Abstract
Aim: Sampsonet al. proposed a method to calculate small dense low-density lipoprotein-cholesterol (sdLDL-C) concentrations using common lipid measurements, but its accuracy remains unresolved. We examined the difference between Sampson’s equation and direct measurement in patients with diabetes.
Methods: sdLDL-C was measured directly by our established homogeneous assay and estimated by Sampson’s equation in patients with diabetes (n=1542) and healthy control subjects (n=673). Large-buoyant (lb)LDL-C was estimated using triglycerides and LDL-C, and sdLDL-C was obtained by subtracting lbLDL-C from LDL-C. The effect of fasting/nonfasting state or lipid-lowering drug therapy on sdLDL-C values was also examined in 30 and 43 patients with diabetes, respectively.
Results: The coefficient of determination (R2) between calculated sdLDL-C and direct measurement was 0.73 and 0.61 for healthy controls and patients with diabetes, respectively. The R2 between calculated sdLDL-C and nonHDL-C or apolipoprotein B was 0.64 and 0.65, respectively. Calculated sdLDL-C was 4–5 mg/dl or 17%–18% higher than the direct measurement. The lower the plasma lipids, especially sdLDL-C, the greater the dissociation between the two methods. Sampson sdLDL-C was also found to give a positive bias when calculated for the nonfasting samples. Statins and pemafibrate significantly reduced sdLDL-C, but their therapeutic effect was underestimated by 5 mg/dl (24%) via Sampson’s equation.
Conclusions: The correlation between Sampson’s equation and direct measurements of sdLDL-C was reduced in patients with diabetes. Furthermore, the correlations with nonHDL-C and apolipoprotein B were even higher than those with direct sdLDL-C. The accuracy of Sampson’s equation decreased with lower sdLDL-C concentrations and was also influenced by diet.
Keywords: Small dense LDL, Cholesterol, Triglycerides, Diabetes, Sampson’s equation
Introduction
Low-density lipoprotein (LDL) is an atherogenic lipoprotein and a strong risk factor for coronary artery disease (CAD) 1) . LDL consists of small dense (sd)LDL and large-buoyant (lb)LDL particles 2) . sdLDL particles are known to promote atherosclerosis more than lbLDL particles 3 , 4) , and the predominance of the sdLDL phenotype (pattern B) is more strongly associated with the development of CAD than the lbLDL phenotype (pattern A) as measured via polyacrylamide gel electrophoresis (PAGE) 2) . We have developed the measurement of sdLDL from a qualitative method (PAGE) to a quantitative method, a fully automated assay that directly measures sdLDL-cholesterol (C) 5) . This assay system was adopted in famous cohort studies, such as the community atherosclerosis risk study 6) , the multi-ethnic study of atherosclerosis 7) , and the Hisayama study 8) , which all have consistently revealed that sdLDL-C is superior to LDL-C in predicting CAD. Recently, Sampson et al. 9) proposed an equation for estimating sdLDL-C by common lipid measurements using our sdLDL-C direct assay as a reference. Briefly, lbLDL-C is estimated using triglycerides (TGs) and LDL-C, and sdLDL-C is obtained by subtracting lbLDL-C from LDL-C. Since plasma TGs are the primary determinants of LDL particle size and buoyancy 3 , 10) and sdLDL-C is part of LDL-C 2) , sdLDL-C should be related to both TG and LDL-C. Nevertheless, many other lipid metabolisms are also involved in the formation of sdLDL particles, including the rate of very-low-density lipoprotein (VLDL) production, VLDL heterogeneity, the VLDL-LDL cascade, and cholesteryl-ester transfer protein activity 3 , 10 , 11) . Diabetes is a typical disease with high sdLDL-C concentrations, and various abnormal lipid metabolisms are involved in diabetic dyslipidemia 12 , 13) . Thus, it is largely unknown whether this simple equation, based solely on TGs and LDL-C concentrations, can be applied to estimate sdLDL-C in a diabetic population.
In the present study, we examined the accuracy of Sampson’s equation for sdLDL-C (Sampson sdLDL-C) in a large number of patients with diabetes and compared it with that of healthy subjects without diabetes. Since TG is the primary variable in this equation and TG increases postprandially, we examined whether Sampson sdLDL is affected by diet to determine the need for fasting measurements. It is also anticipated that Sampson’s equation will be used in future clinical trials to investigate the effect of lipid-lowering manipulations on sdLDL-C. Therefore, we compared the effect of lipid-lowering drugs (statins and pemafibrate) on sdLDL-C values obtained using two different methods in patients with diabetes.
