Abstract
Introduction
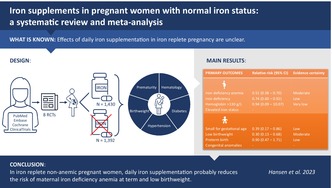
Effects of daily iron supplementation in iron replete pregnancy are unclear. This systematic review aimed to assess benefits and harms of oral iron supplements in pregnant women without anemia and iron deficiency.
Material and methods
We predefined and registered a protocol in PROSPERO (CRD42020186210) and performed the review following the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) methodology. We searched for randomized clinical trials (RCTs) and observational studies comparing daily oral iron supplementation with no iron supplements in non‐anemic iron replete pregnant women. Searches were conducted in MEDLINE (by PubMed), EMBASE (by OVID), Cochrane Library, and ClinicalTrials.gov from inception to September 2022 without language restrictions. Two authors independently screened records, extracted data, and assessed risk of bias using the revised Cochrane risk of bias tool (RoB2). One author read full‐texts, assessed certainty of evidence by GRADE and conducted meta‐analyses using a random‐effects model. Primary outcomes included iron deficiency anemia, iron deficiency, hemoglobin >130 g/L, elevated iron status, small for gestational age newborns, low birthweight newborns, preterm birth, and congenital anomalies.
Results
Eight RCTs (2822 women) but no observational studies were eligible for inclusion. Daily oral iron supplementation in pregnancy probably reduces iron deficiency anemia at term (risk ratio [RR]: 0.51, 95% confidence interval [CI]: 0.38–0.70; 4 RCTs, 1670 women; I 2 = 13%; moderate‐certainty evidence) and the incidence of low birthweight babies (RR: 0.30, 95% CI: 0.13–0.68; 2 RCTs, 361 infants; I 2 = 0%; moderate‐certainty evidence). In addition, it may reduce iron deficiency at term (RR: 0.74, 95% CI: 0.60–0.92; 4 RCTs, 1663 women; I 2 = 58%; low‐certainty evidence) and the incidence of small for gestational age babies (RR: 0.39, 95% CI: 0.17–0.86; 1 RCT, 213 infants; I 2 not estimable; low‐certainty evidence).
Conclusions
Daily iron supplementation in iron replete non‐anemic pregnant women probably reduces the risk of maternal iron deficiency anemia at term and low birthweight.
Keywords: iron, iron deficiency, iron replete, maternal iron deficiency, non‐anemic, pregnant, review
Effects of daily iron supplementation in iron replete pregnancy are unclear. To assess this, a systematic review and meta‐analysis were performed. In iron replete non‐anemic pregnant women, daily iron supplementation probably reduces the risk of maternal iron deficiency anemia at term and low birthweight.
Abbreviations
- CI
confidence interval
- GRADE
Grades of Recommendation, Assessment, Development and Evaluation framework
- Hb
hemoglobin
- LBW
low birthweight
- RCT
randomized clinical trial
- RR
risk ratio
- SGA
small for gestational age
Key message.
Daily iron supplementation in iron replete non‐anemic pregnant women probably reduces the risk of maternal iron deficiency anemia and low birthweight. However, its effect on several clinical infant and maternal outcomes remains unclear and warrants further study.
1. INTRODUCTION
Iron status is defined by the amount of stored and mobilizable body iron. 1 During pregnancy iron is crucial for a range of vital functions including oxygen delivery and fetal organogenesis, 2 and iron requirements increase as gestation proceeds. 3 As iron is prioritized for erythropoiesis, iron deficiency anemia is an end‐stage result of iron deficiency. 4 While anemia is a key contributor to maternal and offspring morbidity, 5 high hemoglobin (Hb) and iron status have also been associated with higher risks of adverse pregnancy outcomes. 6 , 7 This has raised questions to whether or not all women should be advised iron supplements in pregnancy, and especially if it is safe for women with normal Hb and iron status. There are various theories about the potential harmful mechanisms of high iron status; for instance, placental oxidative stress, altered maternal gut microbiome, impaired immunity, and excessive erythropoiesis with increased blood viscosity and compromised placental flow. 6
A Cochrane review has concluded that use of iron supplements in pregnancy reduces the risk of iron deficiency and iron deficiency anemia near term, but also increases the risk of high Hb. No clear beneficial effect on infant and maternal clinical outcomes could be demonstrated. 8 However, a substantial number of women enter pregnancy with normal Hb and iron, but no analyses in the Cochrane review incorporated initial maternal iron status. How the use of iron supplements in non‐anemic iron replete pregnant women effect newborn and maternal health therefore remains unclear. The aim of this systematic review was to assess benefits and harms of daily use of oral iron supplements by non‐anemic pregnant women with normal iron status.
2. MATERIAL AND METHODS
A study protocol was developed prior to study start and registered in the international prospective register of systematic reviews registry (PROSPERO: CRD42020186210). Patients were not involved in the planning or conduct of this systematic review. Reporting of the review adheres to the preferred reporting items for systematic reviews and meta‐analyses (PRISMA) statement. 9 The work was paid for by internal funding only.
