Abstract
Much is known about the generation, removal, and roles of 5-methylcytosine (5mC) in eukaryote DNA, and there is a growing body of evidence regarding N6-methyladenine, but very little is known about N4-methylcytosine (4mC) in the DNA of eukaryotes. The gene for the first metazoan DNA methyltransferase generating 4mC (N4CMT) was reported and characterized recently by others, in tiny freshwater invertebrates called bdelloid rotifers. Bdelloid rotifers are ancient, apparently asexual animals, and lack canonical 5mC DNA methyltransferases. Here, we characterize the kinetic properties and structural features of the catalytic domain of the N4CMT protein from the bdelloid rotifer Adineta vaga. We find that N4CMT generates high-level methylation at preferred sites, (a/c)CG(t/c/a), and low-level methylation at disfavored sites, exemplified by ACGG. Like the mammalian de novo 5mC DNA methyltransferase 3A/3B (DNMT3A/3B), N4CMT methylates CpG dinucleotides on both DNA strands, generating hemimethylated intermediates and eventually fully methylated CpG sites, particularly in the context of favored symmetric sites. In addition, like DNMT3A/3B, N4CMT methylates non-CpG sites, mainly CpA/TpG, though at a lower rate. Both N4CMT and DNMT3A/3B even prefer similar CpG-flanking sequences. Structurally, the catalytic domain of N4CMT closely resembles the Caulobacter crescentus cell cycle–regulated DNA methyltransferase. The symmetric methylation of CpG, and similarity to a cell cycle–regulated DNA methyltransferase, together suggest that N4CMT might also carry out DNA synthesis–dependent methylation following DNA replication.
Keywords: DNA methylation, N4-methylcytosine, metazoan cytosine-N4 DNA methyltransferase, bdelloid rotifer, Adineta vaga
SAM-dependent DNA methyltransferases (MTases) act on DNA and generate three modified bases in cell genomes: 5-methylcytosine (5mC), N4-methylcytosine (N4mC or 4mC), and N6-methyladenine (N6mA or 6mA) (1, 2). The presence of 5mC in eukaryote DNA has been known since the 1950s and 1960s (3, 4), and it plays critical roles in mammalian development and in diseases such as cancer (5, 6, 7, 8). In addition, the action of ten-eleven translocation (TET) enzymes can successively oxidize 5mC to 5-hydroxymethylC, 5-formylC, and 5-carboxylC (9, 10, 11, 12), and some transcription factors preferentially bind to the different oxidized forms (13, 14, 15, 16, 17, 18, 19, 20). The genes and activities of DNA MTases (DNMTs) for generating 5mC in mammalian genomes were cloned and sequenced over a period of about 10 years, starting with Dnmt1 in 1988 (21) and continuing through Dnmt3a/3b (22, 23, 24). [In addition, in rodents (but not primates), Dnmt3c—a gene duplication product of Dnmt3b—is a catalytically active DNA MTase expressed specifically in male germline cells and required for mouse fertility (25, 26, 27).] The catalytic domains of these mammalian DNMTs show striking similarities to bacterial type II DNA cytosine-C5 MTases (28, 29). However, we note that protein sequence similarity alone is insufficient to establish the true enzymatic activity: DNMT2 contains all the conserved DNA MTase motifs (30, 31, 32, 33), but it is a tRNA cytosine-C5 MTase (34). Interestingly, bdelloid rotifers examined to date all lack canonical cytosine-C5 DNA MTases and appear to be missing a TET ortholog as well (35), though we note that Adineta vaga does have an ortholog (UJR25867.1) to the rRNA 5mC MTase of Caenorhabditis elegans (NP_490958.1).
The existence of 6mA in eukaryote DNA was detected immunochemically as early as 1983 (36) and was reported again in mouse embryonic stem cells in 2016 (37), but its presence in the genomes of Drosophila, Arabidopsis, mice, or humans remains a controversial topic (38, 39, 40, 41, 42). Nevertheless, there is clear evidence for the activities of enzymes/protein factors responsible for generating, recognizing, and removing mammalian DNA 6mA, at least in vitro, often (but not always) involving proteins that act on both DNA and RNA. This includes MTases (“writers”) (43, 44, 45, 46, 47, 48, 49), methylation-sensitive DNA-binding proteins (“readers”) (46, 50), and methyl-group removing demethylases (“erasers”) (44, 51, 52, 53, 54, 55, 56). There is a significant amount of 6mA in A. vaga DNA, particularly at a subset of GpA dinucleotides (35). In comparison, among unicellular eukaryotes, including the ciliate Tetrahymena thermophila, the green alga Chlamydomonas reinhardtii, and the early-diverging fungus Saccharomyces cerevesiae, 6mA is enriched in symmetrical ApT dinucleotides (57). Recently an experiment, using forced expression of bacterial DNA adenine-N6 MTases in human cells, demonstrated that 6mA in the context of GATC or GAnTC has a direct effect on gene expression (58).
In contrast to the large body of information on 5mC in eukaryote DNA and the rapidly growing body regarding 6mA, very little is known about 4mC in eukaryote DNA. The rarity of 4mC in eukaryote DNA may reflect in part the facts that—compared to 5mC—4mC is more destabilizing to dsDNA (59) and is harder to repair when it becomes part of a UV-induced cyclobutane dimer with an adjacent pyrimidine (60). The liverwort Marchantia polymorpha apparently has a sperm-specific 4mC DNA MTase (61). There is some evidence for 4mC in the DNA of mammals (62) and plants (63), and a number of papers have reported computational methods for predicting where 4mC is likely to occur (e.g., (64, 65)). However, the gene for the first DNA MTase generating 4mC in a metazoan genome (N4-cytosine methyltransferase, N4CMT) was only reported and partially characterized in 2022, in tiny freshwater invertebrates called bdelloid rotifers (35). The report noted that the catalytic domain of N4CMT in A. vaga—readily recognizable from amino acid sequence motifs—was fused to a chromodomain, which is often associated with recognizing histone modifications (66).
Bdelloid rotifers form the largest and most ancient metazoan taxon (67) and are unique in being asexual animals that produce only females (68), at least under the great majority of conditions (69, 70). Their other distinguishing features include longevity of survival in extreme environments such as anhydrobiosis (71) and freezing (72), as well as extreme resistance to both ionizing radiation (73) and oxidation (74). Bdelloid rotifers use horizontal gene transfer both within and between species (75). They appear to have the highest observed rate of horizontal gene transfer among metazoa (76), which may explain their >60M y success in the apparent absence of sexual recombination. Bdelloid rotifers thus offer an advantageous system for investigating fundamental questions regarding horizontal gene transfer, aging, DNA repair, spread of transposable elements, and the roles of sexual recombination in animal systems (67, 76, 77, 78, 79, 80, 81, 82, 83).
We have a longstanding interest in DNA MTases of the β class (84, 85, 86, 87), of which N4CMT is a member. Accordingly, we have characterized kinetic properties and structural features of this 4mC DNA MTase from the bdelloid rotifer A. vaga (GenBank UJR33503.1).
Results
Relationship of N4CMT to other MTases
As shown in Fig. S1, a search for orthologs to A. vaga N4CMT finds them in two distinct categories. First are the closest relatives, with at least 80% query coverage and 55% identity. These come from other Adineta species, or species of Rotaria, which is another bdelloid rotifer. The second group comes from bacteria, among which the highest query coverage is ∼70%, and the highest identity is under ∼40%. Alignment to the bacterial orthologs nevertheless allows tentative identification of the conserved MTase motifs (Fig. S1 and (84)). In addition, though not surprisingly, the most-conserved residues (66) of the previously identified N4CMT-associated chromodomain (35) are shared by the other bdelloid rotifer orthologs but not by the bacterial MTases—even the few bacterial enzymes that have C-terminal extensions as long as those of the bdelloid orthologs (Fig. S1).
The isolated catalytic domain of N4CMT has the highest activity in vitro
We purified recombinant N4CMT in full-length and as a series of truncations lacking the ∼60-residue N-terminal or C-terminal or both (Fig. 1, A and B). We found that the full-length protein and fragments containing either a complete N- or C-terminus showed low solubility, low yield, and low activity (Fig. S2, A and B and samples 1, 2 and 3 in Fig. 1B). In the original characterization of N4CMT (35), removal of the chromodomain resulted in an increase in enzyme activity due to better solubility. Our results confirm that finding, with 10 to 20% higher activities of samples 6 and 7 compared to those of samples 4 and 5 in Figure 1B. In the remainder of the present study, we used the isolated catalytic domain, including residues 79 to 324 (N4CMT79–324 hereafter) that is active on an oligonucleotide containing a single CpG dinucleotide (sample 7 in Fig. 1B). We also note that the original description of N4CMT reported two alloenzymes, called A and B (35), with seven substitutions between the two, and we have used the A form.
Figure 1.
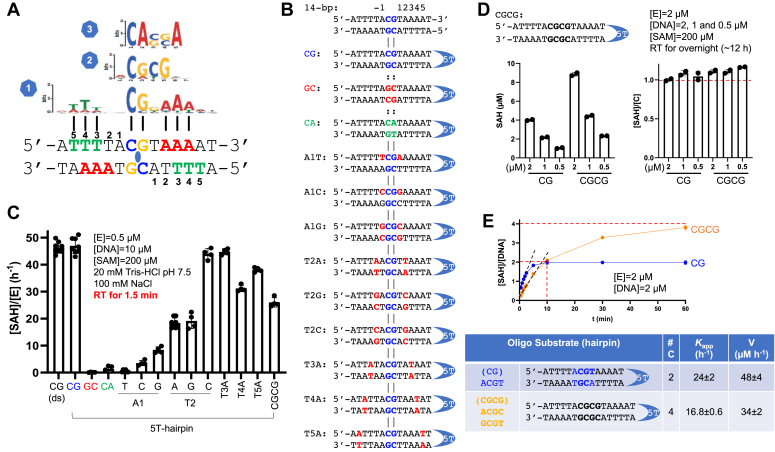
Adineta vaga N4CMT contains an active catalytic domain.A, schematic of Adineta vaga N4CMT. Both N-terminal and C-terminal ∼60 residues contain a putative zinc-binding site (see Fig. S2D), though this is not conserved among N4CMT orthologs (see Fig. S1). B, methyl transfer reactions of N4CMT full-length and N- and/or C-terminal deletions ([E] = 0.5 μM, [DNA] = 10 μM, [SAM] = 200 μM) were incubated for 1.5 min. The bottom 12% PAGE gel shows protein samples used in the assay, with amino acid ranges on the right (FL = full length) (N ≥ 3). C, saturated reactions ([E] = 2 μM, [PCR product] ∼0.15 μM, [SAM] = 200 μM, and overnight at room temperature) generated ∼18 methylation events by N4CMT and one methylation event by CamA, per 990-bp DNA molecule (N ≥ 2). See text for theoretically possible numbers. Sinefungin (SFG) was used as an inhibitor. D, use of anti-methylated-base antibodies in dot blots. Left panel, 6mA-specific antibody recognizes CamA-generated product. Middle panel, 4mC-specific antibody recognizes N4CMT-generated product. Right panel, 5mC-specific antibody recognizes synthetic 5mC-containing oligos. E, 15 out of 19 favored methylation sites contain ACG. F, ACGG is enriched among disfavored ACG sites. 4mC, N4-methylcytosine; 5mC, 5-methylcytosine; 6mA, N6-methyladenine; N4CMT, N4-cytosine methyltransferase.
Next, we measured methyl transfer activity between N4CMT79–324 and a 990-bp PCR product, which contains 79 CpG, 66 CpA, and 68 TpG sites (TpG would have CpA on the complementary strand) (Fig. S2C). The original characterization found genomic 4mC concentrated at both CpG and CpA dinucleotides (35) so, counting both strands, our 990-bp test DNA could in theory be methylated at 292 cytosines. In an overnight saturated reaction in the presence of 200 μM SAM, we observed ∼2.6 μM SAH, the reaction byproduct of SAM. This result is consistent with just ∼17 to 18 methylation events per DNA molecule (Fig. 1C), which is only a tiny fraction (∼6%) of possible sites. This very low level is consistent with the observation that the sequence context of N4CMT is modulated by flanking sequences (35) (and see below). In this assay, we also used CamA as a positive control. CamA is a bacterial DNA 6mA MTase and specifically methylates CAAAAA (the target A is underlined) (88, 89). The 990-bp contains a single CAAAAA site, and under the same conditions, CamA generates one methylation per DNA molecule—specifically, ∼0.16 μM SAH is approximately equivalent in molarity to the 0.15 μM DNA used.
