Abstract
Objectives
To report the incidence, predictors, the impact of bladder perforation (BP), and our protocol of management in patients who underwent trans-urethral resection of bladder tumor (TURBT).
Methods
This is a retrospective study, between 2006 and 2020, on patients who underwent TURBT for non-muscle-invasive bladder cancer (NMIBC). Bladder perforation was defined as any full thickness resection of the bladder wall. Bladder perforations were managed based on their severity and type. Small BP with no or mild symptoms were managed with prolongation of urethral catheters. Those with significant extraperitoneal extravasations were managed by insertion of a tube drain (TD). Abdominal exploration was done for extensive BP and all intraperitoneal extravasations.
Results
Our study included 1,570 patients, the mean age was 58 ± 11 years and 86% were males. Bladder perforation was recorded in 10% (n = 158) of the patients. The perforation was extraperitoneal in 95%, and in 86%, the perforation was associated with no symptoms, mild symptoms, or mild fluid extravasation that required only prolongation of the urethral catheter. On the other hand, active intervention was required for the 21 remaining patients (14%) with TD being the most frequent management. History of previous TURBT (p = 0.001) and obturator jerk (p = 0.0001) were the only predictors for BP.
Conclusions
The overall incidence of bladder perforation is 10%; however, 86% required only prolongation of urethral catheter. Bladder perforation did not affect the probability for tumor recurrence, tumor progression nor radical cystectomy.
Keywords: Bladder perforation, Transurethral resection of bladder tumors, TURB, TURBT, Bladder tumor, Management
Introduction
Bladder tumors are the most common urothelial malignancies, and 75–80% are non-muscle invasive (NMIBC). Trans-urethral resection of bladder tumor (TURBT) is the primary surgical management for NMIBC and is considered one of the most common endourologic procedures [1]. It has been described as a reasonably safe procedure with bleeding and bladder perforation (BP) are the most common complications. Bleeding during TURBT can be controlled at the time of surgery, but BP is a special concern.
Bladder perforation has serious side effects, both in short- and long-term outcomes: BP could abort the procedure, preclude from giving intravesical therapy, and increase the risk of extravasation of tumor cells [2]. The literature has substantial reviews on the predictors of BP [3–5]; however, the impact of BP on bladder tumors was infrequently reported in the literature [4, 6].
In addition to reporting the incidence, predictors, and our protocol of management of BP, we also aimed to report on the impact of BP on bladder tumors outcome. This study included a relatively large series of patients at a single tertiary urology institute.
Patients and methods
After obtaining the institutional review board approval (R.22.12.1975), a retrospective analysis was conducted on the hospital’s data base retrieving all patients who were diagnosed with non-muscle-invasive bladder cancer between January 2006 and December 2020. Patients who were treated with transurethral resection of bladder tumors (TURBT) were further analyzed.
Exclusion criteria: Patients with muscle-invasive bladder cancer, those with bladder tumors that had metastasized at the time of diagnosis, and those with non-cancerous tumors on the final histopathological reports were excluded from the study.
Operative details
Spinal anesthesia was the preferred mode of anesthesia, and general anesthesia was used if the spinal anesthesia was contraindicated or failed. TURBT was done following the standard surgical principles. A single dose of intravesical chemotherapy epirubicin (50 mg) was given in the recovery room to eligible patients apparently with small-volume, low-grade NMIBC. Adjuvant intravesical chemoimmunotherapy was given based on the European guidelines protocol.
Bladder perforation
Our study included not only symptomatic patients with obvious perforations that manifested with extravasation or abdominal distention, but also included silent perforation in asymptomatic patients. BP was defined as any full thickness resection of the bladder wall resulting in documented (gross) extravasation of irrigating fluid or recognition of perivesical fat intraoperatively. Bladder perforations were managed based on the time of recognition, types and its severity.
Intraoperatively, if minor extraperitoneal BP was discovered, the abdomen was checked, and if there were no signs of abdominal distention, the resection was to be performed quickly and hemostasis to be commenced as early as possible. However, if there were signs of abdominal distention, the procedure had to be terminated, and hemostasis was to be performed “if possible”.
