Abstract
Introduction
Ectopic pregnancy is an important health condition which affects up to 1 in 100 women. Women who present with mild symptoms and low serum human chorionic gonadotrophin (hCG) are often treated with methotrexate (MTX), but expectant management with close monitoring is a feasible alternative. Studies comparing the two treatments have not shown a statistically significant difference in uneventful resolution of ectopic pregnancy, but these studies were too small to define whether certain subgroups could benefit more from either treatment.
Material and methods
We performed a systematic review and individual participant data meta‐analysis (IPD‐MA) of randomized controlled trials comparing systemic MTX and expectant management in women with tubal ectopic pregnancy and low hCG (<2000 IU/L). A one‐stage IPD‐MA was performed to assess overall treatment effects of MTX and expectant management to generate a pooled intervention effect. Subgroup analyses and exploratory multivariable analyses were undertaken according to baseline serum hCG and progesterone levels. Primary outcome was treatment success, defined as resolution of clinical symptoms and decline in level of serum hCG to <20 IU/L, or a negative urine pregnancy test by the initial intervention strategy, without any additional treatment. Secondary outcomes were need for blood transfusion, surgical intervention, additional MTX side‐effects and hCG resolution times. Trial registration number: PROSPERO: CRD42021214093.
Results
1547 studies reviewed and 821 remained after duplicates removed. Five studies screened for eligibility and three IPD requested. Two randomized controlled trials supplied IPD, leading to 153 participants for analysis. Treatment success rate was 65/82 (79.3%) after MTX and 48/70 (68.6%) after expectant management (IPD risk ratio [RR] 1.16, 95% confidence interval [CI] 0.95–1.40). Surgical intervention rates were not significantly different: 8/82 (9.8%) vs 13/70 (18.6%) (RR 0.65, 95% CI 0.23–1.14). Mean time to success was 19.7 days (95% CI 17.4–22.3) after MTX and 21.2 days (95% CI 17.8–25.2) after expectant management (P = 0.25). MTX specific side‐effects were reported in 33 MTX compared to four in the expectant group.
Conclusions
Our IPD‐MA showed no statistically significant difference in treatment efficacy between MTX and expectant management in women with tubal ectopic pregnancy with low hCG. Initial expectant management could be the preferred strategy due to fewer side‐effects.
Keywords: expectant management, medical treatment, methotrexate, pregnancy ectopic, pregnancy tubal
Our IPD‐MA showed no statistically significant difference in treatment efficacy between MTX and expectant management in women with tubal EP with low hCG. Initial expectant management could be the preferred strategy due to fewer side‐effects.
Abbreviations
- CI
confidence interval
- EP
ectopic pregnancy
- hCG
human chorionic gonadotrophin
- IPD
individual participant data
- IPD‐MA
individual participant data meta‐analysis
- MTX
methotrexate
- NL
the Netherlands
- PUL
pregnancy of unknown location
- RCT
randomized controlled trial
- RR
risk ratio
- UK
United Kingdom
Key message.
Our individual participant data meta‐analysis shows no evidence of a difference in treatment efficacy between methotrexate and expectant management of tubal ectopic pregnancies with low hCG. Expectant management with close observation could be the preferred initial strategy in these women. This encourages shared decision‐making between the clinician and patient.
1. INTRODUCTION
Ectopic pregnancies affect 1% of all pregnancies 1 and up to 4.7% of patients presenting to early pregnancy emergency services. 2 Although mortality rates for women with ectopic pregnancies have decreased with advances in the diagnosis and surgical innovations, the cost and burden of the disease including multiple investigations, treatments, follow‐up, as well as the psychological impact on women, continue to be high.
The incidence of ectopic pregnancies has been increasing due to increased sensitivity of diagnostic imaging and biochemical algorithms. This enables the detection of small ectopic pregnancies at an early stage which have milder clinical courses. As a result, medical management was introduced into clinical practice 35 years ago. 3 , 4 Methotrexate (MTX) has been widely used for the treatment of women with ectopic pregnancies presenting with mild clinical symptoms and low human chorionic gonadotrophin (hCG) levels.
Expectant management of ectopic pregnancy (EP) has been shown in observational studies to have a high success rate in women with tubal EP and serum hCG levels <1500 IU/L and avoids the risks of medical and surgical treatment. 5 , 6 , 7 , 8 There have been three randomized controlled trials (RCTs), none of which have shown a statistically significant difference between the effectiveness of MTX over expectant management in clinically stable women presenting with low serum hCG levels. 9 , 10 , 11 All studies had relatively small sample sizes and were too small to determine whether certain subgroups of patients could benefit more from either treatment.
An “individual participant data meta‐analysis” (IPD‐MA) is considered a gold standard of systematic review. It obtains raw individual level data, allowing for a wider scope and range of analyses compared with a meta‐analysis, including investigating the impact of participant‐level variables on treatment effectiveness. The aims of this study were to undertake an IPD‐MA to strengthen evidence that compares systemic MTX with expectant management in the treatment of tubal EP and to identify whether any subgroups benefit more from either treatment.
2. MATERIAL AND METHODS
We performed a systematic review and meta‐analysis following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses for Individual Participant Data (PRISMA‐IPD) statement. 12
2.1. Protocol registration
The finalized protocol was registered with PROSPERO, (http://www.crd.york.ac.uk/PROSPERO), with the ID: CRD42021214093.
2.2. Inclusion criteria and identification of studies
All studies meeting the following inclusion criteria were proposed for the IPD‐MA:
Randomized controlled trial;
Compared systemic MTX with expectant management;
Population was women with tubal EP, defined as either:
positively identified on ultrasound scan with a baseline serum hCG <1500 IU/L
or pregnancy of unknown location (PUL) with a plateauing serum hCG concentration <2000 IU/L
Included hemodynamically stable women;
Absence of embryonic cardiac activity;
No evidence of hemoperitoneum;
Approved by the local Institutional Review Board, Ethics Committee, Research Review Board or similar, and participants gave informed consent;
2.3. Exclusion criteria
Studies were excluded for the following reasons:
Investigator(s) fail to provide data on outcomes of interest;
Inclusion criteria required tubal EP with already declining hCG before randomization;
More than 20% attrition or exclusion of patients after randomization;
Incomplete reporting of reasons for withdrawals and protocol violations if no valid reason upon request;
Imbalance in dropouts across groups;
Incomplete reporting of the study's prespecified outcomes;
Study had not begun enrollment of patients at the time of registration of the IPD‐MA protocol
Quasi‐random study designs, to reduce the possibility of bias.
2.4. Literature search
We searched electronic databases and trial registries for published or registered randomized controlled trials for studies on systemic MTX vs expectant management for the resolution of tubal EP up to August 2020. The Cochrane Gynecology and Fertility Group (CGF) Specialized Register of Controlled Trials, Procite platform and The Cochrane Central Register of Studies Online (CRSO Web platform) were searched from inception. MEDLINE Ovid (from 1946), EMBASE Ovid (from 1974), PsychINFO Ovid (1806), CINAHL Ebsco (1982) were searched. The following trial registers were searched: http://www.clinicaltrials.gov, https://wwww/isrctn.com, BioMed Central ISRCTN registry, WHO International Clinical Trials Registry Platform and Cochrane Central Register of Controlled Trials. The electronic search consisted of terms relating to “tubal pregnancy”, “ectopic pregnancy”, “methotrexate”, “expectant management”, “conservative management”, “spontaneous resolution”, randomized controlled trial”, “controlled clinical trial”, “random allocation”, “double‐blind trial”, “single‐blind”, “clinical trial” and “placebos” (Appendix).
All relevant studies were considered for inclusion. Two members of the review team (SAS and MvW) independently performed title and abstract screening using predetermined selection criteria. Full‐text review of the eligible studies following title and abstract screening were conducted by two authors (SAS and MvW). Reference lists of relevant articles and reviews were manually searched to identify further papers.
