Abstract
Background
Medical hospitalizations for people with opioid use disorder (OUD) frequently result in patient-directed discharges (PDD), often due to untreated pain and withdrawal.
Objective
To investigate the association between early opioid withdrawal management strategies and PDD.
Design
Retrospective cohort study using three datasets representing 362 US hospitals.
Participants
Adult patients hospitalized between 2009 and 2015 with OUD (as identified using ICD-9-CM codes or inpatient buprenorphine administration) and no PDD on the day of admission.
Interventions
Opioid withdrawal management strategies were classified based on day-of-admission receipt of any of the following treatments: (1) medications for OUD (MOUD) including methadone or buprenorphine, (2) other opioid analgesics, (3) adjunctive symptomatic medications without opioids (e.g., clonidine), and (4) no withdrawal treatment.
Main Measures
PDD was assessed as the main outcome and hospital length of stay as a secondary outcome.
Key Results
Of 6,715,286 hospitalizations, 127,158 (1.9%) patients had OUD and no PDD on the day of admission, of whom 7166 (5.6%) had a later PDD and 91,051 (71.6%) patients received some early opioid withdrawal treatment (22.3% MOUD; 43.4% opioid analgesics; 5.9% adjunctive medications). Compared to no withdrawal treatment, MOUD was associated with a lower risk of PDD (adjusted odds ratio [aOR] = 0.73, 95%CI 0.68–0.8, p < .001), adjunctive treatment alone was associated with higher risk (aOR = 1.13, 95%CI: 1.01–1.26, p = .031), and treatment with opioid analgesics alone was associated with similar risk (aOR 0.95, 95%CI: 0.89–1.02, p = .148). Among those with PDD, both MOUD (adjusted incidence rate ratio [aIRR] = 1.24, 95%CI: 1.17–1.3, p < .001) and opioid analgesic treatments (aIRR = 1.39, 95%CI: 1.34–1.45, p < .001) were associated with longer hospital stays.
Conclusions
MOUD was associated with decreased risk of PDD but was utilized in < 1 in 4 patients. Efforts are needed to ensure all patients with OUD have access to effective opioid withdrawal management to improve the likelihood they receive recommended hospital care.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11606-023-08059-w.
KEY WORDS: opioid use disorder, patient-directed discharge, opioid withdrawal, epidemiology
INTRODUCTION
US hospitalizations for individuals with opioid use disorder (OUD) increased from 164 to 296 per 100,000 people between 2006 and 2016 and were associated with significant morbidity and mortality.1–3 People with OUD are commonly hospitalized for injection drug-related infections such as endocarditis, overdose, or physical trauma, as well as pneumonia and exacerbations of other chronic medical conditions. Hospitalized patients with OUD frequently leave the hospital prior to completion of medical treatment, designated against medical advice discharge or patient-directed discharge (PDD). PDDs accounted for more than 5% of all discharges for people with OUD and nearly 1 in 5 discharges for individuals with injection drug-associated endocarditis in 2015.4,5 These discharges are associated with increased risk of rehospitalization, higher costs, and worse outcomes compared to planned discharges.6–10
Qualitative studies have shown that PDDs in patients with OUD are driven in part by opioid withdrawal, pain, cravings, and stigma.11,12 Early discharges when opioid withdrawal symptoms are most severe are common but PDDs can occur throughout a hospitalization.4 Patients with OUD frequently remain in the hospital longer than those without OUD for the same conditions due to the care team’s perception that a patient will not adhere to treatments out of the hospital or due to the risk of substance use without monitoring (e.g., intravenous antibiotics).13,14 Additionally, discrimination in post-acute care access for people with OUD limits discharge options and may lengthen hospitalizations.15 Prolonged hospitalizations are challenging for all patients, but especially for those with OUD, whose experiences in the hospital may be particularly difficult due to histories of trauma, loss of autonomy (e.g., movement restrictions, smoking, and substance use prohibitions), and strained communication with hospital staff.11,16,17
Given the growing number of hospitalized people with OUD and the importance of enacting safe discharge plans, it is critical to determine which interventions can reduce PDD. Though inadequately treated withdrawal symptoms are common precipitants of PDD, in our clinical experience, treatment strategies are heterogeneous across hospitals and clinicians. Several evidence-based FDA-approved medications for opioid use disorder (MOUD), namely methadone and buprenorphine, are associated with enhanced treatment retention, sustained abstinence, and reduced overdose and rehospitalization and are recommended by consensus guidelines.18–21 Though management of withdrawal with MOUD has expanded, they remain underused.22–25 Other opioid medications, not specifically approved for the treatment of OUD in the USA, can also effectively control withdrawal symptoms. In Canada, for example, opioid agonists such as extended-release morphine or injectable hydromorphone are approved and recommended treatments for OUD.26–28 In the USA, some experts have called for broader and more aggressive use of full opioid agonists for the management of withdrawal in the hospital, especially in the context of daily fentanyl use which may result in more severe withdrawal symptoms and difficulty initiating buprenorphine due to precipitated withdrawal.29,30 At the same time, others focused on the safety of inpatient opioid dispensing have expressed concerns that in-hospital opioid treatment could accelerate or lead to the development of OUD.31 As a result, some patients with OUD only receive treatment with adjunctive non-opioid medications such as clonidine or dicyclomine which target specific symptoms associated with opioid withdrawal.24
Much of the existing data on OUD and PDD is limited to administrative data sets without clinical granularity to assess withdrawal management strategies.4,7,9,13,32,33 There are several single-site studies which suggest methadone and buprenorphine may be associated with decreased PDD but are limited by sample size and may not be generalizable to all hospital settings.34–38 We therefore used three blended datasets with both clinical and administrative data to examine the association between early opioid withdrawal strategies and PDD as well hospital variation in the use of these strategies in a national sample of US hospitals. We hypothesized substantial variation in withdrawal approaches and that those who receive methadone and buprenorphine will be less likely to experience PDD than those receiving alternative treatments. Additionally, we hypothesized that among those who experienced PDDs, those who received methadone or buprenorphine would remain in the hospital longer.
