Abstract
Background
Little is known about post-discharge outcomes among patients who were discharged alive from hospice.
Objective
To compare healthcare utilization and mortality after hospice live discharge among Medicare patients with and without Alzheimer’s disease and related dementias (ADRD).
Design
Retrospective cohort study using Medicare claims data of a 20% random sample of Medicare fee-for-service (FFS) patients.
Participants
A total of 153,696 Medicare FFS patients experienced live discharge from hospice between 2014 and 2019.
Measures
Two types of burdensome transition (type 1: live discharge from hospice followed by hospitalization and subsequent hospice readmission; type 2: live discharge from hospice followed by hospitalization with the patient deceased in the hospital), acute care utilization, hospice readmission, and mortality in the 30 and 180 days after live discharge and between live discharge and death.
Results
Compared with non-ADRD patients, ADRD patients were less likely to experience burdensome transitions (type 1: adjusted odds ratio [aOR], 0.94; 95% confidence interval [CI], 0.90–0.98; type 2: aOR, 0.70; 95% CI, 0.65–0.75), more likely to have ED visits (aOR, 1.05; 95% CI, 1.01–1.09), less likely to die (aOR, 0.71; 95% CI, 0.69–0.73), and less likely to be readmitted to hospice (aOR, 0.86; 95% CI, 0.84–0.89) 30 days after live discharge. Results of 180-day post-discharge outcomes were largely consistent with results of 30-day outcomes. Among patients who died as of December 31, 2019, ADRD patients were less likely to be hospitalized (aOR, 0.88; 95% CI, 0.85–0.92) and more likely to be readmitted to hospice (aOR, 1.12; 95% CI, 1.08–1.16) between live discharge and death. Significant racial/ethnicity disparities in acute care utilization and mortality after live discharge existed in both ADRD and non-ADRD groups.
Conclusion
ADRD patients had lower mortality, a longer survival time, a lower rate of hospitalization, and an initially lower but gradually increasing rate of hospice readmission than non-ADRD patients after hospice live discharge. These different trajectories warrant further investigation of the eligibility of their initial hospice enrollment. Black patients had significantly worse outcomes after hospice live discharge compared with White patients.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11606-023-08031-8.
KEY WORDS: hospice live discharge, healthcare utilization, mortality, Medicare, end of life
INTRODUCTION
The use of hospice care in the USA has grown rapidly in the past two decades. Medicare spending on hospice increased by more than 400% between 2000 and 20121 and reached $20.9 billion in 2019.2 Prior studies found that hospice use is associated with lower Medicare spending and decreased acute care use (e.g., hospitalization and ED visits) at the end of life.3–5 Although hospice care is designed to support patients and families through the dying process, a considerable proportion of patients experience discharge from hospice prior to death, also known as “live discharge.” In 2019, 17.4% of hospice enrollees had a live discharge.2 Patients could be discharged alive due to condition stabilization or patients’ decision to seek curative care.6–8 In addition, live discharges may also occur due to hospices’ efforts to avoid costs of hospitalization, inadequate counseling regarding the choice of hospice, profit maximization given the per diem-based hospice payment system, or increased market competition between hospice providers.9,10 When discharged alive, patients and family members lose support from the multidisciplinary hospice care team and coordinated provision of medical equipment, medications, and supplies.7,11,12 Therefore, hospice live discharge could lead to care discontinuities, disruptive patient-provider relationship, and burdensome transitions to other care settings.12,13
Prior studies have focused on understanding patient and provider characteristics associated with hospice live discharge.6,7,9,10,13,14 These studies found that hospice characteristics, such as for-profit ownership,9,10 hospice size,9 rural location,10 and patient characteristics, such as race/ethnicity (e.g., Black patients)6,8,10 and certain chronic conditions (e.g., dementia)6,8,10,11, are associated with an increased risk of hospice live discharge. A few studies examined death after live discharge using small samples and found significant variation in survival time among patients after live discharge.15–18 To date, little is known about other patient outcomes after hospice live discharge.
The increasing number of live discharges has led to rising concerns by the Centers for Medicare and Medicaid Services (CMS).2 Starting from 2021, CMS added four quality measures for live discharge as part of the Hospice Care Index to the Hospice Quality Reporting Program, including early live discharge (discharged within the first 7 days of hospice admission), late live discharge (discharged on or after 180 days of hospice admission), and measures for two types of burdensome transition after hospice live discharge (type 1: live discharge from hospice followed by hospitalization and subsequent hospice readmission; type 2: live discharge from hospice followed by hospitalization with the patient deceased in the hospital). 19,20 Understanding post-discharge outcomes has important implications for improving quality of and reducing unnecessary spending on end-of-life (EoL) care.21
Using a national representative sample of Medicare fee-for-service (FFS) patients from 2014 to 2019, we examined healthcare utilization and mortality after hospice live discharge. Due to the distinct disease trajectory, patients with Alzheimer’s disease and related dementias (ADRD) have an increased risk for live discharge.7,9,11 However, no prior study has examined post-discharge outcomes by ADRD status. We therefore compared post-discharge outcomes between patients with and without ADRD.
