Abstract
This study is aimed to evaluate effects of deep learning image reconstruction (DLIR) on image quality in single-energy CT (SECT) and dual-energy CT (DECT), in reference to adaptive statistical iterative reconstruction-V (ASIR-V). The Gammex 464 phantom was scanned in SECT and DECT modes at three dose levels (5, 10, and 20 mGy). Raw data were reconstructed using six algorithms: filtered back-projection (FBP), ASIR-V at 40% (AV-40) and 100% (AV-100) strength, and DLIR at low (DLIR-L), medium (DLIR-M), and high strength (DLIR-H), to generate SECT 120kVp images and DECT 120kVp-like images. Objective image quality metrics were computed, including noise power spectrum (NPS), task transfer function (TTF), and detectability index (d′). Subjective image quality evaluation, including image noise, texture, sharpness, overall quality, and low- and high-contrast detectability, was performed by six readers. DLIR-H reduced overall noise magnitudes from FBP by 55.2% in a more balanced way of low and high frequency ranges comparing to AV-40, and improved the TTF values at 50% for acrylic inserts by average percentages of 18.32%. Comparing to SECT 20 mGy AV-40 images, the DECT 10 mGy DLIR-H images showed 20.90% and 7.75% improvement in d′ for the small-object high-contrast and large-object low-contrast tasks, respectively. Subjective evaluation showed higher image quality and better detectability. At 50% of the radiation dose level, DECT with DLIR-H yields a gain in objective detectability index compared to full-dose AV-40 SECT images used in daily practice.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10278-023-00806-z.
Keywords: Multidetector computed tomography, Deep learning, Image reconstruction, Image enhancement, Radiation dosage
Introduction
Computed tomography (CT) examinations have been broadly applied for clinical diagnosis and treatment in modern medicine as it is a fast, non-invasive imaging method with high resolution. However, with the increasing concern on the potential cancer-inducing risk of ionizing radiation, a conservative approach to reduce radiation exposure as low as reasonably achievable during CT studies is recommended [1]. On the other hand, the reduction in radiation dose leads to an increased image noise and results in a degraded image quality and diagnostic acceptability. To preserve diagnostic confidence of radiologists, several techniques have been employed for CT image reconstruction to reduce the radiation dose while preserve image quality [2, 3]. Filtered back projection (FBP), which yielded high-quality images at a high radiation dose, has been the method of choice for decades, while decreasing the radiation dose would lead to unsatisfied quality of images reconstructed by FBP [4]. With the increasing of computation power, hybrid iterative reconstruction (IR) algorithms, such as adaptive statistical iterative reconstruction-V (ASIR-V), combing FBP and model-based IR have been introduced into CT image reconstruction allowing lower radiation dose while maintaining image quality. Nevertheless, hybrid IR algorithms occasionally resulted in overly-smoothing, artificial-looking images, because hybrid IR algorithms do not model the complete CT system [5]. With the development of artificial intelligence, it is possible to apply artificial intelligence into image reconstruction. One of such image reconstruction algorithms is the deep learning image reconstruction (DLIR) algorithm by GE Healthcare, which employs convolutional neural networks to learn from high-quality labelled FBP CT images and images in clinical settings [6]. DLIR could better distinguish signal and noise to generate better detailed images with more radiation reduction, while the output image characteristics might vary with the input conditions [7].
These image reconstruction algorithms have been used not only in single-energy CT (SECT), but also in dual-energy CT (DECT) recently. To objectively assess their image quality, a task-based image quality assessment has been employed for both SECT [8–14] and DECT [15–18] with metrics such as noise power spectrum (NPS), task-based transfer function (TTF), and detectability index (d′). To employ this tool in DECT, the virtual monoenergetic images (VMI) were generated to mimic corresponding SECT-like images [15–18]. Both DLIR and hybrid IR algorithms can reduce the image noise of VMIs [16, 17], and even more with DLIR [17, 18], but the detectability of VMI using DLIR varied depending on the contrast [18]. Indeed, model-based image reconstruction (MBIR) did yield better objective low-contrast detectability compared to hybrid IR, but worse confidence and subjective low-contrast detectability [19–21]. Since in vitro performance of image reconstruction algorithms does not always translate into clinical performance and acceptability, it is important to correlate the two aspects.
Therefore, this study was aimed to evaluate the effects of DLIR on image quality and clinical acceptance in SECT (120 kVp) and DECT (VMIs at 70 keV) using comprehensive objective imaging metrics and to confirm the results by subjective ratings of multiple human observers, with ASIR-V images as reference standards.
Materials and Methods
Phantom
The workflow of this study is shown in Fig. 1. The ethics approval was not required due to the nature of this phantom study. A 20-cm diameter American College of Radiology quality assurance Gammex 464 phantom was scanned for image quality evaluation. This phantom is composed of four modules. The module 3 is a uniform, water-equivalent material, and was used to compute NPS. The module 1 consists of four inserts of 25-mm-diameter placed into a water-equivalent background material. The bone (955 Hounsfield unit value, HU) and acrylic (120 HU) inserts were used for TTF computation. The module 4 is composed of a series of high contrast patterns of 4- to 12- line pairs per cm. The module 2 is composed of a series of cylinders with 2-mm to 25-mm in diameters of 6 HU. These two modules (module 4 and 2) were used for high-contrast and low-contrast subjective detectability assessments, respectively.
Fig. 1.
Study workflow. The ACR 464 phantom was scanned with two scan modes (SECT and DECT) at three dose levels (5, 10, and 20 mGy) and then reconstructed using six image reconstruction algorithms (FBP, AV-40, AV-100, DLIR-L, DLIR-M, and DLIR-H), which resulted 36 sets of images in total. Objective image quality evaluation (noise power spectrum, task transfer function, and detectability index) and subjective image quality evaluation (image noise, texture, sharpness, overall quality, and low- and high-contrast detectability) have been performed. The results of objective and subjective evaluation have been compared
Image Acquisition and Reconstruction
The phantom was scanned on a 256-slice dual-energy CT scanner (Revolution Apex CT, GE Healthcare). This CT scanner was used because DLIR is currently only available on this scanner. The acquisition parameters are present in Table 1. DECT and SECT scans were performed with the fast kVp switching technology (80/140 kVp) and conventional 120 kVp, respectively, at three different dose levels (volume CT dose indexes, CTDIVol: 5, 10, and 20 mGy). The 20 mGy dose was selected as the reference full dose level for an adult abdomen based on the clinical practice in our institution [22, 23]. The 10 mGy and 5 mGy dose levels were used to reflect the radiation dose reduction conditions. Different combinations of tube current and rotation time were selected to obtain these three dose levels.
Table 1.
Image acquisition parameters
| Scan mode | Tube voltage (kVp) | Milliamperage (mA) | Rotation time (s) | Revolution time (s) | Pitch | CTDIVol (mGy) |
|---|---|---|---|---|---|---|
| SECT | 120 | 90 | 0.8 | 0.8 | 0.98438 | 5.00 |
| SECT | 120 | 180 | 0.8 | 0.8 | 0.98438 | 10.00 |
| SECT | 120 | 215 | 1.227 | 0.7 | 0.51563 | 19.98 |
| DECT | 80/140 | 145 | 0.3 | 0.6 | 0.98438 | 5.00 |
| DECT | 80/140 | 335 | 0.3 | 0.6 | 0.98438 | 10.00 |
| DECT | 80/140 | 370 | 0.5 | 1 | 0.98438 | 19.75 |
For SECT scans, the conventional 120 kVp images were used for evaluation. For the DECT scans, the 120 kVp-like virtual monochromatic images (VMI) were generated around 70 keV [23]. The scan raw data were all reconstructed on advanced workstation (AW, version 4.7, GE Healthcare) with a standard kernel, commonly used in clinical practice, and is currently the only available kernel for DLIR. The images were generated using six different image reconstruction algorithms: FBP, ASIR-V with a blending factor of 40% (AV-40) and 100% (AV-100), and DLIR (TrueFidelity™, GE Healthcare) with a low (DLIR-L), medium (DLIR-M) or high (DLIR-H) strength level. The AV-40 image at 20 mGy dose level was selected as the reference standard for comparison since it is currently employed at our institution in daily clinical practice. Subsequently, thirty-six sets of images were generated in total.
