Abstract
Return to sport following a corticosteroid injection is a complex decision. Multiple considerations should be taken into account, including steroid dose and formulation, involvement of the affected joint in the activity, and intensity of the activity. Research investigating the adverse effects of corticosteroid injections with early initiation of high-intensity activity is limited and has produced mixed results. Rest following injections has typically been recommended to minimize both chondrotoxic effects and systemic absorption. Based on the current research and extensive experience treating professional athletes, we recommend 1 to 2 days of rest of the affected joint or region with a progressive increase of activity following a corticosteroid injection with possible benefits including maximizing the beneficial effects of the injection and a reduced systemic effect.
Level of Evidence
Level V, expert opinion.
Systemic corticosteroids were used in the clinical setting as early as 1949 to treat patients with severe rheumatoid arthritis.1 As the anti-inflammatory effects of corticosteroids were discovered, a range of systemic, intra-articular, intrabursal, and peritendinous analogues were developed, marketed, and made available for patients.2 By the 1980s corticosteroid use in athletes became highly popular and began to be regulated in the Olympics.3 In the 2004 Olympic Games, 9.9% of all athletes competing in sports submitted abbreviated therapeutic use exemptions, a notification of use or intended use of corticosteroids, with 14.3% in the form of intra-articular injections.3 Intra-articular corticosteroid injections have a broad range of indications but are used with the general aims of reducing joint effusion, pain, and inflammation. Mitigating these symptoms may facilitate athletic participation, and, therefore, corticosteroid injections are commonly used in athletes today to treat both acute and chronic symptoms.
Corticosteroid injections may be preferred in athletes with joint complaints due to their relatively quick onset of action, ease of administration, and low level of invasiveness. However, intra-articular corticosteroid injections are not without local and systemic risk. It is thought that premature return to sport may predispose athletes to an increased risk of chondrotoxicity, steroid flare, and systemic dissemination following steroid injection, but there is a lack of data from well-designed clinical trials to support this.4, 5, 6, 7 Furthermore, there remains no consensus on how quickly one should return to activities and sport following an injection. The purpose of this expert opinion article is to review the current literature on intra-articular corticosteroid injections in athletes and provide recommendations on best use practices for these injections in an active population seeking to return to high-demand activity.
General Pharmacodynamics
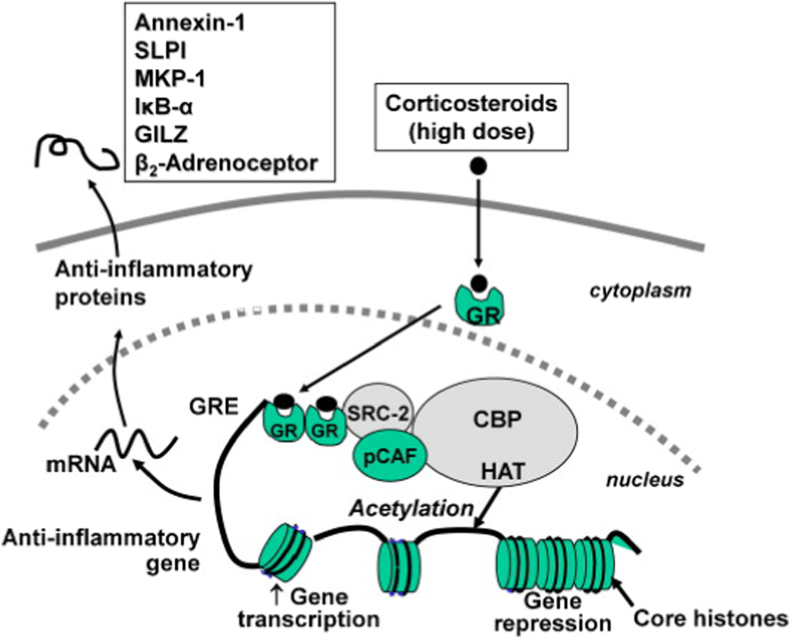
Corticosteroids can reduce pain and inflammation through a variety of mechanisms, including altering gene expression, influencing cascades, and a variety of other local and systemic actions. Gene expression, acting as one of the primary mechanisms of action, occurs after the steroid passes through cellular membranes and binds cytoplasmic steroid receptors. The corticosteroid–receptor complex migrates to the nucleus and affects gene expression by selectively suppressing pro-inflammatory and activating anti-inflammatory signaling pathways (Fig 1).8 Notable targets include mitogen-activated protein kinase phosphatase-1, β2-adrenoceptor, and secretory leukoprotease inhibitor (Fig 1).8 In addition, corticosteroids block the action of phospholipase A2, preventing the formation of arachidonic acid, a pro-inflammatory fatty acid.9 This downregulates the activity of lipoxygenase and cyclooxygenase pathways, leading to a reduction in proinflammatory molecules including leukotriene, prostacyclin, and prostaglandin.6,9,10 These effects often take hours due to the complex steps needed to alter gene expression.11,12 Corticosteroid’s nongenomic effects that influence cascades rather than gene expression can occur as soon as minutes after an injection.11,12 Some of these cascades include the mitogen-activated protein kinase, toll-like receptor 9, and epidermal growth factor receptor pathways.13 Other mechanisms include stabilizing lysosomal membranes of inflammatory cells, inhibiting vasodilation and thus limiting vascular permeability, and altering neutrophil chemotaxis and extravasation.9,14 Despite reducing pain and inflammation, the many mechanisms corticosteroids induce may also influence chondrocyte metabolism. Studies have shown that macromolecule synthesis of proteins like aggrecan, collagen, and fibronectin may be stimulated or reduced by use of corticosteroids depending on the time, dose, and composition used.15,16
Fig 1.