Methods
Patients with diabetes (n=1542), including 51 type 1 diabetes, participated in the “ViNA” cohort study aimed to investigate the prognosis of patients with diabetes at Ebina General Hospital 14) . The ViNA cohort study began on October 1, 2019, and the baseline blood samples were collected from October 2019 to December 2021. The patient characteristics are listed in Table 1 . Of the patients, a history of CAD and stroke was found in 10% and 7.7%, respectively. Three hundred and forty (22%) patients were insulin users. Most patients with type 2 diabetes were treated with the following hypoglycemic agents alone or in combination: a sulfonylurea (n=405, 26%), metformin (n=718,46%), pioglitazone (n=77, 5%), dipeptidase peptidase-4 inhibitors (n=983, 64%), sodium-glucose cotransporter-2 inhibitors (n=395, 25%), or glucagon-like peptide-1 receptor agonist (n=60, 4%). Subjects with hyperlipidemia were treated with statins (n=828, 54%), ezetimibe (n=114, 7%), fibrates (n=111, 7%), or omega-3 fatty acids (n=67, 4%) alone or in combination. All patients are instructed on an appropriate diet proposed by the Japan Diabetes Foundation by a dietitian. The healthy participants (n=673) were staff members of Denka Co. The health status of the participants was assessed in a recent medical checkup. They were basically healthy and did not include diabetes, but hyperlipidemic individuals were allowed to enroll. For convenience, we defined these participants as “healthy controls,” although they also included those with hypertension and mild liver dysfunction. CAD included myocardial infarction, stable angina, and unstable angina, diagnosed by a cardiologist. Stroke included ischemic stroke, cardiogenic cerebral thrombosis, and cerebral hemorrhage, diagnosed by a neurologist or neurosurgeon. Dyslipidemia was diagnosed as LDL-C≧140 mg/dl, fasting TG≧150 mg/dl, HDL-C<40 mg/dl, or use of lipid-lowering drugs. Hypertension was diagnosed with systolic blood pressure≧140 mmHg, diastolic blood pressure≧90 mmHg, or antihypertensive drugs. Liver dysfunction was diagnosed with ALT≧n 40 IU.
Table 1. General clinical characteristics, plasma lipid measurements, and LDL-C and sdLDL-C measured by direct or Samp-son’ estimation methods.
| Diabetic patients | Healthy controls | p | |||
|---|---|---|---|---|---|
| number (male; female) | 1542 (982: 560) | 673 (416: 257) | |||
| mean | SD | mean | SD | ||
| Age year | 65 | 11 | 39 | 11 | <0.0001 |
| BMI kg/m2 | 25.2 | 4.2 | 22.4 | 3.4 | <0.0001 |
| History of CAD n (%) | 161 (10) | 0 | <0.0001 | ||
| Histry of stroke n (%) | 119 (7.7) | 0 | <0.0001 | ||
| Glucose mg/dl | 150 | 41 | 82 | 8 | <0.0001 |
| HbA1c % | 7.3 | 0.8 | 5.3 | 0.2 | <0.0001 |
| eGFR mL/min/1.73m2 | 71 | 20 | 82 | 14 | <0.0001 |
| Lipids (mg/dl) | |||||
| Total-C | 181 | 31 | 202 | 35 | <0.0001 |
| TG | 130 | 104 | 95 | 75 | <0.0001 |
| Ln TG | 4.6 | 0.5 | 4.3 | 0.5 | <0.0001 |
| Hyper-TG >150mg/dl, n (%) | 410 (26) | 88 (13) | <0.0001 | ||
| Severe Hyper-TG >400mg/dl, n (%) | 29 (1.9) | 9 (1.3) | <0.05 | ||
| Direct LDL-TG | 15 | 7 | na | ||
| HDL-C | 54 | 14 | 61 | 16 | <0.0001 |
| TRL-C | 25 | 13 | 19 | 15 | <0.0001 |
| non-HDL-C | 127 | 29 | 141 | 35 | <0.0001 |
| Direct LDL-C | 101 | 25 | 123 | 34 | <0.0001 |
| Sampson LDL-C | 117 | 30 | 124 | 31 | <0.0001 |
| Direct sdLDL-C | 31 | 14 | 29 | 16 | 0.0001 |
| Sampson sdLDL-C | 36 | 12 | 32 | 13 | <0.0001 |
| apolipoprotein B | 87 | 18 | na | ||
| apolipoprotein CIII | 11.2 | 4.4 | na | ||
| apolipoprotein E | 4.1 | 1.3 | na | ||
| Lipoprotein (a) | 20 | 22 | na | ||
Data represent mean±standard deviation (SD) or n (%). P; Diabetic patients vs. healthy controls (Unpaired Student’s t-test, Man-Whitney U-test, or chi-squire test). na = not available, ns = not significant, CAD = coronary artery disease, eGFR = estimated glomerular filtration rate, TG = triglycerides, C = cholesterol, TRL = TG-rich lipoprotein, Ln = natural logarithm
In some patients with diabetes (n=30), nonfasting plasma (1–4 h after breakfast) lipid levels were retrospectively studied on a different day from the blood drawing of the fasting samples. The interval between the two blood samples was 2 months. In another subset of patients with diabetes (n=43), plasma lipid levels were also retrospectively studied before and 2–4 months after statin (n=18) or pemafibrate (0.2 mg/d) (n=25) administration. Statins used were pitavastatin 1 mg/d (n=8), atorvastatin 5–10 mg (n=4), and rosuvastatin (2.5 mg/d) (n=6).
The study complied with the principal of the Declaration of Helsinki. The study was explained in detail to all subjects who agreed to participate, and written informed consent was obtained from all patients with diabetes and healthy controls. This study was approved by the Ebina General Hospital Ethics Committee and by the internal regulations of Denka Co.