Objective Image Evaluation
The objective image evaluations were carried out using imQuest software version 7.1 (Duke University) by a radiologist with 4-year-experience in CT image reading. imQuest is a widely accepted image analysis tool for task-based CT image quality assessment. This tool allows noise evaluation in terms of NPS, resolution evaluation in terms of TTF, and detectability index (d′) computation to stimulate clinical tasks. This tool has been used repeatedly in previous studies and is recognized by American Association of Physicists in Medicine task group report, TG-233 [8–18]. The non-prewhitening with an eye filter and internal noise model was used, integrating human visual systems and internal noise [24].
NPS was measured to show the noise texture and magnitude [10, 25], by placing five square regions of interest (ROIs) in module 3 of the phantom. To improve the measurement statistics [10], the ensemble NPS was computed within 40 consecutive axial slices, which resulted in a total of 200 ROIs. For noise texture evaluation, the average of spatial frequency of NPS (faverage) was measured. For noise magnitude, the square root of the area under the curve (AUC) of the NPS was measured [26]. The spatial frequencies of 0.01 to 0.2 mm−1, and 0.5 to 0.7 mm−1 were employed to present noise magnitude in the low and high spatial frequency ranges, respectively [15].
TTF was computed for spatial resolution using bone and acrylic inserts [7, 10], in module 1. Two circular ROIs were placed around the two inserts, and circular-edge technique was applied to compute the edge spread function [27]. The line-spread function was acquired by derivation of the edge spread function ensemble. To avoid the effect of noise in edge spread function calculation [10], 40 consecutive axial slices were used for the calculation of the spatial frequency at which TTF values reach 50% (f50%).
Detectability index (d′) was calculated as a task-based figure of merit surrogate for imaging performance, which uses a simple template-matching strategy to determine if a given image contains the signal of interest or not [10]. Each task function is defined as a 2D Gaussian form with a full width at half maximum equal to the task diameter. Two tasks were considered, included a small high-contrast feature and a large low-contrast feature [8]. The small high-contrast feature was set as a 1.5-mm-diameter 500 HU feature, mimicking calcifications, or identifying high-contrast tissue boundaries. The large low-contrast feature was defined as a 25-mm-diameter 120 HU feature, modeling a large mass in the liver or a nodule in the lungs. The details of objective image quality evaluation method are presented in Supplementary Note S1.
Subjective Image Evaluation
The subjective image evaluation was performed by six radiologists blinded to the acquisition and reconstruction parameters [28]. The images obtained with SECT and DECT scans at three dose levels using six algorithms resulted in thirty-six sets of images in total. The thirty-six sets of images were read by six radiologists with 1, 3, 3, 4, 4, 6 years of experience in CT image reading, in a random and independent fashion. The images were presented to readers with a default window width and level for four modules of the phantom. Readers were allowed to adjust the window width and level and viewing distance as they preferred and had no time limits to complete the image review. One radiologist with 4-year-experience in CT image reading re-assessed all the images two weeks after the first readout.
Before the assessment of image quality, all readers were trained on how to conduct the analysis. The image quality rating was performed on a five-point scale, from 1 to 5 (worst to best), in terms of image noise, texture, sharpness, and overall quality [29–31]. The image noise referred to mottle or graininess of image. The image texture review included the smoothness, plastic feeling of the images. The sharpness reflected the contour definition and delineation of inserts. The overall image quality was to show reader’s personal preference of the image quality. Readers were told that a value of less than 3 was deemed unsatisfactory for clinical use.
For subjective detectability assessments for high-contrast task and low-contrast task, module 4 and 2 of phantom was used, respectively. Images were displayed in random position and order to avoid memorization bias during readouts. Readers were instructed to tell the number of detectable low-contrast cylinders with a contrast of 6HU from the background (from 0 to 5, 2 to 6 mm in diameter), and the number of clearly displayed high-contrast line pairs (from 0 to 8, 4- to 12- line pairs per cm), but the readers were not asked to localize them [8]. The details of subjective image quality evaluation method are presented in Supplementary Note S2.
Statistical Analysis
The statistical analysis was performed with R language version 4.1.3 (https://www.r-project.org/) within RStudio version 1.4.1106 (https://www.rstudio.com/). The assessment results of image noise and spatial resolution were presented in percentages of variations comparing with corresponding FBP since they were highly correlated to the dose levels, while the detectability and subjective assessment results were compared with the SECT 20 mGy AV-40 image sets, to show the potential of algorithms for clinical practice and dose reduction. The intra- and inter-reader agreements were assessed by linear weighted kappa and Kendall's W test, respectively. Degrees of agreement based on kappa values and Kendall’s W statistics were interpreted using the following criteria: 0 to 0.20, poor; 0.21 to 0.40, fair; 0.41 to 0.60, moderate; 0.61 to 0.80, good; and 0.81 to 1.00, excellent.
Results
Objective Image Assessment
NPS
The absolute values of objective image assessments are presented in Table 2. The NPS curves and corresponding metrics are presented in Figs. 2 and 3. In general, the noise magnitude decreased as the increase of dose level and reconstruction strength (Table 3). Compared to FBP, ASIR-V showed comparable average image noise reduction in SECT and DECT (AV-40: -27.45% vs. -24.58% and AV-100: -63.15% vs. -59.76%), while the DLIR of all strength showed higher average reduction of image noise in DECT than SECT (DLIR-L: -34.66% vs -29.74%, DLIR-M: -46.48% vs -40.37%, and DLIR-H: -59.21% vs -51.19%). The DLIR-H provided additional noise reduction over AV-40 (-59.21% vs. -24.58% in DECT, and -51.19% vs. -27.45% in SECT), but similar noise reduction as AV-100 in DECT (-59.21% vs. -59.76%), and slightly less than AV-100 in SECT (-51.19% vs. -63.15%), using those of FBP as baselines.
Table 2.
Absolute values of objective image assessments
