Abstract
The study aimed to evaluate the keratectasia volume (KEV) before and after corneal cross-linking (CXL) in pediatric patients. This study included 40 eyes of 25 pediatric patients (10–19 years) undergoing standard CXL. The support vector machine (SVM) algorithm was applied to transform mass pixels in corneal topography into a three-dimensioned model to calculate the KEV. The KEV, Kmax, K1, K2, Kave, keratectasia area (KEA), and thinnest corneal thickness (TCT) were determined before CXL and at 3, 6, and 12 months after surgery. The correlation between KEV and other parameters (Kmax, TCT, max decentration, eccentricity, and so on) was calculated. The KEV was 4.75 ± 0.74 preoperatively and 4.43 ± 1.22 postoperatively at last follow-up (p < 0.002). There was strong positive correlation between the KEV and Kmax (r = 0.806, p < 0.0005). The preoperat ive KEV was 4.32 ± 0.69 in mild to moderate keratoconus (Kmax < 58D) and 5.27 ± 0.37 in advanced keratoconus (Kmax > 58D) (p < 0.0005, t-test). Postoperative KEV and K readings remained stable at the early stage, and the KEV showed a more drastic decreasing trend than Kmax at sixth month. Statistical significance was found in the KEV between preoperative and 6 months after surgery (p < 0.0005), but not in Kmax and other parameters. In 83.3% (15 eyes out of 18 eyes) of the eyes, the preoperative KEV was greater than 4.6 in patients with significant flattening after CXL. Compared with K readings, the KEV can be regarded as a more sensitive index to evaluate the postoperative morphological changes after CXL in pediatric patients.
Keywords: The keratectasia volume (KEV), Corneal collagen cross-linking, Topography, Pediatric keratoconus
Introduction
Keratoconus (KC) is described as progressive corneal ectasia caused by non-inflammatory structural changes in stromal collagen, which is characterized by bilateral thinning, asymmetrical degeneration, and conical protrusion of the anterior part of the cornea [1]. Different from adult KC, the disease in younger patients tends to be more aggressive and severe [2, 3] due to age-related glycation-induced cross-linking [4], more eye rubbing [5], etc. The age between 10 and 20 years was confirmed to be a significant factor for corneal scarring and KC progression [6], leading to a higher likelihood of corneal transplantation [7].
Over the past 10 years, compared with glasses, contact lenses, and keratoplasty, corneal cross-linking (CXL) has gained popularity in the treatment of KC, including epithelium-off and trans-epithelial CXL. With the function of forming covalent bonds in the cornea, collagen cross-linking has been exhaustively studied and performed extensively in adults as an effective surgical procedure to halt the progression of KC [8]. However, it is still being studied in adolescents to determine the timing of treatment, the use of higher total power, and the scope of different protocols of CXL [9].
Corneal topography is regarded as an essential component in pediatric KC research, which has been shown to be helpful in preoperative diagnosis [10] and post-operative evaluation [8]. K1, K2, and Kmax are generally used as evaluation parameters to assess the severity of the corneal protrusion and the effect of collagen cross-linking. However, it is challenging to evaluate the extent of ectasia after CXL with one-dimensional data, the curvature values (K1, K2, and Kmax) of a certain point. Accordingly, to address this issue, we considered the possibility of collecting all curvature values of the cornea cone in topography and giving a larger weight to the points with larger curvature value a larger weight, and then summing up to get the theoretical cornea ectasia volume. Additionally, we also wondered whether this new index can be more sensitive than single curvature values (K readings) to evaluate the postoperative morphological changes in pediatric patients.
Recently, the support vector machine (SVM) algorithm, an essential and powerful supervised learning technique, has been used extensively in bioinformatic applications. This algorithm is adept at handling variable graphs and providing recognition subtle patterns [11]. In this study, the SVM algorithm was applied to calculate the keratectasia area (KEA) and keratectasia volume (KEV). Although the calculation of the KEV did not take the real protrusion volume of the cornea into consideration which was affected by the corneal volume or corneal thickness, it spatially integrated the mass curvature values (K readings) and acquired the theoretical ectasia volume on the basis of the SVM method. Therefore, the KEV did not associate with the corneal thickness or corneal volume, and to some extent, the KEV can be regarded as a three-dimensional counterpart of Kmax. Compared with Kmax, KEV takes a large number of curvature values (greater than 47D) into consideration to reflect the flattening extent and evaluate the effect of collagen cross-linking.
Accordingly, in this study, we analyze the one-year topography results of collagen cross-linking in pediatric patients to identify the characterization of the KEV parameter after CXL and its sensitivity of monitoring progression compared with K readings.
Materials and Methods
Patients
This retrospective single-center study included KC pediatric patients without any other eye disorders or eye-related systemic autoimmune diseases. Overall, 25 patients (40 eyes) under 19 years of age included in this study (mean age 16.52 ± 2.22 years), consisting of 16 males and 9 females. These patients underwent CXL from May 1, 2015, to Aug 1, 2018, in the Ophthalmology Department of Shandong Province Hospital. Initially, all the study participants signed informed consent form allowing their data to be used as part of this study. Our research was approved by Ethics Committee in Shandong Province Hospital affiliated to Shandong University.
