Abstract
The Korean Society of Heart Failure (KSHF) Guidelines provide evidence-based recommendations based on Korean and international data to guide adequate diagnosis and management of heart failure (HF). Since introduction of 2017 edition of the guidelines, management of advanced HF has considerably improved, especially with advances in mechanical circulatory support and devices. The current guidelines addressed these improvements. In addition, we have included recently updated evidence-based recommendations regarding acute HF in these guidelines. In summary, Part IV of the KSHF Guidelines covers the appropriate diagnosis and optimized management of advanced and acute HF.
Keywords: Heart failure, Cardiogenic shock, Heart-assist devices, Heart transplantation
INTRODUCTION
Globally, heart failure (HF) is a major cause of morbidity and mortality.1,2) Patients presenting with acute HF are especially at a high risk of adverse outcomes, which necessitates prompt and accurate diagnosis and timely management. Moreover, progressive HF leads to an advanced stage of HF, which is characterized by a devastating clinical course and poor prognosis. Therefore, advanced HF requires timely assessment and management with specialized therapy. In Korea, the introduction of left ventricular assist device (LVAD) improved the management strategy of advanced HF. The Korean Society of Heart Failure (KSHF) had introduced guidelines for the management of acute and chronic HF.3,4) Subsequently, rapid advances improved clinical outcomes remarkably for acute and advanced HF, which indicated the need for updated guidelines. Herein, we have appended latest evidence to the updated guidelines with a focus on acute and advanced HF.
ADVANCED HF
Timely referral of patients with advanced HF to an appropriate center is recommended to assess risk stratification and provide appropriate therapy for advanced HF. (Class I, Level of Evidence C)
Definition
Some patients with HF do not respond to medical therapy and progress to the advanced stage. In advanced HF, symptoms or signs of HF are not improved and worsen even with guideline-directed medical therapy (GDMT); specialized therapy such as mechanical circulatory assist (MCS) devices, heart transplantation, or palliative care are often required.5,6,7) Patients with advanced HF have persistent severe symptoms that limits activities of daily living, impairs the quality of life, and requires frequent administration of intravenous agents for symptom relief. The inclusion criteria for defining the stage of advanced HF is listed in Table 1.7) Left ventricular ejection fraction (LVEF) is not the only mandatory defining factor of HF; in addition, symptom burden and anticipated prognosis of HF are more crucial factors to define this stage. Therefore, HF with preserved LVEF can be classified as advanced HF.
Table 1. European Society of Cardiology criteria defining stage of advanced HF.
| All following criteria should be present despite optimal medical therapy | |
|---|---|
| 1 | Severe and persistent symptoms of heart failure |
| NYHA class III or IV | |
| 2 | Severe cardiac dysfunction defined by at least one of following |
| LVEF ≤30% | |
| Isolated RV failure | |
| Non-operable severe valvular abnormalities | |
| Non-operable severe congenital abnormalities | |
| Severe HF with mildly reduced or preserved EF defined by persistently high BNP or NT-pro-BNP values and significant LV diastolic dysfunction | |
| 3 | Repeated episodes of HF aggravation causing unplanned visits or hospitalization with one of following conditions in the last 12 months |
| Congestion requiring intravenous diuretics or diuretic combination | |
| Low cardiac output requiring inotropes or vasoactive agents | |
| Malignant arrhythmia | |
| 4 | Severe impairment of exercise capacity with inability to exercise or low 6-minute walk test distance (<300 m) or peak VO2 (<12–14 mL/kg/min) or <50% predicted value, estimated to be of cardiac origin |
HF = heart failure; NYHA = New York Heart Association; LVEF = left ventricular ejection fraction; RV = right ventricular; EF = ejection fraction; BNP = brain natriuretic peptide; NT-pro-BNP = N-terminal pro-B-type natriuretic peptide; VO2 = oxygen consumption.
Adapted from McDonagh et al.6) with the permission of the European Society of Cardiology.
Classification
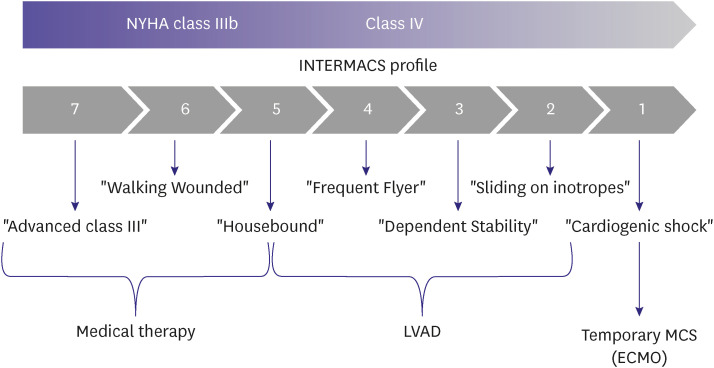
Conventionally, the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) profiles are used to classify patients with advanced HF who require mechanical support such as left ventricular assistive devices and total artificial hearts. The INTERMACS classification ranges from 1–7 profiles; the lower profiles indicate a more urgent requirement for additional circulatory adjuvant treatment (Table 2).8) Patients with severe HF symptoms and/or signs despite GDMT should be referred to a medical center to receive prompt and specialized treatment for HF. Furthermore, medical staff of the primary or secondary hospitals that treat patients with HF should suspect advanced HF and assess whether the findings correspond to the patients’ symptoms (Table 3).
Table 2. Interagency for Mechanically Assisted Circulatory Support profiles.
| Profile | Description |
|---|---|
| 1 | Cardiogenic shock “Crash and Burn” |
| Life-threatening hypotension despite rapidly escalating inotropic or vasopressor support, critical organ hypoperfusion often confirmed by worsening acidosis or lactate level | |
| 2 | Progressive decline “Sliding on Inotropes” |
| Declining function despite inotropic support, showing worsening renal function, malnutrition, inability to achieve euvolemic status. Also declining clinical condition in whom maintenance of inotropic support cannot be tolerated owing to tachyarrhythmia, ischemic, or other conditions. | |
| 3 | Stable but inotrope-dependent “Dependent Stability” |
| Stable perfusion, organ function, nutrition with intravenous inotropic support (or temporary circulatory support device) but showing repeated failure to wean from inotropic support. | |
| 4 | Resting symptoms on oral therapy “Frequent Flyer” |
| Stabilized close to normal volume status with oral therapies but experiencing daily symptoms of congestion at rest or during ADL. Doses of diuretics generally fluctuate at very high levels. | |
| 5 | Exertion intolerant “Housebound” |
| Comfortable without evidence of hypervolemia at rest but unable to perform any activity because of symptoms, living predominantly within the house. | |
| 6 | Exertion limited “Walking Wounded” |
| Comfortable at rest without evidence of hypervolemia and able to perform ADL or minor activities outside the home but fatigues after the first few minutes of any meaningful activity. | |
| 7 | Advanced NYHA class III |
| Stable with a reasonable level of comfortable activity living comfortably with meaningful activity that is limited to mild physical exertion, without current or recent episodes of unstable fluid balance |
ADL = activity of daily living; NYHA = New York Heart Association.
Adapted from Stevenson et al.8) with the permission of the International Society for Heart and Lung Transplantation.
Table 3. Clinical criteria of referral for advanced HF.
| Repeated hospitalization or urgent visit of emergency department within past year |
| Persistent NYHA functional class III or IV symptoms |
| Intolerance of GDMT |
| Need of inotropic agent to relieve HF symptoms |
| Refractory congestion despite medical therapy needing escalation of diuretic dose |
| Persistent or progressive organ failure including renal, hepatic dysfunction |
| Recurrent episodes of ventricular arrhythmia or appropriate ICD shocks |
| Persistent or frequent hypotension (SBP ≤90 mmHg) |
| Persistent hyponatremia |
| Non-responder of CRT |
HF = heart failure; NYHA = New York Heart Association; GDMT = guideline-directed medical therapy; ICD = implantable cardioverter defibrillator; SBP = systolic blood pressure; CRT = cardiac resynchronization therapy.