Measurements
Plasma samples were taken in the morning after overnight fasting. sdLDL-C and LDL-TG concentrations were measured directly in plasma using the homogeneous method established by our group (Denka Co. Tokyo). The principles of these assays have been fully explained previously 5 , 15) . Sampson’s equation for sdLDL-C was as follows: (1) Large-buoyant (lb)LDL-C=1.43×LDL-C-[0.14×{In(triglycerides}×LDL-C)]-8.99. (2) sdLDL-C=LDL-C-lbLDL-C 9) . LDL-C was calculated using Sampson’s equation for LDL-C as follows: LDL-C=Total-C/0.948-HDL-C/0.971-(TG/8.56+TG X nonHDL-C/2140-TG2/16100)-9.44 16) . All blood samples, including direct sdLDL-C and direct LDL-TG, were immediately measured with an automated analyzer without storage in the refrigerator. NonHDL-C was calculated by subtracting HDL-C from Total-C, and TG-rich lipoprotein (TRL)-C was calculated by subtracting LDL-C and HDL-C from Total-C. Direct LDL-C, apolipoproteins (apo), lipoprotein (Lp) (a), HbA1c, and creatinine were measured using commercially available test kits.
Statistics
All continuous variables were expressed as mean±SD or median (interquartile range). Differences between the two groups were examined with Student’s t-test or Mann–Whitney U-test. The Chi-square test was used on categorical variables. The p-trend was estimated using the Jonckheere–Terpstra trend test for differences in sdLDL-C stratified using sdLDL-C quartiles. A simple linear regression analysis was performed to calculate the coefficient of determination (R2) between the two variables. A P-value of less than 0.05 was considered statistically significant. Analyses were performed using JMP software version 15 (SAS Institute, Cary, NC, USA).
Results
Table 1 shows general clinical characteristics and plasma lipid measurements in patients with diabetes and healthy controls. The concentrations of LDL-C and sdLDL-C were directly measured or calculated using Sampson’s equation. Diabetic patients were older and had higher body mass index and hyperglycemia but lower estimated glomerular filtration rate (eGFR) than healthy controls. In the general lipid profile, patients with diabetes had higher TG and TRL-C and lower total-C, HDL-C, and nonHDL-C than healthy controls. The prevalence of hypertriglyceridemia (TG >150 mg/dl) and severe hypertriglyceridemia (TG >400 mg/dl) was higher in patients with diabetes than in healthy controls (26% vs. 13%, 1.9 vs. 1.3%). Direct LDL-C and calculated LDL-C were lower in patients with diabetes than in healthy controls. A possible reason for the lower total-C, LDL-C, and nonHDL-C in patients with diabetes is that more than half of them are taking statins. Contrary to LDL-C, sdLDL-C, determined using the direct method or Sampson’s equation, was significantly higher in patients with diabetes than in healthy controls. LDL-TG, apolipoproteins, and Lp(a) were not measured in the healthy controls, so intergroup comparison of these measurements was not possible.
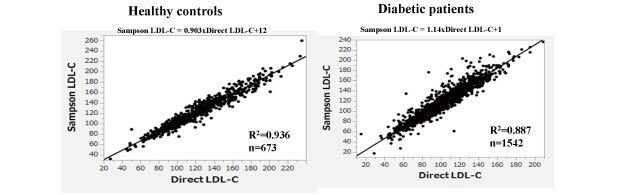
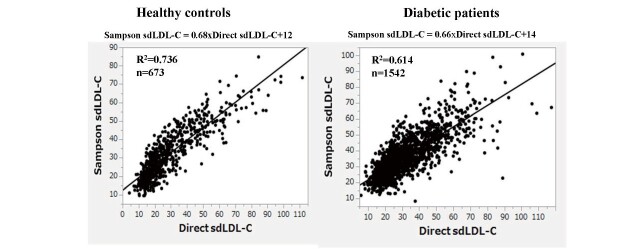
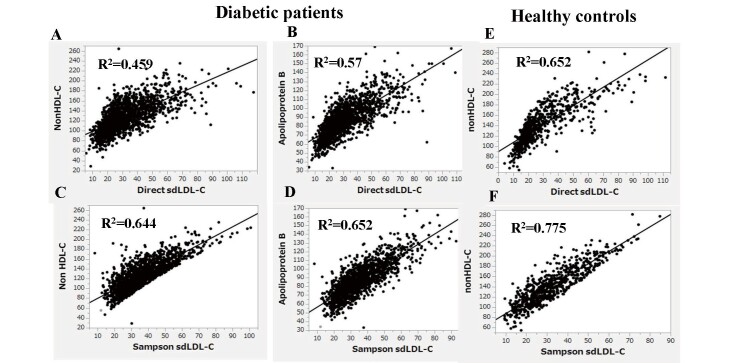
Sampson’s LDL-C equation exhibited a substantially high correlation with direct LDL-C in healthy controls and patients with diabetes (R2=0.936 and 0.887, respectively) ( Supplemental Fig.1 ) . Sampson’s calculated sdLDL-C also showed high correlations with direct sdLDL-C in healthy controls and patients with diabetes (R2=0.614 and 0.736, respectively) ( Fig.1 ) . Nevertheless, both of these correlations were weaker than the LDL-C correlations and even weaker in the patients with diabetes than in the healthy controls, even though the patients with diabetes were more than twice as large. It is well known that nonHDL-C and apo B are strongly correlated with sdLDL-C 17) . Therefore, we tested whether Sampson’s equation could estimate sdLDL-C better than nonHDL-C and apo B in patients with diabetes. As shown in Fig.2 (A, B) , the R2 between direct sdLDL-C and nonHDL-C or apoB was 0.459 and 0.570, respectively. The same analysis was conducted using the Sampson sdLDL-C, and the R2 was obtained as 0.644 to nonHDL-C, and 0.653 to apo B ( Fig.2, C, D ) , both of which were higher than what was calculated between Sampson sdLDL-C and direct method R2 (R2=0.614). We also tested whether Sampson’s equation could estimate sdLDL-C better than nonHDL-C in healthy controls. The R2 between direct sdLDL-C and nonHDL-C was 0.652 ( Fig.2, E ) , whereas that between Sampson sdLDL-C and nonHDL-C was 0.775 ( Fig.2, F ) , which were higher than the correlation between Sampson sdLDL-C and direct method R2 (R2=0.736).
