Abstract
Focus formation in human diploid fibroblasts (HDF cells) is known to require both the simian virus 40 (SV40) large-T and small-t antigens. Similarly, both SV40 proteins were required to stimulate confluent, density-arrested HDF cells to reenter the cell cycle. This study used defective recombinant adenoviruses to examine the roles of the individual SV40 proteins in altering specific steps in the cell cycle. Small-t antigen and, to a lesser extent, large-T antigen increased the level of the S phase cyclin cyclin A but without increasing the activity of associated cyclin kinases unless the two SV40 proteins were coexpressed. The absence of kinase activity reflected the presence in density-arrested cells of high levels of the cyclin-dependent kinase inhibitors p21WAF1 and p27KIP1. We report here that expression of SV40 large-T antigen reduced levels of p21WAF1, while expression of small-t antigen was required to decrease p27KIP1. The separate effects of large-T and small-t antigens on these two inhibitors may explain the joint requirement for the two proteins to drive cell cycle reentry of HDF cells and ultimately transform these cells.
Cellular transformation by simian virus 40 (SV40) is influenced by two viral early proteins, the large-T and small-t antigens (1, 10, 16, 26). Large-T is a key transforming protein that functions through its binding of the cellular tumor suppressors p53 and pRb (13). A DnaJ-like domain is located in the amino-terminal sequences shared by large-T and small-t (11), and this also plays a role in several transformation systems and in the targeting of p107 and p130, pRb family members, for degradation (29–31, 37).
Small-t antigen is necessary for large-T to transform some cell types to anchorage-independent growth (1, 10, 16), and also for focus formation in some established rodent cells (38) and in primary human diploid fibroblasts (HDF cells) (21, 24). The ability of small-t to bind and inhibit protein phosphatase 2A (PP2A) correlates with its ability to enhance transformation by large-T in these systems. Interestingly, small-t can also complement at least two amino-terminal mutations in large-T antigen, allowing focus formation in HDF cells (21). The mechanism for this complementation is presently unknown.
In animal models, the role for small-t antigen has been most apparent in nondividing tissues. Transgenic animals or animals injected with SV40 mutant viruses that lack small-t antigen develop tumors in rapidly dividing tissues, but the absence of small-t strongly reduces the appearance of other types of tumors (2, 3). Along these lines, studies of Chinese hamster lung cells in tissue culture showed that a few cell divisions could replace the need for small-t antigen in anchorage-independent growth assays (14). Experiments like these have reinforced the basic theme that the primary role for small-t antigen in transformation is in the induction of growth of target cells and that the efficiency of transformation by large-T is increased in these cells.
Because of the apparent role for small-t in nondividing target cells and because the earliest steps in the process of cell transformation involve the stimulation of cell cycle progression, it was important to determine the exact effects of small-t or large-T expression in regulating activities that control the cell cycle in normal cells. In this study, HDF cells were used as a model of density-dependent growth arrest. We report here that both small-t and large-T antigens are required to induce cell cycle reentry and that this reflects the reduction in levels of separate cyclin-dependent kinase inhibitors (CKIs) by the viral proteins.
MATERIALS AND METHODS
Cell culture.
Fibroblasts were isolated from newborn-human foreskins by trypsin digestion to separate the dermal and epidermal layers, followed by collagenase treatment to release fibroblasts from the dermal layer. Cells were plated and grown in Dulbecco modified Eagle medium containing 10% fetal bovine serum (FBS). Helper 293 cells were grown in Dulbecco modified Eagle medium plus 10% FBS and used to grow recombinant adenoviruses as previously described (9, 21).
Western blotting.
Cells were washed twice with ice-cold phosphate-buffered saline (PBS), scraped in a volume of 1 ml PBS, and collected by centrifugation in a microcentrifuge. Cells were lysed with cold lysis buffer (50 mM Tris [pH 7.4], 200 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol [DTT], 2% glycerol, 0.5% Nonidet P-40) containing protease and phosphatase inhibitors (0.5 mM phenylmethylsulfonyl fluoride; 10 μg each of leupeptin, pepstatin, and aprotinin per ml; 1 mM NaF; and 1 mM sodium orthovanadate). Lysed cells were vortexed for 15 s and then held on ice for 10 to 15 min with periodic vortexing before removal of insoluble material by centrifugation. Protein concentrations were determined by the Bio-Rad method, and then equal amounts of total protein were loaded onto sodium dodecyl sulfate (SDS)-polyacrylamide gels (11a), separated by electrophoresis, and transferred to Immobilon membranes (Millipore) with a buffer containing 15% methanol, 0.08% SDS, and 1× Laemmli buffer (11a) lacking SDS. After incubation with appropriate primary and secondary antibodies, proteins were visualized by using enhanced chemiluminescence reagents (Pierce Chemical).