CARDIOGENIC SHOCK
1. For patients with cardiogenic shock, the Society for Cardiovascular Angiography and Interventions classification may be used to assess individual risk and predict prognosis. (Class IIb, Level of Evidence C)
2. Supplemental oxygen is recommended for patients with cardiogenic shock and hypoxemia. (Class I, Level of Evidence C)
3. Mechanical ventilation should be considered for patients with cardiogenic shock and persistent respiratory failure despite supplemental oxygen or non-invasive ventilation. (Class IIa, Level of Evidence C)
4. Adequate support with vasoactive agents may be considered for patients with cardiogenic shock to maintain end-organ perfusion. (Class IIb, Level of Evidence C)
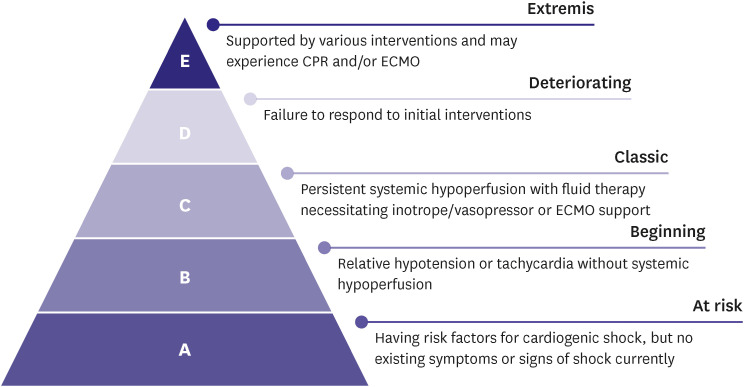
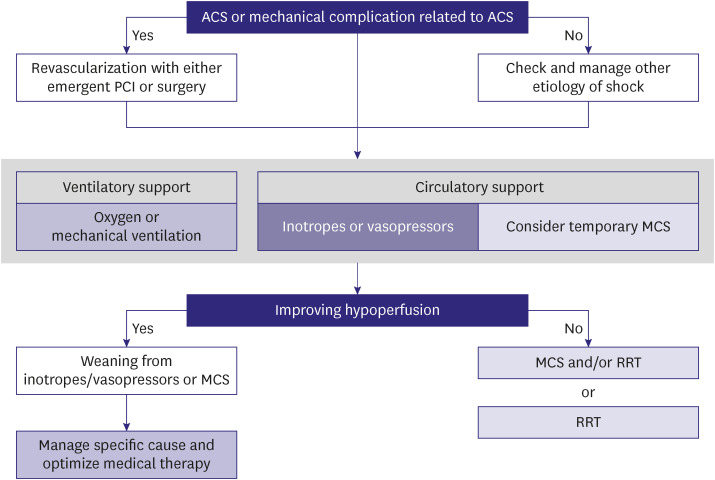
Cardiogenic shock is a severe impairment of cardiac performance that results in inadequate end-organ perfusion and causes life-threatening consequences such as multi-organ failure or mortality. Decline of cardiac function can occur suddenly due to myocardial infarction and myocarditis or gradually due to exacerbation of chronic HF. Identifying tissue hypoxia and alteration of cellular metabolism as indicated by evidence of hypoperfusion on physical examination (including oliguria, altered consciousness, and low pulse pressure) and biochemical studies (including renal dysfunction, metabolic acidosis, and elevated serum lactate) is vital. The Society for Cardiovascular Angiography and Interventions classifies cardiogenic shock into five stages according to the types and intensity of treatment; it is effective in mortality risk stratification (Figure 1).9,10) Treatment for cardiogenic shock should be promptly initiated to restore hemodynamic stability and prevent organ failure; however, early identification and treatment of the causative disease are equally important (Figure 2).6)
Figure 1. Stages of cardiogenic shock. Adapted from Baran et al.9) with the permission of the Society for Cardiovascular Angiography & Interventions.
CPR = cardiopulmonary resuscitation; ECMO = extracorporeal membrane oxygenation.
Figure 2. Treatment algorithm for cardiogenic shock.
ACS = acute coronary syndrome; PCI = percutaneous coronary intervention; MCS = mechanical circulatory support; RRT = renal replacement therapy.
TEMPORARY CIRCULATORY SUPPORT DEVICES
1. Routine use of intraaortic balloon pump (IABP) is not recommended for cardiogenic shock. (Class III, Level of Evidence B)
2. Use of IABP can be considered for cardiogenic shock resulting from acute mitral regurgitation or ventricular septal defect. (Class IIa, Level of Evidence C)
3. Use of extracorporeal membrane oxygenation (ECMO) should be considered for cardiogenic shock refractory to medical therapy. (Class IIa, Level of Evidence C)
4. Extracorporeal cardiopulmonary resuscitation using ECMO is not recommended for patients with unwitnessed, out-of-hospital cardiac arrest, and signs of irreversible neurologic impairment. (Class III, Level of Evidence C)
5. Evaluation of cardiac function recovery to determine adequacy for weaning from ECMO should be assessed by echocardiography, hemodynamic (e.g., CVP, mean arterial blood pressure [BP]), and biochemical parameters (e.g., lactate). (Class IIa, Level of Evidence C)
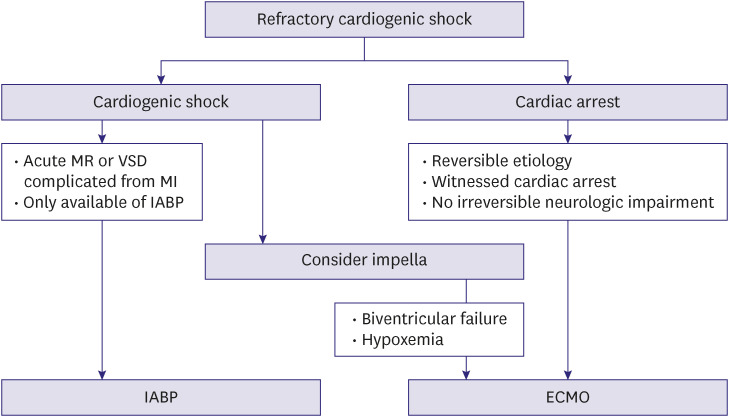
The use of temporary MCS devices should be considered for patients with acute HF who are refractory to fluid and vasoactive/inotropic therapy. The application of specific type of devices should be individualized based on the mechanical characteristics and amount of hemodynamic support required (Figure 3). Current United States and European practice guidelines do not recommend the routine use of intra-aortic balloon pumps; however, these may be helpful for acute MR or VSD, which result from mechanical complication of acute myocardial infarction.5,6) In Korea, the Impella device (Abiomed, Danvers, MA, USA) is unavailable; hence, ECMO is widely used as a bridge to recovery or bridge to LVAD/heart transplantation (HT).
Figure 3. Temporary circulatory support for drug-refractory cardiogenic shock.
MR = mitral regurgitation; VSD = ventricular septal defect; MI = myocardial infarction; IABP = intra-aortic balloon counterpulsation; ECMO = extracorporeal membrane oxygenation.
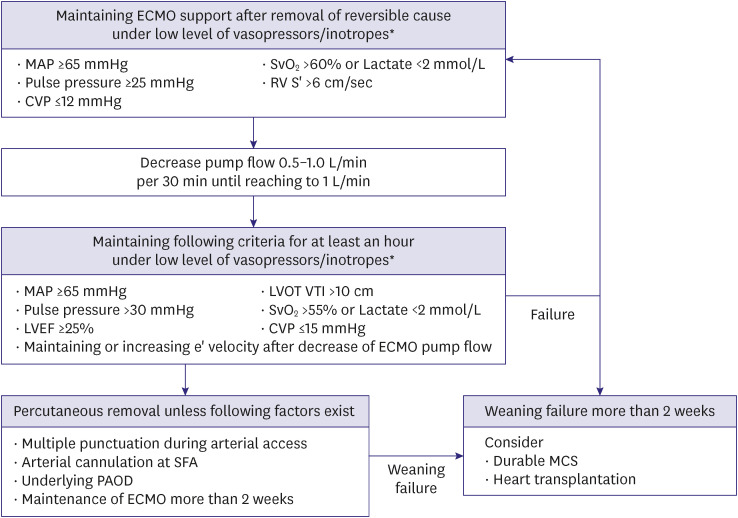
Although clear criteria are yet to be established, weaning from ECMO can be considered in those exhibiting stable hemodynamics with minimal support level of ECMO (<1–1.5 L/min) and vasoactive agents (Figure 4).11) Moreover, the use of echocardiographic parameters may help guide weaning decisions.12,13)
Figure 4. Weaning of percutaneous ECMO.