The inclusion criteria were progressive KC with thinnest corneal thickness (TCT) of no less than 400 μm. The age of patients ranged from 10 to 19 years. The progression criteria were defined as an increase of no less than 1.00 diopter in Kmax, an increase of no less than 1.00 diopter in cylinder, or an increase of no less than 0.5 diopter in spherical equivalent [12].
The exclusion criteria included corneal surgery other than CXL, dry eye, history of corneal trauma, herpetic keratitis, acute or severe corneal infection, and inability to calculate the corneal topography.
Corneal Cross-linking Procedure
All the adolescents underwent standard CXL surgery without preoperative or postoperative systemic narcotic analgesic drug administration under sterile conditions. Initially, proparacaine hydrochloride 0.5% (Alcaine; Alcon Laborato-ries Inc., Geneva, Switzerland) was instilled into the operative eye 3 times at 3-min interval to achieve topical anesthesia. Subsequently, the central 9.0-mm part of the corneal epithelium was removed mechanically with a blunt spatula (Amade-us II, Ziemers Ophthalmic Systems AG, Port, Switzerland).
The 0.1% isotonic riboflavin solution (0.5% in physiological saline solution; Shandong Fangming Pharmaceutical Limited by Share Ltd, Shandong, China) was subsequently administered to the stroma every 3 min for 30 min until the presence of riboflavin was confirmed throughout the cornea as well as the anterior chamber by slit-lamp biomicroscopy during surgery. In addition, a hypotonic riboflavin solution (0.5% in sterile water), rather than an isotonic medicine, was applied to ascertain the adequate thickness of the swollen stroma when the TCT (OCT, Cirrus HD-OCT 4000; Carl Zeiss Meditec Inc., Dublin, CA, USA) was less than 400 μm.
We irradiated the denuded stroma 50 mm away from the apex of cornea with ultraviolet A light (UV-X illumination system version 1000, UVXTM; IROC AG, Zurich, Switzerland), at 365 nm and 3.0 mW/cm [2], and applying riboflavin every 5 min for 30 min.
Postoperatively, a soft bandage contact lens was placed on the cornea for three to five days to promote epithelialization. Additionally, 0.5% levofoxacin eyedrops (Cravit; Santen Pharmaceutical Company, Osaka, Japan) and 0.1% fluorometholone (Fluorometholone Eye Drops; Allergan Pharmaceuticals Ireland, Westport, County Mayo, Ireland) were prescribed as conventional treatment. The former was instilled 4 times daily for 1 week, followed by the latter applied 4 times daily for 2 weeks.
Index Measurement
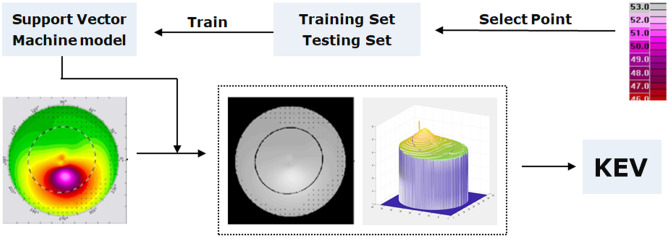
The KEA and KEV were acquired by the SVM method. The basic principle of the SVM model was that different pixels in the picture representing specific curvature values and the corneal topography results can be regarded as the aggregation of a large number of curvature pixels (Fig. 1). Consequently, pixels can be used as intermediate carrier to transform the primitive simple topography to an abstract curvature map by the SVM model implemented in the Matlab 2018b (MathWorks Inc., Natick, MA, USA).
Fig. 1.
The application of support vector machine (SVM) model and topography in calculating Keratectasia volume (KEV)
The concrete calculation process of the KEV can be divided into two steps. The first step is to train an SVM model (the input data are the pixel value, the output data are the curvature values) to convert the color map into the curvature map. The second step is to calculate the KEV through the curvature map.
I. Conversion of the Color Map to Curvature Map
Some pixel values and corresponding curvature values in the right scale bar are selected as training data. In this experiment, a total of 310 pixels (31 curvature values in the right scale bar, 10 corresponding pixels for each curvature value) were selected. Among the 310 pixels, 217 pixels X_train (each curvature value selects 7 corresponding pixels) and their corresponding curvature value Y_train made up the training set (X_train, Y_train), and 93 pixels X_test and their corresponding curvature value Y_test constituted the test set (X_test, Y_test) used to test the performance of the SVM model.
The Libsvm software package is used to train and test the SVM model. After training, the trained SVM model obtained in this experiment had a classification accuracy rate of 100% in the test set; that is to say, the trained SVM model can correctly distinguish the curvature values corresponding to different pixel values.
