Abstract
Exosomes are extracellular vesicles that can be produced by most cells. Exosomes act as important intermediaries in intercellular communication, and participate in a variety of biological activities between cells. Non-coding RNAs (ncRNAs) usually refer to RNAs that do not encode proteins. Although ncRNAs have no protein-coding capacity, they are able to regulate gene expression at multiple levels. Angiogenesis is the formation of new blood vessels from pre-existing vessels, which is an important physiological process. However, abnormal angiogenesis could induce many diseases such as atherosclerosis, diabetic retinopathy and cancer. Many studies have shown that ncRNAs can stably exist in exosomes and play a wide range of physiological and pathological roles including regulation of angiogenesis. In brief, some specific ncRNAs can be enriched in exosomes secreted by cells and absorbed by recipient cells through the exosome pathway, thus activating relevant signaling pathways in target cells and playing a role in regulating angiogenesis. In this review, we describe the physiological and pathological functions of exosomal ncRNAs in angiogenesis, summarize their role in angiogenesis-related diseases, and illustrate potential clinical applications like novel drug therapy strategies and diagnostic markers in exosome research as inspiration for future investigations.
Keywords: Exosome, Non-coding RNA, Exosomal non-coding RNA, Angiogenesis, Therapeutic target
1. Introduction
Extracellular vesicles (EVs), including exosomes and microvesicles, can be secreted by almost every cell [1]. Exosomes are derived from the multivesicular endosome pathways, different from microvesicles formed from the plasma membrane [2]. It has been demonstrated that EVs, including exosomes, are involved in intercellular communication [2] and usually deliver signals by following three ways: binding to receptors on the surface of the cell membrane, transferring surface receptors to target cells, and delivering related functional proteins or RNAs to target cells [3]. Exosomes are nanosized EVs measured 30–150 nm in diameter [4]. At present, the main role of exosomes seems to involve intercellular signal transduction and material transport [5]. Exosomes contain biologically active substances including proteins, lipids, and genetic material [2]. For example, in recent years a variety of non-coding RNAs (ncRNAs) have been found in exosomes, such as microRNA (miRNA), long non-coding RNA (lncRNA), and circular RNA (circRNA) [6]. Therefore, the primary physiological function of exosomes is to serve as intermediaries for the transfer of vesicle material between cells. This action mediates various biological processes such as immune functions [7], intercellular signals, cell growth and differentiation, and gene transcription and expression [8]. However, it is unclear whether the transport of exosome content is random or specific. Interestingly, a novel study demonstrated that a zipcode-like sequence mediated mRNA enrichment in exosomes [9]. In that study, a zipcode-like sequence containing a short CTGCC core domain and a miRNA-1289 binding site in the 3′-untranslated region (3′-UTR) was found to bind directly with miRNA-1289 and increase the levels of specific mRNAs in EVs. Although exosomes were previously thought to have only transport functions, a new study has found that breast cancer-associated exosomes process precursor microRNAs (miRNAs) into mature miRNAs through Dicer, Argonaute2 (AGO2), and trans-activation response RNA-binding protein (TRBP) [10]. Exosomes also exert pathological effects under certain conditions. Taking the tumor microenvironment for example, hypoxia-derived tumor cells can secrete exosomes to activate the NF-κB pathway and upregulate the expression of hypoxia-inducible factor-1α (HIF-1α) to promote tumor cell proliferation [11]. Additionally, non-coding RNA may also participate in this process, the study showed that the 50 miRNAs were found to significantly upregulate in exosomes of hypoxia induced-esophageal squamous cancer cells and further KEGG pathway analysis revealed that they are related to tumor-associated pathways [12]. Moreover, exosomes can also promote tumor metastasis, enhance radiation resistance of tumor and induce tumor angiogenesis [[13], [14], [15]].
Vascular dysfunction and neovascularization are associated with the development and progression of many diseases, including angiogenesis-related, ischemic, inflammatory, and immune diseases and cancer [16]. The formation of new blood vessels is a sophisticated process that includes vasculogenesis and angiogenesis [17]. Vasculogenesis is the early stage of new vessel formation from endothelial progenitor cells, or angioblasts. Angiogenesis is the proliferation and migration of endothelial cells (ECs) from existing mature blood vessels in tissues [18]. Both neovascularization patterns are closely related to the functional status of ECs [19]. Related cytokines, functional proteins, and some RNAs can influence endothelial cell metabolism and thus participate in angiogenesis.
NcRNAs are a large family of RNAs that have been extensively researched in the past. They include lncRNA, miRNA, circRNA, transfer RNA (tRNA), ribosome RNA (rRNA), small interfering RNA (siRNA), small nuclear RNA (snRNA) and piwi-interacting RNA (piRNA) which perform several functions, including amino acid transport, mRNA modification, editing, gene silencing, and recognition [20]. NcRNAs participate in regulating cellular physiological processes through different levels of gene regulation. The aberrant expression of various ncRNAs may induce pathological effects and even become a sign of disease. Recently, the role of lncRNA and miRNA in pathological angiogenesis has been studied in depth [21]. Increasing evidence indicates that lncRNAs serve as molecular switches during cellular differentiation, movement, apoptosis, and reprogramming of cell states by shifting gene expression patterns [22,23]. One of the major functional mechanisms of miRNAs is to regulate post-transcriptional expression by gene silencing [23]. Moreover, depletion of Dicer1 in miRNAs (miRNA-21a, miRNA-125b, miRNA-let-7) suppresses tumor angiogenesis [24]. MiRNAs are involved in diabetic retinopathy, etc [25].
This review describes the roles and mechanisms of several important types of exosomal ncRNAs in angiogenesis and clarifies their participation in pathological angiogenesis. In addition, we discuss the clinical applications of exosomal ncRNAs in angiogenesis-related diseases, as well as their potential capabilities as therapeutic targets and the prospect of related studies.
2. Exosomal miRNAs in angiogenesis
MiRNAs are small ncRNAs with 20-24 nucleotides (nt) in length, made from single-stranded RNAs with a hairpin precursor structure about 70–90 nt in size processed by the Dicer enzyme [23]. MiRNAs could bind to the open reading frame (ORF) region or 3′-UTR of target mRNAs and play a moderating role [26]. MiRNAs have two modes of action: binding closely to the ORF region of mRNA and forming a double-stranded structure that leads to mRNA degradation or loosely binding to the 3′-UTR region of mRNA and inhibiting the post-transcriptional translation of mRNA [27]. Furthermore, miRNAs have been verified to be involved in many biological processes, including cell growth, differentiation, and disease development [28].
MiRNAs are stable in body fluids like blood, urine, saliva, and breast milk [[29], [30], [31]]. In addition, extracellular miRNAs can be loaded into EVs, like exosomes, which prevent their degradation, ensuring their stability [32]. Thus, given the transportation role of exosomes, miRNAs can be delivered by exosomes to specific cells. MiRNAs are not only loaded into exosomes randomly, but also through a sorting mechanism [33], such that some types of miRNAs (miRNA-150, miRNA-142-3p, and miRNA-451) have a higher priority for entering exosomes than other miRNAs [34]. This sorting mechanism may be based on the specific sequences of certain miRNAs, some enzymes, or other proteins [33]. MiRNAs can be transferred between cells by exosomes and participate in many pathological processes such as inflammation, tumor metastasis, and angiogenesis [6]. The role of exosomal miRNAs has attracted greater attention in recent years, and more reports have shown that exosomal miRNAs play an important role in regulating angiogenesis [33]. Exosomal miRNA-214, secreted by human microvascular endothelial cells (HMECs), promoted nearby HMEC migration and blood vessel formation [35]. This study utilized in vivo and in vitro methods to prove that exosomal miRNA-214 could mediate the interaction between endothelial cells. Exosomal miRNA could not only participate in crosstalk between homogeneous cells like endothelial cells, but also involved in signal transduction in different cells. Exosomal miRNA-92a, derived from the K562 leukemic cell line, could be absorbed by human umbilical vein endothelial cells (HUVECs) and induce HUVEC migration and tube formation [36]. However, this result was mainly based on in vitro approaches and how did leukemia cells affect angiogenesis in vivo still need further exploration. Exosomal miRNAs could also change the tumor microenvironment and lead to angiogenesis in cancer [37]. The uptake of related angiogenic exosomal miRNAs by normal ECs could activate the angiogenesis-related signaling pathways and stimulate new blood vessel formation [38]. A recent study showed that exosomes derived from senescent ECs include several pro-angiogenesis miRNAs [39]. The further study revealed that these exosomal miRNAs could regulate endothelium dysfunction and promote angiogenesis of normal ECs. Another research showed that miRNA-155 and miRNA-182 differently expressed between H2O2 treated and normal HUVEC-derived exosomes, and they were involved in cellular senescence and autophagy [40]. Those are interesting findings which suggested that these exosomal miRNAs may be a potential biomarker [39] and therapeutic target [40] of age-related vascular diseases. For example, exosomal miRNA-210 from lung could activate the PI3K/AKT/HIF-1 pathway and promoted angiogenesis [41]. Meanwhile, lung cancer-derived exosomal miRNA-21 could activate the STAT3 pathway, increasing the expression of vascular endothelial growth factor (VEGF) and accelerating angiogenesis in bronchial epithelial cells [42]. Exosomal miRNA-126 was found to upregulate the AKT/ERK signal pathway and inhibit downstream p53 to regulate angiogenesis [25]. On the other hand, exosomal miRNAs could modulate the ECs to take part in the new blood vessels [43]. Furthermore, M2 macrophage-derived exosomal miRNA-155-5p and miRNA-221-5P acted on ECs and induced angiogenesis [44]. Exosomal miRNAs, such as miRNA-132 and miRNA-378, could promote proliferation, migration, and tube formation of ECs, and subsequently regulated sprout formation [45,46]. Similarly, exosomal miRNAs such as exosomal miRNA-210, miRNA-10b, and miRNA-9b increased capillary formation, ECs chemotaxis, and proliferation in response to VEGF [47,48]. Moreover, tumor-derived exosomal miRNAs could target ECs and accelerate tumor angiogenesis. So, it is clear that exosomal miRNA could also regulate angiogenesis by directly affecting ECs.
Taken together, current evidence indicates that exosomal miRNAs play a non-negligible role in angiogenesis. The related mechanisms of exosomal miRNAs in angiogenesis are summarized in Table 1 and Fig. 1, and we look forward to providing more inspiration for potential clinical applications.
Table 1.
The mechanisms of exosomal ncRNAs in angiogenesis.
| Exosomal ncRNAs | Mechanism | Refs. | |
|---|---|---|---|
| miRNA | miRNA-21 | activate STAT3, increase the expression of VEGF | [42] |
| miRNA-126 | regulate AKT/ERK pathway | [25] | |
| miRNA-210 | target SMAD4 and STAT6 in ECs | [49] | |
| miRNA-9 | enhance ECs chemotaxis in response to VEGF | [50] | |
| miRNA-23a | target PHD1, PHD2 and tight junction protein ZO-1 | [51] | |
| miRNA-192 | repression of proangiogenic IL-8, ICAM and CXCL1 | [52] | |
| miRNA-16 | downregulate VEGF expression | [53] | |
| miRNA-130a | regulate PTEN pathway | [54] | |
| miRNA-135b | downregulate the expression of VEGF | [55] | |
| miRNA-6785-5p | inhibit inhibin subunit beta A (INHBA) | [56] | |
| miRNA-378b | inhibit TGF-β receptor III | [57] | |
| miRNA-29a | suppress the expression of VEGF | [58] | |
| miRNA-150-3p | promote tumor angiogenesis | [59] | |
| miRNA-155 | target the c-MYB/VEGF axis of ECs | [60] | |
| miRNA-21-3p | inhibit ECs proliferation and migration | [61] | |
| miRNA-17-3p | target PETN pathway | [62] | |
| miRNA-486-3p | participate in TLR4/NF-κB axis repression | [63] | |
| miRNA-202-5p | target TGF-β receptor II | [64] | |
| lncRNA | lncRNA-p21 | inhibit p53 | [65] |
| lncRNA-01435 | facilitate YY1-mediated HDAC8 expression | [66] | |
| lncRNA OIP5-AS1 | regulate miR-153 and ATG5 | [67] | |
| lncRNA-HOTAIR | target the VEGFA | [68] | |
| lncRNA-H19 | increase the level of VEGF and ICAM-1 in ECs | [69] | |
| lncRNA-NR2F1-AS1 | enhance the production of VEGF in ECs | [70] | |
| lncRNA-PCAT6 | activate IGF-1/ERK pathway | [71] | |
| lncRNA-TUC339 | activate VEGFR/AKT/mTOR pathway | [72] | |
| lncRNA-SNHG16 | regulate miR-195/MFN2 axis | [73] | |
| lncRNA-CCAT2 | activate the Wnt/β-catenin pathway | [74] | |
| lncRNA-UCA1 | regulate miRNA-96-5p/AMOTL2 axis | [75] | |
| circRNA | circRNA -ANRIL | combine with PES1 protein | [76] |
| circRNA-100338 | interact with NOVA2 and activate mTOR pathway | [77] | |
| circRNA-SHKBP1 | upregulate the expression of HUR | [78] | |
| circRNA-PWWP2A | regulate the level of Ang-1 | [79] | |
| circRNA-0005015 | increase the expression of STAT3 | [80] | |
| circRNA-HIPK3 | upregulate the level of VEGF-C | [81] | |
| circRNA-DNMT3B | regulate miR-20b-5p/BAMBI axis | [82] | |
| circRNA-ZNF609 | regulate MEF2A and suppress angiogenesis | [83] | |
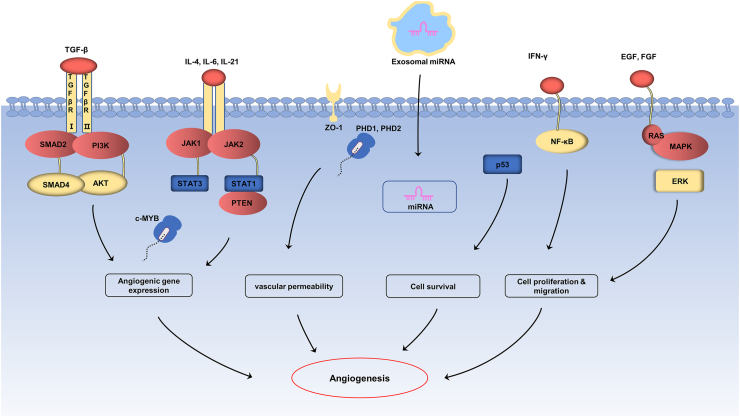
Fig. 1.
