Abstract
Papillomaviruses are small double-stranded DNA viruses that replicate episomally in the nuclei of infected cells. The full-length E1 protein of papillomaviruses is required for the replication of viral DNA. The viral mRNA from which the human papillomavirus type 18 E1 protein is expressed is not known. We demonstrate that in eukaryotic cells, the E1 protein is expressed from polycistronic mRNA containing E6, E7, and E1 open reading frames (ORFs). The translation of adjacent E7 and E1 ORFs is not associated; it is performed by separate populations of ribosomes. The translation of the downstream E1 gene is preceded by ribosome scanning. Scanning happens at least at the 5′ end of the polycistronic mRNA and also approximately 100 bp in front of the E1 gene. Long areas in middle of the mRNA are bypassed by ribosomes, possibly by ribosomal “shunting.” Inactivation of short minicistrons in the upstream area of the E1 gene did not change the expression level of the E1 gene.
Current knowledge about gene expression stresses the importance of the regulated activation of tissue-specific promoters by a myriad of different transcription factors. In addition, regulation of gene expression also occurs at multiple posttranscriptional levels. These include, for example, the regulation of gene activity by changes in protein or mRNA structure, stability, and compartmentalization and other mechanisms, including translational regulation. Our interest in the translational mechanisms of regulation of gene expression was initiated after several attempts to clone the coding sequence of replication protein E1 from human papillomavirus type 6 (HPV6), HPV11, and HPV18 into the eukaryotic expression vector for in vivo DNA replication studies. We observed that papillomavirus replication protein E1 is expressed poorly if the E1 open reading frame (ORF) is cloned without a flanking sequence. As a matter of fact, the expression levels were raised significantly if the E1 700- to 900-bp 5′ flanking sequence was cloned together with the E1 ORF into a vector (36, 37a). This might have been an indication of the existence of the E1-specific promoter in the flanking region or a specific mechanism which would ensure the expression of the E1 protein from the multicistronic messenger. Otherwise, it would have been contradictory to the general paradigm of translational initiation in eukaryotic cells. The area upstream to the E1 ORF contains two long coding sequences for the E6 and E7 proteins and several potential minicistrons. According to the current paradigm, eukaryotic ribosomes most efficiently translate the first cistron in mRNA and only exceptionally continue at the later cistrons with low efficiency (27, 28). The closest known early promoter producing mRNA which would incorporate the E1 ORF of HPV16 and HPV18 is positioned in front of the E6 gene, where it drives the expression of E6 and E7 oncogenes (40, 45). If the E1 protein is expressed from the same mRNA and this mRNA is not modified by splicing to produce the monocistronic message, the E1 ORF would be the third major coding sequence in this mRNA. Therefore, we decided to study the mode of expression of the E1 gene and, in particular, to find out whether HPV18 produces shorter, E1-specific mRNA by splicing or alternative initiation of transcription or whether it is capable of using long polycistronic mRNA for that purpose. We found that the HPV18 E1 protein is translated from the polycistronic mRNA that includes coding sequences for the E6, E7, and E1 proteins, in addition to the smaller cistrons.
MATERIALS AND METHODS
Cell lines and electroporation.
For both RNA analysis and protein expression analysis, we used the COS7 cell line from the European Collection of Animal Cell Cultures (no. 87021302). Some control experiments which measured E1 protein levels by E1-dependent replication were performed with human embryonal kidney cell line 293 (no. 85120602). Cells were grown in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum (Sebak). Plasmid DNA (3 mg of the test plasmid plus 50 mg of carrier DNA) was electroporated into COS7 cells at 180 V with an ElectroPorator (Invitrogen). An exact description of our electroporation method can be found in reference ;[47]. Cells were plated onto 60-mm-diameter dishes and analyzed 24 h later. RNA analysis was performed similarly, except that cells were plated onto 100-mm-diameter dishes. Transfection efficiency was determined in a control plate that was transfected with a β-galactosidase-expressing plasmid (43) by staining of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) positive cells. Transfection efficiency was routinely 40 to 70% and did not vary between parallel controls.
Plasmids.
The basic construct pEPI contained the HPV18 sequence including nucleotides (nt) 105 to 2996 inserted downstream from the cybomegalovirus (CMV) promoter in vector pCG (44) without any leader sequences. The nucleotide numbering of HPV18 throughout this report is based on the EMBL data bank sequence of PAPHPV18 (accession no. X05015). Point mutations and deletions were introduced into this sequence by a double-PCR method (33). The correctness of mutations and surrounding sequences in mutant plasmids was confirmed by sequencing. The exact sequences of all mutants are shown in Table 1.
TABLE 1.
Plasmids and primers used in this studya
| Plasmid or primer | Changed nucleotide(s) | Sequence |
|---|---|---|
| pM26 | 123–910 deleted | GGATC|GCTAATGG |
| pM5 | Insert at 907 | AGTAAcGCAAC |
| pM6 | Insert at 906 | AGTAcAGCAAC |
| pM8 | Changes at 901, 904 | ATCCTAGTAGTAA |
| pM10 | Change at 916 | ACAATAGCTGA |
| pM11 | Change at 907 | CAGTACGCAAC |
| pfsE6B | Frameshift at BamHI site, nt 123 | GGATCgatcCAACA |
| pfsE6X | Frameshift at Xbal site, nt 325 | TCTAGctagAATTA |
| pfsE7 | Frameshift at Mspl site, nt 655 | CCGcgGTT |
| pM7G | Insert at 910 | AAGCAtggACAATGG |
| pM51 | Change at 593 | TAAGTATGGATG |
| pM52 | Change at 622 | ACATGGTATTG |
| pM53 | Change at 712 | ATGAAATGGATGG |
| pM54 | Change at 773 | ACAATGGTGTGT |
| pM55 | Change at 805 | CAGAATGGAGCT |
| pM56 | Changes at 869, 871 | ACACCATGGCCTTT |
| pM41 | Change at 592 | AAGTATACATGGA |
| pM42 | Change at 596 | ATGCATAGACCTA |
| pM43 | Change at 647 | CAAAATAAAATTC |
| pM44 | Change at 669 | TTCTATATCACGA |
| pM45 | Change at 707 | AACGATAAAATAG |
| pM46 | Change at 716 | ATAGATAGAGTTA |
| pM47 | Change at 772 | CACAATATTGTGT |
| pM48 | Change at 781 | GTGTATATGTTGT |
| Primer 6713 | 108–125 | GCGCGCTTTGAGGATCCA |
| Primer 5 | 1216–1198 (complementary) | CTCCCCTAATGGACTGTT |
Nucleotide numbers correspond to those in reference 5. Changed nucleotides are in boldface, and inserted nucleotides are in lowercase.
Western blotting.