Supplemental Fig.1.
Correlation between direct LDL-C and Sampson’s LDL-C equation in healthy controls and in patients with diabetes
Fig.1.
Correlation between direct sdLDL-C and Sampson sdLDL-C in healthy controls and diabetic subjects
Fig.2. Correlation between direct sdLDL-C or Sampson sdLDL-C and nonHDL-C or apolipoprotein B in patients with diabetes (A, B, C, D).
Correlation between direct sdLDL-C or Sampson sdLDL-C and nonHDL-C in healthy control subjects (E, F)
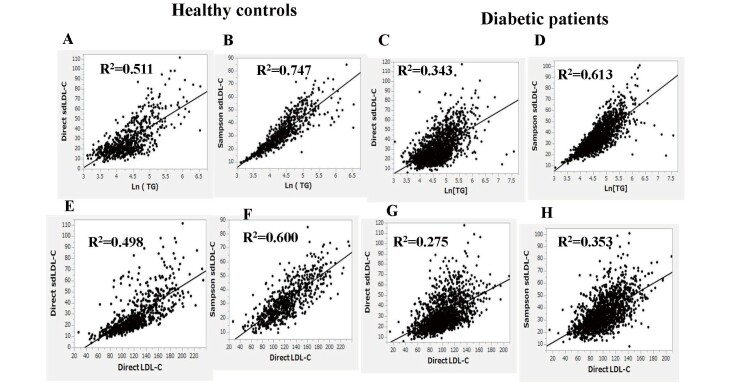
Fig.3 shows the correlation between natural logarithm-transformed (Ln)TG or direct LDL-C and direct sdLDL-C or Sampson sdLDL-C in healthy controls (A, B, E, F) and in patients with diabetes (C, D, G, H). Direct sdLDL-C correlated highly with Ln(TG) or direct LDL-C in healthy controls (R2=0.511 and 0.498, respectively) (A and E), but these correlations were considerably reduced in patients with diabetes (R2=0.343 and 0.275, respectively) (C and G). Sampson sdLDL-C correlated more strongly with Ln(TG) and direct LDL-C than direct sdLDL-C in healthy controls (R2=0.747, 0.600) (B, F) and patients with diabetes (R2=0.613, 0.353) (D, H).
Fig.3.
Correlation between log-transformed (Ln)TG or direct LDL-C and direct sdLDL-C or Sampson sdLDL-C in healthy controls (A, B, E, F) and patients with diabetes (C, D, G, H)
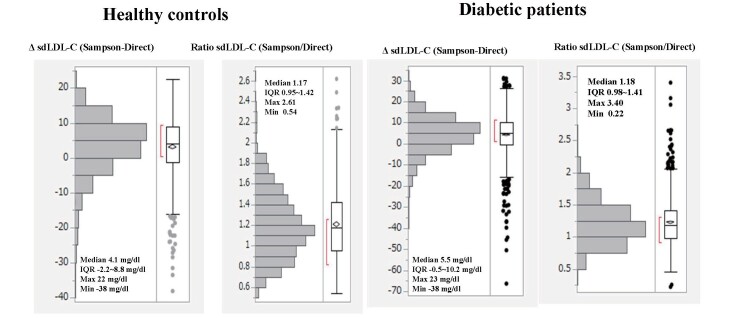
Fig.4 shows the distribution of the difference (Δ) in sdLDL-C (Sampson sdLDL-C minus direct sdLDL-C) or that of Sampson sdLDL-C/direct sdLDL-C ratio in healthy controls and patients with diabetes. The median ΔsdLDL-C was 4.1 mg/dl in healthy controls and 5.5 mg/dl in patients with diabetes, which was significantly higher in patients with diabetes (p<0.0001, Mann–Whitney U-test). Sampson sdLDL-C was significantly (p<0.0001) higher than direct sdLDL-C by 17%–18% in both subjects.
Fig.4.
Distributions of difference (Δ) in sdLDL-C (Sampson minus direct) or distributions of the ratio of Sampson sdLDL-C over direct sdLDL-C in healthy controls and patients with diabetes
Table 2 shows the correlation between the difference or ratio (Sampson sdLDL-C vs. direct sdLDL-C) and the various measurements. In patients with diabetes, ΔsdLDL-C (Sampson-direct) was negatively correlated with TG, HDL-C, TRL-C, direct sdLDL-C, apoB, apoCIII, and eGFR and positively correlated with Lp(a). The ratio of sdLDL-C (Sampson/direct) in patients with diabetes was negatively correlated with total-C, nonHDL-C, direct sdLDL-C, apoB, apoCIII, HbA1c, and eGFR but positively correlated with apoE and Lp(a). In healthy controls, all lipid variables were negatively correlated with ΔsdLDL-C or the ratio of sdLDL-C. Overall, the dissociation between Sampson’s equation and direct measurements of sdLDL-C was greater at lower plasma lipids, with direct sdLDL-C being most strongly and consistently associated with the dissociation.