METHODS
Data Sources and Study Sample
We conducted a retrospective cohort study using a 20% random sample of Medicare FFS patients from 2014 to 2019. We used the Medicare hospice claims file to identify hospice live discharges based on the discharge status codes.22 We implemented a washout period of the first 90 days of 2014 to only include patients who newly started their hospice benefits in the study period.14 This approach allowed us to exclude most hospice stays that might be readmissions following a hospice discharge in 2013.
Using Master Beneficiary Summary File (MBSF), we further categorized all patients with a live discharge into three overlapping sub-cohorts to examine short-term, long-term, and EoL outcomes after live discharge, defined as patients who had continuous enrollment in Medicare Parts A and B or until death in the first 30 days (short-term) or first 180 days (long-term) after live discharge, and patients who died as of December 31, 2019, and had continuous enrollment in Medicare Parts A and B before death (EoL). We used MBSF to extract patient demographics and the Chronic Condition Segment to extract comorbidity information. To estimate healthcare utilization after live discharge, we used Medicare claims files of outpatient, inpatient, and hospice care. Finally, we used the CMS Provider of Services file to identify ownership of hospice providers.23 In rare cases, if a patient had more than one hospice live discharge, we examined the first one in our analysis.
Identifying Patients with ADRD
We used the end-of-year indicator for Alzheimer’s disease and related disorders or senile dementia in the Chronic Conditions Segment of the MBSF to identify patients with an ADRD diagnosis in a given calendar year.24 This indicator was derived using a validated algorithm that used ICD-9 and ICD-10 codes for dementia present within a 3-year lookback period on one or more inpatient, skilled nursing facility, home health, outpatient, or carrier claim.25 This algorithm has been validated and was able to identify over 80% of patients who were known to have ADRD.26
Post-discharge Outcomes
Following previous literature, we focused on transitions to acute care settings after hospice live discharge (i.e., emergency department [ED] visits and hospitalizations), mortality, and hospice readmission as post-discharge outcomes.27–29 For short-term outcomes, we calculated two burdensome transition measures defined by CMS, including (1) hospitalization within 2 days after hospice live discharge, followed by hospice readmission within 2 days of hospital discharge (type 1) and (2) hospitalization within 2 days after hospice live discharge where the patient died during the hospitalization (type 2).22 Other short-term outcomes included any ED visits, any hospitalizations, hospice readmissions, and mortality in the first 30 days after live discharge. Acute care utilization or death within 30 days after hospice live discharge might represent potential quality concerns of hospice care or inappropriate discharge decisions.29
For long-term outcomes, we examined ED visit, hospitalization, hospice readmission, and mortality in the first 180 days after live discharge. Finally, for patients who died as of December 31, 2019, we examined ED visit, hospitalization, and hospice readmissions between live discharge and death as EoL outcomes.
Hospice Stay and Patient Characteristics
We identified patients with an early hospice live discharge within the first 7 days of hospice admission or a late live discharge on or after 180 days of hospice admission and controlled for these variables in the regressions. Other controls of hospice stay characteristics included place of hospice services (home, assisted living, nursing facility, inpatient hospital, inpatient hospice facility, and other [e.g., long-term care hospital and inpatient psychiatric facility]), status of live discharge (e.g., discharge to home with cause, discharge to home due to patient revocation of hospice care, or discharge to home due to condition stabilization), hospice ownership (non-profit, for-profit, government, and other), and services other than routine home care provided during hospice stay (continuous home care, inpatient respite care, and general inpatient care). Use of these services may indicate more complex patient conditions. Patient characteristic controls included patient age in years (under 65, 65–74, 75–84, and 85 and older), sex (male and female), race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, and others), number of chronic conditions (≤ 2, 3–5, 6–8, and ≥ 9) defined by 27 conditions of Chronic Condition Warehouse, dual-eligible enrollment status, end-stage renal disease (ESRD) status, and if patients had any advance care planning claims (starting 2016). We also mapped patient residential zip codes into hospital referral regions (HRRs) and included them as fixed effects to account for variations in provider preference in EoL care and other hospice market characteristics across regions. Finally, we controlled for survival time as number of days when examining EoL outcomes as patients could have different lengths of survival time between live discharge and death.
