Abstract
Aim: The aim of this study was to investigate the effects of continuous cilostazol use on emergency department (ED) visits, hospitalizations, and vascular outcomes in patients with hemodialysis (HD) with peripheral artery disease (PAD).
Methods: This retrospective cohort study recruited 558 adult patients, who had received chronic HD for at least 90 days between January 1, 2008 and December 31, 2012, from the National Health Insurance Research Database. Eligible patients were divided into two groups based on continuing or discontinuing cilostazol treatment. Outcome measures were ED visits, hospitalizations, mortality, and vascular outcomes such as percutaneous transluminal angioplasty, surgical bypass, lower leg amputation, ischemic stroke, hemorrhagic stroke, and cardiovascular events.
Results: Patients with continuous cilostazol use had significantly higher prevalence of stroke, cancer, vintage, and the use of angiotensin receptor blocker and β-blocker, but significantly lower incidence of ischemic stroke and cardiovascular events, as well as lower mortality, than those without continuous cilostazol use (allp<.05). Continuous cilostazol use was independently associated with lower risk of ED visits, hemorrhagic stroke, and cardiovascular events (adjusted hazard ratios: 0.79, 0.29, and 0.67; 95% confidence intervals: 0.62–0.98, 0.10–0.84, and 0.48–0.96, respectively; allp<.05). Continuous cilostazol use was significantly associated with higher ED visit-free and cardiovascular event-free rates (log-rank test;p<.05).
Conclusion: Continuous treatment of cilostazol in patients with HD with PAD significantly decreases the risk of ED visits, hemorrhagic stroke, and cardiovascular events and improves ED visit-free and cardiovascular event-free rates during long-term follow-up.
Keywords: Cilostazol, Emergency department, Hemodialysis, Peripheral artery disease, Vascular outcome
Introduction
Peripheral artery disease (PAD) refers to atherosclerosis-related partial or complete obstruction of the lower limb arteries, resulting in reduced blood flow and oxygen to the leg 1) . Critical limb ischemia (CLI), an advanced stage of PAD, is defined by the presence of ischemic rest pain, ulcers, or gangrene and is noted for its high amputation rate 2) . In addition to amputation, PAD is linked to increased cardiovascular disease, cerebrovascular disease, and mortality 3 - 5) . PAD is prevalent in patients with chronic kidney disease (CKD), especially those on dialysis or with end-stage renal disease (ESRD) 6) . Patients with CKD with PAD have a significantly higher risk of long-term mortality than patients with either disease alone 7) .
Cilostazol, a selective phosphodiesterase 3 inhibitor, is regarded as a vasodilator that provides antiplatelet and antithrombotic effects and is shown to prevent platelet activation and aggregation, inhibit vascular smooth muscle proliferation, and improve endothelial cell function 8) . In patients with PAD with intermittent claudication, cilostazol increases maximal and pain-free walking distance 9 , 10) . Cilostazol also prevents restenosis and re-occlusion and reduces the risk of major amputation after patients with PAD have received endovascular procedures 11 - 13) . The prevention of target lesion restenosis and re-occlusion by cilostazol can still be found in patients with hemodialysis (HD) with PAD after the endovascular procedure 14 , 15) . In addition, cilostazol relieves the clinical symptoms of PAD in patients with HD via improving the lipid-related and endovascular inflammatory biochemical parameters 16) .
Clinical trials have indicated that cilostazol is an effective and safe medication for the secondary prevention of ischemic stroke 17 , 18) . Cilostazol has also remained to be effective in the prevention of stroke in patients with PAD 19) . Nevertheless, the influence of cilostazol on mortality and cardiovascular events in patients with PAD remains controversial. In a small retrospective study of patients with HD with asymptomatic PAD, low-dose cilostazol decreased cardiovascular events and all-cause mortality 20) . A previous study showed that cilostazol decreased major cardiovascular events, stroke, and mortality in patients with HD with PAD 21) . Meanwhile, data of cilostazol on clinical vascular outcomes in patients with chronic HD with prevalent PAD are minimal; to date, no studies have investigated the association between the treatment duration of cilostazol and clinical vascular outcomes in the HD population. Therefore, we designed a study to evaluate the effects of continuous cilostazol use versus the effects of discontinuous cilostazol use, analyzing data from patients with HD with prevalent PAD, including percutaneous transluminal angioplasty (PTA), surgical bypass, lower leg amputation, ischemic stroke, hemorrhagic stroke, cardiovascular events, and mortality. In addition, the current study investigated the emergency department (ED) visits and hospitalizations between the population with continuous cilostazol use and those with discontinuous cilostazol use.
Aim
The present study aimed to investigate the impact of continuous cilostazol use on ED visits, hospitalizations, and clinical vascular outcomes in patients with HD with PAD.
Methods
Data Source
The National Health Insurance Research Database (NHIRD), which is managed by the Health and Welfare Data Science Center under the Ministry of Health and Welfare, Taiwan, contains claims data and detailed information on health services covered by the National Health Insurance (NHI) program. The NHI program was launched in Taiwan in 1985 and now covered more than 99% of the total population of Taiwan. The claims data include demographic data, ambulatory care, records of clinic visits, hospital admissions, dental services, operations, prescriptions, disease status, and dialysis history. Since the NHI system reimburses all dialysis-related expenditures, the claims data provide a comprehensive data source for information on patients with HD. For the present study, we obtained information on all chronic dialysis patients registered in the NHIRD, which classifies diseases according to the International Classification of Diseases, 9th and 10th Revisions, Clinical Modification (ICD-9-CM, ICD-10-CM).
Study Design and Participants
We identified patients with chronic HD in the NHIRD who had received chronic HD therapy for at least 90 days between January 1, 2008 and December 31, 2012. Among the patients who met the criteria, we enrolled 3236 patients with chronic HD who were diagnosed with PAD after HD. We excluded 3 patients who were under 20 years old and 220 patients who had missing demographic data. Among these 3013 patients, 5 patients who shifted to peritoneal therapy, 3 patients who received a kidney transplant, and 5 patients who had died within 3 months were excluded. Of these 3000 adult patients with chronic HD with prevalent PAD, 558 patients who received oral cilostazol treatment were included. The baseline date in the study is the first date of PAD diagnosis. The continuation of cilostazol use was evaluated within 3 months after the baseline date, which we defined as the prescription period. The final study population was further stratified by the status of continuous use of cilostazol for more than 60 days, consisting of 187 patients with chronic HD with prevalent PAD who had continuous cilostazol treatment and 371 patients with chronic HD with prevalent PAD who had discontinuous cilostazol treatment ( Fig.1 ) . Sixty days was used as the cut-off value because some studies have demonstrated that beneficial clinical effects, including secondary stroke prevention and vascular access maturation, appear after 60 days of cilostazol treatment 22 , 23) .
Fig.1.
