Abstract
Crohn's disease (CD) is a type of inflammatory bowel disease (IBD) that manifests mainly as chronic inflammation in different parts of the gastrointestinal tract, and its incidence has come to be increasing in recent years. Ferroptosis, a novel type of programmed cell death, it seems the role of ferroptosis-related biomarkers in CD has not been mentioned. Thus, the role of ferroptosis in CD and its relationship with immune infiltration were explored in this study. The CD dataset was downloaded from the Gene Expression Omnibus database. The validated ferroptosis genes (FRGs) were retrieved from the public FerrDb database. The gene expression matrix of the CD dataset was analyzed with the “limma” package in R language to obtain differentially expressed genes (DEGs) between diseased and healthy samples. Then, intersecting genes between DEGs and FRGs were identified as differentially expressed ferroptosis-associated genes (DE-FRGs). Protein–protein interaction (PPI) network analysis and visualization were carried out with STRING and Cytoscape, and key CD ferroptosis-related genes (CD-FRGs) were identified along with their Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways using the clusterProfiler package. Immune cell infiltration was analyzed with CIBERSORT. The correlation between key CD-FRGs and immune-infiltrated cells in CD was studied by Spearman's correlation method. A total of 37 DE-FRGs and 6 key CD-FRGs (CAV1, CD44, HIF1A, IFNG, TIMP1 and TLR4) were identified. GO and KEGG functional analysis indicated these genes enrichment in programmed cell death and apoptotic process, HIF-1 signaling pathway and IBD. Infiltration matrix analysis of immune cells showed abundant T cells CD4 memory activated, M1 macrophages, M2 macrophages, Mast cells activated and Neutrophils in CD intestinal tissues. The 6 key CD-FRGs were correlated with immune-infiltrated cells in CD based on correlation analysis. Taken together, immune cells with abnormal infiltration can be implicated in CD due to ferroptosis. This study identified 6 key CD-FRGs that may be key biomarkers of ferroptosis in CD; they include CAV1, CD44, HIF1A, IFNG, TIMP1 and TLR4. These findings suggest that the immune response is critical in CD caused by ferroptosis through the interaction between key CD-FRGs and immune infiltrating cells.
Subject terms: Immunology, Gastroenterology
Introduction
Crohn's disease (CD) is a type of inflammatory bowel disease (IBD) that manifests mainly as chronic inflammation in different parts of the gastrointestinal tract, and its incidence has come to be increasing in recent years1. However, the etiology of CD remains unclear, and it has been shown that genetic, immunological and environmental factors increase the risk of its development and progression2. The disease course of CD is progressive and devastating, with not only intestinal destruction but also extraintestinal systemic manifestations, which will seriously affect the quality of life and prognosis of patients3. In the last two decades, the use of biologics such as infliximab, adalimumab and vedolizumab has greatly expanded the therapeutic options for CD4,5. Even though these therapies account for significant advances in the treatment of CD, there are still several patients that are not sensitive to these agents (e.g., anti-TNF antibodies)6. Notably, specific biomarkers can help clinicians benefit in the diagnosis and intervention of CD, as well as play a role in clarifying treatment response and predicting CD relapse. Therefore, it is important to study and explore the precise molecular mechanisms and markers of CD for the development of its therapeutic strategies.
It has been demonstrated in many studies that the progression of IBD is associated with cell death. A growing body of evidence suggests that programmed cell death, including apoptosis, necrosis, and autophagy, is involved in the progression of CD in various ways7–10. Ferroptosis is a newly discovered regulated form of cell death in recent years, which is driven by the lethal build-up of lipid peroxides catalyzed with cellular free iron11. The essential features of Ferroptosis, including iron deposition, glutathione (GSH) depletion, glutathione peroxidase 4 (GPX4) deactivation, and lipid peroxidation, have been found in abundance in the damaged gastrointestinal tract of patients with IBD10. Xu et al. found that Ferrostatin-1, a ferroptosis inhibitor, attenuated the pathological changes of CD-like colitis induced by TNBS in mice12. It has been shown that abnormal ferroptosis has been detected in CD13. Of interest, in terms of dietary habits, it has been shown that dietary polyunsaturated fatty acid (PUFA) is a trigger for GPX4 (which prevents ferroptosis) to limit the inflammatory phenotype of human CD mucosa14. However, studies on ferroptosis in CD are still relatively few and, furthermore, it seems that the role of ferroptosis-related biomarkers in CD has not been mentioned so far.
To investigate whether or how ferroptosis is involved in the progression of CD, microarray data were collected from the GEO database with intestinal tissues of CD patients and healthy individuals. Subsequently, CD-related ferroptosis genes were screened by bioinformatics methods and immuno-infiltration analysis was performed to analyze their correlation. Finally, 37 differentially expressed ferroptosis-related genes (DE-FRGs), and 6 key CD ferroptosis-related genes (CD-FRGs) were identified as potential ferroptosis-related biomarkers of CD.
Materials and methods
Data acquisition
The Gene Expression Omnibus (GEO; www.ncbi.nlm.nih.gov/geo/)15 database was used to search gene microarray data of CD intestinal tissue samples. The GSE1687916 dataset, containing 12 healthy controls and 37 CD intestinal tissue samples was selected. Probes were converted to gene symbols according to the GPL570 platform ([HG-U133_Plus_2] Affymetrix Human Genome U133 Plus 2.0 Array). Ferroptosis-Related Genes (FRGs) that drive, suppress, or mark ferroptosis were retrieved from the public FerrDb database (http://www.zhounan.org/ferrdb)17. The final 365 FRGs obtained after removing duplicate genes were used for subsequent analysis. Two microarray datasets of CD (GSE5907118: 8 active CD vs. 11 healthy controls; GSE95095: 24 active CDs vs. 12 healthy controls) were used to validate the expression of key CD-FRGs. Fold changes greater than 2 or less than 0.5 were considered to be significantly differentially expressed. The p-value was corrected by controlling the false discovery rate (FDR), and the adjusted p-value (Q-value = FDR) was taken to be < 0.05. The "limma" package (http://www.bioconductor.org/packages/release/bioc/html/limma.html) was also applied to identify DEGs with a threshold of |log2 fold change (FC)|≥ 1, adj. P-value (FDR) < 0.05. Results are shown as volcano plots with the key gene labeled.