Transfected cells were lysed in 100 to 150 ml of Laemmli buffer and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (39). Proteins were transferred from the gel to a nitrocellulose filter (Schleiser & Schuell BA85) with a semidry blotting system (Semiphor TE70) from Hoefer Scientific. Transfer was performed in 39 mM glycine–48 mM Tris–0.037% sodium dodecyl sulfate–20% ethanol for 150 min at 0.8 mA/cm2. Filters were blocked, washed, incubated with antibodies and developed with chromogenic or luminescent substrates in accordance with the suppliers’ protocols (NEN Reneissance and Amersham ECL). All experiments were done three to six times. Occasionally, the results were confirmed by immunoprecipitation assays.
RNA extraction and preparation.
Total RNA was extracted from transfected cells by the guanidine thiocyanate method followed by phenol-chloroform extractions (2) and DNase treatment (Promega RQ). The completeness of DNAse treatment was confirmed by simultaneously processing a control sample in which 1 mg of exogeneously added plasmid DNA was digested completely. The control RNA for the estimation of reverse transcription (RT)-PCR efficiency was synthesized in vitro from the T7 bacteriophage promoter of the pCG vector. It contains a deletion within the upstream area (nt 326 to 823). In vitro transcription of control mRNA was performed with T7 RNA polymerase (Fermentas, Vilnius, Lithuania) from a linearized template similarly to the synthesis of other mRNAs described below. It was synthesized in 50 ml in the presence of 80 mM Tris-HCl (pH 7.9), 12 mM MgCl2, 20 mM NaCl, 10 mM dithiothreitol, 500 mM ribonucleoside triphosphate, 100-mg/ml bovine serum albumin, 0.5 U of RNasin, and 9 U of polymerase at 40°C for 45 min.
RT-PCR.
RNA was dissolved in 4 ml of deionized water, denatured at 65°C and annealed to a primer by cooling slowly to 37°C in a commercial first-strand synthesis buffer from GIBCO. After that, the reaction was started by adding 500 mM deoxynucleoside triphosphate mixture and 200 U of reverse transcriptase (GIBCO Superscript). The total volume of the reaction was 20 ml. The reaction was stopped after 1 h by heating at 95°C for 5 min. Finally, 180 ml Tris-EDTA buffer was added and the products were stored at −20°C.
One-hundredth of this product was used as a template for the PCR. The PCR mixture included appropriate commercial buffer, 3 mM MgCl2, 5% glycerol, 200 mM deoxynucleoside triphosphate mixture, and 0.5 U of thermostable polymerase (Eurogentec GoldStar) per 100 ml. We used 50 mM primer 5 for RT and 6 mM primers 5 and 6713 for PCR. The PCR conditions were 1 cycle of 96°C for 3 min, 35 cycles of 94°C for 1 min, 62°C for 1.5 min, and 71°C for 1.5 min in a Robocycler (Stratagene). PCR products were analyzed in a 1.5% agarose gel.
RESULTS
Design of the experiment.
Our group has established transient-replication assays with the replication origins of HPV18, HPV6, and HPV11 (36, 38). The assay is performed by transfection of cells with an origin-containing plasmid and heterologous protein expression vectors that provide viral replication proteins E1 and E2. We have noticed that replication with the monocistronic E1 expression construct is almost undetectable, while constructs with the longer upstream area (including the E7 ORF) gives fairly high levels of replication. Thus, we decided to find out how the E1 protein is expressed. In order to study expression of the E1 protein, we had to solve a major problem: the sensitivity of detection of this weakly expressed protein. The problem was hindered by lack of suitable E1-specific monoclonal antibodies. We decided to insert a foreign epitope tag into the E1 gene of HPV18. This tag is recognized by well-known influenza virus monoclonal antibody 12CA5 (9). The 12-amino-acid tag was inserted after amino acid 4 of the E1 protein. Another epitope tag was cloned into the beginning of the E7 protein (after amino acid 20). This allowed us to compare the expression levels of the two proteins. Both epitope tags were recognized by the same antibody 12CA5, so we were able to compare the expression of both the E1 and E7 proteins simultaneously in a single experiment.
To increase the expression level of the E1 protein, we cloned most of the early region of HPV18 including the E6, E7, and E1 genes into eukaryotic expression vector pCG (44). The original papillomavirus early promoter P105 was replaced with the much stronger CMV promoter from the pCG vector. We maintained the overall structure of viral genes up to the transcription initiation site at P105 and we confirmed by S1 mapping that the transcripts in pCG start at the same position as in the wild-type virus (data not shown). We expect that replacement of the promoter does not cause significant changes in the structure, stability, or translational properties of the mRNA. The resulting construct was called pEPI. Please note that the insertion of the epitope tag within the E1 ORF disrupted the strong E1∧E4 splicing donor at the beginning of the E1 ORF. The possible effects of this change are discussed in the Discussion. We tested E1 protein expression in vivo by electroporation of the respective constructs into COS-7 cells, followed by Western blotting using monoclonal antibody 12CA5 against the epitope. First, we compared the expression of pEPI to that of a monocistronic construct, pM26, in which sequences between the promoter and the E1 gene are deleted. As shown in Fig. 1 (lanes 1 and 2), expression of the E1 protein was from monocistronic construct pM26 was virtually undetectable.
FIG. 1.
Comparison of expression of polycistronic (lane 1) and monocistronic (lane 2) E1 expression constructs. The Western blot shows expression levels of the E1 and E7 proteins in COS7 cells. Lanes 3 and 4 show expression from control plasmids without the CMV promoter-enhancer (lane 3) or with an inverted (lane 4) promoter-enhancer. Deletion (lane 3) or inversion (lane 4) of a 550-bp CMV promoter-enhancer area was done with restriction enzymes EcoRI and BamHI. Lane 5 is a negative control transfected with the vector only. The location of the E6* intron is shown by the triangle above the E6 ORF. Numbers above refer to nucleotide numbers of HPV18 according to reference 5. A nonspecific band appears because the 12CA5 monoclonal antibody cross-reacts with a cellular protein which moves slightly faster than tagged E1 protein.
To eliminate the possibility that the epitope tag influenced the expression of the E1 gene, we also used a parallel model system to check our initial results. Untagged (without an epitope) analogs of pEPI, pM26, and several other plasmids were used in transient-replication assay at limiting plasmid concentrations. We expect that the replication level reflects E1 expression from those plasmids. Indeed, the replication levels exactly coincided with the E1 levels on a Western blot (data not shown). This proves that our model system with tagged epitopes properly reflects E1 expression in eukaryotic cells.
We did not try to find the reason why monocistronic E1 constructs are poorly expressed. Several possibilities are mentioned in the discussion. However, with the work described in this paper, we tried to understand how is it possible to translate the E1 gene from complex polycistronic mRNA.
The E1 protein is translated from polycistronic mRNA.