2.5. Initial contact with trial authors
Members of the review team were also lead investigators of the relevant trials and agreed to provide individual participant data (IPD) from their respective trial. Other trial investigators were approached for input in the manuscript. There were no studies for which IPD was not available on request.
2.6. Contribution and collection of data
IPD was requested on contact with trial investigators and provided in an anonymized and non‐traceable format. Data was stored on a standard format (spreadsheet on STATA) along with explanations, key codes and a data dictionary regarding the data entries. Data was translated to a common language (English) for analysis at the study site in London, UK. Data were checked for consistency, missing or extreme values, missing items, errors and consistency with published reports, and were re‐coded if necessary. Randomization methods and intervention details were cross‐checked against published reports, trial protocols and data collection sheets. Individual trial members checked finalized data for each trial before incorporation into the combined database and continuation of any further analysis. Data‐sharing contracts and collaboration agreements were secured. Anonymized data was emailed on a secure email account, which was deleted after downloaded onto a secure, centralized and customized database at University College London Hospital, London, UK.
2.7. Data integrity
All data was checked separately by SAS and MvW. We checked sequence generation, completeness and balance/imbalance and whether results presented in the publication are confirmed by the data.
2.8. Privacy
Data access, handling, analysis and storage were compliant to the European Union General Data Protection Regulation (GDPR).
2.9. Scoring of risks of bias
Risks of bias for each participating trial were assessed by using the Cochrane collaboration tool [Higgins & Green, 2011] based on the following characteristics:
Random sequence generation;
Allocation concealment;
Blinding of patient participants and study personnel;
Incomplete outcome data;
Selective outcome reporting;
Blinding of outcome assessment;
Other sources of bias.
Each item of bias was scored as Low, High or Unclear according to the Cochrane Handbook. We also undertook a GRADE evaluation of the quality of evidence. Risk of bias and GRADE evaluation for each trial were assessed by three collaborators; SAS. who was independent of the trials included, and MvW and BWM. who were independent of the UK trial. Discrepancies in assessment and the final risk scoring were discussed with all authors of this study.
2.10. Outcomes and subgroups
2.10.1. Planned analysis – study level
Descriptive comparisons between studies were conducted to assess between‐study differences. To avoid bias induced by ignoring missing data, it was assumed to be missing‐at‐random and multiple imputation techniques were used to replace missing data based on observed individual patient characteristics. To preserve any between‐study heterogeneity, any imputation was performed within each original study before the data was pooled and analyzed. We describe the proportion of missing values for each dataset included in the IPD‐MA.
2.10.2. Planned analysis – individual level
Individual participant level information was collected and entered into a database. Outcome variables included age, gravidity, parity, mode of conception, previous miscarriage, previous EP, gestational age, baseline serum hCG and baseline serum progesterone.
Primary outcome:
Treatment success—resolution of clinical symptoms and decline in level of serum hCG to <20 IU/L or a negative urine pregnancy test by the initial intervention strategy (single injection only), without additional medical treatment
Secondary outcomes:
severe intra‐abdominal bleeding requiring blood transfusion
need for surgical intervention
indication for surgical intervention
side‐effects from MTX
number of additional MTX injections given (excluding the single initial injection)
hCG resolution times
2.11. Planned subgroup analyses
We conducted subgroup analyses to compare outcomes according to age, parity, study site, baseline serum hCG and baseline serum progesterone and including only tubal EP visualized on ultrasound scan.
2.12. Statistical analyses
The statistical analyses of this IPD‐MA utilized methods described in the Cochrane Collaboration Handbook. Two people extracted the data independently. We summarized the overall effect of the interventions in relation to each outcome when the data of at least two trials were available. Data were analyzed as intention‐to‐treat. Time to resolution was handled as a continuous outcome. All other outcomes are binary such that the following analysis accounts for these outcomes. Descriptive comparisons were made to assess between‐study differences. Each trial was re‐analyzed separately, and the investigators asked to confirm their individual results. A one‐stage IPD‐MA was then performed to assess overall treatment effects of MTX and expectant management to generate a pooled intervention effect. This included a random intercept (to account for baseline differences between studies) and random slope (to account for differences in treatment effect between studies). A log‐binomial model for dichotomous outcomes yielded a risk ratio (RR) with 95% confidence interval (CI). A quantile random effects model was used for continuous non‐normally distributed outcomes. All analysis included variables that were used for stratified randomization as covariates.
We performed subgroup analyses and explored treatment–covariate interaction for the following patient‐level covariates: female age (cut‐off 30 years), gestational age (GA), parity, hCG and progesterone. These analyses were conducted using a two‐stage approach and were thus based solely on within‐study information as recommended to avoid ecological bias. 13 We performed exploratory multivariable one‐stage IPD‐MA including the covariates for treatment success with associations presented as odds ratio with 95% CI and for time to resolution with associations presented as hazard rate with 95% CI.
2.13. Software for analyses
Statistical analyses were performed using STATA 16.1 (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC) software and R version 3.6.0.
2.14. Ethics statement
All involved studies had institutional review board approval and obtained informed consent from all participants. We approached the Health Research Authority (HRA), who advised that a formal NHS REC (National Health Service Research Ethics Committee) approval was not required, and that a Proportionate Review was sufficient, which we obtained (REC reference: 21/PR/1301). We secured Data Sharing and Collaboration Agreement Contracts with the respective sites (IRAS ID: 293525).
3. RESULTS
3.1. Study selection and IPD
We identified 821 non‐duplicated studies from our electronic search. Following screening of the title, abstract and full‐text screening, five RCTs were assessed for eligibility: Korhonen et al., 14 Van Mello et al., 9 Silva et al., 10 Jurkovic et al., 11 Casikar et al.. 15 The study by Korhonen et al. was excluded because of the use of a very low dosage of MTX (2.5 mg/day for 5 days), which is unlikely to be effective and could be classified as placebo. 16 The study by Silva et al. was also excluded from the systematic review as one of the key inclusion criteria for this study was decreasing hCG levels before randomization, thereby including only women with already failing pregnancies. A third RCT on MTX vs placebo was registered but the authors informed us that they had not recruited any participants and the study had been stopped. 15 Two studies were included for systematic review, Van Mello et al. 9 (NL study) and Jurkovic et al. 11 (UK study), providing IPD for 153 women (Figure 1).
FIGURE 1.
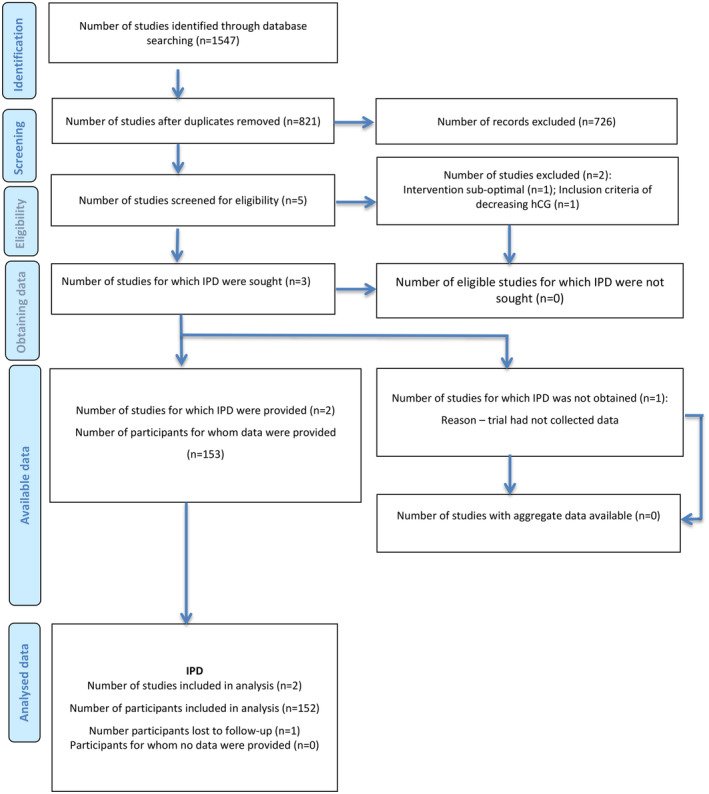
PRISMA IPD flow diagram. IPD, individual participant data.