METHODS
Design, Data Sources, and Population
This was a retrospective cohort study of patients aged 20 or older with OUD admitted to 373 geographically diverse, US academic and community hospitals from January 2009 to September 2015 (the end of International Classification of Diseases 9th Revision, Clinical Modification (ICD-9-CM)). Data were drawn from three non-overlapping datasets: Cerner HealthFacts, HCA Healthcare, and Institute of Health Metrics. This combined data source, which has been described in detail previously, collectively represents a representative mix of US hospitals by size, teaching status, and geographical regions (eMethods 1 in Supplement).25,39 This study was approved by the Harvard Pilgrim Health Care Institute institutional review board.
Identifying Hospitalized Patients with OUD
We identified hospitalized patients with OUD using ICD-9-CM codes for opioid abuse or dependence, opioid adverse effects, or drug poisonings as primary or secondary diagnoses as per the Injury Surveillance Workgroup consensus document (eTable 1 in Supplement).40 We used the broadest administrative definition for OUD, drug poisonings associated with opium, heroin, and opioid analgesics or opioid adverse effect, or abuse/dependence to identify acute adverse events from opioid use or the presence of underlying OUD, consistent with previous research.25 We included principal and secondary diagnosis codes to maximize sensitivity.41 Additionally, to improve case identification, individuals who were administered buprenorphine as inpatients were also included in the cohort. Methadone receipt was not part of the inclusion criteria because it was more commonly used to manage chronic pain than buprenorphine during the study period and we are unable to differentiate between the treatment of OUD and chronic pain in our data. We excluded hospitalizations resulting in PDD on the first day of the hospitalization from the final study cohort to ensure time to observe exposure classification and protect against immortalized time bias.
Measures
Definition of Opioid Withdrawal Management Strategies
Our primary exposure was the type of opioid withdrawal management received during the first two hospital days. We created four-level mutually exclusive categories: first, we classified anyone who received methadone or buprenorphine as having received medication for opioid use disorder (MOUD). Second, we classified anyone who received any other opioid agonist medications but did not receive methadone or buprenorphine as having received other opioid treatment (eTable 2 in Supplement). Third, we defined anyone who received clonidine, dicyclomine, or loperamide, medications commonly used to treat symptoms of withdrawal, but who did not receive methadone, buprenorphine, or any other opioid agonists as having received adjunctive medications. All other patients were categorized as having received no specific opioid withdrawal treatment.
Outcomes
Our primary outcome was disposition status of “against medical advice discharge,” which we defined as PDD to avoid stigmatizing language. Our secondary outcome was hospital length of stay (LOS).
Covariates
We included the following patient characteristics as covariates: age, sex, race/ethnicity, Elixhauser comorbidity score,42 major mental illness, stimulant use, or alcohol use based on ICD-9 codes (eTable 3 in Supplement), intensive care unit (ICU) admission, and mechanical ventilation. For descriptive purposes, we included ICU LOS and, among those with infections, we included infection type (using ICD-9-CM codes) and percent with positive blood culture as well.
Statistical Analyses
We described the sample using mean and standard deviation or median and interquartile range (IQR) for continuous variables and frequencies and proportions for categorical variables. Additionally, we examined variations in the proportion of individuals with OUD who received each withdrawal management approach in each hospital. We used a generalized linear mixed model to fit a logistic regression model to examine the association between opioid withdrawal treatment approach and PDD adjusting for the covariates noted above and having individual hospitals as a random intercept to adjust for between-hospital variation. For the cohort of individuals who had PDDs, we used another mixed model to fit a negative binomial model for the association between opioid withdrawal treatment strategy and hospital LOS, adjusting for the same covariates and treating individual hospitals as random effects. Model results from the three data sources were then compiled using study-level meta-analysis (SLMA).43 Missing data were evaluated and were <1% for gender and race/ethnicity. Therefore, cases with missingness were excluded from specific analyses using those variables but remained in the full cohort. To address variation across hospitals with small samples, as a sensitivity analysis, we repeated the analysis excluding hospitalizations in hospitals that had less than 10 hospitalizations for people with OUD. We also performed a sensitivity analysis including patients with PDD on the day of admission. All statistical analyses were conducted in R version 4.1.0, and p < 0.05 (two-sided) was considered statistically significant. Benjamini–Hochberg (B-H) multiple comparison correction was performed for the regression models and yielded similar results. Both marginal (variance explained by fixed factors) and conditional (variance explained by both fixed and random factors) pseudo-R2 were reported for the regression models as suggested by Nakagawa & Schielzeth (2013).44
RESULTS
Study Cohort
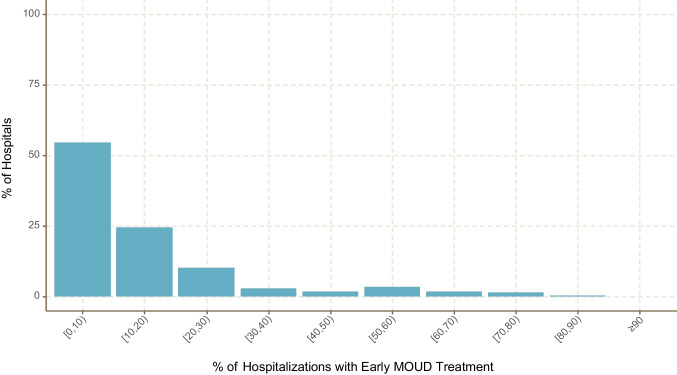
The dataset included a total of 6,715,286 adult hospitalizations, from which a cohort of 127,158 (1.9%) met our inclusion criteria for OUD excluding those with PDD on the day of admission (n = 831) (eFigure 1 in the Supplement). Among the study cohort, 7166 (5.6%) had PDD after the day of admission at a median of 3 days (IQR 2–4). During these hospitalizations, the majority (91,051, 71.6%) received some type of early opioid withdrawal treatment (22.3% received methadone or buprenorphine; 43.4% received other opioid agonists; 5.9% received adjunctive medications) (Table 1). The hospitalizations associated with OUD in the cohort occurred in 362 hospitals which were mainly small (55.7%, <200 beds), non-teaching (67%), and from the South (48.6%) (eTable 4 in Supplement). There were variations in opioid withdrawal management strategies across hospitals. In more than half of the included hospitals (54.4%), <10% of their OUD patients received opioid withdrawal treatment with methadone or buprenorphine (MOUD). In contrast, 6.6% of hospitals provided these medications to 50% or more of patients with OUD (Fig. 1).
