 | 1.21 | <0.001 | |
| PVD | 1.06 | 0.96 | 1.16 | 0.246 | |
| Prior CAD | 1.04 | 0.99 | 1.09 | 0.164 | |
| Prior TIA/Stroke | 0.99 | 0.92 | 1.07 | 0.851 | |
| Prior CABG | 1.02 | 0.94 | 1.10 | 0.665 | |
| Prior PCI | 1.13 | 0.90 | 1.43 | 0.294 | |
| FH of CAD | 0.99 | 0.91 | 1.09 | 0.899 | |
| COPD* | 1.16 | 1.10 | 1.22 | <0.001 | |
| CKD* | 1.17 | 1.10 | 1.24 | <0.001 | |
| Obesity | 0.84 | 0.79 | 0.89 | <0.001 | |
| OSA | 0.94 | 0.88 | 1.01 | 0.089 | |
| Anemia* | 1.25 | 1.19 | 1.32 | <0.001 | |
| Tobacco | 1.00 | 0.96 | 1.05 | 0.865 | |
| Alcohol | 0.95 | 0.86 | 1.04 | 0.286 | |
| CCI¶ | |||||
| 1 | Referent | ||||
| 2 | 1.04 | 0.96 | 1.12 | 0.333 | |
| ≥3* | 1.29 | 1.19 | 1.39 | <0.001 | |
| Disposition | |||||
| Home | Referent | ||||
| Facility/Others* | 1.27 | 1.20 | 1.34 | <0.001 | |
| Length of stay* | 1.006 | 1.003 | 1.008 | <0.001 | |
aOR = adjusted odds ratio; CI = confidence interval; LL = lower limit; UL = upper limit; PVD = peripheral vascular disease; CAD = coronary artery disease; TIA = transient ischemic attack; CABG = coronary artery bypass graft; PCI = percutaneous coronary intervention; FH = family history; COPD = chronic obstructive pulmonary disease; CKD = chronic kidney disease; OSA = obstructive sleep apnea; CCI = Charlson comorbidity index.
*Significant predictors of readmission.
†Represents a quartile classification of the estimated median household income of residents within the patient’s zip code, https://www.hcup-us.ahrq.gov/db/vars/zipinc_qrtl/nrdnote.jsp.
‡The bed size cutoff points divided into small, medium, and large have been done so that approximately one-third of the hospitals in a given region, location, and teaching status combination would fall within each bed size category, https://www.hcup-us.ahrq.gov/db/vars/hosp_bedsize/nrdnote.jsp.
§A hospital is considered to be a teaching hospital if it has an American Medical Association-approved residency program, https://www.hcup-us.ahrq.gov/db/vars/hosp_ur_teach/nrdnote.jsp.
∥International Classification of Diseases, Tenth Revision codes were utilized to identify respective co-morbidities as per Supplementary Table 1.
¶CCI/Deyo comorbidity index was calculated as per Deyo classification.
Figure 3. Predictors of all-cause 30-day readmissions in heart failure patients.
OR = odds ratio (adjusted); CI = confidence interval; CCI = Charlson comorbidity index.
Table 5. Multivariate predictors of readmission with and without heart failure.