Reproduced with permission from Barnes.8 Glucocorticoid activation of anti-inflammatory gene expression: Glucocorticoids bind to cytoplasmic glucocorticoid receptors (GR), which translocate to the nucleus where they bind to glucocorticoid response elements (GRE) in the promoter region of steroid-sensitive genes and also directly or indirectly to coactivator molecules such as CREB-binding protein (CBP), p300/CBP activating factor (pCAF) or steroid receptor coactivator-2 (SRC-2), which have intrinsic histone acetyltransferase (HAT) activity, causing acetylation of lysines on histone H4, which leads to activation of genes encoding anti-inflammatory proteins, such as secretory leukoprotease inhibitor (SLPI), mitogen-activated kinase phosphatase-1 (MKP-1), inhibitor of NF-κB (IκB-α), and glucocorticoid-induced leucine zipper protein (GILZ).
Local Adverse Reactions
Chondrotoxicity
Although corticosteroid injections are commonly used in practice, the long-term effects they have on cartilage remain unclear. In general, the cause of chondrolysis is thought to be multifactorial, including mechanical, chemical, and thermal contributors.17,18 One article reviewed the effects on cartilage in both in vitro and in vivo studies of the most common compositions of corticosteroid injections including hydrocortisone, methylprednisolone, dexamethasone, betamethasone, prednisolone, and triamcinolone.15 While results varied among compounds, overall, the basic science in vitro literature suggests that the deleterious effects on articular cartilage are both time- and dose-dependent.15 Beneficial effects, including increased cell growth and enhanced damage recovery, were seen at low doses and short durations (<1-3 mg/dose or 8-12 mg/cumulative total dose).15 At high doses and longer culture durations, gross cartilage damage and chondrotoxicity predominated (>3 mg/dose or 18-24 mg/cumulative total dose).15 In contrast, a clinical study found that after an intra-articular injection of triamcinolone acetonide (40 mg) every 3 months for 2 years in the affected knee, there was no effect on joint space narrowing or other radiographic parameters, deeming the injections safe.19 There is limited data as to which steroid composition produces the least chondrotoxicity. Composition use is commonly based on availability, cost, and physician preference.
Another area of concern, especially for athletes, is whether physical activity compounds the potentially detrimental effects on cartilage. One animal study involving rats found daily running after 3 weekly knee intra-articular hydrocortisone injections (1.25 mg/kg dose) resulted in a greater rate of subsequent degenerative cartilage lesions than either running or hydrocortisone injections alone.4 They found lesions in femoral articular cartilage were greater in size and number in the injection and running group after the 6-week study period.4 These results suggest that exercise following corticosteroid injections may result in damage to weight-bearing zones of femoral articular cartilage in rats.4 It is unknown whether similar effects would be seen in non–weight-bearing joints such as the shoulder or elbow. Although there is mixed evidence, animal models suggest that it may be wise to avoid immediate exercise following an intra-articular corticosteroid injection, but there is insufficient clinical evidence to provide specific recommendations as to the timing of the resumption of impact activities following an injection and no clinical reports exist related to immediate deleterious cartilage damage following isolated corticosteroid injections.