Table 1.
Characteristics of Hospitalized Patients with OUD by Patient-Directed Discharge Status (n = 127,158)
| Patient-directed discharge | ||||
|---|---|---|---|---|
| Characteristic | Full cohort (n = 127,158) |
Yes (n = 7166, 5.6%) |
No (n = 119,992, 94.4%) |
p-value |
| Age, years (mean ± sd) | 47.3 ± 17.0 | 40.4 ± 12.8 | 47.8 ± 17.1 | <.001 |
| Male sex (%)* | 60,970 (48.0) | 4254 (59.4) | 56,716 (47.3) | <.001 |
| Race/ethnicity (%)* | <.001 | |||
| White | 97,862 (77.8) | 5040 (70.9) | 92,822 (78.2) | |
| Asian | 592 (0.5) | 18 (0.3) | 574 (0.5) | |
| Black | 15,669 (12.4) | 1016 (14.3) | 14,653 (12.3) | |
| Hispanic | 7587 (6.0) | 726 (10.2) | 6861 (5.8) | |
| Other | 4151 (3.3) | 306 (4.3) | 3845 (3.2) | |
| Elixhauser score (mean ± sd) | −0.2 ± 7.5 | −1.4 ± 5.9 | −0.1 ± 7.6 | <.001 |
| ICU admission (%) | 22,616 (17.8) | 1173 (16.4) | 21,443 (17.9) | .001 |
| ICU LOS, days (mean ± sd) | 3.9 ± 8.6 | 2.75 ± 4.94 | 4.0 ± 8.8 | <.001 |
| Hospital LOS, days (mean ± sd) | 6.4 ± 7.5 | 4.33 ± 4.88 | 6.6 ± 7.6 | <.001 |
| Alcohol use | 24,839 (19.5) | 1948 (27.2) | 22,891 (19.1) | <.001 |
| Stimulant use | 9211 (7.2) | 1028 (14.3) | 8183 (6.8) | <.001 |
| Major mental illness | 60,498 (47.6) | 2795 (39.0) | 57,703 (48.1) | <.001 |
| Infection type | ||||
| Septicemia/bacteremia | 7788 (6.1) | 582 (8.1) | 7206 (6.0) | <.001 |
| Pulmonary | 17,552 (13.8) | 835 (11.7) | 16,717 (13.9) | <.001 |
| Genitourinary | 12,255 (9.6) | 448 (6.3) | 11,807 (9.8) | <.001 |
| Intra-abdominal | 4428 (3.5) | 118 (1.6) | 4310 (3.6) | <.001 |
| Skin and soft tissue | 9281 (7.3) | 952 (13.3) | 8329 (6.9) | <.001 |
| Bone/joint | 2027 (1.6) | 157 (2.2) | 1870 (1.6) | <.001 |
| Obstetrics/gynecology | 971 (0.8) | 53 (0.7) | 918 (0.8) | 0.81 |
| Central nervous system | 559 (0.4) | 36 (0.5) | 523 (0.4) | 0.41 |
| Other | 9856 (7.8) | 600 (8.4) | 9256 (7.7) | 0.04 |
| Positive blood culture | 3521 (2.8) | 339 (4.7) | 3182 (2.7) | <.001 |
| Disposition (%) | <.001 | |||
| Death | 1788 (1.4) | 0 | 1788 (1.5) | |
| Hospice | 1187 (0.9) | 0 | 1187 (1.0) | |
| Hospital transfer | 2176 (1.7) | 0 | 2176 (1.8) | |
| Subacute facility | 13,039 (10.3) | 0 | 13,039 (10.9) | |
| Home | 108,968 (85.7) | 7166 (100) | 101,802 (84.8) | |
| Early opioid withdrawal treatment strategy | <.001 | |||
| No treatment | 36,107 (28.4) | 2126 (29.7) | 33,981 (28.3) | |
| MOUD | 28,397 (22.3) | 1905 (26.6) | 26,492 (22.1) | |
| Other opioids | 55,171 (43.4) | 2591 (36.2) | 52,580 (43.8) | |
| Adjunctive agents | 7483 (5.9) | 544 (7.6) | 6939 (5.8) | |
*Percentages are calculated after excluding cases with missing sex and race/ethnicity (<1% of the data)
Figure. 1.
Hospital variation in the use of early treatment medications for opioid use disorder (MOUD) (n = 362 hospitals).
OUD Patients with vs without PDD
Compared to hospitalizations for OUD patients without PDD, those with PDD were younger (mean age 40.4 vs 47.8) and more likely to be male (59.4 vs 47.9%) and non-White (29.1% vs 21.8%), had fewer ICU admissions (16.4% vs 17.9%) and shorter ICU LOS (2.8 vs 4.0 days) and hospital LOS (4.3 vs 6.6), and had more reported alcohol (27.2% vs 19.1%), stimulant use (14.3% vs 6.8%), and skin and soft tissue infections (13.3% vs 6.9%) but fewer major mental illnesses (39.0% vs 48.1%) (Table 1).
