Abstract
Nowadays to cope-up with the emerging global clean-water crisis, wastewater needs to be remediated properly to be used as an alternative source. Here a cost-effective approach has been taken to treat heavily-polluted (BOD-1234.33 mg L−1, COD-1706.64 mg L−1, TDS-6984 mg L−1, and sulfide-140.8 mg L−1 ammonium-134.5 mg L−1) Tannery Waste Water (TWW). Three cyanobacteria were (Arthrospira platensis, Leptolyngbyavalderiana, and Anabaenasphaerica) used as bio-reagents in pilot-scale treatment. Wastewater remediation-potential and biomass-generation capacity were evaluated in various TWW concentrations. The maximum biomass growth and the highest pollution removal percentage was observed when exposed to 50% TWW; although among the tested strain, Arthrospira and Leptolyngbya performed better than Anabaena by showing greater pollution removal potential (BOD 93%, COD 94%, sulfide 99%, ammonium 93%) in one hand and higher biomass production rate (100 mg L−1 Day−1) on the other. DO was increased noticeably by 10–15-fold. Morphological characterizations of tannery wastewater exposed Anabaena revealed unusual thick sheath formation, along with heterocyst and akinete formation in their trichome. Biochemical characterizations of remediating cyanobacteria showed presence of wastewater-accumulated nutrients (N, P, K). Nutrient-loaded biomass improved growth of rice and chickpea seedlings when used as a growth promoter. These facts have been illustrated by factor analysis and discriminant analysis. Cyanobacteria-mediated pilot-scale tannery wastewater treatment would create ecologically and economically-sustainable technology for clean-water production.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-023-03712-x.
Keywords: Phycoremediation, Tannery waste, Cyanobacteria, Correspondence analysis, Factor analysis, Discriminant analysis
Introduction
In developing countries, burgeoning industrialization generates huge amounts of wastewaters (Dey et al. 2021). Among various industries, the tannery industry is one of the most pollution-intensive industry. These industries use various chemicals during processing of leather from animal hides. In addition, the requirement of huge amounts of water in the tanning process necessitates these industries to grow around natural water bodies like river banks or coasts (Lofrano et al. 2013). Thus voluminous, saline, chemical laden and nutrient-rich wastewaters discharged from them mixed directly to nearby water bodies causing environmental hazards (Suresh et al. 2021). Therefore, proper wastewater treatment technologies should be implemented in leather complexes.
Various kinds of chemical and physical treatment processes are practiced in wastewater treatment plants requiring high energy inputs. Again, hazardous sludge generation as a byproduct becomes an unavoidable circumstance of these processes (Rodríguez et al. 2016). These major drawbacks lead the search towards cost-effective and environment friendly treatment technologies. Cultivation of cyanobacteria in wastewater for remediation purposes is an eco-friendly process, an alternative of conventional technology (Atici 2020). This is due to its photosynthesizing capability and rapid growth rate in low-cost nutrient sources of wastewater (Dineshkumar and Sen 2020). Cyanobacteria not only consume inorganic nutrients from wastewater, but also reduce their inorganic and organic loads (Zhou et al. 2013). In contrast to hazardous sludge generation from conventional treatment methods, cyanobacteria mediated treatment methods produce nutrient loaded biomass which further can be used for production of bio-energy, bio-fertilizers, and other value-added products (Ozturk et al. 2019; Derakhshandeh et al. 2019; De Bhowmick et al. 2019).
Vigorous use of chemical fertilizers deteriorates soil quality as well as water bodies. In addition, production of chemical fertilizers needs nonrenewable materials and high energy resources (Chojnacka et al. 2020). On the contrary, the use of organic fertilizer is the need of the time. Wastewater treated cyanobacterial biomass serves as a renewable source of bio-fertilizers (Yadav and Sen 2017; Dineshkumar and Sen 2020; Derakhshandeh et al. 2021). In this process, exposed biomass reduces the energy cost of fertilizer production due to its de-novo growth in the wastewater treatment process.
Till date, uses of cyanobacteria in highly polluted tannery wastewater remediation were confined only under controlled laboratory conditions (Table 1). In the present investigation, an open system was established for pilot-scale tannery wastewater remediation using the cyanobacterial strains (Arthrospira, Leptolyngbya, and Anabaena). This investigation was aimed for amelioration of pollution level of wastewater on one hand and production of nutrient-loaded biomass on the other to be used as growth-promoter of rice and chickpea seedlings. Morphological characterization of TWW treated cyanobacterial filaments were also investigated to understand their growth pattern in polluted environments. Successful implementation of the abovementioned process would create ecologically sustainable technology for clean water production.
Table 1.
Comparisons of different microalgae/cyanobacteria mediated tannery wastewater remediation programs carried out till date
| Name of microalgae or cyanobacteria | Experimental condition | Parameters remediated | Characterization of biomass after remediation | Application of residual biomass | Strength of wastewater | References |
|---|---|---|---|---|---|---|
|
Leptolyngbya Anabaena Arthrospira |
Pilot scale | Color, Odor, DO, BOD, COD, Nitrate, Phosphate, Ammonia, Sulfide, TDS | Light Microscopy, Electron Microscopy, FTIR, EDX, Carbohydrate, Protein, Lipid, NPK content | Seedling growth improvement | 100%, 75%, 50% | Present experiment |
| Chlorella and Oscillatoria |
Laboratory condition and Pilot scale |
TSS, TS, TDS, Chloride, Sulfide, Cr, total Hardness, Ca, Mg, total Alkalinity, Fe, Phosphate, BOD, COD | Dry weight, Chlorophyll, Protein, Carbohydrate, Lipid, Fluorescence microscopy, Light microscopy, | _ | 50%, 60%, 70%, 80%, 90%, 100% | Santhosh et al. (2020) |