Any major extraperitoneal bladder perforation with extensive extravasation and all intraperitoneal perforations were treated by abdominal exploration and the tears were repaired. Cystectomy was performed as an emergency procedure in select patients with a large burden of bladder tumors associated with extensive perforation, or uncontrolled bleeding.
If BP was associated with abdominal distention, the abdomen was screened by ultrasonography (US). No intervention was performed for those who had mild fluid collection limited to the pelvis. However, a moderate amount of extravasation with extension to the upper abdomen was managed by insertion of an abdominal tube drain (TD).
Postoperatively, minor extraperitoneal bladder perforation in asymptomatic patients was managed only by prolongation of the urethral catheter for 3–5 days. In major BP, abdominal TD was removed within 2–3 days and urethral catheter was kept for 7–14 based on the extent of the perforation. Catheters were removed at the outpatient clinic and cystogram was not a routine workup before catheter removal.
The outcomes
The incidence of BP and the predictors were reported using bivariate and multivariate analyses, and the impact of BP on tumor recurrence and progression was studied. Additionally, our protocol in managing BP at a tertiary urology institute was documented in detail.
Statistical analysis
The data were collected using IBM SPSS version 21 (IBM Corp., Armonk, NY, USA). For univariate analysis, frequency and percentage were used to express categorical variables. Mean and standard deviation were used to express the scale variables with normally distributed data. For bivariate analysis, Chi-square (χ 2) test was used for categorical variables. For scale variables, paired sample t-test was used for normally distributed data.
Multivariate analysis with a logistic regression model was generated for variables that yielded significance on bivariate analysis. In all tests, the significance was set at p < 0.05.
Results
Of 1,592 patients with NMIBC who were managed by TURBT, 22 with incomplete files were eliminated from the analysis, leaving 1,570 patients eligible for analysis. The mean age was 58 ± 11 years, males outweighed females (86%), and the median follow up was 42 months (range:10–140). The vast majority of patients had staged T1, and nearly half of them were graded as GII bladder tumors. The other tumor characteristics are shown in Table 1.
Table 1.
Patients’ demographic characteristics of 1570 patients who had TURBT for NMIBC
| Variables | No | (%) |
|---|---|---|
|
Age (Mean ± SD) BMI (Mean ± SD) |
58 ± 11.5 28.2 ± 7.5 |
|
| Gender | ||
| Male | 1350 | (86%) |
| Female | 220 | (14%) |
| Previous TURBT | ||
| No | 1162 | (74) |
| Yes | 408 | (26) |
| Tumor size | ||
| Less than 3 cm | 820 | (53) |
| 3 cm or more | 750 | (47) |
| Tumor grade | ||
| Grade I | 188 | (12) |
| Grade II | 832 | (53) |
| Grade III | 550 | (35) |
| Tumor stage | ||
| Ta | 377 | (24) |
| T1 | 1083 | (69) |
| Primary CIS | 110 | (7) |
| Number of previous TURBT | ||
| De novo | 1162 | (74) |
| One recurrence | 236 | (15) |
| Two recurrence | 94 | (6) |
| Three recurrence | 47 | (3) |
| > Three recurrence | 31 | (2) |
| Tumor site (location) | ||
| Posterior | 346 | (22) |
| Lateral walls | 521 | (33) |
| Anterior and domal | 143 | (9) |
| Trigone and BN | 94 | (6) |
| Multicentric | 466 | (30) |
| Tumor number | ||
| Single | 797 | (51) |
| Multiple | 773 | (49) |
| Bladder perforation | ||
| No | 1412 | (90) |
| Yes | 158 | (10) |
Bladder perforation was recorded in 10% (n = 158) of the patients, 95% of them were extraperitoneal (n = 149/158) and the rest were intraperitoneal. In the majority of the cases [86% (n = 137/158)], the perforation was associated with no symptoms, mild symptoms, or mild fluid extravasation that required only prolongation of the urethral catheter for a median of 5 (range, 3–10) days based on the extension of the BP with no other interventions, Figs. 1 and 2.
Fig. 1.