Factors Associated with PDD
In the adjusted multivariable regression model, early opioid withdrawal treatment strategies were associated with PDD among patients hospitalized with OUD (Table 2). Specifically, compared to those who did not receive any treatment, patients who received MOUD (methadone and/or buprenorphine) had a lower risk of experiencing PDD (adjusted odds ratio [aOR] = 0.73, 95% confidence interval [CI]: 0.68–0.8, p < 0.001), though they appear to be at higher risk for PDD using crude counts (26.6% vs 22.1% in Table 1). There was no significant association between receiving opioid agonists other than methadone or buprenorphine (secondary exposure) and PDD (aOR = 0.95, 95% CI: 0.89–1.02, p = 0.148). Receipt of adjunctive agents alone (tertiary exposure) was significantly associated with an increased risk of PDD (aOR = 1.13, 95%CI: 1.01–1.26, p = 0.031). Older age (aOR = 0.97, 95%CI: 0.97–0.97, p < 0.001), increased comorbidity burden (Elixhauser score, aOR = 0.99, 95%CI: 0.99–1.00, p < 0.001), and major mental illness (aOR = 0.66, 95%CI: 0.63–0.70, p < 0.001) were associated with decreased risk of PDD. On the other hand, male gender (aOR = 1.32, 95%CI: 1.25–1.39, p < 0.001), ICU admission (aOR = 1.10, 95%CI: 1.02–1.18, p = 0.009), alcohol (aOR = 1.17, 95%CI: 1.10–1.25, p < 0.001), and stimulant use (aOR = 1.31, 95%CI: 1.21–1.41, p < 0.001) were associated with increased risk for PDD. For the three datasets, the marginal and conditional pseudo-R2 values ranged from 0.08 to 0.11 and 0.18 to 0.21, respectively, indicating that approximately half of the variance explained in PDD incidence is attributed to the fixed factors (individual-level factors) in the model. The sensitivity analysis including patients with PDD on day 1 revealed a similar protective association for MOUD and PDD; in addition, there was a protective association for opioid agonists and no significant association for adjunctive agents (eTable 5 in Supplement). Moreover, the results of our sensitivity analyses, where hospitals with fewer than 10 hospitalizations (n = 49 hospitals) were excluded, were similar to the primary analysis (eTable 6 in Supplement).
Table 2.
Risk-Adjusted Multivariable Model Results for Patient-Directed Discharge Among Hospitalized Patients with OUD (n = 127,158)*
| Variable | Adjusted OR (95% CI) | P-value |
|---|---|---|
| Early opioid withdrawal treatment | ||
| No treatment | Reference | - |
| MOUD (methadone or buprenorphine) | 0.73 (0.68–0.8) | <.001 |
| Other opioids | 0.95 (0.89–1.02) | 0.15 |
| Adjunctive agents | 1.13 (1.01–1.26) | 0.031 |
| Sociodemographic characteristics | ||
| Age | 0.97 (0.97–0.97) | <.001 |
| Male sex | 1.32 (1.25–1.39) | <.001 |
| Non-White race/ethnicity | 0.94 (0.88–1.00) | 0.07 |
| Clinical characteristics | ||
| ICU admission | 1.1 (1.02–1.18) | 0.009 |
| Elixhauser score | 0.99 (0.99–1.00) | <.001 |
| Alcohol use | 1.17 (1.10–1.24) | <.001 |
| Stimulant use | 1.31 (1.21–1.41) | <.001 |
| Major mental illness | 0.66 (0.63–0.70) | <.001 |
*To adjust for variation across hospitals, individual hospitals were included as random effects
Opioid Withdrawal Treatment Strategies and Hospital LOS
Among those experiencing a PDD, opioid withdrawal management approach was associated with hospital LOS (Table 3). Treatment with MOUD (adjusted incidence rate ratio [aIRR] = 1.24, 95%CI: 1.17–1.3, p < 0.001) and other opioid agonists (aIRR = 1.39, 95%CI: 1.34–1.45, p < 0.001) were associated with increased hospital LOS. Treatment with adjunctive agents was not associated with LOS (aIRR = 1.07, 95%CI: 1.00–1.15, p = 0.059). ICU admission (aIRR = 1.34, 95%CI: 1.28–1.40, p < 0.001), greater Elixhauser scores (aIRR = 1.02, 95%CI: 1.02–1.02, p < 0.001), stimulant use (aIRR = 1.15, 95%CI: 1.09–1.21, p < 0.001), and major mental illness (aIRR = 1.19, 95%CI: 1.15–1.24, p < 0.001) were all associated with increased LOS. For the three datasets, the marginal and conditional pseudo-R2 values ranged from 0.13 to 0.25 and 0.32 to 0.40, respectively, indicating that nearly half of the variance explained in hospital LOS is attributed to the fixed factors (individual-level factors) in the model.
Table 3.
Risk-Adjusted Multivariable Model Results for Hospital Length of Stay Among Hospitalized Patients with OUD Who Have Patient-Directed Discharges (n = 7,116)*
| Variable | Adjusted IRR (95% CI) | P-value |
|---|---|---|
| Early opioid withdrawal treatment | ||
| No treatment | Reference | - |
| MOUD (methadone and buprenorphine) | 1.24 (1.17–1.30) | <.001 |
| Other opioids | 1.39 (1.34–1.45) | <.001 |
| Adjunctive agents | 1.07 (1.00–1.15) | 0.06 |
| Sociodemographic characteristics | ||
| Age | 1.00 (1.00–1.00) | 0.09 |
| Male gender | 1.01 (0.97–1.04) | 0.70 |
| Non-White race/ethnicity | 1.04 (1.00–1.08) | 0.064 |
| Clinical characteristics | ||
| ICU admission | 1.34 (1.28–1.40) | <.001 |
| Elixhauser score | 1.02 (1.02–1.02) | <.001 |
| Alcohol use | 1.01 (0.97–1.05) | 0.75 |
| Stimulant use | 1.15 (1.09–1.21) | <.001 |
| Major mental illness | 1.19 (1.15–1.24) | <.001 |
*To adjust for variation across hospitals, individual hospitals were included as random effects
DISCUSSION
In this cohort of more than 125,000 hospitalizations for patients with OUD from 362 US hospitals, more than 1 in 20 hospitalizations resulted in PDD. Receipt of MOUD (methadone or buprenorphine) in the hospital was associated with lower odds of PDDs compared to no treatment. Receipt of adjunctive medications to control withdrawal symptoms alone was associated with a slightly increased risk of PDD, suggesting that if patients are experiencing severe opioid withdrawal, methadone or buprenorphine may be preferable. Yet there was substantial variation among withdrawal management approaches across hospitals and few hospitals provided methadone or buprenorphine to most patients with OUD.