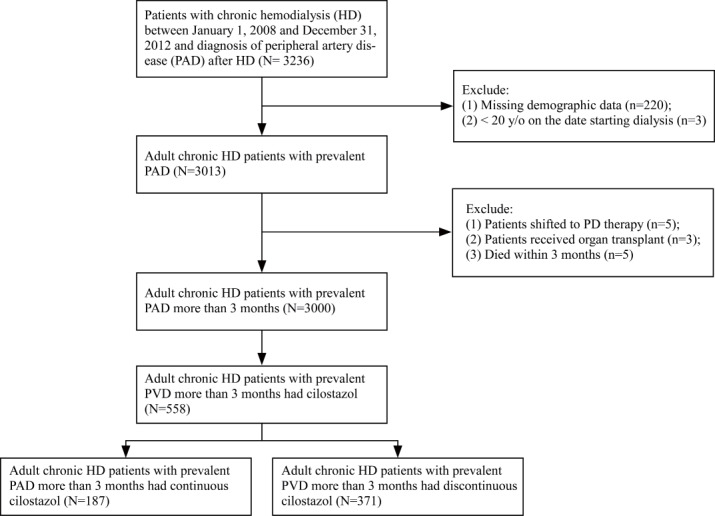
Flowchart of patient selection for the study cohort
Ethical Considerations
This study was performed in line with the principles of the Declaration of Helsinki. Because NHIRD data are deidentified and encrypted, this study was exempt from a full review. The study protocol was approved by the Institutional Review Board (IRB) of Shin Kong Wu Ho-Su Memorial Hospital (approval no 20210305R). Because all patient data from the NHIRD were deidentified, signed informed consent from included patients was waived by the IRB.
Main Outcomes
The primary outcomes were ED visits, hospitalizations, cardiovascular events, and mortality. The secondary outcomes were PTA, surgical bypass, lower leg amputation, ischemic stroke, and hemorrhagic stroke. We set the index date as 3 months after PAD diagnosis, and the index date was the starting point for outcome measures. We regarded the conditions of shift to PD or receiving transplantation as censored data for the analysis when health outcomes were evaluated.
The main outcomes in our study include PTA, surgical bypass, lower leg amputation, ischemic stroke, hemorrhagic stroke, cardiovascular events, and mortality. Most of these outcome events are events that urgently need appropriate clinical management. Previous studies have also shown that dialysis patients, especially patients with chronic HD, are more likely to use emergency services than other patients 24 - 26) . Furthermore, considering that patients with HD with PAD who need to come to the ED owing to clinical symptoms and signs are not necessarily hospitalized after emergent medical treatment, we selected ED visit as one of the main outcomes.
Statistical Analysis
All statistical analyses were performed using SAS software (version 9.4; SAS Institute, Cary, NC, USA). The demographic and clinical characteristics of the study cohort are presented as mean±standard deviation for continuous variables and proportion (n, %) for categorical variables. Student’s t-tests and chi-squared tests were used to compare continuous and categorical variables. Univariate Cox proportional hazard (PH) models were used to estimate the relative risk (crude hazard ratios [HRs]) of outcome events, including ED visits, hospitalizations, PTA, surgical bypass, lower leg amputation, ischemic stroke, hemorrhagic stroke, cardiovascular events, and all-cause mortality in patients with chronic HD with prevalent PAD receiving continuous cilostazol compared with those without continuous cilostazol during the follow-up period, followed by multivariate Cox analysis to estimate adjusted hazard ratios (aHRs). The survival and outcome event-free rates were calculated using the Kaplan–Meier method, and pairs of groups were compared using log-rank tests. We tested the PH assumption by generating time-dependent covariates through modeling interactions between the treatment and the function of survival time that we included in the model 27) . No PH assumptions were violated in our analyses.
In addition, propensity score-based models are applied to control the effects of potential confounders. For estimating the propensity scores for the continuous use of cilostazol, the variables used in the logistic regression models included patient age, gender, and comorbidities (i.e., history of hypertension [HTN], diabetes, hyperlipidemia, coronary artery disease [CAD], congestive heart failure [CHF], stroke, cancer, and amputation). We utilized a set of models across the stratified levels of probability for the continuous use of cilostazol based on the categorization of the propensity score distribution into quartiles 28) to estimate the average treatment effect (ATE). A two-sided p value of <0.05 was considered as statistically significant.
Results
Baseline Characteristics of the Study Population
Table 1 shows the demographic and clinical characteristics of the study population. Among 558 adult patients with chronic HD with diagnosed PAD for more than 3 months, who received cilostazol treatment between 2008 and 2012, 187 patients were treated with cilostazol for more than 60 days without interruption (continuous cilostazol group), and 371 patients were treated with cilostazol but without continuous use for more than 60 days (discontinuous cilostazol group). The mean age of the total population was 70.07±11.90 years, and the mean follow-up period was 4.60±2.32 years. The prevalence of stroke was significantly lower (p=.002) and was higher for cancer (p=.003) in the continuous cilostazol group than in the discontinuous cilostazol group. Patients in the continuous cilostazol group also had higher angiotensin receptor blocker (ARB) (p=.004) and β-blocker use (p=.02) than those in the discontinuous cilostazol group. Dialysis vintage was higher in the continuous cilostazol group than in the discontinuous cilostazol group (p=.001). The distribution of the use of anti-platelet drugs during the observational period was shown in Supplementary Table 1 .
Table 1. Demographic and clinical characteristics of adult chronic HD patients with prevalent PAD between continuous and discontinuous use of cilostazol.