Our initial interest was to find out the structure of the E1-producing mRNA. The first impression was that the area upstream to the E1 ORF could contain an E1-specific promoter. No promoters have previously been identified in this region of HPV18 (21). A late promoter in front of the E1 gene has been described in many HPV types (4, 8, 15, 16, 35). Nevertheless, its activity in undifferentiated cells is extremely low (6, 16, 17) and messages produced from this promoter are unstable in these cells (23). We tested the possible existence of a functional promoter in this region by using two mutant plasmids: pNP and pIP (Fig. 1, lanes 3 and 4). These mutant plasmid have a structure similar to that of pEPI but have a deleted and inverted CMV promoter-enhancer region, respectively. pIP, with the inverted promoter, should retain the CMV enhancer activity that could activate cryptic promoters in the vicinity. The absence of E1 expression from these constructs clearly demonstrates that the CMV promoter in front of the E6 gene is responsible for the E1 expression levels we achieve in our model system.
If the promoter closest to E1 ORF is P105, then the mRNA for expression of the E1 protein would be tricistronic, also containing the E6 and E7 ORFs in front of the E1 ORF. We wanted to test whether this tricistronic pre-mRNA undergoes a splicing event that has not been described previously. The only previously described splicing event within the E6-E7 region of HPV18 is splicing within the E6 gene (42), which is called E6* (shown as a triangle in Fig. 1). We tested the splicing pattern of pEPI mRNAs simultaneously by S1 mapping (data not shown) and RT-PCR (Fig. 2, lane 1). RT-PCR primers were chosen from the ultimate 5′ end of the mRNA and from the beginning of the E1 ORF. They covered all of the E6-E7 area and one-fifth of the E1 gene (see Materials and Methods). No additional splicing products were detected by either method, although the full-length and E6* mRNAs were easily detectable (Fig. 2, lane 1). To confirm that the sensitivity of our RT-PCR was sufficiently high to detect low-level mRNAs, we included an internal control RNA (Fig. 2, lane 2). The in vitro-synthesized control RNA was made from another deletion mutant that can be amplified in a PCR by the same set of primers as pEPI. To prove that our RT-PCR enables us to detect a rare spliced mRNA even if it is present at a single copy per cell, we added 106 molecules (which is approximately equal to the number of transfected COS7 cells in our experiment) of control RNA to our RT-PCR together with total cellular RNA. This control mRNA is an approximate representation of mRNA at a single copy per cell in the background of the total RNA, and it was easily detected in our experiment. Therefore, our RT-PCR is sensitive enough to exclude any possible splicing that could facilitate E1 protein expression from polycistronic mRNA.
FIG. 2.
RT-PCR analysis of total mRNA from COS7 cells transfected with pEPI (lane 1). The area upstream of the E1 ORF was amplified by using PCR primers from the beginning of the E6 ORF (5′ primer, primer 6713) to the beginning of the E1 ORF (3′ primer, primer 5). In lane 2, the same reaction was performed together with 106 molecules of control RNA to control the sensitivity of the method. The control mRNA was synthesized in vitro and added immediately before RT-PCR. The positions of the products of the full-length mRNA, its spliced E6* form, and from the control RNA are shown on the right. The intermediate bands between two bands are heterodimeric DNA molecules. Lanes 3 and 4 are size controls synthesized from DNA. Lane 5 is PCR control without a template. Lane 6 is molecular weight marker λ/Eco 47 I.
Nevertheless, one can imagine that specific degradation of the 5′ end of the mRNA could generate translatable mRNAs, which would not be detected by RT-PCR (if the binding site for the upstream primer is deleted). This unlikely possibility was excluded by a series of S1 mapping experiments. S1 nuclease is an enzyme that detects and cleaves any unpaired nucleotide in an RNA-DNA duplex. Therefore, if we anneal mRNA and a radiolabelled DNA fragment, we are able to detect the first unmatched nucleotide between the mRNA and the DNA.
Our S1 mapping experiments fully covered the same E6-E7 area and detected only full-length and E6* mRNAs (data not shown). This proves that the E1 mRNA starts around nt 105, as in wild-type HPV18, and the only mRNA species that could serve for translation of the HPV18 E1 gene are polycistronic full-length mRNA and its spliced form, E6*.
Translation of the E1 protein is not associated with translation of the E7 protein.
If the E1 protein is produced from polycistronic mRNA, the question of possible translation mechanisms arises. How can ribosomes find an ATG codon that is 800 bp away from the beginning of the mRNA? Normally, ribosomes would approach the ATG by scanning mRNA from its 5′ end. How can ribosomes bypass those numerous ATG codons in E1 mRNA? Firstly, we can discard the influence of the E6 ORF and its ATG codon, because the transcription at P105 starts exactly at the first ATG of the E6 ORF or even some nucleotides later (40). In this context, ribosomes will always have a great chance to bypass the E6 ORF without translating it. Several other strong ATG codons are spliced out in the E6* intron. Thus, we can treat our mRNA as bicistronic mRNAs containing the E7 and E1 ORFs.
Although eukaryotic mRNA is usually monocistronic, translation of polycistronic messages is also possible (22). In most of these cases, the translation of a second gene is linked to the translation of the first gene. Translating ribosomes are (at low efficiency) able to (i) continue scanning the first gene after the stop codon (reinitiation) and (ii) translate through the stop codon without recognizing it (readthrough).
These mechanisms allow translation of the second ORF in a bicistronic message, albeit at lower efficiency (for a review, see reference 1. Frameshift during translation is another widely used way of translating genes from a polycistronic message, resulting in the synthesis of the hybrid protein. In our case, the E7 and E1 ORFs are at the same frame, separated by just 6 nt. We considered the possibility that translation of the E7 ORF and that of the E1 ORF might be linked to each other—the ribosomes translating E7 can continue translating E1 at lower efficiency.
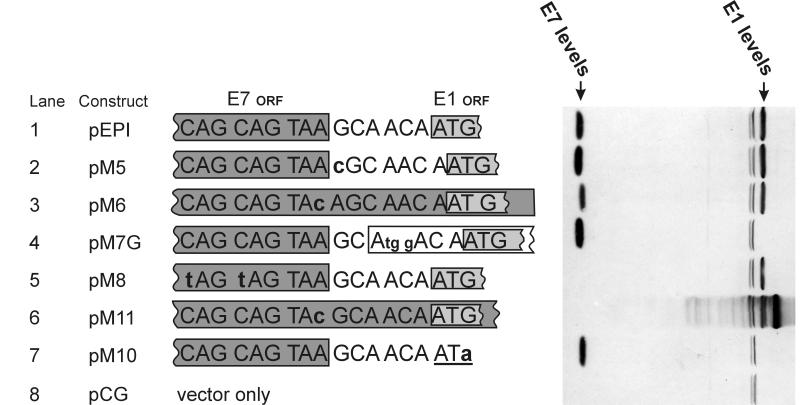
We decided to test these possibilities by making point mutations around the stop codon of the E7 gene and in the 6-bp intergenic space between the E1 and E7 genes (Fig. 3). These point mutations should interfere with the unusual types of translation mechanisms described above. The point mutations we made were the following. pM5 and pM6 (lanes 2 and 3) changed the reading frame between E7 and E1 to avoid translation by readthrough. pM8 (lane 5) placed two additional stop codons at the end of the E7 gene to avoid translation by readthrough. pM11 (lane 6) and pM6 altered the E7 stop codon to avoid possible reinitiation. An important control in this series was pM10, where the first ATG of the E1 gene was destroyed to ensure that this is the real start site for E1 protein translation (lane 7). As shown in Fig. 3, most of these mutations did not interfere with the expression of the E1 protein. The most interesting mutation here was pM11, where E7 and E1 are fused to one big ORF. It produces a fusion protein as expected, but the normal-length E1 protein is made as well, albeit at slightly lower levels (lane 6). This demonstrates that the production of E1 is not associated with the production of the E7 protein. It looks likely that the E1 and E7 genes are translated by different ribosomes or from different mRNAs. The translation of E7 is obviously not associated with translation of E1. Although reinitiation or readthrough after finishing the E7 gene is not entirely excluded, most of the E1 is clearly produced by other mechanisms.