| Variables | Multivariate predictors of readmissions with heart failure only(H) | Multivariate predictors of readmissions except for heart failure(NH) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| aOR | 95% CI | p value | aOR | 95% CI | p value | ||||
| LL | UL | LL | UL | ||||||
| Age | 0.981 | 0.978 | 0.984 | <0.001 | 0.991 | 0.989 | 0.994 | <0.001 | |
| Sex | |||||||||
| Female | Referent | Referent | |||||||
| Male | 1.19 | 1.11 | 1.28 | <0.001 | 0.98 | 0.92 | 1.03 | 0.389 | |
| Median household income (percentile)* | |||||||||
| 0–25 | 1.26 | 1.13 | 1.39 | <0.001 | 1.19 | 1.09 | 1.29 | <0.001 | |
| 26–50 | 1.03 | 0.93 | 1.14 | 0.592 | 1.00 | 0.92 | 1.09 | 0.965 | |
| 51–75 | 1.05 | 0.94 | 1.17 | 0.386 | 0.99 | 0.91 | 1.07 | 0.750 | |
| 76–100 | Referent | Referent | |||||||
| Admission type | |||||||||
| Elective | Referent | Referent | |||||||
| Non-elective | 1.16 | 0.99 | 1.37 | 0.067 | 1.10 | 0.97 | 1.25 | 0.124 | |
| Day of admission | |||||||||
| Weekday | Referent | Referent | |||||||
| Weekend | 1.09 | 1.01 | 1.17 | 0.034 | 0.99 | 0.93 | 1.06 | 0.871 | |
| Bed size of hospital† | |||||||||
| Small | Referent | Referent | |||||||
| Medium | 0.99 | 0.90 | 1.09 | 0.811 | 1.00 | 0.93 | 1.09 | 0.915 | |
| Large | 0.98 | 0.90 | 1.06 | 0.581 | 1.01 | 0.94 | 1.08 | 0.741 | |
| Teaching status of hospital‡ | |||||||||
| Non-metropolitan hospital | Referent | Referent | |||||||
| Metropolitan non-teaching | 1.18 | 1.04 | 1.33 | 0.010 | 1.12 | 1.02 | 1.24 | 0.021 | |
| Metropolitan teaching | 1.19 | 1.07 | 1.33 | 0.001 | 1.15 | 1.05 | 1.25 | 0.001 | |
| Expected primary payer | |||||||||
| Medicare | Referent | Referent | |||||||
| Medicaid | 1.31 | 1.18 | 1.46 | <0.001 | 0.92 | 0.83 | 1.01 | 0.075 | |
| Private insurance | 0.62 | 0.55 | 0.70 | <0.001 | 0.77 | 0.70 | 0.84 | <0.001 | |
| Self-pay | 0.76 | 0.62 | 0.92 | 0.006 | 0.62 | 0.52 | 0.74 | <0.001 | |
| No charge | 1.15 | 0.62 | 2.13 | 0.666 | 1.24 | 0.73 | 2.12 | 0.425 | |
| Other | 0.95 | 0.76 | 1.20 | 0.688 | 0.84 | 0.68 | 1.02 | 0.079 | |
| Comorbidities§ | |||||||||
| Hypertension | 0.98 | 0.90 | 1.07 | 0.679 | 1.02 | 0.95 | 1.09 | 0.587 | |
| Diabetes | 1.07 | 0.99 | 1.16 | 0.105 | 1.03 | 0.96 | 1.09 | 0.463 | |
| Dyslipidemia | 1.04 | 0.96 | 1.13 | 0.289 | 0.95 | 0.89 | 1.01 | 0.114 | |
| Atrial fibrillation or flutter | 1.20 | 1.11 | 1.29 | <0.001 | 1.13 | 1.06 | 1.19 | <0.001 | |
| PVD | 0.87 | 0.75 | 1.00 | 0.054 | 1.17 | 1.06 | 1.30 | 0.003 | |
| Prior CAD | 1.09 | 1.01 | 1.17 | 0.037 | 1.01 | 0.95 | 1.08 | 0.698 | |
| Prior TIA/Stroke | 0.96 | 0.86 | 1.09 | 0.551 | 1.01 | 0.92 | 1.11 | 0.882 | |
| Prior CABG | 1.09 | 0.97 | 1.23 | 0.149 | 0.97 | 0.88 | 1.07 | 0.536 | |
| Prior PCI | 1.34 | 0.98 | 1.84 | 0.071 | 0.99 | 0.73 | 1.34 | 0.937 | |
| FH of CAD | 0.97 | 0.85 | 1.11 | 0.693 | 1.01 | 0.90 | 1.13 | 0.889 | |
| COPD | 1.16 | 1.07 | 1.25 | <0.001 | 1.16 | 1.09 | 1.24 | <0.001 | |
| CKD | 1.48 | 1.35 | 1.61 | <0.001 | 1.01 | 0.94 | 1.08 | 0.820 | |