Statistical Analysis
We compared patient and hospice characteristics and post-discharge outcomes by patients’ ADRD status using χ2 test for categorical variables and Wilcoxon rank sum test for continuous variables. We used multivariable logistic regressions to compare post-discharge outcomes by ADRD status. All regressions were adjusted for thirteen variables of hospice stay and patient characteristics mentioned above, year fixed effects, and HRR fixed effects. We used robust standard errors clustered by hospice provider to account for correlations of patients discharged from the same hospice. The false discovery rate (FDR)–adjusted p-value (q-value) was used to adjust for multiple comparisons for regression analyses (significance threshold, q < 0.05). Prior studies have found that FDR adjustment has greater power to detect true positives than Bonferroni-type adjustments, while still controlling the proportion of type I errors at a specified level.30,31
We conducted several sensitivity and secondary analyses. We estimated proportional hazards competing risk models with death as a competing risk for healthcare utilization after live discharge. We also estimated Cox proportional hazards models for mortality, accounting for time from the beginning of a live discharge to death or end of the study period. Finally, as patients from underrepresented groups are at a higher risk of hospice live discharge,6 we therefore examined post-discharge outcomes by race/ethnicity for non-ADRD and ADRD patients separately to examine any potential disparities.
The Institutional Review Board at Weill Cornell Medicine approved this study. This study is reported using STROBE guidelines.32
RESULTS
Patient Characteristics
This study included 153,696 Medicare FFS beneficiaries with hospice live discharges between 2014 and 2019. The mean [SD] age was 82 [11] years, and 94,553 [61.5%] were female. Among all patients, 100,286 (65.2%) had ADRD (Table 1). Compared with non-ADRD patients, ADRD patients were older (mean age 85 vs 78), were more likely to be female (65.8% vs 53.5%), were more likely to have Medicaid (38.4% vs 32.4%), were less likely to have ESRD (1.3% vs 2.4%), and had a higher number of chronic conditions. The median survival time after live discharge was much longer among ADRD patients compared with non-ADRD patients (140 vs 69 days). All differences were statistically significant (p < .001).
Table 1.
Patient characteristics among hospice live discharges, by ADRD status
| Patient characteristics | All patients (N = 153,696) |
By ADRD status | |
|---|---|---|---|
| Non-ADRD (N = 53,410) |
ADRD (N = 100,286) |
||
| Age (year), % | |||
| <65 | 6.7 | 13.5 | 3.0 |
| 65–74 | 15.7 | 25.3 | 10.6 |
| 75–84 | 28.6 | 29.0 | 28.3 |
| 85 and older | 49.1 | 32.2 | 58.1 |
| Mean age, year (SD) | 82 (11) | 78 (12) | 85 (9) |
| Gender, % | |||
| Male | 38.5 | 46.5 | 34.2 |
| Female | 61.5 | 53.5 | 65.8 |
| Race/Ethnicity, % | |||
| Non-Hispanic White | 81.8 | 81.5 | 82.0 |
| Non-Hispanic Black | 9.5 | 9.4 | 9.5 |
| Hispanic | 5.3 | 5.5 | 5.3 |
| Other | 3.4 | 3.7 | 3.2 |
| Number of chronic conditions, % | |||
| ≤2 | 9.9 | 16.7 | 6.3 |
| 3–5 | 29.4 | 32.7 | 27.7 |
| 6–8 | 34.7 | 33.3 | 35.5 |
| ≥9 | 26.0 | 17.4 | 30.5 |
| Dual enrollment in Medicaid, % | 36.3 | 32.4 | 38.4 |
| End-stage renal disease, % | 1.7 | 2.4 | 1.3 |
| Advance care planning, % | 9.0 | 8.4 | 9.4 |
| Survival time after live discharge, median (IQR) |
110 (27–334) |
69 (18–244) |
140 (36–380) |
ADRD Alzheimer’s disease and related dementias, IQR interquartile range. Other race/ethnicity includes Asian/Pacific Islander, American Indian/Alaska native, unknown, and all others
Hospice Stay Characteristics
Characteristics of hospice stay differed greatly by ADRD status (Table 2). Compared with non-ADRD patients, ADRD patients were more likely to be discharged alive after a long hospice stay (median length of stay: 92 vs 55 days), much more likely to receive hospice care in a long-term care (nursing or assisted living) facility (46.8% vs 15.2%), more likely to get discharged to home because of condition stability (46.0% vs 33.3%), and more likely to receive hospice care from for-profit providers (56.2% vs 50.7%). All differences were statistically significant (p < .001).
Table 2.