2.1. Data sources and searches
With assistance from an information specialist, we searched The Cochrane Library, MEDLINE (by PubMed), and Embase (by Ovid) for observational studies and randomized clinical trials (RCTs) published to September 13, 2022, comparing oral iron supplementation with no iron supplementation in iron replete non‐anemic pregnant women. Search terms and detailed search strategies are provided in Table S1. We conducted the first search on August 27, 2020, and a final updated search on September 13, 2022, respectively. The ClinicalTrials.gov registry was searched for both completed and ongoing studies. In addition, we screened reference lists of the included papers as well as of relevant systematic reviews and meta‐analyses. We applied no time or language restriction.
2.2. Selection criteria
We aimed to include RCTs and observational studies that compared oral iron supplementation with iron‐free supplements, placebo, or no intervention in non‐anemic iron replete pregnant women, determined by Hb and at least one additional indicator of iron status (ferritin, transferrin, transferrin saturation and/or soluble transferrin receptor) measured no more than 20 weeks prior to, or after, conception. We excluded studies published only as abstracts or without original data. A study was included if it used an iron supplement in tablet or capsule formulation containing either iron alone or iron in conjunction with ascorbic‐ and/or folic acid as experimental intervention and compared it to supplements without iron, placebo, or no treatment. We did not include studies examining food‐based interventions, parenteral iron, or studies with regimens where iron was not provided daily. Cointerventions (such as education) were only allowed if both the iron and comparison groups received the same cointerventions.
2.3. Outcomes
All primary and secondary outcomes are summarized in Table S2.
Maternal primary outcomes included iron deficiency anemia at term (≥37 weeks' gestation); iron deficiency at term; Hb > 130 g/L in pregnancy; elevated iron status in pregnancy. Secondary outcomes for mothers included Hb < 70 g/L in pregnancy (severe anemia), gestational diabetes, pre‐eclampsia, gestational hypertension, infections, red blood cell transfusion, postpartum anemia, gastrointestinal side effects, quality of life, and fatigue.
Newborn primary outcomes included small for gestational age (SGA; <10th percentile of weight at birth for gestational age); low birthweight (LBW; <2500 g); preterm birth (before 37 weeks' gestation); and congenital anomalies. Secondary outcomes for newborns included failure to thrive and concentrations of Hb and ferritin in the first 6 months.
For outcomes defined as in pregnancy, we included the information reported closest to term if the incidence was reported at more than one timepoint in a study (eg at both 28‐ and 37‐weeks' gestation). We originally intended to assess iron deficiency anemia in pregnancy both overall and at term, but as the data extracted for these two outcomes ended up being identical, we have chosen to report this as a single outcome: iron deficiency anemia at term.
2.4. Study selection
One author removed duplicates (RH). Titles and abstracts from database searches were independently screened by two authors (RH and EPFS) using the Rayyan QCRI online software (http://rayyan.qcri.org). Records from the ClinicalTrials.gov registry were likewise independently screened (RH and EPFS). Disagreements were resolved by discussion or, if necessary, a third author was consulted (JBS). Full text versions were obtained for all potentially relevant documents and read by one author (RH), who advised a second and third party (EPFS and JBS) when uncertain about a publication's eligibility. If uncertainty could not be solved by discussion, we tried to contact the author of the study for additional information.
2.5. Data extraction
Two authors (RH and EPFS) independently extracted information from each included study and recorded data in identical spreadsheets. If a study reported an outcome in more than one publication, data were included only once and extracted from the publication with the most comprehensive data. Disagreements were resolved by discussion or, if necessary, by a third author (JBS). In addition to maternal and infant outcomes and definitions of these (Table S2), we extracted information about study design, eligibility criteria, location, number of participants, baseline Hb, baseline iron status, and allocated treatment (including type, dose, and duration). When needed, we contacted authors and requested clarifying and/or additional information.
2.6. Assessment of risk of bias and the quality of evidence
Included studies were assessed for risk of bias by two authors (RH and EPFS) independently. We assessed RCTs with the revised Cochrane risk of bias tool (RoB2). 10 We planned to assess observational studies with the risk of bias in non‐randomized studies ‐ of interventions (ROBINS‐I) tool, 11 but found no observational studies that were eligible for inclusion. Disagreements were resolved by discussion or, if necessary, by a third party (JBS). The Grades of Recommendation, Assessment, Development and Evaluation (GRADE) framework 12 and GRADEpro GDT online software (McMaster University, 2020, Ontario, Canada, https://gradepro.org) was used to rate the certainty of the effect and to generate “Summary of findings” tables. Outcomes were rated and downgraded according to the presence or absence of factors (risk of bias, inconsistency, indirectness, imprecision, publication bias) affecting the quality of the body of studies included in each outcome.
2.7. Statistical analysis
We analyzed data using Review Manager software (RevMan Version 5.4, The Cochrane Collaboration, 2020). We used a random‐effects model as we expected some between‐trial differences in study designs (eg participants, intervention). We tested for heterogeneity using the I 2 statistic. Summary risk ratio (RR) with 95% confidence intervals (CIs) were calculated. Analyses were performed by one reviewer (RH) and reviewed by a second and third party (EPFS and JBS). We planned to perform sensitivity analyses based on risk of bias by repeating analyses after excluding studies at high risk. For primary outcomes we planned to conduct subgroup analyses based on daily elemental iron dose (low: ≤30 mg; medium: >30 mg and <60 mg; high: ≥60 mg) and based on gestational age at start of intervention (before/after 20 weeks' gestation).