The antibodies used were as follows: for cyclin A, rabbit polyclonal immunoglobulin G anti-cyclin A H-432 (sc-751; Santa Cruz); for p27, mouse monoclonal anti-p27 (K25020; Transduction Laboratories); and for p21, mouse anti-human p21 (15091A; Pharmingen). Medium from the hybridoma cell line that produces monoclonal antibody PAb419 (6) was used to detect small-t antigen.
Immunoprecipitation kinase assays.
Cells were extracted in cold lysis buffer, and then 100 to 200 μg of each extract was incubated with 15 μg of anti-cyclin A. Immunoprecipitates were collected on prewashed protein A/G agarose beads (Santa Cruz) and washed twice in lysis buffer and twice in kinase buffer (50 mM HEPES [pH 7.4], 10 mM MgCl2, 10 mM MnCl2, 1 mM DTT). Immunoprecipitates were resuspended in 40 μl of kinase buffer containing 1 μg of histone H1 and 15 μCi of [γ-32P]ATP (3,000 Ci/mmol; Amersham) and then incubated at 37°C for 20 to 30 min. Reactions were stopped by the addition of SDS sample buffer, and then the mixtures were boiled for 5 min. Quantitation was done by densitometric analysis on a Molecular Dynamics Personal Densitometer SI or by phosphorimaging.
In vitro degradation of p27.
Subconfluent HDF cells or infected (40-h) confluent cultures were extracted by suspension of PBS-washed cells in cold distilled water, followed by vortexing and one freeze-thaw cycle. Soluble extract protein (40 μg) was then incubated with bacterially expressed and purified His6-p27 for 6 and 9 h as described previously (18). Briefly, degradation mixtures contained 10 mM Tris-HCl (pH 8.3), 10 mM MgCl2, 4 mM DTT, 2 mM ATP, 20 μM phosphocreatine, 80 μg of creatine phosphokinase per ml, and 0.1% bovine serum albumin. The plasmid that expressed recombinant p27 was provided by J. Massague (19, 20). Levels of p27 were determined by Western blot analysis.
Cell cycle analysis by flow cytometry.
Cells were removed from 6-cm-diameter tissue culture plates by trypsinization and pelleted by centrifugation for 10 min at 2,000 rpm in a tabletop IEC centrifuge. Recovered cells were lysed and stained in a solution containing 4 mM sodium citrate (pH 7.8), 100 μg of propidium iodide per ml, 0.5 mg of RNase per ml, 0.1% Triton X-100, and 3% polyethylene glycol 8000. After 20 min at 37°C, an equal volume of hypertonic salt solution (0.35 M sodium chloride, 100 μg of propidium iodide per ml, 0.1% Triton X-100, and 3% polyethylene glycol 8000) was added. Stained nuclei were held overnight at 4°C, passed through nylon filters, and then analyzed on a Becton Dickinson Flow Cytometer with Cell-Quest software.
RESULTS
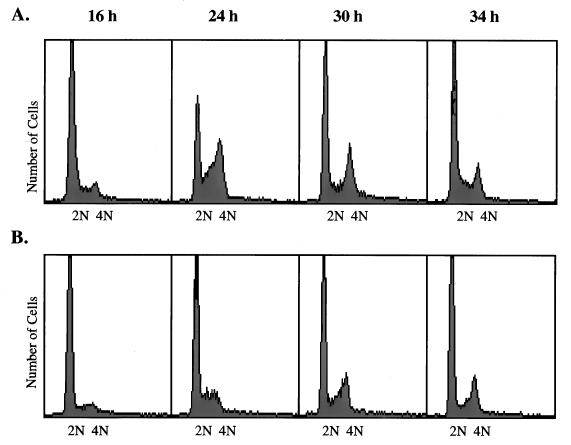
We have shown previously that focus formation by HDF cells requires both the SV40 large-T and small-t antigens (21). Focus formation is an assay in which transformed cells must overcome density arrest (contact inhibition) and overgrow cell monolayers. HDF cells are particularly susceptible to density-dependent growth arrest, and they fail to reenter the cell cycle even in response to addition of fresh medium and serum (24, 36). Figure 1 compares the cell cycle progression of serum-arrested subconfluent (Fig. 1A) and confluent (Fig. 1B) cultures of HDF cells. Readdition of serum to subconfluent cultures resulted in significant levels of S and G2/M phase cells by 24 h. In the experiment shown here, over 60% of the cells had DNA contents beyond diploid by this time. These cells divided and returned to G1 phase by 30 to 34 h after serum addition. In contrast, fewer than 15% of the confluent cells entered S or G2/M following serum addition, and these few did so at a lower rate. Entry into S or G2/M was not simply delayed in these cultures, and incubation for longer periods of time did not increase the numbers of cells that left G1.