Hospice stay characteristics among hospice live discharges, by ADRD status
| Characteristics of hospice stay | All patients (N = 153,696) |
By ADRD status | |
|---|---|---|---|
| Non-ADRD (N = 53,410) |
ADRD (N = 100,286) |
||
| Length of hospice stay (day), median (IQR) |
86 (25–184) |
55 (15–149) |
92 (35–226) |
| Early live discharge (discharged within 7 days of hospice admission), % | 10.5 | 13.6 | 8.2 |
| Late live discharge (discharged on or over 180 days of hospice admission), % | 25.4 | 18.0 | 29.4 |
| Place of services, % | |||
| Home | 59.2 | 78.7 | 48.9 |
| Assisted living | 14.9 | 5.7 | 19.8 |
| Nursing facility | 20.9 | 9.5 | 27.0 |
| Hospital | 2.0 | 2.5 | 1.8 |
| Inpatient hospice facility | 1.6 | 2.2 | 1.3 |
| Other | 1.3 | 1.4 | 1.3 |
| Services other than routine home care provided, % | |||
| Continuous home care | 1.2 | 1.2 | 1.3 |
| Inpatient respite care | 4.6 | 3.8 | 5.1 |
| General inpatient care | 7.4 | 9.3 | 6.4 |
| Hospice ownership, % | |||
| Non-profit | 33.5 | 36.5 | 32.0 |
| For-profit | 54.3 | 50.7 | 56.2 |
| Government | 1.7 | 1.9 | 1.6 |
| Other | 10.5 | 11.0 | 10.3 |
| Status of live discharge, % | |||
| Discharge to home with cause | 1.5 | 1.6 | 1.5 |
| Discharge to home due to patient unavailability | 7.2 | 8.3 | 6.7 |
| Discharge to home due to patient revocation of hospice care | 41.6 | 49.4 | 37.5 |
| Transfer to inpatient care | 4.1 | 4.6 | 3.9 |
| Transfer to other facilities | 3.9 | 2.9 | 4.4 |
| Discharge to home for other reasons (condition stabilization) | 41.6 | 33.3 | 46.0 |
ADRD Alzheimer’s disease and related dementias, IQR interquartile range
Post-discharge Outcomes
ADRD patients had lower rates of burdensome transitions compared with non-ADRD patients (Table 3). ADRD patients had slightly lower rates of ED visit (10.1% vs. 11.5%), hospitalization (29.8% vs. 32.7%), hospice readmission (24.7% vs. 33.3%), and mortality (15.2% vs. 24.7%) 30 days after live discharge compared with non-ADRD patients. All differences were statistically significant (p < .001). A similar pattern was observed 180 days after live discharge. Among patients who died as of December 31, 2019, ADRD patients had higher rates of ED visit and hospice readmission but slightly lower rate of hospitalization between live discharge and death compared with non-ADRD patients.
Table 3.
Post-discharge outcomes among hospice live discharges, by ADRD status
| Post-discharge outcomes | All patients (N = 153,696) |
By ADRD status | |
|---|---|---|---|
| Non-ADRD (N = 53,410) |
ADRD (N = 100,286) |
||
| Burdensome transitions | |||
| Transition type 1 | 9.1 | 10.3 | 8.5 |
| Transition type 2 | 2.8 | 4.0 | 2.2 |
| 30-day post-discharge outcomes, % | |||
| Any ED visits | 10.6 | 11.5 | 10.1 |
| Any hospitalizations | 30.8 | 32.7 | 29.8 |
| Hospice readmission | 27.7 | 33.3 | 24.7 |
| 30-day mortality | 18.5 | 24.7 | 15.2 |
| 180-day post-discharge outcome, % | |||
| Any ED visits | 23.9 | 24.0 | 23.8 |
| Any hospitalizations | 41.5 | 43.5 | 40.5 |
| Hospice readmission | 41.7 | 45.2 | 39.8 |
| 180-day mortality | 42.1 | 50.3 | 37.8 |
| EoL outcomes, % | |||
| Any ED visits | 32.1 | 30.9 | 32.8 |
| Any hospitalizations | 56.1 | 56.9 | 55.7 |
| Hospice readmission | 72.5 | 70.3 | 73.8 |
ADRD Alzheimer’s disease and related dementias, ED emergency department, EoL end of life. Burdensome transition type 1 refers to live discharges from hospice followed by a hospitalization within 2 days and followed by a hospice readmission within 2 days of hospital discharge. Burdensome transition type 2 refers to live discharges from hospice followed by a hospitalization within 2 days, and where the patient also died during the inpatient hospitalization stay.