The trained SVM model is used to transform the color image into a curvature image. The corresponding curvature value can be obtained by inputting the pixel value of the color image into the SVM model, and then, the curvature map can be obtained.
II. Calculation of the KEV
Although the K1, K2, and Kmax in the corneal topography are the most common indicators used to evaluate the severity of KC, they are not quite accurate because they only reflect one-dimensional information of the corneal topography. In this study, the points with curvature values greater than 47D were regarded as outliers. The number of pixels with curvature values greater than 47D (area of lesion, KEA) can be calculated, but it is not accurate for severity assessment of the protrusion. For example, the curvature value of some points is 68D, while that of some points is 48D. Taking only the number of pixels into account, the calculation of the KEA is equivalent to treating the points with a curvature of 68D and the points with a curvature of 48D equally, which means that they have the same degree of response to the disease. However, it is obvious that the point with curvature of 68D is more serious. Therefore, we need a new evaluation index, KEV (not the real volume), which can describe the response of different curvature values to the disease. The larger the curvature value is, the more serious the disease is, so we give a larger weight to the points with larger curvature value. In order to facilitate the processing, the algorithm takes the difference between curvature value and 47D as the weight of this point, and assigns the weight of points with curvature value of less than 47 as 0, then sums the weights of all points to obtain the KEV (KEV can be regarded as the weighted area value or the relative volume value). The algorithm flow chart is shown in Fig. 1.
During the preoperative stage and 1 year after CXL, we used slit lamp microscope, corneal topography (Allegro Topolyzer Vario; Wave Light GmbH, Erlangen, Germany), and endothelial cell density (ECD) of cornea (Specular Microscope SP-3000P; Topcon Corporation, Tokyo, Japan) to measure the morphological transformation in pediatric patients. The significant corneal flattening was defined as the decrease of Kmax by 1.00 diopter one year after CXL (ΔKmax > 1.00D), compared with the preoperative Kmax reading [13].
Statistical Analysis
The correlation of the parameters with the changes in the KEV was calculated by the Pearson correlation test. The changes of the preoperative and postoperative parameters were analyzed using the paired t-test. All statistical analyses were performed using the SPSS ( 26.0) software (IBM Corp., Armonk, NY, USA).
Result
All procedures were successfully completed without complications. No corneal haze or infection was observed by slit lamp biomicroscopy during the 5-day follow-up postoperatively.
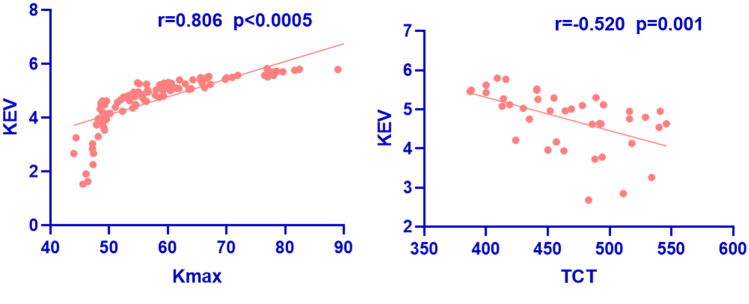
A strong positive correlation was found between the KEV and Kmax in the preoperative and postoperative measurement (r = 0.806, p < 0.05). The preoperative KEV was 4.32 ± 0.69 in mild to moderate KC (Kmax < 58D) and 5.27 ± 0.37 in advanced KC (Kmax > 58D) (p < 0.0005, t-test). In addition, a moderate positive correlation was found between the KEV and max decentration, Q value, eccentricity, and progression indexes (Table 1). We also found a moderate negative correlation between the KEV and TCT (−0.520, p < 0.0005) as shown in Fig. 2. The KEV was negatively related to the flattening rate (−0.635, p < 0.0005).
Table 1.
Correlation between keratectasia volume (KEV) and preoperative parameters
| Parameter | r value | p value |
|---|---|---|
| Kmax | 0.806 | < 0.0005 |
| TCT | −0.520 | 0.001 |
| Corneal volume | 0.178 | 0.273 |
| Max decentration | 0.596 | < 0.0005 |
| Irregularity | 0.277 | 0.084 |
| Q value | 0.620 | < 0.0005 |
| Eccentricity | 0.557 | < 0.0005 |
| Progression index | 0.708 | < 0.0005 |
| ΔKmax | −0.635 | < 0.0005 |
TCT thinnest corneal thickness, p value Pearson correlation test
Fig. 2.
The scatter plot between keratectasia volume (KEV) and Kmax, thinnest corneal thickness (TCT) in preoperative and postoperative measurement
Of the 25 patients (40 eyes), 76.92% completed the 1-year follow-up measurement. The mean follow-up time was 12.13 months (range from 3 to 19 months). The average Kmax, Kave, KEA, and KEV in the preoperative and last follow-up measurement are shown in Table 2. The parameters Kmax, KEV, and KEA exhibited a moderate descending trend, while the Kave remained stable. The KEV was 4.75 ± 0.74 preoperatively and 4.43 ± 1.22 1 year after CXL (p = 0.002, paired t-test), while Kmax was 58.35 ± 10.24 and 57.31 ± 10.11, respectively (p = 0.025, paired t-test).
