Abstract
By using a model of coxsackievirus B4-induced disease, the question of whether tissue damage is due to the virus or to immune-mediated mechanisms was addressed. Both viral replication and T-cell function were implicated in contributing to the severity of disease. Three stages (I to III) of disease, which correspond to periods of high viral titers, low viral titers, and no infectious virus, have been identified. Stage I disease is considered to be primarily the result of viral replication. Immunopathological mechanisms appear to contribute to the severity of stage II and III disease. To investigate the role of T cells in contributing to the severity of disease, viral infection in CD8 knockout (ko) mice and CD4 ko mice was analyzed. CD8 T-cell responses appear to be beneficial during early, viral disease but detrimental in later disease when viral titers are diminishing. CD4 ko mice, unlike the parental strain, survived infection. Viral replication was lower in the CD4 ko mice. Was survival due to decreased viral replication or to the lack of T-helper-cell function? To investigate further the role of T helper cells in contributing to tissue damage, viral infection in two additional ko strains (interleukin-4 [IL-4] ko and gamma interferon ko strains) was examined. A clear correlation between viral replication and the outcome of infection was not observed. The absence of IL-4, which may influence T-helper-cell subset development, was advantageous during early viral disease but deleterious in later disease. The results suggest that T-cell-mediated immunity is both beneficial and detrimental during coxsackievirus B4 infection.
The group B coxsackieviruses, comprising six serotypes (B1 to B6), have been implicated in a variety of diseases such as pancreatitis, type I insulin-dependent diabetes mellitus, myocarditis, and myositis (16, 24, 25, 30). The broad spectrum of diseases associated with the group B viruses reflects the existence of strains, with various degrees of virulence, within a serotype. Although there is a great deal of information on the biochemical, biophysical, and genetic characteristics of the picornaviruses, the mechanisms by which these RNA viruses cause disease are poorly understood. An ongoing question that remains to be resolved is whether tissue damage is due solely to the virus, to immunopathological mechanisms, or to a combination of both. Evidence supporting an immunopathological mechanism during coxsackievirus B3 (CVB3) infection implicates different effector cells such as CD8 T cells (10), CD4 T cells (1, 10), autoantibody-producing B cells (19, 31), and natural killer cells (9). In addition, the type of T-helper-cell response is critical in determining pathogenicity in a myocarditis model (11).
To study the intricate virus-host relationship, we have developed a mouse model of CVB4-induced disease. Using two serologically indistinguishable variants of the B4 serotype, CB4-P and CB4-V, we have shown that the development of mild versus severe disease is dependent on the infecting viral strain (3). The molecular determinants of virulence of CB4-V have been identified. A threonine residue at position 129 of VP1 is a major determinant of virulence (2). An arginine residue at position 16 of VP4 also influences virulence but to a lesser extent than Thr-129 of VP1 (26).
Regardless of the host’s genetic background, the CB4-P variant induces a transient inflammation of the pancreas (pancreatitis) which is followed by repair of the damaged tissues. However, the CB4-V variant induces a severe pancreatitis that can progress to chronic disease, which results in extensive and irreversible destruction of the pancreas. CB4-V infection is also lethal in some strains of mice. The outcome of infection, in B10 strains, is determined by a locus within the major histocompatibility complex (MHC) (23). During CB4-V infection, pancreatic tissue damage is probably due to a combination of mechanisms, including viral cytolysis, autodigestion by pancreatic enzymes, and immunopathology.
In this study, we examined the role of the immune system in contributing to disease during infection with the virulent variant, CB4-V. The approach involved analyzing viral infection in immunologically deficient, knockout (ko) strains of mice. We showed the following: (i) CD8 T-cell responses can be beneficial during early, viral disease but detrimental during later disease; (ii) CD4 T cells contribute to the severity of disease during viral infection; (iii) the absence of interleukin-4 (IL-4) is advantageous during early viral disease but deleterious in later disease; and (iv) the outcome of viral infection can be altered by depletion of specific cellular subsets and by neutralization of specific cytokines.
MATERIALS AND METHODS
Cells and viruses.
The passage history of the CB4-V variant has been described previously (23). After plaque purification, large-scale stocks of virus were grown in LLC-MK2(D) cells. Viral infectivity was assessed by plaque assay.
Mouse strains.
Several strains of mice were used in this study. The two B10 H-2 congenic strains, B10.T(6R) and B10.S(12R), are maintained in our animal facility. The BALB/cByJ mice are bred in the Wadsworth Center’s Animal Core Facility. The ko and transgenic lines are maintained by William Lee at the Wadsworth Center. The CD4 ko (12), IL-4 ko (22), and gamma interferon (IFN-γ) ko (6) strains are on a BALB/c genetic background, while the CD8 ko (7) strain is on a C57BL/6 genetic background. C57BL/6 mice, purchased from the Jackson Laboratory, served as controls. A T-cell receptor transgenic line, D011.10, is also on a BALB/c genetic background (18). Most of the CD4 T cells of the D011.10 strain express a T-cell receptor that recognizes a peptide derived from ovalbumin in the context of an MHC class II (I-Ad) molecule. Two additional mouse strains, BALB/cByJ-Hfh11nu (nude) and BALB/cByJSmn-Prkdcscid (severe combined immunodeficient [SCID]), were purchased from the Jackson Laboratory. Both the nude and the SCID mice are on a BALB/c genetic background. The nude and SCID mice were housed in sterile, filter-top cages and given autoclaved food and water.