FIG. 3.
Western blot analysis of several point mutations in front of the E1 ORF. The nucleotide sequence between the E7 and E1 ORFs is shown. Plasmid pEPI has the wild-type intergenic sequence. Mutated nucleotides are shown in lowercase and boldface. In constructs pM6 and pM11, the E7 ORF overlaps the E1 ORF. Mutant plasmid pM7G in lane 4 added a false initiation codon in front of the real E1 initiation codon.
Synthesis of the full-length E6 and E7 proteins is not required for expression of the E1 protein.
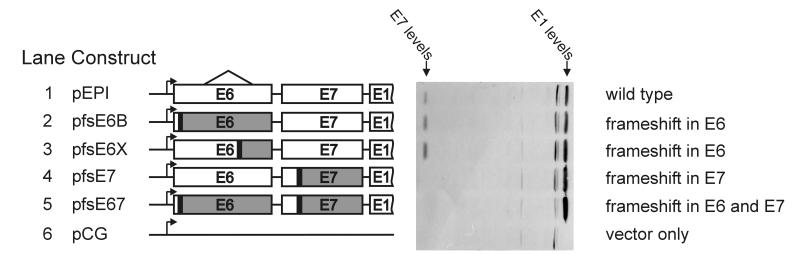
If the area upstream of the E1 ORF is retained in mature mRNA, it should contain some important features that favor the production of E1. The E6 or E7 proteins that are expressed from this region could possibly stimulate translation of the E1 protein. To test the possibility, we disrupted the expression of E6 or E7 by frameshift mutations within E6, E7, or both ORFs (Fig. 4, lane 2 to 5). The mutations in those proteins did not eliminate E1 expression. These mutations confirmed that expression of full-length E6 and E7 is not required for translation of the E1 protein. Moreover, disruption of the E7 ORF even stimulated the expression of E1.
FIG. 4.
Frameshift mutations within preceding ORFs. The frameshifts were generated by Klenow filling in of BamHI (pfsE6B and pfsE67), Xbal (pfsE6X), or Mspl (pfsE7 and pfsE67) restriction sites. The triangle above the E6 ORF shows the location of the E6* intron.
Scanning in front of the E1 gene.
In order to understand the mechanism of E1 gene translation, it is important to know whether the ATG of the E1 gene is approached by normal scanning or is recognized by other mechanisms (direct binding or even backscanning). The best way to solve this question was to introduce an out-of-frame ATG codon in front of the authentic E1 ATG. Scanning ribosomes would be misled by those “false” ATG codons. A mutant plasmid, pM7G, was generated in which the false ATG was placed immediately (4 nt) before the authentic E1 ATG. This mutation completely eliminated the expression of the E1 protein (Fig. 3, lane 4). It is not likely that the effect of M7G was purely due to the change in the primary sequences and not related to scanning. We tested analogous mutant pM7 with a weaker Kozak consensus (ATGA instead of the current ATGG) that differed by only a single nucleotide. Indeed, the M7 with weaker Kozak consensus had a much weaker inhibiting effect on E1 translation (data not shown). This experiment suggests that the E1 start codon is approached by scanning, but it is not clear in which regions this scanning starts. Can the entire mRNA be scanned by ribosomes?
To find out how far in front of the E1 gene ribosomes scan mRNA, we generated a series of mutant plasmids, pM51 to pM56 (Fig. 5). False ATG codons were introduced at increasing distances from the E1 start codon in those mutant plasmids. All of them were in frame with the E7 ORF, so they did not generate any additional minicistrons in front of the E1 ORF. The mutants covered the entire E7 ORF, and one of them, pM51, changed the context of the authentic E7 start codon to make it stronger. We expect that those false ATG codons, which were all in a strong initiation context, should inhibit scanning ribosomes similarly to pM7G. The results are shown in Fig. 5. pM7G, which had already been tested before, again eliminated E1 protein expression (lane 7). The next one (pM56), which was 45 nt away, had only a slight inhibiting effect (lane 6). Other false ATG codons that were further away from the real ATG had no effect (lanes 1 to 5). Thus, it seems that scanning is discontinuous and happens only in front of the E1 gene. This might be an indication of “internal initiation” or “ribosome shunting.”
FIG. 5.
Addition of false ATG codons with a strong context (black bars) into the upstream area of the E1 ORF. These codons are expected to stop scanning ribosomes. Two codons that are shown in lane 8, on pEPI, are two existing strong ATG codons within the E7 ORF. The striped area represents the 12-amino-acid epitope that was added for detection of E7 levels. The numbers in the last column are those of the nucleotides that were mutated to create strong ATGG initiation codons. The numbering is according to reference 5.
Scanning of the polycistronic E1 mRNA starts at its 5′ end, not by internal initiation.
In many picornaviruses, translation of polycistronic mRNAs is performed by internal initiation (32, 34). In this case, ribosomes recognize some internal structures within the mRNA and bind to the mRNA internally. Translation starts directly in the binding region or after short scanning of a few hundred nucleotides. Internal initiation was one of the possible mechanisms we considered for the E1 mRNA. A good way to identify scanning regions in mRNA is by inserting hairpin structures. These hairpin structures cannot be penetrated by scanning ribosomes if their ΔG is less than −61 kcal/mol (25).
Insertion of such hairpins in different areas would allow us to pinpoint regions in polycistronic mRNA that are scanned by E1-translating ribosomes. A similar method has also allowed the identification of a ribosome jumping mechanism in cauliflower mosaic virus (11).
