Abstract
The miR-34a and miR-34b/c encoding genes represent direct targets of the p53 transcription factor, and presumably mediate part of the tumor suppressive effects of p53. Here, we sought to determine their functional relevance by inactivating miR-34a and/or miR-34b/c using a CRISPR/Cas9 approach in the colorectal cancer (CRC) cell line HCT116. Concomitant deletion of miR-34a and miR-34b/c resulted in significantly reduced suppression of proliferation after p53 activation, enhanced migration, invasion and EMT, as well as reduced sensitivity to chemotherapeutics, increased stress-induced autophagic flux, decreased apoptosis and upregulation of autophagy-related genes after 5-FU treatment. However, inactivation of singular miR-34a or miR-34b/c had little effects on the aforementioned processes. RNA-Seq analysis revealed that concomitant deletion of miR-34a/b/c caused EMT signature enrichment, impaired gene repression by the p53-DREAM pathway and elevated autophagy after 5-FU treatment. A gene signature comprised of mRNAs significantly upregulated after combined inactivation of miR-34a and miR-34b/c showed a significant association with the invasive colon cancer subtype CMS4 and poor overall survival in two CRC patient cohorts, and with 5-FU resistance in CRC cell lines. In miR-34a/b/c-deficient cells the upregulated miR-34 target FOXM1 directly induced p62 and ATG9A, which increased autophagy and consequently attenuated apoptosis and rendered the miR-34a/b/c-KO cells more resistant to 5-FU. Inhibition of autophagy by depletion of ATG9A or chloroquine re-sensitized miR-34a/b/c-deficient HCT116 cells to 5-FU. In summary, our findings show a complementary role of miR-34a and miR-34b/c in the regulation of EMT and autophagy which may be relevant for CRC therapy in the future.

Subject terms: Cancer genetics, Experimental models of disease
Introduction
Colorectal cancer (CRC) represents the third most commonly diagnosed cancer type and the second leading cause of cancer death worldwide [1]. While surgery is curative and the first choice for early-stage CRC patients, an estimated 50% to 60% of CRC patients develop colorectal metastases [2], with 80% to 90% of these representing unresectable liver metastases [3]. Fluorouracil (5-FU)-based chemotherapy remains the standard of care for patients with metastatic colorectal cancer (mCRC). However, the overall response rate to 5-FU in mCRC patients is limited to about 50% [4] and resistance to 5-FU inevitably develops [5]. CRC is a complex and heterogeneous disease manifested by distinct epi-genetic and genetic characteristics. Patients with different biological CRC subtypes display a large variation in prognosis and therapy response [6]. Although promising, check-point inhibitor-based or targeted therapy only benefit a fraction of CRC patients [7, 8]. Therefore, there is an urgent need for more profound insights into the molecular mechanisms and gene regulations underlying the response of CRC cells to therapeutic drugs.
MiR-34a and miR-34b/c encoding genes represent direct targets of p53 [9], a tumor suppressive protein that is activated by cellular stresses including DNA damage, oncogene activation and hypoxia [10]. P53 presumably mediates its tumor suppression functions partially through miR-34a/b/c [11]. MiR-34a and miR-34b/c genes are frequently inactivated epigenetically by CpG methylation in CRC [12, 13]. Importantly, combined deletion of Mir34a and Mir34b/c promotes intestinal tumorigenesis and decreases survival in ApcMin/+ mice that inherit a mutant Apc (adenomatous polyposis coli) allele [14]. Furthermore, combined inactivation of Mir34a and p53 promotes colorectal cancer development and progression in mice [15]. In addition, miR-34a suppresses EMT-mediated colorectal cancer invasion and metastasis by inhibiting an IL6R/STAT3/miR-34a feedback loop [16], and miR-34a silencing by DNA-methylation is significantly associated with increased lymph node and liver metastasis in CRC [17]. These findings indicate that miR-34a/b/c have tumor suppressive functions in CRC.
Here, we showed that concomitant inactivation of miR-34a and miR-34b/c in the CRC cell line HCT116 using a CRISPR/Cas9 approach significantly enhanced migration, invasion and EMT, reduced sensitivity to chemotherapeutics and increased stress-induced autophagic flux. RNA-Seq analysis revealed that combined deletion of miR-34a/b/c significantly induced the expression of EMT- and macroautophagy/autophagy-related mRNAs and impaired repression mediated by the DREAM complex. MiR-34a/b/c inhibited autophagy by directly repressing FOXM1 and ATG9A. The downregulation of FOXM1 subsequently repressed p62 and ATG9A, as these represent FOXM1 target genes. In addition, inhibition of autophagy re-sensitized miR-34a/b/c-deficient CRC cells to 5-FU.
Results
Generation and characterization of miR-34a/b/c-deficient HCT116 cell lines
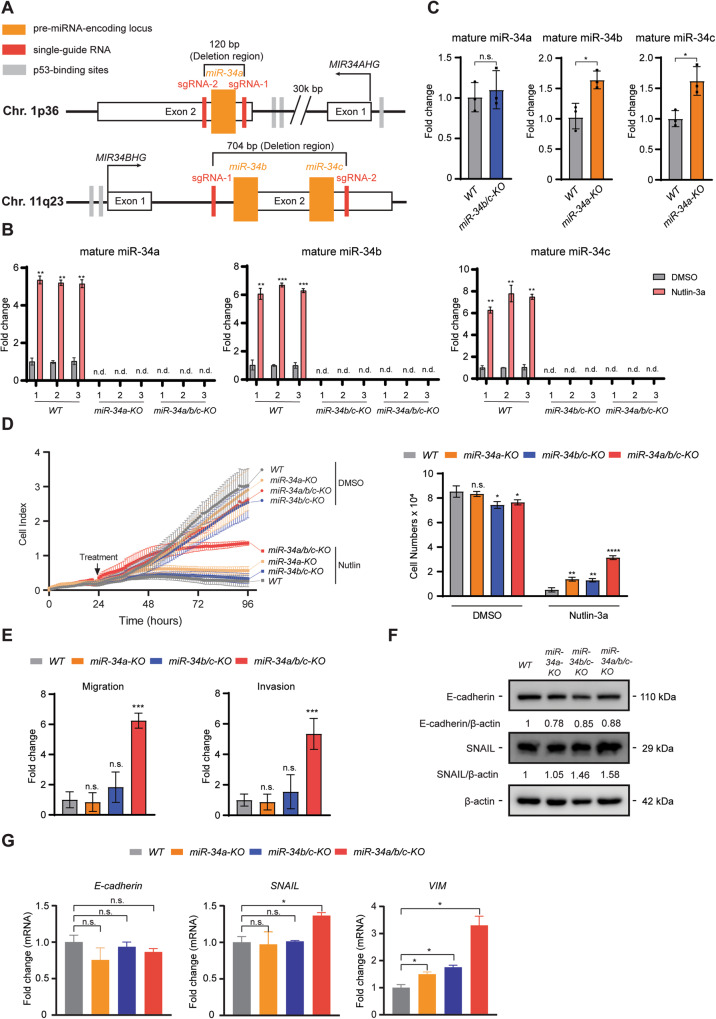
In order to characterize the functions of the three p53-inducible miR-34 family members, the regions encoding the mature miR-34a and miR-34b/c within their host genes were deleted alone or in combination in the CRC cell line HCT116 cell using a CRISPR-Cas9 approach. Single-guide RNAs (sgRNAs) targeting the sequence regions flanking the genomic regions of precursor miR-34a or miR-34b/c are shown in Fig. 1A and Table S1. Three independent single cell derived clones for each genotype (i.e., miR-34a-KO, miR-34b/c-KO and miR-34a/b/c-KO) were obtained. As controls, three independent single cell derived clones for wild-type cells (WT) were also generated by transfecting HCT116 cells with pSpCas9 plasmids not harboring sgRNAs. The deletion of the miR-34a and/or miR-34b/c loci was confirmed by PCR (Fig. S1A–D and Table S2). In addition, the lack of miR-34a and/or miR-34b/c expression was confirmed by qPCR analysis after p53 activation by addition of Nutlin-3a, a highly selective MDM2 inhibitor [18] (Fig. 1B). Interestingly, mature miR-34b and miR-34c showed a significantly elevated expression after miR-34a deletion in HCT116 cells (Fig. 1C), suggesting that the loss of miR-34a functions may be partially compensated by upregulation of miR-34b/c. Deletion of miR-34b/c in HCT116 cells resulted in a marginal increase of mature miR-34a expression (Fig. 1C), presumably since the basal expression levels of miR-34b and miR-34c are relatively low in HCT116 cells when compared to miR-34a [19].
Fig. 1. Deletion of miR-34a/b/c increases cell proliferation, migration and invasion, and promotes EMT in HCT116 cells.
A Schematic illustrations of miR-34a and miR-34b/c genomic location and deletion of the mature miRNA coding regions using a CRISPR-Cas9 approach. MiRNA encoding loci are indicated as orange columns. Single-guide RNAs (sgRNAs) targeted regions are shown as red columns. Gray columns indicate p53-binding sites. B qPCR analysis of mature miR-34 expression after addition of DMSO or 10 μM of Nutlin-3a for 48 h. C qPCR analysis of mature miR-34 expression in miR-34a-KO and miR-34b/c-KO cells. D Proliferation of miR-34-deficient HCT116 cells was determined by real-time cellular impedance assay using an xCELLigence system. Cell numbers were determined at the end point. E Quantification of cellular migration or invasion using Boyden-chamber assays. F Western blot analysis and G qPCR analysis of EMT markers. Results are presented as the mean ± SD (n = 3) for B–E and G with *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, n.s. no significance.
miR-34a/b/c inhibits cellular proliferation in HCT116 cells
Next, we analyzed whether loss of miR-34a/b/c affects cellular proliferation by real-time impedance measurement. Deletion of miR-34a/b/c in HCT116 cells resulted in minor effects on proliferation when compared to WT cells, which however were not consistently statistically significant (Fig. 1D and Fig. S2A). However, when p53 was activated by addition of Nutlin-3a, miR-34-deficient cells were partially refractory to Nutlin-3a when compared to WT cells, with miR-34a/b/c-KO cells displaying the highest rate of proliferation in the presence of Nutlin-3a (Fig. 1D). These results demonstrate that a substantial portion of p53-mediated repression of proliferation is mediated by the combined action of miR-34a/b/c. They also imply that inactivation of a single miR-34 isoform is not sufficient to alleviate inhibition of proliferation by p53.
Loss of miR-34a/b/c promotes EMT, migration and invasion in HCT116 cells
Next, we asked whether miR-34-deficiency affects migration and invasion of HCT116 cells. Indeed, miR-34a/b/c-deficient cells demonstrated significantly increased migration and invasion when compared to WT cells in Boyden-chamber assays (Fig. 1E and Fig. S2B). Increased migration of miR-34a/b/c-KO cells was further confirmed by a wound healing assay with or without treatment of Nutlin-3a (Fig. S2C, D). Since epithelial-mesenchymal transition (EMT) is an important mechanism underlying migration and invasion, EMT markers were tested to determine whether miR-34-deficiency affects EMT. Protein levels of E-cadherin, an epithelial marker, were decreased in miR-34-deficient cells when compared to WT cells, whereas the expression of SNAIL, a mesenchymal marker, was elevated in all miR-34-deficient cells (Fig. 1F). qPCR analysis indicated a significantly higher expression of SNAIL and VIM in miR-34a/b/c-KO cells when compared to WT cells, but for SNAIL not in miR-34a-KO or miR-34b/c-KO cells (Fig. 1G). In addition, mRNA expression of E-cadherin was downregulated in all miR-34-deficent cells, albeit not significantly (Fig. 1G). Therefore, the changes in EMT-related gene expression may at least in part explain the enhanced migration and invasion of miR-34a/b/c-KO cells.