Another aspect that must also be considered is the use of an anesthetic agent in conjunction with a corticosteroid injection.17 Frequently, anesthetic agents such as lidocaine, Marcaine, bupivacaine, or ropivacaine are included with the corticosteroid injection to help with local pain control. However, many in vitro and limited in vivo studies have demonstrated that these may also have short-term, time- and dose-dependent deleterious effects on cartilage.18,20 A review of these agents concluded bupivacaine to be the most toxic of agents at concentrations of 0.5% or greater and ropivacaine to be least toxic at concentrations of 0.75% or lower.18 Limiting the volume and dose of anesthetics used jointly with corticosteroid injections may be beneficial to further reduce deleterious effects on cartilage, but evidence is limited on how these affect cartilage long-term.
Steroid Flare
One of the most common side effects of corticosteroid injections is a postinjection reaction, colloquially known as “steroid flare.” Flare reactions are described as discomfort and pain at the injection site and within the affected joint.21 In most cases, the flare occurs within 24 hours of injection, but an additional 10% of affected patients experience it 24 to 48 hours after.22 Studies have shown the occurrence of patients experiencing a flare reaction typically lies between 1% and 10% and depends on the type of compound, dosage, and route of administration used.21, 22, 23 Although the exact etiology of the flare has not been established, it is thought to be caused by an inflammatory reaction to the trauma of the needle puncture or a transient reaction to the corticosteroid crystals themselves.21 The insoluble steroid crystals form on the synovial membrane, leading to a macrophage-mediated inflammatory response.21 The inflammatory response, known as the steroid flare, causes surplus synovial fluid release from the synovium and may result in a temporary increase in a patient’s pain and swelling. For the athlete, return to sport following the injection should have limited effect on the chance of a flare occurrence as it is thought to be due to the mechanism of or the injection itself. To date, there is no known correlation between the risk of flare reaction and post-injection activity level.
Infection
Although rare, all injections carry a low risk of infection. The most common association with intra-articular injections is septic arthritis with an incidence rate of 2 to 10 cases per 100,000.24 This risk can be minimized, although not entirely mitigated, with adherence to strict aseptic technique when handling, preparing, and administering the injection. Superficial infection or hypersensitivity reactions are also extremely rare, but possible. Characteristics of hypersensitivity reactions or allergies may include hypotension, shortness of breath, hives, and cyanosis.25 Since infection is typically caused by direct inoculation of the bacteria during injection or the injection composition itself, there is no evidence to suggest that immediate, non-contact activity has any impact on infection rate.
Additional Adverse Reactions
Unrelated to activity level, other adverse side effects of intra-articular corticosteroid injections have been reported. Some of these include adrenal suppression, subcutaneous fat atrophy, skin discoloration, insulin tolerance, and hyperglycemia.6,22,23 The effects are often mild and transient, lasting for about 2 to 7 days. Their occurrence is unrelated to activity level and varies between patients, having more dependence on the agent and dosage used, in addition to the patient’s baseline control of diabetes if applicable.
Systemic Absorption
For a corticosteroid injection to be effective, it must bind to target receptors in the joint. This ability may be impacted by the solubility and molecular weight of the steroid as well as post-injection exercise. High-solubility and low-molecular-weight steroids have increased systemic absorption, will spend less time in the joint binding receptors, and will have decreased duration of effects due to this. Therefore, a low-solubility, high molecular weight steroid, such as triamcinolone, betamethasone, or methylprednisolone acetate, is preferred for intra-articular injections to ensure longer-lasting results.6,9,23 Exercising vigorously increases heart rate, promoting blood flow and the circulation of fluid. Blood flow to the synovium and joint brings nutrients and oxygen to the synovial membrane and cartilage and facilitates the dissemination of the injected steroids systemically. Flexion, particularly at the knee, is common in athletic activity and exercise. Knee flexion raises the intra-articular fluid pressure from a few centimeters H2O below atmospheric pressure during extension to 2 to 5 cm H2O above.7 This drives the fluid and associated steroids out of the joint cavity into the permeable synovial lining.7 Because of the potential for exercise and knee motion to release steroids from the joint to the systemic bloodstream, it is thought that immediate exercise should be avoided postinjection to ensure the steroid molecules remain in the joint and bind target receptors for longer periods of time. These effects extend to professional athletes undergoing high-intensity exercise. A delay in return to sport after an injection may be beneficial in allowing the injected corticosteroid to act on the inflamed tissue for elongated periods. However, there is no strong clinical evidence supporting these claims.
