Abstract
Objective:
The aim of this study was to determine the effects of bilateral trunk support during walking on trunk and leg kinematics and neuromuscular responses in children with cerebral palsy (CP).
Design:
Fourteen children with spastic CP (GMFCS level I to III) participated in this study. Children walked on a treadmill under 4 different conditions, i.e., without support (BASELINE), with bilateral support applied to the upper trunk (UTS), the lower trunk (LTS), and combined upper and lower trunk (CTS). The trunk and leg kinematics and muscle activity were recorded.
Results:
Providing bilateral support to the trunk had a significant impact on the displacement of the pelvis and trunk (p<0.003) during walking. Children’s weaker leg showed greater step length (p=0.032) and step height (p=0.012) in CTS compared to BASELINE, and greater step length in UTS (p=0.02) and CTS (p=0.022) compared to LTS. Changes in soleus EMG activity during stance phase of gait mirrored the changes in step length across all conditions.
Conclusion:
Providing bilateral upper or combined upper and lower trunk support during walking may induce improvements in gait performance, which may be due to improved pelvis kinematics. Improving trunk postural control may facilitate walking in children with CP.
Keywords: Children, Cerebral palsy, locomotion, trunk support, posture
1. INTRODUCTION
Cerebral palsy (CP) is the most common physical disability in children1. Many children with CP have poor walking abilities and manipulation skills2. For instance, children with CP often walk with a shorter step length, greater body sway3 and crouch gait pattern4. In addition, children with CP exhibit a higher energy cost during walking than their typical developing peers5, which may be due to less efficient gait patterns6. The abnormal gait patterns deteriorate with age if left untreated or inadequately addressed7. Thus, it is imperative to improve the gait pattern of children with CP through effective intervention approaches.
Treadmill training has been used to improve walking ability in children with CP, although it does not often show improvements in walking function in some of them8. Robotic-assisted devices attached to the treadmill can facilitate leg swing, improve toe walking, and reduce the labor intensity of physical therapists who conduct locomotor training9. While robotic-assisted treadmill training has shown improvements in overground walking speed10, endurance11, and balance12 in some children with CP, a randomized controlled study indicated that the robotic treadmill training was not more effective than conventional treadmill training13. Thus, there is a clear need to develop new intervention approaches to improve gait in children with CP.
Trunk control is a prerequisite for daily activities and walking14. Children with CP often present with compromised trunk control and balance15. Impairments in trunk control significantly limit their walking capacity16. Children with CP often show increased range of motion (RoM) of the thorax, pelvis, and spine in all planes in comparison with typically developed peers during walking17. The increased RoM of the thorax and spine during walking most likely are resulting from not only the compensatory movements for the lower limb impairments18 but also underlying trunk control deficits19. Impaired trunk postural control is one crucial factor contributing to gait impairments in children with CP20. Results from previous studies showed improved stride length and walking speed in children with CP after long-term hippotherapy21 or after trunk stability exercise22. In line with this, providing segmental trunk support that matches their intrinsic level of trunk control has demonstrated improved reaching motor performance in children with CP23. Thus, we postulated that improving trunk postural control by providing external trunk support force might induce improvements in walking performance in children with CP.
The purposes of this study were to determine the effects of lateral segmental trunk support during walking on trunk and leg kinematics and neuromuscular responses in children with CP. We hypothesized that providing lateral trunk support during treadmill walking would improve trunk and leg kinematic performance and enhance muscle activation of trunk and leg muscles, and that the effect of trunk support on gait performance would depend on the trunk level at which the support was provided.
2. METHODS
2.1. Participants
Fourteen children with CP aged 5 to 16 years old recruited from the outpatient clinic of the Shirley Ryan AbilityLab participated in the study (Table 1). Inclusion criteria were as follows: 1) age 4–16 years old; 2) spastic CP with GMFCS level ranges from I to III; 3) able to independently walk or walk with an assistance device as needed; 4) able to signal pain, fear or discomfort reliably; 5) able to follow instructions.
Table 1.
Demographic information for children with cerebral palsy and their normal walking speed.
| Patient number | Age yr, mon | Sex | BW kg | Type of CP | Weaker side | GMFC-s | AFO | AD | Limb length cm | Speed m/s | Length of spring extended cm | Order of testing condition |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 13, 10 | F | 49.7 | diplegia | R | I | R | No | 88 | 1.14 | 11.5 | 5 |
| 2 | 13, 6 | F | 45.2 | quadriplegia | L | I | No | No | 85 | 0.5 | 9.5 | 5 |
| 3* | 11, 5 | F | 38.2 | diplegia | R | I | No | No | 71 | 0.6 | 7 | 1 |
| 4 | 11, 6 | F | 35.5 | diplegia | R | I | No | No | 78 | 0.9 | 8.5 | 4 |
| 5 | 8, 10 | F | 43.1 | diplegia | R | II | No | No | 70 | 0.4 | 9.0 | 5 |
| 6 | 12, 5 | M | 35.3 | diplegia | L | I | No | No | 75 | 0.7 | 9.0 | 3 |
| 7 | 5, 2 | F | 17.1 | diplegia | R | I | No | No | 52 | 0.4 | 8.0 | 3 |
| 8 | 10, 1 | F | 24.6 | triplegia | L | III | Both | RW | 61 | 0.1 | 8.0 | 6 |
| 9 | 8, 5 | F | 25.2 | diplegia | L | II | No | No | 61 | 0.4 | 9.0 | 1 |
| 10 | 12, 8 | M | 64.5 | diplegia | R | II | No | No | 92 | 0.8 | 9.0 | 2 |
| 11 | 14, 11 | F | 48.6 | hemiplegia | R | I | No | No | 90 | 0.8 | 10 | 2 |
| 12* | 15, 8 | M | 75.8 | diplegia | R | I | Both | No | 99 | 0.9 | 10 | 4 |
| 13 | 14, 3 | M | 43.1 | triplegia | R | II | Both | No | 85 | 0.5 | 10 | 3 |
| 14 | 16, 9 | M | 56.2 | diplegia | L | II | Both | No | 95 | 0.4 | 10 | 5 |
Order of testing condition: 1: UTS-LTS-CTS; 2: UTS-CTS-LTS; 3: LTS-UTS-CTS; 4: CTS-UTS-LTS; 5: LTS-CTS-UTS; 6: CTS-LTS-UTS. Assistant device shown in the table indicated that the participant used it in community or home.