| Scenedesmus sp., Chlorella variabilis and Chlorella sorokiniana | Laboratory condition | COD, Ammonium, Phosphorus | Chlorophyll, Dry weight, Carbohydrate, Lipid, Fluorescence intensity | Biofuel | 25%, 40%, 60% | Nagi et al. (2020) |
| Tetraselmis consortium | Pilot scale | BOD, COD, N, P, NH3 − N, Total Organic Carbon | Dry Weight, Turbidity, | – | 100%, 50%, 25% | Pena et al. (2019) |
| Lyngbya | Laboratory condition | BOD, COD, N, P, K, Fe, Cr, Ni, Cd, Pb, Se, As, Cu, Mn, Zn, Hg | SEM | Plant growth improvement | 100% | Mohamed et al. (2019) |
| Chlorella vulgaris and Pseudochlorella pringsheimii | TDS, BOD, COD, Sulphates, Sulfides, Chloride, Na, NH3 − N, NO3 − N, PO4 − P, Carbonate, Bicarbonate, Cr | Chlorophyll, Dry weight, EDAX, SEM, | Biofuel | 100%, 50%, 40%, 30%, 20%, | Saranya and Shanthakumar (2019) | |
| Consortium of Chlorella and Phormidium | Laboratory condition | BOD, COD, TN, TP, TDS, total Cr | Chlorophyll | – | 100% | Das et al. (2018) |
| Scenedesmus | Laboratory condition | Color, Odor, TSS, TDS, TS, BOD, COD, DO, Nitrate, Phosphorus, Cr, Cu, Pb, Zn | FTIR, SEM, EDX | – | 10%, 25%, 50%, 75%, 100% | Ajayan et al. (2015) |
| Lyngbya | Laboratory condition | Chloride, COD, BOD, TDS, Cu, Cr, Fe, Cd, Hg, Pb | Chlorophyll, Protein | – | 100% | Lakshmi and Malliga (2014) |
| Nostoc | Laboratory condition | TSS, TDS, DO, BOD, COD, Pb, Cu, Fe, Na, Cr, Cd, Zn, Ni | AAS | 100% | Jahan et al. (2014) | |
| Spirulina fusiformis |
Laboratory condition and Pilot scale |
Cr, BOD, COD, TS, TDS, TSS | Light microscopy, Chlorophyll, Dry weight, Protein, FTIR | – | 25, 50, 75, 100, 125, 150, 200 and 300 ppm | Pandi et al. (2009) |
| Spirulina platensis | Laboratory condition | Sulphate, Chloride, TS, TSS, BOD, COD, Cr | – | – | 100, 200 and 300 ppm | Shashirekha et al. (2008) |
| Oscillatoria | Laboratory condition | BOD, COD, Nitrate, Cd, Cu, Cr, Zn, Fe, | Dry Weight | – | 100% | Dhamotharan et al. (2016) |
| Spirogyra condensata and Rhizoclonium hieroglyphicum | Laboratory condition | Cr | FTIR | – | 4–500 ppm | Onyancha et al. (2008) |
Materials and methods
Collection and characterization of TWW
TWW was collected from Inlet Pond of treatment plant (22°29ʹ42.6ʺ N and 88°31ʹ29.7ʺ E) situated in the leather industry of Eastern India. This treatment plant treats wastewater from more than 500 tanneries situated across 130 acres area. Wastewater was pooled from the inlet, before going through the treatment process. A 500 L sample was collected in polyethylene bottles and was transferred to the laboratory. To analyze various physicochemical parameters of TWW, viz. Dissolved Oxygen (DO), Biological Oxygen Demand (BOD), Chemical Oxygen Demand (COD), Ammoniacal Nitrogen (NH4−N), Total Dissolved Solids (TDS) and Sulphide, standard methods of APHA (2005) were followed.
Maintenance of cyanobacterial strains
Three cyanobacterial strains were selected i.e., freshwater nonheterocystous Arthrospira platensis (AL/CCU/FW-4), marine nonheterocystous Leptolyngbya valderiana (CUH/AL/MW/CYANO-174), heterocystous Anabaena sphaerica (AL/CCU/FW-1) to compare their ability to remove pollution from TWW. They were obtained from the culture collection of University of Calcutta. Cultures were maintained in modified ASN III medium, BG 11 medium and Aiba & Ogawa’s medium respectively.
Experimentations for simultaneous cultivation of cyanobacteria and remediation study of TWW
Collected TWW was kept in dark for 3 days for settling down of solids and then filtered with thin cotton cloth for further experimental purpose. The filtrate mixed with cost effective media and the final concentration were made up to 75% and 50% TWW. Experiments were set up in PVC tanks (20 cm × 15 cm × 15 cm) containing raw TWW (100%), 75% Tannery Wastewater Media Mixture (TWMM) and 50% TWMM. The media were composed of 2.5 gL−1 Na2CO3, 0.245 gL−1 NO3− and 0.011 gL−1 PO43− salts. However, additional 25 gL−1 NaCl and 7 gL−1 NaHCO3 for Leptolyngbya and Arthrospira were further added, respectively. Initially around 6 g of fresh Arthrospira, Leptolyngbya and Anabaena were added in three separate tanks containing different concentrations of TWW. Culture tanks were maintained in outdoor-open system condition.
Growth estimation of cyanobacteria during outdoor cultivation in TWW
During the 21 days experiment, fresh weights of biomass were taken at a 5 days interval from all three concentrations of wastewater, i.e., 100%, 75% and 50%. Later, they were converted to dry weight. Biomass Productivities (BP) were counted based on the equation
where Bt = Dry weight of biomass after 21 days; B0 = Dry weight of biomass at 0th day and t = 21 days (Gorain et al. 2019).
Determination of remediation parameters after pilot scale wastewater treatment using cyanobacteria
Determination of DO, BOD, COD, Sulfide, TDS, and Ammonia were done after treatment in 50% tannery wastewater. Estimates were carried out at a 5 days interval for 21 days according to standard methods of APHA (2005). Removal Efficiencies (%) of various parameters were measured according to the following method.
where, RP = Removal Percentage, Ci = initial concentration of given parameter and Ce = remaining concentration of the same parameter on a particular sampling day.
Morphological characterization of wastewater treated cyanobacterial mats
Microscopic observations were done for filaments of cyanobacteria during 21 days treatment period with the help of Light Microscope (LM) and Scanning Electron Microscope (SEM) to detect the morphological changes during tannery wastewater treatment indicating their growth pattern. Samples were prepared for SEM and coated with gold using a Quorum (Q 150 TES) gold coater. The photographs were taken at different magnifications using Carl Zeiss EVO 18 (EDS 8100) microscope with Zeiss Inca Penta FETX 3 (Oxford instruments) attachment. In LM, photomicrographs were taken using Canon T2-T2 1, 6 × SLR426115 cameras.
Biochemical characterization of wastewater treated cyanobacteria
After 21 days of experiment, whole cyanobacterial biomass was harvested from each tank using cotton cloth and sun-dried afterwards. The constituents, i.e., carbohydrate (DuBois et al. 1956), protein (Lowry et al. 1951), lipid (Bligh and Dyer 1959) of TWW treated cyanobacterial biomass was analyzed in association with N, P and K content (Singh and Praharaj 2017).