ECMO = extracorporeal membrane oxygenation; MAP = mean arterial pressure; SvO2 = mixed venous oxygen saturation; RV = right ventricular; S’ = tissue Doppler tricuspid lateral peak systolic velocity; CVP = central venous pressure; LVOT = left ventricular outflow tract; VTI = velocity time integral; LVEF = left ventricular ejection fraction; SFA = superficial femoral artery; PAOD = peripheral arterial occlusive disease; MCS = mechanical circulatory support
*Low level of vasopressor/inotropes refers to norepinephrine ≤0.03 μg/min/kg and dobutamine ≤5 μg/min/kg.
LVAD
A LVAD improve survival and the quality of life for patients with advanced HF. The LVAD can be used in following clinical situations: as a bridge to transplantation, bridge to candidacy to determine adequacy of transplantation, and destination therapy. It can be used in patients with refractory cardiogenic shock who are dependent on temporary MCS as well. The LVAD can be implanted in patients with INTERMACS profiles 2–4 and 5, especially those with a potential risk of sudden cardiac death or irreversible organ failure (Figure 5). The outcomes after durable, centrifugal-flow LVAD implantation as a bridge to transplantation is comparable with HT.14) In Korea, hemorrhagic stroke is a leading cause of mortality and bleeding is most common complication during the first year after LVAD implantation.15)
Figure 5. Treatment strategy according to the INTERMACS profile.
NYHA = New York Heart Association; INTERMACS = Interagency Registry for Mechanically Assisted Circulatory Support; LVAD = left ventricular assist device; MCS = mechanical circulatory support; ECMO = extracorporeal membrane oxygenation.
HT
1. HT is recommended to improve survival and quality of life for selected patients with advanced HF. (Class I, Level of Evidence C)
2. Cardiopulmonary exercise test is recommended in patients with advanced HF to guide HT listing. (Class I, Level of Evidence B)
3. Annual evaluation of right heart catheterization is recommended to evaluate hemodynamic status in patients listed for HT. (Class I, Level of Evidence C)
HT is gold standard therapy for patients with advanced HF; it helps improve survival, exercise capacity, and the quality of life.16) In Korea, nearly 200 heart transplants are performed annually and this number has been gradually increasing since 2000.1,17) However, donor shortage is a major limitation of HT18); hence, appropriate recipient selection is important to maximize post-transplant outcome (Table 4). In Korea, the one-year survival after transplantation was reported to reach 90%19) and the International Society for Heart and Lung Transplantation reported a median survival of 12.5 years.20) Cardiopulmonary exercise test helps guide listing for evaluating the candidacy for HT.16,21) HT can be considered if peak oxygen consumption is <14 mL/kg/min for non-beta-blocker (BB) users and <12 mL/kg/min for BB users, achieved during maximal exercise workload represented by a respiratory exchange ratio >1.05.16) Annual right heart catheterizations should be performed to evaluate hemodynamic status in patients who have been listed for HT.16)
Table 4. Common indications and contraindications of heart transplantation.
| Indication | ||
|---|---|---|
| Uncontrolled HF symptoms despite optimal medical and appropriate device therapy | ||
| Persistent NYHA functional class IIIb-IV | ||
| Cardiopulmonary exercise result: | ||
| 1) Peak VO2 ≤14 mL/kg/min (for non-BB users) or ≤12 mL/kg/min (for BB users) | ||
| 2) Peak VO2 ≤50% of predicted for young (<50 years) patients | ||
| 3) VE/VCO2 slope >35 for sub-maximal exercise (RER <1.05) | ||
| Cardiogenic shock not expected to improve | ||
| Acute myocardial infarction | ||
| Acute myocarditis | ||
| Refractory angina despite medical therapy | ||
| Hypertrophic or restrictive cardiomyopathy with severe symptoms | ||
| Uncontrolled ventricular arrhythmia refractory to other therapies | ||
| Congenital heart disease without significant, irreversible pulmonary hypertension | ||
| Non-metastasized intra-cardiac tumor | ||
| Contraindication | ||
| Age | ≥70 years | |
| Obesity | BMI >35 kg/m2 | |
| Infection | Active infection | |
| Cancer | Presence of active malignancy, treated malignancy within 5 years | |
| Diabetes | Uncontrolled diabetes or associated significant organ injury | |
| Peripheral arterial disease | Severe vascular disease not amendable to revascularization | |
| Substance abuse | At least 6 months of abstinence of smoking, alcohol, and illicit drugs | |
| Psychosocial problems | Non-compliance, lack of caregiver support, dementia | |
HF = heart failure; NYHA = New York Heart Association; VO2 = oxygen consumption; BB = beta-blocker; VE/VCO2 = ventilation equivalent of carbon dioxide; RER = respiratory exchange ratio; BMI = body mass index.
PALLIATIVE AND END-OF-LIFE CARE
1. Patients with advanced HF refractory to medical therapy and not indicated for HT or LVAD can benefit from palliative and end-of-life care. (Class IIa, Level of Evidence C)
2. Morphine, antiemetics, diuretics, and oxygen therapy may be considered for palliative care in patients with severe pain and congestive symptoms; GDMT, which lower BP without immediate effect, prescribed for long-term benefit may be continued at a reduced dose or discontinued. (Class Iib, Level of Evidence C)
3. Physician Orders for Life Sustaining Treatment may be prepared after multidisciplinary discussion and review. (Class Iia, Level of Evidence C)
Palliative and end-of-life care can be considered for patients with advanced HF who are not indicated for LVAD implantation or HT, those who are unable to maintain independent daily activities, and those with deteriorating symptoms despite appropriate therapy. Palliative care mainly focuses on providing symptomatic relief to maintain the quality of life and offers psychological support through a multidisciplinary approach that includes the patient, physician, nurse, and other specialists.22,23)
Morphine can reduce breathing difficulty, pain, and anxiety; however, patients should be informed of the following side effects: constipation, nausea, and altered mental status.24,25) Supplemental oxygen and diuretics can help reduce dyspnea or congestive symptoms. BP lowering HF medications with a well-proven efficacy in improving long-term survival can be reduced or withdrawn based on the patient’s condition, thereby reducing risk of falls. A special care plan needs to be established for some patients including desired place for death and deactivation of implanted devices such as pacemakers or implantable cardioverter defibrillators (ICDs); it should consider the legal policies in the country.
ACUTE HF
Definition and aggravating factors
Acute HF, characterized by the rapid onset and deterioration of the symptoms or signs of HF, requires prompt medical attention and often leads to emergency room visits or unexpected hospitalization. If acute HF is suspected, diagnostic tests should be performed immediately and adequate therapy initiated simultaneously. In Korea, among patients with acute HF, approximately 52% were newly-diagnosed with HF and 48% had acute decompensation of pre-existing chronic HF.26) The aggravating factors of HF, albeit not as a cause that leads to worsening events, are diverse (Table 5).