Loss of miR-34a/b/c mediates resistance to chemotherapeutic agents by enhancing autophagic flux
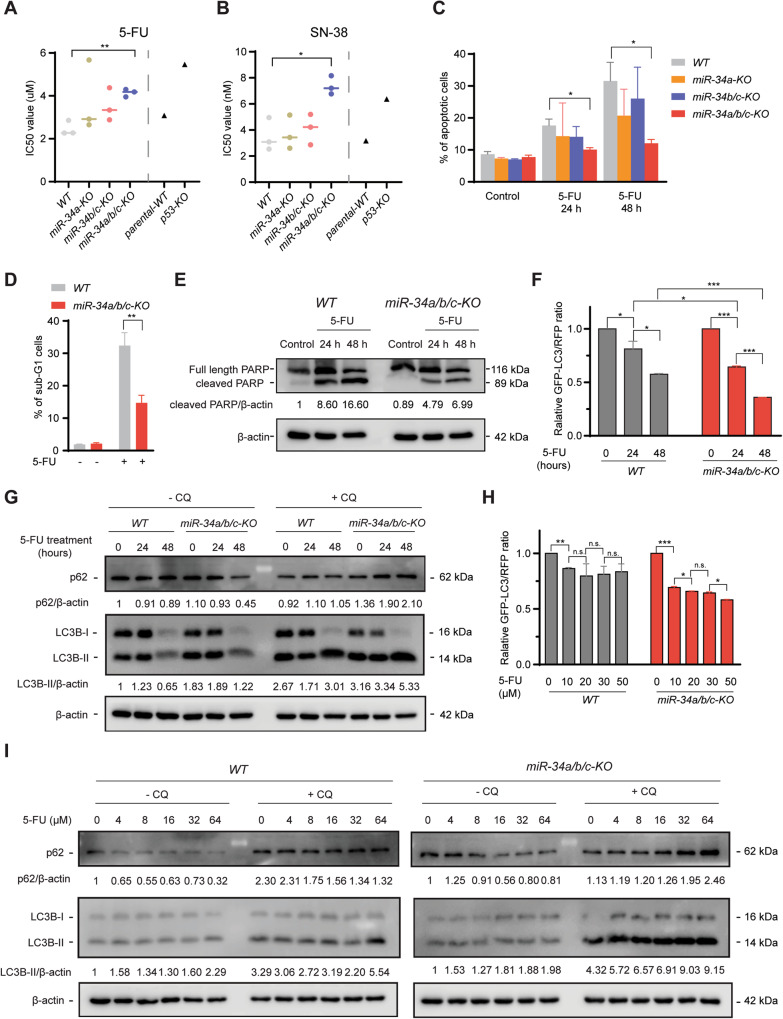
Next, we analyzed whether loss of miR-34 function affects the cellular response to chemotherapeutic agents. 5-FU and SN-38 (the active metabolite of Irinotecan) are widely used chemotherapeutics for treatment of CRC. miR-34-deficient and WT HCT116 cells were treated with 5-FU or SN-38 at a wide range of concentrations, and then subjected to cell viability analysis and IC50 value determination. Intriguingly, only miR-34a/b/c-KO cells displayed a significant, ca. two-fold increase in IC50 values for both 5-FU and SN-38, whereas singular deletion of miR-34a or miR-34b/c resulted in a minor increase (Fig. 2A, B and Fig. S3A, B). P53-KO cells displayed a ca. two-fold increase in IC50 values for both 5-FU and SN-38 when compared to p53-WT cells (Fig. 2A, B and Fig. S3A, B), indicating that only the concomitant loss of miR-34a and miR-34b/c expression has a similar effect as p53-deficiency on the response to chemotherapeutics. As an alternative approach for testing sensitivity towards chemotherapeutic agents, apoptosis was evaluated by detection of Annexin V positive cells using flow cytometry. Only miR-34a/b/c-KO cells displayed a significant reduction in apoptosis when exposed to 5-FU when compared to WT cells (Fig. 2C and Fig. S3C), suggesting that inactivation of both miR-34a and miR-34b/c is required for increased chemo-resistance. The resistance of miR-34a/b/c-KO cells to 5-FU was further corroborated by DNA content analysis using flow cytometry, which showed a decrease of cells in the sub-G1 phase in miR-34a/b/c-KO cells after exposure to 5-FU for 48 h when compared to WT cells (Fig. 2D and Fig. S3D). In addition, miR-34a/b/c-KO cells showed significantly reduced cleavage of PARP, an apoptosis marker, after treatment with 5-FU when compared to WT cells as determined by immunoblotting analysis (Fig. 2E). Taken together, these results indicated the loss of miR-34a/b/c rendered HCT116 cells chemo-resistant by decreasing apoptosis.
Fig. 2. Loss of miR-34a/b/c de-sensitizes HCT116 cells to chemotherapeutic agents by enhancing autophagic flux.
A, B IC50 value determination of HCT116 cells with different miR-34 or p53 genotypes in response to 5-FU or SN-38. The corresponding representative dose-response curves of which the IC50 values were calculated are shown in Fig. S3A, B. Cells were treated with a wide range of concentrations of the indicated therapeutic drugs for 72 h and then subjected to CCK-8 assay and IC50 value determination. C Cells were treated with 5-FU for 24 or 48 h and then apoptosis rates were determined by FITC Annexin V staining and FACS analysis. D Quantification of sub-G1 cell population by FACS analysis after treatment with DMSO or 5-FU for 48 h using PI staining. E Western blot analysis of cleaved-PARP after treatment with DMSO or 5-FU for the indicated periods. F Quantification of GFP-LC3/RFP ratio by FACS analysis of cells stably expressing GFP-LC3-RFP treated with DMSO or 5-FU. Lower GFP-LC3/RFP signal ratio due to increased GFP-LC3 degradation indicates higher autophagic flux. G WT and miR-34a/b/c-KO cells were treated with DMSO or 5-FU for the indicated durations and analyzed by immunoblotting. 20 μM of CQ (chloroquine) was added for 4 h before harvesting cells. H Quantification of GFP-LC3/RFP ratio by FACS analysis of cells stably expressing GFP-LC3-RFP probe treated with DMSO or 5-FU for the indicated concentrations. I Cells were treated with DMSO or 5-FU at the indicated concentrations. 20 μM of CQ (chloroquine) was added for 4 h before harvesting cells. Results are presented as the mean ± SD (n = 3) for A–D and F + H with *p < 0.05, **p < 0.01, ***p < 0.001, n.s. no significance.
Macroautophagy/autophagy, a process in which organelles termed autophagosomes deliver intracellular components to the lysosome for degradation, has been implicated in inhibiting apoptosis and thereby conferring therapy resistance [20]. Autophagy degrades and reduces the abundance of damaged mitochondria and pro-apoptotic proteins (e.g., active caspase 8) to attenuate apoptosis and promote cellular adaptation and survival [21]. Therefore, we asked whether increased autophagy contributes to the chemo-resistance observed in miR-34a/b/c-KO cells. To measure autophagic flux, a measure of autophagic degradation activity, we generated cells stably expressing GFP-LC3-RFP, an established fluorescent autophagic flux probe [22], and used them to estimate autophagy activity by calculating the GFP-LC3/RFP signal ratio. This probe is cleaved into equimolar amounts of GFP-LC3 and RFP by the ATG4 protease and subsequently GFP-LC3 is incorporated into autophagosomes and degraded, while RFP is not degraded by autophagy and remains in the cytosol, thereby serving as an internal control. Consequently, a lower GFP-LC3/RFP signal ratio represents a higher degree of autophagic flux. An example is shown in Fig. S4A, B, where GFP-LC3 signal intensity decreased after 5-FU treatment while RFP intensity remained unchanged. When WT and miR-34a/b/c-KO cells stably expressing a GFP-LC3-RFP probe were treated with 5-FU for 24 or 48 h, it caused a significantly higher level of autophagic flux in miR-34a/b/c-KO cells, since a significantly larger decrease in GFP-LC3/RFP ratio was detected in miR-34a/b/c-KO cells when compared to WT cells (Fig. 2F). As an alternative approach to measure autophagic flux, an immunoblotting analysis was performed to measure the lysosomal turnover of LC3-II, a widely used autophagosome marker, and SQSTM1/p62, a receptor protein for autophagy, in the presence and absence of chloroquine (CQ), a lysosomal inhibitor [23]. In agreement with the results obtained with the GFP-LC3-RFP probe, after treatment of 5-FU for 24 or 48 h, miR-34a/b/c-KO cells displayed a significantly increased turnover of endogenous LC3-II and p62 when compared to WT cells (Fig. 2G), indicating that miR-34a/b/c-KO cells display a higher level of autophagic flux after exposure to 5-FU. In addition, miR-34a/b/c-KO cells consistently displayed an enhanced autophagic flux in response to 5-FU in a dose-dependent manner, whereas WT cells showed less autophagy (Fig. 2H, I). Also higher concentration of 5-FU did not consistently result in increased autophagic flux in WT cells. Collectively, these results show that autophagy is significantly induced in miR-34a/b/c-KO cells after treatment with 5-FU when compared to WT cells. Therefore, autophagy is presumably responsible for the chemo-resistance of miR-34a/b/c-KO cells in response to 5-FU.
Loss of miR-34a/b/c consistently elevates autophagic flux after stress
To investigate whether miR-34a/b/c-KO cells also display a higher autophagic flux when autophagy is induced by alternative means, cells were subjected to starvation of amino acids and serum by cultivation in Earle’s Balanced Salt Solution (EBSS), as well as to treatment of Tunicamycin, which induces ER stress. When cultured in EBSS, miR-34a/b/c-KO cells displayed significantly higher levels of autophagic flux when compared to WT cells, as indicated by the enhanced LC3-II and p62 turnover (Fig. 3A), as well as by the enhanced degradation of GFP-LC3 as shown by FACS analysis (Fig. 3B). Likewise, Tunicamycin treatment also induced significantly higher autophagic flux in miR-34a/b/c-KO cells (Fig. 3C, D). Unexpectedly, accumulation of p62 protein was observed (Fig. 3C). A possible explanation could be a transcriptional activation of p62, which we observed (Fig. S5A). This effect could be mediated by activation of NRF2, a transcription factor that is activated by tunicamycin and is known to regulate p62 [24, 25]. In addition, ectopic expression of pri-miR-34a from an episomal pRTR vector repressed basal autophagy in SW480 (Fig. 3E) and HCT15 cells (Fig. S5B). Since 5-FU also resulted in a significantly elevated autophagic flux in HCT116 p53-KO cells when compared to WT cells (Fig. S5C), the inactivation of miR-34a/b/c at least partially recapitulated the effects of p53 loss on autophagy. Taken together, these results confirmed that miR-34a and miR-34b/c negatively regulate autophagy in HCT116 cells.
Fig. 3. miR-34a/b/c-deficiency increases EBSS- and Tunicamycin-induced autophagic flux, while ectopic expression of miR-34a inhibits autophagy.
A Cells were incubated in complete medium or EBSS for 24 h. 20 μM of chloroquine was added for the last 4 h before Western blot analyses of the indicated proteins. B Cells stably expressing GFP-LC3-RFP were incubated in complete medium or EBSS for 24 h and then subjected to FACS analysis. C WT and miR-34a/b/c-KO cells were treated with DMSO or Tunicamycin for 24 h. 20 μM of chloroquine was added for the last 4 h before Western blot analyses of the indicated proteins. D FACS analysis of cells stably expressing GFP-LC3-RFP treated with DMSO or Tunicamycin for 24 h. E Doxycycline was added as indicated to induce ectopic expression of miR-34a in SW480 cells. 20 μM of chloroquine was added for the last 4 h before Western blot analyses of the indicated proteins. Results are presented as the mean ± SD (n = 3) for B and D with *p < 0.05.