| Variables§ | Total (n = 558) | Continuous cilostazol (n = 187) | Discontinuous cilostazol (n = 371) | p |
|---|---|---|---|---|
| Age (years)§ | 70.1±11.9 | 69.6±11.9 | 70.3±11.9 | 0.94 |
| Age ≥ 65 yrs (%) | 385 (69.0) | 127 (67.9) | 258 (69.5) | 0.69 |
| Gender | 0.57 | |||
| Male (%) | 328 (58.8) | 113 (60.4) | 215 (58.0) | |
| Female (%) | 230 (41.2) | 74 (39.6) | 156 (42.1) | |
| Comorbidities (%) | ||||
| Hypertension | 458 (82.1) | 159 (85.0) | 299 (80.6) | 0.20 |
| Diabetes mellitus | 432 (77.4) | 142 (75.9) | 290 (78.2) | 0.55 |
| Hyperlipidemia | 206 (36.9) | 76 (40.6) | 130 (35.0) | 0.20 |
| CAD | 217 (38.9) | 75 (40.1) | 142 (38.2) | 0.68 |
| CHF | 112 (20.1) | 30 (16.0) | 82 (22.1) | 0.09 |
| Stroke | 146 (26.2) | 34 (18.2) | 112 (30.2) | 0.002 |
| Cancer | 45 (8.1) | 24 (12.8) | 21 (5.7) | 0.003 |
| Amputation | 44 (7.9) | 11 (5.9) | 33 (8.9) | 0.22 |
| Dialysis vintage (year) | 1.6±1.2 | 1.8±1.3 | 1.5±1.2 | 0.001 |
| HD duration# | 13.3 (0.4) | 13.3 (0.4) | 13.3 (0.4) | 0.78 |
| Vascular access | 0.53 | |||
| Fistula | 422 (75.6) | 141 (75.4) | 281 (75.7) | |
| Graft | 126 (22.6) | 41 (21.9) | 85 (22.9) | |
| Else | 10 (1.8) | 5 (2.7) | 5 (1.4) | |
| Medication (%) | ||||
| Anti-HTN drugs | 422 (75.6) | 141 (75.4) | 281 (75.7) | |
| ACE inhibitors | 113 (20.3) | 31 (16.6) | 82 (22.1) | 0.13 |
| ARB | 269 (48.2) | 106 (56.7) | 163 (43.9) | 0.004 |
| B-blockers | 251 (45.0) | 97 (51.9) | 154 (41.5) | 0.02 |
| Calcium antagonists | 334 (60.0) | 112 (59.9) | 222 (59.8) | 0.99 |
| Diuretics | 242 (43.4) | 76 (40.6) | 166 (44.7) | 0.36 |
| Statins | 221 (40.0) | 80 (42.8) | 141 (38.0) | 0.28 |
| OAD | 314 (56.3) | 105 (56.2) | 209 (56.3) | 0.97 |
| Insulin and analogues | 193 (35.0) | 65 (34.8) | 128 (34.5) | 0.95 |
| Aspirin | 266 (47.7) | 86 (46.0) | 180 (48.5) | 0.57 |
| Clopidogrel | 91 (16.3) | 28 (15.0) | 63 (17.0) | 0.54 |
| Warfarin | 91 (16.3) | 7 (3.74) | 19 (5.1) | 0.47 |
| CCPB | 51 (9.1) | 13 (7.0) | 38 (10.2) | 0.20 |
ACE, angiotensin-converting-enzyme; ARB, angiotensin II receptor blockers; CAD, coronary artery disease; CHF, congestive heart failure; OAD, oral anti-diabetic drugs; CCPB, calcium-containing phosphate binder.
§Variables are expressed as mean±standard deviation (SD) for continuous data or n (%) for categorical data. Analyses are applied using Student’s
t-test for continuous data and Chi-square test for categorical data.
#Average frequency per month.
Supplementary Table 1. Distribution of the use of anti-platelet drugs during the observational period.
| Variables§ | Total (n = 558) | Continuous cilostazol (n = 187) | Discontinuous cilostazol (n = 371) | p |
|---|---|---|---|---|
| Aspirin use | ||||
| In the first year after enrollment | 225 (40.3) | 72 (38.5) | 153 (41.2) | 0.53 |
| In the second year after enrollment | 180 (32.3) | 59 (31.6) | 121 (32.6) | 0.80 |
| Clopidogrel use | ||||
| In the first year after enrollment | 115 (20.60) | 72 (19.4) | 43 (23.0) | 0.32 |
| In the second year after enrollment | 96 (17.2) | 64 (17.3) | 32 (17.1) | 0.97 |
§Variables are expressed as n (%) for categorical data. Analyses are applied using Chi-square test for categorical data.
Incidence of Outcome Events between the Study Groups
Table 2 shows the incidence of ED visits, PTA, surgical bypass, lower leg amputation, ischemic and hemorrhagic stroke, cardiovascular events, and all-cause mortality between adult patients with chronic HD with PAD with or without continuous cilostazol use. Patients with discontinuous cilostazol use had a significantly higher incidence of ischemic stroke (p=.02), cardiovascular events (p=.003), and all-cause mortality (p=.02) than those receiving continuous cilostazol use during the follow-up period. Patients with discontinuous cilostazol use also had a higher incidence of lower leg amputation, surgical bypass, hemorrhagic stroke, cardiovascular death, and more ED visits than those with continuous cilostazol use during follow-up, but differences between groups were not significant. The distribution of reasons for hospitalization and emergency department visit was shown in Supplementary Table 2 .
Table 2. Outcome events between chronic HD patients with prevalent PAD receiving continuous vs. discontinuous use of cilostazol.
| Outcome events (%) | Total (n = 558) | Continuous cilostazol (n = 187) | Discontinuous cilostazol (n = 371) | p |
|---|---|---|---|---|
| Emergency department visit | 341 (61.1) | 112 (59.9) | 229 (61.7) | 0.68 |
| Hospitalization | 540 (96.8) | 180 (96.3) | 360 (97.0) | 0.62 |
| PTA | 170 (30.5) | 62 (33.2) | 108 (29.1) | 0.33 |
| Surgical bypass | 52 (9.3) | 13 (7.0) | 39 (10.5) | 0.17 |
| Lower leg amputation | 70 (12.5) | 18 (9.6) | 52 (14.0) | 0.14 |
| Ischemic stroke | 160 (28.7) | 42 (22.5) | 118 (31.8) | 0.02 |
| Hemorrhagic stroke | 33 (5.9) | 4 (2.1) | 29 (6.6) | 0.21 |
| Cardiovascular events | 177 (31.72) | 44 (23.53) | 133 (35.85) | 0.003 |
| All-cause mortality | 306 (54.8) | 90 (48.1) | 216 (58.2) | 0.02 |
PTA, percutaneous transluminal angioplasty. Analyses are applied using Chi-square test.
Supplementary Table 2. Distribution of reasons for hospitalization and emergency department visit between chronic HD patients with prevalent PAD receiving continuous vs. discontinuous use of cilostazol.