We assessed the ACT or NOT trial 17 and concluded that it was not eligible for inclusion, as their study focused on “persisting pregnancy of unknown location” rather than tubal EP. On review of the cases that failed treatment and had unscheduled surgery, the majority had dilation and curettage and less than 2% were eventually diagnosed with an EP. In addition, the reported attrition rate was 39%.
3.2. Study characteristics
The NL study ran between April 2007 and January 2012 and included 73 women with conclusive ultrasound diagnosis of tubal EP with hCG <1500 IU/L or suspected tubal EP (PUL with plateauing serum hCG) <2000 IU/L. 9 This was funded by a grant of the Netherlands Organization for Health Research and Development (ZonMw Clinical fellow grant 90700154). The UK study ran between August 2005 and June 2014 and included 80 women with conclusive ultrasound diagnosis of tubal EP and a serum hCG <1500 IU/L. 11 This did not receive external funding. Baseline characteristics of intervention and comparison groups were similar and all prespecified outcomes were reported in both studies. Further characteristics of both studies are outlined in Table 1.
TABLE 1.
Characteristics of included studies of medical vs expectant management of tubal ectopic pregnancy.
| Study | Van Mello (2012) | Jurkovic (2017) |
| Setting | The Netherlands (NL) | United Kingdom (UK) |
| Study design | Multi‐center, open‐label RCT
|
Multi‐center, placebo controlled RCT
|
| Sample size | 73
|
79
|
| Inclusion criteria | Hemodynamically stable women with tubal EP visible on US and plateauing serum hCG <1500 IU/L or a PUL and a plateauing serum hCG <2000 (persisting PUL) | Clinically stable women with conclusive US diagnosis of tubal EP, baseline serum hCG <1500 IU/L |
| Exclusion criteria | Live ectopic pregnancy, signs of tubal rupture and/or active intra‐abdominal bleeding, contraindication for MTX (eg abnormalities in liver or renal function or at the time of a full blood count) or <18 years of age | Ectopic pregnancy with embryonic heart rate, hemoperitoneum, contraindication to MTX (abnormal full blood count, liver and renal function tests, history of hepatic, renal or pulmonary disease). |
| Diagnostic criteria for TEP | Ectopic ring or ectopic mass and/or pouch of Douglas fluid | NR |
| Intervention | IM MTX 1 mg/kg, (max. 100 mg) ± second MTX if <15% decrease serum hCG in weekly follow‐up, max. 3 injections | Single IM MTX 50 mg/m2 |
| Comparison | Expectant management ± IM MTX if serum hCG increase >15%, max. 3 injections | Single IM 0.9% NaCl IM |
| Treatment success definition | Uneventful decline serum hCG <2 IU/L by initial intervention | Resolution clinical symptoms and decline hCG <20 IU/L or negative UPT without additional intervention |
| Outcomes |
Primary—Treatment success Secondary—additional MTX injections, surgical procedures, treatment complications (i.e. hemorrhage, severe allergic reaction to MTX), mild‐to‐moderate side‐effects of MTX treatment (i.e. nausea, diarrhea, vomiting, buccositis and conjunctivitis), clinical symptoms and serum hCG clearance time |
Primary—Treatment success Secondary—intra‐abdominal bleeding requiring blood transfusion, number of emergency laparotomies performed, proportion of women experiencing significant pelvic pain or gastrointestinal side‐effects and serum hCG resolution times |
| hCG monitoring | Weekly until hCG undetectable | Day 4 and 7 after treatment then every 2 days if static hCG (within 15% of previous reading) or weekly if levels fell >15% |
| Loss to follow‐up | 0 | 1 |
Abbreviations: EP, tubal ectopic pregnancy; FBC, full blood count; IM, intramuscular; MTX, methotrexate; NR, not recorded; POD, pouch of Douglas; PUL, pregnancy of unknown location; SD, standard deviation; UPT, urine pregnancy test; US, ultrasound.
3.3. IPD integrity
There were no issues identified in checking IPD.
3.4. Risk of bias within studies
The details of risks of bias assessments within individual studies are presented in Figures 2 and 3. The UK study had a low risk of bias in randomization method. The NL study had a high number of participating centers and was stratified by center. They used a “block size of four” for randomization and therefore had unequal allocation. The randomization was organized by an independent party and use of “block size of four” was only revealed after the study was finished. We therefore assessed this to have a low risk of bias in randomization. Performance and detection bias in the UK study were assessed as low risk due to blinding of participants, personnel and outcome assessors. Performance and detection bias in the NL study were assessed as high risk. This study was unable to blind participants and personnel as they did not have a licence for placebo production at the time and outcome assessors were also not blinded. Although some outcomes were objective (hCG resolution times and need for blood transfusion) and unlikely to be affected by non‐blinding, other outcomes could have been influenced by knowledge of intervention (need for surgical procedure, side‐effects and additional MTX given). Attrition and reporting bias were low in both studies.
FIGURE 2.
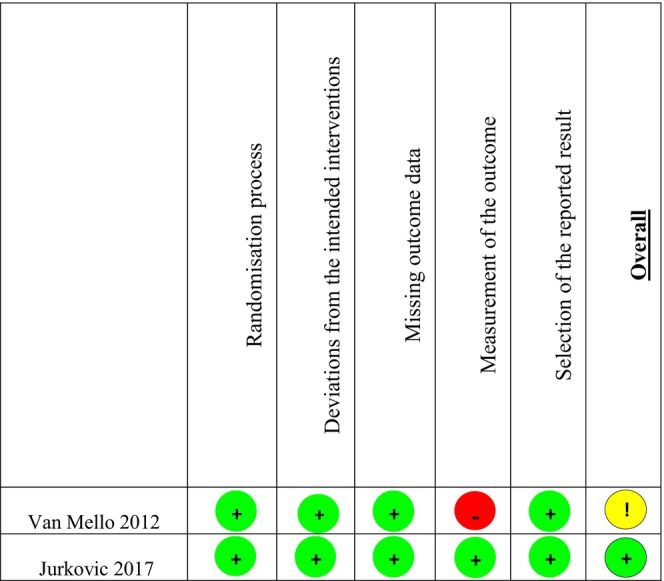
Risk of bias grading of randomized controlled trials.
FIGURE 3.
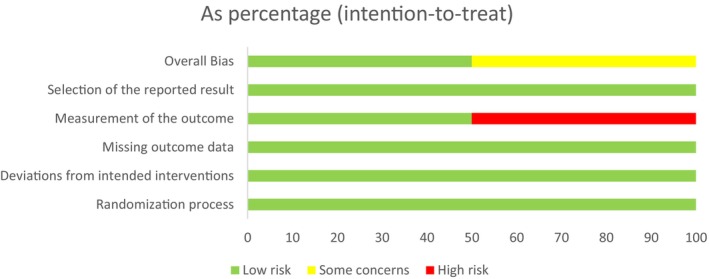
Risk of bias graph. The Cochrane Risk of Bias 2 tool was used to guide and generate this graph.
3.5. GRADE evaluation of studies
A GRADE evaluation of the quality of evidence of the two randomized controlled trials is presented in a Summary of Findings (SoF) in Table 2. Quality of evidence for treatment success was downgraded to low certainty due to serious risk of bias in one study and imprecision due to inadequate confidence in the estimate of effect. Quality of evidence for severe intra‐abdominal bleeding requiring blood transfusion was downgraded to moderate certainty due to imprecision. It was felt that the risk of bias due to lack of blinding in one study did not influence need for blood transfusion. Quality of evidence for surgical intervention, side‐effects and additional MTX given were downgraded to very low certainty. This was due to a serious risk of bias in one study and high imprecision due to inadequate confidence in the estimate of effect and wide CI. Quality of evidence for hCG resolution time was downgraded to moderate certainty due to imprecision. It was felt that the risk of bias due to lack of blinding in one study did not influence the hCG resolution times.