Identification of differentially expressed ferroptosis-related genes (DE-FRGs)
Analysis of the gene expression matrix from the GSE16879 dataset was performed using the "limma" package (http://www.bioconductor.org/packages/release/bioc/html/limma.html) in R language to obtain DEGs between CD and healthy samples. In addition, |log2 FC|> 1 and adj. P-value (FDR) < 0.05 were set as the selection criteria for DEGs. Subsequently, the overlapping genes between DEGs and FRGs were identified as differentially expressed ferroptosis-related genes (DE-FRGs).
Protein–protein interaction (PPI) network construction and module analysis
Analyses of the interactions between different DE-FRGs were performed using the STRING database (http://string-db.org/)19. The PPI network was built and visualized with Cytoscape software 3.9.1. (http://cytoscape.org/)20. The key module in PPI network was identified using the Cytoscape plugin Molecular Complex Detection (MCODE) (version 2.0), a plug-in for clustering based on the topology of a given network to identify densely connected regions21. The overlap of the top 10 genes of the MCC, Maximum Neighborhood Component (MNC), DNMC, Closeness, Degree and Edge Infiltration Component (EPC) algorithms were identified as key Crohn's disease ferroptosis-related genes (CD-FRG) using Cytoscape's plug-in cytoHubba.
Functional enrichment of DE-FRGs and hub CD-FRGs
Using the clusterProfiler package(https://www.bioconductor.org/packages/release/bioc/html/clusterProfiler.html) in R language to identify gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG)22–24 pathways to describe the characteristics of DE-FRG and key CD-FRG and to explore their underlying biological processes (BP), cellular components (CC), molecular functions (MF) and important signaling pathways. The smallest gene set was chosen as 5 and the largest as 5000. P < 0.05 and false detection rate < 0.1 were considered statistically significant.
Evaluation of subtype distribution among immune-infiltrated cells
A normalized gene expression matrix was shown to be transformed by CIBERSORT into a composition of 22 immune cell types based on a deconvolution algorithm25. In this study, CIBERSORT was used to calculate the composition of immune cells in CD and healthy samples. The algorithm used the LM22 signature and performed 1000 permutations.
Analysis of correlations and differences between immune-infiltrated cells
For the assessment of the correlation between different immune cells, Pearson correlation coefficient was obtained from the sample data screened by CIBERSORT, p < 0.05. A rank sum test was used to compare the CD group with the control group.
Correlation between key CD-FRGs and immune-infiltrated cells in CD
Spearman correlation analysis was performed between the component profiles of GSE16879 immune-infiltrated cells analyzed by CIBERSORT and the gene expression profiles of this dataset. Screening and determination of correlation between key CD-FRG and immune-infiltrated cells using Spearman’s correlation coefficient (r) > 0.6 and p < 0.05.
Statistical analysis
Statistical analysis and graphs were performed using Sangerbox online software (http://sangerbox.com/) and GraphPad Prism 6.0 software. Fold changes greater than 2 or less than 0.5 were considered to be significantly differentially expressed. The p-value was corrected by controlling the false discovery rate (FDR), and the adjusted p-value (Q-value = FDR) was taken to be < 0.05. Correlation coefficient r-values > 0.5 were considered to have moderate and above correlation and p < 0.05.
Results
Identification of DE-FRGs in CD
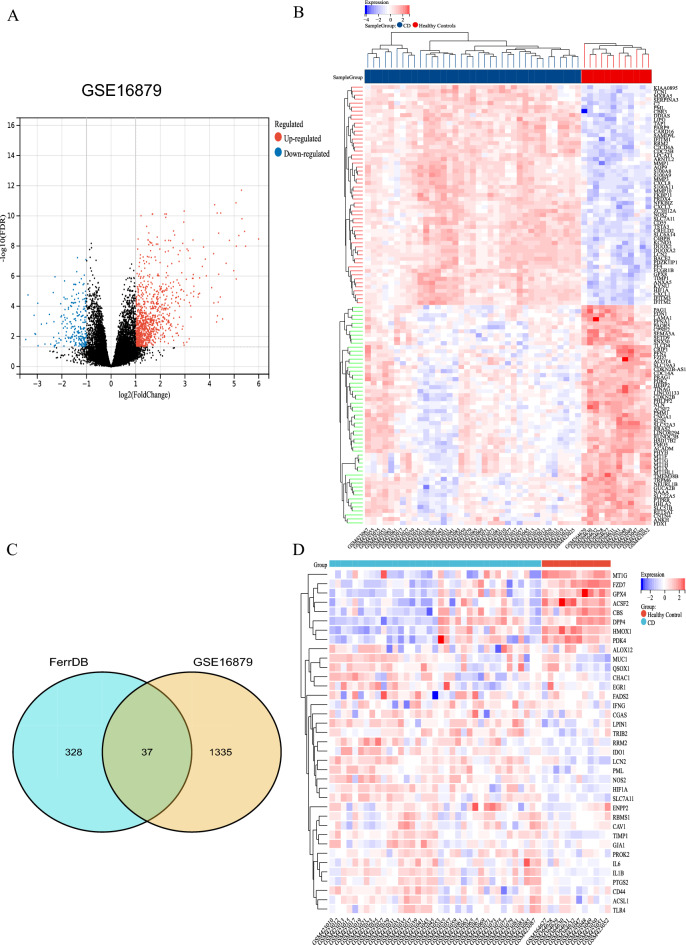
The GSE16879 dataset was retrieved from the GEO database, which contained gene expression profiles from 12 healthy intestinal and 37 CD intestinal biopsy tissue samples. After normalization of microarray results, 1372 DEGs were identified, including 1082 up-regulated genes and 290 down-regulated genes (Fig. 1A,B). A total of 365 FRGs with experimentally validated FRGs were retrieved from FerrDb, a database of regulators, markers and diseases implicated in ferroptosis. After overlapping DEGs and FRGs to take the intersection, a total of 37 FRGs were identified as DE-FRGs (Fig. 1C). Figure 1D showed the expression of 37 DE-FRGs in the CD dataset GSE16879.