The finding that methadone and buprenorphine are associated with decreased PDD is consistent with previous smaller studies.7,34–38 These medications when used at appropriate doses can dramatically decrease withdrawal symptoms and can subsequently facilitate hospital-based care. Additionally, these medications can be continued as outpatients after linkage to appropriate outpatient treatment programs. Though other opioid agonists are indicated for pain, if delivered in adequate doses and frequency they can also control withdrawal. Pain and withdrawal have clearly and repeatedly found to be key concerns and drivers of PDD among hospitalized patients with PDD.11,45 In this study, however, they were not associated with decreased PDD, perhaps due to unmeasured confounding or inability to stratify based on the dose or frequency of opioid agonist treatment. Patients with OUD with acute pain often need higher opioid doses to achieve adequate analgesia even if they receive separate treatment for opioid withdrawal. Opioid agonists were associated with longer time in the hospital among those who ultimately experienced a PDD, suggesting better quality of care. It is also possible this represents confounding by indication where those who received opioid agonist medications had more painful or serious conditions that resulted in longer hospitalizations. The findings that stimulant and alcohol use as well as male sex were associated with PDD suggest a need for tailored interventions targeted to these individuals as well.
The variation in pain and withdrawal management for hospitalized patients with OUD highlights the need for systemic change in caring for this population. This is consistent with a nationwide VA study that found that only 15% of hospitalized patients with OUD received any opioid agonist therapy with a great variation in the frequency of treatment between hospitals (0–43%).24 Addiction consult services can be a cost-effective strategy to improve outcomes for hospitalized patients with OUD.46 Though some hospitals have developed specialized services staffed with addiction medicine physicians, training of hospitalists to deliver MOUD in the hospital has also proven feasible.47 Yet, there are geographic, ethnic, and racial disparities in access to hospital-based OUD treatment.48,49 Efforts are needed to ensure these services are delivered to all patients who need these services using a variety of hospital-based addiction care models.50 In addition, the low rates of MOUD implementation suggest the need for broader investment in implementation strategies and incentives at the local, regional, and federal levels.51
The strengths of this study contribute to an improved understanding of PDDs among people with OUD. The data are from a large and diverse cohort of hospitals that improve generalizability and reproduce prior findings from smaller more homogenous cohorts. In addition, previous studies were single site or included data from many hospitals but lacked inpatient medication administration data. The range of hospitals included permitted analysis of variation in hospital practices, and our data allowed us to include a rich set of patient factors as covariates.
This study also has important limitations, many of which motivate future research. First, although there is heterogeneity in geography and hospital data, this is a convenience sample of hospitals that may not be representative of all hospitals in the USA. Second, we used diagnosis codes to identify individuals with OUD which are subject to misclassification. OUD may be undercoded for some individuals, while others with chronic pain may be misclassified as having OUD without meeting the criteria for OUD.52 Third, though we adjusted for several clinical characteristics and markers of disease severity, this is a retrospective observational study that may limit our causal interpretation due to unaddressed confounding and potentially other sources of bias. For example, we did not include benzodiazepine use disorder as a covariate due to concerns about misclassification. However, this type of study can still deliver valuable information and may provide a better reflection of daily clinical practice compared to the gold standard of randomized controlled trials.53 In particular, due to limitations with the data, we were unable to fully adjust for the reason for clinical presentation and thus residual confounding by indication may impact our findings. For example, individuals with OUD and painful conditions like injuries may be more likely to receive opioid agonist treatments for pain and more likely to remain in the hospital for treatment. Additionally, we are unable to examine admission medication lists to determine if the medications administrated were new initiations or continuations of outpatient medications. Thus, people receiving methadone and buprenorphine, for example, may already be engaged in OUD treatment as outpatients which may be protective against PDD.54 Fourth, we are unable to determine the exact timing, dose, or frequency of administered medications which could impact the likelihood of PDD throughout a hospital stay. In addition, we did not examine the administration of medications for the treatment of alcohol or benzodiazepine withdrawal which could also contribute to PDDs. Fifth, not all hospitals contributed data for each year of our analysis, and we are unsure how some of the variables are collected across different hospitals; for example, whether race/ethnicity is perceived by staff or collected via self-report. Sixth, our data only represent the experience of cohort hospitals until 2015. In recent years, there have been substantial efforts to improve the treatment of OUD in the hospital. Additional research is needed with updated data. Seventh, we excluded individuals who experienced PDDs on the day of admission to protect against misclassification and immortalized time bias. In our cohort, however, PDDs on the first day of admission formed only 10.5% of all PDDs, indicating a minimal potential impact of this exclusion on our results, and our primary finding of a protective effect of MOUD was consistent in a sensitivity analysis that included patients with PDDs on the day of admission. Finally, our datasets did not include patient identifiers to allow us to identify patients with multiple hospitalizations during the study period. This could lead to non-independence in some of the included encounters and underestimation of standard errors and inflated type I error in our results.55
CONCLUSION
Patients with OUD who were hospitalized commonly experienced PDD. Treatment with MOUD was associated with decreased PDD compared to no withdrawal management but was prescribed to less than 1 in 4 patients with OUD. Among patients with PDD, treatment with MOUD or other opioids was associated with longer time receiving treatment in the hospital. Systemic efforts are needed to improve pain and withdrawal management for hospitalized individuals with OUD.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Dr. Alrawashdeh had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
This work was funded by the Centers for Disease Control and Prevention (U54CK000484), the National Institute on Drug Abuse (1K23DA054363 to S. K.), and the National Institute of Allergy and Infectious Diseases (5T32AI052074 to S. K.) as well a Boston University Department of Medicine Career Investment Award (S. K.), and a departmental award from Harvard Medical School/Harvard Pilgrim Health Care Institute (M. A.). The National Institute of Drug Abuse, National Institute of Allergy and Infectious Diseases, and Boston University had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Availability
Aggregate summaries of the data that support the findings of this study are available from the corresponding author, subject to agreement of the clinical owners of the data and reasonable processing fees.