Analysis of mediators of miR-34 function by expression profiling
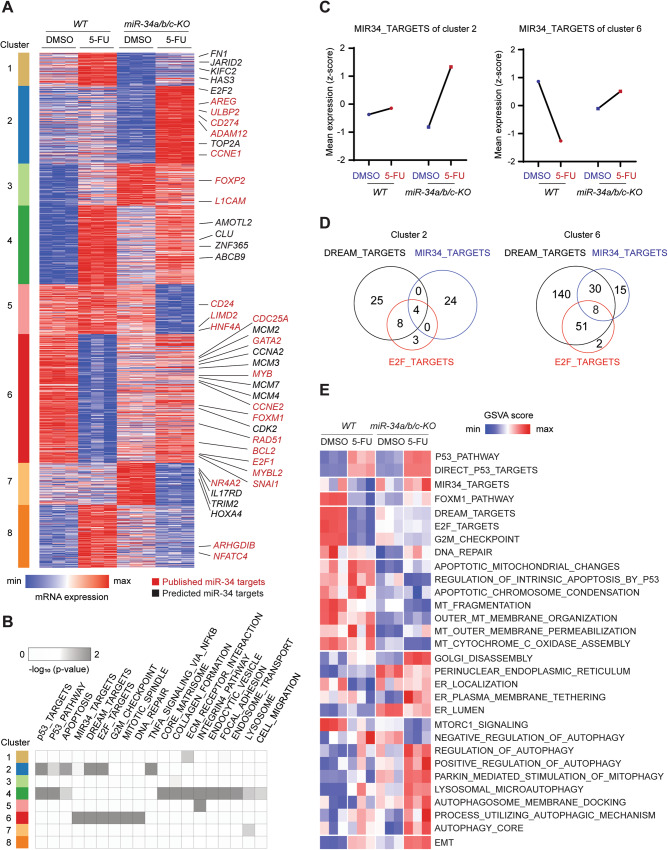
To comprehensively identify mediators of miR-34a and miR-34b/c function by which these microRNAs regulate the aforementioned and other processes, we determined the mRNA expression profiles of HCT116 miR-34a/b/c-KO cells and corresponding WT cells after exposure to 5-FU (4 μM) for 48 h by RNA-Seq analysis. Libraries were generated from RNAs isolated from three biological replicates of 4 states: WT and miR-34a/b/c-KO HCT116 cells treated with 5-FU or, as a control, treated with DMSO. RNA-Seq analysis was performed with more than 30 million paired-end reads per library. Principal component analysis (PCA) revealed that the majority of variations between 5-FU-treated and untreated cells was captured by principal component (PC) 1 in both WT and miR-34a/b/c-KO HCT116 cells, while loss of miR-34a/b/c resulted in significantly altered expression profiles predominantly captured by PC2 (Fig. 4A). Differential RNA expression analysis was performed using DESeq2 and differentially expressed mRNAs (FDR < 0.05 & absolute fold change > 1.5) in unstressed cells or after 5-FU treatment are displayed in volcano plots (Fig. 4B) as well as summarized in Tables S7–9. Deletion of miR-34a/b/c in unstressed HCT116 cells resulted in a significant upregulation of 966 mRNAs and downregulation of 562 mRNAs when compared to WT cells (left panel, Fig. 4B and Table S7). In addition, treatment of 5-FU significantly upregulated 1971 mRNAs and downregulated 1296 mRNAs in WT cells when compared to DMSO control (middle panel, Fig. 4B and Table S8). Treatment of 5-FU significantly upregulated 1675 mRNAs and downregulated 1243 mRNAs in miR-34a/b/c-KO cells when compared to DMSO control (right panel, Fig. 4B and Table S9). Interestingly, the overlap between mRNAs either up- or downregulated (>1.5× fold change) in miR-34a/b/c-KO or wild-type cells after treatment with 5-FU was not complete (Fig. 4C). This suggested that the response to 5-FU treatment, while sharing substantial commonalities that were already indicated by PCA, showed differences that were dependent on miR-34a/b/c. We observed limited overlap between genes showing strong opposing regulation (>1.5x fold change up- or downregulation) (Fig. 4C). Interestingly, among the 8 mRNAs that were downregulated in WT cells and up-regulated in miR-34a/b/c-KO cells, 5 (CCNE2, RAD51AP1, SKA1, ESCO2, EXO1) were related to cell proliferation–related pathways such as DNA replication and repair (EXO1, RAD51AP1), mitosis (ESCO2, SKA1), and cell cycle regulation (CCNE2), suggesting that deletion of miR-34a/b/c may affect cell cycle progression after treatment with 5-FU.
Fig. 4. Comprehensive gene expression analysis of miR-34a/b/c-deficient cells after treatment with 5-FU.
A Principal component analysis of mRNAs expression in WT and miR-34a/b/c-KO HCT116 cells treated with DMSO or 5-FU for 48 h. B Volcano plots showing differential RNA expression with FDR in −log10 scale and fold change in log2 scale. Significantly up- and downregulated genes (FDR < 0.05 & absolute fold change > 1.5) are highlighted in red and blue respectively as indicated. Non-significantly regulated genes are shown in gray. C Venn diagram displaying the number of differentially expressed mRNAs shared between WT and miR-34a/b/c-KO cells (5-FU vs control). D Overrepresentation analysis (ORA) of mRNAs significantly regulated in untreated miR-34a/b/c-KO vs. wild-type cells. E Overrepresentation analysis (ORA) of mRNAs significantly regulated in 5-FU-treated miR-34a/b/c-KO and wild-type cells.
Next, we employed pathway over-representation analysis (ORA) using gene sets from the MSigDB database [26] in order to identify molecular and cellular pathways, showing differential basal expression of their components in untreated miR-34a/b/c-KO vs. wild-type cells (Fig. 4D). The pathways enriched among the mRNAs upregulated in miR-34a/b/c-KO cells were represented by gene sets comprising genes involved in endoplasmic reticulum (ER) and Golgi organization, ER stress, positive regulation of autophagy, as well as extracellular matrix, EMT, and p53 pathway activation. Conversely, gene sets over-represented among the downregulated mRNAs included gene sets representing epithelial cell organization (TIGHT_JUNCTION, CELL_CELL_JUNCTION), as well as gene sets related to cell proliferation, such as E2F_TARGETS, DREAM_TARGETS, and CELL CYCLE. These results largely corroborated our initial observations that miR-34a/b/c-KO cells display a more mesenchymal phenotype, as well as increased autophagy.
Furthermore, we employed ORA to analyze which pathways were significantly altered after 5-FU treatment of miR-34a/b/c-KO and wild-type cells (Fig. 4E). Pathway over-representation among the upregulated mRNAs was similar between miR-34a/b/c-KO and wild-type cells, which included p53 activation, apoptosis, as well as EMT and additional gene sets representing pathways related to cell migration. Interestingly, we observed profound differences in pathway over-representation among the downregulated mRNAs between miR-34a/b/c-KO and wild-type cells after 5-FU treatment. Notably, the downregulation of cell proliferation associated pathways, though still significant, was severely diminished in miR-34a/b/c-KO cells. As noted before (Fig. 4C), these results strongly indicated that deletion of miR-34a/b/c may abrogate the cell cycle arrest observed after 5-FU treatment in WT cells.
Next, we determined how the differential gene expression caused by the loss of miR-34a/b/c may cause the aforementioned alterations observed for specific cellular processes. Therefore, we first determined the set of mRNAs showing genotype-dependent differences (>1.5-fold) in regulation after 5-FU treatment. We performed K-means clustering with the resulting set of 1691 genes showing differential regulation (Fig. 5A and Table S10) and identified the published and predicted miR-34 targets in each cluster (Table S10). Individual predicted or published miR-34a targets did not follow a specific pattern of differential regulation between miR-34a/b/c-KO and wild-type cells and could be found in all of the expression clusters. However, we noted that miR-34 targets were strongly over-represented in cluster 6 (and to a lesser extent in cluster 2), which largely comprised genes involved in cell-cycle regulation (Fig. 5B). These mRNAs were characterized by strongly elevated upregulation in miR-34a/b/c-KO cells or severely diminished repression in miR-34a/b/c-KO cells (Fig. 5C). Although miR-34 targets did not show a uniform type of expression change after treatment with 5-FU, in sum they displayed either activation or de-repression in miR-34a/b/c-KO cells. Interestingly, among the 266 DREAM targets found in clusters 2 and 6, 42 (15.8%) were predicted and/or published miR-34a/b/c targets (Fig. 5D and Table S10), implying that miR-34a/b/c and the DREAM complex share a substantial proportion of targets and presumably cooperatively suppress these genes after p53 activation. Since the DREAM and E2F target gene signatures used here are highly overlapping, which is due to the binding to E2F sites by both E2F and DREAM complexes, we also detected shared targets of E2F and miR-34, such as Cyclin E1/CCNE1, which were upregulated in miR-34a/b/c-KO cells (Fig. 5D, Table S10). Collectively, these data suggest that miR-34 may contribute to the repression of mRNAs, which are also downregulated due to DREAM complex-mediated repression [27].
Fig. 5. Comprehensive identification of differentially regulated miR-34 targets and their association with functional categories.
A Heatmap of RNA expression of mRNAs with statistically significant, genotype-dependent differences (>1.5-fold) in regulation after 5-FU treatment grouped in the indicated transcriptional clusters. Selected miR-34 targets are indicated by color. Red: published; black: predicted. B Heatmap showing enrichment of the indicated pathways and functional categories in the expression clusters as shown in (A). Statistical significance was calculated by Fisher’s exact test. C Line plot representation of mean z-score of normalized RNA expression of all miR-34 targets with statistically significant, genotype-dependent differences in regulation after 5-FU treatment, grouped in the indicated transcriptional clusters. D Venn diagram displaying the number of shared DREAM, E2F and miR-34 targets in the indicated transcriptional clusters. E Heatmap of GSVA analysis of the indicated pathways activities changes caused by miR-34a/b/c inactivation after 5-FU treatment.
Next, we employed Gene Set Variation Analysis (GSVA) [28], a sample-wise pathway enrichment method, to analyze which pathways were significantly altered in miR-34a/b/c-KO vs. wild-type cells after 5-FU treatment, by estimating the variation of different pathway activities in an unsupervised manner (Fig. 5E). Activation of the p53 pathway and the upregulation of direct p53 targets was not significantly altered between miR-34a/b/c-KO and wild-type cells after 5-FU treatment. We observed significantly differential regulation of the miR-34 target set between miR-34a/b/c-KO and wild-type cells, which was downregulated in WT, but upregulated in miR-34a/b/c-KO after 5-FU treatment (Fig. 5E). As already indicated by the ORA analysis, the differential regulation of mRNAs involved in cell proliferation (DREAM_TARGETS, E2F_TARGETS, etc.) was significantly changed in miR-34a/b/c-KO cells, which showed a severely diminished repression compared to WT cells (Fig. 5E). Furthermore, pathways involved in mitochondria and apoptosis were preferentially downregulated in miR-34a/b/c-KO cells after 5-FU treatment. We also observed a differential regulation of genes involved in ER and Golgi organization, as well as gene sets representing various autophagy pathways, and EMT, which either displayed elevated basal levels, and/or increased upregulation in miR-34a/b/c-KO cells.
miR-34a/b/c inhibit multiple key autophagy-related genes
Next, we explored the role of miR-34a and miR-34b/c in the autophagic response to 5-FU treatment. As expected, the pri-miR-34a and pri-miR-34b/c transcripts were significantly induced in WT HCT116 cells when exposed to 5-FU (Fig. S6A). Also, mature miR-34a, miR-34b and miR-34c were significantly upregulated in WT HCT116 cells after 5-FU treatment, but not detectable in miR-34a/b/c-KO HCT116 cells (Fig. S6B). In line with the GSVA results shown in Fig. 5E, qPCR analysis of key autophagy-related mRNAs that are either predicted (ATG13, ULK2) or known (ATG4B [29], ATG5 [30], ATG9A [31], ULK1 [32], XBP1 [33], IRE1A [34]) to be directly inhibited by miR-34, showed that they were either significantly upregulated in miR-34a/b/c-KO cells or downregulated in WT cells after 5-FU treatment (Fig. S6C). In addition, ectopic expression of miR-34a significantly repressed the expression of the aforementioned autophagy-related genes in SW480 cells (Fig. S6D). Therefore, miR-34 presumably inhibits autophagy processes by targeting multiple key autophagy-related mRNAs in HCT116 CRC cells.