Table 5. Common aggravating factors of acute HF.
| Acute coronary syndrome |
| Arrhythmia |
| Uncontrolled hypertension |
| Non-compliance to HF drugs |
| Excessive salt or fluid intake |
| Toxic substance (e.g. alcohol, recreational drugs) |
| Drugs (e.g. NSAIDs, cardiotoxic chemotherapeutic agents, corticosteroids, verapamil) |
| Exacerbation of COPD |
| Pulmonary thromboembolism |
| Active infection |
| Surgery |
| Increased sympathetic drive, stress-related cardiomyopathy |
| Metabolic or hormonal derangements (e.g. adrenal, thyroid dysfunction) |
| Anemia |
| Pregnancy or peripartum abnormalities |
HF = heart failure; NSAID = non-steroidal anti-inflammatory drug; COPD = chronic obstructive pulmonary disease.
Prognosis
Patients presenting with acute HF are at a high risk of in-hospital mortality, which is substantially lowered once they are stabilized and discharged. The Korean Acute Heart Failure (KorAHF) Registry study reported an in-hospital mortality rate of 4.8%.26) The rates of mortality and re-hospitalization of Korean HF population are presented in Table 6.
Table 6. The rates of mortality and re-hospitalization of acute heart failure in Korea.
| Clinical event | 1-month | 3-month | 6-month | 1-year | 2-year | 3-year |
|---|---|---|---|---|---|---|
| Mortality | 3.30% | 8.40% | 12.60% | 18.20% | 27.60% | 36.70% |
| Re-hospitalization | 7.00% | 13.50% | 17.90% | 23.10% | 30.30% | 36.00% |
Diagnosis
Acute HF should be immediately diagnosed during the hospital visit. The diagnosis should aim to assess the hemodynamic profile and investigate reversible causes and aggravating factors, which require prompt treatment (Figure 6). In addition, natriuretic peptides (NPs) measurement and echocardiography should be performed to confirm the diagnosis. NPs measurement can help rule out HF in patients who have symptoms or signs suggestive of HF; although elevated NP levels support a diagnosis of HF, they are not essentially diagnostic because other non-cardiac medical conditions can cause elevated NP levels as well.27,28) Chest radiography and lung ultrasound may help assess acute HF, especially when NP levels cannot be measured. In patients with acute HF, measurement of serum creatinine, blood urea nitrogen, and electrolytes levels is useful for therapeutic management. Elevated liver enzymes suggest poor prognosis.29,30) Hypothyroidism or hyperthyroidism may precipitate HF; hence, thyroid-stimulating hormone levels should be measured. Lactate and hydrogen ion concentration, obtained via arterial blood gas analysis, may help assess hemodynamic condition and predict prognosis. Troponin elevation can be observed in the absence of definite myocardial ischemia or stenotic coronary artery disease in patients with acute HF and provide clinical evidence of acute coronary syndrome as a possible etiology of acute HF.31)
Figure 6. Diagnostic approach to suspected acute HF.
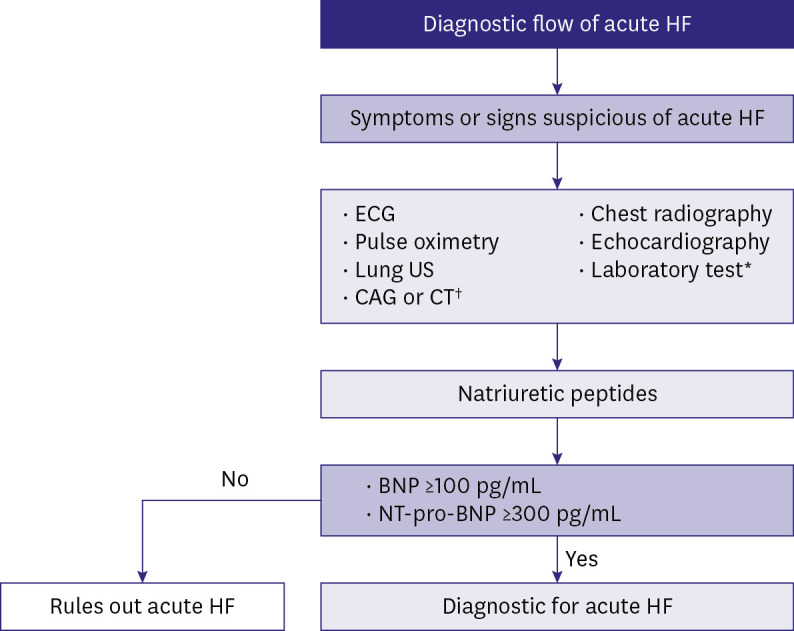
HF = heart failure; US = ultrasound; CAG = coronary angiography; CT = computed tomography; BNP = brain natriuretic peptide; NT-pro-BNP = N-terminal pro-B-type natriuretic peptide.
*Blood tests include troponin, serum creatinine, electrolytes, blood urea nitrogen, thyroid stimulating hormone, liver function test, d-dimer, procalcitonin, arterial blood gas analysis, lactate.
†Coronary angiography can be performed if acute coronary syndrome is suspected, and chest CT if pulmonary embolism is suspected.
Classification
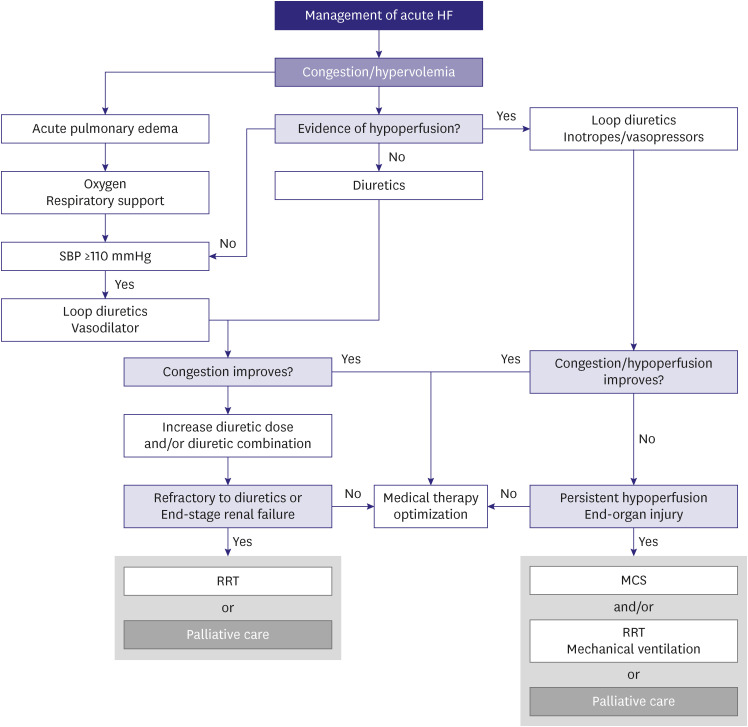
According to clinical status, acute HF can be classified into four subgroups that may overlap with each other (Table 7). Each subgroup is distinguished based on the status of congestion and tissue perfusion, thereby necessitating appropriate management for each group (Figure 7).
Table 7. Subtypes of acute HF.
| Acute decompensated HF | Acute pulmonary edema | Isolated RV failure | Cardiogenic shock | |
|---|---|---|---|---|
| Mechanism | LV dysfunction | Increased afterload | RV dysfunction | Severe cardiac dysfunction |
| Abnormal salt and/or water retention | Diastolic dysfunction | |||
| Valvular heart disease | ||||
| Cause of symptoms | Increased intracardiac filling pressure | Pulmonary fluid redistribution | Increased CVP | Systemic hypoperfusion |
| Fluid accumulation | Low RV output | |||
| Hemodynamic profile | Normal or low BP | Normal or high BP | Low BP | Low BP |
| Increased LVEDP and PCWP | Increased LVEDP and PCWP | Normal or low LVEDP but high RVEDP | Increased or normal LVEDP and PCWP | |
| Normal or low CO | Normal or high CO | Low CO | Low CO | |
| Treatment | Diuretics | Diuretics | Diuretics | Inotropes/vasopressor |
| Inotropes/vasopressor | Vasodilator | Inotropes/vasopressor | MCS | |
| MCS or RRT | MCS or RRT | RRT |
HF = heart failure; LV = left ventricular; RV = right ventricular; CVP = central venous pressure; BP = blood pressure; LVEDP = left ventricular end-diastolic pressure; PCWP = pulmonary capillary wedge pressure; CO = cardiac output; RVEDP = right ventricular end-diastolic pressure; MCS = mechanical circulatory support; RRT = renal replacement therapy.