Data from participants #3 and #12 were excluded from data analysis. Abbreviations: UTS, upper trunk support; LTS, lower trunk support; CTS, combined trunk support; Yr, year; mon, month; BW, body weight; CP, cerebral palsy, GMFC-s, Gross Motor Function Classification System; AFO, ankle-foot orthoses; AD, assistive device; RW, reverse walker; cm, centimeter; m/s, meters per second; F, female; M, male. L, left; R, right;
Exclusion criteria were as follows: 1) orthotics that cross the knees; 2) lower extremity orthopedic surgery within 6 months or Botulinum toxin injection within the past 6 months; 3) severe lower extremity contractures, fractures, osseous instabilities; 4) cardiovascular instability. The study was approved by the Northwestern University Medical School Institute Review Board (Chicago, US). Signed consent forms were obtained from both children and their parents before their participation.
2.2. Experimental Apparatus
An instrumented treadmill (Bertec, Columbus, USA) was used for the walking tasks in this study. Two belts (width=10.5 cm) were attached to the participants’ trunk with one belt being set below the armpits for the upper trunk support (approximately T7–T10), and the other belt set above the pelvis for the lower trunk support (approximately L1–L4). Each belt was connected to two extension springs (stiffness=1.31N/mm) through two ropes from both the left and the right sides of the participant (Fig. 1). Each rope was attached to a fixed aluminum frame that was located at both sides of the treadmill. Four tension/compression loadcells were inserted in series to the springs to record the pulling force applied to the participants during treadmill walking.
Figure 1.
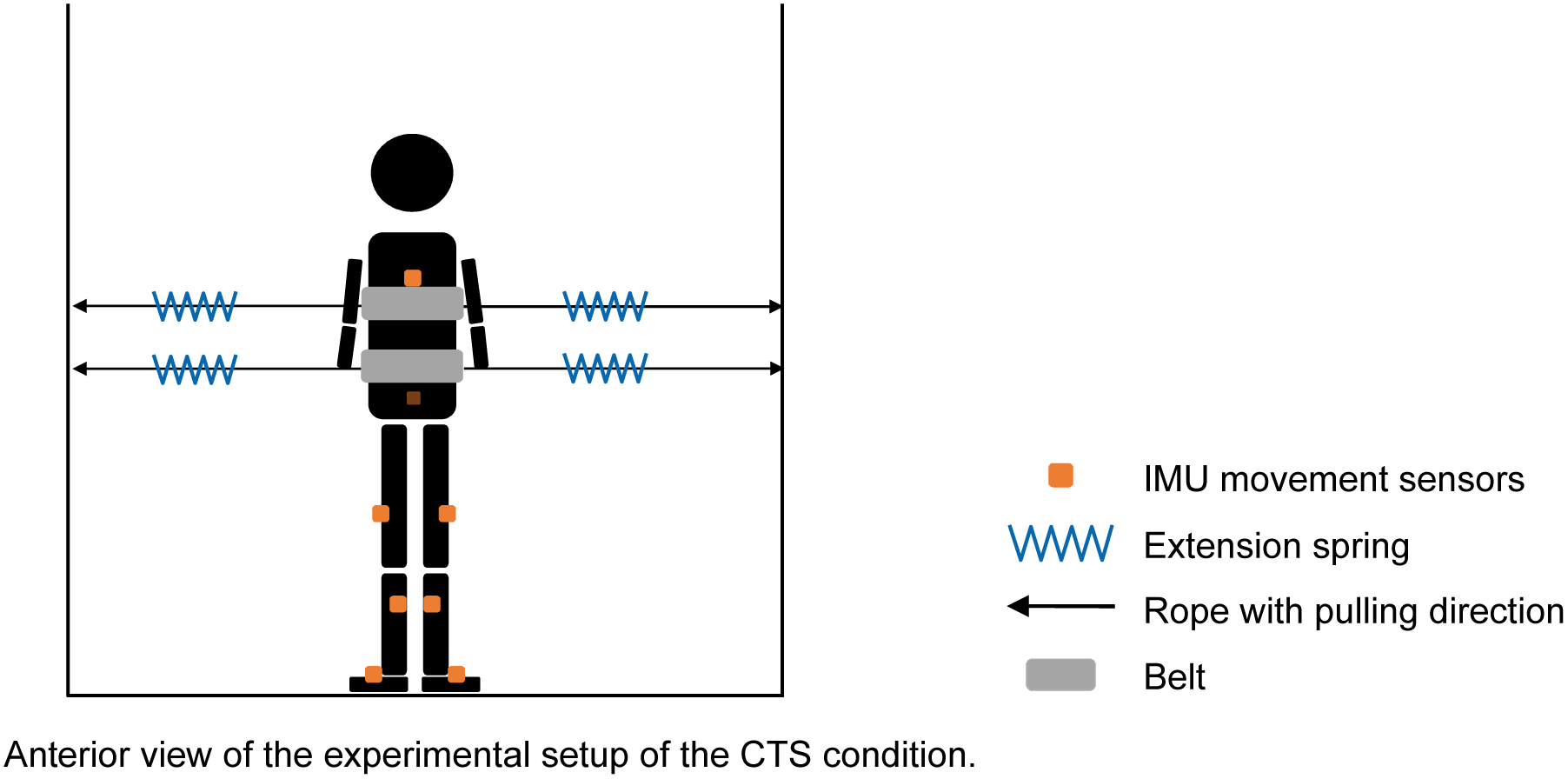
Experimental setup. A child was standing on a treadmill with two belts attached to the trunk (e.g., CTS condition). The saw curves (i.e., blue curves) represent four extension springs that were used in experimental testing conditions (see more details in the protocol). The orange squares represent IMU motion sensors that were attached to body segments for recording kinematic data. The dark orange square represents a motion sensor that was attached posteriorly to the sacrum, which was invisible from the anterior view. Black lines with an arrow represent the ropes and pulling force direction. Abbreviations: CTS, combined trunk support; IMU, inertial measurement unit.