TABLE 2.
Summary of findings: GRADE evaluation of critical outcomes of randomized trials of methotrexate vs expectant management for tubal ectopic pregnancy in clinically stable women with hCG <2000 IU/L.
| Certainty assessment | No. of patients | Effect | Certainty | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Methotrexate | Expectant management | Relative (95% CI) | Absolute (95% CI) | |
| Treatment success (assessed with: uneventful resolution of clinical symptoms and decline of hCG to <20 IU/L) | ||||||||||
| 2 | Serious a | Not serious | Not serious | Serious b | None | 65/82 (79.3%) | 48/70 (68.6%) | RR 1.16 (0.95– 1.40) | 110 more per 1000 (from 34 fewer to 274 more) | ⨁⨁◯◯ Low |
| Severe intra‐abdominal blood loss (assessed with: need for blood transfusion) | ||||||||||
| 2 | Not serious | Not serious | Not serious | Serious b | None | 0/82 (0.0%) | 1/70 (1.4%) | Not estimable | ⨁⨁⨁◯ Moderate | |
| Surgical intervention (assessed with: need for surgical procedure) | ||||||||||
| 2 | Serious a | Not serious | Not serious | Very serious c | None | 8/82 (9.8%) | 13/70 (18.6%) | RR 0.53 (0.23– 1.14) | 87 fewer per 1000 (from 143 fewer to 26 more) | ⨁◯◯◯ Very low |
| Side effects (assessed with: number of side‐effects) | ||||||||||
| 2 | Serious a | Not serious | Not serious | Very serious c | None | 37/82 (45.1%) | 28/70 (40.0%) | RR 1.11 (0.77– 1.62) | 44 more per 1000 (from 92 fewer to 248 more) | ⨁◯◯◯ Very low |
| Additional methotrexate injections given (assessed with: number of additional MTX injections) | ||||||||||
| 1 | Serious | Not serious | Not serious | Very serious c | None | 9/41 (22.0%) | 9/32 (28.1%) | RR 0.78 (0.35– 1.74) | 62 fewer per 1000 (from 183 fewer to 208 more) | ⨁◯◯◯ Very low |
| hCG resolution times (follow‐up: range 4 days to 50 days; assessed with: days) | ||||||||||
| 2 | Not serious | Not serious | Not serious | Serious b | None | 19.7 | 21.2 | ‐ | MD 1.53 days lower (6.06 lower to 3.01 higher) | ⨁⨁⨁◯ Moderate |
Abbreviations: CI, confidence interval; MD, mean difference; RR, risk ratio.
Downgraded one level due to non‐blinding in one study.
Downgraded one level due to inadequate confidence in the estimate of effect.
Downgraded two levels due to inadequate confidence and wide confidence interval in the estimate of effect.
3.6. Results of individual studies
The results of each study are shown in Table 3. Surgical intervention was 20% in the UK study and 7% in the NL study.
TABLE 3.
Individual study data.
| NL (n = 73) | UK (n = 79) | |
|---|---|---|
| Uneventful decline in hCG (%) | ||
| Methotrexate | 31/41 (76) | 34/41 (83) |
| Expectant | 19/32 (59) | 29/38 (76) |
| Blood transfusion | ||
| Methotrexate | 0 | 0 |
| Expectant | 0 | 1 |
| Surgical intervention (%) | 5/73 (7) | 16/79 (20) |
| Methotrexate | 1/41 (2) | 7/41 (17) |
| Expectant | 4/32 (13) | 9/38 (24) |
| Additional MTX (%) | ||
| Methotrexate | 9/41 (22) | 0 |
| Expectant | 9/32 (28) | 0 |
| Resolution time a , mean (95% CI) | ||
| Methotrexate | 22.9 (17.6–28.2) | 20.0 (14.3–25.6) |
| Expectant | 20.3 (16.8–23.7) | 19.2 (15.3–23.1) |
Days
3.7. Aggregate meta‐analysis
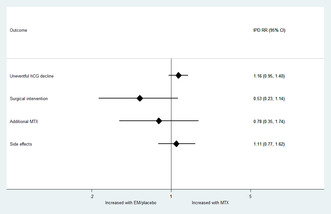
An aggregate meta‐analysis did not identify any statistically significant differences in treatment success, need for surgical intervention, additional MTX or side‐effects overall between MTX and expectant management of tubal EP (Figure 4).
FIGURE 4.
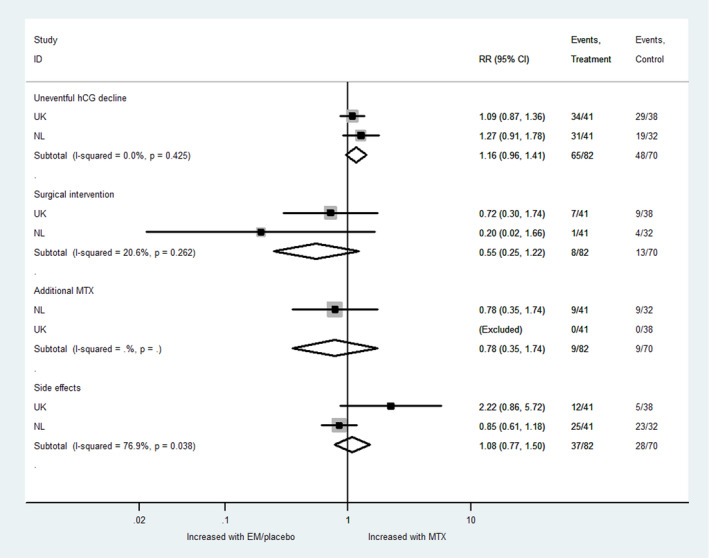
Aggregate meta‐analysis. EM, expectant management; MTX, methotrexate; NL, the Netherlands; RR, risk ratio; UK, United Kingdom.
3.8. IPD meta‐analysis
3.8.1. Results of syntheses
We obtained data from two trials reporting on 153 women with tubal EP and suspected tubal EP who were randomized to MTX or expectant management. Maternal demographic characteristics were similar in both groups and are shown in Table 4. One woman was lost to follow‐up, leaving 152 for analysis.
TABLE 4.
Demographic characteristics of studies combined at baseline.
| MTX (n = 83) | Placebo/expectant (n = 70) | P‐value | |
|---|---|---|---|
| Maternal age a , mean (95% CI) | 30.9 (29.4–32.3) | 31.52 (29.9–32.5) | 0.590 |
| Gestational age b , mean (95% CI in days) e | 7+2 (47.1–55.7) | 7+6 (44.2–49.9) | 0.057 |
| Parity, n (%) | 0.673 | ||
| 0 | 43/81 (53.1) | 42/70 (60.0) | |
| 1 | 23/81 (28.4) | 15/70 (21.4) | |
| 2+ | 15/81 (18.5) | 13/70 (18.6) | |
| Previous miscarriage, n (%) | 0.696 | ||
| 0 | 61/81 (75.3) | 51/70 (72.9) | |
| 1 | 14/81 (17.3) | 12/70 (17.1) | |
| >2 | 6/81 (7.4) | 7/70 (10.0) | |
| Previous EP, n (%) | 8/81 (9.9) | 6/70 (8.6) | 0.783 |
| hCG c , median (Q1–Q3) | 504 (231–960) | 468 (238–906) | 0.929 |
| Progesterone d , median (Q1– Q3) | 9.8 (5.0–24.9) | 12.8 (7.0–19.0) | 0.728 |
Abbreviations: EP, ectopic pregnancy; IQR, interquartile range.
Years.
Weeks and days.