Table 2.
Preoperative and postoperative parameters
| Mean ± SD | p value | ||
|---|---|---|---|
| Parameter | Preoperation | Last follow-up measurement | |
| Kmax (D) | 58.35 ± 10.24 | 57.31 ± 10.11 | 0.025 |
| Kave (D) | 48.44 ± 5.24 | 48.87 ± 6.06 | 0.076 |
| KEA | 21.48 ± 12.48 | 21.62 ± 14.40 | < 0.0005 |
| KEV | 4.75 ± 0.74 | 4.43 ± 1.22 | 0.002 |
KEA keratectasia area, KEV keratectasia volume, p value paired t test
The KEVs were 4.75 ± 0.74, 4.72 ± 0.80, 4.44 ± 1.17, and 4.50 ± 1.35 before CXL and at 3, 6, and 12 months after CXL, respectively. Kmax were 58.35 ± 10.24, 58.81 ± 10.43, 57.63 ± 11.57, and 58.33 ± 9.69, respectively. The postoperative changes with time are shown in Fig. 3. The keratectasia remained stable 3 months after surgery and decreased drastically between 3 and 6 months. It is notable that statistically significance was only found in the KEV between preoperative and 6 months after surgery (p < 0.0005, paired t test), but not in Kmax (p = 0.679). The descending slope from baseline to sixth month was −0.0109, −0.0021 (KEV and Kmax, respectively) (Fig. 3).
Fig. 3.
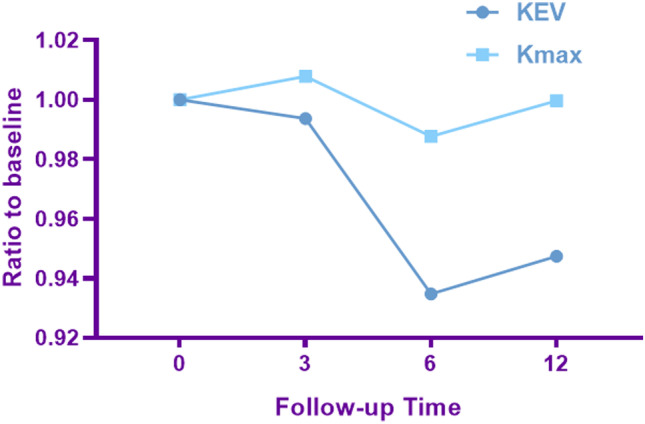
The changes in keratectasia volume(KEV) and Kmax preoperatively and at 3, 6, and 12 months
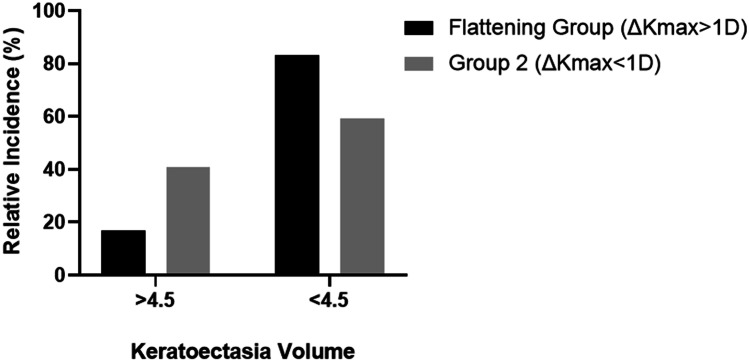
Among the 40 studied eyes, 18 eyes (45%) had significant flattening (ΔKmax ≥ 1D) 1 year after CXL, which were included in flattening group. The maximum flattening was 6.5 D, and the maximum ΔKEV was 2.36. In the flattening group, the mean KEV preoperatively was 4.91, and in group 2 (ΔKmax < 1.00 D), the value was 4.62. The KEV in 15 out of 18 eyes (83.3%) in the flattening group was greater than 4.6, while in group 2, the rate was 59.9% (13 eyes out of 22 eyes). The distribution of preoperative KEV greater than 4.6 in flattening group is shown in Fig. 4. No statistical difference was found in the Kmax and KEV between the flattening group and group 2.
Fig. 4.
The comparison of keratectasia volume (KEV) distribution between flattening group (ΔKmax > 1.00 D) and group 2(ΔKmax < 1.00 D)
Discussion
Due to the dynamic nature of the cornea, the stabilization of the CXL has shown similar effect, but less effective in pediatric patients than adults [14]. Randomized controlled trials in pediatric KC on when to intervene and optimal therapeutic algorithm in this population are scarce. During the course of exploration, no clear and consistent quantitative data was found for the assessment of the ectatic progression [15]. According to the Global Consensus on KC and Ectatic Diseases (2015) [16], the progression is defined by changes in at least two of the following parameters: steepening of the anterior corneal surface, steepening of the posterior corneal surface, and thinning and/or thinning or changes in the pachymetric rate of change.