Infection of mice.
Four- to six-week-old mice were injected intraperitoneally with 104 PFU of virus diluted in phosphate-buffered saline (PBS). Control mice were injected with PBS. All injected mice were monitored daily. If mice became moribund (displayed weight loss, shivering, huddling, and general inactivity), they were euthanized immediately. Mice were sacrificed at various times postinfection (p.i.), and organs were harvested. Tissue homogenates were prepared as previously described (23) and assayed for infectivity by plaque assay. Pancreatic tissues, fixed in phosphate-buffered formalin, were processed for routine histology, followed by staining with hematoxylin and eosin. All animal procedures were approved by the Institutional Animal Care and Use Committee of the Wadsworth Center.
Immunomodulation in vivo.
Monoclonal antibodies were used to deplete specific cellular subsets and to neutralize specific cytokines in vivo. Depletion of CD8 T cells was accomplished by using a monoclonal antibody (2.43 anti-Lyt2.2) against the CD8 marker (29). BALB/cByJ mice were injected intraperitoneally with 1 mg of purified antibody on each of two consecutive days prior to viral infection. To ensure that CD8 T cells were depleted, spleen cells from untreated or antibody-treated mice were stained with the anti-CD8 monoclonal antibody and analyzed by flow cytometry. The CD8 T-cell population was reduced by over 90% in vivo. Neutralization of IFN-γ was accomplished by using a similar protocol. Prior to infection, BALB/cByJ mice were injected intraperitoneally with 1 mg of purified antibody (anti-IFN-γ monoclonal antibody XMG1.2) (4) on each of two consecutive days.
RESULTS
Gender and genetic background influence the outcome of viral infection.
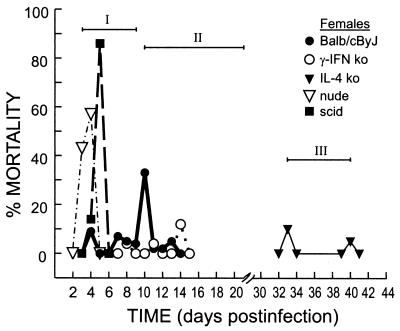
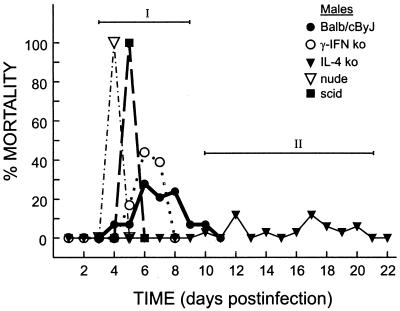
Previous studies focused on CB4-V infection in B10 H-2 congenic strains of male mice (23, 27). We showed that the outcome of infection with CB4-V in B10 strains of male mice is influenced by the MHC. In this report, we examine the role of gender and genetic background on the outcome of viral infection. To test if the host’s genetic background influenced the outcome of infection with CB4-V, we infected three strains of mice (Table 1). Infected mice were monitored daily. If mice became moribund (displayed weight loss, shivering, huddling, and general inactivity), they were euthanized immediately. Male and female B10 mice responded similarly to CB4-V infection. The B10.S(12R) mice survived infection with CB4-V, while the B10.T(6R) mice succumbed to infection within 10 to 14 days. Unlike the case for the B10 strains, a gender difference was observed for BALB/cByJ mice. In addition, infected BALB/cByJ mice died earlier than B10.T(6R) mice. In female mice, mortality peaked at 10 days after infection, with an overall rate of 67% (Fig. 1). In male BALB/cByJ mice, mortality peaked at 6 to 8 days after infection, with a rate of 100% (Fig. 2).
TABLE 1.
Outcome of CB4-V infection in different strains of mice
| Mouse strain | Gender | No. of mice infected | % Mortalitya | Time of death (days p.i.) |
|---|---|---|---|---|
| BALB/cByJ | Male | 29 | 100 | 4–10 |
| Female | 57 | 67 | 4–13 | |
| B10.S(12R) | Male | 20 | 0 | |
| Female | 20 | 0 | ||
| B10.T(6R) | Male | 20 | 100 | 10–14 |
| Female | 20 | 100 | 10–14 |
Percent mortality was determined from the number of mice that became moribund. Moribund mice were euthanized.
FIG. 1.
Outcome of CB4-V infection in immunologically deficient female mice. Mice were infected intraperitoneally with 104 PFU of CB4-V. If mice became moribund, they were euthanized immediately. The percent mortality was determined from the number of mice that became moribund. The times of death allowed the definition of three stages (I, II, and III) of disease.
FIG. 2.
Outcome of CB4-V infection in immunologically deficient male mice. Mice were infected intraperitoneally with 104 PFU of CB4-V. If mice became moribund, they were euthanized immediately. The percent mortality was determined from the number of mice that became moribund. The times of death allowed the definition of two stages (I and II) of disease.