| Reasons (%) | Total (n = 558) | Continuous cilostazol (n = 187) | Discontinuous cilostazol (n = 371) | p |
|---|---|---|---|---|
| For emergency department visit | ||||
| Cardiovascular event | 87 (15.6) | 30 (16.0) | 57 (15.4) | 0.83 |
| Cerebrovascular event | 37 (6.6) | 10 (5.4) | 27 (7.3) | 0.39 |
| Peripheral vascular event | 45 (8.1) | 12 (6.4) | 33 (8.9) | 0.31 |
| For hospitalization | ||||
| Cardiovascular event | 152 (27.2) | 52 (27.8) | 100 (27.0) | 0.83 |
| Cerebrovascular event | 61 (10.9) | 16 (8.6) | 45 (12.1) | 0.20 |
| Peripheral vascular event | 102 (18.3) | 34 (18.2) | 68 (18.3) | 0.97 |
Associations between Continuous Cilostazol Use and Clinical Vascular Outcomes
Table 3 shows the incidence of clinical outcome events and lists the crude HRs and aHRs in patients with chronic HD with PAD with continuous cilostazol use. Two multivariate-adjusted models are presented in Table 3 . One model includes the significant covariates in bivariate testing (i.e., the history of stroke and cancer, the vintage of dialysis, and the use of ARBs and β-blockers). The other model includes all of the possible confounders for the adjustment (i.e., age; gender; the history of HTN, diabetes, hyperlipidemia, CAD, CHF, stroke, cancer, and amputation; the vintage of dialysis; the duration of HD; the type of vascular access; the use of anti-HTN drugs, angiotensin-converting-enzyme inhibitors, ARBs, β-blockers, calcium antagonists, diuretics, statins, oral antidiabetic drugs, insulin and analogs, aspirin, clopidogrel, warfarin, and calcium-containing phosphate binder). The HRs for ED visits, hospitalizations, PTA, surgical bypass, lower leg amputation, ischemic stroke, hemorrhagic stroke, cardiovascular events, and all-cause mortality were 0.74, 0.69, 0.87, 0.51, 0.53, 0.50, 0.21, 0.24, and 0.88 (95% confidence intervals [CIs]: 0.59–0.93, 0.42–1.14, 0.63–1.19, 0.27–0.96, 0.31–0.90, 0.35–0.72, 0.08–0.61, 0.08–0.67, and 0.69–1.13, respectively) in those with continuous cilostazol use. After adjusting for the significant variables listed in Table 1 , continuous cilostazol use remained a significant protective factor for ED visits, hospitalizations, PTA, surgical bypass, lower leg amputation, ischemic stroke, hemorrhagic stroke, cardiovascular events, and all-cause mortality (aHRs: 0.79, 0.66, 1.07, 0.61, 0.69, 0.77, 0.29, 0.67, and 0.99; 95% CIs: 0.62–0.98, 0.39–1.12, 0.78–1.48, 0.32–1.15, 0.39–1.20, 0.54–1.11, 0.10–0.84, 0.48–0.96, and 0.77–1.27, respectively). Table 4 shows the ATEs estimated by using propensity score quartile-stratified Cox PH models and related informations were provided in Supplementary Table 3 .
Table 3. Cox proportional hazards analysis of associations between continuous cilostazol use in chronic HD patients with prevalent PAD and vascular outcome events.
| Event outcome | Crude | Adjusted | |
|---|---|---|---|
| HR (95% CI) | Model 1§ HR (95% CI) | Model 2§§ HR (95% CI) | |
| Emergency department visits | |||
| Continuous cilostazol use | 0.74 (0.59-0.93) | 0.75 (0.60-0.95) | 0.79 (0.62-0.98) |
| Hospitalization | |||
| Continuous cilostazol use | 0.69 (0.42-1.14) | 0.68 (0.40-1.14) | 0.66 (0.39-1.12) |
| PTA | |||
| Continuous cilostazol use | 0.87 (0.63-1.19) | 1.00 (0.72-1.37) | 1.07 (0.78-1.48) |
| Surgical bypass | |||
| Continuous cilostazol use | 0.51 (0.27-0.96) | 0.56 (0.30-1.07) | 0.61 (0.32-1.15) |
| Lower leg amputation | |||
| Continuous cilostazol use | 0.53 (0.31-0.90) | 0.64 (0.37-1.10) | 0.69 (0.39-1.20) |
| Ischemic stroke | |||
| Continuous cilostazol use | 0.50 (0.35-0.72) | 0.58 (0.40-0.82) | 0.77 (0.54-1.11) |
| Hemorrhagic stroke | |||
| Continuous cilostazol use | 0.21 (0.08-0.61) | 0.24 (0.08-0.69) | 0.29 (0.10-0.84) |
| Cardiovascular events | |||
| Continuous cilostazol use | 0.24 (0.08-0.67) | 0.53 (0.38-0.75) | 0.67 (0.48-0.96) |
| All-cause mortality | |||
| Continuous cilostazol use | 0.88 (0.69-1.13) | 0.87 (0.67-1.12) | 0.99 (0.77-1.27) |
PTA, percutaneous transluminal angioplasty
§Adjusted for the history of stroke and cancer; the vintage of dialysis; and the use of ARB and β-blockers.
§§Adjusted for age; gender; the history of hypertension, diabetes, hyperlipidemia, CAD, CHF, stroke, cancer, and amputation; the vintage of
dialysis; the duration of HD; the type of vascular access; and the use of anti-HTN drugs, ACE inhibitors, ARB, β-blockers, calcium antagonists, diuretics, statins, OAD, insulin and analogues, aspirin, clopidogrel, warfarin, and CCPB.
Table 4. Propensity score quartile-stratified Cox proportional hazard models of associations between continuous cilostazol use in chronic HD patients with prevalent PAD and vascular outcome events.
| Event outcome | Average treatment effect |
|---|---|
| Emergency department visits | |
| Continuous cilostazol use | 0.83 |
| Hospitalization | |
| Continuous cilostazol use | 0.70 |
| PTA | |
| Continuous cilostazol use | 1.18 |
| Surgical bypass | |
| Continuous cilostazol use | 0.56 |
| Lower leg amputation | |
| Continuous cilostazol use | 0.78 |
| Ischemic stroke | |
| Continuous cilostazol use | 0.61 |
| Hemorrhagic stroke | |
| Continuous cilostazol use | 0.40 |
| Cardiovascular events | |
| Continuous cilostazol use | 0.58 |
| All-cause mortality | |
| Continuous cilostazol use | 0.95 |
PTA, percutaneous transluminal angioplasty
Supplementary Table 3. Propensity score quartile-stratified Cox proportional hazard models of associations between continuous cilostazol use in chronic HD patients with prevalent PAD and vascular outcome events.
| Event outcome | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Emergency department visits | ||||
| Continuous cilostazol use | 1.01 (0.57-1.78) | 0.87 (0.56-1.36) | 0.70 (0.45-1.08) | 0.73 (0.48-1.14) |
| Hospitalization | ||||
| Continuous cilostazol use | 0.81 (0.23-2.88) | 0.73 (0.23-2.35) | 0.58 (0.22-1.53) | 0.68 (0.28-1.62) |
| PTA | ||||
| Continuous cilostazol use | 0.98 (0.48-2.02) | 0.55 (0.27-1.10) | 1.95 (1.05-3.63) | 1.24 (0.67-2.27) |
| Surgical bypass | ||||
| Continuous cilostazol use | 0.35 (0.05-2.68) | 0.47 (0.13-1.70) | 0.54 (0.15-1.97) | 0.86 (0.29-2.56) |
| Lower leg amputation | ||||
| Continuous cilostazol use | 1.12 (0.41-3.00) | 0.44 (0.14-1.32) | 0.99 (0.34-2.90) | 0.57 (0.17-1.94) |
| Ischemic stroke | ||||
| Continuous cilostazol use | 0.75 (0.39-1.44) | 0.68 (0.35-1.31) | 0.28 (0.10-0.79) | 0.72 (0.39-1.32) |
| Hemorrhagic stroke | ||||
| Continuous cilostazol use | 0.47 (0.06-3.69) | 0.16 (0.02-1.29) | 0.80 (0.15-4.10) | 0.15 (0.02-1.15) |
| Cardiovascular events | ||||
| Continuous cilostazol use | 0.75 (0.40-1.40) | 0.57 (0.30-1.08) | 0.31 (0.12-0.81) | 0.67 (0.37-1.20) |
| All-cause mortality | ||||
| Continuous cilostazol use | 1.03 (0.58-1.82) | 0.72 (0.44-1.18) | 1.16 (0.71-1.87) | 0.88 (0.53-1.46) |
PTA, percutaneous transluminal angioplasty.