Currently, Kmax is regarded as the most common quantitative index to detect and document the progression of KC. In general, a change of more than 1D in Kmax was defined as Kmax progression [15]. However, Kmax, which is a one-dimensional parameter, can only provide the curvature value of one point, reflecting the status of the corneal apex rather than the ectatic corneal cone. In addition, Kmax may remain unchanged or even decrease when progression occurs [15, 17]. Compared with Kmax, KEV attained the curvature value of 310 pixels(Kreading > 47D) and was assigned different values, which take the ectatic cone area into consideration. Several other parameters have been used to evaluate the progression of KC, including the index of surface variance (ISV), the index of height decentration (IHD), visual acuity, manifest refraction, and central corneal thickness. The evaluation efficacy of these parameters has not been verified or has been found to be unreliable for its subjectivity [18–21].
The machine learning model has been extensively applied in the diagnosis of KC. Previous studies performed discriminant analysis and used a classification tree to improve the sensitivity and specificity for the differential diagnosis among KC, subclinical KC, abnormal eyes, and normal eyes [22–24]. Arbelaez et al. [25] used a SVM model and increased the diagnosis sensitivity to 96.0% in abnormal eyes, 95.0% in eyes with KC, 92.0% in eyes with subclinical KC, and 97.2% in normal eyes. The SVM model was trained with the thickness, curvature, and height data of both the anterior and posterior corneal surface and pachymetry, which improved the detection rate of subclinical KC. However, this algorithm targets diagnosis of KC and cannot be applied in the evaluation of ectatic progression and efficacy of CXL.
In the present study the SVM model was trained with 217 pixels and their correlated values (K reading), which was equivalent to establishing a theoretical corneal surface made up of curvature values to calculate the virtual ectasia volume without regard to corneal thickness. It shows a reduction in the KEV from 4.75 ± 0.74 before surgery to 4.43 ± 1.22 at the last follow-up measurement (mean follow-up time: 12.13 months, range from 3 to 19 months). The decline of the KEV probably implied significant corneal flattening 1 year after CXL. The calculation of the KEV focuses on the corneal curvature factor (> 47D) without considering the corneal thickness, which is appropriate for evaluating the flattening status of cornea. From the standpoint of pathophysiology, the flattening may correlate with the depth-dependent stiffness effect [26] and increased extracellular stromal matrix [27].
According to a study by Perez-Straziota et al. [3], more than 60% pediatric patients showed regression of Kmax one year after standard CXL and the average flattening was 1.5D. In this study, we found that there was a strong positive correlation between the KEV and Kmax (r = 0.806, p < 0.0005). Moreover, there was a statistically significant decrease in the KEV with the progression of KC (p < 0.0005). The preoperative KEV value was 4.32 ± 0.69 in mild to moderate KC (Kmax < 58D) and 5.27 ± 0.37 in advanced KC (Kmax < 58D). In this study, the preoperative and last follow-up observation for Kmax was 58.35 ± 10.24 and 57.31 ± 10.11 respectively (p < 0.0005, paired t-test), which was in accordance with changes in the KEV.
Caporossi et al. [28] studied pediatric topography features in different stages after CXL; they found that the mean Kreading decreased gradually at 3, 6, 12, and 36 months. In this study, the morphological changes in different stages after CXL exhibited an interesting trend. The KEV and Kmax remained stable at 3 months and clearly decreased thereafter. The initial change of the KEV may be correlated with epithelial debridement [29, 30]. Starting from the third month, the reduction of the KEV and Kmax showed a continuous flattening effect due to new collagen synthesis and corneal apex recentering [28, 30]. These findings explain the results that the refractive outcomes remained stable or worsen slightly at the third month and clearly improved at the sixth month in previous studies [29, 31].
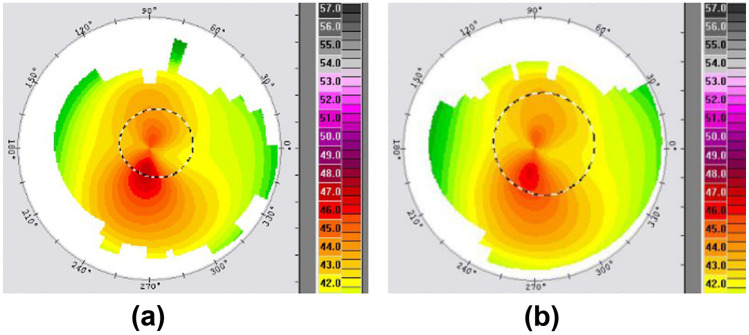
The KEV takes more curvature values into consideration and transforms a large amount of data to visible values instead of only considering a single maximum Kreading index. The data in Fig. 5 show a marked flattening effect and reduction of the KEV 6 months after surgery, while the change of Kmax was not noticeable. Also, as shown in Fig. 3, the KEV showed a more remarkable decrease at 6 months than Kmax. In addition, a statistically significant reduction was only found in the KEV 6 months after CXL (p < 0.0005), which indicates that the KEV is a more sensitive parameter than Kmax to evaluate the morphological changes of the cornea after CXL.