We characterized the methylation products of the PCR-amplified DNA segment using three available antibodies: anti-6mA, anti-5mC, and anti-4mC (Fig. 1D) and got the expected results in all three cases. First, none of these antibodies recognized our unmodified PCR product (lane 3 in Fig. 1D). Second, the 4mC-specific antibody detected only the N4CMT-modified DNA (lane 4), whereas the 6mA-specific antibody detected only the CamA-modified DNA (lane 5). Third, the 5mC-specific antibody recognized neither the CamA- nor the N4CMT-generated products.
We next performed NAME-seq, a sequencing method that can detect 4mC at single-base resolution, to identify N4CMT methylated sites on our PCR product (90). We identified 19 sites having >50% methylation, and all 19 sites are CpG dinucleotides (Figs. 1E and S3). Among the 19 sites, 15 occur on ACG trinucleotides, indicating N4CMT favors Ade 5′ to the CpG dinucleotides. We also investigated other ACG sites having low methylation percentage (<30%) and found ACGG motif (with a 3′ Gua) was enriched in these poorly methylated sites (Fig. 1E). Among the six symmetrically methylated CpG sites, five are palindromes—four ACGT and one GCGC—and only one occurs at ACGC (or GCGT) (Fig. S3).
N4CMT79–324 exhibits weak binding of SAM
We first optimized the enzymatic activity of N4CMT79–324 using as substrate a 14-bp oligo containing a single CpG (Fig. 2A). We carried out the reactions at room temperature (∼22 °C), both because A. vaga is grown at 20 to 24 °C (91) and because the original N4CMT characterization was done at 25 °C (35). N4CMT79–324, at an enzyme concentration of 0.5 μM, is in the linear range for a 15-min reaction time (Fig. 2A), has an optimal activity at 100 mM NaCl, is sensitive to ionic strength (sodium chloride concentrations above 200 mM; Fig. 2B), and has a narrow pH range of 7.5 to 8.0 (Fig. 2C). These experiments were performed in the presence of 40 μM SAM. We next measured the N4CMT79–324 kinetic parameters at the optimal conditions of pH 7.5, 100 mM NaCl, and reaction time of 9 min by varying concentrations of DNA substrate, in the presence of 40 μM SAM, yielding a kcat of 17 h−1 and KM for DNA of 1.3 μM (Fig. 2D). However, varying SAM concentration, even up to 200 μM, did not saturate the reaction (in contrast with the expected hyperbolic relationship); that is, the binding affinity for SAM (as reflected by the KM value) is >155 μM (Fig. 2E). For comparison, the original study (35) used 80 μM SAM, with addition of a second equal amount of SAM during the 16 h reaction. We thus repeated the kinetics, in the presence of 200 μM SAM, which increased the kcat nearly four-fold (compared to 40 μM SAM) to 65 h−1 but gave a KM for DNA of 1.8 μM, just 1.4× higher (Fig. 2F). The catalytic efficiency thus improved at the higher SAM level by ∼2.8× (comparing kcat/KM from 13 to 36 h−1 μM−1). The binding order of the two substrates (DNA and SAM) did not matter to N4CMT79–324 (Fig. 2G).
Figure 2.
Activity of N4CMT79–324on an ACGT-containing DNA oligonucleotide.A, N4CMT79–324 activity, presented as the accumulation of byproduct SAH in a bioluminescence assay, after varied reaction times (N = 2). B and C, effects on N4CMT79–324 activity of NaCl (N ≥ 3) and pH (N = 4). The CBTP buffer contains a mixture of 10 mM citric acid and 10 mM BisTris propane. D–F, dependence of the velocity of byproduct SAH formation per enzyme molecule [SAH]/[E] on substrate concentration was analyzed according to the Michaelis–Menten equation. D, N4CMT79–324 has a kcat value of 17 h−1, in the presence of 40 μM SAM (N = 2). E, N4CMT79–324 has a KM (SAM) value of >155 μM, which is very large (low affinity) compared to most MTases (N = 3). F, N4CMT79–324 has improved catalytic rate (kcat) and catalytic efficiency (kcat/KM), in the presence of a high SAM concentration (200 μM) (N = 2). G, N4CMT79–324 uses a random sequential reaction, in terms of the order of binding of the two substrates DNA or SAM (respectively preincubated with enzyme) (N = 2). N4CMT, N4-cytosine methyltransferase.
Most characterized SAM-dependent MTases have low-μM KM values (high affinity) for SAM. For example, the two RNA MTases PCIF1 and MettL5 have KM values of ∼1 μM for SAM (92). However, several other characterized MTases have low-binding affinities (high KM values) for SAM, including human MettL16 (KM > 400 μM) (92) and human PRDM9 (KM = 120–170 μM) (93). MettL16 is unique in that its binding to the mRNA substrates (including the MAT2A 3′ UTR) increases under SAM-limiting conditions (94). Thus, the sensitivity of MettL16 to SAM levels results in a negative feedback loop, such that increasing SAM levels lead to reduced expression of MAT2A, which encodes an isozyme of the SAM synthetase (94). Although PRDM9 exhibits weak binding of SAM, its high kcat value appears to make it the most active histone lysine MTase (93). For comparison, the SAM concentration in human lymphoblasts is ∼60 μM (95) and in yeast is ∼100 μM (96), though those are whole-cell measurements and N4CMT presumably acts in the nucleus. We do not yet know the biological significance of the weak SAM binding by N4CMT, in part, because the role of 4mC methylation in A. vaga is not yet clear.
N4CMT79–324 preferentially recognizes a symmetrical 5′-YACGTR-3′ sequence
To investigate the N4CMT substrate sequence specificity, we designed a 14-bp palindrome containing a central CpG site. The sequence matches one of the three MEME-CHIP sequence motifs derived from A. vaga (35) (motif 1 in Fig. 3A), containing symmetric ACGT (the most preferred site in NAME-seq; Fig. 1E). For convenience, we also designed a hairpin DNA containing a 5-thymine (5T) loop (Fig. 3B). N4CMT79–324 has equal activity on the ACGT containing double-stranded and 5T-hairpin substrates (Fig. 3C), so we introduced sequence variations in the context of the 5T-hairpin substrate and made the following observations. First, replacing CpG with either GpC or CpA reduced N4CMT79–324 methylation activity to background levels at the initial reaction time of 1.5 min (for longer time points, see Fig. 4C). Second, substituting the first 5′ A outside of CpG (on both strands) to the other three possible bases significantly reduced activity, with the A-to-T being the most severe. Third, substituting the second 5′ base away from CpG from T to C has no impact on activity, whereas changing that base to A or G resulted in ∼50% reduction. Fourth, the substitutions of the 5′ T base at positions 3, 4, or 5 to A retained significant activity. We conclude that N4CMT79–324 has a preference for the symmetrical sequence YACGTR (where Y = C or T and R = A or G), at least in the context of our in vitro assay system, and this is reflected in the high activities of hairpin substrates T2C, T3A, T4A, and T5A in Figure 3, B and C.
Figure 3.
Substrate sequence specificity of N4CMT79–324in the context of symmetric sequences.A, the 14-bp palindromic oligo matches sequence motif 1 derived from Adineta vaga by Rodriguez et al (35). B, tested variations of symmetric sequence in the context of 5T-hairpin. C, activities of N4CMT79–324 on oligos with varied sequences, at initial reaction time of 1.5 min (N ≥ 4). Oligo names are defined in (B). D and E, under single turnover conditions, where the enzyme is present at or above the DNA substrate concentration, cytosines on both strands (CpG and CGCG) are methylated, after either saturated overnight reactions (panel D; N = 2) or a 1 h time course (panel E; N = 2). Summary of Kapp and the velocity of the reaction, from fitting the linear ranges in panel E (indicated by the dashed lines). 5T, 5-thymine; N4CMT, N4-cytosine methyltransferase.
Figure 4.
Substrate sequence specificity of N4CMT79–324in the context of asymmetric sequences.A, early time points (0–5 min) of reaction, monitoring the first and second methylation events of ACGT sequence under near single turnover condition (N = 2). B, comparison of kinetic parameters (Km and kcat) and catalytic efficiency (kcat/KM) of N4CMT79–324 on varied concentrations of four oligo substrates with three indicated sequences. The reaction time was 1.5 min for unmethylated ACGT (N = 4) and 5 min for hemi-ACGT and ACGG (N = 2). C, comparison of three oligonucleotide substrates (unmethylated ACGT, hemi-ACGT, and ACAT) within the favored CpG-flanking sequences (N = 2). Summary of Kapp and the velocity of the reaction, from fitting the linear ranges in panel C and D (indicated by the dashed lines). N4CMT, N4-cytosine methyltransferase.
Next, we expanded the central CpG site to CGCG (motif 2 in Fig. 3A). This sequence is methylated, but the activity is lower than the single CpG site at the initial reaction time of 1.5 min (Fig. 3C), probably because the ACGC or GCGT differ from the optimal sequence of ACGT. However, under single turnover conditions where the enzyme is present at or above the DNA substrate concentration, after either saturated overnight reactions (Fig. 3D) or a 1 h time course (Fig. 3E), we observed complete methylation for oligos containing CpG (two methylation events) or CGCG (four methylation events). In other words, the reaction product SAH had the same molarity as the number of cytosine residues in the DNA substrate used in the reaction, with both strands having been methylated in vitro.
N4CMT79–324 has lower catalytic efficiency on asymmetrical sequences
We investigated asymmetric sequences including hemimethylated ACGT, ACAT, and ACGG (the most-disfavored site in NAME-seq, Fig. 1F). Using the unmethylated substrate having a single ACGT site with two possible target cytosines (CpG/CpG; Fig. 4A), the first methylation event was complete by ∼1.2 min, and the second methylation event was slower with ∼50% completion at 2.5 min and near-completion by 5 min. The slower reaction exhibited by N4CMT79–324 on a hemimethylated CpG site is due to a ∼5.6-fold weaker affinity for the substrate (Km = 10 μM on hemi-CpG versus 1.8 μM on unmodified CpG) and a ∼1.9-fold slower reaction rate (kcat = 30 h−1 versus 56 h−1) than for the unmethylated substrate (Fig. 4B). In other words, N4CMT79–324 shows more than 10-fold loss in catalytic efficiency (comparing kcat/Km value of ∼3 h−1 μM−1 for hemimethylated CpG and ∼31 h−1 μM−1 for unmethylated CpG) driven by the existing methylation on one strand. Furthermore, the disfavored ACGG site, with a guanine on the 3′ side of CpG, has even further-reduced catalytic efficiency (kcat/KM = 1.7 h−1 μM−1). We note that the opposite strand of ACGG is CCGT, which is the second most-preferred site after ACGT (Figs. 1E and S3).
In addition, we reanalyzed methylation of ACAT/ATGT (a single cytosine), in comparison to ACGT/ACGT either unmethylated or hemimethylated. The single ACAT oligo, having the same flanking sequences as ACGT in the comparable substrate, displayed a ∼4.4 fold slower reaction rate than that of CpG (Kapp = 5.4 h−1 versus 24 h−1) (Fig. 4C). The CpA reaction was linear for 5 min and reached completion at 30 min. Interestingly, hemimethylated-CpG has approximately the same reaction rate as that of CpA/TpG.
Ionic strength has opposing effects on solubility/stability and activity of N4CMT79–324
As shown in Figure 2B, the activity of N4CMT79–324 is sensitive to ionic strength and is diminished at or above 250 mM NaCl, probably due to inhibition of protein-DNA binding (97). However, the enzyme was purified and stored at 0.5 or 1 M NaCl to keep it from precipitating (Fig. S2F). We used three biophysical methods to measure the effects of NaCl on oligomeric states of N4CMT79–324. Analytical size-exclusion chromatography (Fig. S2F) and sedimentation velocity analytical ultracentrifugation (SV-AUC) studies at 500 mM NaCl revealed exclusive presence of monomers at three protein concentrations above 2 μM (Fig. 5A). At the concentration of 19 μM, N4CMT79–324 was 97% monomers and 3% dimers to tetramers. However, reducing NaCl from 500 mM to 250 mM resulted in a major dimer subpopulation (Fig. 5B). Similarly, as monitored by dynamic light scattering, reducing NaCl to 250 mM resulted in higher molecular weight populations (dimers, oligomers, or polymers), whereas doubling the ionic strength to 1 M NaCl did not affect the size distribution seen at 500 mM (Figs. 5C and S4). Most importantly, at 100 mM NaCl (where the enzyme is the most active, Fig. 2B), the specific activity per enzyme monomer increased with enzyme concentration in a nonlinear fashion (Fig. 5D), which we suggest reflects association/dissociation of monomers/dimers, a possible allosteric regulatory mechanism. This is consistent with the observation that tested MTases in the β class are only active as multimers (usually homodimers) because the two subunits divide their labors in terms of SAM binding (for catalysis) and DNA recognition (for specificity) (87).
Figure 5.