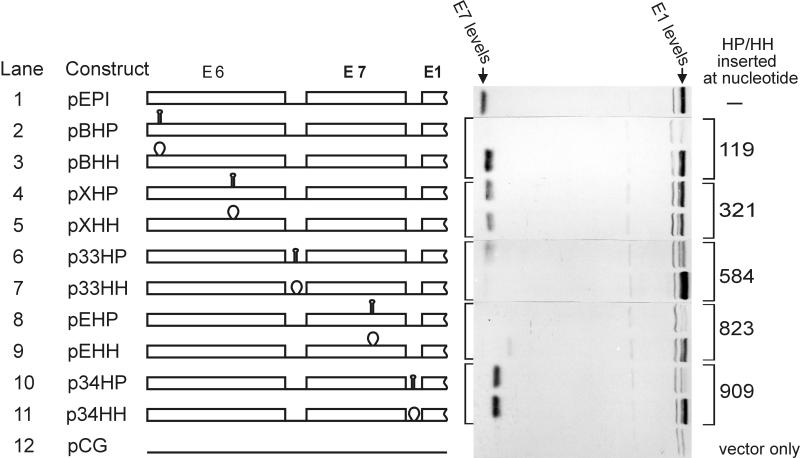
We inserted 20-bp inverted repeats to create HP (identical to the hp7 described in reference (25)) into five sites in the area upstream of the E1 gene. Their positions and the respective levels of the E1 protein expression are shown in Fig. 6. Together with hairpin mutant HP, we always created a control mutant HH also. In HH-type mutants, the same 20-bp sequence was not inverted but inserted as two direct repeats at the same position as in the respective HP mutant. These HH mutants served as controls in which we did not disrupt any important primary or secondary structural elements of the mRNA. As shown in Fig. 6, of five hairpin structures, three caused significant reductions in the level of E1 expression. The only hairpin that certainly did not inhibit E1 synthesis was in a previously described intron within the E6 gene (lanes 4 and 5). This result was expected because the intron was spliced out in the wild-type virus (40), as well as in our model system (Fig. 2, lane 1), before translation of the mRNA. The results obtained with the hairpin p33HP region around the start of the E7 ORF are unclear. The levels of control mutant p33HH showed an increase above the normal level of E1. At the same time, p33HH reduced E7 protein expression for an unknown reason. We noticed already that a decrease in E7 levels can increase E1 protein levels, and that could be how p33HH indirectly affects E1 levels. Another unexpected result is the discrepancy between the results produced by hairpin EHP (Fig. 6, lane 8) and the artificial ATG mutants in the previous experiment (Fig. 5, lane 6). The hairpin seems to inhibit at a distance of 80 bp in front of the E1 start codon, whereas the artificially introduced ATG codon was not inhibitory at a distance of 30 nt. One of the possible reasons for this disagreement could be the effect of hairpin to local secondary structure of the mRNA. 40 nucleotides were inserted to generate EHP hairpin, while only 2 point mutations were generated for “false” ATG mutant (pM56).
FIG. 6.
Western blot analysis of E1 protein expression from constructs with hairpins. The positions of inserted stable hairpins (HP) or their analogs not forming hairpins (HH) are shown. The hairpins are supposed to inhibit scanning ribosomes. The numbers in the last column are according to reference 5.
The interesting fact is that a hairpin at the ultimate beginning of the mRNA inhibited E1; as well as E7; expression. This confirms that 40S ribosome subunits need to scan through from the beginning of the polycistronic mRNA to reach the start codon of the E1 gene. As expected from false ATG results (Fig. 3, lane 4), the hairpin immediately in front of the E1 gene also inhibited the E1 gene translation. The fact that the hairpin in 5′ end of the mRNA inhibits expression of both the E7 and E1 proteins is not in agreement with an internal initiation mechanism. We also tested the hypothesis of internal initiation by inserting a 1,000-bp-long bacterial gene (Pseudomonas putida xylS) into the BamHI site at the beginning of the mRNA region (data not shown). In case of internal initiation, it would not interfere with the translation of downstream genes. In our experiment, addition of sequences to the beginning of the mRNA completely eliminated expression from the E7 and E1 genes. Thus, we discarded the hypothesis of internal initiation and looked for other possible mechanisms for translation of the E1 gene.
Minicistrons are not involved in translation of the E1 mRNA.
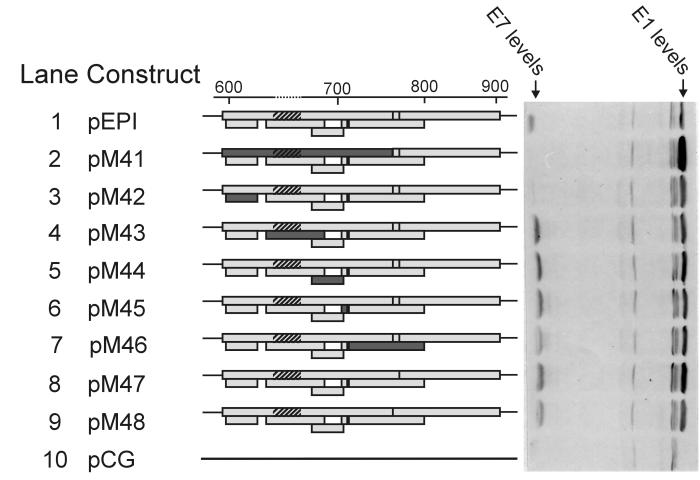
In some cases, translation of downstream genes is preceded by translation of small minicistrons. Products of those minicistrons could regulate the translation of downstream genes (13). As the mechanism for passing of ribosomes through the E7 gene area was still unclear, we decided to mutate all of the start codons in this area. This area contains seven ATG codons, of which two are in the same reading frame with the E7 gene. The initiation context around those ATG codons is not optimal but still relatively good (except for two ATGs in a very poor context). We mutated all of the ATG codons one by one, generating seven different mutant plasmids (M42 to M48) plus one in which the E7 start codon itself (M41) was destroyed. Surprisingly, all minicistrons within the E7 gene could be destroyed without affecting the E1 protein levels (lane 2 to 8). Therefore, the E1-translating ribosomes are able to pass all of the upstream area without having to recognize those numerous ATG codons in the E7 region. Thus, ribosome jumping seems to be the most likely mechanism for the translation of polycistronic E1 mRNA.
Generally, the mutations within the E7 gene did not affect E1 protein levels (Fig. 5 and 7). The only exceptions were mutation M41, which destroyed the genuine start codon of the E7 gene (Fig. 7, lane 2), and frameshift mutations within the E7 gene (Fig. 4, lanes 4 and 5) and that increased the levels of E1 protein. This effect could be explained by the ribosome shunting hypothesis (see Discussion).
FIG. 7.
Western blot showing E1 levels with mutated minicistrons in the upstream area. Shown is the upstream area with the E7 ORF and five minicistrons in it. The inactivated minicistrons are darkest. The striped region represents the 12-amino-acid epitope that was added for detection of E7 levels. In pM47 and pM48, two ATG initiation codons that were in frame with the E7 ORF were mutated. In pM41 (lane 2), the authentic E7 start codon was mutated. The numbers above refer to nucleotide numbers of HPV18 according to reference 5.