FOXM1 induces autophagy and transactivates p62 and ATG9A
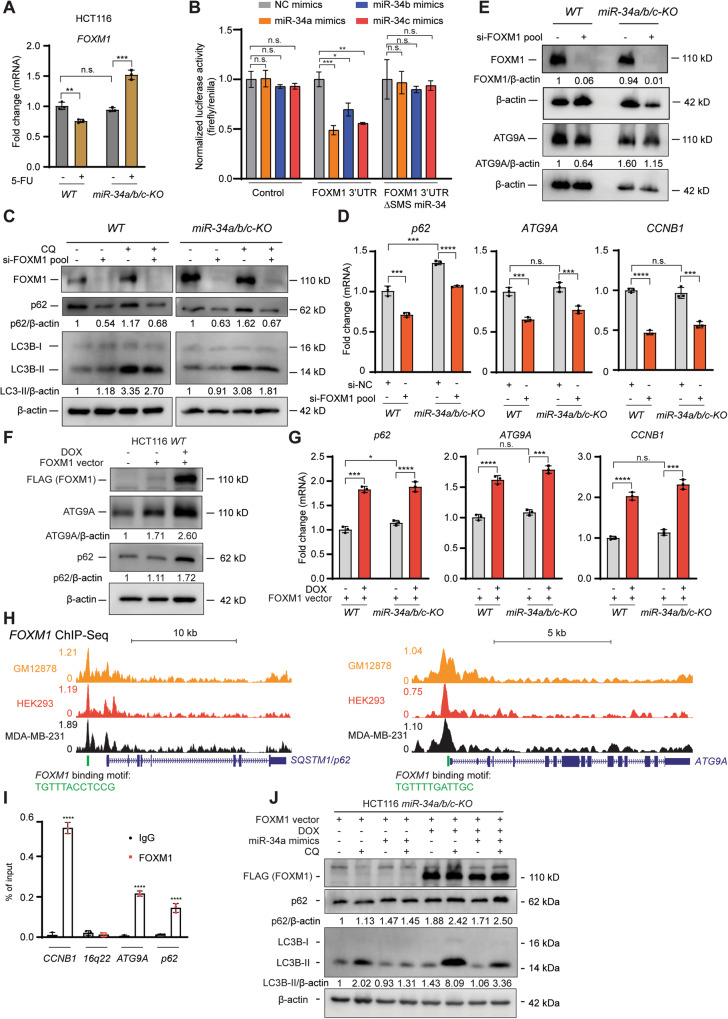
Among the known miR-34 targets that were differentially upregulated in miR-34a/b/c-KO cells after 5-FU treatment (Fig. 5A), FOXM1 appeared to be a potential mediator of autophagy, since it had been previously linked to the regulation of autophagy [35, 36]. Since the upregulation of FOXM1 may cause the increased expression of the autophagy signatures observed in miR-34a/b/c-KO cells after 5-FU treatment (Fig. 5E), we explored whether FOXM1 mediates the effects of miR-34 on autophagy. First, the upregulation of FOXM1 in miR-34a/b/c-KO cells after 5-FU treatment was confirmed by qPCR (Fig. 6A). We further confirmed that FOXM1 mRNA is a target of miR-34a/b/c by a dual luciferase reporter assay in HCT116 miR-34a/b/c-KO cells (Fig. 6B). The luciferase activity of a human FOXM1 3’-UTR reporter was repressed after co-transfection of miR-34a/b/c mimics, whereas that of a reporter with a mutant miR-34 seed-matching sequence (SMS) was refractory (Fig. 6B). The miR-34a/b/c-mediated repression of FOXM1 mRNA was corroborated by querying the METAmiR34TARGET database [37], which shows that FOXM1 mRNA was repressed after ectopic expression of miR-34a/b/c in various cell lines (Fig. S7A). Next, the miR-34-mediated repression of FOXM1 mRNA and protein was confirmed by ectopic expression of pri-miR-34a in SW480 cells (Fig. S7B, C). Furthermore, depletion of FOXM1 by a specific siRNA pool (Fig. 6C and Fig. S7D) significantly reduced autophagic flux in both WT and miR-34a/b/c-KO cells, which was indicated by the decreased turnover of endogenous LC3-II and p62 (Fig. 6C), supporting the assumption that miR-34a/b/c repress autophagic flux by downregulating FOXM1. Unexpectedly, depletion of FOXM1 also repressed p62 (Fig. 6C). p62 is a cargo receptor of autophagy and therefore should accumulate if autophagy is inhibited due to the decreased autophagy-mediated degradation of p62 [23, 38]. Therefore, the repression of p62 resulting from the depletion of FOXM1 cannot be attributed to autophagy inhibition but instead suggests that FOXM1 may transactivate p62 and potentially other autophagy-related genes. Indeed, depletion of FOXM1 repressed the p62 mRNA and also downregulated ATG9A at the mRNA and protein level (Fig. 6D, E). CCNB1 (Cyclin B1), a bona fide FOXM1 target [39], was also significantly repressed after depletion of FOXM1 (Fig. 6D). In addition, ectopic expression of FOXM1 in HCT116 WT cells significantly increased the expression of p62 and ATG9A at protein and mRNA levels (Fig. 6F-G). Furthermore, FOXM1 occupancy at the promoter of p62 and ATG9A was detected by publicly available FOXM1 ChIP-Seq data (Fig. 6H) from the Cistrome Data Browser [40]. Since a FOXM1 binding motif (Fig. S7E) was also identified under the corresponding FOXM1 ChIP-Seq peaks (Fig. 6H), FOXM1 presumably binds directly to the promoter of the p62 and ATG9A genes and regulates their expression. The occupancy of FOXM1 at the promoter regions of p62 and ATG9A was confirmed by qChIP (Fig. 6I). Importantly, ectopic expression of FOXM1 significantly induced autophagic flux in miR-34a/b/c-KO cells, and was sufficient to reverse the miR-34a mimics-mediated repression of autophagic flux (Fig. 6J), supporting that miR-34 represses autophagy by targeting FOXM1. Since miR-34a/b/c represent direct targets of p53, we propose that the p53-miR-34 axis negatively regulates autophagy by suppressing the expression of several autophagy-related genes via a coherent feed-forward regulation, in which miR-34 repress autophagy by directly targeting FOXM1 and ATG9A mRNAs, as well as by indirectly repressing p62 and ATG9A gene expression via targeting FOXM1 (see Graphical abstract).
Fig. 6. Upregulation of FOXM1 mediates enhancing effects of miR-34a/b/c loss on autophagy by regulating p62 and ATG9A.
A qPCR analysis of FOXM1 in WT or miR-34a/b/c-KO HCT116 cells after treatment with DMSO or 5-FU for 48 h. B Dual luciferase reporter assay was performed 48 h after miR-34a/b/c-KO HCT116 cells were transfected with indicated miRNA mimics and reporter plasmids. C Immunoblotting analysis of autophagic flux of cells transfected with 10 nM si-FOXM1 pools (siRNA pools specifically targeting FOXM1). WT and miR-34a/b/c-KO cells were transfected with si-NC or si-FOXM1 pool for 48 h. 20 μM of chloroquine was added for 4 h before harvesting cells for Western blot analysis. D qPCR analysis of indicated mRNAs in WT or miR-34a/b/c-KO HCT116 cells after transfection with si-NC or si-FOXM1 pool for 48 h. E Western blot analysis of ATG9A protein after transfection with si-NC or si-FOXM1 pool for 48 h. F Immunoblotting analysis of indicated proteins after HCT116 cells transfected with FOXM1 vector and addition of doxycycline (DOX) for 48 h. G qPCR analysis of indicated mRNAs in WT or miR-34a/b/c-KO HCT116 cells after transfection with FOXM1 vector and addition of DOX for 48 h. H Cistrome Data Browser representation of FOXM1 ChIP-Seq profiles at the genomic regions of p62 and ATG9A. I ChIP-qPCR analysis of FOXM1 occupancy at the promoter regions of p62 and ATG9A. Chromatin was enriched by anti-FOXM1 or anti-rabbit-IgG antibodies. CCNB1 and 16q22 served as positive and negative control, respectively. J Immunoblotting analysis of autophagic flux of HCT116 miR-34a/b/c-KO cells co-transfected with FOXM1 expression vector and NC mimics or miR-34a mimics. 20 μM of chloroquine (CQ) was added for 4 h before harvesting cells for Western blot analysis.
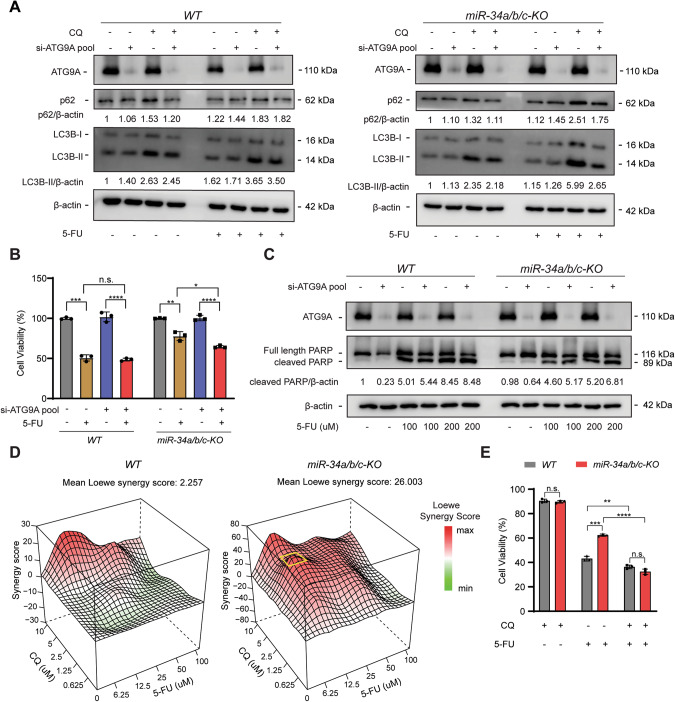
Silencing of ATG9A in miR-34a/b/c-KO cells inhibits autophagic flux and re-sensitizes to 5-FU
We hypothesized that if increased autophagy accounts for the decreased 5-FU sensitivity in miR-34a/b/c-KO cells, then inhibition of autophagy should reverse this effect. To test this hypothesis, ATG9A, a key mediator of autophagy, was silenced using an siRNA pool specifically targeting ATG9A mRNA. ATG9A was downregulated by ca. 85% at the mRNA level and also effectively at the protein level (Fig. S8A, B). Furthermore, ATG9A protein levels were increased in miR-34a/b/c-KO cells but decreased in WT cells after 5-FU treatment, suggesting autophagy induction after 5-FU treatment may indeed be attenuated by the inhibitory effects of miR-34 on autophagy-related genes (Fig. S8B). Surprisingly, depletion of ATG9A only marginally repressed 5-FU-induced autophagic flux in WT cells, but significantly repressed 5-FU-induced autophagic flux in miR-34a/b/c-KO cells (Fig. 7A), suggesting ATG9A may be a prominent mediator of autophagy in miR-34a/b/c-KO cells but not in WT cells, presumably since ATG9A was already repressed in WT cells after 5-FU treatment but significantly upregulated in miR-34a/b/c-KO cells after 5-FU treatment (Fig. S8B). In addition, cell viability assays showed that knockdown of ATG9A significantly re-sensitized miR-34a/b/c-KO cells to 5-FU, but had little effect in WT cells (Fig. 7B). Furthermore, depletion of ATG9A increased protein levels of cleaved-PARP, an apoptosis marker, in miR-34a/b/c-KO cells to a significantly greater degree when compared to WT cells after treatment with 5-FU (Fig. 7C). Taken together, these results suggest that ATG9A plays a pivotal role in the acquired resistance of miR-34a/b/c-KO cells to 5-FU presumably by enhancing autophagy and attenuating apoptosis.
Fig. 7. Inhibition of autophagy re-sensitized miR-34a/b/c-KO cells to 5-FU.
A WT or miR-34a/b/c-KO cells were transfected with 10 nM si-NC or si-ATG9A pool for 24 h and then subjected to DMSO or 5-FU treatment for 48 h. 20 μM of chloroquine was added for the last 4 h before harvesting cells for Western blot analysis of the indicated proteins. B Cells were transfected with 10 nM si-NC or si-ATG9A for 48 h, and then re-seeded in 96-well plates and incubated for 24 h. After that, cells were treated with DMSO or 5-FU for 72 h before subjected to cell viability determination. C Western blot analysis of cleaved-PARP in DMSO or 5-FU treated cells transfected with si-NC or si-ATG9A for 24 h. D Analysis of synergistic effects of combined treatment of 5-FU and CQ in WT or miR-34a/b/c-KO cells. Cells were treated for 48 h with 5-FU and/or CQ as indicated and then subjected to cell viability analysis and Loewe synergy score estimation. The most synergistic concentrations are highlighted with a yellow square. E Cell viability assays showing cytotoxicity of 6.25 μM of 5-FU or 2.5 μM of CQ or their combination. Results are presented as the mean ± SD (n = 3) for B and E with *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, n.s. no significance.