The mechanisms of exosomal miRNAs in angiogenesis. Exosomal miRNAs target angiogenesis related pathways (TLR-4/NF– NF-κB, JAK/STAT, TGF-β/SMAD4, PI3K/AKT, PTEN and MAPK/ERK pathway) in ECs or act on downstream target genes (PHD1, PHD2, c-MYB) to increase the expression of angiogenesis related factors (VEGF, TGF-β, HIF-1α) and promote proliferation of endothelial cells, leading to angiogenesis.
3. Exosomal lncRNAs in angiogenesis
In contrast to miRNAs, lncRNAs are ncRNAs longer than 200 nt, the largest category among the ncRNAs [84]. LncRNAs are known to take part in many physiological and pathological processes: for example, fibrosis in various organs such as liver, heart, lung, and kidney [85], regulation of synaptogenesis in the human nervous system through modulation of gene expression [86], and adjustment of reprogramming of human induced pluripotent stem cells [86]. In other words, lncRNAs are involved in gene regulation at multiple levels, incorporating epigenetic, transcriptional, and post-transcriptional regulation [87].
LncRNAs have four classical modes of action [88]. The first mode of action is as signals: in particular situations, some lncRNAs will be transcribed and participate in specific signal pathways as signal transduction molecules [88]. For example, lncRNA-p21 located upstream of the cyclin-dependent kinase inhibitor 1A (CDKN1A) gene, was reported as a transcription inhibitor of the classic p53 pathway [65]. The second mode of action is as decoys: when these lncRNAs are transcribed, they will bind directly to RNA or proteins, blocking molecular action and signaling pathways [88]. For instance, lncRNA PANDA could bind directly with the apoptotic program nuclear transcription factor NF-YA and thereby block the inhibit cells apoptosis to some extent [89]. The third mode of action is as guides: some types of lncRNAs could guide binding protein binding with transcription product and regulate the transcription of downstream molecules [88] such as lncRNA-HOTTIP [90]. The riboprotein body complex could be guided to a specific target by this pattern. The last mode of action is a molecular scaffold: lncRNAs can act as a platform upon which other relative molecules can assemble [88].
A wide range of lncRNAs have also been found in exosomes [91], where they play an important role in angiogenesis [92]. Studies have proved that tumor-derived exosomal lncRNAs could stimulate tumor angiogenesis by activating angiogenic cells [93]. Furthermore, exosomes secreted by different types of cells, such as mesenchymal stem cells and endothelial cells, were involved in tumor angiogenesis [94,95]. According to Wang et al., lncRNA HITT and HIF-1α form a loop to regulate angiogenesis and tumor growth in colon cancer [96]. Exosomal lncRNA OIP5-AS1 induces the angiogenesis in osteosarcoma by regulating miRNA-153 and autophagy-related protein 5 (ATG5) [67]. According to these results, some exosomal lncRNAs could affect relative protein level like ATG5 to regulate tumor cells autophagy and thereby promote tumor angiogenesis. Exosomal lncRNAs could also regulate angiogenesis by affecting the expression of angiogenic factors [97]. For instance, LncRNA HOTAIR was highly expressed in glioma cells [98] and transferred from glioma cells to ECs, where it prompted tumor angiogenesis by increasing the expression of VEGFA [68]. In addition, exosomal lncRNAs can also act directly on the ECs involved in angiogenesis [43]. Researchers recently identified lncRNA H19-enriched CD90+ liver cancer cells-derived exosomes, which were delivered to ECs, where they enhanced the production of VEGF [69]. Thus, some kind of exosomal lncRNAs are capable of activating ECs, which means that they can promote ECs proliferation and migration, as well as increase the level of pro-angiogenic factors such as VEGF. Meanwhile, overexpression of lncRNA HOTAIR and MALAT1 was found in ethanol-induced endothelial cell-derived exosomes, which related to alcohol-induced tumor angiogenesis. This might suggest that ECs-derived exosomal lncRNAs could induce angiogenesis by targeting other ECs [99], and that exosomal lncRNAs could serve as competing endogenous RNA (ceRNA) [100]. CeRNAs are RNA transcripts with miRNA binding sites that competitively bind miRNA and inhibit miRNA regulation on target genes, including mRNAs, lncRNAs, pseudogenes, and circRNAs [100]. CeRNAs regulate and communicate with each other by competing for binding to specific miRNAs. They are called miRNA sponges since they could block miRNAs from their original targets by binding with them [101]. For example, lncRNA NR2F1-AS1 acts as a sponge by binding to miRNA-338-3p and suppressing the expression of insulin-like growth factor-1 (IGF-1), thus increasing the expression of IGF-1 and activating the IGF-1/IGF-1R/ERK pathway, both of which play an important role in the angiogenesis of breast cancer [70]. Furthermore, M2 macrophage-induced lncRNA PCAT6 upregulates VEGF receptor (VEGFR) through ceRNA and activates VEGFR/AKT/mTOR signaling pathway to accelerate angiogenesis in breast cancer [71].
To summarize, exosomal lncRNAs participate in angiogenesis via multiple mechanisms, such as regulating the proliferation of ECs and enhancing VEGF expression; acting as ceRNA for miRNAs and thereby suppressing miRNA-mediated inhibition of angiogenesis, and activating related signal pathways in ECs (Fig. 2).
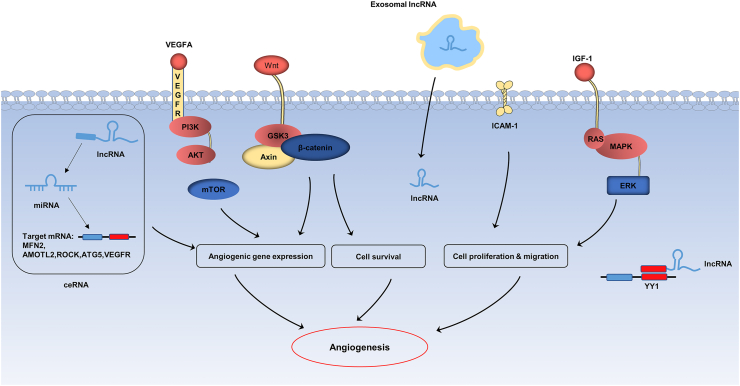
Fig. 2.
The mechanisms of exosomal lncRNAs in angiogenesis. Exosomal lncRNAs participate in the regulation of angiogenesis through a variety of mechanisms, including the regulation of ECs related signaling pathways (mTOR, Wnt/β-catenin and ERK pathway), the increase of target gene expression by ceRNA mechanism, and the regulation of ECs proliferation, differentiation and migration (ICAM-1, YY-1 and HDAC8).
4. Exosomal circRNAs in angiogenesis
CircRNAs are prevalent in eukaryotic cells and have a closed-loop structure, making them more stable than other ncRNAs [102]. As a novel member of the ncRNAs, circRNAs have multiple functions in gene regulation [103]. New research shows that circRNAs can act as miRNA sponges and inhibit miRNA function [104], regulate transcription of cells (cell nucleus) [105], take part in splicing of the target gene, and interact with RBPs [106]. Moreover, recent studies have shown the stability and enrichment in exosomes of circRNAs [107]. Over one thousand circRNAs can be found in human serum exosomes [108], and they play a role in many human systems, such as the nervous [109,110], immune [111], and hematopoietic systems [112], and also participate in pathological angiogenesis [113].
In recent years, the roles of exosomal circRNAs in tumor angiogenesis and cardiovascular disease have been studied extensively [[78], [114], [115]]. Exosomal circRNAs could regulate angiogenesis by interacting with RBPs; for example, Holdt et al. found that circRNA-ANRIL could induce nucleolar stress and regulate ribosomal RNA maturation by combining with PES1 protein (a protein-coding gene) [76]. After combining with PES1 protein, circRNA-ANRIL modulated pre-rRNA processing and ribosome synthesis in macrophages and vascular smooth muscle cells, thereby serving as a protective factor in atherosclerosis. Moreover, Huang et al. demonstrated that HCC cell-derived exosomal circRNA-100,338 might regulate tumor angiogenesis through interacting with the RBP NOVA2 [77]. Their results showed that transferring the exosomal circRNA-100,338 to recipient receptor cells could increase angiogenesis and activate the mTOR pathway. Similar to lncRNA, exosomal circRNA could also act as miRNA sponge to participate in the regulation of angiogenesis [116]. Exosomal circ-SHKBP1 produced by gastric cancer cells could sponge miRNA-582-3p and upregulate the expression of HUR (downstream target gene of miRNA-582-3p). HUR activated the translation of VEGF and increased tumor angiogenesis [78]. Meanwhile, circRNA-PWWP2A could be delivered from pericytes to ECs through exosomes, and inhibited miRNA-579 by acting as an endogenous miRNA-579 sponge. CircRNA-PWWP2A was proved to regulate vascularization via the circRNA-PWWP2A-miRNA-579-angiopoietin 1(Ang-1) axis [79].
Second, the n6-methyladenosinde (m6A) modification mechanism also participates in the regulation of circRNA and angiogenesis [117]. M6A is the most common modification of RNAs in eukaryotic cells, and regulates RNA transcription, processing, splicing, degradation and translation [[118], [119], [120]]. The functions of the m6A modification are mediated by three kinds of methylase called “writers,” “erasers,” and “readers” [121]. “Writers” are involved in the formation of the methyltransferase complex [122], “erasers” act as demethylases, and “readers” serve as binding proteins [123]. For example, METTL14 and ALKBH5 (“erasers”) inhibited YTHDF3 (“readers”) by controlling each other’s expression and then regulated the m6A modification of transforming growth factor–β(TGF-β), which played an important role in the angiogenesis of diabetic retinopathy [124]. A recent study showed that m6A-modified circRNAs could also be endoribonuclease-cleaved via a YTHDF2-HRSP12-RNase P/MRP axis [125]. However, these studies did not address exosome pathways. Another study revealed the role of m6A modification in exosomal circRNA. In this study, m6A methylase METTL3 and ALKBH5 could regulate the molecular sponge effect of circ-0008542 in osteoblast exosomes [126]. Notably, m6A modification is also present in other types of ncRNAs [127], but how m6A modification plays its biological role by regulating ncRNAs through exosomes in angiogenesis remains to be further studied.
In conclusion, circRNA could assemble in exosomes and be delivered to recipient cells. After exosomes were ingested by the recipient cells, circRNA played a regulatory role in the nucleus or cytoplasm as follows: acting as a miRNA sponge, interacting with RNA-binding proteins, and depending on pathways of RNA modifications such as m6A methylation. We summarized the mechanisms of exosomal circRNA in angiogenesis (Table 1 and Fig. 3).
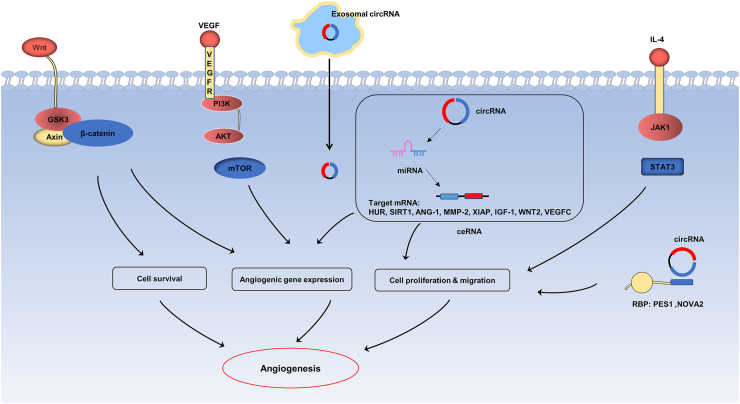
Fig. 3.
The mechanisms of exosomal circRNAs in angiogenesis. Exosomal circRNAs mainly participate in angiogenesis through the mechanism of ceRNA, but also modulate related signaling pathways (mTOR, Wnt/β-catenin and JAK/STAT pathway) and interact with RNA-binding proteins (PES1, NOVA2) to regulate angiogenesis and tube formation.
5. Other exosomal ncRNAs in angiogenesis
Other types of exosomal ncRNAs have been shown to play essential roles in angiogenesis, including transfer RNA-derived small RNA (tsRNA), snRNA, and siRNA.
TsRNAs are small ncRNAs with 18–40 nt in length, produced by precursor or mature tRNAs [128]. Many studies have revealed that the expression of tsRNAs is controlled under certain physiological conditions and plays a vital role in many biological processes and diseases such as genetic, metabolic, and cancer [129,130]. Many types of ncRNAs exist stably in exosomes and play diverse roles in physiological and pathological processes [6]. A study by Dou et al. showed that MSC-secreted exosomal tsRNA-21109 might suppress M1 macrophage polarization by reducing the expression of Ras, mitogen-activated protein kinase (MAPK), and transforming growth factor-β (TGF-β) signal pathways and thereby stimulate the inflammatory response in systemic lupus erythematosus [131]. TsRNAs are also found involved in angiogenesis. Ischemia is defined as the interruption of tissue blood supply [132], and angiogenesis could also be found in ischemic tissue. According to Li et al., two tsRNA fragments generated from tRNA-Val and tRNA-Gly were highly expressed in the brain ischemia and hindlimb ischemia mouse model [133]. Their study proved that tsRNAs upregulated in the model possessed the capacity to inhibit angiogenesis and significantly inhibit the proliferation, migration, and tube formation of ECs. Moreover, a recent study showed that tsRNA contributed to angiogenesis in moyamoya disease (MMD) [134], a cerebrovascular disease characterized by chronic, progressive stenosis or occlusion [135]. Researchers found four differentially expressed (DE)-tsRNAs: tsRNA-Glu-TTC-010, tsRNA-Glu-CTC-003, tsRNA-Val-AAC-017, and tsRNA-Arg-CCT-008. Further research revealed that DE-tsRNAs activated the HIF-1 pathway and then played a compensatory role in angiogenesis of MMD [134].