Figure 1.
Screening of Differentially Expressed Ferroptosis-Related Genes (DE-FRGs). (A) Volcano plots showing significantly differentially expressed genes. |Log2FC|≥ l and P < 0.05 were taken for filtering. Blue dots indicate genes significantly down-regulated, red dots indicate genes significantly up-regulated, and black dots indicate genes without any significant differences shown. (B) Heatmap showing the expression of DEGs in GSE16879. Blue indicates lower gene expression and red indicates higher gene expression. (C) Display of DE-FRG by Venn diagram. (D) Heatmap showing the expressions of the 37 DE-FRGs in CD dataset GSE16879.
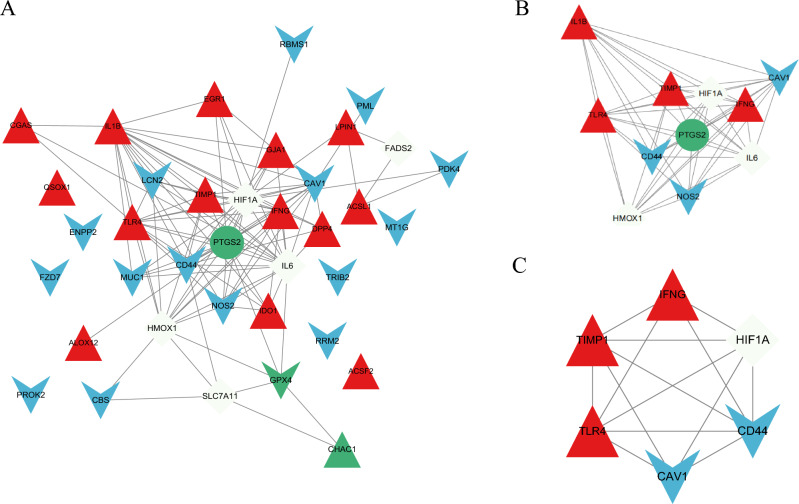
Construction of PPI network, analysis of modules and identification of key CD-FRGs
The PPI construction for DE-FRGs was performed with the STRING database and the results were subsequently visualized with Cytoscape (Fig. 2A). The MCODE plug-in recognized the most densely connected regions (11 nodes and 51 edges) of the PPI network (Fig. 2B). The top 10 genes were obtained by 6 algorithms of Cytoscape plug-in cytoHubba (Table 1). Subsequently, the final six crossover genes obtained by overlapping the results derived from the MCC, DNMC, MNC, Degree, Closeness and EPC algorithms were chosen as the key CD-FRGs, which included CAV1, CD44, HIF1A, IFNG, TIMP1 and TLR4 (Fig. 2C). Of note, all these key CD-FRGs were upregulated in patients with CD. Table 2 indicated the details of these 6 key CD-FRGs.
Figure 2.
Construction and module analysis of PPI network. (A) PPI network of DE-FRGs (37 nodes and 106 edges). (B) Identification of the most densely connected regions (11 nodes and 51 edges) in PPI network through MCODE. (C) Identification of key CD-FRGs by 6 algorithms of cytoscape plugin cytoHubba. Red triangle indicates the driver of ferroptosis, blue V indicates the suppressor of ferroptosis, green circle indicates the marker of ferroptosis, green triangle both driver and marker of ferroptosis, green V indicates both suppressor and marker of ferroptosis, white diamond indicates the both driver and suppressor of ferroptosis.
Table 1.
The top 10 hub genes rank in cytoHubba.
| MCC | DNMC | MNC | Degree | EPC | Closeness | Overlap |
|---|---|---|---|---|---|---|
| CAV1 | CAV1 | CAV1 | CAV1 | CAV1 | CAV1 | CAV1 |
| CD44 | CD44 | CD44 | CD44 | CD44 | CD44 | CD44 |
| HIF1A | GJA1 | HIF1A | HIF1A | HIF1A | HIF1A | HIF1A |
| HMOX1 | HIF1A | HMOX1 | HMOX1 | HMOX1 | HMOX1 | IFNG |
| IFNG | IDO1 | IFNG | IFNG | IFNG | IFNG | TIMP1 |
| IL1B | IFNG | IL1B | IL1B | IL1B | IL1B | TLR4 |
| IL6 | LCN2 | IL6 | IL6 | IL6 | IL6 | |
| PTGS2 | NOS2 | PTGS2 | PTGS2 | PTGS2 | PTGS2 | |
| TIMP1 | TIMP1 | TIMP1 | TIMP1 | TIMP1 | TIMP1 | |
| TLR4 | TLR4 | TLR4 | TLR4 | TLR4 | TLR4 |
Table 2.
The top 6 hub genes and their functions.
| Gene symbol | Description | Function |
|---|---|---|
| CAV1 | Caveolin-1 | May act as a scaffolding protein within caveolar membranes. Interacts directly with G-protein alpha subunits and can functionally regulate their activity |
| CD44 | CD44 antigen | Receptor for hyaluronic acid (HA). Adhesion with HA plays an important role in cell migration, tumor growth and progression |
| HIF1A | Hypoxia-inducible factor 1-alpha | Functions as a master transcriptional regulator of the adaptive response to hypoxia |
| IFNG | Interferon gamma | Produced by lymphocytes activated by specific antigens or mitogens. IFN-gamma, in addition to having antiviral activity, has important immunoregulatory functions |
| TIMP1 | Metalloproteinase inhibitor 1 | Metalloproteinase inhibitor that functions by forming one to one complexes with target metalloproteinases, such as collagenases, and irreversibly inactivates them by binding to their catalytic zinc cofactor |
| TLR4 | Toll-like receptor 4 | Cooperates with LY96 and CD14 to mediate the innate immune response to bacterial lipopolysaccharide (LPS). Acts via MYD88, TIRAP and TRAF6, leading to NF-kappa-B activation, cytokine secretion and the inflammatory response |
Validation of the key CD-FRGs expression in CD
We selected two additional microarray datasets (GSE59071 and GSE95095) for validation to determine whether key CD-FRGs are differentially expressed in the CD datasets. A total of 751 DEGs were found in GSE59071 and 11,480 DEGs were found in GSE95095 (Fig. 3A,B). In the datasets GSE59071 and GSE95095, the 6 previously screened key CD-FRGs were validated to be upregulated DEGs.