Associations Between ADRD and Post-discharge Outcomes
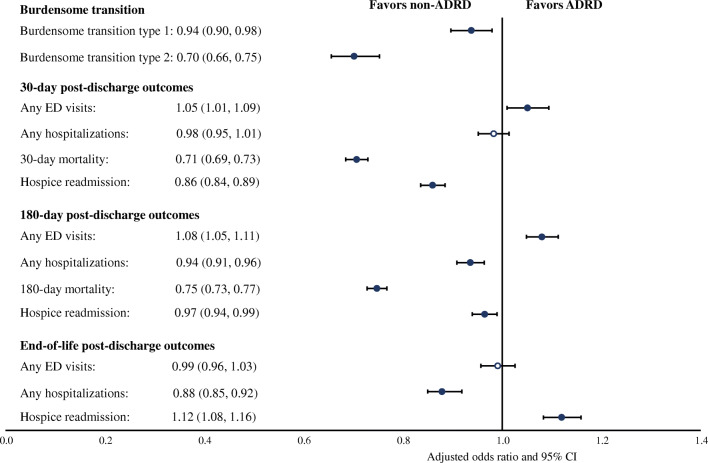
After adjusting for all covariates, having an ADRD diagnosis was associated with lower odds of burdensome transitions (type 1: adjusted odds ratio [aOR], 0.94; 95% confidence interval [CI], 0.90–0.98; type 2: aOR, 0.70; 95% CI, 0.66–0.75), lower odds of death (aOR, 0.71; 95% CI, 0.69–0.73), lower odds of hospice readmission (aOR, 0.86; 95% CI, 0.84–0.89), and higher odds of ED visits (aOR, 1.05; 95% CI, 1.01–1.09) within 30 days after live discharge (Fig. 1). Findings were similar for outcomes in 180 days after live discharge, except that having an ADRD diagnosis was associated with lower odds of hospitalization (Fig. 1). Between live discharge and death, having an ADRD diagnosis was associated with higher odds of hospice readmission (aOR, 1.12; 95% CI, 1.08–1.16), but lower odds of hospitalization (aOR, 0.88; 95% CI, 0.85–0.92) after adjusting for patient and hospice characteristics and survival time (Fig. 1).
Fig. 1.
Association between ADRD status and post-discharge outcomes among patients who were discharged alive from hospice. Notes: ED, emergency department. Burdensome transition type 1 refers to live discharges from hospice followed by a hospitalization within 2 days and followed by a hospice readmission within 2 days of hospital discharge. Burdensome transition type 2 refers to live discharges from hospice followed by a hospitalization within 2 days, and where the patient also died during the inpatient hospitalization stay. Odds ratios were estimated from logistic regressions where each post discharge outcome was the dependent variable and ADRD status was the independent variable, adjusting for patient and hospice stay characteristics, year fixed effects, and hospital referral region fixed effects. Standard errors are clustered at hospice provider level. Filled symbols indicate that the odds ratios were statistically significant (false discovery rate [FDR]–adjusted p value or q value < 0.05).
Secondary and Sensitivity Analyses
Analyses using competing risk models and Cox proportional hazard regression models found similar results as those using logistic regressions (eTables 1). Among both ADRD and non-ADRD groups, patients from the underrepresented groups, especially Black patients, had higher rates of acute care use, higher mortality, and lower hospice readmission after live discharge (eTables 2-4 and eFigures 1-3).
DISCUSSION
We found that ADRD patients were less likely to experience burdensome transitions, be hospitalized, or die within 30 and 180 days after hospice live discharge compared to non-ADRD patients, although they had more ED visits during these periods. ADRD patients were also less likely to be readmitted to hospice within 30 and 180 days after a live discharge, but more likely to have hospice readmissions before death. We identified significant racial/ethnicity disparities in post-discharge outcomes among both ADRD and non-ADRD patients. Patients from underrepresented groups, especially Black patients, were more likely to use acute care and had higher mortality after live discharge.
Results from this study provide several implications for improving EoL care among hospice live discharges. First, transitions to acute care settings were common after hospice live discharge. Previous studies found that transitions to acute care near EoL were associated with adverse health outcomes,33 lower patient and family satisfaction,34 and higher costs.18 The reason for these transitions is poorly understood. It may indicate a lack of communication between hospice providers and patients and family members about advance care planning.35 It might be also driven by hospices’ financial incentive to avoid inpatient care when they are near the hospice inpatient cap imposed by CMS. The inpatient cap limits the number of inpatient days (general or respite) to 20% of a hospice’s total patient care days. A hospice must refund to Medicare any payment amounts more than the inpatient cap.36 More research is needed to understand the causes of transitions and develop strategies to reduce them.
The prolonged survival time after hospice live discharge among ADRD patients raises the concern of their eligibility and appropriateness of the initial hospice enrollment. The disease trajectory of ADRD is highly unpredictable and ADRD patients were more likely to have stabilized conditions during a hospice stay and experience live discharges.6,7,11 Our findings show additionally that the post-discharge trajectory also differed by ADRD status. Compared with non-ADRD patients, the physical condition of ADRD patients may decline at a slower pace after live discharge, indicated by lower 30- and 180-day mortality and similar or lower hospitalization rate. The lower mortality and prolonged survival time of ADRD patients after hospice live discharge requires better decision-making regarding when to start hospice benefits and improved planning and care coordination following a hospice live discharge. The finding that ADRD patients used more ED visits after live discharge is consistent with prior evidence,37 possibly resulting from the challenges of reconnecting to their previous primary care providers and vulnerability to interrupted care after hospice live discharge.