The effects of ionic strength on protein stability and activity of N4CMT79–324.A, sedimentation coefficient distribution profiles in 500 mM NaCl at three different protein concentrations, 19.1 μM (blue), 6.4 μM (black), and 2.1 μM (red). B, sedimentation coefficient distribution profiles at two concentrations of NaCl, 500 mM (red) and 250 mM (green). C, effects of NaCl on oligomeric states, as assessed by DLS, indicated by an increased molecular radius. D, the change in specific activity per enzyme molecule as a function of enzyme concentration (N = 2). E, first derivative curve of thermal shift assay showing the effects of varying concentration of NaCl on protein melting temperature (Tm). The peak minimum of these curves corresponds to the protein Tm. DLS, dynamic light scattering; N4CMT, N4-cytosine methyltransferase.
On the other hand, we visually observed protein precipitation at low ionic strength (e.g., 100 mM NaCl), which is supported by the observation that the protein melting temperature (Tm) decreased to 38.8 °C, from 42.3 °C at 250 mM and 45.6 °C at 500 mM NaCl (Fig. 5E). Thus, ionic strength has opposing effects: high salt is needed for protein solubility/stability while low salt concentration is required for activity of N4CMT79–324. It is possible that, under the reaction conditions of 100 mM NaCl, not every enzyme molecule is active owing to partial aggregation or precipitation. For comparison, the effective concentration of K+ (the major cation) in Escherichia coli cytoplasm ranges from about 150 to 750 mM, depending on the growth environment (98).
Structure of N4CMT79–324 in complex with sinefungin
Given that known MTases in the β class are only active as multimers (87), we were interested in determining the quaternary structure of N4CMT79–324. We crystallized N4CMT79–324 in the presence of sinefungin (adenosyl ornithine), a SAM analog typically used as a pan inhibitor of SAM-dependent MTases (99) (Fig. 1C). N4CMT79–324 crystallized in space group C2221 and diffracted X-rays to 1.49 Å resolution (Fig. 6A). In addition, we used a construct comprising residues 61 to 324, which exhibited the same activity as that of N4CMT79–324 (Fig. 1B) and the same solution behavior of dependence of ionic strength. N4CMT61–324 crystallized in two space groups, C2 and P1, and diffracted X-rays to resolutions of 1.89 Å and 2.17 Å, respectively (Fig. 6, B and C). Despite crystallizing in three different space groups, N4CMT displayed the same dimerization interactions in all three cases, mediated either by a 2-fold crystallographic symmetry in C2221 and C2 (Fig. 6, A and B) or by a noncrystallographic symmetry involving two molecules within the P1 crystallographic unit (Fig. 6C).
Figure 6.
N4CMT exists as a dimer in crystals.A, N4CMT79–324 dimer is formed by the 2-fold symmetry in space group C2221. B, N4CMT61–324 dimer is formed by the 2-fold symmetry in space group C2. C, N4CMT61–324 dimer is formed by a noncrystallographic symmetry (NCS) in space group P1. The buried dimer surface is ∼1400 to 1800 Å2. D and E, a water channel (indicated by the two red dots on white background at the center of the molecule in panel D or a vertical yellow tube in panel E) runs through the center of dimer. F and G, six pairs of charge–charge interactions on either side of dimer interface. G and H, Phe206 of the neighboring molecule is inserted into a hydrophobic pocket which is formed by six hydrophobic/aromatic residues, forming a “mortise and tenon” joint, where Phe206 is the tenon. N4CMT, N4-cytosine methyltransferase.
Interface analysis of molecular assemblies (100, 101) identified the dimer interface to be about 1870 Å2 in the C2221 space group (Fig. 6A), including six pairs of salt bridges, 32 hydrogen bonds, and 290 nonbonded interactions (both solvent-mediated interactions and van der Waals contacts). Three features of this interface stand out. First, there is a water channel running through the middle of the interface (Fig. 6, D and E), generating many water-mediated interactions along the stream. Second, there are six pairs of charge–charge interactions, two reciprocal Arg172-Glu186 pairs lining one side of the interface (Fig. 6F), and two reciprocal Arg207-Glu236 and two reciprocal Arg244-Asp194 pairs lining the other side of the interface (Fig. 6G). As noted above, dimer formation is sensitive to the ionic strength, and ions can probably diffuse into the channel and modulate dimer dissociation. Third, in addition to the charge–polar interactions, Phe206—the residue N-terminal prior to ion-pairing Arg207—is inserted into a hydrophobic cage of the neighboring monomer and is completely enclosed by the six residues Ile191, Trp243, Ile264, Leu234, Phe229, and Trp215 (Fig. 6H). Analogous “mortise and tenon” joints have been seen in some other protein families (102). Significantly, the hydrophobic cage (mortise) into which the Phe (tenon) of the neighboring molecule inserted is conserved in at least six other dimeric class β MTases (see below).
Comparison to other structurally characterized dimeric MTases
The homodimeric subunit structures of class β MTases have been observed previously (reviewed in (87)), including M.PvuII of Proteus vulgaris (the only other structurally characterized N4mC MTase) (85), M.RsrI of Rhodobacter sphaeroides (103, 104), M1.MboII of Moraxella bovis (105), THA0409 of Thermus thermophilus HB8 (106), and M1.HpyAVI of Helicobacter pylori (107) (examples shown in Fig. 7). However, none of these class β MTases were structurally characterized in complex with DNA, and so the significance of the dimeric character was not grasped until the structural determinations of M.EcoP15I of E. coli (108) and CcrM of Caulobacter crescentus (86). Dimerization allows the enzyme to divide functional tasks between the two subunits. In the case of CcrM, each monomer contacts primarily a different DNA strand. Specifically, the MTase domain of one subunit binds the target strand, recognizes the target sequence, and catalyzes methyl transfer, while the C-terminal domain of the second subunit binds the nontarget strand forming a strand separation bubble in the dsDNA (86).
Figure 7.
Examples of dimeric class β MTases with “mortise and tenon” subunit interfaces involving Phe/Tyr insertion into the neighboring subunit’s hydrophobic cage.A, N4CMT, (B) CcrM, (C) M1.MboIIa, (D) M1.HpyAVI, (E) M.PvuII, (F) M.EcoP15I and (G) M.TthA0409. In each case, the top panel shows the dimer interface with two inserted residues highlighted by two red circles. The bottom panel illustrates the hydrophobic/aromatic residues (colored in green) forming the cage and the inserted tenon residue is in cyan. MT, methyltransferase; N4CMT, N4-cytosine methyltransferase.
Comparing the dimer interfaces, while each enzyme has its unique charged/polar intersubunit interactions, the Phe insertion into the neighboring subunit’s hydrophobic cage is also observed in CcrM and MboII (Fig. 7, B and C); the Phe “tenon” is conserved at the same sequence position in N4CMT and CcrM (see Fig. 8A). In HpyAVI, the inserted residue is Tyr122 (Fig. 7D). The corresponding aromatic residue is substituted by Val153 in PvuII, Ile237 in EcoP15I, and Ile163 in THA0409 (Fig. 7, E–G). As shown in Fig. S1, the equivalent of N4CMT tenon Phe206 is fully conserved among MTases showing overall relatedness to N4CMT.
Figure 8.
N4CMT interaction with sinefungin.A, sequence alignment of β MTases N4CMT and CcrM. As is characteristic of β MTases, catalytic motif IV is more amino-proximal than SAM-binding motif I. In CcrM, the C-terminal addition is for binding of the nontarget DNA strand, and the loops immediately after strand β2, motif IV (DPPY), strand β3, and strand β4 recognize the target DNA sequence. Labels above the sequences are as follows: s for sinefungin binding, c for the six pairs of charge–charge interactions in the dimer interface, and d for dimer hydrophobic cage insertion (“mortise and tenon” joint). The seven small circles indicate substitutions between the two isoenzymes of N4CMT, with red circles for nonconservative substitutions and black circles for conservative substitutions. The secondary structural elements of N4CMT are indicated above the sequence with arrows for β-strands (1–8) and cylinders for α-helices (A–I). Helices (B, D, and E) are on one side of the β-sheet, and the helices (G, H, and I) are on the opposite side. B, schematic diagram of N4CMT secondary structures, with strands (1–8) shown as blue arrows and helices (A–I) as rectangles. Residues binding sinefungin are circled, and residues for dimer interactions are in squares. The locations of sequence motifs (I–X) are indicated in red. C, the adenosyl moiety of sinefungin is visible from the surface. Two orientations of N4CMT-bound sinefungin superimposed with omit electron density contoured at 5σ above the mean. D, D88, D301, and H253 interact with the adenine and ribose rings. E, hydrophobic/aromatic residues enclose the adenine ring. F, aromatic residues encircle the ribose ring. G, polar interactions involve the terminal carboxylate group of the ornithine moiety. H, the positively charged ε amino group of sinefungin is oriented towards the water network surrounding the active-site SPPY. I, superimposition of N4CMT with DNA-bound CcrM. J, a model of the target cytosine bound in the active site of N4CMT. MT, methyltransferase; N4CMT, N4-cytosine methyltransferase.
N4CMT interactions with sinefungin
Focusing next on the monomer structures (Fig. 8B), we observed nearly every residue from 79 to 324 (with broken density at residues 220–225) of N4CMT79–324 in space group C2221. For N4CMT61–324, the first seven residues (61, 62, 63, 64, 65, 66, 67) were disordered, and residues 69 to 80 formed an additional helix, away from both the active site (where sinefungin binds) and the dimer interface (Fig. 6, B and C). In addition, two loops, residues 111 to 136 and residues 166 to 174, were disordered (lack of electron densities; dashed lines in Fig. 6, B and C). These two loops are located right next to each other and immediately after strands β2 and β3 (loop-2D and loop-3E, colored green in Fig. 8B). The corresponding loops in CcrM are responsible for recognizing specific DNA sequences (86). In addition, the distortedness of loop-3E (residues 166–174) also reduces the dimer interface surface area (Fig. 6, B and C).
Turning next to the complete and higher-resolution (1.49 Å) structure of N4CMT79–324, as expected, sinefungin binds in the SAM-binding site, and its adenosyl moiety is solvent-exposed on the surface (Fig. 8C). The three moieties of sinefungin form extensive hydrogen bonds (H-bonds) and hydrophobic–aromatic interactions with N4CMT. Two negatively charged aspartates, Asp88 and Asp301, form hydrogen bonds respectively with the adenosyl N6 amino group and the two hydroxyl oxygen atoms of the sinefungin ribose (Fig. 8D). The Asp301 interactions are consistent with expectations for a Class I MTase (109). In addition, His253 forms an H-bond with one of the ribose hydroxyl oxygen atoms. Besides the polar interactions, the adenine ring is surrounded by Ile302, Tyr130, Pro109, Trp142, and Phe280 (Fig. 8E), while the ribose ring is fenced in by Tyr306, Tyr127, and Pro109 (Fig. 8F). From the ornithine moiety, the terminal carboxylate group H-bonds with the side chains of Ser283 and Thr285 and the main chain amide nitrogen atom of Phe257 (Fig. 8G). The Arg313 guanidium group participates in and stabilizes the H-bond network. Sinefungin carries a formal positive charge on the ε amino group, in place of the transferrable methyl group of SAM, which points to a network of water molecules coordinated by the highly conserved (Fig. S1) active site S107PPY110 motif IV, along with Tyr127, Asp132, and Glu211 (Fig. 8H). The water molecule in the center (labeled as #1 in Fig. 8H) is coordinated tetrahedrally with four H-bonds to the Ser107 hydroxyl oxygen, the main chain carbonyl oxygen of Pro108, Nε of sinefungin, and a second water molecule (#2), which is further H-bonded to water #3. We note that nothing in these structural elements provides an obvious explanation for the low N4CMT SAM affinity.
Superimposing the ternary structure of DNA-sinefungin-CcrM onto the binary structure of sinefungin-N4CMT (1.9 Å RMSD between 211 pairs of Cα atoms) revealed nearly perfect fitting of the DPPY motif IV of CcrM to the SPPY motif IV of N4CMT, as well as of the two enzyme-bound sinefungin molecules (Fig. 8I). The target adenine of CcrM is positioned with its N6 amino group (the methyl acceptor during the methyl transfer) near water molecule #1 and has the equivalent H-bonds (like water #1 in N4CMT) with Asp31 and Pro32 of the DPPY motif and Nε of sinefungin. Substituting the adenine with a cytosine in silico resulted in a model implying that the cytosine ring stacks with Tyr110 of the N4CMT SPPY motif, while the polar edge of the cytosine (O2, N3, and N4) occupies (or is near to) the water positions #3 (O2), #2 (N3), and #1 (N4) (Fig. 8J). This arrangement positions the target N4 atom in line with the transferrable methyl group and sulfur atom of SAM (in the place of sinefungin), consistent with the SN2 reaction mechanism used by SAM-dependent MTases (109, 110).