Outcome of viral infection in immunodeficient mice.
The fact that gender influenced the outcome of infection in BALB/cByJ mice suggests that hormonal and immune responses can influence disease progression. The following experiments focused on the role of the immune system in the development of disease. To examine the role of T cells in contributing to the severity of viral disease and, hence, to the outcome of infection, several strains of immunodeficient mice were infected with CB4-V (Table 2). All of the immunodeficient strains are on a BALB/c genetic background. BALB/cByJ mice served as controls. SCID mice (which lack T and B cells) and nude mice (which lack T cells) succumbed to viral infection within 3 to 5 days. However, infection of mice lacking CD4 T cells resulted in a survival rate of 100%. To test whether attenuated disease was due to the absence of CD4 T cells or to lack of specific CD4 T cells, we infected a transgenic strain, D011.10, that has CD4 T cells but of a restricted repertoire. Most of the CD4 T cells in the D011.10 strain express a T-cell receptor that recognizes a peptide derived from ovalbumin in the context of an MHC class II (I-Ad) molecule (18). A survival rate of 100% was observed in CB4-V-infected D011.10 mice. Gender did not influence the outcome of infection in SCID, nude, CD4 ko, and D011.10 mice.
TABLE 2.
Outcome of CB4-V infection in immunologically deficient mice
| Gender | Mouse strain | No. infected | % Mortalitya | Time of death (days p.i.) |
|---|---|---|---|---|
| Male | BALB/cByJ | 29 | 100 | 4–10 |
| SCID | 7 | 100 | 4–5 | |
| Nude | 7 | 100 | 3–4 | |
| CD4 ko | 11 | 0 | ||
| IL-4 ko | 34 | 47 | 14–21 | |
| IFN-γ ko | 18 | 100 | 5–7 | |
| D011.10 | 8 | 0 | ||
| Female | BALB/cByJ | 57 | 67 | 4–13 |
| SCID | 7 | 100 | 4–5 | |
| Nude | 7 | 100 | 3–4 | |
| CD4 ko | 16 | 0 | ||
| IL-4 ko | 20 | 21 | 33–40 | |
| IFN-γ ko | 24 | 21 | 8–14 | |
| D011.10 | 8 | 0 |
Percent mortality was determined from the number of mice that became moribund. Moribund mice were euthanized.
Since CD4 T cells contributed to pathology in this model system, additional experiments focused on CB4-V infection in two additional strains of ko mice (IL-4 ko and IFN-γ ko mice). As was observed in BALB/cByJ mice, gender influenced the outcome of infection in IL-4 ko and IFN-γ ko mice. In these strains, male mice succumbed earlier and had a higher mortality rate than female mice. In the analysis of viral infection in female mice, mortality was observed at three distinct time periods (Fig. 1). The times of death allowed us to identify three stages of disease: stage I (3 to 9 days p.i.), stage II (10 to 21 days p.i.), and stage III (after 21 days p.i.). Nude and SCID mice succumbed to viral infection during stage I, while peak mortality for BALB/cByJ and IFN-γ ko mice occurred during stage II. Mortality in infected IL-4 ko mice was confined to stage III. In infected female mice, a lack of IFN-γ resulted in a decrease in mortality during stage I and II disease, while a lack of IL-4 caused a 21% mortality rate in stage III disease (Table 3).
TABLE 3.
CB4-V infection results in a multistage disease
| Mouse strain | Gender | Treatment | % Mortalitya in stage:
|
||
|---|---|---|---|---|---|
| I (3–9 days) | II (10–21 days) | III (22–40 days) | |||
| BALB/cByJ | Male | None | 100 | ||
| CD4 ko | Male | None | 0 | 0 | 0 |
| IL-4 ko | Male | None | 0 | 47 | 0 |
| IFN-γ ko | Male | None | 100 | ||
| BALB/cByJ | Male | Anti-IFN-γ | 100 | ||
| BALB/cByJ | Male | Anti-CD8 | 50 | 0 | 0 |
| C57BL/6 | Male | None | 0 | 61 | 0 |
| CD8 ko | Male | None | 69 | 0 | 0 |
| BALB/cByJ | Female | None | 24 | 42 | 0 |
| CD4 ko | Female | None | 0 | 0 | 0 |
| IL-4 ko | Female | None | 0 | 0 | 21 |
| IFN-γ ko | Female | None | 4 | 17 | 0 |
| BALB/cByJ | Female | Anti-IFN-γ | 0 | 0 | 0 |
Percent mortality was determined from the number of mice that became moribund. Moribund mice were euthanized.
In the analysis of the outcome of viral infection in male mice, mortality was observed at two distinct times (Fig. 2). Nude, SCID, BALB/cByJ, and IFN-γ ko mice succumbed to viral infection during the first week, while IL-4 ko mice became moribund during the second week. There was essentially no difference in the outcomes of infection of male BALB/cByJ mice and the IFN-γ ko mice. Both strains succumbed during stage I disease. However, a lack of IL-4 resulted in survival of stage I disease, with a 47% mortality rate during stage II disease.