3. RESULTS
3.1. Selection process
We identified 4960 citations through the electronic and manual searches (Figure 1). After excluding duplicate citations, we screened the titles and abstracts of 3142 publications (2696, 198, and 248 identified in the first and updated database searches, respectively) and screened 211 records identified in the ClinicalTrials.gov registry. Eight additional relevant publications were identified from reference lists. Of the 3361 screened records we excluded 3234 as irrelevant and a total of 127 publications were assessed in full text. We tried to contact authors when we believed that additional information could lead to inclusion. However, the majority of the contacted authors either did not respond to our request or replied that they had no current access to data. Among all assessed publications, we found no observational studies eligible for inclusion. Subsequently, eight unique randomized trials (in nine publications) 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 could be included in the systematic review.
FIGURE 1.
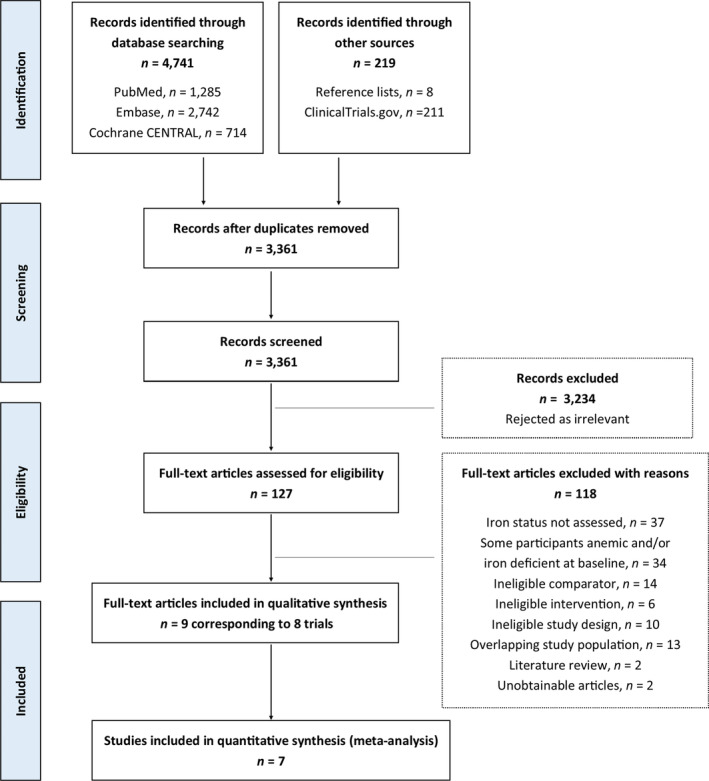
Preferred reporting items for systematic reviews and meta‐analyses (PRISMA) flow diagram.
3.2. Characteristics
The eight trials included 2822 participants, of whom 1430 were randomly allocated to receive iron supplements vs 1392 randomly allocated to receive no iron. Characteristics of all included trials are summarized in Table 1. Three trials had been conducted in Iran 17 , 18 , 19 , 21 and the remaining in Finland, 13 Italy, 14 Sweden, 15 USA 16 and China, 20 respectively. Two trials provided us with additional outcome data 16 , 20 and another three responded but could not provide us with the requested outcome data. 15 , 19 , 21 One of the latter trials 15 did not report any of our predefined primary or secondary outcomes and could therefore not contribute with data to any meta‐analyses. In all the included trials interventions were initiated no later than 20 weeks' gestation. One trial administered treatment twice daily 13 and the remaining once daily. The total daily elemental iron dose ranged from 30 to 200 mg. In one trial, folic acid was given to all participants in addition to iron and placebo treatment, 20 whereas the remaining trials used iron‐only interventions. One trial stated that iron supplements were given to almost all participants at some point after 28 weeks' gestation regardless of initial allocation, 16 and another that all participants regardless of initial allocation were allowed to buy over‐the‐counter iron‐containing supplements and that physicians were free to use clinical judgment in treating those who became anemic. 20
TABLE 1.
Characteristics of the included studies.