FIG. 1.
Cell cycle progression in confluent and subconfluent HDF cells. (A) Confluent plates of HDF cells were subcultured 1:6 and then placed in serum-free medium for 48 h. Medium containing 10% FBS was added to the arrested cells, which were harvested by trypsinization 16, 24, 30, and 34 h later. Cells were collected by centrifugation, fixed, and stained for flow cytometry as described in Materials and Methods. (B) Confluent plates of HDF cells were placed in serum-free medium for 48 h and then stimulated with medium containing 10% FBS. Cells were collected and stained at the same times as shown for panel A.
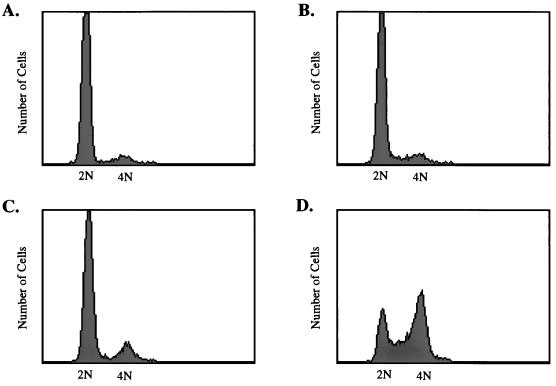
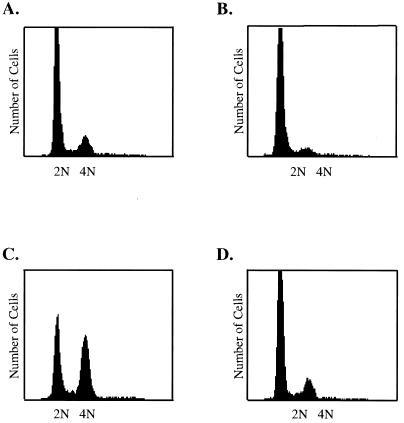
In an effort to mimic the ability of transformed cells to overgrow cell monolayers, we first asked whether both SV40 proteins were required for the induction of cell cycle progression in confluent, density-arrested HDF cells. Recombinant adenoviruses that express the individual SV40 protein were used, because these allow efficient expression of recombinant proteins by entire cultures of cells in short periods of time. These viruses, Ad-LT and Ad-st, have been used in previous studies (9, 33) and express either large-T or small-t antigen, respectively, from the cytomegalovirus (CMV) promoter inserted in place of the adenovirus E1 A/B genes. HDF cells were grown to confluence, held in medium with reduced serum for 48 h, and then infected with 20 PFU of Ad-LT or Ad-st per cell singly or in combination. As shown in Fig. 2, only cells that were coinfected with Ad-LT and Ad-st were able to progress through the cell cycle to the S and G2/M stages.
FIG. 2.
Induction of cell cycle progression by large-T plus small-t antigens. Confluent monolayers of HDF cells were placed in serum-free medium for 48 h and then infected with 20 PFU of Ad-CMV (A), Ad-LT (B), or Ad-ST (C) per cell or coinfected with 20 PFU each of Ad-ST and Ad-LT per cell (D). Medium removed from cells at the beginning of the infection was replaced after the 1-h infection period. At 32 h postinfection, cells were harvested, fixed, and stained for flow cytometry as described in Materials and Methods.