Fig. 5.
a The preoperative topography. b The topography acquired at the sixth month after CXL. KEV was 2.85, 1.63, and Kmax was 47.14, 46.36, respectively
Customized ablation was recommended to transform the corneal irregularity of the KC into a toric conoid, and the higher flattening rate after surgery would decrease the predictability of simultaneous ablation and CXL. Tobias Koller et al. found that a Kmax greater than 58.00D would predict more flattening and more failures after CXL [13, 32, 33]. According to our study, 45% of the eyes (18 out of 40) with a significant flattening rate (ΔKmax > 1.0D) 1 year after CXL were included in the flattening group. The mean KEV preoperatively was 4.91 in the flattening group and 4.62 in group 2 (ΔKmax less than 1.00 D). We found that there was a moderate negative correlation between the KEV and flattening rate (−0.635, p < 0.0005). In addition, a KEV greater than 4.6 occurred more frequently in the flattening group. Indeed, in 15 out of 18 eyes (83.3%), the KEV in the flattening group was greater than 4.6, while in group 2, the rate was 59.9% (13 eyes out of 22 eyes). A KEV greater than 4.6 is probably a predictor for postoperative significant flattening in pediatric patients. However, this difference was not statistically significant and a likely reason may be the small sample size.
The TCT is an indispensable tool to detect KC in the preoperative screening of laser surgery [34]. David P. Piñero et al. found that the minimum pachymetry values showed a significant decreasing trend with the progression of the KC in preoperative diagnosis [35]. Although the calculation of the KEV did not take the real protrusion volume of the cornea or corneal thickness into consideration, the cornea theoretical ectasia volume did, we found that there was a negative correlation between the preoperative KEV and TCT (−0.520, p < 0.0005). Thus, the progression of the KC meant thinner pachymetry and larger keratectasia volume. Whether the TCT changed after corneal cross-linking remains controversial [36] and we speculate that the KEV and TCT could be applied in monitoring the progression of KC. No significant correlation was found between the KEV and corneal volume.
Univariate and multivariate detection systems are used in topography to qualitatively and quantitatively analyze the geometric characterization of a KC cornea [37]. However, to the best of our knowledge, there is no index that can assist in the diagnosis of the KC-suspect eyes with 100% specificity and sensitivity. We wonder whether the KEV can be applied in preoperative diagnosis to improve the early detection rates of KC. In addition, long-term and larger, prospective studies are required to support further research of KEV on the postoperative effect in various ages.
Conclusion
In conclusion, we found that the KEV in pediatric patients remained stable at 3 months and then showed a significant decrease at 6 months postoperatively. The KEV is a more sensitive index to evaluate the extent of protrusion and observe the morphological characteristics of cornea after collagen cross-linking compared with Kmax. A KEV greater than 4.6 may be a predictor to estimate the flattening rate in pediatric patients one year after collagen cross-linking in advance.
Funding
This work was supported by Shandong Provincial Hospital, Cheeloo College of Medicine, Shandong University, China.
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiangjun Wang, Email: 358799515@qq.com.
Bo Zhang, Email: 1486364635@qq.com.
Zhiwei Li, Email: zhiweiovs@qq.com.
Mengyao Li, Email: 13046030680@163.com.
Jia Wang, Email: wangjiaallan@163.com.
Guoying Mu, Email: mgyeyes@163.com.