| Trial | Country | Design |
Treatment; no of patients I: Iron C: Comparator T: Total |
Duration of intervention | Iron intervention | Comparator intervention |
Adherence assessment I: Iron C: Comparator |
Plan in case of treatment failure |
BaselineHb (g/L) a I: Iron C: Comparator |
Baseline ferritin (ng/mL)
a
I: Iron C: Comparator |
|---|---|---|---|---|---|---|---|---|---|---|
| Puolakka et al. (1980) 13 | Finland | RCT, non‐blinded |
I: 16 C: 16 T: 32 |
Start: 16 weeks' gestation Stop: 1 month postpartum |
Ferrous sulfate, 100 mg, twice daily | No treatment | Not described | Not described |
I: 119 ± 7 C: 121 ± 6 |
Geometric mean (±1 SD range): I: 97 (55–171) C: 86 (50–150) |
| Tura et al. (1989) 14 | Italy | RCT, non‐blinded |
I: 136 C: 118 T: 254 |
Start: 12–16 weeks' gestation Stop: 2 months postpartum | Capsules (preparation not specified), 40 mg elemental iron, once daily | No treatment |
Participants taking more or less than intended I: 6% C: 9% |
Not described |
I: 128 ± 8 C: 129 ± 9 |
I: 63 ± 27 C: 63 ± 38 |
| Tholin et al. (1995) 15 | Sweden | RCT, double‐blind |
I: 27 C: 21 T: 48 |
Start: 20 weeks’ gestation throughout pregnancy (stop not specified) | Ferrous sulfate, 100 mg elemental iron, once daily | Placebo, not further described, once daily | Not described | Not described |
Median (range): I: 128 (111–150) C: 126 (119–142) |
Median (range): I: 43 (17–102) C: 42 (23–212) |
| Cogswell et al. b , c (2003) 16 | USA | RCT, double‐blind |
I: 146 C: 129 T: 275 |
Start: <20 weeks' gestation throughout pregnancy (stop not specified) | Ferrous sulfate, 30 mg elemental iron, once daily | Placebo, gelatin capsules identical to iron intervention, once daily |
Mean dose taken (pill count by investigator) I: 63% C: 65% |
Iron supplements and/or medical evaluation |
I: 129 ± 9 C: 127 ± 10 |
Antilog of mean (25th and 75th percentile): I: 45 (30–60) C: 49 (34–77) |
| Ziaei et al. (2008) 17 , 18 | Iran | RCT, double‐blind |
I: 122 C: 122 T: 244 |
Start: 20 weeks' gestation Stop: delivery |
Ferrous sulfate, 50 mg elemental iron, once daily | Placebo, not further described, once daily | Mentioned but not described | Not described |
I: 140 ± 6 C: 139 ± 5 |
I: 28 ± 11 C: 28 ± 10 |
| Falahi et al. (2011) 19 | Iran | RCT, triple‐blind |
I: 70 C: 78 T: 148 |
Start: <20 weeks' gestation Stop: delivery | Ferrous sulfate, 60 mg elemental iron, once daily | Placebo, indistinguishable from iron intervention, once daily | Not described | Not described |
I: 130 ± 10 C: 131 ± 9 |
I: 37 ± 21 C: 32 ± 20 |
| Zhao et al. d , e (2015) 20 | China | RCT, double‐blind |
I: 881 C: 876 T: 1757 |
Start: 10–20 weeks' gestation Stop: delivery |
Ferrous sulfate, 60 mg elemental iron, once daily, and 0.4 mg folate, once daily | Placebo (starch, dextrin, sucrose, and magnesium stearate), once daily, and 0.4 mg folate, once daily |
Mean capsules consumed (self‐reported) I: 85% C: 84% |
Individualized (up to physicians' clinical judgment) |
I: 124 ± 8 C: 123 ± 8 |
I: 50 ± 45 C: 48 ± 34 |
| Alizadeh & Salehi (2016) 21 | Iran | RCT, double‐blind |
I: 32 C: 32 T: 64 |
Start: 16–20 weeks' gestation Stop: delivery |
Ferrous sulfate, 50 mg elemental iron, once daily | Placebo, not further described, once daily | Not described | Not described |
I: 137 ± 4 C: 136 ± 4 |
I: 34 ± 14 C: 37 ± 17 |
Abbreviations: Hb, hemoglobin; RCT, randomized clinical trial.
Reported as mean ± SD and if not available then as specified in table.
Some included data were provided by the author upon request.
At 28 weeks' gestation, the following algorithm was applied: continued initial intervention if non‐iron deficient and non‐anemic; additional iron supplements if iron deficient (dose depending on severity); excluded and referred for medical evaluation in case of severe anemia or non‐iron deficient anemia. Consequently, only 15 iron participants and 15 placebo participants continued with initial intervention whereas most participants received additional iron supplements.
Included data are subgroup data for non‐anemic iron replete participants provided by the author upon request (asked for subgroup data for only iron replete non‐anemic participants). Hb and ferritin cutoff defined by author.
All women were free to obtain over‐the‐counter iron‐containing supplements. Physicians were blinded to the supplement group and free to use clinical judgment in treating anemic women. Local clinical practice was to prescribe medicinal iron therapy and/or to make dietary recommendations.
3.3. Risk of bias and the quality of evidence
Figure 2 illustrates the summarized risk of bias for the included trials. One trial had low risk of bias in all five domains and was also the trial contributing with the most comprehensive data. 20 One non‐blinded trial did not evaluate adherence to the allocated intervention 13 and was assessed to be at high risk of bias because it is plausible that participants allocated to no iron could have used iron supplements on their own or their caregiver's initiative. This trial only contributed with data for a single secondary outcome (maternal red blood cell transfusion). The remaining trials had some concerns of risk of bias, most commonly arising from the randomization process, missing outcome data that could not be accounted for, or due to no mention or use of a predefined analysis plan (Figure 2). Certainty of the evidence ranged from very low to moderate. The most common reasons for downgrading quality of the evidence for an outcome included substantial heterogeneity, imprecision, and risk of bias in the trials contributing with data.
FIGURE 2.
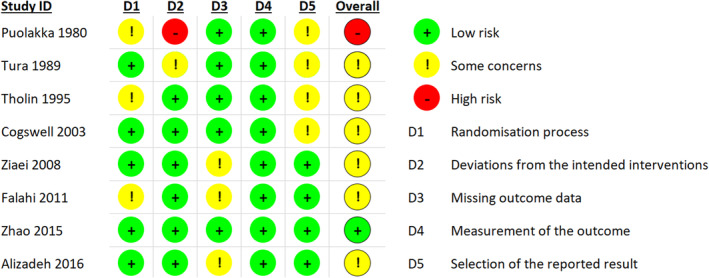
Summary of risk of bias for each included trial assessed by the revised Cochrane risk‐of‐bias tool (RoB2). With this tool, the risk of bias of each included study is judged according to five domains (D1–D5) and overall, as “low risk”, “some concerns”, or “high risk”.