| Metric | SECT | DECT | ||||
|---|---|---|---|---|---|---|
| 5 mGy | 10 mGy | 20 mGy | 5 mGy | 10 mGy | 20 mGy | |
| Noise (Hu) | ||||||
| FBP | 24.4 | 17.4 | 11.9 | 22.3 | 16.6 | 12.4 |
| AV40 | 17.6 | 12.6 | 8.7 | 17.3 | 12.5 | 9.1 |
| AV100 | 8.5 | 6.3 | 4.7 | 9.2 | 6.5 | 5 |
| DLIR-L | 16 | 12.4 | 8.8 | 15.4 | 10.9 | 7.6 |
| DLIR-M | 13.2 | 10.6 | 7.6 | 12.7 | 8.9 | 6.2 |
| DLIR-H | 10.2 | 8.7 | 6.5 | 9.7 | 6.8 | 4.7 |
| FBP | 0.315 | 0.283 | 0.252 | 0.299 | 0.283 | 0.268 |
| AV40 | 0.220 | 0.220 | 0.173 | 0.189 | 0.173 | 0.173 |
| AV100 | 0.126 | 0.126 | 0.126 | 0.126 | 0.110 | 0.110 |
| DLIR-L | 0.252 | 0.252 | 0.205 | 0.268 | 0.220 | 0.173 |
| DLIR-M | 0.252 | 0.236 | 0.173 | 0.205 | 0.189 | 0.094 |
| DLIR-H | 0.220 | 0.220 | 0.142 | 0.094 | 0.094 | 0.079 |
| f average | ||||||
| FBP | 0.319 | 0.315 | 0.306 | 0.306 | 0.303 | 0.296 |
| AV40 | 0.288 | 0.284 | 0.272 | 0.274 | 0.269 | 0.263 |
| AV100 | 0.193 | 0.193 | 0.188 | 0.184 | 0.183 | 0.181 |
| DLIR-L | 0.304 | 0.301 | 0.287 | 0.288 | 0.284 | 0.278 |
| DLIR-M | 0.295 | 0.293 | 0.277 | 0.277 | 0.274 | 0.268 |
| DLIR-H | 0.280 | 0.282 | 0.263 | 0.259 | 0.257 | 0.247 |
| TTF bone f 50% | ||||||
| FBP | 0.429 | 0.431 | 0.429 | 0.402 | 0.395 | 0.400 |
| AV40 | 0.434 | 0.437 | 0.433 | 0.407 | 0.400 | 0.404 |
| AV100 | 0.442 | 0.445 | 0.440 | 0.415 | 0.407 | 0.409 |
| DLIR-L | 0.432 | 0.436 | 0.435 | 0.426 | 0.425 | 0.426 |
| DLIR-M | 0.431 | 0.436 | 0.435 | 0.425 | 0.425 | 0.427 |
| DLIR-H | 0.430 | 0.435 | 0.434 | 0.425 | 0.425 | 0.427 |
| TTF acrylic f 50% | ||||||
| FBP | 0.430 | 0.411 | 0.414 | 0.368 | 0.370 | 0.363 |
| AV40 | 0.408 | 0.403 | 0.424 | 0.404 | 0.391 | 0.372 |
| AV100 | 0.375 | 0.404 | 0.438 | 0.458 | 0.447 | 0.392 |
| DLIR-L | 0.460 | 0.436 | 0.452 | 0.458 | 0.470 | 0.487 |
| DLIR-M | 0.457 | 0.431 | 0.447 | 0.464 | 0.475 | 0.493 |
| DLIR-H | 0.450 | 0.432 | 0.443 | 0.470 | 0.482 | 0.498 |
| d' 1.5 mm 500 Hu | ||||||
| FBP | 23.273 | 32.245 | 44.789 | 23.906 | 31.337 | 40.689 |
| AV40 | 28.020 | 38.587 | 53.144 | 26.532 | 35.701 | 47.638 |
| AV100 | 38.308 | 52.199 | 68.981 | 33.580 | 46.913 | 61.748 |
| DLIR-L | 33.531 | 42.947 | 56.858 | 32.587 | 44.921 | 62.805 |
| DLIR-M | 38.990 | 48.442 | 62.591 | 37.349 | 52.552 | 74.175 |
| DLIR-H | 47.371 | 56.258 | 69.854 | 45.355 | 64.249 | 90.022 |
| d' 25 mm 120 Hu | ||||||
| FBP | 275.268 | 382.729 | 477.089 | 280.637 | 357.741 | 444.233 |
| AV40 | 312.741 | 426.642 | 533.312 | 294.353 | 381.316 | 486.420 |
| AV100 | 368.858 | 508.831 | 614.655 | 326.332 | 443.314 | 567.957 |
| DLIR-L | 353.110 | 459.127 | 553.239 | 343.244 | 454.915 | 601.506 |
| DLIR-M | 387.314 | 495.557 | 587.152 | 373.240 | 504.970 | 674.945 |
| DLIR-H | 433.851 | 550.363 | 627.794 | 416.384 | 574.662 | 756.503 |
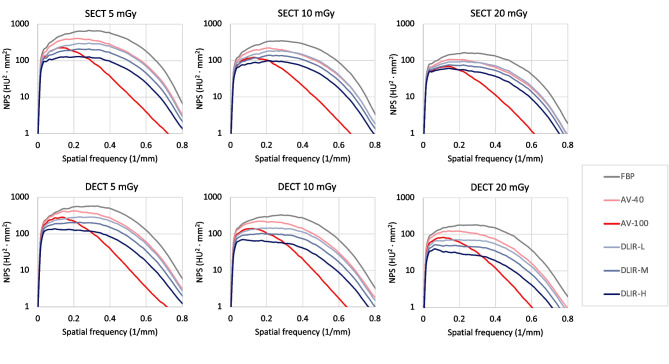
Fig. 2.
NPS curves. NPS curves were obtained with six image reconstruction algorithms (FBP, AV-40, AV-100, DLIR-L, DLIR-M, and DLIR-H) at three dose levels (5, 10, and 20 mGy). top, SECT; bottom, DECT. from left to right, 5, 10, and 20 mGy. Note the AV-100 algorithm obviously reduced the noise in the high spatial frequency range
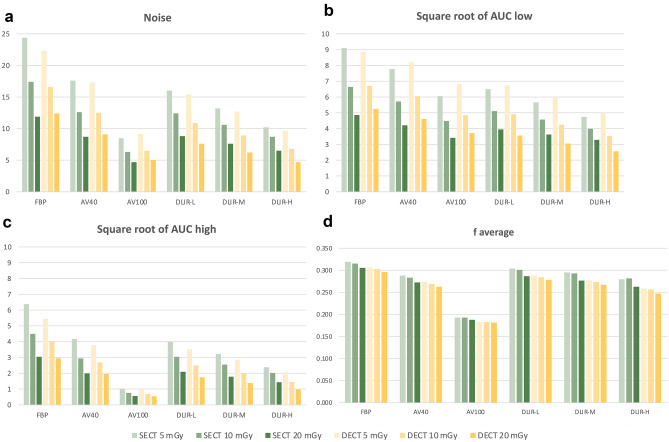
Fig. 3.
NPS metrics. a Noise, b Square root of AUC low, c Square root of AUC high, d f average Note the AV-100 algorithm obviously reduced the noise in the high spatial frequency range, which resulted in large shifts toward lower frequencies in f peak, and f average
Table 3.
Percentage variations of noise magnitude and noise texture relative to FBP images
| Reconstruction | SECT | DECT | ||||||
|---|---|---|---|---|---|---|---|---|
| 5 mGy | 10 mGy | 20 mGy | average | 5 mGy | 10 mGy | 20 mGy | average | |
| Noise (HU) | ||||||||
| FBP | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| AV-40 | -27.87% | -27.59% | -26.89% | -27.45% | -22.42% | -24.70% | -26.61% | -24.58% |
| AV-100 | -65.16% | -63.79% | -60.50% | -63.15% | -58.74% | -60.84% | -59.68% | -59.76% |
| DLIR-L | -34.43% | -28.74% | -26.05% | -29.74% | -30.94% | -34.34% | -38.71% | -34.66% |
| DLIR-M | -45.90% | -39.08% | -36.13% | -40.37% | -43.05% | -46.39% | -50.00% | -46.48% |
| DLIR-H | -58.20% | -50.00% | -45.38% | -51.19% | -56.50% | -59.04% | -62.10% | -59.21% |
| Square root of AUC NPS low | ||||||||
| FBP | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| AV-40 | -14.52% | -13.76% | -13.34% | -13.87% | -7.51% | -9.64% | -12.33% | -9.83% |
| AV-100 | -33.46% | -32.46% | -29.69% | -31.87% | -22.81% | -27.49% | -29.24% | -26.52% |
| DLIR-L | -28.65% | -23.00% | -18.90% | -23.52% | -23.96% | -26.75% | -32.13% | -27.61% |
| DLIR-M | -37.86% | -31.08% | -25.59% | -31.51% | -32.80% | -36.60% | -41.83% | -37.08% |
| DLIR-H | -47.89% | -39.95% | -32.45% | -40.10% | -43.54% | -47.23% | -51.12% | -47.30% |
| Square root of AUC NPS high | ||||||||
| FBP | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| AV-40 | -34.51% | -34.44% | -34.45% | -34.47% | -30.48% | -32.63% | -34.10% | -32.40% |
| AV-100 | -84.07% | -83.11% | -81.41% | -82.86% | -82.24% | -82.80% | -81.93% | -82.32% |
| DLIR-L | -37.37% | -32.34% | -30.92% | -33.54% | -34.96% | -37.55% | -40.74% | -37.75% |
| DLIR-M | -49.42% | -43.23% | -41.39% | -44.68% | -47.64% | -50.15% | -53.28% | -50.36% |
| DLIR-H | -62.58% | -55.16% | -52.68% | -56.80% | -62.23% | -63.88% | -66.67% | -64.26% |
| f average | ||||||||
| FBP | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| AV-40 | -9.73% | -10.10% | -10.93% | -10.25% | -10.62% | -11.06% | -11.17% | -10.95% |
| AV-100 | -39.63% | -38.82% | -38.54% | -39.00% | -40.08% | -39.62% | -38.64% | -39.44% |
| DLIR-L | -4.77% | -4.60% | -6.27% | -5.21% | -5.97% | -6.15% | -5.83% | -5.98% |
| DLIR-M | -7.62% | -7.05% | -9.46% | -8.04% | -9.63% | -9.56% | -9.53% | -9.57% |
| DLIR-H | -12.38% | -10.69% | -14.09% | -12.39% | -15.61% | -15.20% | -16.42% | -15.74% |
Negative values indicated reduction
In terms of noise reduction distribution as spatial frequency, the noise in both the low and high spatial frequency ranges decreased with the increase of DLIR strength and ASIR-V blending factor (Table 3). Compared to FBP, the noise in the low spatial frequency range was decreased with AV-100 by an average reduction of -31.87% and -26.52% in SECT and DECT, respectively, which is lower than that of -40.10% and -47.30% with DLIR-H. In contrast, the AV-100 provided an average reduction of noise in the high spatial frequency range of -82.86% and -82.32% in SECT and DECT, respectively, much higher than that of -62.23% and -64.26% with DLIR-H, compared to FBP. AV-40 exhibited the same characteristics as AV-100: higher noise reduction in the high frequency range (-34.47% in SECT and -32.40% in DECT), but much lower noise reduction in the low frequency range (-13.87% in SECT and -9.83% in DECT).