Study of plant growth parameters after applying nutrient-laden cyanobacterial biomass
The experiment was carried out using Oryza sativa indica MTU1010 and Cicer arietinum Anuradha plants. O. sativa seeds and C. arietinum seeds were procured from Chinsurah Rice Research Centre, Chinsurah, West Bengal and Pulse and Oilseed Research Station, Murshidabad, West Bengal respectively. Each pot was filled with 100 g of soil mixed with 1.5 g of either cyanobacterial biomass or Chemical Fertilizer (CF). After 21 days, plants were harvested and several parameters like, no. of leaves, plant heights, root lengths, fresh weights and dry weights were determined. Chlorophyll content was measured using Arnon’s method in Hitachi U-2900 UV/Vis spectrophotometer (Tokyo, Japan). Seed germination percentage and vigor index were also counted for both plants. Selected cyanobacterial biomass (fresh weight 2gL−1) mixed with tap water were applied on 100 seeds of each plant. Two separate control sets were prepared considering two separate plants using only tap water. Seeds of both plants were soaked on tissue paper overnight before the germination test. Germination rate and vigor index were calculated over a 7-day period. These experiments were done to compare the plant-growth improvement potentials between CF and cyanobacterial biomass.
Statistical analysis
All experiments were conducted three times. The differences among phycoremediation sets were analyzed statistically using Two Way ANOVA in Graph pad prism 9.0.2 respectively. Differences between plant growth parameters were determined statistically employing a two-sample t test with significance level p < 0.05. Correspondence analysis was carried out using PAST to draw a comparative account among fertilizers. Factor analyses and discriminant analyses were done to represent the plant growth improvements in response to different fertilizers. They were done using StatistiXL. Schematic representation of workflow has been represented in Fig. 1.
Fig. 1.
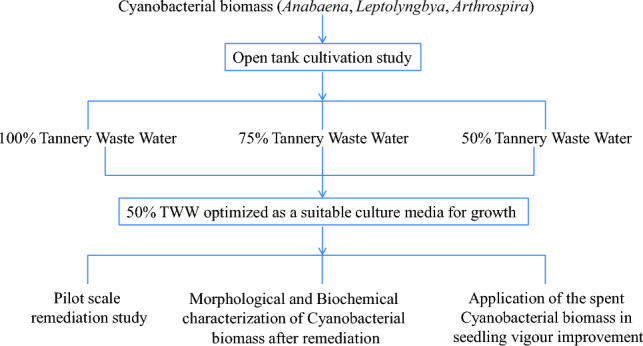
Schematic representation of the entire workflow
Results and discussion
Composition of TWW
The wastewater used in our study was alkaline in nature, with high organic loads, and with complex physico-chemical components as shown in Table 2. All the parameters in the TWW effluent were well beyond their permissible limits as also experienced by previous authors (Das et al. 2018; Ajayan et al. 2015). However, organic loads and TDS were excessively higher in our studied TWW.
Table 2.
Characteristics of raw TWW
| Parameters (mg L−1) | Raw TWW | Safe limits |
|---|---|---|
| DO | 1.42 ± 0.349 | |
| BOD | 1234 ± 1.78 | 100 (BIS 1994) |
| COD | 3325 ± 2.13 | 250 (BIS 1994) |
| Nitrate Nitrogen | 10 ± 0.77 | 10 (U.S. Environmental Protection agency) |
| Phosphate | 1.1 ± 0.10 | 1 (BIS 1994) |
| Ammoniacal Nitrogen | 342 ± 0.93 | 12 (WHO 1981) |
| TDS | 8400 ± 4.1 | 2100 (BIS 1994) |
| Sulfide | 25 ± 0.96 | 10 (WHO 1981) |
| Chromium | 1.8 | 2 (BIS 1994) |
| Cadmium | < 0.01 | 0.6 (Chowdhury et al. 2015) |
| Iron | 2 | 0.3 (WHO 1981) |
| Lead | < 0.1 | 2 (Chowdhury et al. 2015) |
Optimization of suitable TWW concentration for open tank culture of cyanobacteria
Out of three concentrations investigated (100%, 75%, 50%), 50% dilution showed maximum cyanobacterial growth rate followed by 75% and 100% TWW (Fig. 2). This growth improvement may have occurred due to higher nutrient accumulation by cyanobacteria in 50% TWW. Comparatively, the growth performance of Arthrospira and Leptolyngbya was better than that of Anabaena. In this study, at 50% TWW, the N: P ratio was 30:1 inducing better growth. According to a previous report (Crawford 2008), this N/P ratio is effective for the advantageous growth of nonheterocystous cyanobacteria. Similar results obtained in our experiment for nonheterocystous genera. Besides, Leptolyngbya (Phormidium) being the salt tolerant marine species and Arthrospira (Spirulina) having the ability to thrive in alkaline water grew better than Anabaena in wastewater. Kottangodan et al. (2019) reported similar steady growth of marine Phormidium sp. in 30% diluted industrial wastewater. Decreased growth performance of Anabaena in wastewater was also reported by Abedi et al. (2019). It has been assumed that lower acclimatization ability of Anabaena was responsible for this decreased growth performance.