SnRNA as a type of ncRNA is mainly involved in the processing of mRNA precursors [136]. Exosomal snRNA could activate Toll-like receptor 3 (TLR3) in lung epithelial cells, change tumor microenvironment, and promote tumor metastasis [137]. SiRNAs are double-strand RNAs 19–24 nt in length, which can serve as an RNA-induced silencing complex (RISC) and play a part in gene silencing in the post-transcriptional stage [138]. Recently, the feasibility of exosomes as carriers to inhibit tumor angiogenesis was verified by Zhang, et al., who proved that exosomes with hepatocyte growth factor (HGF) siRNA significantly suppressed tumor angiogenesis of gastric cancer in vitro and in vivo via the HGF/cMET axis [139]. Furthermore, another study demonstrated that bone mesenchymal stem cells (BMSCs)‐derived exosomal siRNA enhanced the proliferative ability of ECs, stimulated angiogenesis, and inhibited osteonecrosis of the femoral head by suppressing related gene expression including FGF2, WNT-11, and S100A9 [140].
In summary, current evidence indicates that diverse exosomal ncRNAs play an indispensable role in the progression of angiogenesis. The modes of action of exosomal ncRNAs involved in the regulation of angiogenesis have been described (Fig. 4). Notably, other ncRNAs such as small nucleolar RNA (snoRNA) and PIWI-interacting RNA (piRNA) also play critical roles in physiological or pathological processes through exosomes [141,142], while their roles and mechanisms in angiogenesis need to be further studied.
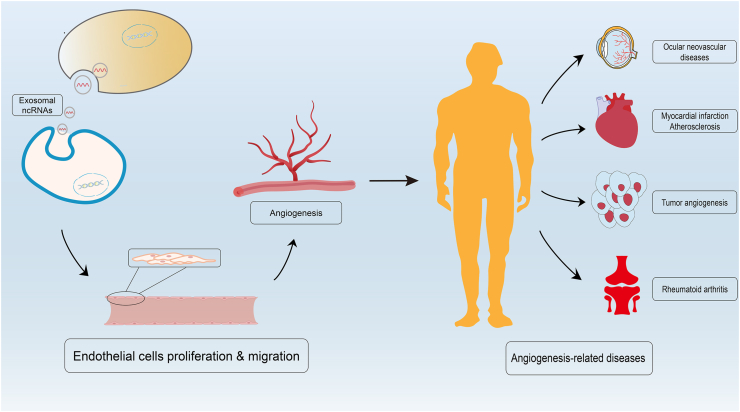
Fig. 4.
The process of exosomal ncRNAs involved in the regulation of angiogenesis and angiogenesis-related diseases. Exosomal ncRNAs derived from donor cells can be absorbed by target cells and activate angiogenesis related signal pathways. After that, the activated endothelial cells will proliferate and migrate to form new blood vessels which also called angiogenesis. Abnormal angiogenesis will cause a series of pathological changes and ultimately induce angiogenesis-related diseases like ocular neovascular diseases, atherosclerosis, rheumatoid arthritis and cancer.
6. Exosomal ncRNAs in tumor angiogenesis
Tumor angiogenesis is one of the common characteristics of cancer, essential for the growth and development of tumor cells, and plays a vital role in tumor invasion and metastasis [143]. The formation of tumor blood vessels is dependent on the regulation of the tumor microenvironment [144]. High expression of angiogenic factors, inflammatory cytokines, and HIF-1α in the tumor microenvironment induces tumor angiogenesis [145]. In addition, cancer-derived exosomes are involved in tumor angiogenesis, and some of the ncRNAs in cancer-derived exosomes are closely related to them [146]. Further elucidation of the mechanism of exosomes in tumor proliferation, invasion and angiogenesis is helpful for future clinical application such as cancer diagnosis and novel therapeutic target [147].
Lung cancer cells could secret exosomal miRNA-23a to promote angiogenesis by inhibiting the tight junction protein ZO-1 [51]. It has been reported that breast cancer (BC) cells-derived exosomal miRNA-210 can stimulate angiogenesis [148]. In particular, researchers have revealed that miRNA-210 could be delivered from hepatocellular carcinoma (HCC) cells to ECs via exosomes and thus promote angiogenesis by targeting SMAD4 and STAT6 pathways [49]. Meanwhile, exosomal miRNA-155 secreted by HCC cells in hypoxia could accelerate tube formation in ECs [149]. In addition to the exosomal miRNAs that can promote tumor angiogenesis mentioned above, there are also exosomal miRNAs could suppress tumor angiogenesis. A study by Lee et al. showed that miRNA-16 and miRNA-100 in exosomes derived from mesenchymal stem cells (MSCs) suppress angiogenesis by reducing the production of VEGF in BC cells [53]. Valencia et al. reported that exosomal miRNA-192 could also suppress the expression of angiogenic factors such as IL-8, ICAM, and CXCL1, consequently impairing tumor-induced angiogenesis in lung cancer [52].
In addition, lncRNA-H19 is highly expressed in exosomes released by CD90+liver cancer cells and is proved to be involved in regulating tumor angiogenesis [69]. Exosomal lncRNA-TUC339 from HCC cells could regulate M2 macrophage polarization and participate in the migration of liver cancer cells [72]. Moreover, Huang et al. found that highly metastatic HCC cells-secreted exosomal circRNA-100338 was enriched in HUVECs [77].
Exosomal miRNA-25-3p is related to tumorigenesis and angiogenesis in colorectal cancer [150,151]. Similarly, exosomal ncRNAs participate in gastric carcinoma (GC) angiogenesis. Investigators have shown that GC cells-derived exosomal miRNA-let-7 promotes tumor angiogenesis by regulating HMGA2 and RAS [152]; meanwhile, miRNA-130a has been highly expressed in GC cells-secreted exosomes and could be delivered to ECs where they induced angiogenesis [153]. In addition, exosomal circRNA-SHKBP1 from GC cells could act as miRNA sponge and enhance angiogenesis via miRNA-582-3p/HUR/VEGF axis [78].
7. Exosomal ncRNAs in ocular neovascular diseases
Ocular neovascularization diseases are divided into two main types, retinal and choroidal neovascularization [154]. Various diseases that cause vision loss are associated with ocular neovascularization [155,156].
7.1. Diabetic retinopathy
Diabetic retinopathy (DR) is one of the complications of diabetes. Multiple mechanisms are involved in the development of DR, including dysfunction of multiple cell-signaling pathways, inflammation and oxidative stress resulting from hyperglycemia, and dyslipidemia [156]. DR is divided into two types: non-proliferative DR (NPDR) and proliferative DR (PDR). The exosomes in plasma of patients with diabetic retinopathy were found to upregulate the expression of angiogenic factors, revealing that exosomes may be involved in the delivery of angiogenic factors and participate in DR [157]. In addition, many in vitro and in vivo experiments have shown that exosomal ncRNAs are closely related to DR [158,62]. Maisto et al. found that VEGF was increased, and the level of anti-angiogenic miRNAs was decreased in photoreceptor cells exosomes of a high glucose model used to simulate DR [159]. Moreover, Cao et al. indicated that MSC-derived exosomal lncRNA-SNHG7 was involved in DR retinal angiogenesis [158]. MiRNA-15a was found to be produced by pancreatic cells and delivered to the retina through exosomes in the blood circulation, where they induced oxidative stress and promoted type 2 diabetes (T2D)-induced DR [160]. Researchers also reported that circRNA-cPWWP2A could be transferred by exosomes from pericytes to ECs in the retina and mediate the function of diabetic retinal microvasculogenesis [79].
7.2. Retinopathy of prematurity
Retinopathy of prematurity (ROP) is one of the leading causes of vision loss in children [161]. Despite some differences in mechanisms, the oxygen-induced retinopathy (OIR) mouse model is widely used in the study of retinal neovascular diseases, especially ROP [162]. Scott and Fruttiger found that the reduction of circRNA-ZNF609 suppressed pathologic angiogenesis in the OIR mouse model [83]. Likewise, the role of microglia in the pathophysiology of retinal diseases has been studied extensively [163]. Xu et al. used intravitreal injections with microglial cells-derived exosomes in the OIR mouse model and found smaller avascular areas and fewer neovascular tufts in treated eyes. Further research revealed that microglial cells-derived exosomal miRNA-24-3p downregulated the levels of VEGF and TGF- β in photoreceptors [164]. Novel clinical research proved that intravitreal injections of MSCs-secreted exosomes could effectively protect hyperoxia-induced retinopathy [165].
7.3. Age-related macular degeneration
Age-related macular degeneration (AMD) is divided into dry AMD and wet AMD according to the clinical features and pathological changes [166]. Wet AMD is characterized by choroidal neovascularization [167]. In the past decades, many studies implicated aging, circulation disorders, photodamage and oxidative damage, inflammatory responses, and related genetic changes in the pathogenesis of AMD [168]. Oxidative stress was proved to be one of the most important factors in AMD [169]. Shah et al. showed that oxidative-injured retinal pigment epithelium (RPE) cells could secrete exosomes with oxidative stress signals to normal RPE cells and contributed to angiogenesis [170]; additionally, RPE and retinal astroglial cells (RACs) are involved in AMD [171]. Researchers demonstrated that RACs-derived exosomes could reduce angiogenesis via anti-angiogenic components, including proteins, lipids, mRNA, and miRNA [172], and that RPE cells and RACs could serve as feasible therapeutic targets for AMD. Furthermore, a bioinformatics study revealed that AMD-derived exosomes acted as miRNA cargo and played a key role in the pathological angiogenesis of AMD, such as exosomal miRNA-126 and miRNA-410 [173], which might be a novel diagnostic and therapeutic approach for AMD.
8. Exosomal ncRNAs in other angiogenesis-related diseases
In addition to the diseases mentioned above, there are many other angiogenesis-related diseases including cardiovascular diseases, rheumatoid arthritis, and diabetic microangiopathy.
Hypoxic-treated cardiomyocytes were found to release exosomes containing circRNA-HIPK3 to cardiac microvascular ECs [174]. This finding indicated that exosomal circRNA could target ECs. Interestingly, a recent study also supported this conclusion. Wang et al. found that vascular ECs in a high glucose environment could release exosomal circRNA-0077930 to regulate senescence in vascular smooth muscle cells [175].
Angiogenesis also participates in the inflammatory process of autoimmune diseases such as rheumatoid arthritis, spondyloarthropathies, systemic lupus erythematosus, systemic sclerosis, and atherosclerosis [176]. As proof, exosomes from IL-1β-treated fibroblast-like synoviocytes have been found to lead osteoarthritic changes in chondrocytes [177]. Exosomes derived from rheumatoid models have also been shown to induce the secretion of COX-2 and promote angiogenesis [178].
Atherosclerosis is generally recognized as a chronic, lipid-induced inflammatory disease associated with endothelial cell damage [179]. Results from a study by Chen et al. suggest that human leukemia monocytic cell line (THP-1)-derived exosomes could promote the apoptosis of vascular ECs by transporting lncRNA-GAS5 [180]. In another study, Cheng et al. found high expression of miRNA-1 and miRNA-208 in exosomes from the urine of patients with acute myocardial infarction [181]. Moreover, higher levels of exosomal miRNAs were found in a rat model of myocardial infarction [182]. Furthermore, a cardio-protective miRNA-214 has been shown to be upregulated in the heart after ischemia and secreted by exosomes from human ECs [35]. The ncRNAs associated with angiogenesis-related diseases were summarized in Table 2. The ncRNAs mentioned in the review and the corresponding Gene Symbol and ENSEMBL ID are summarized in Supplementary Table S1.
Table 2.