Figure 3.
Validation of expression of key CD-FRGs in CD. (A,B) DEGs from the GSE59071 and GSE95095 datasets are shown, respectively. red dots represent up-regulated genes, blue dots represent down-regulated genes, and black dots indicate genes without any significant differences shown.
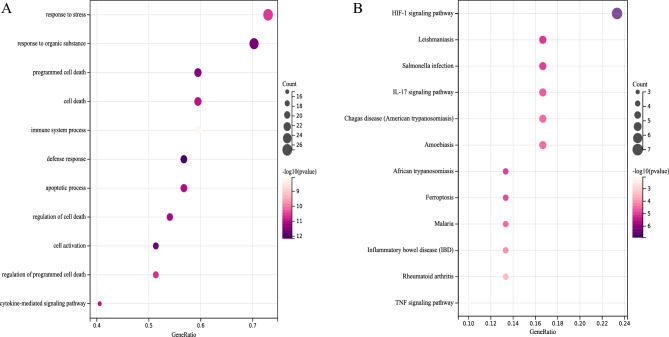
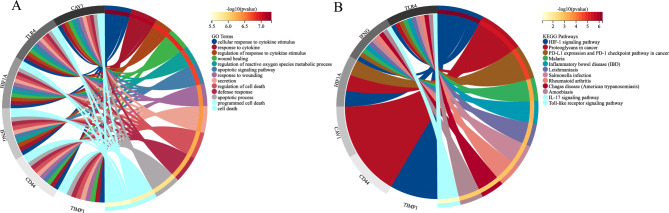
Functional enrichment of DE-FRGs and key CD-FRGs
For predicting the biological functions of DE-FRGs, functional enrichment analysis was performed. GO analysis indicated that DE-FRGs were enriched mainly in programmed cell death, immune system process, apoptotic process and regulation of cell death (Fig. 4A); whereas KEGG pathway analysis indicated DE-FRGs were significantly enriched in HIF-1 signaling pathway, IL-17 signaling pathway, Ferroptosis and IBD (Fig. 4B). GO analysis revealed that key CD-FRGs were enriched significantly in regulation of reactive oxygen species metabolic process, programmed cell death and apoptotic process (Fig. 5A); whereas KEGG pathway analysis indicated key CD-FRGs were significantly enriched in HIF-1 signaling pathway and IBD (Fig. 5B). The results of functional enrichment analysis obtained for GO terms or KEGG revealed that these CD genes were associated with either programmed cell death or IBD.
Figure 4.
Functional enrichment analysis for DE-FRGs associated with CD. (A) Bubble diagram showing DE-FRG enriched with GO terms. (B) Bubble diagram showing DE-FRG enriched with KEGG pathways22–24. The darker the color and the larger the bubbles, the more significant the difference.
Figure 5.
Functional enrichment analysis of key CD-FRGs related to CD. (A) GO terms of key CD-FRGs. (B) KEGG enrichment22–24 of key CD-FRGs. Genes are linked by colored bands to their assigned pathway terms and sorted according to the observed log10 P values, which are shown in decreasing intensity of the red squares next to the selected genes.
Distribution of immune-infiltrated cells
Screening of the microarray with CIBERSORT inverse convolution method at P < 0.05 resulted in 12 healthy intestinal tissues and 37 CD intestinal tissue groups on the heat map. Memory CD4 + T cells activated, M1 macrophages, M2 macrophages, Mast cells activated and Neutrophils were all more abundant in intestinal tissues of patients with CD than in healthy controls, while CD8 + T cells, regulatory T cells (Tregs), Eosinophils and resting NK cells were less expressed in the intestinal tissues of CD patients than in the healthy control group (Fig. 6A). The distribution of the 22 immune cells in each sample was detailed in Fig. 6B.
Figure 6.
Infiltration of immune-related cells in CD and healthy control samples. (A) Amount of immune cells in each sample. (B) Relative percentages of 22 immune cell subpopulations from CD samples in the GSE16879 dataset.
Analysis of the correlation and differences in immune-infiltrated cells
A positive correlation was found between memory resting CD4 + T cells and resting Dendritic cells (r = 0.72, p < 0.0001), activated Mast cells and activated dendritic cells (r = 0.58, p < 0.001), as well as activated Mast cells and Neutrophils (r = 0.52, p < 0.01) (Fig. 7A). Instead, a negative correlation was detected between naïve CD4 + T cells and memory resting CD4 + T cells (r = − 0.66, p < 0.0001), follicular helper T cells and Neutrophils (r = − 0.65, p < 0.0001), activated dendritic cells and resting dendritic cells (r = − 0.51, p < 0.01), as well as Mast cells resting and Mast cells activated (r = − 0.78, p < 0.0001) (Fig. 7A).
Figure 7.
Differences and correlation analysis of immune cells in CD. (A) Correlation analysis of immune cells in CD. Green indicates positive correlation, blue indicates negative correlation; the higher the absolute value, the stronger the correlation between immune cells. (B) Violin diagram displaying the proportion of differences between healthy and CD samples for each immune cell type; light green corresponds to healthy samples and pink to CD samples, P < 0.05.
The differences in immune-infiltrated cells were shown by violin plots between the intestinal tissues of healthy individuals and CD patients and were statistically significant at p < 0.05. Activated memory CD4 + T cells, M1 macrophages, M2 macrophages, Mast cells activated and Neutrophils were differentially elevated in the intestinal tissues of CD patients, in contrast to CD8 + T cells, Tregs, Eosinophils and resting NK cells (Fig. 7B).