Adapted from McDonagh et al.6) with the permission of the European Society of Cardiology.
Figure 7. Therapeutic algorithm of acute HF.
HF = heart failure; SBP = systolic blood pressure; RRT = renal replacement therapy; MCS = mechanical circulatory support.
MONITORING ACUTE HF
1. Vital signs and oxygen saturation should be continuously monitored in patients with acute HF. (Class I, Level of Evidence C)
2. Volume status and fluid balance should be assessed by symptoms (orthopnea) and signs (jugular venous distention, peripheral edema, and body weight gain). (Class I, Level of Evidence C)
3. Renal function and serum electrolytes should be assessed frequently when initiating treatment with intravenous diuretics or renin-angiotensin-aldosterone system blockers. (Class I, Level of Evidence C)
4. Hemodynamic assessment using pulmonary artery catheter can be considered for refractory symptoms despite standard therapy with uncertainty of volume or perfusion status, low systolic BP, worsening renal function, and need for inotropes or MCS or HT. (Class Iia, Level of Evidence C)
5. Measurement of brain natriuretic peptide (BNP) or N-terminal pro-B-type natriuretic peptide (NT-pro-BNP) and/or cardiac troponin levels is recommended to predict prognosis and assess HF severity. (Class I, Level of Evidence A)
More than 90% of patients presenting with acute HF have dyspnea; it is an important surrogate to evaluate treatment efficacy. Orthopnea predicts elevated pulmonary capillary wedge pressure (PCWP) with approximately 90% sensitivity.32) Along with symptom improvement, measurement of body weight, jugular venous pressure (JVP), pulmonary rales on auscultation, and assessment of peripheral edema provide important evidence for treatment effect.33) Daily measurement of body weight with a standardized scale at a certain time is recommended. Since JVP reflects right atrial pressure, PCWP can be indirectly assessed by JVP measurement, which is an efficient method to evaluate volume status.32,33,34,35) Pulmonary congestion can be evaluated via auscultation and chest radiography. Loop diuretics and renin-angiotensin-aldosterone system blockers can reduce glomerular filtration rate and result in electrolyte imbalance; hence, assessment of renal function and serum electrolyte levels are important. Routine use of pulmonary artery catheter is not recommended for patients with acute HF36,37); however, it may help guide management of patients with HF with uncertain hemodynamic and/or volume status, refractoriness of initial therapy, persistent hypotension, progressively worsening renal function, and those considering MCS or HT. The CHAMPION (CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III HF Patients) trial reported that individualized adjustment of diuretics doses through pulmonary artery pressure monitoring may reduce the risk of HF-related hospitalization by 28% in patients with New York Heart Association functional class III symptom.38,39) The subsequent GUIDE-HF (haemodynamic-GUIDEd management of Heart Failure) study did not report significant benefits of pulmonary artery pressure monitoring in HF with mild symptoms; hence, further studies are needed to clarify the merits of pulmonary artery pressure monitoring.40)
DIURETICS
1. Intravenous loop diuretics should be used to improve symptoms for all patients admitted for acute HF with symptoms or signs of volume overload. (Class I, Level of Evidence C)
2. The recommended initial doses of intravenous loop diuretics are 20–40 mg of furosemide for non-users of diuretics and an equivalent dose or more for those already on diuretics for acute exacerbation of pre-existing HF. (Class I, Level of Evidence B)
3. Measurement of urine sodium and urine output can be helpful to assess diuretic response. (Class Iia, Level of Evidence C)
4. Combination therapy of loop diuretics and thiazides can be considered for those with inadequate response to loop diuretics. (Class IIa, Level of Evidence C)
Diuretics prevent the renal absorption of salt and water and consequently, promote their excretion, thereby relieving fluid accumulation and congestion. Diuretics should be administered in the early course of management of acute HF in all patients presenting with symptoms or signs of congestion and volume overload, regardless of LVEF. Although the level of evidence for mortality was low because of the lack of randomized controlled trials, most HF trials were conducted on the basis of sufficient diuretic usage. Diuretic therapy is targeted at achieving and maintaining euvolemic status at the lowest dose. Reduced dose or treatment withdrawal should be considered after achievement of euvolemic or hypovolemic status because excessive diuretics can cause hypotension and renal dysfunction.41)
Loop diuretics are first-line agents because of their rapid action and strong diuretic effect, especially via the intravenous route, which is beneficial in the early presentation of acute HF. These diuretics can be initiated at a low dose and gradually increased if the diuretic effect is insufficient, while monitoring the response. An initial dose of 20–40 mg of furosemide or 10–20 mg of torsemide may be intravenously administered for patients with de novo acute HF. Furosemide can be administered as a twice or three times daily bolus injection or as a continuous infusion. If the patient is on oral diuretics before exacerbation, intravenous administration of an equivalent or twice the dose of the total daily oral dose can be administered.
Diuretic responses are targeted at a weight loss of 0.75–1.0 kg/day with sufficient urine output. The response should be assessed by estimating the spot urine sodium excretion (>50–79 mEq/L at after 2 or 6 hours) and hourly urine output (>100–150 mL during first 6 hours) after initiation of diuretic therapy.41,42,43) In case of insufficient response, the diuretic dose can be doubled until it reaches the maximal dose. If diuretic response remains unsatisfactory (hourly urine volume <100 mL) despite the doubled dose, a combination therapy of other diuretic classes including acetazolamide and thiazides may be considered.44,45,46) Diuretic doses should gradually be reduced upon achieving euvolemia, while maintaining the lowest dose of diuretics that can prevent congestion. Concomitant therapy with angiotensin receptor-neprilysin inhibitors (ARNIs), mineralocorticoid receptor antagonists (MRAs) and sodium-glucose cotransporter-2 inhibitors should be monitored for excessive diuresis as these agents also have diuretic properties.47,48)
VASODILATORS AND OTHER DRUGS
1. Intravenous nitroglycerin or nitroprusside may be considered in patients with acute HF and systolic BP >110 mmHg to help improve symptoms and congestion. (Class Iib, Level of Evidence B)
2. Prophylactic anticoagulation therapy is recommended to prevent deep vein thrombosis and pulmonary thromboembolism in patients not on anticoagulants and where it is not contraindicated. (Class I, Level of Evidence A)
3. Routine use of opiates is not recommended to control symptoms except in selected patients with severe pain or anxiety. (Class III, Level of Evidence C)
Intravenous vasodilators
Intravenous vasodilators, nitrates or nitroprusside, induce arterial and venous dilation, reduce preload and afterload, and subsequently, lead to increased stroke volume, decreased congestion, and symptom relief. Although evidence supporting the use of these agents is lacking, certain patients with acute HF may be considered suitable for receiving intravenous vasodilators especially those with acute pulmonary edema and increased afterload manifested as high BP, coronary ischemia, or significant MR.49,50) However, vasodilators may increase the risk of hypotension, tachyphylaxis; therefore, adequate hemodynamic monitoring is essential. The criterion for systolic BP of 110 mmHg to initiate vasodilator therapy lacks robust evidence and is primarily based on expert opinion derived from large-scale randomized trials.
Venous thromboprophylaxis
Anticoagulation therapy is recommended for patients hospitalized for worsening HF and other medical problems in pre-existing HF to decrease the risk of deep vein thrombosis and pulmonary embolism for those who do not have contraindications to and are not already on anticoagulant therapy. Unfractionated heparin, low-molecular-weight heparins, warfarin, or approved direct oral anticoagulants can be used.
