See article vol. 30: 884-906
Accumulated epidemiological evidence has demonstrated that the consumption of fish is associated with reduced risk of atherosclerotic cardiovascular disease (ASCVD) 1) . The mechanism underlying the suppression of ASCVD by fish consumption is assumed to depend on the cardioprotective effect of omega-3 fatty acids. The effect of long-term use of eicosapentaenoic acid (EPA), one of the omega-3 fatty acids, on the prevention of major coronary events in patients with hypercholesterolaemia was demonstrated in Japan EPA Lipid Intervention Study (JELIS) 2) . Recently, reduction of cardiovascular events with icosapent ethyl-intervention trial (REDUCE-IT) has also proven the cardiovascular effect of EPA in strong-statin era 3) . Beyond their triglyceride-lowering effects, fish-derived omega-3 fatty acids appear to have diverse effects including anti-inflammatory properties. Previously, the INTERLIPID study, which compared lipid profiles and multiple dietary factors in the Japanese and the Japanese–Americans with similar genetic backgrounds, demonstrated a positive association between fish-derived omega-3 fatty acid intake and circulating high-density lipoprotein cholesterol (HDL-C) levels 4) . In this issue, Okami Y. et al have revealed that the intake of fish-derived omega-3 fatty acids was also associated with HDL subclass distribution using comprehensive lipid profile data in the INTERLIPID study provided by nuclear magnetic resonance (NMR) spectroscopy 5) . The present findings could provide a new explanation for the atheroprotective effects of fish-derived omega-3 fatty acids.
Over the years, numerous epidemiological evidence has demonstrated that low levels of circulating HDL-C are associated with an increased risk for atherosclerotic cardiovascular disease (ASCVD). On the contrary, in the Framingham Offspring Study, low HDL-C was not an ASCVD risk factor when levels of low-density lipoprotein cholesterol and triglycerides were not elevated 6) . Recently, a large contemporary biracial cohort has proven that low HDL-C was associated with increased ASCVD risk in White but not Black adults 7) . Moreover, several studies have revealed that very high HDL-C levels are paradoxically associated with high ASCVD mortality 8) . Under these circumstances, establishment of novel measures for HDL evaluation has garnered a lot of interest.
Until now, enough evidence suggests that HDL particle concentration can be superior to HDL-C as a predictor of ASCVD incidence 9) . On the contrary, the data regarding the relationship between HDL subclasses and ASCVD risk are conflicting ( Fig.1 ) . HDLs are heterogeneous particles that differ in size, density, and charge; they are formed as small, dense, lipid-poor discoid particles, which can be classified as HDL3. After interaction with lecithin:cholesterol acyltransferase (LCAT), which generates hydrophobic core of cholesteryl esters, discoid HDL becomes large, light, lipid-rich spherical particles, HDL2 10) . Most of the previous reports found that greater concentration of blood HDL2 subfracion has inverse association with the ASCVD risk, while that of HDL3 subfraction appears to be associated with increased risk 11) . A plausible reason for the association of lower HDL2 to increased ASCVD risk can be cholesterol efflux capacity of larger HDL 11) . HDL biogenesis and maturation is coordinated by ATP-binding cassette transporter A1 (ABCA1) and G1 (ABCG1), and scavenger receptor class B type I (SR-BI); ABCA1 initially mediates the export of cellular cholesterol and phospholipids to nascent HDL and ABCG1 and SR-BI subsequently provide additional cholesterol to larger HDL 12) . The Chicago Healthy Aging study revealed that HDL particle size was significantly associated with HDL efflux capacity 13) . In the hypertriglyceridemia state, a reduction in the number of large HDL particles, and concomitant impairment of SR-BI-dependent efflux was observed 14) . Large HDL2 also decreased in patients with insulin resistance and type 2 diabetes 15) suggesting that impairment of HDL maturation has occurred in the pathological conditions. Conversely, contradictory results have also been reported wherein lower HDL3 subfracion is associated with increased ASCVD risk 16) . From the aspect of biological activities, small, dense, protein-rich HDL particles are thought to display potent atheroprotective properties 10) . It has been demonstrated that HDL3 particles are closely associated with paraoxonase 1 (PON1) and are strong antioxidants 17) . These discrepancy in the relationship between HDL particle size and ASCVD risk may be accounted for by different techniques for evaluation of HDL subclasses, such as ultracentrifugation, gel electrophoresis, ion mobility, and NMR spectroscopy. Moreover, because HDL particles are highly heterogeneous in structure, composition, metabolism, and biological activity, measurement of particle size may be insufficient to assess HDL characterization for ASCVD risk discrimination. For instance, phospholipids are major components of the mature HDL, accounting for 40%–60% of the total HDL lipids, and phospholipid content of HDL particle is a major factor determining cholesterol removing capacity of HDL 18 , 19) . Alternatively, cholesterol-overloaded, larger HDL particle was associated with the progression of carotid atherosclerosis and increased risk of cardiovascular death 20 , 21) .