To examine the role of CD8 T cells in contributing to the severity of disease, viral infection in CD8 ko mice was analyzed (Table 3). The CD8 ko strain was on a C57BL/6 genetic background. CB4-V-infected C57BL/6 mice survived stage I disease but developed a mortality rate of 61% during stage II disease. In CB4-V-infected CD8 ko mice, a mortality rate of 69% was observed during stage I disease, and the remaining mice survived stage II disease.
Immunomodulation in vivo.
To test the validity of the ko mouse models, monoclonal antibodies were used to neutralize specific cytokines and to deplete specific cellular subsets in vivo. Initial experiments focused on the neutralization of IFN-γ. The outcome of infection in mice pretreated with the anti-IFN-γ antibody was similar to that observed in the IFN-γ ko mice (Table 3). Since CD8 T cells affected the outcome of CB4-V infection in C57BL/6 mice, we tested whether CD8 T cells also influenced disease severity during infection of BALB/cByJ mice. Depletion of CD8 T cells, in vivo, was accomplished by using a monoclonal antibody against CD8. This protocol results in a 90% reduction of the CD8 T-cell population in the spleen (data not shown). Depletion of CD8 T cells resulted in a twofold reduction in mortality in infected male BALB/cByJ mice during stage I disease (Table 3).
Viral replication in BALB/cByJ mice.
Previous studies showed that while CB4-V replicates in several organs, viral titers are highest in the pancreas (23). Histological analysis revealed that CB4-V infection results in extensive damage to the exocrine pancreas, while the endocrine tissues appear to be unaffected at the light microscopic level (27). The assumption is that CB4-V-induced mortality is due to dysfunction of the exocrine pancreas. The function of the exocrine pancreas is to synthesize and secrete digestive enzymes which aid in the digestion and absorption of food in the small intestine. Therefore, CB4-V-infected mice may be unable to digest and absorb food. To test if exocrine pancreatic insufficiency contributed to morbidity and mortality in CB4-V-infected mice, female BALB/cByJ mice were given supplements of pancreatic enzymes (pancreatin, 1 mg/ml) in their water. During the 18-day follow-up, the mice did not succumb to viral infection. During the same period, the mortality rate in untreated, infected mice was 67%. These results suggest that exocrine pancreatic insufficiency contributed to morbidity and mortality during CB4-V infection.
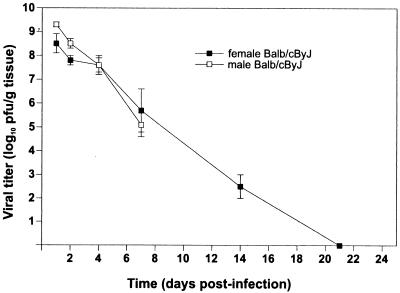
Since male BALB/cByJ mice succumbed to CB4-V infection earlier and with a higher mortality rate than female mice, we investigated whether this difference was attributable to altered viral replication. A kinetic study of viral replication in the pancreatic tissues of male and female BALB/cByJ mice was undertaken (Fig. 3). During the first 2 days of infection, viral replication in male mice was 10-fold higher than that in female mice. However, from day 4 to 7 after infection, viral replication was similar in male and female mice. Due to the extensive mortality in infected male mice, viral titers were not determined beyond 7 days after infection. In female mice, infectious virus was cleared by 21 days after infection.
FIG. 3.
Viral replication in pancreatic tissues of CB4-V-infected BALB/cByJ mice. Groups of three to five (male and female) mice were infected intraperitoneally with 104 PFU of CB4-V. Pancreatic tissues were harvested at different times p.i. Homogenates of individual samples were assayed for viral infectivity by plaque assay. Mean values (and standard deviations) are shown.
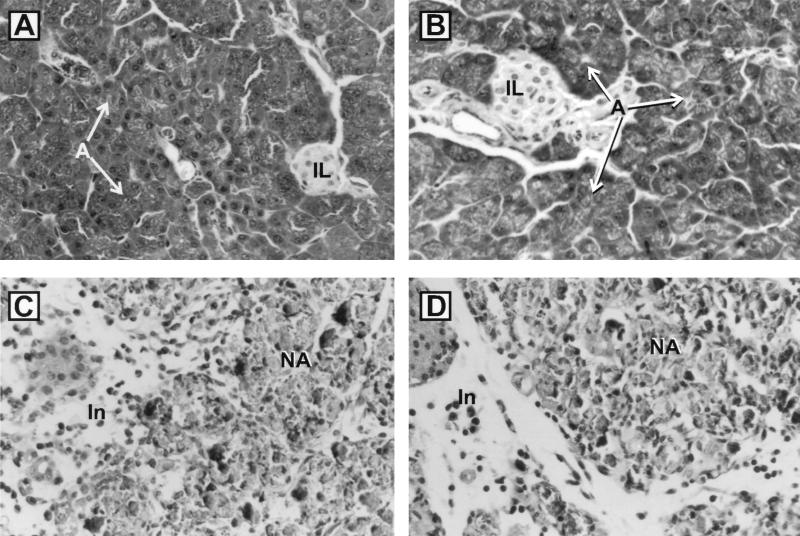
To determine if the increased viral replication, early in infection, correlated with more extensive tissue injury, a histological assessment of pancreatic tissues was undertaken (Fig. 4). At the light microscopic level, tissue injury appeared to be similar in male and female mice. Mice developed severe pancreatitis with extensive destruction of the exocrine tissue. The islets of Langerhans appeared to be intact. At 2 to 7 days after infection, extensive acinar cell necrosis with interlobular edema was observed. A generalized inflammation was evident. The inflammatory infiltrate consisted primarily of mononuclear cells.