Discussion
Here, we report characterization of the in vitro enzymatic and structural properties of the catalytic region of the DNA cytosine-N4 MTase from the bdelloid rotifer A. vaga, building on earlier work by others (35). N4CMT methylates CpG dinucleotides on both DNA strands, generating hemimethylated intermediates and eventually fully methylated CpG/CpG. In addition, N4CMT methylates CpA/TpG, though at a lower rate. These properties are reminiscent of mammalian de novo 5mC MTase DNMT3A, which, in addition to CpG, could also act on non-CpG dinucleotides (mainly CpA) (111). N4CMT (prefers YACG; Y = T or C) and DNMT3A [(T/c)(A/c)CG] are even similar in preferring a thymine (T) at the −2 flanking position of CpG (112). This symmetric methylation of CpG, yielding 4mC, suggests a similar postreplication maintenance mechanism to that for the CpG methylation of cytosine-C5 in mammals. However, we observed an order of magnitude lower catalytic efficiency by N4CMT79–324 on hemimethylated CpG, as compared to unmethylated CpG, in the context of the highly favored palindromic ACGT sequence. At disfavored and asymmetric sequences, such as ACGG, the low level methylation does remain hemimethylated.
Like the two mammalian de novo MTases DNMT3A and DNMT3B, there are two isoenzymes of N4CMT in A. vaga, and they differ in seven residues with three in the catalytic domain and four in the C-terminal domain (35) (Fig. 8A). While four of these differences are conservative substitutions (260Ile/Leu, 338Val/Ile, 397Ala/Val, and 434Ile/Leu), three are altered from polar and aromatic to polar (209Tyr/Asn), polar to hydrophobic (221Ser/Ala), and hydrophobic to charged residues (461Leu/Arg). In the current structures, lacking DNA, Tyr209 (two residues away from the tenon Phe206) is involved in dimer interaction, Ser221 after strand β5 is located in the putative DNA-binding surface, and Ile260 of helix αG is part of an intramolecular hydrophobic interior core (Fig. 8B). The differences between the two isoenzymes of N4CMT in their expression of life cycle and sequence preferences remain to be investigated.
Our observation that N4CMT (at least its catalytic domain) is highly similar to the C. crescentus cell cycle–regulated DNA MTase (CcrM) raises the question of whether the function and/or expression of N4CMT is cell cycle–regulated. The possible cell cycle regulation of N4CMT is linked to the question of whether DNA cytosine-N4 methylation is DNA synthesis–dependent, as 5mC methylation is in mammals (113, 114, 115, 116, 117). One major difference between N4CMT and CcrM involves the C-terminal domain, which is dispensable for N4CMT in vitro DNA methylation activity, whereas the deletion of the corresponding C-terminal domain in CcrM results in loss of enzymatic activity (118). Further studies will be needed to address these questions and to further understand the process of adaptation of epigenetic regulators imported into eukaryotes via horizontal gene transfer (119).
In A. vaga chromosomal DNA, 4mCpG is found preferentially at the ends of transposable elements (TEs) (35) and may thus play a TE-silencing role analogous to that of 5mC in mammals (120, 121, 122). The mechanism of such 4mC methylation-dependent TE silencing would be partially distinct from that involving 5mC, as 4mC deamination leads to rapidly removed uracil rather than the more highly mutagenic thymine-guanine (T:G) mismatch. In mammals, two additional proteins function in DNA methylation–mediated machinery: DNMT3L in de novo methylation in germ cells (123, 124) and UHRF1 in maintenance methylation (125, 126), with both of them linking DNA methylation to histone modifications. In somatic cells, the inactive DNMT3B isoforms can replace the function of DNMT3L and facilitate de novo methylation (127, 128). It remains to be seen whether functional homologs of DNMT3L (or DNMT3B3) and UHRF1 exist in A. vaga. A preliminary search reveals a candidate ortholog for human UHRF1 (accession # UJR31201), but it is fairly weak (33% query cover, 25% identity) and remains to be tested. Most importantly, there is a need to understand whether DNA methylation at cytosine-N4 modulates the fundamental processes of horizontal gene transfer, aging, DNA repair, spread of transposable elements, sexual recombination, and tolerance of extreme environments in bdelloid rotifers.
Experimental procedures
N4CMT constructs and protein purification
Bdelloid rotifer A. vaga N4CMT full-length cDNA (GenBank UJR33503.1) was codon optimized for E. coli expression and synthesized by GenScript. The fragments of N4CMT (Fig. S2A) were cloned into a modified pET-30a vector with a C-terminal His6 tag. Plasmids were transformed into E. coli strain BL21-Codon-Plus.
An overnight culture grown in MDAG medium (129) was inoculated into lysogeny broth at 1:1000 dilution fold and shaken at 37 °C until the A600nm reached 0.4∼0.6. At that point, the temperature was reduced to 16 °C. When A600nm reached 0.8∼1.0, protein expression was induced with 0.2 mM IPTG and incubated for another 18 h. Cells were harvested, resuspended, and lysed by sonification in lysis buffer [0.5 M NaCl, 20 mM imidazole, 20 mM Tris–HCl pH 8.0, 5% glycerol, 0.5 mM tris(2-carboxyethyl)phosphine (TCEP)], supplemented with 0.1 mM PMSF. The cell lysates were clarified by centrifugation at 45,000g for 1 h, the supernatants were applied to a 5-ml HisTrap HP column (GE Healthcare), and the target N4CMT protein fragment was eluted with a linear gradient of 20 to 500 mM imidazole and eluted at ∼150 mM imidazole.
Fractions containing the target protein were pooled, diluted three times with 20 mM Tris–HCl pH 8.0, 5% glycerol, 0.5 mM TCEP (resulting in ∼150 mM NaCl), and loaded onto a HiTrap SP column (GE Healthcare). The bound proteins were eluted with a linear gradient of 0.1 to 1 M NaCl and eluted at about 0.5 M NaCl. Fractions containing the target proteins were pooled and further purified by HiLoad Superdex 200 16/60 (GE Healthcare) in buffer containing 1 M NaCl, 20 mM Tris–HCl pH 7.0, 5% glycerol, and 0.5 mM TCEP. Fractions with the target protein were pooled, concentrated, flash-frozen in liquid nitrogen, and stored in −80 °C prior to use.
In vitro DNA methylation
The DNA methylation activity was measured using a Promega bioluminescence assay (MTase-Glo) (130) in which the generated SAH is converted to ATP in two steps, and the generated ATP is detected through a luciferase reaction. The concentration of SAH was calibrated based on the SAH standard in a linear regression plot of the luminescence signal of the Promega assay (see Fig. S3 of (131) or Fig. S1 of (48)).
Typically, the methylation reaction was conducted at room temperature in a total volume of 20 μl in the optimal reaction buffer (100 mM NaCl, 20 mM Tris–HCl pH 7.5, 1 mM DTT). In general, 2 × (N4CMT and SAM) was preincubated at room temperature (∼22 °C) for about 3 min and the reaction was started by adding the same volume of 2 × DNA substrate. Reactions were stopped by adding TFA to a final concentration of 0.1% v/v. A 5 μl aliquot of the terminated reaction mixture was transferred to a 384 low-volume microwell plate, and the luminescence signal was measured with a Synergy 4 multimode microplate reader (BioTek).
dsDNA oligonucleotide substrates
A 4 mM ssDNA oligo, containing the palindromic DNA sequence 5′-ATTTTACGTAAAT-3′, was self-annealed in 50 mM NaCl, 20 mM Tris–HCl pH 7.5 to form 2 mM dsDNA and was used for characterizing the optimal reaction conditions for N4CMT79–324 (Fig. 2).
Reactions were conducted with 0.5 μM N4CMT79–324, 40 μM SAM, and 10 μM DNA for varied reaction times (Fig. 2A), NaCl concentrations (Fig. 2B), and buffer pH values (Fig. 2C). Reactions lasted for 9 min for variations of NaCl and pH.
For varying DNA concentrations, reactions were performed with 40 μM SAM for 9 min (Fig. 2D) or 200 μM SAM for 1.5 min (Fig. 2F), with 0.5 μM N4CMT79–324 in the reaction buffer. For varying SAM concentrations, reactions were conducted in the reaction buffer with 0.5 μM N4CMT79–324 and 10 μM DNA for 1.5 min (Fig. 2E). For varying enzyme concentrations, reactions were carried out with 10 μM DNA and 200 μM SAM for 1.5 min in the reaction buffer (Fig. 5D).
Hairpin DNA substrates
A series of ssDNA (100 μM) containing a 5T loop (Fig. 3B) were self-annealed in 50 mM NaCl and 20 mM Tris–HCl pH 7.5. DNA methylation reactions were carried out in the reaction buffer with 0.5 μM N4CMT79–324, 200 μM SAM, and 10 μM DNA for 1.5 min (Fig. 3C). Under the near-single turnover condition with 2 μM N4CMT79–324, 2 μM DNA (or less), and 200 μM SAM, reactions were performed for varying times in the reaction buffer (Fig. 3, D–E).
PCR-amplified DNA substrates
A 990 bp DNA product containing the coding sequence of N4CMT79–395 was generated by PCR (Fig. S2C). Methylations of the 990 bp DNA substrate by N4CMT79–324 or CamA of Clostridioides difficile (89) were carried out in the reaction buffer using 2 μM enzyme, 200 μM SAM, and 115 nM or 150 nM PCR product (Fig 1, C and D). Reactions were stopped by adding TFA to a final concentration of 0.1% v/v, and methylation was calculated by measuring the generated product SAH using the Promega bioluminescence assay (MTase-Glo).
The overnight methylation product generated by N4CMT79––395 was further purified using the DNA Clean and Concentrator kit from ZYMO RESEARCH (Cat: D4013), prior to dot blotting assays. While some reduction in methylation level in overnight reactions may result from the instability of SAM (132), the Promega assay (MTase-Glo) we used in this study does not detect SAH-like breakdown products of SAM (Fig. S2G).
Methylation assay by dot blotting
Methylation assays were performed by dot blotting, following procedures that we previously described (48). Briefly, 15 ng DNA oligos or 10 ng of PCR product (with and without enzyme treatment) were serially diluted 2-fold in TE buffer, mixed with 1 M NaOH/25 mM EDTA, and denatured at 95 °C for 10 min. The samples were neutralized with 2 M ammonium acetate (pH 7.0, on ice) and loaded onto an Amersham Hybond-N+ membrane (RPN119B, GE Healthcare) using a Bio-Dot apparatus (#170-6545, Bio-Rad). The membrane was air-dried, cross-linked with UV for 1 min, blocked in Tris-buffered saline and 0.1% Tween 20 supplemented with 5% nonfat dry milk for 1 h at room temperature, and then used for antibody incubations.
The primary antibodies were anti-6mA (D9D9W) (Cell Signaling Technology; #56593; 1:1000), anti-5mC (Millipore Sigma; MABE1081; 1:1000), and anti-4mC (New England Biolabs; BL_382; 1:500). The anti-4mC antibody was a gift from Drs Richard J. Roberts and Iain Murray (133). The secondary antibodies were horseradish peroxidase (HRP)-conjugated anti-rabbit IgG antibody (Cell Signaling Technology; #7074; 1:5000) and donkey anti-mouse IgG H&L (Abcam; ab205724; 1:5000). The signals were detected with Clarity Western ECL substrate (Bio-Rad Laboratories, #1705061) and imaged using a ChemiDoc imaging system (Bio-Rad Laboratories). For positive controls of 6mA and 5mC oligonucleotides, we extended the length of DNA oligos using T4 Polynucleotide Kinase (New England Biolabs; M0201) and T4 DNA ligase (New England Biolabs; M0202).
NAME-seq workflow and data analysis
NAME-seq was performed following the protocol (90). Briefly, 600 ng of N4CMT methylated and unmethylated PCR product (two replicates were performed for each condition) were denatured by incubating at 95 °C for 5 min and on ice for 5 min. The denatured DNA was treated with 1 M sodium nitrite (NaNO2) and 2.3% acetic acid (AcOH) at 37 °C for 2 h. Nitrite-treated DNA was purified using Oligo Clean & Concentrator Kits (Zymo Research, D4060). To the nitrite-treated DNA solution, we added 1.25 μl 10 mM dNTPs (NEB, N0447S), 4 μl 10 μM Biotin-R2-N9 primer (5′-[biotin]C AGA CGT GTG CTC TTC CGA TCT NNN NNN NNN-3′), 5 μl 10X DNA polymerase buffer according to manufacturer's instructions and adjusted the final volume to 50 μl with water (Sigma-Aldrich, W4502). The mixed solution without DNA polymerase was incubated at 94 °C for 5 min and 4 °C for 5 min. The program was then paused and 1.5 μl 5 U/μl KF-exo- (NEB, M0212L) was added to the solution. The solution was mixed and incubated at 4 °C for 15 min, raised to 37 °C at a ramp rate of 0.1 °C/s, and incubated at 37 °C for 90 min to finish the first strand synthesis. Next, the enzyme activity was killed by incubating the tube at 70 °C for 10 min.