Opiates
Opiates reduce dyspnea and anxiety and can be used as sedatives in patients with non-invasive positive pressure ventilation. However, routine use of opiates for acute HF is not recommended because some retrospective studies have reported that morphine increased the risk of mechanical ventilation, prolonged the length of hospital stay, increased admissions to intensive care units, and increased mortality.51,52)
Digoxin
Digoxin can be considered in patients with atrial fibrillation intolerable to BBs and a higher heart rate (>110 beats/min) despite BB use. However, since the metabolism of digoxin can be influenced by multiple factors including other drugs or renal function, serum level of digoxin should be measured.
INOTROPES AND VASOPRESSORS
1. Inotropes may be considered to maintain systemic perfusion in patients with hypotension and evidence of end-organ hypoperfusion despite standard therapy and adequate fluid resuscitation. (Class Iib, Level of Evidence C)
2. Routine administration of inotropes is not recommended unless symptomatic hypotension and evidence of systemic hypoperfusion are observed. (Class III, Level of Evidence C)
3. Vasopressors may be used to maintain hemodynamics and end-organ perfusion for patients with cardiogenic shock. (Class Iib, Level of Evidence B)
In some patients, acute HF can be accompanied by a decrease in end-organ perfusion due to low cardiac output and systemic BP. In such patients, inotropes and/or vasopressors may be necessary to maintain adequate hemodynamics and restore systemic perfusion. Each agent has different properties or mechanism of action; hence, they should be used accordingly (Table 8).53) Inotropes/vasopressors should be initiated at low doses and gradually increased, and the hemodynamic response should be monitored to maintain adequate blood pressure (mean BP 65–70 mmHg). Results from the KorAHF registry study suggested that use of inotropes/vasopressors in patients with systolic BP >90 mmHg was associated with poor prognosis.54) Since these agents may increase the risk of arrhythmia and myocardial ischemia, they should be used in minimal doses and for a short-term, if possible, until end-organ perfusion improvement.55)
Table 8. Hemodynamic properties of common inotropes and vasopressors.
| Agent | Common infusion rate | Mechanism of action | Hemodynamic effect | |||||
|---|---|---|---|---|---|---|---|---|
| α1 | β1 | β2 | Dopamine | CO | SVR | |||
| Vasopressors | ||||||||
| Dopamine | 0.5–20 μg/kg/min | ~+++ | + ~ +++ | ~+ | ++ ~ +++ | ↑ ~ ↑↑ | ↑ ~ ↑↑ | |
| Norepinephrine | 0.05–0.4 μg/kg/min | ++++ | ++ | + | ↑ | ↑↑ | ||
| Epinephrine | 0.01–0.5 μg/kg/min | ++++ | ++++ | +++ | ↑↑ | ↑↑ | ||
| Phenylephrine | 0.1–10 μg/kg/min | +++ | ↑↑ | |||||
| Inodilators | ||||||||
| Dobutamine | 2–20 μg/kg/min | + | ++++ | ++ | ↑↑ | ↓ | ||
| Milrinone | 0.125–0.75 μg/kg/min | Phosphodiesterase-3 inhibition | ↑ | ↓ | ||||
| Isoproterenol | 2–20 μg/min | ++++ | +++ | ↑↑ | ↓ | |||
CO = cardiac output; SVR = systemic vascular resistance.
Adapted from van Diepen et al.53) with the permission of the American Heart Association.
Inotropes
Dobutamine mainly acts on β1 receptors to increase myocardial contractility and consequently, cardiac output. However, dobutamine increases the risk of arrhythmia and myocardial ischemia.56,57) Dobutamine acts on β2 receptor of peripheral vessels causes vasodilation and subsequent reduction of BP, which require hemodynamic monitoring. Milrinone, a type-3-phosphodiesterase inhibitor, increases myocardial contractility and reduces pulmonary vascular resistance. Since milrinone does not act on β1 receptors, it can be used with BBs. However, milrinone decreases systemic BP and should be cautiously used especially in patients with shock.
Vasopressors
Norepinephrine, epinephrine, and dopamine are commonly used vasopressors, which increase both myocardial contractility and systemic vascular resistance. Vasopressors are often combined with inotropes for patients with cardiogenic shock and hypotension. Evidence supporting the superiority of one specific agent over another is lacking. In the SOAP (Sepsis Occurrence in Acutely Ill Patients) II trial, dopamine use significantly increased the risk of arrhythmia in patients with shock and norepinephrine was reportedly favorable in terms of mortality for a subgroup of patients with cardiogenic shock.58) Another study reported that norepinephrine was favorable over epinephrine regarding heart rate and lactic acidosis, without differences in hemodynamic profiles in patients with myocardial infarction-induced cardiogenic shock.59) Therefore, based on available evidence, norepinephrine may be prioritized over various vasopressors for cardiogenic shock necessitating vasoactive agents.
PRE- AND POST-DISCHARGE MANAGEMENT
1. Patients admitted for HF should be evaluated for the achievement of adequate decongestion before discharge. (Class I, Level of Evidence C)
2. GDMT should be initiated and preferably optimized before discharge. (Class I, Level of Evidence C)
3. Early follow-up evaluation within 1–2 weeks after discharge is recommended to assess volume status and drug tolerance. (Class I, Level of Evidence C)
The early period of post-hospitalization discharge for worsening HF refers a vulnerable period that demonstrates a high risk of mortality and readmission.60) Therefore, it is necessary to ensure that the precipitating factors are appropriately corrected and symptoms and/or signs of HF are improved before discharge. Approximately 30% of patients discharged from HF hospitalization have residual congestion and a higher risk of first-year mortality compared with those without congestion.61) Therefore, appropriate decongestion before discharge through diuretic therapy is mandatory.
Furthermore, optimization of medical therapy, evaluation for clinical need of device therapy (e.g. ICD), and appropriate patient education for diet and physical exercise are important.62) Patients admitted for acute HF should be initiated on evidence-based medical therapy prior to discharge unless it is contraindicated.63) In Korea, the at-discharge prescription rates of renin-angiotensin system blockers, BBs, and MRAs were 68.8%, 52.2%, and 46.6%, respectively,26) which is suboptimal. Medical therapy with these agents at discharge is reported to improve clinical outcomes; hence, physicians should check whether these medications are prescribed.63,64) Furthermore, in-hospital initiation of ARNI can reduce NT-pro-BNP levels and reduce the risk of HF-related adverse clinical outcomes.65) Therefore, early initiation of ARNI should be considered.
CONCLUSION
The diagnosis and management of acute and advanced HF is rapidly advancing recently. Early diagnosis and optimization of therapy is cornerstone for acute HF, and adequate assessment and management in accordance with the patient’s profile is crucial in patients with advanced HF. This Part 4 of the HF guidelines adapted latest evidence and provided optimal approach focused on the patients with acute or advanced HF.
ACKNOWLEDGMENTS
This article has been published jointly, with consent, in both Korean Circulation Journal and International Journal of Heart Failure.
Also, the final version of this guideline was endorsed by Korean Society of Cardiology, Korean Society of Lipid and Atherosclerosis, Korean Association of Clinical Cardiology, Korean Society of Hypertension, Korean Society of Heart Failure, Korean Society of Echocardiography, Korean Society of Interventional Cardiology, Korean Heart Rhythm Society, and Korean Society of CardioMetabolic Syndrome.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Cho JH, Kang SM.
- Funding acquisition: Cho JH, Yoo BS, Kang SM.
- Supervision: Cho JH, Kang SM.
- Writing - original draft: Hyun J.
- Writing - review & editing: Cho JY, Youn JC, Kim D, Cho DH, Park SM, Jung MH, Cho JH, Park SM, Choi JO, Chung WJ, Yoo BS, Kang SM.