The SECT and DECT images exhibited almost identical shifts in faverage for all scan protocols comparing to FBP (Table 3). The faverage shifted toward lower frequencies with increasing DLIR strength and ASIR-V blending factor. The shift of faverage in SECT and DECT with DLIR-H (-12.39% and -15.74%) were lower than that of faverage with AV-100 (-39.00% and -39.44%).
TTF
The TTF curves of bone and acrylic inserts and their corresponding metrics are presented in Figs. 4 and 5. For the bone insert, the TTF50% values obtained with DLIR and ASIR-V algorithms were not different compared to those with FBP in both SECT and DECT (Table 4), and were not affected by the dose level. With the acrylic insert, all DLIR images improved TTF50% values compared with FBP and ASIR-V in both SECT and DECT. ASIR-V images also improved TTF50% values in DECT compared with those of FBP images.
Fig. 4.
TTF curves. TTF curves were obtained with six image recnstruction algorithms (FBP, AV-40, AV-100, DLIR-L, DLIR-M, and DLIR-H) at three dose levels (5, 10, and 20 mGy). top, SECT; bottom, DECT. from left to right, 5, 10, and 20 mGy. a Bone insert. the f50% values obtained with DLIR and ASIR-V algorithms were not different compared to those obtained with FBP in both SECT and DECT, and were not obviously affected by the dose level. b Acrylic insert. All DLIR images improved f50% values compared with FBP and ASIR-V in both SECT and DECT. ASIR-V images also improved f50% values in DECT compared with FBP images
Fig. 5.
TTF metrics. a TTF bone f 50%, b TTF acrylic f 50%
Table 4.
Percentage variations of TTF relative to FBP images
| Reconstruction | SECT | DECT | ||||||
|---|---|---|---|---|---|---|---|---|
| 5 mGy | 10 mGy | 20 mGy | average | 5 mGy | 10 mGy | 20 mGy | average | |
| Bone TTF f50% | ||||||||
| FBP | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| AV-40 | 1.18% | 1.21% | 1.04% | 0.00% | 1.81% | 1.24% | 0.91% | 0.00% |
| AV-100 | 3.04% | 3.08% | 2.65% | 1.14% | 3.74% | 3.22% | 2.29% | 1.32% |
| DLIR-L | 0.89% | 1.14% | 1.44% | 2.92% | 6.39% | 7.59% | 6.60% | 3.08% |
| DLIR-M | 0.67% | 1.01% | 1.37% | 1.16% | 6.29% | 7.63% | 6.64% | 6.86% |
| DLIR-H | 0.44% | 0.86% | 1.31% | 1.02% | 6.27% | 7.60% | 6.69% | 6.85% |
| Acrylic TTF f50% | ||||||||
| FBP | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| AV-40 | -5.07% | -1.93% | 2.51% | 0.00% | 9.73% | 5.81% | 2.51% | 0.00% |
| AV-100 | -12.77% | -1.67% | 5.85% | -1.50% | 24.43% | 20.91% | 8.02% | 6.02% |
| DLIR-L | 6.94% | 5.96% | 9.10% | -2.86% | 24.46% | 27.02% | 33.97% | 17.79% |
| DLIR-M | 6.29% | 4.80% | 7.95% | 7.33% | 26.14% | 28.50% | 35.67% | 28.48% |
| DLIR-H | 4.58% | 4.94% | 7.08% | 6.35% | 27.77% | 30.29% | 37.20% | 30.10% |
Negative values indicated reduction and positive values indicated improvement
Detectability Index
The detectability indexes for small high-contrast feature (1.5-mm-diameter, 500 HU) and large low-contrast feature (25-mm-diameter, 120 HU) are presented in Fig. 6. The detectability indexes were increased with the increasing dose level (Fig. 7). To provide insights for clinical practice, the detectability indexes were compared with that of SECT 20 mGy AV-40 images (Table 5). For the small high-contrast feature, the SECT 10 mGy DLIR-H images provided comparable detectability (5.86%), and the DECT 10 mGy DLIR-H images showed higher detectability (20.90%), comparing to the SECT 20 mGy AV-40 images. Similarly, for the large low-contrast feature, the SECT 10 mGy DLIR-H images (3.20%) and DECT 10 mGy DLIR-H images (7.75%) provided comparable detectability, comparing to the SECT 20 mGy AV-40 images. For both features, the DECT 20 mGy AV-40 images showed lower detectability (-10.36% and -8.79%), but DECT 20 mGy AV-100 and DECT 20 mGy DLIR images all showed increased detectability and values increased with the reconstruction strength, comparing to the SECT 20 mGy AV-40 images.
Fig. 6.
Detectability index. a Small high-contrast feature and, b Large low-contrast feature
Fig. 7.
Detectability index and dose level. Comparisons of detectability index (d′) values obtained with a, c SECT and b, d DECT scan modes for a, b small high-contrast feature, and c, d large low-contrast feature
Table 5.
Percentage variations of d’ relative to SECT 20 mGy AV-40 images
| Reconstruction | SECT | DECT | ||||
|---|---|---|---|---|---|---|
| 5 mGy | 10 mGy | 20 mGy | 5 mGy | 10 mGy | 20 mGy | |
| d’ (1.5 mm, 500 HU) | ||||||
| FBP | -56.21% | -39.33% | -15.72% | -55.02% | -41.03% | -23.44% |
| AV-40 | -47.28% | -27.39% | n/a | -50.07% | -32.82% | -10.36% |
| AV-100 | -27.92% | -1.78% | 29.80% | -36.81% | -11.73% | 16.19% |
| DLIR-L | -36.90% | -19.19% | 6.99% | -38.68% | -15.47% | 18.18% |
| DLIR-M | -26.63% | -8.85% | 17.78% | -29.72% | -1.11% | 39.57% |
| DLIR-H | -10.86% | 5.86% | 31.44% | -14.66% | 20.90% | 69.39% |
| d’ (25 mm, 120 HU) | ||||||
| FBP | -48.39% | -28.24% | -10.54% | -47.38% | -32.92% | -16.70% |
| AV-40 | -41.36% | -20.00% | n/a | -44.81% | -28.50% | -8.79% |
| AV-100 | -30.84% | -4.59% | 15.25% | -38.81% | -16.88% | 6.50% |
| DLIR-L | -33.79% | -13.91% | 3.74% | -35.64% | -14.70% | 12.79% |
| DLIR-M | -27.38% | -7.08% | 10.10% | -30.01% | -5.31% | 26.56% |
| DLIR-H | -18.65% | 3.20% | 17.72% | -21.92% | 7.75% | 41.85% |
SECT 20 mGy AV-40 images was considered as the standard radiation dose and representative reconstruction method for an adult abdomen with single-energy CT in clinical practice. Negative values indicated reduction and positive values indicated improvement
Subjective Image Assessment
Subjective Image Quality Assessment
The subjective image quality assessment showed good to excellent intra-reader agreement and fair to good inter-reader agreement (Table 6). The results of subjective image quality assessment are presented in Fig. 8 and Table 7. The image noise and overall quality rating usually increased with increasing dose level, while the texture and sharpness rating did not show obvious increase trend with increasing dose level. The image noise rating increased with increasing DLIR strength and ASIR-V blending factor, but the other subjective image quality rating did not present an increase trend with increasing DLIR strength or ASIR-V blending factor. Notably, the DECT 10 mGy DLIR-H images showed better image quality than SECT 20 mGy AV-40 images in terms of image noise (10.00%), texture (5.88%), sharpness (5.00%), and overall quality (5.00%).
Table 6.
Intra-reader agreements of subjective image quality evaluation
| Rating | Intra-reader agreement | Inter-reader agreement | ||
|---|---|---|---|---|
| Weighted kappa | P value | Kendall’s W statistics | P value | |
| Image noise | 0.743 | < 0.001 | 0.660 | < 0.001 |
| Texture | 0.689 | < 0.001 | 0.389 | < 0.001 |
| Sharpness | 0.748 | < 0.001 | 0.446 | < 0.001 |
| Overall quality | 0.674 | < 0.001 | 0.563 | < 0.001 |
| High-contrast detectability | 0.735 | < 0.001 | 0.421 | < 0.001 |
| Low-contrast detectability | 0.813 | < 0.001 | 0.745 | < 0.001 |
Fig. 8.
Subjective image quality and detectability assessment. Bar plots of a image noise, b image texture, c border sharpness, d overall image quality, e high-contrast detectability, and f low-contrast detectability subjectively evaluated by six radiologists
Table 7.