Expression of exosomal ncRNAs in angiogenesis-related diseases.
| Angiogenesis-related diseases | Exosomal ncRNAs | Expression | Refs. |
|---|---|---|---|
| Lung cancer | miRNA-9 | ↑ | [50] |
| miRNA-21 | ↑ | [42] | |
| miRNA-23a | ↑ | [51] | |
| miRNA-126 | ↑ | [183] | |
| miRNA-210 | ↑ | [41] | |
| Breast cancer | miRNA-192 | ↑ | [52] |
| miRNA-210 | ↑ | [148] | |
| miRNA-16 | ↑ | [53] | |
| miRNA-100 | ↑ | [53] | |
| Hepatocellular carcinoma | lncRNA-TUC339 | ↑ | [72] |
| miRNA-21 | ↑ | [184] | |
| miRNA-210 | ↑ | [49] | |
| circRNA-100338 | ↑ | [77] | |
| miRNA-155 | ↑ | [149] | |
| miRNA-378b | ↑ | [57] | |
| lncRNA-SNHG16 | ↑ | [185] | |
| miRNA-130a | ↑ | [186] | |
| Gastric cancer and colorectal cancer | circRNA-0044366 | ↑ | [187] |
| miRNA-135b | ↑ | [55] | |
| circRNA-SHKBP1 | ↑ | [78] | |
| Pancreatic cancer | miRNA-27a | ↑ | [188] |
| miRNA-501-3p | ↑ | [59] | |
| lncRNA-CCAT1 | ↑ | [189] | |
| lncRNA-UCA1 | ↑ | [75] | |
| Diabetic retinopathy | miRNA-17-3p | ↓ | [62] |
| miRNA-486-3p | ↓ | [62] | |
| miRNA-202-5p | ↓ | [64] | |
| miRNA-377-3p | ↓ | [190] | |
| lncRNA SNHG7 | ↓ | [191] | |
| circRNA-cPWWP2A | ↑ | [79] | |
| Retinopathy of prematurity | miRNA-24-3p | ↓ | [164] |
| Age-related macular degeneration | miRNA-486-5p | ↑ | [192] |
| miRNA-626 | ↑ | [192] | |
| miRNA-885-5p | ↓ | [192] | |
| Myocardial infarction | miRNA-214 | ↑ | [182] |
| Atherosclerosis | miRNA-21-3p | ↑ | [61] |
| miRNA-155 | ↑ | [193] | |
| lncRNA GAS5 | ↑ | [180] | |
| Rheumatoid arthritis | miRNA-150-5p | ↓ | [194] |
| circRNA-EDIL3 | ↓ | [195] | |
| Diabetic microangiopathy | circRNA-PWWP2A | ↑ | [79] |
9. Potential clinical application of exosomal ncRNAs
The stable expression and enrichment of ncRNAs in exosomes has been confirmed through a large number of studies. Exosomal ncRNAs are widely studied in disease diagnosis and prognosis, drug resistance research, and new therapeutic targets [196,197]. Many studies have proved that exosomal miRNAs are abnormally expressed in certain types of cancer: for example, miRNA-17 and miRNA-21 were upregulated in colon, lung, stomach, prostate, and pancreatic cancer, and miRNA-155 was overexpressed in breast, lung, and colon tumors [198].
The blood levels of lncRNA-POU3F3 were identified as a diagnostic marker in esophageal squamous cell carcinoma [199], while lncRNA-MALAT-1 was overexpressed in prostate cancer [200]. Meanwhile, the overexpression of circRNA-IARS were identified in pancreatic cancer tissues and blood [201]. Due to the significant differential expression between patients and healthy groups, there is no doubt that exosomal ncRNA could be a promising biomarker in cancer. Besides, miRNA-122-5p from serum exosomes were found differentially expressed between multiple sclerosis patients and healthy individuals [202]. The exosomes with miRNA-223, miRNA-339 and miRNA-21 from platelets could suppress the PDGFR-β in SMCs and thereby proved to be biomarkers of atherosclerotic thrombosis [203]. Similarly, exosomal ncRNAs could also be biomarkers for early diagnosis of ischemic diseases [204].
Exosomal miRNA-21 is a proven biomarker of treatment outcome in non-small cell lung cancer (NSCLC); inhibiting miRNA-21 increases the sensitivity to chemotherapy of NSCLC by suppressing PI3K/AKT pathway [205,206]. Unlike miRNA, the specificity of lncRNA in tissue expression and the stability of circRNA in exosomes make them better biomarkers for molecular diagnosis and disease prognosis [107]. For example, exosomal lncRNA-SNHG15 is an indicator of poor prognosis in HCC [207].
Given the abilities of miRNAs to target multiple genes, targeting miRNA could be a promising therapeutic approach. There are two miRNA-based therapeutic modalities: miRNA mimics and anti -miRNA [208]. MiRNA mimics are small, double-stranded RNA molecules that can mimic miRNAs [208]. A previous study showed that miRNA-210 mimic enhanced angiogenesis in myocardial infarction mouse model [209]. Anti-miRNAs are antisense oligonucleotides with the complementary reverse sequences of miRNAs. They play a role in inhibiting miRNAs by interacting and combining with them. Costa et al. used anti-miRNA gene therapy approach to inhibit the proliferation and tumor size of glioblastoma in mice [210]. Although further research is needed to evaluate its clinical efficacy, this result suggested that miRNA targeted therapy is a promising new treatment. Moreover, exosomes, as intercellular mediators, have potential applications as drug carriers [211]. Dong et al. found that in the OIR model, using an exosome loading system with VEGF inhibitors to inhibit angiogenesis was superior to using VEGF inhibitors alone [212]. Actually, exosomes have been used in several clinical therapeutic tests such as NSCLC and colorectal cancer since 2000s [213,214]. It is feasible to target exosomes as vectors and exosomal miRNAs as new therapeutic strategies. In 2020, Peng et al. found the correlation of plasma exosomal miRNAs and efficacy of immunotherapy between NSCLC patient and healthy individuals, and predicted the possible miRNA therapeutic targets [215]. A clinical trial demonstrated the potential function of miRNAs derived from adipose extracellular vesicles in type 2 diabetes with pioglitazone treatment [216]. Besides, there was study showed that MSC-derived exosomes played an angiogenic effect under concentration of 100 μg/mL, while other result showed that lower concentration of MSC exosomes (10 μg/mL) maybe more effective than high concentration in tube formation [94,217]. The different results may be attributed to the differences in exosome size, purity, and surface molecules during the isolation of the MSC-derived exosomes [218,219]. The targets and mechanisms, as well as clinical therapeutic effects of exosomal ncRNAs should be further studied on this basis. Moreover, tumor-released exosomal ncRNAs were found to interfere with the sensitivity to radiotherapy and efficiency of treatment [220]. Consequently, exosomal ncRNAs are of great significance in the study of drug resistance.
10. Prospect and challenges of exosome research
Currently, the study of exosomes has been developed extensively; however, challenges still exist. EVs are divided into exosomes and microvesicles. Microvesicles are generated by budding from the plasma membrane, while exosomes are produced via the multivesicular endosome pathway. Microvesicles and exosomes have similar size, density, and biological composition; therefore, current methods cannot effectively distinguish them [221]. In addition, the relative ratio of exosomes and microvesicles released by cells is highly variable, depending on the cell type, environmental conditions, and other factors (such as infection or artificially induced).
Although currently no technology can completely isolate and purify exosomes [222], there are still some feasible isolation and purification techniques based on exosome characteristics [223]: 1) density-gradient separation enhances the purity of exosomes by further separating particles by density and effectively removing non-exosomes; 2) size-exclusion chromatography separates exosomes via a stationary phase composed of a porous polymer and is mainly used to separate exosomes from plasma; 3) immunological isolation based on immune affinity interactions between proteins present on the membrane of exosomes and their antibodies. Till now, the most common method of exosome isolation is ultracentrifugation, which is complex, tedious and time-consuming [211]. However, Heller et al. showed a novel alternating current electrokinetic microarray chip device which makes the isolation of exosomes convenient and fast [224]. It also makes exosome analysis suitable for point-of-care diagnostic applications. Such study revealed the application prospect of exosome research.
Notably, study indicated that exosomes secreted from red blood cells are more likely to be absorbed by the liver and bone while melanoma-derived exosomes are mainly uptake by the lung and spleen [225]. It suggested that exosomes derived from different cell may have different targets. Indeed, the mechanism of exosomes and surface receptors of target cells deserves in-depth study. Since the prospects for the treatment of angiogenesis diseases based on exosomal ncRNAs have been mentioned above, we should also set sights on the best way of exosome delivery and exosome concentration of exosome-based therapies [93].
Despite their great potential, our understanding of exosomal ncRNAs is still in the preliminary stages. The current understanding of the physiology, diversity, internalization, and molecular transport of exosomes is too limited to accurately draw conclusions about the mechanisms by which exosomes interact with and modify recipient cells [226]. Moreover, the interaction mechanisms between donor cells, recipient cells, and exosomes are unclear, such as how recipient cells recognize and ingest the donor cells-derived exosomes and whether the transport of exosome content is random or specific. Therefore, it is necessary to conduct comprehensive research, including biogenesis formation and sorting, to make progress in the field of exosomal ncRNAs [[227], [228], [229]].
11. Conclusion
Increasing attention has been paid to the physiological and pathological roles of exosomal ncRNAs. As a frontier research area, exosomal ncRNAs revealed increasing research value. This review described the roles and mechanisms of several main exosomal ncRNAs in angiogenesis. Exosomes as EVs exist widely in various body fluids and could mediate cell to cell communication. NcRNAs, as one of the components of exosomes, can be expressed stably in exosomes and can play an accurate role in regulation. In addition to the roles and mechanisms of exosomal ncRNAs in angiogenesis, we should also attach great importance to their potential clinical applications, such as in disease diagnosis based on their stable expressions in exosomes. Exosomal ncRNAs also play an important role in pharmacology, for instance, exosomes can serve as a new drug delivery vector, exosomal non-coding RNAs can act as a novel therapeutic target and drug resistance research. Therefore, further studies should emphasize the use of ncRNAs as clinical therapeutic targets in tumor angiogenesis and ocular neovascular disease. On the other hand, the study of exosomal ncRNAs as disease biomarkers is very valuable and important, and may greatly improve the diagnosis of some angiogenesis-related diseases. In summary, the study of exosomal ncRNAs in the future is promising.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
Dr Yedi Zhou was supported by National Natural Science Foundation of China {82271110}, Natural Science Foundation of Hunan Province {2022JJ30869}, The Science and Technology Innovation Program of Hunan Province {2021SK53526}, Scientific Research Project of Hunan Provincial Health Commission {202207022574}.
Zicong Wang was supported by the Fundamental Research Funds for the Central Universities of Central South University {2023ZZTS0882}.
Data availability statement
No data was used for the research described in the article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e18626.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Abels E.R., Breakefield X.O. Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cell. Mol. Neurobiol. 2016;36:301–312. doi: 10.1007/s10571-016-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Niel G., D’Angelo G., Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 3.Ratajczak J., Wysoczynski M., Hayek F., Janowska-Wieczorek A., Ratajczak M.Z. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20:1487–1495. doi: 10.1038/sj.leu.2404296. [DOI] [PubMed] [Google Scholar]
- 4.Andaloussi S.E., Mager I., Breakefield X.O., Wood M.J. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013;12:347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 5.Schorey J.S., Bhatnagar S. Exosome function: from tumor immunology to pathogen biology. Traffic. 2008;9:871–881. doi: 10.1111/j.1600-0854.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J.J., Lötvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 7.Rezaie J., Etemadi T., Feghhi M. The distinct roles of exosomes in innate immune responses and therapeutic applications in cancer. Eur. J. Pharmacol. 2022;933 doi: 10.1016/j.ejphar.2022.175292. [DOI] [PubMed] [Google Scholar]