2.3. Protocol
Participants walked on a treadmill and wore an overhead harness, used for safety only. Participants were allowed to hold onto the front or side rail (s) for safety and were also required to keep attachments and holding strategy consistent for all testing sessions (see Table 1). The treadmill speed was set at their self-selected speed, which was determined based on their overground walking speed before the data collection, and was kept the same for all testing sessions. Before data collection, participants also walked at their fast speed for 30 strides. EMG data during this time period were recorded and used to normalize the EMG data in other conditions.
Before the application of trunk support, participants walked on a treadmill for one minute at their comfortable speed (BASELINE), then, walked on the treadmill under three trunk supporting conditions: 1) Upper Trunk Support, UTS, a support force was provided to the trunk bilaterally through the belt positioned at the upper level of the trunk; 2) Lower Trunk Support, LTS, a support force was provided to the trunk bilaterally through the belt positioned at the lower level of the trunk; 3) Combined Trunk Support, CTS, a support force was provided bilaterally through the belts positioned at both the upper and lower levels of the trunk (see CTS in Fig. 1a). The order of the three testing conditions was randomized across participants. Each test condition lasted for one minute with a one-minute standing break inserted between conditions. A pretension force (i.e., the spring was pre-stretched 7–11.5 cm, which was adjusted based on participants’ tolerance, Table 1) was applied and remained the same on both sides and all three testing conditions.
2.4. Data Recording
The trunk and bilateral lower limb kinematics were recorded (sampling frequency was 100 Hz) using Xsens wearable inertial measurement unit (IMU) system (Xsens, Enschede, Netherlands). We attached 8 inertial sensors on both feet, shanks, thighs, sacrum, and sternum through straps (see Fig. 1b). The placement of these sensors was determined based on the instructions of Xsens system. The displacement data of the C7, pelvis, hip, knee, and ankle joints, as well as the angular displacement data of the hip, knee, and ankle joints were obtained from the sensors using software provided by the Xsens system. Ground reaction forces (GRF) were recorded using an instrumented split-belt treadmill (Bertec, Columbus, Ohio) with a 1000 Hz sampling rate.
Surface electromyographic (EMG) activity was recorded by using Trigno wireless EMG system (Delsys Inc., Boston, USA) with the signals amplified (×1000) and band-pass filtered (20–450 Hz), and then sampled with an A/D board (National Instruments, Austin, USA) at 500 Hz using a customized LabVIEW program (National Instruments, Austin, USA). The kinematic, kinetic, and EMG data were synchronized using the same trigger signals. The EMGs of following 12 muscles were recorded from the more affected (weaker) body side: tibialis anterior (TA), soleus (SOL), gastrocnemius medialis (MG), rectus femoris (RF), vastus medialis (VM), medial hamstring - semitendinosus (MH), hip adductor – adductor magnus (ADD), hip abductor - gluteus medius (ABD), rectus abdominis (RA), external oblique abdominis (OBL), erector spinae - longissimus (ES), and upper trapezius (TRAP). The weaker body side was determined based on children’s self-reported and confirmed by a licensed physical therapist using Manual Muscle Test. The locations of EMG electrodes were positioned on the middle of the muscle belley.
2.5. Data Analysis
All analyses of kinematic, kinetic, and EMG data were conducted using MATLAB. Specifically, kinematic data obtained from the Xsens IMU system were lowpass filtered using a Butterworth 4th order filter with a 10 Hz cutoff frequency. Gait cycles were first identified according to the foot contact data provided by the Xsens IMU system and then confirmed by visual inspections. All variables were averaged over the middle 30 steps in each condition for each participant, with the exception of one child (GMFCS level III), for whom, data from only 10 steps were used due to slow walking speed.
Kinematic variables including step length, step height, peak hip flexion during swing phase, peak hip extension during stance phase, hip range of motion (RoM) in the sagittal plane, peak knee extension during mid-stance phase, pelvis and C7 displacement in the mediolateral (ML) direction over a gait cycle, single-leg support time, stance time, and swing time were calculated. Specifically, step length of each leg was calculated as the distance between the two ankles in the anterior-posterior (AP) direction at the instant of foot contact. Step height was defined as the maximum vertical displacement of the ankle over a step cycle. The step length was further normalized to the limb length (i.e., the height from floor to great trochanter of each child). Peak knee extension during mid-stance was defined as the maximum knee extension during the 33%−66% of stance phase.
EMG data were high-pass filtered using a 4th-order Butterworth filter with a 10 Hz cutoff frequency, and notch filtered (55–65 Hz and 115–125 Hz), rectified, and smoothed (40 Hz). The integrated muscle activity during the single-limb stance phase and swing phase were calculated and normalized to the integrated muscle activity of each muscle during fast-speed walking of each child. The GRF data were re-sampled down to 500 Hz (in order to synchronize to EMG data), low-pass filtered using a 2nd-order Butterworth filter with a 30 Hz cutoff frequency. Peak propulsive force was defined as the maximum anterior GRF during 31–100% of the stance phase (propulsion phase). We included GRF data only from 8 children, and GRF data from other children were not included because these children had too many steps in which the standing foot was across over the two force plates.
2.6. Statistical Analysis
The Shapiro-Wilk W test was used for the assessment of normal distribution. Mauchly’s test was used for assessing violations of sphericity. Greenhouse-Geisser correction was used when data violated sphericity assumption. Effects of trunk support (BASELINE, UTS, LTS, and CTS) on all variables were analyzed using repeated measures one-way ANOVAs. Post-hoc tests with Least Significant Difference (LSD) correction were used to compare differences between conditions24. All statistical analyses were performed using IBM SPSS with the level of significance at p<0.05 with the power greater than 0.8.