Missing data = 8 MTX, 10 placebo/expectant.
IU/L.
nmol/L.
The IPD RR are presented in Table 5 and Figure 5. These are similar to the aggregate RR. The primary outcome, treatment success or uneventful decline in hCG was not significantly different between MTX and expectant management (IPD RR 1.16, 95% CI 0.95–1.40, I 2 = 0%, low certainty of evidence). This implies that if the success rate following expectant management is 69%, the success rate following MTX is expected to be between 66% and 94%. The mean time to hCG resolution was 19.7 days (95% CI 17.4–22.3) after MTX and 21.2 days (95% CI 17.8–25.2) after expectant management (P = 0.25).
TABLE 5.
Combined outcomes.
| Outcome | MTX (n = 82) | Placebo/expectant (n = 70) | IPD RR (95% CI) |
|---|---|---|---|
| Including all data | |||
| Uneventful decline in hCG, n (%) | 65/82 (79.3) | 48/70 (68.6) | 1.16 (0.95–1.40) |
| Including only tubal EP seen on scan | |||
| Uneventful decline in hCG, n (%) | 41/49 (83.7) | 33/45 (73.3) | 1.14 (0.92–1.42) |
| Additional MTX (one study) | 9/41 (22.0) | 9/32 (28.1) | 0.78 (0.35–1.74) |
| Surgical intervention | 8/82 (9.8) | 13/70 (18.6) | 0.53 (0.23–1.14) |
| Any side‐effects | 37/82 (45.1) | 28/70 (40.0) | 1.11 (0.77–1.62) |
Abbreviations: ACI, confidence interval; hCG, human chorionic gonadotrophin; IPD, individual participant data; MTX, methotrexate; RR, relative risk.
FIGURE 5.
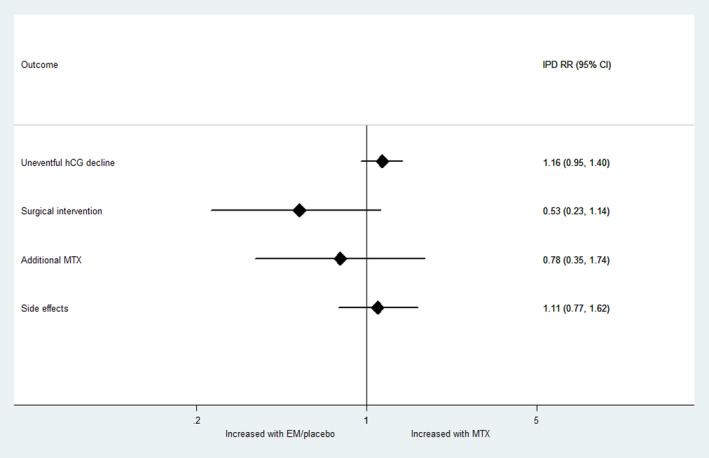
Individual Participant Data (IPD) risk ratio. EM, expectant management; MTX, methotrexate.
In the NL study, 15 were tubal EP visualized on ultrasound, and 58 were PUL. A sensitivity analysis including tubal EP visualized on scan only and excluding PULs suggested a similar result (IPD RR 1.14, 95% CI 0.92–1.42).
Only the NL study used additional MTX in their treatment protocol, of which 9/41 (22%) had additional treatment in the MTX group vs 9/32 (28%) in the expectant management group (RR 0.78, 95% CI 0.35–1.74).
Surgical intervention was required in 8/82 (9.8%) in the MTX group and 13/70 (18.6%) in the expectant group (RR 0.53, 95% CI 0.23–1.14). Of those who had surgical intervention, 9/13 (69%) were tubal EP visualized on ultrasound scan, and 4/13 (31%) were PUL. All women requiring surgery had confirmed tubal EP at laparoscopy. The commonest indication for surgery in the MTX group was abdominal pain with evidence of intra‐abdominal bleeding on ultrasound scan (6/8, 75%). The commonest indications for surgery in the expectant management group were abdominal pain with evidence of intra‐abdominal bleeding on ultrasound scan (4/13, 31%), rising hCG (4/13, 31%) and abdominal pain only (3/13, 23%).
One woman (1.4%) in the expectant group required a blood transfusion of two units with no women needing it in the MTX group. We could therefore not analyze this difference between the groups.
There was no difference in reported side‐effects between MTX and expectant management, although the commonest side‐effects in both groups were abdominal pain and vaginal bleeding, which could be due to the natural presentation of EP. Those given MTX reported more side‐effects specific to MTX,, including nausea, vomiting, diarrhea, mucositis conjunctivitis and photosensitivity. These side‐effects were reported 33 times compared with four times (nausea only) in the expectant management group.
The main IPD results were also calculated as risk differences (Figure S1).
3.8.2. Treatment–covariate interaction (subgroup analyses)
There was no significant difference in the treatment effect on uneventful decline of hCG according to maternal age, gestational age, parity, baseline serum hCG or progesterone (Figure 6). The heterogeneity measure I 2 was 0% for all subgroups, except for hCG: hCG <1000, I 2 = 26 and ≥1000, I 2 = 48%.
FIGURE 6.
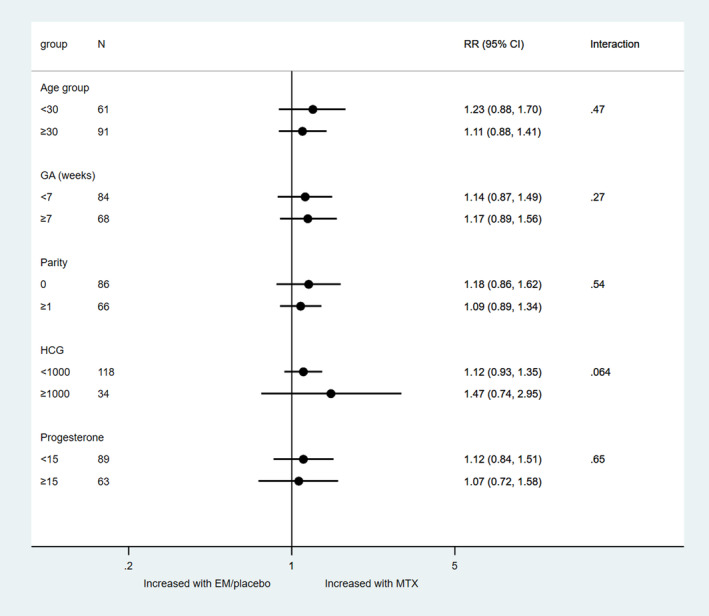
Treatment‐covariate interaction for uneventful decline of human chorionic gonadotrophin. EM, expectant management; GA, gestational age; MTX, methotrexate; RR, risk ratio.
There was no significant difference in the treatment effect on surgical intervention according to maternal age, gestational age, parity, baseline serum hCG or progesterone (Figure 7). The heterogeneity measure I 2 was 0% for all subgroups, except for hCG: hCG <1000, I 2 = 51% and ≥1000, I 2 = 74%.
FIGURE 7.
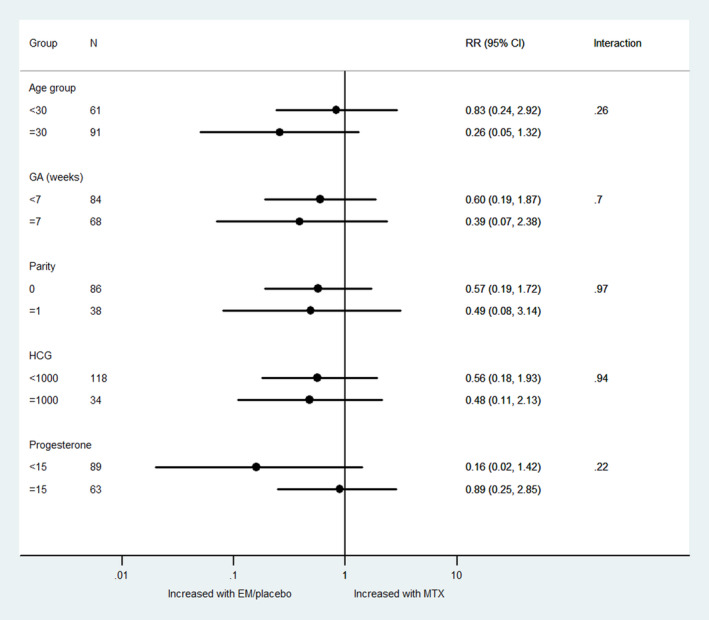
Treatment‐covariate interaction for surgical intervention. EM, expectant management; GA, gestational age; MTX, methotrexate; RR, risk ratio.