Subjective evaluation results
| Reconstruction | SECT 5 mGy | SECT 10 mGy | SECT 20 mGy | DECT 5 mGy | DECT 10 mGy | DECT 20 mGy |
|---|---|---|---|---|---|---|
| Image noise (1 to 5) | ||||||
| FBP | 1.33 ± 0.52 | 2.17 ± 0.98 | 2.67 ± 1.03 | 1.50 ± 0.55 | 2.17 ± 0.75 | 2.17 ± 0.75 |
| AV-40 | 1.83 ± 1.17 | 2.33 ± 1.03 | 3.33 ± 0.82 | 2.00 ± 1.10 | 2.50 ± 0.84 | 3.00 ± 0.63 |
| AV-100 | 2.17 ± 0.75 | 2.50 ± 1.05 | 3.67 ± 1.03 | 2.00 ± 0.63 | 3.00 ± 0.89 | 3.33 ± 1.21 |
| DLIR-L | 2.17 ± 0.75 | 2.67 ± 1.03 | 3.17 ± 1.17 | 2.33 ± 0.82 | 2.83 ± 0.98 | 3.50 ± 0.84 |
| DLIR-M | 2.67 ± 1.03 | 3.00 ± 0.89 | 3.33 ± 1.03 | 2.33 ± 0.82 | 3.17 ± 0.75 | 3.67 ± 1.03 |
| DLIR-H | 2.83 ± 0.41 | 3.33 ± 0.82 | 4.00 ± 1.10 | 2.67 ± 1.03 | 3.67 ± 1.37 | 4.17 ± 1.33 |
| Texture (1 to 5) | ||||||
| FBP | 2.00 ± 0.89 | 2.50 ± 0.55 | 2.83 ± 0.41 | 2.67 ± 1.37 | 2.67 ± 0.52 | 2.67 ± 0.82 |
| AV-40 | 2.17 ± 0.75 | 2.67 ± 0.82 | 2.83 ± 0.41 | 2.83 ± 1.47 | 2.67 ± 1.03 | 3.17 ± 0.75 |
| AV-100 | 2.50 ± 0.84 | 2.17 ± 1.17 | 3.33 ± 0.82 | 2.50 ± 1.38 | 2.83 ± 0.41 | 2.83 ± 0.98 |
| DLIR-L | 2.83 ± 0.41 | 3.33 ± 0.52 | 3.00 ± 0.63 | 3.00 ± 0.89 | 3.00 ± 0.63 | 3.67 ± 0.52 |
| DLIR-M | 2.83 ± 0.98 | 3.17 ± 0.41 | 3.00 ± 0.63 | 2.83 ± 1.17 | 3.00 ± 0.63 | 3.33 ± 0.82 |
| DLIR-H | 3.33 ± 0.52 | 3.00 ± 0.63 | 3.50 ± 0.55 | 3.00 ± 1.10 | 3.00 ± 0.63 | 3.17 ± 0.75 |
| Sharpness (1 to 5) | ||||||
| FBP | 1.67 ± 0.52 | 2.67 ± 0.52 | 3.17 ± 0.75 | 2.50 ± 1.38 | 2.67 ± 0.82 | 2.67 ± 0.82 |
| AV-40 | 2.33 ± 0.82 | 2.67 ± 0.52 | 3.33 ± 0.82 | 2.83 ± 1.17 | 2.50 ± 1.05 | 3.00 ± 0.73 |
| AV-100 | 2.00 ± 0.00 | 2.33 ± 1.03 | 3.50 ± 0.55 | 2.33 ± 1.03 | 2.67 ± 0.82 | 3.00 ± 0.89 |
| DLIR-L | 2.83 ± 0.41 | 3.33 ± 0.52 | 3.17 ± 0.75 | 3.00 ± 0.89 | 3.17 ± 0.75 | 3.67 ± 0.52 |
| DLIR-M | 2.67 ± 0.82 | 3.00 ± 0.00 | 3.00 ± 0.63 | 2.83 ± 1.17 | 3.17 ± 0.75 | 3.83 ± 0.98 |
| DLIR-H | 3.17 ± 0.41 | 3.17 ± 0.98 | 3.50 ± 0.55 | 3.00 ± 1.26 | 3.50 ± 0.84 | 3.33 ± 0.82 |
| Overall (1 to 5) | ||||||
| FBP | 1.67 ± 0.52 | 2.50 ± 0.55 | 3.00 ± 0.63 | 2.00 ± 1.10 | 2.33 ± 0.52 | 2.67 ± 0.82 |
| AV-40 | 2.00 ± 0.63 | 2.50 ± 0.55 | 3.33 ± 0.82 | 2.83 ± 1.47 | 2.67 ± 1.03 | 3.17 ± 0.75 |
| AV-100 | 2.17 ± 0.41 | 2.67 ± 1.03 | 3.83 ± 0.75 | 2.33 ± 0.82 | 2.83 ± 0.41 | 3.33 ± 0.82 |
| DLIR-L | 2.83 ± 0.41 | 3.50 ± 0.55 | 3.33 ± 0.82 | 2.50 ± 0.55 | 3.00 ± 0.63 | 3.83 ± 0.75 |
| DLIR-M | 2.83 ± 0.75 | 3.00 ± 0.63 | 3.17 ± 0.75 | 2.83 ± 1.33 | 3.00 ± 0.63 | 3.83 ± 0.98 |
| DLIR-H | 3.33 ± 0.52 | 3.33 ± 0.82 | 3.83 ± 0.75 | 3.00 ± 1.10 | 3.50 ± 0.84 | 3.50 ± 0.55 |
| High-contrast detectability (0 to 8) | ||||||
| FBP | 3.50 ± 0.55 | 3.50 ± 0.55 | 3.83 ± 0.41 | 3.50 ± 0.84 | 3.50 ± 0.55 | 3.67 ± 0.52 |
| AV-40 | 3.50 ± 0.55 | 3.50 ± 0.55 | 4.00 ± 0.00 | 3.83 ± 0.41 | 4.00 ± 0.00 | 4.17 ± 0.41 |
| AV-100 | 3.67 ± 0.52 | 3.50 ± 0.55 | 4.00 ± 0.00 | 3.83 ± 0.41 | 4.00 ± 0.00 | 4.00 ± 0.00 |
| DLIR-L | 3.50 ± 0.55 | 3.50 ± 0.55 | 3.83 ± 0.41 | 3.83 ± 0.41 | 4.00 ± 0.00 | 4.17 ± 0.41 |
| DLIR-M | 3.50 ± 0.55 | 3.50 ± 0.55 | 4.00 ± 0.00 | 4.00 ± 0.00 | 4.00 ± 0.00 | 4.00 ± 0.00 |
| DLIR-H | 3.33 ± 0.52 | 3.33 ± 0.52 | 4.00 ± 0.00 | 4.00 ± 0.00 | 4.17 ± 0.41 | 4.00 ± 0.00 |
| Low-contrast detectability (0 to 5) | ||||||
| FBP | 0.17 ± 0.41 | 0.50 ± 0.55 | 2.17 ± 0.98 | 0.33 ± 0.52 | 1.00 ± 0.89 | 1.17 ± 0.75 |
| AV-40 | 0.50 ± 0.55 | 0.83 ± 0.41 | 2.67 ± 1.03 | 1.17 ± 0.75 | 1.17 ± 1.17 | 1.67 ± 1.03 |
| AV-100 | 0.83 ± 0.41 | 1.33 ± 1.03 | 2.83 ± 1.47 | 1.17 ± 0.75 | 1.33 ± 1.03 | 2.17 ± 0.98 |
| DLIR-L | 0.83 ± 0.41 | 1.33 ± 0.82 | 2.33 ± 1.51 | 0.67 ± 0.82 | 1.83 ± 1.17 | 2.33 ± 1.03 |
| DLIR-M | 1.00 ± 0.63 | 1.50 ± 0.55 | 2.50 ± 1.64 | 1.00 ± 1.10 | 1.83 ± 0.75 | 2.50 ± 0.55 |
| DLIR-H | 1.83 ± 0.41 | 1.83 ± 0.75 | 2.83 ± 1.33 | 1.17 ± 0.98 | 2.67 ± 1.03 | 2.83 ± 1.17 |
SECT 20 mGy AV-40 images was considered as the standard radiation dose and representive reconstruction method for an adult abdomen with single-energy CT in clinical practice. Negative values indicated reduction and positive values indicated improvement. Present as mean ± standard deviation
Subjective Detectability Assessment
The subjective detectability for high-contrast and low-contrast tasks were compared to the SECT 20 mGy AV-40 images (Fig. 9 and Table 7). SECT 10 mGy images could not provide comparable high-contrast (3.33 vs. 4.00, -16.75%) and low-contrast (1.83 vs. 2.67, -31.46%) detectability even with DLIR-H. In contrast, DECT 10 mGy DLIR-H images provided comparable detectability for both high-contrast (4.17 vs. 4.00, 4.25%) and low-contrast (2.67 vs. 2.67, 0.00%) task, comparing with SECT 20 mGy AV-40 images. DLIR algorithms provided the same detectability in both SECT and DECT at the dose level of 20 mGy, while ASIR-V algorithms performed slightly worse in DECT than in SECT.