3. RESULTS
3.1. Step Length and Step Height
Foot trajectories in four conditions from one child are shown in Figure 2a. This child demonstrated an increase in stride length in the AP direction on the weaker leg for the CTS and UTS conditions, although the increment was smaller for the UTS condition, and an increase in step height for the LTS condition, in comparison to BASELINE. A similar trend was shown for the stronger side, however, the increment was smaller than that of the weaker side.
Figure 2.

(a) Foot trajectories in the anterior-posterior (AP) and vertical directions from one representative child with diplegia CP (13 years, GMFCS level I) in 4 different conditions (i.e., baseline, UTS, LTS, and CTS). Each curve shown in the figure was the average across 10 strides for each condition. (b) Group average of step length of both legs for 4 conditions. (c) Group average of step height of both legs for 4 conditions. Bars and whiskers represent mean and standard error for all conditions. Asterisk (*) indicates a significant difference between two conditions. Abbreviations: AP, anterior-posterior; UTS, upper trunk support; LTS, lower trunk support; CTS, combined trunk support; m, meter.
The group average of step length and step height of both legs in four conditions are shown in Figure 2b and 2c (data from 12 participants were analyzed with data from two participants were excluded, one participant seemed to need more time to familiarize the experimental setup and showed a walking pattern that was very different from the rest participants, data from one participant were considered as outlier, which was defined as the outliers because the values were at a 1.5 interquartile range (IQR) above the third quartile values (Q3))). ANOVAs indicated that providing bilateral trunk support had a significant effect on step length [F(3, 33) = 3.60, p = 0.02] and step height [F(3, 33) = 4.05, p = 0.015] of the weaker leg. Post-hoc analysis indicated that participants showed greater step length of the weaker leg in the CTS condition than in BASELINE [i.e., 52% (45–58%) of limb length vs. 46% (40–52%) of limb length, mean (95% confidence interval, CI), p = 0.032, 12% increase], and greater than in LTS [45% (36–53%) of limb length, p = 0.022] condition. Post-hoc analysis also indicated a significant difference in step length of the weaker leg between the LTS and UTS conditions [i.e., 45% (36–53%) vs. 49.2% (42–57%), p = 0.02]. Moreover, participants showed a higher step height of the weaker leg in the CTS condition [0.096 m (0.079–0.112)] than in BASELINE [0.086 m (0.066–0.105), p = 0.014, 11.6% increase]. Additionally, the step length of the stronger leg in the CTS condition [45% (37.8–52.2%) of limb length] tended to increase from BASELINE [39.2% (31.5–46.8%) of limb length], although this was not significant [F(3, 33) = 2.50, p = 0.077, 15% increase]. The change in the step height of the stronger leg was not significant across conditions, p = 0.261.
3.2. Kinematics of Pelvis and Trunk
Providing lateral support to the trunk had a significant effect on the displacement of the pelvis [F(3, 33) = 7.648), p = 0.001], and trunk [(F(3, 33) = 4.617, p = 0.008, ANOVA]. Post-hoc analysis indicated a significant decrease in pelvis displacement from BASELINE to the CTS condition (p = 0.009) and from the LTS to CTS conditions (p = 0.003), Table 2. In addition, a significant difference in trunk displacement was detected between the LTS and CTS conditions (p = 0.002).
Table 2.
Group average of the pelvis and trunk displacements in the mediolateral (ML) direction over a gait cycle for all testing conditions.
| Displacement in ML direction | BASELINE | UTS | LTS | CTS | P-values | |
|---|---|---|---|---|---|---|
| (cm) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | ANOVA | Post-hoc comparison |
| pelvis | 8.0 (6.1, 10.0) | 6.7 (5.3, 8.1) | 8.5 (6.7, 10.3) | 4.7 (3.5, 6.0) | 0.001 | 0.009* (BASELINE/CTS) 0.003* (LTS/CTS) |
| C7 | 9.6 (6.3, 12.8) | 9.4 (6.3, 12.5) | 12.0 (8.8, 15.2) | 6.8 (4.6, 8.9) | 0.008 | 0.294 (BASELINE/CTS) 0.002* (LTS/CTS) |
Note. The asterisk indicates a significant difference between two conditions. Abbreviations: ML, mediolateral; UTS, Upper Trunk Support; LTS, Lower Trunk Support; CTS, Combined Trunk Support; 95% CI, 95% confidence interval.
The bilateral trunk support also had a significant impact on the hip RoM in the sagittal plane of the stronger side [F(3, 33) = 4.65, p = 0.008], and the peak hip flexion angle of both limbs [stronger side, F(3, 33) = 4.394; p = 0.01, weaker side, F(3, 33) = 3.912, p = 0.017, Table 3]. Post-hoc analysis indicated that participants showed significantly greater hip RoM of the stronger side in the CTS than that in BASELINE and in the LTS. The peak hip flexion of the stronger leg was greater in the UTS and CTS than that in the LTS. The peak hip flexion of the weaker limb was greater in the CTS than that in BASELINE and the LTS. Additionally, ANOVAs revealed that no significant difference was observed for the hip RoM of the weaker side (Table 2) or the mid-stance peak knee extension (stronger, p = 0.113; weaker, p = 0.116). No significant differences in propulsive forces, single-limb support time, stance time, and swing time were detected (p > 0.05).
Table 3.