FIG. 4.
Histopathology of pancreatic tissues from CB4-V-infected BALB/cByJ mice. Mice were infected intraperitoneally with 104 PFU of CB4-V or mock infected with PBS. Pancreatic tissues were harvested at various times p.i., processed for histology, and stained with hematoxylin and eosin. (A) Mock-infected female; (B) mock-infected male. Panels A and B show normal pancreas. (C) CB4-V-infected female at day 7 p.i.; (D) CB4-V-infected male at day 7 p.i. Panels C and D show complete destruction of acini, with necrosis, and inflammatory cell infiltrate. Abbreviations: IL, islet of Langerhans; A, acini with zymogen granules; NA, necrotic acini; In, inflammatory cells. Magnification, ×306.
Viral replication in immunodeficient mice.
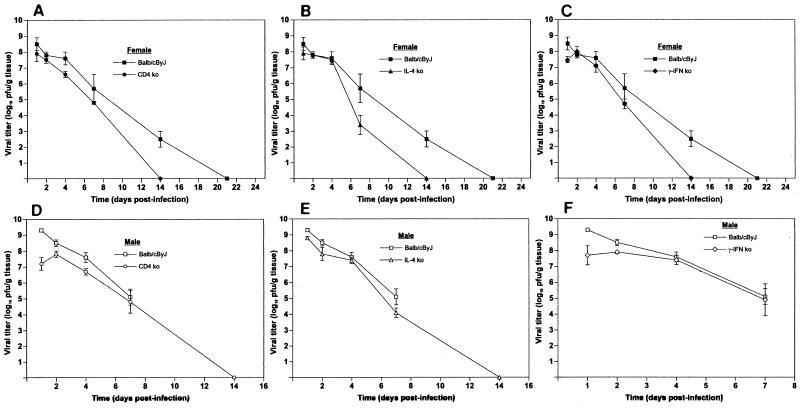
Since the outcome of viral infection was altered in some strains of immunodeficient mice, we investigated whether this was due to a difference in viral replication. A kinetic study of viral replication in the pancreatic tissues of CD4 ko, IL-4 ko, and IFN-γ ko mice was undertaken (Fig. 5). Unlike the case for BALB/cByJ mice, gender did not influence viral replication in the ko strains.
FIG. 5.
Comparison of viral replication in the pancreatic tissues of BALB/cByJ mice and various immunologically deficient strains of mice. Groups of three to five mice were infected intraperitoneally with 104 PFU of CB4-V. Pancreatic tissues were harvested at different times p.i. Homogenates of individual samples were assayed for viral infectivity by plaque assay. Mean values (and standard deviations) are shown. (A) BALB/cByJ and CD4 ko female mice; (B) BALB/cByJ and IL-4 ko female mice; (C) BALB/cByJ and IFN-γ ko female mice; (D) BALB/cByJ and CD4 ko male mice; (E) BALB/cByJ and IL-4 ko male mice; (F) BALB/cByJ and IFN-γ ko male mice.
Early in infection, viral replication in the CD4 ko and the IL-4 ko female mice was similar to that observed in the control mice. After 3 to 4 days of infection, viral replication in the CD4 ko and the IL-4 ko female mice was lower and viral clearance was faster than in the control mice (Fig. 5A and B). At 1 day after infection, viral titers were lower in the IFN-γ ko female mice than in the control mice. From 2 to 7 days after infection, viral replication in the IFN-γ ko female mice was similar to that seen in BALB/cByJ mice (Fig. 5C). Infectious virus was cleared more quickly in the IFN-γ ko female mice than in the control mice.
As was observed for female mice, viral replication in the CD4 ko and the IL-4 ko male mice was lower than that seen in BALB/cByJ mice (Fig. 5D and E). The kinetics of viral replication in the IFN-γ ko male mice paralleled that observed in the female mice. Initially (1 to 2 days p.i.), viral titers were lower than those in control mice. From 4 to 7 days after infection, viral replication in the IFN-γ ko male mice was similar to that seen in BALB/cByJ mice (Fig. 5F). Since IFN-γ ko and BALB/cByJ male mice succumbed to viral infection very early, viral titers were not determined beyond 7 days p.i.
A clear correlation between viral replication and the outcome of infection in the ko strains was not observed. For example, lower viral replication in the IFN-γ ko mice did not alter the time of death of male and female mice or the overall mortality of male mice (Fig. 5; Table 3). However, lower viral replication in the IL-4 ko mice was associated with a delay in the time of death and a decrease in mortality of both male and female mice.