Fig. 9.
Objective and subjective detectability assessment. Heatmaps of percentage of variation of objective and subjective detectability assessment compared to SECT 20 mGy AV-40 images. a objective detectability index for small high-contrast feature, b detectability index for small high-contrast feature, c subjective detectability assessment results for high-contrast task, d subjective detectability assessment results for low-contrast task
Discussion
Our study presented the image quality and detectability improvement with DLIR in both SECT and DECT, compared with the commonly used ASIR-V in routine clinical applications. DLIR could reduce the image noise in a more balanced manner in both the low and high spatial frequency ranges compared with FBP and ASIR-V algorithms, and provide improved TTF value compared with ASIR-V. DLIR provided higher detectability index for both small high-contrast feature and large low-contrast feature in DECT than those with ASIR-V. At 50% (10 mGy) dose level, DLIR-H images in DECT had similar image quality and diagnostic ability in terms of subjective detectability assessments, and objective detectability index estimation as the AV-40 images in SECT obtained at 20 mGy dose level that are commonly used in daily practice.
The noise magnitude evaluation indicated that DLIR provided deeper noise reduction in the low spatial frequency range compared to ASIR-V, which was similar to those of previous studies [18, 29]. On the other hand, AV-100 had higher noise reduction in the high spatial frequency range than DLIR. Similar characteristics of hybrid IR were observed in a previous study [32]. More reduction in high spatial frequency range than that in low spatial frequency range caused a shift of faverage toward lower frequencies. This unbalanced image noise decreasing affected the image texture and potentially resulted in lower detectability of diagnostic tasks [7]. In contrast, DLIR showed lower shifts of faverage, since DLIR decreased noise in a more balanced manner regarding both low and high spatial frequency ranges. Therefore, the DLIR could preserve the image texture, which was similar to that reconstructed using FBP, and provide images that are not too overly-smoothing or artificial-looking to radiologists [12, 18].
In the current study, the TTF50% values obtained in DECT images processed by DLIR did not show obvious change compared to those of FBP for the bone insert (955 HU). while these values were higher for the acrylic insert (120 HU). In a previous study, the DECT images processed by DLIR showed higher spatial resolution than those of SECT with the same algorithm for a contrast object of 300 HU, but the TTF50% values resulting from DLIR were lower than those obtained by FBP at contrast of 60 HU [18]. These results indicated that the spatial resolution of DECT images processed by DLIR showed different characteristics depending on the object contrast. SECT images processed by FBP have linear properties, and the spatial resolution of these images did not change with the object contrast [27]. As a contrast, the spatial resolution of DECT images produced by FBP differed depending on the object contrast [15], since the noise reduction was performed on the raw data of DECT images with FBP [15, 18]. We suspected that the varying properties of DLIR on the spatial resolution of DECT images was related to the fact that DLIR was applied to the original images in which noise reduction had already been performed [18].
We further subjectively assessed the image quality. The visual assessment indicated a higher noise magnitude of images obtained with FBP than those with hybrid IR and DLIR for the same dose level, which was in accordance with subjective image noise rating, and supported by objective measurements. Images processed by AV-100 were smoothed out. Similar results have been described in SECT images [13]. However, image texture, sharpness and overall quality rating did not obviously differ visually among reconstruction algorithms. Since the phantom images might not feature the same exact characteristics as the anatomical structures present in patients [17], we considered that subjective assessments on clinical image might better reflect the preference of the radiologists [28]. Indeed, within clinical abdominal images, radiologists have showed their preference for DLIR images both with SECT mode [14, 30, 33–37], and DECT mode [38–44].
The results of subjective detectability assessment were not always in accordance with the corresponding objective detectability indexes. Objective assessment showed higher detectability index for both small high-contrast feature and large low-contrast feature with 20 mGy images with all DLIR reconstructions in SECT and DECT. However, with DECT 10 mGy DLIR-H images, objective assessments showed more improvements than subjective evaluation in both high-contrast (20.90% vs 4.17%) and low-contrast (7.75% vs 0.00%) tasks. Meanwhile, with DECT 20 mGy DLIR-H images, the differences between objective and subjective assessment were even more obvious in both high-contrast (69.39% vs 0.00%) and low-contrast (41.85% vs 6.25%) tasks. These results indicated that objective in vitro performance improvements did not always translate into clinical acceptability to naked eyes.
Therefore, we believe that the subjective evaluation should be considered important before applying DLIR algorithms in clinical practice. Previous studies using MBIR algorithm [19] and the deep learning reconstruction (DLR) by Canon in SECT have demonstrated that subjective evaluation did not always show corresponding improvements in detection tasks as objective assessments suggested [20, 21]. A recent study implied dramatic detectability improvement of DECT images with DLIR in terms of objective assessment without subjective validation [18], which left the doubt whether these promising objective improvements could translate into clinical acceptance of DLIR algorithm in DECT images. Our study also indicated that although there were improvements in image quality perceived by human observers for DLIR algorithms, the improvements were not as large as the objective measurements in both SECT and DECT images.
The detectability index varied depending on the contrast of the object being investigated. The detectability indexes of DECT 10 mGy DLIR-H images were higher than those of SECT 20 mGy AV-40 images, for both small high-contrast (500 HU) feature and large low-contrast (120 HU) feature. We believe that DLIR-H did not decrease the diagnostic performance of these two detection tasks when the dose level was reduced by half. The results of subjective detectability assessments supported the objective measurements. At 50% of the radiation dose, only DECT images processed by DLIR-H were deemed to be of similar detectability for both high-contrast and low-contrast tasks, compared with those of SECT 20 mGy AV-40 images. Similar results were obtained in SECT that DLIR-H could provide higher detectability index with approximately half the dose reduction for features with objective contrast of 10 HU, 120 HU, and 500 HU [17]. However, DLIR-H were not recommended in DECT images for objective contrast of 60 HU, simulating soft tissue or organs [18]. Meanwhile, DLIR-M were recommended for objective contrast of 300 HU, to improve image quality of CT angiography and bone images [18]. The detectability index estimation relied on NPS and TTF [10]. Considering the dependence of spatial resolution on objective contrast, it is not strange that the detectability index might differ according to tasks in DECT images. Therefore, further investigation is needed to reveal the potential influence of DLIR on spatial resolution and detectability index of different object contrast in DECT images. On the other hand, since the objective metrics are not always in accordance with the subjective evaluation, investigations with clinical images might provide insights for this contrast-depending variation, to directly guide the usage of DLIR in diagnostic tasks with different contrast.
There were several limitations in our study. First, our study was performed with a phantom instead of clinical images. There were only two lesions used in our study to mimic calcifications or identifying high-contrast tissue boundaries. To confirm the results of this study, further clinical studies are needed. Second, only standard reconstruction kernel was available for DLIR in DECT. The influence of different reconstruction kernels on the image quality reconstructed using DLIR needs further investigation. Third, our study was performed with only one CT system. Other manufacturers provide different deep learning-based algorithms for clinical use and may induce different characteristics of images from those obtained in current study [45]. Finally, it is also important to note that the DECT assessment performed in this study is very limited in scope, focusing only on VMIs similar to SECT acquisitions.
Conclusion
To summarize, compared to the ASIR-V at 40% strength commonly used in clinical practice, DLIR further reduces image noise in a more balanced manner in both SECT and DECT, and showed better objective and subjective detectability. DLIR-H has the potential to provide similar or better image quality and preserve the diagnostic performance at 50% of the radiation dose compared to images used in clinical practice. Nevertheless, the spatial resolution and detectability of DECT images processed with DLIR varied depending on the contrast of the object being investigated, and further clinical investigation is needed to guide the clinical use of DLIR in DECT. The potential to provide additional 50% radiation dose reduction with DLIR might accelerate the clinical acceptance of DECT.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to express their gratitude to Dr. Zhen Pan for her assistance in image quality assessment, and Dr. Shiqi Mao for his advice on data visualization.