| Obesity | 0.87 | 0.79 | 0.95 | 0.002 | 0.83 | 0.77 | 0.89 | <0.001 | |
| OSA | 0.90 | 0.81 | 1.00 | 0.055 | 0.97 | 0.89 | 1.06 | 0.465 | |
| Anemia | 1.15 | 1.07 | 1.24 | <0.001 | 1.32 | 1.24 | 1.40 | <0.001 | |
| Tobacco | 1.02 | 0.95 | 1.09 | 0.677 | 1.00 | 0.94 | 1.06 | 0.995 | |
| Alcohol | 1.01 | 0.88 | 1.15 | 0.919 | 0.91 | 0.81 | 1.03 | 0.134 | |
| CCI∥ | |||||||||
| 1 | Referent | Referent | |||||||
| 2 | 0.90 | 0.81 | 1.01 | 0.076 | 1.13 | 1.03 | 1.24 | 0.007 | |
| ≥3 | 1.13 | 1.01 | 1.26 | 0.041 | 1.40 | 1.27 | 1.53 | <0.001 | |
| Disposition | |||||||||
| Home | Referent | Referent | |||||||
| Facility/Others | 1.18 | 1.09 | 1.28 | <0.001 | 1.32 | 1.24 | 1.41 | <0.001 | |
| Length of stay | 0.99 | 0.99 | 1.00 | 0.049 | 1.01 | 1.01 | 1.01 | <0.001 | |
aOR = adjusted odds ratio; CI = confidence interval; LL = lower limit; UL = upper limit; PVD = peripheral vascular disease; CAD = coronary artery disease; TIA = transient ischemic attack; CABG = coronary artery bypass graft; PCI = percutaneous coronary intervention; FH = family history; COPD = chronic obstructive pulmonary disease; CKD = chronic kidney disease; OSA = obstructive sleep apnea; CCI = Charlson comorbidity index.
(H)Patients readmitted because of heart failure, and non-heart failure-related readmissions were excluded.
(NH)Patients readmitted because of non-heart failure, and heart failure-related readmissions were excluded.
*Represents a quartile classification of the estimated median household income of residents within the patient’s zip code, https://www.hcup-us.ahrq.gov/db/vars/zipinc_qrtl/nrdnote.jsp.
†The bed size cutoff points divided into small, medium, and large have been done so that approximately one-third of the hospitals in a given region, location, and teaching status combination would fall within each bed size category, https://www.hcup-us.ahrq.gov/db/vars/hosp_bedsize/nrdnote.jsp.
‡A hospital is considered to be a teaching hospital if it has an American Medical Association-approved residency program, https://www.hcup-us.ahrq.gov/db/vars/hosp_ur_teach/nrdnote.jsp.
§International Classification of Diseases, Tenth Revision codes were utilized to identify respective co-morbidities as per Supplementary Table 1.
∥CCI/Deyo comorbidity index was calculated as per Deyo classification.
DISCUSSION
We examined the 2019 NRD dataset to determine readmission characteristics in HF patients. The readmission rate was 21.2% in HF patients. Half of the readmissions were due to cardiac causes with HF being the most common cause for readmission in 39%. The etiology of non-cardiac admissions included pulmonology, infection, gastro-intestinal and renal causes. Predictors of all-cause readmissions were male sex, lower socioeconomic status, nonelective admissions, metropolitan non-teaching, and teaching hospitalizations, AFF, COPD, CKD, anemia, CCI ≥3, discharge status to other facilities, and length of stay.