DNA after the first random priming extension was purified twice with 1.0X Ampure XP beads (Beckman, A63881) according to the manufacturer's instructions and eluted with 50 μl of 10 mM Tris–HCl, pH 7.5 to remove the unextended biotin primers. An aliquot (5 μl) of Dynabeads MyOne Streptavidin C1 beads (Invitrogen, 65001) were washed and eluted in 50 μl 2× B&W buffer (10 mM Tris–HCl pH 7.5, 1 mM EDTA, and 2 M NaCl) and combined with 50 μl eluted DNA. The beads–DNA mixture was incubated at room temperature with constant rotation for 30 min. The DNA-bound beads were collected and washed with the following buffers: once with 2× B&W buffer, twice with fresh prepared 0.1 N NaOH (incubated for 2 min during each wash), once with 2X B&W buffer, and once with 10 mM Tris–HCl, pH 7.5.
The second random priming extension was performed on streptavidin beads by the following steps: the washed beads were suspended in 50 μl random priming buffer (1.25 μl 10 mM dNTPs (NEB, N0447S), 4 μl 10 μM R1-N9 primer (5′- C TAC ACG ACG CTC TTC CGA TCT NNN NNN NNN-3′), 5 μl 10X DNA polymerase buffer, and the final volume was adjusted to 50 μl with water). The mixed solution, without DNA polymerase, was incubated at 94 °C for 5 min and 4 °C for 5 min. The program was then paused and 1.5 μl of 5 U/μl KF-exo- (NEB, M0212L) was added to the solution. The solution was mixed and incubated at 4 °C for 15 min, raised to 37 °C at a ramp rate of 0.1 °C/s, and incubated at 37 °C for 30 min. The enzyme activity was then killed by incubating the tube at 70 °C for 10 min. Beads were collected and eluted in 50 μl Bst-3.0 DNA polymerase mixture (5 μl Isothermal Amplification Buffer II, 3 μl 100 mM MgSO4, 1.25 μl dNTP, 1 μl 8 U/μl Bst-3.0 DNA polymerase (NEB, M0374S), and 39.75 μl water) and incubated at 65 °C for 30 min to complete the second strand synthesis.
Beads were next collected and resuspended using 50 μl Phusion elution buffer (10 μl 5× Phusion HF buffer, 1.25 μl dNTP, 4 μl 10 μM R2-22nt primer (5′-CAG ACG TGT GCT CTT CCG ATC T-3′), 1 μl 2 U/μl Phusion High-Fidelity DNA Polymerase (NEB, M0530S), and 33.75 μl water). The solution was incubated at 94 °C for 5 min, 55 °C for 10 min, and 72 °C for 30 min to elute dsDNA from streptavidin beads. The supernatant was transferred to a new tube and treated with 1 μl Exonuclease I (NEB, M0293S) at 37 °C for 15 min and 70 °C for 10 min to remove excessive R2-22nt primer. Exonuclease I–treated DNA was then purified using 1.0X Ampure XP beads to remove the short fragments. The final indexed libraries were constructed using NEBNext Multiplex Oligos for Illumina (NEB, E7335S/E7500S/E7710S/E7730S) and Q5 High-Fidelity 2X Master Mix (NEB, M0492S) (cycle number was determined by qPCR with PowerUP SYBR Green Master Mix using R1-22nt (5′-CTA CAC GAC GCT CTT CCG ATC T-3′) and R2-22nt primers (Applied Biosystems, A25742)). Samples were sequenced using an Illumina NextSeq 500.
NAME-seq primary analysis was performed using the analysis workflow from the original report (https://github.com/TaoLabBCM/NAME-seq). Cytosine sites with fewer than 100× coverage were filtered out. Methylation percentage and Z score of C-to-T ratio was calculated using a custom Python script. Differential enrichment of 4mC methylation motifs in the PCR product (four bases upstream and downstream of identified 4mCpG sites were used to generate input FASTA, four bases upstream and downstream of all CpN sites were used to generate control FASTA) was determined using MEME-suite (v5.4.1) (https://meme-suite.org/meme/) (134).
Dynamic light scattering
N4CMT79–324 and N4CMT61–395 proteins were prepared in buffers containing 10 mM Tris–HCl pH 7.5, 0.5 mM TCEP, with different NaCl concentrations (Figs. 5C and S4). A 20 μl portion of the 10 μM N4CMT79–324 preparation was transferred to a 384-well plate, centrifuged at 3000g for 5 min, and loaded for dynamic light scattering analysis using a DynaPro PlateReader-II (Wyatt Technology). Experiments were conducted at 25 °C, and each sample was set to acquire data for 10 s. The generated data were analyzed and plotted using DYNAMICS 7.1.9 software (https://www.wyatt.com/products/software/dynamics.html).
SV-AUC experiment
Purified N4CMT79–324 was injected onto an analytical size-exclusion chromatography column (S200 10/300 Gl) pre-equilibrated with 10 mM Tris pH 7.5, 0.5 M NaCl, and 0.5 mM TCEP. The peak fractions of the chromatogram were used to prepare three different concentrations of N4CMT79–324 (with A280 = 0.9, 0.3, and 0.1 corresponding to ∼19.1, 6.4, and 2 μM, respectively). SV-AUC experiments were performed using a Beckman-Coulter XL-A equipped with an An-60 Ti four hole-rotor, housed in the Protein and Monoclonal Antibody Production Core, Baylor College of Medicine. The rotor was precooled and equilibrated at 4 °C in the vacuum chamber for ∼3 h. The absorbance scans were monitored at 280 nm at 1 min intervals, at 40,000 rpm at 4 °C. The scans were analyzed using the c(s) distribution analysis module in the program SEDFIT (135). The values of partial specific volume of protein, solvent density, and viscosity were calculated using Sednterp (Alliance Protein Laboratories). A second SV-AUC run was carried out using the same experimental conditions except at a lower protein concentration (1 μM) and the reference buffer having 0.25 M NaCl. All data were plotted using Origin 6.0 software (https://www.originlab.com).
Thermal shift assay
Thermal shift assays were performed using a CFX Opus 96 Real Time PCR system (#12011319) and Invitrogen SYPRO Orange Protein Gel Stain, SO (catalog number S6651). N4CMT79–324 at 1 μM was prepared in three different buffers (all with 10 mM Tris pH 7.5, 0.5 mM TCEP), varying in NaCl concentration (0.1 M, 0.25 M, and 0.5 M). Each protein sample was mixed with SO (5×: final assay concentration in each well), and the mixture was heated from 20 to 95 °C with increments of 0.5 °C per min, to generate the protein thermal melting curves. The negative controls (buffer + SO) were included in the same experiments. The data were acquired and exported using CFX Maestro software (https://www.bio-rad.com/en-us/product/cfx-maestro-software-for-cfx-real-time-pcr-instruments), and derivative plots were plotted using Graphpad Prism 9.0 (https://www.graphpad.com/features).
X-ray crystallography
Crystallization screens were carried out at about 20 °C for N4CMT61–324 (pXC2317) or N4CMT79–324 (pXC2309) using crystallization screening kits (Hampton research). N4CMT protein at 169.5 μM (5 mg/ml) was preincubated with 600 μM sinefungin (above the KM value for SAM) on ice in 500 mM NaCl, 20 mM Tris–HCl pH 7.5, 0.5 mM TCEP for 30 min. An Art Robbins Gryphon Crystallization Robot was used to set up a 0.4-μl sitting-drops (0.2 μl protein solution + 0.2 μl well solution) over 70 μl crystallization solution. The starting NaCl concentration in the crystallization mixture was 250 mM.
Crystals for N4CMT79–324–sinefungin complex were grown from 0.2 M potassium citrate tribasic monohydrate, 20% PEG 3350. The N4CMT61–324–sinefungin complexes in P1 space group were crystallized in 0.2 M sodium thiocyanate, 20% PEG 3350 and in C2 space group in 0.2 M sodium chloride, 0.1 M Bis–Tris pH 6.5, 25% PEG 3350. All crystals were soaked in cryoprotectants made from the mother liquors supplemented with 20% (v/v) ethylene glycol and flash frozen in liquid nitrogen.
Diffraction data were collected at the Southeast Regional Collaborative Access Team (SER-CAT) beamline 22ID of the Advanced Photon Source at Argonne National Laboratory and the AMX beamline 17-ID-1 of National Synchrotron Light Source II at Brookhaven National Laboratory (136) (Table S1). At SER-CAT, crystallographic datasets were processed with HKL2000 (137) and at 17-ID-1, with DIALS (138) and AIMLESS (139). The N4CMT–sinefungin complex crystal structures were solved by molecular replacement using a search model of CcrM (PDB: 6PBD) (86) or an Alphafold predicted model (140) from the COLAB server (https://colab.research.google.com/github/phenix-project/Colabs/blob/main/alphafold2/AlphaFold2.ipynb). Structure quality was analyzed during PHENIX refinements (141), together with manual inspection and manipulation using COOT (142). Final structure models were validated by the PDB validation server (143). Structure images were prepared by PyMol (Schrödinger, LLC).
COOT was utilized to superimpose the structure of N4CMT79–324–sinefungin onto the ternary structure of the CcrM–sinefungin–DNA (PDB: 6PBD). First, the Secondary-Structure Matching (SSM) and then Least-squares (LSQ) options in COOT were primarily used for the superimposition. Second, further small rotations and translations were made such that the adenosine moiety of sinefungin in CcrM exactly overlaid with that moiety in the N4CMT structure. Third, the flipped adenine was substituted with cytosine and slight manual rotational and translational adjustments of the cytosine were made in COOT before exporting to PyMol.
The PISA server (http://www.ebi.ac.uk/pdbe/prot_int/pistart.html) was used to identify symmetry-related molecules as a biological dimer assembly and to calculate the areas of dimer interfaces. PISA was utilized to write out the PDB of the recognized dimer as input to PDBsum server (http://www.ebi.ac.uk/thornton-srv/databases/pdbsum/) for specific interacting residues at the interface. The water channel volume in N4CMT79–324 dimer in C2221 space group was calculated by using the average radius and length of the channel using values from the Caver Web server (https://loschmidt.chemi.muni.cz/caverweb).
Data availability
The experimental data that support the findings of this study are contained within the article. The authors have deposited the X-ray structure (coordinates) and the source data (structure factor file) of N4CMT-sinefungin to the PDB, and these will be released upon article publication under accession numbers PDB 8S9M (C2221), PDB 8S9N (C2), and PDB 8S9O (P1).
Supporting information
This article contains supporting information.
Conflict of interest
The authors declare that they have no conflicts of interests with the contents of this article.
Acknowledgments
We thank Yu Cao of MDACC for technical assistance. We thank Richard J. Roberts and Iain Murray of New England Biolabs for the gift of the anti-4mC antibody. We thank Xiangpeng Kong for assistance of access to 17-ID-1 beamtime. We thank the beamline scientists of Southeast Regional Collaborative Access Team (SER-CAT) at the Advanced Photon Source (APS), Argonne National Laboratory and 17-ID-1 of the National Synchrotron Light Source II, Brookhaven National Laboratory. The use of SER-CAT is supported by its member institutions and equipment grants (S10_RR25528, S10_RR028976, and S10_OD027000) from the US National Institutes of Health. Use of the APS was supported by the U.S. Department of Energy (DOE), Office of Science, Office of Basic Energy Sciences, under contract W-31-109-Eng-38. This research also used resources 17-ID-1 of the National Synchrotron Light Source II, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract No. DE-SC0012704. The use of protein and monoclonal antibody production core at Baylor College of Medicine is supported by P30 Cancer Center Support Grant NCI-CA125123.
Author contributions
J. Z., J. R. H., G. K., Q. C., X. L., F. M., T. W., and R. M. B. investigation; R. M. B. and X. C. writing–review and editing; X. Z. supervision; X. Z. and X. C. conceptualization; X. Z. project administration; X. C. funding acquisition; X. C. writing–original draft; X. C. methodology.
Funding and additional information
The work was supported by US National Institutes of Health grant R35GM134744 (to X. C.), Cancer Prevention and Research Institute of Texas grant RR160029 (to X. C., who is a CPRIT Scholar in Cancer Research), and the Cockrell Foundation Fellowship (to J. Z.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Biography

Dr Jujun Zhou is a postdoctoral scientist at the University of Texas MD Anderson Cancer Center. He studies enzyme kinetics, molecular mechanism, and small molecular inhibition of DNA methyltransferases from bacteria to human. While there is considerable knowledge about 5-methylcytosine in DNA, little is known about N4-methylcytosine (4mC) in eukaryotes. Here, he characterized the biochemical and structural features of the first discovered metazoan DNA methyltransferase that generates 4mC from Bdelloid rotifers.