Fig.1.
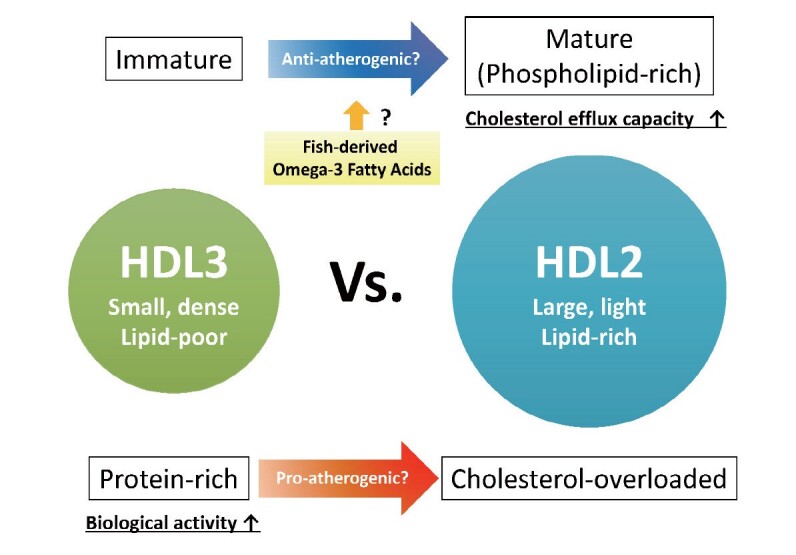
The complex relationship between HDL particle size and atherosclerosis
In the present issue of the JAT, the INTERLIPID study has found that high consumption of fish among Japanese was related to lower the quantity of the smallest HDL particles. Although precise mechanisms underlying the modification of HDL particle size by omega-3 fatty acids remain unclear, previous studies also demonstrate that the increase of fish consumption correlated with the increased concentration of large HDL particles 22) . On the other hand, recent studies have revealed that EPA also modified biological activities of HDL. The orally administered EPA was efficiently incorporated into the HDL particles, increased activity of anti-oxidative enzyme, PON1, and augmented anti-inflammatory properties and cholesterol efflux capacity of HDL 23) . In addition, the EPA-enriched HDL particles exhibit cardioprotective properties via the production of anti-inflammatory lipid metabolites, resolvin E3 24) . However, because the INTERLIPID study was a cross-sectional study, the authors were not able to conclude if the effect of fish-derived omega-3 fatty acids intake on HDL subclass distribution is beneficial or not against atherosclerosis. Further investigations are required to elucidate whether HDL modification induced by fish consumption actually contributes to the reduction of ASCVD incidence.
Conflict of Interest
The Division of Evidence-based Laboratory Medicine, Kobe University Graduate School of Medicine, was established by an endowment fund from the Sysmex Corporation.