Fig. 2.
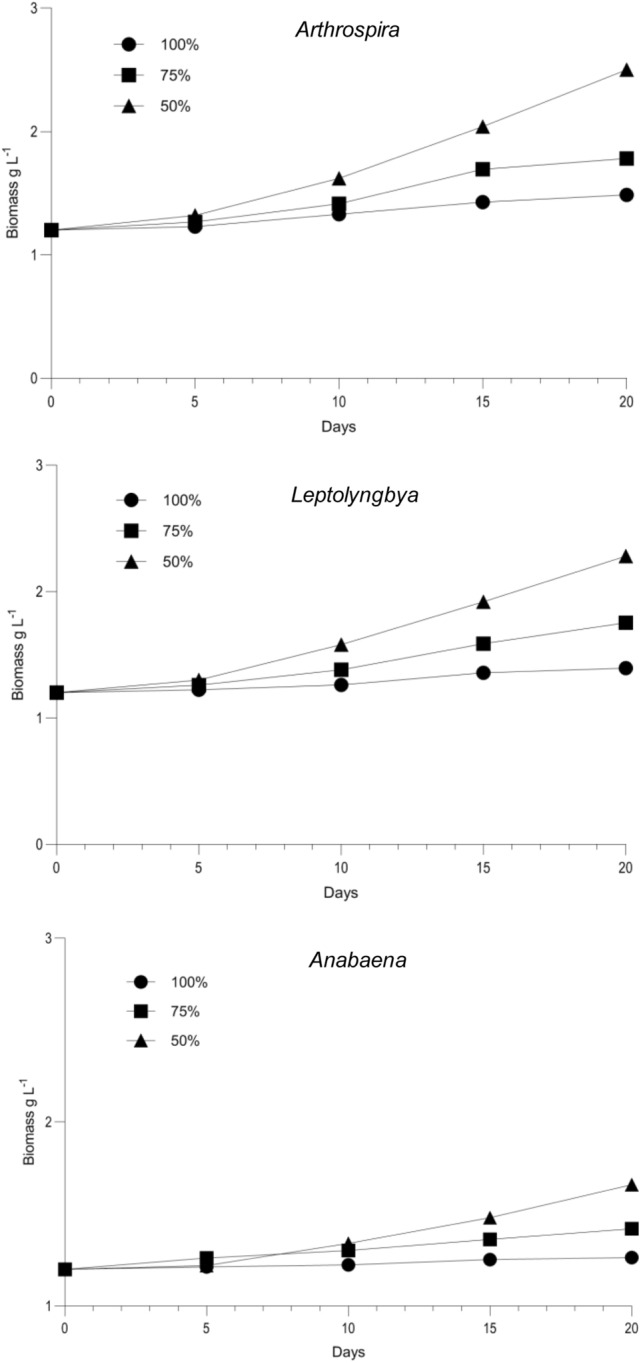
Growth study of Arthrospira, Leptolyngbya and Anabaena in different concentrations (100%, 75%, and 50%) of TWW
Improvement of water quality
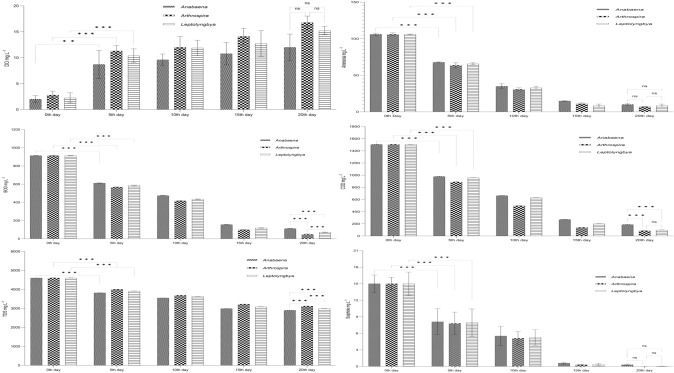
As TWW in 50% dilution induced better growth performance out of the other experimental dilutions, this concentration was chosen for water quality assessment. From the results it became evident that the pollution level lowered due to cyanobacterial treatment (Fig. 3). For all three experimental taxa, the DO level increased significantly (p < 0.001) within 5 days of treatment. It was seen in the results that DO content of wastewater was increased by 6 folds (14 mg L−1) after 21 days of exposure. Conventional bacterial wastewater treatment processes are unable to bring such drastic change. The release of oxygen through cyanobacterial photosynthesis is generally responsible for this enormous DO enhancement (Zhang et al. 2018). Efficacy of any wastewater treatment process is also judged by its potency of organic matter removal from wastewater. Within the first 5 days of treatment, significant (p < 0.001) reduction in organic load has occurred in all experimental sets. Maximum reductions of organic loads were done by Arthrospira (> 94%) followed by Leptolyngbya (> 92%) and Anabaena (87%). Performance of Anabaena was significantly (p < 0.001) lower than Arthrospira and Leptolyngbya. Cyanobacteria achieved this higher organic load removal capacity through higher organic carbon assimilation for their growth. Das et al. (2018) and Shashirekha et al. (2011) reported a similar phenomenon of organic load reduction by Phormidium and Spirulina from wastewater.
Fig. 3.
Increase in DO content and decrease in BOD, COD, TDS, Sulfide and Ammonium content in 50% TWW concentration
Various organic and inorganic components dissolved in wastewater contribute to TDS contents. Acceptable level of TDS in the environment is 2100 mg L−1. Owing to the use of various salt components in experimental wastewater media, comparatively little TDS reduction had taken place in all three experimental sets (Arthrospira 3117 mg L−1; Leptolyngbya 2790 mg L−1; Anabaena 3098 mg L−1). No significant TDS reduction took place after 5 days of experiment. However, among three sets, marine Leptolyngbya lowered TDS content to almost acceptable limit. Leptolyngbya had utilized dissolved components as nutrients for their cellular growth and in this way, they were able to reduce TDS contents from wastewater. Ajayan et al. (2015) has also speculated a similar reason for TDS removal efficiency of cyanobacteria from industrial wastewater.
Sulfide induced pungent odor emission has remained a constant health risk for tannery plant workers and nearby localities. In our remediation process, cyanobacteria reduced sulfide more than 99% after 20 days of exposure, however significant reduction had occurred within 5 days of experiment. Among the studied genera, Leptolyngbya showed maximum removal capacity than the other two genera (Arthrospira and Anabaena). According to Hamilton et al. (2018), cyanobacteria can reduce sulfide content from sulfide rich environments through cellular consumption. In this work, apart from other parameters, NH4−N was also efficiently removed as depicted in Fig. 3. Ammonium removal rates were higher in all three cyanobacteria after 20 days of exposure (Arthrospira 7 mg L−1; Leptolyngbya 8 mg L−1; Anabaena 10 mg L−1); although significant removal took place within 5 days of exposure. It is a well-known fact that cyanobacteria prefer reduced nitrogen sources, like ammonium over nitrate, as assimilation of nitrate requires an energy driven process (Ge and Champagne 2016). Percent reduction of all parameters were correlated with biomass growth and displayed separately for all three genera i.e., Anabaena, Arthrospira and Leptolyngbya in Fig. 4a, Fig. 4b and Fig. 4c, respectively.
Fig. 4.