DISCUSSION
The group B coxsackieviruses are associated with several human diseases that have an autoimmune component, e.g., type I insulin-dependent diabetes mellitus (25), idiopathic chronic pancreatitis (20, 21, 32), myocarditis (28), and idiopathic dilated cardiomyopathy (28). The question of whether tissue damage, in various animal models, is due solely to the virus, to immunopathological mechanisms, or to a combination of both needs to be resolved. Several studies have focused on CVB3-induced myocarditis. Whether myocardial damage is due to the effects of the virus or the immune system is controversial. Data supporting the idea of virus-induced tissue damage are derived from studies of CVB3-infected SCID mice (which lack B and T cells), which develop virus-induced lysis of myocytes (5). In addition, histological observations support a role for viral replication in mediating cardiac tissue injury (15). Data supporting the idea that the immune system contributes to tissue damage come from studies of CVB3 infection in immunologically deficient mice (1, 8, 10) and in mice that had been depleted of specific cell populations (13) and from adoptive transfer experiments (11).
In our model of CVB4-induced pancreatitis, we have identified three stages of disease based on the time of death of infected mice. Stage I (or early) disease, spanning 3 to 9 days after infection, was characterized by high titers of virus in pancreatic tissues. Stage I disease is considered to be primarily the result of viral replication. Stage II (or intermediate) disease, spanning 10 to 21 days p.i., was characterized by low viral titers in pancreatic tissues. Stage III (or late) disease was characterized by the absence of infectious virus and represents a time period of 22 to 40 days after infection. Immunopathological mechanisms appeared to contribute to the severity of stage II and III disease.
In the analysis of viral infection in B10 strains and BALB/cByJ mice, gender influenced the outcome of infection in the BALB/cByJ but not the B10 strains. Male BALB/cByJ mice succumbed to viral infection during early disease, while female mice had mortality rates of 24 and 42% during stage I and stage II disease, respectively. The question arose as to whether the difference in the outcome of infection was due to altered viral replication in male and female mice. During the first 2 days of infection, viral replication was greater in male mice than in female mice. From 4 to 7 days after infection, viral replication was similar in male and female BALB/cByJ mice. The initial increase in viral replication may have been due to a gender difference in the level of viral receptors, since for CVB3 testosterone has been shown to enhance the expression of the receptor (14). If pancreatic tissue damage was due solely to virus replication, then one would expect that tissue damage in male mice would be greater than that observed in female mice. Histological analysis revealed that pancreatic tissue damage was similar in male and female mice, suggesting that, in addition to virus replication, other mechanisms must contribute to tissue injury.
To begin to investigate the role of the immune response in contributing to tissue damage, viral infection of immunodeficient mice was analyzed. Initial studies focused on the use of SCID mice, which lack both T- and B-cell function, and nude mice, which lack T-cell function. CB4-V infection of nude and SCID mice resulted in 100% mortality very early in infection. These data suggest that functional adaptive immune responses are required for survival of early viral disease.
To dissect the roles of specific immunological compartments during infection, attention has been focused on the use of transgenic ko mice. By analyzing viral infection in various ko strains, one can begin to identify specific responses that are protective or deleterious at different stages of disease. The limitation of this approach is the issue of compensatory immune dysregulation in the ko strains. The problem can be partially controlled by comparing the ko strain to the parental strain in which the specific cellular subset had been depleted or the specific cytokine had been neutralized by in vivo administration of the appropriate monoclonal antibody. We were able to verify the results for some of our ko strains by this approach.
Our studies focused on the role of T cells in contributing to the severity of CB4-V-induced disease. We examined viral infection in CD4 ko mice. CD4 ko mice essentially lack T helper cells (12). Viral infection of the CD4 ko mice resulted in 100% survival, suggesting that CD4 T cells contribute to disease severity in this model system. To test whether the attenuated disease was due to the absence of CD4 T cells or to a lack of specific CD4 T cells, we infected a transgenic strain, D011.10, that has CD4 T cells but of a restricted repertoire. A survival rate of 100% was observed for CB4-V-infected D011.10 mice. While these data suggest that attenuation in this model system is due to a lack of specific CD4 T cells, we also observed that viral replication was lower in the CD4 ko strain than in the BALB/cByJ control mice. In addition, virus was cleared faster in the CD4 ko mice than in the BALB/cByJ mice. Was survival due to a difference in the ability of CB4-V to replicate in the ko strain or due to the lack of specific T helper cells? This question was pursued by analyzing infection in two additional strains (IL-4 ko and IFN-γ ko) of mice.
T helper cells can be divided into subsets, Th1 and Th2, based on the cytokines that they produce and their biological activities (17). IFN-γ is critical in the development of Th1 cells, while IL-4 is critical in the development of Th2 cells. In the ko mice, the assumption is that T-cell development is skewed in favor of a Th1 response in the IL-4 ko mice and in favor of a Th2 response in the IFN-γ ko mice. A clear correlation between viral replication and the outcome of infection was not observed. In the IL-4 ko mice, viral replication was generally lower and infectious virus was cleared faster than in the BALB/cByJ mice, suggesting that a polarized T-helper-cell response may help to contain viral replication. The IL-4 ko mice survived stage I of infection and developed some mortality during stage II (males) and stage III (females) of disease. While a polarized T-helper-cell response may be beneficial for containing viral infection, the same response appeared to be detrimental during later disease. For IFN-γ ko male mice, the outcome of infection was similar to that observed in the BALB/cByJ mice (100% mortality in stage I) even though viral replication in the ko mice was initially lower than that observed in the BALB/cByJ mice. The data suggest that male BALB/cByJ mice do not mount an adequate immune response early in infection and succumb to viral disease. The situation is more complex for female mice, since the outcome of infection in the IFN-γ ko mice differed from that in the control mice. Some mortality was observed in the IFN-γ ko female mice during stages I and II, while no mortality was observed in stage III. These data suggest that a Th2 response may be protective during stage III disease. In female mice, IFN-γ contributes, in part, to disease severity during stages I and II, but the significance of this result is unclear.