Synergistic effects of 5-FU and chloroquine in miR-34a/b/c-KO cells
We next used CQ (chloroquine) as an alternative autophagy inhibitor to determine whether it may also re-sensitize miR-34a/b/c-deficient cells towards 5-FU as observed for ATG9A inhibition. Indeed, we found that the combination of 5-FU and CQ resulted in a synergistic cytotoxicity in miR-34a/b/c-KO cells, whereas an additive cytotoxicity was observed in WT cells (Fig. 7D), as determined by the SynergyFinder 2.0 [41] algorithm. To validate the synergy map results we treated cells with a combination of two drugs at the concentration corresponding to the highest synergistic score area (highlighted by a yellow square in Fig. 7D). Indeed, by combined treatment with 2.5 μM of CQ and 6.25 μM of 5-FU, a significantly greater reduction in cell viability was achieved in miR-34a/b/c-KO cells than in WT cells (Fig. 7E). Therefore, the 5-FU-resistance of CRC cells with defects in the p53/miR-34a/b/c pathway may be alleviated by combining 5-FU-based treatment with CQ.
Clinical relevance of miR-34a/b/c-KO-derived gene signatures
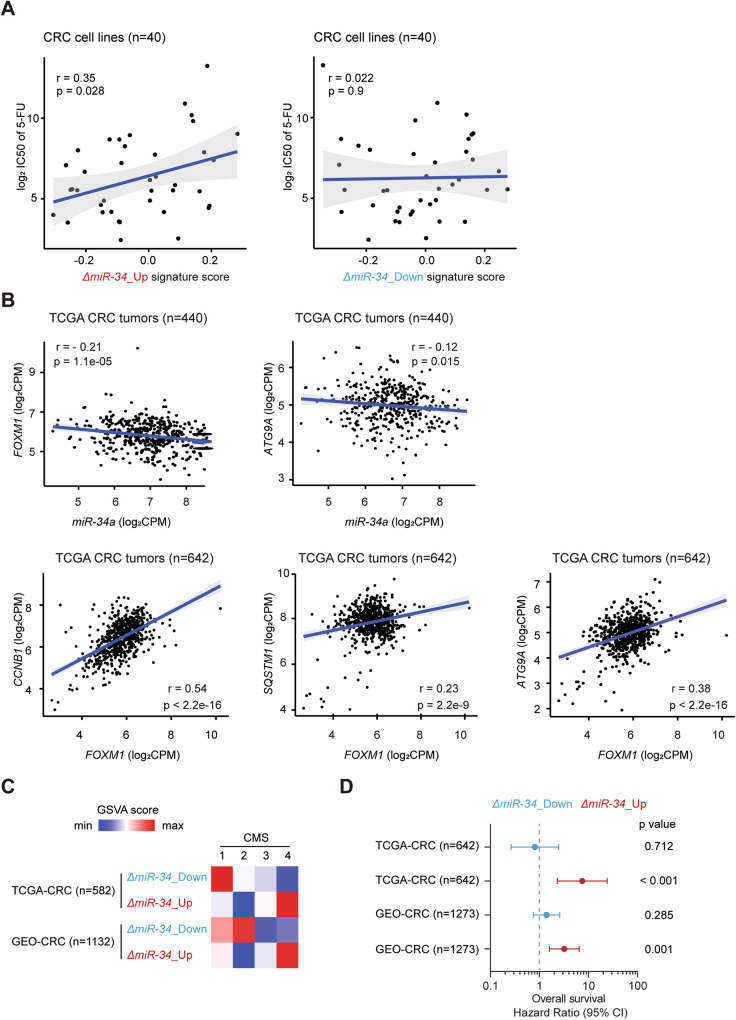
Next, we explored the relation of miR-34a/b/c-KO-derived signatures with the chemotherapeutic response of CRC cells by interrogating the Genomics of Drug Sensitivity in Cancer (GDSC) datasets [42]. For this, we defined two miR-34a/b/c-KO-derived signatures (ΔmiR-34_Up and ΔmiR-34_Down), which comprise the significantly up- and downregulated genes in miR-34a/b/c-KO cells compared to WT cells (Table S7). Single sample miR-34a/b/c-KO-derived signature scores of CRC cell lines were computed by the GSVA algorithm in an unsupervised manner. Interestingly, the ΔmiR-34_Up signature score showed a significant, positive association with the IC50 values of 5-FU in CRC cell lines, whereas ΔmiR-34_Down signature score showed no significant association (Fig. 8A). Therefore, the observed resistance of miR-34a/b/c-deficient HCT116 cells to 5-FU may also be found in other CRC cell lines that exhibit a similar expression pattern.
Fig. 8. Clinical association analysis of selected genes and miR-34a/b/c-KO-derived gene signatures.
A Scatter plots showing the correlations of the indicated miR-34a/b/c-KO-derived signatures scores with IC50 values of 5-FU in CRC cell lines. Two-sided Pearson correlation coefficient r and p values are indicated. B Scatter plots showing correlations between selected gene expressions in the TCGA-CRC patient cohort. Pearson correlation coefficient r and p values are indicated. C Associations between miR-34a/b/c-KO-derived signatures scores with CMS subtypes in the indicated CRC patient cohorts. D Cox regression model analysis of the associations between miR-34a/b/c-KO-derived signatures scores with overall survival in the indicated CRC patient cohorts.
Next, we analyzed whether the regulatory relationships between miR-34, FOXM1, p62 and ATG9A identified above are conserved in primary CRCs. For this, we analyzed RNA expression data deposited in the TCGA database (TCGA-CRC) (n = 642) [43]. Expression of mature miR-34a displayed a significantly negative correlation with both FOXM1 and ATG9A, whereas FOXM1 showed a significantly positive correlation with p62 and ATG9A as well as CCNB1 (Fig. 8B), a bona fide FOXM1 target. Therefore, the FOXM1-mediated regulation of p62 and ATG9 is presumably conserved in primary CRCs. Taken together, these results suggest that the regulation of autophagy by miR-34a via repression of FOXM1 may be relevant in primary CRCs.
Finally, we sought to determine whether the miR-34a/b/c-KO-derived gene signatures are associated clinical parameters in primary CRC patient cohorts. Samples from the TCGA-CRC and a large, integrated GEO-CRC (n = 1273) patient cohort [44] were included in this analysis. MiR-34a/b/c-KO-derived signature scores of CRC patient samples were again computed by the GSVA algorithm. In line with the repression of EMT by miR-34 [45], ΔmiR-34_Up signature scores were highest in CMS4 (Fig. 8C), the consensus molecular subtype (CMS) characteristic for mesenchymal-like CRCs, which is associated with the worst overall patient survival [6]. Consistent with this finding, a Cox proportional-hazards model analysis showed that ΔmiR-34_Up signature scores were significantly associated with poor overall patient survival in both CRC cohorts, whereas ΔmiR-34_Down signature scores showed no significant association (Fig. 8D), supporting a tumor suppressive role of miR-34a/b/c. Furthermore, ΔmiR-34_Up signature scores were also significantly associated with poor overall patient survival in 17 out of 33 TCGA cancer types (Fig. S9), indicating that these findings may also be relevant to other tumor entities as p53 and miR-34a/b/c inactivation are common in many types of tumors.
Discussion
Here, we demonstrated a complementary role of miR-34a and miR-34b/c as only concomitant deletion of both isoforms resulted in significantly reduced suppression of proliferation after p53 activation, enhanced migration, invasion and EMT, as well as reduced sensitivity to chemotherapeutics. The latter was due to increased stress-induced autophagic flux and upregulation of autophagy-related genes after 5-FU treatment, which also resulted in a decreased rate of apoptosis. Furthermore, inhibition of autophagy re-sensitized miR-34a/b/c-KO cells to 5-FU. Genome-wide gene expression analysis revealed that deletion of miR-34a/b/c in the HCT116 cell line resulted in impaired functions mediated by the gene-repressive effect of the p53-DREAM axis and enhanced autophagy after exposure to 5-FU, which is presumably due to the upregulation of FOXM1, a miR-34 target and transcription factor that transactivates key factors involved in cell cycle and autophagy processes. Notably, FOXM1 was required for increased autophagy, presumably by transactivating p62 and ATG9A. Since ATG9A is an established miR-34 target, we propose a feed-forward loop in which miR-34 represses autophagy by directly targeting FOXM1 and ATG9A mRNAs, as well as by indirectly repressing p62 and ATG9A gene expression via targeting FOXM1. Since a gene signature comprised of genes significantly upregulated as a result of the combined deletion of miR-34a and miR-34b/c was significantly associated with poor prognosis as well as 5-FU resistance, and the combination of chemotherapeutics with autophagy inhibition resulted in synergistic effects in a miR-34-deficient context, these findings are presumably of clinical relevance.
The combined inactivation of miR-34a and miR-34b/c in unstressed HCT116 cells resulted in enhanced migration and invasion, which was accompanied by elevated EMT. These observations were further corroborated by an RNA-Seq analysis, which showed enrichment of EMT pathways in miR-34a/b/c-KO cells, and by detection of a significant correlation between ΔmiR-34_Up signature score and poor survival of CRC patients. However, singular deletion of either miR-34a or miR-34b/c showed no significant effects on migration, invasion or EMT. Collectively, these data suggest a complementary role of miR-34a and miR-34b/c in repressing these processes.
By inactivating miR-34a and miR-34b/c alone or in combination using a genetic approach, we showed that the combined deletion of miR-34a and miR-34b/c has a significant effect on promoting stress-induced autophagic flux and reducing sensitivity to chemotherapeutics, whereas abrogation of endogenous expression of either of them was not able to render cells chemo-resistant.
In this study, we employed two approaches to estimate autophagic flux, which is a more reliable measure of autophagy activity than measuring the steady level of LC3 [46, 47]. On one hand, the turnover of LC3-II and p62 was determined to measure autophagy using immunoblotting assays. In addition, cells stably expressing a GFP-LC3-RFP autophagy probe were generated to measure the cumulative degradation of GFP-LC3 in a quantitative manner. By utilizing these two methods, we showed that miR-34a/b/c-deficient cells consistently displayed higher autophagic flux when stressed by chemotherapeutics, starvation or endoplasmic reticulum stress. Therefore, miR-34a/b/c presumably plays a pivotal role in suppressing stress-induced autophagy.
Genome-wide gene expression profiling analysis revealed an upregulation of autophagy-related pathways in miR-34a/b/c-deficient cells after 5-FU treatment, thus corroborating the enhanced autophagic flux observed in miR-34a/b/c-KO cells.
Interestingly, we also observed a strongly diminished repression of genes related to cell proliferation in miR-34a/b/c-KO cells after 5-FU treatment. A large number of these genes was previously identified as direct targets of the DREAM complex, which suggested a compromised DREAM complex activity in miR-34a/b/c-KO cells. Since the DREAM complex can regulate both E2F targets via binding to E2F binding sites, as well as FOXM1 targets via binding to CHR binding sites [48], the repression of both E2F and FOXM1 targets was also abrogated in miR-34a/b/c-KO cells. Interestingly, miR-34 regulates several upstream signaling components that affect DREAM activity, which showed either diminished repression or upregulation in miR-34a/b/c-KO cells after 5-FU treatment, such as CCNE1 (Cyclin E1) [49] and CCNE2 (Cyclin E2) [50]. Upregulation of CCNE1 and CCNE2 by the loss of miR-34a/b/c would presumably increase phosphorylation of p130 via CDK2 and result in disassembly of the DREAM complex [48].
Interestingly, we noted that miR-34a/b/c and the DREAM complex share a substantial proportion of targets and presumably cooperatively suppress these genes after p53 activation. Among these were several transcription factors that affect DREAM complex function, such as MYBL2 (B-MYB) [51], E2F1 [51], as well as FOXM1 [52], which are direct targets of miR-34 that showed upregulation in miR-34a/b/c-KO cells after 5-FU treatment. Upregulation of B-MYB by the loss of miR-34a/b/c presumably competes with DREAM complex for the MuvB complex and facilitate the formation of B-MYB-MuvB-FOXM1 complex since binding of B-MYB to MuvB is necessary for recruiting FOXM1 [53]. Moreover, the upregulation of E2F1 in miR-34a/b/c-KO cells likely counteracts the repression of E2F targets by DREAM.
FOXM1 represents a key factor for cell cycle progression [48], as well as a prognostic marker of CRC [54]. The miR-34-dependent upregulation of FOXM1 in miR-34a/b/c-KO cells after 5-FU treatment presumably competed with the DREAM complex for the MuvB core complex, thereby switching the MuvB-based complexes from DREAM repressor to B-MYB-MuvB-FOXM1 activators and exerting opposite functions [27, 48]. Taken together, the combinatorial effects of loss of miR-34a/b/c on these regulations are likely contribute to either abrogated repression or activation of DREAM, E2F and FOXM1 target genes after activation of p53.