We found that patients of underrepresented groups in both ADRD and non-ADRD groups, especially Black patients, were more likely to receive acute care after live discharge and had higher mortality compared with White patients, highlighting the persistent racial disparities in EoL care quality. Previous studies found that Black patients had greater preferences for life-sustaining treatment at EoL.16,38 However, the higher mortality among minority patients we found in this study may indicate limited benefits of these intensive treatment. We also found that fewer Black patients who were discharged alive were readmitted to hospice before death compared with White patients. This is consistent with prior research that decedents of underrepresented groups used less hospice but more acute care at EoL compared with White decedents.39 Hospice should work with other providers and family caregivers to coordinate and maintain the continuity of care and meet health needs of these patients after live discharge.
Given the increasing number of live discharges from hospice and costly EoL care, payers should hold hospice accountable for high-intensity post-discharge care. Our findings point to deficiencies in the current hospice payment scheme. In contrast to bundled payment implemented in inpatient and other post-acute care settings where providers assume accountability for patients’ Medicare spending across an entire care episode, hospice providers are paid on a per diem basis covering services during a hospice stay without financial incentives to improve care after live discharge. The incorporation of burdensome transitions in the Hospice Quality Reporting program is a promising start. However, burdensome transitions only consider patient outcomes shortly after live discharge. Many patients, especially ADRD patients, have prolonged survival time after live discharge. Policymakers should further explore ways to incentivize longer term care coordination between hospice and other providers, and to better scrutinize hospice enrollment for certain patient subgroups such as those with ADRD.
LIMITATIONS
This study has several limitations. First, we used claims data to identify ADRD patients, which might be a less precise approach than other approaches based on strict clinical assessment criteria.40,41 We were not able to implement these alternative approaches to ascertain dementia as they rely on data from patient cognitive assessment, which are not available in the Medicare claims data. Medicare claims also do not have information on the stage of dementia. Second, our study excluded Medicare Advantage patients. Although hospice care for Medicare Advantage patients is covered by the traditional Medicare program, we could not observe healthcare utilization and other outcomes after live discharge if they were paid by Medicare Advantage plans. Third, we only examined healthcare utilization measures as quality outcomes. Other important outcomes, such as patient satisfaction, were not examined due to data limitations. Finally, we combined patients from racial/ethnic groups with small sample sizes, such as Asian/Pacific Islander and American Native. These patients may have different patterns of healthcare utilization and mortality after hospice live discharge.
CONCLUSIONS
ADRD patients had lower mortality, a longer survival time, a lower rate of hospitalization, and an initially lower but gradually increasing rate of hospice readmission than non-ADRD patients after hospice live discharge. Underrepresented patients had a higher rate of acute care utilization and higher mortality in both ADRD and non-ADRD groups. As the number of hospice live discharge increases, it is of great policy and clinical importance to develop interventions to improve EoL care and reduce disparities. Future studies need to examine how post-discharge outcomes change after they are added to the Hospice Quality Reporting program and improve the design of the Medicare hospice benefit to meet the EoL needs of ADRD patients.
Supplementary Information
(DOCX 206 kb)
Funding
This study was supported by the National Institute on Aging of the National Institutes of Health (grant No. R00AG064030 to Dr. Zhang; grant No. K01AG066946 to Dr. Li) and the Physicians Foundation Center for the Study of Physician Practice and Leadership at Weill Cornell Medical College.
Declarations
Conflict of Interest
The authors report no conflicts of interest.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH or the Physicians Foundation.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Medicare Payment Advisory Commission. March 2015 Report to the Congress: Medicare Payment Policy. https://www.medpac.gov/document/http-www-medpac-gov-docs-default-source-reports-mar2015_entirereport_revised-pdf/. Accessed 07/01, 2022
- 2.Medicare Payment Advisory Commission. March 2021 Report to the Congress: Medicare Payment Policy. https://www.medpac.gov/document/march-2021-report-to-the-congress-medicare-payment-policy/. Accessed 07/01, 2022.