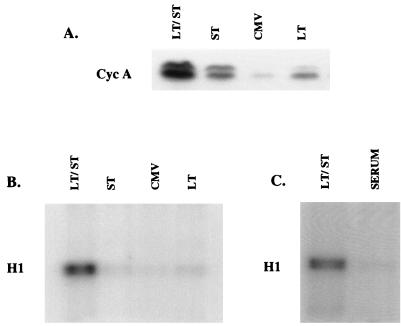
Early studies identified the cyclin A promoter as one target of the transactivating function of small-t antigen (21). Consequently, we examined the ability of the individual viral proteins to increase levels of cyclin A in confluent HDF cells. As shown in Fig. 3A, levels of cyclin A protein increased in cells infected with either Ad-LT or Ad-st, and small-t was more effective than large-T in this regard. The two proteins acted synergistically, and coinfection with Ad-LT and Ad-st led to dramatic increases in the levels of cyclin A protein. Cells at confluence expressed cyclin E, and no changes in the levels of cyclin E were found in these experiments (data not shown).
FIG. 3.
Induction of cyclin A and cyclin A-associated kinase activity by SV40 tumor antigens. Confluent monolayers of HDF cells were stimulated with medium containing 10% FBS or infected with Ad-CMV (CMV), Ad-LT (LT), Ad-ST (ST), or a combination of Ad-ST and Ad-LT (LT/ST) as described for Fig. 2. At 27 h postinfection, cells were extracted with buffer containing 0.5% Nonidet P-40 and protease and phosphatase inhibitors. (A) Equal amounts of each extract were used for Western blot analysis of cyclin A (Cyc A). (B and C) Equal portions of each extract were used for immunoprecipitation with anti-cyclin A antibodies, and then immunoprecipitated proteins were assayed for kinase activity with histone H1 and [32P]ATP as substrates.
Cyclin A-associated kinase activity was also measured by immunoprecipitation-kinase assay with histone H1 as a substrate (Fig. 3B). Coinfection with the two viruses stimulated high levels of the kinase activity. Surprisingly, when compared to control infections (Ad-CMV), small-t was unable to activate cyclin A-associated kinase activity, even though its expression led to substantially increased cyclin A levels. Ad-LT also failed to activate cyclin A-associated kinase activity, but this was less surprising given the smaller amounts of cyclin A protein present in Ad-LT-infected cells. Finally, addition of fresh serum to confluent HDF cells failed to activate cyclin A-associated kinase activity (Fig. 3C), a finding that was consistent with the inability of serum to induce cell cycle progression in these cells.
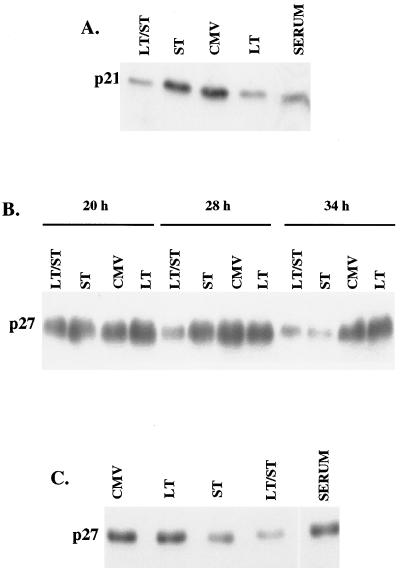
The absence of detectable cyclin A-associated kinase activity in Ad-LT- or Ad-st-infected cells, as well as in serum-stimulated cells, suggested that one or more CKIs might be present in these cells. Accordingly, extracts were tested for the presence of several CKIs, results for two of which are shown here. The first, p21WAF1, also known as p21CIP1, is known to be present in confluent cells and is particularly responsive to transcriptional induction by p53 (4, 5, 7, 35). As shown in Fig. 4A, p21 was present at high levels in confluent HDF cells, and levels of this inhibitor were reduced when cells were infected for 28 h with the Ad-LT, but not the Ad-st, virus. Serum addition was also able to decrease p21 levels.
FIG. 4.
Effects of large-T and small-t antigens on cyclin inhibitors p21WAF1 and p27KIP1. Confluent monolayers of HDF cells were serum stimulated or infected as described for Fig. 2 and 3. The experiments shown in the three panels were performed at different times. (A) Cells were extracted at 27 h postinfection, and then 50 μg of protein from each extract was used for Western blot analysis of the CKI p21WAF1. (B) Cells were extracted at 20, 28, and 34 h postinfection, and then extracts were analyzed for p27KIP1 as for panel A. (C) Cells were treated with serum or infected for 32 h and then were extracted for p27KIP1 analysis.