Moreover, FOXM1 is a known inducer of autophagy [35, 36], a target of miR-34a [52] and presumably a prominent effector of miR-34 in this context. Here, we showed that FOXM1 is also directly targeted by miR-34b and miR-34c. The elevated autophagy resulting from the deletion of miR-34a/b/c is presumably, at least in part, caused by the upregulation of FOXM1 as depletion of FOXM1 significantly repressed autophagic flux, whereas ectopic expression of FOXM1 significantly induced autophagic flux.
Here, we showed that FOXM1 induced autophagy, presumably through transactivating its target genes, SQSTM1/p62 and ATG9A. P62 is one of the most prominent autophagy receptors [55]. Importantly, p62 is at the crossroads of autophagy and the ubiquitin-proteasome system, linking these two major quality control systems responsible for degradation of proteins and organelles in eukaryotic cells via its LC3-binding domain and ubiquitin-associated domain respectively [56]. The upregulation of p62 by the de-regulation of miR-34/FOXM1 axis presumably not only increases autophagy, but also affects the proteasome system, which could perturb cellular homeostasis. ATG9A is the only transmembrane protein of the core autophagy machinery [57]. ATG9A-containing vesicles form seeds that establish contact sites to facilitate the de novo formation of autophagosomes [58, 59]. Thus, the miR-34-dependent downregulation of ATG9A may presumably impair autophagosome formation and expansion, and thereby inhibit autophagy. Importantly, the expression of mature miR-34a showed a negative correlation with FOXM1 and ATG9A, whereas FOXM1 displayed a significant, positive correlation with p62 and ATG9A expression in the TCGA-CRC patient cohort, indicating that such regulatory connections may also exist in primary CRCs.
CQ (chloroquine) and its derivative hydroxy-chloroquine are drugs widely used to treat malaria [60], amebiasis [61] and rheumatic diseases [62]. Importantly, CQ is a potent autophagy inhibitor that blocks autophagy at a late step by preventing autophagosome and lysosome fusion [63] and was therefore tested in clinical trials for use as an anti-tumor drug [64, 65]. Interestingly, CQ was shown to potentiate the cytotoxicity of 5-FU in colon cancer and pancreatic cancer cell lines [66, 67]. Here, we showed that a combination of 5-FU and CQ resulted in a synergistic effect specifically in a miR-34-deficient context whereas an additive effect was observed in miR-34a/b/c-proficient CRC cells. Therefore, the inactivation of miR-34a/b/c may sensitize tumor cells to autophagy inhibition. In the future, it should be tested whether combinations of other autophagy inhibitors and 5-FU-related drugs also elicit a synergistic toxicity towards tumor cells with defects in the p53/miR-34a/b/c pathway.
Materials and methods
Cell culture and treatments
The colorectal cancer cell lines HCT116, SW480 and HCT15 were cultured in McCoy’s 5A medium, with 10% fetal bovine serum and 1% penicillin/streptomycin, at 5% CO2 and 37 °C. For conditional pri-miR-34a expression from pRTR vectors, doxycycline (Sigma-Aldrich, St. Louis, MO) was dissolved in water and used at a final concentration of 100 ng/ml. To select for cells harboring pRTR vectors, cell pools were cultured at a final concentration of 4 μg/ml puromycin. pCW57.1-FOXM1c (obtained from Addgene, a gift from Adam Karpf; Plasmid #68810) was used to ectopically express FLAG-tagged human FOXM1c in cells in a doxycycline-inducible manner [68]. Hsa-miR-34a/b/c-5p mimics and corresponding negative control mimics were purchased from Qiagen (Hilden, Germany). FlexiTube GeneSolution GS2305 for FOXM1 (consisting of a pool of 4 different siRNAs for FOXM1) and control siRNAs were purchased from Qiagen (Hilden, Germany). Two siRNAs (#s35505 and #s35506) targeting ATG9A and the corresponding negative control siRNAs were purchased from ThermoFisher Scientific (Waltham, MA, USA).
RNA isolation and real-time polymerase chain reaction (qPCR) analysis
Total RNA from cultured cells was isolated using High Pure RNA Isolation Kit (Roche) according to manufacturer’s protocol. One microgram total RNA was then used to generate cDNA using Verso cDNA Synthesis Kit (Thermo Scientific). qPCR analysis of mRNA was performed using LightCycler 480 (Roche) and the Fast SYBR Green Master Mix (Applied Biosystems). Mature miRNAs were isolated using miRNeasy Mini Kit (QIAGEN). Sequence information of the primers is provided in Table S3.
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) in HCT116 cells was performed according to protocol provided in the iDeal ChIP-qPCR kit (Diagenode, Belgium). The sequence information of the qChIP primers used here is provided in Table S4. 16q22 region was used as a negative control in qChIP assay, which is devoid of enriched FOXM1 signal (Fig. S7F) as determined by public FOXM1 ChIP-seq data from the Cistrome Data Browser [40].
CRISPR-Cas9-mediated deletion of miR-34
Two single-guide RNAs (sgRNAs) targeting flanks of pre-miRNA encoding locus (Table S1) were designed using the CRISPR design tool at benchling.com. Each of them was cloned via two complementary DNA oligonucleotides into the BbsI sites of pSpCas9(BB)-2A-GFP [69] to generate sgRNA expression plasmids, as described previously [70]. HCT116 cells were then transfected with 2.5 μg of each sgRNA-pSpCas9(BB)-2A-GFP plasmid, or transfected with “empty” pSpCas9(BB)-2A-GFP harboring no sgRNA. After transfection for 48 h, GFP-positive cells were sorted into 96-wells using a FACSARIA cell sorter (BD Biosystems) and expanded as single-cell clones for two weeks. Cells transfected with “empty” pSpCas9(BB)-2A-GFP vectors were treated in a similar manner to obtain wild-type single-cell clones. Subsequently, genomic DNA of individual single-cell clones were screened by genotyping PCR for appropriate deletions of pre-miRNA encoding regions using two pairs of genotyping screening primers listed in Table S2. Clones with deletion of both pre-miRNA encoding alleles were analyzed by qPCR to confirm the loss of mature miRNAs expression.
Modified Boyden-chamber assay
Migration and invasion assays using modified Boyden-chambers were performed as described previously [16]. In brief, 1 × 105 cells in serum-free medium were seeded in the upper chamber (8.0 μM pore size membrane; Corning), whereas medium containing 10% fetal bovine serum was placed in lower chamber as chemoattractant. For migration assay, cells were cultured for 24 h. For invasion assay, chamber membrane was first coated with 100 μl Matrigel matrix (Corning) at a concentration of 300 μg/ml in serum-free medium. Subsequently, cells were seeded and cultured for 48 h. Subsequently, non-motile cells at the top of the filter were removed and the cells in the bottom chamber were fixed with ice-cold methanol for 20 min at room temperature and stained with 0.5% crystal violet for 30 min. Fold change of migrated cells were calculated by normalizing to corresponding control groups.
Wound healing assay
Cell-free gap was created by seeding and culturing cells in Culture-inserts (80241; IBIDI, Martinsried, Germany) until confluent cell monolayer was formed. Cells were treated with 10 μg/mL mitomycin C (M4287; Sigma-Aldrich, Germany) for 2 h before removing Culture-inserts to create cell-free gap. After washing twice with HBSS to remove mitomycin C and detached cells, cells were filled with medium. The cell-free gap was monitored immediately and after 36 h by phase contrast microscope and corresponding pictures were taken.
Western blot analysis
Cells were lysed in RIPA lysis buffer with complete mini protease inhibitors (Roche, Basel, Switzerland) and PhosSTOP Phosphatase Inhibitor Cocktail Tablets (Roche). Lysates were sonicated and then centrifuged at 13,000 rpm for 20 min at 4 °C. Protein concentration was measured with BCA Protein Assay Kit (Thermo Fisher Scientific) according to manufacturer’s instructions. 30 μg protein per lane were separated by 12% SDS-PAGE gels and transferred to PVDF membranes (Millipore). ECL (Millipore) system was used and imaged through LI-COR Odyssey FC imaging system (Bad Homburg, Germany). Western blot signals were quantified using Image Studio (LI-COR). Antibodies are list in Table S5.
Apoptosis detection with FITC Annexin V staining
Apoptosis analysis was carried out by flow cytometry with FITC Annexin V Apoptosis Detection Kit I (556547; BD Pharmingen™) according to manufacturer’ instructions. In brief, supernatant containing apoptotic cells was collected before harvesting cells by trypsinization (EDTA-free). Cells were then washed twice and resuspended in 1× binding buffer at a concentration of 1 × 106 cells/ml. One hundred microloters of solution (1 × 105 cells) were incubated with 5 µl of FITC Annexin V and 5 µl Propidium Iodide (PI) for 15 min at room temperature in the dark before adding 400 µl of 1× binding buffer. Samples were analyzed within 1 h by flow cytometry using an Accuri C6 flow cytometry instrument (BD Biosciences).
Apoptosis evaluation with cell cycle analysis by Propidium Iodide staining
Cells were seeded in 6-well plates at a density of 2 × 105 cells per well. After 24 h, cells were treated as indicated for 48 h. Both supernatant and attached cell fractions were collected and combined. Cells were washed twice with HBSS and fixed with ice-cold 70% ethanol overnight at −20°C. Fixed samples were washed once with HBSS and then resuspended by Propidium Iodide (PI) staining solution. Cell-cycle distribution was measured using an Accuri C6 flow cytometry instrument (BD Biosciences) and analyzed with the CFlow software. Sub-G1 cell population represents apoptotic cells.
Cell viability assay
Cell viability was determined by Cell Counting Kit-8 (CCK-8) (Dojindo EU GmbH) according to manufacturer’s instructions. Briefly, 3000 cells per well were seeded into 96-well plates and treated with the indicated cytostatic agents for the indicated durations. 10% CCK-8 solution was added to each well at end point and incubated for 2 h. Absorbance was measured at 450 nm on a Berthold Orio II Microplate Luminometer (Berthold, Bad Wildbad, Germany). GraphPad Prism (v9.31; GraphPad Software, USA) was used to generate dose-response curves and estimated corresponding half-maximal inhibitory concentration (IC50) values of indicated drugs.
Assessment of cell proliferation by real‑time impedance measurement
Cell proliferation was determined by real-time cellular impedance measurement using a xCELLigence Real-Time Cell Analyzer (RTCA) (Roche Diagnostics GmbH, Penzberg, Germany) as described previously [71]. Cells were seeded at a density of 3000 cells per well of the E-plate and treated as indicated after 24 h. Impedance was measured every 60 min for 96 h and reported as a dimensionless parameter called Cell Index by RTCA software . The magnitude of Cell Index is dependent on cell number, cell morphology and cell size and on the strength of cell adherence to the substrate coating the plate [72]. Because the Cell Index does not solely depend on cell number, the results of impedance measurements were validated by end-point cell number counting. Therefore, cells were simultaneously seeded into 96-well plates and treated in the same manner. Cells at the end point were counted using a Neubauer-chamber.
Autophagic flux assay with GFP-LC3-RFP probe
Cells stably expressing GFP-LC3-RFP were generated by transfected of a GFP-LC3-RFP plasmid (obtained from Addgene, a gift from Noboru Mizushima; Plasmid #84573) with lipofectamine LTX (Invitrogen) followed by puromycin selection for two weeks. Cells were stressed as indicated and subjected to flow cytometry analysis using an Accuri C6 instrument (BD Biosciences) to assess GFP and RFP fluorescence intensities.