References
- 1.Park JJ, Lee CJ, Park SJ, et al. Heart failure statistics in Korea, 2020: a report from the Korean Society of Heart Failure. Int J Heart Fail. 2021;3:224–236. doi: 10.36628/ijhf.2021.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. Eur J Heart Fail. 2020;22:1342–1356. doi: 10.1002/ejhf.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim MS, Lee JH, Kim EJ, et al. Korean guidelines for diagnosis and management of chronic heart failure. Korean Circ J. 2017;47:555–643. doi: 10.4070/kcj.2017.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim MS, Lee JH, Cho HJ, et al. KSHF guidelines for the management of acute heart failure: Part III. Specific management of acute heart failure according to the etiology and co-morbidity. Korean Circ J. 2019;49:46–68. doi: 10.4070/kcj.2018.0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895–1032. doi: 10.1161/CIR.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 6.McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 7.Crespo-Leiro MG, Metra M, Lund LH, et al. Advanced heart failure: a position statement of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20:1505–1535. doi: 10.1002/ejhf.1236. [DOI] [PubMed] [Google Scholar]
- 8.Stevenson LW, Pagani FD, Young JB, et al. INTERMACS profiles of advanced heart failure: the current picture. J Heart Lung Transplant. 2009;28:535–541. doi: 10.1016/j.healun.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Baran DA, Grines CL, Bailey S, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: This document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv. 2019;94:29–37. doi: 10.1002/ccd.28329. [DOI] [PubMed] [Google Scholar]
- 10.Jentzer JC, van Diepen S, Barsness GW, et al. Cardiogenic shock classification to predict mortality in the cardiac intensive care unit. J Am Coll Cardiol. 2019;74:2117–2128. doi: 10.1016/j.jacc.2019.07.077. [DOI] [PubMed] [Google Scholar]
- 11.Keebler ME, Haddad EV, Choi CW, et al. Venoarterial extracorporeal membrane oxygenation in cardiogenic shock. JACC Heart Fail. 2018;6:503–516. doi: 10.1016/j.jchf.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 12.Kim D, Jang WJ, Park TK, et al. Echocardiographic predictors of successful extracorporeal membrane oxygenation weaning after refractory cardiogenic shock. J Am Soc Echocardiogr. 2021;34:414–422.e4. doi: 10.1016/j.echo.2020.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Kim D, Park Y, Choi KH, et al. Prognostic implication of RV coupling to pulmonary circulation for successful weaning from extracorporeal membrane oxygenation. JACC Cardiovasc Imaging. 2021;14:1523–1531. doi: 10.1016/j.jcmg.2021.02.018. [DOI] [PubMed] [Google Scholar]
- 14.Mehra MR, Goldstein DJ, Uriel N, et al. Two-year outcomes with a magnetically levitated cardiac pump in heart failure. N Engl J Med. 2018;378:1386–1395. doi: 10.1056/NEJMoa1800866. [DOI] [PubMed] [Google Scholar]
- 15.Park Y, Kim D, Yang JH, Cho YH, Choi JO, Jeon ES. Clinical outcome in patients with end-stage heart failure who underwent continuous-flow left ventricular assist devices in a single center. Korean J Intern Med. 2022;37:340–349. doi: 10.3904/kjim.2021.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehra MR, Canter CE, Hannan MM, et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: a 10-year update. J Heart Lung Transplant. 2016;35:1–23. doi: 10.1016/j.healun.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 17.Kim IC, Youn JC, Kobashigawa JA. The past, present and future of heart transplantation. Korean Circ J. 2018;48:565–590. doi: 10.4070/kcj.2018.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee HY, Oh BH. Heart transplantation in Asia. Circ J. 2017;81:617–621. doi: 10.1253/circj.CJ-17-0162. [DOI] [PubMed] [Google Scholar]
- 19.Kim D, Choi JO, Oh J, et al. The Korean Organ Transplant Registry (KOTRY): second official adult heart transplant report. Korean Circ J. 2019;49:724–737. doi: 10.4070/kcj.2018.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khush KK, Cherikh WS, Chambers DC, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-sixth adult heart transplantation report - 2019; focus theme: donor and recipient size match. J Heart Lung Transplant. 2019;38:1056–1066. doi: 10.1016/j.healun.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim KJ, Cho HJ, Kim MS, et al. Focused update of 2016 Korean Society of Heart Failure guidelines for the management of chronic heart failure. Int J Heart Fail. 2019;1:4–24. doi: 10.36628/ijhf.2019.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sahlollbey N, Lee CK, Shirin A, Joseph P. The impact of palliative care on clinical and patient-centred outcomes in patients with advanced heart failure: a systematic review of randomized controlled trials. Eur J Heart Fail. 2020;22:2340–2346. doi: 10.1002/ejhf.1783. [DOI] [PubMed] [Google Scholar]
- 23.Hill L, Prager Geller T, Baruah R, et al. Integration of a palliative approach into heart failure care: a European Society of Cardiology Heart Failure Association position paper. Eur J Heart Fail. 2020;22:2327–2339. doi: 10.1002/ejhf.1994. [DOI] [PubMed] [Google Scholar]
- 24.Johnson MJ, McDonagh TA, Harkness A, McKay SE, Dargie HJ. Morphine for the relief of breathlessness in patients with chronic heart failure--a pilot study. Eur J Heart Fail. 2002;4:753–756. doi: 10.1016/s1388-9842(02)00158-7. [DOI] [PubMed] [Google Scholar]
- 25.Oxberry SG, Bland JM, Clark AL, Cleland JG, Johnson MJ. Repeat dose opioids may be effective for breathlessness in chronic heart failure if given for long enough. J Palliat Med. 2013;16:250–255. doi: 10.1089/jpm.2012.0270. [DOI] [PubMed] [Google Scholar]
- 26.Lee SE, Lee HY, Cho HJ, et al. Clinical characteristics and outcome of acute heart failure in Korea: results from the Korean Acute Heart Failure Registry (KorAHF) Korean Circ J. 2017;47:341–353. doi: 10.4070/kcj.2016.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Januzzi JL, Jr, Chen-Tournoux AA, Christenson RH, et al. N-terminal pro-B-type natriuretic peptide in the emergency department: the ICON-RELOADED study. J Am Coll Cardiol. 2018;71:1191–1200. doi: 10.1016/j.jacc.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 28.Mueller C, McDonald K, de Boer RA, et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail. 2019;21:715–731. doi: 10.1002/ejhf.1494. [DOI] [PubMed] [Google Scholar]
- 29.Takagi K, Kimmoun A, Sato N, Mebazaa A. Management of acute heart failure during an early phase. Int J Heart Fail. 2020;2:91–110. doi: 10.36628/ijhf.2019.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nikolaou M, Parissis J, Yilmaz MB, et al. Liver function abnormalities, clinical profile, and outcome in acute decompensated heart failure. Eur Heart J. 2013;34:742–749. doi: 10.1093/eurheartj/ehs332. [DOI] [PubMed] [Google Scholar]
- 31.Wettersten N. Biomarkers in acute heart failure: diagnosis, prognosis, and treatment. Int J Heart Fail. 2021;3:81–105. doi: 10.36628/ijhf.2020.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drazner MH, Hellkamp AS, Leier CV, et al. Value of clinician assessment of hemodynamics in advanced heart failure: the ESCAPE trial. Circ Heart Fail. 2008;1:170–177. doi: 10.1161/CIRCHEARTFAILURE.108.769778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stevenson LW, Perloff JK. The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA. 1989;261:884–888. [PubMed] [Google Scholar]
- 34.Chakko S, Woska D, Martinez H, et al. Clinical, radiographic, and hemodynamic correlations in chronic congestive heart failure: conflicting results may lead to inappropriate care. Am J Med. 1991;90:353–359. doi: 10.1016/0002-9343(91)80016-f. [DOI] [PubMed] [Google Scholar]