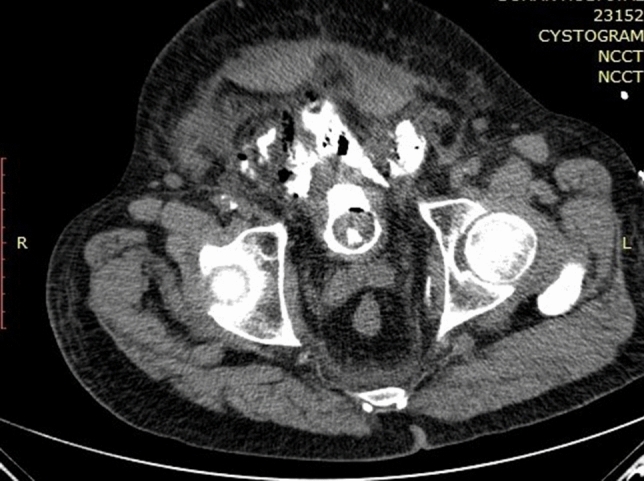
65-year-old male who came to the ER with an acute abdomen 5 days post TURBT and after 2 days of removal of the urethral catheter. CT cystogram axial view showing extravasation of the contrast outside the UB in the extraperitoneal space
Fig. 2.
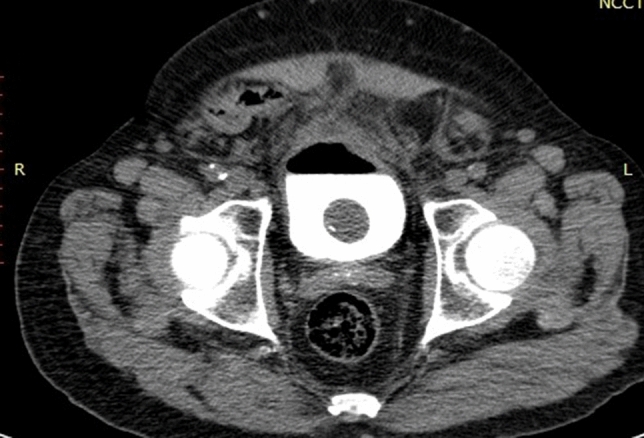
In the same patient, CT cystogram axial view with post indwelling urethral catheter for 10 days after the initial presentation showing the contrast in the UB with resolving of the extravasation
On the other hand, active intervention was required for the 21 remaining patients (14%); drain for 13, bladder repair in 6, and urgent cystectomy for only 2 patients.
On bivariate analysis, none of the following were predictors for BP: age, body mass index, gender, or tumor number/location. However, statistically significant predictors were the history of previous TURBT with the odds increasing as the number of previous TURBT increased (p = 0.001), tumor stage (p = 0.045), and occurrence of obturator jerk (p = 0.0001), Table 2.
Table 2.
Bivariate and multivariate analysis of the possible predictors of perforation in patients with non-muscle-invasive bladder cancer (N = 1570)
| Parameters | Bivariate analysis | Multivariate regression | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Occurrence of bladder perforation | Odds ratio | p-value | Odds ratio | 95% CI | p-value | ||||
| No N: 1412 (%) |
Yes N: 158 (%) |
||||||||
| Gender | |||||||||
| Male | 1219 | (90) | 131 | (10) | 0.2 | ||||
| Female | 193 | (87) | 27 | (13) | |||||
| Previous TURBT | |||||||||
| No | 1073 | (92) | 89 | (8) | 13 | 0.001 | 2 | 1.4– | 0.001 |
| Yes | 339 | (83) | 69 | (17) | 2.8 | ||||
| Number of previous TURBT | |||||||||
| De novo | 1055 | (91) | 107 | (9) | |||||
| One recurrence | 205 | (87) | 31 | (13) | |||||
| Two recurrences | 88 | (84) | 6 | (16) | 19 | 0.001 | 0.08 | ||
| Three recurrences | 39 | (84) | 8 | (16) | |||||
| > Three recurrence | 25 | (81) | 6 | (19) | |||||
| Tumor number | |||||||||
| Single | 725 | (91) | 71 | (9) | 3.4 | 0.12 | 0.06 | ||
| Multiple | 687 | (88) | 87 | (12) | |||||
| Tumor size | |||||||||
| Less than 3 cm | 735 | (89) | 85 | (11) | |||||
| 3 cm or more | 677 | (90) | 73 | (10) | 0.7 | ||||
| Tumor grade | |||||||||
| Grade I | 170 | (91) | 18 | (9) | |||||
| Grade II | 754 | (91) | 78 | (9) | 0.6 | ||||
| Grade III | 488 | (89) | 62 | (11) | |||||
| Tumor stage | |||||||||
| Ta | 354 | (97) | 23 | (6) | |||||
| T1 | 956 | (90) | 127 | (11) | 7 | 0.045 | |||
| Primary CIS | 102 | (95) | 8 | (7) | |||||
| Tumor site (location) | |||||||||
| Posterior | 315 | (91) | 31 | (9) | 0.08 | ||||
| Lateral walls | 459 | (87) | 62 | (13) | |||||
| Anterior and domal | 123 | (86) | 20 | (14) | |||||
| Trigone and BN | 86 | (93) | 8 | (7) | |||||
| Multicentric | 429 | (92) | 37 | (8) | |||||
| Obturator jerk | |||||||||
| No | 1404 | (90) | 149 | (10) | 30 | 0.0001 | 10 | 3.8– | 0.0001 |
| Yes | 8 | (47) | 9 | (53) | 27.2 | ||||
Bold values indicate statistically significant values