Declarations
Conflict of Interest
Dr. Larochelle reports consulting funds for research paid to his institution by OptumLabs. Dr. Kimmel reports receiving consulting fees from the Massachusetts Department of Public Health as well as the American Association of Addiction Psychiatry for trainings on harm reduction and substance use disorder treatment. None of the other authors have any conflicts of interest to disclose.
Disclaimer
This research was supported in part by HCA Healthcare. The views expressed in this publication represent those of the author(s) and do not necessarily represent the official views of HCA Healthcare or any of its affiliated entities.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Owens PL, Weiss AJ, Barrett ML. Hospital Burden of Opioid-Related Inpatient Stays: Metropolitan and Rural Hospitals, 2016. www.hcup-us.ahrq.gov/sidoverview.jsp.Accessed 25 Feb 2021. [PubMed]
- 2.Ronan MV, Herzig SJ. Hospitalizations Related To Opioid Abuse/Dependence And Associated Serious Infections Increased Sharply, 2002-12. Health Aff (Millwood). 2016;35(5):832-7. 10.1377/hlthaff.2015.1424. [DOI] [PMC free article] [PubMed]
- 3.Song Z. Mortality Quadrupled Among Opioid-Driven Hospitalizations, Notably Within Lower-Income And Disabled White Populations. Health Aff (Millwood). 2017;36(12):2054-2061. 10.1377/hlthaff.2017.0689. [DOI] [PMC free article] [PubMed]
- 4.Kimmel SD, Kim JH, Kalesan B, Samet JH, Walley AY, Larochelle MR. Against Medical Advice Discharges in Injection and Non-injection Drug Use-associated Infective Endocarditis: A Nationwide Cohort Study. Clin Infect Dis. 2021;73(9):e2484-e2492. 10.1093/cid/ciaa1126. [DOI] [PMC free article] [PubMed]
- 5.Weiss AJ, Elixhauser A, Barrett ML, Steiner CA, Bailey MK, O’Malley L. HCUP Statistical Brief #219. Rockville, MD: Agency for Healthcare Research and Quality; December 2016.
- 6.Eaton EF, Westfall AO, McClesky B, et al. In-Hospital Illicit Drug Use and Patient-Directed Discharge: Barriers to Care for Patients With Injection-Related Infections. Open Forum Infect Dis. 2020;7(3):ofaa074. 10.1093/ofid/ofaa074. [DOI] [PMC free article] [PubMed]
- 7.Ti L, Ti L. Leaving the Hospital Against Medical Advice Among People Who Use Illicit Drugs: A Systematic Review. Am J Public Health. 2015;105(12):e53-9. 10.2105/ajph.2015.302885. [DOI] [PMC free article] [PubMed]
- 8.Saitz R, Ghali WA, Moskowitz MA. The impact of leaving against medical advice on hospital resource utilization. J Gen Intern Med. 2000;15(2):103-7. 10.1046/j.1525-1497.2000.12068.x. [DOI] [PMC free article] [PubMed]
- 9.Spooner KK, Salemi JL, Salihu HM, Zoorob RJ. Discharge Against Medical Advice in the United States, 2002-2011. Mayo Clin Proc. 2017;92(4):525-535. 10.1016/j.mayocp.2016.12.022. [DOI] [PubMed]
- 10.Choi M, Kim H, Qian H, Palepu A. Readmission rates of patients discharged against medical advice: a matched cohort study. PLoS ONE. 2011;6(9):e24459. 10.1371/journal.pone.0024459. [DOI] [PMC free article] [PubMed]
- 11.Simon R, Snow R, Wakeman S. Understanding why patients with substance use disorders leave the hospital against medical advice: A qualitative study. Subst Abus. 2020;41(4):519-525. 10.1080/08897077.2019.1671942. [DOI] [PubMed]
- 12.Pollini RA, Paquette CE, Drvar T, et al. A qualitative assessment of discharge against medical advice among patients hospitalized for injection-related bacterial infections in West Virginia. Int J Drug Policy. 2021;94:103206. 10.1016/j.drugpo.2021.103206. [DOI] [PMC free article] [PubMed]
- 13.Kim JH, Fine DR, Li L, et al. Disparities in United States hospitalizations for serious infections in patients with and without opioid use disorder: A nationwide observational study. PLoS Med. 2020;17(8):e1003247. 10.1371/journal.pmed.1003247. [DOI] [PMC free article] [PubMed]
- 14.Suzuki J, Johnson J, Montgomery M, Hayden M, Price C. Outpatient Parenteral Antimicrobial Therapy Among People Who Inject Drugs: A Review of the Literature. Open Forum Infect Dis. 2018;5(9):ofy194. 10.1093/ofid/ofy194. [DOI] [PMC free article] [PubMed]
- 15.Kimmel SD, Rosenmoss S, Bearnot B, Larochelle M, Walley AY. Rejection of Patients With Opioid Use Disorder Referred for Post-acute Medical Care Before and After an Anti-discrimination Settlement in Massachusetts. J Addict Med. 2021;15(1):20–26. 10.1097/ADM.0000000000000693. [DOI] [PMC free article] [PubMed]
- 16.Bearnot B, Mitton JA, Hayden M, Park ER. Experiences of care among individuals with opioid use disorder-associated endocarditis and their healthcare providers: Results from a qualitative study. J Subst Abuse Treat. 2019;102:16-22. 10.1016/j.jsat.2019.04.008. [DOI] [PubMed]
- 17.McNeil R, Small W, Wood E, Kerr T. Hospitals as a 'risk environment': an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66. 10.1016/j.socscimed.2014.01.010. [DOI] [PMC free article] [PubMed]
- 18.Weinstein ZM, Wakeman SE, Nolan S. Inpatient Addiction Consult Service: Expertise for Hospitalized Patients with Complex Addiction Problems. Med Clin North Am. 2018;102(4):587-601. 10.1016/j.mcna.2018.03.001. [DOI] [PMC free article] [PubMed]
- 19.Wakeman SE, Metlay JP, Chang Y, Herman GE, Rigotti NA. Inpatient Addiction Consultation for Hospitalized Patients Increases Post-Discharge Abstinence and Reduces Addiction Severity. J Gen Intern Med. 2017;32(8):909-916. 10.1007/s11606-017-4077-z. [DOI] [PMC free article] [PubMed]