References
- 1.Rabinowitz YS: Keratoconus[J]. Surv Ophthalmol, 297–319, 1998 [DOI] [PubMed]
- 2.Reeves SW, Stinnett S, Adelman RA, Afshari NA. Risk factors for progression to penetrating keratoplasty in patients with keratoconus. Am J Ophthalmol. 2005;140:607–611. doi: 10.1016/j.ajo.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 3.Perez-Straziota C, Gaster RN, Rabinowitz YS. Corneal cross-linking for pediatric keratcoconus review. Cornea. 2018;37(6):802–809. doi: 10.1097/ICO.0000000000001579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daxer A, Misof K, Grabner B, Ettl A, Fratzl P. Collagen fibrils in the human corneal stroma: structure and aging. Invest Ophthalmol Vis Sci. 1998;39(3):644–648. [PubMed] [Google Scholar]
- 5.Léoni-Mesplié S, Mortemousque B, Mesplié N, Touboul D, Praud D, Malet F, Colin J. Epidemiological aspects of keratoconus in children[J] Journal francais d'ophtalmologie. 2012;35(10):776–785. doi: 10.1016/j.jfo.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Barr JT, Wilson BS, Gordon MO, Rah MJ, Riley C, Kollbaum PS, Zadnik K. CLEK Study Group: Estimation of the incidence and factors predictive of corneal scarring in the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study. Cornea. 2006;25(1):16–25. doi: 10.1097/01.ico.0000164831.87593.08. [DOI] [PubMed] [Google Scholar]
- 7.Léoni-Mesplié S, Mortemousque B, Touboul D, Malet F, Praud D, Mesplié N, Colin J. Scalability and severity of keratoconus in children. Am J Ophthalmol. 2012;154(1):56–62.e1. doi: 10.1016/j.ajo.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 8.Shalchi Z, Wang X, Nanavaty MA. Safety and efficacy of epithelium removal and transepithelial corneal collagen crosslinking for keratoconus. Eye (Lond) 2015;29(1):15–29. doi: 10.1038/eye.2014.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kankariya VP, Kymionis GD, Diakonis VF, Yoo SH. Management of pediatric keratoconus - evolving role of corneal collagen cross-linking: an update. Indian J Ophthalmol. 2013;61(8):435–440. doi: 10.4103/0301-4738.116070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ambrosio R, Jr, Alonso RS, Luz A, Coca Velarde LG. Corneal-thickness spatial profile and corneal-volume distribution: tomographic indices to detect keratoconus. J Cataract Refract Surg. 2006;32:1851–1859. doi: 10.1016/j.jcrs.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 11.Wang MFZ, Fernandez-Gonzalez R. (Machine-)learning to analyze in vivo microscopy: support vector machines. Biochim Biophys Acta Proteins Proteom. 1865;1719–1727:2017. doi: 10.1016/j.bbapap.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Hersh PS, Greenstein SA, Fry KL. Corneal collagen crosslinking for keratoconus and corneal ectasia: one-year results. J Cataract Refract Surg. 2011;37:149–160. doi: 10.1016/j.jcrs.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 13.Koller T, Pajic B, Vinciguerra P, Seiler T. Flattening of the cornea after collagen crosslinking for keratoconus. J Cataract Refract Surg. 2011;37(8):1488–1492. doi: 10.1016/j.jcrs.2011.03.041. [DOI] [PubMed] [Google Scholar]
- 14.Mukhtar S, Ambati BK. Pediatric keratoconus: a review of the literature. Int Ophthalmol. 2018;38(5):2257–2266. doi: 10.1007/s10792-017-0699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duncan JK, Belin MW, Borgstrom M: Assessing progression of keratoconus: novel tomographic determinants. Eye Vis (Lond), 3:6, 2016 [DOI] [PMC free article] [PubMed]
- 16.Gomes JA, Tan D, Rapuano CJ, Belin MW, Ambrósio R, Jr, Guell JL, Malecaze F, Nishida K, Sangwan VS. Global consensus on keratoconus and ectatic diseases. Cornea. 2015;34(4):359–369. doi: 10.1097/ICO.0000000000000408. [DOI] [PubMed] [Google Scholar]
- 17.Mahmoud AM, Nuñez MX, Blanco C, Koch DD, Wang L, Weikert MP, Frueh BE, Tappeiner C, Twa MD, Roberts CJ. Expanding the cone location and magnitude index to include corneal thickness and posterior surface information for the detection of keratoconus. Am J Ophthalmol. 2013;156(6):1102–1111. doi: 10.1016/j.ajo.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 18.Kanellopoulos AJ, Moustou V, Asimellis G. Evaluation of visual acuity, pachymetry and anterior-surface irregularity in keratoconus and crosslinking intervention follow-up in 737 cases. J Kerat Ect Cor Dis. 2013;2(3):95–103. [Google Scholar]
- 19.Suzuki M, Amano S, Honda N, Usui T, Yamagami S, Oshika T. Longitudinal changes in corneal irregular astigmatism and visual acuity in eyes with keratoconus. Jpn J Ophthalmol. 2007;51(4):265–269. doi: 10.1007/s10384-007-0453-2. [DOI] [PubMed] [Google Scholar]