On multivariate regression model, history of previous TURBT (OR = 2 [CI = 1.4–2.8], p = 0.001) and occurrence of obturator jerk (OR = 10, [CI = 3.8–27] p = 0.0001) sustained their significance, Table 2.
There were no statistical differences in the occurrence of bladder tumor recurrence (39/368 = 10.5% vs 119/1202 = 9.9%, p = 0.6), tumor progression (22/231 = 9.5% vs 29/1339 = 8.5%, p = 0.5), nor the probability for radical cystectomy (19/230 = 8.3% vs 139/1340 = 10.4%, p = 0.3) between bladder perforation vs non-perforation groups, respectively.
Discussion
In our series, we recorded 10% incidence of BP, and the literature reported a wide range of BP incidence: 1–10% [4, 7, 8]. This wide range depends on the definition of BP, documentation, and the tools for diagnosis. We defined BP as any full thickness resection of the bladder wall resulting in documented (gross) extravasation of irrigating fluid or recognition of perivesical fat intraoperatively, and our protocol was to document any perforation, even in asymptomatic patients. For that reason, we recorded a quite higher incidence than those who reported only symptomatic BP. On the other hand, our incidence is considered low if compared to the series with an incidence of 50% that performed a routine postoperative cystogram, a method that discovers all tiny and trivial perforations which sometimes are not visible to surgeons [9]
Despite this wide range in the reporting of the incidence of BP, there is agreement in the literature that the majority of BP are extraperitoneal, mild and asymptomatic which can be treated only with prolongation of urethral catheters [8, 10, 11]. Similarly, in our research, 95% of BP were extraperitoneal, and 86% of the cases required only prolongation of the urethral catheter.
Bladder perforation can be identified at the time of resection, and the patient may have abdominal tenderness and distention, tachycardia, or low blood pressure that can be observed by the anesthesiologist. A high degree of suspicion is required by the surgeon to detect BP early as seen by a deep hole in the bladder wall and yellow, perivesical fat or inadequate bladder filling. In cases of very large defects with intraperitoneal extravasation, the surgeon may even notice inadequate bladder filling. In our series, and in most of the published series, the vast majority of the bladder tumors were in the posterior and lateral walls. Subsequently, it is expected that extraperitoneal perforation is more common than intraperitoneal perforation which we reported in our series at only 5% [12]. Anterior and domal bladder tumors were reported in only 9% of the entire series, and this could explain that fact. Bladder perforation could be accompanied by escape of irrigation fluids outside the bladder to the extraperitoneal space. With the escape of only a minimal amount of irrigation fluids, the problem is usually self-limited and does not require any active intervention. This occurred in 86% in our series, and there is agreement in the literature that BP does not require active intervention in the vast majority of patients [10, 12].