- 20.Trowbridge P, Weinstein ZM, Kerensky T, et al. Addiction consultation services - Linking hospitalized patients to outpatient addiction treatment. J Subst Abuse Treat. 2017;79:1-5. 10.1016/j.jsat.2017.05.007. [DOI] [PMC free article] [PubMed]
- 21.Calcaterra SL, Martin M, Bottner R, et al. Management of opioid use disorder and associated conditions among hospitalized adults: A Consensus Statement from the Society of Hospital Medicine. J Hosp Med. 2022;17(9):744-756. 10.1002/jhm.12893. [DOI] [PMC free article] [PubMed]
- 22.Englander H, Weimer M, Solotaroff R, et al. Planning and Designing the Improving Addiction Care Team (IMPACT) for Hospitalized Adults with Substance Use Disorder. J Hosp Med. 2017;12(5):339–342. 10.12788/jhm.2736. [DOI] [PMC free article] [PubMed]
- 23.Englander H, Dobbertin K, Lind BK, et al. Inpatient Addiction Medicine Consultation and Post-Hospital Substance Use Disorder Treatment Engagement: a Propensity-Matched Analysis. J Gen Intern Med. 2019;34(12):2796-2803. 10.1007/s11606-019-05251-9. [DOI] [PMC free article] [PubMed]
- 24.Priest KC, Lovejoy TI, Englander H, Shull S, McCarty D. Opioid Agonist Therapy During Hospitalization Within the Veterans Health Administration: a Pragmatic Retrospective Cohort Analysis. J Gen Intern Med. 2020;35(8):2365-2374. 10.1007/s11606-020-05815-0. [DOI] [PMC free article] [PubMed]
- 25.Alrawashdeh M, Klompas M, Kimmel S, et al. Epidemiology, Outcomes, and Trends of Patients With Sepsis and Opioid-Related Hospitalizations in U.S. Hospitals. Crit Care Med. 2021; Publish Ahead of Print(12):2102–2111. 10.1097/ccm.0000000000005141. [DOI] [PMC free article] [PubMed]
- 26.British Columbia Centre on Substance Use and British Columbia Ministry of Health. A Guideline for the Clinical Management of Opioid Use Disorder. http://www.bccsu.ca/care-guidance-publications/.Accessed 27 May 2019.
- 27.Priest KC, Gorfinkel L, Klimas J, Jones AA, Fairbairn N, McCarty D. Comparing Canadian and United States opioid agonist therapy policies. Int J Drug Policy. 2019;74:257-265. 10.1016/j.drugpo.2019.01.020. [DOI] [PMC free article] [PubMed]
- 28.Kimmel S, Bach P, Walley AY. Comparison of Treatment Options for Refractory Opioid Use Disorder in the United States and Canada: a Narrative Review. J Gen Intern Med. 2020;35(8):2418-2426. 10.1007/s11606-020-05920-0. [DOI] [PMC free article] [PubMed]
- 29.Kleinman RA, Wakeman SE. Treating Opioid Withdrawal in the Hospital: A Role for Short-Acting Opioids. Ann Intern Med. 2022;175(2):283-284. 10.7326/M21-3968. [DOI] [PubMed]
- 30.Thakrar AP. Short-Acting Opioids for Hospitalized Patients With Opioid Use Disorder. JAMA Intern Med. 2022;182(3):247-248. 10.1001/jamainternmed.2021.8111. [DOI] [PubMed]
- 31.Herzig SJ, Mosher HJ, Calcaterra SL, Jena AB, Nuckols TK. Improving the Safety of Opioid Use for Acute Noncancer Pain in Hospitalized Adults: A Consensus Statement From the Society of Hospital Medicine. J Hosp Med. 2018;13(4):263–271. 10.12788/jhm.2980. [DOI] [PMC free article] [PubMed]
- 32.Onukwugha E, Alfandre D. Against Medical Advice Discharges Are Increasing for Targeted Conditions of the Medicare Hospital Readmissions Reduction Program. J Gen Intern Med. 2019;34(4):515-517. 10.1007/s11606-018-4765-3. [DOI] [PMC free article] [PubMed]
- 33.Compton P, Aronowitz SV, Klusaritz H, Anderson E. Acute pain and self-directed discharge among hospitalized patients with opioid-related diagnoses: a cohort study. Harm Reduct J. 2021;18(1):131. 10.1186/s12954-021-00581-6. [DOI] [PMC free article] [PubMed]
- 34.Nolan NS, Marks LR, Liang SY, Durkin MJ. Medications for Opioid use Disorder Associated With Less Against Medical Advice Discharge Among Persons Who Inject Drugs Hospitalized With an Invasive Infection. J Addict Med. 2020;10.1097/ADM.0000000000000725. [DOI] [PMC free article] [PubMed]
- 35.Wang SJ, Wade E, Towle J, et al. Effect of Inpatient Medication-Assisted Therapy on Against-Medical-Advice Discharge and Readmission Rates. Am J Med. 2020;133(11):1343-1349. 10.1016/j.amjmed.2020.04.025. [DOI] [PubMed]
- 36.Chan AC, Palepu A, Guh DP, et al. HIV-positive injection drug users who leave the hospital against medical advice: the mitigating role of methadone and social support. J Acquir Immune Defic Syndr. 2004;35(1):56-9. 10.1097/00126334-200401010-00008. [DOI] [PubMed]
- 37.Santos CJ, Shofer FS, Lowenstein M, Perrone J. Discharges "Against Medical Advice" in Patients With Opioid-related Hospitalizations. J Addict Med. 2020;10.1097/ADM.0000000000000688. [DOI] [PubMed]
- 38.Suzuki J, Robinson D, Mosquera M, et al. Impact of Medications for Opioid Use Disorder on Discharge Against Medical Advice Among People Who Inject Drugs Hospitalized for Infective Endocarditis. Am J Addict. 2020;29(2):155-159. 10.1111/ajad.13000. [DOI] [PMC free article] [PubMed]
- 39.Rhee C, Dantes R, Epstein L, et al. Incidence and Trends of Sepsis in US Hospitals Using Clinical vs Claims Data, 2009-2014. JAMA. 2017;318(13):1241-1249. 10.1001/jama.2017.13836. [DOI] [PMC free article] [PubMed]
- 40.Injury Surveillance Workgroup 7: Consensus Recommendations for National and State Poisoning Surveillance. https://www.safestates.org/page/ISWReports.Accessed 12 Sept 2018.