Summary of findings including quality of evidence assessments have been summarized for primary and secondary outcomes in Tables 2 and 3, respectively. Forest plots can be seen in Figures S1, S2, S3, S4, S5, S6.
TABLE 2.
Summary of results: Maternal and infant primary outcomes.
| Outcomes | Anticipated absolute effects a (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Risk with no oral iron supplements | Risk with oral iron supplements | ||||
| Maternal iron deficiency anemia at term (37 weeks' gestation or more) | 177 per 1.000 | 90 per 1.000 (67–124) | RR 0.51 (0.38–0.70) | 1670 (4 RCTs) b | ⨁⨁⨁◯ MODERATE c |
| Maternal iron deficiency at term (37 weeks' gestation or more) | 672 per 1.000 | 497 per 1.000 (403–618) | RR 0.74 (0.60–0.92) | 1663 (4 RCTs) b | ⨁⨁◯◯ LOW c , d |
| Maternal high Hb concentrations during pregnancy (more than 130 g/L) | 106 per 1.000 | 100 per 1.000 (10–1.000) | RR 0.94 (0.09–10.07) | 1346 (3 RCTs) e | ⨁◯◯◯ VERY LOW c , f , g , h |
| Small for gestational age | 177 per 1.000 | 69 per 1.000 (30–152) | RR 0.39 (0.17–0.86) | 213 (1 RCT) i | ⨁⨁◯◯ LOW j , k |
| Low birthweight (less than 2500 g) | 121 per 1.000 | 36 per 1.000 (16–82) | RR 0.30 (0.13–0.68) | 361 (2 RCTs) l | ⨁⨁⨁◯ MODERATE m |
| Preterm birth (before 37 weeks' gestation) | 98 per 1.000 | 88 per 1.000 (46–167) | RR 0.90 (0.47–1.71) | 361 (2 RCTs) l | ⨁⨁◯◯ LOW g , m |
Note: GRADE Working Group grades of evidence. High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. Maternal elevated iron status and congenital anomalies are not included in the table as no trials reported these outcomes.
Abbreviations: CI, confidence interval; Hb, hemoglobin; RCTs, randomized clinical trials; RR, risk ratio.
The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
Several trials contributing data had design limitations.
Moderate/high heterogeneity: I 2 = 58%.
High heterogeneity: I 2 = 83%.
Wide 95% CI.
Cogswell et al. 16 reported higher incidence of elevated Hb concentration at term in the placebo group. However, the incidence of elevated Hb at 28 weeks' gestation was similar between groups, whereas the conflicting estimate from term was affected by substantial loss to follow‐up. Excluding the trial from the meta‐analysis gave the following result: RR: 2.19, 95% CI: 1.64–2.93, I 2 0%; 2 RCTs, 1194 women; moderate‐certainty evidence.
Cogswell et al. (2003). 16
The trial contributing data had design limitations.
Small sample size.
Both trials contributing data had design limitations.
TABLE 3.
Summary of results: maternal and infant secondary outcomes.
| Outcomes | Anticipated absolute effects a (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) Comments | |
|---|---|---|---|---|---|
| Risk with no oral iron supplements | Risk with oral iron supplements | ||||
| Maternal severe anemia during pregnancy (Hb concentration below 70 g/L) | 0 per 1.000 | 0 per 1.000 (0–0) | Not estimable | 1407 (4 RCTs) b | ⨁⨁⨁◯ MODERATE c |
| Gestational hypertension | 0 per 1.000 | 0 per 1.000 (0–0) | RR 3.34 (0.14–80.63) | 148 (1 RCT) d | ⨁◯◯◯ VERY LOW e , f |
| Maternal red blood cell transfusion (intra‐ and/or postpartum) | 63 per 1.000 | 21 per 1.000 (1–476) | RR 0.33 (0.01–7.62) | 32 (1 RCT) g | ⨁◯◯◯ VERY LOW e , f |
| Maternal postpartum anemia (Hb concentration <110 g/L) | 80 per 1.000 | 33 per 1.000 (10–105) | RR 0.41 (0.13–1.31) | 223 (1 RCT) h | ⨁⨁◯◯ LOW e , i |
Note: GRADE Working Group grades of evidence. High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. The following secondary outcomes are not included in the table as no trials reported them: gestational diabetes, pre‐eclampsia, maternal infections, gastrointestinal side effects, maternal quality of life, maternal fatigue, newborn failure to thrive, newborn Hb concentration in the first 6 months, and newborn ferritin concentration in the first 6 months.
Abbreviations: CI, confidence interval; Hb, hemoglobin; RR, risk ratio.
The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
Cogswell et al. (2003), 16 Ziaei et al. (2008) 17 , Zhao et al. (2015), 20 Alizadeh & Salehi (2016). 21
Several trials contributing data had design limitations.
Falahi et al. (2011). 19
The trial had design limitations.
Wide 95% CI, small sample size and few events.