There were center differences in reported MTX specific side‐effects, with most seen in the NL study and only one side‐effect in the UK study (diarrhea in the MTX group). Therefore, we could not evaluate treatment–covariate interaction.
3.8.3. Exploratory multivariable analysis
In exploratory multivariable IPD analysis we found nulliparity, serum hCG >1000 IU/L and progesterone >15 nmol/L to be negatively associated with treatment success (Table 6). Serum hCG >1000 IU/L was the only variable found to be negatively associated with resolution time.
TABLE 6.
Multivariable analysis of factors associated with treatment success.
| Variable | OR (95% CI) | HR (95% CI) |
|---|---|---|
| MTX vs expectant | 1.93 (0.87–4.29) | 1.38 (0.93–2.06) |
| Study: NL vs UK | 0.51 (0.21–1.21) | 0.87 (0.57–1.31) |
| Age per year | 1.03 (0.97–1.11) | 1.01 (0.98–1.04) |
| GA per week | 1.02 (0.96–1.10) | 1.00 (0.94–1.07) |
| Parity | 1.75 (1.04–2.93) | 1.10 (0.91–1.33) |
| hCG | ||
| >1000 vs ≤1000 | 0.28 (0.12–0.66) | 0.50 (0.29–0.84) |
| Progesterone | ||
| >15 vs ≤15 | 0.44 (0.20–0.99) | 0.68 (0.46–1.02) |
Abbreviations: GA, gestational age; hCG, human chorionic gonadotrophin; HR, hazard ratio; MTX, methotrexate; NL, the Netherlands; OR, odds ratio; UK, United Kingdom.
Those who had surgical intervention were too small a group for an exploratory multivariable analysis.
4. DISCUSSION
Our IPD‐MA findings did not show a significant difference in treatment success between MTX and expectant management of tubal EP in clinically stable women with hCG <2000 IU/L. There was no covariate interaction or a difference in treatment success according to maternal age, gestational age, parity, baseline serum hCG or progesterone. In an exploratory multivariable analysis, a serum hCG ≤1000 IU/L and progesterone ≤15 nmol/L were associated with a higher success rate in both groups. Given no significant difference between MTX and expectant management in these women, this encourages shared decision‐making between the clinician and patient, factoring in the latter's voice, values and preferences.
Our study is the first to provide an IPD‐MA of MTX vs expectant management of tubal EP, obtaining all IPD from eligible RCTs. We observed robust and recommended methodology, assessed the risk of bias of included trials, graded quality of evidence and strengthened clinical data of interest to inform future practice.
Our main limitation is that there were only two trials of limited sample size. However, these are the only eligible trials available, highlighting the paucity of data. One trial did not have a placebo comparison, introducing performance, bias as surgical intervention could be more readily applied to women with mild clinical symptoms in the expectant group and participants were more likely to report side‐effects in the MTX group. There were also selection criteria differences in that the NL study had a higher proportion of PUL, presumed to be tubal EP due to plateauing serum hCG levels, and the UK study had positively identified all tubal EP on ultrasound. When analyzing only tubal EP seen on ultrasound scan we still did not find a significant difference between the two groups, although this was a smaller sample. The selection criteria difference could explain why the surgical intervention rate was higher in the UK study than the NL study, as studies have shown that the majority of PULs resolve spontaneously without intervention. 18 Another explanation for surgical intervention difference between the studies could be the use of additional MTX in the NL study only. This could have increased the pharmacological effectiveness of MTX or allowed more time for the EP to resolve. As there was no option to administer additional doses of MTX in the UK study, clinicians were compelled to offer surgical intervention to women with rising hCG.
Our findings corroborate an aggregate meta‐analysis that did not find evidence in differences in resolution, need for surgery or time to resolution between expectant and medical management of EP. 19 An RCT of 23 participants also found no difference between single dose MTX and placebo (saline solution), with a treatment success of 90% vs 92.3%, respectively. 10 The higher treatment success rates in this study could be due to inclusion of participants who already had declining hCG levels introducing bias into treatment effectiveness. The success rate of expectant management in our study was similar to retrospective studies which where success rates ranged between 63% and 75%. 6 , 8 , 20 , 21 , 22 , 23
Our study showed that an hCG level <1000 mIU/mL was associated with higher success rates and shorter resolution time in both groups. Several studies on MTX noted a higher treatment success with lower hCG levels. 20 , 24 , 25 , 26 , 27 Similarly, studies on expectant management favor a lower hCG for successful resolution6, 14, 22, 23, 28 with the subgroup of women with initial hCG <1500 IU/L having a 77% success rate. 8 Our mean time to resolution of 19.7 days with MTX and 21.2 days with expectant management was similar to that of other studies. 10 , 23 , 29
Our study showed that women with progesterone ≤15 nmol/L were more likely to have treatment success than those with progesterone >15 nmol/L. Several papers have attempted to determine the influence of progesterone levels on the resolution of EP. One study found that all women with a progesterone <32 nmol/L were successfully treated with a single dose of MTX compared with 45% when progesterone >32 nmol/L. 30 Another found a 97% positive predictive value for successful resolution with single dose MTX if progesterone <22 nmol/L. 31 Elson et al. identified a 90% success rate for expectant management if progesterone <10 nmol/L with hCG <1500 IU/L. 8
MTX has long been used for treatment for EP in different regimens and administration routes. 16 However, it has a strong dose‐related potential for toxicity and adverse effects include stomatitis, conjunctivitis, photosensitivity, bone marrow suppression, pulmonary fibrosis, liver cirrhosis, renal failure and gastric ulceration. Women are also advised to avoid conceiving 3 months after administration. 32 Its effectiveness ranges between 65% and 95%33, 34, 35, 36, 37 with studies comparing different MTX regimens to each other rather than to a control. The variation in effectiveness is also due to differences in selection criteria, diagnosis of tubal EP and definitions of treatment success. In studies where tubal EP is not visualized on ultrasound scan, one must be cautious caution not to use MTX to treat miscarriages or normally sited live pregnancies. 38 , 39 There could be scope for use in ectopic pregnancies with higher hCG. 27 , 40 , 41 , 42 A recent multi‐center RCT compared MTX and Gefitinib with MTX and placebo to treat tubal EP with hCG 1000–5000 IU/L. Although there was no difference in need for surgical intervention between the two treatments, resolution of tubal EP was 70% vs 71%, respectively. 39
Our IPD‐MA highlights the need to standardize outcome definitions and reporting to compare studies more effectively. The recent development of a core outcome set for treatment of ectopic pregnancies for future investigators will enable this. 43 All future studies should state clear diagnostic criteria for an EP to eliminate uncertainty as to what condition is being treated, whether a true tubal EP or a PUL, the latter of which could be a miscarriage or live normally sited pregnancy too small to visualize on ultrasound scan. Advances in ultrasound have enabled accurate detection of tubal EP with studies demonstrating high sensitivity, specificity and positive predictive values2, 44, 45, 46 and we should therefore define tubal EP as a scan diagnosis. Further high‐quality RCTs are needed, in particular to determine whether MTX reduces surgical intervention and need for blood transfusion. We should also assess the effectiveness of MTX in hCG between 1000 and 5000 IU/L to identify subgroups that may benefit.