Group average of hip sagittal range of motion (RoM), peak extension angles, and peak flexion angles for all testing conditions.
| Joint angles | BASELINE | UTS | LTS | CTS | P-values | |
|---|---|---|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | ANOVA | Post-hoc comparison | |
| Hip (strong) | ||||||
| Sagittal ROM (⁰) | 41.5 (36.5, 46.5) | 44.7 (38.2, 51.3) | 41.8 (35.1, 48.6) | 49.0 (44.9, 53.1) | 0.008 | 0.001* (BASELINE/CTS) 0.021* (LTS/CTS) |
| Peak extension of stance (⁰) | −8.6 (−12.3, −4.9) | −8.6 (−13.9, −3.3) | −9.2 (−15.6, −2.8) | −12.3 (−15.6, −2.8) | 0.231 | NA |
| Peak flexion of swing (⁰) | 31.6 (28.4, 35.5) | 33.9 (30.4, 41) | 30.1 (28.2, 32.3) | 34.8 (31.1, 41.4) | 0.01 | 0.05 (BASELINE/CTS) 0.043* (UTS/LTS) 0.013* (LTS/CTS) |
| Hip (weak) | ||||||
| Sagittal ROM (⁰) | 39.3 (37.1, 41.6) | 40.0 (34.8, 46) | 40.1 (33.8, 46.5) | 43.5 (37.8, 49.2) | 0.338 | NA |
| Peak extension of stance (⁰) | −6.6 (−9.4, −3.8) | −5.6 (−10.4, −0.9) | −7.5 (−13.4, −1.6) | −6.9 (−12.4, −1.5) | 0.843 | NA |
| Peak flexion of swing (⁰) | 32.3 (30.2, 34) | 34.0 (30.2, 38.6) | 32.0 (28.4, 35.1) | 36.7 (32, 40.3) | 0.017 | 0.033* (BASELINE/CTS) 0.004* (LTS/CTS) |
Note. Positive numbers represent flexion; negative numbers represent extension. The asterisk indicates a significant difference between two conditions. Abbreviations: UTS, Upper Trunk Support; LTS, Lower Trunk Support; CTS, Combined Trunk Support; 95% CI, 95% confidence interval; RoM, Range of Motion.
3.3. Integrated EMG
The group averages of integrated EMG of all recorded muscles during the single-leg stance phase are illustrated in Figure 3. One-way ANOVA revealed significant differences of the integrated EMG across conditions for SOL [F(3, 30) = 5.61, p = 0.004], VM [F(3, 33) = 4.84, p = 0.007], and ADD [F(3, 33) = 4.90, p = 0.006] (Fig. 3). Post-hoc analysis indicated that participants showed significantly greater SOL activity in the CTS [82% (69–94%), mean (CI)] than in BASELINE [71% (59–83%), p=0.008], greater SOL activity in the CTS than in the LTS [64% (48–80%), p = 0.005], and greater SOL activity in the UTS [76% (61–91%)] than in the LTS (p = 0.027). The VM activity was greater in the UTS [72% (51–93%), p = 0.02], the LTS [61% (48–73%), p = 0.03], and the CTS [68% (51–84%), p = 0.02] than in BASELINE [53% (42–64%)]. In addition, post-hoc analysis indicated that participants showed greater ADD activity in the UTS [69% (57–82%), p = 0.001] and the LTS [69% (59–80%), p = 0.003] than in BASELINE [52% (43–62%)]. No significant difference was observed for other muscles (p > 0.05).
Figure 3.
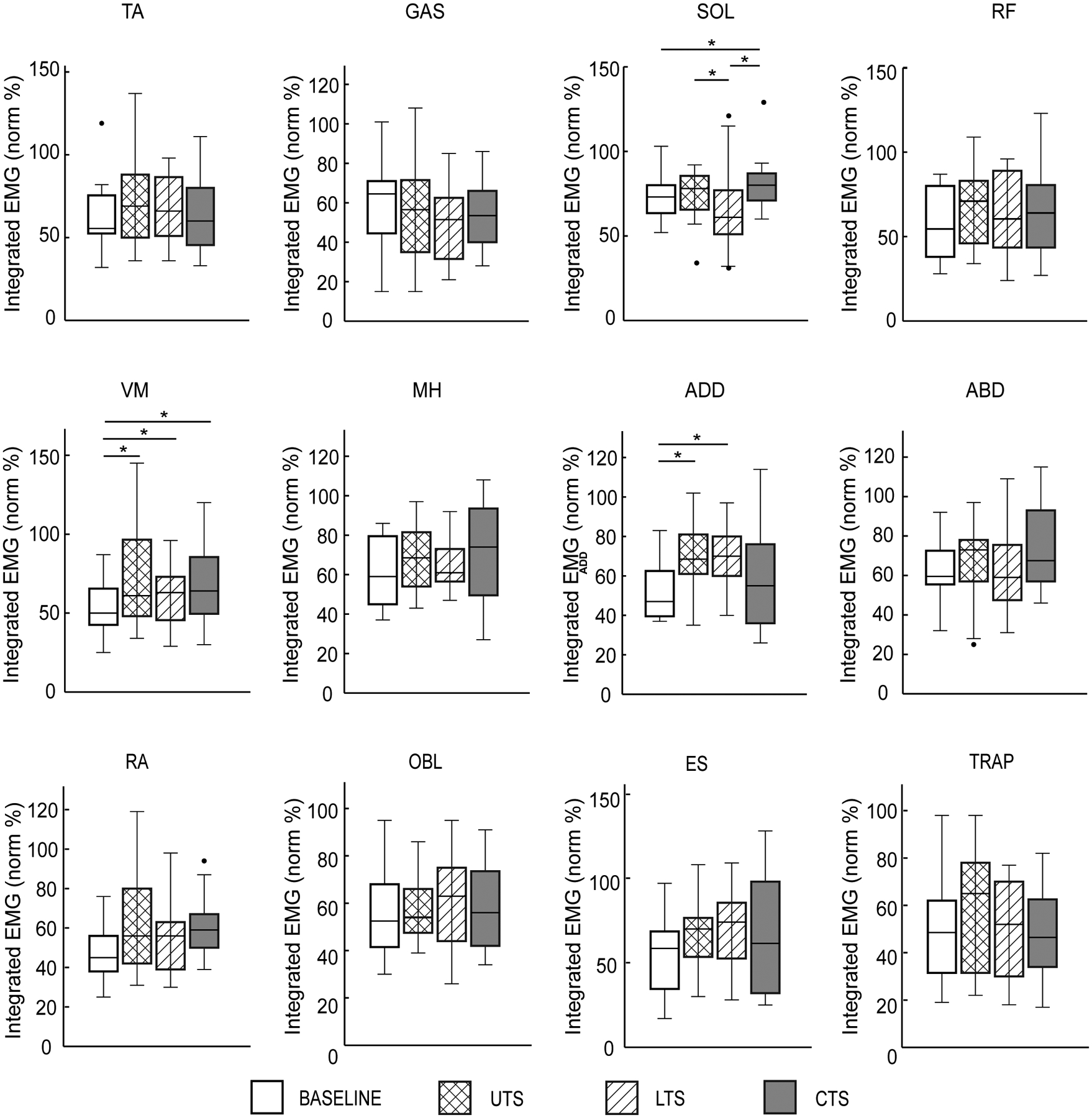
Group average of integrated EMGs of 12 muscles from the weaker side during the single-leg stance phase for each condition. Black dots represent outliers. Abbreviations: TA, tibialis anterior; SOL, soleus, MG, gastrocnemius medialis; RF, rectus femoris; VM, vastus medialis; MH, medial hamstring; ADD, hip adductor; ABD, hip abductor; RA, rectus abdominis; OBL, external oblique abdominis; ES, erector spinae; TRAP, trapezius. Bars and whiskers represent mean and standard error for all conditions. Asterisk (*) indicates a significant difference between two conditions.