Current Literature on Athletes
Current literature tends to support waiting 1 to 2 days after an injection to return to activities and sport, but strong clinical evidence is lacking. Relative rest of the involved area after an injection has been found to be beneficial and is often recommended among physicians.4,26,27 Although none of these studies have been focused on professional athletes in particular, one study following patients after a single intra-articular knee injection of triamcinolone hexacetonide (40 mg) and 2 mL of lidocaine found that although there was no difference at 3 weeks between a non-rest cohort and 24 hours of rest cohort, from 12 to 25 weeks the rested cohort had a greater degree of improvement in pain, stiffness, knee circumference, walking time, and C-reactive protein measures than the non-rested group.27 However, another study reported no benefits for rest at 48 hours and at 10 months postinjection when measuring pain/tenderness, joint swelling, range of motion, and an overall status questionnaire.28 It is possible that the difference in study findings is due to variability in injection type and the different time points evaluated.23
When focusing on professional and high-level athletes, the International Olympic Committee Medical Commission, the Union Cycliste Internationale, and World Anti-Doping Agency (WADA) do not allow injections during competition but do so primarily from an anti-doping standpoint.3,29, 30, 31 Glucocorticoids fall on WADA’s list of “prohibited in competition” substances meaning they are prohibited during the period before midnight on the day before competition until the end of the competition and the sample collection process.29 While both the International Olympic Committee and Union Cycliste Internationale follows guidelines from WADA, the Union Cycliste Internationale additionally requires 8 days of rest and no competition for the rider following a glucocorticoid injection.30, 31, 32
Return to Sport Progression
When returning to sport, it is important to recognize that there is a spectrum of physical activity that can occur and that the injection site and pathology treated may dictate the timing of these activities. Ardern et al.33 in the World Congress in Sports Physical Therapy explored the 3 different stages of performance. Return to participation entails a modified or lower level than the original level of sport. Return to sport entails a return to baseline, but not participating at the patient's desired level and return to performance entails performing at least at preinjury levels. The goal of receiving a corticosteroid injection is to reduce symptoms allowing a return to play at a high level. When planning a return to performance, all stakeholders must be active participants in the process: the athlete, physician, and sports medicine staff. The StARRT (i.e., Strategic Assessment of Risk and Risk Tolerance) Framework looks at 3 steps to consider: assessment of health risk, assessment of activity risk, and assessment of risk tolerance.34 The health risk assesses current tissue health and reaction to the injection. Activity risk looks at the sport involved and how it involves the location of where the injection was performed, and risk tolerance considers timing concerns related to the season versus championship play, for example. These 3 factors are considered by all stakeholders for a successful return to performance. Although there is limited evidence for specific guidance, it is recommended to follow a progressive increase in activity after injection. The first 24 hours after injection should consist of relative rest to allow for sufficient medication absorption and monitoring of any adverse reactions. If for example, the site of the injection is not engaged during sport, then modifications of this timing constraint are acceptable. For lower-extremity injections, 24 to 48 hours after the injection, progressive load-bearing activities can occur such as general cardiorespiratory exercise on a bicycle/elliptical or bodyweight exercises. Advancing to full activities generally occurs as tolerated depending upon symptom resolution imparted from the effects of the injection.35
Recommendations
The senior author (B.J.C.) has extensive experience with treating active patients, including elite and professional athletes. Taking into consideration personal experience and the findings discussed in this article, the senior author’s preference, when possible, is for athletes of all levels to engage in relative rest of the injected region for 1 to 2 days and then return to sport with a progressive increase of activity after intra-articular injection. The benefits of rest following an injection may include maximizing the clinical benefits and a reduction in the systemic absorption of the corticosteroid, thereby maintaining a more local intra-articular effect. Furthermore, consideration of which and how much local anesthetic is to be used in conjunction with the corticosteroid is important to further reduce the risk of chondrotoxicity. However, as data remain limited, it is important that decisions on whether or not to pursue a corticosteroid injection, with or without a local anesthetic, and how long an athlete should rest after an injection are made jointly and on an individual basis with the physician, athlete, and relevant members of their team. Notably, in high stake decision-making related to the duration an athlete is to remain out of competition following a corticosteroid injection, consensual decision-making weighing the risks and benefits to an expedited return to high-level activities remains in the purview of the physician–patient relationship.