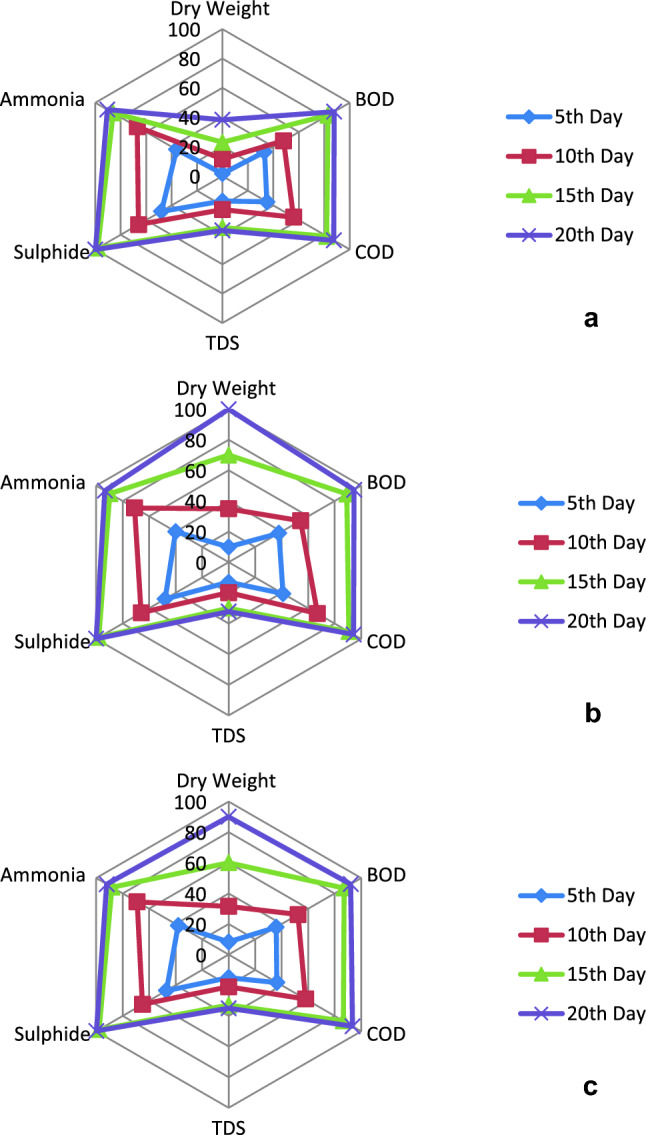
Biomass growth (Dry weight) in relation to percentage reductions of several wastewater remediation parameters (BOD, COD, TDS, Sulfide, Ammonia) according to a shared scale in radar charts (a Anabaena, b Arthrospira, c Leptolyngbya)
Morphological changes in cyanobacterial biomass and filaments during remediation period
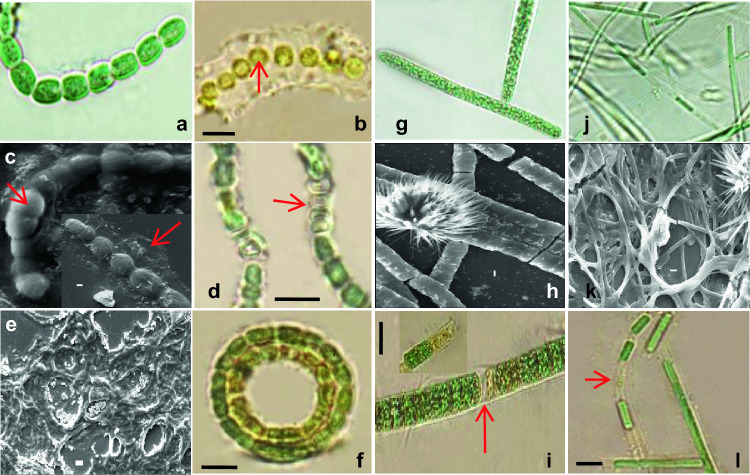
Abedi et al. (2019) reported similar stressed behavior of Anabaena in wastewater during their work and they suspected that toxic compounds in TWW are responsible for it. Acclimatization behavior of cyanobacterial biomass was observed with LM and SEM and represented in Fig. 5. Biomass of all three TWW grown cyanobacteria became slightly more clumped and yellowish green in color. In Anabaena, on the 7th day of treatment 20–25% vegetative cells were transformed into heterocyst (Fig. 5c, d) and plenty of trichomes became coiled (Fig. 5e, f). Reduced nitrogen concentration in 50% TWW may have triggered heterocyst differentiation from vegetative cells in Anabaena as previously suggested by Komárek and Johansen (2015). Coiling of trichomes and akinetes formation are also frequent incidents in Anabaena under altered nutrient composition (Zapomělová et al. 2008). On 14th day onwards, they released extracellular thick sheath to their surroundings and their 50% cells were rounded up to form akinetes, sometimes in chains (Fig. 5b). Braune (1980) explained that thick sheath formation in Anabaena results from condensation and thickening of fibrillar sheaths under a stressed environment. Trichomes of Arthrospira are usually devoid of sheath but in our investigation colorless or yellowish sheath were found covering trichomes after exposure (Fig. 5i). Sili et al. (2012) suggested this sheath formation as an acclimatization method in Arthrospira. In all studied cyanobacteria, yellowish cells were observed after the entire treatment period (Fig. 5i). This could correspond with chlorophyll and phycobiliproteins reduction (Walter et al. 2020). Again, due to the fragmentation process, filaments of both Arthrospira and Leptolyngbya produce several smaller fragments (Fig. 5i, l). Formation of needle shaped sodium chloride crystals on the surface of both Arthrospira and Leptolyngbya has been observed in SEM study (Fig. 5h, k). High sodium chloride content of the studied TWW was responsible for these needle structures. Needle shaped crystal formation from saturated sodium chloride solution has also been experienced by the previous author (Townsend et al. 2018).
Fig. 5.
Morphological changes in cyanobacterial filaments in response to 50% TWW; a–f (a control Anabaena, b, c red arrows indicate thick sheath and akinete formation within filament, c, d red arrows indicate heterocyst formation within filament, e, f red arrows indicate coiled filaments); g–i (g control Arthrospira, h flower shaped salt crystals have been formed above trichome, i red arrow indicates yellowish fragment with unusual sheath formation); j–l (j control Leptolyngbya, k clumped filaments with flower shaped salt crystals, l red arrow indicates thin sheath); Scale bar (a, b, d, f, g, i, j, l 5 µm and c, e, h, k 1 µm)
Biochemical properties of TWW remediated cyanobacterial biomass
To know the altered biochemical composition of potent cyanobacteria, after treating TWW, different biochemical analyses were performed. Levels of various biochemical components differed among Leptolyngbya Biomass (LB), Arthrospira Biomass (AB) and Chemical Fertilizer as revealed by different biochemical analyses and represented in Table S1. In this research, wastewater treated cyanobacteria were rich in carbohydrate (9%-11%) and protein content (21%-23%) whereas Chemical Fertilizer (CF) was rich in NPK content. Correspondence Analysis (Fig. 6a) carried out based on the biochemical components of fertilizers, further reflected the above-mentioned fact, i.e., CF contained more NPK content whereas cyanobacterial biomass (LB and AB) contained higher Carbohydrate−Protein−Lipid content.