- 3.Gozalo P, Plotzke M, Mor V, Miller SC, Teno JM. Changes in Medicare costs with the growth of hospice care in nursing homes. N Engl J Med. 2015;372(19):1823–31. doi: 10.1056/NEJMsa1408705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aldridge MD, Moreno J, McKendrick K, Li L, Brody A, May P. Association Between Hospice Enrollment and Total Health Care Costs for Insurers and Families, 2002-2018. JAMA Health Forum. 2022;3(2):e215104. 10.1001/jamahealthforum.2021.5104 [DOI] [PMC free article] [PubMed]
- 5.Zuckerman RB, Stearns SC, Sheingold SH. Hospice Use, Hospitalization, and Medicare Spending at the End of Life. J Gerontol B Psychol Sci Soc Sci. 2016;71(3):569–80. doi: 10.1093/geronb/gbv109. [DOI] [PubMed] [Google Scholar]
- 6.Luth EA, Russell DJ, Brody AA, et al. Race, Ethnicity, and Other Risks for Live Discharge Among Hospice Patients with Dementia. J Am Geriatr Soc. 2020;68(3):551–558. doi: 10.1111/jgs.16242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luth EA, Russell DJ, Xu JC, et al. Survival in hospice patients with dementia: the effect of home hospice and nurse visits. J Am Geriatr Soc. 2021;69(6):1529–1538. doi: 10.1111/jgs.17066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russell D, Diamond EL, Lauder B, et al. Frequency and Risk Factors for Live Discharge from Hospice. J Am Geriatr Soc. 2017;65(8):1726–1732. doi: 10.1111/jgs.14859. [DOI] [PubMed] [Google Scholar]
- 9.Teno JM, Plotzke M, Gozalo P, Mor V. A national study of live discharges from hospice. J Palliat Med. 2014;17(10):1121–7. doi: 10.1089/jpm.2013.0595. [DOI] [PubMed] [Google Scholar]
- 10.Wu S, Volker DL. Live Discharge From Hospice: A Systematic Review. J Hosp Palliat Nurs. 2019;21(6):482–488. doi: 10.1097/NJH.0000000000000547. [DOI] [PubMed] [Google Scholar]
- 11.Hunt LJ, Harrison KL. Live discharge from hospice for people living with dementia isn't "graduating"-It's getting expelled. J Am Geriatr Soc. 2021;69(6):1457–1460. doi: 10.1111/jgs.17107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wladkowski SP, Wallace CL. Current Practices of Live Discharge from Hospice: Social Work Perspectives. Health Soc Work. 2019;44(1):30–38. doi: 10.1093/hsw/hly040. [DOI] [PubMed] [Google Scholar]
- 13.Dolin R, Hanson LC, Rosenblum SF, Stearns SC, Holmes GM, Silberman P. Factors Driving Live Discharge From Hospice: Provider Perspectives. J Pain Symptom Manage. 2017;53(6):1050–1056. doi: 10.1016/j.jpainsymman.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Dolin R, Holmes GM, Stearns SC, et al. A Positive Association Between Hospice Profit Margin And The Rate At Which Patients Are Discharged Before Death. Health Aff (Millwood). 2017;36(7):1291–1298. doi: 10.1377/hlthaff.2017.0113. [DOI] [PubMed] [Google Scholar]
- 15.Johnson KS, Elbert-Avila K, Kuchibhatla M, Tulsky JA. Characteristics and outcomes of hospice enrollees with dementia discharged alive. J Am Geriatr Soc. 2012;60(9):1638–44. doi: 10.1111/j.1532-5415.2012.04117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson KS, Kuchibhatla M, Tanis D, Tulsky JA. Racial differences in hospice revocation to pursue aggressive care. Arch Intern Med. 2008;168(2):218–24. doi: 10.1001/archinternmed.2007.36. [DOI] [PubMed] [Google Scholar]
- 17.Carlson MD, Herrin J, Du Q, et al. Impact of hospice disenrollment on health care use and medicare expenditures for patients with cancer. J Clin Oncol. 2010;28(28):4371–5. doi: 10.1200/JCO.2009.26.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlson MD, Herrin J, Du Q, et al. Hospice characteristics and the disenrollment of patients with cancer. Health Serv Res. 2009;44(6):2004–21. doi: 10.1111/j.1475-6773.2009.01002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plotzke M, Groover K, Harrison Z, Massuda C. Construction and Performance of the Hospice Care Index Claims-Based Quality Measure. J Pain Symptom Manag. 2022;63(5):876–876. doi: 10.1016/j.jpainsymman.2022.02.072. [DOI] [Google Scholar]
- 20.Centers for Medicare and Medicaid Services. Hospice Quality Reporting Program: Current Measures. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Hospice-Quality-Reporting/Current-Measures#:~:text=The%20Hospice%20Care%20Index%20(HCI)%20captures%20care%20processes%20occurring%20throughout,calculated%20from%20Medicare%20claims%20data. Accessed 07/01, 2022
- 21.Bazell C, Caplen MA, Coates W, Pelizzari PM, Pyenson B. Hospice Medicare Margins: Analysis of Patient and Hospice Characteristics, Utilization, and Cost. https://www.milliman.com/en/insight/hospice-medicare-margins-analysis-of-patient-and-hospice-characteristics-utilization-an. Accessed 07/01, 2022
- 22.Centers for Medicare and Medicaid Services. Hospice Quality Reporting Program Quality Measure Specifications User's Manual. https://www.cms.gov/files/document/hqrp-qm-users-manual-v100oct2021.pdf. Accessed 07/01, 2022