Footnotes
The authors report no conflicts of interest in the authorship and publication of this article. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
References
- 1.Hench P.S., Kendall E.C., Slocumb C.H., Polley H.F. Adrenocortical hormone in arthritis. Ann Rheum Dis. 1949;8:97–104. doi: 10.1136/ard.8.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nichols A.W. Complications associated with the use of corticosteroids in the treatment of athletic injuries. Clin J Sport Med. 2005;15:370–375. doi: 10.1097/01.jsm.0000179233.17885.18. [DOI] [PubMed] [Google Scholar]
- 3.Fitch K. Glucocorticoids at the Olympic Games: State-of-the-art review. Br J Sports Med. 2016;50:1267. doi: 10.1136/bjsports-2016-096664. [DOI] [PubMed] [Google Scholar]
- 4.Gogia P.P., Brown M., Al-Obaidi S. Hydrocortisone and exercise effects on articular cartilage in rats. Arch Phys Med Rehabil. 1993;74:163–467. doi: 10.1016/0003-9993(93)90105-j. [DOI] [PubMed] [Google Scholar]
- 5.Fadale P.D., Wiggins M.E. Corticosteroid injections: Their use and abuse. J Am Acad Orthop Surg. 1994;2:133–140. doi: 10.5435/00124635-199405000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Olafsen N.P., Herring S.A., Orchard J.W. Injectable corticosteroids in sport. Clin J Sport Med. 2018;28:451–456. doi: 10.1097/JSM.0000000000000603. [DOI] [PubMed] [Google Scholar]
- 7.Lu Y., Levick J.R., Wang W. The mechanism of synovial fluid retention in pressurized joint cavities. Microcirculation. 2005;12:581–595. doi: 10.1080/10739680500253527. [DOI] [PubMed] [Google Scholar]
- 8.Barnes P.J. Mechanisms and resistance in glucocorticoid control of inflammation. J Steroid Biochem Mol Biol. 2010;120:76–85. doi: 10.1016/j.jsbmb.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 9.Bedi A., Trinh T.Q., Olszewski A.M., Maerz T., Ramme A.J. nonbiologic injections in sports medicine. JBJS Rev. 2020;8 doi: 10.2106/JBJS.RVW.19.00052. [DOI] [PubMed] [Google Scholar]
- 10.Jelsema T.R., Tam A.C., Moeller J.L. Injectable ketorolac and corticosteroid use in athletes: A systematic review. Sports Health. 2020;12:521–527. doi: 10.1177/1941738120946008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stellato C. Post-transcriptional and nongenomic effects of glucocorticoids. Proc Am Thorac Soc. 2004;1:255–263. doi: 10.1513/pats.200402-015MS. [DOI] [PubMed] [Google Scholar]
- 12.Croxtall J.D., Van Hal P.T.W., Choudhury Q., Gilroy D.W., Flower R.J. Different glucocorticoids vary in their genomic and non-genomic mechanism of action in a549 cells. Br J Pharmacol. 2002;135:511–519. doi: 10.1038/sj.bjp.0704474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panettieri R.A., Schaafsma D., Amrani Y., Koziol-White C., Ostrom R., Tliba O. Non-genomic effects of glucocorticoids: An updated view. Trends Pharmacol Sci. 2019;40:38–49. doi: 10.1016/j.tips.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coutinho A.E., Chapman K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335:2–13. doi: 10.1016/j.mce.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wernecke C., Braun H.J., Dragoo J.L. The effect of intra-articular corticosteroids on articular cartilage: A systematic review. Orthop J Sports Med. 2015;3 doi: 10.1177/2325967115581163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fubini S.L., Todhunter R.J., Burton-Wurster N., Vernier-Singer M., Macleod J.N. Corticosteroids alter the differentiated phenotype of articular chondrocytes. J Orthop Res. 2001;19:688–695. doi: 10.1016/S0736-0266(00)00060-7. [DOI] [PubMed] [Google Scholar]
- 17.Provencher M., Navaie M., Solomon D.J., Smith J.C., Romeo A.A., Cole B.J. Joint chondrolysis. J Bone Joint Surg Am. 2011;93:2033–2044. doi: 10.2106/JBJS.J.01931. [DOI] [PubMed] [Google Scholar]
- 18.Jayaram P., Kennedy D.J., Yeh P., Dragoo J. Chondrotoxic effects of local anesthetics on human knee articular cartilage: A systematic review. PM R. 2019;11:379–400. doi: 10.1002/pmrj.12007. [DOI] [PubMed] [Google Scholar]