ANOVAs revealed a significant effect of trunk support on the integrated EMG of ADD [F(3, 33) = 5.80, p = 0.003] during swing phase. Post-hoc analysis indicated that the ADD activity was greater in the UTS (p < 0.001) and CTS (p = 0.007) than that in BASELINE.
4. DISCUSSION
We examined the kinematic and EMG responses of children with CP to bilateral trunk support protocols at different segmental levels during treadmill walking. We found that providing bilateral trunk support may improve gait performance in children with CP. Specifically, children with CP showed improvements in step length and step height on the weaker side, hip RoM on the stronger side, and peak hip flexion during swing phase of gait for both sides when both the upper and lower trunk support were applied. Children with CP also showed increases in muscle activity of SOL, VM, ADD of the weaker limb during the single-leg support phase, and in ADD of the weaker limb during swing phase when trunk support was applied in the UTS and CTS conditions.
4.1. Effect of Trunk Support on Lower Limb Motor Performance
Applying bilateral support to the trunk during treadmill walking may facilitate leg swing in children with CP. Many children with CP show impairments in walking performance, such as shorter step length compared to their typically developed peers3, and excessive trunk movements in all planes19. The impairments in walking performance may be partially due to the impairments in trunk control, one crucial factor contributing to gait impairment in children with CP20, and trunk control was the strongest predictor for gait capacity in children with CP25. Results from this study indicate that providing bilateral trunk support during walking may facilitate trunk postural control and improve pelvis movement. For instance, on average, the displacement of C7 and pelvis in the ML direction reduced 29% and 41%, respectively, with the application of both upper and lower trunk support in comparison to baseline, see Table 2. The improvements in trunk and pelvis movement may provide a dynamic stable supporting platform for the swing leg, resulting in improvements in step length and step height. These results are partially consistent with previous long-term intervention studies22, which indicated that long-term hippotherapy may induce improvements in trunk control26 and gait in children with CP21. It was suggested that improving trunk postural control may improve pelvis kinematics, indicated as improved pelvic anterior tilt after hippotherapy27, resulting in improvement in gait after hippotherapy. Thus, these results suggest that improving trunk postural control, which was achieved by providing lateral trunk support force in this case, may facilitate leg swing in children with CP.
In addition, results from this study suggest that the trunk level where the support force was applied may have an impact on the effect of facilitating leg swing during walking in children with CP. For instance, while applying trunk support force to the upper trunk or both the upper and lower trunk may improve leg swing, resulting in improvements in step length and step height, applying trunk support force to the lower trunk only was less effective in facilitating leg swing. One possible reason is that the moment arm of the trunk support force that was applied to the lower trunk relative to the pelvis was smaller than that of the trunk support force that was applied to the upper trunk. Thus, while the magnitude of the trunk support force was comparable between the two conditions, the moment generated by the lower trunk support force relative to the pelvis was much smaller than that generated by the upper trunk force. In other words, the moment generated by the lower trunk support force might be not large enough to provide effective support to the upper trunk during walking.
4.2. Effect of Trunk Support on iEMG of Leg Muscles
Applying bilateral trunk support may induce enhanced muscle activity of the weaker leg during stance phase of gait in children with CP. Children with CP often demonstrate muscle weakness of the trunk and lower limbs in comparison to their typically developed peers28. In the present study, muscle activity of VM, SOL, and ADD of the weaker leg significantly increased with the application of trunk support. One possible mechanism is that improved trunk control may allow participants to shift more weight onto the weaker leg during stance phase of gait. Thus, sensory afferents from Golgi tendon organs in extensor muscles and cutaneous afferents from the sole of the foot may be enhanced in response to increased leg loading29. The enhanced afferents may increase the amplitude of leg extensors muscle activity, e.g., VM and SOL, through spinal reflex pathways29. In this study, we did not observe a significant change in peak knee extension angle during stance phase of gait, which might be because that most participants were high function patients who did not show much impairments in knee extension, e.g., crouch gait, or the effect of trunk supporting force on knee joint extension angle during stance phase might be only modest to reach a significance. In addition, increased activation of SOL and ADD may contribute to a more stable single-leg stance of the weaker leg, which may facilitate swing performance of the contralateral leg, resulting in an increase in hip RoM of the stronger leg.
4.3. Potential Clinical Applications
Results from this study may have potential clinical applications. For instance, results from this study suggest that improved trunk postural control may facilitate leg swing during walking in children with CP. Thus, physical therapists may develop targeted intervention approaches to improve trunk postural control in children with CP, such as applying either a perturbation or supporting force to the trunk during walking, which may induce improvements in walking performance.