- 41.Hume B, Gabella B, Hathaway J, et al. Assessment of Selected Overdose Poisoning Indicators in Health Care Administrative Data in 4 States, 2012. Public Health Rep. 2017;132(4):488-495. 10.1177/0033354917718061. [DOI] [PMC free article] [PubMed]
- 42.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. [DOI] [PubMed]
- 43.Wolfson M, Wallace SE, Masca N, et al. DataSHIELD: resolving a conflict in contemporary bioscience--performing a pooled analysis of individual-level data without sharing the data. Int J Epidemiol. 2010;39(5):1372-82. 10.1093/ije/dyq111. [DOI] [PMC free article] [PubMed]
- 44.Nakagawa S, Schielzeth H, O'Hara RB. A general and simple method for obtainingR2from generalized linear mixed-effects models. Methods Ecol Evol. 2013;4(2):133-142. 10.1111/j.2041-210x.2012.00261.x.
- 45.Biancarelli DL, Biello KB, Childs E, et al. Strategies used by people who inject drugs to avoid stigma in healthcare settings. Drug Alcohol Depend. 2019;198:80-86. 10.1016/j.drugalcdep.2019.01.037. [DOI] [PMC free article] [PubMed]
- 46.Barocas JA, Savinkina A, Adams J, et al. Clinical impact, costs, and cost-effectiveness of hospital-based strategies for addressing the US opioid epidemic: a modelling study. Lancet Public Health. 2022;7(1):e56-e64. 10.1016/S2468-2667(21)00248-6. [DOI] [PMC free article] [PubMed]
- 47.Calcaterra SL, McBeth L, Keniston AM, Burden M. The Development and Implementation of a Hospitalist-Directed Addiction Medicine Consultation Service to Address a Treatment Gap. J Gen Intern Med. 2022;37(5):1065-1072. 10.1007/s11606-021-06849-8. [DOI] [PMC free article] [PubMed]
- 48.Chang JE, Franz B, Cronin CE, Lindenfeld Z, Lai AY, Pagan JA. Racial/ethnic disparities in the availability of hospital based opioid use disorder treatment. J Subst Abuse Treat. 2022;138:108719. 10.1016/j.jsat.2022.108719. [DOI] [PubMed]
- 49.Priest KC, King CA, Englander H, Lovejoy TI, McCarty D. Differences in the delivery of medications for opioid use disorder during hospitalization by racial categories: A retrospective cohort analysis. Subst Abus. 2022;43(1):1251-1259. 10.1080/08897077.2022.2074601. [DOI] [PMC free article] [PubMed]
- 50.Englander H, Jones A, Krawczyk N, et al. A Taxonomy of Hospital-Based Addiction Care Models: a Scoping Review and Key Informant Interviews. J Gen Intern Med. 2022;37(11):2821-2833. 10.1007/s11606-022-07618-x. [DOI] [PMC free article] [PubMed]
- 51.Englander H, Davis CS. Hospital Standards of Care for People with Substance Use Disorder. N Engl J Med. 2022;387(8):672-675. 10.1056/NEJMp2204687. [DOI] [PubMed]
- 52.Howell BA, Abel EA, Park D, Edmond SN, Leisch LJ, Becker WC. Validity of Incident Opioid Use Disorder (OUD) Diagnoses in Administrative Data: a Chart Verification Study. J Gen Intern Med. 2021;36(5):1264-1270. 10.1007/s11606-020-06339-3. [DOI] [PMC free article] [PubMed]
- 53.Yang W, Zilov A, Soewondo P, Bech OM, Sekkal F, Home PD. Observational studies: going beyond the boundaries of randomized controlled trials. Diabetes Res Clin Pract. 2010;88 Suppl 1:S3-9. 10.1016/S0168-8227(10)70002-4. [DOI] [PubMed]
- 54.Tierney HR, Rowe CL, Coffa DA, Sarnaik S, Coffin PO, Snyder HR. Methadone treatment and patient-directed hospital discharges among patients with opioid use disorder: Observations from general medicine services at an urban, safety-net hospital. Drug Alcohol Depend Rep. 2022;310.1016/j.dadr.2022.100066. [DOI] [PMC free article] [PubMed]
- 55.Finch WH, Bolin JE, Kelley K. Multilevel Modeling Using R. CRC Press/Taylor & Grancis Group; 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Aggregate summaries of the data that support the findings of this study are available from the corresponding author, subject to agreement of the clinical owners of the data and reasonable processing fees.