Reviewed by members of the JBC Editorial Board. Edited by Karin Musier-Forsyth
Contributor Information
Robert M. Blumenthal, Email: Robert.Blumenthal@utoledo.edu.
Xing Zhang, Email: XZhang21@mdanderson.org.
Xiaodong Cheng, Email: XCheng5@mdanderson.org.
Supporting information
References
- 1.Roberts R.J. How restriction enzymes became the workhorses of molecular biology. Proc. Natl. Acad. Sci. U. S. A. 2005;102:5905–5908. doi: 10.1073/pnas.0500923102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anton B.P., Roberts R.J. Beyond restriction modification: epigenomic roles of DNA methylation in prokaryotes. Annu. Rev. Microbiol. 2021;75:129–149. doi: 10.1146/annurev-micro-040521-035040. [DOI] [PubMed] [Google Scholar]
- 3.Wyatt G.R. Recognition and estimation of 5-methylcytosine in nucleic acids. Biochem. J. 1951;48:581–584. doi: 10.1042/bj0480581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srinivasan P.R. Kinetics of incorporation of 5-methylcytosine in HeLa cells. Biochim. Biophys. Acta. 1962;55:553–556. doi: 10.1016/0006-3002(62)90992-7. [DOI] [PubMed] [Google Scholar]
- 5.Bestor T.H. The DNA methyltransferases of mammals. Hum. Mol. Genet. 2000;9:2395–2402. doi: 10.1093/hmg/9.16.2395. [DOI] [PubMed] [Google Scholar]
- 6.Goll M.G., Bestor T.H. Eukaryotic cytosine methyltransferases. Annu. Rev. Biochem. 2005;74:481–514. doi: 10.1146/annurev.biochem.74.010904.153721. [DOI] [PubMed] [Google Scholar]
- 7.Jones P.A., Baylin S.B. The epigenomics of cancer. Cell. 2007;128:683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones P.A., Ohtani H., Chakravarthy A., De Carvalho D.D. Epigenetic therapy in immune-oncology. Nat. Rev. Cancer. 2019;19:151–161. doi: 10.1038/s41568-019-0109-9. [DOI] [PubMed] [Google Scholar]
- 9.Tahiliani M., Koh K.P., Shen Y., Pastor W.A., Bandukwala H., Brudno Y., et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito S., D'Alessio A.C., Taranova O.V., Hong K., Sowers L.C., Zhang Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature. 2010;466:1129–1133. doi: 10.1038/nature09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He Y.F., Li B.Z., Li Z., Liu P., Wang Y., Tang Q., et al. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science. 2011;333:1303–1307. doi: 10.1126/science.1210944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito S., Shen L., Dai Q., Wu S.C., Collins L.B., Swenberg J.A., et al. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science. 2011;333:1300–1303. doi: 10.1126/science.1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hashimoto H., Olanrewaju Y.O., Zheng Y., Wilson G.G., Zhang X., Cheng X. Wilms tumor protein recognizes 5-carboxylcytosine within a specific DNA sequence. Genes Dev. 2014;28:2304–2313. doi: 10.1101/gad.250746.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L., Zhou Y., Xu L., Xiao R., Lu X., Chen L., et al. Molecular basis for 5-carboxycytosine recognition by RNA polymerase II elongation complex. Nature. 2015;523:621–625. doi: 10.1038/nature14482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin S.G., Zhang Z.M., Dunwell T.L., Harter M.R., Wu X., Johnson J., et al. Tet3 reads 5-carboxylcytosine through its CXXC domain and is a potential guardian against neurodegeneration. Cell Rep. 2016;14:493–505. doi: 10.1016/j.celrep.2015.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang D., Hashimoto H., Zhang X., Barwick B.G., Lonial S., Boise L.H., et al. MAX is an epigenetic sensor of 5-carboxylcytosine and is altered in multiple myeloma. Nucleic Acids Res. 2017;45:2396–2407. doi: 10.1093/nar/gkw1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren R., Horton J.R., Zhang X., Blumenthal R.M., Cheng X. Detecting and interpreting DNA methylation marks. Curr. Opin. Struct. Biol. 2018;53:88–99. doi: 10.1016/j.sbi.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang J., Horton J.R., Li J., Huang Y., Zhang X., Blumenthal R.M., et al. Structural basis for preferential binding of human TCF4 to DNA containing 5-carboxylcytosine. Nucleic Acids Res. 2019;47:8375–8387. doi: 10.1093/nar/gkz381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howard M.J., Foley K.G., Shock D.D., Batra V.K., Wilson S.H. Molecular basis for the faithful replication of 5-methylcytosine and its oxidized forms by DNA polymerase beta. J. Biol. Chem. 2019;294:7194–7201. doi: 10.1074/jbc.RA118.006809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang J., Zhang X., Blumenthal R.M., Cheng X. Detection of DNA modifications by sequence-specific transcription factors. J. Mol. Biol. 2020;432:1661–1673. doi: 10.1016/j.jmb.2019.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bestor T., Laudano A., Mattaliano R., Ingram V. Cloning and sequencing of a cDNA encoding DNA methyltransferase of mouse cells. The carboxyl-terminal domain of the mammalian enzymes is related to bacterial restriction methyltransferases. J. Mol. Biol. 1988;203:971–983. doi: 10.1016/0022-2836(88)90122-2. [DOI] [PubMed] [Google Scholar]
- 22.Lei H., Oh S.P., Okano M., Juttermann R., Goss K.A., Jaenisch R., et al. De novo DNA cytosine methyltransferase activities in mouse embryonic stem cells. Development. 1996;122:3195–3205. doi: 10.1242/dev.122.10.3195. [DOI] [PubMed] [Google Scholar]
- 23.Okano M., Xie S., Li E. Cloning and characterization of a family of novel mammalian DNA (cytosine-5) methyltransferases. Nat. Genet. 1998;19:219–220. doi: 10.1038/890. [DOI] [PubMed] [Google Scholar]
- 24.Okano M., Bell D.W., Haber D.A., Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99:247–257. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- 25.Barau J., Teissandier A., Zamudio N., Roy S., Nalesso V., Herault Y., et al. The DNA methyltransferase DNMT3C protects male germ cells from transposon activity. Science. 2016;354:909–912. doi: 10.1126/science.aah5143. [DOI] [PubMed] [Google Scholar]
- 26.Jain D., Meydan C., Lange J., Claeys Bouuaert C., Lailler N., Mason C.E., et al. Rahu is a mutant allele of Dnmt3c, encoding a DNA methyltransferase homolog required for meiosis and transposon repression in the mouse male germline. PLoS Genet. 2017;13 doi: 10.1371/journal.pgen.1006964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dura M., Teissandier A., Armand M., Barau J., Lapoujade C., Fouchet P., et al. DNMT3A-dependent DNA methylation is required for spermatogonial stem cells to commit to spermatogenesis. Nat. Genet. 2022;54:469–480. doi: 10.1038/s41588-022-01040-z. [DOI] [PubMed] [Google Scholar]
- 28.Posfai J., Bhagwat A.S., Roberts R.J. Sequence motifs specific for cytosine methyltransferases. Gene. 1988;74:261–265. doi: 10.1016/0378-1119(88)90299-5. [DOI] [PubMed] [Google Scholar]
- 29.Posfai J., Bhagwat A.S., Posfai G., Roberts R.J. Predictive motifs derived from cytosine methyltransferases. Nucleic Acids Res. 1989;17:2421–2435. doi: 10.1093/nar/17.7.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okano M., Xie S., Li E. Dnmt2 is not required for de novo and maintenance methylation of viral DNA in embryonic stem cells. Nucleic Acids Res. 1998;26:2536–2540. doi: 10.1093/nar/26.11.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vilain A., Apiou F., Dutrillaux B., Malfoy B. Assignment of candidate DNA methyltransferase gene (DNMT2) to human chromosome band 10p15.1 by in situ hybridization. Cytogenet. Cell Genet. 1998;82:120. doi: 10.1159/000015083. [DOI] [PubMed] [Google Scholar]
- 32.Yoder J.A., Bestor T.H. A candidate mammalian DNA methyltransferase related to pmt1p of fission yeast. Hum. Mol. Genet. 1998;7:279–284. doi: 10.1093/hmg/7.2.279. [DOI] [PubMed] [Google Scholar]
- 33.Dong A., Yoder J.A., Zhang X., Zhou L., Bestor T.H., Cheng X. Structure of human DNMT2, an enigmatic DNA methyltransferase homolog that displays denaturant-resistant binding to DNA. Nucleic Acids Res. 2001;29:439–448. doi: 10.1093/nar/29.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goll M.G., Kirpekar F., Maggert K.A., Yoder J.A., Hsieh C.L., Zhang X., et al. Methylation of tRNAAsp by the DNA methyltransferase homolog Dnmt2. Science. 2006;311:395–398. doi: 10.1126/science.1120976. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez F., Yushenova I.A., DiCorpo D., Arkhipova I.R. Bacterial N4-methylcytosine as an epigenetic mark in eukaryotic DNA. Nat. Commun. 2022;13:1072. doi: 10.1038/s41467-022-28471-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Achwal C.W., Iyer C.A., Chandra H.S. Immunochemical evidence for the presence of 5mC, 6mA and 7mG in human, Drosophila and mealybug DNA. FEBS Lett. 1983;158:353–358. doi: 10.1016/0014-5793(83)80612-7. [DOI] [PubMed] [Google Scholar]
- 37.Wu T.P., Wang T., Seetin M.G., Lai Y., Zhu S., Lin K., et al. DNA methylation on N(6)-adenine in mammalian embryonic stem cells. Nature. 2016;532:329–333. doi: 10.1038/nature17640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schiffers S., Ebert C., Rahimoff R., Kosmatchev O., Steinbacher J., Bohne A.V., et al. Quantitative LC-MS provides No evidence for m(6) dA or m(4) dC in the genome of mouse embryonic stem cells and tissues. Angew. Chem. Int. Ed. Engl. 2017;56:11268–11271. doi: 10.1002/anie.201700424. [DOI] [PubMed] [Google Scholar]
- 39.Douvlataniotis K., Bensberg M., Lentini A., Gylemo B., Nestor C.E. No evidence for DNA N (6)-methyladenine in mammals. Sci. Adv. 2020;6 doi: 10.1126/sciadv.aay3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Musheev M.U., Baumgartner A., Krebs L., Niehrs C. The origin of genomic N(6)-methyl-deoxyadenosine in mammalian cells. Nat. Chem. Biol. 2020;16:630–634. doi: 10.1038/s41589-020-0504-2. [DOI] [PubMed] [Google Scholar]
- 41.Bochtler M., Fernandes H. DNA adenine methylation in eukaryotes: enzymatic mark or a form of DNA damage? Bioessays. 2021;43 doi: 10.1002/bies.202000243. [DOI] [PubMed] [Google Scholar]
- 42.Kong Y., Cao L., Deikus G., Fan Y., Mead E.A., Lai W., et al. Critical assessment of DNA adenine methylation in eukaryotes using quantitative deconvolution. Science. 2022;375:515–522. doi: 10.1126/science.abe7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woodcock C.B., Yu D., Hajian T., Li J., Huang Y., Dai N., et al. Human MettL3-MettL14 complex is a sequence-specific DNA adenine methyltransferase active on single-strand and unpaired DNA in vitro. Cell Discov. 2019;5:63. doi: 10.1038/s41421-019-0136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kweon S.M., Chen Y., Moon E., Kvederaviciute K., Klimasauskas S., Feldman D.E. An adversarial DNA N(6)-methyladenine-sensor network preserves polycomb silencing. Mol. Cell. 2019;74:1138–1147. doi: 10.1016/j.molcel.2019.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hao Z., Wu T., Cui X., Zhu P., Tan C., Dou X., et al. N(6)-Deoxyadenosine methylation in mammalian mitochondrial DNA. Mol. Cell. 2020;78:382–395. doi: 10.1016/j.molcel.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu D., Horton J.R., Yang J., Hajian T., Vedadi M., Sagum C.A., et al. Human MettL3-MettL14 RNA adenine methyltransferase complex is active on double-stranded DNA containing lesions. Nucleic Acids Res. 2021;49:11629–11642. doi: 10.1093/nar/gkab460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qi S., Mota J., Chan S.H., Villarreal J., Dai N., Arya S., et al. RNA binding to human METTL3-METTL14 restricts N(6)-deoxyadenosine methylation of DNA in vitro. Elife. 2022;11 doi: 10.7554/eLife.67150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu D., Zhou J., Chen Q., Wu T., Blumenthal R.M., Zhang X., et al. Enzymatic characterization of in vitro activity of RNA methyltransferase PCIF1 on DNA. Biochemistry. 