As was observed with the BALB/cByJ mice, the outcome of viral infection in the IL-4 ko and the IFN-γ ko mice was influenced by gender. Male mice succumbed earlier and had higher mortality rates than female mice. If viral replication were solely responsible for the severity of disease, then one would expect that replication would be higher in the ko male mice. The kinetics of viral replication in the ko male mice was similar to that observed in the female mice, suggesting that viral replication is not solely responsible for the severity of disease in this model system.
Viral infection in CD8 ko mice was also analyzed. CD8 ko mice essentially lack cytotoxic T cells (7). CD8 T cells contributed, in part, to mortality at different stages of disease in BALB/cByJ and C57BL/6 mice. A lack of CD8 T cells was deleterious during stage I disease in C57BL/6 mice. This finding is consistent with the idea that stage I disease is due to viral replication and that CD8 T cells are required to clear virus-infected cells.
In summary, the severity of CVB4-induced disease depends on several factors, including viral replication and T-cell function. While T-cell-mediated immunity appears to be beneficial in early, viral disease, the same responses seem to be detrimental in later disease when viral titers are diminishing. Ongoing studies are focused on whether the deleterious T-cell responses are the result of exaggerated (uncontrolled?) immune responses or the result of autoimmunity.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant DK43929 from the National Institute of Diabetes and Digestive and Kidney Diseases and by the American Heart Association.
We thank the staff of the Department of Pathology at the Wadsworth Center for processing tissue samples for histology. The secretarial assistance of Maryellen Carl is greatly appreciated.
REFERENCES
- 1.Blay R, Simpson K, Leslie K, Huber S A. Coxsackievirus-induced disease: CD4+ cells initiate both myocarditis and pancreatitis in DBA/2 mice. Am J Pathol. 1989;135:899–907. [PMC free article] [PubMed] [Google Scholar]
- 2.Caggana M, Chan P, Ramsingh A. Identification of a single amino acid residue in the capsid protein VP1 of coxsackievirus B4 that determines the virulent phenotype. J Virol. 1993;67:4797–4803. doi: 10.1128/jvi.67.8.4797-4803.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapman N M, Ramsingh A I, Tracy S. Genetics of coxsackievirus virulence. Curr Top Microbiol Immunol. 1997;223:227–258. doi: 10.1007/978-3-642-60687-8_11. [DOI] [PubMed] [Google Scholar]
- 4.Cherwinski H M, Schumacher J H, Brown K D, Mosmann T R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987;166:1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow L H, Beisel K W, McManus B M. Enteroviral infection of mice with severe combined immunodeficiency. Evidence for direct viral pathogenesis of myocardial injury. Lab Invest. 1992;66:24–31. [PubMed] [Google Scholar]
- 6.Dalton D K, Pitts-Meek S, Keshav S, Figari I S, Bradley A, Stewart T A. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259:1739–1745. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 7.Fung-Leung W, Schilham M W, Rahemtulla A, Kundig T M, Vollenweider M, Potter J, van Ewijk W, Mak T W. CD8 is needed for development of cytotoxic T cells but not helper T cells. Cell. 1991;65:443–449. doi: 10.1016/0092-8674(91)90462-8. [DOI] [PubMed] [Google Scholar]
- 8.Gebhard J R, Perry C M, Harkins S, Lane T, Mena I, Asensio V C, Campbell I L, Whitton J L. Coxsackievirus B3-induced myocarditis: perforin exacerbates disease, but plays no detectable role in virus clearance. Am J Pathol. 1998;153:417–428. doi: 10.1016/S0002-9440(10)65585-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Godeny E K, Gauntt C J. Murine natural killer cells limit coxsackievirus B3 replication. J Immunol. 1987;139:913–918. [PubMed] [Google Scholar]
- 10.Henke A, Huber S, Stelzner A, Whitton J L. The role of CD8+ T lymphocytes in coxsackievirus B3-induced myocarditis. J Virol. 1995;69:6720–6728. doi: 10.1128/jvi.69.11.6720-6728.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huber S A, Pfaeffle B. Differential Th1 and Th2 cell responses in male and female BALB/c mice infected with coxsackievirus group B type 3. J Virol. 1994;68:5126–5132. doi: 10.1128/jvi.68.8.5126-5132.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Killeen N, Sawada S, Littman D R. Regulated expression of human CD4 rescues helper T cell development in mice lacking expression of endogenous CD4. EMBO J. 1993;12:1547–1553. doi: 10.1002/j.1460-2075.1993.tb05798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leslie K, Blay R, Haisch C, Lodge A, Weller A, Huber S. Clinical and experimental aspects of viral myocarditis. Clin Microbiol Rev. 1989;2:191–203. doi: 10.1128/cmr.2.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyden D C, Olszewski J, Feran M, Job L P, Huber S A. Coxsackievirus B3-induced myocarditis. Effects of sex steroids on viremia and infectivity of cardiocytes. Am J Pathol. 1987;126:432–438. [PMC free article] [PubMed] [Google Scholar]