5. CONCLUSION
Our study did not identify a significant difference in treatment success, need for surgical intervention or tubal EP resolution time between expectant and medical management of clinically stable tubal EP with low hCG. Serum hCG <1000 IU/L and progesterone <15 nmol/L were associated with a higher success rate in both groups. However, only two trials were eligible for inclusion, and we therefore propose the need for more well‐designed RCTs to better determine who would benefit from either treatment. Our result of no significant difference encourages shared decision‐making between the clinician and patient. At present, expectant management should be offered as the preferred initial strategy for clinically stable women with tubal EP presenting with low hCG levels, particularly if <1000 IU/L. This can have positive implications for policy makers, service providers and service users in reducing cost, reducing adverse effects and offering patients a wider choice of treatment options. The American College of Obstetricians and Gynecologists, Royal College of Obstetricians and Gynaecologists and National Institute for Health and Care Excellence guidelines on EP have already endorsed the use expectant management of EP when clinically safe. 47 , 48 , 49
AUTHOR CONTRIBUTIONS
SAS conducted the systematic review, risk of bias assessment, obtained Data Sharing contracts, collated the data, interpreted the data and drafted the article. MVW was responsible for the methodology of the study, conducted the systematic review, risk of bias assessment, analysis and interpretation of data and revised the article. NVM provided IPD for the NL study, supervised the study and revised the article. BWM was responsible for the conception of the study, conducted risk of bias of assessment, supervised the study and revised the article. JAR was a researcher in the UK study and secured a data‐sharing agreement with King's College Hospital. DJ was overall supervisor of the study, provided IPD for the UK study, contributed to interpretation of data and revised the article critically for intellectual content. All authors approved the final version.
CONFLICT OF INTEREST STATEMENT
BWM is supported by a NHMRC Investigatorgrant (GNT1176437). BWM reports consultancy for ObsEva and Merck and travel support from Merck. The other authors do not have any conflict of interests to declare.
Supporting information
Figure S1
Appendix S1
Appendix S2
ACKNOWLEDGMENTS
The authors thank Amsterdam University Medical Center, Amsterdam, Netherlands, King's College Hospital, London, UK and the authors of the original studies for their collaboration and provision of IPD.
Solangon SA, Van Wely M, Van Mello N, Mol BW, Ross JA, Jurkovic D. Methotrexate vs expectant management for treatment of tubal ectopic pregnancy: An individual participant data meta‐analysis. Acta Obstet Gynecol Scand. 2023;102:1159‐1175. doi: 10.1111/aogs.14617
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
REFERENCES
- 1. O'Herlihy C. Centre for Maternal and Child Enquiries (CMACE). Deaths in early pregnancy. Saving mother's lives: reviewing maternal deaths to make motherhood safer: 2006‐2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG. 2011;118(Suppl.1):81‐84. [DOI] [PubMed] [Google Scholar]
- 2. Dooley WM, Chaggar P, De Braud LV, Bottomley C, Jauniaux E, Jurkovic D. Effect of morphological type of extrauterine ectopic pregnancy on accuracy of preoperative ultrasound diagnosis. Ultrasound Obstet Gynecol. 2019;54:538‐544. [DOI] [PubMed] [Google Scholar]
- 3. Rodi IA, Sauer MV, Gorrill MJ, et al. The medical treatment of unruptured ectopic pregnancy with methotrexate and citrovorum rescue: preliminary experience. Fertil Steril. 1986;46:811‐813. [DOI] [PubMed] [Google Scholar]
- 4. Sauer MV, Gorrill MJ, Rodi IA, et al. Nonsurgical management of unruptured ectopic pregnancy: an extended clinical trial. Fertil Steril. 1987;48:752‐755. [PubMed] [Google Scholar]
- 5. Cacciatore B, Korhonen J, Stenman UH, Ylostalo P. Transvaginal sonography and serum hCG in monitoring of presumed ectopic pregnancies selected for expectant management. Ultrasound Obstet Gynecol. 1995;5:297‐300. [DOI] [PubMed] [Google Scholar]
- 6. Trio D, Strobelt N, Picciolo C, Lapinski RH, Ghidini A. Prognostic factors for successful expectant management of ectopic pregnancy. Fertil Steril. 1995;63:469‐472. [DOI] [PubMed] [Google Scholar]
- 7. Banerjee S, Aslam N, Woelfer B, Lawrence A, Elson J, Jurkovic D. Expectant management of early pregnancies of unknown location: a prospective evaluation of methods to predict spontaneous resolution of pregnancy. Br J Obstet Gynecol. 2001;108:158‐163. [DOI] [PubMed] [Google Scholar]
- 8. Elson J, Tailor A, Banerjee S, Salim R, Hillaby K, Jurkovic D. Expectant management of tubal ectopic pregnancy: prediction of successful outcome using decision tree analysis. Ultrasound Obstet Gynecol. 2004;23:552‐556. [DOI] [PubMed] [Google Scholar]
- 9. Van Mello N, Mol F, Verhoeve H, et al. Methotrexate or expectant management in women with an ectopic pregnancy or pregnancy of unknown location and low serum hCG concentrations? A randomized comparison. Hum Reprod. 2012;28:60‐67. [DOI] [PubMed] [Google Scholar]
- 10. Silva P, Araujo Junior E, Cecchino G, Elito Junior J, Camano L. Effectiveness of expectant management versus methotrexate in tubal ectopic pregnancy: a double‐blind randomized trial. Arch Gynecol Obstet. 2015;291:939‐943. [DOI] [PubMed] [Google Scholar]
- 11. Jurkovic D, Memtsa M, Sawyer E, et al. Single‐dose systemic methotrexate vs expectant management for treatment of tubal ectopic pregnancy: a placebo‐controlled randomized trial. Ultrasound Obstet Gynecol. 2017;49:171‐176. [DOI] [PubMed] [Google Scholar]
- 12. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Riley RD, Debray TPA, Fisher D, et al. Individual participant data meta‐analysis to examine interactions between treatment effect and participant‐level covariates: statistical recommendations for conduct and planning. Stat Med. 2020;39:2115‐2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Korhonen J, Stenman UH, Ylöstalo P. Low‐dose oral methotrexate with expectant management of ectopic pregnancy. Obstet Gynecol. 1996;88(5):775‐778. [DOI] [PubMed] [Google Scholar]
- 15. Casikar I, Lu C, Reid S, et al. Methotrexate vs placebo in early tubal ectopic pregnancy: a multi‐ Centre double‐blind randomised trial. Rev Recent Clin Trials. 2012;7:238‐243. [DOI] [PubMed] [Google Scholar]
- 16. Hajenius J, Mol F, Mol B, Bossuyt P, Ankum W, van der Veen F. Interventions for tubal ectopic pregnancy. Cochrane Database Syst Rev. 2007;1:CD000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barnhart KT, Hansen KR, Stephenson MD, et al. Effect of an active vs expectant management strategy on successful resolution of pregnancy among patients with a persisting pregnancy of unknown location: the ACT or NOT randomized clinical trial. Jama. 2021;326:390‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kirk E, Condous G, Van Calster B, Van Huffel S, Timmerman D, Bourne T. Rationalizing the follow‐up of pregnancies of unknown location. Hum Reprod. 2007;22:1744‐1750. [DOI] [PubMed] [Google Scholar]