HF-related emergency department (ED) visits totaled 1.1 million between 2006 to 2014, according to a large population-based US sample study, and 91.5% of these patients ultimately were admitted.15) HF-related ED visits and hospitalizations were most prevalent in the patients ≥65 years of age, with a mean of 71.6 and 72.1 years, respectively.15) The NRD-based analysis of 2013 demonstrated 73.4% of HF hospitalizations were ≥ 65 years of age.9) The mean age of our HF patient cohort was 68.6 years, but readmissions occurred more frequently in younger patients compared to non-readmissions (67.4 vs. 68.9 years). Our study cohort was male predominant (54.2%) and male sex was an independent predictor of readmission in HF patients, similar to previous studies.16) However, in a meta-analysis published by Saito et al.17) male gender was not a predictor of readmission in HF patients. Our study cohort primarily consisted of patients from lower-income quartiles involving HF-specific, and non-HF-related readmissions, consistent with prior studies using the 2010–2017 NRD datasets.11) Most of the admissions in our analysis were non-elective (95.2%) consistent with previous studies.11,15) Majority of the index admissions in our study were during weekdays (77.1%). Interestingly, a study by Shah et al.18) found a great number of admissions and discharges on weekdays, and Friday was independently associated with the highest 30-day HF-readmission rates. HF admissions were more prevalent in large bed-size hospitals (50.8%) and metropolitan teaching hospitals (65.2%), similar to previous studies.9,10) In our analysis, 52.1% of readmissions occurred in patients with large-hospital index admissions. Joynt and Jha19) reported discharges from small hospitals (28.4%) had higher readmissions compared to large-size hospital discharges (25.2%, p<0.001). Kaneko et al.20) concluded that the medium-volume and high-volume groups had lower in-hospital mortality for HF patients vs. low-volume group. Contrary to this finding, the work from Kumbhani et al.21) suggested that hospital volume correlated significantly with well-defined HF management protocols but did not impact 30-day mortality or readmission rates. These conflicting data are likely due to the use of databases comprised of heterogenous patient profiles. Though readmitted patients were mostly Medicare enrollees in our analysis, a significant number of Medicaid enrollees were also included (17.9%), which is higher than reported by Khan et al.11) and Jackson et al.15)
HTN, DM, smoking, obesity, and hyperlipidemia are well-established risk factors for HF. Nyjo et al.22) reported that almost 95% of patients with HF have CCI ≥3. Our study revealed a higher prevalence of AFF, PVD, CAD, prior CABG, COPD, CKD, and anemia in patients readmitted with HF than non-readmitted patients (Table 1). We found higher readmissions with CCI ≥3, consistent with the prior studies.9,10) Furthermore, in an analysis by Testa et al.23) CCI was not associated with long-term mortality in HF patients, questioning the role of CCI in predicting mortality. We found most of the patients in the readmitted cohort had single readmission only in 30-day follow-up. Only small number had either 4 or more than 4 readmissions in this 30-day follow-up. Historically, Arora et al.9) had shown similar distribution of the readmission frequencies.