Correlation between key CD-FRGs and immune-infiltrated cells in CD
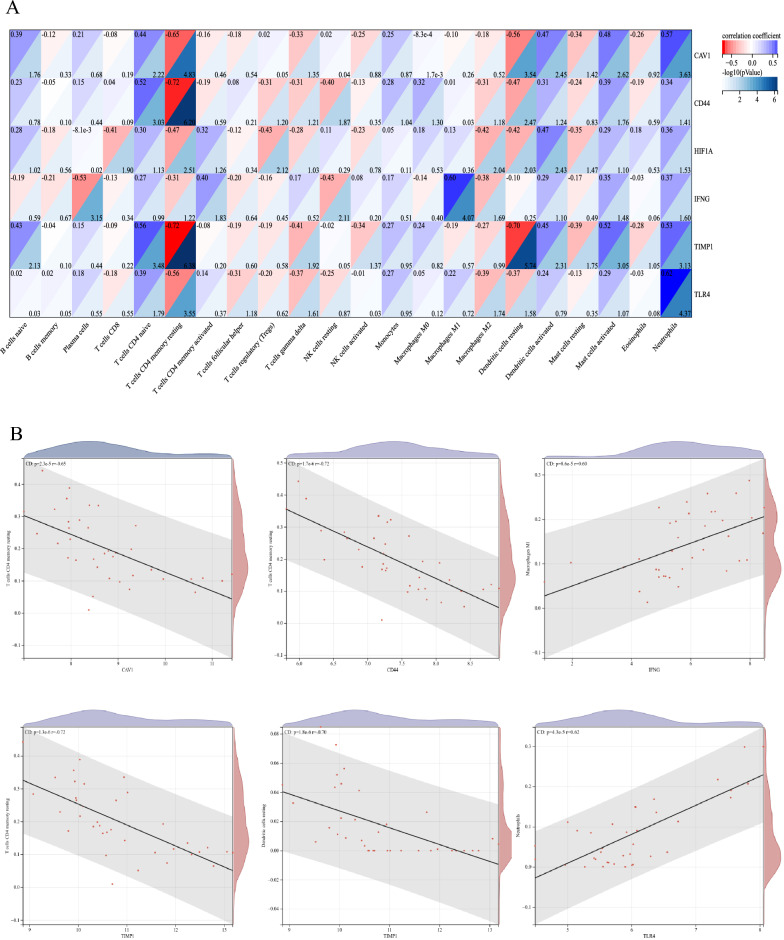
The correlation between key CD-FRGs and immune-infiltrated cells in CD, which differed between CD and healthy controls, was performed by Spearman correlation (Fig. 8). Memory resting CD4 + T cells exhibited a negative correlation with CAV1 (r = − 0.65, p = 2.3e − 5). Memory resting CD4 + T cells displayed a negative correlation with CD44 (r = − 0.72, p = 1.7e − 6). Memory resting CD4 + T cells showed a negative correlation with TIMP1 (r = − 0.72, p = 1.3e − 6). Neutrophils exhibited a positive correlation with TLR4 (r = 0.62, p = 4.3e − 5). M1 macrophages exhibited a positive correlation with IFNG (r = 0.60, p = 8.6e − 5). Resting dendritic cells displayed a negative correlation with TIMP1 (r = − 0.70, p = 1.8e − 6). Accordingly, these genes were closely correlated to the immune-infiltrated cells in CD.
Figure 8.
Correlation between key CD-FRG and immune-infiltrated cells in CD. (A) The darker the blue hue, the smaller the P value. (B) Correlation analysis of key CD-FRG and immune-infiltrated cells, P < 0.05.
Discussion
Bioinformatics has not only helped to broaden the field of complicated multigene diseases, but has also helped to determine several genes that cause CD, which has provided novel insights into CD pathogenesis. In the present study, total of 37 DE-FRGs were found to be significantly expressed in CD intestinal tissues, whereas 6 key CD-FRGs were found, but further studies are still needed to investigate how or to what extent they participate in the pathogenesis of CD. Functional enrichment analysis of GO terms indicated that DE-FRGs were enriched mainly in programmed cell death, immune system process, apoptotic process, and regulation of cell death. KEGG pathway analysis indicated that DE-FRGs were mainly enriched in HIF-1 signaling pathway, IL-17 signaling pathway, Ferroptosis and IBD. Among the PPI network of DE-FRGs, high scores were obtained for 6 of the 37 genes (CAV1, CD44, HIF1A, IFNG, TIMP1 and TLR4) in the 6 algorithms of cytoHubba. GO terms analysis revealed that these 6 key CD-FRGs were highly enriched in regulation of reactive oxygen species metabolic process, programmed cell death and apoptotic process, as well as in KEGG of HIF-1 signaling pathway and IBD. Defective apoptosis has long been thought to be associated with IBD, and the cause of CD pathogenesis is thought to be an excessive T-cell response due to an intrinsic defect in the T-cell apoptosis pathway26. Impaired regulation of cell death will disrupt the intestinal epithelial cell barrier and trigger a range of intestinal diseases, including CD27. The important role of IL-17 signaling pathway in CD has been demonstrated by numerous studies6,28. It has been demonstrated that HIF-1α stabilizers can be used to treat IBD29. These results for both GO terms and the KEGG pathway indicated that the DE-FRGs or key CD-FRGs found in the present study may be involved inin the progression of CD through the above pathways.
Caveolin-1 (CAV1) is a scaffold structural protein of caveolae involved in the regulation of angiogenesis, cellular signaling pathways and inflammation mediated by endothelial cells30. Caveolin-1 has been shown to inhibit fibroblast autophagy by regulating Sequestosome 1, which decreases intestinal fibrosis in CD31. As a suppressor of ferroptosis, CAV1 has been shown to indicate ferroptosis in acute immune-mediated liver injury by attenuating nitrogen stress32. Therefore, it is speculated that CAV1 may affect intestinal fibrosis in CD by alleviating ferroptosis, but further studies are still needed to validate it.