- 19. Colombo GE, Leonardi M, Armour M, et al. Efficacy and safety of expectant management in the treatment of tubal ectopic pregnancy: a systematic review and meta‐analysis. Hum Reprod Open. 2020;4:hoaa044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ylöstalo P, Cacciatore B, Sjöberg J, Kääriäinen M, Tenhunen A, Stenman UH. Expectant management of ectopic pregnancy. Obstet Gynecol. 1992;80:345‐348. [PubMed] [Google Scholar]
- 21. Nowak‐Markwitz E, Michalak M, Olejnik M, Spaczynski M. Cutoff value of human chorionic gonadotropin in relation to the number of methotrexate cycles in the successful treatment of ectopic pregnancy. Fertil Steril. 2009;92:1203‐1207. [DOI] [PubMed] [Google Scholar]
- 22. Kirk E, Van Calster B, Condous G, et al. Ectopic pregnancy: using the hCG ratio to select women for expectant or medical management. Acta Obstet Gynecol Scand. 2011;90:264‐272. [DOI] [PubMed] [Google Scholar]
- 23. Mavrelos D, Memtsa M, Helmy S, Derdelis G, Jauniaux E, Jurkovic D. β‐hCG resolution times during expectant management of tubal ectopic pregnancies. BMC Womens Health. 2015;15:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Helmy S, Bader Y, Pablik E, et al. Cut‐off value of initial serum β‐hCG level predicting a successful MTX therapy in tubal ectopic pregnancy: a retrospective cohort study. Eur J Obstet Gynecol Reprod Biol. 2014;179:175‐180. [DOI] [PubMed] [Google Scholar]
- 25. Cohen A, Bibi G, Almog B, Tsafrir Z, Levin I. Second‐dose methotrexate in ectopic pregnancies: the role of beta human chorionic gonadotropin. Fertil Steril. 2014;102:1646‐1649. [DOI] [PubMed] [Google Scholar]
- 26. Hakim H, Yaich R, Halouani S, Jouou S, Arfaoui R, Rachdi R. Non‐surgical Management of Ectopic Pregnancies. Austin J Womens Health. 2019;6:1035. [Google Scholar]
- 27. Zhang J, Zhang Y, Gan L, Liu XY, Du SP. Predictors and clinical features of methotrexate therapy for ectopic pregnancy. BMC Pregnancy Childbirth. 2020;20:654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dooley W, De Braud L, Memtsa M, Thanatsis N, Jauniaux E, Jurkovic D. Physical resolution of tubal ectopic pregnancy on ultrasound imaging following successful expectant management. Reprod Biomed Online. 2020;40:880‐886. [DOI] [PubMed] [Google Scholar]
- 29. Helmy S, Mavrelos D, Sawyer E, et al. Serum human chorionic gonadotropin (β‐ hCG) clearance curves in women with successfully expectantly managed tubal ectopic pregnancies: a retrospective cohort study. PLoS One. 2015;10:e0130598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ransom MX, Garcia AJ, Bohrer M, Corsan GH, Kemmann E. Serum progesterone as a predictor of methotrexate success in the treatment of ectopic pregnancy. Obstet Gynecol. 1994;83:1033‐1037. [DOI] [PubMed] [Google Scholar]
- 31. Corsan GH, Karacan M, Qasim S, Bohrer MK, Ransom MX, Kemmann E. Identification of hormonal parameters for successful systemic single‐dose methotrexate therapy in ectopic pregnancy. Hum Reprod. 1995;10:2719‐2722. [DOI] [PubMed] [Google Scholar]
- 32. Lloyd ME, Carr M, McElhatton P, Hall GM, Hughes RA. The effects of methotrexate on pregnancy, fertility and lactation. Quart J Med. 1999;92:551‐563. [DOI] [PubMed] [Google Scholar]
- 33. Saraj AJ, Wilcox JG, Najmabadi S, Stein S, Johnson MB, Paulson RJ. Resolution of hormonal markers of ectopic gestation: a randomized trial comparing single‐dose intramuscular methotrexate with salpingostomy. Obstet Gynecol. 1998;92:989‐994. [DOI] [PubMed] [Google Scholar]
- 34. Sowter MC, Farquhar CM, Petrie KJ, Gudex G. A randomised trial comparing single dose systemic methotrexate and laparoscopic surgery for the treatment of unruptured tubal pregnancy. BJOG. 2001;108:192‐203. [DOI] [PubMed] [Google Scholar]
- 35. Rozenberg P, Chevret S, Camus E, et al. Medical treatment of ectopic pregnancies: a randomized clinical trial comparing methotrexate–mifepristone and methotrexate–placebo. Hum Reprod. 2003;18:1802‐1808. [DOI] [PubMed] [Google Scholar]
- 36. Alleyassin A, Khademi A, Aghahosseini M, Safdarian L, Badenoosh B, Akbari HE. Comparison of success rates in the medical management of ectopic pregnancy with single‐dose and multiple‐dose administration of methotrexate: a prospective, randomized clinical trial. Fertil Steril. 2006;85:1661‐1666. [DOI] [PubMed] [Google Scholar]
- 37. Song T, Kim MK, Kim M, Jung YW, Yun BS, Seong SJ. Single‐dose versus two‐dose administration of methotrexate for the treatment of ectopic pregnancy: a randomized controlled trial. Hum Reprod. 2016;31:332‐338. [DOI] [PubMed] [Google Scholar]
- 38. Fridman D, Hawkins E, Dar P, et al. Methotrexate administration to patients with presumed ectopic pregnancy leads to methotrexate exposure of intrauterine pregnancies. J Ultrasound Med. 2019;38:675‐684. [DOI] [PubMed] [Google Scholar]
- 39. Horne AW, Tong S, Moakes CA, et al. Combination of gefitinib and methotrexate to treat tubal ectopic pregnancy (GEM3): a multicentre, randomised, double‐blind, placebo‐controlled trial. Lancet. 2023;401:655‐663. [DOI] [PubMed] [Google Scholar]
- 40. Mol F, Mol BW, Ankum WM, van der Veen F, Hajenius PJ. Current evidence on surgery, systemic methotrexate and expectant management in the treatment of tubal ectopic pregnancy: a systematic review and meta‐analysis. Hum Reprod Update. 2008;14:309‐319. [DOI] [PubMed] [Google Scholar]
- 41. Richardson A. Medical management of ectopic pregnancy: a 10‐year case series. Hum Fertil (Camb). 2012;15:116‐120. [DOI] [PubMed] [Google Scholar]
- 42. Xiao C, Shi Q, Cheng Q, Xu J. Non‐surgical management of tubal ectopic pregnancy: a systematic review and meta‐analysis. Medicine (Baltimore). 2021;100:e27851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chong KY, Solangon S, Barnhart K, et al. A core outcome set for future research in ectopic pregnancy—an international consensus development study. Fertil Steril. 2023;119:804‐812. [DOI] [PubMed] [Google Scholar]
- 44. Kirk E, Damen A, Papageorghiou AT, et al. Why are some ectopic pregnancies characterized as pregnancies of unknown location at the initial transvaginal ultrasound examination? Acta Obstet Gynecol Scand. 2008;87:1150‐1154. [DOI] [PubMed] [Google Scholar]
- 45. Ofili‐Yebovi D, Cassik P, Lee C, Elson J, Hillaby K, Jurkovic D. The efficacy of ultrasound‐based protocol for the diagnosis of tubal ectopic pregnancy. Ultrasound Obstet Gynecol. 2003;22:5. [Google Scholar]
- 46. Condous G, Okaro E, Khalid A, et al. The accuracy of transvaginal ultrasonography for the diagnosis of ectopic pregnancy prior to surgery. Hum Reprod. 2005;20:1404‐1409. [DOI] [PubMed] [Google Scholar]
- 47. American College of Obstetricians and Gynecologists . ACOG Practice Bulletin No. 193: tubal ectopic pregnancy. Obstet Gynecol. 2018;131:e91‐e103. [DOI] [PubMed] [Google Scholar]
- 48. Elson CJ, Salim R, Potdar N, et al. Diagnosis and management of ectopic pregnancy. BJOG. 2016;123:e15‐e55. [DOI] [PubMed] [Google Scholar]
- 49. Webster K, Eadon H, Fishburn S, Kumar G, Committee G. Ectopic pregnancy and miscarriage: diagnosis and initial management: summary of updated NICE guidance. BMJ. 2019;367:l6283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Appendix S1
Appendix S2
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.