If the escape of fluid irrigation in the extraperitoneal space is quite large, Ultrasound can be used not only to diagnose fluid extravasation, but also can guide tube insertion for drainage. This method was the most common modality of intervention used in our series (60%). In contrast to the extraperitoneal space, the intraperitoneal space is open, and escape of irrigation fluid into this space is not self-limited and requires active intervention. Only 8 cases (5%) in our series required exploration, bladder repair was performed in six of them, and only two required urgent cystectomies. Most of the published series reported that exploration is the method of choice for intraperitoneal BP, however, insertion of a tube for drainage was reported infrequently in the literature [13] for intraperitoneal BP.
Factors that may predispose a patient to BP can be classified into four categories: patient, urinary bladder, tumor, and technical factors. Our analysis did not indicate a correlation between patient’s factors (such as age, gender and BMI) and the occurrence of BP, however, Herkommer et al. [7] reported female gender, and low BMI, and Golan et al. [5] reported elderly patients were predictors of BP occurrence. Bladder perforation was proved not to be due to deficient structure of the bladder wall itself. If the natural bladder wall was not a predictor, repeated resection could weaken the bladder wall due to fibrosis replacing the muscles and predispose the patient to BP.
In our series, we found that history of previous resection was an independent risk factor for the occurrence of BP (p = 0.001), with the odds increasing as the number of previous TURBT increased. This could be because bladder recurrence is more common on the tumor bed, and the same results were reported by [5] who found that BP is more likely to occur in heavily pretreated bladders.
Tumor characteristics could play a role in the occurrence of BP, and in our study, tumor stage was a predictor for BP on bivariate analysis, but it did not sustain its significance on multivariate analysis. However, tumor staging was reported by another study to be a predictor for BP (p-value = 0. 0.029)8. In that study, Herkommer et al. [7] included T2 bladder tumors that could be associated with deeper resections which increase the possibility of bladder perforation.
We could not find a correlation between tumor location, as reported by El Hayek et al. [9], where tumor location in the dome was a predisposing factor for bladder perforation.
No doubt that technical factors may play a role in the occurrence of BP. Obturator jerk (OJ) was an independent risk factor for BP in our study which sustained its significance in multivariate analysis (odds, 10 [CI = 3.8–27], p = 0.001). Because OJ was reported frequently in medical studies as a risk factor for BP [8, 14], researchers studied different surgical techniques to minimize the incidence of BP. Prospective studies and meta-analysis revealed that bipolar electrocautery has fewer complications than monopolar, but it is not superior to monopolar with respect to OJ and BP [15, 16]. On the other hand, there are differences reported between different types of bipolar Gyrus PlasmaKinetic system and Olympus TUR in saline (TURis) system [17]. Laser resection and en-block TUR resection using 980 nm laser fiber were reported to reduce the incidence of BP [18, 19]. Anesthetic techniques may play a role in the occurrence of OJ and BP. The results from systematic reviews on obturator nerve block reported that it lowered the obturator jerk (p = 0.001), and BP (p = 0.02) [14, 20].
Lee et al. [6] reported a few cases of tumor recurrence and progression after BP; however, the total cases of BP were 45 cases only, and the results were not solid to be compared. In our analysis, BP did not affect tumor recurrence, progression nor the probability of radical cystectomy. The impact of BP on survival was also infrequently reported in the literature. In a series that constituted 340 patients (7% = 26) who had BP, Skolarikos et al. [2] reported that open exploration after BP was an independent risk factor for extravesical recurrence and survival (p = 0.001). The possibility of microscopic tumor cell seeding on the bladder wall as a result of perforation could be the explanation; however, this matter requires more investigation. On the other hand, Golan et al. [5] reported that despite the potential comorbidities of BP, this adverse event does not seem to substantially increase the risk of extravesical tumor seeding.
It is to be mentioned that BP is a serious complication, and all measures are recommended to be taken to minimize its occurrence. Several measures proposed in the literature were focused on reduction of the likelihood of occurrence of obturator jerk, reducing the diathermy current, avoiding over-distention of the bladder, and laser use [8]. The presence or absence of detrusor muscle is a validated quality control indicator for the indicated cases (Tis & TaLG/G1-2 tumors, and TURBTs for random biopsies and re-TURBTs of scar excluded) [21]
The main limitation of our study is that it is a retrospective study. Additionally, TURBT were performed by different surgeons with varying levels of experience. Additionally, we do not evaluate time to recurrence, time to progression, and time to cystectomy. We did not include patients with T2 bladder tumor because it is a known risk factor for perforation.