Abbreviations
- ASIR-V
Adaptive statistical iterative reconstruction-V
- AUC
Area under the curve
- DECT
Dual-energy computed tomography
- DLIR
Deep learning image reconstruction
- DLR
Deep learning reconstruction
- FBP
Filtered back-projection
- HU
Hounsfield unit value
- IR
Iterative reconstruction
- MBIR
Model-based image reconstruction
- NPS
Noise power spectrum
- ROI
Region of interest
- SECT
Single-energy computed tomography
- VMI
Virtual monoenergetic images
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Jingyu Zhong, Hailin Shen, Yong Chen, Yihan Xia, Yue Xing, Yangfan Hu, Xiang Ge, Defang Ding, and Zhenming Jiang. The first draft of the manuscript was written by Jingyu Zhong and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by National Natural Science Foundation of China (82271934, 82101986), Yangfan Project of Science and Technology Commission of Shanghai Municipality (22YF1442400, 20YF1427200), Shanghai Science and Technology Commission Science and Technology Innovation Action Clinical Innovation Field (18411953000), Medicine and Engineering Combination Project of Shanghai Jiao Tong University (YG2021QN08, YG2019ZDB09), Research Fund of Tongren Hospital, Shanghai Jiao Tong University School of Medicine (TRKYRC-XX202204, TRGG202101, TRYJ2021JC06, 2020TRYJ(LB)06, 2020TRYJ(JC)07), Guangci Innovative Technology Launch Plan of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine (2022–13).
Data Availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Declarations
Ethics Approval
Institutional Review Board approval was not required because of the nature of our study, which was a phantom study.
Consent to Participate
Written informed consent was not required for this study because of the nature of our study, which was a phantom study.
Consent to Publish
Consent to publish was not required because of the nature of our study, which was a phantom study.
Competing Interests
Mr. Wei Lu and Dr. Jianying Li are employees of GE Healthcare. However, they neither had access nor control on the data acquisition and analysis. All other authors of this manuscript have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jingyu Zhong and Hailin Shen contributed equally to this work.
Contributor Information
Jingyu Zhong, Email: wal_zjy@163.com, Email: ZJY4623@shtrhospital.com.
Hailin Shen, Email: hailinshen@163.com.
Yong Chen, Email: shaoer.cy@gmail.com.
Yihan Xia, Email: 1870369408@qq.com.
Xiaomeng Shi, Email: colin-shi@hotmail.com.
Wei Lu, Email: Tony.Lu@geahk.ge.com.
Jianying Li, Email: Jianying.Li@med.ge.com.
Yue Xing, Email: xingyuesjtu@163.com, Email: XY4445@shtrhospital.com.
Yangfan Hu, Email: huyangfan11@126.com, Email: HYF4660@shtrhospital.com.
Xiang Ge, Email: gexianghuainan@163.com, Email: XG4622@shtrhospital.com.
Defang Ding, Email: dingdefen@163.com, Email: DDF4249@shtrhospital.com.
Zhenming Jiang, Email: jiangzhenmean@163.com, Email: JZM3723@shtrhospital.com.
Weiwu Yao, Email: yaoweiwuhuan@163.com, Email: YWW4142@shtrhospital.com.
References
- 1.Garba I, Zarb F, McEntee MF, Fabri SG. Computed tomography diagnostic reference levels for adult brain, chest and abdominal examinations: A systematic review. Radiography (Lond) 2021;27(2):673–681. doi: 10.1016/j.radi.2020.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Geyer LL, Schoepf UJ, Meinel FG, et al. State of the art: Iterative CT reconstruction techniques. Radiology. 2015;276(2):339–357. doi: 10.1148/radiol.2015132766. [DOI] [PubMed] [Google Scholar]
- 3.Willemink MJ, Noël PB. The evolution of image reconstruction for CT-from filtered back projection to artificial intelligence. Eur Radiol. 2019;29(5):2185–2195. doi: 10.1007/s00330-018-5810-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fält T, Söderberg M, Hörberg L, et al. (2019) Simulated dose reduction for abdominal CT with filtered back projection technique: effect on liver lesion detection and characterization. AJR Am J Roentgenol. 2019;212(1):84–93. doi: 10.2214/AJR.17.19441. [DOI] [PubMed] [Google Scholar]
- 5.Mileto A, Guimaraes LS, McCollough CH, Fletcher JG, Yu L. State of the art in abdominal CT: the limits of iterative reconstruction algorithms. Radiology. 2019;293(3):491–503. doi: 10.1148/radiol.2019191422. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Z, Seeram E. The use of artificial intelligence in computed tomography image reconstruction - A literature review. J Med Imaging Radiat Sci. 2020;51(4):671–677. doi: 10.1016/j.jmir.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Chen B, Christianson O, Wilson JM, Samei E. Assessment of volumetric noise and resolution performance for linear and nonlinear CT reconstruction methods. Med Phys. 2014;41(7):071909. doi: 10.1118/1.4881519. [DOI] [PubMed] [Google Scholar]
- 8.Samei E, Richard S. Assessment of the dose reduction potential of a model-based iterative reconstruction algorithm using a task-based performance metrology. Med Phys. 2015;42(1):314–323. doi: 10.1118/1.4903899. [DOI] [PubMed] [Google Scholar]
- 9.Christianson O, Chen JJ, Yang Z, et al. (2015) An improved index of image quality for task-based performance of CT iterative reconstruction across three commercial implementations. Radiology. 2015;275(3):725–734. doi: 10.1148/radiol.15132091. [DOI] [PubMed] [Google Scholar]
- 10.Samei E, Bakalyar D, Boedeker KL, et al. Performance evaluation of computed tomography systems: summary of AAPM task group 233. Med Phys. 2019;46(11):e735–e756. doi: 10.1002/mp.13763. [DOI] [PubMed] [Google Scholar]
- 11.Greffier J, Larbi A, Frandon J, Moliner G, Beregi JP, Pereira F. Comparison of noise-magnitude and noise-texture across two generations of iterative reconstruction algorithms from three manufacturers. Diagn Interv Imaging. 2019;100(7–8):401–410. doi: 10.1016/j.diii.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Greffier J, Frandon J, Larbi A, Beregi JP, Pereira F. CT iterative reconstruction algorithms: a task-based image quality assessment. Eur Radiol. 2020;30(1):487–500. doi: 10.1007/s00330-019-06359-6. [DOI] [PubMed] [Google Scholar]
- 13.Greffier J, Hamard A, Pereira F, et al. Image quality and dose reduction opportunity of deep learning image reconstruction algorithm for CT: a phantom study. Eur Radiol. 2020;30(7):3951–3959. doi: 10.1007/s00330-020-06724-w. [DOI] [PubMed] [Google Scholar]
- 14.Bornet PA, Villani N, Gillet R, et al. Clinical acceptance of deep learning reconstruction for abdominal CT imaging: objective and subjective image quality and low-contrast detectability assessment. Eur Radiol. 2022;32(5):3161–3172. doi: 10.1007/s00330-021-08410-x. [DOI] [PubMed] [Google Scholar]
- 15.Masuda S, Sugisawa K, Minamishima K, Yamazaki A, Jinzaki M. Assessment of the image quality of virtual monochromatic spectral computed tomography images: a phantom study considering object contrast, radiation dose, and frequency characteristics. Radiol Phys Technol. 2021;14(1):41–49. doi: 10.1007/s12194-020-00597-w. [DOI] [PubMed] [Google Scholar]
- 16.Greffier J, Si-Mohamed S, Dabli D, et al. Performance of four dual-energy CT platforms for abdominal imaging: a task-based image quality assessment based on phantom data. Eur Radiol. 2021;31(7):5324–5334. doi: 10.1007/s00330-020-07671-2. [DOI] [PubMed] [Google Scholar]
- 17.Greffier J, Dabli D, Hamard A, et al. Impact of dose reduction and the use of an advanced model-based iterative reconstruction algorithm on spectral performance of a dual-source CT system: a task-based image quality assessment. Diagn Interv Imaging. 2021;102(7–8):405–412. doi: 10.1016/j.diii.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Masuda S, Yamada Y, Minamishima K, Owaki Y, Yamazaki A, Jinzaki M. Impact of noise reduction on radiation dose reduction potential of virtual monochromatic spectral images: Comparison of phantom images with conventional 120 kVp images using deep learning image reconstruction and hybrid iterative reconstruction. Eur J Radiol. 2022;149:110198. doi: 10.1016/j.ejrad.2022.110198. [DOI] [PubMed] [Google Scholar]
- 19.Laurent G, Villani N, Hossu G, et al. Full model-based iterative reconstruction (MBIR) in abdominal CT increases objective image quality, but decreases subjective acceptance. Eur Radiol. 2019;29(8):4016–4025. doi: 10.1007/s00330-018-5988-8. [DOI] [PubMed] [Google Scholar]
- 20.Akagi M, Nakamura Y, Higaki T, et al. Deep learning reconstruction improves image quality of abdominal ultra-high-resolution CT. Eur Radiol. 2019;29(11):6163–6171. doi: 10.1007/s00330-019-06170-3. [DOI] [PubMed] [Google Scholar]
- 21.Singh R, Digumarthy SR, Muse VV, et al. Image quality and lesion detection on deep learning reconstruction and iterative reconstruction of submillisievert chest and abdominal CT. AJR Am J Roentgenol. 2020;214(3):566–573. doi: 10.2214/AJR.19.21809. [DOI] [PubMed] [Google Scholar]
- 22.The National Health Commission of People’s Republic of China. Diagnostic reference levels for adults in X-ray computed tomography. Accessed via http://www.nhc.gov.cn/wjw/pcrb/201810/d3bb2f7acef248f0a1347a2da93cb41f.shtml on Apr 2022.