CD44 (CD44 Molecule) is a cell surface glycoprotein which has long been researched as a cancer molecule owing to its vital function in the biological activities of normal cells and in the pathological actions of cancer cells (e.g., cell proliferation, adhesion, and migration)33. Liu et al. revealed that CD44 expression inhibited ferroptosis in cancer cells in an OTUB1-dependent manner34. It has been suggested that the absence of specific CD44 variable splice exons in macrophages prevents CD35. It remains to be investigated whether CD44, a ferroptosis inhibitor, could exacerbate CD by reducing ferroptosis in CD.
Hypoxia-inducible factor 1-alpha (HIF1A) serves as a transcription factor that modulates cellular adaptation to hypoxia levels and provides support for development and function of the intestinal barrier36. The study by Mimouna et al. demonstrated that co-activation of autophagy and HIF1A expression may be a new approach to address adherent invasive E. coli (AIEC) infection in CD patients37. HIF1A has been shown to be not only a driver38 but also an inhibitor39 of ferroptosis. Thus, it is crucial to maintain the balance of HIF1A in CD to control ferroptosis in CD..
Interferon gamma (IFN-γ, namely IFNG) is a pro-inflammatory factor which regulates numerous immune-related genes40. Interferon γ (IFN-γ) released from CD8 + T cells impairs the uptake of cystine by tumor cells, thereby promoting lipid peroxidation and ferroptosis in tumor cells41. IFNN has long been thought to be a driver of inflammatory factors in CD and to exacerbate its course42,43, and coupled with its driving role in ferroptosis, we speculated that it may promote CD by exacerbating ferroptosis.
Tissue inhibitor of metalloproteinase 1 (TIMP1) is one of the four members of the glycoproteome (TIMP1-4), whose main feature is to mediate extracellular matrix turnover. Since the main cellular sources of TIMP-1 are macrophages and fibroblasts, regulation of these cells is essential for the prevention of colonic fibrosis in patients with CD44,45. Shi et al. demonstrated that silencing TIMP1 inhibited ferroptosis in cardiac microvascular endothelial cells through downregulation of transferrin receptor 1 (TFR-1)46. However, whether TIMP1 can be involved in CD progression by affecting ferroptosis in fibroblasts remains undescribed and needs to be further explored.
Toll-like receptor 4 (TLR4) is thought to play a key role in the pathogenesis of IBD and has been identified as an effective target for the treatment of IBD47,48. It has been demonstrated that knockdown of TLR4 significantly improves left ventricular remodeling in heart failure by inhibiting ferroptosis in cardiomyocytes49. Therefore, we venture to speculated that TLR4 may be able to exacerbate CD by promoting ferroptosis in intestinal cells.
Abnormal immune cell infiltration is a hallmark of CD50. Abnormal infiltration of immune cells is closely related to ferroptosis in intestinal tissues, and a study by Tang et al. found that intervention with OTSSP167, a selective inhibitor of MELK, significantly inhibited ferroptosis in intestinal tissues and suppressed macrophage infiltration and M1 polarization, thereby reducing the secretion of pro-inflammatory factors51. Earlier studies have shown that soluble caveolin-1-Ig inhibits T cell proliferation and cytokine production in response to recall antigen or alloantigen-presenting cells (APC), and in combination with our study, CAV1 was negatively correlated with memory resting CD4 + T cells, suggesting that CAV1 may be involved in CD progression by inhibiting T cell proliferation52.CD44 has been shown to block apoptosis of CD4 + T cells that activate colitis35,53. The inhibitor of ferroptosis, CD44, was negatively correlated with memory resting CD4 + T cells in the present study, which may suggest that CD44 exacerbates CD in the intestine by inhibiting apoptosis or ferroptosis of CD4 + T cells. It has been demonstrated that the microenvironment of IFN-γ polarizes macrophages and induces intestinal Epithelial-mesenchymal transition (EMT) affecting CD fibrosis54, which in addition combined with the positive correlation of IFNG with macrophage M1 in our study may suggest that IFNG could be involved in the pathogenesis of CD through macrophages. One study showed that the anti-inflammatory factor interleukin-10 can act on dendritic cells and induce their production of TIMP155, with our study, this suggests that TIMP1 and dendritic cells may interact to affect CD. Li et al. found that ferroptosis coordinates neutrophil recruitment to damaged myocardium by promoting neutrophils adhesion to coronary endothelial cells via the TLR4/TRIF/I type IFN signaling pathway56, and combined with the positive association of TLR4 and neutrophils in our study, suggests that TLR4 may affect CD by regulating ferroptosis of neutrophils in the intestine. However, how HIF1A mediates ferroptosis causing CD through these immune infiltrated cells still needs to be explored. Experiments with these CD-FRGs specific immune cell knock-out may contribute to unraveling the underlying mechanisms in the future.
There are also some limitations in this study. First, it was a secondary mining and analysis of previously published datasets. Thus, the results of the experiments may vary from the findings of earlier experiments, probably due to bias in the data analysis because of a small sample size or other reasons. Second, since the CIBERSORT deconvolution algorithm relies on limited genetic data, different diseases have variability in susceptibility factors and disease phenotypes, which will lead to inaccurate results. Despite this, our study could still provide compelling evidence and new research ideas for further investigation of the role of these immune-infiltrated cells or immune-related genes in ferroptosis of CD.
Conclusion
Taken together, immune cells with abnormal infiltration can be implicated in CD due to ferroptosis. This study identified 6 key CD-FRGs that may be key biomarkers of ferroptosis in CD; they include CAV1, CD44, HIF1A, IFNG, TIMP1 and TLR4. These findings suggest that the immune response is critical in CD caused by ferroptosis through the interaction between key CD-FRGs and immune infiltrating cells.
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Author contributions
X.T. and W.H. participated in the design of the study, carried out the study,performed the statistical analysis, and drafted the manuscript. Y.W. helped to carried out the study. T.F. reviewed and edited the manuscript. All authors read and approved the final manuscript.
Data availability
The datasets generated and/or analyzed during the current study are available in the [GEO] repository, [https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE16879/GSE59071/GSE95095].
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xiaoting Tang and Weitao Hu.