Despite that, our study included a considerable number of patients who were treated at a single tertiary urology center. This provides insight in our protocol in managing bladder perforation in a relatively larger number of patients.
Conclusion
We recorded a total of 10% incidence of bladder perforation with TURBT for NMIBC. History of previous bladder resection and obturator jerk were the only independent risk factors for the occurrence of BP. In the vast majority of patients (86%), the symptoms were mild and did not require any active interventions, but only prolongation of urethral catheter. Abdominal drainage was the most common procedure for those who required intervention. Bladder perforation did not affect bladder tumor recurrence, progression nor the probability for radical cystectomy.
Author contributions
YO: Manuscript writing, E: Project development, Data analysis, DET: Data Collection, MZ: Data Collection, RTA: Radiology imaging extraction and putting comments, DS: Data Collection, AM: Manuscript reviewing, Bedeir Ali-El Dein: Manuscript reviewing.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
The data that support the findings of this study are available on request from the corresponding author [Mohamed Elawdy].
Declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Research involving Human Participants and/or Animals
No.
Informed consent
Not required, retrospective study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yasser Osman, Email: y_osman99@yahoo.com.
Mohamed Elawdy, Email: mmelawdy@gmail.com.
Diaa-Eldin Taha, Email: drdiaaeldin@gmail.com.
Mohamed H. Zahran, Email: zahranmha@yahoo.com
Rasha T. Abouelkheir, Email: dr_rasha_taha@hotmail.com
Doaa Elsayed Sharaf, Email: doaa90@hotmail.com.
Ahmed Mosbah, Email: amosbah@hotmail.com.
Bedeir Ali-El Dein, Email: balieldein@yahoo.com.
References
- 1.Lee F, Patel HRS, Emberton M. The ‘top 10’ urological procedures: a study of hospital episodes statistics 1998–99. BJU Int. 2002;90(1):1–6. doi: 10.1046/j.1464-410X.2002.02833.x. [DOI] [PubMed] [Google Scholar]
- 2.Skolarikos A, Chrisofos M, Ferakis N, Papatsoris A, Dellis A, Deliveliotis C. Does the management of bladder perforation during transurethral resection of superficial bladder tumors predispose to extravesical tumor recurrence? J Urol. 2005;173(6):1908–1911. doi: 10.1097/01.ju.0000158450.71497.ae. [DOI] [PubMed] [Google Scholar]
- 3.Poletajew S, et al. Prediction of the risk of surgical complications in patients undergoing monopolar transurethral resection of bladder tumour - a prospective multicentre observational study. Arch Med Sci. 2020;16(4):863–870. doi: 10.5114/aoms.2019.88430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Comploj E, et al. Perforation during TUR of bladder tumours influences the natural history of superficial bladder cancer. World J Urol. 2014;32(5):1219–1223. doi: 10.1007/s00345-013-1197-x. [DOI] [PubMed] [Google Scholar]
- 5.Golan S, Baniel J, Lask D, Livne PM, Yossepowitch O. Transurethral resection of bladder tumour complicated by perforation requiring open surgical repair - clinical characteristics and oncological outcomes. BJU Int. 2011;107(7):1065–1068. doi: 10.1111/j.1464-410X.2010.09696.x. [DOI] [PubMed] [Google Scholar]
- 6.Lee J, Suh J, Jeong CW, Kwak C, Kim HH, Ku JH. Efficacy of the treatment of intraperitoneal bladder perforation during transurethral resection of bladder tumor with the urethral catheter alone: retrospective analysis of over 15 years using the clinical data warehouse system. Urol Int. 2022;106(2):138–146. doi: 10.1159/000517332. [DOI] [PubMed] [Google Scholar]
- 7.Herkommer K, Hofer C, Gschwend JE, Kron M, Treiber U. Gender and body mass index as risk factors for bladder perforation during primary transurethral resection of bladder tumors. J Urol. 2012;187(5):1566–1570. doi: 10.1016/j.juro.2011.12.114. [DOI] [PubMed] [Google Scholar]