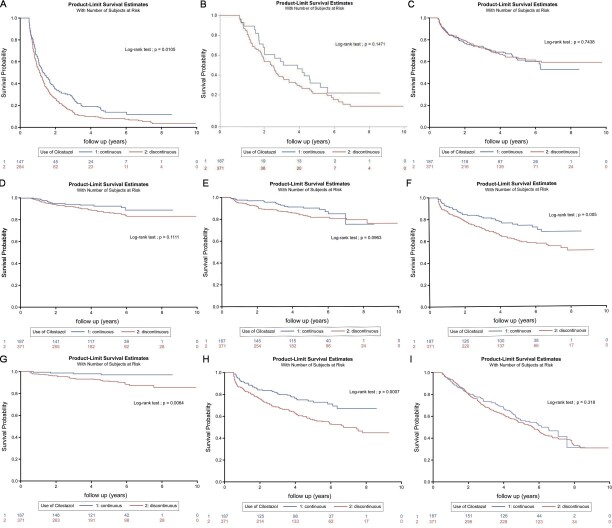
Fig.2 depicts the Kaplan–Meier curves for (A) emergency department visit (B) hospitalization (C) PTA (D) surgical bypass (E) amputation of lower leg extremities (F) ischemic stroke (G) hemorrhagic stroke (H) cardiovascular events and (I) survival curve in chronic HD patients with prevalent PAD between continuous and discontinuous use of cilostazol over the 10-year follow-up period. ED visit-, ischemic stroke-, hemorrhagic stroke-, and cardiovascular event-free rates were higher in patients with chronic HD with PAD who had continuous use of cilostazol (11.47%, 68.42%, 97.09%, and 66.99%, respectively) than in those with discontinuous use of cilostazol (3.32%, 51.33%, 85.68%, and 44.74%, respectively) ( Fig.2A, F, G, and H ; log-rank test, p<.05).
Fig.2.
Kaplan–Meier curves for (A) emergency department visit, (B) hospitalization, (C) PTA, (D) surgical bypass, (E) amputation of lower leg extremities, (F) ischemic stroke, (G) hemorrhagic stroke, (H) cardiovascular events, and (I) survival curve in patients with chronic HD with prevalent PAD between continuous and discontinuous use of cilostazol over the 10-year follow-up period
Discussion
In summary, results of the present study demonstrated that the incidence of clinical vascular events and mortality is significantly lower in patients with HD with PAD who received continuous cilostazol treatment. Continuous treatment with cilostazol, relative to discontinuous cilostazol use, significantly decreased the risk of ED visits, hemorrhagic stroke, and cardiovascular events in patients with chronic HD with PAD. Patients with continuous treatment of cilostazol also had a better ED visit-free rate, ischemic stroke-free rate, hemorrhagic-free rate, and cardiovascular event-free rate during long-term follow-up.
Patients with HD visit the ED more frequently than the general population because they tend to be frail and elderly and have multiple comorbidities 29 , 30) . Frequent ED visits influence the quality of dialysis patient care, the burden of ED service, medical resources, and medical costs. However, knowledge regarding ED visits of patients with HD with PAD is insufficient. Patients with HD with PAD are believed to have more frequent ED visits because PAD serves as an important marker for advanced systemic atherosclerosis, which increases the risk for CAD, cerebrovascular disease, vascular dysfunction, morbidity, and mortality 3 - 5) . In the present study, reduced HRs indicated that continuous use of cilostazol decreased ED visits during long-term follow-up in the HD with PAD population. The percentage of patients who visited the ED did not differ significantly between the two groups. However, the HR revealed that continuous cilostazol use significantly reduced ED visits by 21%. Considering that the Cox PH model not only analyzed whether an event occurred but also took the time-to-event into consideration, one of the possible reasons for this reduction is that cilostazol may delay the onset of emergency clinical symptoms in patients.
In contrast, the effect of continuous cilostazol use was not significant with respect to hospitalization in our study. A previous study 31) showed that the majority of ED patients triaged as urgent accounted for nearly 70% of ED patients, and that more than 20% of urgent patients after primary management in the ED still need admission. Therefore, the events with the urgent need for appropriate clinical management, such as difficulty walking accompanied by discomfort, weakness, cramping, other symptoms in the hips or lower extremities, and patients with HD with PAD who possibly visit the ED with symptoms and signs, were not necessarily hospitalized. But even so, considering that the ED visit is a vaguer outcome than hospitalization, the reason that continuous cilostazol use reduces the risk of ED visit but not hospitalization still needs further exploration.
Revascularization is an essential therapy for patients with CLI to improve limb perfusion distal to the area of arterial stenosis or occlusion and to alleviate symptoms and salvage the affected limb 32) . Historically, an open surgical bypass was considered standard therapy for CLI, but additional evidence indicated that endovascular revascularization is an effective and safe therapy for CLI 33 , 34) . In a previous study of patients with chronic HD with CLI, no significant differences were found in clinical outcomes, such as overall survival, major amputation, and major adverse limb events, between surgical bypass and endovascular therapy 35) . Despite this, endovascular therapy may be a better option for patients with HD than surgical bypass because perioperative complications are less frequent with endovascular therapy than with surgical revascularization. In fact, endovascular therapy has been regarded as first-line therapy for patients with chronic HD with CLI, and surgical bypass was performed in patients with vessels unsuitable for endovascular therapy 36) . In the present study, continuous cilostazol use significantly decreased the incidence of surgical bypass but not the incidence of PTA only before adjustment of all variables. Given that continuous cilostazol use could possibly help decelerate the progression of CLI and reduce or eliminate the need for surgical bypass, it should be investigated in further studies.
Cilostazol had a beneficial effect on ulcer wound healing 37) and prevention of lower leg amputation among patients with PAD after lower extremity revascularization, even in those with ESRD or the dialysis population 12 , 13 , 38) . The effect of cilostazol in patients with HD with PAD who have not undergone revascularization remains unclear. In the present study, continuous cilostazol use significantly decreased the incidence of lower leg amputation among patients with HD with PAD only before the adjustment of all variables. Further studies are needed to provide data supporting that continuous cilostazol use decreases the progression of CLI and improves wound ulcer healing in the population of patients with HD and PAD.