DISCUSSION
In this paper, we demonstrate that the E1 protein of HPV18 can be produced from polycistronic mRNA. This was confirmed by various experiments like promoter mutagenesis (Fig. 1), RT-PCR (Fig. 2), and S1 mapping. The most interesting conclusion is that the E1 mRNA has to be polycistronic to be expressed. Monocistronic E1 expression construct pM26 was inactive (or extremely inefficient) for expression of the E1 protein. Although the mechanism by which expression from monocistronic mRNA was inhibited was not studied in this work, the fact itself indicates that complicated regulation is involved in the expression of the papillomavirus E1 protein. It also explains why many researchers have had low yields in HPV replication assays if monocistronic E1 and E2 constructs were used.
Another interesting question was the translation mechanism of the E1 ORF in E6/E7/E1 mRNA. Being polycistronic, it is a complicated template for ribosomes. The classical eukaryotic mRNA is monocistronic and the start site of its translation is localized by a scanning process (for reviews, see references 24, 29, and 30). Scanning is performed by 40S ribosomal subunits in a complex with several cellular translation factors. Normally, scanning starts from the ultimate 5′ end of the mRNA and does not continue after translation of the first cistron. Nevertheless, the existence of polycistronic eukaryotic messages has been demonstrated before. A variety of mechanisms has been proposed for translation of such exceptional mRNAs. Ribosomal frameshift is a common mechanism for translation of overlapping proteins of retroviruses (20), and nonoverlapping genes can be translated by reinitiation (3, 18) or internal initiation (32, 34) of ribosomes. Sometimes ribosomes might be less sensitive to weak initiation codons and ignore preceding ORFs by leaky scanning (10, 27). In cauliflower mosaic virus and adenovirus, ribosome jumping mechanisms have been proposed to move over some parts of mRNA without scanning (11, 48). Which mechanism could be responsible for translation of the HPV18 E1 gene? Combining all of our experiments, we were able to exclude any unusual translation mechanism except ribosome jumping. So far, ribosome jumping has been described for pararetroviruses of plants (11, 12) and for adenovirus (48). It has been suggested that ribosome jumping is favored in cells under stress conditions (e.g., by lack of serum or mitogens), when the RNA-helicase complex eIF-4F is less active. In early stages of infection, papillomaviruses infect basal and suprabasal cells of the skin and mucosa. Although we have no experimental data on eIF-4F concentrations in such papillomavirus-infected cells, we expect that its activity is low because those cells are deprived of mitogens. Therefore, it seems likely that papillomaviruses have adapted to use of ribosome jumping during evolution. The important determinant of the ribosome jumping mechanism is a strong secondary structure within the bypassed region of the translated mRNA and a short minicistron preceding the secondary structure (7, 37). Similar elements are characteristic of the mRNA region preceding the E1 ORF.
Why should the E1 protein be expressed in such an inefficient and complicated way? We can find many examples of small viruses that use complicated mechanisms to regulate their gene expression. The most prominent examples are human immunodeficiency virus type 1 (20) and other retroviruses, repetitive element LINE-1 from humans and rats (18, 31), hepatitis A virus (14), hepatitis B virus (10), hepatitis C virus (46), duck hepatitis B virus (3), and cauliflower mosaic virus (41). Often, the replication proteins or DNA polymerases of small viruses are produced by unusual translation mechanisms. These are the last genes in polycistronic messages being preceded by other genes that should be produced at higher levels. The replication proteins themselves are required at minor levels, and the use of polycistronic messages is probably the easiest way to achieve the expression of two or three proteins at desired ratios.
Naturally, translational regulation is not the only way of adjusting viral protein levels. A majority of the E1 protein that we see in our experiments is translated to E1∧E4 protein in vivo due to the strong conserved splicing donor at the beginning of the E1 ORF. In our model system, we inactivated this splice donor site to increase the levels of detectable E1 protein. We expect that only a small fraction of those polycistronic mRNAs contain the full-length E1 gene and that the majority of early mRNAs are spliced to form the E1∧E4 ORF (4, 19). Nevertheless, we do not expect that splicing at this E1∧E4 donor site changes the mechanism by which ribosomes find the E1 start codon. We have repeated the same experiments with spliceable constructs, in both monocistronic (like pM26) and polycistronic (like pEPI and pM5 to pM11) configurations and seen similar results.
Extending the results of our current work, we hypothesize that monocistronic E1 mRNA is inhibited in early stages of the viral infection cycle by an unknown mechanism of translational inhibition. Consequently, this explains the expression of viral replication protein E1 from the polycistronic message. The late promoter is in front of the E1 gene in several HPVs (15, 16). If this late promoter were conserved in HPV18, it would allow upregulation of E1 levels in a late stage of replication cycle. The use of a less efficient polycistronic message could prevent the premature expression of large quantities of the E1 protein and possibly prevent overreplication of the virus too early in its life cycle. The expressions of the viral E6, E7, and E1 proteins from polycistronic mRNA are obviously highly regulated and associated with each other. We show that the translation of the E7 protein and that of the E1 protein are not directly associated (Fig. 3 and 4). Nevertheless, the inactivation of the E7 ORF stimulates expression of the E1 ORF (Fig. 4, lane 4 and 5, and Fig. 7, lane 2). One of the possible explanations for this is the disruption of secondary structure elements during translation of the E7 gene. Translating ribosomes are able to dissolve strong secondary structures. If certain secondary structure is important for the expression of the E1 gene, then it is understandable why the translation of the E7 gene inhibits the translation of the E1 gene. An alternate possibility is that premature termination of the E7 ORF simply increases the intergenic distance between the E7 and E1 ORFs. As known before (26), longer intergenic distances are more favorable for reinitiation. Thus, the translation of the following E1 ORF would be increased by additional ribosomes that would normally translate the E7 ORF.
However, how is the E7 protein expressed? It is also at least 500 nt away from the beginning of the mRNA and is preceded by several strong ATG codons that might inhibit scanning ribosomes. From our experiments we see that the expression of the E7 gene is dependent on scanning similarly to the E1 gene—the hairpin in the beginning of the mRNA inhibits expression from both the E1 and E7 genes (Fig. 6, lanes 2 and 3). Most of the strong initiation codons in front of the E7 gene are located within E6* intron and do not interfere with the scanning of ribosomes if a spliced form of the polycistronic mRNA is used. The only remaining initiation codon, at nt 469, is in a relatively weak context. The influence of the first ORF, the E6 ORF, in this mRNA is also insignificant for downstream genes because its ATG starts at the first nucleotide of the mRNA and is thus in an extremely unfavorable context for translation initiation. Furthermore, the P105 promoter produces a subset of shorter mRNAs that start several nucleotides later and do not include the start of the E6 gene (40). Thus, the subset of longer and unspliced mRNAs could be used for expression of the E6 gene, and shorter and spliced mRNAs could be used for expression of the E7, E1∧E4, and E1 genes. Therefore, the combination of alternative splicing and complicated translation mechanisms could ensure balanced expression of the four proteins from the P105 promoter in early stages of papillomavirus infection. We should also keep in mind possible regulatory effects of the viral E2 protein and its binding sites in the context of the full viral genome. The regulation of early transcription by the E2 protein can add additional complexity to the expression pattern of the early proteins.