RNA-Seq analysis
Total RNAs from HCT116 cells were isolated using a High Pure RNA Isolation Kit (Roche) with an on-column DNase digestion according to the manufacturer’ protocol. Random primed cDNA libraries were constructed and sequenced using the NovaSeq 6000 (Illumina, San Diego, CA, USA) platform by GATC (Konstanz, Germany). Each sample was covered by at least 30 million paired-end read pairs of 150 bp length. RNA-Seq FASTQ files were processed using the RNA-Seq module implemented in the CLC Genomics Workbench v20.0.2 software (Qiagen Bioinformatics, Dusseldorf, Germany) and mapped to the GRCh38/hg38 human reference genome and its associated gene and transcript annotation (ENSEMBL) using the settings mismatch cost = 2, insertion cost = 2, deletion cost = 3, length fraction = 0.8, and similarity fraction = 0.8. RNA-Seq data were filtered to exclude weakly expressed transcripts with less than 20 mapped exon reads in all samples from the analysis and subjected to upper quartile normalization using the R/Bioconductor RUVSeq (remove unwanted variation from RNA-Seq data, Version 1.18.0) package [73]. Differential gene expression analysis was performed with DESeq2 (Version 1.24.0) [74] after normalization using the RUVg approach to remove variation between RNA samples resulting from differences in library preparation. Principal component analysis (PCA) was performed using the PCA functionality of the EDASeq R package as implemented in RUVSeq. For the identification of miR-34 targets, we used recently published lists of miR-34 targets and of the top 1000 ranked miR-34a targets, which were generated using the METAmiR34TARGET website [37]. Pathway over-representation analysis (ORA) using a hypergeometric testing method was performed via the enricher function implemented in clusterProfiler 4.0 [75]. Gene sets were obtained from the Molecular Signatures database (MSigDB) [76]. AUTOPHAGY_CORE gene set was obtained from Bordi M et al. [77]. Sample-wise variations of different pathway activities were estimated in a non-parametric, unsupervised manner via the GSVA package [28]. EdgeR was used to test the interaction effects between genotype and treatment condition and determine the set of mRNAs showing genotype-dependent differences in regulation after 5-FU treatment [78].
Analysis of gene expression and clinical data from public databases
For the analysis of human colorectal cancer (CRC) samples, we retrieved expression and clinical data of the TCGA-CRC samples from GDC portal [79] and a large integrated CRC samples from GEO repository [44]. CMS (consensus molecular subtypes) [6] classifications of CRC samples were determined using the CMScaller R package v.2.0.1 [80]. 5-FU sensitivity data of CRC cell lines were obtained from the Genomics of Drug Sensitivity in Cancer (GDSC) database [42], and corresponding gene expression data of CRC cell lines were obtained from the Cancer Cell Line Encyclopedia (CCLE) [81]. The Cox proportional-hazards regression model was applied to investigate the hazard ratio for assessing the association between patients’ overall survival time and miR-34-a/b/c-KO-derived signatures scores in CRC patient cohorts.
3′-UTR dual reporter assay
The full length human FOXM1 3′-UTR was PCR-amplified from cDNA obtained from HCT116 cells. The PCR product was cloned into pGL3-control-MCS [82]. To delete the miR-34a/b/c-5p seed-matching sequence (SMS) in the FOXM1 3′-UTR a QuikChange II XL Site-Directed Mutagenesis Kit (Stratagene, San Diego, CA, USA) was used according to the manufacturer’s instructions. All plasmids were verified by Sanger sequencing. The oligonucleotides used for cloning and mutagenesis were listed in Table S6. For the dual reporter assays, HCT116 miR-34a/b/c-KO cells were seeded into a 12-well plate at 3 × 104 cells/well and cultivated for 24 h before transfection. Transfections were performed using HiPerFect Transfection Reagent (Qiagen) with 10 nM of indicated miRNA mimics and 100 ng of indicated reporter vectors and 20 ng Renilla plasmid as normalization control. After 48 h of incubation with the indicated treatments, luciferase activity was measured with a Dual Luciferase Reporter assay kit (Promega) according to manufacturer’s instructions using an Orion II Microplate Luminometer (Berthold, Germany) and the Simplicity software package.
Drug combination synergy scores analysis
To analyze the synergistic effects of the combination between CQ and 5-FU, synergy scores were calculated by SynergyFinder 2.0 [41] with the Loewe model [83], using the dose-response matrix derived from cell viability assays.
Statistical analysis
Statistical analyses were performed with Prism 9 (GraphPad Software, San Diego, CA, USA) or R (version 4.2.2). Each set of experiments was repeated at least three times. Student’s t test (unpaired, two-tailed) was used to determine statistical significance of differences between two groups of samples. P values less than 0.05 were considered statistically significant (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; n.s. not significant). Benjamini–Hochberg method was used to adjust for multiple testing error and calculate false discovery rate (FDR).
Supplementary information
Acknowledgements
We thank Ursula Götz for technical assistance, Esra Cetin for the generation of miR-34a-gRNA-plasmids and Raffaele Conca (Dr. von Haunersches Children’s Hospital, Munich) for FACS sorting. This work was supported by grants of the Rudolf-Bartling-Stiftung to HH. Zekai Huang is a recipient of a China Scholarship Council fellowship.
Author contributions
HH conceived, planned and supervised the project; HH, MK and ZH designed experiments; ZH performed experiments and analyzed results; MK performed bioinformatics analyses; HH, ZH and MK wrote the manuscript. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
RNA expression profiling data obtained in this study were deposited in the Gene Expression Omnibus website (accession no. GSE227230). All data, analytic methods, and study materials will be made available to other researchers upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41418-023-01193-2.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Van Cutsem E, Nordlinger B, Adam R, Köhne CH, Pozzo C, Poston G, et al. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer. 2006;42:2212–21. doi: 10.1016/j.ejca.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Dawood O, Mahadevan A, Goodman KA. Stereotactic body radiation therapy for liver metastases. Eur J Cancer. 2009;45:2947–59. doi: 10.1016/j.ejca.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23–30. doi: 10.1200/JCO.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 5.Vodenkova S, Buchler T, Cervena K, Veskrnova V, Vodicka P, Vymetalkova V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: past, present and future. Pharmacol Ther. 2020;206:107447. doi: 10.1016/j.pharmthera.2019.107447. [DOI] [PubMed] [Google Scholar]
- 6.Guinney J, Dienstmann R, Wang X, de Reyniès A, Schlicker A, Soneson C, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350–6. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weng J, Li S, Zhu Z, Liu Q, Zhang R, Yang Y, et al. Exploring immunotherapy in colorectal cancer. J Hematol Oncol. 2022;15:95. doi: 10.1186/s13045-022-01294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie YH, Chen YX, Fang JY. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct Target Ther. 2020;5:22. doi: 10.1038/s41392-020-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hermeking H. p53 enters the microRNA world. Cancer Cell. 2007;12:414–8. doi: 10.1016/j.ccr.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 10.Hernández Borrero LJ, El-Deiry WS. Tumor suppressor p53: biology, signaling pathways, and therapeutic targeting. Biochim Biophys Acta Rev Cancer. 2021;1876:188556. doi: 10.1016/j.bbcan.2021.188556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hermeking H. The miR-34 family in cancer and apoptosis. Cell Death Differ. 2010;17:193–9. doi: 10.1038/cdd.2009.56. [DOI] [PubMed] [Google Scholar]
- 12.Vogt M, Munding J, Grüner M, Liffers ST, Verdoodt B, Hauk J, et al. Frequent concomitant inactivation of miR-34a and miR-34b/c by CpG methylation in colorectal, pancreatic, mammary, ovarian, urothelial, and renal cell carcinomas and soft tissue sarcomas. Virchows Arch. 2011;458:313–22. doi: 10.1007/s00428-010-1030-5. [DOI] [PubMed] [Google Scholar]
- 13.Lodygin D, Tarasov V, Epanchintsev A, Berking C, Knyazeva T, Körner H, et al. Inactivation of miR-34a by aberrant CpG methylation in multiple types of cancer. Cell Cycle. 2008;7:2591–600. doi: 10.4161/cc.7.16.6533. [DOI] [PubMed] [Google Scholar]
- 14.Jiang L, Hermeking H. miR-34a and miR-34b/c suppress intestinal tumorigenesis. Cancer Res. 2017;77:2746–58. doi: 10.1158/0008-5472.CAN-16-2183. [DOI] [PubMed] [Google Scholar]
- 15.Öner MG, Rokavec M, Kaller M, Bouznad N, Horst D, Kirchner T, et al. Combined inactivation of TP53 and MIR34A promotes colorectal cancer development and progression in mice via increasing levels of IL6R and PAI1. Gastroenterology. 2018;155:1868–82. doi: 10.1053/j.gastro.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Rokavec M, Öner MG, Li H, Jackstadt R, Jiang L, Lodygin D, et al. IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J Clin Investig. 2014;124:1853–67. doi: 10.1172/JCI73531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siemens H, Neumann J, Jackstadt R, Mansmann U, Horst D, Kirchner T, et al. Detection of miR-34a promoter methylation in combination with elevated expression of c-Met and β-catenin predicts distant metastasis of colon cancer. Clin Cancer Res. 2013;19:710–20. doi: 10.1158/1078-0432.CCR-12-1703. [DOI] [PubMed] [Google Scholar]
- 18.Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–8. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 19.Tazawa H, Tsuchiya N, Izumiya M, Nakagama H. Tumor-suppressive miR-34a induces senescence-like growth arrest through modulation of the E2F pathway in human colon cancer cells. Proc Natl Acad Sci USA. 2007;104:15472–7. doi: 10.1073/pnas.0707351104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poillet-Perez L, Sarry JE, Joffre C. Autophagy is a major metabolic regulator involved in cancer therapy resistance. Cell Rep. 2021;36:109528. doi: 10.1016/j.celrep.2021.109528. [DOI] [PubMed] [Google Scholar]
- 21.Mariño G, Niso-Santano M, Baehrecke EH, Kroemer G. Self-consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol. 2014;15:81–94. doi: 10.1038/nrm3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaizuka T, Morishita H, Hama Y, Tsukamoto S, Matsui T, Toyota Y, et al. An autophagic flux probe that releases an internal control. Mol Cell. 2016;64:835–49. doi: 10.1016/j.molcel.2016.09.037. [DOI] [PubMed] [Google Scholar]
- 23.Yoshii SR, Mizushima N. Monitoring and measuring autophagy. Int J Mol Sci. 2017;18:1865. [DOI] [PMC free article] [PubMed]
- 24.Jain A, Lamark T, Sjøttem E, Larsen KB, Awuh JA, Øvervatn A, et al. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J Biol Chem. 2010;285:22576–91. doi: 10.1074/jbc.M110.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bellezza I, Scarpelli P, Pizzo SV, Grottelli S, Costanzi E, Minelli A. ROS-independent Nrf2 activation in prostate cancer. Oncotarget. 2017;8:67506–18. doi: 10.18632/oncotarget.18724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liberzon A, Subramanian A, Pinchback R, Thorvaldsdóttir H, Tamayo P, Mesirov JP. Molecular signatures database (MSigDB) 3.0. Bioinformatics. 2011;27:1739–40. doi: 10.1093/bioinformatics/btr260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engeland K. Cell cycle arrest through indirect transcriptional repression by p53: I have a DREAM. Cell Death Differ. 2018;25:114–32. doi: 10.1038/cdd.2017.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hänzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinformatics. 2013;14:7. doi: 10.1186/1471-2105-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Y, Ni Z, Yan X, Dai X, Hu C, Zheng Y, et al. Targeting the MIR34C-5p-ATG4B-autophagy axis enhances the sensitivity of cervical cancer cells to pirarubicin. Autophagy. 2016;12:1105–17. doi: 10.1080/15548627.2016.1173798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng X, Xu Q, Zhang Y, Shen M, Zhang S, Mao F, et al. miR-34a inhibits progression of neuroblastoma by targeting autophagy-related gene 5. Eur J Pharmacol. 2019;850:53–63. doi: 10.1016/j.ejphar.2019.01.071. [DOI] [PubMed] [Google Scholar]