The second CKI studied was p27KIP1, an inhibitor that is particularly important in contact inhibition (19). Levels of p27 were high in confluent cells and decreased only in Ad-st- or doubly infected cultures (Fig. 4B and C). Expression of small-t alone was sufficient to decrease levels of p27, but coexpression of both large-T and small-t antigens resulted in a more rapid decline in the levels of this inhibitor (Fig. 4B).
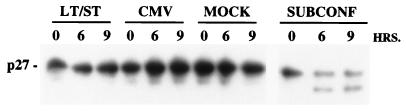
In an effort to determine the mechanism through which small-t antigen reduced p27 levels, degradation of His6-tagged p27 was monitored in vitro. One of the best-known mechanisms for regulating p27 levels occurs at the level of protein stability. As reported by others (18), extracts from subconfluent, cycling cells promoted the degradation of the recombinant p27 molecule (Fig. 5). A prominent degradation fragment was apparent after incubation for 6 or 9 h, and the total level of p27 was reduced. Extracts from density-arrested cells had no effect on p27 levels in the same time periods. Under the conditions used here, small-t did not appear to promote the degradation of p27, at least in vitro.
FIG. 5.
Failure of infected-cell extracts to degrade p27 in vitro. Confluent cultures of HDF cells were infected with Ad-LT plus Ad-ST for 40 h, and then cell extracts made with water were used to assay cell-free degradation of recombinant p27. Negative controls in this experiment were Ad-CMV-infected or uninfected (MOCK) confluent cultures, while extracts of subconfluent cells (SUBCONF) were used as a positive control. Levels of p27 were determined by Western blot analysis, following incubation periods of 0, 6 or 9 h under the conditions described in Materials and Methods.
In summary, both large-T expression and fresh serum addition decreased the levels of p21, while small-t antigen expression alone could trigger a decline in p27. Assuming that the joint role for large-T and small-t in cell cycle reentry reflects a requirement for reduction of the levels of both inhibitors, we predicted that the expression of small-t antigen (but not of large-T antigen) in the presence of serum should also allow cell cycle progression. This was, in fact, found to be the case (Fig. 6). When confluent cells were infected with 20 PFU of Ad-st per cell and then placed in medium containing 10% serum, significant numbers of cells left G1 and accumulated in the G2/M stage of the cell cycle (Fig. 6C). Nearly 75% of all cells could be driven into the cell cycle when cells were infected with higher multiplicities of Ad-st or with both Ad-st and Ad-LT in the presence of serum (data not shown). Cells infected with Ad-LT were completely unable to reenter the cell cycle, even in the presence of fresh serum (Fig. 6D). The failure of large-T and serum to cooperate in growth induction was of interest because we had observed previously that LT by itself was unable to cause focus formation in HDF cells, even when high serum levels were maintained throughout the 4- to 6-week assay period (data not shown). This reinforces the concept that small-t would be needed for regulation of the CKI p27 for cell growth and eventual focus formation to occur.
FIG. 6.
Induction of HDF cell cycle progression by small-t antigen in the presence of serum. Confluent monolayers of HDF cells were infected with Ad-ST or the small-t mutant virus Ad-C103S and then fed with fresh medium containing 10% FBS at the end of the infection period. Cells were harvested, fixed, and stained for fluorescence-activated cell sorter analysis at 32 h postinfection. The patterns shown are for serum-stimulated cells (A), cells infected with Ad-ST in the absence of serum (B), cells infected with Ad-ST in the presence of 10% serum (C), and cells infected with Ad-LT in the presence of 10% serum (D).
DISCUSSION
The first step in the process of transformation of growth-arrested cells is reentry into the growth cycle. The results presented here argue that, for many cells types, the combined action of CKIs may block cell cycle progression, thus requiring more than a single stimulus for a response. In the case of SV40, the joint requirement for small-t and large-T antigens may be explained by their independent actions on the inhibitors p21WAF1 and p27KIP1. In HDF cells, a system in which both viral proteins are required for focus formation, large-T antigen expression decreases p21 levels while small-t antigen expression decreases p27 levels. HDF cells are particularly sensitive to density arrest and are not readily stimulated at confluence to undergo even a single round of cell cycling. This behavior contrasts with that of many established rodent cell lines, in which density arrest occurs but is easily overridden by the addition of medium containing fresh serum. The fact that SV40 large-T antigen is often sufficient to cause focus formation in such established rodent cells may reflect this less tight regulation of growth and confluence. It may be that p27 plays a less significant role in density-dependent growth arrest of cell lines that respond both to serum and to large-T antigen alone.