Another question that has been raised by our experiments is why a monocistronic construct does not produce the full-length E1 protein. The most likely explanation is that the monocistronic E1 mRNAs are less stable or are preferably spliced to make alternative, shorter mRNAs. Nevertheless, our preliminary experiments do not confirm that hypothesis. The RT-PCR analysis shows equal mRNA levels with polycistronic messages if sampled from within different regions of the E1 gene. Another possibility is that active inhibition of translation is responsible for poor expression of monocistronic messages. The issue needs further experiments that exceed the scope of the current study.
As mentioned, our system reflects only early stages of the papillomavirus infection cycle, infection and latency in basal and suprabasal cells. In later stages, when the replication of the viral DNA probably requires much more E1 protein, the hypothetical late promoter in front of E1 takes over the synthesis of the E1-specific mRNA. Then the mechanisms of E1 (and abundant E1∧E4) expression might be completely different from the mechanism described by us.
ACKNOWLEDGMENTS
We thank Jüri Parik and the ribosome group for continuous methodological support and Mike Romanos, Jaanus Remme, Juhan Sedman, Tanel Tenson, and Arnold Kristjuhan for critical reading of the manuscript.
This work was partly supported by grants from the Estonian Science Fund (no. 1134), the International Science Foundation (LD 6000 and LKL 100), and the EC Copernicus project (CIPA-CT94-0154).
REFERENCES
- 1.Atkins J F, Weiss R B, Gesteland R F. Ribosome gymnastics—degree of difficulty 9.5, style 10.0. Cell. 1990;62:413–423. doi: 10.1016/0092-8674(90)90007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates and Wiley-Interscience; 1992. [Google Scholar]
- 3.Chang L J, Pryciak P, Ganem D, Varmus H E. Biosynthesis of the reverse transcriptase of hepatitis B viruses involves de novo translational initiation not ribosomal frameshifting. Nature. 1989;337:364–368. doi: 10.1038/337364a0. [DOI] [PubMed] [Google Scholar]
- 4.Chow L T, Nasseri M, Wolinsky S M, Broker T R. Human papillomavirus types 6 and 11 mRNAs from genital condylomata acuminata. J Virol. 1987;61:2581–2588. doi: 10.1128/jvi.61.8.2581-2588.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cole S T, Danos O. Nucleotide sequence and comparative analysis of the human papillomavirus type 18 genome. Phylogeny of papillomaviruses and repeated structure of the E6 and E7 gene products. J Mol Biol. 1987;193:599–608. doi: 10.1016/0022-2836(87)90343-3. [DOI] [PubMed] [Google Scholar]
- 6.DiLorenzo T P, Steinberg B M. Differential regulation of human papillomavirus type 6 and 11 early promoters in cultured cells derived from laryngeal papillomas. J Virol. 1995;69:6865–6872. doi: 10.1128/jvi.69.11.6865-6872.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dominguez D, Ryabova L, Pooggin M, Schmidt-Puchta W, Futterer J, Hohn T. Ribosome shunting in cauliflower mosaic virus. Identification of an essential and sufficient structural element. J Biol Chem. 1998;273:3669–3678. doi: 10.1074/jbc.273.6.3669. [DOI] [PubMed] [Google Scholar]
- 8.Doorbar J, Parton A, Hartley K, Banks L, Crook T, Stanley M, Crawford L. Detection of novel splicing patterns in a HPV16-containing keratinocyte cell line. Virology. 1990;178:254–262. doi: 10.1016/0042-6822(90)90401-c. [DOI] [PubMed] [Google Scholar]
- 9.Field J, Nikawa J, Broek D, MacDonald B, Rodgers L. Purification of a RAS-responsive adenylyl cyclase complex from Saccharomyces cerevisiae by use of an epitope addition method. Mol Cell Biol. 1988;8:2159–2165. doi: 10.1128/mcb.8.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fouillot N, Tlouzeau S, Rossignol J M, Jean-Jean O. Translation of the hepatitis B virus P gene by ribosomal scanning as an alternative to internal initiation. J Virol. 1993;67:4886–4895. doi: 10.1128/jvi.67.8.4886-4895.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fütterer J, Kiss-Laszlo Z, Hohn T. Nonlinear ribosome migration on cauliflower mosaic virus 35S RNA. Cell. 1993;73:789–802. doi: 10.1016/0092-8674(93)90257-q. [DOI] [PubMed] [Google Scholar]
- 12.Fütterer J, Potrykus I, Bao Y, Li L, Burns T M, Hull R, Hohn T. Position-dependent ATT initiation during plant pararetrovirus rice tungro bacilliform virus translation. J Virol. 1996;70:2999–3010. doi: 10.1128/jvi.70.5.2999-3010.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geballe A, Morris D. Initiation codons within 5′-leaders of mRNAs as regulators of translation. Trends Biochem Sci. 1994;19:159–164. doi: 10.1016/0968-0004(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 14.Glass M J, Jia X Y, Summers D F. Identification of the hepatitis A virus internal ribosome entry site: in vivo and in vitro analysis of bicistronic RNAs containing the HAV 5′ noncoding region. Virology. 1993;193:842–852. doi: 10.1006/viro.1993.1193. [DOI] [PubMed] [Google Scholar]
- 15.Higgins G D, Uzelin D M, Phillips G E, McEvoy P, Marin R, Burrell C J. Transcription patterns of human papillomavirus type 16 in genital intraepithelial neoplasia: evidence for promoter usage within the E7 open reading frame during epithelial differentiation. J Gen Virol. 1992;73:2047–2057. doi: 10.1099/0022-1317-73-8-2047. [DOI] [PubMed] [Google Scholar]
- 16.Hummel M, Hudson J B, Laimins L A. Differentiation-induced and constitutive transcription of human papillomavirus type 31b in cell lines containing viral episomes. J Virol. 1992;66:6070–6080. doi: 10.1128/jvi.66.10.6070-6080.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hummel M, Lim H B, Laimins L A. Human papillomavirus type 31b late gene expression is regulated through protein kinase C-mediated changes in RNA processing. J Virol. 1995;69:3381–3388. doi: 10.1128/jvi.69.6.3381-3388.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ilves H, Kahre O, Speek M. Translation of the rat LINE bicistronic RNAs in vitro involves ribosomal reinitiation instead of frameshifting. Mol Cell Biol. 1992;12:4242–4248. doi: 10.1128/mcb.12.9.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inagaki Y, Tsunokawa Y, Takebe N, Nawa H, Nakanishi S, Terada M, Sugimura T. Nucleotide sequences of cDNAs for human papillomavirus type 18 transcripts in HeLa cells. J Virol. 1988;62:1640–1646. doi: 10.1128/jvi.62.5.1640-1646.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacks T, Power M D, Masiarz F R, Luciw P A, Barr P J, Varmus H E. Characterization of ribosomal frameshifting in HIV-1 gag-pol expression. Nature. 1988;331:280–283. doi: 10.1038/331280a0. [DOI] [PubMed] [Google Scholar]