- 8.Doyle L.M., Wang M.Z. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 2019;8 doi: 10.3390/cells8070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolukbasi M.F., Mizrak A., Ozdener G.B., Madlener S., Ströbel T., Erkan E.P., et al. miR-1289 and “Zipcode”-like sequence enrich mRNAs in microvesicles. Mol. Ther. Nucleic Acids. 2012;1:e10. doi: 10.1038/mtna.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melo S.A., Sugimoto H., O’Connell J.T., Kato N., Villanueva A., Vidal A., et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014;26:707–721. doi: 10.1016/j.ccell.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J., Yang P., Chen F., Tan Y., Huang C., Shen H., et al. Hypoxic colorectal cancer-derived extracellular vesicles deliver microRNA-361-3p to facilitate cell proliferation by targeting TRAF3 via the noncanonical NF-κB pathways. Clin. Transl. Med. 2021;11:e349. doi: 10.1002/ctm2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen F., Chu L., Li J., Shi Y., Xu B., Gu J., et al. Hypoxia induced changes in miRNAs and their target mRNAs in extracellular vesicles of esophageal squamous cancer cells. Thorac Cancer. 2020;11:570–580. doi: 10.1111/1759-7714.13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L., Yu D. Exosomes in cancer development, metastasis, and immunity. Biochim. Biophys. Acta Rev. Canc. 2019;1871:455–468. doi: 10.1016/j.bbcan.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L., Jiao G., Ren S., Zhang X., Li C., Wu W., et al. Exosomes from bone marrow mesenchymal stem cells enhance fracture healing through the promotion of osteogenesis and angiogenesis in a rat model of nonunion. Stem Cell Res. Ther. 2020;11:38. doi: 10.1186/s13287-020-1562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen F., Xu B., Li J., Yang X., Gu J., Yao X., et al. Hypoxic tumour cell-derived exosomal miR-340-5p promotes radioresistance of oesophageal squamous cell carcinoma via KLF10. J. Exp. Clin. Cancer Res. 2021;40:38. doi: 10.1186/s13046-021-01834-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rohlenova K., Veys K., Miranda-Santos I., De Bock K., Carmeliet P. Endothelial cell metabolism in Health and disease. Trends Cell Biol. 2018;28:224–236. doi: 10.1016/j.tcb.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Naito H., Iba T., Takakura N. Mechanisms of new blood-vessel formation and proliferative heterogeneity of endothelial cells. Int. Immunol. 2020;32:295–305. doi: 10.1093/intimm/dxaa008. [DOI] [PubMed] [Google Scholar]
- 18.Carmeliet P., Jain R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eelen G., de Zeeuw P., Treps L., Harjes U., Wong B.W., Carmeliet P. Endothelial cell metabolism. Physiol. Rev. 2018;98:3–58. doi: 10.1152/physrev.00001.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szymański M., Barciszewska M.Z., Zywicki M., Barciszewski J. Noncoding RNA transcripts. J. Appl. Genet. 2003;44:1–19. [PubMed] [Google Scholar]
- 21.Zhao Z., Sun W., Guo Z., Zhang J., Yu H., Liu B. Mechanisms of lncRNA/microRNA interactions in angiogenesis. Life Sci. 2020;254 doi: 10.1016/j.lfs.2019.116900. [DOI] [PubMed] [Google Scholar]
- 22.Ulitsky I., Bartel D.P. lincRNAs: genomics, evolution, and mechanisms. Cell. 2013;154:26–46. doi: 10.1016/j.cell.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu T.X., Rothenberg M.E. MicroRNA. J. Allergy Clin. Immunol. 2018;141:1202–1207. doi: 10.1016/j.jaci.2017.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen S., Xue Y., Wu X., Le C., Bhutkar A., Bell E.L., et al. Global microRNA depletion suppresses tumor angiogenesis. Genes Dev. 2014;28:1054–1067. doi: 10.1101/gad.239681.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tiwari A., Mukherjee B., Dixit M. MicroRNA key to angiogenesis regulation: MiRNA biology and therapy. Curr. Cancer Drug Targets. 2018;18:266–277. doi: 10.2174/1568009617666170630142725. [DOI] [PubMed] [Google Scholar]
- 26.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 27.Huntzinger E., Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat. Rev. Genet. 2011;12:99–110. doi: 10.1038/nrg2936. [DOI] [PubMed] [Google Scholar]
- 28.Finnegan E.F., Pasquinelli A.E. MicroRNA biogenesis: regulating the regulators. Crit. Rev. Biochem. Mol. Biol. 2013;48:51–68. doi: 10.3109/10409238.2012.738643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallo A., Tandon M., Alevizos I., Illei G.G. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS One. 2012;7 doi: 10.1371/journal.pone.0030679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lv L.L., Cao Y., Liu D., Xu M., Liu H., Tang R.N., et al. Isolation and quantification of microRNAs from urinary exosomes/microvesicles for biomarker discovery. Int. J. Biol. Sci. 2013;9:1021–1031. doi: 10.7150/ijbs.6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou Q., Li M., Wang X., Li Q., Wang T., Zhu Q., et al. Immune-related microRNAs are abundant in breast milk exosomes. Int. J. Biol. Sci. 2012;8:118–123. doi: 10.7150/ijbs.8.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato-Kuwabara Y., Melo S.A., Soares F.A., Calin G.A. The fusion of two worlds: non-coding RNAs and extracellular vesicles--diagnostic and therapeutic implications. Int. J. Oncol. 2015;46:17–27. doi: 10.3892/ijo.2014.2712. (Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J., Li S., Li L., Li M., Guo C., Yao J., et al. Exosome and exosomal microRNA: trafficking, sorting, and function. Dev. Reprod. Biol. 2015;13:17–24. doi: 10.1016/j.gpb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guduric-Fuchs J., O’Connor A., Camp B., O’Neill C.L., Medina R.J., Simpson D.A. Selective extracellular vesicle-mediated export of an overlapping set of microRNAs from multiple cell types. BMC Genom. 2012;13:357. doi: 10.1186/1471-2164-13-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Balkom B.W., de Jong O.G., Smits M., Brummelman J., den Ouden K., de Bree P.M., et al. Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood. 2013;121:3997–4006. doi: 10.1182/blood-2013-02-478925. s1-15. [DOI] [PubMed] [Google Scholar]
- 36.Umezu T., Ohyashiki K., Kuroda M., Ohyashiki J.H. Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene. 2013;32:2747–2755. doi: 10.1038/onc.2012.295. [DOI] [PubMed] [Google Scholar]
- 37.Sun Z., Shi K., Yang S., Liu J., Zhou Q., Wang G., et al. Effect of exosomal miRNA on cancer biology and clinical applications. Mol. Cancer. 2018;17:147. doi: 10.1186/s12943-018-0897-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olejarz W., Kubiak-Tomaszewska G., Chrzanowska A., Lorenc T. Exosomes in angiogenesis and anti-angiogenic therapy in cancers. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21165840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shaban S.A., Rezaie J., Nejati V. Exosomes derived from senescent endothelial cells contain distinct pro-angiogenic miRNAs and proteins. Cardiovasc. Toxicol. 2022;22:592–601. doi: 10.1007/s12012-022-09740-y. [DOI] [PubMed] [Google Scholar]
- 40.Mahbubfam S., Rezaie J., Nejati V. Crosstalk between exosomes signaling pathway and autophagy flux in senescent human endothelial cells. Tissue Cell. 2022;76 doi: 10.1016/j.tice.2022.101803. [DOI] [PubMed] [Google Scholar]
- 41.Cui H., Seubert B., Stahl E., Dietz H., Reuning U., Moreno-Leon L., et al. Tissue inhibitor of metalloproteinases-1 induces a pro-tumourigenic increase of miR-210 in lung adenocarcinoma cells and their exosomes. Oncogene. 2015;34:3640–3650. doi: 10.1038/onc.2014.300. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y., Luo F., Wang B., Li H., Xu Y., Liu X., et al. STAT3-regulated exosomal miR-21 promotes angiogenesis and is involved in neoplastic processes of transformed human bronchial epithelial cells. Cancer Lett. 2016;370:125–135. doi: 10.1016/j.canlet.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 43.Ni Y.Q., Lin X., Zhan J.K., Liu Y.S. Roles and functions of exosomal non-coding RNAs in vascular aging. Aging Dis. 2020;11:164–178. doi: 10.14336/AD.2019.0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang Y., Guo Z., Chen W., Wang X., Cao M., Han X., et al. M2 macrophage-derived exosomes promote angiogenesis and growth of pancreatic ductal adenocarcinoma by targeting E2F2. Mol. Ther. 2021;29:1226–1238. doi: 10.1016/j.ymthe.2020.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anand S., Majeti B.K., Acevedo L.M., Murphy E.A., Mukthavaram R., Scheppke L., et al. MicroRNA-132-mediated loss of p120RasGAP activates the endothelium to facilitate pathological angiogenesis. Nat. Med. 2010;16:909–914. doi: 10.1038/nm.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee D.Y., Deng Z., Wang C.H., Yang B.B. MicroRNA-378 promotes cell survival, tumor growth, and angiogenesis by targeting SuFu and Fus-1 expression. Proc. Natl. Acad. Sci. U. S. A. 2007;104:20350–20355. doi: 10.1073/pnas.0706901104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fasanaro P., D’Alessandra Y., Di Stefano V., Melchionna R., Romani S., Pompilio G., et al. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J. Biol. Chem. 2008;283:15878–15883. doi: 10.1074/jbc.M800731200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Plummer P.N., Freeman R., Taft R.J., Vider J., Sax M., Umer B.A., et al. MicroRNAs regulate tumor angiogenesis modulated by endothelial progenitor cells. Cancer Res. 2013;73:341–352. doi: 10.1158/0008-5472.CAN-12-0271. [DOI] [PubMed] [Google Scholar]
- 49.Lin X.J., Fang J.H., Yang X.J., Zhang C., Yuan Y., Zheng L., et al. Hepatocellular carcinoma cell-secreted exosomal MicroRNA-210 promotes angiogenesis in vitro and in vivo. Mol. Ther. Nucleic Acids. 2018;11:243–252. doi: 10.1016/j.omtn.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhuang G., Wu X., Jiang Z., Kasman I., Yao J., Guan Y., et al. Tumour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. EMBO J. 2012;31:3513–3523. doi: 10.1038/emboj.2012.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsu Y.L., Hung J.Y., Chang W.A., Lin Y.S., Pan Y.C., Tsai P.H., et al. Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene. 2017;36:4929–4942. doi: 10.1038/onc.2017.105. [DOI] [PubMed] [Google Scholar]
- 52.Valencia K., Luis-Ravelo D., Bovy N., Antón I., Martínez-Canarias S., Zandueta C., et al. miRNA cargo within exosome-like vesicle transfer influences metastatic bone colonization. Mol. Oncol. 2014;8:689–703. doi: 10.1016/j.molonc.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee J.K., Park S.R., Jung B.K., Jeon Y.K., Lee Y.S., Kim M.K., et al. Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS One. 2013;8 doi: 10.1371/journal.pone.0084256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gu X., Wang X.Q., Lin M.J., Liang H., Fan S.Y., Wang L., et al. Molecular interplay between microRNA-130a and PTEN in palmitic acid-mediated impaired function of endothelial progenitor cells: effects of metformin. Int. J. Mol. Med. 2019;43:2187–2198. doi: 10.3892/ijmm.2019.4140. [DOI] [PubMed] [Google Scholar]
- 55.Bai M., Li J., Yang H., Zhang H., Zhou Z., Deng T., et al. miR-135b delivered by gastric tumor exosomes inhibits FOXO1 expression in endothelial cells and promotes angiogenesis. Mol. Ther. 2019;27:1772–1783. doi: 10.1016/j.ymthe.2019.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56.Chen Z., Xie Y., Chen W., Li T., Chen X., Liu B. microRNA-6785-5p-loaded human umbilical cord mesenchymal stem cells-derived exosomes suppress angiogenesis and metastasis in gastric cancer via INHBA. Life Sci. 2021;284 doi: 10.1016/j.lfs.2021.119222. [DOI] [PubMed] [Google Scholar]
- 57.Chen W., Huang L., Liang J., Ye Y., He S., Niu J. Hepatocellular carcinoma cells-derived exosomal microRNA-378b enhances hepatocellular carcinoma angiogenesis. Life Sci. 2021;273 doi: 10.1016/j.lfs.2023.121426. [DOI] [PubMed] [Google Scholar]
- 58.Zhang H., Bai M., Deng T., Liu R., Wang X., Qu Y., et al. Cell-derived microvesicles mediate the delivery of miR-29a/c to suppress angiogenesis in gastric carcinoma. Cancer Lett. 2016;375:331–339. doi: 10.1016/j.canlet.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 59.Yin Z., Ma T., Huang B., Lin L., Zhou Y., Yan J., et al. Macrophage-derived exosomal microRNA-501-3p promotes progression of pancreatic ductal adenocarcinoma through the TGFBR3-mediated TGF-β signaling pathway. J. Exp. Clin. Cancer Res. 2019;38:310. doi: 10.1186/s13046-019-1313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Deng T., Zhang H., Yang H., Wang H., Bai M., Sun W., et al. Exosome miR-155 derived from gastric carcinoma promotes angiogenesis by targeting the c-MYB/VEGF Axis of endothelial cells. Mol. Ther. Nucleic Acids. 2020;19:1449–1459. doi: 10.1016/j.omtn.2020.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Zhu J., Liu B., Wang Z., Wang D., Ni H., Zhang L., et al. Exosomes from nicotine-stimulated macrophages accelerate atherosclerosis through miR-21-3p/PTEN-mediated VSMC migration and proliferation. Theranostics. 2019;9:6901–6919. doi: 10.7150/thno.37357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li W., Jin L.Y., Cui Y.B., Xie N. Human umbilical cord mesenchymal stem cells-derived exosomal microRNA-17-3p ameliorates inflammatory reaction and antioxidant injury of mice with diabetic retinopathy via targeting STAT1. Int. Immunopharm. 2021;90 doi: 10.1016/j.intimp.2020.107010. [DOI] [PubMed] [Google Scholar]
- 63.Li W., Jin L., Cui Y., Nie A., Xie N., Liang G. Bone marrow mesenchymal stem cells-induced exosomal microRNA-486-3p protects against diabetic retinopathy through TLR4/NF-κB axis repression. J. Endocrinol. Invest. 2021;44:1193–1207. doi: 10.1007/s40618-020-01405-3. [DOI] [PubMed] [Google Scholar]
- 64.Gu S., Liu Y., Zou J., Wang W., Wei T., Wang X., et al. Retinal pigment epithelial cells secrete miR-202-5p-containing exosomes to protect against proliferative diabetic retinopathy. Exp. Eye Res. 2020;201 doi: 10.1016/j.exer.2020.108271. [DOI] [PubMed] [Google Scholar]
- 65.Huarte M., Guttman M., Feldser D., Garber M., Koziol M.J., Kenzelmann-Broz D., et al. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell. 2010;142:409–419. doi: 10.1016/j.cell.2010.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fu W., Liang D., Wu X., Chen H., Hong X., Wang J., et al. Long noncoding RNA LINC01435 impedes diabetic wound healing by facilitating YY1-mediated HDAC8 expression. iScience. 2022;25 doi: 10.1016/j.isci.2022.104006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li Y., Lin S., Xie X., Zhu H., Fan T., Wang S. Highly enriched exosomal lncRNA OIP5-AS1 regulates osteosarcoma tumor angiogenesis and autophagy through miR-153 and ATG5. Am J Transl Res. 2021;13:4211–4223. [PMC free article] [PubMed] [Google Scholar]
- 68.Ma X., Li Z., Li T., Zhu L., Li Z., Tian N. Long non-coding RNA HOTAIR enhances angiogenesis by induction of VEGFA expression in glioma cells and transmission to endothelial cells via glioma cell derived-extracellular vesicles. Am J Transl Res. 2017;9:5012–5021. [PMC free article] [PubMed] [Google Scholar]