Among all mono- or dual antiplatelet regimens, cilostazol has been demonstrated to have the best long-term secondary protection after transient ischemic attack (TIA) and ischemic stroke 39 , 40) . In addition, cilostazol improves overall stroke and hemorrhagic stroke and reduces the incidence of fatal stroke in patients with previous TIA 41) . The optimal time to prevent recurrent stroke or hemorrhagic events is during the chronic phase of a stroke 42) . As such, these previous studies support the neuroprotective effects of cilostazol against ischemic or hemorrhagic injury. The neuroprotective potential of cilostazol may be dependent on its anti-inflammatory and antiapoptotic effects and endothelial and blood–brain barrier protection 43 , 44) . As for patients with HD with PAD, cilostazol also improves stroke events after endovascular therapy 21) . In the present study, continuous cilostazol use reduced the incidence of hemorrhagic stroke in patients with chronic HD with PAD significantly more than that in those with discontinuous cilostazol use.
The precise role of cilostazol in preventing cardiovascular events is controversial. According to a previous study, the usage of cilostazol may be associated with an increased risk of hospitalization due to heart failure 45) . However, adjunctive cilostazol based dual antiplatelet medication is associated with a lower risk of major adverse cardiovascular events (MACE), target lesions or vessel revascularization, cardiovascular mortality, and all-cause mortality after stent placement 46 - 49) . Cilostazol administration has also been shown to prevent MACE after endovascular therapy in patients with HD with PAD 21) . Results of the present study revealed that continuous cilostazol treatment significantly reduced cardiovascular events in the HD with PAD population.
Although a meta-analysis found that cilostazol use is associated with improved primary patency and lower risk of major amputation and target lesion revascularization in patients with PAD after peripheral endovascular interventions, the mortality associated with cilostazol use in the general population as part of antiplatelet regimens remains unclear 13) . In the current study, the continuous use of cilostazol did not result in the better survival outcomes in patients with chronic HD with prevalent PAD than those without continuous use of cilostazol. However, patients with continuous use of cilostazol had fewer cardiovascular and cerebrovascular events than those without.
The present study has a few limitations, including that the NHIRD lacks detailed information on certain factors that may affect vascular outcomes, such as smoking, ankle-brachial index, laboratory data, severity and location of PAD, and reasons for discontinuing cilostazol. Although the baseline differences between patients with continuous and discontinuous use of cilostazol were adjusted appropriately, the existence of residual biases that may have affected individual and collective outcomes cannot be ruled out. In addition, because NHIRD is an administrative healthcare database that was used instead of actual patient data, the number of days covered by the prescriptions were assumed to be consistent with the actual number of days of drug use. Furthermore, due to the lack of information regarding out-of-pocket healthcare services, whether a given patient was continuously receiving medication may be misclassified. Finally, all results of this population-based study were from Taiwan and are not generalizable to other populations, ethnic backgrounds, or geographic locations. Hence, future large-scale population studied conducted in other geographic areas is warranted to confirm the findings of this study.
Conclusions
This population-based retrospective cohort study revealed that continuous cilostazol use reduces the risk of unfavorable clinical outcomes in patients with HD with PAD, including ED visits, hemorrhagic stroke, and cardiovascular events. Therefore, we propose that patients with chronic HD with PAD receiving cilostazol should not have their treatment interrupted unless a contraindication to cilostazol usage is present.
Funding Statement
The study was supported by a grant under a cooperative project between Shin Kong Wu Ho-Su Memorial Hospital and National Yang-Ming University in Taiwan (109GB006-2) and a grant from the Shin-Kong Wu Ho-Su Memorial Hospital Research Foundation (Number 2020SKHADR038). The funding source played no role in this study.
Acknowledgements
None.
Conflicts of Interest
The authors declare no competing financial interests.
Author Contributions
Conception and design: C-KW and Y-YC
Analysis and interpretation: C-KW and Y-YC
Data collection: C-KW, Y-BY, and Y-YC
Writing the article: C-KW
Assistance in writing the article: C-HL, NY, and Y-YC
Critical revision of the article: C-KW, C-HL, and Y-YC
Final approval of the article: C-KW, C-HL, NY, Z-KK, Y-BY, and Y-YC
Statistical analysis: Z-KK, Y-YC
Overall responsibility: Y-YC
Data Sharing Statement
The data that support the findings of this study are available from the Health and Welfare Data Science Center (HWDC), Ministry of Health and Welfare (MOHW) (http://www.mohw.gov.tw/cht/DOS/) for the researchers in Taiwan. Data are available at http://dep.mohw.gov.tw/DOS/np-2497-113.html (Chinese only currently) with the permission of HWDC, Department of Statistics, MOHW.
