Abstract
Ticks are vectors and reservoirs of a variety of pathogens including protozoa, bacteria and viruses which cause tick-borne diseases (TBDs) in humans and livestock. TBDs pose serious constraints to the improvement of livestock production in tropical and subtropical regions of the world. Despite their wide distribution, information on the tick and pathogen relationship is scarce in Tanzania. We used nested PCR and sequencing to screen pathogens of public and veterinary health importance in ticks collected by flagging from four districts of Tanzania. In total, 2021 ticks comprising nine species were identified. DNA from ticks was pooled according to tick species, developmental stage, and location, then screened for Babesia bigemina, Babesia bovis, Theileria parva and Coxiella burnetii. Out of 377 pools, 34.7% were positive for at least one pathogen. Theileria parva was the most abundant with a minimum infection rate (MIR) of 2.8%, followed by B. bigemina (MIR = 1.8%) and B. bovis (MIR = 0.8%). Multiple pathogens detection was observed in 7.2% of the tested pools. However, PCR screening of individual tick DNA revealed that only 0.3% of the examined pools had co-infection. DNA of C. burnetii was never detected in any tick DNA pool. The MIR of tick-borne pathogens (TBPs) differed significantly among districts, seasons, tick species, and tick developmental stages. Sequence analysis showed that B. bigemina RAP-1a, B. bovis SBP-4, and T. parva p104 genes were conserved among pathogens in the four districts. Despite the absence of C. burnetii in ticks, considering its pathogenic potential, it is essential to continue monitoring for its possible recurrence in ticks. This information adds to the knowledge of TBPs epidemiology and will contribute to the scientific basis for planning future control strategies.
Supplementary information
The online version contains supplementary material available at 10.1007/s10493-023-00816-0.
Keywords: Ticks, Tick-borne pathogens, Epidemiology, Tanzania
Introduction
Ticks (Acari: Ixodoidea) represent a major threat to human and animal health worldwide due to their major role as vectors and reservoirs of a variety of zoonotic protozoan, bacterial and viral pathogens. Therefore, ticks play a critical role in maintaining tick-borne pathogens (TBPs) in nature (Bekloo et al. 2018). Tick-borne diseases (TBDs) impede the growth of the livestock sector and impose major constraints on the health and management of livestock in the tropic and subtropical regions globally (Jongejan and Uilenberg 2004). Severe effects of ticks and TBDs are observed mostly in rural populations where livestock is an essential source of income and food supply.
In East Africa, East Coast fever (ECF), babesiosis, and anaplasmosis are the major TBDs affecting livestock health and causing production losses (Ringo et al. 2020) whereas Q fever is among the most frequently reported tick-borne zoonoses (Crump et al. 2013; Njeru et al. 2016). ECF is a devastating disease associated with high mortality rates in cattle populations, caused by Theileria parva (Zobba et al. 2020) and transmitted by Rhipicephalus appendiculatus or Rhipicephalus zambeziensis ticks (Meneghi et al. 2016). The disease is prevalent in eastern, southern, and central Africa where its vectors are present (Adjou Moumouni et al. 2015).
In Tanzania and sub-Saharan Africa at large, bovine babesiosis is mainly caused by two distinct protozoa, Babesia bigemina and Babesia bovis (Lynen et al. 2008; Adjou Moumouni et al. 2015; Heylen et al. 2023). Bovine babesiosis is an acute disease that becomes persistent in animals that survive the infection and is characterized by fever, listlessness, anorexia, dehydration, and progressive hemolysis (Zintl et al. 2005). Whereas B. bigemina is transovarially transmitted by Rhipicephalus (Boophilus) decoloratus and Rhipicephalus (Boophilus) microplus ticks, B. bovis is efficiently transmitted only by R. (B.) microplus. Although B. bigemina is more widespread than B. bovis, the latter parasite is responsible for much heavier losses in susceptible livestock (Lynen et al. 2008).
Coxiella burnetii is an intracellular Gram-negative bacterium that occurs worldwide but not in New Zealand (Mediannikov et al. 2010). Its infection in humans and animals leads to Q fever and coxiellosis, respectively. Because of its potential for rapid spread and highly infectious nature, hence its effects on global public and veterinary health, C. burnetii has attracted significant attention for research purposes. Domestic animals such as sheep, goats, and cattle are considered the latent source of infection in humans. The infected animal sheds the bacterium through the placenta and amniotic fluids which may contaminate the environment (Maurin et al. 1999). The main transmission route of C. burnetii is via inhalation of contaminated particles. However, ticks are considered the natural primary vector of C. burnetii as they maintain the infection in domestic animals. Several tick species have been reported to carry natural infection and shed a significant number of viable C. burnetii in their feces (Maurin et al. 1999). This means, inhaling tick excreta can be a significant source of infection. Previous studies have confirmed the circulation of tick-borne zoonoses including C. burnetii in human and livestock samples in northern Tanzania (Crump et al. 2013)—despite this fact, no surveillance has been done to establish its prevalence in ticks.
Information on the infection rate of pathogens in ticks, and the genetic diversity of the circulating pathogens are important variables in understanding the epidemiology and control of TBDs. The risk of transmission of TBPs is determined by the prevalence of ticks in the environment and by the probability of an encounter between an infected tick and a susceptible host. Unfortunately, very few studies have identified TBPs of veterinary importance in ticks in Tanzania, and the majority of these few are using ticks collected while they are feeding on hosts. The procedure of measuring tick abundance and risk of pathogens using feeding ticks was challenged by Gray et al. (2021), because preferences for a given host and individual susceptibility of each vertebrate to carry ticks bias the data obtained. Estrada-Peña et al. (2021) emphasize the use of questing ticks to estimate the risk of pathogens as the reliable procedure that provides an unbiased view of the actual infection rates in the field.
Therefore, this study was carried out to determine and understand the epidemiology and genetic diversities of some TBPs circulating in ticks in Tanzania mainland using DNA-based PCR and sequencing.
Materials and methods
Description of the study area
Tick collection sites were located in Longido (2°42′S, 36°42′E), Gairo (6°14′S, 36°87′E), Mvomero (8°10′S, 28°37′E) and Monduli (3°20′S, 36°15′E) districts in Tanzania. Monduli and Longido districts are located in northern Tanzania. The area has a semi-arid ecosystem with average annual rainfall of 600–700 mm which falls mostly between March and May and in November and December. The climate is tropical sub-humid with average temperatures of 26 °C annually (Warwick et al. 2016).
Mvomero district has a bimodal rainfall distribution, with a long wet season from March to May and a short wet season from October to December. The climate is humid to sub-humid, annual rainfall ranges from 600 to 2000 mm. Average annual temperatures range from 20 to 30 °C. Gairo district is found in central Tanzania and experiences a total annual rainfall of 1200 mm, with a long rainfall season starting from December to February. The climate is tropical sub-humid with annual average temperature of 25–30 °C (Nonga et al. 2012).