- 19.Raynauld J.P., Buckland-Wright C., Ward R., et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: A randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2003;48:370–377. doi: 10.1002/art.10777. [DOI] [PubMed] [Google Scholar]
- 20.Gomoll A.H., Kang R.W., Williams J.M., Bach B.R., Cole B.J. Chondrolysis after continuous intra-articular bupivacaine infusion: An experimental model investigating chondrotoxicity in the rabbit shoulder. Arthroscopy. 2006;22:813–819. doi: 10.1016/j.arthro.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Alsop R.J., Khondker A., Hub J.S., Rheinstadter M.C. The lipid bilayer provides a site for cortisone crystallization at high cortisone concentrations. Sci Rep. 2016;6 doi: 10.1038/srep22425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar N., Newman R.J. Complications of intra- and peri-articular steroid injections. Br J Gen Pract. 1999;49:465–466. [PMC free article] [PubMed] [Google Scholar]
- 23.Cole B.J., Schumacher R.H. Injectable corticosteroids in modern practice. J Am Acad Orthop Surg. 2005;13:37–46. doi: 10.5435/00124635-200501000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Mohamed M., Patel S., Plavnik K., Liu E., Casey K., Hossain M.A. Retrospective analysis of septic arthritis caused by intra-articular viscosupplementation and steroid injections in a single outpatient center. J Clin Med Res. 2019;11:480–483. doi: 10.14740/jocmr3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karsh J., Yang W.H. An anaphylactic reaction to intra-articular triamcinolone: A case report and review of the literature. Ann Allergy Asthma Immunol. 2003;90:254–258. doi: 10.1016/S1081-1206(10)62151-5. [DOI] [PubMed] [Google Scholar]
- 26.Charalambous C., Paschalides C., Sadiq S., Tryfonides M., Hirst P., Paul A.S. Weight bearing following intra-articular steroid injection of the knee: Survey of current practice and review of the available evidence. Rheumatol Int. 2002;22:185–187. doi: 10.1007/s00296-002-0213-z. [DOI] [PubMed] [Google Scholar]
- 27.Chakravarty K., Pharoah P.D.P., Scott D.G.I. A randomized controlled study of post-injection rest following intra-articular steroid therapy for knee synovitis. Br J Rheumatol. 1994;33:464–468. doi: 10.1093/rheumatology/33.5.464. [DOI] [PubMed] [Google Scholar]
- 28.Chatham W., Williams G., Moreland L., et al. Intraarticular corticosteroid injections: should we rest the joints? Arthritis Care Res. 1989;2:70–74. doi: 10.1002/anr.1790020209. [DOI] [PubMed] [Google Scholar]
- 29.wada-ama.org [Internet]. World Anti-Doping Code International Standard Prohibited List; c2023. https://www.wada-ama.org/en/resources/world-anti-doping-program/prohibited-list
- 30.wada-ama.org [Internet]. International Olympic Committee Anti-Doping Rules Applicable to the XXIV Olympic Winter Games Beijing 2022; c2021-2023. https://www.wada-ama.org/en/resources/ioc-anti-doping-rules-xxiv-olympic-winter-games-beijing-2022-november-2021
- 31.uci.org [Internet]. Part 14 Anti-Doping Rules (“UCI ADR”); c2023. https://www.uci.org/rules-and-procedures/60WVbRlrvTB6h9zACK2fe8
- 32.uci.org [Internet]. Part 13 Medical Rules; c2022-2023. https://www.uci.org/medical-rules/654MthQKzzl4lyYEHE2DdH
- 33.Ardern C.L., Glasgow P., Schneiders A., et al. 2016 consensus statement on return to sport from the First World Congress in Sports Physical Therapy, Bern. Br J Sports Med. 2016;50:853–864. doi: 10.1136/bjsports-2016-096278. [DOI] [PubMed] [Google Scholar]
- 34.Shrier I. Strategic Assessment of Risk and Risk Tolerance (StARRT) framework for return-to-play decision-making. Br J Sports Med. 2015;49:1311–1315. doi: 10.1136/bjsports-2014-094569. [DOI] [PubMed] [Google Scholar]
- 35.Nepple J.J., Matava M.J. Soft tissue injections in the athlete. Sports Health. 2009;1:396–404. doi: 10.1177/1941738109343159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.