References
- 1.Roda G, et al. Crohn's disease. Nat. Rev. Dis. Primers. 2020;6(1):22. doi: 10.1038/s41572-020-0156-2. [DOI] [PubMed] [Google Scholar]
- 2.Torres J, et al. Crohn's disease. Lancet. 2017;389(10080):1741–1755. doi: 10.1016/S0140-6736(16)31711-1. [DOI] [PubMed] [Google Scholar]
- 3.Ananthakrishnan AN, et al. Environmental triggers in IBD: A review of progress and evidence. Nat. Rev. Gastroenterol. Hepatol. 2018;15(1):39–49. doi: 10.1038/nrgastro.2017.136. [DOI] [PubMed] [Google Scholar]
- 4.Feagan BG, et al. Ustekinumab as induction and maintenance therapy for Crohn's Disease. N. Engl. J. Med. 2016;375(20):1946–1960. doi: 10.1056/NEJMoa1602773. [DOI] [PubMed] [Google Scholar]
- 5.Dulai PS, et al. The real-world effectiveness and safety of vedolizumab for moderate-severe Crohn's disease: Results from the US VICTORY consortium. Am. J. Gastroenterol. 2016;111(8):1147–1155. doi: 10.1038/ajg.2016.236. [DOI] [PubMed] [Google Scholar]
- 6.Schmitt H, Neurath MF, Atreya R. Role of the IL23/IL17 pathway in Crohn's Disease. Front. Immunol. 2021;12:622934. doi: 10.3389/fimmu.2021.622934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nunes T, Bernardazzi C, de Souza HS. Cell death and inflammatory bowel diseases: Apoptosis, necrosis, and autophagy in the intestinal epithelium. Biomed. Res. Int. 2014;2014:218493. doi: 10.1155/2014/218493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunther C, et al. Apoptosis, necrosis and necroptosis: cell death regulation in the intestinal epithelium. Gut. 2013;62(7):1062–1071. doi: 10.1136/gutjnl-2011-301364. [DOI] [PubMed] [Google Scholar]
- 9.Azzman N. Crohn's disease: Potential drugs for modulation of autophagy. Medicina (Kaunas) 2019;55(6):224. doi: 10.3390/medicina55060224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao W, Zhang T, Wu H. Emerging pathological engagement of ferroptosis in gut diseases. Oxid. Med. Cell Longev. 2021;2021:4246255. doi: 10.1155/2021/4246255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang X, Stockwell BR, Conrad M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021;22(4):266–282. doi: 10.1038/s41580-020-00324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu J, et al. Ferrostatin-1 alleviated TNBS induced colitis via the inhibition of ferroptosis. Biochem. Biophys. Res. Commun. 2021;573:48–54. doi: 10.1016/j.bbrc.2021.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Zhang D, et al. Evidence of pyroptosis and ferroptosis extensively involved in autoimmune diseases at the single-cell transcriptome level. J. Transl. Med. 2022;20(1):363. doi: 10.1186/s12967-022-03566-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayr L, et al. Dietary lipids fuel GPX4-restricted enteritis resembling Crohn's disease. Nat. Commun. 2020;11(1):1775. doi: 10.1038/s41467-020-15646-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30(1):207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arijs I, et al. Mucosal gene expression of antimicrobial peptides in inflammatory bowel disease before and after first infliximab treatment. PLoS ONE. 2009;4(11):e7984. doi: 10.1371/journal.pone.0007984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou N, Bao J. FerrDb: A manually curated resource for regulators and markers of ferroptosis and ferroptosis-disease associations. Database (Oxford) 2020 doi: 10.1093/database/baaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vanhove W, et al. Strong upregulation of AIM2 and IFI16 inflammasomes in the mucosa of patients with active inflammatory bowel disease. Inflamm. Bowel Dis. 2015;21(11):2673–2682. doi: 10.1097/MIB.0000000000000535. [DOI] [PubMed] [Google Scholar]
- 19.Franceschini A, et al. STRING v9.1: Protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013;41:D808–15. doi: 10.1093/nar/gks1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smoot ME, et al. Cytoscape 2.8: new features for data integration and network visualization. Bioinformatics. 2011;27(3):431–2. doi: 10.1093/bioinformatics/btq675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bandettini WP, et al. MultiContrast Delayed Enhancement (MCODE) improves detection of subendocardial myocardial infarction by late gadolinium enhancement cardiovascular magnetic resonance: A clinical validation study. J. Cardiovasc. Magn. Reson. 2012;14:83. doi: 10.1186/1532-429X-14-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanehisa M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 2019;28(11):1947–1951. doi: 10.1002/pro.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanehisa M, et al. KEGG: Integrating viruses and cellular organisms. Nucleic Acids Res. 2021;49(D1):D545–D551. doi: 10.1093/nar/gkaa970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newman AM, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods. 2015;12(5):453–457. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verstege MI, te Velde AA, Hommes DW. Apoptosis as a therapeutic paradigm in inflammatory bowel diseases. Acta Gastroenterol. Belg. 2006;69(4):406–412. [PubMed] [Google Scholar]
- 27.Foerster EG, et al. How autophagy controls the intestinal epithelial barrier. Autophagy. 2022;18(1):86–103. doi: 10.1080/15548627.2021.1909406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nirula A, et al. Effect of IL-17 receptor A blockade with brodalumab in inflammatory diseases. Rheumatology (Oxford) 2016;55(suppl 2):ii43–ii55. doi: 10.1093/rheumatology/kew346. [DOI] [PubMed] [Google Scholar]
- 29.Bhat S, Rieder F. Hypoxia-Inducible factor 1-alpha stabilizers in the treatment of inflammatory bowel diseases: Oxygen as a novel IBD therapy? J. Crohns Colitis. 2022 doi: 10.1093/ecco-jcc/jjac092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chidlow JH, et al. Endothelial caveolin-1 regulates pathologic angiogenesis in a mouse model of colitis. Gastroenterology. 2009;136(2):575–84 e2. doi: 10.1053/j.gastro.2008.10.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu M, et al. Caveolin-1 alleviates Crohn's disease-induced intestinal fibrosis by inhibiting fibroblasts autophagy through modulating sequestosome 1. Inflamm. Bowel Dis. 2022;28(6):923–935. doi: 10.1093/ibd/izab342. [DOI] [PubMed] [Google Scholar]