Fig. 6.
a Correspondence analysis plot based on the biochemical components of both cyanobacterial biomass and chemical fertilizers (TP Total Phosphate, TK Total Potassium, TN Total Nitrate); Factor analyses plots based on the plant growth improvement parameters after application of spent cyanobacterial biomass (b Chickpea, c Rice); Discriminant analyses plots based on the germination and vigor index of plants after application of spent cyanobacterial biomass (d Chickpea, e Rice)
Role of wastewater treated cyanobacterial biomass in seedling growth improvement
The TWW treatment plant of our study did not contain detectable chromium in their wastewater due to a separate chromium recovery process. Therefore, cyanobacteria cultivated in this TWW were devoid of any detectable amount of chromium. Considering their rich carbon and nutrient source and renewable nature (Derakhshandeh et al. 2021), they were employed to improve growth in Rice and Chickpea seedlings. In Chickpea, both AB and LB increased several plant growth parameters, like, no. of leaves, chlorophyll content and fresh weight by > 25%, > 13%, and > 10% respectively which were significantly (p < 0.05) higher than control soil (Table S2). However, the length of the plant remained unaltered (± 35 cm) when treated with AB or LB. There was a significant increase in root length by 22% in AB treated plants. Both AB and LB increased growth parameters magnificently in Chickpea plants but between them AB improved Chickpea plant growth in a more luxurious way. In Rice, compared to control soil, AB and LB treated soil showed significant augment in plant height (> 30%), root length (> 14%), chlorophyll content (> 18%) and fresh weight (> 8%) respectively (Table S2). There was no significant difference between leaf numbers of plants raised in control soil and treated soils (CF, AB, and LB). Both AB and LB performed as well as CF in enhancing the growth parameters of the Rice plant; however, AB showed greater resemblance with CF. Though cyanobacteria were low in NPK content, their seedling growth capability was like chemical fertilizer. This is due to the rich content of vitamins and micronutrients (Motta and Maggiore 2013; Wuang et al. 2016; Renuka et al. 2016). Nutrients provided by the wastewater grown cyanobacterial biomass were able to improve the growth of Rice and Chickpea plants. Besides, cyanobacterial biomass increased the carbon content of soil which also helped to improve the growth of plants. Several authors reported earlier about this sort of utilization of wastewater treated biomass as biofertilizer and suspected similar reasons behind the enhanced plant growth performances (Mulbry et al. 2005; Motta and Maggiore 2013; Wuang et al. 2016; Dineshkumar et al. 2018). An increase in plant growth in our study may also have occurred due to the release of plant growth promoters or metabolites by both cyanobacteria and inhabitant soil bacteria. Again, exopolysaccharide produced from cyanobacteria might further play a significant role in increasing nutrient availability in soil. Factor analyses were carried out to represent the above-mentioned comparison in a more clarified way (Fig. 6b and c along with Tables S3 and S4).
Effect of TWW treated cyanobacterial biomass on seed germination and vigor index
Germination rate and vigor index give ideas about field performance of any plant. Therefore, in addition to the growth parameter studies, seed germination and vigor index were also checked in both Rice and Chickpea plants (Table S5). To know the effect of four treatment groups (Control, CF, LB, and AB) on both Rice and Chickpea plants, Discriminant analyses were performed based on seed germination and vigor index (Fig. 6d and e). Previous factor analyses results based on plant growth parameters, showed that Chickpea had a better performance than Rice. In contrast to that result, here Rice showed better activity (Germination rate ± 95%; Vigor Index, ± 256) than Chickpea (Germination rate, ± 93%; Vigor Index, ± 120). In Discriminant plots; CF, AB and LB were closely placed to each other while the Control group was distantly placed from all three of them. Germination rate and vigor index were better in Control, i.e., in water treated sets than fertilizer treated sets. Similar reduced performance of fertilizers in seed germination has also been reported by earlier authors (Wuang et al. 2016). This may be due to the slow-release patterns of fertilizers which create their effects on later growth stages of plants but not in the initial germination stage.
Conclusion
A cost-effective approach has been taken to treat tannery wastewater using cyanobacteria as bio-reagent at outdoor-open system condition. Commercial-grade salt and tap water-based media was used instead of expensive salts and distilled water-based media. Among varied concentrations of wastewater, 50% concentration induced maximum cyanobacterial growth due to its suitable nutrient ratios. Among the studied strains, it was revealed that nonheterocystous cyanobacteria were more suitable for phycoremediation purpose. Interestingly, freshwater strain removed more organic loads from wastewater whereas marine strain was more capable in removing of total dissolved solids (Ajayan et al. 2015; Liu et al 2016; Singh et al. 2017; Das et al. 2018; Kottangodanet al. 2019). On the other hand, heterocystous genus was more susceptible to pollutants, forming a large no. of heterocyst and akinetes as stress response with minimum vegetative growth. Biomass was harvested using low-cost cloth filtration process. Following the resource recovery concept, biomass was reused for growth improvement in Rice and Chickpea seedling. Better growth improvement was observed for Chickpea seedling compared to rice seedling. Arthrospira biomass induced seedling growth improvement in a better way than Leptolyngbya biomass. However, assessment through long-term field assay is further needed to establish their role in plant growth improvement. It is challenging nowadays to handle wastewater while maintaining ecological equilibrium. However, it will be simpler to use this zero-waste technology through by-product usage. This combined approach of tannery wastewater remediation along with residual biomass utilization as plant growth-promoter would create ecologically and economically sustainable technology for clean water production.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 Supplementary information includes tables of statistical analyses, fertilizer and algal biomass biochemical properties, plant growth parameters, details of statistical analyses, and additional references of Tables 1 and 2. (DOCX 33 KB)
Acknowledgements
Iman Dey would like to thank CRNN; Dept. of Botany, University of Calcutta and DST-FIST for providing infrastructural facilities and UGC, India (UGC-Ref. No.: 723) for providing financial support to conduct the research work.
Author contributions
Prof. Ruma Pal gave the concept of investigation; Iman Dey has done all the experimental works and wrote the full manuscript; Finally, Prof. Ruma Pal critically analyzed and edited the whole manuscript.