2022;61:1005–1013. doi: 10.1021/acs.biochem.2c00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hsu K.W., Lai J.C., Chang J.S., Peng P.H., Huang C.H., Lee D.Y., et al. METTL4-mediated nuclear N6-deoxyadenosine methylation promotes metastasis through activating multiple metastasis-inducing targets. Genome Biol. 2022;23:249. doi: 10.1186/s13059-022-02819-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woodcock C.B., Horton J.R., Zhou J., Bedford M.T., Blumenthal R.M., Zhang X., et al. Biochemical and structural basis for YTH domain of human YTHDC1 binding to methylated adenine in DNA. Nucleic Acids Res. 2020;48:10329–10341. doi: 10.1093/nar/gkaa604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jia G., Fu Y., Zhao X., Dai Q., Zheng G., Yang Y., et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 2011;7:885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zou S., Toh J.D., Wong K.H., Gao Y.G., Hong W., Woon E.C. N(6)-Methyladenosine: a conformational marker that regulates the substrate specificity of human demethylases FTO and ALKBH5. Sci. Rep. 2016;6 doi: 10.1038/srep25677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang X., Wei L.H., Wang Y., Xiao Y., Liu J., Zhang W., et al. Structural insights into FTO's catalytic mechanism for the demethylation of multiple RNA substrates. Proc. Natl. Acad. Sci. U. S. A. 2019;116:2919–2924. doi: 10.1073/pnas.1820574116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Z., Zhao S., Nelakanti R.V., Lin K., Wu T.P., Alderman M.H., et al. N6-methyladenine in DNA antagonizes SATB1 in early development. Nature. 2020;583:625–630. doi: 10.1038/s41586-020-2500-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tian L.F., Liu Y.P., Chen L., Tang Q., Wu W., Sun W., et al. Structural basis of nucleic acid recognition and 6mA demethylation by human ALKBH1. Cell Res. 2020;30:272–275. doi: 10.1038/s41422-019-0233-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang M., Yang S., Nelakanti R., Zhao W., Liu G., Li Z., et al. Mammalian ALKBH1 serves as an N(6)-mA demethylase of unpairing DNA. Cell Res. 2020;30:197–210. doi: 10.1038/s41422-019-0237-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beh L.Y., Debelouchina G.T., Clay D.M., Thompson R.E., Lindblad K.A., Hutton E.R., et al. Identification of a DNA N6-adenine methyltransferase complex and its impact on chromatin Organization. Cell. 2019;177:1781–1796. doi: 10.1016/j.cell.2019.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Broche J., Kohler A.R., Kuhnel F., Osteresch B., Chandrasekaran T.T., Adam S., et al. Genome-wide deposition of 6-methyladenine in human DNA reduces the viability of HEK293 cells and directly influences gene expression. Commun. Biol. 2023;6:138. doi: 10.1038/s42003-023-04466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Butkus V., Klimasauskas S., Petrauskiene L., Maneliene Z., Janulaitis A., Minchenkova L.E., et al. Synthesis and physical characterization of DNA fragments containing N4-methylcytosine and 5-methylcytosine. Nucleic Acids Res. 1987;15:8467–8478. doi: 10.1093/nar/15.20.8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamamoto J., Oyama T., Kunishi T., Masutani C., Hanaoka F., Iwai S. A cyclobutane thymine-N4-methylcytosine dimer is resistant to hydrolysis but strongly blocks DNA synthesis. Nucleic Acids Res. 2014;42:2075–2084. doi: 10.1093/nar/gkt1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walker J., Zhang J., Liu Y., Vickers M., Dolan L., Nakajima K., et al. Extensive N4 cytosine methylation is essential for Marchantia sperm function. bioRxiv. 2021 doi: 10.1101/2021.02.12.428880. [preprint] [DOI] [Google Scholar]
- 62.Ye P., Luan Y., Chen K., Liu Y., Xiao C., Xie Z. MethSMRT: an integrative database for DNA N6-methyladenine and N4-methylcytosine generated by single-molecular real-time sequencing. Nucleic Acids Res. 2017;45:D85–D89. doi: 10.1093/nar/gkw950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Usai G., Vangelisti A., Simoni S., Giordani T., Natali L., Cavallini A., et al. DNA modification patterns within the transposable elements of the fig (Ficus carica L.) genome. Plants (Basel) 2021;10:451. doi: 10.3390/plants10030451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu L., Zhang Y., Xue L., Liu F., Chen Q., Luo J., et al. Systematic analysis and accurate identification of DNA N4-methylcytosine sites by deep learning. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.843425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zheng P., Zhang G., Liu Y., Huang G. MultiScale-CNN-4mCPred: a multi-scale CNN and adaptive embedding-based method for mouse genome DNA N4-methylcytosine prediction. BMC Bioinformatics. 2023;24:21. doi: 10.1186/s12859-023-05135-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blus B.J., Wiggins K., Khorasanizadeh S. Epigenetic virtues of chromodomains. Crit. Rev. Biochem. Mol. Biol. 2011;46:507–526. doi: 10.3109/10409238.2011.619164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Terwagne M., Nicolas E., Hespeels B., Herter L., Virgo J., Demazy C., et al. DNA repair during nonreductional meiosis in the asexual rotifer Adineta vaga. Sci. Adv. 2022;8 doi: 10.1126/sciadv.adc8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Flot J.F., Hespeels B., Li X., Noel B., Arkhipova I., Danchin E.G., et al. Genomic evidence for ameiotic evolution in the bdelloid rotifer Adineta vaga. Nature. 2013;500:453–457. doi: 10.1038/nature12326. [DOI] [PubMed] [Google Scholar]
- 69.Laine V.N., Sackton T.B., Meselson M. Genomic signature of sexual reproduction in the bdelloid rotifer Macrotrachella quadricornifera. Genetics. 2022;220 doi: 10.1093/genetics/iyab221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vakhrusheva O.A., Mnatsakanova E.A., Galimov Y.R., Neretina T.V., Gerasimov E.S., Naumenko S.A., et al. Genomic signatures of recombination in a natural population of the bdelloid rotifer Adineta vaga. Nat. Commun. 2020;11:6421. doi: 10.1038/s41467-020-19614-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lapinski J., Tunnacliffe A. Anhydrobiosis without trehalose in bdelloid rotifers. FEBS Lett. 2003;553:387–390. doi: 10.1016/s0014-5793(03)01062-7. [DOI] [PubMed] [Google Scholar]
- 72.Shmakova L., Malavin S., Iakovenko N., Vishnivetskaya T., Shain D., Plewka M., et al. A living bdelloid rotifer from 24,000-year-old Arctic permafrost. Curr. Biol. 2021;31:R712–R713. doi: 10.1016/j.cub.2021.04.077. [DOI] [PubMed] [Google Scholar]
- 73.Gladyshev E., Meselson M. Extreme resistance of bdelloid rotifers to ionizing radiation. Proc. Natl. Acad. Sci. U. S. A. 2008;105:5139–5144. doi: 10.1073/pnas.0800966105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krisko A., Leroy M., Radman M., Meselson M. Extreme anti-oxidant protection against ionizing radiation in bdelloid rotifers. Proc. Natl. Acad. Sci. U. S. A. 2012;109:2354–2357. doi: 10.1073/pnas.1119762109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Debortoli N., Li X., Eyres I., Fontaneto D., Hespeels B., Tang C.Q., et al. Genetic exchange among bdelloid rotifers is more likely due to horizontal gene transfer than to meiotic Sex. Curr. Biol. 2016;26:723–732. doi: 10.1016/j.cub.2016.01.031. [DOI] [PubMed] [Google Scholar]
- 76.Simion P., Narayan J., Houtain A., Derzelle A., Baudry L., Nicolas E., et al. Chromosome-level genome assembly reveals homologous chromosomes and recombination in asexual rotifer Adineta vaga. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abg4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Meadow N.D., Barrows C.H., Jr. Studies on aging in a bdelloid rotifer. I. The effect of various culture systems on longevity and fecundity. J. Exp. Zool. 1971;176:303–313. doi: 10.1002/jez.1401760306. [DOI] [PubMed] [Google Scholar]
- 78.Snell T.W. Rotifers as models for the biology of aging. Int. Rev. Hydrobiol. 2014;99:84–95. doi: 10.1002/iroh.201301707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Olah Z., Bush A.I., Aleksza D., Galik B., Ivitz E., Macsai L., et al. Novel in vivo experimental viability assays with high sensitivity and throughput capacity using a bdelloid rotifer. Ecotoxicol. Environ. Saf. 2017;144:115–122. doi: 10.1016/j.ecoenv.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 80.Hecox-Lea B.J., Mark Welch D.B. Evolutionary diversity and novelty of DNA repair genes in asexual Bdelloid rotifers. BMC Evol. Biol. 2018;18:177. doi: 10.1186/s12862-018-1288-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Datki Z., Galik-Olah Z., Bohar Z., Zadori D., Fulop F., Szatmari I., et al. Kynurenic acid and its analogs are beneficial physiologic attenuators in bdelloid rotifers. Molecules. 2019;24:2171. doi: 10.3390/molecules24112171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nowell R.W., Wilson C.G., Almeida P., Schiffer P.H., Fontaneto D., Becks L., et al. Evolutionary dynamics of transposable elements in bdelloid rotifers. Elife. 2021;10 doi: 10.7554/eLife.63194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gribble K.E. Brachionus rotifers as a model for investigating dietary and metabolic regulators of aging. Nutr. Healthy Aging. 2021;6:1–15. doi: 10.3233/NHA-200104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Malone T., Blumenthal R.M., Cheng X. Structure-guided analysis reveals nine sequence motifs conserved among DNA amino-methyltransferases, and suggests a catalytic mechanism for these enzymes. J. Mol. Biol. 1995;253:618–632. doi: 10.1006/jmbi.1995.0577. [DOI] [PubMed] [Google Scholar]
- 85.Gong W., O'Gara M., Blumenthal R.M., Cheng X. Structure of PvuII DNA-(cytosine N4) methyltransferase, an example of domain permutation and protein fold assignment. Nucleic Acids Res. 1997;25:2702–2715. doi: 10.1093/nar/25.14.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Horton J.R., Woodcock C.B., Opot S.B., Reich N.O., Zhang X., Cheng X. The cell cycle-regulated DNA adenine methyltransferase CcrM opens a bubble at its DNA recognition site. Nat. Commun. 2019;10:4600. doi: 10.1038/s41467-019-12498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Woodcock C.B., Horton J.R., Zhang X., Blumenthal R.M., Cheng X. Beta class amino methyltransferases from bacteria to humans: evolution and structural consequences. Nucleic Acids Res. 2020;48:10034–10044. doi: 10.1093/nar/gkaa446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oliveira P.H., Ribis J.W., Garrett E.M., Trzilova D., Kim A., Sekulovic O., et al. Epigenomic characterization of Clostridioides difficile finds a conserved DNA methyltransferase that mediates sporulation and pathogenesis. Nat. Microbiol. 2020;5:166–180. doi: 10.1038/s41564-019-0613-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou J., Horton J.R., Blumenthal R.M., Zhang X., Cheng X. Clostridioides difficile specific DNA adenine methyltransferase CamA squeezes and flips adenine out of DNA helix. Nat. Commun. 2021;12:3436. doi: 10.1038/s41467-021-23693-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li X., Xhang Z., Yin J., Wu T.P. Quantitative profiling of DNA 6mA at single-base resolution using NAME-seq. Research Square. 2022 doi: 10.21203/rs.3.rs-2286301/v1. [preprint] [DOI] [Google Scholar]
- 91.Ricci C. Culturing of some bdelloid rotifers. Hydrobiologia. 1984;112:45–51. [Google Scholar]
- 92.Yu D., Kaur G., Blumenthal R.M., Zhang X., Cheng X. Enzymatic characterization of three human RNA adenosine methyltransferases reveals diverse substrate affinities and reaction optima. J. Biol. Chem. 2021;296 doi: 10.1016/j.jbc.2021.100270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Eram M.S., Bustos S.P., Lima-Fernandes E., Siarheyeva A., Senisterra G., Hajian T., et al. Trimethylation of histone H3 lysine 36 by human methyltransferase PRDM9 protein. J. Biol. Chem. 2014;289:12177–12188. doi: 10.1074/jbc.M113.523183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pendleton K.E., Chen B., Liu K., Hunter O.V., Xie Y., Tu B.P., et al. The U6 snRNA m(6)A methyltransferase METTL16 regulates SAM synthetase Intron retention. Cell. 2017;169:824–835.e14. doi: 10.1016/j.cell.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.German D.C., Bloch C.A., Kredich N.M. Measurements of S-adenosylmethionine and L-homocysteine metabolism in cultured human lymphoid cells. J. Biol. Chem. 1983;258:10997–11003. [PubMed] [Google Scholar]
- 96.Chen Y., Tan T. Enhanced S-adenosylmethionine production by increasing ATP levels in baker's yeast (Saccharomyces cerevisiae) J. Agric. Food Chem. 2018;66:5200–5209. doi: 10.1021/acs.jafc.8b00819. [DOI] [PubMed] [Google Scholar]