- 15.McManus B M, Chow L H, Wilson J E, Anderson D R, Gulizia J M, Gauntt C J, Klingel K E, Beisel K W, Kandolf R. Direct myocardial injury by enterovirus: a central role in the evolution of murine myocarditis. Clin Immunol Immunopathol. 1993;68:159–169. doi: 10.1006/clin.1993.1113. [DOI] [PubMed] [Google Scholar]
- 16.Melnick J L. Enteroviruses: polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses. In: Fields B N, Knipe D M, Howley P M, et al., editors. Fields virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 655–705. [Google Scholar]
- 17.Mosmann T R, Coffman R L. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 18.Murphy K M, Heimberger A B, Loh D Y. Induction by antigen of intrathymic apoptosis of CD4+CD8+ TCRlo thymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 19.Neu N, Beisel K W, Traystman M D, Rose N R, Craig S W. Autoantibodies specific for the cardiac myosin isoform are found in mice susceptible to coxsackievirus B3-induced myocarditis. J Immunol. 1987;138:2488–2492. [PubMed] [Google Scholar]
- 20.Nishimori I, Okazaki K, Yamamoto Y, Morita M, Tamura S. Specific cellular immune responses to pancreatic antigen in chronic pancreatitis and Sjogren’s syndrome. J Clin Immunol. 1993;13:265–271. doi: 10.1007/BF00919385. [DOI] [PubMed] [Google Scholar]
- 21.Nishimori I, Yamamoto Y, Okazaki K, Morita M, Onodera M, Kino J, Tamura S. Identification of autoantibodies to a pancreatic antigen in patients with idiopathic chronic pancreatitis and Sjogren’s syndrome. Pancreas. 1994;9:374–381. doi: 10.1097/00006676-199405000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Noben-Trauth N, Kropf P, Muller I. Susceptibility to Leishmania major infection in interleukin-4-deficient mice. Science. 1996;271:987–990. doi: 10.1126/science.271.5251.987. [DOI] [PubMed] [Google Scholar]
- 23.Ramsingh A, Slack J, Silkworth J, Hixson A. Severity of disease induced by a pancreatropic Coxsackie B4 virus correlates with the H-2Kq locus of the major histocompatibility complex. Virus Res. 1989;14:347–358. doi: 10.1016/0168-1702(89)90027-0. [DOI] [PubMed] [Google Scholar]
- 24.Ramsingh A I. Coxsackieviruses and pancreatitis. Frontiers Biosci. 1997;2:e53–e62. doi: 10.2741/a227. [DOI] [PubMed] [Google Scholar]
- 25.Ramsingh A I, Chapman N M, Tracy S. Coxsackieviruses and diabetes. BioEssays. 1997;19:793–800. doi: 10.1002/bies.950190909. [DOI] [PubMed] [Google Scholar]
- 26.Ramsingh A I, Collins D N. A point mutation in the VP4 coding sequence of coxsackievirus B4 influences virulence. J Virol. 1995;69:7278–7281. doi: 10.1128/jvi.69.11.7278-7281.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramsingh A I, Lee W, Collins D N, Armstrong L. Differential recruitment of B and T cells in coxsackievirus B4-induced pancreatitis is influenced by a capsid protein. J Virol. 1997;71:8690–8697. doi: 10.1128/jvi.71.11.8690-8697.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rose N R, Hill S L. The pathogenesis of postinfectious myocarditis. Clin Immunol Immunopathol. 1996;80:S92–S99. doi: 10.1006/clin.1996.0146. [DOI] [PubMed] [Google Scholar]
- 29.Sarmiento M, Glasebrook A L, Fitch F W. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T cell-mediated cytolysis in the absence of complement. J Immunol. 1980;125:2665–2672. [PubMed] [Google Scholar]
- 30.Tracy S, Chapman N M, Romero J, Ramsingh A I. Genetics of coxsackievirus B cardiovirulence and inflammatory heart muscle disease. Trends Microbiol. 1996;4:175–179. doi: 10.1016/0966-842x(96)10026-3. [DOI] [PubMed] [Google Scholar]
- 31.Wolfgram L J, Beisel K W, Rose N R. Heart-specific autoantibodies following murine coxsackievirus B3 myocarditis. J Exp Med. 1985;161:1112–1121. doi: 10.1084/jem.161.5.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida K, Toki F, Takeuchi T, Watanabe S, Shiratori K, Hayashi N. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Digest Dis Sci. 1995;40:1561–1568. doi: 10.1007/BF02285209. [DOI] [PubMed] [Google Scholar]