4.4. Limitations
This study has the following limitations. First, due to the limited number of A/D board channels, EMG signals only from one side of the body (i.e., the weaker side) were recorded. Second, the sample size was relatively small and participants were heterogeneous, although, it was still large enough to detect significant differences in primary outcomes. Further studies with a larger cohort of homogeneous participants are needed. Third, the majority of participants are high-function children with CP (i.e., the GMFC levels were I and II). It is unknown whether similar responses in kinematics and EMG for those low function children with CP (e.g., the GMFC levels III) could be observed. Further studies are needed to examine the kinematics and EMG responses in children with CP who show more impairments in trunk postural control (e.g., these children with GMFC level III). Fourth, for safety, participants were allowed to hold onto handrails during walking, which might have an impact on the effects of trunk support on walking performance. However, there was no significant difference in the change in step length from baseline to the condition of CTS between the participants who held onto handrails (n = 5) and the participants who did not (n=7) (t test, p=0.67). The same was true when comparing the difference in step length between the condition of UTS and LTS (p=0.65), although it was not the case between the condition of LTS and CTS (p=0.039). In addition, if one participant preferred to hold onto the handrails, the participant was required to hold onto the handrails for all conditions. Thus, we do not believe that using the handrails systematically impacted our results. Fifth, five participants wore AFO during test, which might affect their trunk postural control30. However, these participants wore AFO for all the testing conditions. Thus, wearing AFO might not systematically impact our results. Sixth, in this study, we only tested the acute effect of the trunk supporting force on the trunk motor performance using a short-term experimental protocol (e.g., the force was applied only for 1 minute). We do not know how children with CP will adapt to the repeated application of the lateral supporting force for a long period of time and whether repeated application of the lateral supporting force through a long-term training will induce functional improvements in trunk postural control and walking in children with CP. However, using this short-term experimental protocol allows for the study of long-term motor learning in a more controlled and timely way. Finally, these tasks were conducted during treadmill walking, it is unknown whether the improved walking performance could be transferred to overground walking.
5. CONCLUSION
Providing bilateral trunk support during treadmill walking may improve gait performances, suggesting that improved trunk postural control may facilitate walking performance in children with CP. These findings suggest that intervention approaches targeting trunk postural control may be used in clinical training to improve not only trunk postural control but also gait performance in children with CP.
What is Known:
Trunk postural control is a prerequisite for daily activities and walking. Children with cerebral palsy (CP) often present with compromised trunk control and balance. Impairments in trunk postural control significantly limit their walking capacity.
What is New:
Providing bilateral support to the trunk had a significant impact on the displacement of the pelvis and trunk during walking. In addition, providing bilateral upper or combined upper and lower trunk support during walking may induce improvements in gait performance, which may be due to improved pelvis kinematics, in children with CP. Improving trunk postural control may facilitate walking in children with CP.
Acknowledgments:
This work was supported by the National Institute of Health (R01NS115487).
List of abbreviations:
- ANOVA
Analysis of variance
- ABD
hip abductor - gluteus medius
- ADD
hip adductor – magnus
- AP
anterior-posterior
- CI
confidence interval
- CP
cerebral palsy
- CTS
combined upper and lower trunk support
- EMG
Electromyography
- ES
erector spinae – longissimus
- GRF
ground reaction force
- GMFCS
Gross Motor Function Classification System
- IMU
inertial measurement unit
- LTS
the lower trunk support
- MG
gastrocnemius medialis
- MH
medial hamstring
- OBL
external oblique abdominis
- RA
rectus abdominis
- RF
rectus femoris
- RoM
range of motion
- SOL
soleus
- TA
tibialis anterior
- TRAP
trapezius
- UTS
upper trunk support
- VM
vastus medialis
Footnotes
Conflict of interest statement:
None of the authors have potential conflicts of interest to be disclosed.
Availability of data:
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Reddihough DS, Collins KJ. The epidemiology and causes of cerebral palsy. Aust J Physiother. 2003;49(1):7–12. [DOI] [PubMed] [Google Scholar]
- 2.Woollacott MH, Shumway-Cook A. Postural dysfunction during standing and walking in children with cerebral palsy: what are the underlying problems and what new therapies might improve balance? Neural Plast. 2005;12(2–3):211–219; discussion 263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heyrman L, Feys H, Molenaers G, et al. Three-dimensional head and trunk movement characteristics during gait in children with spastic diplegia. Gait Posture. Sep 2013;38(4):770–776. [DOI] [PubMed] [Google Scholar]
- 4.Rethwilm R, Bohm H, Dussa CU, Federolf P. Excessive Lateral Trunk Lean in Patients With Cerebral Palsy: Is It Based on a Kinematic Compensatory Mechanism? Front Bioeng Biotechnol. 2019;7:345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnston TE, Moore SE, Quinn LT, Smith BT. Energy cost of walking in children with cerebral palsy: relation to the Gross Motor Function Classification System. Dev Med Child Neurol. Jan 2004;46(1):34–38. [DOI] [PubMed] [Google Scholar]