Data availability
All data are included within manuscript. Any other information about data will be made available on request.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest in publishing this article. No conflicts, informed consent, human or animal rights are applicable to this work. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abedi S, Astaraei FR, Ghobadian B, Tavakoli O, Jalili H, Greenwell HC, Cummins I, Chivasa S. Decoupling a novel Trichormus variabilis Synechocystis sp. interaction to boost phycoremediation. Sci Rep. 2019;9(1):2511. doi: 10.1038/s41598-019-38997-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajayan KV, Selvaraju M, Unnikannan P, Sruthi P. Phycoremediation of tannery wastewater using microalgae Scenedesmus species. Int J Phytorem. 2015;17(10):907–916. doi: 10.1080/15226514.2014.989313. [DOI] [PubMed] [Google Scholar]
- American Public Health Association . Standard methods for the examination of water & wastewater. Washington: American Public Health Association; 2005. APHA standard methods for the examination of water and wastewater. [Google Scholar]
- Atici T. Production and collection of microalgae isolated from freshwater reserves in Central Anatolia. Turkey Türlervehabitatlar. 2020;1(1):37–44. [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bureau of Indian Standards (BIS) (1994) Quality tolerance for water for Tanning industry IS: 4221, New Delhi
- Braune W. Structural aspects of akinete germination in the cyanobacterium Anabaena variabilis. Arch Microbiol. 1980;126:257–261. doi: 10.1007/BF00409929. [DOI] [Google Scholar]
- Chojnacka K, Moustakas K, Witek-Krowiak A. Bio-based fertilizers: A practical approach towards circular economy. Bioresour Technol. 2020;295:122223. doi: 10.1016/j.biortech.2019.122223. [DOI] [PubMed] [Google Scholar]
- Chowdhury M, Mostafa MG, Biswas TK, Mandal A, Saha AK. Characterization of the effluents from leather processing industries. Environ Process. 2015;2:173–187. doi: 10.1007/s40710-015-0065-7. [DOI] [Google Scholar]
- Crawford KA (2008) The effects of nutrient ratios and forms on the growth of Microcystis aeruginosa and Anabaena flos-aquae. Graduate College Dissertations and Theses. 59. https://scholarworks.uvm.edu/graddis/59
- Das C, Ramaiah N, Pereira E, Naseera K. Efficient bioremediation of tannery wastewater by monostrains and consortium of marine Chlorella sp. and Phormidium sp. Int J Phytoremediation. 2018;20(3):284–292. doi: 10.1080/15226514.2017.1374338. [DOI] [PubMed] [Google Scholar]
- De Bhowmick G, Sarmah AK, Sen R. Performance evaluation of an outdoor algal biorefinery for sustainable production of biomass, lipid and lutein valorizing flue-gas carbon dioxide and wastewater cocktail. Bioresour Technol. 2019;283:198–206. doi: 10.1016/j.biortech.2019.03.075. [DOI] [PubMed] [Google Scholar]
- Derakhshandeh M, Atici T, Un UT. Lipid extraction from microalgae Chlorella and Synechocystis sp. using glass microparticles as disruption enhancer. Energy Environ. 2019;30(8):1341–1355. doi: 10.1177/0958305X19837463. [DOI] [Google Scholar]
- Derakhshandeh M, Atici T, TezcanUn U. Evaluation of wild-type microalgae species biomass as carbon dioxide sink and renewable energy resource. Waste Biomass Valoriz. 2021;12:105–121. doi: 10.1007/s12649-020-00969-8. [DOI] [Google Scholar]
- Dey I, Banerjee S, Bose R, Pal R. Spatiotemporal variations in the composition of algal mats in wastewater treatment ponds of tannery industry. Environ Monit Assess. 2021;193(6):359. doi: 10.1007/s10661-021-09144-5. [DOI] [PubMed] [Google Scholar]
- Dhamotharan R, Murugesan S, Yoganandam M (2016) Bioremediation of tannery effluent using cyanobacterium. Biosci Biotechnol Res Asia 5(1)
- Dineshkumar R, Sen R. A sustainable perspective of microalgal biorefinery for co-production and recovery of high-value carotenoid and biofuel with CO2 valorization. Biofuels Bioprod Biorefin. 2020;14(4):879–897. doi: 10.1002/bbb.2107. [DOI] [Google Scholar]
- Dineshkumar R, Kumaravel R, Gopalsamy J, Sikder MNA, Sampathkumar P. Microalgae as bio-fertilizers for rice growth and seed yield productivity. Waste Biomass Valoriz. 2018;9:793–800. doi: 10.1007/s12649-017-9873-5. [DOI] [Google Scholar]
- DuBois M, Gilles KA, Hamilton JK, Rebers PT, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28(3):350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Ge S, Champagne P. Nutrient removal, microalgal biomass growth, harvesting and lipid yield in response to centrate wastewater loadings. Water Res. 2016;88:604–612. doi: 10.1016/j.watres.2015.10.054. [DOI] [PubMed] [Google Scholar]
- Gorain PC, Paul I, Bhadoria PS, Pal R. An integrated approach towards agricultural wastewater remediation with fatty acid production by two cyanobacteria in bubble column photobioreactors. Algal Res. 2019;42:101594. doi: 10.1016/j.algal.2019.101594. [DOI] [Google Scholar]
- Hamilton TL, Klatt JM, De Beer D, Macalady JL. Cyanobacterial photosynthesis under sulfidic conditions: insights from the isolate Leptolyngbya sp. strain hensonii. ISME J. 2018;12(2):568–584. doi: 10.1038/ismej.2017.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahan MAA, Akhtar N, Khan NMS, Roy CK, Islam R, Nurunnabi M. Characterization of tannery wastewater and its treatment by aquatic macrophytes and algae. Bangladesh J Sci Ind Res. 2014;49(4):233–242. doi: 10.3329/bjsir.v49i4.22626. [DOI] [Google Scholar]
- Komárek J, Johansen JR. Freshwater algae of North America. Academic Press; 2015. Filamentous cyanobacteria; pp. 135–235. [Google Scholar]
- Kottangodan N, Das C, Ram A, Meena RM, Ramaiah N. Phycoremediation of hazardous mixed industrial effluent by a marine strain of Phormidium sp. Clean: Soil, Air, Water. 2019;47(6):1800264. [Google Scholar]
- Lakshmi K, Malliga P. Treatment of tannery effluent using cyanobacterium (Lyngbya sp.) with coirpith. Int J Sci Res. 2014;4(9):414–416. [Google Scholar]
- Liu C, Subashchandrabose S, Ming H, Xiao B, Naidu R, Megharaj M. Phycoremediation of dairy and winery wastewater using Diplosphaera sp. MM1. J Appl Phycol. 2016;28:3331–3341. doi: 10.1007/s10811-016-0894-4. [DOI] [Google Scholar]
- Lofrano G, Meriç S, Zengin GE, Orhon D. Chemical and biological treatment technologies for leather tannery chemicals and wastewaters: a review. Sci Total Environ. 2013;461:265–281. doi: 10.1016/j.scitotenv.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. doi: 10.1016/S0021-9258(19)52451-6. [DOI] [PubMed] [Google Scholar]
- Mohamed RS, Soumya R, Jenifer G, Ketut GDP, Jenny S, Malliga P (2019) Biological treatment of tannery effluent using cyanobacteria and NRK for sansevieria trifasciata plant growth. J Glob Res 141–147