Collection of ticks from pastures
Questing ticks were collected from the pasture by dragging from February 2021 to October 2022. Dragging was performed along 90 line transects which were randomly selected (approximately 23 transects from each district). A white flag of 1 m2 was dragged along transect lines, averaging 90 m (Short and Norval 1981). After collection, the ticks were placed in 100 ml universal bottles with cotton wool dampened with sterile water. The bottles were placed in cool contained ice packs and transported to the laboratory for analysis. In each district, sampling was done twice (dry and rainy season).
Tick identification and DNA extraction
The collected ticks were morphologically examined using a stereo microscope (80-fold magnification and identified to species level based on morphological features (Walker et al. 2014). DNA from individual ticks was extracted by the TIANamp Genomic DNA Kit (DP130227; Tiangen Biotech, Beijing, China) in accordance with manufacturer’s instructions. The DNA of each tick was divided into two equal volumes. One set of DNA was stored in Eppendorf tubes and frozen at − 20 °C until needed for further analysis. The second set of DNA was pooled into five according to tick species, developmental stage, and location of the collection.
PCR amplification for detection of piroplasmids (Babesia/Theileria) and Coxiella like organisms
Each of the pooled tick DNA sample was screened with species-specific nested PCR (nPCR) primers (Table 1) for the presence of B. bigemina rhoptry-associated protein-1a (RAP-1a), B. bovis spherical body protein-4 (SBP-4), T. parva 104 kDa antigen (p104) and C. burnetii htpB genes (To et al. 1996; Skilton et al. 2002; Odongo et al. 2010; Terkawi et al. 2011). PCR amplification of both pathogens consisted of 16 µl of nuclease-free water which was added to a crystallized PCR premix (Accupower PCR Premix; Bioneer, South Korea), 2 µl of DNA template, and 1 µl (10 pmol) of each primer (total 20 µl reaction). Except for C. burnetii which used single PCR, 1 µl of DNA of each pathogen obtained from the first round of PCR was used as a template for the second round of nPCR, respectively.
Table 1.
Sequences of primer sets used for detection of Theileria parva, Babesia bovis, Babesia bigemina and Coxiella burnetii DNA in tick pools
| Pathogen | Assays | Oligonucleotide sequences (5′ > 3′) | Product size (bp) | References |
|---|---|---|---|---|
| Theileria parva p104 | PCR | ATTTAAGGAACCTGACGTGACTGC | 486 | Skilton et al. (2002) |
| TAAGATGCCGACTATTAATGACACC | ||||
| nPCR | GGCCAAGGTCTCCTTCAGAATACG | 277 | Odongo et al. (2010) | |
| TGGGTGTGTTTCCTCGTCATCTGC | ||||
| Babesia bovis SBP-4 | PCR | AGTTGTTGGAGGAGGCTAAT | 907 | Terkawi et al. (2011) |
| TCCTTCTCGGCGTCCTTTTC | ||||
| nPCR | GAAATCCCTGTTCCAGAG | 503 | ||
| TCGTTGATAACACTGCAA | ||||
| Babesia bigemina RAP-1a | PCR | GAGTCTGCCAAATCCTTAC | 879 | |
| TCCTCTACAGCTGCTTCG | ||||
| nPCR | AGCTTGCTTTCACAACTCGCC | 412 | ||
| TTGGTGCTTTGACCGACGACAT | ||||
| Coxiella burnetii htpB | PCR | GCGGGTGATGGTACCACAACA | 501 | To et al. (1996) |
| GGCAATCACCAATAAGGGCCG |
Primary PCR amplifications of T. parva, B. bigemina and B. bovis were performed with an initial denaturation at 95 °C for 5 min, followed by 35 cycles of denaturation at 94 °C (30 s), annealing at 55 °C (1 min), and extension at 72 °C (1 min), followed by a final extension at 72 °C for 10 min. The cycling conditions for the second amplifications of B. bigemina and B. bovis were the same as that of primary amplification except for B. bovis whose annealing temperature was 50 °C. In the case of T. parva, the second amplification comprised of an initial denaturation at 95 °C for 5 min, 25 cycles of denaturation at 94 °C for 30 s, annealing at 58 °C for 1 min, extension at 72 °C for 1 min plus a final extension at 72 °C for 20 min. The PCR cycling condition of C. burnetii comprised 94 °C for 3 min, followed by 36 cycles of 94 °C for 1 min, 56 °C for 1 min, and 72 °C for 1 min, and then a final extension at 72 °C for 4 min. The reactions were performed using an automatic thermal cycler (Takara).
PCR products of each gene was visualized by UV light in a 1.5% agarose gel containing 3 µl GelRed (Biotium, Fremont, CA, USA). In the case of the pools which had multiple pathogens detection, nPCR of the respective individual ticks were performed using the stored set of individual tick DNA.
Sequencing of the PCR-positive samples
In total, 13 samples (B. bigemina, n = 3; B. bovis, n = 5; and T. parva, n = 5) were randomly selected for DNA sequencing. Purification of the DNA of selected samples was done using Roche High PCR Purification Kit (Bioneer) as per the manufacturer’s protocol. The concentration of PCR products was checked by a Nanodrop system (Thermo-Scientific, UK). Each of the purified samples was sent to Macrogen (Europe) for automated nucleotide sequencing by Sanger dideoxy method with both the forward and reverse primers.
Sequence and phylogenetic analyses
The returned sequences were edited in Geneious prime software v.2022.01 (created by Biomatters) (Kearse et al. 2012). Consensus sequences of each isolate were compared for identities and similarities to other published sequences available in GenBank using the basic local alignment search tool (BLAST) and comparison with sequences deposited in the non-redundant National Center for Biotechnology Information (NCBI) database (https://blast.ncbi.nlm.nih.gov/). Pairwise/multiple sequence alignments were done using CLUSTALX (Thompson et al. 1997). Aligned sequences were trimmed to the same length (with gaps) from which phylogenetic trees were constructed. Phylogenetic trees were constructed using MEGA v.11.0 (Tamura et al. 2021) with DNA sequences obtained from this study and those from the same pathogens already available in the GenBank. The evolutionary models for individual DNA sequence alignments were determined using the Akaike information criterion test in jModelTest v.3.7 (Darriba et al. 2012). Maximum likelihood method was used for phylogenetic tree analysis of B. bovis (SBP-4), T. parva (p104) and B. bigemina (RAP-1a) gene. The bootstrap consensus tree inferred from 1000 replicates (Tamura et al. 2004) was taken to represent the evolutionary history of the taxa analyzed.