- 69.Conigliaro A., Costa V., Lo Dico A., Saieva L., Buccheri S., Dieli F., et al. CD90+ liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Mol. Cancer. 2015;14:155. doi: 10.1186/s12943-015-0426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Q., Li T., Wang Z., Kuang X., Shao N., Lin Y. lncRNA NR2F1-AS1 promotes breast cancer angiogenesis through activating IGF-1/IGF-1R/ERK pathway. J. Cell Mol. Med. 2020;24:8236–8247. doi: 10.1111/jcmm.15499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dong F., Ruan S., Wang J., Xia Y., Le K., Xiao X., et al. M2 macrophage-induced lncRNA PCAT6 facilitates tumorigenesis and angiogenesis of triple-negative breast cancer through modulation of VEGFR2. Cell Death Dis. 2020;11:728. doi: 10.1038/s41419-020-02926-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li X., Lei Y., Wu M., Li N. Regulation of macrophage activation and polarization by HCC-derived exosomal lncRNA TUC339. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19102958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang R., Ma X., Jiang L., Xia W., Li H., Zhao N., et al. Decreased lncRNA SNHG16 accelerates oxidative stress induced pathological angiogenesis in human retinal microvascular endothelial cells by regulating miR-195/mfn2 Axis. Curr. Pharmaceut. Des. 2021;27:3047–3060. doi: 10.2174/1381612827666210202141541. [DOI] [PubMed] [Google Scholar]
- 74.Lang H.L., Hu G.W., Zhang B., Kuang W., Chen Y., Wu L., et al. Glioma cells enhance angiogenesis and inhibit endothelial cell apoptosis through the release of exosomes that contain long non-coding RNA CCAT2. Oncol. Rep. 2017;38:785–798. doi: 10.3892/or.2017.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guo Z., Wang X., Yang Y., Chen W., Zhang K., Teng B., et al. Hypoxic tumor-derived exosomal long noncoding RNA UCA1 promotes angiogenesis via miR-96-5p/AMOTL2 in pancreatic cancer. Mol. Ther. Nucleic Acids. 2020;22:179–195. doi: 10.1016/j.omtn.2020.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Holdt L.M., Stahringer A., Sass K., Pichler G., Kulak N.A., Wilfert W., et al. Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nat. Commun. 2016;7 doi: 10.1038/ncomms12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang X.Y., Huang Z.L., Huang J., Xu B., Huang X.Y., Xu Y.H., et al. Exosomal circRNA-100338 promotes hepatocellular carcinoma metastasis via enhancing invasiveness and angiogenesis. J. Exp. Clin. Cancer Res. 2020;39:20. doi: 10.1186/s13046-020-1529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xie M., Yu T., Jing X., Ma L., Fan Y., Yang F., et al. Exosomal circSHKBP1 promotes gastric cancer progression via regulating the miR-582-3p/HUR/VEGF axis and suppressing HSP90 degradation. Mol. Cancer. 2020;19:112. doi: 10.1186/s12943-020-01208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu C., Ge H.M., Liu B.H., Dong R., Shan K., Chen X., et al. Targeting pericyte-endothelial cell crosstalk by circular RNA-cPWWP2A inhibition aggravates diabetes-induced microvascular dysfunction. Proc. Natl. Acad. Sci. U. S. A. 2019;116:7455–7464. doi: 10.1073/pnas.1814874116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang S.J., Chen X., Li C.P., Li X.M., Liu C., Liu B.H., et al. Identification and characterization of circular RNAs as a new class of putative biomarkers in diabetes retinopathy. Invest. Ophthalmol. Vis. Sci. 2017;58:6500–6509. doi: 10.1167/iovs.17-22698. [DOI] [PubMed] [Google Scholar]
- 81.Shan K., Liu C., Liu B.H., Chen X., Dong R., Liu X., et al. Circular noncoding RNA HIPK3 mediates retinal vascular dysfunction in diabetes mellitus. Circulation. 2017;136:1629–1642. doi: 10.1161/CIRCULATIONAHA.117.029004. [DOI] [PubMed] [Google Scholar]
- 82.Zhu K., Hu X., Chen H., Li F., Yin N., Liu A.L., et al. Downregulation of circRNA DMNT3B contributes to diabetic retinal vascular dysfunction through targeting miR-20b-5p and BAMBI. EBioMedicine. 2019;49:341–353. doi: 10.1016/j.ebiom.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu C., Yao M.D., Li C.P., Shan K., Yang H., Wang J.J., et al. Silencing of circular RNA-ZNF609 ameliorates vascular endothelial dysfunction. Theranostics. 2017;7:2863–2877. doi: 10.7150/thno.19353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Loewer S., Cabili M.N., Guttman M., Loh Y.H., Thomas K., Park I.H., et al. Large intergenic non-coding RNA-RoR modulates reprogramming of human induced pluripotent stem cells. Nat. Genet. 2010;42:1113–1117. doi: 10.1038/ng.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang Z., Jiang S., Shang J., Jiang Y., Dai Y., Xu B., et al. LncRNA: shedding light on mechanisms and opportunities in fibrosis and aging. Ageing Res. Rev. 2019;52:17–31. doi: 10.1016/j.arr.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 86.Bernard D., Prasanth K.V., Tripathi V., Colasse S., Nakamura T., Xuan Z., et al. A long nuclear-retained non-coding RNA regulates synaptogenesis by modulating gene expression. EMBO J. 2010;29:3082–3093. doi: 10.1038/emboj.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dykes I.M., Emanueli C. Transcriptional and post-transcriptional gene regulation by long non-coding RNA. Dev. Reprod. Biol. 2017;15:177–186. doi: 10.1016/j.gpb.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang K.C., Chang H.Y. Molecular mechanisms of long noncoding RNAs. Mol. Cell. 2011;43:904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hung T., Wang Y., Lin M.F., Koegel A.K., Kotake Y., Grant G.D., et al. Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nat. Genet. 2011;43:621–629. doi: 10.1038/ng.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gupta R.A., Shah N., Wang K.C., Kim J., Horlings H.M., Wong D.J., et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim K.M., Abdelmohsen K., Mustapic M., Kapogiannis D., Gorospe M. Wiley Interdiscip Rev RNA; 2017. RNA in Extracellular Vesicles; p. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Casado-Díaz A., Quesada-Gómez J.M., Dorado G. Extracellular vesicles derived from mesenchymal stem cells (MSC) in regenerative medicine: applications in skin wound healing. Front. Bioeng. Biotechnol. 2020;8:146. doi: 10.3389/fbioe.2020.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Todorova D., Simoncini S., Lacroix R., Sabatier F., Dignat-George F. Extracellular vesicles in angiogenesis. Circ. Res. 2017;120:1658–1673. doi: 10.1161/CIRCRESAHA.117.309681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liang X., Zhang L., Wang S., Han Q., Zhao R.C. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J. Cell Sci. 2016;129:2182–2189. doi: 10.1242/jcs.170373. [DOI] [PubMed] [Google Scholar]
- 95.Banfi C., Brioschi M., Wait R., Begum S., Gianazza E., Pirillo A., et al. Proteome of endothelial cell-derived procoagulant microparticles. Proteomics. 2005;5:4443–4455. doi: 10.1002/pmic.200402017. [DOI] [PubMed] [Google Scholar]
- 96.Wang X., Li L., Zhao K., Lin Q., Li H., Xue X., et al. A novel LncRNA HITT forms a regulatory loop with HIF-1α to modulate angiogenesis and tumor growth. Cell Death Differ. 2020;27:1431–1446. doi: 10.1038/s41418-019-0449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Behera J., Kumar A., Voor M.J., Tyagi N. Exosomal lncRNA-H19 promotes osteogenesis and angiogenesis through mediating Angpt1/Tie2-NO signaling in CBS-heterozygous mice. Theranostics. 2021;11:7715–7734. doi: 10.7150/thno.58410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sun G., Wang Y., Zhang J., Lin N., You Y. MiR-15b/HOTAIR/p53 form a regulatory loop that affects the growth of glioma cells. J. Cell. Biochem. 2018;119:4540–4547. doi: 10.1002/jcb.26591. [DOI] [PubMed] [Google Scholar]
- 99.Lamichhane T.N., Leung C.A., Douti L.Y., Jay S.M. Ethanol induces enhanced vascularization bioactivity of endothelial cell-derived extracellular vesicles via regulation of MicroRNAs and long non-coding RNAs. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-14356-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tay Y., Rinn J., Pandolfi P.P. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505:344–352. doi: 10.1038/nature12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Thomson D.W., Dinger M.E. Endogenous microRNA sponges: evidence and controversy. Nat. Rev. Genet. 2016;17:272–283. doi: 10.1038/nrg.2016.20. [DOI] [PubMed] [Google Scholar]
- 102.Salzman J. Circular RNA expression: its potential regulation and function. Trends Genet. 2016;32:309–316. doi: 10.1016/j.tig.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li X., Yang L., Chen L.L. The biogenesis, functions, and challenges of circular RNAs. Mol. Cell. 2018;71:428–442. doi: 10.1016/j.molcel.2018.06.034. [DOI] [PubMed] [Google Scholar]
- 104.Hansen T.B., Jensen T.I., Clausen B.H., Bramsen J.B., Finsen B., Damgaard C.K., et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 105.Li Z., Huang C., Bao C., Chen L., Lin M., Wang X., et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat. Struct. Mol. Biol. 2015;22:256–264. doi: 10.1038/nsmb.2959. [DOI] [PubMed] [Google Scholar]
- 106.Du W.W., Zhang C., Yang W., Yong T., Awan F.M., Yang B.B. Identifying and characterizing circRNA-protein interaction. Theranostics. 2017;7:4183–4191. doi: 10.7150/thno.21299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang Y., Liu J., Ma J., Sun T., Zhou Q., Wang W., et al. Exosomal circRNAs: biogenesis, effect and application in human diseases. Mol. Cancer. 2019;18:116. doi: 10.1186/s12943-019-1041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang S., Dong Y., Gong A., Kong H., Gao J., Hao X., et al. Exosomal circRNAs as novel cancer biomarkers: challenges and opportunities. Int. J. Biol. Sci. 2021;17:562–573. doi: 10.7150/ijbs.48782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rybak-Wolf A., Stottmeister C., Glažar P., Jens M., Pino N., Giusti S., et al. Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol. Cell. 2015;58:870–885. doi: 10.1016/j.molcel.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 110.Westholm J.O., Miura P., Olson S., Shenker S., Joseph B., Sanfilippo P., et al. Genome-wide analysis of drosophila circular RNAs reveals their structural and sequence properties and age-dependent neural accumulation. Cell Rep. 2014;9:1966–1980. doi: 10.1016/j.celrep.2014.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Robbins P.D., Morelli A.E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Preußer C., Hung L.H., Schneider T., Schreiner S., Hardt M., Moebus A., et al. Selective release of circRNAs in platelet-derived extracellular vesicles. J. Extracell. Vesicles. 2018;7 doi: 10.1080/20013078.2018.1424473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sun L.L., Li W.D., Lei F.R., Li X.Q. The regulatory role of microRNAs in angiogenesis-related diseases. J. Cell Mol. Med. 2018;22:4568–4587. doi: 10.1111/jcmm.13700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang W., Wang Y., Piao H., Li B., Huang M., Zhu Z., et al. Circular RNAs as potential biomarkers and therapeutics for cardiovascular disease. PeerJ. 2019;7 doi: 10.7717/peerj.6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vahabi A., Rezaie J., Hassanpour M., Panahi Y., Nemati M., Rasmi Y., et al. Tumor Cells-derived exosomal CircRNAs: novel cancer drivers, molecular mechanisms, and clinical opportunities. Biochem. Pharmacol. 2022;200 doi: 10.1016/j.bcp.2022.115038. [DOI] [PubMed] [Google Scholar]
- 116.Yu M., Yu J., Zhang Y., Sun X., Sun R., Xia M., et al. A novel circRNA-miRNA-mRNA network revealed exosomal circ-ATP10A as a biomarker for multiple myeloma angiogenesis. Bioengineered. 2022;13:667–683. doi: 10.1080/21655979.2021.2012553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen Y.G., Chen R., Ahmad S., Verma R., Kasturi S.P., Amaya L., et al. N6-Methyladenosine modification controls circular RNA immunity. Mol. Cell. 2019;76:96–109.e9. doi: 10.1016/j.molcel.2019.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bartosovic M., Molares H.C., Gregorova P., Hrossova D., Kudla G., Vanacova S. N6-methyladenosine demethylase FTO targets pre-mRNAs and regulates alternative splicing and 3'-end processing. Nucleic Acids Res. 2017;45:11356–11370. doi: 10.1093/nar/gkx778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Du H., Zhao Y., He J., Zhang Y., Xi H., Liu M., et al. YTHDF2 destabilizes m(6)A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat. Commun. 2016;7 doi: 10.1038/ncomms12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhao X., Yang Y., Sun B.F., Shi Y., Yang X., Xiao W., et al. FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res. 2014;24:1403–1419. doi: 10.1038/cr.2014.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu J., Yue Y., Han D., Wang X., Fu Y., Zhang L., et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat. Chem. Biol. 2014;10:93–95. doi: 10.1038/nchembio.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu N., Dai Q., Zheng G., He C., Parisien M., Pan T. N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature. 2015;518:560–564. doi: 10.1038/nature14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pamudurti N.R., Bartok O., Jens M., Ashwal-Fluss R., Stottmeister C., Ruhe L., et al. Translation of CircRNAs. Mol. Cell. 2017;66:9–21.e7. doi: 10.1016/j.molcel.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Panneerdoss S., Eedunuri V.K., Yadav P., Timilsina S., Rajamanickam S., Viswanadhapalli S., et al. Cross-talk among writers, readers, and erasers of m(6)A regulates cancer growth and progression. Sci. Adv. 2018;4:eaar8263. doi: 10.1126/sciadv.aar8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Park O.H., Ha H., Lee Y., Boo S.H., Kwon D.H., Song H.K., et al. Endoribonucleolytic cleavage of m(6)A-containing RNAs by RNase P/MRP complex. Mol. Cell. 2019;74:494–507.e8. doi: 10.1016/j.molcel.2019.02.034. [DOI] [PubMed] [Google Scholar]