- Motta SR, Maggiore T. Evaluation of nitrogen management in maize cultivation grows on soil amended with sewage sludge and urea. Eur J Agron. 2013;45:59–67. doi: 10.1016/j.eja.2012.10.007. [DOI] [Google Scholar]
- Mulbry W, Westhead EK, Pizarro C, Sikora L. Recycling of manure nutrients: use of algal biomass from dairy manure treatment as a slow release fertilizer. Bioresour Technol. 2005;96(4):451–458. doi: 10.1016/j.biortech.2004.05.026. [DOI] [PubMed] [Google Scholar]
- Nagi M, He M, Li D, Gebreluel T, Cheng B, Wang C. Utilization of tannery wastewater for biofuel production: new insights on microalgae growth and biomass production. Sci Rep. 2020;10(1):1–14. doi: 10.1038/s41598-019-57120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyancha D, Mavura W, Ngila JC, Ongoma P, Chacha J. Studies of chromium removal from tannery wastewaters by algae biosorbents, Spirogyra condensata and Rhizoclonium hieroglyphicum. J Hazard Mater. 2008;158(2–3):605–614. doi: 10.1016/j.jhazmat.2008.02.043. [DOI] [PubMed] [Google Scholar]
- Ozturk BY, Asikkutlu B, Akkoz C, Atici T. Molecular and morphological characterization of several cyanobacteria and Chlorophyta species isolated from lakes in Turkey. Turk J Fish Aquat Sci. 2019;19(8):635–643. [Google Scholar]
- Pandi M, Shashirekha V, Swamy M. Bioabsorption of chromium from retan chrome liquor by cyanobacteria. Microbiologic Res. 2009;164(4):420–428. doi: 10.1016/j.micres.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Pena ADCC, Bertoldi CF, Fontoura JTD, Trierweiler LF, Gutterres M (2019) Consortium of microalgae for tannery effluent treatment. Brazilian Archives of Biology and Technology, 62
- Renuka N, Prasanna R, Sood A, Ahluwalia AS, Bansal R, Babu S, Singh R, Shivay L, Nain L. Exploring the efficacy of wastewater-grown microalgal biomass as a biofertilizer for wheat. Environ Sci Pollut Res. 2016;23:6608–6620. doi: 10.1007/s11356-015-5884-6. [DOI] [PubMed] [Google Scholar]
- Rodríguez R, Espada JJ, Pariente MI, Melero JA, Martínez F, Molina R. Comparative life cycle assessment (LCA) study of heterogeneous and homogeneous Fenton processes for the treatment of pharmaceutical wastewater. J Clean Prod. 2016;124:21–29. doi: 10.1016/j.jclepro.2016.02.064. [DOI] [Google Scholar]
- Santhosh S, Rajalakshmi AM, Navaneethakrishnan M, Jenny Angel S, Dhandapani R. Lab-scale degradation of leather industry effluent and its reduction by Chlorella sp. SRD3 and Oscillatoria sp. SRD2: a bioremediation approach. Appl Water Sci. 2020;10(5):1–11. doi: 10.1007/s13201-020-01197-0. [DOI] [Google Scholar]
- Saranya D, Shanthakumar S. Green microalgae for combined sewage and tannery effluent treatment: performance and lipid accumulation potential. J Environ Manage. 2019;241:167–178. doi: 10.1016/j.jenvman.2019.04.031. [DOI] [PubMed] [Google Scholar]
- Shashirekha V, Sridharan MR, Swamy M. Biosorption of trivalent chromium by free and immobilized blue green algae: kinetics and equilibrium studies. J Environ Sci Health Part A. 2008;43(4):390–401. doi: 10.1080/10934520701795608. [DOI] [PubMed] [Google Scholar]
- Shashirekha V, Sridharan MR, Swamy M. Bioremediation of tannery effluents using a consortium of blue–green algal species. Clean: Soil, Air, Water. 2011;39(9):863–873. [Google Scholar]
- Sili C, Torzillo G, Vonshak A. Arthrospira (spirulina). Ecology of cyanobacteria II: their diversity in space and time. Springer; 2012. pp. 677–705. [Google Scholar]
- Singh U, Praharaj CS. Practical manual-chemical analysis of soil and plant samples. Kanpur, Uttar Pradesh, India: ICAR-Indian Institute of Pulses Research; 2017. [Google Scholar]
- Singh AK, Sharma N, Farooqi H, Abdin MZ, Mock T, Kumar S. Phycoremediation of municipal wastewater by microalgae to produce biofuel. Int J Phytorem. 2017;19(9):805–812. doi: 10.1080/15226514.2017.1284758. [DOI] [PubMed] [Google Scholar]
- Suresh G, Balasubramanian B, Ravichandran N, Ramesh B, Kamyab H, Velmurugan P, Siva GV, Ravi AV. Bioremediation of hexavalent chromium-contaminated wastewater by Bacillus thuringiensis and Staphylococcus capitis isolated from tannery sediment. Biomass Convers Biorefinery. 2021;11:383–391. doi: 10.1007/s13399-020-01259-y. [DOI] [Google Scholar]
- Townsend ER, van Enckevort WJ, Tinnemans P, Blijlevens MA, Meijer JA, Vlieg E. Additive induced formation of ultrathin sodium chloride needle crystals. Cryst Growth Des. 2018;18(2):755–762. doi: 10.1021/acs.cgd.7b01170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter J, Leganés F, Aro EM, Gollan PJ. The small Ca2+-binding protein CSE links Ca2+ signalling with nitrogen metabolism and filament integrity in Anabaena sp. PCC 7120. BMC Microbiol. 2020;20(1):1–14. doi: 10.1186/s12866-020-01735-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (1981) Drinking-water and sanitation, 1981-1990: a way to health, a WHO contribution to the international drinking water supply and sanitation decade. World Health Organization
- Wuang SC, Khin MC, Chua PQD, Luo YD. Use of Spirulina biomass produced from treatment of aquaculture wastewater as agricultural fertilizers. Algal Res. 2016;15:59–64. doi: 10.1016/j.algal.2016.02.009. [DOI] [Google Scholar]
- Yadav G, Sen R. Microalgal green refinery concept for biosequestration of carbon-dioxide vis-à-vis wastewater remediation and bioenergy production: Recent technological advances in climate research. J CO2 Util. 2017;17:188–206. doi: 10.1016/j.jcou.2016.12.006. [DOI] [Google Scholar]
- Zapomělová E, Hrouzek P, Řeháková K, Šabacká M, Stibal M, Caisová L, Komárková J, Lukešová A. Morphological variability in selected heterocystous cyanobacterial strains as a response to varied temperature, light intensity and medium composition. Folia Microbiol. 2008;53:333–341. doi: 10.1007/s12223-008-0052-8. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Yu Z, Zhu L, Ye T, Zuo J, Li X, Xiao B, Jin S. Vertical-algal-biofilm enhanced raceway pond for cost-effective wastewater treatment and value-added products production. Water Res. 2018;139:144–157. doi: 10.1016/j.watres.2018.03.076. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Schideman L, Yu G, Zhang Y. A synergistic combination of algal wastewater treatment and hydrothermal biofuel production maximized by nutrient and carbon recycling. Energy Environ Sci. 2013;6(12):3765–3779. doi: 10.1039/c3ee24241b. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 Supplementary information includes tables of statistical analyses, fertilizer and algal biomass biochemical properties, plant growth parameters, details of statistical analyses, and additional references of Tables 1 and 2. (DOCX 33 KB)
Data Availability Statement
All data are included within manuscript. Any other information about data will be made available on request.