Table 2. Correlation of the difference or ratio (Sampson sdLDL-C vs. direct sdLDL-C) with the various measurements.
| Measurements | Diabetic patients | Healthy controls | ||||||
|---|---|---|---|---|---|---|---|---|
| ΔsdLDL-C | Ratio of sdLDL-C | ΔsdLDL-C | Ratio of sdLDL-C | |||||
| r | p | r | p | r | p | r | p | |
| Total-C | -0.2 | ns | -0.43 | <0.0001 | -0.25 | <0.0001 | -0.37 | <0.0001 |
| TG | -0.23 | <0.05 | -0.06 | ns | -0.23 | <0.0001 | -0.13 | <0.001 |
| Direct LDL-TG | -0.08 | ns | -0.12 | ns | na | na | ||
| Direct LDL-C | -0.04 | ns | -0.16 | <0.0001 | -0.18 | <0.0001 | -0.3 | <0.0001 |
| HDL-C | -0.37 | <0.0001 | -0.36 | <0.0001 | -0.1 | <0.05 | 0.15 | <0.0001 |
| TRL-C | -0.28 | <0.05 | -.09 | ns | -0.19 | <0.0001 | -0.11 | <0.01 |
| nonHDL-C | -0.04 | ns | -0.39 | <0.0001 | -0.21 | <0.0001 | -0.3 | <0.0001 |
| Direct sdLDL-C | -0.73 | <0.0001 | -0.75 | <0.0001 | -0.62 | <0.0001 | -0.54 | <0.0001 |
| Sampson sdLDL-C | 0.1 | ns | 0.2 | ns | 0.13 | <0.001 | 0.13 | <0.001 |
| apo B | -0.32 | <0.0001 | -0.5 | <0.0001 | na | na | ||
| apo CIII | -0.36 | <0.0001 | -0.45 | <0.0001 | na | na | ||
| apo E | 0.13 | ns | 0.25 | <0.05 | na | na | ||
| Lp(a) | 0.3 | <0.01 | 0.3 | <0.0001 | na | na | ||
| HbA1c | -0.04 | ns | -0.23 | <0.05 | -0.03 | ns | -0.03 | ns |
| eGFR | -0.45 | <0.0001 | -0.2 | <0.0001 | -0.04 | ns | -0.02 | ns |
| ΔsdLDL-C | 0.92 | <0.0001 | 0.85 | <0.0001 | ||||
Δ: difference in sdLDL-C (Sampson minus direct), Ratio: Sampson sdLDL-C/direct sdLDL-C, na= not available, ns = not significant, apo = apolipoprotein.
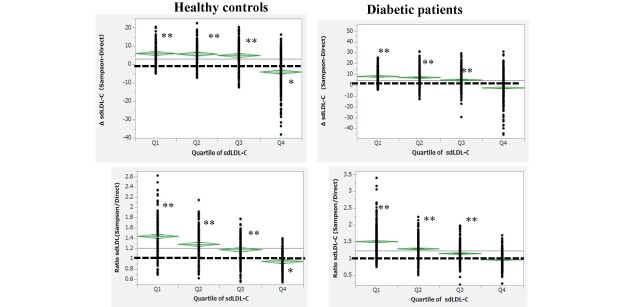
The difference (Δ) and ratio (Sampson vs. direct) of sdLDL-C stratified by quartiles (Q1, Q2, Q3, and Q4) of direct sdLDL-C concentrations in healthy controls and patients with diabetes are shown in Fig.5 . Concentrations in the direct sdLDL-C quartiles were as follows: Q1, 2.4–18.1; Q2, 18.2–23.4; Q3, 23.4–36; and Q4, 36.2–111.6 mg/dl for healthy controls and Q1, 5.9–21.2; Q2, 21.3–27.9; Q3, 28–39; and Q4, 39.1–117.6 mg/dl for patients with diabetes. ΔsdLDL-C and the ratio of Sampson sdLDL-C to direct sdLDL-C were all increased with lower quartiles of direct sdLDL-C (p-trend<0.01). ΔsdLDL-C in healthy controls was <0 in Q4 (p<0.001) but >0 in Q1, Q2, and Q3 (p<0.0001). In patients with diabetes, ΔsdLDL-C was ~0 in Q4 but significantly (p<0.0001) >0 in Q1, Q2, and Q3. The ratio of Sampson sdLDL-C to direct sdLDL-C in Q4 was ~1-fold but higher in the lower quartile in both groups and 40%–50% higher in Q1 than in Q4.
Fig.5. Difference (Δ) or the ratio of sdLDL-C (Sampson vs. direct) stratified by the quartile of direct sdLDL-C concentrations (Q1,2,3,4) in healthy controls and patients with diabetes.