- 20.Sefic Kasumovic S, Racic-Sakovic A, Kasumovic A, Pavljasevic S, Duric-Colic B, Cabric E, Mavija M, Lepara O, Jankov M. Assessment of the tomographic values in keratoconic eyes after collagen crosslinking procedure. Med Arch. 2015;69(2):91–94. doi: 10.5455/medarh.2015.69.91-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanellopoulos AJ, Asimellis G. Revisiting keratoconus diagnosis and progression classification based on evaluation of corneal asymmetry indices, derived from Scheimpflug imaging in keratoconic and suspect cases. Clin Ophthalmol. 2013;7:1539–1548. doi: 10.2147/OPTH.S44741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maeda N, Klyce SD, Smolek MK, Thompson HW. automated keratoconus screening with corneal topography analysis. Invest Ophthalmol Vis Sci. 1994;35:2749–2757. [PubMed] [Google Scholar]
- 23.Saad A, Gatinel D. Topographic and tomographic properties of forme fruste keratoconus corneas. Invest Ophthalmol Vis Sci. 2010;51:5546–5554. doi: 10.1167/iovs.10-5369. [DOI] [PubMed] [Google Scholar]
- 24.Uçakhan ÖÖ, Cetinkor V, Özkan M, Kanpolat A. Evaluation of Scheimpflug imaging parameters in subclinical keratoconus, keratoconus, and normal eyes. J Cataract Refract Surg. 2011;37:1116–1124. doi: 10.1016/j.jcrs.2010.12.049. [DOI] [PubMed] [Google Scholar]
- 25.Arbelaez MC, Versaci F, Vestri G, Barboni P, Savini G. Use of a support vector machine for keratoconus and subclinical keratoconus detection by topographic and tomographic data. Ophthalmology. 2012;119(11):2231–2238. doi: 10.1016/j.ophtha.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Kohlhaas M, Spoerl E, Schilde T, Unger G, Wittig C, Pillunat LE. Biomechanical evidence of the distribution of cross-links in corneas treated with riboflavin and ultraviolet A light. J Cataract Refract Surg. 2006;32(2):279–283. doi: 10.1016/j.jcrs.2005.12.092. [DOI] [PubMed] [Google Scholar]
- 27.Mazzotta C, Balestrazzi A, Traversi C, Baiocchi S, Caporossi T, Tommasi C, Caporossi A. Treatment of progressive keratoconus by riboflavin-UVA-induced cross-linking of corneal collagen: ultrastructural analysis by Heidelberg Retinal Tomograph II in vivo confocal microscopy in humans. Cornea. 2007;26(4):390–397. doi: 10.1097/ICO.0b013e318030df5a. [DOI] [PubMed] [Google Scholar]
- 28.Caporossi A, Mazzotta C, Baiocchi S, Caporossi T, Denaro R, Balestrazzi A. Riboflavin-UVA-induced corneal collagen cross-linking in pediatric patients. Cornea. 2012;31(3):227–231. doi: 10.1097/ICO.0b013e31822159f6. [DOI] [PubMed] [Google Scholar]
- 29.Vinciguerra P, Albé E, Frueh BE, Trazza S, Epstein D. Two-year corneal cross-linking results in patients younger than 18 years with documented progressive keratoconus. Am J Ophthalmol. 2012;154(3):520–526. doi: 10.1016/j.ajo.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 30.Mazzotta C, Caporossi T, Denaro R, Bovone C, Sparano C, Paradiso A, Baiocchi S, Caporossi A. Morphological and Functional Correlations in Riboflavin Uv a Corneal Collagen Cross-Linking for Keratoconus. Acta Ophthalmol. 2012;90:259–265. doi: 10.1111/j.1755-3768.2010.01890.x. [DOI] [PubMed] [Google Scholar]
- 31.Magli A, Forte R, Tortori A, Capasso L, Marsico G, Piozzi E. Epithelium-off corneal collagen cross-linking versus transepithelial cross-linking for pediatric keratoconus. Cornea. 2013;32(5):597–601. doi: 10.1097/ICO.0b013e31826cf32d. [DOI] [PubMed] [Google Scholar]
- 32.Koller T, Iseli HP, Donitzky C, Ing D, Papadopoulos N, Seiler T. Topography-guided surface ablation for forme fruste keratoconus. Ophthalmology. 2006;113(12):2198–2202. doi: 10.1016/j.ophtha.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 33.Kanellopoulos AJ, Binder PS. Collagen cross-linking (CCL) with sequential topography-guided PRK: a temporizing alternative for keratoconus to penetrating keratoplasty. Cornea. 2007;26(7):891–895. doi: 10.1097/ICO.0b013e318074e424. [DOI] [PubMed] [Google Scholar]
- 34.Ambrósio R, Jr, Klyce SD, Wilson SE. Corneal topographic and pachymetric screening of keratorefractive patients. J Refract Surg. 2003;19(1):24–29. doi: 10.3928/1081-597X-20030101-05. [DOI] [PubMed] [Google Scholar]
- 35.Piñero DP, Alió JL, Alesón A, Escaf Vergara M, Miranda M. Corneal volume, pachymetry, and correlation of anterior and posterior corneal shape in subclinical and different stages of clinical keratoconus. J Cataract Refract Surg. 2010;36(5):814–825. doi: 10.1016/j.jcrs.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 36.Kobashi H, Rong SS. Corneal collagen cross-linking for keratoconus: systematic review. Biomed Res Int. 2017;2017:8145651. doi: 10.1155/2017/8145651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cavas-Martínez F, De la Cruz Sánchez E, Nieto Martínez J, Fernández Cañavate FJ, Fernández-Pacheco DG: Corneal topography in keratoconus: state of the art. Eye Vis (Lond), 3:5, 2016 [DOI] [PMC free article] [PubMed]