References
- 1).Morley RL, Sharma A, Horsch AD, Hinchliffe RJ. Peripheral artery disease. BMJ, 2018; 360: j5842 [DOI] [PubMed] [Google Scholar]
- 2).Farber A, Eberhardt RT. The current state of critical limb ischemia: a systematic review. JAMA Surg, 2016; 151: 1070-1077 [DOI] [PubMed] [Google Scholar]
- 3).Olinic DM, Spinu M, Olinic M, Homorodean C, Tataru DA, Liew A, Schernthaner GH, Stanek A, Fowkes G, Catalano M. Epidemiology of peripheral artery disease in Europe: VAS Educational Paper. Int Angiol, 2018; 37: 327-334 [DOI] [PubMed] [Google Scholar]
- 4).Virtanen J, Varpela M, Biancari F, Jalkanen J, Hakovirta H. Association between anatomical distribution of symptomatic peripheral artery disease and cerebrovascular disease. Vascular, 2020; 28: 295-300 [DOI] [PubMed] [Google Scholar]
- 5).Agnelli G, Belch JJF, Baumgartner I, Giovas P, Hoffmann U. Morbidity and mortality associated with atherosclerotic peripheral artery disease: A systematic review. Atherosclerosis, 2020; 293: 94-100 [DOI] [PubMed] [Google Scholar]
- 6).Bourrier M, Ferguson TW, Embil JM, Rigatto C, Komenda P, Tangri N. Peripheral artery disease: its adverse consequences with and without CKD. Am J Kidney Dis, 2020; 75: 705-712 [DOI] [PubMed] [Google Scholar]
- 7).Liew YP, Bartholomew JR, Demirjian S, Michaels J, Schreiber MJ. Combined effect of chronic kidney disease and peripheral arterial disease on all-cause mortality in a high-risk population. Clin J Am Soc Nephr, 2008; 3: 1084-1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Rogers KC, Oliphant CS, Finks SW. Clinical efficacy and safety of cilostazol: a critical review of the literature. Drugs, 2015; 75: 377-395 [DOI] [PubMed] [Google Scholar]
- 9).Bedenis R, Stewart M, Cleanthis M, Robless P, Mikhailidis DP, Stansby G. Cilostazol for intermittent claudication. Cochrane Database Syst Rev, 2014; 10: CD003748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Robless P, Mikhailidis DP, Stansby GP. Cilostazol for peripheral arterial disease. Cochrane Database Syst Rev, 2007; 1: CD003748 [DOI] [PubMed] [Google Scholar]
- 11).de Donato G, Setacci F, Mele M, Giannace G, Galzerano G, Setacci C. Restenosis after coronary and peripheral intervention: efficacy and clinical Impact of cilostazol. Ann Vasc Surg, 2017; 41: 300-307 [DOI] [PubMed] [Google Scholar]
- 12).Nanto K, Iida O, Takahara M, Soga Y, Suzuki K, Hirano K, Kawasaki D, Shintani Y, Suematsu N, Yamaoka T, Uematsu M. Effect of cilostazol following endovascular intervention for peripheral artery disease. Angiology, 2015; 66: 774-778 [DOI] [PubMed] [Google Scholar]
- 13).Megaly M, Abraham B, Saad M, Mekaiel A, Soukas P, Banerjee S, Shishehbor MH. Outcomes with cilostazol after endovascular therapy of peripheral artery disease. Vasc Med, 2019; 24: 313-323 [DOI] [PubMed] [Google Scholar]
- 14).Ishii H, Kumada Y, Toriyama T, Aoyama T, Takahashi H, Tanaka M, Kamoi D, Kawamura Y, Yamada S, Hayashi M, Yasuda Y, Yuzawa Y, Maruyama S, Matsuo S, Matsubara T, Murohara T. Effects of oral cilostazol 100 mg BID on long-term patency after percutaneous transluminal angioplasty in patients with femoropopliteal disease undergoing hemodialysis: a retrospective chart review in Japanese patients. Clin Ther, 2010; 32: 24-33 [DOI] [PubMed] [Google Scholar]
- 15).Ishii H, Kumada Y, Toriyama T, Aoyama T, Takahashi H, Yamada S, Yasuda Y, Yuzawa Y, Maruyama S, Matsuo S, Matsubara T, Murohara T. Cilostazol improves long-term patency after percutaneous transluminal angioplasty in hemodialysis patients with peripheral artery disease. Clin J Am Soc Nephrol, 2008; 3: 1034-1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Shiohira S, Yoshida T, Sugiura H, Yoshida S, Mitobe M, Shimada K, Ohba T, Tsuchiya K, Kabaya T, Nitta K. Effect of the antiplatelet agent cilostazol on endovascular inflammatory biochemical parameters and the clinical symptoms of peripheral artery disease and restless legs syndrome in hemodialysis patients. Clin Exp Nephrol, 2011; 15: 893-899 [DOI] [PubMed] [Google Scholar]
- 17).Gotoh F, Tohgi H, Hirai S, Terashi A, Fukuuchi Y, Otomo E, Shinohara Y, Itoh E, Matsuda T, Sawada T, Yamaguchi T, Nishimaru K, Ohashi Y. Cilostazol stroke prevention study: A placebo-controlled double-blind trial for secondary prevention of cerebral infarction. J Stroke Cerebrovasc Dis, 2000; 9: 147-157 [DOI] [PubMed] [Google Scholar]
- 18).Shinohara Y, Katayama Y, Uchiyama S, Yamaguchi T, Handa S, Matsuoka K, Ohashi Y, Tanahashi N, Yamamoto H, Genka C, Kitagawa Y, Kusuoka H, Nishimaru K, Tsushima M, Koretsune Y, Sawada T, Hamada C; CSPS 2 group. Cilostazol for prevention of secondary stroke (CSPS 2): an aspirin-controlled, double-blind, randomised non-inferiority trial. Lancet Neurol, 2010; 9: 959-968 [DOI] [PubMed] [Google Scholar]
- 19).Lee WH, Chu CY, Hsu PC, Su HM, Lin TH, Voon WC, Lai WT, Sheu SH. Cilostazol for primary prevention of stroke in peripheral artery disease: a population-based longitudinal study in Taiwan. Thromb Res, 2013; 132: 190-195 [DOI] [PubMed] [Google Scholar]
- 20).Lim PS, Jeng Y, Wu MY, Pai MA, Wu TK, Chen CH. Role of Cilostazol Therapy in hemodialysis patients with asymptomatic peripheral arterial disease: A retrospective cohort study. Biomed Res Int, 2016; 2016: 8236903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Ishii H, Aoyama T, Takahashi H, Kumada Y, Kamoi D, Sakakibara T, Umemoto N, Suzuki S, Tanaka A, Ito Y, Murohara T. Treatment with cilostazol improves clinical outcome after endovascular therapy in hemodialysis patients with peripheral artery disease. J Cardiol, 2016; 67: 199-204 [DOI] [PubMed] [Google Scholar]
- 22).Russell TE, Kasper GC, Seiwert AJ, Comerota AJ, Lurie F. Cilostazol may improve maturation rates and durability of vascular access for hemodialysis. Vasc Endovascular Surg, 2017; 51: 120-124 [DOI] [PubMed] [Google Scholar]
- 23).de Havenon A, Sheth KN, Madsen TE, Johnston KC, Turan TN, Toyoda K, Elm JJ, Wardlaw JM, Johnston SC, Williams OA, Shoamanesh A, Lansberg MG. Cilostazol for secondary stroke prevention: history, evidence, limitations, and possibilities. Stroke, 2021; 52: e635-e645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Miller JB, Brauer E; Rao H, Wickenheiser K, Dev S, Omino R, Stokes-Buzzelli S. The most frequent ED patients carry insurance and a significant burden of disease. Am. J. Emerg. Med., 2013; 31: 16-19 [DOI] [PubMed] [Google Scholar]