The regulation of p27 levels by small-t may also play a role in other small-t-dependent systems. Small-t-dependent focus formation has also been observed in mouse 10T1/2 cells (18a, 30, 38), rat 3Y1 cells (32), and rat embryo fibroblasts (38). Several cell lines for which small-t is not required in focus formation do depend on small-t for anchorage-independent growth (1, 14, 23). It is interesting that the cell cycle arrest that occurs when normally adherent cells are placed under nonadherent conditions involves the inhibitors p21 and p27 (17). This suggests that reductions of p21 by large-T and of p27 by small-t could account for the need for both of these proteins for SV40 to induce anchorage-independent growth in relevant target cells.
The mechanism through which large-T causes a decline in p21 levels may well involve its binding of the tumor suppressor protein p53. The expression of p21 is known to reflect levels of p53 present in cells, and in fact, one of the ways in which p21 was first identified was as a p53-responsive gene (5). Formal proof of this possibility will require the study of large-T mutants with reduced or no binding to p53. Assuming that this is the case, it seems unlikely that large-T regulation of p21 levels is a key feature of the transforming activity of large-T. Binding of large-T antigen to p53 is often not required for transformation or tumorigenesis, although p53 binding plays a significant role in protecting cells from apoptosis (15, 22, 27, 30). Also, in the HDF focus formation assay, it is unlikely that large-T is required to regulate p21 protein levels, at least in the first few days of the assay, because 10% serum is present during this initial period until cells achieve confluence (21).
The mechanism through which small-t affects levels of p27 is completely unknown. Much of the regulation of p27 is believed to be at either the translational (8) or the posttranslational (18, 25) level. The half-life of p27 is reduced in subconfluent cells relative to density-arrested ones, and this can be demonstrated by using cell extracts (18). Although we were able to reproduce this finding for subconfluent cells, extracts from cells expressing small-t did not promote p27 degradation, and consequently, other mechanisms through which small-t might function will need to be explored. For example, small-t might affect the translational efficiency of the p27 message or the efficiency of its transcription, a possibility suggested by the known ability of small-t to function as a transcriptional regulator (12, 21, 34). It may also be the case that small-t will promote p27 degradation, but with reaction requirements that have not yet been duplicated in cell extracts.
The reduction of p27 levels by small-t is a novel addition to the known activities of small-t antigen that contribute to the enhancement of transformation by SV40. Earlier studies showed that the binding and inhibition of PP2A was essential in this regard (16, 21), and this is likely to reflect the activation of key cellular enzymes such as MEK, MAPK (9, 28), and the Na/H antiporter (9). It will be interesting to determine whether the reduction in p27 levels is completely separate from these other functions of small-t. The recent demonstration that the half-life of p27 was influenced by its phosphorylation (25) suggests a possible connection between PP2A inhibition and p27 stability, a potential mechanism that is currently being tested in this laboratory.