Statistical analysis
Pearson’s χ2 test was applied to analyze the MIR of each TBP according to independent variables such as tick developmental stages, tick species, season or study area, using SPSS v.22 (α = 0.05). The MIR of a pathogen was calculated by the following formula: MIR = X/(Y × Z) × 100%, where X is the number of positive pools, Y is the total number of pools tested and Z is the size of the pool. This formula assumes that only one tick is infected in a positive pool (Andreassen et al. 2012).
Results
Tick species identification
In total, 2021 hard ticks were collected within three tick genera namely Rhipicephalus, Hyalomma, and Amblyomma. The genus Rhipicephalus, with a prevalence of 82% (1658/2021), was the most prevalent, followed by the genus Amblyomma 14% (282/2021) and Hyalomma 4% (81/2021). In total, nine tick species were identified, and the most common was R. appendiculatus (n = 500, 24.7%), R. (B.) decoloratus (n = 428, 21.2%), R. (B.) microplus (n = 263, 13.0%), A. variegatum (n = 262, 13.0%), R. evertsi evertsi (n = 244, 12.1%), R. pulchellus (n = 172, 8.5%), H. albiparmatum (n = 81, 4.0%), R. praetextatus (n = 51, 2.5%) and Amblyomma lepidum (n = 20, 1.0%).
Tick species proportion varied among districts in the dry and wet seasons. Except for R. (B.) decoloratus (in Longido and Monduli) and H. albiparmatum (in Gairo and Monduli) which were more abundant in the dry season, and R. praetextatus which had relatively the same abundance (%) in the two seasons, all other tick species (˃ 70%) were more abundant in the wet season. However, the number of some species, such as A. lepidum collected in this study was too small to assess their seasonal dynamics. The details of the tick demographics are shown in Table 2.
Table 2.
Rhipicephalus (Boophilus), Amblyomma and Hyalomma tick species collected from pasture during dry and wet seasons in Longido, Gairo, Mvomero and Monduli districts, Tanzania
| Location (district) | Season | R. appendiculatus | R. pulchellus | R. (B.) decoloratus | R. (B.) microplus | R. evertsi | R. praetextatus | A. variegatum | A. lepidum | H. albiparmatum | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Longido | Dry | 8 | 26 | 24 | 8 | 5 | 4 | 0 | 0 | 0 | 75 |
| Wet | 134 | 27 | 0 | 0 | 6 | 7 | 8 | 0 | 0 | 152 | |
| Gairo | Dry | 28 | 0 | 69 | 39 | 33 | 0 | 42 | 18 | 52 | 281 |
| Wet | 64 | 0 | 97 | 51 | 95 | 0 | 38 | 0 | 25 | 370 | |
| Monduli | Dry | 14 | 48 | 31 | 0 | 3 | 17 | 54 | 0 | 4 | 171 |
| Wet | 116 | 71 | 11 | 0 | 15 | 23 | 69 | 2 | 0 | 307 | |
| Mvomero | Dry | 24 | 0 | 74 | 55 | 33 | 0 | 33 | 0 | 0 | 219 |
| Wet | 112 | 0 | 122 | 110 | 54 | 0 | 18 | 0 | 0 | 416 | |
| Total no. ticks (%) | 500 (24.7) | 172 (8.5) | 428 (21.2) | 263 (13) | 244 (12.1) | 51 (2.5) | 262 (13) | 20 (1) | 81 (4) | 2021 (100) |
The proportion of tick developmental stages differed significantly among tick species and locations. Rhipicephalus appendiculatus had the highest (n = 195, 47%) percentage of nymphs collected, followed by R. evertsi evertsi (n = 80, 19%), A. variegatum (n = 65, 16%), H. albiparmatum (n = 30, 7%), R. pulchellus (n = 25, 6%), A. lepidum (n = 6, 1%) and R. praetextatus (n = 5, 1%). No nymphal stages of R. (B.) decoloratus or R. (B.) microplus were collected. The highest percentage of nymphs was found in Longido (54%) followed by Gairo (28%), Monduli (14%), and Mvomero (12%). The proportion of nymphal stages did not differ significantly among wet (21%) and dry (23%) seasons.
Pathogens detected in the ticks and infection rates
Theileria parva was the most abundant (MIR = 2.8%), followed by B. bigemina (1.8%) and B. bovis (0.8%) (Table 3). DNA of C. burnetii was never detected in any tick pool.
Table 3.
Minimum infection rates (MIR, %) of tick-borne pathogen DNA in pools of Rhipicephalus (Boophilus), Amblyomma and Hyalomma ticks (size n = 5 each) by PCR in Longido, Gairo, Mvomero and Monduli districts, Tanzania
| Location (district) | Tick species | No. tick pools tested |
No. positive pools (MIR %) | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Theileria parva | Coxiella burnetii | Babesia bigemina | Babesia bovis | Co-detections | ||||
| Longido | R. (B.) decoloratus | 3 | 0 (0) | 0 (0) | 2 (13.3) | 0 (0) | 0 (0) | 2 (13.3) |
| R. appendiculatus | 28 | 5 (3.6) | 0 (0) | 0 (0) | 0 (0) | 6 (4.3) T. parva + B. bigemina | 11 (7.9) | |
| R. (B.) microplus | 2 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| R. evertsi evertsi | 1 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| R. praetextatus | 2 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| A. variegatum | 1 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Total Longido | 37 | 5 (2.7) | 0 (0) | 2 (1) | 0 (0) | 6 (3.2) | 13 (7) | |
| Gairo | R. (B.) decoloratus | 33 | 0 (0) | 0 (0) | 16 (9.7) | 0 (0) | 7 (4.2) T. parva + B. bigemina | 18 (13.9) |
| R. (B.) microplus | 18 | 0 (0) | 0 (0) | 0 (0) | 11 (12.2) | 1 (1.1) B. bigemina + B. bovis | 12 (13.3) | |
| R. appendiculatus | 18 | 3 (3.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (3.3) | |
| R. evertsi evertsi | 25 | 1 (08) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0.8) | |
| A. variegatum | 16 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (5) T. parva + B. bigemina | 4 (5) | |
| A. lepidum | 3 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| H. albiparmatum | 15 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Total Gairo | 128 | 4 (0.6) | 0 (0) | 16 (2.5) | 11 (1.7) | 12 (1.9) | 43 (6.7) | |
| Mvomero | R. (B.) decoloratus | 39 | 0 (0) | 0 (0) | 10 (5.1) | 0 (0) | 0 (0) | 10 (5.1) |
| R. (B.) microplus | 33 | 0 (0) | 0 (0) | 0 (0) | 5 (3.3) | 4 (2.4) T. parva + B. bigemina + B. bovis | 9 (5.5) | |
| R. appendiculatus | 26 | 17 (13.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 17 (13.1) | |
| R. evertsi evertsi | 17 | 4 (4.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (4.7) | |
| A. variegatum | 10 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Total Mvomero | 125 | 21 (3.4) | 0 (0) | 10 (1.6) | 5 (0.8) | 4 (0.6) | 40 (6.4) | |
| Monduli | R. (B.) decoloratus | 8 | 0 (0) | 0 (0) | 5 (12.5) | 0 (0) | 0 (0) | 5 (12.5) |
| R. evertsi evertsi | 3 | 1 (6.7) | 0 (0) | 1 (6.7) | 0 (0) | 1 (6.7) T. parva + B. bigemina | 3 (20) | |
| R. appendiculatus | 24 | 18 (15) | 0 (0) | 0 (0) | 0 (0) | 2 (0) T. parva + B. bigemina | 20 (16.7) | |
| R. pulchellus | 23 | 4 (3.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (3.5) | |
| R. praetextatus | 8 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| A. variegatum | 20 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (3) T. parva + B. bigemina | 3 (3) | |
| H. albiparmatum | 1 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Total Monduli | 87 | 23 (5.3) | 0 (0) | 6 (1.4) | 0 (0) | 6 (1.4) | 35 (8) | |
| Overall total | 377 | 85 (4.5) | 0 (0) | 34 (1.8) | 16 (0.8) | 28 (1.5) | 131 (6.9) | |
The MIR of TBPs differed significantly among districts, seasons, tick species and tick developmental stages. Except for B. bigemina whose MIR did not differ among districts, the MIR of T. parva and B. bovis varied significantly. Higher MIR of T. parva was found in Mvomero (3.4%), Longido (2.7%), and Monduli (5.3%) than in Gairo (0.6%) district. Babesia bovis was detected only in Gairo (MIR = 1.7%) and Mvomero (0.8%).