- 126.Wang W., Qiao S.C., Wu X.B., Sun B., Yang J.G., Li X., et al. Circ_0008542 in osteoblast exosomes promotes osteoclast-induced bone resorption through m6A methylation. Cell Death Dis. 2021;12:628. doi: 10.1038/s41419-021-03915-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ma S., Chen C., Ji X., Liu J., Zhou Q., Wang G., et al. The interplay between m6A RNA methylation and noncoding RNA in cancer. J. Hematol. Oncol. 2019;12:121. doi: 10.1186/s13045-019-0805-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhu L., Liu X., Pu W., Peng Y. tRNA-derived small non-coding RNAs in human disease. Cancer Lett. 2018;419:1–7. doi: 10.1016/j.canlet.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 129.Kim H.K., Fuchs G., Wang S., Wei W., Zhang Y., Park H., et al. A transfer-RNA-derived small RNA regulates ribosome biogenesis. Nature. 2017;552:57–62. doi: 10.1038/nature25005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen Q., Yan M., Cao Z., Li X., Zhang Y., Shi J., et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science. 2016;351:397–400. doi: 10.1126/science.aad7977. [DOI] [PubMed] [Google Scholar]
- 131.Dou R., Zhang X., Xu X., Wang P., Yan B. Mesenchymal stem cell exosomal tsRNA-21109 alleviate systemic lupus erythematosus by inhibiting macrophage M1 polarization. Mol. Immunol. 2021;139:106–114. doi: 10.1016/j.molimm.2021.08.015. [DOI] [PubMed] [Google Scholar]
- 132.Anaya-Prado R., Toledo-Pereyra L.H., Lentsch A.B., Ward P.A. Ischemia/reperfusion injury. J. Surg. Res. 2002;105:248–258. doi: 10.1006/jsre.2002.6385. [DOI] [PubMed] [Google Scholar]
- 133.Li Q., Hu B., Hu G.W., Chen C.Y., Niu X., Liu J., et al. tRNA-derived small non-coding RNAs in response to ischemia inhibit angiogenesis. Sci. Rep. 2016;6 doi: 10.1038/srep20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li L., Liu P., Wang R., Huang Y., Luo J., Jiao L., et al. Pathophysiological significance of neutrophilic transfer RNA-derived small RNAs in asymptomatic moyamoya disease. Cells. 2021;10 doi: 10.3390/cells10051086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shang S., Zhou D., Ya J., Li S., Yang Q., Ding Y., et al. Progress in moyamoya disease. Neurosurg. Rev. 2020;43:371–382. doi: 10.1007/s10143-018-0994-5. [DOI] [PubMed] [Google Scholar]
- 136.Morais P., Adachi H., Yu Y.T. Spliceosomal snRNA epitranscriptomics. Front. Genet. 2021;12 doi: 10.3389/fgene.2021.652129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liu Y., Gu Y., Han Y., Zhang Q., Jiang Z., Zhang X., et al. Tumor exosomal RNAs promote lung pre-metastatic niche formation by activating alveolar epithelial TLR3 to recruit neutrophils. Cancer Cell. 2016;30:243–256. doi: 10.1016/j.ccell.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 138.Alshaer W., Zureigat H., Al Karaki A., Al-Kadash A., Gharaibeh L., Hatmal M.M., et al. siRNA: mechanism of action, challenges, and therapeutic approaches. Eur. J. Pharmacol. 2021;905 doi: 10.1016/j.ejphar.2021.174178. [DOI] [PubMed] [Google Scholar]
- 139.Zhang H., Wang Y., Bai M., Wang J., Zhu K., Liu R., et al. Exosomes serve as nanoparticles to suppress tumor growth and angiogenesis in gastric cancer by delivering hepatocyte growth factor siRNA. Cancer Sci. 2018;109:629–641. doi: 10.1111/cas.13488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang C., Su Y., Ding H., Yin J., Zhu Z., Song W. Mesenchymal stem cells-derived and siRNAs-encapsulated exosomes inhibit osteonecrosis of the femoral head. J. Cell Mol. Med. 2020;24:9605–9612. doi: 10.1111/jcmm.15395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wajahat M., Bracken C.P., Orang A. Emerging functions for snoRNAs and snoRNA-derived fragments. Int. J. Mol. Sci. 2021:22. doi: 10.3390/ijms221910193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.El-Andaloussi S., Lee Y., Lakhal-Littleton S., Li J., Seow Y., Gardiner C., et al. Exosome-mediated delivery of siRNA in vitro and in vivo. Nat. Protoc. 2012;7:2112–2126. doi: 10.1038/nprot.2012.131. [DOI] [PubMed] [Google Scholar]
- 143.Viallard C., Larrivée B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis. 2017;20:409–426. doi: 10.1007/s10456-017-9562-9. [DOI] [PubMed] [Google Scholar]
- 144.Jiang X., Wang J., Deng X., Xiong F., Zhang S., Gong Z., et al. The role of microenvironment in tumor angiogenesis. J. Exp. Clin. Cancer Res. 2020;39:204. doi: 10.1186/s13046-020-01709-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Stewart M., Talks K., Leek R., Turley H., Pezzella F., Harris A., et al. Expression of angiogenic factors and hypoxia inducible factors HIF 1, HIF 2 and CA IX in non-Hodgkin’s lymphoma. Histopathology. 2002;40:253–260. doi: 10.1046/j.1365-2559.2002.01357.x. [DOI] [PubMed] [Google Scholar]
- 146.Dai J., Su Y., Zhong S., Cong L., Liu B., Yang J., et al. Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduct. Targeted Ther. 2020;5:145. doi: 10.1038/s41392-020-00261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rahbarghazi R., Jabbari N., Sani N.A., Asghari R., Salimi L., Kalashani S.A., et al. Tumor-derived extracellular vesicles: reliable tools for Cancer diagnosis and clinical applications. Cell Commun. Signal. 2019;17:73. doi: 10.1186/s12964-019-0390-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kosaka N., Iguchi H., Hagiwara K., Yoshioka Y., Takeshita F., Ochiya T. Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic microRNAs regulate cancer cell metastasis. J. Biol. Chem. 2013;288:10849–10859. doi: 10.1074/jbc.M112.446831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Matsuura Y., Wada H., Eguchi H., Gotoh K., Kobayashi S., Kinoshita M., et al. Exosomal miR-155 derived from hepatocellular carcinoma cells under hypoxia promotes angiogenesis in endothelial cells. Dig. Dis. Sci. 2019;64:792–802. doi: 10.1007/s10620-018-5380-1. [DOI] [PubMed] [Google Scholar]
- 150.Zeng Z., Li Y., Pan Y., Lan X., Song F., Sun J., et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat. Commun. 2018;9:5395. doi: 10.1038/s41467-018-07810-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cheng W.C., Liao T.T., Lin C.C., Yuan L.E., Lan H.Y., Lin H.H., et al. RAB27B-activated secretion of stem-like tumor exosomes delivers the biomarker microRNA-146a-5p, which promotes tumorigenesis and associates with an immunosuppressive tumor microenvironment in colorectal cancer. Int. J. Cancer. 2019;145:2209–2224. doi: 10.1002/ijc.32338. [DOI] [PubMed] [Google Scholar]
- 152.Wang M., Yu F., Ding H., Wang Y., Li P., Wang K. Emerging function and clinical values of exosomal MicroRNAs in cancer. Mol. Ther. Nucleic Acids. 2019;16:791–804. doi: 10.1016/j.omtn.2019.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang F., Sun Y., Shi J. Programmed death-ligand 1 monoclonal antibody-linked immunoliposomes for synergistic efficacy of miR-130a and oxaliplatin in gastric cancers. Nanomedicine (Lond) 2019;14:1729–1744. doi: 10.2217/nnm-2019-0073. [DOI] [PubMed] [Google Scholar]
- 154.Campochiaro P.A. Retinal and choroidal neovascularization. J. Cell. Physiol. 2000;184:301–310. doi: 10.1002/1097-4652(200009)184:3<301::AID-JCP3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 155.Campochiaro P.A. Ocular neovascularization. J Mol Med (Berl). 2013;91:311–321. doi: 10.1007/s00109-013-0993-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Eid S., Sas K.M., Abcouwer S.F., Feldman E.L., Gardner T.W., Pennathur S., et al. New insights into the mechanisms of diabetic complications: role of lipids and lipid metabolism. Diabetologia. 2019;62:1539–1549. doi: 10.1007/s00125-019-4959-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tokarz A., Szuścik I., Kuśnierz-Cabala B., Kapusta M., Konkolewska M., Żurakowski A., et al. Extracellular vesicles participate in the transport of cytokines and angiogenic factors in diabetic patients with ocular complications. Folia Med. Cracov. 2015;55:35–48. [PubMed] [Google Scholar]
- 158.Cao X., Xue L.D., Di Y., Li T., Tian Y.J., Song Y. MSC-derived exosomal lncRNA SNHG7 suppresses endothelial-mesenchymal transition and tube formation in diabetic retinopathy via miR-34a-5p/XBP1 axis. Life Sci. 2021;272 doi: 10.1016/j.lfs.2021.119232. [DOI] [PubMed] [Google Scholar]
- 159.Maisto R., Trotta M.C., Petrillo F., Izzo S., Cuomo G., Alfano R., et al. Resolvin D1 modulates the intracellular VEGF-related miRNAs of retinal photoreceptors challenged with high glucose. Front. Pharmacol. 2020;11:235. doi: 10.3389/fphar.2020.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kamalden T.A., Macgregor-Das A.M., Kannan S.M., Dunkerly-Eyring B., Khaliddin N., Xu Z.H., et al. Exosomal MicroRNA-15a transfer from the pancreas augments diabetic complications by inducing oxidative stress. Antioxidants Redox Signal. 2017;27:913–930. doi: 10.1089/ars.2016.6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kong L.K., Fry M., Al-Samarraie M., Gilbert C., Steinkuller P.G. An update on progress and the changing epidemiology of causes of childhood blindness worldwide. Journal of Aapos. 2012;16:501–507. doi: 10.1016/j.jaapos.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 162.Connor K.M., Krah N.M., Dennison R.J., Aderman C.M., Chen J., Guerin K.I., et al. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat. Protoc. 2009;4:1565–1573. doi: 10.1038/nprot.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Silverman S.M., Wong W.T. Microglia in the retina: roles in development, maturity, and disease. Annu Rev Vis Sci. 2018;4:45–77. doi: 10.1146/annurev-vision-091517-034425. [DOI] [PubMed] [Google Scholar]
- 164.Xu W.Q., Wu Y., Hu Z.C., Sun L.J., Dou G.R., Zhang Z.F., et al. Exosomes from microglia attenuate photoreceptor injury and neovascularization in an animal model of retinopathy of prematurity. Mol. Ther. Nucleic Acids. 2019;16:778–790. doi: 10.1016/j.omtn.2019.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Moisseiev E., Anderson J.D., Oltjen S., Goswami M., Zawadzki R.J., Nolta J.A., et al. Protective effect of intravitreal administration of exosomes derived from mesenchymal stem cells on retinal ischemia. Curr. Eye Res. 2017;42:1358–1367. doi: 10.1080/02713683.2017.1319491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Takahashi K., Ishibashi T., Ogura Y., Yuzawa M., Working Grp Establishing Diag C. Classification and diagnostic criteria of age-related macular degeneration. Nippon. Ganka Gakkai Zasshi. 2008;112:1076–1084. [PubMed] [Google Scholar]
- 167.Tong Y., Zhou Y.L., Wang Y.X., Zhao P.Q., Wang Z.Y. Retinal pigment epithelium cell-derived exosomes: possible relevance to CNV in wet-age related macular degeneration. Med. Hypotheses. 2016;97:98–101. doi: 10.1016/j.mehy.2016.10.027. [DOI] [PubMed] [Google Scholar]
- 168.Nowak J.Z. Age-related macular degeneration (AMD): pathogenesis and therapy. Pharmacol. Rep. 2006;58:353–363. [PubMed] [Google Scholar]
- 169.Datta S., Cano M., Ebrahimi K., Wang L., Handa J.T. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog. Retin. Eye Res. 2017;60:201–218. doi: 10.1016/j.preteyeres.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Shah N., Ishii M., Brandon C., Ablonczy Z., Cai J.W., Liu Y.T., et al. Extracellular vesicle-mediated long-range communication in stressed retinal pigment epithelial cell monolayers. Biochim. Biophys. Acta-Mol. Basis Dis. 2018;1864:2610–2622. doi: 10.1016/j.bbadis.2018.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ramírez J.M., Ramírez A.I., Salazar J.J., de Hoz R., Triviño A. Changes of astrocytes in retinal ageing and age-related macular degeneration. Exp. Eye Res. 2001;73:601–615. doi: 10.1006/exer.2001.1061. [DOI] [PubMed] [Google Scholar]
- 172.Hajrasouliha A.R., Jiang G.M., Lu Q.X., Lu H.Y., Kaplan H.J., Zhang H.G., et al. Exosomes from retinal astrocytes contain antiangiogenic components that inhibit laser-induced choroidal neovascularization. J. Biol. Chem. 2013;288:28058–28067. doi: 10.1074/jbc.M113.470765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.ElShelmani H., Wride M.A., Saad T., Rani S., Kelly D.J., Keegan D. The role of deregulated MicroRNAs in age-related macular degeneration pathology. Transl. Vis. Sci. Technol. 2021;10:12. doi: 10.1167/tvst.10.2.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang Y., Zhao R., Liu W., Wang Z., Rong J., Long X., et al. Exosomal circHIPK3 released from hypoxia-pretreated cardiomyocytes regulates oxidative damage in cardiac microvascular endothelial cells via the miR-29a/IGF-1 pathway. Oxid. Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/7954657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang S., Zhan J., Lin X., Wang Y., Wang Y., Liu Y. CircRNA-0077930 from hyperglycaemia-stimulated vascular endothelial cell exosomes regulates senescence in vascular smooth muscle cells. Cell Biochem. Funct. 2020;38:1056–1068. doi: 10.1002/cbf.3543. [DOI] [PubMed] [Google Scholar]