ACKNOWLEDGMENTS
This work was supported by NIH grant R01 CA21327 to K.R. We are grateful for assistance in the purchase of new laboratory equipment from funds of the Lester G. Woods Foundation, administered by the Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
REFERENCES
- 1.Bouck N, Beales N, Shenk T, Berg P, di Mayorca G. New region of the simian virus 40 genome required for efficient viral transformation. Proc Natl Acad Sci USA. 1978;75:2473–2477. doi: 10.1073/pnas.75.5.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carbone M, Lewis A M, Matthews B J, Levine A S, Dixon K. Characterization of hamster tumors induced by simian virus 40 small t deletion mutants as true histiocytic lymphomas. Cancer Res. 1989;49:1565–1571. [PubMed] [Google Scholar]
- 3.Choi Y, Lee I, Ross S R. Requirement for the simian virus 40 small t antigen in tumorigenesis in transgenic mice. Mol Cell Biol. 1988;8:3382–3390. doi: 10.1128/mcb.8.8.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dulic V, Kaufmann W K, Wilson S J, Tlsty T D, Lees E, Harper J W, Elledge S J, Reed S I. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell. 1994;76:1013–1023. doi: 10.1016/0092-8674(94)90379-4. [DOI] [PubMed] [Google Scholar]
- 5.El-Deiry W S, Tokino T, Velculescu V E, Levy D B, Parson R, Trent J M, Lin D, Mercer W E, Kinzler K W, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 6.Harlow E, Crawford L V, Pim D C, Williamson N M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981;39:861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harper J W, Adami G R, Wei N, Keyomarski K, Elledge S J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 8.Hengst L, Reed S I. Translational control of p27Kip1 accumulation during the cell cycle. Science. 1996;271:1861–1864. doi: 10.1126/science.271.5257.1861. [DOI] [PubMed] [Google Scholar]
- 9.Howe A K, Gaillard S, Bennett J S, Rundell K. Cell cycle progression in monkey cells expressing simian virus 40 small t antigen from adenovirus vectors. J Virol. 1998;72:9637–9644. doi: 10.1128/jvi.72.12.9637-9644.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jog P, Joshi B, Dhamankar V, Imperiale M J, Rutila J, Rundell K. Mutational analysis of simian virus 40 small t antigen. J Virol. 1990;64:2895–2900. doi: 10.1128/jvi.64.6.2895-2900.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelley W L, Landry S J. Chaperone power in a virus? Trends Biochem Sci. 1994;19:2767–2768. doi: 10.1016/0968-0004(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 11a.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 12.Loeken M, Bikel I, Livingston D M, Brady J. Trans-activation of RNA polymerase II and III promoters by SV40 small t antigen. Cell. 1988;55:1171–1177. doi: 10.1016/0092-8674(88)90261-9. [DOI] [PubMed] [Google Scholar]
- 13.Manfredi J J, Prives C. The transforming activity of simian virus 40 large tumor antigen. Biochim Biophys Acta. 1994;1198:65–83. doi: 10.1016/0304-419x(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 14.Martin R, Setlow V, Edwards C, Vembu D. The roles of the simian virus 40 tumor antigens in transformation of Chinese hamster lung cells. Cell. 1979;17:635–643. doi: 10.1016/0092-8674(79)90271-x. [DOI] [PubMed] [Google Scholar]
- 15.McCarthy S A, Symonds H S, Van Dyke T. Regulation of apoptosis in transgenic mice by simian virus 40 T antigen-mediated inactivation of p53. Proc Natl Acad Sci USA. 1994;91:3979–3983. doi: 10.1073/pnas.91.9.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mungre S, Enderle K, Turk B, Porras A, Wu Y-Q, Mumby M C, Rundell K. Mutations which affect the inhibition of protein phosphatase 2A by simian virus 40 small-t antigen in vitro decrease viral transformation. J Virol. 1994;68:1675–1681. doi: 10.1128/jvi.68.3.1675-1681.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orend G, Hunter T, Ruoslahti E. Cytoplasmic displacement of cyclin E-cdk2 inhibitors p21Cip1 and p27Kip1 in anchorage-independent cells. Oncogene. 1998;16:2575–2583. doi: 10.1038/sj.onc.1201791. [DOI] [PubMed] [Google Scholar]
- 18.Pagano M, Tam S W, Theodoras A M, Beer-Romero P, Del Sal G, Chau V, Yew P R, Draetta G F, Rolfe M. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269:682–685. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- 18a.Phillips, B., and K. Rundell. Unpublished data.