Babesia bigemina was the only pathogen whose MIR varied significantly with the season and it was higher in the dry season (MIR = 3.4%) than in the wet (1%) season. The highest MIR of T. parva, B. bigemina, and B. bovis was found in R. appendiculatus (MIR = 6.5%), R. (B.) decoloratus (7.7%) and R. (B.) microplus (6.0%) ticks, respectively. Except for T. parva which was detected in both adult ticks and nymphs, the two species of Babesia were detected only in adult ticks of the subgenus Boophilus. A trend towards higher MIR was observed in adult ticks than in nymphs.
The details of how the MIR of each of the pathogens differed among various variables are shown in Table 4.. Representative images of PCR-positive gel electrophoresis for B. bigemina, B. bovis, T. parva, and C. burnetii are shown in Supplementary figures S1–S4.
Table 4.
Minimum infection rates of Theileria parva, Babesia bigemina, and Babesia bovis for pools of Rhipicephalus (Boophilus), Amblyomma and Hyalomma ticks (size n = 5 each) by season, location, tick developmental stages and tick from Longido, Gairo, Mvomero and Monduli districts, Tanzania
| Variable | No. pools for variable | No. positive pools (MIR %) | P | ||
|---|---|---|---|---|---|
| T. parva | B. bigemina | B. bovis | |||
| Season | |||||
| Wet | 226 | 22 (2.9) | 11 (1.) | 14 (1.2) | T. parva = 0.45; B. bigemina = 0.001; B. bovis = 0.34 |
| Dry | 151 | 27 (2.7) | 23 (3) | 3(0.4) | |
| Location (district) | |||||
| Longido | 37 | 5 (2.7) | 2 (1.1) | 0 | T. parva = 0.003; B. bigemina = 0.41; B. bovis = 0.009 |
| Gairo | 128 | 4 (0.6) | 16 (2.5) | 11 (1.7) | |
| Mvomero | 125 | 9 (1.4) | 10 (1.6) | 5 (0.8) | |
| Monduli | 87 | 35 (20) | 6 (1.4) | 0 | |
| Tick developmental stage | |||||
| Nymph | 83 | 9 (2.2) | 0 | 0 | T. parva = 0.22; B. bigemina < 0.001; B. bovis = 0.017 |
| Adult | 293 | 44 (3) | 34 (2.3) | 16 (1.1) | |
| Tick species | |||||
| R. appendiculatus | 96 | 31 (6.5) | 0 | 0 | T. parva < 0.001; B. bigemina < 0.001; B. bovis < 0.001 |
| R. (B.) decoloratus | 83 | 1 (0.2) | 32 (7.7) | 0 | |
| R. (B.) microplus | 53 | 0 | 1 (0.4) | 16 (6) | |
| A. variegatum | 47 | 7 (3) | 0 | 0 | |
| R. evertsi evertsi | 46 | 4 (1.7) | 1 (0.4) | 0 | |
| R. pulchellus | 23 | 9 (7.8) | 0 | 0 | |
| H. albiparmatum | 17 | 0 | 0 | 0 | |
| R. praetextatus | 8 | 1 (2.5) | 0 | 0 | |
| A. lepidum | 4 | 0 | 0 | 0 | |
Multiple pathogen detections
There were multiple pathogen detections, which involved double and triple infections, in 28 (7.4%) of the DNA pools (Table 3). However, PCR screening of the individual tick DNA revealed that only six (0.3%) of the examined ticks were co-infected. Four (66.7%) of the co-infections involved T. parva and B. bigemina in R. appendiculatus and (A) variegatum (nymph) from Gairo and Monduli districts. Two of the co-infections were of (B) bigemina and B. bovis in R. (B.) microplus, from Mvomero district.
Comparative sequence analyses of piroplasmids (Babesia/Theileria)
All B. bigemina, B. bovis and T. parva sequences in this study were of the expected sizes. Nucleotide identity among the three B. bigemina (RAP-1a) gene sequences (ON221511–ON221513) was 99.7%. These sequences shared 99.5–100% nucleotide similarities with sequences from Tanzania (MG210824, MN 807,306), Uganda (MG426201, MG426202), and Turkey (KT220512). Furthermore, the percentage nucleotide similarities of all five B. bovis SBP-4 gene sequences (OM981234–OM981238) was 99.8% among themselves. These sequences showed 93.8–97.3% nucleotide similarities to sequences from Kenya (KP347555), Benin (KX685402), and Indonesia (KY484530).
The five T. parva (p104) gene sequences from this study (ON157060–ON157064) showed 99.6–100% nucleotide identity among themselves. These sequences had 97.8–99.6% nucleotide similarities with sequences from Uganda (MN810052), Tanzania (MN807321, MG700532), and Kenya (KP347566).