References
- 1).He K, Song Y, Daviglus ML, Liu K, Van Horn L, Dyer AR and Greenland P: Accumulated evidence on fish consumption and coronary heart disease mortality: a meta-analysis of cohort studies. Circulation, 2004; 109: 2705-2711 [DOI] [PubMed] [Google Scholar]
- 2).Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K and Japan EPAlisI: Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet, 2007; 369: 1090-1098 [DOI] [PubMed] [Google Scholar]
- 3).Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT, Jr., Juliano RA, Jiao L, Granowitz C, Tardif JC, Ballantyne CM and Investigators R-I: Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N Engl J Med, 2019; 380: 11-22 [DOI] [PubMed] [Google Scholar]
- 4).Okuda N, Ueshima H, Okayama A, Saitoh S, Nakagawa H, Rodriguez BL, Sakata K, Choudhury SR, Curb JD, Stamler J and Group IR: Relation of long chain n-3 polyunsaturated fatty acid intake to serum high density lipoprotein cholesterol among Japanese men in Japan and Japanese-American men in Hawaii: the INTERLIPID study. Atherosclerosis, 2005; 178: 371-379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Okami Y, Chan Q, Miura K, Kadota A, Elliott P, Masaki K, Okayama A, Okuda N, Yoshita K, Miyagawa N, Okamura T, Sakata K, Saitoh S, Sakurai M, Nakagawa H, Stamler J, Ueshima H, Intermap and Groups IR: Small High-Density Lipoprotein and Omega-3 Fatty Acid Intake Differentiates Japanese and Japanese-Americans: The INTERLIPID Study. J Atheroscler Thromb, 2023; 30: 884-906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Bartlett J, Predazzi IM, Williams SM, Bush WS, Kim Y, Havas S, Toth PP, Fazio S and Miller M: Is Isolated Low High-Density Lipoprotein Cholesterol a Cardiovascular Disease Risk Factor? New Insights From the Framingham Offspring Study. Circ Cardiovasc Qual Outcomes, 2016; 9: 206-212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Zakai NA, Minnier J, Safford MM, Koh I, Irvin MR, Fazio S, Cushman M, Howard VJ and Pamir N: Race-Dependent Association of High-Density Lipoprotein Cholesterol Levels With Incident Coronary Artery Disease. J Am Coll Cardiol, 2022; 80: 2104-2115 [DOI] [PubMed] [Google Scholar]
- 8).Liu C, Dhindsa D, Almuwaqqat Z, Ko YA, Mehta A, Alkhoder AA, Alras Z, Desai SR, Patel KJ, Hooda A, Wehbe M, Sperling LS, Sun YV and Quyyumi AA: Association Between High-Density Lipoprotein Cholesterol Levels and Adverse Cardiovascular Outcomes in High-risk Populations. JAMA Cardiol, 2022; 7: 672-680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Singh K, Chandra A, Sperry T, Joshi PH, Khera A, Virani SS, Ballantyne CM, Otvos JD, Dullaart RPF, Gruppen EG, Connelly MA, Ayers CR and Rohatgi A: Associations Between High-Density Lipoprotein Particles and Ischemic Events by Vascular Domain, Sex, and Ethnicity: A Pooled Cohort Analysis. Circulation, 2020; 142: 657-669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Camont L, Chapman MJ and Kontush A: Biological activities of HDL subpopulations and their relevance to cardiovascular disease. Trends Mol Med, 2011; 17: 594-603 [DOI] [PubMed] [Google Scholar]
- 11).Superko HR, Pendyala L, Williams PT, Momary KM, King SB, 3rd and Garrett BC: High-density lipoprotein subclasses and their relationship to cardiovascular disease. J Clin Lipidol, 2012; 6: 496-523 [DOI] [PubMed] [Google Scholar]