The concentration of direct sdLDL-C was as follows: Q1, 2.4–18.1; Q2, 18.2–23.4; Q3, 23.4–36; and Q4, 36.2–111.6 mg/dl for healthy controls, and Q1, 5.9–21.2; Q2, 21.3–27.9; Q3, 28–39; and Q4, 39.1–117.6 mg/dl for patients with diabetes. ΔsdLDL-C and the ratio of sdLDL-C are all increased with lower quartiles of direct sdLDL-C (p-trend <0.01 estimated using the Jonckheere–Terpstra trend test). *p<0.001 and **p<0.0001 vs. 0 mg/dl for ΔsdLDL and 1 for the ratio of sdLDL-C (paired t-test)
Table 3 compares fasting and nonfasting values of direct sdLDL-C and Sampson sdLDL-C in 30 patients with diabetes. TG values were significantly (p<0.05) higher in nonfasting than in fasting plasma. Direct LDL-C and Sampson LDL-C values were slightly lower in nonfasting than in fasting, but the difference was not statistically significant. Sampson sdLDL-C levels were significantly (p<0.0001) higher in nonfasting than in fasting, whereas direct sdLDL-C levels did not change.
Table 3. Comparison between fasting and non-fasting values of direct sdLDL-C and Sampson sdLDL-C in 30 diabetic patients.
| Fasting | Non-fasting | Δ | |
|---|---|---|---|
| TG | 114 (50) | 181 (124) | 67 (100)** |
| Direct LDL-C | 102 (25) | 97 (17) | -6 (17) |
| Sampson LDL-C | 118 (30) | 115 (26) | -2 (22) |
| Direct sdLDL-C | 30 (9) | 33 (13) | 3 (9) |
| Sampson sdLDL-C | 35 (10) | 40 (10) | 5 (6)*** |
Δ: difference (Non-fasting minus fasting). *p<0.05 and ***P<0.0001 (Paired t-test).
Table 4 lists the changes in TG, direct LDL-C, direct sdLDL-C, Sampson sdLDL-C, and difference in sdLDL-C (Sampson-direct) in patients with diabetes treated with statins (n=18) or pemafibrate (n=25). Statins significantly reduced LDL-C, direct sdLDL-C, and Sampson sdLDL-C and slightly reduced TG. Direct sdLDL-C and Sampson sdLDL-C were comparable at baseline, but Sampson sdLDL-C after statin treatment was 4 mg/dl higher than the direct measurement. Pemafibrate substantially reduced TG, direct sdLDL-C, and Sampson sdLDL-C without affecting LDL-C. Direct sdLDL-C and Sampson sdLDL-C were comparable at baseline, but Sampson sdLDL-C after pemafibrate was 5 mg/dl higher than the direct measurement. Taken together, the reduction in sdLDL-C with statins and pemafibrate was 21 mg/dl via direct measurement and 15 mg/dl via Sampson’s estimate. Thus, Sampson’s equation underestimated the drug-induced reduction in sdLDL-C by 5 mg/dl (24%).
Table 4. Comparison between direct sdLDL-C and Sampson sdLDL-C by treatment with statins or pemafibrate in diabetic patients.
| Statins (n = 18) | Pemafibrate (n = 25) | Combined (n = 43) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| before | after | Δ | before | after | Δ | before | after | Δ | |
| TG | 187 (26) | 165 (75) | 22 (74)*** | 236 (22) | 133 (74) | 102 (66)*** | 216 (112) | 147 (75) | 68 (80)*** |
| LDL-C | 125 (24) | 88 (20) | 36 (26)*** | 105 (22) | 99 (20) | 5 (15) | 113 (24) | 95 (21) | 18 (25)*** |
| Direct sdLDL-C | 54 (10) | 32 (10) | 22 (9)*** | 46 (19) | 26 (14) | 20 (11)*** | 50 (16) | 28 (13) | 21 (10)*** |
| Sampson sdLDL-C | 55 (14) | 38 (9) | 17 (12)*** | 51 (18) | 36 (13) | 14 (9)*** | 53 (17) | 37 (12) | 15 (10)*** |
| ΔsdLDL-C | 0 (14) | 5 (5) | -4 (11)** | 4 (12) | 14 (9) | -5 (11)*** | 2 (13) | 15 (10) | -5 (11)*** |
Δ: difference in sdLDL-C (before-after). **p<0.01 and ***P<0.0001 (Paired t-test). Plasma lipid levels were also retrospectively studied before and 2-4 months after statin or pemafibrate (0.2 mg/d) administration. Statins used were pitavastatin 1 mg/d (n = 8), atorvastatin 5-10 mg (n = 4), and rosuvastatin (2.5 mg/d) (n = 6).
Discussion
Sampson’s equation exhibited a significant correlation with sdLDL-C in both healthy controls and patients with diabetes. Nevertheless, the correlation between the calculated sdLDL-C and direct sdLDL-C was modest compared with those between the calculated LDL-C and direct LDL-C. Sampson et al. 9) reported that the coefficient of determination between direct LDL-C and estimated lbLDL-C or estimated sdLDL-C was 0.869 and 0.745, respectively. Sampson’s equation is designed primarily to estimate lbLDL-C 9) , and because LDL-C is more strongly correlated with lbLDL-C than with sdLDL-C, the sdLDL-C equation is not as accurate as the LDL-C equation. NonHDL-C and apoB are highly associated with direct sdLDL-C 17) and are also regulated by TG and LDL-C. The correlation of Sampson sdLDL-C with direct sdLDL-C was higher than those of apoB or nonHDL-C. It should be noted, however, that Sampson’s correlation of sdLDL-C with non-HDL-C or apoB is even higher than that found with direct sdLDL-C. Sampson’s equation may reflect nonHDL-C and apoB rather than direct sdLDL-C.