Phylogenetic analyses
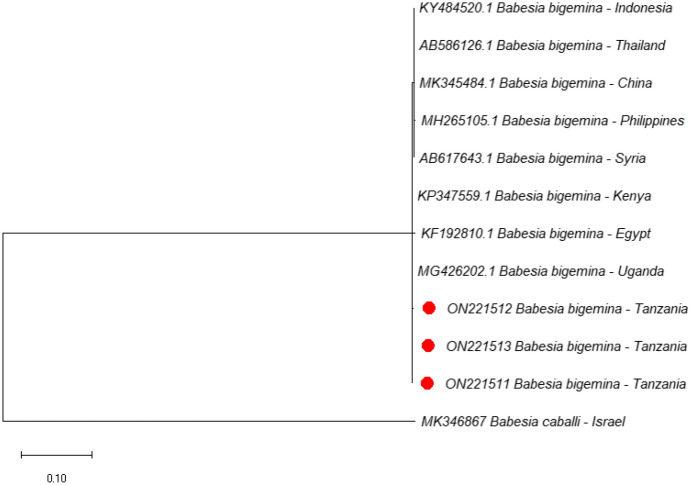
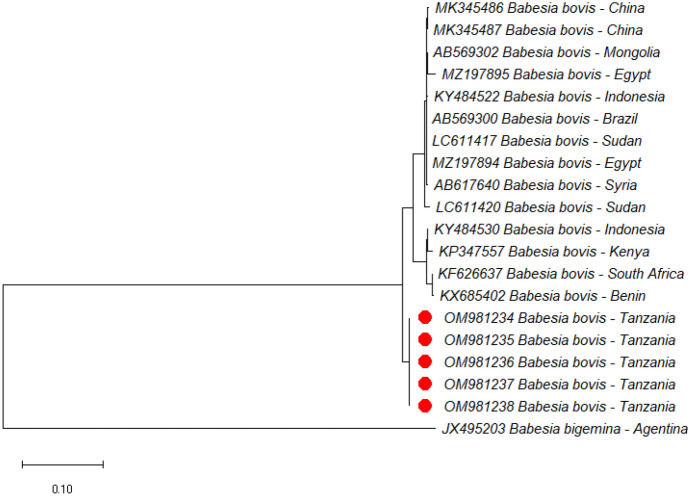
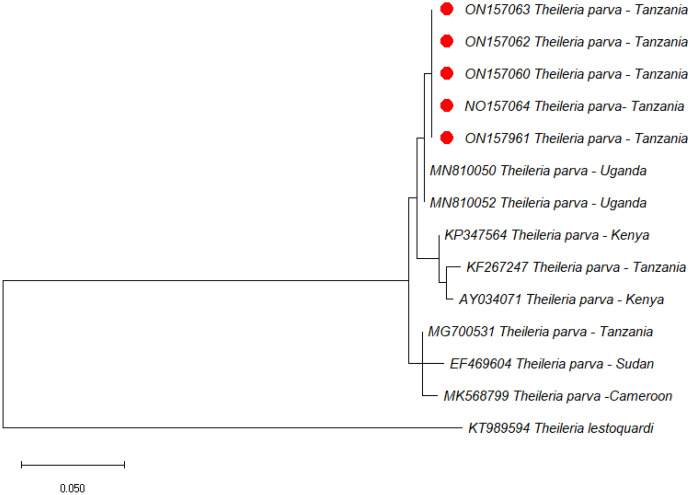
In the current study, phylogenetic trees of B. bigemina, B. bovis and T. parva were constructed based on RAP-1a, SBP-4, and p104 genes, respectively, using sequences from the NCBI GenBank. All three sequences of B. bigemina clustered in the same clade with sequences from Uganda (MG426202), Kenya (KP347559), and Egypt (KF192810) (Fig. 1). On the other hand, all the five SBP-4 sequences of B. bovis from this study clustered together forming a separate clade. Furthermore, five sequences of T. parva isolated in this study clustered together forming a separate clade but were close to other sequences from Uganda (MN810050 and MN810052). Other p104 sequences from Tanzania (KF267247 and MG700531) previously deposited in GenBank were placed in different clades. Representative images of the phylogenetic trees of B. bigemina, B. bovis and T. parva are shown in Figs. 1, 2 and 3.
Fig. 1.
Phylogenetic analysis by maximum likelihood method based on Babesia bigemina RAP-1a gene sequences. Sequences from this study are marked with red dots. The RAP-1a gene of Babesia bovis (KT312810) was used as an outgroup
Fig. 2.
Phylogenetic analysis by maximum likelihood method based on Babesia bovis SBP-4 gene sequences. Sequences from this study are marked with red dots. The SBP-4 gene of Babesia bigemina (JX495203) was used as an outgroup
Fig. 3.
Phylogenetic analysis by Neighbor-joining method based on Theileria parva p104 gene sequences. Sequences from this study are marked with red dots. The p104 gene of Theileria lestoquardi (KT989594) was used as an outgroup
The gene sequences produced from the current study were deposited in NCBI’s GenBank with the following GenBank accession numbers: ON221511–ON221513, ON157060–ON157064 and OM981234–OM981238.
Discussion
This study reports tick species diversity and molecular detection of B. bigemina, B. bovis and T. parva in questing ticks as well as phylogenetic analysis of these pathogens but it could not detect C. burnetii from four districts of northern and central Tanzania. The presence of various tick species observed in the study sites may increase the risk of transmission of TBPs and the incidence of diseases in livestock and human populations. Some of the TBPs in the current study were detected in tick species that are not known to be their biological vectors. However, the detection of TBP DNAs in such tick species does not necessarily mean that the ticks can transmit the infection.
In line with previous reports (Nonga et al. 2012), this study indicates that R. appendiculatus is the dominant tick species in Tanzania. Furthermore, R. appendiculatus had the highest proportion of nymphs. The climate of these study areas is cool and moist, in such conditions R. appendiculatus reproduces most successfully as the climate is favorable to permit its cyclic activity and two consecutive generations can be completed in a year (Lynen et al. 2007).
Rhipicephalus (B.) decoloratus was the second most frequent species encountered in this study. A relatively high proportion of this tick species was in Gairo and Mvomero compared to Longido and Monduli districts. The high abundance of this tick species in these districts could be explained by Gairo and Mvomero having a conducive climate (> 800 mm rainfall annually) which favors the survival of this tick species relative to Longido and Monduli, which have a lower annual rainfall of 600–700 mm (Walker et al. 2014). Another species of the subgenus Boophilus observed in this study was R. (B.) microplus. However, this species was never collected in Monduli and only eight ticks were collected in Longido district. Furthermore, no nymphs of R. (B.) decoloratus / R. (B.) microplus were collected in this study. The absence of nymphal stages of these tick species can be attributed to these ticks being one-host ticks where once the larvae attach to the host they never drop to the vegetation until they become adults. This may have reduced the chances of collecting nymphs on the vegetation.
This study has recorded two Amblyomma species, A. variegatum and A. lepidum, the former being more prevalent than the later (14 vs. 1.0%). Unlike A. lepidum, which needs more specialized environmental conditions for survival, A. variegatum is the most widespread species among Amblyomma species covering the subhumid and low–high altitude areas of Tanzania (Lynen et al. 2007).