Puolakka et al. (1980). 13
Tura et al. (1989). 14
Wide 95% CI and small sample size.
3.4. Primary outcomes
Four trials reported incidences of maternal iron deficiency anemia at term. 14 , 16 , 19 , 20 Three of the trials 16 , 19 , 20 used similar definitions of iron deficiency anemia (Hb < 110 g/L and ferritin <12–15 ng/mL) while the definition in the fourth was different 14 (Hb ≤ 115 g/L and ferritin <40 ng/mL or transferrin saturation <20%). The meta‐analysis showed lower risk of iron deficiency anemia at term in favor of oral iron treatment (pooled RR: 0.51, 95% CI: 0.38–0.70; 4 RCTs, 1670 women; I 2 = 13%; moderate‐certainty evidence) (Table 2; Figure S1).
Maternal iron deficiency at term was reported in four trials. 14 , 16 , 19 , 20 Three trials used similar definitions of iron deficiency (ferritin <12 ng/mL 16 , 19 and ferritin <15 ng/mL 20 ) while the definition in the fourth was different (Hb > 115 g/L, ferritin <40 ng/mL and/or transferrin saturation <20%). 14 The meta‐analysis showed lower risk of iron deficiency at term in favor of oral iron treatment (pooled RR: 0.74, 95% CI: 0.60–0.92; 4 RCTs, 1663 women; I 2 = 58%; low‐certainty evidence) (Table 2; Figure S2).
Three trials reported incidences of high Hb (>130 g/L) in pregnancy. 16 , 20 , 21 The meta‐analysis showed no between group difference (pooled RR: 0.94, 95% CI: 0.09–10.07; 3 RCTs, 1346 women; I 2 = 83%; very low certainty evidence) (Table 2; Figure S3). The estimate from one of the trials (unpublished data provided by the author) was affected by substantial loss to follow up: this trial found a higher incidence of elevated Hb concentration in the placebo group at term (but a similar incidence between groups at 28 weeks). Excluding the trial from the meta‐analysis gave the following result: RR: 2.19, 95% CI: 1.64–2.93, I 2 0%; 2 RCTs, 1194 women; moderate‐certainty evidence.
One trial reported incidences of SGA infants, defined by the trialists as <10th percentile of weight at birth for gestational age. 16 The risk of SGA newborns was lower in mothers who received iron (pooled RR: 0.39, 95% CI: 0.17–0.86; 1 RCT, 213 infants; I2 not estimable; low‐certainty evidence) (Table 2).
The incidences of LBW neonates (<2500 g) was reported in two trials. 16 , 19 The meta‐analysis showed lower risk of LBW infants in favor of oral iron treatment (pooled RR: 0.30, 95% CI: 0.13–0.68; 2 RCTs, 361 infants; I 2 = 0%; moderate‐certainty evidence) (Table 2; Figure S4).
Two trials reported incidences of infants born preterm (before 37 weeks’ gestation). 16 , 19 The meta‐analysis showed no between group difference (pooled RR: 0.90, 95% CI: 0.47–1.71; 2 RCTs, 361 infants; I 2 = 0%; low‐certainty evidence) (Table 2; Figure S5).
3.5. Secondary outcomes
Severe maternal anemia in pregnancy (Hb < 70 g/L) was reported in four trials. 16 , 18 , 20 , 21 Among 727 women treated with daily oral iron supplements and 680 women receiving placebo, there were no events of severe anemia (Table 3; Figure S6).
One trial reported incidences of gestational hypertension. 19 The risk of gestational hypertension was similar across groups (pooled RR: 3.34, 95% CI: 0.14–80.63; 1 RCT, 148 women; I 2 not estimable; very low certainty evidence) (Table 3).
The prevalence of red blood cell transfusion intra‐ and postpartum was reported in one trial. 13 The risk of transfusion did not differ between groups (pooled RR: 0.33, 95% CI: 0.0–7.62; 1 RCT, 32 women; I 2 not estimable; very low certainty evidence) (Table 3).
One trial reported incidences of postpartum anemia. 14 The risk of postpartum anemia was similar between groups (pooled RR: 0.41, 95% CI: 0.13–1.31; 1 RCT, 223 women; I 2 not estimable; low‐certainty evidence) (Table 3).
3.6. Outcomes not reported
The remaining predefined outcomes for newborns (congenital anomalies, hematological indices, measures for failure of thrive such as physical growth) and mothers (elevated iron status, gestational diabetes, preeclampsia, infections, quality of life, fatigue, gastrointestinal side‐effects) were not reported in any trial.
3.7. Subgroup and sensitivity analysis
The number of trials included in the primary analyses was too small for any subgroup analyses by iron dosing to be meaningful. Subgroup analyses by gestational age at start of intervention was not possible as all trials started intervention no later than at 20 weeks' gestation. It was not possible to perform the planned sensitivity analyses based on risk of bias, as no outcome was reported in ≥ two trials with ≥ one trial at high risk of bias. One trial had a markedly larger sample size 20 and provided significant weight in the meta‐analysis of maternal iron deficiency anemia, iron deficiency, high Hb, and severe anemia in pregnancy. When excluding this trial from the analyses (post hoc sensitivity analysis), the effect estimates lost statistical significance, although the trend remained similar (data not shown). An additional post hoc sensitivity analysis was performed for high Hb in pregnancy as described in section 3.2.