- 23.Chen Y, Zhong J, Wang L, et al. Multivendor comparison of quantification accuracy of iodine concentration and attenuation measurements by dual-energy CT: a phantom study. AJR Am J Roentgenol. 2022;219(5):827–839. doi: 10.2214/AJR.22.27753. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto K, Jinzaki M, Tanami Y, Ueno A, Yamada M, Kuribayashi S. Virtual monochromatic spectral imaging with fast kilovoltage switching: improved image quality as compared with that obtained with conventional 120-kVp CT. Radiology. 2011;259(1):257–262. doi: 10.1148/radiol.11100978. [DOI] [PubMed] [Google Scholar]
- 25.Solomon J, Samei E. Correlation between human detection accuracy and observer model-based image quality metrics in computed tomography. J Med Imaging (Bellingham) 2016;3(3):035506. doi: 10.1117/1.JMI.3.3.035506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kijewski MF, Judy PF. The noise power spectrum of CT images. Phys Med Biol. 1987;32(5):565–575. doi: 10.1088/0031-9155/32/5/003. [DOI] [PubMed] [Google Scholar]
- 27.Urikura A, Yoshida T, Nakaya Y, Nishimaru E, Hara T, Endo M. Deep learning-based reconstruction in ultra-high-resolution computed tomography: can image noise caused by high definition detector and the miniaturization of matrix element size be improved? Phys Med. 2021;81:121–129. doi: 10.1016/j.ejmp.2020.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Richard S, Husarik DB, Yadava G, Murphy SN, Samei E. Towards task-based assessment of CT performance: system and object MTF across different reconstruction algorithms. Med Phys. 2012;39(7):4115–4122. doi: 10.1118/1.4725171. [DOI] [PubMed] [Google Scholar]
- 29.Cheng Y, Abadi E, Smith TB, et al. Validation of algorithmic CT image quality metrics with preferences of radiologists. Med Phys. 2019;46(11):4837–4846. doi: 10.1002/mp.13795. [DOI] [PubMed] [Google Scholar]
- 30.Greffier J, Dabli D, Frandon J, et al. Comparison of two versions of a deep learning image reconstruction algorithm on CT image quality and dose reduction: A phantom study. Med Phys. 2021;48(10):5743–5755. doi: 10.1002/mp.15180. [DOI] [PubMed] [Google Scholar]
- 31.Lyu P, Neely B, Solomon J, et al. Effect of deep learning image reconstruction in the prediction of resectability of pancreatic cancer: Diagnostic performance and reader confidence. Eur J Radiol. 2021;141:109825. doi: 10.1016/j.ejrad.2021.109825. [DOI] [PubMed] [Google Scholar]
- 32.Lee S, Choi YH, Cho YJ, et al. Noise reduction approach in pediatric abdominal CT combining deep learning and dual-energy technique. Eur Radiol. 2021;31(4):2218–2226. doi: 10.1007/s00330-020-07349-9. [DOI] [PubMed] [Google Scholar]
- 33.Euler A, Solomon J, Marin D, Nelson RC, Samei E. A third-generation adaptive statistical iterative reconstruction technique: phantom study of image noise, spatial resolution, lesion detectability, and dose reduction potential. AJR Am J Roentgenol. 2018;210(6):1301–1308. doi: 10.2214/AJR.17.19102. [DOI] [PubMed] [Google Scholar]
- 34.Jensen CT, Liu X, Tamm EP, et al. Image quality assessment of abdominal CT by use of new deep learning image reconstruction: initial experience. AJR Am J Roentgenol. 2020;215(1):50–57. doi: 10.2214/AJR.19.22332. [DOI] [PubMed] [Google Scholar]
- 35.Ichikawa Y, Kanii Y, Yamazaki A, et al. Deep learning image reconstruction for improvement of image quality of abdominal computed tomography: comparison with hybrid iterative reconstruction. Jpn J Radiol. 2021;39(6):598–604. doi: 10.1007/s11604-021-01089-6. [DOI] [PubMed] [Google Scholar]
- 36.Cao L, Liu X, Li J, et al. A study of using a deep learning image reconstruction to improve the image quality of extremely low-dose contrast-enhanced abdominal CT for patients with hepatic lesions. Br J Radiol. 2021;94(1118):20201086. doi: 10.1259/bjr.20201086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nam JG, Hong JH, Kim DS, Oh J, Goo JM. Deep learning reconstruction for contrast-enhanced CT of the upper abdomen: similar image quality with lower radiation dose in direct comparison with iterative reconstruction. Eur Radiol. 2021;31(8):5533–5543. doi: 10.1007/s00330-021-07712-4. [DOI] [PubMed] [Google Scholar]
- 38.Fair E, Profio M, Kulkarni N et al (2022) Image quality evaluation in dual-energy CT of the chest abdomen and pelvis in obese patients with deep learning image reconstruction. J Comput Assist Tomogr 46(4):604–611. 10.1097/RCT.0000000000001316 [DOI] [PubMed]
- 39.Noda Y, Kawai N, Nagata S et al (2022) Deep learning image reconstruction algorithm for pancreatic protocol dual-energy computed tomography: image quality and quantification of iodine concentration. Eur Radiol 32(1):384–394. 10.1007/s00330-021-08121-3 [DOI] [PubMed]
- 40.Sato M, Ichikawa Y, Domae K et al (2022) Deep learning image reconstruction for improving image quality of contrast-enhanced dual-energy CT in abdomen. Eur Radiol 32(8):5499–5507. 10.1007/s00330-022-08647-0 [DOI] [PubMed]
- 41.Xu JJ, Lönn L, Budtz-Jørgensen E, Hansen KL, Ulriksen PS (2022) Quantitative and qualitative assessments of deep learning image reconstruction in low-keV virtual monoenergetic dual-energy CT. Eur Radiol 32(10):7098–7107. 10.1007/s00330-022-09018-5 [DOI] [PubMed]
- 42.Xu JJ, Lönn L, Budtz-Jørgensen E, Jawad S, Ulriksen PS, Hansen KL (2023) Evaluation of thin-slice abdominal DECT using deep-learning image reconstruction in 74 keV virtual monoenergetic images: an image quality comparison. Abdom Radiol (NY). 10.1007/s00261-023-03845-w [DOI] [PubMed]
- 43.Noda Y, Kawai N, Kawamura T et al (2022) Radiation and iodine dose reduced thoraco-abdomino-pelvic dual-energy CT at 40 keV reconstructed with deep learning image reconstruction. Br J Radiol 95(1134):20211163. 10.1259/bjr.20211163 [DOI] [PMC free article] [PubMed]
- 44.Fukutomi A, Sofue K, Ueshima E et al (2023) Deep learning image reconstruction to improve accuracy of iodine quantification and image quality in dual-energy CT of the abdomen: a phantom and clinical study. Eur Radiol 33(2):1388–1399. 10.1007/s00330-022-09127-1 [DOI] [PubMed]
- 45.Kawashima H, Ichikawa K, Takata T, Seto I (2022) Comparative assessment of noise properties for two deep learning CT image reconstruction techniques and filtered back projection. Med Phys 49(10):6359–6367. 10.1002/mp.15918 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.