Tick population dynamics vary according to seasonal and location effects and have been linked to outbreaks of various TBDs (Lynen et al. 2008). Although many of the tick species in this study were found in both seasons, the total count of each species indicated a significant seasonal variation. As previously reported (Yawa et al. 2018), higher abundances were found in the wet than in the dry season. According to Walker et al. (2014), despite different tick species requiring different microclimates, most have higher reproduction activity during the wet season.
Although T. parva seems to have the highest MIR compared to other TBPs in this study, it is still low compared to that found in other studies (Bazarusanga et al. 2011) that used a similar pool size. In endemic areas like Tanzania where most of the infected ticks acquire infection from traditionally managed pastoral cattle, the lower rate of infection of T. parva in ticks is not uncommon (Swai et al. 2006), because host-to-tick transmission from traditionally managed animals is usually low (Medley et al. 1993). In addition, the grazing system in the study areas is free-range, where young cattle which usually have high parasitemia of T. parva are kept indoors in their first year of life to prevent contact with ticks and predators. As such, the ticks may feed only on low parasitemic adult-carrier cattle.
The MIR of pathogens detected in tick pools in the four study sites did not appear to differ in terms of numbers. However, the detection of B. bovis only in Gairo and Mvomero districts seems to correlate with the geographic abundance and distribution of its vector, R. (B.) microplus (a vector of both B. bigemina and B. bovis) which was mostly collected in the two districts. The only tick of the subgenus Boophilus collected mostly in Longido and Monduli district was R. (B.) decoloratus, which can only efficiently transmit B. bigemina (Lynen et al. 2008).
Apart from the pathogens detected in this study, A. variegatum is the main vector of Ehrlichia ruminantium which causes heart water in cattle and R. pulchellus is a vector of Theileria taurotragi, the cause of benign bovine theileriosis. Rhipicehalus evertsi evertsi transmits Anaplasma marginale, the cause of bovine anaplasmosis, and can also release toxins that cause paralysis in cattle and sheep. Furthermore, R. praetextatus can transmit Rickettsia conorii and Nairobi sheep disease virus to humans and sheep, respectively (Walker et al. 2014).
The absence of C. burnetii DNA in all the ticks examined disagree with previous studies (Oswe et al. 2018) but corroborate Pilloux et al. (2018) who also reported a zero prevalence of the bacterium in tick pools. Coxiella burnetii is infrequently detected especially in flagging ticks (Knap et al. 2019). Although ticks play a minor role in Q fever transmission, it has also be noted that centers of attention in which ticks may act as the natural reservoir for C. burnetii seem to exist; only, these foci are hard to identify (Körner et al. 2021; Celina and Cerný 2022).
Co-infections of epidemiologically important pathogens in hard ticks have been previously reported and it varies primarily depending on geographic area and the number of pathogens screened (Rocha et al. 2022). This study has shown that 0.3% of the collected ticks were co-infected by various TBPs. In line with the previous reports (Moutailler et al. 2016; Klitgaard et al. 2019), which showed a higher percentage of co-infection in adult questing ticks than in questing nymphs, 83% of the co-infected ticks in this study were adult questing ticks. According to Rocha et al. (2022), adult ticks are more likely to be co-infected than nymphs because they may have had additional blood feeding. Ticks co-infected with multiple pathogens greatly increase the risk of co-infections in the vertebrate host, which would result in more complex clinical manifestations and could be misdiagnosed.
Results from the phylogenetic trees show that B. bigemina RAP-1a, B. bovis SBP-4 and T. parva p104 gene sequences isolated in the current study are conserved among B. bigemina, B. bovis and T. parva, respectively. These results disagree with Adjou Moumouni et al. (2015) from Kenya, where p104 genes of T. parva were clustered in different clades. The clustering of all the isolates of B. bigemina, B. bovis and T. parva from this study into their respective clades suggests their genetic relatedness. It is therefore possible that they may have a similar evolution despite coming from diverse geographical areas (Bekloo et al. 2018).
The major limitation with this study was the pooling of tick DNA samples instead of processing individual ticks. This was done mainly to avoid processing of large number of samples and also to reduce costs. As a result of this limitation, the multiple pathogens detected in a single tick pool did not necessarily mean that individual ticks were co-infected as it could also be caused by the simultaneous detection of several mono-infected ticks (AL-Hosary et al. 2021). To confirm this, all the tick pools with multiple infections were further screened individually using the second set of DNA to confirm the co-infection. However, despite this limitation, pooling of ticks for molecular detection of pathogens used in the current study is a useful common practice (Barghash et al. 2016), especially in resource-scarce settings (Speybroeck et al. 2012). Pooling of ticks for molecular detection of pathogens has also been previously used in different wild and domestic animals (Krishnamoorthy et al. 2021).
Conclusion
This study revealed a diversity of tick species and TBPs affecting cattle in the study area. The infection rate of TBPs in ticks greatly differed among tick species, season, location, and tick developmental stages. Theileria parva was the most prevalent pathogens in questing ticks compared to the other detected pathogens (B. bigemina and B. bovis). These pathogens are phylogenetically similar among themselves, but differed from pathogens of other regions. The absence of C. burnetii in tick pools suggests an extremely low role of ticks as vector and reservoir of the bacterium in study areas. Nevertheless, considering its pathogenic potential, it is essential to continue monitoring for the possible recurrence of the bacterium in ticks in the future. This information adds to the knowledge of TBPs epidemiology in Tanzania and will be useful in the formulation of control strategies.
Supplementary information
Below is the link to the electronic supplementary material.
Supplementary material 1 (DOCX 379.2 kb)
Author contributions
The study was designed by NJ and QC. The manuscript was written by IH and finalized by QC. Data collected by IH and analysed by IH and MS. All authors reviewed the manuscript.
Funding
This work was supported by the CAMS Innovation Fund for Medical Sciences (CIFMS) (Grant No. 2019-I2M-5-042).
Data availability
Not applicable.
Declarations
Competing interest
The authors declare no competing interests.
Ethical approval
This study was approved by the regional and district veterinary authorities in Tanzania (Reference no. is DGC/V.10/1 VOL III/143).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Isihaka Haji, Email: isihaka.haji@sua.ac.tz.
Qijun Chen, Email: qijunchen759@syau.edu.cn.