4. DISCUSSION
This systematic review identified eight trials reporting effects of oral iron supplementation in iron replete non‐anemic women and highlights that the evidence in this field is sparse. The evidence quality of outcomes ranged from very low to moderate, and most trials had some risk of bias. Several reported outcomes suffered from low numbers of participants as well as design limitations in the trials leading to limited confidence in the pooled effect estimates. Compared to no iron supplements, daily oral iron supplementation in pregnancy probably reduces maternal iron deficiency anemia at term and may reduce maternal iron deficiency at term. Furthermore, daily oral iron supplements in pregnancy may reduce newborn SGA and probably reduces newborn LBW. Among 727 women treated with daily oral iron supplements and 680 women receiving placebo, there were no events of severe anemia. The evidence is very uncertain about the effect of iron supplementation on maternal high Hb. Several outcomes that we aimed to assess showed no between‐group difference (i.e., preterm birth, gestational hypertension, maternal red blood cell transfusion, and postpartum anemia) or were not reported in any trials.
As expected, the amount of original research on the topic for our review was sparse and there were inconsistencies between study designs regarding settings, dosing regimens, and outcome measures reported. Although baseline ferritin concentrations indicate that several participants had acceptable iron status, baseline ferritin seem to have varied both within and between trials (Table 1). Most trials allowed baseline ferritin concentrations as low as 10–20 ng/mL, 15 , 16 , 17 , 18 , 19 , 20 , 21 which is a substantially lower limit than 70 ng/mL that has been suggested to reflect the amount of body iron required to complete pregnancy without developing iron deficiency. 22 , 23 As previously mentioned, excessive iron could theoretically do harm by negative influence on oxidative stress, microbiome, immunity, erythropoiesis, and ultimately placental flow. 6 Nevertheless, the studies we included mainly focused on beneficial effects of iron, whereas none reported data for relevant adverse outcomes such as gestational diabetes, preeclampsia, and gastrointestinal side‐effects. Regarding the review process, the risk that we oversaw eligible published material is small as we applied no time or language restrictions in the searches and contacted authors for additional information when relevant. However, a more limited number of outcomes with even more thorough prioritization could have improved the directness and implications of our findings. Furthermore, subgroup analyses on primary outcomes based on dosing was planned but not conducted, as we believe that the number of trials was too small for this to be meaningful.
Whereas physicians internationally agree that iron deficiency anemia in pregnancy should be treated, there is no current consensus as to whether preventive iron supplements should be given to iron replete non‐anemic women or not. Subsequently, iron policies for pregnant women vary between nations, and routine iron supplementation is being practiced in several countries, including countries where most women are expected to be iron replete in early pregnancy. As we believe it is important to assess the consequences of iron supplements in those who from a biological perspective probably benefit least, this review focused exclusively on its effect in iron replete non‐anemic women. To the best of our knowledge, this has never been the topic of a systematic review before. Two previous reviews including a Cochrane review have stated that iron supplementation in pregnant women result in a lower incidence of iron deficiency and iron deficiency anemia at delivery, but that its effects on clinical maternal and infant outcomes is unclear. 8 , 24 What distinguishes our and these prior reviews is that we considered initial maternal iron status and focused only on those who started out with normal Hb and iron status. Consistent with the previous reviews, we found that iron supplementation may reduce the risk of iron deficiency anemia (moderate‐certainty evidence) and iron deficiency (low‐certainty evidence) at term, although the absolute risk of iron deficiency at term was high in both groups: 49.4% in iron supplemented mothers and 67.2% in non‐supplemented mothers, respectively, despite 30–60 mg elemental iron daily from ≤20 weeks' gestation. In contrast to the two previous reviews 8 , 24 we found moderate‐quality evidence of reduced risk of LBW infants in iron treated mothers, although this was based on a limited number of infants (n = 361) and only two trials.
5. CONCLUSION
The number of trials exploring benefits and harms of daily oral iron supplements in pregnant iron replete non‐anemic women is limited. However, we did find evidence suggesting that iron supplements probably have beneficial effects in means of preventing maternal iron deficiency anemia and newborn LBW. The evidence for the influence of iron supplements on maternal high Hb is uncertain, and if a potential increase has any clinical importance remains unknown. Our finding of no difference in a range of additional obstetric and perinatal outcomes does not suggest that there truly is no difference, but rather that we lack the evidence to tell if there are differences or not. This is further underlined by the fact that several of our predefined outcomes were not reported in any trials. A substantial number of women enter pregnancy with normal Hb and iron status, and therefore, we believe that large‐scale, high‐quality, blinded RCTs that further explore the effects of iron supplements in pregnancy in these women are warranted.
AUTHOR CONTRIBUTIONS
All authors were involved in the conception and design of the study. Screening of records, data collection and risk of bias assessments were carried out by RH and EPFS. RH conducted meta‐analyses and assessed certainty of the evidence. All authors were involved in the interpretation of the data. RH drafted the manuscript and all authors reviewed and approved the final version.
Supporting information
Figure S1.
Figure S2.
Figure S3.
Figure S4.
Figure S5.
Figure S6.
Table S1.
Table S2.
Captions.
ACKNOWLEDGMENTS
The authors kindly thank trialists who upon request provided us additional study information, and the Copenhagen University Library for valuable input to the search strategy development.