- 31.Pang J, Xiong H, Lin P, Lai L, Yang H, Liu Y, et al. Activation of miR-34a impairs autophagic flux and promotes cochlear cell death via repressing ATG9A: implications for age-related hearing loss. Cell Death Dis. 2017;8:e3079. doi: 10.1038/cddis.2017.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao HX, Miao CF, Sang LN, Huang YM, Zhang R, Sun L, et al. Circ_0009910 promotes imatinib resistance through ULK1-induced autophagy by sponging miR-34a-5p in chronic myeloid leukemia. Life Sci. 2020;243:117255. doi: 10.1016/j.lfs.2020.117255. [DOI] [PubMed] [Google Scholar]
- 33.Bartoszewska S, Cabaj A, Dąbrowski M, Collawn JF, Bartoszewski R. miR-34a and IRE1A/XBP-1(S) form a double-negative feedback Loop to regulate hypoxia-induced EMT, metastasis, chemo-resistance and autophagy. Cancers. 2019;33:1143. doi: 10.3390/cancers15041143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krammes L, Hart M, Rheinheimer S, Diener C, Menegatti J, Grässer F, et al. Induction of the endoplasmic-reticulum-stress response: microRNA-34a targeting of the IRE1α-branch. Cells. 2020;9:1442. [DOI] [PMC free article] [PubMed]
- 35.Hamurcu Z, Delibaşı N, Nalbantoglu U, Sener EF, Nurdinov N, Tascı B, et al. FOXM1 plays a role in autophagy by transcriptionally regulating Beclin-1 and LC3 genes in human triple-negative breast cancer cells. J Mol Med. 2019;97:491–508. doi: 10.1007/s00109-019-01750-8. [DOI] [PubMed] [Google Scholar]
- 36.Lin JZ, Wang WW, Hu TT, Zhu GY, Li LN, Zhang CY, et al. FOXM1 contributes to docetaxel resistance in castration-resistant prostate cancer by inducing AMPK/mTOR-mediated autophagy. Cancer Lett. 2020;469:481–9. doi: 10.1016/j.canlet.2019.11.014. [DOI] [PubMed] [Google Scholar]
- 37.Rokavec M, Huang Z, Hermeking H. Meta-analysis of miR-34 target mRNAs using an integrative online application. Comput Struct Biotechnol J. 2023;21:267–74. doi: 10.1016/j.csbj.2022.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bjørkøy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, et al. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol. 2005;171:603–14. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laoukili J, Kooistra MR, Brás A, Kauw J, Kerkhoven RM, Morrison A, et al. FoxM1 is required for execution of the mitotic programme and chromosome stability. Nat Cell Biol. 2005;7:126–36. doi: 10.1038/ncb1217. [DOI] [PubMed] [Google Scholar]
- 40.Zheng R, Wan C, Mei S, Qin Q, Wu Q, Sun H, et al. Cistrome Data Browser: expanded datasets and new tools for gene regulatory analysis. Nucleic Acids Res. 2019;47:D729–d735. doi: 10.1093/nar/gky1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ianevski A, Giri AK, Aittokallio T. SynergyFinder 2.0: visual analytics of multi-drug combination synergies. Nucleic Acids Res. 2020;48:W488–w493. doi: 10.1093/nar/gkaa216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iorio F, Knijnenburg TA, Vis DJ, Bignell GR, Menden MP, Schubert M, et al. A Landscape of Pharmacogenomic Interactions in Cancer. Cell. 2016;166:740–54. doi: 10.1016/j.cell.2016.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.The Cancer Genome Atlas (TCGA) consortium. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–7. [DOI] [PMC free article] [PubMed]
- 44.Martinez-Romero J, Bueno-Fortes S, Martín-Merino M, Ramirez de Molina A, De Las Rivas J. Survival marker genes of colorectal cancer derived from consistent transcriptomic profiling. BMC Genomics. 2018;19:857. doi: 10.1186/s12864-018-5193-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siemens H, Jackstadt R, Hünten S, Kaller M, Menssen A, Götz U, et al. miR-34 and SNAIL form a double-negative feedback loop to regulate epithelial-mesenchymal transitions. Cell Cycle. 2011;10:4256–71. doi: 10.4161/cc.10.24.18552. [DOI] [PubMed] [Google Scholar]
- 46.Mizushima N, Murphy LO. Autophagy assays for biological discovery and therapeutic development. Trends Biochem Sci. 2020;45:1080–93. doi: 10.1016/j.tibs.2020.07.006. [DOI] [PubMed] [Google Scholar]
- 47.Tanida I, Minematsu-Ikeguchi N, Ueno T, Kominami E. Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy. 2005;1:84–91. doi: 10.4161/auto.1.2.1697. [DOI] [PubMed] [Google Scholar]
- 48.Fischer M, Schade AE, Branigan TB, Müller GA, DeCaprio JA. Coordinating gene expression during the cell cycle. Trends Biochem Sci. 2022. [DOI] [PubMed]
- 49.Han Z, Zhang Y, Yang Q, Liu B, Wu J, Zhang Y, et al. miR-497 and miR-34a retard lung cancer growth by co-inhibiting cyclin E1 (CCNE1) Oncotarget. 2015;6:13149–63. doi: 10.18632/oncotarget.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y, et al. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447:1130–4. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zauli G, Voltan R, di Iasio MG, Bosco R, Melloni E, Sana ME, et al. miR-34a induces the downregulation of both E2F1 and B-Myb oncogenes in leukemic cells. Clin Cancer Res. 2011;17:2712–24. doi: 10.1158/1078-0432.CCR-10-3244. [DOI] [PubMed] [Google Scholar]
- 52.Xu X, Chen W, Miao R, Zhou Y, Wang Z, Zhang L, et al. miR-34a induces cellular senescence via modulation of telomerase activity in human hepatocellular carcinoma by targeting FoxM1/c-Myc pathway. Oncotarget. 2015;6:3988–4004. doi: 10.18632/oncotarget.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sadasivam S, Duan S, DeCaprio JA. The MuvB complex sequentially recruits B-Myb and FoxM1 to promote mitotic gene expression. Genes Dev. 2012;26:474–89. doi: 10.1101/gad.181933.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li D, Wei P, Peng Z, Huang C, Tang H, Jia Z, et al. The critical role of dysregulated FOXM1-PLAUR signaling in human colon cancer progression and metastasis. Clin Cancer Res. 2013;19:62–72. doi: 10.1158/1078-0432.CCR-12-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lamark T, Svenning S, Johansen T. Regulation of selective autophagy: the p62/SQSTM1 paradigm. Essays Biochem. 2017;61:609–24. doi: 10.1042/EBC20170035. [DOI] [PubMed] [Google Scholar]
- 56.Pohl C, Dikic I. Cellular quality control by the ubiquitin-proteasome system and autophagy. Science. 2019;366:818–22. doi: 10.1126/science.aax3769. [DOI] [PubMed] [Google Scholar]
- 57.Guardia CM, Tan XF, Lian T, Rana MS, Zhou W, Christenson ET, et al. Structure of human ATG9A, the only transmembrane protein of the core autophagy machinery. Cell Rep. 2020;31:107837. doi: 10.1016/j.celrep.2020.107837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sawa-Makarska J, Baumann V, Coudevylle N, von Bülow S, Nogellova V, Abert C, et al. Reconstitution of autophagosome nucleation defines Atg9 vesicles as seeds for membrane formation. Science. 2020;369:eaaz7714. [DOI] [PMC free article] [PubMed]
- 59.Maeda S, Yamamoto H, Kinch LN, Garza CM, Takahashi S, Otomo C, et al. Structure, lipid scrambling activity and role in autophagosome formation of ATG9A. Nat Struct Mol Biol. 2020;27:1194–201. doi: 10.1038/s41594-020-00520-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McIntosh HM. Chloroquine or amodiaquine combined with sulfadoxine-pyrimethamine for treating uncomplicated malaria. Cochrane Database Syst Rev. 2001;4:Cd000386. doi: 10.1002/14651858.CD000386. [DOI] [PubMed] [Google Scholar]
- 61.Cohen HG, Reynolds TB. Comparison of metronidazole and chloroquine for the treatment of amoebic liver abscess. A controlled trial. Gastroenterology. 1975;69:35–41. doi: 10.1016/S0016-5085(19)32633-2. [DOI] [PubMed] [Google Scholar]
- 62.Dörner T. Therapy: Hydroxychloroquine in SLE: old drug, new perspectives. Nat Rev Rheumatol. 2010;6:10–1. doi: 10.1038/nrrheum.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mauthe M, Orhon I, Rocchi C, Zhou X, Luhr M, Hijlkema KJ, et al. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy. 2018;14:1435–55. doi: 10.1080/15548627.2018.1474314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ferreira PMP, Sousa RWR, Ferreira JRO, Militão GCG, Bezerra DP. Chloroquine and hydroxychloroquine in antitumor therapies based on autophagy-related mechanisms. Pharmacol Res. 2021;168:105582. doi: 10.1016/j.phrs.2021.105582. [DOI] [PubMed] [Google Scholar]
- 65.Kinsey CG, Camolotto SA, Boespflug AM, Guillen KP, Foth M, Truong A, et al. Protective autophagy elicited by RAF→MEK→ERK inhibition suggests a treatment strategy for RAS-driven cancers. Nat Med. 2019;25:620–7. doi: 10.1038/s41591-019-0367-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sasaki K, Tsuno NH, Sunami E, Kawai K, Hongo K, Hiyoshi M, et al. Resistance of colon cancer to 5-fluorouracil may be overcome by combination with chloroquine, an in vivo study. Anticancer Drugs. 2012;23:675–82. doi: 10.1097/CAD.0b013e328353f8c7. [DOI] [PubMed] [Google Scholar]
- 67.Hashimoto D, Bläuer M, Hirota M, Ikonen NH, Sand J, Laukkarinen J. Autophagy is needed for the growth of pancreatic adenocarcinoma and has a cytoprotective effect against anticancer drugs. Eur J Cancer. 2014;50:1382–90. doi: 10.1016/j.ejca.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 68.Barger CJ, Zhang W, Hillman J, Stablewski AB, Higgins MJ, Vanderhyden BC, et al. Genetic determinants of FOXM1 overexpression in epithelial ovarian cancer and functional contribution to cell cycle progression. Oncotarget. 2015;6:27613–27. doi: 10.18632/oncotarget.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8:2281–308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaller M, Götz U, Hermeking H. Loss of p53-inducible long non-coding RNA LINC01021 increases chemosensitivity. Oncotarget. 2017;8:102783–102800. doi: 10.18632/oncotarget.22245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Siemens H, Jackstadt R, Kaller M, Hermeking H. Repression of c-Kit by p53 is mediated by miR-34 and is associated with reduced chemoresistance, migration and stemness. Oncotarget. 2013;4:1399–415. doi: 10.18632/oncotarget.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hamidi H, Lilja J, Ivaska J. Using xCELLigence RTCA instrument to measure cell adhesion. Bio Protoc. 2017;7:e2646. [DOI] [PMC free article] [PubMed]
- 73.Risso D, Ngai J, Speed TP, Dudoit S. Normalization of RNA-seq data using factor analysis of control genes or samples. Nat Biotechnol. 2014;32:896–902. doi: 10.1038/nbt.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu T, Hu E, Xu S, Chen M, Guo P, Dai Z, et al. clusterProfiler 4.0: a universal enrichment tool for interpreting omics data. Innovation. 2021;2:100141. doi: 10.1016/j.xinn.2021.100141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liberzon A, Birger C, Thorvaldsdóttir H, Ghandi M, Mesirov JP, Tamayo P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015;1:417–25. doi: 10.1016/j.cels.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bordi M, De Cegli R, Testa B, Nixon RA, Ballabio A, Cecconi F. A gene toolbox for monitoring autophagy transcription. Cell Death Dis. 2021;12:1044. doi: 10.1038/s41419-021-04121-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen Y, Lun AT, Smyth GK. From reads to genes to pathways: differential expression analysis of RNA-Seq experiments using Rsubread and the edgeR quasi-likelihood pipeline. F1000Res. 2016;5:1438. doi: 10.12688/f1000research.8987.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grossman RL, Heath AP, Ferretti V, Varmus HE, Lowy DR, Kibbe WA, et al. Toward a shared vision for cancer genomic data. N Engl J Med. 2016;375:1109–12. doi: 10.1056/NEJMp1607591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eide PW, Bruun J, Lothe RA, Sveen A. CMScaller: an R package for consensus molecular subtyping of colorectal cancer pre-clinical models. Sci Rep. 2017;7:16618. doi: 10.1038/s41598-017-16747-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–7. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Welch C, Chen Y, Stallings RL. MicroRNA-34a functions as a potential tumor suppressor by inducing apoptosis in neuroblastoma cells. Oncogene. 2007;26:5017–22. doi: 10.1038/sj.onc.1210293. [DOI] [PubMed] [Google Scholar]
- 83.Loewe S. The problem of synergism and antagonism of combined drugs. Arzneimittelforschung. 1953;3:285–90. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA expression profiling data obtained in this study were deposited in the Gene Expression Omnibus website (accession no. GSE227230). All data, analytic methods, and study materials will be made available to other researchers upon reasonable request.