References
- Adjou Moumouni PF, Aboge GO, Terkawi MA, Masatani T, Cao S, Kamyingkird K, Jirapattharasate C, Zhou M, Wang G, Liu M, Iguchi A, Vudriko P, Ybanez AP, Inokuma H, Shirafuji-umemiya R, Suzuki H, Xuan X. Molecular detection and characterization of Babesia bovis, Babesia bigemina, Theileria species and Anaplasma marginale isolated from cattle in Kenya. Parasit Vectors. 2015;8:495. doi: 10.1186/s13071-015-1106-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AL-Hosary A, Raileanu C, Tauchmann O, Fischer S, Nijhof AM, Silaghi C. Tick species identification and molecular detection of tick-borne pathogens in blood and ticks collected from cattle in Egypt. Ticks Tick Borne Dis. 2021 doi: 10.1016/j.ttbdis.2021.101676. [DOI] [PubMed] [Google Scholar]
- Andreassen A, Jore S, Cuber P, Dudman S, Tengs T, Isaksen K, Olav HH, Viljugrein H, Ånestad G, Ottesen P, Vainio K. Prevalence of tick borne encephalitis virus in tick nymphs in relation to climatic factors on the southern coast of Norway. Parasit Vectors. 2012;5:177. doi: 10.1186/1756-3305-5-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barghash SM, Hafez AA, Darwish AM, El-Naga TRA. Molecular detection of pathogens in ticks infesting camels in Matrouh Governorate. Egypt J Bacteriol Parasitol. 2016;7:2. doi: 10.4172/2155-9597.1000269. [DOI] [Google Scholar]
- Bazarusanga T, Marcotty AM, Ahouandjinou KI, Ntumba T, Katendi C, Geysen D. Estimation of the Theileria parva entomological inoculation rate (EIR) by means of tick burden and proportion of infected questing ticks in three different farming systems in Rwanda. Int J Voc Tech Educ. 2011;3:99–106. doi: 10.1108/13598540410550082. [DOI] [Google Scholar]
- Bekloo AJA, Ramzgouyan MR, Shirian S, Tajedin L, Bakhshi H, Faghihi F, Sedaghat M, Telmadarraiy Z. Molecular characterization and phylogenetic analysis of Anaplasma spp. and Ehrlichia spp. isolated from various ticks in the southeastern and northwestern regions of Iran. Vector Borne Zoonotic Dis. 2018;18:252–257. doi: 10.1089/vbz.2018.2271. [DOI] [PubMed] [Google Scholar]
- Celina SS, Cerný J. Coxiella burnetii in ticks, livestock, pets and wildlife: a mini-review. Front Vet Sci. 2022;9:1068129. doi: 10.3389/fvets.2022.1068129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump JA, Morrissey AB, Nicholson WL, Massung RF, Stoddard RA, et al. Etiology of severe non-malaria febrile illness in northern Tanzania: a prospective cohort study. PLoS Negl Trop Dis. 2013;7:e2324. doi: 10.1371/journal.pntd.0002324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darriba D, Taboada GL, Doallo R, Doallo D. jModelTest 2: more models new heuristics and parallel computing. Nat Methods. 2012;9(8):772–772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meneghi D, Stachurski F, Adakal H. Experiences in tick control by acaricide in the traditional cattle sector in Zambia and Burkina Faso: possible environmental and public health implications. Front Public Heal. 2016;4:239. doi: 10.3389/fpubh.2016.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Peña A, Cevidanes A, Sprong H, Millán J. Pitfalls in tick and tick-borne pathogens research, some recommendations and a call for data sharing. Pathogens. 2021;10:712. doi: 10.3390/pathogens10060712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JS, Kahl O, Zintl A. What we do still need to know about Ixodes ricinus? Ticks Tick Borne Dis. 2021 doi: 10.1016/j.ttbdis.2021.101682. [DOI] [PubMed] [Google Scholar]
- Heylen DJA, Kumsa B, Kimbita E, Frank MN, Muhanguzi D, Jongejan F, Adehan SB, Toure A, AboagyeAntwi F, Ogo NI, Julef N, Craford D, Fourie J, Labuchange M, Madder M. Tick-borne pathogens and body condition of cattle in smallholder rural livestock production systems in East and West Africa. Parasit Vectors. 2023;16:117. doi: 10.1186/s13071-023-05709-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongejan F, Uilenberg G. The global importance of ticks. Parasitology. 2004;129:1057–1058. doi: 10.1017/S0031182004005967. [DOI] [PubMed] [Google Scholar]
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klitgaard K, Kjaer LJ, Isbrand A, Hansen MF, Bodker R. Multiple infections in questing nymphs and adult female Ixodes ricinus ticks collected in a recreational forest in Denmark. Ticks Tick Borne Dis. 2019;10:1060–1065. doi: 10.1016/j.ttbdis.2019.05.016. [DOI] [PubMed] [Google Scholar]
- Knap N, Žele D, Glinšek Biškup U, Avšič-Županc T, Vengušt G. The prevalence of Coxiella burnetii in ticks and animals in Slovenia. BMC Vet Res. 2019;15:368. doi: 10.1186/s12917-019-2130-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körner S, Makert GR, Ulbert S, Pfeffer M, Mertens-Scholz K. The prevalence of Coxiella burnetii in hard ticks in Europe and their role in Q fever transmission Revisited—a systematic review. Front Vet Sci. 2021;8:655715. doi: 10.3389/fvets.2021.655715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy P, Sudhagar S, Goudar AL, Jacob SS, Suresh KP. Molecular survey and phylogenetic analysis of tick-borne pathogens in ticks infesting cattle from two south indian states. Vet Parasitol Reg Stud Reports. 2021;25:100595. doi: 10.1016/j.vprsr.2021.100595. [DOI] [PubMed] [Google Scholar]
- Lynen G, Zeman P, Bakuname C, Di Giulio G, Mtui P, Sanka P, Jongejan F. Cattle ticks of the genera Rhipicephalus and Amblyomma of economic importance in Tanzania: distribution assessed with GIS based on an extensive field survey. Exp Appl Acarol. 2007;43:303–319. doi: 10.1007/s10493-007-9123-9. [DOI] [PubMed] [Google Scholar]
- Lynen G, Zeman P, Bakuname C, Di Giulio G, Mtui P, Sanka P, Jongejan F. Shifts in the distributional ranges of Boophilus ticks in Tanzania: evidence that a parapatric boundary between Boophilus microplus and B. decoloratus follows climate gradients. Exp Appl Acarol. 2008;44:147–164. doi: 10.1007/s10493-008-9134-1. [DOI] [PubMed] [Google Scholar]