The preponderance of sdLDL particles is a feature of diabetic dyslipidemia 12 , 13) , and insulin resistance is closely related to the generation of sdLDL particles through increased production of VLDL1 10 - 12) . However, VLDL1 production does not necessarily correlate with the degree of hypertriglyceridemia because other TRLs such as VLDL2 and chylomicrons are also involved in hypertriglyceridemia 18) . The VLDL-LDL cascade, which is regulated by TG hydrolysis, is another determinant of TG concentration 18 , 19) . However, defects in TG removal increase VLDL but decrease LDL 19) . Thus, Sampson’s presumption based on TG and LDL-C would dissociate with direct sdLDL if impaired TG removal is the primary mechanism of hypertriglyceridemia. As shown in Fig.3 , the high correlation between Sampson sdLDL-C and TG or LDL-C is a natural result because TG and LDL-C are included in the equation. Conversely, the weaker correlation of these lipids with direct sdLDL-C than with Sampson sdLDL-C may suggest that other mechanisms of sdLDL generation are involved that cannot be explained by TG or LDL-C concentrations. The relationship between direct sdLDL-C and TG or LDL-C in patients with diabetes was more modest than in healthy controls, suggesting that mechanisms independent of TG and LDL-C are more deeply involved. Interestingly, the lower the eGFR, the greater the dissociation between direct sdLDL-C and Sampson sdLDL-C in patients with diabetes ( Table 2 ) . Renal dysfunction associated with diabetes leads to abnormal lipoprotein metabolism, resulting in the development of severe and complex dyslipidemia 14) . This may be an additional explanation for the lower ability to estimate sdLDL-C in patients with diabetes than in healthy controls. The difference between Sampson’s estimation and direct sdLDL-C was more pronounced at lower concentrations of general lipids and sdLDL-C. This may be because Sampson’s equation is completely dependent on lipid levels, which limits its ability to accurately measure sdLDL-C, especially in the low lipid range.
Sampson’s LDL-C equation can be applied to severe high triglyceride samples 16) , but this equation, like Friedewald’s LDL-C equation 20) , is established based on fasting plasma. Sampson’s sdLDL-C equation includes Sampson’s LDL-C 9) , which means that fasting plasma is required. LDL particles have a longer residence time in the blood than TRLs, and sdLDL particles have an even longer residence time than lbLDL particles 21) . It is well accepted that LDL-C has no significant circadian rhythm, which suggests that sdLDL-C does not change during the day. In fact, we recently reported that direct sdLDL-C concentrations do not change significantly in a day even in the presence of postprandial hypertriglyceridemia 22) . Consistent with our previous results 22 , 23) , the present study confirmed that the values of direct sdLDL-C were similar in fasting and nonfasting plasmas. Therefore, fasting is not necessary as long as sdLDL-C is measured using the direct method. Conversely, since TG is an essential component of Sampson’s sdLDL-C estimation, fasting is necessary for estimating sdLDL-C via Sampson’s equation. Finally, we found that Sampson’s equation underestimates the reduction in sdLDL-C by the two lipid-lowering agents to the same extent. This may be due to the nature of Sampson’s equation, which overestimates sdLDL-C in the low range.
Conclusion
Although Sampson’s equation may be as useful as the direct sdLDL-C measurement, it is highly relevant to nonHDL-C or apoB and its specificity must be well verified. Additionally, it should be noted that the equation is based on fasting, and its accuracy is blunted in patients with diabetes and in the low sdLDL-C range.
Acknowledgements
We would like to thank Drs Takeshi Hirashima, Ema Aoki, Natsuko Suzuki and Taito Oshima, and Mrs. Miyuki Tokudome in Ebina General Hospital for their cooperation in data collection.
COI
Tsutomu Hirano receives advisor fee from Denka Co., and lecture free from Kowa Co. Yasuki Ito is an employee of Denka Co. Ltd.