- 32.Deng G, et al. Caveolin-1 dictates ferroptosis in the execution of acute immune-mediated hepatic damage by attenuating nitrogen stress. Free Radic. Biol. Med. 2020;148:151–161. doi: 10.1016/j.freeradbiomed.2019.12.026. [DOI] [PubMed] [Google Scholar]
- 33.Weng X, et al. The membrane receptor CD44: Novel insights into metabolism. Trends Endocrinol. Metab. 2022;33(5):318–332. doi: 10.1016/j.tem.2022.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Liu T, et al. The deubiquitylase OTUB1 mediates ferroptosis via stabilization of SLC7A11. Cancer Res. 2019;79(8):1913–1924. doi: 10.1158/0008-5472.CAN-18-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wittig BM, et al. Absence of specific alternatively spliced exon of CD44 in macrophages prevents colitis. Mucosal. Immunol. 2018;11(3):846–860. doi: 10.1038/mi.2017.98. [DOI] [PubMed] [Google Scholar]
- 36.Yin J, et al. The role of hypoxia-inducible factor 1-alpha in inflammatory bowel disease. Cell Biol. Int. 2022;46(1):46–51. doi: 10.1002/cbin.11712. [DOI] [PubMed] [Google Scholar]
- 37.Mimouna S, et al. HIF1A regulates xenophagic degradation of adherent and invasive Escherichia coli (AIEC) Autophagy. 2014;10(12):2333–2345. doi: 10.4161/15548627.2014.984275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zou Y, et al. A GPX4-dependent cancer cell state underlies the clear-cell morphology and confers sensitivity to ferroptosis. Nat. Commun. 2019;10(1):1617. doi: 10.1038/s41467-019-09277-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang M, et al. Clockophagy is a novel selective autophagy process favoring ferroptosis. Sci. Adv. 2019;5(7):eaaw2238. doi: 10.1126/sciadv.aaw2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mandai M, et al. Dual faces of IFNgamma in cancer progression: A role of PD-L1 induction in the determination of pro- and antitumor immunity. Clin. Cancer Res. 2016;22(10):2329–2334. doi: 10.1158/1078-0432.CCR-16-0224. [DOI] [PubMed] [Google Scholar]
- 41.Wang W, et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569(7755):270–274. doi: 10.1038/s41586-019-1170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat. Rev. Immunol. 2003;3(7):521–533. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 43.Strober W, et al. Proinflammatory cytokines underlying the inflammation of Crohn's disease. Curr. Opin. Gastroenterol. 2010;26(4):310–317. doi: 10.1097/MOG.0b013e328339d099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Talapka P, et al. Structural and molecular features of intestinal strictures in rats with Crohn's-like disease. World J. Gastroenterol. 2016;22(22):5154–5164. doi: 10.3748/wjg.v22.i22.5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suga H, et al. Tracking the elusive fibrocyte: Identification and characterization of collagen-producing hematopoietic lineage cells during murine wound healing. Stem Cells. 2014;32(5):1347–1360. doi: 10.1002/stem.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi P, et al. Neutrophil-like cell membrane-coated siRNA of lncRNA AABR07017145.1 therapy for cardiac hypertrophy via inhibiting ferroptosis of CMECs. Mol. Ther. Nucleic Acids. 2022;27:16–36. doi: 10.1016/j.omtn.2021.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dai W, et al. Phytochemicals targeting Toll-like receptors 4 (TLR4) in inflammatory bowel disease. Chin. Med. 2022;17(1):53. doi: 10.1186/s13020-022-00611-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tam JSY, et al. Toll-like receptor 4 (TLR4) antagonists as potential therapeutics for intestinal inflammation. Indian J. Gastroenterol. 2021;40(1):5–21. doi: 10.1007/s12664-020-01114-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen X, et al. Role of TLR4/NADPH oxidase 4 pathway in promoting cell death through autophagy and ferroptosis during heart failure. Biochem. Biophys. Res. Commun. 2019;516(1):37–43. doi: 10.1016/j.bbrc.2019.06.015. [DOI] [PubMed] [Google Scholar]
- 50.Yu B, et al. Diagnostic and predictive value of immune-related genes in Crohn's Disease. Front. Immunol. 2021;12:643036. doi: 10.3389/fimmu.2021.643036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang B, et al. Pharmacological inhibition of MELK restricts ferroptosis and the inflammatory response in colitis and colitis-propelled carcinogenesis. Free Radic. Biol. Med. 2021;172:312–329. doi: 10.1016/j.freeradbiomed.2021.06.012. [DOI] [PubMed] [Google Scholar]
- 52.Ohnuma K, et al. Blockade of CD26-mediated T cell costimulation with soluble caveolin-1-Ig fusion protein induces anergy in CD4+T cells. Biochem. Biophys. Res. Commun. 2009;386(2):327–332. doi: 10.1016/j.bbrc.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 53.Visekruna A, et al. Intestinal development and homeostasis require activation and apoptosis of diet-reactive T cells. J. Clin. Investig. 2019;129(5):1972–1983. doi: 10.1172/JCI98929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Macias-Ceja DC, et al. IFNgamma-treated macrophages induce EMT through the WNT pathway: Relevance in Crohn's Disease. Biomedicines. 2022;10(5):1093. doi: 10.3390/biomedicines10051093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao L, et al. Efferocytosis fuels malignant pleural effusion through TIMP1. Sci. Adv. 2021 doi: 10.1126/sciadv.abd6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li W, et al. Ferroptotic cell death and TLR4/Trif signaling initiate neutrophil recruitment after heart transplantation. J. Clin. Investig. 2019;129(6):2293–2304. doi: 10.1172/JCI126428. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in the [GEO] repository, [https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE16879/GSE59071/GSE95095].