- 176.Elshabrawy H.A., Chen Z., Volin M.V., Ravella S., Virupannavar S., Shahrara S. The pathogenic role of angiogenesis in rheumatoid arthritis. Angiogenesis. 2015;18:433–448. doi: 10.1007/s10456-015-9477-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Withrow J., Murphy C., Liu Y., Hunter M., Fulzele S., Hamrick M.W. Extracellular vesicles in the pathogenesis of rheumatoid arthritis and osteoarthritis. Arthritis Res. Ther. 2016;18:286. doi: 10.1186/s13075-016-1178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kato T., Miyaki S., Ishitobi H., Nakamura Y., Nakasa T., Lotz M.K., et al. Exosomes from IL-1β stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes. Arthritis Res. Ther. 2014;16:R163. doi: 10.1186/ar4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Falk E. Pathogenesis of atherosclerosis. J. Am. Coll. Cardiol. 2006;47:C7–C12. doi: 10.1016/j.jacc.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 180.Chen L., Yang W., Guo Y., Chen W., Zheng P., Zeng J., et al. Exosomal lncRNA GAS5 regulates the apoptosis of macrophages and vascular endothelial cells in atherosclerosis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0185406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cheng Y., Wang X., Yang J., Duan X., Yao Y., Shi X., et al. A translational study of urine miRNAs in acute myocardial infarction. J. Mol. Cell. Cardiol. 2012;53:668–676. doi: 10.1016/j.yjmcc.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Aurora A.B., Mahmoud A.I., Luo X., Johnson B.A., van Rooij E., Matsuzaki S., et al. MicroRNA-214 protects the mouse heart from ischemic injury by controlling Ca2⁺ overload and cell death. J. Clin. Invest. 2012;122:1222–1232. doi: 10.1172/JCI59327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Grimolizzi F., Monaco F., Leoni F., Bracci M., Staffolani S., Bersaglieri C., et al. Exosomal miR-126 as a circulating biomarker in non-small-cell lung cancer regulating cancer progression. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-15475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhou Y., Ren H., Dai B., Li J., Shang L., Huang J., et al. Hepatocellular carcinoma-derived exosomal miRNA-21 contributes to tumor progression by converting hepatocyte stellate cells to cancer-associated fibroblasts. J. Exp. Clin. Cancer Res. 2018;37:324. doi: 10.1186/s13046-018-0965-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Li S., Qi Y., Huang Y., Guo Y., Huang T., Jia L. Exosome-derived SNHG16 sponging miR-4500 activates HUVEC angiogenesis by targeting GALNT1 via PI3K/Akt/mTOR pathway in hepatocellular carcinoma. J. Physiol. Biochem. 2021;77:667–682. doi: 10.1007/s13105-021-00833-w. [DOI] [PubMed] [Google Scholar]
- 186.Yang H., Zhang H., Ge S., Ning T., Bai M., Li J., et al. Exosome-derived miR-130a activates angiogenesis in gastric cancer by targeting C-MYB in vascular endothelial cells. Mol. Ther. 2018;26:2466–2475. doi: 10.1016/j.ymthe.2018.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 187.Li S., Li J., Zhang H., Zhang Y., Wang X., Yang H., et al. Gastric cancer derived exosomes mediate the delivery of circRNA to promote angiogenesis by targeting miR-29a/VEGF axis in endothelial cells. Biochem. Biophys. Res. Commun. 2021;560:37–44. doi: 10.1016/j.bbrc.2021.04.099. [DOI] [PubMed] [Google Scholar]
- 188.Shang D., Xie C., Hu J., Tan J., Yuan Y., Liu Z., et al. Pancreatic cancer cell-derived exosomal microRNA-27a promotes angiogenesis of human microvascular endothelial cells in pancreatic cancer via BTG2. J. Cell Mol. Med. 2020;24:588–604. doi: 10.1111/jcmm.14766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yu Q., Zhou X., Xia Q., Shen J., Yan J., Zhu J., et al. Long non-coding RNA CCAT1 that can be activated by c-Myc promotes pancreatic cancer cell proliferation and migration. Am J Transl Res. 2016;8:5444–5454. [PMC free article] [PubMed] [Google Scholar]
- 190.Jiang L., Cao H., Deng T., Yang M., Meng T., Yang H., et al. Serum exosomal miR-377-3p inhibits retinal pigment epithelium proliferation and offers a biomarker for diabetic macular edema. J. Int. Med. Res. 2021;49 doi: 10.1177/03000605211002975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cao X., Xue L.D., Di Y., Li T., Tian Y.J., Song Y. MSC-derived exosomal lncRNA SNHG7 suppresses endothelial-mesenchymal transition and tube formation in diabetic retinopathy via miR-34a-5p/XBP1 axis. Life Sci. 2021:272. doi: 10.1016/j.lfs.2021.119232. [DOI] [PubMed] [Google Scholar]
- 192.Elbay A., Ercan, Akbas F., Bulut H., Ozdemir H. Three new circulating microRNAs may be associated with wet age-related macular degeneration. Scand. J. Clin. Lab. Invest. 2019;79:388–394. doi: 10.1080/00365513.2019.1637931. [DOI] [PubMed] [Google Scholar]
- 193.Zheng B., Yin W.N., Suzuki T., Zhang X.H., Zhang Y., Song L.L., et al. Exosome-mediated miR-155 transfer from smooth muscle cells to endothelial cells induces endothelial injury and promotes atherosclerosis. Mol. Ther. 2017;25:1279–1294. doi: 10.1016/j.ymthe.2017.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 194.Chen Z., Wang H., Xia Y., Yan F., Lu Y. Therapeutic potential of mesenchymal cell-derived miRNA-150-5p-expressing exosomes in rheumatoid arthritis mediated by the modulation of MMP14 and VEGF. J. Immunol. 2018;201:2472–2482. doi: 10.4049/jimmunol.1800304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhang J., Zhang Y., Ma Y., Luo L., Chu M., Zhang Z. Therapeutic potential of exosomal circRNA derived from synovial mesenchymal cells via targeting circEDIL3/miR-485-3p/PIAS3/STAT3/VEGF functional module in rheumatoid arthritis. Int. J. Nanomed. 2021;16:7977–7994. doi: 10.2147/IJN.S333465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Guo C., Liu J., Zhou Q., Song J., Zhang Z., Li Z., et al. Exosomal noncoding RNAs and tumor drug resistance. Cancer Res. 2020;80:4307–4313. doi: 10.1158/0008-5472.CAN-20-0032. [DOI] [PubMed] [Google Scholar]
- 197.Cheng J., Meng J., Zhu L., Peng Y. Exosomal noncoding RNAs in Glioma: biological functions and potential clinical applications. Mol. Cancer. 2020;19:66. doi: 10.1186/s12943-020-01189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Garzon R., Marcucci G., Croce C.M. Targeting microRNAs in cancer: rationale, strategies and challenges. Nat. Rev. Drug Discov. 2010;9:775–789. doi: 10.1038/nrd3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tong Y.S., Wang X.W., Zhou X.L., Liu Z.H., Yang T.X., Shi W.H., et al. Identification of the long non-coding RNA POU3F3 in plasma as a novel biomarker for diagnosis of esophageal squamous cell carcinoma. Mol. Cancer. 2015;14:3. doi: 10.1186/1476-4598-14-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ren S., Wang F., Shen J., Sun Y., Xu W., Lu J., et al. Long non-coding RNA metastasis associated in lung adenocarcinoma transcript 1 derived miniRNA as a novel plasma-based biomarker for diagnosing prostate cancer. Eur. J. Cancer. 2013;49:2949–2959. doi: 10.1016/j.ejca.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 201.Li J., Li Z., Jiang P., Peng M., Zhang X., Chen K., et al. Circular RNA IARS (circ-IARS) secreted by pancreatic cancer cells and located within exosomes regulates endothelial monolayer permeability to promote tumor metastasis. J. Exp. Clin. Cancer Res. 2018;37:177. doi: 10.1186/s13046-018-0822-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Selmaj I., Cichalewska M., Namiecinska M., Galazka G., Horzelski W., Selmaj K.W., et al. Global exosome transcriptome profiling reveals biomarkers for multiple sclerosis. Ann. Neurol. 2017;81:703–717. doi: 10.1002/ana.24931. [DOI] [PubMed] [Google Scholar]
- 203.Tan M., Yan H.B., Li J.N., Li W.K., Fu Y.Y., Chen W., et al. Thrombin stimulated platelet-derived exosomes inhibit platelet-derived growth factor receptor-beta expression in vascular smooth muscle cells. Cell. Physiol. Biochem. 2016;38:2348–2365. doi: 10.1159/000445588. [DOI] [PubMed] [Google Scholar]
- 204.Wang W., Li Z., Feng J. The potential role of exosomes in the diagnosis and therapy of ischemic diseases. Cytotherapy. 2018;20:1204–1219. doi: 10.1016/j.jcyt.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 205.Li B., Ren S., Li X., Wang Y., Garfield D., Zhou S., et al. MiR-21 overexpression is associated with acquired resistance of EGFR-TKI in non-small cell lung cancer. Lung Cancer. 2014;83:146–153. doi: 10.1016/j.lungcan.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 206.Ma Y., Xia H., Liu Y., Li M. Silencing miR-21 sensitizes non-small cell lung cancer A549 cells to ionizing radiation through inhibition of PI3K/Akt. BioMed Res. Int. 2014;2014 doi: 10.1155/2014/617868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zhang J.H., Wei H.W., Yang H.G. Long noncoding RNA SNHG15, a potential prognostic biomarker for hepatocellular carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2016;20:1720–1724. [PubMed] [Google Scholar]
- 208.van Rooij E., Marshall W.S., Olson E.N. Toward microRNA-based therapeutics for heart disease: the sense in antisense. Circ. Res. 2008;103:919–928. doi: 10.1161/CIRCRESAHA.108.183426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hu S., Huang M., Li Z., Jia F., Ghosh Z., Lijkwan M.A., et al. MicroRNA-210 as a novel therapy for treatment of ischemic heart disease. Circulation. 2010;122:S124–S131. doi: 10.1161/CIRCULATIONAHA.109.928424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Costa P.M., Cardoso A.L., Custódia C., Cunha P., Pereira de Almeida L., Pedroso de Lima M.C. MiRNA-21 silencing mediated by tumor-targeted nanoparticles combined with sunitinib: a new multimodal gene therapy approach for glioblastoma. J. Contr. Release. 2015;207:31–39. doi: 10.1016/j.jconrel.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 211.He C., Zheng S., Luo Y., Wang B. Exosome theranostics: biology and translational medicine. Theranostics. 2018;8:237–255. doi: 10.7150/thno.21945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Dong X., Lei Y., Yu Z., Wang T., Liu Y., Han G., et al. Exosome-mediated delivery of an anti-angiogenic peptide inhibits pathological retinal angiogenesis. Theranostics. 2021;11:5107–5126. doi: 10.7150/thno.54755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Morse M.A., Garst J., Osada T., Khan S., Hobeika A., Clay T.M., et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J. Transl. Med. 2005;3:9. doi: 10.1186/1479-5876-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Dai S., Wei D., Wu Z., Zhou X., Wei X., Huang H., et al. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol. Ther. 2008;16:782–790. doi: 10.1038/mt.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Peng X.X., Yu R., Wu X., Wu S.Y., Pi C., Chen Z.H., et al. Correlation of plasma exosomal microRNAs with the efficacy of immunotherapy in EGFR/ALK wild-type advanced non-small cell lung cancer. J Immunother. Cancer. 2020;8 doi: 10.1136/jitc-2019-000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Nunez Lopez Y.O., Casu A., Kovacova Z., Petrilli A.M., Sideleva O., Tharp W.G., et al. Coordinated regulation of gene expression and microRNA changes in adipose tissue and circulating extracellular vesicles in response to pioglitazone treatment in humans with type 2 diabetes. Front. Endocrinol. (Lausanne) 2022;13 doi: 10.3389/fendo.2022.955593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Anderson J.D., Johansson H.J., Graham C.S., Vesterlund M., Pham M.T., Bramlett C.S., et al. Comprehensive proteomic analysis of mesenchymal stem cell exosomes reveals modulation of angiogenesis via nuclear factor-KappaB signaling. Stem Cell. 2016;34:601–613. doi: 10.1002/stem.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Nikfarjam S., Rezaie J., Zolbanin N.M., Jafari R. Mesenchymal stem cell derived-exosomes: a modern approach in translational medicine. J. Transl. Med. 2020;18:449. doi: 10.1186/s12967-020-02622-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Joo H.S., Suh J.H., Lee H.J., Bang E.S., Lee J.M. Current knowledge and future perspectives on mesenchymal stem cell-derived exosomes as a new therapeutic agent. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21030727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Yue X., Lan F., Xia T. Hypoxic glioma cell-secreted exosomal miR-301a activates Wnt/β-catenin signaling and promotes radiation resistance by targeting TCEAL7. Mol. Ther. 2019;27:1939–1949. doi: 10.1016/j.ymthe.2019.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Jeppesen D.K., Fenix A.M., Franklin J.L., Higginbotham J.N., Zhang Q., Zimmerman L.J., et al. Reassessment of exosome composition. Cell. 2019;177:428. doi: 10.1016/j.cell.2019.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Thery C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018;7 doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Li P., Kaslan M., Lee S.H., Yao J., Gao Z.Q. Progress in exosome isolation techniques. Theranostics. 2017;7:789–804. doi: 10.7150/thno.18133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ibsen S.D., Wright J., Lewis J.M., Kim S., Ko S.Y., Ong J., et al. Rapid isolation and detection of exosomes and associated biomarkers from plasma. ACS Nano. 2017;11:6641–6651. doi: 10.1021/acsnano.7b00549. [DOI] [PubMed] [Google Scholar]
- 225.Yáñez-Mó M., Siljander P.R., Andreu Z., Zavec A.B., Borràs F.E., Buzas E.I., et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles. 2015;4 doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Rezaie J., Feghhi M., Etemadi T. A review on exosomes application in clinical trials: perspective, questions, and challenges. Cell Commun. Signal. 2022;20:145. doi: 10.1186/s12964-022-00959-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Mathieu M., Martin-Jaular L., Lavieu G., Thery C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019;21:9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 228.Thery C. Exosome EXPLOSION! Scientist. 2011;25:36–40. [Google Scholar]
- 229.Wei H., Chen Q., Lin L., Sha C., Li T., Liu Y., et al. Regulation of exosome production and cargo sorting. Int. J. Biol. Sci. 2021;17:163–177. doi: 10.7150/ijbs.53671. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.