- 97.Semenyuk P., Muronetz V. Protein interaction with charged macromolecules: from model polymers to unfolded proteins and post-translational modifications. Int. J. Mol. Sci. 2019;20:1252. doi: 10.3390/ijms20051252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cayley S., Lewis B.A., Guttman H.J., Record M.T., Jr. Characterization of the cytoplasm of Escherichia coli K-12 as a function of external osmolarity. Implications for protein-DNA interactions in vivo. J. Mol. Biol. 1991;222:281–300. doi: 10.1016/0022-2836(91)90212-o. [DOI] [PubMed] [Google Scholar]
- 99.Zheng W., Ibanez G., Wu H., Blum G., Zeng H., Dong A., et al. Sinefungin derivatives as inhibitors and structure probes of protein lysine methyltransferase SETD2. J. Am. Chem. Soc. 2012;134:18004–18014. doi: 10.1021/ja307060p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Krissinel E., Henrick K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 2007;372:774–797. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 101.Laskowski R.A. PDBsum new things. Nucleic Acids Res. 2009;37:D355–359. doi: 10.1093/nar/gkn860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Leyton D.L., Johnson M.D., Thapa R., Huysmans G.H., Dunstan R.A., Celik N., et al. A mortise-tenon joint in the transmembrane domain modulates autotransporter assembly into bacterial outer membranes. Nat. Commun. 2014;5:4239. doi: 10.1038/ncomms5239. [DOI] [PubMed] [Google Scholar]
- 103.Scavetta R.D., Thomas C.B., Walsh M.A., Szegedi S., Joachimiak A., Gumport R.I., et al. Structure of RsrI methyltransferase, a member of the N6-adenine beta class of DNA methyltransferases. Nucleic Acids Res. 2000;28:3950–3961. doi: 10.1093/nar/28.20.3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thomas C.B., Scavetta R.D., Gumport R.I., Churchill M.E. Structures of liganded and unliganded RsrI N6-adenine DNA methyltransferase: a distinct orientation for active cofactor binding. J. Biol. Chem. 2003;278:26094–26101. doi: 10.1074/jbc.M303751200. [DOI] [PubMed] [Google Scholar]
- 105.Osipiuk J., Walsh M.A., Joachimiak A. Crystal structure of MboIIA methyltransferase. Nucleic Acids Res. 2003;31:5440–5448. doi: 10.1093/nar/gkg713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Morita R., Ishikawa H., Nakagawa N., Kuramitsu S., Masui R. Crystal structure of a putative DNA methylase TTHA0409 from Thermus thermophilus HB8. Proteins. 2008;73:259–264. doi: 10.1002/prot.22158. [DOI] [PubMed] [Google Scholar]
- 107.Ma B., Ma J., Liu D., Guo L., Chen H., Ding J., et al. Biochemical and structural characterization of a DNA N6-adenine methyltransferase from Helicobacter pylori. Oncotarget. 2016;7:40965–40977. doi: 10.18632/oncotarget.9692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gupta Y.K., Chan S.H., Xu S.Y., Aggarwal A.K. Structural basis of asymmetric DNA methylation and ATP-triggered long-range diffusion by EcoP15I. Nat. Commun. 2015;6:7363. doi: 10.1038/ncomms8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schubert H.L., Blumenthal R.M., Cheng X. Many paths to methyltransfer: a chronicle of convergence. Trends Biochem. Sci. 2003;28:329–335. doi: 10.1016/S0968-0004(03)00090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang Z., Liu F., Steeves A.H., Kulik H.J. Quantum mechanical description of electrostatics provides a Unified picture of catalytic action across methyltransferases. J. Phys. Chem. Lett. 2019;10:3779–3787. doi: 10.1021/acs.jpclett.9b01555. [DOI] [PubMed] [Google Scholar]
- 111.Gowher H., Jeltsch A. Enzymatic properties of recombinant Dnmt3a DNA methyltransferase from mouse: the enzyme modifies DNA in a non-processive manner and also methylates non-CpG [correction of non-CpA] sites. J. Mol. Biol. 2001;309:1201–1208. doi: 10.1006/jmbi.2001.4710. [DOI] [PubMed] [Google Scholar]
- 112.Gao L., Emperle M., Guo Y., Grimm S.A., Ren W., Adam S., et al. Comprehensive structure-function characterization of DNMT3B and DNMT3A reveals distinctive de novo DNA methylation mechanisms. Nat. Commun. 2020;11:3355. doi: 10.1038/s41467-020-17109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kishikawa S., Murata T., Ugai H., Yamazaki T., Yokoyama K.K. Control elements of Dnmt1 gene are regulated in cell-cycle dependent manner. Nucleic Acids Res. Suppl. 2003 doi: 10.1093/nass/3.1.307. [DOI] [PubMed] [Google Scholar]
- 114.Patel K., Dickson J., Din S., Macleod K., Jodrell D., Ramsahoye B. Targeting of 5-aza-2'-deoxycytidine residues by chromatin-associated DNMT1 induces proteasomal degradation of the free enzyme. Nucleic Acids Res. 2010;38:4313–4324. doi: 10.1093/nar/gkq187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hervouet E., Nadaradjane A., Gueguen M., Vallette F.M., Cartron P.F. Kinetics of DNA methylation inheritance by the Dnmt1-including complexes during the cell cycle. Cell Div. 2012;7:5. doi: 10.1186/1747-1028-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Barra V., Schillaci T., Lentini L., Costa G., Di Leonardo A. Bypass of cell cycle arrest induced by transient DNMT1 post-transcriptional silencing triggers aneuploidy in human cells. Cell Div. 2012;7:2. doi: 10.1186/1747-1028-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schneider K., Fuchs C., Dobay A., Rottach A., Qin W., Wolf P., et al. Dissection of cell cycle-dependent dynamics of Dnmt1 by FRAP and diffusion-coupled modeling. Nucleic Acids Res. 2013;41:4860–4876. doi: 10.1093/nar/gkt191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Reich N.O., Dang E., Kurnik M., Pathuri S., Woodcock C.B. The highly specific, cell cycle-regulated methyltransferase from Caulobacter crescentus relies on a novel DNA recognition mechanism. J. Biol. Chem. 2018;293:19038–19046. doi: 10.1074/jbc.RA118.005212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Arkhipova I.R., Yushenova I.A., Rodriguez F. Shaping eukaryotic epigenetic systems by horizontal gene transfer. Bioessays. 2023;45 doi: 10.1002/bies.202200232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yoder J.A., Walsh C.P., Bestor T.H. Cytosine methylation and the ecology of intragenomic parasites. Trends Genet. 1997;13:335–340. doi: 10.1016/s0168-9525(97)01181-5. [DOI] [PubMed] [Google Scholar]
- 121.Greenberg M.V.C., Bourc'his D. The diverse roles of DNA methylation in mammalian development and disease. Nat. Rev. Mol. Cell Biol. 2019;20:590–607. doi: 10.1038/s41580-019-0159-6. [DOI] [PubMed] [Google Scholar]
- 122.Zhou W., Liang G., Molloy P.L., Jones P.A. DNA methylation enables transposable element-driven genome expansion. Proc. Natl. Acad. Sci. U. S. A. 2020;117:19359–19366. doi: 10.1073/pnas.1921719117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bourc/'his D., Xu G.L., Lin C.S., Bollman B., Bestor T.H. Dnmt3L and the establishment of maternal genomic imprints. Science. 2001;294:2536–2539. doi: 10.1126/science.1065848. [DOI] [PubMed] [Google Scholar]
- 124.Ooi S.K.T., Qiu C., Bernstein E., Li K., Jia D., Yang Z., et al. DNMT3L connects unmethylated lysine 4 of histone H3 to de novo methylation of DNA. Nature. 2007;448:714–717. doi: 10.1038/nature05987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bostick M., Kim J.K., Esteve P.O., Clark A., Pradhan S., Jacobsen S.E. UHRF1 plays a role in maintaining DNA methylation in mammalian cells. Science. 2007;317:1760–1764. doi: 10.1126/science.1147939. [DOI] [PubMed] [Google Scholar]
- 126.Sharif J., Muto M., Takebayashi S., Suetake I., Iwamatsu A., Endo T.A., et al. The SRA protein Np95 mediates epigenetic inheritance by recruiting Dnmt1 to methylated DNA. Nature. 2007;450:908–912. doi: 10.1038/nature06397. [DOI] [PubMed] [Google Scholar]
- 127.Zeng Y., Ren R., Kaur G., Hardikar S., Ying Z., Babcock L., et al. The inactive Dnmt3b3 isoform preferentially enhances Dnmt3b-mediated DNA methylation. Genes Dev. 2020;34:1546–1558. doi: 10.1101/gad.341925.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xu T.H., Liu M., Zhou X.E., Liang G., Zhao G., Xu H.E., et al. Structure of nucleosome-bound DNA methyltransferases DNMT3A and DNMT3B. Nature. 2020;586:151–155. doi: 10.1038/s41586-020-2747-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Studier F.W. Protein production by auto-induction in high density shaking cultures. Protein Expr. Purif. 2005;41:207–234. doi: 10.1016/j.pep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 130.Hsiao K., Zegzouti H., Goueli S.A. Methyltransferase-Glo: a universal, bioluminescent and homogenous assay for monitoring all classes of methyltransferases. Epigenomics. 2016;8:321–339. doi: 10.2217/epi.15.113. [DOI] [PubMed] [Google Scholar]
- 131.Zhou J., Horton J.R., Menna M., Fiorentino F., Ren R., Yu D., et al. Systematic design of adenosine analogs as inhibitors of a Clostridioides difficile-specific DNA adenine methyltransferase required for normal sporulation and persistence. J. Med. Chem. 2023;66:934–950. doi: 10.1021/acs.jmedchem.2c01789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Parks L.W., Schlenk F. The stability and hydrolysis of S-adenosylmethionine; isolation of S-ribosylmethionine. J. Biol. Chem. 1958;230:295–305. [PubMed] [Google Scholar]
- 133.Kong H., Lin L.F., Porter N., Stickel S., Byrd D., Posfai J., et al. Functional analysis of putative restriction-modification system genes in the Helicobacter pylori J99 genome. Nucleic Acids Res. 2000;28:3216–3223. doi: 10.1093/nar/28.17.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bailey T.L., Johnson J., Grant C.E., Noble W.S. The MEME suite. Nucleic Acids Res. 2015;43:W39–49. doi: 10.1093/nar/gkv416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schuck P., Perugini M.A., Gonzales N.R., Howlett G.J., Schubert D. Size-distribution analysis of proteins by analytical ultracentrifugation: strategies and application to model systems. Biophys. J. 2002;82:1096–1111. doi: 10.1016/S0006-3495(02)75469-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schneider D.K., Soares A.S., Lazo E.O., Kreitler D.F., Qian K., Fuchs M.R., et al. Amx - the highly automated macromolecular crystallography (17-ID-1) beamline at the NSLS-II. J. Synchrotron Radiat. 2022;29:1480–1494. doi: 10.1107/S1600577522009377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Otwinowski Z., Borek D., Majewski W., Minor W. Multiparametric scaling of diffraction intensities. Acta Crystallogr. A. 2003;59:228–234. doi: 10.1107/s0108767303005488. [DOI] [PubMed] [Google Scholar]
- 138.Beilsten-Edmands J., Winter G., Gildea R., Parkhurst J., Waterman D., Evans G. Scaling diffraction data in the DIALS software package: algorithms and new approaches for multi-crystal scaling. Acta Crystallogr. D Struct. Biol. 2020;76:385–399. doi: 10.1107/S2059798320003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Evans P.R., Murshudov G.N. How good are my data and what is the resolution? Acta Crystallogr. D Biol. Crystallogr. 2013;69:1204–1214. doi: 10.1107/S0907444913000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jumper J., Evans R., Pritzel A., Green T., Figurnov M., Ronneberger O., et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Afonine P.V., Grosse-Kunstleve R.W., Echols N., Headd J.J., Moriarty N.W., Mustyakimov M., et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D Biol. Crystallogr. 2012;68:352–367. doi: 10.1107/S0907444912001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Emsley P., Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 143.Read R.J., Adams P.D., Arendall W.B., 3rd, Brunger A.T., Emsley P., Joosten R.P., et al. A new generation of crystallographic validation tools for the protein data bank. Structure. 2011;19:1395–1412. doi: 10.1016/j.str.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The experimental data that support the findings of this study are contained within the article. The authors have deposited the X-ray structure (coordinates) and the source data (structure factor file) of N4CMT-sinefungin to the PDB, and these will be released upon article publication under accession numbers PDB 8S9M (C2221), PDB 8S9N (C2), and PDB 8S9O (P1).