- 23.Centers for Medicare and Medicaid Services. Provider of Services Current Files. https://www.cms.gov/Research-Statistics-Data-and-Systems/Downloadable-Public-Use-Files/Provider-of-Services. Accessed 07/01, 2022
- 24.Rahman M, White EM, Thomas KS, Jutkowitz E. Assessment of Rural-Urban Differences in Health Care Use and Survival Among Medicare Beneficiaries With Alzheimer Disease and Related Dementia. JAMA Netw Open. 2020;3(10):e2022111. doi: 10.1001/jamanetworkopen.2020.22111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chronic Condition Data Warehouse. Chronic Conditions. https://www2.ccwdata.org/web/guest/condition-categories-chronic. Accessed 06/01, 2022
- 26.Taylor DH, Jr, Fillenbaum GG, Ezell ME. The accuracy of medicare claims data in identifying Alzheimer's disease. J Clin Epidemiol. 2002;55(9):929–37. doi: 10.1016/s0895-4356(02)00452-3. [DOI] [PubMed] [Google Scholar]
- 27.MacKenzie MA, Hanlon A. Health-Care Utilization After Hospice Enrollment in Patients With Heart Failure and Cancer. Am J Hosp Palliat Care. 2018;35(2):229–235. doi: 10.1177/1049909116688209. [DOI] [PubMed] [Google Scholar]
- 28.Russell D, Luth EA, Ryvicker M, Bowles KH, Prigerson HG. Live Discharge From Hospice Due to Acute Hospitalization: The Role of Neighborhood Socioeconomic Characteristics and Race/Ethnicity. Med Care. 2020;58(4):320–328. doi: 10.1097/MLR.0000000000001278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Medicare and Medicaid Services. Draft Measure Specifications: Transitions from Hospice Care, Followed by Death or Acute Care. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/MMS/Downloads/Development-of-Draft-HQRP-Transitions-Measure-Specifications.pdf. Accessed 01/01, 2022
- 30.Glickman ME, Rao SR, Schultz MR. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J Clin Epidemiol. 2014;67(8):850–7. doi: 10.1016/j.jclinepi.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Korthauer K, Kimes PK, Duvallet C, et al. A practical guide to methods controlling false discoveries in computational biology. Genome Biol. 2019;20(1):118. doi: 10.1186/s13059-019-1716-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gallo V, Egger M, McCormack V, et al. STrengthening the Reporting of OBservational studies in Epidemiology--Molecular Epidemiology STROBE-ME: an extension of the STROBE statement. J Clin Epidemiol. 2011;64(12):1350–63. doi: 10.1016/j.jclinepi.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 33.Phongtankuel V, Scherban BA, Reid MC, et al. Why Do Home Hospice Patients Return to the Hospital? A Study of Hospice Provider Perspectives. J Palliat Med. 2016;19(1):51–56. doi: 10.1089/jpm.2015.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dolin R, Hanson LC, Rosenblum SF, Stearns SC, Holmes GM, Silberman P. Factors Driving Live Discharge From Hospice: Provider Perspectives. J Pain Symptom Manag. 2017;53(6):1050–1056. doi: 10.1016/j.jpainsymman.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Horn E, Teno J, Plotzke M, Gozalo P, Christian TJ. A National Study of Live Hospice Discharges Between 2000 and 2012. J Pain Symptom Manag. 2015;49(2):431–432. doi: 10.1016/j.jpainsymman.2014.11.227. [DOI] [PubMed] [Google Scholar]
- 36.Medicare Payment Advisory Commission. Hospice Services Payment System. https://www.medpac.gov/wp-content/uploads/2021/11/medpac_payment_basics_21_hospice_final_sec.pdf. Accessed 11/20, 2022
- 37.Williamson LE, Evans CJ, Cripps RL, Leniz J, Yorganci E, Sleeman KE. Factors Associated With Emergency Department Visits by People With Dementia Near the End of Life: A Systematic Review. J Am Med Dir Assoc. 2021;22(10):2046–2055. doi: 10.1016/j.jamda.2021.06.012. [DOI] [PubMed] [Google Scholar]
- 38.Ornstein KA, Roth DL, Huang J, et al. Evaluation of Racial Disparities in Hospice Use and End-of-Life Treatment Intensity in the REGARDS Cohort. JAMA Netw Open. 2020;3(8):e2014639. doi: 10.1001/jamanetworkopen.2020.14639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin PJ, Zhu Y, Olchanski N, et al. Racial and Ethnic Differences in Hospice Use and Hospitalizations at End-of-Life Among Medicare Beneficiaries With Dementia. JAMA Netw Open. 2022;5(6):e2216260. doi: 10.1001/jamanetworkopen.2022.16260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee E, Gatz M, Tseng C, et al. Evaluation of Medicare Claims Data as a Tool to Identify Dementia. J Alzheimers Dis. 2019;67(2):769–778. doi: 10.3233/JAD-181005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gianattasio KZ, Wu Q, Glymour MM, Power MC. Comparison of Methods for Algorithmic Classification of Dementia Status in the Health and Retirement Study. Epidemiology. 2019;30(2):291–302. doi: 10.1097/EDE.0000000000000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 206 kb)