The etiology of readmissions in HF patients was due to cardiovascular causes in nearly half of the 30-day readmissions in our study, with acute HF (39%) being the most common. Similarly, Arora et al.9) reported 34.5% readmissions owing to HF in the US in 2013. Khan et al.11) reported an increasing trend of adjusted HF-specific, all-cause 30- and 90-day readmissions from 2010 to 2017. Other cardiovascular causes of readmissions in our study were CAD, AFF, valvular heart disease, and HTN. Pulmonary diseases (12.4%) were the second most common cause for readmission (including pneumonia and influenza), comparable to previous studies.10,15) Infections are associated with higher rates of HF exacerbation and higher mortality24) which we also found in our study. One potential explanation for this may be related to acute systemic inflammation impairing cardiac function secondary to demand ischemia as well as sepsis-induced myocardial dysfunction.25)
We found that common predictors for all-cause readmissions and HF-specific readmissions were male sex, AFF, CKD, anemia, CCI ≥3, and discharge to facilities in index admission. AFF was more prevalent in HF patients with readmissions and was an independent predictor of all-cause readmission. However, studies by Saito et al.17) and Fleming et al.26) found no role of atrial fibrillation in HF readmissions. Our study showed higher odds of HF-related readmissions in patients with COPD, a finding previously reported by Ruigómez et al.27) This finding is likely related to HF and COPD sharing similar risk factors such as smoking, advanced age, and systemic inflammation.28) Multiple studies have reported CKD as a significant predictor of HF readmissions.17,20,26,27,28) It may be explained by a higher prevalence of traditional cardiovascular and uremia-related risk factors in such patients.29) Anand and Gupta30) reported anemia was associated with poor outcomes in HF patients. Importantly, we found higher odds of HF readmissions in anemic patients in our study potentially due to the neurohormonal and proinflammatory cytokine activation and renal dysfunction resulting in anemia of chronic disease in HF patients.31)
Lower-income quartile HF patients had higher odds of readmissions in our analysis which was previously reported by Khan et al.11) Medicare patients represent the majority of the population admitted with HF.31) Conversely, we observed Medicaid patients having significantly higher odds of readmissions due to HF. We believe that this may be due to poor healthcare accessibility and social determinants of health in these patients. We found that HF-specific, non-HF and all-cause readmissions were significantly associated with prolonged LOS, and a greater number of discharges to nursing homes or rehab facilities after the index admissions in our study, similar to the previous reports.9,10)
We cannot exclude that errors in coding may have occurred, such as under-reporting of primary and secondary diagnoses, which may have impacted the overall accuracy of our HF dataset. Additionally, we limited our analysis on patients readmitted within 30 days of their index admission. The patient profile and predictors of readmission may be different in patients readmitted beyond the 30-day time frame which also warrants further investigation. Furthermore, our dataset lacks key diagnostic testing information such as laboratory data, including cardiac biomarkers, cardiac imaging, and functional assessments such as the New York Heart Association class that would aid in prognostication of these patients.
In conclusions, we found that HF remains the most common cause of readmissions for HF patients. Readmission rates, causes of readmissions, and predictors of readmissions were similar to the previously published reports on HF readmissions utilizing large inpatient datasets. In addition to high comorbidity index, and low socioeconomic status, hospital resource utilization is a significant predictor of readmissions in HF patients for all-cause and HF-specific readmissions. Our research demonstrated that patients with HF are equally likely to be readmitted due to cardiac and non-cardiac causes. This supports the critical need to optimally manage traditional cardiovascular risk factors as well as common comorbid conditions to mitigate the risk of readmission and mortality.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Jain A, Arora S, Patel V, Raval M, Modi K, Arora N, Desai R, Bozorgnia B, Bonita R.
- Data curation: Jain A, Arora S.
- Formal analysis: Jain A, Arora S.
- Investigation: Jain A, Arora S.
- Methodology: Jain A, Arora S.
- Project administration: Jain A, Arora S, Bozorgnia B, Bonita R.
- Software: Jain A, Arora S.
- Supervision: Jain A, Arora S, Bozorgnia B, Bonita R.
- Validation: Jain A, Arora S, Bozorgnia B, Bonita R.
- Visualization: Jain A, Arora S.
- Writing - original draft: Jain A, Arora S, Patel V, Raval M, Modi K, Arora N, Desai R, Bozorgnia B, Bonita R.
- Writing - review & editing: Jain A, Arora S, Patel V, Raval M, Modi K, Arora N, Desai R, Bozorgnia B, Bonita R.