- 35.Butman SM, Ewy GA, Standen JR, Kern KB, Hahn E. Bedside cardiovascular examination in patients with severe chronic heart failure: importance of rest or inducible jugular venous distension. J Am Coll Cardiol. 1993;22:968–974. doi: 10.1016/0735-1097(93)90405-p. [DOI] [PubMed] [Google Scholar]
- 36.Binanay C, Califf RM, Hasselblad V, et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. 2005;294:1625–1633. doi: 10.1001/jama.294.13.1625. [DOI] [PubMed] [Google Scholar]
- 37.Shah MR, Hasselblad V, Stevenson LW, et al. Impact of the pulmonary artery catheter in critically ill patients: meta-analysis of randomized clinical trials. JAMA. 2005;294:1664–1670. doi: 10.1001/jama.294.13.1664. [DOI] [PubMed] [Google Scholar]
- 38.Costanzo MR, Stevenson LW, Adamson PB, et al. Interventions linked to decreased heart failure hospitalizations during ambulatory pulmonary artery pressure monitoring. JACC Heart Fail. 2016;4:333–344. doi: 10.1016/j.jchf.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 39.Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–666. doi: 10.1016/S0140-6736(11)60101-3. [DOI] [PubMed] [Google Scholar]
- 40.Lindenfeld J, Zile MR, Desai AS, et al. Haemodynamic-guided management of heart failure (GUIDE-HF): a randomised controlled trial. Lancet. 2021;398:991–1001. doi: 10.1016/S0140-6736(21)01754-2. [DOI] [PubMed] [Google Scholar]
- 41.Rohde LE, Rover MM, Figueiredo Neto JA, et al. Short-term diuretic withdrawal in stable outpatients with mild heart failure and no fluid retention receiving optimal therapy: a double-blind, multicentre, randomized trial. Eur Heart J. 2019;40:3605–3612. doi: 10.1093/eurheartj/ehz554. [DOI] [PubMed] [Google Scholar]
- 42.Mebazaa A, Yilmaz MB, Levy P, et al. Recommendations on pre-hospital & early hospital management of acute heart failure: a consensus paper from the Heart Failure Association of the European Society of Cardiology, the European Society of Emergency Medicine and the Society of Academic Emergency Medicine. Eur J Heart Fail. 2015;17:544–558. doi: 10.1002/ejhf.289. [DOI] [PubMed] [Google Scholar]
- 43.Damman K, Ter Maaten JM, Coster JE, et al. Clinical importance of urinary sodium excretion in acute heart failure. Eur J Heart Fail. 2020;22:1438–1447. doi: 10.1002/ejhf.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mullens W, Verbrugge FH, Nijst P, et al. Rationale and design of the ADVOR (Acetazolamide in Decompensated Heart Failure with Volume Overload) trial. Eur J Heart Fail. 2018;20:1591–1600. doi: 10.1002/ejhf.1307. [DOI] [PubMed] [Google Scholar]
- 45.Cox ZL, Hung R, Lenihan DJ, Testani JM. Diuretic strategies for loop diuretic resistance in acute heart failure: the 3T trial. JACC Heart Fail. 2020;8:157–168. doi: 10.1016/j.jchf.2019.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mullens W, Dauw J, Martens P, et al. Acetazolamide in acute decompensated heart failure with volume overload. N Engl J Med. 2022;387:1185–1195. doi: 10.1056/NEJMoa2203094. [DOI] [PubMed] [Google Scholar]
- 47.Vardeny O, Claggett B, Kachadourian J, et al. Reduced loop diuretic use in patients taking sacubitril/valsartan compared with enalapril: the PARADIGM-HF trial. Eur J Heart Fail. 2019;21:337–341. doi: 10.1002/ejhf.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mullens W, Damman K, Harjola VP, et al. The use of diuretics in heart failure with congestion - a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019;21:137–155. doi: 10.1002/ejhf.1369. [DOI] [PubMed] [Google Scholar]
- 49.Cotter G, Metzkor E, Kaluski E, et al. Randomised trial of high-dose isosorbide dinitrate plus low-dose furosemide versus high-dose furosemide plus low-dose isosorbide dinitrate in severe pulmonary oedema. Lancet. 1998;351:389–393. doi: 10.1016/S0140-6736(97)08417-1. [DOI] [PubMed] [Google Scholar]
- 50.Levy P, Compton S, Welch R, et al. Treatment of severe decompensated heart failure with high-dose intravenous nitroglycerin: a feasibility and outcome analysis. Ann Emerg Med. 2007;50:144–152. doi: 10.1016/j.annemergmed.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 51.Peacock WF, Hollander JE, Diercks DB, Lopatin M, Fonarow G, Emerman CL. Morphine and outcomes in acute decompensated heart failure: an ADHERE analysis. Emerg Med J. 2008;25:205–209. doi: 10.1136/emj.2007.050419. [DOI] [PubMed] [Google Scholar]
- 52.Gil V, Domínguez-Rodríguez A, Masip J, Peacock WF, Miró Ò. Morphine use in the treatment of acute cardiogenic pulmonary edema and its effects on patient outcome: a systematic review. Curr Heart Fail Rep. 2019;16:81–88. doi: 10.1007/s11897-019-00427-0. [DOI] [PubMed] [Google Scholar]
- 53.van Diepen S, Katz JN, Albert NM, et al. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2017;136:e232–e268. doi: 10.1161/CIR.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 54.Kang J, Cho HJ, Lee HY, et al. Effects of widespread inotrope use in acute heart failure patients. J Clin Med. 2018;7:368. doi: 10.3390/jcm7100368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chioncel O, Parissis J, Mebazaa A, et al. Epidemiology, pathophysiology and contemporary management of cardiogenic shock - a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2020;22:1315–1341. doi: 10.1002/ejhf.1922. [DOI] [PubMed] [Google Scholar]
- 56.Maack C, Eschenhagen T, Hamdani N, et al. Treatments targeting inotropy. Eur Heart J. 2019;40:3626–3644. doi: 10.1093/eurheartj/ehy600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ahmad T, Miller PE, McCullough M, et al. Why has positive inotropy failed in chronic heart failure? Lessons from prior inotrope trials. Eur J Heart Fail. 2019;21:1064–1078. doi: 10.1002/ejhf.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Backer D, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362:779–789. doi: 10.1056/NEJMoa0907118. [DOI] [PubMed] [Google Scholar]
- 59.Levy B, Clere-Jehl R, Legras A, et al. Epinephrine versus norepinephrine for cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2018;72:173–182. doi: 10.1016/j.jacc.2018.04.051. [DOI] [PubMed] [Google Scholar]
- 60.Greene SJ, Fonarow GC, Vaduganathan M, Khan SS, Butler J, Gheorghiade M. The vulnerable phase after hospitalization for heart failure. Nat Rev Cardiol. 2015;12:220–229. doi: 10.1038/nrcardio.2015.14. [DOI] [PubMed] [Google Scholar]
- 61.Chioncel O, Mebazaa A, Maggioni AP, et al. Acute heart failure congestion and perfusion status - impact of the clinical classification on in-hospital and long-term outcomes; insights from the ESC-EORP-HFA Heart Failure Long-Term Registry. Eur J Heart Fail. 2019;21:1338–1352. doi: 10.1002/ejhf.1492. [DOI] [PubMed] [Google Scholar]
- 62.Naylor M, Brooten D, Jones R, Lavizzo-Mourey R, Mezey M, Pauly M. Comprehensive discharge planning for the hospitalized elderly. A randomized clinical trial. Ann Intern Med. 1994;120:999–1006. doi: 10.7326/0003-4819-120-12-199406150-00005. [DOI] [PubMed] [Google Scholar]
- 63.Gayat E, Arrigo M, Littnerova S, et al. Heart failure oral therapies at discharge are associated with better outcome in acute heart failure: a propensity-score matched study. Eur J Heart Fail. 2018;20:345–354. doi: 10.1002/ejhf.932. [DOI] [PubMed] [Google Scholar]
- 64.Prins KW, Neill JM, Tyler JO, Eckman PM, Duval S. Effects of beta-blocker withdrawal in acute decompensated heart failure: a systematic review and meta-analysis. JACC Heart Fail. 2015;3:647–653. doi: 10.1016/j.jchf.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Velazquez EJ, Morrow DA, DeVore AD, et al. Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;380:539–548. doi: 10.1056/NEJMoa1812851. [DOI] [PubMed] [Google Scholar]