- 19.Polyak K, Kato J, Solomon M J, Sherr C J, Massague J, Roberts J M, Koff A. p27Kip1, a cyclin-cdk inhibitor, links transforming growth factor-β and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- 20.Polyak K, Lee M-H, Erdjument-Bromage H, Koff A, Roberts J, Tempst P, Massague J. Cloning of p27 Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994;78:59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- 21.Porras A, Bennett J, Howe A, Tokos K, Bouck N, Henglein B, Sathyamangalam S, Thimmapaya B, Rundell K. A novel simian virus 40 early-region domain mediates transactivation of the cyclin A promoter by small-t antigen and is required for transformation in small-t antigen-dependent assays. J Virol. 1996;70:6902–6908. doi: 10.1128/jvi.70.10.6902-6908.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quartin R S, Cole C N, Pipas J M, Levine A J. The amino-terminal functions of the simian virus 40 large T antigen are required to overcome wild-type p53 mediated growth arrest of cells. J Virol. 1994;68:1334–1341. doi: 10.1128/jvi.68.3.1334-1341.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubin H, Figge J, Bladon M T, Chen L B, Ellman M, Bikel I, Farrell M, Livingston D M. Role of small t antigen in the acute transforming activity of SV40. Cell. 1982;30:469–480. doi: 10.1016/0092-8674(82)90244-6. [DOI] [PubMed] [Google Scholar]
- 24.Rundell K, Gaillard S, Porras A. Small-t and large-T antigens cooperate to drive cell proliferation. Dev Biol Stand. 1998;94:127–133. [PubMed] [Google Scholar]
- 25.Sheaff R J, Groudine M, Gordon M, Roberts J M, Clurman B E. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev. 1997;11:1464–1478. doi: 10.1101/gad.11.11.1464. [DOI] [PubMed] [Google Scholar]
- 26.Sleigh M, Topp W, Hanich R, Sambrook J. Mutants of SV40 with an altered small t protein are reduced in their ability to transform cells. Cell. 1978;14:79–88. doi: 10.1016/0092-8674(78)90303-3. [DOI] [PubMed] [Google Scholar]
- 27.Sompayrac L, Danna K J. The amino-terminal 147 amino acids of SV40 large T antigen transform secondary rat embryo fibroblasts. Virology. 1991;181:412–415. doi: 10.1016/0042-6822(91)90516-e. [DOI] [PubMed] [Google Scholar]
- 28.Sontag E, Fedorov S, Kamibayashi C, Robbins D, Cobb M, Mumby M. The interaction of SV40 small t antigen with protein phosphatase 2A stimulates the Map kinase pathway and induces cell proliferation. Cell. 1993;75:887–897. doi: 10.1016/0092-8674(93)90533-v. [DOI] [PubMed] [Google Scholar]
- 29.Srinivasan A, McClellan A J, Vartikar J, Marks I, Cantalupo P, Li Y, Whyte P, Rundell K, Brodsky J L, Pipas J M. The amino-terminal transforming region of simian virus 40 large T and small t antigens functions as a J domain. Mol Cell Biol. 1997;17:4761–4773. doi: 10.1128/mcb.17.8.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srinivasan A, Peden K W C, Pipas J M. The large tumor antigen of simian virus 40 encodes at least two distinct transforming functions. J Virol. 1989;63:5459–5463. doi: 10.1128/jvi.63.12.5459-5463.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stubdal H, Zalvide J, Campbell K S, Schweitzer C, Roberts T M, DeCaprio J A. Inactivation of pRB-related proteins p130 and p107 mediated by the J domain of simian virus 40 large T antigen. Mol Cell Biol. 1997;17:4979–4990. doi: 10.1128/mcb.17.9.4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sugano S, Yamaguchi N, Shimojo H. Small t protein of simian virus 40 is required for dense focus formation in a rat cell line. J Virol. 1982;41:1073–1076. doi: 10.1128/jvi.41.3.1073-1075.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swaminathan S, Thimmapaya B. Transactivation of adenovirus E2-early promoter by E1A and E4 6/7 in the context of viral chromosome. J Mol Biol. 1996;258:736–746. doi: 10.1006/jmbi.1996.0283. [DOI] [PubMed] [Google Scholar]
- 34.Wang W-B, Bikel I, Marsilio E, Newsome D, Livingston D M. Transrepression of RNA polymerase II promoters by the simian virus 40 small t antigen. J Virol. 1994;68:6180–6187. doi: 10.1128/jvi.68.10.6180-6187.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiong Y, Hannon G J, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 36.Yoshizumi M, Hsieh C-M, Zhou F, Tsai J-C, Patterson C, Perrella M A, Lee M-E. The ATF site mediates downregulation of the cyclin A gene during contact inhibition in vascular endothelial cells. Mol Cell Biol. 1995;15:3266–3272. doi: 10.1128/mcb.15.6.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zalvide J, Stubdal H, DeCaprio J A. The J domain of simian virus 40 large T antigen is required to functionally inactivate RB family members. Mol Cell Biol. 1998;18:1408–1415. doi: 10.1128/mcb.18.3.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu J, Rice P W, Gorsch L, Abate M, Cole C N. Transformation of a continuous rat embryo fibroblast cell line requires three separate domains of simian virus 40 large T antigen. J Virol. 1992;66:2780–2791. doi: 10.1128/jvi.66.5.2780-2791.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]