- 8.Panagoda PI, Vasdev N, Gowrie-Mohan S. Avoiding the obturator Jerk during TURBT. Curr Urol. 2018;12(1):1–5. doi: 10.1159/000447223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El Hayek OR, et al. Evaluation of the incidence of bladder perforation after transurethral bladder tumor resection in a residency setting. J Endourol. 2009;23(7):1183–1186. doi: 10.1089/end.2008.0406. [DOI] [PubMed] [Google Scholar]
- 10.Shoshany O, Mano R, Margel D, Baniel J, Yossepowitch O. Presence of detrusor muscle in bladder tumor specimens-predictors and effect on outcome as a measure of resection quality. Urologic Oncology: Seminars and Original Investigations. 2014;32(1):40.e17–40.e22. doi: 10.1016/j.urolonc.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Nieder AM, Meinbach DS, Kim SS, Soloway MS. Transurethral bladder tumor resection: Intraoperative and postoperative complications in a residency setting. J Urol. 2005;174(6):2307–2309. doi: 10.1097/01.ju.0000181797.19395.03. [DOI] [PubMed] [Google Scholar]
- 12.Pansadoro A, Franco G, Laurenti C, Pansadoro V. Conservative treatment of intraperitoneal bladder perforation during transurethral resection of bladder tumor. Urology. 2002;60(4):682–684. doi: 10.1016/S0090-4295(02)01843-5. [DOI] [PubMed] [Google Scholar]
- 13.Manikandan R, Lynch N, Grills RJ. Percutaneous peritoneal drainage for intraperitoneal bladder perforations during transurethral resection of bladder tumors. J Endourol. 2003;17(10):945–947. doi: 10.1089/089277903772036343. [DOI] [PubMed] [Google Scholar]
- 14.Krishan A, Bruce A, Khashaba S, Abouelela M, Ehsanullah SA. Safety and efficacy of transurethral resection of bladder tumor comparing spinal anesthesia with spinal anesthesia with an obturator nerve block: a systematic review and meta-analysis. J Endourol. 2021;35(3):249–258. doi: 10.1089/end.2020.1054. [DOI] [PubMed] [Google Scholar]
- 15.Osman Y, Harraz AM. A review comparing experience and results with bipolar versus monopolar resection for treatment of bladder tumors. Curr Urol Rep. 2016;17(3):1–6. doi: 10.1007/s11934-016-0579-1. [DOI] [PubMed] [Google Scholar]
- 16.Cui Y, Chen H, Liu L, Chen J, Qi L, Zu X. Comparing the efficiency and safety of bipolar and monopolar transurethral resection for non-muscle invasive bladder tumors: a systematic review and meta-analysis. J Laparoendosc Adv Surg Tech. 2016;26(3):196–202. doi: 10.1089/lap.2015.0507. [DOI] [PubMed] [Google Scholar]
- 17.Kaynar M, et al. Comparison of two different bipolar energy resources in transurethral resection of bladder tumors. Urol Int. 2021;105(3–4):304–308. doi: 10.1159/000512380. [DOI] [PubMed] [Google Scholar]
- 18.Xu J, Wang C, Ouyang J, Sun J, Hu C. Efficacy and safety of transurethral laser surgery versus transurethral resection for non-muscle-invasive bladder cancer: a meta-analysis and systematic review. Urol Int. 2020;104(9–10):810–823. doi: 10.1159/000506655. [DOI] [PubMed] [Google Scholar]
- 19.Tao W, et al. The clinical study of en bloc transurethral resection with 980nm laser for treatment of primary non-muscle invasive bladder cancer. J Xray Sci Technol. 2020;28(3):563–571. doi: 10.3233/XST-190616. [DOI] [PubMed] [Google Scholar]
- 20.Bolat D, et al. Impact of nerve stimulator-guided obturator nerve block on the short-term outcomes and complications of transurethral resection of bladder tumour: a prospective randomized controlled study. Can Urol Assoc J. 2015;9(11–12):E780–E784. doi: 10.5489/cuaj.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akand M, Muilwijk T, Raskin Y, De Vrieze M, Joniau S, Van Der Aa F. Quality control indicators for transurethral resection of non-muscle-invasive bladder cancer. Clin Genitourin Cancer. 2019;17(4):e784–e792. doi: 10.1016/J.CLGC.2019.04.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author [Mohamed Elawdy].