- 25).Morris JN, Howard EP, Steel K, Schreiber R, Fries BE, Lipsitz LA, Goldman B. Predicting risk of hospital and emergency department use for home care elderly persons through a secondary analysis of cross-national data. BMC Health Serv. Res., 2014; 14: 1-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Lin YC, Hsu HK, Lai TS, Chiang WC, Lin SL, Chen YM, Chen CC, Chu TS. Emergency department utilization and resuscitation rate among patients receiving maintenance hemodialysis. J Formos Med Assoc, 2019; 118: 1652-1660 [DOI] [PubMed] [Google Scholar]
- 27).Ng’andu NH. An empirical comparison of statistical tests for assessing the proportional hazards assumption of Cox’s model. Stat Med, 1997; 16: 611-626 [DOI] [PubMed] [Google Scholar]
- 28).Hedeker, D., & Leon, A. C. Propensity score stratification for observational comparison of repeated binary outcomes. Statistics and its Interface, 2011; 4: 489-498 [Google Scholar]
- 29).Garcia-Canton C, Rodenas A, Lopez-Aperador C, Rivero Y, Anton G, Monzon T, Diaz N, Vega N, Loro JF, Santana A, Esparza N. Frailty in hemodialysis and prediction of poor short-term outcome: mortality, hospitalization and visits to hospital emergency services. Ren Fail, 2019; 41: 567-575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Lin YC, Hsu HK, Lai TS, Chiang WC, Lin SL, Chen YM, Chen CC, Chu TS. Emergency department utilization and resuscitation rate among patients receiving maintenance hemodialysis. J Formos Med Assoc, 2019; 118: 1652-1660 [DOI] [PubMed] [Google Scholar]
- 31).Hooker EA, Mallow PJ, Oglesby MM. Characteristics and trends of emergency department visits in the united states. J Emerg Med, 2019; 56: 344-351 [DOI] [PubMed] [Google Scholar]
- 32).Morcos R, Louka B, Tseng A, Misra S, McBane R, Esser H, Shamoun F. The evolving treatment of peripheral arterial disease through guideline-directed recommendations. J Clin Med, 2018; 7: 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Sachs T, Pomposelli F, Hamdan A, Wyers M, Schermerhorn M. Trends in the national outcomes and costs for claudication and limb threatening ischemia: angioplasty vs bypass graft. J Vasc Surg, 2011; 54: 1021-1031 [DOI] [PubMed] [Google Scholar]
- 34).Mustapha JA, Anose BM, Martinsen BJ, Pliagas G, Ricotta J, Boyes CW, Lee MS, Saab F, Adams G. Lower extremity revascularization via endovascular and surgical approaches: A systematic review with emphasis on combined inflow and outflow revascularization. SAGE Open Med, 2020; 8: 2050312120929239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Shiraki T, Iida O, Takahara M, Soga Y, Mii S, Okazaki J, Kuma S, Yamaoka T, Kamoi D, Shintani Y, Ishikawa T, Kitano I, Uematsu M. Comparison of clinical outcomes after surgical and endovascular revascularization in hemodialysis patients with critical limb ischemia. J Atheroscler Thromb, 2017; 24: 621-629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Okamoto S, Iida O, Mano T. Current perspective on hemodialysis patients with peripheral artery disease. Ann Vasc Dis, 2017; 10: 88-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Furuyama T, Onohara T, Yamashita S, Yoshiga R, Yoshiya K, Inoue K, Morisaki K, Kyuragi R, Matsumoto T, Maehara Y. Prognostic factors of ulcer healing and amputation-free survival in patients with critical limb ischemia. Vascular, 2018; 26: 626-633 [DOI] [PubMed] [Google Scholar]
- 38).Neel JD, Kruse RL, Dombrovskiy VY, Vogel TR. Cilostazol and freedom from amputation after lower extremity revascularization. J Vasc Surg, 2015; 61: 960-964 [DOI] [PubMed] [Google Scholar]
- 39).Niu PP, Guo ZN, Jin H, Xing YQ, Yang Y. Antiplatelet regimens in the long-term secondary prevention of transient ischaemic attack and ischaemic stroke: an updated network meta-analysis. BMJ Open, 2016; 6: e009013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Xie W, Zheng F, Zhong B, Song X. Long-term antiplatelet mono- and dual therapies after ischemic stroke or transient ischemic attack: network meta-analysis. J Am Heart Assoc, 2015; 4: e002259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Wang W, Zhang L, Liu W, Zhu Q, Lan Q, Zhao J. Antiplatelet agents for the secondary prevention of ischemic stroke or transient ischemic attack: a network meta-analysis. J Stroke Cerebrovasc Dis, 2016; 25: 1081-1089 [DOI] [PubMed] [Google Scholar]
- 42).Shi L, Pu J, Xu L, Malaguit J, Zhang J, Chen S. The efficacy and safety of cilostazol for the secondary prevention of ischemic stroke in acute and chronic phases in Asian population--an updated meta-analysis. BMC Neurol, 2014; 14: 251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).Edrissi H, Schock SC, Cadonic R, Hakim AM, Thompson CS. Cilostazol reduces blood brain barrier dysfunction, white matter lesion formation and motor deficits following chronic cerebral hypoperfusion. Brain Res, 2016; 1646: 494-503 [DOI] [PubMed] [Google Scholar]
- 44).Takagi T, Imai T, Mishiro K, Ishisaka M, Tsujimoto M, Ito H, Nagashima K, Matsukawa H, Tsuruma K, Shimazawa M, Yoshimura S, Kozawa O, Iwama T, Hara H. Cilostazol ameliorates collagenase-induced cerebral hemorrhage by protecting the blood-brain barrier. J Cereb Blood Flow Metab, 2017; 37: 123-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Wu CK, Lin JW, Wu LC, Chang CH. Risk of heart failure hospitalization associated with cilostazol in diabetes: a nationwide case-crossover study. Front Pharmacol, 2019; 9: 1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46).Ding XL, Xie C, Jiang B, Gao J, Zhang LL, Zhang H, Zhang JJ, Miao LY. Efficacy and safety of adjunctive cilostazol to dual antiplatelet therapy after stent implantation: an updated meta-analysis of randomized controlled trials. J Cardiovasc Pharmacol Ther, 2013; 18: 222-228 [DOI] [PubMed] [Google Scholar]
- 47).Jang JS, Jin HY, Seo JS, Yang TH, Kim DK, Kim DS, Kim DK, Seol SH, Kim DI, Cho KI, Kim BH, Park YH, Je HG, Jeong YH, Kim WJ, Lee JY, Lee SW. A meta-analysis of randomized controlled trials appraising the efficacy and safety of cilostazol after coronary artery stent implantation. Cardiology, 2012; 122: 133-143 [DOI] [PubMed] [Google Scholar]
- 48).Chen J, Meng H, Xu L, Liu J, Kong D, Chen P, Gong X, Bai J, Zou F, Yang Z, Li C, Eikelboom JW. Efficacy and safety of cilostazol based triple antiplatelet treatment versus dual antiplatelet treatment in patients undergoing coronary stent implantation: an updated meta-analysis of the randomized controlled trials. J Thromb Thrombolysis, 2015; 39: 23-24 [DOI] [PubMed] [Google Scholar]
- 49).Bangalore S, Singh A, Toklu B, DiNicolantonio JJ, Croce K, Feit F, Bhatt DL. Efficacy of cilostazol on platelet reactivity and cardiovascular outcomes in patients undergoing percutaneous coronary intervention: insights from a meta-analysis of randomised trials. Open Heart, 2014; 1: e000068 [DOI] [PMC free article] [PubMed] [Google Scholar]