- 12).Toh R: Assessment of HDL Cholesterol Removal Capacity: Toward Clinical Application. J Atheroscler Thromb, 2019; 26: 111-120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Mutharasan RK, Thaxton CS, Berry J, Daviglus ML, Yuan C, Sun J, Ayers C, Lloyd-Jones DM and Wilkins JT: HDL efflux capacity, HDL particle size, and high-risk carotid atherosclerosis in a cohort of asymptomatic older adults: the Chicago Healthy Aging Study. J Lipid Res, 2017; 58: 600-606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Asztalos BF, Horvath KV, Mehan M, Yokota Y and Schaefer EJ: Influence of HDL particles on cell-cholesterol efflux under various pathological conditions. J Lipid Res, 2017; 58: 1238-1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Garvey WT, Kwon S, Zheng D, Shaughnessy S, Wallace P, Hutto A, Pugh K, Jenkins AJ, Klein RL and Liao Y: Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes, 2003; 52: 453-462 [DOI] [PubMed] [Google Scholar]
- 16).Kim DS, Burt AA, Rosenthal EA, Ranchalis JE, Eintracht JF, Hatsukami TS, Furlong CE, Marcovina S, Albers JJ and Jarvik GP: HDL-3 is a superior predictor of carotid artery disease in a case-control cohort of 1725 participants. J Am Heart Assoc, 2014; 3: e000902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Bergmeier C, Siekmeier R and Gross W: Distribution spectrum of paraoxonase activity in HDL fractions. Clin Chem, 2004; 50: 2309-2315 [DOI] [PubMed] [Google Scholar]
- 18).Fournier N, Paul JL, Atger V, Cogny A, Soni T, de la Llera-Moya M, Rothblat G and Moatti N: HDL phospholipid content and composition as a major factor determining cholesterol efflux capacity from Fu5AH cells to human serum. Arterioscler Thromb Vasc Biol, 1997; 17: 2685-2691 [DOI] [PubMed] [Google Scholar]
- 19).Iino T, Toh R, Nagao M, Shinohara M, Harada A, Murakami K, Irino Y, Nishimori M, Yoshikawa S, Seto Y, Ishida T and Hirata KI: Effects of Elaidic Acid on HDL Cholesterol Uptake Capacity. Nutrients, 2021; 13: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Qi Y, Fan J, Liu J, Wang W, Wang M, Sun J, Liu J, Xie W, Zhao F, Li Y and Zhao D: Cholesterol-overloaded HDL particles are independently associated with progression of carotid atherosclerosis in a cardiovascular disease-free population: a community-based cohort study. J Am Coll Cardiol, 2015; 65: 355-363 [DOI] [PubMed] [Google Scholar]
- 21).Teis A, Cediel G, Amigo N, Julve J, Aranyo J, Andres-Cordon J, Puig-Jove C, Castelblanco E, Gual-Capllonch F, Ferrer-Sistach E, Vallejo N, Junca G, Lopez-Ayerbe J, De Antonio M, Domingo M, Santiago-Vacas E, Codina P, Mauricio D, Lupon J, Alonso N and Bayes-Genis A: Particle size and cholesterol content of circulating HDL correlate with cardiovascular death in chronic heart failure. Sci Rep, 2021; 11: 3141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Lankinen M, Kolehmainen M, Jaaskelainen T, Paananen J, Joukamo L, Kangas AJ, Soininen P, Poutanen K, Mykkanen H, Gylling H, Oresic M, Jauhiainen M, Ala-Korpela M, Uusitupa M and Schwab U: Effects of whole grain, fish and bilberries on serum metabolic profile and lipid transfer protein activities: a randomized trial (Sysdimet). PLoS One, 2014; 9: e90352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Tanaka N, Ishida T, Nagao M, Mori T, Monguchi T, Sasaki M, Mori K, Kondo K, Nakajima H, Honjo T, Irino Y, Toh R, Shinohara M and Hirata K: Administration of high dose eicosapentaenoic acid enhances anti-inflammatory properties of high-density lipoprotein in Japanese patients with dyslipidemia. Atherosclerosis, 2014; 237: 577-583 [DOI] [PubMed] [Google Scholar]
- 24).Tanaka N, Irino Y, Shinohara M, Tsuda S, Mori T, Nagao M, Oshita T, Mori K, Hara T, Toh R, Ishida T and Hirata KI: Eicosapentaenoic Acid-Enriched High-Density Lipoproteins Exhibit Anti-Atherogenic Properties. Circ J, 2018; 82: 596-601 [DOI] [PubMed] [Google Scholar]