References
- 1).Siri-Tarino PW, Krauss RM. The early years of lipoprotein research: from discovery to clinical application. J Lipid Res, 2016; 57: 1771-1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Austin MA, Breslow JL, Hennekens CH, Buring JE, Willett WC, Krauss RM. Low-density lipoprotein subclass patterns and risk of myocardial infarction. JAMA, 1988; 260: 1917-1921 [PubMed] [Google Scholar]
- 3).Berneis KK, Krauss RM. Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res, 2002; 43: 1363-1379 [DOI] [PubMed] [Google Scholar]
- 4).Krauss RM. Small dense low-density lipoprotein particles: clinically relevant? Curr Opin Lipidol, 2022; 33: 160-166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Ito Y, Fujimura M, Ohta M, Hirano T. Development of a homogeneous assay for measurement of small dense LDL cholesterol. Clin Chem, 2011; 57: 57-65 [DOI] [PubMed] [Google Scholar]
- 6).Hoogeveen RC, Gaubatz JW, Sun W, Dodge RC, Crosby JR, Jiang J, Couper D, Virani SS, Kathiresan S, Boerwinkle E, Ballantyne CM. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: the Atherosclerosis Risk In Communities (ARIC) study. Arterioscler Thromb Vasc Biol, 2014; 34: 1069-1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Tsai MY, Steffen BT, Guan W, McClelland RL, Warnick R, McConnell J, Hoefner DM, Remaley AT. New automated assay of small dense low-density lipoprotein cholesterol identifies risk of coronary heart disease: the Multi-ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol, 2014; 34: 196-201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Higashioka M, Sakata S, Honda T, Hata J, Yoshida D, Hirakawa Y, Shibata M, Goto K, Kitazono T, Osawa H, Ninomiya T. Small Dense Low-Density Lipoprotein Cholesterol and the Risk of Coronary Heart Disease in a Japanese Community. J Atheroscler Thromb, 2020; 27: 669-682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Sampson M, Wolska A, Warnick R, Lucero D, Remaley AT. A New Equation Based on the Standard Lipid Panel for Calculating Small Dense Low-Density Lipoprotein-Cholesterol and Its Use as a Risk-Enhancer Test. Clin Chem, 2021; 67: 987-997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Packard CJ. Triacylglycerol-rich lipoproteins and the generation of small, dense low-density lipoprotein. Biochem Soc Trans, 2003; 31: 1066-1069 [DOI] [PubMed] [Google Scholar]
- 11).Guérin M, Le Goff W, Lassel TS, Van Tol A, Steiner G, Chapman MJ. Atherogenic role of elevated CE transfer from HDL to VLDL(1) and dense LDL in type 2 diabetes: impact of the degree of triglyceridemia. Arterioscler Thromb Vasc Biol, 2001; 21: 282-288 [DOI] [PubMed] [Google Scholar]
- 12).Verges B. Pathophysiology of diabetic dyslipidaemia: where are we? Diabetologia, 2015; 58: 886-899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Hirano T. Pathophysiology of Diabetic Dyslipidemia. J Atheroscler Thromb, 2018; 25: 771-782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Hirano T, Satoh N, Kodera R, Hirashima T, Suzuki N, Aoki E, Oshima T, Hosoya M, Fujita M, Hayashi T, Ito Y. Dyslipidemia in diabetic kidney disease classified by proteinuria and renal dysfunction: A cross-sectional study from a regional diabetes cohort. J Diabetes Investig, 2022; 13: 657-667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Ito Y, Ohta M, Ikezaki H, Hirao Y, Machida A, Schaefer EJ, Furusyo N. Development and population results of a fully automated homogeneous assay for LDL triglyceride. JALM, 2018; 2: 746-756 [DOI] [PubMed] [Google Scholar]
- 16).Sampson M, Ling C, Sun Q, Harb R, Ashmaig M, Warnick R, Sethi A, Fleming JK, Otvos JD, Meeusen JW, Delaney SR, Jaffe AS, Shamburek R, Amar M, Remaley AT. A New Equation for Calculation of Low-Density Lipoprotein Cholesterol in Patients With Normolipidemia and/or Hypertriglyceridemia. JAMA Cardiol, 2020 May 1; 5(5): 540-548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Hayashi T, Koba S, Ito Y, Hirano T. Method for estimating high sdLDL-C by measuring triglyceride and apolipoprotein B levels. Lipids Health Dis, 2017 26; 16: 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Björnson E, Packard CJ, Adiels M, Andersson L, Matikainen N, Söderlund S, Kahri J, Sihlbom C, Thorsell A, Zhou H. Investigation of human apoB48 metabolism using a new, integrated non-steady-state model of apoB48 and apoB100 kinetics. J Intern Med, 2019; 285: 562-577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Gaw A, Packard CJ, Lindsay GM, Griffin BA, Caslake MJ, Lorimer AR, Shepherd J. J Overproduction of small very low density lipoproteins (Sf 20-60) in moderate hypercholesterolemia: relationships between apolipoprotein B kinetics and plasma lipoproteins. J Lipid Res, 1995; 36: 158-171 [PubMed] [Google Scholar]
- 20).Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem, 1972; 18: 499-502 [PubMed] [Google Scholar]
- 21).Thongtang N, Diffenderfer MR, Ooi EMM, Barrett PHR, Turner SM, Le NA, Brown WV, Schaefer EJ. Metabolism and proteomics of large and small dense LDL in combined hyperlipidemia: effects of rosuvastatin. J Lipid Res, 2017; 58: 1315-1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Hayashi T, Ai M, Goto S, Nakamura M, Nagaike H, Suzuki R, Abe Y, Ohta M, Ito Y, Hirano T. Circadian Rhythm of Subspecies of Low-Density Lipoprotein-Cholesterol and High-Density Lipoprotein-Cholesterol in Healthy Subjects and Patients with Type 2 Diabetes. J Atheroscler Thromb, 2023; 30; 3-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Hirano T, Kodera R, Hirashima T, Suzuki N, Aoki E, Hosoya M, Oshima T, Hayashi T, Koba S, Ohta M, Satoh N, Ito Y. Metabolic Properties of Low-density Lipoprotein (LDL) Triglycerides in Patients with Type 2 Diabetes, Comparison with Small Dense LDL-Cholesterol. J Atheroscler Thromb, 2022; 29: 762-774 [DOI] [PMC free article] [PubMed] [Google Scholar]