SUPPLEMENTARY MATERIALS
ICD-10 codes for baseline characteristics and comorbidities
ICD-10 codes for admission diagnosis from NRD (2019)
References
- 1.Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 2.McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 3.McMurray JJ, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 4.McMurray JJ, Packer M. How should we sequence the treatments for heart failure and a reduced ejection fraction?: a redefinition of evidence-based medicine. Circulation. 2021;143:875–877. doi: 10.1161/CIRCULATIONAHA.120.052926. [DOI] [PubMed] [Google Scholar]
- 5.Dixit NM, Shah S, Ziaeian B, Fonarow GC, Hsu JJ. Optimizing guideline-directed medical therapies for heart failure with reduced ejection fraction during hospitalization. US Cardiol. 2021;15:e07 [Google Scholar]
- 6.Starling RC, Naka Y, Boyle AJ, et al. Results of the post-U.S. Food and Drug Administration-approval study with a continuous flow left ventricular assist device as a bridge to heart transplantation: a prospective study using the INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support) J Am Coll Cardiol. 2011;57:1890–1898. doi: 10.1016/j.jacc.2010.10.062. [DOI] [PubMed] [Google Scholar]
- 7.Jorde UP, Kushwaha SS, Tatooles AJ, et al. Results of the destination therapy post-food and drug administration approval study with a continuous flow left ventricular assist device: a prospective study using the INTERMACS registry (Interagency Registry for Mechanically Assisted Circulatory Support) J Am Coll Cardiol. 2014;63:1751–1757. doi: 10.1016/j.jacc.2014.01.053. [DOI] [PubMed] [Google Scholar]
- 8.Moertl D, Altenberger J, Bauer N, et al. Disease management programs in chronic heart failure: position statement of the Heart Failure Working Group and the Working Group of the Cardiological Assistance and Care Personnel of the Austrian Society of Cardiology. Wien Klin Wochenschr. 2017;129:869–878. doi: 10.1007/s00508-017-1265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arora S, Patel P, Lahewala S, et al. Etiologies, trends, and predictors of 30-day readmission in patients with heart failure. Am J Cardiol. 2017;119:760–769. doi: 10.1016/j.amjcard.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 10.Arora S, Lahewala S, Hassan Virk HU, et al. Etiologies, trends, and predictors of 30-day readmissions in patients with diastolic heart failure. Am J Cardiol. 2017;120:616–624. doi: 10.1016/j.amjcard.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 11.Khan MS, Sreenivasan J, Lateef N, et al. Trends in 30- and 90-day readmission rates for heart failure. Circ Heart Fail. 2021;14:e008335. doi: 10.1161/CIRCHEARTFAILURE.121.008335. [DOI] [PubMed] [Google Scholar]
- 12.Agency for Healthcare Research and Quality. Overview of the Nationwide Readmissions Database (NRD) [Internet] Rockville: Agency for Healthcare Research and Quality; 2019. [cited 2023 March 9]. Available from: https://www.hcup-us.ahrq.gov/nrdoverview.jsp. [Google Scholar]
- 13.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 14.Glasheen WP, Cordier T, Gumpina R, Haugh G, Davis J, Renda A. Charlson comorbidity index: ICD-9 update and ICD-10 translation. Am Health Drug Benefits. 2019;12:188–197. [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson SL, Tong X, King RJ, Loustalot F, Hong Y, Ritchey MD. National burden of heart failure events in the United States, 2006 to 2014. Circ Heart Fail. 2018;11:e004873. doi: 10.1161/CIRCHEARTFAILURE.117.004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robertson J, McElduff P, Pearson SA, Henry DA, Inder KJ, Attia JR. The health services burden of heart failure: an analysis using linked population health data-sets. BMC Health Serv Res. 2012;12:103. doi: 10.1186/1472-6963-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saito M, Negishi K, Marwick TH. Meta-analysis of risks for short-term readmission in patients with heart failure. Am J Cardiol. 2016;117:626–632. doi: 10.1016/j.amjcard.2015.11.048. [DOI] [PubMed] [Google Scholar]