Hansen R, Sejer EPF, Holm C, Schroll JB. Iron supplements in pregnant women with normal iron status: A systematic review and meta‐analysis. Acta Obstet Gynecol Scand. 2023;102:1147‐1158. doi: 10.1111/aogs.14607
REFERENCES
- 1. World Health Organization . Iron deficiency anaemia assessment, prevention, and control. A Guide for Programme Managers. WHO; 2001. [Google Scholar]
- 2. Georgieff MK. Iron deficiency in pregnancy. Am J Obstet Gynecol. 2020;223:516‐524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bothwell TH. Iron requirements in pregnancy and strategies to meet them. Am J Clin Nutr. 2000;72:257S‐264S. [DOI] [PubMed] [Google Scholar]
- 4. Daru J, Allotey J, Peña‐Rosas JP, Khan KS. Serum ferritin thresholds for the diagnosis of iron deficiency in pregnancy: a systematic review. Transfus Med. 2017;27:167‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization . Global health risks. WHO; 2009. [Google Scholar]
- 6. Dewey KG, Oaks BM. U‐shaped curve for risk associated with maternal hemoglobin, iron status, or iron supplementation. Am J Clin Nutr. 2017;106:1694S‐1702S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Young MF, Oaks BM, Tandon S, Martorell R, Dewey KG, Wendt AS. Maternal hemoglobin concentrations across pregnancy and maternal and child health: a systematic review and meta‐analysis. Ann N Y Acad Sci. 2019;1450:47‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peña‐Rosas JP, De‐Regil LM, Garcia‐Casal MN, Dowswell T. Daily oral iron supplementation during pregnancy. Cochrane Database Syst Rev. 2015;2015:CD004736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 11. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE handbook for grading quality of evidence and strength of recommendations (updated October 2013). The GRADE Working Group, 2013. https://gdt.gradepro.org/app/handbook/handbook.html [Google Scholar]
- 13. Puolakka J, Jänne O, Pakarinen A, Järvinen PA, Vihko R. Serum ferritin as a measure of iron stores during and after normal pregnancy with and without iron supplements. Acta Obstet Gynecol Scand Suppl. 1980;95:43‐51. [DOI] [PubMed] [Google Scholar]
- 14. Tura S, Carenza L, Baccarani M, et al. Therapy and iron supplements with ferritin iron during pregnancy. Randomized prospective study of 458 cases. Recenti Prog Med. 1989;80:607‐614. [PubMed] [Google Scholar]
- 15. Tholin K, Sandström B, Palm R, Hallmans G. Changes in blood manganese levels during pregnancy in iron supplemented and non supplemented women. J Trace Elem Med Biol. 1995;9:13‐17. [DOI] [PubMed] [Google Scholar]
- 16. Cogswell ME, Parvanta I, Ickes L, Yip R, Brittenham GM. Iron supplementation during pregnancy, anemia, and birth weight: a randomized controlled trial. Am J Clin Nutr. 2003;78:773‐781. [DOI] [PubMed] [Google Scholar]
- 17. Ziaei S, Mehrnia M, Faghihzadeh S. Iron status markers in nonanemic pregnant women with and without iron supplementation. Int J Gynecol Obstet. 2008;100:130‐132. [DOI] [PubMed] [Google Scholar]
- 18. Ziaei S, Janghorban R, Shariatdoust S, Faghihzadeh S. The effects of iron supplementation on serum copper and zinc levels in pregnant women with high‐normal hemoglobin. Int J Gynaecol Obstet. 2008;100:133‐135. [DOI] [PubMed] [Google Scholar]
- 19. Falahi E, Akbari S, Ebrahimzade F, Gargari BP. Impact of prophylactic iron supplementation in healthy pregnant women on maternal iron status and birth outcome. Food Nutr Bull. 2011;32:213‐217. [DOI] [PubMed] [Google Scholar]
- 20. Zhao G, Xu G, Zhou M, et al. Prenatal iron supplementation reduces maternal anemia, iron deficiency, and iron deficiency anemia in a randomized clinical trial in rural China, but iron deficiency remains widespread in mothers and neonates. J Nutr. 2015;145:1916‐1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alizadeh L, Salehi L. Is routine iron supplementation necessary in pregnant women with high hemoglobin? Iran Red Crescent Med J. 2016;18:e22761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Milman N, Byg KE, Bergholt T, Eriksen L, Hvas AM. Body iron and individual iron prophylaxis in pregnancy – should the iron dose be adjusted according to serum ferritin? Ann Hematol. 2006;85:567‐573. [DOI] [PubMed] [Google Scholar]
- 23. Khambalia AZ, Collins CE, Roberts CL, et al. Iron deficiency in early pregnancy using serum ferritin and soluble transferrin receptor concentrations are associated with pregnancy and birth outcomes. Eur J Clin Nutr. 2016;70:358‐363. [DOI] [PubMed] [Google Scholar]
- 24. Cantor AG, Bougatsos C, Dana T, Blazina I, McDonagh M. Routine iron supplementation and screening for iron deficiency anemia in pregnancy: a systematic review for the U.S. preventive services task force. Ann Intern Med. 2015;162:566‐576. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.
Figure S2.
Figure S3.
Figure S4.
Figure S5.
Figure S6.
Table S1.
Table S2.
Captions.