- 21.Karlen S, Beard P. Identification and characterization of novel promoters in the genome of human papillomavirus type 18. J Virol. 1993;67:4296–4306. doi: 10.1128/jvi.67.7.4296-4306.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaufman R J, Murtha P, Davies M V. Translational efficiency of polycistronic mRNAs and their utilization to express heterologous genes in mammalian cells. EMBO J. 1987;6:187–193. doi: 10.1002/j.1460-2075.1987.tb04737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kennedy I M, Haddow J K, Clements J B. A negative regulatory element in the human papillomavirus type 16 genome acts at the level of late mRNA stability. J Virol. 1991;65:2093–2097. doi: 10.1128/jvi.65.4.2093-2097.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kozak M. An analysis of vertebrate mRNA sequences: intimations of translational control. J Cell Biol. 1991;115:887–903. doi: 10.1083/jcb.115.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kozak M. Circumstances and mechanisms of inhibition of translation by secondary structure in eucaryotic mRNAs. Mol Cell Biol. 1989;9:5134–5142. doi: 10.1128/mcb.9.11.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kozak M. Effects of intercistronic length on the efficiency of reinitiation by eucaryotic ribosomes. Mol Cell Biol. 1987;7:3438–3445. doi: 10.1128/mcb.7.10.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozak M. Evaluation of the “scanning model” for initiation of protein synthesis in eucaryotes. Cell. 1980;22:7–8. doi: 10.1016/0092-8674(80)90148-8. [DOI] [PubMed] [Google Scholar]
- 28.Kozak M. How do eucaryotic ribosomes select initiation regions in messenger RNA? Cell. 1978;15:1109–1123. doi: 10.1016/0092-8674(78)90039-9. [DOI] [PubMed] [Google Scholar]
- 29.Kozak M. The scanning model for translation: an update. J Cell Biol. 1989;108:229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kozak M. Structural features in eukaryotic mRNAs that modulate the initiation of translation. J Biol Chem. 1991;266:19867–19870. [PubMed] [Google Scholar]
- 31.McMillan J P, Singer M F. Translation of the human LINE-1 element, L1Hs. Proc Natl Acad Sci USA. 1993;90:11533–11537. doi: 10.1073/pnas.90.24.11533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meerovitch K, Sonenberg N. Internal initiation of picornavirus RNA translation. Semin Virol. 1993;4:217–227. [Google Scholar]
- 33.Mikaelian I, Sergeant A. A general and fast method to generate multiple site directed mutations. Nucleic Acids Res. 1992;20:376. doi: 10.1093/nar/20.2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oh S K, Sarnow P. Gene regulation: translational initiation by internal ribosome binding. Curr Opin Genet Dev. 1993;3:295–300. doi: 10.1016/0959-437X(93)90037-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palermo-Dilts D A, Broker T R, Chow L T. Human papillomavirus type 1 produces redundant as well as polycistronic mRNAs in plantar warts. J Virol. 1990;64:3144–3149. doi: 10.1128/jvi.64.6.3144-3149.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plumpton M, Sharp N A, Liddicoat L H, Remm M, Tucker D O, Hughes F J, Russell S M, Romanos M A. A high capacity assay for inhibitors of human papillomavirus DNA replication. Bio/Technology. 1995;13:1210–1214. doi: 10.1038/nbt1195-1210. [DOI] [PubMed] [Google Scholar]
- 37.Pooggin M, Hohn T, Fütterer J. Forced evolution reveals the importance of short open reading frame A and secondary structure in the cauliflower mosaic virus 35S RNA leader. J Virol. 1998;72:4157–4169. doi: 10.1128/jvi.72.5.4157-4169.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37a.Remm, M. Unpublished data.
- 38.Remm M, Brain R, Jenkins J. The E2 binding sites determine the efficiency of replication for the origin of human papillomavirus type 18. Nucleic Acids Res. 1992;20:6015–6021. doi: 10.1093/nar/20.22.6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schägger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 40.Schneider-Gädicke A, Schwarz E. Different human cervical carcinoma cell lines show similar transcription patterns of human papillomavirus type 18 early genes. EMBO J. 1986;5:2285–2292. doi: 10.1002/j.1460-2075.1986.tb04496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schultze M, Hohn T, Jiricny J. The reverse transcriptase gene of cauliflower mosaic virus is translated separately from the capsid gene. EMBO J. 1990;9:1177–1185. doi: 10.1002/j.1460-2075.1990.tb08225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smotkin D, Prokoph H, Wettstein F O. Oncogenic and nononcogenic human genital papillomaviruses generate the E7 mRNA by different mechanisms. J Virol. 1989;63:1441–1447. doi: 10.1128/jvi.63.3.1441-1447.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spaete R R, Mocarski E S. Regulation of cytomegalovirus gene expression: α and β promoters are trans activated by viral functions in permissive human fibroblasts. J Virol. 1985;56:135–143. doi: 10.1128/jvi.56.1.135-143.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanaka M, Herr W. Differential transcriptional activation by Oct-1 and Oct-2: interdependent activation domains induce Oct-2 phosphorylation. Cell. 1990;60:375–386. doi: 10.1016/0092-8674(90)90589-7. [DOI] [PubMed] [Google Scholar]
- 45.Thierry F, Heard J M, Dartmann K, Yaniv M. Characterization of a transcriptional promoter of human papillomavirus 18 and modulation of its expression by simian virus 40 and adenovirus early antigens. J Virol. 1987;61:134–142. doi: 10.1128/jvi.61.1.134-142.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsukiyama-Kohara K, Iizuka N, Kohara M, Nomoto A. Internal ribosome entry site within hepatitis C virus RNA. J Virol. 1992;66:1476–1483. doi: 10.1128/jvi.66.3.1476-1483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ustav M, Stenlund A. Transient replication of BPV-1 requires two viral polypeptides encoded by the E1 and E2 open reading frames. EMBO J. 1991;10:449–457. doi: 10.1002/j.1460-2075.1991.tb07967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yueh A, Schneider R. Selective translation initiation by ribosome jumping in adenovirus-infected and heat-shocked cells. Genes Dev. 1996;10:1557–1567. doi: 10.1101/gad.10.12.1557. [DOI] [PubMed] [Google Scholar]