- 18.Shah M, Patnaik S, Patel B, et al. The day of the week and acute heart failure admissions: Relationship with acute myocardial infarction, 30-day readmission rate and in-hospital mortality. Int J Cardiol. 2017;249:292–300. doi: 10.1016/j.ijcard.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Joynt KE, Jha AK. Who has higher readmission rates for heart failure, and why? Implications for efforts to improve care using financial incentives. Circ Cardiovasc Qual Outcomes. 2011;4:53–59. doi: 10.1161/CIRCOUTCOMES.110.950964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaneko H, Itoh H, Yotsumoto H, et al. Impact of hospital volume on clinical outcomes of hospitalized heart failure patients: analysis of a nationwide database including 447,818 patients with heart failure. BMC Cardiovasc Disord. 2021;21:49. doi: 10.1186/s12872-021-01863-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumbhani DJ, Fonarow GC, Heidenreich PA, et al. Association between hospital volume, processes of care, and outcomes in patients admitted with heart failure: insights from get with the guidelines-heart failure. Circulation. 2018;137:1661–1670. doi: 10.1161/CIRCULATIONAHA.117.028077. [DOI] [PubMed] [Google Scholar]
- 22.Nyjo S, Leigh J, Jackson C, Dubey G, Sankaranarayanan R, Douglas H. P1470: Heart failure patients with high Charlson co-morbidity scores can be managed safely in a day-case ambulatory heart failure unit without the need for hospital admission. Eur Heart J. 2017;38:ehx502.P1470 [Google Scholar]
- 23.Testa G, Cacciatore F, Galizia G, et al. Charlson comorbidity index does not predict long-term mortality in elderly subjects with chronic heart failure. Age Ageing. 2009;38:734–740. doi: 10.1093/ageing/afp165. [DOI] [PubMed] [Google Scholar]
- 24.Alon D, Stein GY, Korenfeld R, Fuchs S. Predictors and outcomes of infection-related hospital admissions of heart failure patients. PLoS One. 2013;8:e72476. doi: 10.1371/journal.pone.0072476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colombo PC, Ganda A, Lin J, et al. Inflammatory activation: cardiac, renal, and cardio-renal interactions in patients with the cardiorenal syndrome. Heart Fail Rev. 2012;17:177–190. doi: 10.1007/s10741-011-9261-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fleming LM, Gavin M, Piatkowski G, Chang JD, Mukamal KJ. Derivation and validation of a 30-day heart failure readmission model. Am J Cardiol. 2014;114:1379–1382. doi: 10.1016/j.amjcard.2014.07.071. [DOI] [PubMed] [Google Scholar]
- 27.Ruigómez A, Michel A, Martín-Pérez M, García Rodríguez LA. Heart failure hospitalization: An important prognostic factor for heart failure re-admission and mortality. Int J Cardiol. 2016;220:855–861. doi: 10.1016/j.ijcard.2016.06.080. [DOI] [PubMed] [Google Scholar]
- 28.de Miguel Díez J, Chancafe Morgan J, Jiménez García R. The association between COPD and heart failure risk: a review. Int J Chron Obstruct Pulmon Dis. 2013;8:305–312. doi: 10.2147/COPD.S31236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lisowska A, Musiał WJ. Heart failure in patients with chronic kidney disease. Rocz Akad Med Bialymst. 2004;49:162–165. [PubMed] [Google Scholar]
- 30.Anand IS, Gupta P. Anemia and iron deficiency in heart failure: current concepts and emerging therapies. Circulation. 2018;138:80–98. doi: 10.1161/CIRCULATIONAHA.118.030099. [DOI] [PubMed] [Google Scholar]
- 31.Khera R, Kondamudi N, Zhong L, et al. Temporal trends in heart failure incidence among Medicare beneficiaries across risk factor strata, 2011 to 2016. JAMA Netw Open. 2020;3:e2022190. doi: 10.1001/jamanetworkopen.2020.22190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ICD-10 codes for baseline characteristics and comorbidities
ICD-10 codes for admission diagnosis from NRD (2019)