- Maurin M, Raoult D. Q Fever. Clin Microbiol Rev. 1999;12:518–553. doi: 10.1128/CMR.12.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mediannikov O, Fenollar F, Socolovschi C, Diatta G, Bassene H, Molez JF, Sokhna C, Trape JF, Raoult D. Coxiella burnetii in humans and ticks in rural Senegal. PLoS Negl Trop Dis. 2010;4:e654. doi: 10.1371/journal.pntd.0000654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medley GF, Perry B, Young AAS. Preliminary analysis of the transmission dynamics of Theileria parva in eastern Africa. Parasitology. 1993;106:251–264. doi: 10.1017/s0031182000075077. [DOI] [PubMed] [Google Scholar]
- Moutailler S, Valiente Moro C, Vaumourin E, Michelet L, Tran FH, Devillers E, Cosson JF, Gasqui P, Van VT, Mavingui P. Co-infection of ticks: the rule rather than the exception. PLoS Negl Trop Dis. 2016;10:e0004539. doi: 10.1371/journal.pntd.0004539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njeru J, Henning K, Pletz MW, HELLER R, Neubauer H. Q fever is an old and neglected zoonotic disease in Kenya: a systematic review. BMC Public Health. 2016;16:297. doi: 10.1186/s12889-016-2929-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonga E, Muwonge A, Mdegela H. Tick infestations in extensively grazed cattle and efficacy trial of high-cis cypermethrin pour-on preparation for control of ticks in Mvomero district in Tanzania. BMC Vet Res. 2012;8:2–8. doi: 10.1186/1746-6148-8-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odongo DO, Sunter JD, Kiara HK, Skilton RA, Bishop RP. A nested PCR assay exhibits enhanced sensitivity for the detection of Theileria parva infections in bovine blood samples from carrier animals. Parasitol Res. 2010;106:357–365. doi: 10.1007/s00436-009-1670-z. [DOI] [PubMed] [Google Scholar]
- Oswe M, Odhiambo R, Mutai B, Nyakoe N, Awinda G, Waitumbi JN. Zoonotic pathogens in ticks collected from livestock in Kenya. Open J Prev Med. 2018;8:248–259. doi: 10.4236/ojpm.2018.88021. [DOI] [Google Scholar]
- Pilloux L, Baumgartner A, Jaton K, Lienhard R, Ackermann-Gäumann R, Beuret C, Greub G. Prevalence of Anaplasma phagocytophilum and Coxiella burnetii in Ixodes ricinus ticks in Switzerland: an underestimated epidemiologic risk. New Microbes New Infect. 2018;27:22–26. doi: 10.1016/j.nmni.2018.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringo A, Rizk MA, Adjou Moumouni PF, Liu M, Galon EM, Li., Ji S, Tumwebaze M, Byamukama B, Thekisoe O, Xuan X. Molecular detection and characterization of tick-borne haemoparasites among cattle on Zanzibar Island, Tanzania. Acta Trop. 2020;211:105598. doi: 10.1016/j.actatropica.2020.105598. [DOI] [PubMed] [Google Scholar]
- Rocha SC, Velásquez CV, Aquib A, Al-Nazal A, Parveen N. Transmission cycle of tick-borne infections and co-infections, animal models and diseases. Pathogens. 2022;11:1309. doi: 10.3390/pathogens11111309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short NJ, Norval RA. The seasonal activity of Rhipicephalus appendiculatus Neumann 1901 (Acarina: Ixodidae) in the highveld of Zimbabwe Rhodesia. J Parasitol. 1981;67:77–84. doi: 10.2307/3280782. [DOI] [PubMed] [Google Scholar]
- Skilton RA, Bishop RP, Katende JM, Mwaura S, Morzaria SP. The persistence of Theileria parva infection in cattle immunized using two stocks which differ in their ability to induce a carrier state: analysis using a novel blood spot PCR assay. Parasitology. 2002;124:265–276. doi: 10.1017/s0031182001001196. [DOI] [PubMed] [Google Scholar]
- Speybroeck N, Williams CJ, Lafia KB, Devleesschauwer B, Berkvens D. Estimating the prevalence of infections in vector populations using pools of samples. Med Vet Entomol. 2012;26:361–371. doi: 10.1111/j.1365-2915.2012.01015.x. [DOI] [PubMed] [Google Scholar]
- Swai ES, Karimuribo ED, Rugaimukamu EA, Kambarage DM. Factors influencing the distribution of questing ticks and the prevalence estimation of T. parva infection in brown ear ticks in the Tanga region. Tanzan J Vector Ecol. 2006;31:224–228. doi: 10.3376/1081-1710(2006)31[224:FITDOQ]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Tamura K, Nei M, Kumar S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Nat Acad Sci (USA) 2004;101:11030–11035. doi: 10.1073/pnas.0404206101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Kumar S. MEGA 11: molecular evolutionary genetics analysisversion11. Mol Biol Evol. 2021;8:3022–3027. doi: 10.1093/molbev/msab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terkawi MA, Huyen NX, Shinuo C, Inpankaew T, Maklon K, Aboulaila M. Molecular and serological prevalence of Babesia bovis and Babesia bigemina in water buffaloes in the northeast region of Thailand. Vet Parasitol. 2011;178:201–207. doi: 10.1016/j.vetpar.2011.01.041. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To H, Kako N, Zhang GQ, Otsuka H, Ogawa M, Ochiai O, Nguyen SAV, Yamaguchi T, Fukushi H, Nagaoka N, Akiyama M, Amano K, Hirai K. Q fever pneumonia in children in Japan. J Clin Microbiol. 1996;34:647–651. doi: 10.1128/jcm.34.3.647-651.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AR, Bouattour A, Camicas JL, Estrada-Peña A, Horak IG, Latif AA, Pegram RG, Preston PM. Ticks of domestic animals in Africa: a guide to identification of species. Edinburgh Scotland, UK: Bioscience Reports; 2014. p. 227. [Google Scholar]
- Warwick BT, Bak E, Baldassarre J, Gregg E, Martinez R, Kioko J, Saningo K, Kiffner C. Abundance estimations of ixodid ticks on Boran cattle and somali sheep in northern Tanzania. Int J Acarol. 2016;42:12–17. doi: 10.1080/01647954.2015.1109708. [DOI] [Google Scholar]
- Yawa M, Nyangiwe N, Muchenje V, Kadzere CT, Mpendulo TC, Marufu MC. Ecological preferences and seasonal dynamics of ticks (Acari: Ixodidae) on and of bovine hosts in the Eastern Cape Province South Africa. Exp Appl Acarol. 2018 doi: 10.1007/s10493-018-0234-2. [DOI] [PubMed] [Google Scholar]
- Zintl A, Gray JS, Skerrett HE, Mulcahy G. Possible mechanisms underlying age-related resistance to bovine babesiosis. Parasit Immunol. 2005;27:115–120. doi: 10.1111/j.1365-3024.2005.00748.x. [DOI] [PubMed] [Google Scholar]
- Zobba R, Chisub V, Parpaglia MLP, Spezzigud A, Masala G, Schianchia E, Alberti A. Molecular characterization and phylogenetic analysis of Babesia and Theileria spp. in sardinian ruminants. Vet Parasitol Reg Stud Reports. 2020;22:100453. doi: 10.1016/j.vprsr.2020.100453. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 (DOCX 379.2 kb)
Data Availability Statement
Not applicable.