- 6.Van de Walle P, Hallemans A, Truijen S, et al. Increased mechanical cost of walking in children with diplegia: the role of the passenger unit cannot be neglected. Res Dev Disabil. Nov-Dec 2012;33(6):1996–2003. [DOI] [PubMed] [Google Scholar]
- 7.Johnson DC, Damiano DL, Abel MF. The evolution of gait in childhood and adolescent cerebral palsy. J Pediatr Orthop. May-Jun 1997;17(3):392–396. [PubMed] [Google Scholar]
- 8.Valentin-Gudiol M, Mattern-Baxter K, Girabent-Farres M, Bagur-Calafat C, Hadders-Algra M, Angulo-Barroso RM. Treadmill interventions with partial body weight support in children under six years of age at risk of neuromotor delay. Cochrane Database Syst Rev. Dec 7 2011(12):CD009242. [DOI] [PubMed] [Google Scholar]
- 9.Willoughby KL, Dodd KJ, Shields N. A systematic review of the effectiveness of treadmill training for children with cerebral palsy. Disabil Rehabil. 2009;31(24):1971–1979. [DOI] [PubMed] [Google Scholar]
- 10.Carvalho I, Pinto SM, Chagas DDV, Praxedes Dos Santos JL, de Sousa Oliveira T, Batista LA. Robotic Gait Training for Individuals With Cerebral Palsy: A Systematic Review and Meta-Analysis. Arch Phys Med Rehabil. Nov 2017;98(11):2332–2344. [DOI] [PubMed] [Google Scholar]
- 11.Meyer-Heim A, Ammann-Reiffer C, Schmartz A, et al. Improvement of walking abilities after robotic-assisted locomotion training in children with cerebral palsy. Arch Dis Child. Aug 2009;94(8):615–620. [DOI] [PubMed] [Google Scholar]
- 12.Dodd KJ, Foley S. Partial body-weight-supported treadmill training can improve walking in children with cerebral palsy: a clinical controlled trial. Dev Med Child Neurol. Feb 2007;49(2):101–105. [DOI] [PubMed] [Google Scholar]
- 13.Druzbicki M, Rusek W, Snela S, et al. Functional effects of robotic-assisted locomotor treadmill thearapy in children with cerebral palsy. J Rehabil Med. Apr 2013;45(4):358–363. [DOI] [PubMed] [Google Scholar]
- 14.Kavanagh JJ, Morrison S, Barrett RS. Coordination of head and trunk accelerations during walking. Eur J Appl Physiol. Jul 2005;94(4):468–475. [DOI] [PubMed] [Google Scholar]
- 15.Nashner LM, Shumway-Cook A, Marin O. Stance posture control in select groups of children with cerebral palsy: deficits in sensory organization and muscular coordination. Exp Brain Res. 1983;49(3):393–409. [DOI] [PubMed] [Google Scholar]
- 16.Curtis DJ, Butler P, Saavedra S, et al. The central role of trunk control in the gross motor function of children with cerebral palsy: a retrospective cross-sectional study. Dev Med Child Neurol. Apr 2015;57(4):351–357. [DOI] [PubMed] [Google Scholar]
- 17.Swinnen E, Goten LV, De Koster B, Degelaen M. Thorax and pelvis kinematics during walking, a comparison between children with and without cerebral palsy: A systematic review. NeuroRehabilitation. 2016;38(2):129–146. [DOI] [PubMed] [Google Scholar]
- 18.Perry J Gait Analysis: Normal and Pathological Function. J Sports Sci Med. 2010;9(2):353–353. [Google Scholar]
- 19.Heyrman L, Feys H, Molenaers G, et al. Altered trunk movements during gait in children with spastic diplegia: compensatory or underlying trunk control deficit? Res Dev Disabil. Sep 2014;35(9):2044–2052. [DOI] [PubMed] [Google Scholar]
- 20.Balzer J, Marsico P, Mitteregger E, van der Linden ML, Mercer TH, van Hedel HJA. Influence of trunk control and lower extremity impairments on gait capacity in children with cerebral palsy. Disabil Rehabil. Sep 24 2017:1–7. [DOI] [PubMed] [Google Scholar]
- 21.Kwon JY, Chang HJ, Lee JY, Ha Y, Lee PK, Kim YH. Effects of hippotherapy on gait parameters in children with bilateral spastic cerebral palsy. Arch Phys Med Rehabil. May 2011;92(5):774–779. [DOI] [PubMed] [Google Scholar]
- 22.El Shemy SA. Trunk endurance and gait changes after core stability training in children with hemiplegic cerebral palsy: A randomized controlled trial. J Back Musculoskelet Rehabil. 2018;31(6):1159–1167. [DOI] [PubMed] [Google Scholar]
- 23.Santamaria V, Rachwani J, Saavedra S, Woollacott M. Effect of Segmental Trunk Support on Posture and Reaching in Children With Cerebral Palsy. Pediatr Phys Ther. fall 2016;28(3):285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayter AJ. The Maximum Familywise Error Rate of Fishers Least Significant Difference Test. J Am Stat Assoc. Dec 1986;81(396):1000–1004. [Google Scholar]
- 25.Balzer J, Marsico P, Mitteregger E, van der Linden ML, Mercer TH, van Hedel HJA. Influence of trunk control and lower extremity impairments on gait capacity in children with cerebral palsy. Disabil Rehabil. Dec 2018;40(26):3164–3170. [DOI] [PubMed] [Google Scholar]
- 26.Shurtleff TL, Standeven JW, Engsberg JR. Changes in dynamic trunk/head stability and functional reach after hippotherapy. Arch Phys Med Rehabil. Jul 2009;90(7):1185–1195. [DOI] [PubMed] [Google Scholar]
- 27.Zadnikar M, Kastrin A. Effects of hippotherapy and therapeutic horseback riding on postural control or balance in children with cerebral palsy: a meta-analysis. Dev Med Child Neurol. Aug 2011;53(8):684–691. [DOI] [PubMed] [Google Scholar]
- 28.Wiley ME, Damiano DL. Lower-extremity strength profiles in spastic cerebral palsy. Dev Med Child Neurol. Feb 1998;40(2):100–107. [DOI] [PubMed] [Google Scholar]
- 29.Duysens J, Clarac F, Cruse H. Load-regulating mechanisms in gait and posture: comparative aspects. Physiol Rev. Jan 2000;80(1):83–133. [DOI] [PubMed] [Google Scholar]
- 30.Swinnen E, Baeyens JP, Van Mulders B, Verspecht J, Degelaen M. The influence of the use of ankle-foot orthoses on thorax, spine, and pelvis kinematics during walking in children with cerebral palsy. Prosthet Orthot Int. Apr 2018;42(2):208–213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


